Abstract
Human immunodeficiency virus TAT plays an important role in the dysregulation of cytokine production associated with the neurological disorders that follow HIV infection. IL-1β is one of the important inflammatory cytokines secreted by immune-activated monocytes/macrophages. Previous reports have shown that extracellular TAT stimulates IL-1β expression in monocytes/macrophages. However, little is known about the mechanisms and possible TAT-responsive elements within the IL-1β promoter. The present study shows that TAT increases the production of IL-1β in human monocytes; PLC-PKC pathway-dependent phosphorylation of p44/42 and JNK MAP kinases participates partially in IL-1β induction by TAT; specific C/EBP and NF-kB transcription factor binding elements within the IL-1β promoter are involved in TAT regulation of IL-1β production. This study identifies a signaling mechanism for HIV-1-induced IL-1β production in human monocytes that may be involved in the neuropathogenesis of HIV-associated dementia.
Keywords: HIV, TAT, Interleukin 1β, p44/42, JNK, NF-κB, phospholipase C, protein kinase C
Introduction
HIV-associated dementia is a neurological disorder that is estimated to develop in 20% of advanced AIDS cases, despite the availability of highly active antiretroviral treatment (HAART) (Aquaro et al., 2008, Wiley et al. 1991, Price et al., 1988). HIV-associated dementia is characterized by the infiltration of peripheral monocytes/macrophages into the central nervous system (CNS), the formation of multinucleated giant cells and neuronal death (Gray et al, 2001). The pathogenesis of HIV-associated dementia is complex and not fully understood. Neuron death and neuronal damage within the brains of AIDS patients with dementia are not caused by direct infection of neurons by HIV-1, but are primarily consequences of the cumulative toxic effects of soluble factors. A good portion of these soluble factors, including virus proteins, excess chemokines or pro-inflammatory cytokines, are produced by activated monocytes and infiltrated monocytes/macrophages (Gallo et al, 1989; Nath, 2002; Nuovo et al, 1996; Tyor et al, 1992).
One key pro-inflammatory cytokine that is believed to drive the neuroinflammatory process is Interleukin-1β (IL-1β), which is up-regulated in Alzheimer's disease, Parkinson's disease, multiple sclerosis and other neurodegenerative disorders (Griffin et al, 1995; Griffin et al, 2006; Rothwell et al, 2000). IL-1β signaling through the Interleukin-1 receptor leads to the transcription of pro-inflammatory cytokines Interferon-γ (IFN-γ), Tumor necrosis factor-α (TNF-α), Interleukin-6 (IL-6), neutrophil recruiting chemokines CXCL1 and CXCL2, and increases glia activity, blood-brain-barrier permeability, and leukocyte invasion, all of which are common events following brain injury (Allan et al, 2005; Ambrosino et al 1991; Moynagh, 2005). Elevated levels of IL-1β have been found in the sera and peripheral blood monocytes/macrophages of patients infected with HIV-1, and in the cerebrospinal fluid (CSF) of HIV-associated dementia patients. The levels of cytokine production correlate with the severity of neurologic symptoms (Brabers et al, 2006; Gallo et al, 1989; Merrill et al, 1989; Nuovo et al, 1996; Tyor et al, 1992).Enhanced IL-1β production during HIV infection has been attributed partially to HIV proteins, including the HIV accessory protein, TAT, in a number of cell types such as T cells and monocytes/macrophages (Brabers et al, 2006; Nath et al, 1999; Pu et al, 2003).
The HIV-1-TAT protein, which is well known for its intracellular transactivating activity and is essential for efficient HIV-1 viral replication, can be actively released from HIV-infected T cells and monocytes/macrophages (Ensoli et al, 1993; Westendorp et al, 1995). Injection of this extracellular form of TAT leads to macrophage infiltration and neuronal death, neurological symptoms that are observed in the brains of HIV-associated dementia patients (Jones et al, 1998). TAT may exert its neurotoxic effects directly on neurons, which eventually lead to apoptosis of neurons (Kinga, 2006; Kruman et al, 1998). TAT also may interact with certain cell receptors on a variety of cell types, such as chemokine receptors, the vascular endothelial growth factor (VEGF) receptor, and the beta-integrin receptor (Albini, 1998; Albini, 1996; Kinga, 2006). TAT ligation of these specific surface molecules activates several cellular protein kinases, such as focal adhesion kinase (FAK) associated phosphoinositide-3 Kinase (PI3K), mitogen activated protein kinases (MAPKs), and protein kinase C (PKC) (Albini et al, 1996; Ganju et al, 1998; Zidovetzki et al, 1998), which may stimulate excessive production of neurotoxic mediators from monocytes/macrophages and other cells, triggering neurodegenerative processes and damage in brain.
The regulation of IL-1β synthesis occurs at both the transcriptional and posttranscriptional level (Cogswell et al, 1994; Zhao et al, 2001; Zhang et al, 1993). Depending on the cell type and stimuli used, different transcription factors play a role in IL-1β induction. For example, in monocytes, nuclear factor-kB (NF-κB), NF-IL6/CCAAT enhancer-binding protein (C/EBP), or cAMP response element-binding proteins (CREB) are required for the induction of IL-1β transcription in response to variety of stimuli (Cogswell et al, 1994; Zhang et al, 1993). These transcription factors may also be activated by extracellular TAT in variety of cells following the interaction of TAT with various cell surface receptors. Although there are reports that have shown that extracellular TAT induces IL-1β production in human monocytes/macrophages and revealed the important role of IL-1β and monocytes/macrophages in neuronal injury and HIV-associated dementia pathogenesis (Brabers et al, 2006; Kinga et al, 2006; Nath et al, 1999; Zhao et al, 2001), the molecular mechanism by which TAT induces IL-1β release from monocytes/macrophages is still poorly understood. The present study was conducted to examine the effects of extracellular TAT on IL-1β expression in human monocytic cells. The molecular mechanism involved suggested that TAT induced IL-1β production through the PLC-PKC cascade-dependent p42 / p44 and c-Jun N-terminal kinase (JNK) MAP kinase activation.
Material and Methods
Recombinant TAT and reagents
The recombinant HIV-1 TAT proteins, TAT72, derived from the first exon of TAT, was a gift from Dr. Avindra Nath (Johns Hopkins Medical Center, Baltimore, MD) and HIV-1 TAT86, a C-terminal truncated 86 amino acids form of TAT, was obtained from the National Institutes of Health AIDS Reference and Reagent Program. Unless otherwise specified, the TAT protein used in these experiments refers to TAT72. Inhibitors SP600125, SB203580, GF109203 were purchased from Biomol (Plymouth Meeting, PA). U0126, U73122, and LY294002 were from Cayman (Ann Arbor, MI).
Monocyte isolation and cell culture
Peripheral blood mononuclear cells (PBMCs) were isolated from venous blood drawn from healthy donors. PBMCs were isolated by Ficoll-Paque density gradient. Isolated PBMCs were purified using anti-CD14 microbeads and a magnetic cell separation system (Miltenyi Biotec, Bergisch Gladbach, Germany), according to the instruction manual. The human primary monocyte and monocytic cell line, THP-1, was grown in RPMI-1640 supplemented with 10% fetal bovine serum, 2 mM L-glutamine, and streptomycin (100 µg /ml) and penicillin (100IU/ml).
