Abstract
Human endometrium is a highly regenerative tissue undergoing more than 400 cycles of growth, differentiation, and shedding during a woman's reproductive years. Endometrial regeneration is likely mediated by adult stem/progenitor cells. This study investigated key stem cell properties of individual clonogenic epithelial and stromal cells obtained from human endometrium. Single-cell suspensions of endometrial epithelial or stromal cells were obtained from hysterectomy tissues from 15 women experiencing normal menstrual cycles, and were cultured at clonal density (10 cells/cm2) or limiting dilution. The adult stem cell properties—self-renewal, high proliferative potential, and differentiation of single epithelial and stromal cells—were assessed by harvesting individual colonies and undertaking serial clonal culture, serial passaging, and culture in differentiation-induction media, respectively. Lineage differentiation markers were examined by RT-PCR, immunocytochemistry, and flow cytometry. Rare single human endometrial EpCAM+ epithelial cells and EpCAM− stromal cells demonstrated self-renewal by serially cloning >3 times and underwent >30 population doublings over 4 mo in culture. Clonally derived epithelial cells differentiated into cytokeratin+ gland-like structures in three dimensional culture. Single stromal cells were multipotent, as their progeny differentiated into smooth muscle cells, adipocytes, chondrocytes, and osteoblasts. Stromal clones expressed mesenchymal stem cell (MSC) markers ITGB1 (CD29), CD44, NT5E (CD73), THY1 (CD90), ENG (CD105), PDGFRB (CD140B), MCAM (CD146) but not endothelial or hemopoietic markers PECAM1 (CD31), CD34, PTPRC (CD45). Adult human endometrium contains rare epithelial progenitors and MSCs, likely responsible for its immense regenerative capacity, which may also have critical roles in the development of endometriosis and endometrial cancer. Human endometrium may provide a readily available source of MSCs for cell-based therapies.
Keywords: adult stem cells, clonal assays, differentiation, endometrial stem cell, epithelial progenitor cell, female reproductive tract, human endometrium, mesenchymal stem cell, uterus
Human endometrium contains rare epithelial progenitors and mesenchymal stem cells that self-renew, differentiate, and are highly proliferative.
INTRODUCTION
The human endometrium is a dynamic remodeling tissue undergoing more than 400 cycles of regeneration, differentiation, and shedding during a woman's reproductive years [1–3]. Each month, 4–7 mm of mucosal tissue grows within 4–10 days in the first half or proliferative stage of the menstrual cycle [3]. Endometrial regeneration also follows parturition, extensive resection, and occurs in postmenopausal women taking estrogen replacement therapy [1]. This level of new tissue growth is at least equivalent to the cellular turnover in other highly regenerative organs, such as blood-forming tissue of the bone marrow, epidermis, and intestinal epithelium, where adult stem cells replenish lost cells to maintain tissue homeostasis [4, 5].
Adult stem cells are rare, undifferentiated cells present in adult tissues and organs. They are extremely difficult to identify in tissues, as they are rare, lack distinguishing morphological features, and specific adult stem cell markers are currently unavailable. Adult stem cells are therefore defined by their functional properties: substantial self-renewal, high proliferative potential, and ability to differentiate into one or more lineages [6, 7]. These functions are highly regulated by the stem cell niche to ensure an appropriate balance between stem cell replacement and provision of sufficient differentiated mature cells for tissue and organ function [7, 8].
There is increasing interest in the concept that endometrial stem/progenitor cells may be responsible for the highly regenerative capacity of human endometrium. It has been hypothesized that both epithelial and stromal adult stem cells exist in the basal layer of human endometrium, since regeneration occurs from this layer after the top two-thirds or functional layer is shed at menstruation, and the endometrium comprises glandular tissue supported by an extensive vascularized stroma [1, 9]. Initial evidence from cell cloning studies suggests that adult stem cells are likely present in human endometrium [10, 11], but subsequent studies have focused on various subpopulations of epithelial and/or stromal cells rather than individual cells [12–15]. The pluripotency marker, POU5F1 (formerly Oct-4) has been observed in some cells in human endometrial stroma [16], but the identity and stem cell function of these cells was not examined. To date, adult stem cell activity of individual human endometrial epithelial and stromal cells has not been investigated.
Disorders of uterine endometrial proliferation are common, leading to endometriosis, endometrial hyperplasia, and endometrial cancer. Despite their common occurrence and the substantial public health burden that these diseases present [17], little is known about their pathogenesis [18–20]. We hypothesize that endometrial stem or progenitor cells play key roles in the initiation of these endometrial proliferative disorders [1]. In endometriosis, endometrial stem/progenitor cells may be shed into the pelvic cavity by retrograde menstruation to establish endometriotic growths [1]. Endometrial epithelial progenitors or their immediate progeny may be targets of early genetic or epigenetic alterations, leading to the emergence of endometrial cancer stem cells that initiate and maintain endometrial cancer [1, 19]. In this study, we report the isolation of individual epithelial progenitor cells and mesenchymal stem cells (MSCs) in human endometrium and the characterization of their adult stem cell properties of self-renewal, high proliferative potential, and differentiation.
MATERIALS AND METHODS
Human Tissues
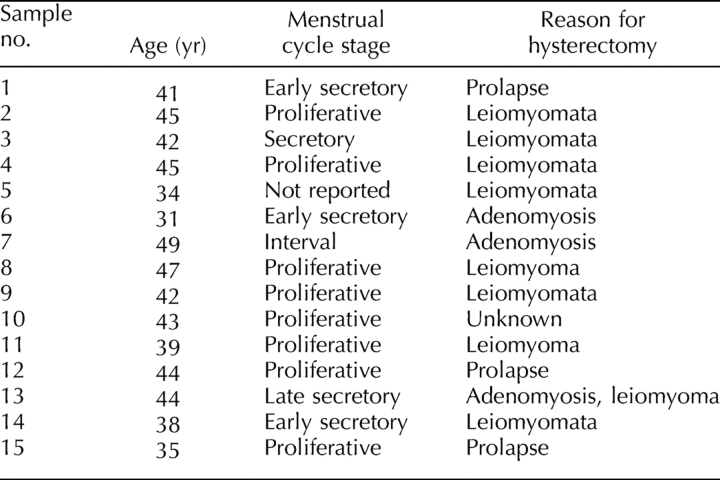
Human endometrium was obtained from hysterectomy samples collected from 15 cycling women, aged 31–49 yr (mean, 41.3 ± 1.3 [±SEM]) undergoing surgery for fibroids and/or adenomyosis and prolapse (Table 1), and who had not taken exogenous hormones for 3 mo prior to surgery. Endometrial tissue was collected distal to submucosal fibroids if they were present. The study protocol was approved by the Southern Health Human Research and Ethics Committee B, and informed written consent was obtained from each patient. The women reported the date of their last menstrual period, and menstrual cycle stage was confirmed from pathology reports assessed by experienced histopathologists, according to well-established histological criteria for the normal menstrual cycle [21]. Patient details, including menstrual cycle stage at time of hysterectomy, are listed in Table 1. Full thickness endometrium attached to 5 mm myometrium was collected in medium containing Hepes-buffered Dulbecco modified Eagle medium (DMEM)/Hams F-12 (F-12) (Invitrogen, Carlsbad, CA) containing antibiotics and 5% newborn calf serum (CSL, Parkville, Australia) [10], stored at 4°C, and processed within 2–18 h.
TABLE 1.
Details regarding patients in this study.
