Abstract
Uterine natural killer (uNK) cells accumulate at the maternal-fetal interface during gestation and are thought to have an important role during pregnancy in both mice and humans. While the cell surface phenotype of human uNK cells is increasingly well defined, less is known regarding the cell surface expression profile of murine uNK cells both before and during gestation. Herein, we demonstrate that murine NK1.1+ (KLRB1C) endometrial NK (eNK) cells, derived from virgin mice, and NK1.1+ decidual NK (dNK) cells, obtained from pregnant mice, belong to the B220+ (PTPRC) CD11c+ (ITGAX) subset of NK cells. While B220 expression was low on NK1.1+ eNK cells, it was increased on a subset of NK1.1+ dNK cells at Embryonic Day 10.5. Endometrial NK and dNK cells also differed somewhat in their expression patterns of two activation markers, namely, CD69 and inducible costimulator (ICOS). The eNK cells acquired a B220hiICOS+ dNK cell surface phenotype when cultured in vitro in the presence of uterine cells and murine interleukin 15. Thus, the cell surface profiles generated for both NK1.1+ eNK cells and dNK cells demonstrate that they belong to the recently described B220+CD11c+ subset of NK cells, which are potent cytokine producers.
Keywords: immunology, natural killer cells, pregnancy, uterus
NK1.1+ murine uterine natural killer cells belong to the newly identified B220+CD11c+ subset of NK cells, which demonstrate enhanced NK cell effector functions.
INTRODUCTION
In addition to their fundamental role in host defense, natural killer (NK) cells are thought to have an important role during pregnancy [1–3]. Natural killer cells are present in the uterus both before and during gestation in mice and humans [3, 4]. In mice, it is thought that NK progenitor cells migrate mainly from secondary lymphoid organs to the uterus, at which point they undergo further differentiation [5]. In humans, it has been proposed that, in addition to the recruitment of NK cells from the periphery, some uterine NK (uNK) cells may arise from hematopoietic stem cells present in the endometrium [6–12]. In mice, decidual NK (dNK) cell numbers peak near midgestation and begin to decline shortly thereafter [13, 14]. Few NK cells can be detected in the mouse uterus at the end of pregnancy [15]. In humans, dNK cell numbers are highest in the first trimester of pregnancy, when they are thought to comprise the majority of decidual lymphocytes [16–18]. Decidual NK cell numbers begin to decline during the second trimester, and, similar to mice, few human NK cells are present in the uterus at term [1, 19].
Studies [20–22] utilizing NK cell-deficient mouse models demonstrated that mice deficient in NK cells display implantation site anomalies, incomplete uterine spiral artery remodeling, and impaired decidualization of uterine stromal cells. Subsequent studies [23, 24] revealed that interferon γ (IFNγ, IFNG) was the key cytokine produced by murine dNK cells that supported pregnancy-associated vascularization of the uterus and the process of decidualization. Human dNK cells have been shown to secrete cytokines, chemokines, and angiogenic factors and are believed to regulate trophoblast invasion [10, 25–30]. Thus, uNK cells are thought to help establish a successful pregnancy through constructive effects on vascularization and placentation.
Human NK cells can be divided into two functionally distinct subsets based on levels of CD56 (NCAM1) expression [31, 32]. CD56bright NK cells are thought to be effective cytokine producers but poorly cytotoxic [32]. In contrast, CD56dim NK cells are thought to be poor cytokine producers yet highly cytotoxic [33]. During a normal human pregnancy, the vast majority of uNK cells display a CD56brightCD16 (FCGR3)− cell surface phenotype [16, 18, 34]. A functional homologue of human CD56 does not exist in mice; thus, it has been difficult to compare NK cell subsets between the two species.
Recently, several studies [35–37] have demonstrated that peripheral B220+CD11c+NK1.1+ cells belong to the NK cell lineage. B220+CD11c+NK1.1+ NK cells were shown to produce greater amounts of IFNγ than conventional NK cells in response to certain stimuli [35, 37]. While it has been suggested that B220+CD11c+NK1.1+ cells correspond to activated NK cells [37], it has also been proposed that these cells may be analogous to human CD56bright NK cells [35]. Given that CD56bright cells represent the vast majority of NK cells found in human decidua during pregnancy and in an attempt to delineate a potential physiologic role for these cells, we examined whether murine uNK cells belong to the B220+CD11c+NK1.1+ subset of NK cells.
MATERIALS AND METHODS
Mice
Six- to seven-wk-old female C57BL/6NCr mice were purchased from the National Cancer Institute (Frederick, MD). Mice in estrus were mated with males of proven fertility overnight. The morning on which the vaginal plug was detected is referred to as Embryonic Day (E) 0.5. All procedures described herein were reviewed and approved by the animal studies committee at Washington University and were performed in accord with institutional animal care and use committee approval.
Uterine Cell Isolation
Virgin or pregnant mice at E10.5 were euthanized and perfused with 20 ml of PBS (Sigma-Aldrich, St. Louis, MO) containing 1 μg/ml heparin (Sigma-Aldrich), and the uteri were subsequently isolated. The mesometrium was removed from the uteri, which were cut directly below the oviduct and above the cervix. The uteri were subsequently placed in dishes containing 4°C Hanks balanced salt solution (HBSS; Invitrogen, Carlsbad, CA). Uteri derived from virgin mice were cut longitudinally, minced into small pieces of approximately 1 mm3, and transferred to 50-ml conical tubes containing 4°C HBSS. Interimplantation sites were removed from uteri derived from pregnant mice. The remaining implantation sites were cut along the antimesometrial edge. The fetus and placenta were removed from each site, and the placenta was retained in a separate dish. The uteri, including the myometrium and the mesometrial lymphoid aggregate of pregnancy, were then minced into small pieces as already described and transferred to a 50-ml conical tube containing 4°C HBSS. The decidua basalis was subsequently removed from the placenta, minced into small pieces, and added to the uterine tissue already dissected from the pregnant mice.
Dissected uteri derived from virgin and pregnant mice were washed twice in 4°C HBSS to remove blood contamination. The tissue was then digested in 10 ml of HBSS containing 2 mg/ml collagenase type I (Invitrogen). Uteri were digested at 37°C for 60 min with intermittent vortexing. After digestion, the cells were pelleted and resuspended in 5 ml of ice-cold fetal bovine serum (FBS) (Sigma-Aldrich). They were then pelleted again and resuspended in PBS containing 10% FBS. The cells were passed through a 70-μm cell strainer (BD Falcon; Fisher Scientific, Pittsburgh, PA), counted, and stained for analysis by multiparameter flow cytometry. Staining of each sample with 7-amino-actinomycin D (7-AAD) demonstrated that cell viability after collagenase digestion was routinely >75% live cells.
