Abstract
Dietary supply of nutrients, both periconception and during pregnancy, influence the growth and development of the fetus and offspring and their health into adult life. Despite the importance of research efforts surrounding the developmental origins of health and disease hypothesis, the biological mechanisms involved remain elusive. Mitochondria are of major importance in the oocyte and early embryo, particularly as a source of ATP generation, and perturbations in their function have been related to reduced embryo quality. The present study examined embryo development following periconception exposure of females to a high-protein diet (HPD) or a low-protein diet (LPD) relative to a medium-protein diet (MPD; control), and we hypothesized that perturbed mitochondrial metabolism in the mouse embryo may be responsible for the impaired embryo and fetal development reported by others. Although the rate of development to the blastocyst stage did not differ between diets, both the HPD and LPD reduced the number of inner cell mass cells in the blastocyst-stage embryo. Furthermore, mitochondrial membrane potential was reduced and mitochondrial calcium levels increased in the 2-cell embryo. Embryos from HPD females had elevated levels of reactive oxygen species and ADP concentrations, indicative of metabolic stress and, potentially, the uncoupling of oxidative phosphorylation, whereas embryos from LPD females had reduced mitochondrial clustering around the nucleus, suggestive of an overall quietening of metabolism. Thus, although periconception dietary supply of different levels of protein is permissive of development, mitochondrial metabolism is altered in the early embryo, and the nature of the perturbation differs between HPD and LPD exposure.
Keywords: embryo, metabolism, mitochondria, nutrition
The supply of high (25%) or low (9%) levels of dietary protein to the female mouse prior to conception is permissive of embryo development but has differential effects on the metabolism and mitochondria of 2-cell embryos.
INTRODUCTION
During the early 1990s, with the emergence of the Barker hypothesis and what is now termed the developmental origins of health and disease hypothesis, came the comprehension that a low weight at birth predisposes offspring to reduced development and health into adult life. With this, our knowledge concerning the impact of maternal diet and lifestyle choices during pregnancy on subsequent offspring has evolved significantly (for recent reviews, see [1, 2]). At the forefront of this research, various studies using rodent models clearly demonstrate that maternal dietary protein supply during pregnancy can program adverse health outcomes, such as hypertension [3, 4], heart disease [5], and diabetes [6]. In contrast, implications of dietary protein supply before and at the time of conception specifically are comparatively less well understood but also likely to elicit effects that have ramifications on growth, metabolism, and health during development [7].
Our awareness about the preimplantation stage of development as a crucial window of time has been highlighted by studies in humans undergoing assisted reproduction [8–11] and recent studies in animal models [12, 13]. With regards to dietary protein supply during this periconception period, imbalances in protein supply (i.e., high or low levels) affect fertilization [14], embryo development rates [15, 16], and embryo quality [7, 17]. The supply of high levels of protein or urea periconception increases reproductive tract ammonium levels [17, 18], allowing inferences to be drawn from studies investigating ammonium exposure during embryo development that show reduced blastocyst cell numbers and increased apoptosis [19]. The developing fetus and placenta also are influenced by periconception dietary protein supply, including a reduction in pregnancy rates [15], altered fetal development and abnormalities [15, 20, 21], reduced placental weight and size [7, 22], and altered imprinted gene expression in fetal tissue [15, 23]. Although evidence to date implicates a low-protein diet (LPD) in rodents with perturbed embryo, fetal, and offspring development, comparatively less work has focused on the consequences of a high-protein diet (HPD) similar to that consumed in popular weight-loss programs [24]. Thus, the link between imbalanced dietary protein levels and impaired development are clear, but the mechanisms contributing to this remain to be elucidated.
During the early stages of development, the embryo undergoes dynamic changes in its metabolic requirements as its mitochondria evolve and mature in both structure and function to facilitate cellular homeostasis. As a consequence, the preimplantation-stage embryo is susceptible to changes in the environment, including nutrient supply. Optimal oocyte and embryo metabolism contributes to embryo quality and subsequent development postimplantation [25], and when mitochondrial function is inhibited in the embryo directly, poor fetal outcomes result [26]. Furthermore, studies in numerous species have linked mitochondrial parameters, such as number, distribution, and membrane potential, with embryo competence [27–30]. The present study examined periconception maternal dietary protein supply and hypothesized that altered mitochondrial structure and metabolic function measured in 2-cell embryos from these females contribute to perturbations in embryo development and, potentially, could disrupt subsequent fetal and offspring development and health, as described by others [7, 20, 31].
MATERIALS AND METHODS
Animals and Dietary Intervention
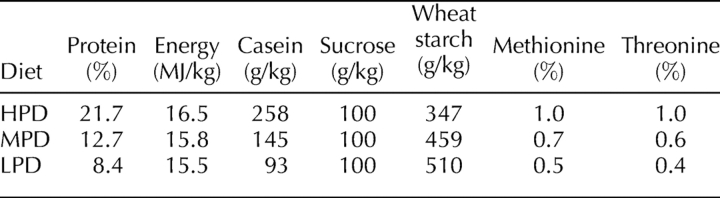
From 5 wk of age, outbred female Swiss mice (Laboratory Animal Services) were fed one of three diets for 3–4 wk before oocyte or embryo collection, with the same length of dietary exposure across all diets for any one measure. The diets were formulated to supply 7% total fat, 4.7% crude fiber, and between 15.5 and 16.5 MJ/kg of digestible energy. The protein concentration was 21.7% for the HPD, 12.7% for the medium-protein diet (MPD; control), and 8.4% for the LPD (Agro-Nutritional Research Laboratory), achieved by altering the dietary composition of wheat starch and casein (Table 1), similar to the Southampton-style diet [32]. The semipure diets were manufactured by Specialty Feeds and were sterilized by gamma radiation. All experimental procedures were conducted in accordance with the National Research Council publication Guide for Care and Use of Laboratory Animals and were approved by the University of Adelaide Animal Ethics Committee. All animals were housed in groups, and the number of animals housed together was kept constant for each diet.
TABLE 1.
Nutritional values for the major constituents of the high- (HPD), medium- (MPD), and low- (LPD) protein experimental diets.
Physiological Data Collection
Body weight was recorded for individual females at the commencement of the experiment and then weekly thereafter until the completion of the feeding period (n = 22–23 females/diet). Following euthanasia by cervical dislocation, the liver, ovary, pancreas, and fat (retroperitoneal and peritoneal deposits) were dissected from these mice and the weights recorded. From this cohort, weekly feed intake was determined over a 3-wk period for four groups of mice (n = 3 females) per diet using metabolism cages to enable the measurement of residual feed for group-housed females, and no difference was found in the amount of feed consumed for groups on each diet (data not shown). Blood samples also were taken from these mice via suborbital bleeding, and plasma metabolites were measured using enzymatic calorimetry and a COBAS Mira automated sample system for the measurement of glucose, free fatty acids (FFA), and triglycerides (TG), using the assay kits, as described previously [33].
Media Composition
The media used for embryo culture were G1.2 and G2.2 sequential medium (Vitrolife), and G-MOPS was used as a collection and handling medium [34]. All other chemicals were from Sigma unless specified otherwise.
Ovulation Rate
Following the dietary intervention for 3–4 wk, female Swiss outbred mice (age, 8–9 wk) were treated via intraperitoneal injection with 5 IU of equine chorionic gonadotropin (eCG; Folligon; Intervet) and, 44 h later, with 5 IU of human chorionic gonadotropin (hCG; Pregnyl; Organon). Ovulated cumulus-oocyte complexes (COCs) were retrieved 15 h after hCG administration from the oviduct by cutting it in close proximity to the COC mass, releasing the COCs into G-MOPS medium (n = 7–8 females/diet). The number of COCs was counted for each female; this number included any COCs remaining in the oviduct, which was determined by examining the flattened oviduct on a microscope slide under a dissecting microscope.