Plasmid constructs and PCR mutagenesis
Plasmid pGL3-Basic (Promega, Madison, WI) was used to generate serial constructs of the human IL-1β promoter. The Forward primers with restriction sites used to amplify different IL-1β promoter fragments from genomic DNA are: 5-TCT CAC GCG TCT CTG TTT GTG GTC CCC TCT C-3 (pIL1β-4016, −4016 to +38), 5-CTC CAC GCG TTA CCG TAT GTT CTC TGC CCC A-3 (pIL1β-606, −606 to +38), 5-CTC TAC GCG TCC ACT TTG TCC CAC ATA TAC T-3 (pIL1β-360, −360 to +38), 5-CTC TAC GCG TTG TCA TAG TTT GCT ACT CCT T-3 (pIL1β-225, −225 to +38), 5-CTC TAC GCG TCT AAG AAG CTT CCA CCA ATA C-3 (pIL1β-155, −155 to +38), 5-CTC TAC GCG TAG AAA TTT CTC AGC CTC CTAC-3 (pIL1β-88, −88 to +38). The reverse primer was 5' TCT CAG ATC TAG CAG CCT GTT GTG CCT TGTG-3'. The PCR products were digested with MluI and BglII and cloned into pGL3-Basic. Site-directed mutations were introduced by mutated primers using the Phusion™ Site-Directed Mutagenesis Kit (NEB, Beverly, MA), according to the manufacturer’s instructions. The primer sequences, with the mutations underlined, are: NFκB 286/296mt, 5-TCC ACA TTA AAA TAC GTT TAA TTA ACA CGT TAG AAG AATG-3, 5-CAT CAA CTG CAC AAC GAT TGT CAG GAA AAC AA-3; NFκB 403/413mt, 5-TTG TAC TTA AGT TTA TTG CTA GCA CAC TTA CAG ATG GAT-3, 5-AGA AGT GAA TGA AGA AAA GTA TGT GCA TGT ATA-3; CEBP 32/40mt, 5-GTT TTT ATG GCT TTT AAG GGC AGA AGT AGG AGG-3, 5-AGC GAG GGA GAA ACT GGC AGA TAC CAA ACC-3; CEBP 82/90mt, 5-TGT TGA ATA CCT GCT TTT ACG GTC AAG TTA AAG GAA-3, 5-GAG AAA TTT CTC AGC CTC CTA CTT CTG CTT TT-3; plasmids were isolated and sequencing was carried out to identify those that contained the mutated sequences of interest.
THP-1 cell electroporation and Luciferase assays
THP-1 cells (2 × 107 cells) were washed with cold PBS, resuspended in serum-free medium and electroporated at 350 V and 950 µF (Gene Pulser II Electroporation System, Bio-Rad, Hercules, CA) with or without 20 µg of the IL-1β luciferase reporter and 2 µg of the internal control plasmid pRL-TK. Cells were washed and resuspended in complete media and cultivated for 24 h. Then, each transfection was divided into two equal portions that were incubated at 37 °C for another 12 hours in serum-free medium, in the presence or absence of TAT protein. Luciferase activity was measured with a Dual luciferase assay kit (Promega, Madison, Wis.) in a Turner luminometer-96 (Turner Designs, Sunnyvale, Calif.). The values obtained were normalized to the Renilla luciferase levels.
ELISA quantitation of cytokines
Cells plated in 24-well plates (2 × 105 cells/well) were incubated overnight in serum-free medium, exposed to 100 ng TAT for 12 h (unless stated otherwise), and IL-1β levels in cell culture medium were determined by ELISA (Ebioscience, San Diego, CA), according in the manufacturer’s instructions. For blocking studies, inhibitors, or control vehicles (0.1% DMSO), were added 2 h prior and maintained throughout the period of stimulation.
RNA Isolation and RT-PCR
Total RNA was extracted using the RNeasy Plus mini kit (Qiagen, Valencia, CA) and transcribed. The cDNA was used as a template in RT-PCR with IL-1β-specific primers: forward, 5-TTG AAG CTG ATG GCC CTA-3; reverse, 5-TGC TCA GGT CAT TCT CCT-3, the GAPDH primers: forward, 5-ACC ACA GTC CAT GCC ATC AC-3; reverse, 5-TCC ACC ACC CTG TTG CTG TA-3 were included as an internal control.
Protein extraction and Western blot analyses
The p38, p44/42, JNK antibodies and phosphorylated p44/42, p38, and JNK antibodies were from Cell Signaling (Beverly, MA). Protein was fractionated in a 10% SDS-PAGE and transferred to nitrocellulose membranes. Membranes were blocked for 1 hour with 5% BSA in TBST, blotted overnight with appropriate primary antibodies, 1 hour with the HRP-conjugated-secondary antibody, washed, visualized using SuperSignal West Pico Substrate (Pierce, Rockford, IL) and recorded by Chemi Doc XRS imaging system (Bio-Rad, Hercules, CA).
Nonradioactive electrophoretic mobility shift assays
The oligodeoxynucleotide sequences coding for the NF-kB, C/EBP cis-element within the IL-1β promoter used for the nonradioactive EMSA assay are shown with the mutations underlined: wt NFκB-286, 5-CTA ACG TGG GAA AAT CCA GGA AAA TCC-3; mutant NFκB b-286, 5-CTA ACG TAC TAA AAT CCA GGA AAA TCC-3; wt nfkb-413, 5-TGT AAG TGG GAA GAT TCC TAA ACT TAA-3; mutant NFκB -413, 5-TGT AAG TAC TAA GAT TAA TAA ACT TAA-3; wtc/ebp-82, 5-AAC TTG ATT GTG AAA TCA GGT ATT CA-3; mutant c/ebp-82, 5-AAC TTG ACC GTA AAA GCA GGT ATT CA-3; wtc/ebp-32, 5-CTT CTG CTT TTG AAA GCC ATA AAA AC-3; mutant c/ebp-32, 5-CTT CTG CCC TTA ACA GCC ATA AAA AC-3. Digoxigenin labeling of the oligodeoxynucleotides, DNA–protein binding reactions and electrophoretic mobility shift assays were performed as described by the manufacturer’s instructions (Roche, Indianapolis, IN).
Statistical analysis
Quantitative data are presented as the mean±S.D. of three independent experiments. Multiple group comparisons were analyzed by one-way ANOVA. For all tests, p<0.05 was considered statistically significant.
Results
HIV-1 TAT induced IL-1β production in human monocytes
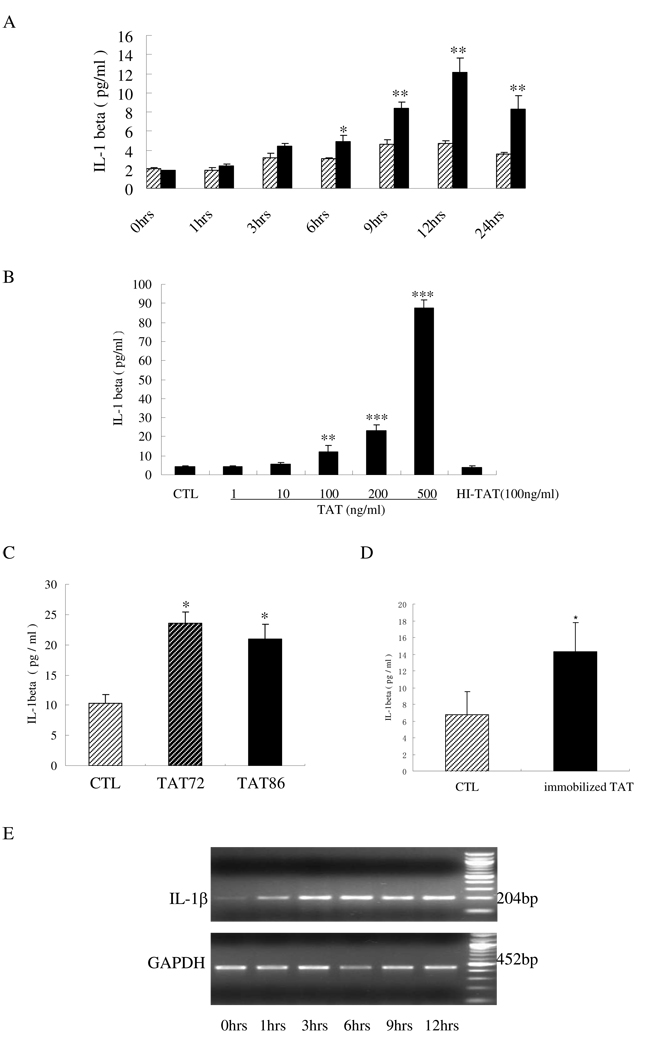
The effects of soluble TAT on IL-1β production by human monocytes were examined by ELISA and RT-PCR. Human primary monocytes were treated with HIV TAT at a concentration of 100 ng/ml for different amounts of time, reaching up to 24 hours. TAT induced an elevation of IL-1β production in a time-dependent manner and peaked with ~2.57-fold increase after 12 hours of stimulation (Fig.1A). RT-PCR results showed that IL-1β mRNA in human monocytes with TAT (100 ng/ml) stimulation reached the highest level within 3 hours, and stabilized for at least 12 hours (Fig.1E). The efficiency by which TAT enhances IL-1β production by human monocytes was also tested in this study. The stimulation of human monocytes by increasing concentrations of TAT clearly increased IL-1β production in a dose-dependent manner, after 12 hours of stimulation. The fold changes were ~1.30-fold, ~2.9-fold, ~5.54-fold and ~19.1-fold, when TAT was used at concentrations of 10 ng/ml, 100 ng/ml, 200 ng/ml and 500 ng/ml, respectively. The concentration of 100 ng/ml was expected to be comparable with TAT levels in peripheral blood because an earlier study using dot-blot analysis demonstrated that the concentration of HIV-1 TAT in sera from HIV-positive individuals was at levels up to 10 nM (Xiao et al., 2000). Therefore, the concentration of 100 ng/ml TAT (~14 nM) was used in all subsequent experiments. These results indicated that soluble TAT was able to induce IL-1β production in monocytes and that a change at the mRNA level was involved in TAT regulation of IL-1β expression in monocytes.