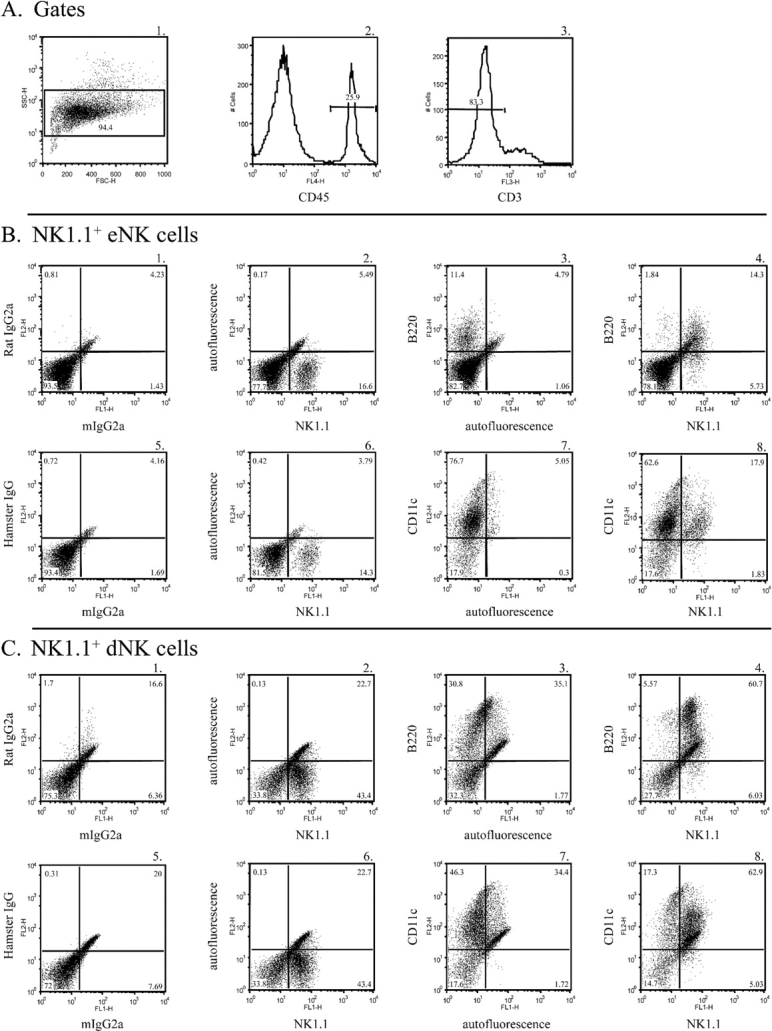
As a control for collagenase treatment, murine splenocytes were either conventionally isolated or isolated by collagenase digestion. Briefly, splenocytes were isolated from adult C57BL/6 mice by mechanically disrupting the spleen and passing the splenocytes through a 70-μm cell filter. Alternately, spleens were minced, and collagenase digested as already described. The red blood cells from both cell preparations were lysed using Tris-buffered ammonium chloride (0.14 M NH4Cl and 0.017 M Tris [pH 7.2]). The cells were washed and stained with the indicated antibody as described herein, and expression was examined by flow cytometry. Live gates were based on 7-AAD fluorescence. The percentages of positive cells for the indicated antibodies are largely similar between conventionally isolated and collagenase-treated splenocytes (Supplemental Fig. S1 available at www.biolreprod.org).
Antibodies and Flow Cytometry Reagents
The following antibodies and staining reagents were purchased from eBioscience (San Diego, CA): fluorescein isothiocyanate (FITC)-conjugated mouse IgG2a, FITC-conjugated rat IgG2b, FITC-conjugated Armenian hamster IgG, FITC-conjugated anti-mouse NK1.1 (KLRB1C), FITC-conjugated anti-mouse major histocompatibility complex (MHC) class II (I-A/I-E), FITC-conjugated anti-mouse CD69, FITC-conjugated anti-mouse/rat inducible costimulator (ICOS), FITC-conjugated anti-mouse CD45 (LCA), phycoerythrin (PE)-conjugated rat IgG2a, PE-conjugated mouse IgG2a, PE-conjugated rat IgG2b, PE-conjugated rat IgM, PE-conjugated Armenian hamster IgG, PE-conjugated anti-mouse NK1.1, PE-conjugated anti-mouse NKp46 (NCR1), PE-conjugated anti-mouse pan-NK cells (CD49b, ITGA2, and DX5), PE-conjugated anti-mouse CD69, PE-conjugated anti-mouse ICOS, PE-conjugated anti-mouse CD11c (ITGAX), PE-conjugated anti-mouse CD11b (ITGAM), PE-conjugated anti-mouse/human/rat CD27, PE-conjugated anti-mouse CD45 (LCA), PE-conjugated streptavidin, biotin-conjugated rat IgG2a, biotin-conjugated anti-mouse/human CD45R (B220), PerCP-Cy5.5-conjugated Armenian hamster IgG, PerCP-Cy5.5-conjugated anti-mouse CD3 (CD3E), and allophycocyanin (APC)-conjugated anti-mouse CD45 (LCA). The PE-conjugated anti-mouse CD122, anti-mouse CD16 (FCGR3)/CD32 (FCGR2B)-2.4G2, and 7-AAD were purchased from BD Biosciences (San Jose, CA). The FITC-conjugated Dolichos biflorus agglutinin (DBA) was purchased from Sigma-Aldrich.
Flow Cytometry
A total of 3.5 × 105 uterine cells were incubated in PBS/10% FBS containing 5% normal mouse serum (Sigma-Aldrich), 5% normal rat serum (Sigma-Aldrich), and 2 μg/ml anti-mouse CD16/CD32 (2.4G2) antibody for 15 min at 4°C to block nonspecific antibody binding. The cells were pelleted and resuspended in 100 μl of PBS/10% FBS containing the indicated antibodies or FITC-conjugated DBA lectin for 30 min at 4°C. All antibodies and the FITC-DBA lectin staining reagent were titered to determine optimal concentrations. Each staining cocktail also contained an APC-conjugated anti-mouse CD45 (LCA) antibody and a PerCP-Cy5.5-conjugated anti-mouse CD3 antibody. The cells were washed three times with PBS. Cells stained with a biotinylated primary antibody were then incubated in PE-conjugated streptavidin for 30 min at 4°C. The cells were washed three times in PBS and ultimately resuspended in PBS/10% FBS for analysis. To demonstrate the specificity of DBA lectin binding, the DBA reagent was preincubated with either 100 mM N-acetyl-d-galactosamine or 100 mM of an irrelevant sugar (d-glucose) for 15 min at 4°C before adding the lectin to the uterine cells. Data were collected using a BD FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA) and analyzed with FlowJo software (TreeSTar, Inc., Ashland, OR). The data shown in all figures are gated to include only CD45+ cells and to exclude all CD3+ cells within the CD45+ gate. Thus, the cells shown in all figures are CD45+ and CD3−. All samples in each experiment were run in duplicate. To address cell viability in the duplicate sample, 7-AAD was substituted for the PerCPCy5.5 anti-mouse CD3 antibody. The data obtained from each sample in the pair were compared and yielded identical results (data not shown). At least 15 implantation sites were pooled for each experiment in which dNK cells were analyzed. In addition, uteri from at least 10 virgin female mice were pooled for each experiment in which endometrial NK (eNK) cells were analyzed. All flow cytometry experiments were conducted at least three times each, and the results shown are from one representative experiment.
In Vitro Culture of eNK Cells
Uterine cell suspensions were generated from virgin mice as already described. A total of 8 × 105 cells were plated per well in a six-well plate in 2 ml of RPMI-1640 media containing 10% FBS, 2 mM l-glutamine (Cambrex Bio Science, Walkerville, MD), 1 mM sodium pyruvate (Cambrex Bio Science), 0.5% sodium bicarbonate (Cambrex Bio Science), 50 μg/ml penicillin/streptomycin (Cambrex Bio Science), 100 μM β-mercaptoethanol (Sigma-Aldrich), and 100 ng/ml murine interleukin (IL) 15 (eBioscience). Cells were cultured for 48 h at 37°C and 5% CO2. The cells were then harvested using cell dissociation solution (Sigma-Aldrich), stained, and analyzed by flow cytometry as already described. On the day the in vitro uterine cell cultures were harvested for analysis, virgin mice were euthanized to use as controls in the flow cytometry experiments. This experiment was conducted at least three times, and the results shown are from one representative experiment.
RESULTS
B220 and CD11c Expression on NK1.1+ eNK and dNK Cells
To determine whether NK1.1+ eNK and dNK cells display a B220+CD11c+ cell surface phenotype, we analyzed uterine cells isolated from either virgin mice or pregnant mice at E10.5 by multiparametric flow cytometry. First, a forward- and side-scatter gate was placed around the uterine cell population (Fig. 1, panel A1). Next, we gated on CD45+ cells within the forward- and side-scatter gate as shown in Figure 1, panel A2. Finally, although NK1.1 has been shown to be a useful NK cell marker in C57BL/6 mice [38, 39], it is also expressed on NKT cells [40]. However, these two cell populations can be distinguished by expression of the CD3 (CD3E) molecule, which is present on NKT cells but absent on conventional NK cells. Thus, a final gate was drawn within the CD45+ gate to exclude CD3+ cells (Fig. 1, panel A3). The data presented herein represent CD45+CD3− cells.