Embryo Collection
Embryos were collected from female mice following dietary intervention for 3–4 weeks. Females were treated via intraperitoneal injection with 5 IU of eCG and, 48 h later, with 5 IU of hCG. Following the intraperitoneal injection of hCG, female mice were housed with males (CBA/C57BL6 F1 hybrid strain) overnight, and mating was confirmed the following morning by the presence of a vaginal plug. Embryos at the 2-cell stage were flushed from the oviduct of mated female mice 43 h post-hCG injection and were collected separately for each individual in G-MOPS medium. Subsequently, the retrieved 2-cell embryos underwent mitochondria or metabolic analysis or were cultured to the blastocyst stage (discussed later).
Embryos also were collected at the blastocyst stage of development to measure the number of trophectoderm (TE) and inner cell mass (ICM) cells at 90 h post-hCG injection by flushing from the uterine horns in G-MOPS medium. Blastocysts for each individual female were kept separate (n = 3–4 females/diet).
Embryo Culture and Assessment of Embryo Morphology
Two-cell embryos collected from the oviduct were cultured to the blastocyst stage in G1.2 and G2.2 sequential medium with 5% added human serum albumin (Vitrolife) for 24 h and 48 h, respectively (n = 9–19 females/diet). All embryo culture media were equilibrated at 37°C in 6% CO2, 5% O2, and 89% N2 for at least 4 h before embryo culture, which was conducted in 20-μl drops under mineral oil. Embryo morphology was assessed at 66 and 90 h post-hCG injection (24 and 48 h after the commencement of culture) using a phase-contrast microscope. Embryos were classified using the following criteria: two to eight cells, morula (compact structure), blastocyst (blastocoele cavity comprises less than three quarters of the embryo), expanded blastocyst, and hatching blastocyst (clear herniation of the zona pellucida).
Differential Staining Procedure for Blastocyst- Stage Embryos
Blastocyst-stage embryos, collected in vivo, were assessed using a differential staining protocol adapted from that described by Gardner et al. [35] to determine the allocation of cells to the TE and ICM. Briefly, the zona pellucida was removed by incubating blastocysts in 0.5% pronase, followed by incubation in 10 mM picricsulfonic acid (TNBS) at 4°C for 10 min. Embryos were transferred to 0.1 mg/ml of anti-dinitrophenyl-bovine serum albumin for 10 min at 37°C and then placed into 10 μg/ml of propidium iodide in guinea pig serum for 5 min at 37°C. Blastocysts were then stained in 6 μg/ml of bisbenzimide in ethanol overnight and washed in 100% ethanol before mounting in glycerol on a microscope slide the following day. Stained blastocysts were visualized using a fluorescent microscope under a ultraviolet filter, allowing TE (appearing pink) and ICM (appearing blue) cell nuclei to be counted independently.
Fluorescent Staining of Mitochondria in 2-Cell Embryos
Fluorescent ionophores (Molecular Probes) were used to examine the mitochondria in the in vivo-derived, 2-cell mouse embryos from females fed one of the three experimental diets.
Mitochondrial membrane potential (MMP) was determined using the mitochondrial stain 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolyl-carbocynanine iodide (JC-1) [29]. Embryos from three to four females per diet (n = 16–27 embryos/diet) were stained with 1.5 mM JC-1 for 15 min at 37°C in the dark. They were washed in G-MOPS medium (containing no protein or phenol red) before immediate imaging using a confocal microscope (Nikon C1 Confocal Scanning Head, Nikon TE2000E).
The distribution of active mitochondria and the mitochondrial calcium levels were determined simultaneously in embryos by dual labeling with MitoTracker Green FM and Rhod 2AM, respectively (n = 31–36 embryos/diet) [36, 37]. Embryos from four to six females per diet were coincubated with 100 nM MitoTracker Green FM and 5 μM Rhod 2AM for 15 min at 37°C in the dark. They were washed in G-MOPS medium (containing no protein or phenol red) before immediate imaging using a confocal microscope (Nikon C1 Confocal Scanning Head, Nikon TE2000E).
Assessment and Quantification of Fluorescent Images
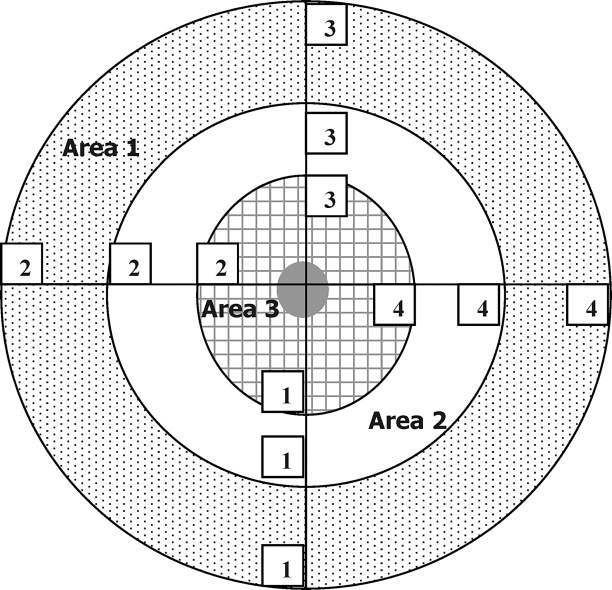
All images were analyzed using the Adobe Photoshop Pro software package (Version 13; Adobe Systems, Inc.) and a method adapted from that described by Barnett et al. [38]. The spatial organization and function of mitochondria in the embryo is related to metabolic and ionic regulation and to embryo competence, so it was examined in the following way [30, 39]. For analysis of MMP, the staining intensity (pixel intensity) was determined in four different regions (regions 1–4) within three different areas (areas 1–3) of each of the cells of the 2-cell embryos, as depicted in Figure 1. The average membrane potential in each of the three areas was expressed as a ratio of red intensity to mean green pixel intensity for each area. For analysis of mitochondrial distribution and mitochondrial calcium levels, fluorescent pixel intensity for each dye in four different regions (regions 1–4) in two different areas (area 1, cortex; area 3, adjacent to the nucleus) was determined, averaged, and expressed as a mean fluorescence intensity in the two areas.
FIG. 1.
Template for analysis of fluorescently labeled embryos imaged using confocal microscopy. Figure represents one of the cells of a 2-cell embryo and is repeated for the other cell. Area 1 = outer area, Area 2 = intermediate area, and Area 3 = perinuclear area. Numbers 1–4 in the cell where fluorescence readings were taken.
ATP and ADP Production by 2-Cell Embryos
Production of ATP and ADP by individual 2-cell mouse embryos was determined using a method described by Leese et al. [40]. Briefly, 2-cell embryos from three to four females per diet (n = 12–17 embryos/diet) were recovered 43 h post-hCG administration and placed in warm G-MOPS medium. Individual embryos were placed into 20-nl drops of G-MOPS containing 1 mg/ml of Ficoll under mineral oil. Immediately after, 4 nl of 3 M perchloric acid were added to each drop and incubated at 37°C for 10 min in the dark. Finally, 16 nl of ice-cold potassium sulfate were added to each drop to complete the deproteinization extraction process. Each drop was analyzed to determine ATP and ADP concentration using coupled reactions catalyzed by hexokinase and glucose-6-phosphate dehydrogenase and by lactate dehydrogenase and pyruvate kinase, respectively (Roche Diagnostics) [40]. Data are expressed as picomoles per embryo.
Pyruvate Uptake by 2-Cell Embryos
Pyruvate metabolism was determined using a quantitative microfluorescence assay [41]. Individual embryos from thee to four females per diet were placed in 200-nl drop of G-MOPS under oil and incubated at 37°C for 4 h (n = 25–33 embryos/diet). After 4 h, embryos were removed from the drops, and the media were analyzed using assay cocktails based on the enzymatic conversion of pyruvate and NADH by lactate dehydrogenase (Roche Diagnostics) to lactate and NAD to determine the concentration of pyruvate. Data are expressed as the uptake of pyruvate in picomoles per embryo per hour.