Fig 1.
HIV TAT-induced IL-1β production in human monocytes. A) Monocytes were isolated and cultured in 24-well plates (2 × 105 cells/well) without (slash bars) or with (black bars) TAT addition (100 ng/ml) for the indicated times; B) human primary monocytes (2 × 105 cells) were stimulated with the indicated concentrations of TAT (1–500 ng/ml), or heat-inactivated TAT (HI-TAT) at 100 ng/ml, or without TAT (CTL) for 12 hours; C) THP-1 cells cultured in 24-well plates (2 × 105 cells) were treated without TAT (CTL), with TAT72 (100 ng/ml), or with TAT86 (100 ng/ml) for 12 hours; D) TAT72 (200 ng) was immobilized in wells by a 2-hour 37°C incubation. After 3 washes with culture media, THP-1 cells (2 × 105 cells) were added and cultured with immobilized TAT, or without TAT (CTL) for an additional 12 hours. Cell culture supernatants were collected and the levels of IL-1β production in all tests were quantified by ELISA. The values are shown as the means+SD of three experiments. Differences between treated cells and normal control cells were considered significant at P < 0.05. *, p<0.05; **, p<0.01; or ***, p<0.001. E) Primary monocytes (2 × 105 cells) were treated with 100 ng/ml TAT for the indicated times (0–12 hours) followed by the measurement of IL-1β and GAPDH mRNA by RT-PCR.
The induction of IL-1β was also confirmed in the THP-1 monocytic cell line. A ~2.31-fold increase in IL-1β production was obtained when THP-1 cells were stimulated with 100 ng/ml TAT for 12 hours. Another recombinant 86 amino acid form of HIV TAT, TAT86, was tested and resulted in a ~2.1-fold change, when used at a concentration of 100 ng/ml. Pre-immobilization of TAT in culture wells resulted in IL-1β elevation in THP-1 monocytes. Though this increase is smaller, it is still significant compared to that observed with the soluble TAT (Fig. 1C). Furthermore, after heat-inactivation at 100°C for 30 minutes and addition onto the monocytes for 12 hours (Fig.1B), TAT completely lost the capacity to induce IL-1β production, demonstrating that this induction was not due to heat-stable components, such as endotoxin contamination.
P44/42 and JNK kinases of MAP kinase, but not p38 and PI3K, were involved in the regulation of IL-1β expression by TAT with human monocytes
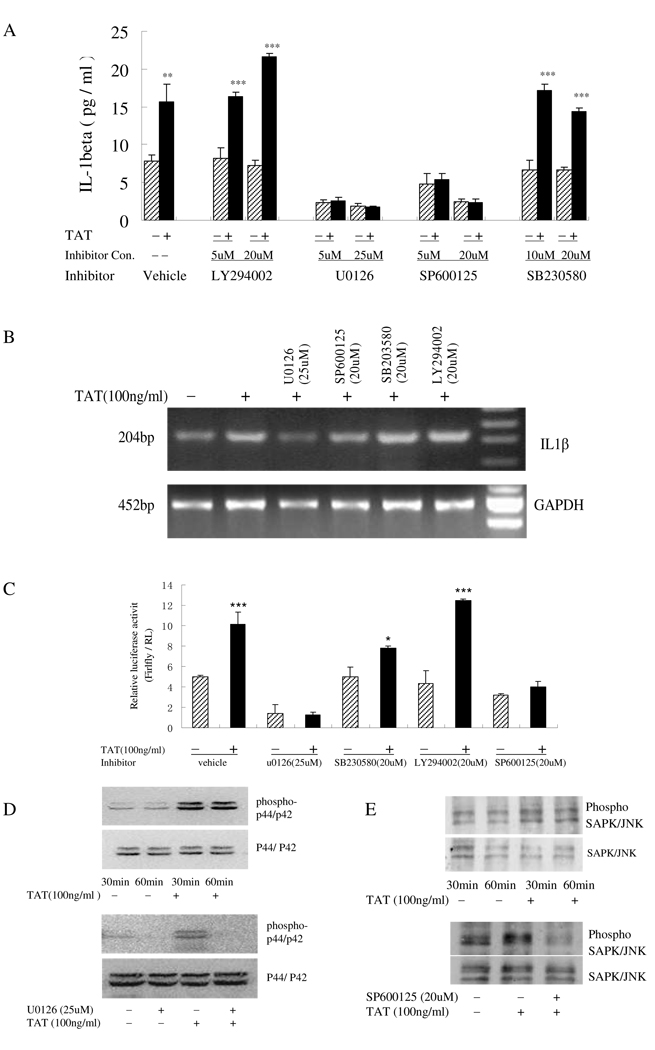
Past studies have demonstrated that mitogen-activated protein kinases (MAPKs) and phosphoinositide 3-kinase (PI3K) were involved in the IL-1β regulation in response to multiple stimuli (Kim et al., 2004). The roles of c-jun N-terminal kinase (JNK), p38, p44/42 MAPKs and PI3K, with regards to the regulation of TAT-induced IL-1β production in monocytes, were examined using a pharmacological approach. Monocytes were pretreated with either the JNK inhibitor SP600125, the P38 inhibitor SB203580, the p44/42 inhibitor U0126 or the PI3-K Inhibitor LY294002, followed by stimulation with TAT for 12 hours. Supernatant from treated cells was collected and analyzed for IL-1β content by ELISA. As shown in Fig. 2A, at lower and higher concentrations, the p38 inhibitor SB203580 (10 µM or 20 µM) and PI3k inhibitor LY294002 (5 µM or 20 µM) had no effect on the increase in IL-1β expression. Elevation of IL-1β production was reduced following the dose increase of JNK inhibitor SP600125 from 5 µM to 20 µM, thus underscoring the possible role of JNK in the TAT-induced IL-1β production in monocytes. However, in the presence of various concentrations of the p44/42 kinase inhibitor U0126 (5 µM or 25 µM), TAT-induced IL-1β production was decreased by 80–90%, a similar range as that observed in monocytes with only inhibitor treatment, which had ~20–40% basal-level expression. These data indicated that the activity of MAPK family member p44/42 might regulate both basal and TAT-induced IL-1β expression.
Fig 2.
Activation of p44/42 and JNK MAP kinase pathways involved in TAT-induced IL-1β release by human monocytes. A) Primary human monocytes (2 × 105 cells) pretreated with U0126 (5 µM and 25 µM), SB230580 (10 µM and 20 µM), SP600125 (5 µM and 20 µM), LY294002 (5 µM and 20 µM) for 2 h were stimulat with (+) or without (−) 100 ng/ml HIV TAT for an additional 12 hours. IL-1β in cell supernatants was analyzed by ELISA. B) RT-PCR for IL-1β and GAPDH was performed on total RNA from monocytes after a 3-hour treatment with 100 ng/ml TAT following a 2-hour pretreatment with U0126 (25 µM), SB230580 (20 µM), SP600125 (20 µM) or LY294002 (20 µM). GAPDH was included as a loading control. C) THP-1 cells were transfected with the luciferase reporter constructs containing −4016 bp to +38 bp of the IL-1β promoter with an internal control Renilla luciferase reporter, RL-TK, and cultured with (+) or without (−) 100 ng/ml TAT for another 12 hours after a 2-hour inhibitor treatment. Cells were then assayed for luciferase activity. All values of the ELISA results are the means+SD of three experiments. Differences between TAT-treated cells and normal control cells were considered significant at P < 0.05. *, p<0.05; **, p<0.01; or ***, p<0.001.D and E) Cell lysate from monocytes treated with (+) or without (−) TAT (100 ng/ml) for 30 minutes and 60 minutes (up panel) or treated with TAT (100 ng/ml) for 1 hour with (+) or without (−) inhibitors U0126 (25 µM) or SP600125 (20 µM) (lower panel) were prepared and subjected to Western blotting using phospho-JNK or phospho-p44/p4 Abs. Blots were re-probed with Abs to total p42/44 or JNK as loading control. Blots shown are representative of three separate experiments.
