Abstract
Two isoforms of phosphoprotein phosphatase 1, PPP1CC1 and PPP1CC2, are translated from alternatively spliced transcripts of a single gene, Ppp1cc, and differ only at their extreme C-termini. While PPP1CC1 expression is almost ubiquitous, PPP1CC2 is largely restricted to testicular germ cells and mature spermatozoa. Targeted deletion of Ppp1cc leads to sterility of −/− males due to a combination of gross structural defects in developing spermatids resulting in apoptosis and faulty spermiation. Because PPP1CC2 is the only PP1 isoform that demonstrates high-level expression in wild-type meiotic and postmeiotic male germ cells, we have tested whether its loss in Ppp1cc−/− males is largely responsible for manifestation of this phenotype by expressing PPP1CC2 transgenically in the testis of Ppp1cc−/− mice (rescue mice). Herein, we demonstrate that PPP1CC2 expression in the Ppp1cc−/− testis is antiapoptotic, thus reestablishing spermatid development and spermiation. However, because aberrant flagellar morphogenesis is incompletely ameliorated, rescue males remain infertile. Because these results suggest that expression of PPP1CC2 in developing germ cells is essential but insufficient for normal spermatogenesis to occur, appropriate spatial and temporal expression of both PPP1CC isoforms in the testis during spermatogenesis appears to be necessary to produce structurally normal fertility-competent spermatozoa.
Keywords: sperm, spermatid, spermatogenesis, testis
An isoform of phosphoprotein phosphatase 1 (PPP1CC2) rescues spermatogenesis but not sperm motility and fertility.
INTRODUCTION
Spermatogenesis is the complex sequence of events occurring within the seminiferous tubules of the testis to produce spermatozoa [1, 2]. The fourth and final phase of this process (spermiogenesis) encompasses the morphological transformation of haploid products of meiosis, called spermatids, into highly differentiated spermatozoa. Spermatids are evident by Day 22 postpartum in male mice. Within this phase, round spermatids differentiate morphologically into elongating spermatids that undergo radical condensation to become mature testicular spermatozoa. The mature testicular spermatozoa consist of 1) a streamlined head containing a membrane-bound acrosome and a nucleus with highly condensed transcriptionally inactive chromatin and 2) a highly evolved compartmentalized flagellum through which a central axonemal engine, anchored peripherally to unique outer dense fibers (ODFs), runs its entire length. By virtue of flagellar compartmentalization, the axoneme and surrounding ODFs are exposed to successive differentially structured regulatory environments. These include a proximal mitochondrial sheath-bound compartment known as the midpiece, then more distally a long fibrous sheath-encapsulated principal piece, and finally most distally a tiny end piece. The somatic cells of the testis, Sertoli cells and Leydig cells, have an essential role in supporting spermatogenesis through complex signaling networks.
Morphologically mature sperm, released into the lumen of the seminiferous tubule by a process called spermiation, are transported through the efferent ducts into the epididymides, where they undergo additional maturation steps to acquire their competency to swim and to bind and fertilize eggs. Epididymal sperm maturation is an added feature of gamete development in mammals, involving a series of biochemical and physiological modifications that include the following: 1) remodeling of the sperm plasma membrane, 2) changes in the composition and cellular localization of proteins, 3) acquisition and alteration of glycoproteins, and 4) changes in pH and in the levels of the second messengers, Ca2+ and cAMP [1–5]. These mediators of cellular regulation are thought to modulate sperm function through changes in protein phosphorylation. Until recently, investigations of the role of protein phosphorylation on male germ cell development and function have focused mainly on the protein kinases, especially cAMP-dependent protein kinase A [6], while little attention has been paid to the influence of protein phosphatases on these processes [7, 8].
The predominant phosphoprotein phosphatase catalytic subunit in both testicular germ cells and epididymal spermatozoa is phosphoprotein phosphatase 1 catalytic subunit C2 (PPP1CC2, previously called PP1γ2), one of four highly conserved type 1 serine/threonine protein phosphatases (the other three being PPP1CA, PPP1CB, and PPP1CC1, previously called PP1α, PP1β, and PP1γ1, respectively) [9]. The PPP1CC isoforms, PPP1CC1 and PPP1CC2, are products of alternatively spliced transcripts of a single gene, Ppp1cc. These polypeptides differ from each other at their extreme C-termini, with PPP1CC2 having a unique 21-amino acid carboxyl-terminal extension [9]. The PPP1CC2 isoform, with its virtually unaltered C-terminus, is only found in mammals. Inhibition of PPP1CC2 in sperm by treatment with PPP1C inhibitors results in initiation and stimulation of motility [10, 11]. These highly correlative results have led to the suggestion that PPP1CC2 has an important role in the regulation of mammalian sperm motility. In mice, targeted disruption of Ppp1cc, which eliminates both PPP1CC1 and PPP1CC2 expression, results in male infertility due to aberrant spermiogenesis. Disruption of this phase is highlighted by impaired sperm head and tail morphogenesis, leading to the absence of germ cells in the epididymides due to a combination of spermatid apoptosis and dysfunctional spermiation [12]. Mutant female mice are fertile and appear normal, suggesting that the other PPP1C proteins, PPP1CA and PPP1CB, can substitute for the loss of the PPP1CC isoforms in all cells and tissues where either is expressed except testis. At least one, and possibly both, of the PPP1CC isoforms is indispensable for normal spermatogenesis. Because PPP1CC2 expression is predominant in testicular meiotic and postmeiotic germ cells [13], it was relevant to determine whether the expression of PPP1CC2 in Ppp1cc-null mice could reestablish normal spermiogenesis in the mutant testis and thus restore fertility to these males. A transgene construct encoding PPP1CC2 driven by the Pgk2 promoter, the same promoter that was used to fully rescue fertility in Ace−/− (angiotensin-converting enzyme −/−) mice [14], was therefore utilized in an attempt to rescue fertility in Ppp1cc−/− males.
MATERIALS AND METHODS
Generation of the PPP1CC2 Rescue Plasmid
The SacI-Xbal fragment of the human Pgk2 promoter was amplified from the plasmid pCR2.1 (a kind gift from Dr. John McCarrey, Department of Biology, University of Texas at San Antonio, San Antonio, TX) by PCR with forward and reverse primers (5′-CTCGAGCTCGAGGTTTTTACATATCA-3′ and 5′-CTCTCTAGAGACAATATAAAGACATA-3′, respectively). This fragment was subsequently inserted between the SacI-Xbal sites of pBluescript SK+ (Stratagene, La Jolla, CA). A 1-kilobase (kb) fragment comprising the start to the stop codon of rat testicular Ppp1cc2 cDNA was ligated between the BamHl-XhoI sites (Fig. 1A). The SV40 poly A signal was amplified from a pcDNA4.0 plasmid using the forward and reverse primers 5′-CTCCTCGAGTCTCATGCTGGAGTTCT-3′ and 5′-CTCGGTACCACCATGATTACGCCAAG-3′, respectively, followed by ligation between the XhoI-Kpnl sites of pBluescript SK+. The DNA fragment containing the Pgk2 promoter, the Ppp1cc2 cDNA, and the SV40 poly A signal was excised from the vector by digestion with BamHl and Kpnl, and the 1.7-kb fragment was gel purified. The purified fragment was then microinjected into the pronuclei of fertilized B6/SJL eggs, and the injected eggs were implanted into the uteri of pseudopregnant mothers. Both microinjection and embryo implantation were carried out at the Transgenic Facility of Case Western Reserve University (Cleveland, Ohio). Transgenic mouse production and use at Kent State University follow approved institutional animal care and use committee protocols adapted from the National Research Council publication Guide for the Care and Use of Laboratory Animals.