Reactive Oxygen Species Production by 2-Cell Embryos
The level of reactive oxygen species (ROS) was determined using a 2′,7′-dichlorodihydrofluorescein diacetate (DCDHFDA) fluorescent assay as described previously by Nasr-Esfahani et al. [42]. Embryos from three females per diet (n = 8–19 embryos/diet) were stained for 30 min in 1 μM DCDHFDA (dissolved in dimethyl sulfoxide and stored under nitrogen), then washed in G-MOPS, and the level of 2′,7′-dichlorofluorescein (DCF) was measured using fluorescence spectroscopy. The level of cellular uptake and hydrolysis of the dye was controlled for by incubating two or three embryos from each diet with 5(and 6)carboxyl 2′,7′-dichlorodihydrofluorescein diacetate (CDCFDA) for 20 min and then measuring the level of fluorescence of 5(and 6)carboxy-2′,7′-dichlorofluorescein (CDCF) using fluorescence spectroscopy. The relative fluorescence for each embryo was then determined comparative to that of the control embryos for the corresponding diet and expressed as mean fluorescence units.
Mitochondrial Transition Pore Opening in 2-Cell Embryos
Opening of the mitochondrial transition pore (mPTP) was monitored by analyzing the change in Rhod 2AM staining intensity in 2-cell embryos following exposure to increasing doses of Ca2+. Embryos were incubated with 5 μM of the fluorescent stain Rhod 2AM for 15 min in G-MOPS medium, minus calcium, and then washed. They were mounted in this medium in groups of five on a glass-bottomed Fluoro dish FD35 (World Precision Instruments, Inc.) and were imaged using a confocal microscope (Nikon C1 Confocal Scanning Head, Nikon TE2000E), with the gain settings maintained constant for all subsequent imaging. Immediately following this, embryos were incubated for 5 min with 75 μM of the calcium ionophore A23187 (Sigma) to permeabilize the plasma membrane to extracellular calcium Ca2+ [39], and then in the same field of view, embryos were imaged. Next, embryos were sequentially dosed with increasing concentrations of Ca2+, as determined in preliminary experiments in embryos (data not shown) and described for isolated mitochondria [43]. The equivalent of 30, 60, and 90 nM Ca2+ was supplied at 2-min intervals, and the embryos were imaged after each addition. All images were analyzed using the Adobe Photoshop Pro software package. The fluorescent pixel intensity for the Rhod 2AM dye was determined in four different regions (regions 1–4) of the cortex for each cell of each embryo and then averaged, and expressed as a mean fluorescence intensity for each embryo. Two replicate experiments of n ≥ 5 embryos per diet were analyzed (n = 4 females/diet).
To ensure that the change in fluorescent staining was related to mPTP opening, control embryos were incubated with the Rhod 2AM dye as described and then imaged. Subsequently, embryos were incubated with the A23187 ionophore and with 40 μg/ml of cyclosporin A (CsA) (Sigma) to prevent mPTP opening [44]. Embryos were imaged following CsA exposure and at three subsequent time points following dosage with 30, 60, and 90 nM Ca2+. All images were analyzed as described above.
Statistics
Data are expressed as the mean ± SEM unless stated otherwise. Statistical analysis was performed using a univariate general linear model in SPSS (Version 13; SPSS, Inc.). The model used included diet as a fixed factor and maternal identification number as a random effect. Analysis of all mitochondria parameters (membrane potential, mitochondria distribution, and mitochondrial calcium) and metabolic measurements (ATP, ADP, pyruvate, and ROS) were weighted for the number of 2-cell embryos recovered per female. Differences in mPTP opening in embryos as a consequence of diet were determined, using the univariate model described above, at each calcium concentration.
RESULTS
Body and Tissue Weights
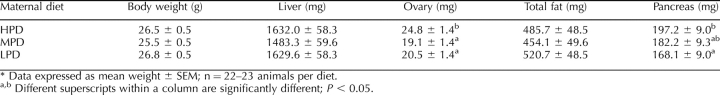
The average final body weight of females fed different levels of dietary protein for three to four weeks did not differ significantly between the three diets, nor was a difference found in the weight of combined fat deposits from the peritoneal and intraperitoneal area or in liver weight (Table 2). Pancreas weight was greatest in those from HPD females relative to LPD females, but this weight did not differ from that of the control, MPD females (Table 2). Ovary weight was significantly greater when females consumed the HPD relative to those consuming the MPD and the LPD (Table 2), and this difference remained significant when body weight was taken into account (0.92 × 10−3% vs. 0.72 × 10−3% and 0.79 × 10−3%, respectively; P < 0.05).
TABLE 2.
Average body weight, and liver, ovary, fat, and pancreas weights for Swiss female mice fed a high (HPD), medium (MPD), or low (LPD) level of dietary protein for 3–4 wk.*.
Plasma Analysis
Plasma concentration of TG, FFA, and glucose were determined, because abnormal levels are associated with insulin resistance and impaired glucose metabolism. No significant difference was found in the concentration of any of these metabolites in the plasma from mice fed the different diets (TG: HPD, 2.5 ± 0.24 mmol/L; MPD, 2.3 ± 0.28 mmol/L; LPD, 2.6 ± 0.26 mmol/L; FFA: HPD, 2.8 ± 0.16 mEq/L; MPD, 2.9 ± 0.18 mEq/L; LPD, 2.8 ± 0.17 mEq/L; glucose: HPD, 10.1 ± 0.64 mmol/L; MPD, 9.1 ± 0.74 mmol/L; LPD, 9.5 ± 0.70 mmol/L).
Ovulation Rate, Embryo Development, and Blastocyst Cell Counts
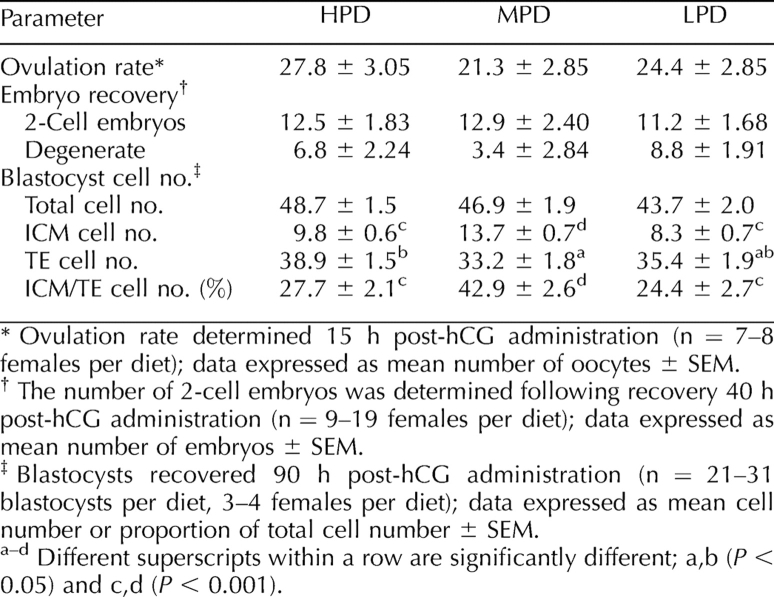
To determine the effect of maternal dietary protein supply on early oocyte and embryo development, the number of COCs ovulated and the number of 2-cell embryos recovered was determined. No significant difference was found between diets in the number of COCs ovulated following hCG administration (Table 3). This trend continued following fertilization, with an average of 12 two-cell embryos recovered per female, irrespective of diet (Table 3). Although not statistically significant, the number of degenerate oocytes and embryos recovered at this time tended to be less when females consumed the MPD relative to the HPD or LPD. No difference was found between diets in the rate of embryo development in culture from the in vivo-derived, 2-cell stage to the blastocyst stage (data not shown).
TABLE 3.
The influence of maternal dietary protein supply on ovulation rate, 2-cell embryo recovery, and average blastocyst cell number.