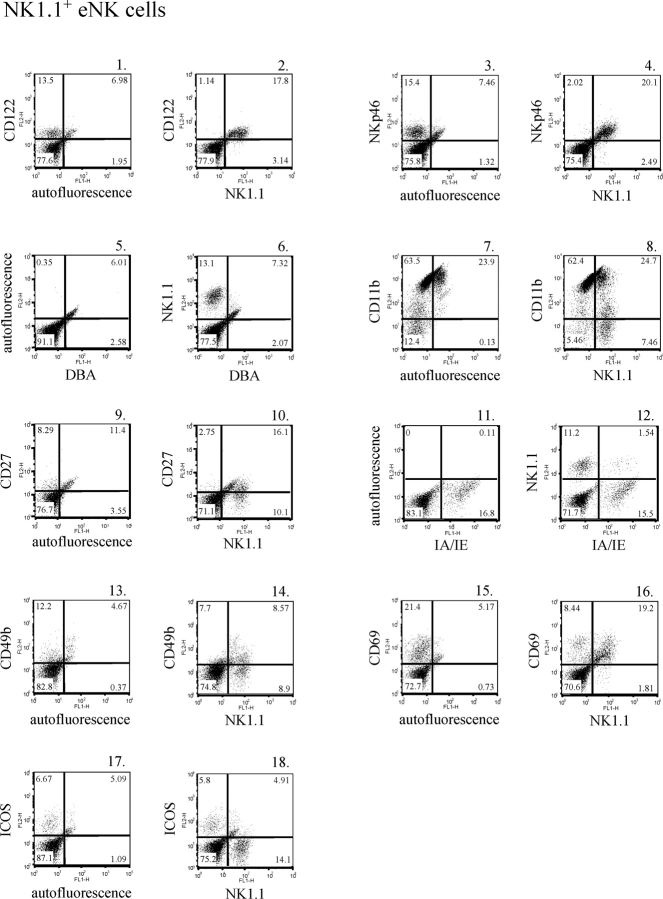
FIG. 1.
B220 and CD11c are expressed on NK1.1+ eNK and dNK cells. A) A forward-scatter (FSC) and side-scatter (SSC) gate was placed around the uterine cell population (panel A1). Next, a CD45+ gate was placed within the forward- and side-scatter gate (panel A2). A final gate was drawn within the CD45+ gate to exclude CD3+ cells (panel A3). The data depict CD45+CD3− cells. B and C) Endometrial NK cells (panel B) and dNK cells (panel C) at E10.5 were isolated and stained with either isotype control antibodies (panels B1 and B5 and panels C1 and C5), an FITC-conjugated anti-NK1.1 antibody (panels B2 and B6 and panels C2 and C6), a biotin-conjugated anti-B220 antibody (panels B3 and C3), a PE-conjugated anti-CD11c antibody (panels B7 and C7), a biotin-conjugated anti-B220 antibody and an FITC-conjugated anti-NK1.1 antibody (panels B4 and C4), or a PE-conjugated anti-CD11c antibody and an FITC-conjugated anti-NK1.1 antibody (panels B8 and C8).
We were able to detect the NK1.1 marker on a population of uterine cells derived from both virgin and pregnant mice (Fig. 1, panels B2 and C2, respectively). As expected, the number of NK1.1+ cells was greater in the pregnant uterus than the virgin uterus. Thus, a population of eNK and dNK cells expressed the NK1.1 marker, and we subsequently examined the expression of other peripheral NK cell surface markers on the NK1.1+ uNK cells.
We examined B220 protein expression on NK1.1+ eNK cells and dNK cells at E10.5. B220 was detected on NK1.1+ eNK and dNK cells (Fig. 1, panels B2, B3, and B4 and panels C2, C3, and C4, respectively). The majority of NK1.1+ cells present in both the virgin and pregnant mouse uteri were B220+, and, conversely, the majority of B220+ cells were NK1.1+. B220 expression increased on a subset of NK1.1+ dNK cells compared with eNK cells. Thus, the majority of NK1.1+ eNK cells and dNK cells at E10.5 express the B220 protein.
We also detected the integrin subunit CD11c on NK1.1+ eNK and dNK cells (Fig. 1, panels B6, B7, and B8 and panels C6, C7, and C8, respectively). Again, the vast majority of NK1.1+ eNK and dNK cells expressed CD11c protein. Thus, NK1.1+ eNK cells and dNK cells display a B220+CD11c+ cell surface phenotype.
Expression Profile of Peripheral NK Cell Markers on NK1.1+ eNK and dNK Cells
As previously mentioned, the cell surface profile of human uNK cells is better defined than that of murine uNK cells both before and during pregnancy. Thus, we profiled eNK cells and dNK cells at E10.5 for the presence of a panel of cell surface markers expressed on peripheral NK cells. In mice, cells committed to the NK cell lineage express CD122, the IL2/IL15 receptor common β subunit [41, 42]. Virtually all NK1.1+ eNK and dNK cells expressed CD122 (Fig. 2, panels 1 and 2, and Fig. 3, panels 1 and 2, respectively). For Figures 2, 3, and 4, the isotype control data and data depicting cells stained with an anti-NK1.1 antibody alone are presented as corresponding Supplemental Figures S2, S3, and S4. It has been suggested that the natural cytotoxicity receptor NKp46 (NCR1), which is specifically expressed on NK cells, may be used as an NK cell marker across species [43–46]. We examined NKp46 expression and found that NK1.1+ eNK and dNK cells also expressed this protein (Fig. 2, panels 3 and 4, and Fig. 3, panels 3 and 4, respectively). Thus, NKp46 also defines NK1.1+ uNK cells.
FIG. 2.
Cell surface markers expressed on NK1.1+ eNK cells. Endometrial NK cells were isolated and stained with a PE-conjugated anti-CD122 antibody (panel 1), a PE-conjugated anti-NKp46 antibody (panel 3), FITC-conjugated DBA lectin (panel 5), a PE-conjugated anti-CD11b antibody (panel 7), a PE-conjugated anti-CD27 antibody (panel 9), an FITC-conjugated anti-MHC II (IA/IE) antibody (panel 11), a PE-conjugated anti-CD49b antibody (panel 13), a PE-conjugated anti-CD69 antibody (panel 15), a PE-conjugated anti-ICOS antibody (panel 17), a PE-conjugated anti-CD122 antibody and an FITC-conjugated anti-NK1.1 antibody (panel 2), a PE-conjugated anti-NKp46 antibody and an FITC-conjugated anti-NK1.1 antibody (panel 4), FITC-conjugated DBA lectin and a PE-conjugated anti-NK1.1 antibody (panel 6), a PE-conjugated anti-CD11b antibody and an FITC-conjugated anti-NK1.1 antibody (panel 8), a PE-conjugated anti-CD27 antibody and an FITC-conjugated anti-NK1.1 antibody (panel 10), a PE-conjugated anti-NK1.1 antibody and an FITC-conjugated anti-MHC II antibody (panel 12), a PE-conjugated anti-CD49b antibody and an FITC-conjugated anti-NK1.1 antibody (panel 14), a PE-conjugated anti-CD69 antibody and an FITC-conjugated anti-NK1.1 antibody (panel 16), or a PE-conjugated anti-ICOS antibody and an FITC-conjugated anti-NK1.1 antibody (panel 18).
FIG. 3.