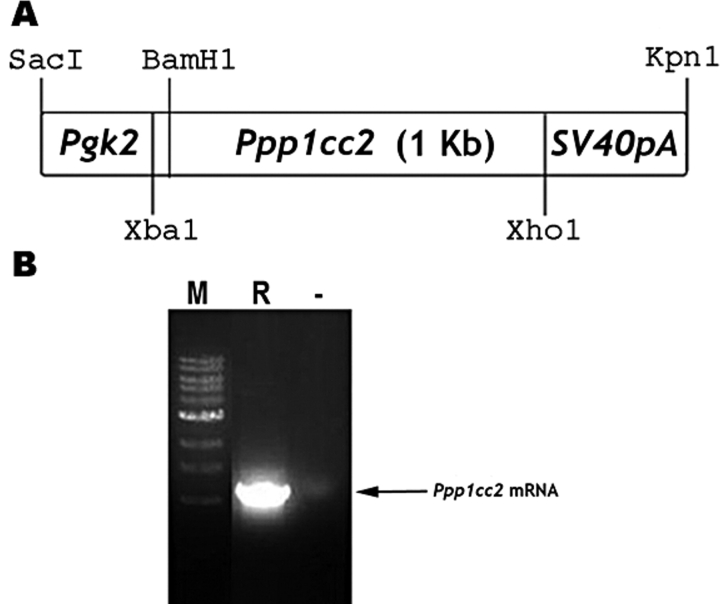
FIG. 1.
Design of PPP1CC2-rescue transgenic construct and genotyping transgenic mice. A) The Pgk2-Ppp1cc2 cDNA-SV40 poly A (pA) transgene. B) An RT-PCR was used to detect the presence of transgenic mRNA of expected length (1.3 kb) in Ppp1cc2-rescue testis. M, marker; R, rescue; −, negative control.
DNA samples isolated from ear punches of 21-day-old progeny (n = 290) were analyzed by PCR for the presence of the transgene using the Pgk2 forward primer 5′-GCGCACACCTCAGGACTATT-3′ and the SV40 reverse primer 5′-CTCGGTACCACCATGATTACGCCAAG-3′. The transgenic-positive B6/SJL founder mice were mated either with Ppp1cc-null CD1 females or Ppp1cc+/− males (Ppp1cc-null mice were obtained from Dr. Susan Varmuza, University of Toronto, Toronto, ON). PPP1CC2 transgene-positive Ppp1cc−/− males were obtained by crossing PPP1CC2 transgene-positive Ppp1cc+/− males to Ppp1cc−/− females.
Expression of the Transgene
An RT-PCR was used to detect expression of the transgene in the testis. Briefly, 50–100 mg of testes was homogenized in 1 ml of TRI REAGENT (Sigma-Aldrich, St. Louis, MO), and DNA-free RNA was isolated according to the manufacturer's directions. The RNA samples were reverse transcribed at 42°C for 20 min using the RT Primer Mix (Qiagen, Gaithersburg, MD), followed by a 3-min incubation at 95°C to inactivate the RT. The PCR was then performed using the same conditions used to detect the transgenic Ppp1cc2 gene using the forward primer 5′-ATGGCGGATATCGACAA-3′ and reverse primer 5′-CGAACAACTCCAGCATGAGA-3′. The 1040-base pair product was detected by ethidium bromide staining following electrophoresis through 1% agarose.
Protein Extract Preparation and Western Blot Analysis
Mouse testes and brain were homogenized in 1 ml of HB+ buffer using a Model Pro 200 tissue homogenizer (Pro Scientific Inc., Oxford, CT) as described previously [13]. Epididymal sperm extruded (as described in the next subsection) in PBS was centrifuged for 3 min at 300 × g, and the sperm pellet was resuspended in 1% SDS and boiled for 5 min, followed by centrifugation at 16 000 × g for 20 min. The supernatant was boiled after addition of 6× sample buffer and separated by 12% SDS-PAGE, and gels were blotted to polyvinylidene fluoride membranes (Immobilon-P; Millipore Corporation, Bedford, MA). These were probed with a 1:1000 dilution of an affinity-purified rabbit polyclonal anti-PPP1CC2 antibody directed against the PPP1CC2 unique C-terminal region, followed by a peroxidase-conjugated goat anti-rabbit secondary antibody [13]. Blots were developed by enhanced chemiluminescence.
Sperm Extrusion Methods from Testis, Caput, and Cauda Epididymis
Testicular sperm from the rescue animals were isolated using the methods described by Chakrabarti et al. [13]. In brief, testes were decapsulated in PBS. Seminiferous tubules were untangled manually using fine forceps. Dark regions of the tubule, as observed by transillumination, containing mature sperm were teased open, and the suspension was fixed in 3.7% paraformaldehyde in PBS.
Sperm from both caput and caudal epididymis were isolated by carefully removing both regions separately, followed by squeezing sperm from both regions after piecing both the caput and caudal epididymis with a fine-tip needle. The sperm were extruded into PBS, followed by fixation in two volumes of 3.7% paraformaldehyde in PBS. The fixed sperm were then observed under a microscope (Leica Microsystems, Wetzlar, Germany) using the differential interference contrast (DIC) optics.
Statistical Analysis of Testicular, Caput, and Cauda Epididymal Sperm Head and Flagellar Bends from Rescue Mice
Two slides each of extruded and fixed testicular, caput, and cauda epididymal sperm were prepared from a single rescue male from line A. From each slide, 15 randomly selected fields were observed by light microscopy using DIC optics, and the numbers of straight sperm, sperm with 180° hairpin bends at the connecting piece, and sperm with 180° hairpin bends at the midpiece/principal piece junction were determined. The significance of statistical differences of the means of each morphological phenotype between testicular, caput, and caudal sperm was determined by Tukey HSD test following one-way ANOVA.
Sperm Count and Motility Assessment
Caudae epididymides were lightly minced and incubated in potassium simplex optimized medium [15] for 15 min at 37°C in 5% CO2 in air to allow the sperm to swim out and disperse into the medium. A measured portion of the sperm suspension was briefly centrifuged at 600 × g, resuspended in medium containing 0.02% sodium azide, and counted with a hemocytometer. Sperm motility was assessed by computer-assisted semen analysis (CASA) and other higher-resolution videotape analyses [16].
Fertility Analysis
Experimental rescue and wild-type (control) male mice were mated with wild-type CD1 females over a period of 5 wk, and the number of offspring in each litter was recorded. CD1 females that failed to become pregnant when mated with experimental males were subsequently tested for fertility by mating to wild-type CD1 (control) males.
Histology and Immunohistochemistry
Tissues were embedded in paraffin and sectioned and then stained with hematoxylin-eosin. For immunostaining, paraffin was removed from sections by sequential washings in citrosol, alcohol, and PBS. Sections were blocked by incubation in 10% goat serum and 2.5% bovine serum albumin overnight and then were washed and incubated with the aforementioned anti-PPP1CC2 antibody (1:100 dilution) for 5 h at room temperature. After washing, sections were incubated with a Cy3-conjugated secondary antibody (1:250 dilution) for 1 h at room temperature. After washing, the sections were mounted, viewed, and photographed using a FluoView 500 fluorescence microscope (Olympus, Center Valley, PA).
Ultrastructural Analysis of Epididymal Spermatozoa by Transmission Electron Microscopy
Caudae epididymides were fixed by immersion in 2.5% glutaraldehyde in 0.1 M sodium cacodylate. Following fixation, the epididymides were minced and immersed in the same fixative for 15 min at 4°C. After washing in 0.1 M sodium cacodylate buffer, the tissue blocks were postfixed in 1.33% OsO4 for 90 min at 4°C and then dehydrated through a graded series of ethanols and infiltrated with and subsequently embedded in Epon/Araldite (Structure Probe, Inc./SPI Supplies, West Chester, PA). Ultrathin 85-nm-thick sections were cut and placed onto grids, followed by staining for 5 min in 10% uranyl acetate in methanol and then in Reynold lead citrate for 2 min. Sections were viewed and photographed with a Philips 400 transmission electron microscope (Philips Technologies, Cheshire, CT) [16].
