Abstract
T regulatory (Treg) cells are implicated in maternal immune tolerance of the conceptus at implantation; however, the antigenic and regulatory signals controlling Treg cells in early pregnancy are undefined. To examine the role of male seminal fluid in tolerance induction, the effect of exposure to seminal fluid at mating on responsiveness to paternal alloantigens was examined using paternal tumor cell grafts and by delayed-type hypersensitivity (DTH) challenge on Day 3.5 postcoitum. Exposure to seminal fluid inhibited rejection of paternal tumor cells, independently of fertilization and embryo development, while seminal fluid from major histocompatability complex (MHC)-dissimilar males was less effective. Similarly, mating with intact males suppressed the DTH response to paternal alloantigens in an MHC-specific fashion. Excision of the seminal vesicle glands diminished the tolerance-inducing activity of seminal fluid. Mating with intact males caused an increase in CD4+CD25+ cells expressing FOXP3 in the para-aortic lymph nodes draining the uterus, beyond the estrus-associated peak in cycling mice. The increase in CD4+CD25+ cells was abrogated when males were vasectomized or seminal vesicles were excised. Collectively, these data provide evidence that exposure to seminal fluid at mating promotes a state of functional tolerance to paternal alloantigens that may facilitate maternal acceptance of the conceptus at implantation, and the effects of seminal fluid are likely to be mediated by expansion of the Treg cell pool. Both seminal plasma and sperm components of the seminal fluid are necessary to confer full tolerance and elicit the Treg cell response, potentially through provision of immune-deviating cytokines and antigens, respectively.
Keywords: female reproductive tract, immunology, pregnancy, seminal fluid, seminal vesicles, T regulatory cells, tolerance
Seminal fluid transmission to mice at mating expands the population of immune-suppressive T regulatory cells and induces a state of functional immune tolerance to paternal alloantigens to facilitate establishment of pregnancy.
INTRODUCTION
Survival of the semiallogeneic fetus in pregnancy depends on adaptations in both the innate and adaptive immune compartments [1, 2]. A transient state of peripheral immune tolerance is one key mechanism, since the maternal immune system is not ignorant of paternal major histocompatability complex (MHC) and other conceptus antigens [3–5]. This tolerance is mediated, at least in part, by regulatory T (Treg) cells [6], a unique subpopulation of T cells that are potent suppressors of the generation and effector function of type 1 (cell-mediated) immune responses. Treg cells comprise 5%–10% of CD4+ T cells in rodents [7, 8], and are identified on the basis of their constitutive expression of the interleukin 2 receptor, CD25 [9], and the transcription factor, FOXP3 [10].
Treg cells generated in peripheral tissues require antigen-driven activation and proliferation to mediate their full suppressive function [11, 12]. The events associated with activation and expansion of the Treg cell pool during early pregnancy are not defined, and the nature and origin of the eliciting antigens are unclear [12]. In mice, there is some evidence that paternal alloantigens associated with the gestational tissues serve in this role, acting together with antigen-nonspecific hormonal factors to facilitate expansion of Treg cell populations [13]. Several studies in T-cell receptor-transgenic systems specific for paternal H-Y antigen [4], paternal MHC class I [5], or employing chicken egg ovalbumin (OVA) as a model paternal antigen [14] infer that T-cell activation and proliferation occurs in response to antigens shed by fetal or placental trophoblast cells, and cross-presentation by maternal dendritic cells may be involved [14]. However, this is not reconciled with studies showing that mice expressing transgenic T-cell receptors reactive with paternal MHC exhibit functional tolerance and show evidence of T cell anergy even prior to embryo implantation [3]. The increase in Treg cells that occurs in early pregnancy can partly be attributed to elevated estrogen associated with ovulation [15], but this is not a complete explanation, since there is evidence of antigen specificity in the Treg cell response even at this early time point [16, 17].
Female reproductive tissues are first exposed to paternal antigens during transmission of seminal fluid to the female reproductive tract at mating [18]. This raises the questions of whether the female immune response can recognize and respond to male antigens in seminal fluid, and whether mating constitutes an opportunity for initial activation of the maternal immune response. The kinetics of expansion in CD4+CD25+ Treg cell populations within days after mating [6], and the necessity for Treg cell function prior to embryo implantation [19], suggests that expansion of the Treg cell pool occurs independently of the products of conception, and is, instead, consistent with a role for seminal fluid.