When embryos were collected at the blastocyst stage, those from the HPD females had the same total cell number but significantly greater TE cell numbers and reduced ICM cell numbers in comparison with embryos from MPD females. Similarly, the number of ICM cells was reduced in embryos from LPD females. This translates to a significant reduction in the proportion of ICM:TE cells from 42.9% for MPD females, versus 27.7% and 24.4% for HPD females and LPD females, respectively (Table 3).
MMP in 2-Cell Mouse Embryos
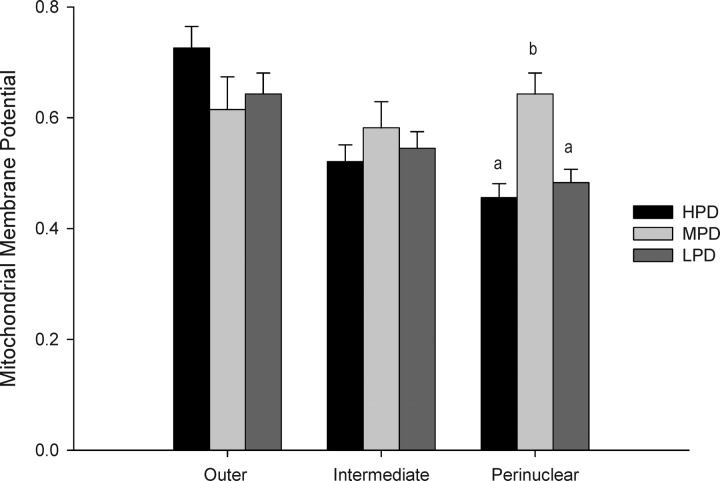
The effect of maternal dietary intervention on the mitochondria of the early embryo was assessed. No difference between maternal diets was found in MMP of the intermediate region or the outer region of the embryo (Fig. 2). A significant reduction in MMP, however, was measured in the area closest to the nucleus (perinuclear) in embryos from HPD or LPD females relative to embryos from MPD females. Furthermore, a decreasing gradient of MMP from the outside of the embryo toward the nucleus was measured in embryos from HPD females (0.73 to 0.46) and LPD females (0.64 to 0.48); however, this MMP gradient remained relatively constant across the whole embryo when derived from MPD females (0.62 to 0.64) (Fig. 2).
FIG. 2.
The effect of maternal dietary protein on mitochondrial membrane potential in the outer, intermediate, and perinuclear regions of the 2-cell embryo. Different lowercase letters within regions are significantly different (P < 0.001; n = 16–27 embryos/diet, n = 3–4 females/diet). Membrane potential is expressed as a ratio of the average red fluorescence (high potential) relative to average green fluorescence (low potential) in the three areas of the embryo.
Mitochondrial Distribution in 2-Cell Mouse Embryos
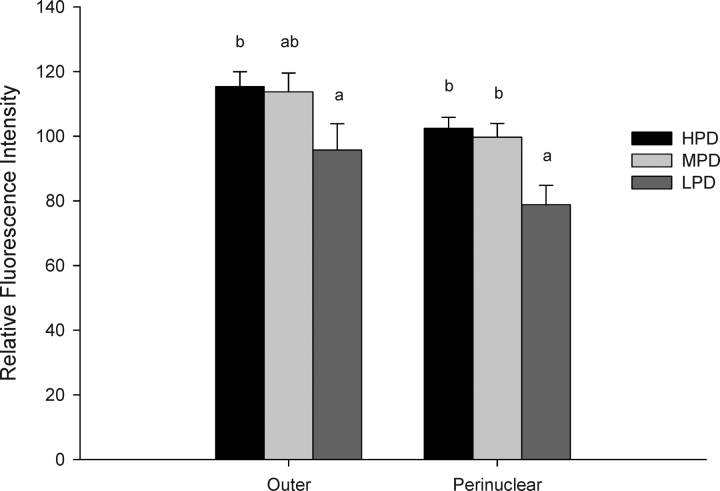
Significantly less mitochondria were found in the perinuclear region of embryos from LPD females relative to embryos from MPD and HPD females (P < 0.001), and significantly less were found in the outer region in embryos from LPD females relative to HPD females (P < 0.001) (Fig. 3).
FIG. 3.
The effect of maternal dietary protein supply on the distribution of active mitochondria in the outer and perinuclear regions of the 2-cell embryo. Different lowercase letters within areas are significantly different (P < 0.001; n = 31–36 embryos/diet, n = 4–6 females/diet).
Mitochondrial Calcium in 2-Cell Mouse Embryos
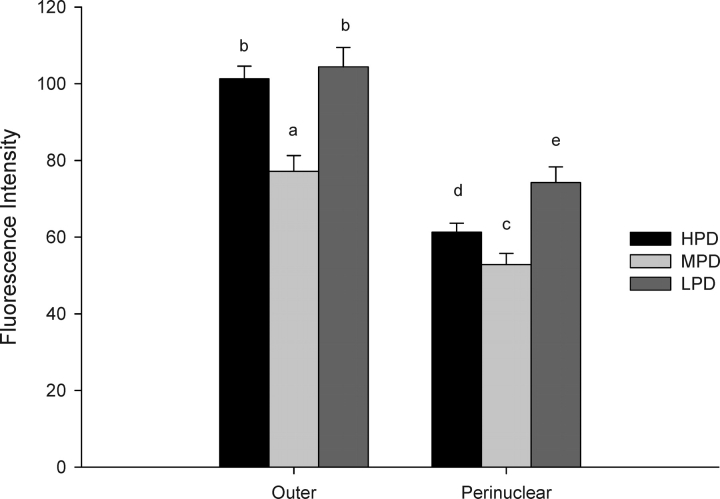
The intensity of staining for mitochondrial calcium was greater in the outer region of the embryo relative to those in the perinuclear area for all dietary treatments. Within either the outer or the perinuclear region, the levels were greater for both the HPD and LPD embryos relative to those determined for the control and MPD embryos (Fig. 4).
FIG. 4.
The effect of maternal dietary protein supply on mitochondrial calcium levels in the outer and perinuclear regions of the 2-cell embryo. Different lowercase letters within areas are significantly different (a,b P < 0.001, c–e P < 0.05; n = 31–36 embryos/diet, n = 4–6 females/diet).
ATP and ADP Production, Pyruvate Uptake, and Production of ROS by 2-Cell Embryos
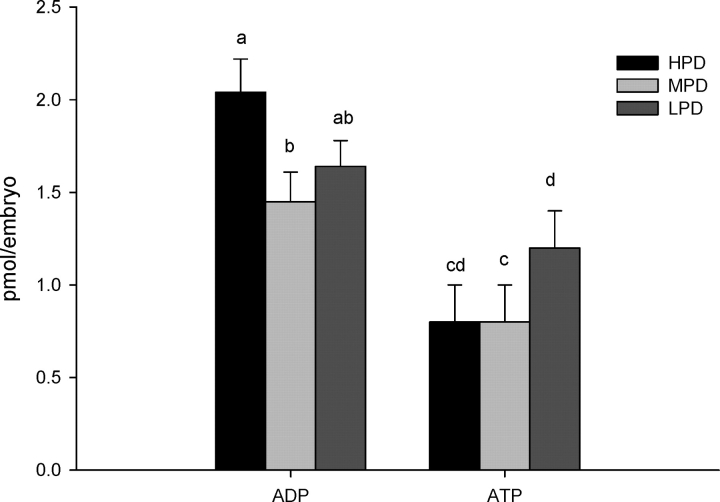
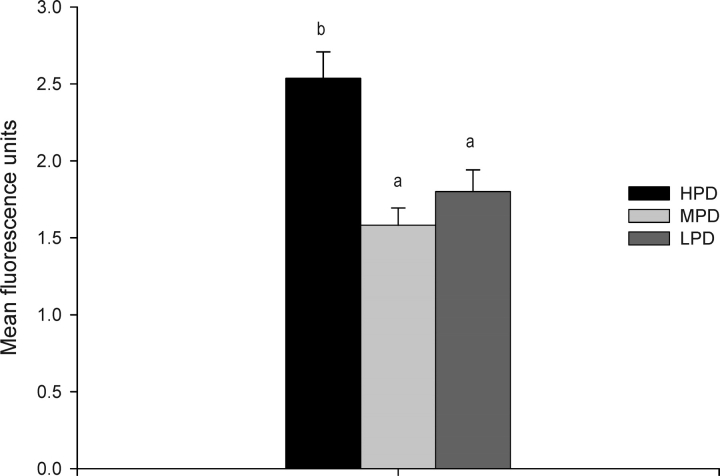
Maternal consumption of an HPD resulted in a significant increase of ADP levels in 2-cell embryos relative to those of the control MPD embryos, and ATP levels tended to be greater in LPD embryos relative to MPD embryos (P = 0.07) (Fig. 5). No significant difference in pyruvate uptake was detected in 2-cell embryos collected from females fed any of three different levels of dietary protein (HPD, 5.2 ± 0.35 pmol/embryo; MPD, 4.6 ± 0.45 pmol/embryo; LPD, 5.3 ± 0.60 pmol/embryo; n = 25–33 embryos/diet). To determine the levels of oxidative stress, the levels of ROS were measured, and significantly greater levels were found in embryos from females fed the HPD compared to embryos from the LPD or MPD (Fig. 6).