Human endometrial tissue was scraped from the myometrium and dissociated into single-cell suspensions using collagenase type 3 (300 μg/ml; Worthington Biochemical Corp., Freehold, NJ), 40 μg/ml deoxyribonuclease type I (Roche Diagnostics, Mannheim, Germany), and mechanical methods for 50–60 min [10]. Leukocytes were removed with anti-PTPRC (CD45)-coated Dynabeads (Dynal Biotech, Oslo, Norway). Purified epithelial and stromal cell suspensions were then obtained by selecting epithelial cells with a further round of magnetic bead sorting using anti-EpCAM-coated Dynabeads [10]. Both epithelial and stromal cell preparations were >95% pure.
Clonal Cell Culture, Self-Renewal, and Proliferative Potential Assays
Purified, freshly isolated epithelial and stromal cells were cultured separately at clonal density (8–20 cells/cm2, 500-1200 cells/dish) in 12 ml DMEM/F-12 medium containing 10% fetal calf serum (FCS; CSL), 2 mM glutamine (Invitrogen), and antibiotic-antimycotic on fibronectin-coated 100-mm petri dishes (10 μg/ml; Becton Dickinson Biosciences, Bedford, MA) in triplicate, and in limiting dilution or at 1 cell/well in 96-well plates (100 μl medium/well using a stock cell suspension of 10 cells/ml) and incubated at 37°C in 5% CO2. The culture medium for epithelial cells was also supplemented with 10 ng/ml epidermal growth factor (GroPep, Adelaide, Australia) to promote growth of epithelial clones [10]. A seeding density of 8–20/cm2 was chosen based on our previous cloning efficiency data [10] to ensure that no more than 10 well-separated, nonoverlapping clones/plate were obtained. Plates were examined twice/week to ensure clones were established from single cells, and individual colonies were monitored until harvest. For the limiting dilution analysis, freshly isolated epithelial and stromal cells were seeded in 100-μl volumes into wells of 96-well plates with 8 replicates/cell concentration for 4 patient samples in serial dilution from 256 to 0.5 cells/well from stock cell suspensions of 2560–5 cells/ml. Due to the low number of cells in culture plates, medium changes were done every 14 days, except for the higher dilutions in the 96-well plates, where weekly or more frequent changes were required. Following fixation in 10% formalin and staining with 0.5% toluidine blue, colony efficiency assays were performed using Poisson distribution statistics by determining the percentage of wells without cell clones (>50 cells) after 30 days in culture using limiting dilution software tools in the statmod software package for R computing environment (available at http://cran.r-project.org/). The R version (R2.7.0) of the limdil software (http://bioinf.wehi.edu.au/software/elda/index.html) was used for analysis.
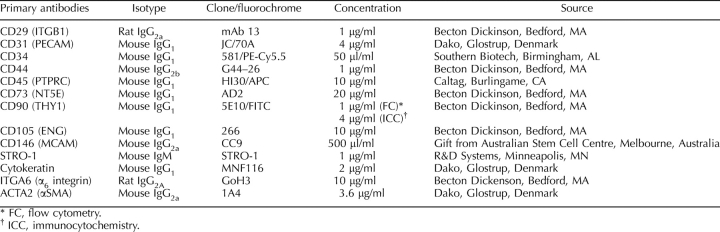
Nonoverlapping single epithelial or stromal clones were harvested from culture dishes using 0.025% trypsin (Invitrogen) and cloning rings (Sigma-Aldrich, St. Louis, MO) after 20–35 days in culture, or from individual wells containing a single colony (Fig. 1, A and B). Six to twelve large clones containing 4–8 × 103 epithelial or stromal cells, or 6–12 small clones comprising 0.5–2 × 103 cells, were collected from each patient sample for analysis of adult stem cell properties. A small proportion of the cells in the harvested clones were cultured on coverslips for cytokeratin or α6 integrin (IGTA6), and THY1 (CD90) immunocytochemistry analysis (Table 2) [10] to confirm that individual clones were epithelial or stromal, respectively, prior to undertaking adult stem cell assays.
FIG. 1.
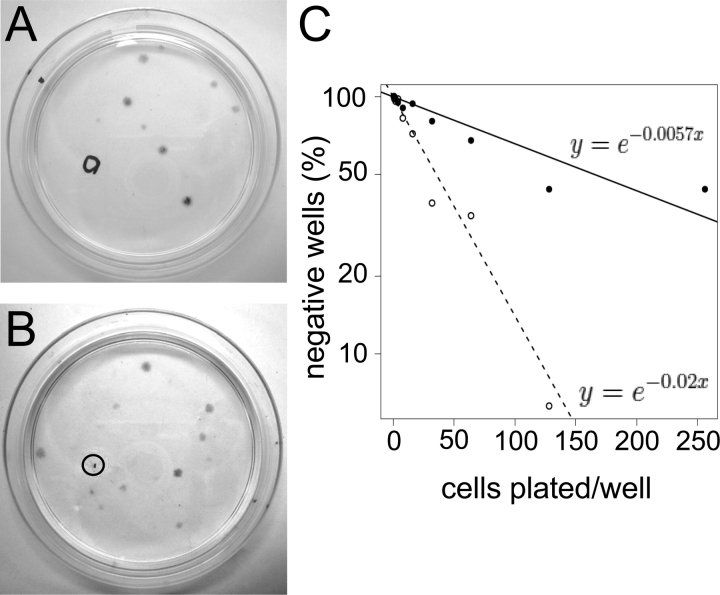
Human endometrial cell clones. Typical 6-cm cloning plates of freshly isolated single cell suspensions of epithelial (A) and stromal (B) cells seeded at 20 and 10 cells/cm2, respectively, showing well-separated individual colonies (CFU). Circles indicate removal of a clone for analysis. C) Limiting dilution analysis showing frequency of epithelial (black circles) and stromal (open circles) clones in endometrial cell suspensions by limiting dilution. Data are from four patient samples (nos. 11, 12, 14, and 15), with eight replicates/sample using Poisson distribution analysis. The frequency of stromal CFU was significantly greater than for epithelial (P < 0.0001).
TABLE 2.
Antibodies used to phenotype human endometrial cells by flow cytometry and immunocytochemistry.
Self-renewal of epithelial and stromal cells was assessed by serial cloning individual large and small clones generated by single epithelial or stromal cells. Cells from individual clones were reseeded at cloning density (5–10 cells/cm2) in 100-mm petri dishes to generate secondary clones. Two stromal and two epithelial clones were harvested per primary clone 14 and 21 days later, respectively, since clonogenic stromal cells proliferated more rapidly than clonogenic epithelial cells. Each clone was subcloned into duplicate 100 mm culture plates if >600 cells were harvested from the primary clone, or subcloned into a single dish for secondary or higher-order clones containing <500 cells. The secondary clones were recloned in a similar manner to generate tertiary clones (Fig. 2), and recloning of individual clones continued until cloning activity was exhausted. Some cells from each clone were plated on coverslips, and their epithelial and stromal phenotype confirmed by immunocytochemistry for cytokeratin and THY1 (CD90) (Table 2), respectively.
FIG. 2.