This study aimed to determine whether seminal fluid has a role in activating Treg cell populations and eliciting functional immune tolerance to male alloantigens at the time of embryo implantation. A paternal tumor graft model and a delayed-type hypersensitivity (DTH) assay were utilized to investigate the significance of the seminal plasma and sperm components of seminal fluid, and male partner MHC status, in eliciting a functional tolerogenic response. The effect of seminal fluid on populations of CD4+CD25+ Treg cells in lymph nodes (LNs) draining the uterus and systemically was examined by flow cytometry. Our findings show that both the sperm and seminal plasma components of seminal fluid act in concert to induce a state of functional tolerance acting to suppress the type-1 immune response to male alloantigens, and this is associated with expansion of the CD4+CD25+ Treg cell pool beyond the estrogen-regulated increase at ovulation. We conclude that male seminal fluid contributes to the events through which maternal immune tolerance is established at the outset of pregnancy.
MATERIALS AND METHODS
Mice and Surgical Treatments
BALB.K (H-2k) and C57BL/B10.BR (B10; H-2k) mice were purchased from the Animal Resource Centre (Perth, Australia). BALB/c (H-2d), C57BL/6 (B6; H-2b), and (CBA × C57BL/6) F1 (CBB6F1; H-2b/k) mice were purchased from the University of Adelaide Central Animal House. All mice were housed under specific pathogen-free conditions at the University of Adelaide Medical School Animal House on a 12L:12D cycle, and were administered food and water ad libitum. All experiments were conducted in accordance with the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes, with approval from the University of Adelaide Animal Ethics Committee.
Some females underwent surgery 2–4 wk prior to mating to ligate the oviductal-uterine junction after flurothane anesthesia. Vasectomized and seminal vesicle-excised (SVX) male mice were prepared surgically, as previously described [20], and were allowed 2 wk to recover, then proven capable of mating by caging with females and detection of vaginal plugs and sperm deposition, respectively, prior to experimental use. For matings, one to three adult (8–12 wk) intact or uterine-ligated female mice were caged with intact, vasectomized, or SVX stud males and checked daily for vaginal plugs. For female mice caged with SVX males, vaginal smears were taken each morning and examined for the presence of sperm. The day of detection of a vaginal plug or sperm-positive smear was designated Day 0.5 postcoitum (pc). Mated females were removed from males and housed in groups of one to three females per cage.
Tumor Cell Lines and Tumor Challenge Experiments
Tumor challenge experiments utilized JR-5 fibrosarcoma cells or Lewis lung tumor (LLT) cells, both provided by Drs. Lindsay Dent and John Finlay-Jones (Flinders University, Adelaide, SA, Australia). JR-5 tumors were originally induced by subcutaneous inoculation of 3-methylcholanthrene into male BALB/c mice [21], and LLT cells arose spontaneously as a carcinoma of the lung in a B6 mouse [22]. Tumors were propagated by serial passage in BALB/c and B6 mice, respectively, with culture for one to three passages in vitro prior to experimental use. For in vitro culture, both JR-5 and LLT cells were cultured in RPMI-1640 containing 10% fetal calf serum (FCS) and antibiotics (RPMI-FCS) at 37°C in 5% CO2, and were harvested using EDTA (20 mM in PBS for 10 min at 37°C). Expression of MHC class I antigens was confirmed by flow cytometric analysis in cultured cells, or cells recovered from solid tumors excised from mice and mechanically disaggregated with the aid of fine scissors and a manually operated glass homogenizer.
For tumor challenge experiments, adult, virgin intact or uterine-ligated BALB.K or B10 mice at estrus or at Day 3.5 pc after mating with intact, vasectomized, or SVX BALB/c, B6, or CBA × B6 F1 males were administered 1 × 105 JR-5 cells or 5 × 105 LLT cells in 50 μl PBS by s.c. injection into the right ventral flank. Mice were killed by cervical dislocation 13 days later (Day 16.5 pc), and tumor growth was quantified by making two measurements each of the length and width of tumors using digital calipers (Mitutoyo Corp., Kawasaki, Japan), and calculating the average diameter as the mean of these four readings. The incidence of pregnancy was noted, and late gestation pregnancy parameters were measured by excision of the intact uterus and counting total, viable, and resorbing implantation sites.