FIG. 5.
Amount of ADP and ATP for individual in vivo-derived, 2-cell mouse embryos from females fed different levels of dietary protein. Different lowercase letters are significantly different (a,b P < 0.05, c,d P = 0.07; n = 12–17 embryos/diet, n = 3–4 females/diet).
FIG. 6.
ROS production by individual 2-cell, in-vivo-derived mouse embryos from females fed different levels of dietary protein. Different lowercase letters are significantly different (P < 0.001; n = 8–19 embryos/diet, n = 3 females/diet).
Mitochondrial Transition Pore Opening in 2-Cell Embryos
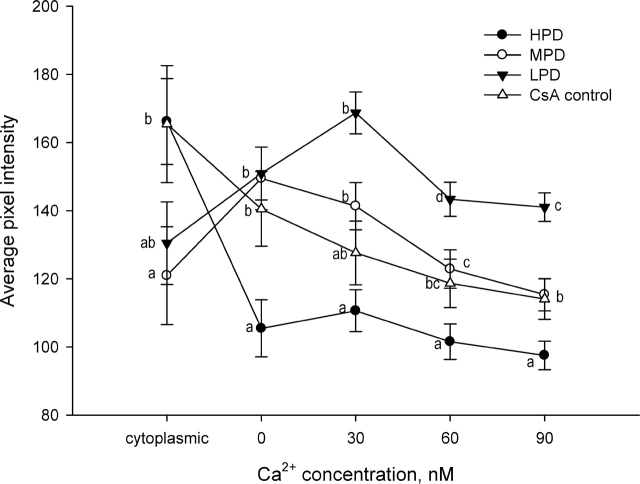
When embryos were placed into a medium without Ca2+, embryos from females fed the HPD had greater mitochondrial calcium levels than those in the MPD and LPD embryos (Fig. 7), similar to what was observed earlier in medium with 1 mM calcium (Fig. 4). Following incubation of embryos with the calcium ionophore A23187 to permeabilize the phospholipid membrane, however, embryos from the MPD and LPD females maintained significantly higher levels of calcium compared to those from the HPD females, which underwent a significant decrease in mitochondrial Ca2+ levels (Fig. 7). This difference between HPD embryos and MPD and LPD embryos in mitochondrial calcium remained with increasing doses of Ca2+. The levels of calcium in MPD embryos decreased after administration of 30 nM Ca2+, whereas the levels of calcium in LPD embryos decreased after 60 nM Ca2+. The levels of calcium for the control CsA embryos did not differ significantly from those for the MPD embryos throughout the experiment.
FIG. 7.
Change in mitochondrial calcium levels in 2-cell embryos from females fed different levels of dietary protein in response to sequential Ca2+ exposure from 0 to 90 nM. Different superscripts within calcium concentrations are significantly different (P < 0.05; n = 11–14 embryos/diet, n = 4 females/diet). Cyclosporin A (CsA): control treatment, to prevent mitochondrial transition pore opening.
DISCUSSION
The present study investigated the influence of periconception dietary protein supply on preimplantation embryo development and proposed that perturbations in embryo mitochondrial structure and metabolism are causative mechanisms underpinning altered embryo development. We have shown that changes in the mitochondria and in embryo metabolism do, in fact, occur in response to high or low levels of dietary protein, yet these changes are permissive of blastocyst formation. This development, however, is perturbed, as indicated by reduced ICM number and proportion in blastocysts recovered from females fed LPD or HPD relative to females fed the MPD control diet in the present study. This change in ICM is consistent with other findings in rodents associated with periconception diet [7, 17] and ammonium concentration in culture [12], despite embryos having a similar morphological appearance and development rate. Other studies have examined these diets or ammonium levels and found altered blastocyst gene expression and fetal development [12, 19, 20] as well as reduced implantation and placental size [7, 16, 19, 20]. To our knowledge, this is the first examination of mitochondrial and metabolic changes in early mouse embryos as a consequence of periconception dietary protein, and it is proposed that this is a potential mechanism contributing to impaired embryo development described here and to the adverse fetal development and health described by others [7, 20, 31].
Diet and Mitochondrial Calcium in Embryos
Maternal HPD or LPD increased the level of mitochondrial calcium in 2-cell embryos relative to levels measured in embryos from control females (MPD). In general, examination of mitochondrial calcium in oocytes and embryos has been limited; however, intracellular calcium plays a vital role in the completion of meiosis in the oocyte and the process of fertilization [45]. In various cell types, the majority of intracellular calcium is sequestered by mitochondria and sarcoplasmic/endoplasmic reticulum, and uptake into the mitochondria requires an electrochemical gradient [46]. Regulated accumulation of calcium in the mitochondria is essential for control of ATP generation via oxidative metabolism, because this stimulates various calcium-sensitive mitochondrial dehydrogenases, which in turn promotes proton extrusion and maintenance of the respiratory transport chain [47, 48]. Elevated mitochondrial calcium is damaging to the cell, however, and it has been implicated in apoptosis and necrosis [49]. Excessive mitochondrial calcium leads to the opening of nonselective permeability transition pores (mPTP) located within the inner mitochondrial membrane, which allows permeability of solutes from within the mitochondria. Thus, the regulation of mitochondrial calcium levels is of great importance to normal cellular functioning, and evidence in many cell types suggests that a threshold level of calcium is required for optimal mitochondrial functioning. Therefore, the elevated mitochondrial calcium levels in the embryos measured in our study relative to control (MPD) embryos may be related to the altered development observed.
Diet and MMP in Embryos
Exposure to an HPD or LPD affected MMP in 2-cell embryos similarly—that is, both diets reduced MMP in the perinuclear region of the embryo relative to levels measured in embryos from MPD females. The formation of MMP across the inner mitochondrial membrane enables oxidative phosphorylation and drives the conversion of ADP to ATP via a series of respiratory chain enzymes. The MMP, or ΔΨm, has been examined in a number of species at various stages of oocyte and embryo development, and differences in the extent of the MMP reflect variations in mitochondrial activity and function [29, 50, 51]. In human eggs and embryos, a positive relationship exists between MMP and embryo development on Day 3 of culture, a negative relationship exists between MMP and maternal age, and MMP is reduced in in vitro-aged oocytes relative to MMP in freshly recovered oocytes [30]. Acton et al. [50], however, report an increase in MMP in arrested mouse 2-cell embryos and fragmented human 8-cell embryos. These findings could be explained by the loss of metabolic regulation that likely occurs in dying or stressed embryos, and a greater MMP in early mouse embryos generally is correlated with positive embryo development. Thus, the decreased MMP reported here for mouse embryos from HPD and LPD females suggests a reduction in the proton gradient and, therefore, oxidative phosphorylation in the region surrounding the nucleus. Furthermore, others have reported a reduction in MMP in mouse oocytes that is accompanied by increased calcium levels [39], as described in the present paper. Despite the similarly disrupted calcium homeostasis and MMP described for embryos from HPD and LPD females, other parameters differed in these embryos, indicating that different mechanisms may be responsible for the perturbation in cell allocation in the blastocyst-stage embryo.