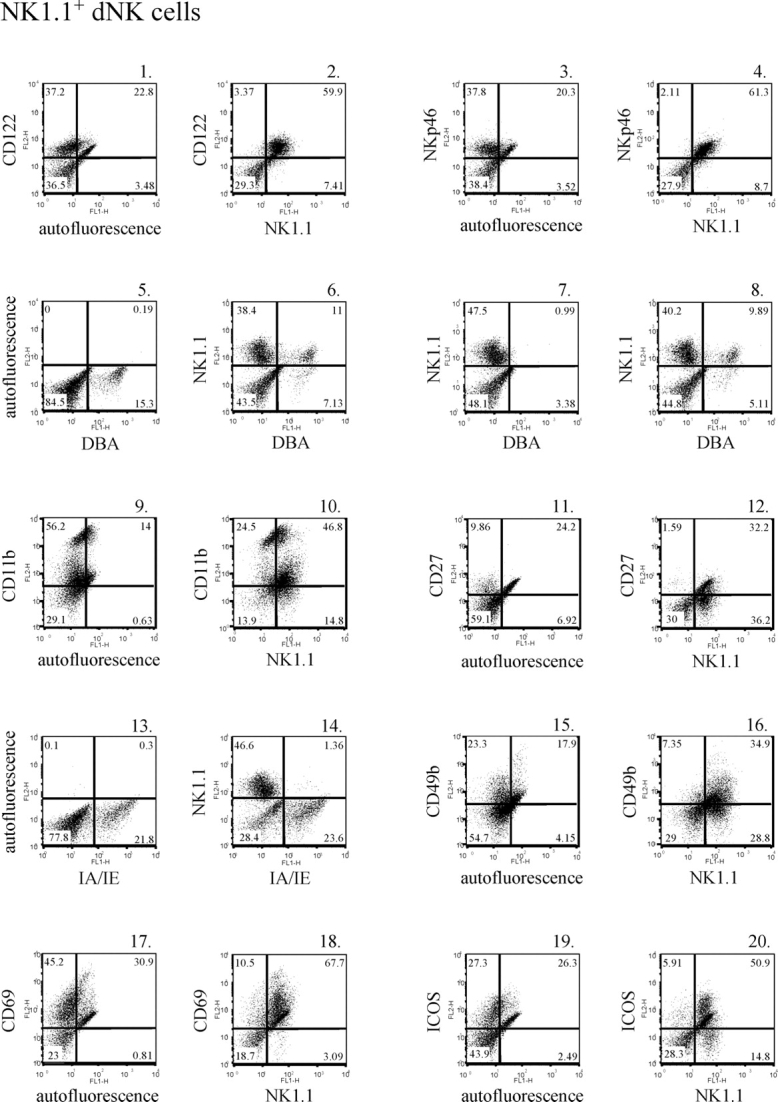
Cell surface markers expressed on NK1.1+ dNK cells. Decidual NK cells were isolated and stained with a PE-conjugated anti-CD122 antibody (panel 1), a PE-conjugated anti-NKp46 antibody (panel 3), FITC-conjugated DBA lectin (panel 5), a PE-conjugated anti-CD11b antibody (panel 9), a PE-conjugated anti-CD27 antibody (panel 11), an FITC-conjugated anti-MHC II (IA/IE) antibody (panel 13), a PE-conjugated anti-CD49b antibody (panel 15), a PE-conjugated anti-CD69 antibody (panel 17), a PE-conjugated anti-ICOS antibody (panel 19), a PE-conjugated anti-CD122 antibody and an FITC-conjugated anti-NK1.1 antibody (panel 2), a PE-conjugated anti-NKp46 antibody and an FITC-conjugated anti-NK1.1 antibody (panel 4), FITC-conjugated DBA lectin and a PE-conjugated anti-NK1.1 antibody (panel 6), a PE-conjugated anti-CD11b antibody and an FITC-conjugated anti-NK1.1 antibody (panel 10), a PE-conjugated anti-CD27 antibody and an FITC-conjugated anti-NK1.1 antibody (panel 12), a PE-conjugated anti-NK1.1 antibody and an FITC-conjugated anti-MHC II (IA/IE) antibody (panel 14), a PE-conjugated anti-CD49b antibody and an FITC-conjugated anti-NK1.1 antibody (panel 16), a PE-conjugated anti-CD69 antibody and an FITC-conjugated anti-NK1.1 antibody (panel 18), a PE-conjugated anti-ICOS antibody and an FITC-conjugated anti-NK1.1 antibody (panel 20), FITC-conjugated DBA lectin and a PE-conjugated anti-NK1.1 antibody preincubated with 100 mM N-acetyl-d-galactosamine (panel 7), or FITC-conjugated DBA lectin and a PE-conjugated anti-NK1.1 antibody preincubated with 100 mM d-glucose (panel 8).
Dolichos biflorus agglutinin binds N-acetyl-d-galactosamine glycoconjugates and has been shown to specifically stain murine uNK cells [47, 48]. We examined the ability of both NK1.1+ eNK and dNK cells to bind DBA lectin. Endometrial NK cells did not react with DBA lectin (Fig. 2, panels 5 and 6). In contrast, we were able to detect DBA lectin reactivity on a subset of dNK cells (Fig. 3, panels 5 and 6). To demonstrate binding specificity, the DBA reagent was preincubated with either 100 mM N-acetyl-d-galactosamine (Fig. 3, panel 7) or 100 mM of an irrelevant sugar (d-glucose) (Fig. 3, panel 8) before adding the DBA to the uterine cells. The presence of N-acetyl-d-galactosamine completely blocked DBA lectin binding to the NK1.1+ dNK cells, whereas d-glucose had no effect. Thus, roughly 20% of the NK1.1+ dNK cells isolated demonstrated DBA lectin reactivity.
The B220+CD11c+NK1.1+ peripheral NK cell population recently described in mice was shown to express CD11b (ITGAM) and CD27 [35, 37]. The integrin subunit CD11b and the tumor necrosis factor (TNF) receptor family member CD27 were both expressed on a portion of NK1.1+ eNK and dNK cells (Fig. 2, panels 7 and 8 and panels 9 and 10, respectively, and Fig. 3, panels 9 and 10 and panels 11 and 12, respectively). Not all NK1.1+ eNK and dNK cells appeared to express these proteins.
While MHC II protein expression was detected in both the virgin and pregnant mouse uteri, few NK1.1+ NK cells expressed MHC II molecules (Fig. 2, panels 11 and 12, and Fig. 3, panels 13 and 14, respectively). Thus, few NK1.1+ cells derived from virgin uteri expressed MHC II protein, and pregnancy did not lead to a significant increase in the number of NK1.1+ dNK cells that express MHC II. CD49b (recognized by the monoclonal antibody DX5) is an α2 integrin that is expressed on most NK1.1+CD3− NK cells. CD49b was detected on a subset of both eNK and dNK cells (Fig. 2, panels 13 and 14, and Fig. 3, panels 15 and 16, respectively). It appeared that some NK1.1+ eNK and dNK cells do not express CD49b.
We next examined markers associated with NK cell activation. CD69 (very early activation antigen) is expressed on leukocytes during activation, and its expression has been detected on both eNK and dNK cells in humans [18, 49–51]. Similarly, we found that CD69 is expressed on the majority of murine NK1.1+ eNK and dNK cells (Fig. 2, panels 15 and 16, and Fig. 3, panels 17 and 18, respectively). In addition, CD69 expression increased in the uterus as the result of pregnancy. We also examined the expression of ICOS on uNK cells. ICOS has been shown to support NK cell function, and its expression is increased on activated NK cells [52]. Low levels of ICOS were detected in the virgin uterus (Fig. 2, panel 17). Very few NK1.1+ eNK cells appeared to express this protein (Fig. 2, panel 18). In contrast, uterine ICOS expression increased during gestation, and a subset of NK1.1+ dNK cells were ICOS+. Moreover, the majority of ICOS+ cells were NK1.1+ (Fig. 3, panels 19 and 20).
In Vitro Culture of NK1.1+ eNK Cells with Murine IL15 Leads to a dNK Cell Surface Phenotype
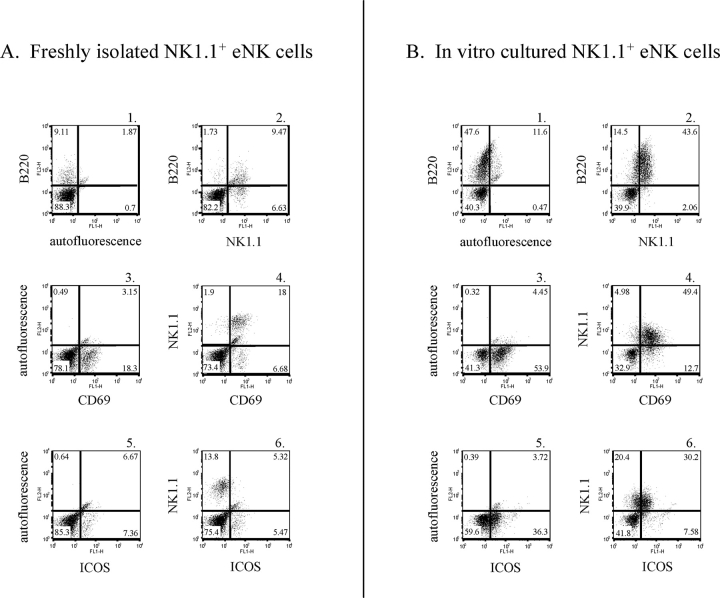
The data presented thus far demonstrate that the cell surface phenotypes of NK1.1+ eNK cells and dNK cells at E10.5 differ primarily by their levels of B220 and ICOS expression. In addition, CD69 expression is somewhat higher on a subset of dNK cells compared with eNK cells. Interleukin 15 is required for NK cell development and proliferation and is present in the uterus of both mice and humans during gestation [53–58]. Moreover, IL15 has been shown in vitro to expand the B220+CD11b+ population of NK cells [59]. To determine if eNK cells possessed the ability to adopt a dNK cell surface phenotype, we generated a uterine single-cell suspension from virgin mice and cultured the uterine population in vitro in the presence of murine IL15 and uterine cells for 48 h. Virgin female mice were euthanized on the same day the in vitro cell culture was harvested to act as a control in the flow cytometry experiment. B220 expression increased on in vitro-cultured NK1.1+ eNK cells compared with freshly isolated eNK cells (Fig. 4, panels A1 and A2 vs. panels B1 and B2). CD69 expression levels were similar on in vitro-cultured NK1.1+ eNK cells compared with controls (Fig. 4, panels A3 and A4 vs. panels B3 and B4). Finally, ICOS expression was induced on the in vitro-cultured NK1.1+ eNK cells compared with controls, which expressed little to no ICOS protein (Fig. 4, panels A5 and A6 vs. panels B5 and B6). NK1.1 protein expression was lower on eNK cells cultured in vitro compared with freshly isolated eNK cells. This reasons for this phenomenon were unclear.