DTH Experiments
Adult virgin BALB.K mice were evaluated to determine the stage of estrous cycle by phase-contrast examination of vaginal smears prepared at 0900–1000 h. Four sequential stages of the cycle were identifiable on the basis of the cellular composition of the smear: proestrus (>50% intact, live epithelial cells); estrus (100% cornified epithelial cells); metestrus (∼50% leukocytes and ∼50% cornified epithelial cells); or diestrus (>70% leukocytes ± cornified or intact epithelial cells). Mice in diestrus were primed by administration of 1 × 107 BALB/c spleen cells s.c. into both the left and right dorsal flank at 1200–1400 h (Day 0). Prior to injection, spleen cells were incubated with mitomycin C (0.5 mg/ml; Sigma-Aldrich) for 20 min at 37°C, then washed three times in Hanks Balanced Salt Solution (HBSS). On the afternoon of the next day, immunized females were placed with either BALB/c males or BALB.K males. Mice that mated on the first, second, or third night (identified by detection of a vaginal plug on the mornings of Days 2–4) were included in the experiment. On Day 3.5 pc (Days 6–8 after priming), or on Day 7 after priming (unmated virgin controls), all mice were challenged by injection of the left footpad with 1 × 107 mitomycin C-treated BALB/c spleen cells in 25 μl HBSS. The right footpad was injected with 25 μl HBSS. Immediately prior to challenge and 24 h after challenge, footpad thickness was measured as the average of five replicate readings made using a constant pressure caliper (Mitutoyo Corp.). The DTH response was calculated as the difference in microns between the left and right footpad thickness values.
Flow Cytometry
Adult virgin B6 mice at defined stages of the estrous cycle (as described above) or Day 3.5 pc after mating with intact, vasectomized, or SVX BALB/c males, were administered 2% Avertin anesthesia (2% 2.2.2-tribromomethanol; Sigma-Aldrich), and blood was collected by cardiac puncture and diluted 1:2 in PBS containing heparin (Sigma-Aldrich). Peripheral blood mononuclear cells were collected after centrifugation over Lympholyte (Cedarlane, Hornby, Canada) according to the manufacturer's instructions. Single-cell suspensions were prepared from excised para-aortic (ileac) LNs, mesenteric LNs, and the spleen by mechanical dispersion between glass microscope slides. Cells were washed in RPMI-FCS and resuspended in 0.1% FCS/PBS (fluorescence-activated cell sorting [FACS] buffer) at 107 cells/ml. Aliquots of 106 cells were incubated with anti-Fc-γIIR antibody (BD Pharmingen, BD Biosciences, San Diego, CA) to block nonspecific binding (15 min/4°C) prior to incubation with PE anti-CD3 (145–2C11), allophycocyanin anti-CD4 (L3T4), and biotin anti-CD25 (7D5) (all from BD Pharmingen) for 30 min at 4°C. Cells were washed in FACS buffer and incubated with PE-Cy5 streptavidin (BD Pharmingen) for 30 min at 4°C. After further washing in FACS buffer, the cells were resuspended in 300 μl FACS buffer and analyzed using a FACS Canto with FACS Diva software (both from BD Biosciences). CD4+CD25+ cells were quantified as a percent of total CD4+ cells after gating on the CD3+ T-lymphocyte population and excluding debris and dead cells. The absolute number of CD4+CD25+ cells in para-aortic LN was calculated as follows: absolute CD4+CD25+ cell number = total cell number × % CD4+ cells × CD4+CD25+ cells (% CD4+ cells), where total cell number is mean values from identical treatment groups in our previous study [23].