IL-1β mRNA regulation by TAT in response to MAPKs and PI3K inhibitors was determined by semi-quantitative RT-PCR. The IL-1β mRNA levels observed in TAT-treated monocytes were decreased following the use of JNK inhibitor SP600125 (20 µM) or p44/42 inhibitor U0126 (25 µM), suggesting that these inhibitors are implicated in regulation at the mRNA level. The p38 inhibitor SB230580 and PI3K inhibitor LY294002 at concentrations of 20 µM did not affect IL-1β mRNA abundance (Fig. 2B).
Whether or not IL-1β transcription can be regulated by TAT, and whether or not JNK, p38, p44/42 and PI3k kinases are involved in regulation, were verified by dual-luciferase reporter assays utilizing the IL-1β promoter in THP-1 cells. The transcriptional activity of the 4kb IL-1β promoter increased by ~1.95-fold after 12 hours of TAT treatment. As seen, there was no reduction in the activity of the IL-1β promoter in the presence of the PI3k or p38 inhibitor, as compared to that without inhibitors. Pre-treatment with JNK inhibitor SP600125 clearly decreased the TAT-induced, but not the basal transcriptional activity of IL-1β promoter. By contrast, in the presence of the p44/42 kinase inhibitor U0126, the TAT-induced IL-1β promoter transcription activity was markedly reduced below the basal level, which is consistent with the results from the direct IL-1β production assay (Fig. 2C).
Whether JNK or p44/42 was actually activated by TAT and whether this activation was inhibited by JNK or p44/42 inhibitor were confirmed by analysis of JNK and p44/42 phosphorylation by western blot after monocytes were treated with TAT alone, the inhibitor alone, or the inhibitor and TAT. Both p44/42 and JNK exhibited basal-level phosphorylation in the absence of TAT, which was increased upon TAT treatment and sustained for at least 1 hour (Fig. 2D and 2E). Treatment of cells with p44/42 inhibitor U0126 (25 µM) prior to TAT exposure inhibited basal and TAT-induced p44/42 phosphorylation (Fig. 2D). JNK inhibitor SP600125, which inhibits JNK phosphorylation of c-Jun, and to a lesser degree inhibits MAPK kinase 4 (MKK4) phosphorylation of JNK (Bennett et al, 2001), had a modest reduction of JNK phosphorylation when used at a concentration of 20 µM (Fig. 2E). Taken together, all the above data suggests that the regulation of IL-1β expression by TAT starts from the transcriptional level and is regulated at least in part by MAPK family members JNK and p44/42, but not p38 and PI3K kinase.
P44/42 and JNK MAP kinases acted downstream of phospholipase C-protein kinase C and protein kinase C in TAT-induced IL-1β production in monocytes
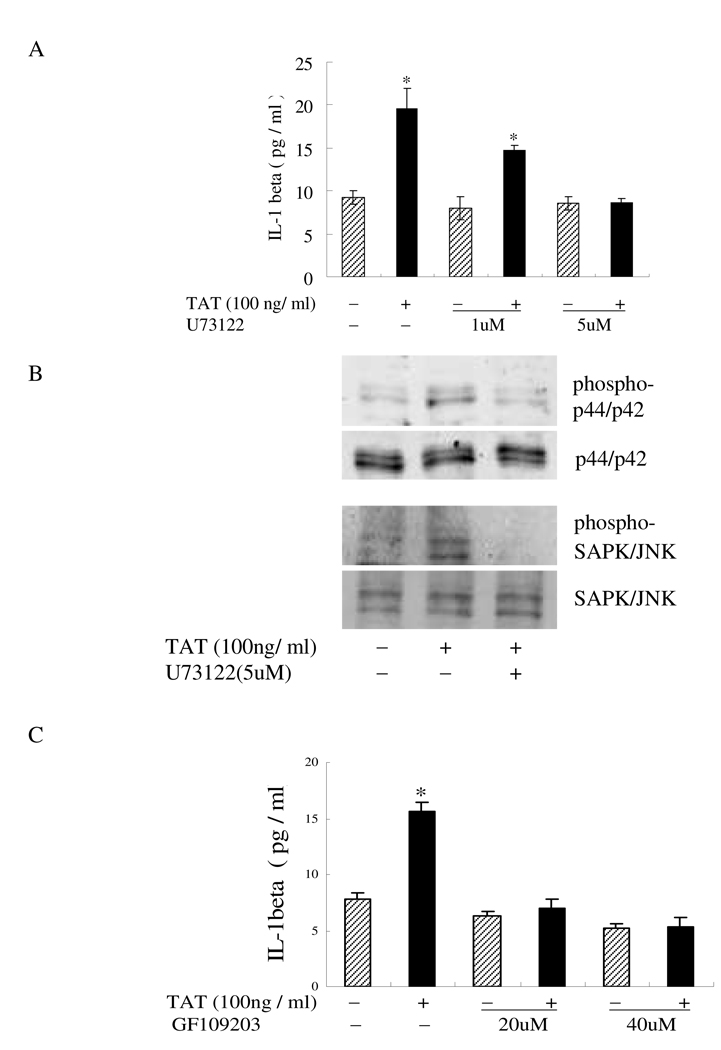
The MAPK pathway has been shown to be activated by the phospholipase C (PLC) and protein kinase C (PKC) pathway. Since the above results showed that both the p44/42 and JNK MAPK regulated TAT-induced IL-1β production, TAT may activate these two MAP kinases through the activation of PLC/PKC signaling molecules. To confirm this hypothesis, IL-1β production was measured after the monocytes were treated by TAT for 12 hours, following pretreatment with PLC inhibitor U73122 (5 µM) or PKC inhibitor GF109203 (20 µM). The induction of IL-1β by TAT was potently inhibited by both U73122 (Fig. 3A) and GF109203 (Fig. 3C) in a dose-dependent manner, which indicated that PLC and PKC mediated the TAT-induced IL-1β increase in monocytes. To determine whether PLC/PKC activates the MAPK pathways in TAT-treated monocytes, human monocytes were pretreated with the PLC inhibitor U73122 or PKC inhibitor GF109203 for 30 minutes before a 1-hour stimulation with HIV-TAT. The following analysis of p44/42 or JNK phosphorylation was confirmed by western blot. Pretreatment of cells with U73122 and GF109203 inhibited TAT-induced p44/42 (Fig. 3B) or JNK phosphorylation (Fig. 3D). These results indicated that p44/42 and JNK kinases might act as downstream targets of PLC and PKC in regulating IL-1β induction by TAT. Overall, these results suggest that the PLC/PKC pathway may be involved in the induction of IL-1β by TAT; p44/42 and JNK MAP kinases acted downstream of the phospholipase C/protein kinase C pathways in TAT-induced IL-1β production in monocytes.
Fig 3.
Involvement of PLC and PKC in TAT-induced IL-1β production. A and C) Monocytes (2 × 105 cells) treated with TAT (100 ng/ml) for 12 hours following a 2-hour pretreatment with (+) or without (−) U73122 at a concentration of 1 µM or 5 µM (A), or GF109203 at a concentration of 20 µM or 40 µM (C). IL-1β in cell supernatants was determined by ELISA. The values are the mean±SD of three experiments. Differences between TAT-treated cells and normal control cells were considered significant at P < 0.05. *, p<0.05; **, p<0.01; or ***, p<0.001. B and D) Monocytes (2 × 106 cells) pretreated with 5 µM U73122 (B) or 40 µM GF109203 (D) for 2 h were followed by HIV TAT (100 ng/ml) stimulation for an additional 30 min. Cell lysate was subjected to Western blotting using Abs to phosphor-p44/42 and phosphor-JNK. Blots were re-probed using anti-p44/42 or anti-JNK antibodies as a loading control. The blots shown are a representative of three separate experiments.