Perturbed Embryo Metabolism: HPD
To examine more closely the functional significance of elevated mitochondrial calcium and reduced MMP, pyruvate utilization and levels of ATP and ADP were measured for individual embryos. The mouse embryo has an absolute requirement for pyruvate during the first cleavage stage [52] to drive the tricarboxylic acid cycle and, ultimately, to generate ATP. Although maternal exposure to an HPD did not influence pyruvate uptake by the embryos, the level of ADP was significantly increased. This has been described for mouse oocytes, in which mitochondrial injury induced by photosensitization led to structural changes in the mitochondria in the absence of metabolic alterations, yet TE cell numbers in the blastocyst and fetal development were compromised [53]. Altered ADP levels in the absence of changes in pyruvate uptake are suggestive of a partial uncoupling of mitochondrial metabolism, whereby the functioning of electron transport is disconnected from production of ATP and the phosphorylation of ADP to ATP via ATP synthase is disengaged from the remaining electron-transport chain enzymes.
The level of ROS also was significantly elevated in embryos from HPD females. The ROS arise either from the process of lipid peroxidation or directly as a consequence of perturbed mitochondrial function (for review, see [54]). Mitochondrial metabolism of pyruvate generates ROS via NADH production [55]; thus, the constant but low metabolic levels within the first few cleavage stages minimize ROS production but enable energy production to be sustained at levels sufficient to maintain development [56]. Excess generation of ROS in oocytes and embryos has been linked to reduced developmental outcomes [57], and elevated ROS has been reported for dietary [58] and various media components during in vitro culture [59]. Thus, the data indicate that embryos from HPD females are under metabolic stress, with elevated ROS levels and partial uncoupling of mitochondrial metabolism, which is not yet translated to detectable differences in energy (pyruvate) utilization at the 2-cell stage.
Taken together, these findings lead us to propose the involvement of permeability transition pores (mPTP) located within the inner mitochondrial membrane to explain the metabolic response to an HPD. An overload of mitochondrial calcium triggers the opening of the mPTP, releasing ions (e.g., calcium) from the matrix. The mPTP opening causes the inner MMP to collapse, leading to an uncoupling of oxidative phosphorylation and perturbed ATP synthesis [60–62]. The production of ROS also is stimulated by mPTP opening and, in fact, further promotes mPTP opening itself [47]. A consequence of continual increases in mitochondrial calcium is mitochondrial swelling, rupture of the outer mitochondrial membrane, and apoptosis [63, 64]. Interestingly, an HPD has been shown to increase the apoptotic index in mouse embryos [17]. The different pattern of calcium staining intensity in response to dosing with increasing concentrations of calcium (indicative of mPTP) in the HPD embryos compared to the MPD and LPD embryos is suggestive of a potential mechanism for the perturbed development in these embryos. Further examination of the extent of mPTP opening and functioning in embryos from HPD females would enhance our understanding of its potential role in regulating embryo development.
Perturbed Embryo Metabolism: LPD
Despite similarities in mitochondrial calcium levels and MMP between embryos from LPD and HPD females, the metabolic response to the different diets clearly was not the same. LPD embryos had significantly less active mitochondria in the perinuclear region of the embryo, in contrast to MPD embryos. Perinuclear clustering of mitochondria has been described during normal hamster embryo development from the 2-cell through the 8-cell stage [65] and has been associated with normal embryo development in the mouse [28]. This grouping of mitochondria around the nucleus, in contrast to the homogenous distribution described in oocytes, is indicative of changes in energy demands, such as to supply ATP for processes of nuclear replication during cleavage development [65]. The decreased perinuclear localization of active mitochondria in LPD embryos is reminiscent of the distribution described for hamster embryos, in which perturbed cytoplasmic distribution was described for cultured embryos compared with the perinuclear localization in control, in vivo-derived embryos [66].
The production of ATP tended to be greater in LPD embryos relative to the controls, despite the relatively reduced MMP. Intuitively, one would expect a decrease in ATP levels, but it has been demonstrated elsewhere that MMP and ATP levels are independent, at least in mouse oocytes [39]. Furthermore, with the context of the apparent reduced number of mitochondria around the nucleus, it is feasible that an overall ramping down of metabolism has occurred in these embryos and that ATP is not being utilized. As was described for the HPD embryos, despite the measurable changes in mitochondria at this stage of development, the extent to which metabolic functions, such as pyruvate utilization, are affected may be less readily detectable at the 2-cell stage.
In summary, the present study examined whether periconception exposure to an HPD or LPD influenced mitochondrial metabolism in the early mouse embryo. Both diets reduced the number of ICM cells in the blastocyst-stage embryo, and in the 2-cell embryo, MMP was reduced and mitochondrial calcium levels increased. Embryos from HPD females had elevated ROS levels and ADP concentration, which are indicative of metabolic stress that likely involves the opening of mPTP and the uncoupling of oxidative phosphorylation. Embryos from LPD females had reduced mitochondrial clustering around the nucleus, which is suggestive of an overall quietening of metabolism. It may be interesting to further examine embryo development and metabolism in response to dietary protein supply in naturally cycling mice to eliminate any concerns regarding the effect of superovulation regimes. Finally, studies to independently explore the response to an HPD or LPD will enhance our understanding of the seemingly different metabolic mechanisms involved in the perturbed but permissive development and how this influences later development.
Acknowledgments
The authors would like to thank Dr. Darryl Russell for his assistance in examining ovulation rate.
Footnotes
1Supported by the Australian NHMRC Program grant (250306). M.L. is a recipient of an R.D. Wright Fellowship from the NHMRC.