FIG. 4.
Murine IL15 induces a dNK cell surface phenotype on eNK cells. Uterine cell suspensions were generated from virgin mice and cultured in vitro for 48 h in media supplemented with 100 ng/ml murine IL15. Freshly isolated eNK cells (A) and in vitro-cultured eNK cells (B) were stained with a biotin-conjugated anti-B220 antibody (panels A1 and B1), an FITC-conjugated anti-CD69 antibody (panels A3 and B3), an FITC-conjugated anti-ICOS antibody (panels A5 and B5), a biotin-conjugated anti-B220 antibody and an FITC-conjugated anti-NK1.1 antibody (panels A2 and B2), an FITC-conjugated anti-CD69 antibody and a PE-conjugated anti-NK1.1 antibody (panels A4 and B4), or an FITC-conjugated anti-ICOS antibody and a PE-conjugated anti-NK1.1 antibody (panels A6 and B6).
DISCUSSION
Human uNK cells are becoming better defined with respect to cell surface markers expressed and effector functions elicited. Less is known regarding the cell surface profile of murine uNK cells and more specifically the cell surface phenotype of peripheral NK cells that migrate to the mouse uterus. However, a recent study by Yadi et al. [60] has provided insight into the distinct receptor profile of murine uNK cells.
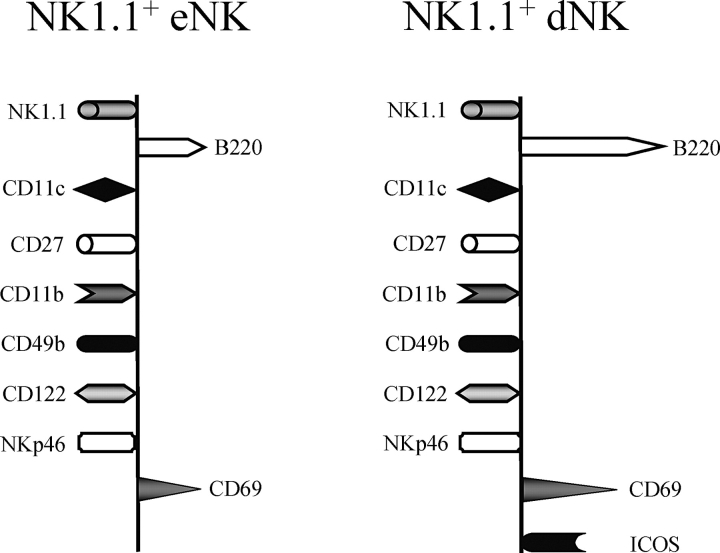
Herein, we demonstrate that both murine NK1.1+ eNK and dNK cells display a B220+CD11c+ cell surface phenotype. This profile is similar to the recently described subset of peripheral NK cells in mice, which are potent IFNγ producers [35–37]. The peripheral B220+CD11c+NK1.1+ NK cell subset was also shown to express CD27 and CD11b [35, 37]. We demonstrated that a portion of B220+CD11c+NK1.1+ eNK and dNK cells also expressed CD27 and CD11b. CD27 is found on immature NK cells and has been used as a marker to define functional subsets within the mature murine NK cell population [61]. CD27+ NK cells were shown to have enhanced effector functions compared with CD27− cells [61]. Therefore, the cell surface phenotype of NK1.1+ eNK and dNK cells generated thus far is similar to that described for peripheral NK cells that are effective cytokine producers. Most important, the positive effects of uNK cells on placentation and vascularization are largely mediated by growth factors, cytokines, chemokines, and angiogenic factors secreted by these cells [1, 3].
The NK cell precursors and immature NK cells express little to no B220, whereas approximately 10%–30% of mature NK1.1+ CD49b+ NK cells in the bone marrow and spleen express this protein [37]. In contrast to what is seen in the periphery, we found that the majority of NK1.1+ eNK and dNK cells expressed B220. Thus, the uterus appears to be enriched in B220+NK1.1+ NK cells both before and during gestation. We also demonstrated that B220 expression dramatically increased on a subpopulation of NK1.1+ NK cells as the result of pregnancy. Previous investigations have demonstrated increased B220 expression on activated NKT cells [62]. In addition, B220 protein expression was reported to be upregulated on splenocytes cultured in the presence of IL2 and in response to TLR stimulation [37]. It was suggested that this protein acts as an NK cell activation marker. At the time of blastocyst implantation and uterine decidualization, uNK cells become activated [4]. We speculate that the increase in B220 expression on NK1.1+ NK cells at E10.5 reflects the activation status of dNK cells.
We demonstrated that NK1.1+ eNK and dNK cells in the mouse express CD122 and NKp46 and that a large subset of these cells also express CD49b. In contrast, very few NK1.1+ eNK cells expressed MHC II molecules. Moreover, pregnancy and thus uNK cell activation did not lead to a significant increase in the number of dNK cells expressing MHC II. In humans, activated NK cells can express MHC II proteins and act as antigen-presenting cells [63]. Recently, activated murine NK cells were also shown to upregulate MHC II expression [35, 37]. It was suggested that MHC II acts as an NK cell activation marker, with specific stimuli leading to the upregulation of this protein [37]. While NK cell activation may lead to MHC II upregulation on peripheral NK cells, we did not find a significant increase in the number of NK1.1+ dNK cells that express MHC II.
Histologically, DBA lectin has been used to identify uNK cells in implantation sites, and more recently it has been used to identify these cells in flow cytometry studies [48, 60, 64–66]. This reagent specifically stains uNK cells but largely does not stain peripheral NK cells [48, 67]. Histological and flow cytometry studies [48, 60] demonstrated that uNK cells derived from virgin mice do not bind DBA lectin, and our data reaffirm this observation. The DBA-reactive uNK cells appear early during gestation at approximately Day 5 [15, 48, 67]. Yadi et al. [60] recently identified two uNK cell populations in the uterus at E9.5. The larger subset displayed a distinct cell surface phenotype in part because the uNK cells (CD122+CD3−) were NK1.1−DX5−DBA+. The majority of mature NK cells express NK1.1 and DX5 [42, 68, 69]. The smaller subset resembled peripheral NK cells, as they were DBA− NK1.1+DX5+. Although we isolated DBA+ uNK cells, our enzymatic procedure did not allow for the isolation of the uNK cell population described by Yadi et al. [60], as we found that virtually all NK1.1+ dNK cells were also CD122+. It appears that we have mainly isolated immature uNK cells given that the dNK cells were NK1.1+CD122+ and that approximately 80% of the dNK cells were DBA−. The larger more fragile DBA+ uNK cells do not appear to survive this isolation procedure. The origin of DBA+ uNK cells is unknown. In mice, it is thought that the self-renewing progenitors of uNK cells traffic to the uterus from the periphery [5]. It is tempting to speculate that NK1.1+ uNK cells, specifically those of the B220+CD11c+ subset, migrate from the periphery to the uterus, where they may further differentiate into DBA+ uNK cells.
