Abstract
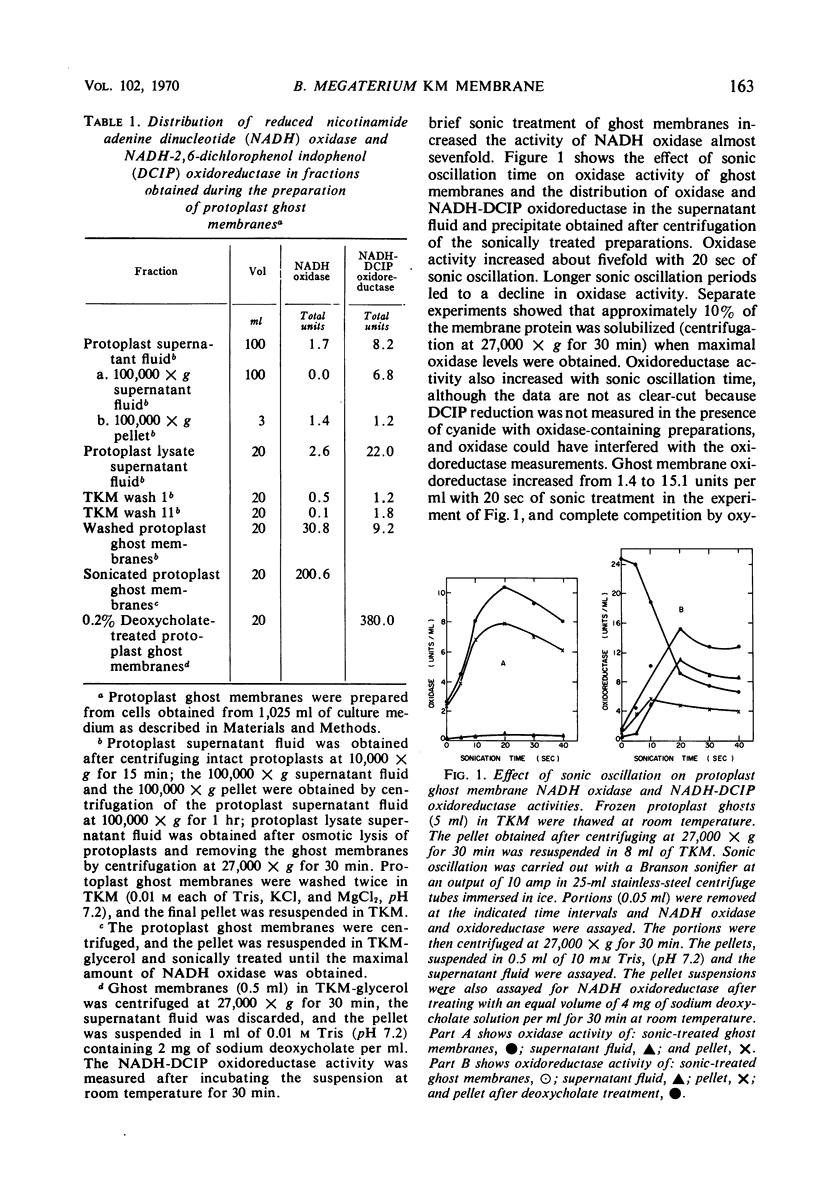
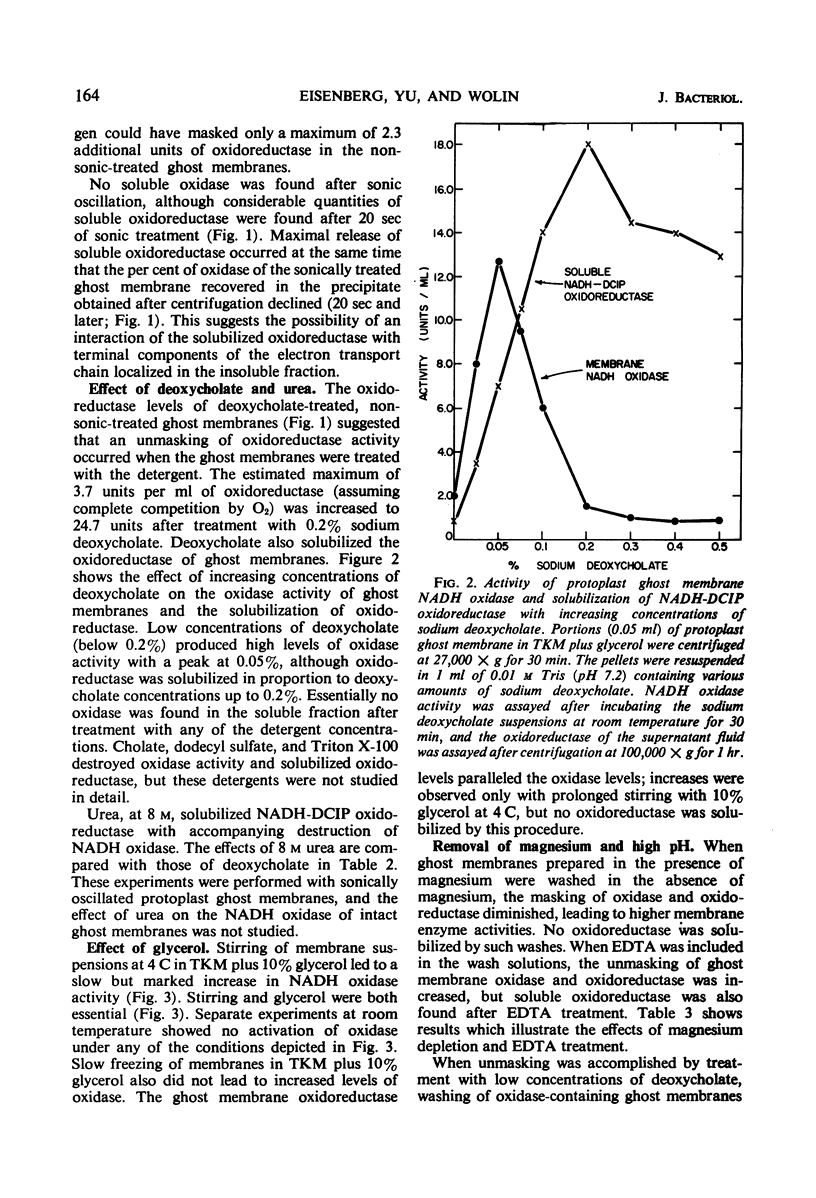
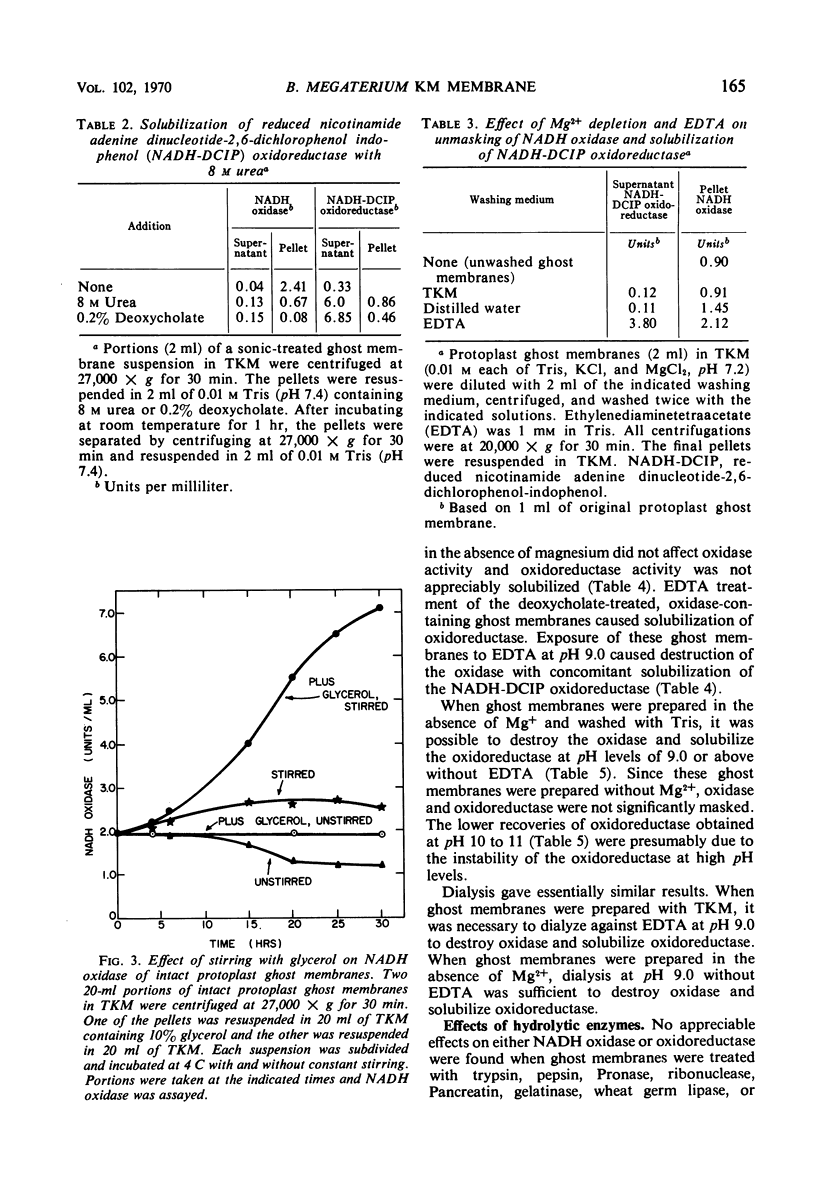
Solubilization of a reduced nicotinamide adenine dinucleotide (NADH)-2,6 dichlorophenol indophenol (DCIP) oxidoreductase associated with the membrane NADH oxidase system of Bacillus megaterium KM was effected by treatment with 0.2% sodium deoxycholate, 8 m urea, or buffer (pH 9.0) in the presence of ethyl-enediaminetetraacetate. These treatments inactivated membrane NADH oxidase. It was found that membrane NADH oxidase and NADH-DCIP oxidoreductase were masked in membranes. Several procedures, including brief sonic oscillation, treatment with 0.05% deoxycholate, prolonged stirring at 4 C with 10% glycerol, and washing in the absence of Mg2+, unmasked the oxidase and oxidoreductase activities. It was necessary to study the masking and unmasking of these activities to quantitate adequately the effects of solubilization procedures. Further information on the localization of oxidase and oxidoreductase in subcellular fractions and the effects of electron transport inhibitors on NADH oxidation was also obtained.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronson A. Adsorption of polysomes to bacterial membranes. J Mol Biol. 1966 Feb;15(2):505–514. doi: 10.1016/s0022-2836(66)80124-9. [DOI] [PubMed] [Google Scholar]
- Aspen A. J., Wolin M. J. Solubilization and reconstitution of a particulate hydrogenase from Vibrio succinogenes. J Biol Chem. 1966 Sep 25;241(18):4152–4156. [PubMed] [Google Scholar]