REFERENCES
- Symonds ME.Integration of physiological and molecular mechanisms of the developmental origins of adult disease: new concepts and insights. Proc Nutr Soc 2007; 66: 442–450. [DOI] [PubMed] [Google Scholar]
- Vickers MH, Krechowec SO, Breier BH.Is later obesity programmed in utero? Curr Drug Targets 2007; 8: 923–934. [DOI] [PubMed] [Google Scholar]
- Langley-Evans SC, Welham SJ, Sherman RC, Jackson AA.Weanling rats exposed to maternal low-protein diets during discrete periods of gestation exhibit differing severity of hypertension. Clin Sci (Lond) 1996; 91: 607–615. [DOI] [PubMed] [Google Scholar]
- Thone-Reineke C, Kalk P, Dorn M, Klaus S, Simon K, Pfab T, Godes M, Persson P, Unger T, Hocher B.High-protein nutrition during pregnancy and lactation programs blood pressure, food efficiency, and body weight of the offspring in a sex-dependent manner. Am J Physiol Regul Integr Comp Physiol 2006; 291: R1025–R1030. [DOI] [PubMed] [Google Scholar]
- Fernandez-Twinn DS, Ekizoglou S, Wayman A, Petry CJ, Ozanne SE.Maternal low-protein diet programs cardiac beta-adrenergic response and signaling in 3-mo-old male offspring. Am J Physiol Regul Integr Comp Physiol 2006; 291: R429–R436. [DOI] [PubMed] [Google Scholar]
- Petry CJ, Dorling MW, Pawlak DB, Ozanne SE, Hales CN.Diabetes in old male offspring of rat dams fed a reduced protein diet. Int J Exp Diabetes Res 2001; 2: 139–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong WY, Wild AE, Roberts P, Willis AC, Fleming TP.Maternal undernutrition during the preimplantation period of rat development causes blastocyst abnormalities and programming of postnatal hypertension. Development 2000; 127: 4195–4202. [DOI] [PubMed] [Google Scholar]
- Boerjan ML, den Daas JH, Dieleman SJ.Embryonic origins of health: long-term effects of IVF in human and livestock. Theriogenology 2000; 53: 537–547. [DOI] [PubMed] [Google Scholar]
- Cetin I, Cozzi V, Antonazzo P.Fetal development after assisted reproduction—a review. Placenta 2003; 24(suppl B):S104–S113. [DOI] [PubMed] [Google Scholar]
- Cheung AP.Assisted reproductive technology: both sides now. J Reprod Med 2006; 51: 283–292. [PubMed] [Google Scholar]
- Kurinczuk JJ.Safety issues in assisted reproduction technology. From theory to reality—just what are the data telling us about ICSI offspring health and future fertility and should we be concerned? Hum Reprod 2003; 18: 925–931. [DOI] [PubMed] [Google Scholar]
- Zander DL, Thompson JG, Lane M.Perturbations in mouse embryo development and viability caused by ammonium are more severe after exposure at the cleavage stages. Biol Reprod 2006; 74: 288–294. [DOI] [PubMed] [Google Scholar]
- Rooke JA, McEvoy TG, Ashworth CJ, Robinson JJ, Wilmut I, Young LE, Sinclair KD.Ovine fetal development is more sensitive to perturbation by the presence of serum in embryo culture before rather than after compaction. Theriogenology 2007; 67: 639–647. [DOI] [PubMed] [Google Scholar]
- Blanchard T, Ferguson J, Love L, Takeda T, Henderson B, Hasler J, Chalupa W.Effect of dietary crude-protein type on fertilization and embryo quality in dairy cattle. Am J Vet Res 1990; 51: 905–908. [PubMed] [Google Scholar]
- Powell K, Rooke JA, McEvoy TG, Ashworth CJ, Robinson JJ, Wilmut I, Young LE, Sinclair KD.Zygote donor nitrogen metabolism and in vitro embryo culture perturbs in utero development and IGF2R expression in ovine fetal tissues. Theriogenology 2006; 66: 1901–1912. [DOI] [PubMed] [Google Scholar]
- Sinclair KD, Kuran M, Gebbie FE, Webb R, McEvoy TG.Nitrogen metabolism and fertility in cattle: II. Development of oocytes recovered from heifers offered diets differing in their rate of nitrogen release in the rumen. J Anim Sci 2000; 78: 2670–2680. [DOI] [PubMed] [Google Scholar]
- Gardner DK, Stilley KS, Lane M.High-protein diet inhibits inner cell mass formation and increases apoptosis in mouse blastocysts developed in vivo by increasing the levels of ammonium in the reproductive tract. Reprod Fertil Dev 2004; 16: 190 [Google Scholar]
- McEvoy TG, Robinson JJ, Aitken RP, Findlay PA, Robertson IS.Dietary excesses of urea influence the viability and metabolism of preimplantation sheep embryos and may affect fetal growth among survivors. Anim Reprod Sci 1997; 47: 71–90. [DOI] [PubMed] [Google Scholar]
- Lane M, Gardner DK.Ammonium induces aberrant blastocyst differentiation, metabolism, pH regulation, gene expression and subsequently alters fetal development in the mouse. Biol Reprod 2003; 69: 1109–1117. [DOI] [PubMed] [Google Scholar]
- Gardner DK, Hewitt E, Linck D.Diet affects embryo imprinting and fetal development. Hum Reprod 2004; 19(suppl 1): i27 [Google Scholar]
- Sinawat S, Hsaio WC, Flockhart JH, Kaufman MH, Keith J, West JD.Fetal abnormalities produced after preimplantation exposure of mouse embryos to ammonium chloride. Hum Reprod 2003; 18: 2157–2165. [DOI] [PubMed] [Google Scholar]
- Rees WD, Hay SM, Buchan V, Antipatis C, Palmer RM.The effects of maternal protein restriction on the growth of the rat fetus and its amino acid supply. Br J Nutr 1999; 81: 243–250. [PubMed] [Google Scholar]
- Kwong WY, Miller DJ, Ursell E, Wild AE, Wilkins AP, Osmond C, Anthony FW, Fleming TP.Imprinted gene expression in the rat embryo-fetal axis is altered in response to periconceptional maternal low protein diet. Reproduction 2006; 132: 265–277. [DOI] [PubMed] [Google Scholar]
- Ma Y, Pagoto SL, Griffith JA, Merriam PA, Ockene IS, Hafner AR, Olendzki BC.A dietary quality comparison of popular weight-loss plans. J Am Diet Assoc 2007; 107: 1786–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett DK, Bavister BD.What is the relationship between the metabolism of preimplantation embryos and their developmental competence? Mol Reprod Dev 1996; 43: 105–133. [DOI] [PubMed] [Google Scholar]
- Mitchell M, Cashman KS, Gardner DK, Thompson JG, Lane M.Disruption of mitochondrial malate-aspartate shuttle activity in mouse blastocysts impairs viability and fetal growth. Biol Reprod 2009; 80: 295–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Shourbagy SH, Spikings EC, Freitas M, St John JC.Mitochondria directly influence fertilization outcome in the pig. Reproduction 2006; 131: 233–245. [DOI] [PubMed] [Google Scholar]
- Nagai S, Mabuchi T, Hirata S, Shoda T, Kasai T, Yokota S, Shitara H, Yonekawa H, Hoshi K.Correlation of abnormal mitochondrial distribution in mouse oocytes with reduced developmental competence. Tohoku J Exp Med 2006; 210: 137–144. [DOI] [PubMed] [Google Scholar]
- Van Blerkom J, Davis P, Mathwig V, Alexander S.Domains of high-polarized and low-polarized mitochondria may occur in mouse and human oocytes and early embryos. Hum Reprod 2002; 17: 393–406. [DOI] [PubMed] [Google Scholar]
- Wilding M, Dale B, Marino M, di Matteo L, Alviggi C, Pisaturo ML, Lombardi L, De Placido G.Mitochondrial aggregation patterns and activity in human oocytes and preimplantation embryos. Hum Reprod 2001; 16: 909–917. [DOI] [PubMed] [Google Scholar]
- Watkins AJ, Ursell E, Panton R, Papenbrock T, Hollis L, Cunningham C, Wilkins A, Perry VH, Sheth B, Kwong WY, Eckert JJ, Wild AE, Hanson MA, Osmond C, Fleming TP.Adaptive responses by mouse early embryos to maternal diet protect fetal growth but predispose to adult onset disease. Biol Reprod 2008; 78: 299–306. [DOI] [PubMed] [Google Scholar]
- Langley SC, Jackson AA.Increased systolic blood pressure in adult rats induced by fetal exposure to maternal low protein diets. Clin Sci (Lond) 1994; 86: 217–222. [DOI] [PubMed] [Google Scholar]