Northern Blot
A 30-μl mixture containing 20 μg of total RNA, 2 μl of 10× 3-(N-morpholino)propanesulfonic acid (MOPS) buffer, 4 μl of HCHO (37% solution; Amresco, Solon, OH), 10 μl of deionized formamide (Amresco), and 1 μl of ethidium bromide (200 μg/ml) was heated at 85°C for 10 min and chilled on ice for another 10 min. A 2-μl aliquot of 10× gel-loading buffer (50% glycerol, 10 mM edetic acid [EDTA] [pH 8.0], 0.25% w/v bromophenol blue, and 0.25% w/v xylene cyanol FF) was added to each sample, and samples were vortexed and briefly centrifuged. Samples were loaded onto 1% agarose/MOPS gel (SeaKem GTG Agarose, Cambrex, NJ) and electrophoresed at 70 V. The gel was denatured in a solution of 0.05 M NaOH and 1.5 M NaCl for 30 min, neutralized in a solution of 0.5 M Tris-HCl (pH 7.4) and 1.5 M NaCl, and then equilibrated in 20× saline-sodium citrate (SSC) for 45 min. The gel was transferred to Hybond-XL nylon membrane (GE Healthcare, Piscataway, NJ) in 10× SSC for 16 h. The membrane containing RNA was then baked at 85°C for 2 h, after which it was prehybridized in 8 ml of modified Church buffer (1 mM EDTA [pH 8.0], 0.5 M NaHPO4 [pH 7.2], and 5% SDS) for 1 h at 65°C in a water bath. Fresh Church buffer containing a probe of Ppp1cc2 cDNA between exon 5 and exon 7 and actin cDNA labeled with P32-deoxycytidine triphosphate (MP Biomedicals, Solon, OH) was used for hybridization overnight at 65°C in a water bath. The membrane was then washed in 1× SSC/0.1% SDS three times for 2 min each at room temperature, followed by washing in 0.1× SSC/0.1% SDS twice for 5 min in a 65°C water bath. After washing, the membrane was dried at room temperature, covered with plastic wrap, exposed to film in a cassette with an intensifying screen overnight, and then developed in a Typhoon automated film developer (GE Healthcare).
RESULTS
Transgenic Expression of PPP1CC2 in the Ppp1cc−/− Mouse
Ppp1cc2 transgenic mice carrying the construct shown in Figure 1A were produced as described in Materials and Methods. The promoter of the testis-specific Pgk2 gene was used to drive transcription of rat Ppp1cc2 in the mouse testis based on the following tripartite rationale. First, the Pgk2 promoter is well characterized and has been used successfully to rescue fertility by driving germ cell transcription of transgenic Ace in sterile Ace-null males [14]. Second, the Pgk2 promoter would drive transcription of the Ppp1cc2 transgene in an appropriate fashion to allow for elevated translation of transgenic Ppp1cc2 in cells in which the level of wild-type PPP1CC2 protein is normally high (late spermatocytes and spermatids). Third, because the Ppp1cc endogenous promoter expresses transcripts in most somatic tissues (PPP1CC2 is the product of an alternatively spliced transcript particularly abundant in developing male germ cells), its use would run the risk of expressing PPP1CC2 everywhere, possibly producing complicating phenotypes.
Transgene-positive mice were identified by PCR of DNA isolated from ear punches as described in Materials and Methods. Five lines that transmitted the transgene were established. The RT-PCR was used to detect Ppp1cc2 transgene mRNA expression (Fig. 1B). These transgene-positive mice were crossed as described in Materials and Methods to produce Ppp1cc2 transgene-positive Ppp1cc−/− (rescue) males. Several lines of rescue mice showed testicular Ppp1cc2 mRNA expression equivalent to the level expressed in the testis of fertile Ppp1cc+/− mice (Supplemental Fig. S1 available at www.biolreprod.org).
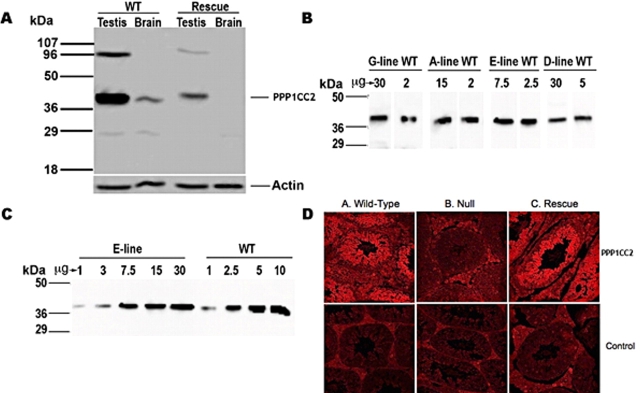
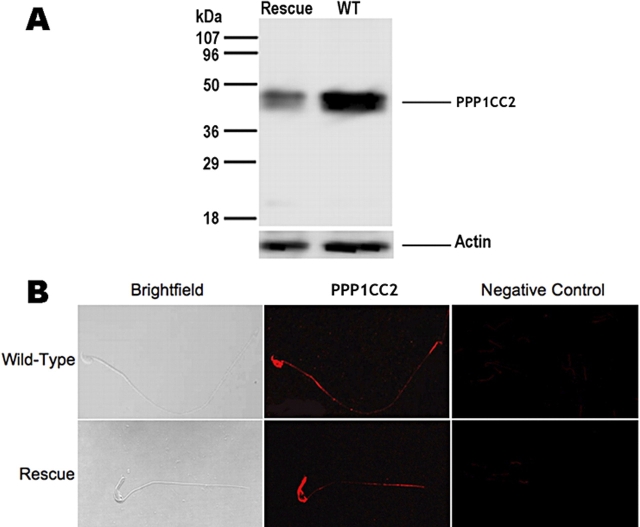
Transgene-positive Ppp1cc+/+ male and female mice exhibited a wild-type phenotype, as did transgene-positive Ppp1cc−/− females. In addition, the presence of the Ppp1cc2 transgene did not appear to affect the development or life span of any male, and the general health characteristics of rescue mice were comparable to those of wild-type and Ppp1cc−/− mice. Surprisingly, wild-type (Ppp1cc+/+) and heterozygous (Ppp1cc+/−) mice with or without the transgene expressed approximately the same amount of PPP1CC2 in testis protein extracts as assessed by immunoreactivity on Western blots (data not shown). However, significant transgenic PPP1CC2 protein expression was evident in rescue testes in which endogenous expression was absent (Fig. 2A). These findings suggested that an upper boundary of PPP1CC2 protein expression exists and is tightly controlled. Moreover, as expected, transgenic Ppp1cc2 mRNA expression was restricted to the testis (Fig. 2A) owing to the use of the spermatocyte-specific Pgk2 promoter to drive transcription of the transgene. Western blot analysis of testis extracts from rescue males showed that four transgenic lines expressed significant amounts of PPP1CC2 in the testis (Fig. 2B), with the E-line rescue mice expressing the greatest amount, approximately one third of the steady-state level of wild type (Fig. 2C). Finally, immunohistochemical analysis of testis sections from these mice demonstrated that transgenic PPP1CC2 was mainly restricted to meiotic and postmeiotic germ cells, roughly comparable to the staining pattern in wild-type testis [13] (Fig. 2D).