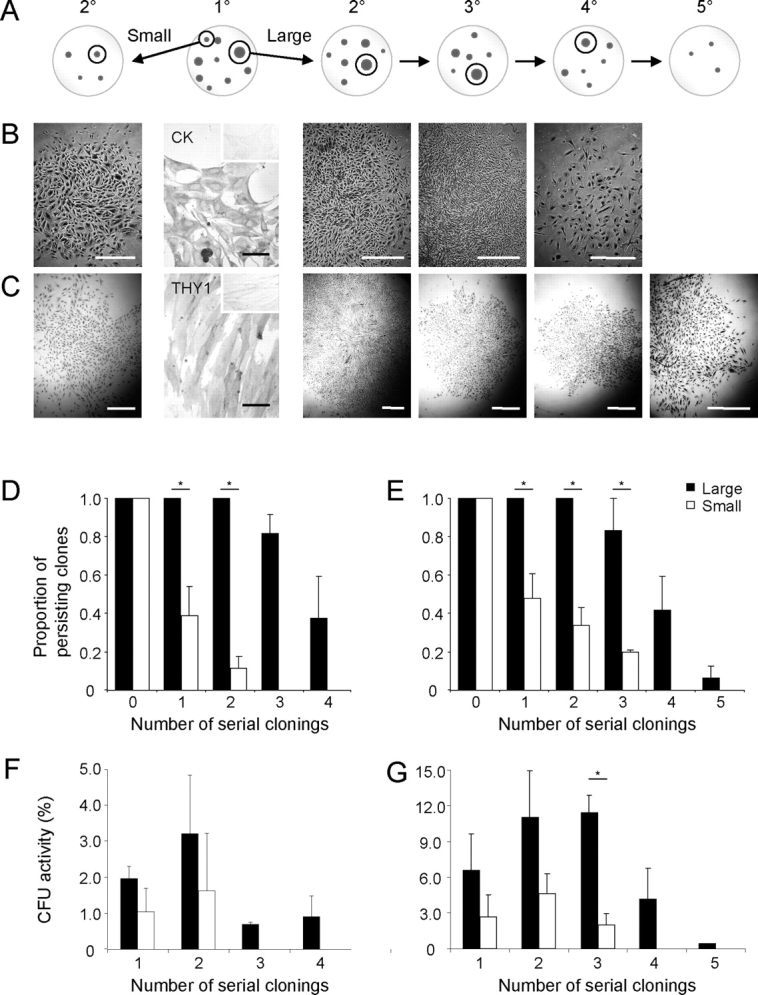
Serial cloning analysis for measuring self-renewal of human endometrial large and small primary (1°) epithelial and stromal CFU. A) Schematic showing serial cloning strategy. The initial cloning plate (seeded at 10–20 cells/cm2), second panel with two cloning rings selecting the largest (>4000 cells), and a small to medium (<2000 cells) CFU. These clones were individually replated at 5–10 cells/cm2 and cultured for 14 days. Serial clonal passaging (2°–5°) is depicted as cloning plates containing representative clones, with selection of typical large clones for the subsequent round of cloning indicated within cloning rings, until CFU activity was exhausted (4°/5°). Typical endometrial epithelial (B) and stromal (C) colonies formed at each round of serial cloning. Endometrial epithelial colonies were cytokeratin+ (CK) and stromal colonies THY1 (CD90+) (second panels). Inserts are isotype controls. Rate of clonal extinction is shown for both large and small CFU for each round of serial cloning for epithelial (D) and stromal (E) CFU. Percentage of CFU in large and small epithelial (F) and stromal (G) CFU at each round of serial cloning. Results are means ± SEM (n = 3 patient samples [from nos. 7–9 for epithelial and nos. 1–4 for stromal); averages of three to five small and large CFU/cell type/patient sample). *Significant difference between large and small CFU (P < 0.05). Bars = 1 mm for clones (C); bars = 50 μm for immunostained images, including insets (B and C).
Proliferative potential of individual colony-forming epithelial and stromal cells was assessed by serially passaging [22, 23] 3–5 large and 3–5 small primary clones per patient sample at bulk culture seeding density (2000 cells/cm2) until senescence. Progressively larger wells and flasks were used with each passage, as each clone formed an individual cell line. Cells were passaged every 7–10 days when cultures reached 80–100% confluence. Once sufficient cells were obtained for each cell line, duplicate cultures were established for each clone and excessive cells were frozen in 10% DMSO/90% FCS and stored in liquid nitrogen. Cell counts were done at each passage, and cumulative population doublings (cPDs) calculated from the formula: cPD = ln(cumulative cell yield)/ln2 [22]. At each passage, a small proportion of cells was seeded at clonal density (25–50 cells/cm2) in 60-mm plates, and the cloning efficiency at each passage was calculated: (no. clones/no. cells seeded) × 100 [22].
In Vitro Differentiation
Clonally derived epithelial cells.
Large primary or secondary epithelial clones were expanded in culture and 2 × 105 cells were then cultured in 1:1 dilution of Matrigel (Becton Dickinson) in DMEM/F12/10% FCS medium in eight-well chamber slides [24] placed over a monolayer of endometrial stromal cells cultured in the same medium in 5% CO2 at 37°C for 25 days. Culture medium was changed twice weekly. Differentiation into gland-like structures was monitored microscopically, and cultures were immunostained with anti-human cytokeratin (2 μg/ml; Dako, Carpinteria, CA). An isotype-matched IgG negative control (Table 2) was included for each sample examined. The area of gland-like structures was measured using Zeiss AxioVision 4.6 image analysis software.
Clonally derived stromal cells.
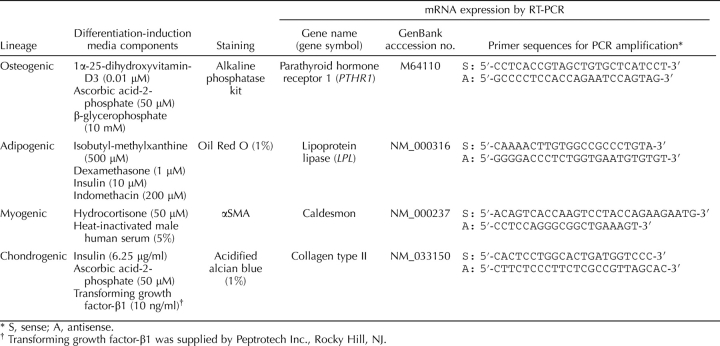
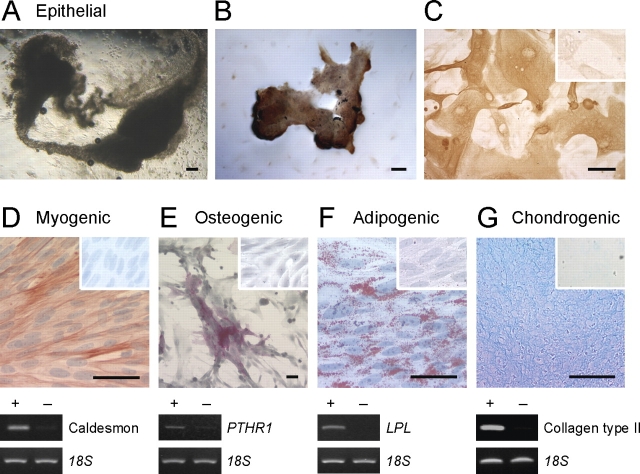
Large primary or secondary stromal clones were isolated, expanded in culture in serum-containing medium (DMEM/F12/10% FCS) to generate 2–3 × 106 cells, seeded at 5000–10 000 cells/cm2 into 25-cm2 flasks and 13-mm gelatin-coated Thermanox coverslips (Nalge Nunc, Naperville, IL), and cultured in specific differentiation-induction media (Table 3) for 4 wk using standard methods [25, 26]. Media were changed every 2–3 days. Undifferentiated control cells were cultured concurrently in low serum medium (DMEM/F12/1% FCS%/1% antibibiotic/1% glutamine) for the same incubation time. Control media were changed as regularly as the differentiation induction media. Total RNA was isolated from cultures to assess cell lineage-specific genes by RT-PCR, and cells on coverslips were stained by histochemical or immunocytochemical methods [12]. Isotype-matched IgG-negative controls were included for each antibody (Table 2). Osteogenic differentiation was assessed using an alkaline phosphatase kit (Sigma-Aldrich) and parathyroid hormone receptor 1 (PTHR1) mRNA using primers listed in Table 3 [12]. Adipogenic differentiation into lipid-laden cells was detected with Oil Red O and the adipogenic lineage-specific gene, lipoprotein lipase (LPL) (Table 3) [12]. Myogenic differentiation was induced by culturing cells in medium supplemented with heat-inactivated 5% male human serum (Red Cross Blood Service, Melbourne, Victoria, Australia) and 50 μM hydrocortisone (Sigma-Aldrich). Smooth muscle cells were detected by smooth muscle actin (αSMA; 3.6 μg/ml, clone 1A4; Dako) immunocytochemistry and expression of caldesmon mRNA (Table 3) [12]. Chondrogenic differentiation was detected in 5-μm paraffin sections of formalin-fixed cell pellets incubated in 1% acidified Alcian blue, and by expression of collagen type II mRNA (Table 3) [12].