A recent study [70] demonstrated differential expression of certain NK cell receptors on human eNK and dNK cells. Specifically, eNK cells were shown to lack NKp30 and chemokine receptors, whereas dNK cells express these proteins. The eNK cells stimulated with IL15 demonstrated a marked increase in NKp30 and NKp44 protein expression. Moreover, it was shown that eNK cells were functionally static until stimulated with IL15, at which point they acquired the ability to mediate NK cell effector functions [70]. Analogous results were obtained in a separate study [71] using IL12 and IL15. Similarly, we demonstrated that murine NK1.1+ eNK cells and dNK cells differ in their expression of certain cell surface proteins. NK1.1+ eNK cells expressed low levels of B220 and little to no ICOS, whereas a subset of NK1.1+ dNK cells expressed high levels of B220 and were ICOS+. In vitro culture of eNK cells with IL15 and uterine cells led to a B220hiICOS+ cell surface phenotype on the NK1.1+ NK cells. Thus, eNK cells may be an early form of certain dNK cells. We cannot conclude that the upregulation of the aforementioned cell surface proteins was a direct effect of IL15. The eNK cells were not purified; they were cultured in the presence of uterine cells. Thus, the effects of IL15 on eNK cells may in part be mediated by effects of this cytokine on the uterine cells present in the culture.
Uterine stromal cells produce IL15, and its expression is upregulated by progesterone, which is important in the decidualization process [56–58, 72, 73]. In addition, immune cells present in the uterus have been shown to secrete this cytokine [56, 74, 75]. Other potential stimulators of eNK cells are also present in the nonpregnant endometrium. MICA, ULBP2, and ULBP3, which are NKG2D (KLRK1) ligands, have been detected on human endometrial cells [70, 76]. We and others have demonstrated that eNK cells express the activation marker CD69 [77]. If, similar to humans, murine endometrial cells in the nonpregnant uterus express IL15 and NKG2D ligands, in theory a minimal amount of eNK cell stimulation could occur, an amount that potentially could lead to low levels of B220 and CD69 protein expression on CD11c+NK1.1+ eNK cells. In humans, findings suggest that eNK cells mediate low levels of cytotoxicity [78]. Although, in mice, eNK cells have been shown to be immature and nongranulated [15, 79, 80]. Alternately, CD69 could act to keep NK cells in the uterus, as it has been shown to retain lymphocytes in lymphoid tissue [81]. We cannot rule out the possibility that the cell isolation procedure led to the expression of the CD69 marker on eNK cells. However, ICOS was not upregulated under the same purification conditions.
Thus, the cell surface phenotype of NK1.1+ eNK and dNK cells, shown in Figure 5, is similar to the recently identified B220+CD11c+ peripheral NK cell population found to secrete higher amounts of IFNγ than conventional NK cells. Given that the majority of NK1.1+ uNK cells present in the uterus are also B220+CD11c+, it is tempting to speculate that the peripheral pool of NK cells with this cell surface phenotype are those that migrate to the uterus. However as previously mentioned, if B220 acts as a uNK cell activation marker, then the presence of IL15 or NKG2D ligands in the virgin uterus could potentially lead to subthreshold stimulation of the uNK cells, with induction of low levels of B220 protein expression at the cell surface. Further studies are warranted to distinguish between these two possibilities.
FIG. 5.
Cell surface profile of murine NK1.1+ eNK and dNK cells. NK1.1+ eNK and dNK cells belong to the B220+CD11c+ subset of NK cells. NK1.1+ eNK cells and dNK cells similarly expressed most cell surface markers examined except for B220, CD69, and ICOS, which were upregulated as the result of pregnancy.
Supplementary Material
Acknowledgments
We thank Dr. Anne Croy and Dr. Leonidas Carayannopoulos for their invaluable advice and thoughtful discussions. We also thank Sarah Frazier for technical assistance.
Footnotes
1Supported by NIH grants 5K12HD00145908 and 2P60DK02057931 (to J.K.R.).
REFERENCES
- Croy BA, van den Heuvel MJ, Borzychowski AM, Tayade C.Uterine natural killer cells: a specialized differentiation regulated by ovarian hormones. Immunol Rev 2006; 214: 161–185. [DOI] [PubMed] [Google Scholar]
- Moffett A, Loke C.Immunology of placentation in eutherian mammals. Nat Rev Immunol 2006; 6: 584–594. [DOI] [PubMed] [Google Scholar]
- Manaster I, Mandelboim O.The unique properties of human NK cells in the uterine mucosa. Placenta 2008; 29(suppl A):S60–S66. [DOI] [PubMed] [Google Scholar]
- Croy BA, Esadeg S, Chantakru S, van den Heuvel M, Paffaro VA, He H, Black GP, Ashkar AA, Kiso Y, Zhang J.Update on pathways regulating the activation of uterine natural killer cells, their interactions with decidual spiral arteries and homing of their precursors to the uterus. J Reprod Immunol 2003; 59: 175–191. [DOI] [PubMed] [Google Scholar]
- Chantakru S, Miller C, Roach LE, Kuziel WA, Maeda N, Wang WC, Evans SS, Croy BA.Contributions from self-renewal and trafficking to the uterine NK cell population of early pregnancy. J Immunol 2002; 168: 22–28. [DOI] [PubMed] [Google Scholar]
- Moffett-King A.Natural killer cells and pregnancy. Nat Rev Immunol 2002; 2: 656–663. [DOI] [PubMed] [Google Scholar]
- Hanna J, Wald O, Goldman-Wohl D, Prus D, Markel G, Gazit R, Katz G, Haimov-Kochman R, Fujii N, Yagel S, Peled A, Mandelboim O.CXCL12 expression by invasive trophoblasts induces the specific migration of CD16- human natural killer cells. Blood 2003; 102: 1569–1577. [DOI] [PubMed] [Google Scholar]
- Sentman CL, Meadows SK, Wira CR, Eriksson M.Recruitment of uterine NK cells: induction of CXC chemokine ligands 10 and 11 in human endometrium by estradiol and progesterone. J Immunol 2004; 173: 6760–6766. [DOI] [PubMed] [Google Scholar]
- Kitaya K, Nakayama T, Daikoku N, Fushiki S, Honjo H.Spatial and temporal expression of ligands for CXCR3 and CXCR4 in human endometrium. J Clin Endocrinol Metab 2004; 89: 2470–2476. [DOI] [PubMed] [Google Scholar]
- Hanna J, Goldman-Wohl D, Hamani Y, Avraham I, Greenfield C, Natanson-Yaron S, Prus D, Cohen-Daniel L, Arnon TI, Manaster I, Gazit R, Yutkin V, et al. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat Med 2006; 12: 1065–1074. [DOI] [PubMed] [Google Scholar]
- Lynch L, Golden-Mason L, Eogan M, O'Herlihy C, O'Farrelly C.Cells with haematopoietic stem cell phenotype in adult human endometrium: relevance to infertility? Hum Reprod 2007; 22: 919–926. [DOI] [PubMed] [Google Scholar]
- Keskin DB, Allan DS, Rybalov B, Andzelm MM, Stern JN, Kopcow HD, Koopman LA, Strominger JL.TGFbeta promotes conversion of CD16+ peripheral blood NK cells into CD16- NK cells with similarities to decidual NK cells. Proc Natl Acad Sci U S A 2007; 104: 3378–3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado SR, McBey BA, Yamashiro S, Fujita J, Kiso Y, Croy BA.Accounting for the peripartum loss of granulated metrial gland cells, a natural killer cell population, from the pregnant mouse uterus. J Leukoc Biol 1996; 59: 262–269. [PubMed] [Google Scholar]
- Kather A, Chantakru S, He H, Minhas K, Foster R, Markert UR, Pfeffer K, Croy BA.Neither lymphotoxin alpha nor lymphotoxin beta receptor expression is required for biogenesis of lymphoid aggregates or differentiation of natural killer cells in the pregnant mouse uterus. Immunology 2003; 108: 338–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peel S.Granulated metrial gland cells. Adv Anat Embryol Cell Biol 1989; 115: 1–112. [DOI] [PubMed] [Google Scholar]