FIG. 2.
The presence of PPP1CC2 in rescue mouse testis. A) Transgenic PPP1CC2 is restricted to testis and is not expressed in brain. B) The amount of PPP1CC2 expressed in each rescue line testis is estimated by Western blot analysis: E-line rescue mice express approximately one third of wild-type (WT) PPP1CC2 expression; D-line rescue mice, approximately one sixth; A-line rescue mice, approximately one seventh; and G-line rescue mice, approximately one fifteenth. C) Concentration curve/Western blot comparing E-line rescue mice with wild-type protein expression. D) Immunohistochemistry of wild-type, Ppp1cc−/− (Null), and E-line (Rescue) testes. Sections were probed with rabbit anti-PPP1CC2 antibody, followed by goat anti-rabbit Cy3-labeled secondary antibody. Slides were viewed with the FluoView 500 fluorescence microscope at 20× magnification.
Effects of Ppp1cc2 Transgene Expression in Ppp1cc−/− Mice
Previous findings have demonstrated that spermiogenesis is clearly impaired in Ppp1cc−/− mice [12], in which the lumen of the seminiferous tubules is almost devoid of late elongated spermatids and testicular spermatozoa, and virtually no mature sperm are found in the epididymal lumen. In contrast, light microscopic analysis of hematoxylin-stained rescue testis sections revealed that the testicular architecture is similar to that of wild-type mice, and the lumina of the seminiferous tubules contain what appear to be numerous mature sperm tails (Fig. 3A). Many spermatozoa were evident in the lumina of the caudae epididymides from all lines of rescue mice as well (Fig. 3B). Thus, the presence of PPP1CC2 in the testis of rescue mice restores spermiogenesis and testicular/epididymal sperm numbers to qualitatively normal levels.
FIG. 3.
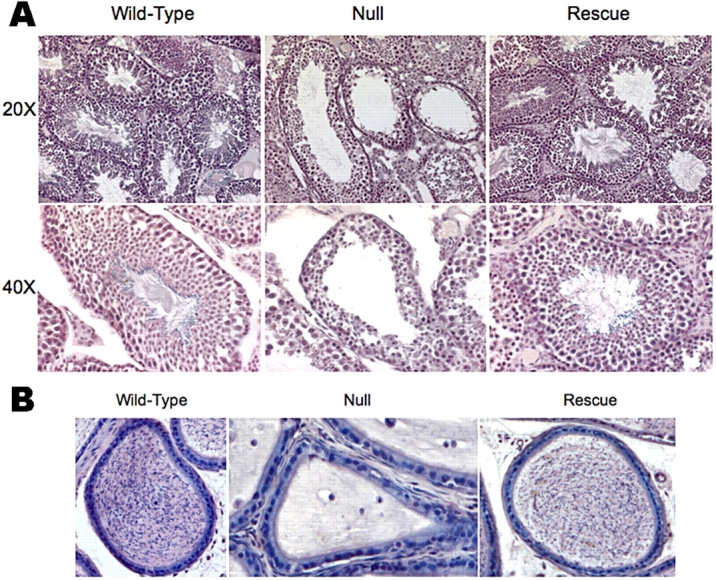
Hematoxylin-eosin staining of experimental mouse testes and caudae epididymides. A) Testes cross-sections from wild-type (WT), null, and E-line rescue mice were stained with hematoxylin-eosin. The stained sections were viewed with an Olympus IX70 microscope at 20× and 40× magnifications. Note the seemingly wild-type architecture of the rescue testis at these magnifications. B) Hematoxylin-eosin stained cross-sections through the lumina of the caudae epididymides from wild-type, null, and E-line rescue mice were viewed with the Olympus IX70 microscope at 40×. Note the dearth of sperm in the null epididymis.
Sperm Morphology Phenotypes and Sperm Motility Assessment in Transgene Positive-Ppp1cc−/− Mice
The absence of PPP1CC1 and PPP1CC2 expression in the testis causes a drastic impairment of spermiogenesis, in which mitochondrial sheath formation is disrupted or absent in the few testicular sperm of Ppp1cc−/− mice and sperm head shape is highly irregular (Fig. 4A). In contrast, testicular sperm heads appear to be normally hook-shaped in rescue mice (Fig. 4, B and C). However, most mitochondrial sheaths appear to contain irregularities, including gaps, especially at their proximal, distal, or both ends (Fig. 4, B and C). In addition, most of these sperm contained residual cytoplasmic irregularities at the head/connecting piece junction (Fig. 4, B and C), suggesting some abnormality in the condensation process.
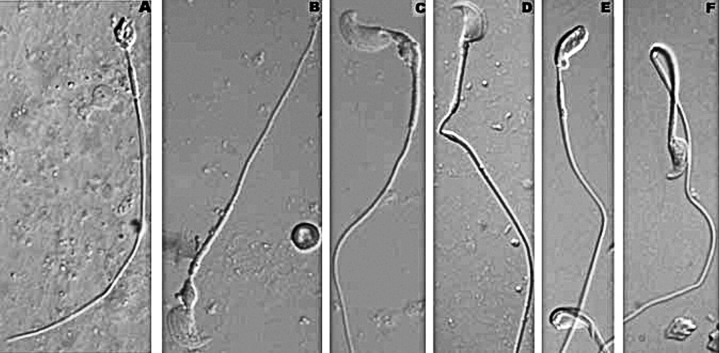
FIG. 4.
The DIC imaging of testicular, caput, and cauda epididymal spermatozoon. A) Typical testicular sperm from Ppp1cc−/− mouse. Note rounded deformed head and the mitochondrial sheath abbreviated at both ends. B) Typical testicular sperm from rescue mouse containing a hook-shaped head, a cytoplasmic remnant at the head/connecting piece junction, a distally shortened mitochondrial sheath, and a straight flagellum. C) Testicular sperm from rescue mouse showing gross similarities to sperm in B but displaying non-hairpin bending at the head/connecting piece junction and in the principal piece. Note the distally shortened mitochondrial sheath, leaving a small gap between the mitochondrial sheath and the beginning of the principal piece. D) Rescue sperm from the caput epididymis. Note ∼90° sharp bend at a gap in the mitochondrial sheath near the distal end of the midpiece. E) Cauda epididymal sperm from rescue mouse. Note the gap in the mitochondrial sheath near the head/connecting piece junction of upper sperm, as well as the head/connecting piece junction hairpins, where the heads appear to be engulfed in cytoplasm with the proximal midpiece. F) Cauda epididymal sperm exhibiting hairpin bend at the midpiece/principal piece junction. All sperm were viewed at 100× magnification.
When we examined caput (Fig. 4D) and cauda epididymal (Fig. 4, E and F) rescue sperm, we detected two phenotypes not observed previously in sperm extruded from the testes of the same animal: many of the epididymal sperm had heads that folded back at the connecting piece at a 180° angle (hairpinning), and these appeared to be attached to the proximal end of the midpiece within a common membrane-bound cytoplasmic pouch (Fig. 4E). In many of these cases, the mitochondrial sheath appeared gapped or absent at what is normally its proximal end (Fig. 4E). A few sperm also showed a similar hairpin phenotype at the midpiece/principal piece junction (Fig. 4F). In most of these cases, the mitochondrial sheath did not appear to extend to the distal end of the midpiece.