TABLE 3.
Mesenchymal differentiation induction and detection of lineage specific markers.
RT-PCR Analysis
Total RNA was isolated using Trizol (Invitrogen) with genomic DNA removed. RNA quality was assessed by spectrophotometry, and the nucleotide:protein ratio (260:280) was within acceptable boundaries of 1.8 and 2.1. A 1-μg aliquot was reverse transcribed into cDNA with AMV reverse transcriptase (Roche, Penzberg, Germany) at 42°C for 1 h. Complementary DNA was amplified using GoTaq Green Master Mix (Promega) in a Gene Amp PCR System 2700 (Applied Biosystems, Foster City, CA). Primer sequences are shown in Table 3; 18S RNA was the loading control. Reaction products were analyzed by 1.5% agarose gel electrophoresis and ethidium bromide. The sequence of each product was confirmed by automatic sequencing.
Flow Cytometry
Cells derived from secondary stromal clones were incubated with directly conjugated or unconjugated antibodies to MSC surface markers (Table 2) or matched-isotype control IgG for 45 min at 4°C, followed by fluorescein isothiocyanate-conjugated goat anti-mouse IgM (10 μg/ml; Southern Biotech, Birmingham, AL), Alexa Fluor 488-conjugated chicken anti-rat IgG (10 μg/ml; Molecular Probes, Eugene, OR), or PE-conjugated sheep anti-mouse Ig F(ab′)2 fragments (10 μl/ml; Chemicon Australia, Melbourne, Victoria, Australia). Cells were incubated with propidium iodide (Sigma-Aldrich) and analyzed by flow cytometry using a Mo-Flo Cytometer (Cytomation, Fort Collins, CO) [12].
Statistical Analysis
Data are shown as mean ± SEM from n patient samples. Unpaired t-tests were used to compare the significance between two groups. Data for cell yield were log transformed prior to t-test analysis. Results were considered statistically significant at a P value <0.05.
RESULTS
In Vitro Self-Renewal of Endometrial Colony Forming Units/Cells
To assess the adult stem cell activity of individual endometrial cells, single-cell suspensions of freshly isolated endometrium (n = 15) were separated into EpCAM+ epithelial cells and EpCAM− stromal cells and seeded at much lower cloning densities (10–20 cells/cm2; Fig. 1, A and B) than in our previous studies [10], or at <1 cell/well in 96-well plates (n = 3) to obtain epithelial and stromal cell colony forming cells (CFU). We also conducted limiting dilution assays in 96-well plates on 4 patient samples. Poisson distribution analysis demonstrated that the frequency of clonogenic epithelial cells was 1/174 (confidence interval [CI] 1/235, 1/130) and 1/48 (CI 1/61, 1/39) for stromal cells (Fig. 1C), in broad agreement with cloning efficiencies obtained on culture dishes [10].
Self-renewal was assessed using a serial cloning strategy, since single CFU undergoing self-renewing cell divisions during colony establishment will form new clones on recloning [27]. We harvested and recloned at least 5 individual large and small clones per patient sample at 5–10 cells/cm2 (Fig. 2, A–C), and demonstrated that large endometrial epithelial CFU exhibited significantly greater self-renewal capacity than small CFU, undergoing 2.9 ± 0.5 versus 0.5 ± 0.3 (P = 0.0048; n = 3) rounds of serial cloning. Similarly, large stromal CFU underwent 3.3 ± 0.4 rounds of cloning compared with small CFU (0.9 ± 0.2; P = 0.0054; n = 4). All large epithelial and stromal CFU were able to serially clone ≥3 rounds (Fig. 2, D and E), significantly more than small CFU, where 39% of epithelial clones and 47% of stromal clones were able to initiate CFU on the second round, while 36% of large epithelial and 41% of large stromal CFU initiated clones on the third round, and 7% of large stromal CFU did so on the fourth (Fig. 2, D and E). Large epithelial and stromal CFU formed densely packed clones for the first three rounds of serial cloning (Fig. 2, B and C, central panels), but initiated smaller, less dense clones on further recloning, which appeared similar to primary small clones (Fig. 2, B and C, far right panels). The proportion of persisting clones [28] from the original large CFU was significantly greater for both cell types compared with the small CFU (Fig. 2, D and E). There was a trend for increasing CFU activity in epithelial secondary clones and also for stromal clones for secondary and tertiary clones (Fig. 2, F and G), but this increase was mainly small compared with large clones, indicating that the large CFU produce more differentiated progeny with decreasing proliferative potential at each serial cloning step.
The number of PDs undergone by individual large epithelial or stromal CFU during 3–4 subclonings was 35–45 (data not shown). A total of 12–13 PDs of the original CFU produced a primary clone between 4 × 103 and 8 × 103cells, and the size of subsequent subclones ranged between 0.5 × 103 and 1 × 103 cells, representing a further 9–10 PDs for the second, third, and fourth subclonings. This number of PDs for individual large CFU cultured at clonal density is similar to that obtained from single large CFU cultured to senescence at bulk culture densities (see below, Table 4).
TABLE 4.
Total cell output from individual human endometrial large and small CFU.
Proliferative Potential of Endometrial CFU
Proliferative potential was determined by serially passaging three to five individual large and small human epithelial and stromal CFU from three different patient samples until senescence to determine the total cell output of single endometrial CFU. Large CFU could be cultured for more than 4 mo, and produced significantly more progeny than small CFU (Fig. 3). The yield from single large epithelial CFU ranged from 8.3 × 109 to 9.2 × 1011 cells, while large stromal CFU produced between 5.5 × 106 and 6.1 × 1012 cells before reaching senescence (Table 4), significantly more than the respective small epithelial and stromal CFU. Some small CFU survived in culture for 1–2 mo, but many could not be passaged. Large CFU generated six to seven orders of magnitude more cells than small CFU for both cell types (Table 4). The number of PDs—one indicator of the proliferative potential of individual CFU—was significantly greater for large epithelial (34.5 ± 2.8; n = 3) and large stromal (30.4 ± 2.3; n = 3) CFU than the respective small CFU (P < 0.005 and P < 0.001, respectively) (Table 4). When small colonies reached senescence, large CFU had already undergone twice the number of PDs compared with small CFU (Fig. 3), indicating that the generation time was half that for small CFU. Since CFU maintain the proliferative capacity of cell cultures, we assessed their number at each passage to determine the number of cell generations [22] and demonstrated that large CFU underwent >3- to 4-fold more cell generations than did small CFU (Table 4).
FIG. 3.