- De Blasio MJ, Dodic M, Jefferies AJ, Moritz KM, Wintour EM, Owens JA.Maternal exposure to dexamethasone or cortisol in early pregnancy differentially alters insulin secretion and glucose homeostasis in adult male sheep offspring. Am J Physiol Endocrinol Metab 2007; 293: E75–E82. [DOI] [PubMed] [Google Scholar]
- Lane M, Gardner DK.Preparation of gametes, in vitro maturation, in vitro fertilization and embryo recovery and transfer. Gardner DK, Lane M, Watson AJ.A Laboratory Guide to the Mammalian Embryo New York:Oxford University Press;2004: 24–40. [Google Scholar]
- Gardner DK, Lane MW, Lane M.EDTA stimulates cleavage stage bovine embryo development in culture but inhibits blastocyst development and differentiation. Mol Reprod Dev 2000; 57: 256–261. [DOI] [PubMed] [Google Scholar]
- Liu L, Hammar K, Smith PJ, Inoue S, Keefe DL.Mitochondrial modulation of calcium signaling at the initiation of development. Cell Calcium 2001; 30: 423–433. [DOI] [PubMed] [Google Scholar]
- Stojkovic M, Machado SA, Stojkovic P, Zakhartchenko V, Hutzler P, Goncalves PB, Wolf E.Mitochondrial distribution and adenosine triphosphate content of bovine oocytes before and after in vitro maturation: correlation with morphological criteria and developmental capacity after in vitro fertilization and culture. Biol Reprod 2001; 64: 904–909. [DOI] [PubMed] [Google Scholar]
- Barnett DK, Clayton MK, Kimura J, Bavister BD.Glucose and phosphate toxicity in hamster preimplantation embryos involves disruption of cellular organization, including distribution of active mitochondria. Mol Reprod Dev 1997; 48: 227–237. [DOI] [PubMed] [Google Scholar]
- Van Blerkom J, Davis P, Alexander S.Inner mitochondrial membrane potential (DeltaPsim), cytoplasmic ATP content and free Ca2+ levels in metaphase II mouse oocytes. Hum Reprod 2003; 18: 2429–2440. [DOI] [PubMed] [Google Scholar]
- Leese HJ, Biggers JD, Mroz EA, Lechene C.Nucleotides in a single mammalian ovum or preimplantation embryo. Anal Biochem 1984; 140: 443–448. [DOI] [PubMed] [Google Scholar]
- Leese HJ, Barton AM.Pyruvate and glucose uptake by mouse ova and preimplantation embryos. J Reprod Fertil 1984; 72: 9–13. [DOI] [PubMed] [Google Scholar]
- Nasr-Esfahani MH, Aitken JR, Johnson MH.Hydrogen peroxide levels in mouse oocytes and early cleavage stage embryos developed in vitro or in vivo. Development 1990; 109: 501–507. [DOI] [PubMed] [Google Scholar]
- Pastorino JG, Marcineviciute A, Cahill A, Hoek JB.Potentiation by chronic ethanol treatment of the mitochondrial permeability transition. Biochem Biophys Res Commun 1999; 265: 405–409. [DOI] [PubMed] [Google Scholar]
- Thouas GA, Trounson AO, Wolvetang EJ, Jones GM.Mitochondrial dysfunction in mouse oocytes results in preimplantation embryo arrest in vitro. Biol Reprod 2004; 71: 1936–1942. [DOI] [PubMed] [Google Scholar]
- Dumollard R, Marangos P, Fitzharris G, Swann K, Duchen M, Carroll J.Sperm-triggered [Ca2+] oscillations and Ca2+ homeostasis in the mouse egg have an absolute requirement for mitochondrial ATP production. Development 2004; 131: 3057–3067. [DOI] [PubMed] [Google Scholar]
- Duchen MR.Roles of mitochondria in health and disease. Diabetes 2004; 53(suppl 1):S96–S102. [DOI] [PubMed] [Google Scholar]
- Hajnoczky G, Csordas G, Das S, Garcia-Perez C, Saotome M, Sinha Roy S, Yi M.Mitochondrial calcium signaling and cell death: approaches for assessing the role of mitochondrial Ca2+ uptake in apoptosis. Cell Calcium 2006; 40: 553–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack JG, Halestrap AP, Denton RM.Role of calcium ions in regulation of mammalian intramitochondrial metabolism. Physiol Rev 1990; 70: 391–425. [DOI] [PubMed] [Google Scholar]
- Ankarcrona M, Dypbukt JM, Bonfoco E, Zhivotovsky B, Orrenius S, Lipton SA, Nicotera P.Glutamate-induced neuronal death: a succession of necrosis or apoptosis depending on mitochondrial function. Neuron 1995; 15: 961–973. [DOI] [PubMed] [Google Scholar]
- Acton BM, Jurisicova A, Jurisica I, Casper RF.Alterations in mitochondrial membrane potential during preimplantation stages of mouse and human embryo development. Mol Hum Reprod 2004; 10: 23–32. [DOI] [PubMed] [Google Scholar]
- Van Blerkom J.Mitochondria in human oogenesis and preimplantation embryogenesis: engines of metabolism, ionic regulation and developmental competence. Reproduction 2004; 128: 269–280. [DOI] [PubMed] [Google Scholar]
- Biggers JD, Whittingham DG, Donahue RP.The pattern of energy metabolism in the mouse oocyte and zygote. Proc Natl Acad Sci U S A 1967; 58: 560–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thouas GA, Trounson AO, Jones GM.Developmental effects of sublethal mitochondrial injury in mouse oocytes. Biol Reprod 2006; 74: 969–977. [DOI] [PubMed] [Google Scholar]
- Gutierrez J, Ballinger SW, Darley-Usmar VM, Landar A.Free radicals, mitochondria, and oxidized lipids: the emerging role in signal transduction in vascular cells. Circ Res 2006; 99: 924–932. [DOI] [PubMed] [Google Scholar]
- Dumollard R, Ward Z, Carroll J, Duchen MR.Regulation of redox metabolism in the mouse oocyte and embryo. Development 2007; 134: 455–465. [DOI] [PubMed] [Google Scholar]
- Leese HJ.Quiet please, do not disturb: a hypothesis of embryo metabolism and viability. Bioessays 2002; 24: 845–849. [DOI] [PubMed] [Google Scholar]
- Johnson MH, Nasr-Esfahani MH.Radical solutions and cultural problems: could free oxygen radicals be responsible for the impaired development of preimplantation mammalian embryos in vitro? Bioessays 1994; 16: 31–38. [DOI] [PubMed] [Google Scholar]
- Wakefield SL, Lane M, Schulz SJ, Hebart ML, Thompson JG, Mitchell M.Maternal supply of omega-3 polyunsaturated fatty acids alter mechanisms involved in oocyte and early embryo development in the mouse. Am J Physiol Endocrinol Metab 2008; 294: E425–E434. [DOI] [PubMed] [Google Scholar]
- Karja NW, Kikuchi K, Fahrudin M, Ozawa M, Somfai T, Ohnuma K, Noguchi J, Kaneko H, Nagai T.Development to the blastocyst stage, the oxidative state, and the quality of early developmental stage of porcine embryos cultured in alteration of glucose concentrations in vitro under different oxygen tensions. Reprod Biol Endocrinol 2006; 4: 54–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crompton M.Mitochondria and aging: a role for the permeability transition? Aging Cell 2004; 3: 3–6. [DOI] [PubMed] [Google Scholar]
- Lemasters JJ, Nieminen AL, Qian T, Trost LC, Herman B.The mitochondrial permeability transition in toxic, hypoxic and reperfusion injury. Mol Cell Biochem 1997; 174: 159–165. [PubMed] [Google Scholar]
- Maragos WF, Korde AS.Mitochondrial uncoupling as a potential therapeutic target in acute central nervous system injury. J Neurochem 2004; 91: 257–262. [DOI] [PubMed] [Google Scholar]
- Hirsch T, Marzo I, Kroemer G.Role of the mitochondrial permeability transition pore in apoptosis. Biosci Rep 1997; 17: 67–76. [DOI] [PubMed] [Google Scholar]
- Smaili SS, Hsu YT, Youle RJ, Russell JT.Mitochondria in Ca2+ signaling and apoptosis. J Bioenerg Biomembr 2000; 32: 35–46. [DOI] [PubMed] [Google Scholar]
- Barnett DK, Kimura J, Bavister BD.Translocation of active mitochondria during hamster preimplantation embryo development studied by confocal laser scanning microscopy. Dev Dyn 1996; 205: 64–72. [DOI] [PubMed] [Google Scholar]
- Lane M, Bavister BD.Calcium homeostasis in early hamster preimplantation embryos. Biol Reprod 1998; 59: 1000–1007. [DOI] [PubMed] [Google Scholar]