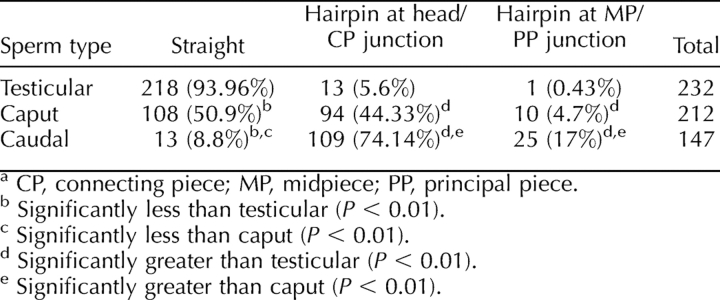
It is possible that many of the hairpin flagella are by-products of shear forces acting on already weakened regions of the rescue sperm flagella during passive transit from the testis to the epididymis. If this is truly the case, then the numbers of head/connecting piece junction and midpiece/principal piece junction hairpins should not differ significantly between caput and cauda epididymal sperm but should show significant differences between epididymal sperm from either the cauda or caput and testicular sperm. A quantitative analysis of sperm morphologies (see Materials and Methods) from the testes, caput, and caudae epididymides of a rescue male (Table 1) indeed demonstrated that the numbers of head/connecting piece junction and midpiece/principal piece junction hairpins in both the caput and cauda epididymis differed significantly from the numbers of testicular sperm exhibiting either phenotype (P < 0.01 for both). However, the numbers of head/connecting piece junction and midpiece/principal piece junction hairpins seen in the caput were significantly fewer (P < 0.01) than the numbers of sperm displaying these phenotypes in the cauda epididymis. A highly significant increase in both hairpin phenotypes in caudal vs. caput sperm suggested that a biological process that takes place in the cauda but not in the caput might increase mechanical shear in the flagellum. An example of a process that would increase shear force in the flagellum is the development of initial flagellar motility in the cauda epididymis.
TABLE 1.
Sperm tail bend phenotype.a
To test whether rescue sperm were motile, lightly minced caudae epididymides were incubated briefly in a medium that supports progressive sperm motility in vitro to allow sperm to move into the medium. High-resolution videotape studies demonstrated that a small percentage of the rescue sperm displayed motility of variably poor quality (data not shown). However, most of these motile sperm exhibited so little forward (progressive) movement that quantifiable parameters of progressive motility were below the detectable limit of the sperm motion analysis (CASA) system used (data not shown).
Expression and Localization of PPP1CC2 in Spermatozoa from Rescue Mice
We also tested to see if PPP1CC2 protein expressed in testicular germ cells of rescue mice was properly incorporated into epididymal spermatozoa. Western blot analysis of caudal sperm protein extracts from these mice showed that immunoreactive PPP1CC2 was indeed present, although, as expected, at lower levels than in extracts prepared from wild-type sperm (Fig. 5A). Immunocytochemical analysis demonstrated that PPP1CC2 localization was distributed along the tail and the head in a similar fashion to the pattern seen in wild-type caudal sperm (Fig. 5B).
FIG. 5.
Transgenic PPP1CC2 detection in caudal spermatozoon from A-line rescue mouse (A). Immunocytochemistry of PPP1CC2 in rescue sperm shows the presence of PPP1CC2 along the head and tail, similar to wild type (WT) in B. Slides were viewed using the FluoView 500 fluorescence microscope at 60× magnification.
Ultrastructural Analysis of Cauda Epididymal Sperm from Rescue Mice
As already demonstrated, hairpin phenotypes rarely observed in testicular sperm were significantly more frequent in caput and cauda epididymal sperm of rescue mice. To examine these hairpin phenotypes in more detail, we used transmission electron microscopy (TEM) of cauda epididymal sperm in situ.
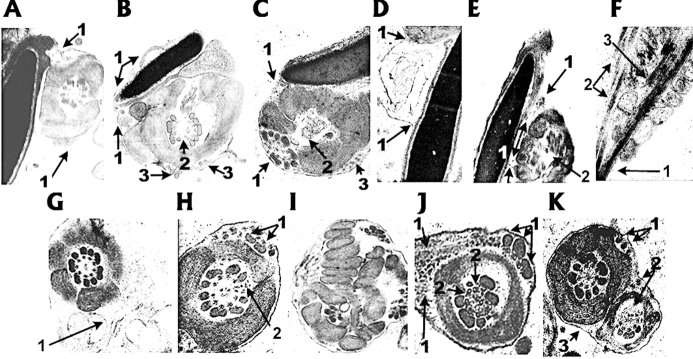
When the ultrastructure of rescue sperm in the cauda epididymis was inspected by TEM, head/connecting piece junction hairpins were readily evident (Fig. 6, A–C and E). Plasma membrane with some cytoplasmic content loosely surrounded both the heads and proximal midpieces in these sections. This residual cytoplasm was often appended asymmetrically around the outer circumference of the proximal midpiece, where extruded ODFs and other cytoplasmic remnants were observed (Fig. 6B). We also discerned that the heads of these sperm were elongated (Fig. 6, A–E), in contrast to the rounded heads of Ppp1cc−/− testicular sperm (Fig. 4A), and that they contained nuclei with completely condensed chromatin (Fig. 6, A–E). In some cases, concave deformities were observed in the proximal acrosome (Fig. 6, A and E).
FIG. 6.
Ultrastucture of cauda epididymal Ppp1cc2-rescue sperm in situ. A) Longitudinal section through sperm head abutting transverse section of the midpiece: 1) Arrows point to plasma membrane extending from sperm head and surrounding one side of the midpiece; note the absence of intervening plasma membrane between the head and midpiece and the distorted mitochondrion. B) Longitudinal section through sperm head abutting transverse section of the midpiece. 1) Arrows point to membranous structures extending from the head and beginning to encircle the midpiece containing a poorly formed mitochondrial sheath. 2) Arrow indicates disruption of the axoneme along the longitudinal plane created by MT doublet/ODF pairs three and eight connected to the central singlet pair of MTs by radial spokes; however, none of the ODFs or associated MT doublets appear to be extruded at this level of section of the midpiece. 3) Arrows pointing to ODFs external to the mitochondrial sheath. Their presence indicates that they were extruded into the peripheral cytoplasm distally, perhaps through weak spots or discontinuities in the mitochondrial and/or fibrous sheaths. C) Longitudinal section through sperm head abutting transverse section of the midpiece. 1) Arrows point to plasma membrane extending from sperm head and arching around one side of the midpiece. 2) Arrow indicates the absence of entire ODF/axonemal complex from within the highly disorganized mitochondrial sheath; the ODF/axonemal complex appears to have extruded through a large gap in the poorly formed mitochondrial sheath. 3) Arrow indicates unidentified cytoplasmic content. D) 1) Arrows indicate plasma membrane connecting center of sperm head to the midpiece (top). Note unidentified cytoplasmic content within the plasma membrane between the head and midpiece. E) Longitudinal section through sperm head abutting oblique section of the midpiece. 1) Both single and double-headed arrows show what appear to be thin membranous connections between the head and midpiece. 2) Arrow indicates disorganized/absent ODF/axonemal complex. 3) Arrow points to a gap between nucleus and acrosome. F) Longitudinal section through distal end of a shortened mitochondrial sheath. 1) Distal extent of a shortened mitochondrial sheath. 2) Broken ODFs extruding through a gap between the principal piece and shortened distal end of a mitochondrial sheath. 3) Broken ODF internal to the mitochondrial sheath on only one side of the axoneme. G) 1) Section through a normal mitochondrial sheath and an ODF/axonemal complex contained within asymmetrically surrounding cytoplasm. H) The midpiece containing a mitochondrial sheath circling the full complement of ODFs and MT doublets. 1) Arrows indicate extruded ODFs as in B. 2) Arrow indicates a gap in the circle of ODF/MT doublet complexes filled with debris. I) Section through thoroughly unorganized mitochondrial sheath of Ppp1cc−/− testicular sperm. J) Transverse section through the principal piece of rescue sperm. 1) Arrows indicate regions of asymmetric cytoplasmic bulge with granular content (left) and three extruded ODF/MT doublet-dynein arm complexes (right). 2) Arrows indicate opposite direction of dynein arms of adjacent MT doublets three (leftward) and four (rightward); note also that ODF associated with MT doublet four is present but reduced in size. K) Cross-section through the midpiece and adjacent principal piece bounded by a single plasma membrane. 1) Arrows point to extruded ODFs in a cytoplasmic bulge around the midpiece. 2) Long arrow points to missing ODF/MT doublet complexes four through seven from the principal piece section; short arrow indicates possible extruded ODF. 3) Arrow indicates continuity of plasma membrane extending around the midpiece and the principal piece cross-sections. Original magnification ×15 000 (B and E), ×20 000 (D), ×30 000 (A, C, F, G, I, and K), ×35 000 (J), and ×50 000 (H).