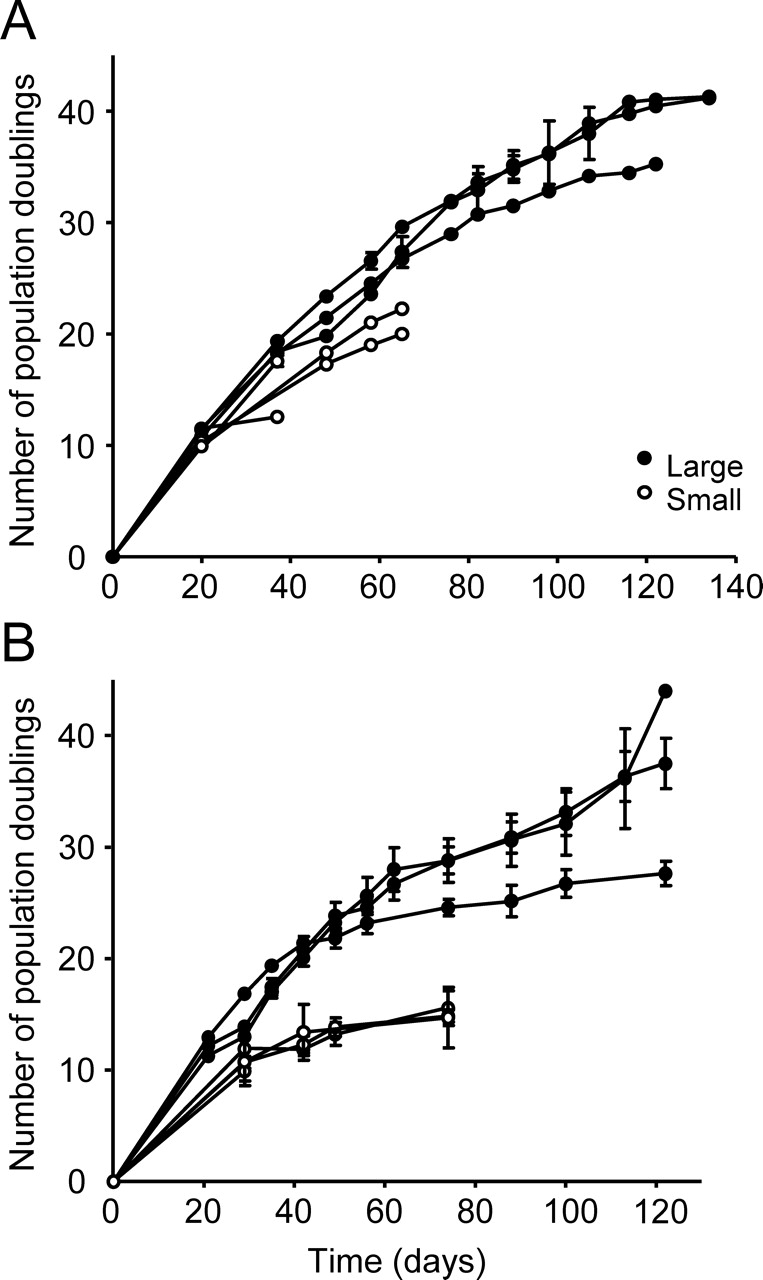
Proliferative potential of human endometrial large and small epithelial and stromal CFU. Growth curves from single primary large and small epithelial (A) and stromal (B) CFU from individual patient samples passaged at 2000 cells/cm2 illustrating differences in cell proliferation rates and total cell output. Each curve is the mean ± SEM of n = 3–5 clones derived from an individual patient sample (n = 3 patients, nos. 10, 12, and 13 for epithelial; n = 4 patients, nos. 1–4 for stromal).
Differentiation of Endometrial CFU
To examine the differentiation potential of single endometrial CFU, we harvested individual secondary epithelial and stromal clones (Fig. 2) and expanded them in culture to produce sufficient cells for differentiation induction. Small CFU were not examined as insufficient numbers of cells were produced (Table 4). Clonally derived epithelial cells formed small spheroid cytokeratin+ structures in three-dimensional Matrigel cultures (data not shown), but when a stromal feeder layer was included below the Matrigel layer (Fig. 4, A and B), much larger cytokeratin+ (Fig. 4, B and C) gland-like structures developed, indicating the importance of niche cells. These structures were 15× larger than similarly cultured fresh endometrial cells, and 12× larger than without the endometrial stromal feeder layer, suggesting that single endometrial epithelial CFU have the capacity to differentiate into mature “glands” in vitro.
FIG. 4.
Multilineage differentiation of single-cell-derived large human endometrial epithelial CFU and stromal CFU. A–C) Clonally derived epithelial cells (large CFU) cultured in 50% Matrigel above an endometrial stromal cell feeder layer for 4 wk differentiated into epithelial gland-like structures (A) observed by phase contrast microscopy, and (B and C) immunoreactive for cytokeratin. Inset in C is the isotype control. D–G) Clonally derived stromal cells (two clones) were cultured as monolayers or cell pellets (chondrogenic) for 4 wk in differentiation-induction media for mesenchymal lineages and assessed for lineage-specific markers by histochemistry, immunocytochemistry (upper panels), and RT-PCR (lower panels). D) Myogenic differentiation to smooth muscle cells, positive for αSMA and caldesmon. E) Osteogenic differentiation indicated by alkaline phosphatase reactivity and expression of parathyroid hormone receptor 1 (PTHR1). F) Adipogenic differentiation visualized by Oil Red O staining of lipid droplets and expression of lipoprotein lipase (LPL). G) Chondrogenic differentiation shown in a paraffin section of a micromass cell pellet stained with Alcian blue, and collagen type II expression. Cells cultured in control culture media for 4 wk and stained for lineage markers are shown as insets (D–G) for each lineage and as (−) for RT-PCR analysis; 18S mRNA was the internal control. Shown are results from a single patient sample representative of three (patient sample nos. 4, 5, 6, and 13). Bars = 50 μm (including insets).
Large secondary stromal clones, originating from a single stromal CFU, demonstrated multipotency as their progeny differentiated into four mesenchymal lineages when cultured in typical differentiation-induction media. In myogenic medium, clonally derived stromal cells differentiated into αSMA- and caldesmon-expressing cells (Fig. 4D), suggesting smooth muscle rather than myofibroblast differentiation. In osteogenic medium, clonally derived stromal cells strongly expressed alkaline phosphatase and PTHR1, an osteogenic lineage-specific marker, indicative of osteogenic differentiation (Fig. 4E). Most clonally derived stromal cells cultured in adipogenic differentiation media accumulated lipid droplets, as observed by phase contrast microscopy and confirmed with Oil Red O staining. They also expressed the adipocyte gene, LPL (Fig. 4F). Clonally derived stromal cells cultured as a pelleted micromass for chondrogenic differentiation showed strong Alcian blue staining of cartilaginous matrix in paraffin-embedded sections (Fig. 4G), and expressed the chondrocyte-specific marker, collagen type II. Control cultures failed to form sufficient extracellular cartilaginous matrix to produce pellets. Similarly, mesenchymal lineage-specific markers were not observed in any control cultures (Fig. 4, D–G). These data suggest that single stromal CFU have MSC properties.