To evaluate FOXP3 expression, para-aortic LN cells prepared as above were incubated with Fc Block (BD Pharmingen) to block CD16/CD32 receptors, and then incubated with fluorescein isothiocyanate (FITC) anti-CD3 (17A2), PE anti-CD4 (RM4–5) (both from BD Pharmingen), and PE-Cy7 anti CD25 (PC61; eBiosciences). Cells were subsequently fixed and permeabilized using a FOXP3 staining buffer set (eBiosciences) according to the manufacturer's instructions. Following further treatment with Fc Block, cells were incubated with allophycocyanin anti-FOXP3 antibody (FJK-16s; eBiosciences), then analyzed using a BD FACS Canto with FACS Diva software. FOXP3+ cells were quantified as a percent of total CD4+CD25+ or CD4+CD25− cells after gating on the CD3+ T-lymphocyte population and excluding debris and dead cells. Epitope mapping shows FJK-16s reactivity with amino acids 75–125 of the mouse FOXP3 protein (manufacturer's product notes), and specificity for FOXP3 is further demonstrated by the absence of FJK-16s staining in mice with a null mutation in the Foxp3 gene [24].
For quantification of MHC class I expression, JR-5 or LLT cells were incubated with monoclonal antibody anti-H-2Kb (HB176; American Type Culture Collection, Manassas, VA), followed by FITC goat anti-rat (Dako, Glostrup, Denmark), or FITC anti-H-2Kd (SF1–1.1; BD Pharmingen), and analyzed using a FACScan with CellQuest software (Becton Dickinson) using forward and side scatter to exclude debris and dead cells.
Statistical Analysis
All data were analyzed using SPSS version 13 (SPSS, Chicago, IL). Categorical data (proportion of mice with tumors) were compared by chi-square test. ANOVA and independent samples t-test were used to compare differences between treatment groups in tumor size, footpad thickness, and cell numbers. The relationship between tumor size and number of viable implantation sites was assessed using Pearson two-tailed bivariate correlation analysis. Statistical significance in differences between groups or correlation was concluded when P < 0.05.
RESULTS
Seminal Fluid Induces Tolerance to Male Partner MHC Antigens
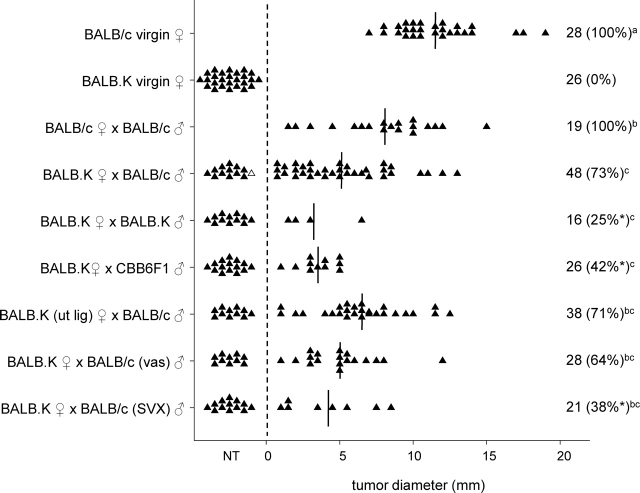
Initially, we investigated whether seminal fluid exposure at mating can induce functional tolerance to paternal alloantigens. A model system was established employing congenic mice in the BALB series, which differ at only the MHC gene locus. Intact or uterine-ligated BALB.K (H-2k) females were mated with intact BALB/c (H-2d) or BALB.K males and then challenged with BALB/c JR-5 fibrosarcoma cell grafts on Day 3.5 pc. JR-5 cells were selected from a panel of candidate BALB/c tumor cells on the basis of their formation of solid tumors with linear growth kinetics over a 2-wk period, poor metastatic potential, and stable expression of MHC class I (data not shown). JR-5 cells consistently developed a single solid tumor when injected s.c. into virgin BALB/c mice (28/28, 100% tumor growth), but were rejected from virgin BALB.K mice (24/24, 0% tumor growth). Pregnancy did not alter the incidence of tumor development, since JR-5 cells grew in BALB/c females mated with BALB/c males and injected with JR-5 cells on Day 3.5 pc (19/19, 100% tumor growth). However, tumors were smaller in pregnant BALB/c mice compared with virgin BALB/c mice (P = 0.003) (Fig. 1).
FIG. 1.