Proximal NF-kB and C/EBP sites within the human IL-1β promoter are involved in the regulation of IL-1β by HIV-1 TAT
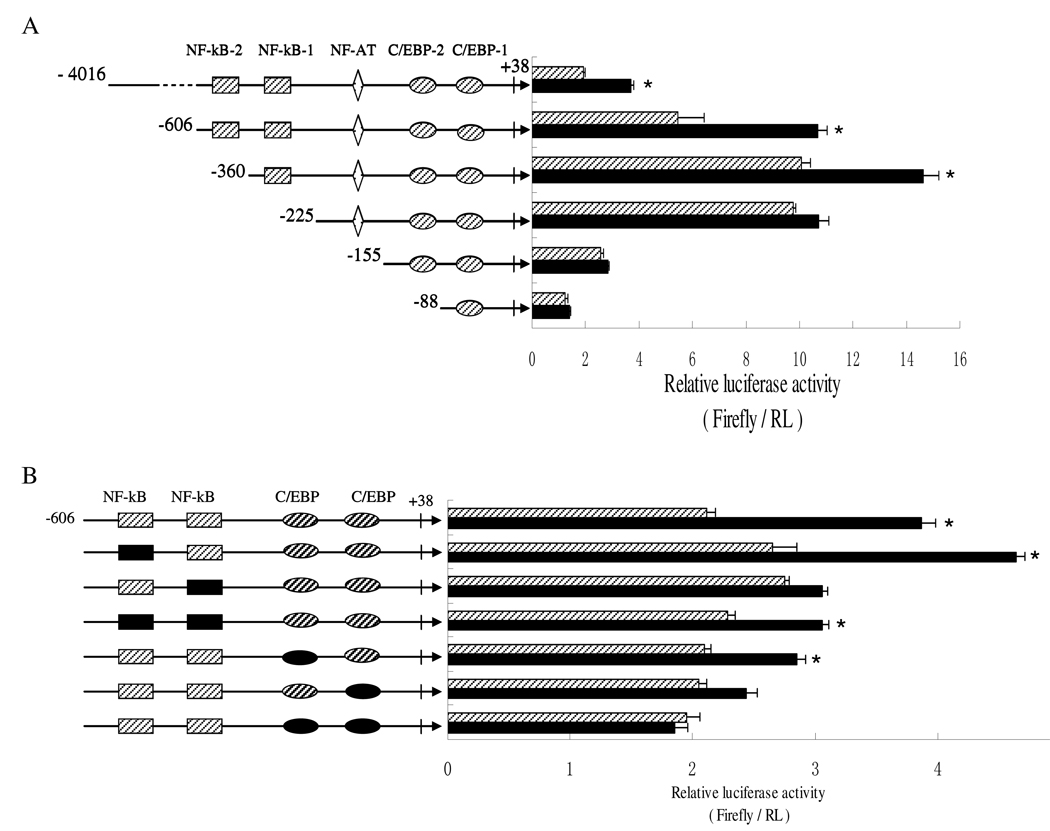
To identify the regulatory elements contributing to the TAT-induced IL-1β expression within the IL-1β promoter, a series of firefly luciferase reporter plasmids, containing various 5'-flanking fragments of the IL-1β promoter or site-specific mutations, were transiently transfected into the human monocytic cell line THP-1. The responses of these reporters to HIV TAT were determined by measuring luciferase activity by dual luciferase assay. As shown in Fig. 4A, deletion of −4016 bp to −606 and further to −360 bp increased gradually both basal and TAT-induced-luciferase activity, with same trend. This pattern of activity suggests that the promoter sequences between −4016 bp and −360 bp may harbor several negative elements. Further 5' truncation to –225 bp, eliminating all the distal regions of the IL-1β promoter and both NF-κB sites at −413/−403 bp and −296/−286 bp, was not associated with further change in basal activity as compared to the activity of the promoter with the deletion to −360 bp. However, this deletion resulted in a critical reduction of promoter activity with TAT treatment. The removal of sequences further down to −155 and −88 caused dramatic decreases in both basal and TAT-induced activity. These results suggested that −360 to +38, located in proximal region of IL-1β, were involved in TAT regulation of IL-1β expression.
Fig 4.
NF-κB and C/EBP transcription factor-binding elements within the IL-1β promoter are involved in TAT regulation of IL-1β expression. A and B) Luciferase reporter assays with the indicated IL-1β promoter and Renilla luciferase RL-TK reporter as an internal control was performed in THP-1 cell with (black bar) or without (slash bar) 100 ng/ml TAT for 12 hours. Assays were performed in triplicate and expressed as relative luciferase activity (Firefly / Renilla). Results are expressed as the mean±SD of three independent experiments. Differences between TAT-treated cells and normal control cells were considered significant at P < 0.05. *, p<0.05; **, p<0.01; or ***, p<0.001. C and D) Monocytes were treated with or without TAT for 2 hours. Nuclear extracts were isolated and analyzed for binding to DIG-labeled probes corresponding to the NFκB-binding elements −413 to −403 (C, lanes 1–4) and −296 to −286 (C, lane 5–10) and C/EBP elements −40 to −32 (D, lane 1–4) and −90 to −82 (D, lane 5–8) of the human IL-1β promoter. Specificity for the probe was confirmed by a competition test with either 50-fold excess unlabeled mutated (mt 50×) NFκB probe (C, lane 3 and 7), wild type (wt 50×) NFκB probe (C, lane 4 and 8), wild type (wt 50×) C/EBP (D, lane 3 and 7), or mutated (mt 50×) C/EBP probe (D, lane 4 and 8). Cells were also treated with TAT for 1hour after 2-hour pretreatment with either 25 µM U0126 (lane 9) or 20 µM SP600125 (lane 10). Arrows indicate specific binding bands. The results shown are representative of two separate experiments.
As indicated in Fig. 4B, specific mutations were introduced into two NF-κB sites at −413/−403 bp and −296/−286 bp, and two C/EBP sites at −90/−82 bp and −40/−32 bp, within the context of the proximal promoter (−606 to +38). Neither the NF-kB, nor the C/EBP site-specific mutations, led to significant changes in the basal activity of the IL-1β promoter. The −296/−286 NF-kB site mutation resulted in ~90 % loss in TAT-induced activity of the IL-1β promoter. There was no significant change in promoter activity between the −296/−286 NF-kB single mutation and the double mutation at −413/−403 bp and −296/−286 bp. Either of these mutations at the two C/EBP sites also presented partial loss of TAT-induced promoter activity by 50–60%. The double mutation of two C/EBP elements almost abolished the induction of promoter activity completely.
EMSA experiments and band quantification with IL-1β gene-specifc NF-kB and C/EBP sequences are shown in Fig. 4C and Fig. 4D, respectively. EMSA results demonstrated that both NF-kB and both C/EBP response elements had basal binding, using the nuclear extract from human monocytes (Fig. 4C, lane 1 and 5 for NF-kB; Fig. 4D, lane 1 and lane 5 for C/EBP). TAT increased the formation of the DNA-protein binding complex with the −296/−286 NF-kB sequence (Fig. 4C, lane 6) and −32/−40 C/EBP sequence (Fig. 4D, lane 6) by 50.1% and 88.4%, respectively. The p44/42 inhibitor U0126 and JNK inhibitor SP600125 slightly reduced the binding complex formed with the −296/−286 NF-kB oligo (Fig. 4C, lane 9 and 10). It suggested that the proximal −296/−286 NF-kB site and −32/−40 C/EBP site may be involved in TAT regulation of IL-1β promoter activity. There is a chance that p44/42 and JNK contributed partially to the proximal −296/−286 NF-kB response element involved in IL-1β promoter regulation by TAT stimulation.