Most of the proximal midpieces of these sperm contained additional abnormalities, including disorganized gapped mitochondrial sheaths, deformed mitochondria, disordered doublet microtubules (MT doublets), and/or misaligned ODFs (Fig. 6, A–C and E). However, in contrast to Ppp1cc−/− testicular sperm, in which the number of ODFs in the midpiece was usually highly increased [13], the numbers of ODFs and MT doublets internal to the mitochondrial sheaths of rescue sperm were generally normal or were occasionally decreased by one (Fig. 6, B, C, and F). In the latter case, it seemed probable that absent ODF/MT doublet complexes protruded through abnormal gaps in the more distal mitochondrial sheaths during initiation of motility (Fig. 6F).
In other cross-sections through these more distal regions of the midpieces of rescue sperm, two primary abnormalities were evident (Fig. 6, H, J, and K). First, extruded ODF/MT doublet complexes were present in unshed asymmetrical cytoplasmic bulges peripheral to the mitochondrial sheath (Fig. 6H). Also in this cross-section, the shape of the circle of nine ODFs and attached MT doublets appears distorted by a gap, possibly created by the breakdown of extra MTs between ODF/MT doublet complexes three and four. Second, in sections where the mitochondrial sheath, ODFs, and axonemal MTs appeared normal, asymmetrical cytoplasm often surrounded the mitochondrial sheath (Fig. 6G). However, the appearance of some normal mitochondrial structures in rescue sperm was in stark contrast to the much larger number of mitochondrial anomalies seen in Ppp1cc−/− testicular sperm (Fig. 6I).
Tail disorganization appeared to be no less radical in the principal pieces of rescue sperm tails (Fig. 6, J and K), in which extruded ODFs associated with MT doublets and attached dynein arms were evident in abnormal asymmetric cytoplasmic appendages, and not only were the axonemal MT doublets and associated periaxonemal ODFs reduced in number, but also dynein arms of different doublets sometimes faced in opposing directions (Fig. 6J). It was not unusual to see two different flagellar cross-sections bounded by a single membrane, perhaps representative of the more distal flagellar hairpin bends. Within the principal piece portions of these cross-sections, extrusion of ODFs four through seven and associated MT doublets was common, while extruded ODFs were also observed in the midpiece portion of many of these sections (Fig. 6K). The presence of these ultrastructural anomalies in the majority of caudal rescue sperm further increased the probability that hairpin phenotypes were exacerbated by the initiation of motility in the cauda epididymis. However, because normally high levels of PPP1CC2 activity in the wild-type caput epididymis are known to inhibit the initiation of sperm motility [11], we cannot rule out the possibility that the appearance of these phenotypes at a significantly higher level in caput vs. testicular rescue sperm might indicate that lower catalytic activity of PPP1CC2 in the rescue caput epididymis leads to premature initiation of rescue sperm motility.
To test the possibility that the disorder we saw in caudal rescue sperm results from the initiation of axonemal motility in sperm with structurally unstable tails, we examined testicular rescue sperm by TEM (Supplemental Fig. S2). Although the results are based on a small number of micrographs, they bear out the contention that more ultrastructural features of Ppp1cc−/− spermatids/testicular sperm appear to be rescued by expression of transgenic PPP1CC2 than are evident from examination of caudal rescue sperm ultrastructure. Thus, it seems likely that the initiation of motility in caudal (and perhaps caput) rescue sperm instigates the extrusion of structurally unstable ODFs and attached MT doublets through abnormal gaps between poorly developed mitochondrial gyres and/or fibrous sheath ribs.
DISCUSSION
Targeted disruption of the gene ultimately responsible for expression of both PPP1CC protein isoforms, PPP1CC1 and PPP1CC2, results in male infertility stemming from impaired spermatogenesis, particularly in the spermiogenic phase, in which (presumably) spermatid apoptosis is widespread [12]. Although the other protein phosphatase 1 isoforms, PPP1CA and PPP1CB, are substantially upregulated in the Ppp1cc−/− testis [13], neither is able to substitute for the absent PPP1CC proteins in restoring spermatogenesis and male fertility. This is somewhat surprising because the primary sequences of the four PP1 isoforms in mammals are not only almost identical but also highly homologous to their counterparts in yeast and other organisms. In fact, any of the four mammalian PPP1C subunits can complement the single PPP1C isoform in yeast [17]. It is also notable that, although PPP1CC1 is expressed in almost all tissues, while the PPP1CC2 protein is testis restricted (and is the only PPP1C isoform present in mammalian spermatozoa), the absence of PPP1CC1 has no apparent detrimental effect on any process other than (possibly) testicular function. Because PPP1CC2 is more highly expressed than any other of the PP1 isoforms in testicular germ cells, we hypothesized that its spermatocyte- and spermatid-restricted expression in the Ppp1cc−/− testis might be able to restore spermatogenesis and fertility in males. Thus, our approach to test this hypothesis was to drive transcription of PPP1CC2 from the spermatocyte-specific promoter of Pgk2 (Fig. 1) so that PPP1CC2 protein expression would be restored to secondary spermatocytes and spermatids. Previous investigations have shown that the Pgk2 promoter is able to drive transcription of the germinal isoform of Ace in Ace-null mice, thus fully restoring their fertility [14].
Apparently, as a consequence of Ppp1cc2 transgene expression in the Ppp1cc−/− mouse, PPP1CC2 spatial expression approximates wild-type protein expression in the testis (Fig. 2D) and in caudal sperm (Fig. 5), while qualitatively normal testis architecture within the seminiferous tubules of PPP1CC2-rescue mice is restored (Fig. 3A). In addition, spermatozoa derived by swim-out from the cauda epididymis of the rescue mice were abundant (∼2 × 106 sperm/cauda epididymis compared with ∼1 × 107 sperm/cauda epididymis of wild-type mice). Furthermore, while the epididymis of the Ppp1cc-null mouse was virtually devoid of spermatozoa, the lumen of the epididymis of the rescue mouse appeared to have substantial numbers of spermatozoa in it (Fig. 3B). However, and contrary to our initial expectations, transgenic expression of PPP1CC2 in the Ppp1cc−/− testis failed to restore fertility of rescue males. Motility analysis showed that the great majority of caudal spermatozoa derived from PPP1CC2-rescue mice and incubated in medium that supports vigorous progressive motility in wild-type sperm were immotile, while those that were motile lacked progressive movement. In addition, high-resolution light and electron microscopic observations of caudal rescue sperm provided a variety of structural causes for the lack of sperm motility (and thus infertility).