The effect of seminal fluid exposure at mating on rejection of paternal tumor cell grafts in BALB.K female mice. Intact or uterine-ligated BALB/c or BALB.K females, either virgin or Day 3.5 pc after mating with intact, vasectomized (vas) or SVX BALB/c, BALB.K or CBB6F1 males, were administered 1 × 105 JR-5 tumor cells, and the occurrence and size of any resulting tumor was measured 13 days later. Symbols represent individual mice. The number of mice per group and the percent tumor growth (in parentheses) is shown. Vertical lines indicate mean values for mice with tumors. The effect of treatment on proportion of mice with tumors was analyzed by chi-square analysis (*P < 0.05, compared with BALB.K ♀ × BALB/c ♂ group). The effect of treatment on tumor size was analyzed by ANOVA and post hoc Sidak t-test. Different superscript letters indicate significant differences between groups (P < 0.05). NT, no tumor; ut lig, uterine ligated.
In contrast to virgin BALB.K females, tumor cell graft rejection was impaired in the majority of BALB.K females following mating with intact BALB/c males and administration of JR-5 cells on Day 3.5 pc (35/48, 73% tumor growth) (Fig. 1). Tumor growth was independent of whether or not pregnancy ensued and conceptus tissue was present, since 31/48 (65%) of mated BALB.K females administered JR-5 cells did not have implantation sites at autopsy on Day 16.5 pc, indicating that viable pregnancy was not necessary for female tolerance of tumor cells. A similar rate of pregnancy failure was seen in BALB/c females mated with BALB/c males, where 7/19 (37%) mice administered JR-5 cells had no implantation sites on Day 16.5 pc (P = 0.088, chi-square test). Pregnancy loss rates in both groups were substantially greater than in BALB.K or BALB/c mice not given tumor cells (<10%; data not shown), indicating that the presence of tumor cells interferes with either embryo implantation or pregnancy maintenance. There was no correlation between tumor size and number of viable implantation sites in either BALB/c or BALB.K mice, or both data sets combined (R = 0.064).
In another group of BALB.K females, the uterus was ligated at the oviductal junction 2 wk prior to mating in order to prevent conception and embryo formation, while allowing seminal fluid access to the uterine cavity. When uterine-ligated BALB.K females were mated with BALB/c males and challenged with JR-5 cells on Day 3.5 pc, tumor cell rejection was inhibited (27/38, 71% tumor growth). The proportion of mated uterine-ligated BALB.K mice that developed tumors, as well as the size of tumors, were similar to mated intact BALB.K females (Fig. 1). This shows that tolerance to paternal tumor challenge is induced by seminal fluid at mating, and that neither conception nor the presence of embryos is necessary.
The effect of male partner strain on seminal fluid induction of tolerance to tumor cell challenge was examined next. Compared with BALB.K females mated with BALB/c males, tolerance to JR-5 tumor cell grafts occurred less frequently in BALB.K females mated with CBB6F1 (H-2b/k) males (11/26, 42%; P = 0.031), or BALB.K females mated with BALB.K males (4/16, 25%; P = 0.003) (Fig. 1). This indicates that development of tolerance after seminal fluid exposure is maximized when the male partner is of the same genotype as the tumor cell graft, and implicates male MHC antigens in tolerance activation.
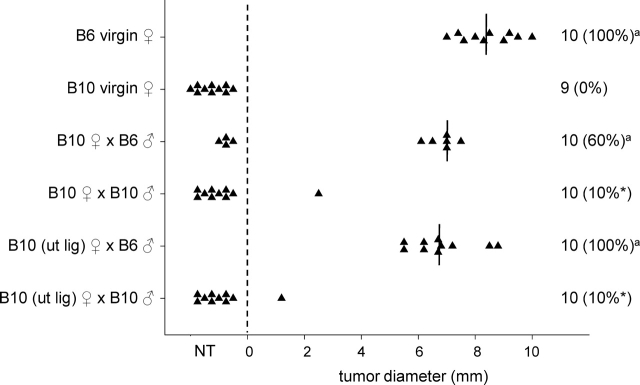
A second congenic model was evaluated in order to ensure that the tolerance-inducing effect of seminal fluid was not limited to BALB.K mice. Intact or uterine-ligated B10 (H-2d) females were mated with intact B6 (H-2k) or B10 males, and challenged with B6 LLT cells on Day 3.5 pc. LLT cells formed solid tumors with linear growth kinetics over a 2-wk period, and showed poor metastatic potential and stable expression of MHC class I (data not shown). While virgin B10 mice all rejected LLT tumor cells (0/9, 0% tumor growth), rejection was inhibited in the majority of B10 females after mating with intact B6 males (6/10, 60% tumor growth) (Fig. 2). In B10 uterine-ligated females mated with B6 males, tumor cell graft rejection was also inhibited (10/10, 100% tumor growth; P < 0.001 compared with virgin B10.BR mice) (Fig. 2). Compared with B10 females mated with B6 males, tolerance to LLT challenge occurred less frequently in both intact B10 females mated with B10 males (1/10, 10%; P = 0.028), and in uterine-ligated B10 females mated with B10 males (1/10, 10%; P < 0.001) (Fig. 2). This indicates that, in B10 mice, as in BALB.K mice, exposure to seminal fluid is sufficient to induce tolerance to tumor cell grafts in a partner MHC-specific manner.