Phenotype of Clonally Derived Large Stromal CFU
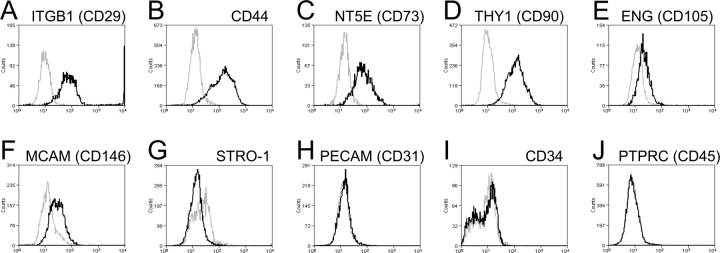
Large secondary stromal CFU were then examined for typical MSC phenotypic surface markers. Flow cytometric analysis showed that the majority of clonally derived human endometrial stromal cells expressed high levels of the surface markers, ITGB1 (CD29), CD44, NT5E (CD73), and THY1 (CD90); approximately 20% expressed ENG (CD105) and MCAM (CD146), and were negative for PECAM1 (CD31), CD34, and PTPRC (CD45) (Fig. 5), indicating that they have a similar phenotype to bone marrow and fat MSCs, although they lacked Stro-1 expression (Fig. 5).
FIG. 5.
Phenotyping of human endometrial stromal secondary CFU for typical MSC surface markers. A–G) Single-parameter histograms for individual MSC markers and (H–J) MSC exclusion markers, representative of two to three patient samples (nos. 2, 4, and 5) (black lines). Gray lines indicate background fluorescence obtained with isotype control IgG or IgM. The x axis represents fluorescence intensity, and the y axis represents cell counts.
DISCUSSION
The human endometrium is a cyclically regenerating mucosal tissue comprising glands and an extensive vascularized stroma. Our findings show, for the first time, that rare individual endometrial cells with colony forming activity (large CFU) display adult stem cell properties of self-renewal, differentiation, and high proliferative potential in vitro. This suggests that they are responsible for monthly endometrial tissue regeneration, preparing the endometrium for steroid hormone-initiated differentiation into a receptive environment for embryo implantation. Both epithelial progenitor cell and MSC-like populations were identified. The entire endometrial functionalis layer, which is shed each month during menstruation, is likely replenished from these endometrial epithelial and stromal CFU, postulated to reside in the basalis. The small CFU, a more numerous population, have less proliferative potential, and are likely transit-amplifying cells, defined as proliferative stem cell progeny fated for differentiation, that also contribute to the rapid monthly growth of the endometrial mucosa.
Emerging evidence suggests that human and mouse endometrium may contain adult stem cells. Reports have identified clonogenic cells [10], side population (SP) cells [13, 29], and some cells that can differentiate into chondrocytes [15] or adipocytes [30] in human endometrium, while mouse endometrium contains label-retaining cells (LRC) [31, 32]. However, identification of CFU, SP cells, or LRC alone in adult tissues is not sufficient to prove the existence of adult stem cells [33]. It is necessary to demonstrate key adult stem cell properties of individual putative stem cells. In the present study, we show that single, freshly isolated human endometrial epithelial cells initiating large epithelial CFU undergo substantial self-renewal in serial cloning or replating assays, have high proliferative potential, producing billions of cells, and undergo unilineage differentiation into gland-like organoids in vitro. These properties suggest that endometrial progenitor cells initiate the rare, large epithelial CFU of human endometrium. We also found that rare, single, freshly isolated endometrial stromal cells self-renew, have high proliferative potential, and undergo multilineage differentiation into four mesenchymal lineages in vitro, suggesting that they are similar to bone marrow MSCs [34]. Large endometrial stromal CFU undergo substantial self-renewal, producing tertiary or higher order clones, indicating that they are mesenchymal stem/progenitor cells [35]. Small epithelial and stromal CFU may be initiated by more differentiated transit-amplifying cell populations that progressively acquire differentiation markers as they undergo several rounds of proliferation to produce small clones [10], in a similar manner to that demonstrated for epidermal and hemopoietic stem cell hierarchies [1, 4].
Various human endometrial stromal populations can be induced to differentiate into one or more mesenchymal lineages when cultured under appropriate differentiation-inducing conditions [12, 15, 30]. In adipogenic or chondrogenic media, an undefined proportion of cells differentiated into adipocytes or chondrocytes [15, 30]. Furthermore, endometrial cells cultured from a single sample of menstrual blood also differentiated into five mesenchymal lineages and into a single neural lineage [36]. However, human endometrial stroma comprises a heterogeneous population of cells, and it is uncertain which cells are represented in these experiments. These studies highlight an unresolved question about whether nonclonogenic endometrial stromal cells are multipotent and have multilineage differentiation capacity. Only some of the stromal cells appeared to differentiate, and they may represent the 15% of clonogenic cells present in these cultures [30]. Studies on the MCAM (CD146)+PDGFR+ endometrial stromal cell population enriched 8 fold for stromal CFU, demonstrated that most cells differentiated into 4 mesenchymal lineages (adipogenic, myogenic, chondrogenic, and osteogenic) [12], while the MCAM (CD146) −PDGFR− cells lacked this capacity, suggesting that the clonogenic cells were responsible for mesenchymal differentiation. In contrast, the present study demonstrates that large endometrial stromal CFU, which comprise 0.02% of fresh endometrial stromal cells [10], underwent multilineage differentiation at the single-cell level. More comprehensive quantitative studies are required to determine if a larger proportion of endometrial stromal cells differentiate into one or several lineages. However, such studies await the identification of a marker that enriches endometrial MSCs to purity, to allow for comparisons between prospectively isolated pure populations of MSCs and non-MSCs for multipotency.
Epithelial stem/progenitor cells have been identified in some human tissues with varying rates of cellular turnover, including rapidly proliferating epidermis [37], ocular surface [22] and intestine [38], occasionally remodelling breast epithelium [33], and relatively static prostate [39] epithelium. Human clonogenic epithelial cells typically produce several types of colony, which have different capacities for self-renewal, differentiation and proliferative potential. Similar to endometrial epithelial CFU, only the rarer and largest epidermal keratinocyte (holoclones) [37] and prostate epithelial [39] CFU demonstrated adult stem cell activity. A progressive loss of serial cloning activity and conversion to small CFU was observed for large endometrial epithelial tertiary and higher order CFU, similar to epidermal holoclones [37], suggesting progressive differentiation of self-renewing epithelial CFU. In contrast, the number of mammosphere CFU [33] remained constant over 5 serial cloning passages suggesting that sphere culturing is superior to 2D culture for maintaining self-renewing human epithelial stem/progenitor cells in vitro. It is currently unknown whether clonogenic endometrial epithelial cells can be cultured as spheres. The proliferative potential (cell generations) of large endometrial epithelial CFU (40) is 2- to 4-fold less than epidermal (120–160) [37] and ocular keratinocyte CFU (80–100) [22], reflecting differences in cellular turnover between the continuously proliferating epidermal tissues and cyclically regenerating endometrial epithelium, which undergoes rapid expansion in the first 7–10 days of each menstrual cycle [1]. Clonally derived endometrial epithelial CFU differentiated into cytokeratin-expressing gland-like structures likely mediated by endometrial stromal cell signals, that together with the Matrigel extracellular matrix provided a stem cell “niche” permissive for the differentiation into simple, pseudostratified epithelium that lines endometrial glands. Unlike epidermis, mammary gland or prostate there is no basal cell layer of a different cell phenotype, nor are several derivatives produced, like ocular goblet cells. Together our data suggest that human endometrium contains a rare epithelial progenitor that self renews, has high proliferative potential and is probably unipotential.