Discussion
The underlying mechanism of HIV-1-mediated neuropathogenesis and dementia is still unclear and remains the subject of active research. Up-regulated expression of chemokines and cytokines within the brain has been recognized as an important response to HIV infection, producing related neuroinflammation and various neurodegenerative diseases (Brabers et al, 2006; Gallo et al, 1989; Merrill et al, 1989; Nuovo et al, 1996; Tyor et al, 1992; Allan et al, 2005; Ambrosino et al 1991; Moynagh, 2005). HIV TAT is known to accumulate within the nuclei of infected cells and is also secreted into the plasma of HIV-infected patients where it can exert its effects on uninfected bystander cells. Secreted TAT can act in an autocrine or paracrine manner on neighboring cells, altering their normal functionality directly through its activity on cells (Zauli, 1995), or indirectly through regulating the expression of pro-inflammatory mediators like TNF-α and IL-1β (Bennasser, 2002; Kinga, 2006). The molecular mechanism underlying TAT-mediated biological effects remains a major subject in AIDS pathogenesis research. In this study, the molecular mechanism underlying the regulation of IL-1β in human monocytes by HIV-1 TAT was explored and the results suggested that HIV TAT induced IL-1β transcription by PLC/PKC-dependent regulation of mitogen-activated protein Kinases (MAPKs). The levels of the pro-inflammatory mediator IL-1β are increased in the CSF and plasma of individuals infected with HIV-1, as compared with that in healthy individuals (Brabers et al, 2006; Gallo et al, 1989; Merrill et al, 1989; Tyor et al, 1992). Transient exposure to HIV TAT has been reported to result in IL-1β induction in human monocytes and astrocytes (Nath et al, 1999). Results from this work on monocytes were consistent with these observations, showing a release of low level IL-1β in monocytes that was increased by HIV TAT stimulation in a time- and dose-dependent manner. The change in IL-1β expression induced by HIV TAT was also observed at the mRNA level in monocytes. Transcription and cleavage of pro-IL-1β protein into mature IL-1β were two major steps in IL-1β expression regulation (Zhao et al, 2001). The transcriptional regulation of IL-1β mRNA abundance in monocytes by TAT stimulation was supported by luciferase reporter gene assays utilizing an IL-1β promoter. However, this finding cannot rule out the possibility of other regulatory mechanisms at the RNA or protein level, such as mRNA stability or pro-IL-1β protein cleavage, may be involved in the TAT-induced IL-1β increase. The observed effects of TAT were not due to heat-stable components such as endotoxin contamination, which was ensured by the results showing that biologically inactive TAT prepared by heat-inactivation failed to induce an IL-1β increase. The pre-immobilization of TAT in the culture wells, preventing the penetration of TAT into the cells, also resulted in IL-1β induction similar to that with soluble TAT, indicating the possibility that the effects of TAT may be exerted at or near the membrane level. However, the characterization of the actual action of TAT deserves further study.
Regulation of IL-1β induction in monocytic cells following HIV TAT stimulation may involve the activation of a complex cascade of signaling pathways, including MAPKs and PI3K. The use of specific kinase inhibitors allowed the dissection of TAT-mediated kinase activation and was applied to investigate the role of p44/42, p38 and JNK, three major members of the MAPKs family, and PI3K in the TAT regulation of IL-1β expression in monocytic cells. PI3K has been reported to be involved in HIV gp120 induction of IL-1β in monocytes (Cheung et al, 2008) and TAT mediated PI3k/Akt pathway activation (Deregibus et al, 2002). However, results from this study showed that the PI3K was not involved in TAT regulation of IL-1β expression in monocytes. Consistent with previous reports that TAT utilizes selective members of MAPKs in the induction of different cytokines (Mischiati et al, 1999; Singh, et al, 2005), p44/42, p38 and JNK were differentially employed by TAT in the regulation of IL-1β expression in monocytes. The TAT-induced IL-1β production, IL-1β mRNA level and promoter activity were affected by the JNK and p44/42 inhibitors, but not the p38 inhibitor, indicating that p44/42 and JNK, but not p38 MAPKs, were involved in TAT regulation of IL-1β expression in monocytes. It was noteworthy that the inhibition of p44/42 using inhibitor U0126 not only inhibited basal-level expression of IL-1β, but decreased the TAT-induced IL-1β expression to the level drastically, below the basal level in monocytes. This suggested that p44/42 might be on the convergence point of the signaling pathways that regulate both basal and TAT-induced IL-1β expression. Phosphorylation analysis by western blot revealed a sustained basal phosphorylation of JNK and p44/42, which was increased dramatically following TAT treatment and reduced by specific inhibitors in monocytes. The sustained basal phosphorylation of p44/42 and JNK MAPKs is supposed to be related to normal cell physiological activity, which maintaining low-level basal expression of IL-1β in monocytes might be included. The increase of MAPK activation was responsible for some TAT-induced physiological changes within the cells, including the increase in IL-1β expression. Considering the complex mechanism underlying IL-1β regulation, inhibition of TAT-induced IL-1β expression to a level below the basal by p44/42 inhibitor U0126 may be attributed to the cooperation between the members of the p44/42 MAPK pathway and other signaling molecules that regulate both basal and TAT-induced IL-1β expression.
MAPKs have been reported to be the downstream targets of two predominant signaling pathways, the PKC and calcium pathways, which have been reported to be activated by extracellular TAT (Conant et al, 1996; Zidovetzki R, 1998; Brigino et al, 1997). Removal of extracellular calcium by chelators have been reported to have no effects on IL-1β production by transient exposure to TAT in monocytes (Nath et al, 1999). Therefore, the PKC pathway might be an alternative to the calcium-dependent pathway in TAT-induced IL-1β induction. The involvement of PKC was supported by the reduction of TAT-induced IL-1β release in the presence of specific PKC inhibitor GF109203. Phospholipase C (PLC) is the common starting point of the PKC and calcium pathways and a major downstream effector of G-coupled protein receptors, such as chemokine receptors (Rhee, 2001; Arai et al, 1996). Inhibition of IL-1β was obtained when PLC inhibitor U73122 was used to inhibit this pathway. PLC and PKC inhibition also reduced the p44/42 and JNK phosphorylation induced by TAT. Thus, the activation of p44/42 and JNK MAPKs seems to couple with PLC /PKC and TAT-induced IL-1β production.
NF-κB and C/EBP transcription factors are known to regulate IL-1β gene transcriptional activity and be involved in TAT regulation of gene expression (Tsukada et al, 1994; Hiscott et al, 1993, Cogswell et al, 1994; Zhang et al, 1993). Neither the NF-kB nor C/EBP site-specific mutations described previously led to a significant change in the basal activity of the IL-1β promoter using Dual luciferase assays. NF-κB transcription factor has been described to be activated by HIV TAT in various cell types, including monocytes (Demarchi et al, 1996; Blazquez et al, 1999, Conant et al, 1996). The deletion and site-specific mutation of the −296/−286 NF-kB-binding element resulted in incomplete inhibition of TAT-induced IL-1β promoter activity. Furthermore, the EMSA experiment demonstrated that basal binding of the specific −296/−286 NF-kB site DNA-protein complex was slightly increased after exposure to TAT. These results provided evidence that this NF-kB element was partially involved in TAT regulation of IL-1β promoter activity in the context of −606 to +38. The p44/42 and JNK inhibitor resulted in the reduction of binding affinity of NF-kB complexes and provided additional evidence that the JNK and p44/42 was involved in TAT regulation of IL-1β induction. The C/EBP transcription factor has been reported to regulate transcription, either alone or in cooperation with a lot of transcription factors, and participate in the chromatin-remodeling complex (Ramji et al, 2002; Erickson et al, 2001; Smale et al, 2002). Results from site-specific mutations showed that both the C/EBP mutations decreased TAT induction of IL-1β promoter activity. Although basal binding with the two C/EBP element sequences was observed by EMSA, the increase of binding affinity of the DNA complex was only found with the −90/−82 and not with −40/−32 C/EBP element. As seen, TAT-induced increase of IL-1β promoter activity did not necessarily rely on the increase of C/EBP DNA binding. On the whole, results that mutations of either C/EBP element only partially reduced the TAT-induced activity suggests that there is a chance that C/EBP is involved in the regulation of the IL-1β promoter activity observed in this study, which might be attributed to the cooperation of C/EBP with other transcription factors or additional mechanisms such as nucleosome remodeling within the IL-1β promoter locus.
This study does not suggest that the signaling pathways and described transcription factors provide a complete scheme of TAT regulation of IL-1β gene expression. There is little chance that the induction of IL-1β by TAT stimulation depends solely on a distinct signaling pathway or single transcription factor, as this work presented. Considering the extremely complex nature of transcription regulation and the limitations of the recombinant TAT protein, chemical inhibitors and proximal promoter used in this study, the presence of other signaling pathways and additional regulatory elements within the promoter cannot be ruled out.