- Broberg P. L., Smith L. The cytochrome system of Bacillus megaterium KM. The presence and some properties of two CO-binding cytochromes. Biochim Biophys Acta. 1967 May 9;131(3):479–489. doi: 10.1016/0005-2728(67)90007-2. [DOI] [PubMed] [Google Scholar]
- KING T. E. RECONSTITUTION OF RESPIRATORY CHAIN ENZYME SYSTEMS. XII. SOME OBSERVATIONS ON THE RECONSTITUTION OF THE SUCCINATE OXIDASE SYSTEM FROM HEART MUSCLE. J Biol Chem. 1963 Dec;238:4037–4051. [PubMed] [Google Scholar]
- Kaback H. R. The role of the phosphoenolpyruvate-phosphotransferase system in the transport of sugars by isolated membrane preparations of Escherichia coli. J Biol Chem. 1968 Jul 10;243(13):3711–3724. [PubMed] [Google Scholar]
- Kagawa Y., Racker E. Partial resolution of the enzymes catalyzing oxidative phosphorylation. 8. Properties of a factor conferring oligomycin sensitivity on mitochondrial adenosine triphosphatase. J Biol Chem. 1966 May 25;241(10):2461–2466. [PubMed] [Google Scholar]
- Kopaczyk K., Asai J., Green D. E. Reconstitution of the repeating unit of the mitochondrial inner membrane. Arch Biochem Biophys. 1968 Jul;126(1):358–379. doi: 10.1016/0003-9861(68)90592-4. [DOI] [PubMed] [Google Scholar]
- Mizushima S., Ishida M., Miura T. Subfractionation of protoplast membrane and enzyme localization in Bacillus megaterium. J Biochem. 1966 Sep;60(3):256–261. doi: 10.1093/oxfordjournals.jbchem.a128431. [DOI] [PubMed] [Google Scholar]