Testicular rescue sperm have normal sickle-shaped heads, as opposed to deformed rounded heads in Ppp1cc−/− testicular sperm (Fig. 4), strongly suggesting that PPP1CC2 activity regulates sperm head morphogenesis. However, one flagellar aberration seen in testicular sperm from Ppp1cc−/− mice, a malformed, abbreviated, or gapped mitochondrial sheath, is only partially ameliorated in testicular rescue sperm, while irregularly shaped residual cytoplasmic bulges are frequently observed at the head/connecting piece junction (Fig. 4). These findings have provided obvious reasons for the loss of movement in rescue sperm and suggest that either PPP1CC1 has a major role in flagellar morphogenesis or PPP1CC2 activity is insufficient in the rescue testis, in which the level of transgenic PPP1CC2 protein expressed is at best one third of wild type, to completely rescue flagellar morphogenesis.
Approximately 44% and 75% of the heads of Ppp1cc2-rescue spermatozoa extruded from the caput and caudae epididymides, respectively, are bent backwards and partially encircle the circumference of the mitochondrial sheath; these are tethered to the proximal midpiece within unremoved residual cytoplasm, while another ∼5% and ∼17%, respectively, of these sperm exhibit a 180° hairpin bend at the junction of the midpiece and the principal piece (Fig. 4 and Table 1). A strikingly similar phenotype to the head/connecting piece junction hairpin abnormality seen herein has been reported recently in mutant sperm lacking SPEM1, a protein expressed in spermatids and localized to the cytoplasmic droplet [18]. This defect has been attributed to the improper removal of this residual cytoplasm in the newly formed spermatozoa during spermiation. Whether expression of SPEM1 or other still unidentified polypeptides required for proper removal of residual cytoplasm is compromised in rescue spermatozoa remains to be determined.
Surprisingly, neither hairpin phenotype is exhibited to a significant degree (∼6% and ∼0.4% for the head/connecting piece junction phenotype and the midpiece/principal piece junction phenotype, respectively) in testicular rescue sperm (Table 1). Most important, caput and caudal rescue sperm are also significantly different from each other (P < 0.01) for both hairpin phenotypes. The reason for this continuum of phenotypic variation along the male genital tract has several possible explanations: 1) As is the case in the Spem1-null sperm, cytoplasm that is normally removed from the junction between the head and neck of the condensing spermatid is insufficiently loosened so that the sperm head and proximal midpiece become abnormally encapsulated within this residual material in the epididymis, possibly due to mechanical shear forces arising first from transit of sperm from the testis to the caput epididymis and then exacerbated by the initiation of sperm motility in the cauda epididymis; 2) Or, transit of sperm from the testis to the caput epididymis might have little (if any) role in creating either flagellar bend phenotype: instead, PPP1CC2 catalytic activity in caput rescue sperm (and possibly the testis) that is lower than that in wild type could permit limited flagellar activity in the testis and caput that is then amplified in the cauda epididymis as PPP1CC2 activity is further suppressed [11]. Electron micrographs of rescue spermatids and testicular sperm appear to reinforce these explanations for the incidence of flagellar bending phenotypes in testicular vs. epididymal rescue sperm, as the tail ultrastructure of testicular rescue germ cells is improved compared with that of caudal rescue sperm (Supplemental Fig. S2).
While these simple explanations need to be more completely tested, it is notable that spermatozoa recovered from selenium-deficient mice or mice lacking the serum selenoprotein P1 or the putative selenoprotein receptor, apolipoprotein E receptor 2 [19–21], display principal piece and midpiece ODF abnormalities similar to those of rescue mice. Striking similarities include thinning of the midpiece, particularly at its distal end, where the mitochondrial sheath is abbreviated distally, as well as hairpin bends of the flagellum at the midpiece/principal piece junction accompanied by peripheral extrusion of ODF/MT doublet complexes four through seven through the gap created between the annulus and the prematurely terminated mitochondrial sheath. This suggests that Ppp1cc2-rescue mice may be defective in selenium transport and metabolism. It is possible that expression of the putative selenoprotein P1 (SEPP1) receptor in Sertoli cells is compromised or in some way functionally defective in rescue mice. The rare testicular spermatozoa that could be recovered from Ppp1cc−/− mice are characterized by disorganized or missing mitochondrial sheaths. Mitochondrial sheath abnormalities, while less prominent, still persist in rescue spermatozoa (Figs. 4 and 6). As stated earlier, thinning of the mitochondrial sheath at varying positions in the midpiece is frequently observed in spermatozoa from rescue mice. Studies in progress show that the level of the selenoprotein phospholipid hydroperoxide glutathione peroxidase, an abundant protein in mitochondrial sheaths [19], is substantially reduced in both Ppp1cc−/− and Ppp1cc2-rescue testis (Vijayaraghavan, unpublished results). Further work is required to examine whether either or both PPP1CC isoforms in Sertoli cells or Leydig cells have a role in selenium import into and the expression of selenoproteins in the testis.
Unless PPP1CC1 has a major role in directing spermiogenesis, the exact reason for the lack of complete rescue of sperm structure and function in PPP1CC2 mice is not entirely clear. However, several testable possibilities exist. It could be that low levels of PPP1CC2 protein expression in Ppp1cc2-rescue testis are insufficient to sustain normal spermatogenesis and sperm maturation. However, it should be emphasized that the sperm phenotype is essentially the same in all lines of transgenic mice (expressing varying low levels of PPP1CC2 compared with wild type). Surprisingly, the presence of the transgene fails to increase PPP1CC2 protein levels in +/+ and +/− backgrounds (data not shown). It appears that a homeostatic mechanism might operate to ensure no more than an optimum level of PPP1CC2 translation from its mRNA. Such a mechanism can be at the level of initiation of protein synthesis or in the breakdown of excess protein by proteolysis. However, the reason why mRNA derived from the transgene does not translate into higher protein levels in the Ppp1cc−/− background is puzzling, as several rescue lines express testicular levels of Ppp1cc2 mRNA equivalent to those of fertile Ppp1cc+/− mice (Supplemental Fig. S1). One possibility is that the stability of the mRNA derived from the transgene is less than that of the message derived from the endogenous gene. The transcript derived from the transgene lacks both the 5′ and 3′ untranslated regions (UTRs) present in the mRNA derived from the endogenous Ppp1cc gene. Also, only a limited number of genes are transcribed in spermatids. Generally, translation in spermatids is from preexisting mRNA [22]. Thus, the reduced stability of the mRNA from the Ppp1cc2 transgene may compromise PPP1CC2 protein levels in spermatozoa. To address this concern, we have engineered a new transgene under the same Pgk2 promoter that includes the cDNA for both Ppp1cc1 and Ppp1cc2 and the bona fide 5′ and 3′ UTRs present in the Ppp1cc gene.
Another possibility for the lack of a complete rescue is that normal spermatogenesis may require expression of PPP1CC2 at stages of germ cell development earlier than when the Pgk2 promoter becomes normally active. Transcription of the sperm-specific Pgk2 gene apparently occurs in secondary spermatocytes [23]. Fluorescence immunohistochemical analysis of testis sections shows that a strong signal for PPP1CC2 first appears in primary spermatocytes and remains strong through all further stages of germ cell development (Fig. 2), although Western blot analysis also has shown that a weak signal for PPP1CC2 is detected in the testis of 8-day-old mice (corresponding to spermatogonial/somatic cell expression) [13]. Thus, PPP1CC2 may be essential for gene expression and for other biochemical processes in primary spermatocytes. Therefore, it is conceivable that a lack of PPP1CC2 at the primary spermatocyte stage (or earlier) of germ cell differentiation in rescue mice could result in the abnormal assembly of the sperm flagellum during spermiogenesis. The mRNAs for a number of proteins that are synthesized in haploid spermatids are transcribed earlier in primary and secondary spermatocytes [22, 24]. Among these are proteins of the ODFs, which are unique features of the flagella of mammalian spermatozoa. It is possible that PPP1CC2, a PP1 isoform found only in mammals, may be involved in an isoform-specific manner in the morphogenesis of the unique features of the mammalian sperm flagellum. Finally, as noted earlier, we cannot rule out the requirement of a function for the PPP1CC proteins in Sertoli cells and/or Leydig cells. That is, one or both PPP1CC isoforms may be part of a signaling system that must operate between Sertoli cells and developing germ cells to ensure normal spermatogenesis.