Acknowledgement
This research was supported in part by grants from the Hawaii Community Foundation (20071383) and the National Institutes of Health (S11NS043499-01A2, MH079717-01A2 and G12RR003061).
Abbreviations used in this paper
- HIV
human immunodeficiency virus
- IL-1β
Interleukin-1β
- NF-kB
nuclear factor-kB
- C/EBP
CAAT enhancer-binding protein
- PLC
phospholipase C
- PKC
protein kinase C
- JNK
c-Jun N-terminal kinase
- PI3K
phosphoinositide-3 Kinase
- MAPK
mitogen activated protein kinase.
Reference
- Albini A, Soldi R, Giunciuglio D, Giraudo E, Benelli R, Primo L. The angiogenesis induced by HIV-1 Tat protein is mediated by the Flk-1/KDR receptor on vascular endothelial cells. Nat. Med. 1996;2:1371–1375. doi: 10.1038/nm1296-1371. [DOI] [PubMed] [Google Scholar]
- Albini A, Ferrini S, Benelli R, Sforzini S, Giunciuglio D, Aluigi MG, Proudfoot AE, Alouani S, Wells TN, Mariani G, Rabin RL, Farber JM, Noonan DM. HIV-1 TAT protein mimicry of chemokines. Proc Natl Acad Sci U S A. 1998;95:13153–13158. doi: 10.1073/pnas.95.22.13153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan SM, Tyrrell PJ, Rothwell NJ. Interleukin-1 and neuronal injury. Nat Rev Immunol. 2005;5:629–640. doi: 10.1038/nri1664. [DOI] [PubMed] [Google Scholar]
- Ambrosino C, Ruocco MR, Chen X, Mallardo M, Baudi F, Trematerra S, Quinto I, Venuta S, Scala G. HIV-1 Tat Induces the Expression of the Interleukin-6 (IL6) Gene by Binding to the IL6 Leader RNA and by Interacting with CAAT Enhancer-binding Protein beta /NF-IL6) Transcription Factors. J. Biol. Chem. 1997;272:14883–14892. doi: 10.1074/jbc.272.23.14883. [DOI] [PubMed] [Google Scholar]
- Aquaro S, Svicher V, Ronga L, Perno CF, Pollicita M. HIV-1-associated dementia during HAART therapy. Recent Pat CNS Drug Discov. 2008;3(1):23–33. doi: 10.2174/157488908783421438. [DOI] [PubMed] [Google Scholar]
- Arai H, Charo IF. Differential regulation of G-protein-mediated signaling by chemokine receptors. J. Biol. Chem. 1996;271:21814–21819. doi: 10.1074/jbc.271.36.21814. [DOI] [PubMed] [Google Scholar]
- Bennasser Y, Badou A, Tkaczuk J, Bahraoui E. Signaling pathways triggered by HIV-1 Tat in human monocytes to induce TNF-α. Virology. 2002;303:174–180. doi: 10.1006/viro.2002.1676. [DOI] [PubMed] [Google Scholar]
- Bennett BL, Sasaki DT, Murray BW, O'Leary EC, Sakata ST, Xu W, Leisten JC, Motiwala A, Pierce S, Satoh Y. SP600125, an anthrapyrazolone inhibitor of jun n-terminal kinase. Proc Natl Acad Sci USA. 2001;98:13681–13686. doi: 10.1073/pnas.251194298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazquez MV, Macho A, Ortiz C, Lucena C, Lopez-Cabrera M, Sanchez-Madrid F, Munoz E. Extracellular HIV type 1 Tat protein induces CD69 expression through NF-κB activation: possible correlation with cell surface Tat-binding proteins. AIDS Res. Hum. Retroviruses. 1999;15:1209. doi: 10.1089/088922299310304. [DOI] [PubMed] [Google Scholar]
- Brabers NA, Nottet HS. Role of the pro-inflammatory cytokines TNF-α and IL-1β in HIV-1-associated dementia. Eur. J. Clin. Invest. 2006;36:447–458. doi: 10.1111/j.1365-2362.2006.01657.x. [DOI] [PubMed] [Google Scholar]
- Brigino E, Haraguchi S, Koutsonikolis A, Cianciolo GJ, Owens U, Good RA, Day NK. Interleukin 10 is induced by recombinant HIV-1 Nef protein involving the calcium/calmodulin-dependent phosphodiesterase signal transduction pathway. Proc. Natl. Acad. Sci. USA. 1997;94:3178–3182. doi: 10.1073/pnas.94.7.3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung R, Ravyn V, Wang L, Ptasznik A, Collman RG. Signaling mechanism of HIV-1 gp120 and virion-induced IL-1beta release in primary human macrophages. J Immunol. 2008;180:6675–6684. doi: 10.4049/jimmunol.180.10.6675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogswell JP, Godlevski MM, Wisely GB, Clay WC, Leesnitzer LM, Ways JP, Gray JG. NF-kB regulates IL-1beta transcription through a consensus NF-kB binding site and a nonconsensus CRE-like site. J. Immunol. 1994;153:712–723. [PubMed] [Google Scholar]
- Conant C, Ma M, Nath A, Major EO. Extracellular human immunodeficiency virus type 1 Tat protein is associated with an increase in both NF-κB binding and protein kinase C activity in primary human astrocytes. J. Virol. 1996;70:1384. doi: 10.1128/jvi.70.3.1384-1389.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demarchi F, d'Adda di Fagagna F, Falaschi A, Giacca M. Activation of transcription factor NF- kappa B by the Tat protein of human immunodeficiency virus type 1. J. Virol. 1996;70:4427–4437. doi: 10.1128/jvi.70.7.4427-4437.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deregibus MC, Cantaluppi V, Doublier S, Brizzi MF, Deambrosis I, Albini A, Camussi G. HIV-1-Tat protein activates phosphatidylinositol 3-kinase/ AKT-dependent survival pathways in Kaposi's sarcoma cells. J Biol Chem. 2002;277:25195–25202. doi: 10.1074/jbc.M200921200. [DOI] [PubMed] [Google Scholar]
- Ensoli B, Buonaguro L, Barillari G, Fiorelli V, Gendelman R, Morgan RA, Wingfield P, Gallo RC. Release, uptake, and effects of extracellular human immunodeficiency virus type-1 Tat protein on cell growth and viral transactivation. J. Virol. 1993;67:277–287. doi: 10.1128/jvi.67.1.277-287.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson RL, Hemati N, Ross SE, MacDougald OA. p300 coactivates the adipogenic transcription factor CCAAT/enhancer-binding protein alpha. J Biol Chem. 2001;276:16348–16355. doi: 10.1074/jbc.m100128200. [DOI] [PubMed] [Google Scholar]
- Gallo P, Frei K, Rordorf C, Lazdins J, Tavolato B, Fontana A. Human immunodeficiency virus type 1 infection of the central nervoussystem: an evaluation of cytokines in cerebrospinal fluid. J. Neuroimmunol. 1989;23:109–116. doi: 10.1016/0165-5728(89)90029-5. [DOI] [PubMed] [Google Scholar]
- Ganju RK, Munshi N, Nair BC, Liu ZY, Gill P, Groopman JE. Human immunodeficiency virus tat modulates the Flk-1/KDR receptor mitogen activated protein kinases, and components of focal adhesion in Kaposi’s sarcoma cells. J Virol. 1998;72:6131–6137. doi: 10.1128/jvi.72.7.6131-6137.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray F, Adle-Biassette H, Chretien F, Lorin de la Grandmaison G, Force G, Keohane C. Neuropathology and neurodegeneration in human immunodeficiency virus infection: pathogenesis of HIV-induced lesions of the brain:correlations with HIV-associated disorders, and modifications according to treatments. Clin. Neuropathol. 2001;20:146–155. [PubMed] [Google Scholar]
- Griffin WS, Sheng JG, Roberts GW, Mrak RE. Interleukin-1 expression in different plaque types in Alzheimer’s disease: significance in plaque evolution. J Neuropathol Exp Neurol. 1995;54:276–281. doi: 10.1097/00005072-199503000-00014. [DOI] [PubMed] [Google Scholar]