FIG. 2.
The effect of seminal fluid exposure at mating on rejection of paternal tumor cell grafts in B10 female mice. Intact or uterine-ligated B6 or B10 females, either virgin or Day 3.5 pc after mating with intact B6 or B10 males, were administered 5 × 105 LLT cells, and the occurrence and size of any resulting tumor was measured 13 days later. Symbols represent individual mice. The number of mice per group and the percent tumor growth (in parentheses) is shown. Vertical lines indicate mean values for mice with tumors. The effect of treatment on proportion of mice with tumors was analyzed by chi-square analysis (*P < 0.05, compared with B10 ♀ × B6 ♂ group). aNo effect of treatment on tumor size was evident when data were analyzed by ANOVA and post hoc Sidak t-test. NT, no tumor; ut lig, uterine ligated.
Seminal Plasma Is Required for Activation of Tolerance at Mating
The requirement for the sperm and seminal plasma components of seminal fluid to induce tolerance to tumor cell grafts was examined next, utilizing both vasectomized males, and males from which the seminal vesicle glands were surgically excised (SVX males) to remove the majority of the seminal plasma from the ejaculate. Compared with BALB.K females mated with intact BALB/c males, tolerance to JR-5 challenge occurred less frequently in BALB.K females mated with SVX BALB/c males (8/21, 38%; P = 0.026), but was unchanged in BALB.K females mated with vasectomized BALB/c males (18/28, 64%) (Fig. 1). This shows that tolerance to tumor cell grafts after mating can occur without transmission of sperm, but that factors of seminal vesicle origin in the seminal plasma are essential for induction of tolerance.
Exposure to Seminal Fluid at Mating Inhibits DTH Responses to Male MHC Antigens
Female tolerance of paternal tumor cell grafts after insemination suggests hyporesponsiveness in the type 1 immune response. In another experimental approach to examine the effect of seminal fluid on the female type 1 immune response to male partner MHC antigens, a DTH assay was utilized. Adult virgin female BALB.K mice were immunized s.c. with 1 × 107 BALB/c spleen cells, then, 2–4 days later, mated with intact, vasectomized, or SVX BALB/c males, or intact BALB.K males. On Day 3.5 pc (6–8 days after priming), all mice were challenged in the footpad with BALB/c spleen cells. Mating with BALB/c males reduced footpad swelling at challenge, indicating a diminished DTH reaction compared with control unmated mice (P = 0.020) (Fig. 3). In contrast, no reduction in DTH response was observed when immunized BALB.K females were mated with BALB.K males, or with vasectomized or SVX BALB/c males.
FIG. 3.