- Starkey PM, Sargent IL, Redman CW.Cell populations in human early pregnancy decidua: characterization and isolation of large granular lymphocytes by flow cytometry. Immunology 1988; 65: 129–134. [PMC free article] [PubMed] [Google Scholar]
- King A, Wellings V, Gardner L, Loke YW.Immunocytochemical characterization of the unusual large granular lymphocytes in human endometrium throughout the menstrual cycle. Hum Immunol 1989; 24: 195–205. [DOI] [PubMed] [Google Scholar]
- Nishikawa K, Saito S, Morii T, Hamada K, Ako H, Narita N, Ichijo M, Kurahayashi M, Sugamura K.Accumulation of CD16-CD56+ natural killer cells with high affinity interleukin 2 receptors in human early pregnancy decidua. Int Immunol 1991; 3: 743–750. [DOI] [PubMed] [Google Scholar]
- Santoni A, Carlino C, Stabile H, Gismondi A.Mechanisms underlying recruitment and accumulation of decidual NK cells in uterus during pregnancy. Am J Reprod Immunol 2008; 59: 417–424. [DOI] [PubMed] [Google Scholar]
- Guimond MJ, Luross JA, Wang B, Terhorst C, Danial S, Croy BA.Absence of natural killer cells during murine pregnancy is associated with reproductive compromise in TgE26 mice. Biol Reprod 1997; 56: 169–179. [DOI] [PubMed] [Google Scholar]
- Guimond MJ, Wang B, Croy BA.Engraftment of bone marrow from severe combined immunodeficient (SCID) mice reverses the reproductive deficits in natural killer cell-deficient tg epsilon 26 mice. J Exp Med 1998; 187: 217–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood JD, Minhas K, di Santo JP, Makita M, Kiso Y, Croy BA.Ultrastructural studies of implantation sites from mice deficient in uterine natural killer cells. Placenta 2000; 21: 693–702. [DOI] [PubMed] [Google Scholar]
- Ashkar AA, Croy BA.Interferon-gamma contributes to the normalcy of murine pregnancy. Biol Reprod 1999; 61: 493–502. [DOI] [PubMed] [Google Scholar]
- Ashkar AA, Di Santo JP, Croy BA.Interferon gamma contributes to initiation of uterine vascular modification, decidual integrity, and uterine natural killer cell maturation during normal murine pregnancy. J Exp Med 2000; 192: 259–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito S, Nishikawa K, Morii T, Enomoto M, Narita N, Motoyoshi K, Ichijo M.Cytokine production by CD16-CD56bright natural killer cells in the human early pregnancy decidua. Int Immunol 1993; 5: 559–563. [DOI] [PubMed] [Google Scholar]
- Saito S, Kasahara T, Sakakura S, Enomoto M, Umekage H, Harada N, Morii T, Nishikawa K, Narita N, Ichijo M.Interleukin-8 production by CD16-CD56bright natural killer cells in the human early pregnancy decidua. Biochem Biophys Res Commun 1994; 200: 378–383. [DOI] [PubMed] [Google Scholar]
- Jokhi PP, King A, Sharkey AM, Smith SK, Loke YW.Screening for cytokine messenger ribonucleic acids in purified human decidual lymphocyte populations by the reverse-transcriptase polymerase chain reaction. J Immunol 1994; 153: 4427–4435. [PubMed] [Google Scholar]
- Sharkey AM, King A, Clark DE, Burrows TD, Jokhi PP, Charnock-Jones DS, Loke YW, Smith SK.Localization of leukemia inhibitory factor and its receptor in human placenta throughout pregnancy. Biol Reprod 1999; 60: 355–364. [DOI] [PubMed] [Google Scholar]
- Li XF, Charnock-Jones DS, Zhang E, Hiby S, Malik S, Day K, Licence D, Bowen JM, Gardner L, King A, Loke YW, Smith SK.Angiogenic growth factor messenger ribonucleic acids in uterine natural killer cells. J Clin Endocrinol Metab 2001; 86: 1823–1834. [DOI] [PubMed] [Google Scholar]
- Hiby SE, Walker JJ, O'Shaughnessy KM, Redman CW, Carrington M, Trowsdale J, Moffett A.Combinations of maternal KIR and fetal HLA-C genes influence the risk of preeclampsia and reproductive success. J Exp Med 2004; 200: 957–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanier LL, Le AM, Civin CI, Loken MR, Phillips JH.The relationship of CD16 (Leu-11) and Leu-19 (NKH-1) antigen expression on human peripheral blood NK cells and cytotoxic T lymphocytes. J Immunol 1986; 136: 4480–4486. [PubMed] [Google Scholar]
- Cooper MA, Fehniger TA, Turner SC, Chen KS, Ghaheri BA, Ghayur T, Carson WE, Caligiuri MA.Human natural killer cells: a unique innate immunoregulatory role for the CD56(bright) subset. Blood 2001; 97: 3146–3151. [DOI] [PubMed] [Google Scholar]
- Nagler A, Lanier LL, Cwirla S, Phillips JH.Comparative studies of human FcRIII-positive and negative natural killer cells. J Immunol 1989; 143: 3183–3191. [PubMed] [Google Scholar]
- King A, Balendran N, Wooding P, Carter NP, Loke YW.CD3- leukocytes present in the human uterus during early placentation: phenotypic and morphologic characterization of the CD56++ population. Dev Immunol 1991; 1: 169–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasius AL, Barchet W, Cella M, Colonna M.Development and function of murine B220+CD11c+NK1.1+ cells identify them as a subset of NK cells. J Exp Med 2007; 204: 2561–2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caminschi I, Ahmet F, Heger K, Brady J, Nutt SL, Vremec D, Pietersz S, Lahoud MH, Schofield L, Hansen DS, O'Keeffe M, Smyth MJ, et al. Putative IKDCs are functionally and developmentally similar to natural killer cells, but not to dendritic cells. J Exp Med 2007; 204: 2579–2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosshenrich CA, Lesjean-Pottier S, Hasan M, Richard-Le Goff O, Corcuff E, Mandelboim O, Di Santo JP.CD11cloB220+ interferon-producing killer dendritic cells are activated natural killer cells. J Exp Med 2007; 204: 2569–2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett J, Jr, Bosma GC, Bosma MJ, Bennett M, Kumar V.Transplantable progenitors of natural killer cells are distinct from those of T and B lymphocytes. Proc Natl Acad Sci U S A 1986; 83: 3427–3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanier LL, Phillips JH, Hackett J, Jr, Tutt M, Kumar V.Natural killer cells: definition of a cell type rather than a function. J Immunol 1986; 137: 2735–2739. [PubMed] [Google Scholar]
- Bendelac A, Rivera MN, Park SH, Roark JH.Mouse CD1-specific NK1 T cells: development, specificity, and function. Annu Rev Immunol 1997; 15: 535–562. [DOI] [PubMed] [Google Scholar]
- Ikawa T, Kawamoto H, Fujimoto S, Katsura Y.Commitment of common T/natural killer (NK) progenitors to unipotent T and NK progenitors in the murine fetal thymus revealed by a single progenitor assay. J Exp Med 1999; 190: 1617–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosmaraki EE, Douagi I, Roth C, Colucci F, Cumano A, Di Santo JP.Identification of committed NK cell progenitors in adult murine bone marrow. Eur J Immunol 2001; 31: 1900–1909. [DOI] [PubMed] [Google Scholar]
- Sivori S, Vitale M, Morelli L, Sanseverino L, Augugliaro R, Bottino C, Moretta L, Moretta A.p46, a novel natural killer cell-specific surface molecule that mediates cell activation. J Exp Med 1997; 186: 1129–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storset AK, Kulberg S, Berg I, Boysen P, Hope JC, Dissen E.NKp46 defines a subset of bovine leukocytes with natural killer cell characteristics. Eur J Immunol 2004; 34: 669–676. [DOI] [PubMed] [Google Scholar]
- Walzer T, Blery M, Chaix J, Fuseri N, Chasson L, Robbins SH, Jaeger S, Andre P, Gauthier L, Daniel L, Chemin K, Morel Y, et al. Identification, activation, and selective in vivo ablation of mouse NK cells via NKp46. Proc Natl Acad Sci U S A 2007; 104: 3384–3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walzer T, Jaeger S, Chaix J, Vivier E.Natural killer cells: from CD3(−)NKp46(+) to post-genomics meta-analyses. Curr Opin Immunol 2007; 19: 365–372. [DOI] [PubMed] [Google Scholar]