Multipotent mesenchymal stromal cells (MSCs), defined as plastic adherent with a characteristic surface phenotype capable of differentiating into osteoblasts, adipocytes, and chondroblasts in vitro [40], have been identified in several human tissues, including bone marrow [25], adipose [26], dental pulp [41], placenta, skeletal muscle, and pancreas [42], and, now, in endometrium. Similar to endometrial MSCs, colony size variation is a feature of bone marrow MSCs, with large CFU demonstrating defining MSC properties [43]. There are limited data on human MSC self-renewal, although it has been demonstrated in vivo for dental pulp MSCs [41] and in vitro for human bone marrow [44]. Large endometrial stromal CFU can be regarded as MSCs, as they generate quaternary clones, more than the minimum definition of adult stem cell status (secondary clones) [35]. As for hemopoietic stem cells [28], persisting endometrial CFU diminished with serial cloning, and at a slower rate for large compared with small endometrial stromal CFU. The extensive proliferative capacity of human endometrial stromal cells has been exploited to provide feeder layers for supporting human embryonic stem cell cell culture [45]. Similarly, large endometrial stromal CFU demonstrate substantial proliferative capacity (30 PDs), greater than most human bone marrow, dental pulp, and adipose CFU-F (<20 PDs) [41, 46, 47], which have similar capacity to that of small endometrial stromal CFU (13 PDs), and less than that of human fetal muscle cells (40 PDs) [42]. Thus, human endometrium contains a subpopulation of multipotent mesenchymal stromal cells that self-renew and have high proliferative potential, suggesting that they are MSCs. Similar to MSCs in other tissues [42, 48], human endometrial MSCs are found in a perivascular niche [12].
Importantly, previous data have demonstrated reconstitution of endometrial tissue from xenografted human endometrial cells [49]. Single-cell suspensions of unfractionated human endometrial cells injected directly beneath the kidney capsule of NOG mice generated endometrial tissue comprising glands that proliferated in response to estrogen, and stroma that differentiated into decidual cells on further administration of progesterone. Withdrawal of hormones resulted in a menstruation-like event in the reconstituted endometrial tissue [49]. Transplantation of human endometrial tissue fragments into immunocompromised mice has served as a mouse model for investigating endometriosis [50]. The survival and remodeling of the transplanted cells and tissue suggests that endometrial epithelial progenitors and MSCs, present in the original transplant, may be responsible for ectopic endometrial tissue growth. It also suggests their possible role in the pathophysiology of endometriosis [1]. It is important that future studies examine the capacity of clonally derived endometrial CFU to reconstitute endometrial tissue in vivo using similar xenotransplantation models as reported by Masuda et al. [49]. Consideration will need to be given to recapitulate stem cell niche conditions to enable transplanted stem/progenitor cells to generate endometrial tissue.
Our study has demonstrated adult stem cell activity for two cell types in human endometrium: an epithelial progenitor and an MSC. However, the stem cell assays used to identify these adult stem cells are retrospective, and do not allow their prospective isolation from endometrium, nor do they identify their location in endometrial tissue. We speculated that both endometrial epithelial and stromal CFU would be found in the basalis layer that remains during menstruation, and from which the new functionalis regenerates [1]. Interestingly, our recent discovery of markers that partially purify endometrial MSCs showed that they are located in a perivascular niche in both the functionalis and basalis blood vessels [12]. However, there are currently no known markers for endometrial epithelial progenitors, and their location is currently unknown.
Our demonstration of adult stem/progenitor cell and transit-amplifying cell activity in human endometrium provides the impetus for discovery of endometrial epithelial stem/progenitor cell markers and more specific MSC markers to distinguish these cell types, as the assays that we have developed provide a means for assessing the effectiveness of potential markers [12]. It will then be possible to determine their expression of steroid hormone receptors, their location in normal endometrium, and to investigate the role of endometrial stem/progenitor cells in the pathogenesis of common gynecological diseases associated with abnormal endometrial regeneration. Such knowledge will enhance our understanding of endometriosis, adenomyosis, endometrial hyperplasia, and endometrial cancer, and has the potential to provide new therapeutic options targeting key endometrial stem/progenitor cell functions in preference to current hormonal manipulations. Endometrial MSCs may also provide an alternative readily available source of autologous MSCs for cell-based therapies in tissue engineering applications.
Acknowledgments
The authors acknowledge Nicki Sam and Nancy Taylor for collection of the tissue, gynecologists at Southern Health and Waverley Private Hospital for the provision of hysterectomy tissue, and technical assistance from Saddaf Naqvi.
Footnotes
1Supported by National Health Medical and Research Council (NHMRC) of Australia grant 284344, Cancer Council Victoria grant 491079, NHMRC RD Wright Career Development Award 465121 to C.E.G., and Monash University Graduate Scholarships to K.E.S. and H.P.T.N.
REFERENCES
- Gargett CE.Uterine stem cells: what is the evidence? Hum Reprod Update 2007; 13: 87–101. [DOI] [PubMed] [Google Scholar]
- Jabbour HN, Kelly RW, Fraser HM, Critchley HOD.Endocrine regulation of menstruation. Endocrine Rev 2006; 27: 17–46. [DOI] [PubMed] [Google Scholar]
- McLennan CE, Rydell AH.Extent of endometrial shedding during normal menstruation. Obstet Gynecol 1965; 26: 605–621. [PubMed] [Google Scholar]
- Fuchs E, Segre JA.Stem cells: a new lease on life. Cell 2000; 100: 143–155. [DOI] [PubMed] [Google Scholar]
- Li L, Xie T.Stem cell niche: structure and function. Annu Rev Cell Dev Biol 2005; 21: 605–631. [DOI] [PubMed] [Google Scholar]
- Eckfeldt CE, Mendenhall EM, Verfaillie CM.The molecular repertoire of the ‘almighty' stem cell. Nature Rev Molec Cell Biol 2005; 6: 726–737. [DOI] [PubMed] [Google Scholar]
- Shostak S.(Re)defining stem cells. BioEssays 2006; 28: 301–308. [DOI] [PubMed] [Google Scholar]
- Moore KA, Lemischka IR.Stem cells and their niches. Science 2006; 311: 1880–1885. [DOI] [PubMed] [Google Scholar]
- Padykula HA.Regeneration of the primate uterus: the role of stem cells. Ann N Y Acad Sci 1991; 622: 47–56. [DOI] [PubMed] [Google Scholar]
- Chan RWS, Schwab KE, Gargett CE.Clonogenicity of human endometrial epithelial and stromal cells. Biol Reprod 2004; 70: 1738–1750. [DOI] [PubMed] [Google Scholar]
- Schwab KE, Chan RW, Gargett CE.Putative stem cell activity of human endometrial epithelial and stromal cells during the menstrual cycle. Fertil Steril 2005; 84(suppl 2):1124–1130. [DOI] [PubMed] [Google Scholar]
- Schwab KE, Gargett CE.Co-expression of two perivascular cell markers isolates mesenchymal stem-like cells from human endometrium. Hum Reprod 2007; 22: 2903–2911. [DOI] [PubMed] [Google Scholar]