- Reaveley D. A., Rogers H. J. Some enzymic activities and chemical properties of the mesosomes and cytoplasmic membranes of Bacillus licheniformis 6346. Biochem J. 1969 Jun;113(1):67–79. doi: 10.1042/bj1130067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryter A., Frehel C., Ferrandes B. Comportement des mésosomes lors de l'attaque de Bacillus subtilis par le lysozyme en milieu hyper- ou hypotonique. C R Acad Sci Hebd Seances Acad Sci D. 1967 Oct 23;265(17):1259–1262. [PubMed] [Google Scholar]
- SCHLESSINGER D. PROTEIN SYNTHESIS BY POLYRIBOSOMES ON PROTOPLAST MEMBRANES OF B. MEGATERIUM. J Mol Biol. 1963 Nov;7:569–582. doi: 10.1016/s0022-2836(63)80103-5. [DOI] [PubMed] [Google Scholar]
- Salton M. R., Freer J. H., Ellar D. J. Electron transport components localized in a lipid-depleted sheet isolated from Micrococcus lysodeikticus membranes by deoxycholate extraction. Biochem Biophys Res Commun. 1968 Dec 30;33(6):909–915. doi: 10.1016/0006-291x(68)90398-7. [DOI] [PubMed] [Google Scholar]
- WACHSMAN J. T., STORCK R. Propionate induced lysis of protoplasts of Bacillus megaterium. J Bacteriol. 1960 Nov;80:600–606. doi: 10.1128/jb.80.5.600-606.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIBULL C. The nature of the ghosts obtained by lysozyme lysis of Bacillus megaterium. Exp Cell Res. 1956 Feb;10(1):214–221. doi: 10.1016/0014-4827(56)90087-8. [DOI] [PubMed] [Google Scholar]
- WHITTAM R. The asymmetrical stimulation of a membrane adenosine triphosphatase in relation to active cation transport. Biochem J. 1962 Jul;84:110–118. doi: 10.1042/bj0840110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita S., Racker E. Reconstitution of the mitochondrial oxidation chain from individual components. J Biol Chem. 1968 May 10;243(9):2446–2447. [PubMed] [Google Scholar]