- Damjanov A, Damjanov I.Isolation of serine protease from granulated metrial gland cells of mice and rats with lectin from Dolichos biflorus. J Reprod Fertil 1992; 95: 679–684. [DOI] [PubMed] [Google Scholar]
- Paffaro VA, Jr, Bizinotto MC, Joazeiro PP, Yamada AT.Subset classification of mouse uterine natural killer cells by DBA lectin reactivity. Placenta 2003; 24: 479–488. [DOI] [PubMed] [Google Scholar]
- Testi R, D'Ambrosio D, De Maria R, Santoni A.The CD69 receptor: a multipurpose cell-surface trigger for hematopoietic cells. Immunol Today 1994; 15: 479–483. [DOI] [PubMed] [Google Scholar]
- Searle RF, Jones RK, Bulmer JN.Phenotypic analysis and proliferative responses of human endometrial granulated lymphocytes during the menstrual cycle. Biol Reprod 1999; 60: 871–878. [DOI] [PubMed] [Google Scholar]
- Mselle TF, Meadows SK, Eriksson M, Smith JM, Shen L, Wira CR, Sentman CL.Unique characteristics of NK cells throughout the human female reproductive tract. Clin Immunol 2007; 124: 69–76. [DOI] [PubMed] [Google Scholar]
- Ogasawara K, Yoshinaga SK, Lanier LL.Inducible costimulator costimulates cytotoxic activity and IFN-gamma production in activated murine NK cells. J Immunol 2002; 169: 3676–3685. [DOI] [PubMed] [Google Scholar]
- Waldmann TA, Tagaya Y.The multifaceted regulation of interleukin-15 expression and the role of this cytokine in NK cell differentiation and host response to intracellular pathogens. Annu Rev Immunol 1999; 17: 19–49. [DOI] [PubMed] [Google Scholar]
- Kennedy MK, Glaccum M, Brown SN, Butz EA, Viney JL, Embers M, Matsuki N, Charrier K, Sedger L, Willis CR, Brasel K, Morrissey PJ, et al. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J Exp Med 2000; 191: 771–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehniger TA, Caligiuri MA.Interleukin 15: biology and relevance to human disease. Blood 2001; 97: 14–32. [DOI] [PubMed] [Google Scholar]
- Ye W, Zheng LM, Young JD, Liu CC.The involvement of interleukin (IL)-15 in regulating the differentiation of granulated metrial gland cells in mouse pregnant uterus. J Exp Med 1996; 184: 2405–2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada S, Okada H, Sanezumi M, Nakajima T, Yasuda K, Kanzaki H.Expression of interleukin-15 in human endometrium and decidua. Mol Hum Reprod 2000; 6: 75–80. [DOI] [PubMed] [Google Scholar]
- Kitaya K, Yasuda J, Yagi I, Tada Y, Fushiki S, Honjo H.IL-15 expression at human endometrium and decidua. Biol Reprod 2000; 63: 683–687. [DOI] [PubMed] [Google Scholar]
- Nakajima S, Hida S, Taki S.IL-15 inhibits pre-B cell proliferation by selectively expanding Mac-1+B220+ NK cells. Biochem Biophys Res Commun 2008; 369: 1139–1143. [DOI] [PubMed] [Google Scholar]
- Yadi H, Burke S, Madeja Z, Hemberger M, Moffett A, Colucci F.Unique receptor repertoire in mouse uterine NK cells. J Immunol 2008; 181: 6140–6147. [DOI] [PubMed] [Google Scholar]
- Hayakawa Y, Smyth MJ.CD27 dissects mature NK cells into two subsets with distinct responsiveness and migratory capacity. J Immunol 2006; 176: 1517–1524. [DOI] [PubMed] [Google Scholar]
- Koyasu S.CD3+CD16+NK1.1+B220+ large granular lymphocytes arise from both alpha-beta TCR+CD4-CD8- and gamma-delta TCR+CD4-CD8- cells. J Exp Med 1994; 179: 1957–1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roncarolo MG, Bigler M, Haanen JB, Yssel H, Bacchetta R, de Vries JE, Spits H.Natural killer cell clones can efficiently process and present protein antigens. J Immunol 1991; 147: 781–787. [PubMed] [Google Scholar]
- Stewart IJ, Webster AJ.Lectin histochemical studies of mouse granulated metrial gland cells. Histochem J 1997; 29: 885–892. [DOI] [PubMed] [Google Scholar]
- Tayade C, Hilchie D, He H, Fang Y, Moons L, Carmeliet P, Foster RA, Croy BA.Genetic deletion of placenta growth factor in mice alters uterine NK cells. J Immunol 2007; 178: 4267–4275. [DOI] [PubMed] [Google Scholar]
- Herington JL, Bany BM.Effect of the conceptus on uterine natural killer cell numbers and function in the mouse uterus during decidualization. Biol Reprod 2007; 76: 579–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco J, Stephenson K, Yamada AT, Croy BA.Time-course analyses addressing the acquisition of DBA lectin reactivity in mouse lymphoid organs and uterus during the first week of pregnancy. Placenta 2008; 29: 1009–1015. [DOI] [PubMed] [Google Scholar]
- Williams NS, Moore TA, Schatzle JD, Puzanov IJ, Sivakumar PV, Zlotnik A, Bennett M, Kumar V.Generation of lytic natural killer 1.1+, Ly-49- cells from multipotential murine bone marrow progenitors in a stroma-free culture: definition of cytokine requirements and developmental intermediates. J Exp Med 1997; 186: 1609–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Iizuka K, Kang HS, Dokun A, French AR, Greco S, Yokoyama WM.In vivo developmental stages in murine natural killer cell maturation. Nat Immunol 2002; 3: 523–528. [DOI] [PubMed] [Google Scholar]
- Manaster I, Mizrahi S, Goldman-Wohl D, Sela HY, Stern-Ginossar N, Lankry D, Gruda R, Hurwitz A, Bdolah Y, Haimov-Kochman R, Yagel S, Mandelboim O.Endometrial NK cells are special immature cells that await pregnancy. J Immunol 2008; 181: 1869–1876. [DOI] [PubMed] [Google Scholar]
- Eriksson M, Meadows SK, Wira CR, Sentman CL.Unique phenotype of human uterine NK cells and their regulation by endogenous TGF-beta. J Leukoc Biol 2004; 76: 667–675. [DOI] [PubMed] [Google Scholar]
- Dunn CL, Critchley HO, Kelly RW.IL-15 regulation in human endometrial stromal cells. J Clin Endocrinol Metab 2002; 87: 1898–1901. [DOI] [PubMed] [Google Scholar]
- Zourbas S, Dubanchet S, Martal J, Chaouat G.Localization of pro-inflammatory (IL-12, IL-15) and anti-inflammatory (IL-11, IL-13) cytokines at the foetomaternal interface during murine pregnancy. Clin Exp Immunol 2001; 126: 519–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizargity P, Bonney EA.Dendritic cells: a family portrait at mid-gestation. Immunology 2009; 126: 565–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskarin G, Redzovic A, Rubesa Z, Mantovani A, Allavena P, Haller H, Vlastelic I, Rukavina D.Decidual natural killer cell tuning by autologous dendritic cells. Am J Reprod Immunol 2008; 59: 433–445. [DOI] [PubMed] [Google Scholar]
- Basu S, Pioli PA, Conejo-Garcia J, Wira CR, Sentman CL.Estradiol regulates MICA expression in human endometrial cells. Clin Immunol 2008; 129: 325–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassiliadou N, Bulmer JN.Expression of CD69 activation marker by endometrial granulated lymphocytes throughout the menstrual cycle and in early pregnancy. Immunology 1998; 94: 368–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RK, Bulmer JN, Searle RF.Cytotoxic activity of endometrial granulated lymphocytes during the menstrual cycle in humans. Biol Reprod 1997; 57: 1217–1222. [DOI] [PubMed] [Google Scholar]
- Parr EL, Parr MB, Zheng LM, Young JD.Mouse granulated metrial gland cells originate by local activation of uterine natural killer lymphocytes. Biol Reprod 1991; 44: 834–841. [DOI] [PubMed] [Google Scholar]
- Kiso Y, McBey BA, Mason L, Croy BA.Histological assessment of the mouse uterus from birth to puberty for the appearance of LGL-1+ natural killer cells. Biol Reprod 1992; 47: 227–232. [DOI] [PubMed] [Google Scholar]
- Shiow LR, Rosen DB, Brdickova N, Xu Y, An J, Lanier LL, Cyster JG, Matloubian M.CD69 acts downstream of interferon-alpha/beta to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature 2006; 440: 540–544. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.