- Kato K, Yoshimoto M, Kato K, Adachi S, Yamayoshi A, Arima T, Asanoma K, Kyo S, Nakahata T, Wake N.Characterization of side-population cells in human normal endometrium. Hum Reprod 2007; 22: 1214–1223. [DOI] [PubMed] [Google Scholar]
- Schwab KE, Hutchinson P, Gargett CE.Identification of surface markers for prospective isolation of human endometrial stromal colony-forming cells. Hum Reprod 2008; 23: 934–943. [DOI] [PubMed] [Google Scholar]
- Wolff EF, Wolff AB, Du H, Taylor HS.Demonstration of multipotent stem cells in the adult human endometrium by in vitro chondrogenesis. Reprod Sci 2007; 14: 524–533. [DOI] [PubMed] [Google Scholar]
- Matthai C, Horvat R, Noe M, Nagele F, Radjabi A, van TM, Huber J, Kolbus A.Oct-4 expression in human endometrium. Mol Hum Reprod 2006; 12: 7–10. [DOI] [PubMed] [Google Scholar]
- Simoens S, Hummelshoj L, D'Hooghe T.Endometriosis: cost estimates and methodological perspective. Hum Reprod Update 2007; 13: 395–404. [DOI] [PubMed] [Google Scholar]
- Giudice LC, Kao LC.Endometriosis. Lancet 2004; 364: 1789–1799. [DOI] [PubMed] [Google Scholar]
- Di Cristofano A, Ellenson LH.Endometrial carcinoma. Ann Rev Pathol Mech Dis 2007; 2: 57–85. [DOI] [PubMed] [Google Scholar]
- Amant F, Moerman P, Neven P, Timmerman D, Van LE, Vergote I.Endometrial cancer. Lancet 2005; 366: 491–505. [DOI] [PubMed] [Google Scholar]
- Noyes RW, Hertig AT, Rock J.Dating the endometrial biopsy. Fertil Steril 1950; 1: 3–25. [DOI] [PubMed] [Google Scholar]
- Pellegrini G, Golisano O, Paterna P, Lambiase A, Bonini S, Rama P, De Luca M.Location and clonal analysis of stem cells and their differentiated progeny in the human ocular surface. J Cell Biol 1999; 145: 769–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A, Simmons PJ, Kaur P.Identification and isolation of candidate human keratinocyte stem cells based on cell surface phenotype. Proc Natl Acad Sci U S A 1998; 95: 3902–3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinehart CA, Jr, Lyn-Cook BD, Kaufman DG.Gland formation from human endometrial epithelial cells in vitro. In Vitro Cell Dev Biol 1988; 24: 1037–1041. [DOI] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR.Multilineage potential of adult human mesenchymal stem cells. Science 1999; 284: 143–147. [DOI] [PubMed] [Google Scholar]
- Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH.Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell 2002; 13: 4279–4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaberg RM, van der Kooy D.Stem and progenitor cells: the premature desertion of rigorous definitions. Trends Neurosci 2003; 26: 125–131. [DOI] [PubMed] [Google Scholar]
- Marley SB, Lewis JL, Gordon MY.Progenitor cells divide symmetrically to generate new colony-forming cells and clonal heterogeneity. Br J Haematol 2003; 121: 643–648. [DOI] [PubMed] [Google Scholar]
- Tsuji T, Yoshimoto M, Takahashi K, Noda Y, Nakahata T, Heike T.Side population cells contributed to the genesis of human endometrium. Fertil Steril 2008; 90(suppl 4):1528–1537. [DOI] [PubMed] [Google Scholar]
- Dimitrov R, Timeva T, Kyurkchiev D, Stamenova M, Shterev A, Kostova P, Zlatkov V, Kehayov I, Kyurkchiev S.Characterization of clonogenic stromal cells isolated from human endometrium. Reprod 2008; 135: 551–558. [DOI] [PubMed] [Google Scholar]
- Chan RW, Gargett CE.Identification of label-retaining cells in mouse endometrium. Stem Cells 2006; 24: 1529–1538. [DOI] [PubMed] [Google Scholar]
- Cervello I, Martinez-Conejero JA, Horcajadas JA, Pellicer A, Simon C.Identification, characterization and co-localization of label-retaining cell population in mouse endometrium with typical undifferentiated markers. Hum Reprod 2007; 22: 45–51. [DOI] [PubMed] [Google Scholar]
- Dontu G, Abdallah WM, Foley JM, Jackson KW, Clarke MF, Kawamura MJ, Wicha MS.In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev 2003; 17: 1253–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaananen HK.Mesenchymal stem cells. Ann Med 2005; 37: 469–479. [DOI] [PubMed] [Google Scholar]
- Reynolds BA, Rietze RL.Neural stem cells and neurospheres—re-evaluating the relationship. Nat Meth 2005; 2: 333–336. [DOI] [PubMed] [Google Scholar]
- Patel AN, Park E, Kuzman M, Benetti F, Silva FJ, Allickson JG.Multipotent menstrual blood stromal stem cells: isolation, characterization, and differentiation. Cell Transplant 2008; 17: 303–311. [DOI] [PubMed] [Google Scholar]
- Barrandon Y, Green H.Three clonal types of keratinocyte with different capacities for multiplication. Proc Natl Acad Sci U S A 1987; 84: 2302–2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead RH, Demmler K, Rockman SP, Watson NK.Clonogenic growth of epithelial cells from normal colonic mucosa from both mice and humans. Gastroenterol 1999; 117: 858–865. [DOI] [PubMed] [Google Scholar]
- Hudson DL, O'Hare M, Watt FM, Masters JRW.Proliferative heterogeneity in the human prostate: evidence for epithelial stem cells. Lab Invest 2000; 80: 1243–1250. [DOI] [PubMed] [Google Scholar]
- Dominici M, Le BK, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E.Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006; 8: 315–317. [DOI] [PubMed] [Google Scholar]
- Gronthos S, Brahim J, Li W, Fisher LW, Cherman N, Boyde A, DenBesten P, Robey PG, Shi S.Stem cell properties of human dental pulp stem cells. J Dental Res 2002; 81: 531–535. [DOI] [PubMed] [Google Scholar]
- Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, Norotte C, Teng PN, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 2008; 3: 301–313. [DOI] [PubMed] [Google Scholar]
- DiGirolamo CM, Stokes D, Colter D, Phinney DG, Class R, Prockop DJ.Propagation and senescence of human marrow stromal cells in culture: a simple colony-forming assay identifies samples with the greatest potential to propagate and differentiate. Br J Haematol 1999; 107: 275–281. [DOI] [PubMed] [Google Scholar]
- Colter DC, Class R, DiGirolamo CM, Prockop DJ.Rapid expansion of recycling stem cells in cultures of plastic-adherent cells from human bone marrow. Proc Natl Acad Sci U S A 2000; 97: 3213–3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JB, Lee JE, Park JH, Kim SJ, Kim MK, Roh SI, Yoon HS.Establishment and maintenance of human embryonic stem cell lines on human feeder cells derived from uterine endometrium under serum-free condition. Biol Reprod 2005; 72: 42–49. [DOI] [PubMed] [Google Scholar]
- Gronthos S, Zannettino ACW, Hay SJ, Shi ST, Graves SE, Kortesidis A, Simmons PJ.Molecular and cellular characterisation of highly purified stromal stem cells derived from human bone marrow. J Cell Sci 2003; 116: 1827–1835. [DOI] [PubMed] [Google Scholar]
- Kern S, Eichler H, Stoeve J, Kluter H, Bieback K.Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells 2006; 24: 1294–1301. [DOI] [PubMed] [Google Scholar]
- Shi S, Gronthos S.Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. J Bone Mineral Res 2003; 18: 696–704. [DOI] [PubMed] [Google Scholar]
- Masuda H, Maruyama T, Hiratsu E, Yamane J, Iwanami A, Nagashima T, Ono M, Miyoshi H, Okano HJ, Ito M, Tamaoki N, Nomura T, et al. Noninvasive and real-time assessment of reconstructed functional human endometrium in NOD/SCID/γcnull immunodeficient mice. Proc Natl Acad Sci U S A 2007; 104: 1925–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull ML, Prentice A, Wang DY, Butt RP, Phillips SC, Smith SK, Charnock-Jones DS.Nimesulide, a COX-2 inhibitor, does not reduce lesion size or number in a nude mouse model of endometriosis. Hum Reprod 2005; 20: 350–358. [DOI] [PubMed] [Google Scholar]