- Griffin WS, Liu L, Li Y, Mrak RE, Barger SW. Interleukin-1 mediates Alzheimer and Lewy body pathologies. J Neuroinflammation. 2006;3:5. doi: 10.1186/1742-2094-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiscott J, Marois J, Garoufalis J, D'Addario M, Roulston A, Kwan I, Pepin N, Lacoste J, Nguyen H, Bensi G, et al. Characterization of a functional NF-kappa B site in the human interleukin 1 beta promoter: evidence for a positive autoregulatory loop. Mol Cell Biol. 1993;13(10):6231–6240. doi: 10.1128/mcb.13.10.6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones M, Olafson K, Del Bigio MR, Peeling J, Nath A. Intraventricular injection of human immunodeficiency virus type 1 (HIV-1) tat protein causes inflammation, gliosis, apoptosis, and ventricular enlargement. J. Neuropathol. Exp. Neurol. 1998;57:563–570. doi: 10.1097/00005072-199806000-00004. [DOI] [PubMed] [Google Scholar]
- Kim SH, Smith CJ, Van Eldik LJ. Importance of MAPK pathways for microglial pro-inflammatory cytokine I IL-1β production. Neurobiol Aging. 2004;25(4):431–439. doi: 10.1016/S0197-4580(03)00126-X. [DOI] [PubMed] [Google Scholar]
- Kinga JE, Eugenina EA, Bucknera CM, Berman JW. HIV tat and neurotoxicity. Microbes and Infection. 2006;8:1347–1357. doi: 10.1016/j.micinf.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Kruman PH, Nath A, Mattson MP. HIV-1 protein tat induces apoptosis of hippocampal neurons by a mechanism involving caspase activation, calcium overload, and oxidative stress. Exp. Neurol. 1998;154:276–288. doi: 10.1006/exnr.1998.6958. [DOI] [PubMed] [Google Scholar]
- Merrill JE, Koyanagi Y, Chen IS. Interleukin-1 and tumor necrosis factor can be induced from mononuclear phagocytes by human immunodeficiency virus type 1 binding to the CD4 receptor. J. Virol. 1989;63:4404–4408. doi: 10.1128/jvi.63.10.4404-4408.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mischiati C, Pironi F, Milani D, Giacca M, Mirandola P, Capitani S, Zauli G. Extracellular HIV-1 Tat protein differentially activates the JNK and ERK/MAPK pathways in CD4 T cells. AIDS. 1999;13:1637–1645. doi: 10.1097/00002030-199909100-00006. [DOI] [PubMed] [Google Scholar]
- Moynagh PN. The interleukin-1 signalling pathway in astrocytes: key contributor to inflammation in the brain. J. Anat. 2005;207:265–269. doi: 10.1111/j.1469-7580.2005.00445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath A, Conant K, Chen P, Scott C, Major EO. Transient Exposure to HIV-1 Tat Protein Results in Cytokine Production in Macrophages and Astrocytes. J Biol Chem. 1999;274:17098–17102. doi: 10.1074/jbc.274.24.17098. [DOI] [PubMed] [Google Scholar]
- Nath A. Human immunodeficiency virus(HIV) proteins in neuropathogenesis of HIV dementia. J Infect Dis. 2002;186:193–198. doi: 10.1086/344528. [DOI] [PubMed] [Google Scholar]
- Nuovo GJ, Alfieri ML. AIDS dementia is associated with massive, activated HIV-1 infection and concomitant expression of several cytokines. Mol. Med. 1996;2:358–366. [PMC free article] [PubMed] [Google Scholar]
- Price RW, Brew BJ. The AIDS dementia complex. J Infect Dis. 1988;158(5):1079–1083. doi: 10.1093/infdis/158.5.1079. [DOI] [PubMed] [Google Scholar]
- Pu H, Tian J, Flora G, Lee YW, Nath A, Hennig B, Toborek M. HIV-1 Tat protein upregulates inflammatory mediators and induces monocyte invasion into the brain. Mol Cell Neurosci. 2003;24:224–237. doi: 10.1016/s1044-7431(03)00171-4. [DOI] [PubMed] [Google Scholar]
- Rhee SG. Regulation of phosphoinositide-specific phospholipase C. Annu Rev Biochem. 2001;70:281–312. doi: 10.1146/annurev.biochem.70.1.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell NJ, Luheshi GN. Interleukin 1 in the brain: biology, pathology and therapeutic target. Trends Neurosci. 2000;23:618–625. doi: 10.1016/s0166-2236(00)01661-1. [DOI] [PubMed] [Google Scholar]
- Ramji DP, Foka P. CCAAT/enhancer-binding proteins: structure, function and regulation. Biochem J. 2002;365:561–575. doi: 10.1042/BJ20020508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh IN, Hage El, Campbell ME, Lutz SE, Knapp PE, Nath A, Hauser KF. Differential involvement of p38 and JNK MAP kinases in HIV-1 Tat and gp120-induced apoptosis and neurite degeneration in striatal neurons. Neuroscience. 2005;135:781–790. doi: 10.1016/j.neuroscience.2005.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smale ST, Fisher AG. Chromatin structure and gene regulation in the immune system. Annu. Rev. Immunol. 2002;20:427–462. doi: 10.1146/annurev.immunol.20.100301.064739. [DOI] [PubMed] [Google Scholar]
- Tsukada J, Saito K, Waterman WR, Webb AC, Auron PE. Transcription factors NF-IL6 and CREB recognize a common essential site in the human prointerleukin 1b gene. Mol Cell Biol. 1994;14:7285. doi: 10.1128/mcb.14.11.7285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyor WR, Glass JD, Griffin JW, Becker PS, McArthur JC, Bezman L, Griffin DE. Cytokine expression in the brain during the acquired immunodeficiency syndrome. Ann. Neurol. 1992;31:349–360. doi: 10.1002/ana.410310402. [DOI] [PubMed] [Google Scholar]
- Westendorp MO, Frank R, Ochsenbauer C, Stricker K, Dhein JH, Walczak KM, Krammer PH. Sensitization of T cells to CD95-mediated apoptosis by HIV-1 Tat and gp120. Nature. 1995;375:497–500. doi: 10.1038/375497a0. [DOI] [PubMed] [Google Scholar]
- Wiley CA, Masliah E, Morey M, Lemere C, DeTeresa R, Grafe M, Hansen L, Terry R. Neocortical damage during HIV infection. Ann. Neurol. 1991;29:651–657. doi: 10.1002/ana.410290613. [DOI] [PubMed] [Google Scholar]
- Xiao H, Neuveut C, Tiffany HL, Benkirane M, Rich EA, Murphy PM, Jeang KT. Selective CXCR4 antagonism by Tat: implications for in vivo expansion of coreceptor use by HIV-1. Proc Natl Acad Sci U S A. 2000;97(21):11466–11471. doi: 10.1073/pnas.97.21.11466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zauli G, La Placa M, Vignoli M, Re MC, Gibellini D, Furlini G, Milani D, Marchisio M, Mazzoni M, Capitani S. An autocrine loop of HIV Tat protein responsible for the improved survival/proliferation capacity of permanently Tat-transfected cells and required for optimal human immunodeficiency virus type 1 long terminal repeat transactivating activity. J Acquir Immune Defic Syndr. 1995;10:306–316. [PubMed] [Google Scholar]
- Zhang Y, Rom WN. Regulation of the interleukin-1 beta (IL-1 beta) gene by mycobacterial components and lipopolysaccharide is mediated by two nuclear factor-IL6 motifs. Mol Cell Biol. 1993;13:3831–3837. doi: 10.1128/mcb.13.6.3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao ML, Kim MO, Morgello S, Lee SC. Expression of inducible nitric oxide synthase, interleukin-1 and caspase-1 inHIV-1 encephalitis. J Neuroimmunol. 2001;115:182–191. doi: 10.1016/s0165-5728(00)00463-x. [DOI] [PubMed] [Google Scholar]
- Zidovetzki R, Wang JL, Chen P, Jeyaseelan R, Hofman F. Human immunodeficiency virus Tat protein induces interleukin 6 mRNA expression in human brain endothelial cells via protein kinase C and cAMP-dependent protein kinase pathways. AIDS Res Hum Retroviruses. 1998;4:825–833. doi: 10.1089/aid.1998.14.825. [DOI] [PubMed] [Google Scholar]