Efforts are under way to create transgenic mice whose expression of PPP1CC2 is driven by its endogenous promoter and to develop conditional knockout mice in which one or both of the PPP1CC isoforms can be deleted in specific cell types in the testis. These studies should shed further light on the role and requirement of the unique PPP1CC2 isoform in spermatogenesis in mammals.
Supplementary Material
Acknowledgments
We thank Dr. Gary E. Olson (Department of Cell and Developmental Biology, Vanderbilt University, Nashville, TN) for his comments regarding this manuscript, Dr. Douglas Kline (Department of Biological Sciences, Kent State University, Kent, OH) for his helpful expertise, Dr. Mike Model (Department of Biological Sciences, Kent State University, Kent, OH) for his assistance in confocal fluorescence microscopy, and members of the Vijayaraghavan laboratory for useful discussions.
Footnotes
1Supported by NIH grants HD 38520 to S.V. and HD31164 to S.H.P.
REFERENCES
- Bedford JM, Hoskins DD.The mammalian spermatozoon: morphology, biochemistry and physiology. Lamming GE.Marshall's Physiology of Reproduction New York:Churchill Livingstone;1990: 379–568. [Google Scholar]
- Jones R.Plasma membrane structure and remodeling during sperm maturation in the epididymis. J Reprod Fertil Suppl 1998; 53: 73–84. [PubMed] [Google Scholar]
- Vijayaraghavan S, Hoskins DD.Regulation of bovine sperm motility and cyclic adenosine 3′,5′-monophosphate by adenosine and its analogues. Biol Reprod 1986; 34: 468–477. [DOI] [PubMed] [Google Scholar]
- Vijayaraghavan S, Bhattacharya A, Hoskins DD.Calcium uptake by bovine epididymal spermatozoa is regulated by the redox state of the mitochondrial pyridine nucleotide. Biol Reprod 1989; 40: 744–751. [DOI] [PubMed] [Google Scholar]
- Vijayaraghavan S, Critchlow LM, Hoskins DD.Evidence for a role for cellular alkalinization in the cyclic adenosine 3′,5′-monophosphate mediated initiation of motility in bovine caput spermatozoa. Biol Reprod 1985; 3: 489–500. [DOI] [PubMed] [Google Scholar]
- Burton KA, McKnight KS.PKA, germ cells and fertility. Physiology 2007; 22: 40–46. [DOI] [PubMed] [Google Scholar]
- Chakrabarti R, Cheng L, Puri P, Soler D, Vijayaraghavan S.Protein phosphatase PP1γ2 in sperm morphogenesis and epididymal initiation of sperm motility. Asian J Androl 2007; 9: 445–452. [DOI] [PubMed] [Google Scholar]
- Vijayaraghavan S, Chakrabarti R, Myers K.Regulation of sperm function by protein phosphatase PP1γamma2. Soc Reprod Fertil Suppl 2007; 63: 111–121. [PubMed] [Google Scholar]
- Kitagawa Y, Sasaki K, Shima H, Shibuya M, Sugimura T, Nagao M.Protein phosphatases possibly involved in rat spermatogenesis. Biochem Biophys Res Commun 1990; 171: 230–235. [DOI] [PubMed] [Google Scholar]
- Smith GD, Wolf DP, Trautman KC, da Cruz e Silva EF, Greengard P, Vijayaraghavan S.Primate sperm contain protein phosphatase 1, a biochemical mediator of motility. Biol Reprod 1996; 3: 719–727. [DOI] [PubMed] [Google Scholar]
- Vijayaraghavan S, Stephens DT, Trautman K, Smith GD, Khatra B, da Cruz e Silva EF, Greengard P.Sperm motility development in the epididymis is associated with decreased glycogen synthase kinase-3 and protein phosphatase-1 activity. Biol Reprod 1996; 54: 709–718. [DOI] [PubMed] [Google Scholar]
- Varmuza S, Jurisicova A, Okano K, Hudson J, Boekelheide K, Shipp EB.Spermiogenesis is impaired in mice bearing a targeted mutation in the protein phosphatase 1c gamma gene. Dev Biol 1999; 205: 98–110. [DOI] [PubMed] [Google Scholar]
- Chakrabarti R, Kline D, Lu J, Orth J, Pilder S, Vijayaraghavan S.Analysis of Ppp1cc-null mice suggests a role for PP1γamma2 in sperm morphogenesis. Biol Reprod 2007; 76: 992–1001. [DOI] [PubMed] [Google Scholar]
- Kessler SP, Gomos JB, Scheidemantel TS, Rowe TM, Smith HL, Sen GC.The germinal isozyme of angiotensin-converting enzyme can substitute for the somatic isozyme in maintaining normal renal structure and functions. J Biol Chem 2002; 277: 4271–4276. [DOI] [PubMed] [Google Scholar]
- Summers MC, McGinnis LK, Lawitts JA, Raffin M, Biggers JD.IVF of mouse ova in a simple optimized medium supplemented with amino acids. Hum Reprod 2000; 15: 1791–1801. [DOI] [PubMed] [Google Scholar]
- Pilder SH, Olds-Clarke P, Orth JM, Jester WF, Dugan L.Hst7: a male sterility mutation perturbing sperm motility, flagellar assembly, and mitochondrial sheath differentiation. J Androl 1997; 18: 663–671. [PubMed] [Google Scholar]
- Gibbons GA, Kozubowski L, Tatchell K, Shenolikar S.Expression of human protein phosphatase-1 in Saccharomyces cerevisiae highlights the role of phosphatase isoforms in regulating eukaryotic functions. J Biol Chem 2007; 282: 21838–21847. [DOI] [PubMed] [Google Scholar]
- Zheng H, Stratton CJ, Morozumi K, Jin J, Yanagimachi R, Yan W.Lack of Spem1 causes aberrant cytoplasm removal, sperm deformation, and male infertility. Proc Natl Acad Sci U S A 2007; 104: 6852–6857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson GE, Winfrey VP, Nagdas SK, Hill KE, Burk RF.Selenoprotein P is required for mouse sperm development. Biol Reprod 2005; 73: 201–211. [DOI] [PubMed] [Google Scholar]
- Andersen OM, Yeung CH, Vorum H, Wellner M, Andreassen TK, Erdmann B, Mueller EC, Herz J, Otto A, Cooper TG, Willnow TE.Essential role of the apolipoprotein E receptor-2 in sperm development. J Biol Chem 2003; 278: 23989–23995. [DOI] [PubMed] [Google Scholar]
- Olson GE, Winfrey VP, Hill KE, Burk RF.Sequential development of flagellar defects in spermatids and epididymal spermatozoa of selenium-deficient rats. Reproduction 2004; 127: 335–342. [DOI] [PubMed] [Google Scholar]
- Iguchi N, Tobias JW, Hecht NB.Expression profiling reveals meiotic male germ cell mRNAs that are translationally up- and down-regulated. Proc Natl Acad Sci U S A 2006; 103: 7712–7717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang LP, Stroud J, Eddy CA, Walter CA, McCarrey JR.Multiple elements influence transcriptional regulation from the human testis-specific PGK2 promoter in transgenic mice. Biol Reprod 1999; 60: 1329–1337. [DOI] [PubMed] [Google Scholar]
- Hecht NB.Regulation of ‘haploid expressed genes' in male germ cells. J Reprod Fertil 1990; 88: 679–693. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.