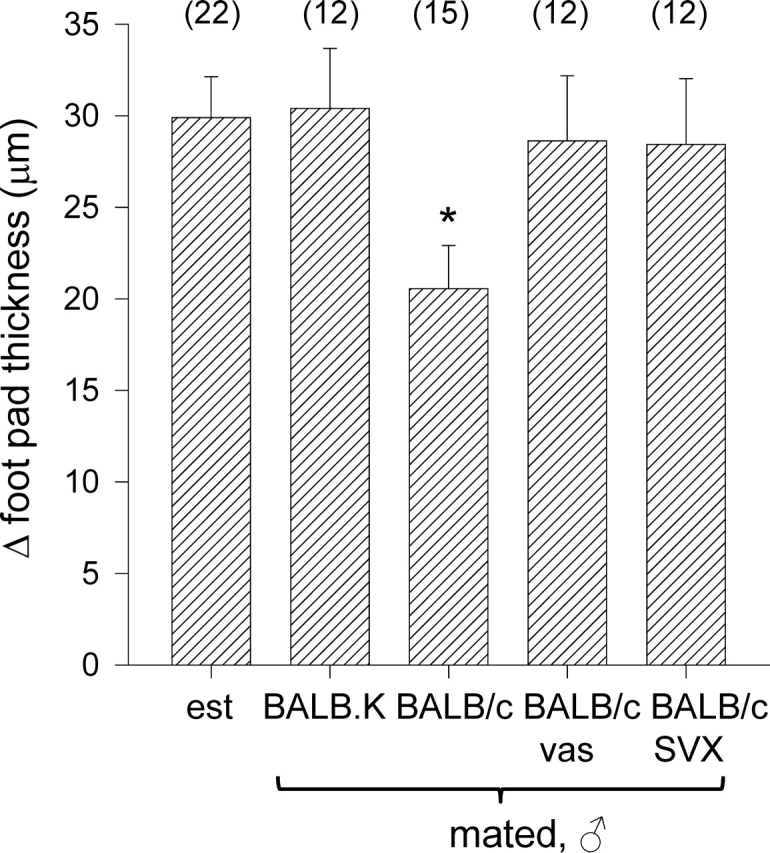
The effect of seminal fluid exposure at mating on DTH responses in BALB.K female mice. BALB.K females were immunized by s.c. injection with BALB/c spleenocytes, left unmated (control; est, estrus) or mated 3–5 days later with either intact BALB.K males or intact, vasectomized (vas), or SVX BALB/c males, then challenged by s.c. injection to the footpad on Day 3.5 pc with BALB/c spleenocytes. Footpad thickness was measured 24 h later. Data are change in footpad thickness (mean ± SEM), calculated as the difference in thickness between spleenocyte-challenged and control footpads. The effect of treatment on DTH response was analyzed by ANOVA and post hoc Sidak t-test (*P < 0.05, compared with unmated virgin control group).
These data show that natural insemination can inhibit the type I immune response to paternal MHC antigens. The effect is dependent on mating with a male of the same MHC haplotype as the immunizing MHC antigen, and appears to require both the seminal plasma and sperm fractions of the ejaculate.
Exposure to Seminal Fluid at Mating Causes Expansion of CD4+CD25+FOXP3+ Cell Populations
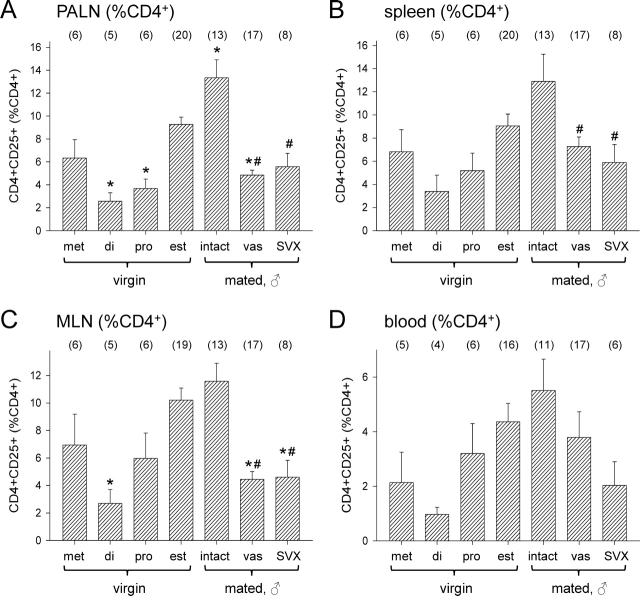
To investigate whether the immune tolerance induced by seminal fluid exposure is potentially mediated by Treg cells, and the requirement for seminal plasma and sperm components of seminal fluid in eliciting any Treg cell response, CD4+CD25+ cells were analyzed by flow cytometry in para-aortic LN, mesenteric LN, peripheral blood, and spleens recovered from B6 mice on Day 3.5 pc after mating with intact, vasectomized, or SVX BALB/c males. For comparison, CD4+CD25+ cells were also analyzed in tissues recovered from virgin mice at defined phases of the estrous cycle (Fig. 4, A and B).
FIG. 4.
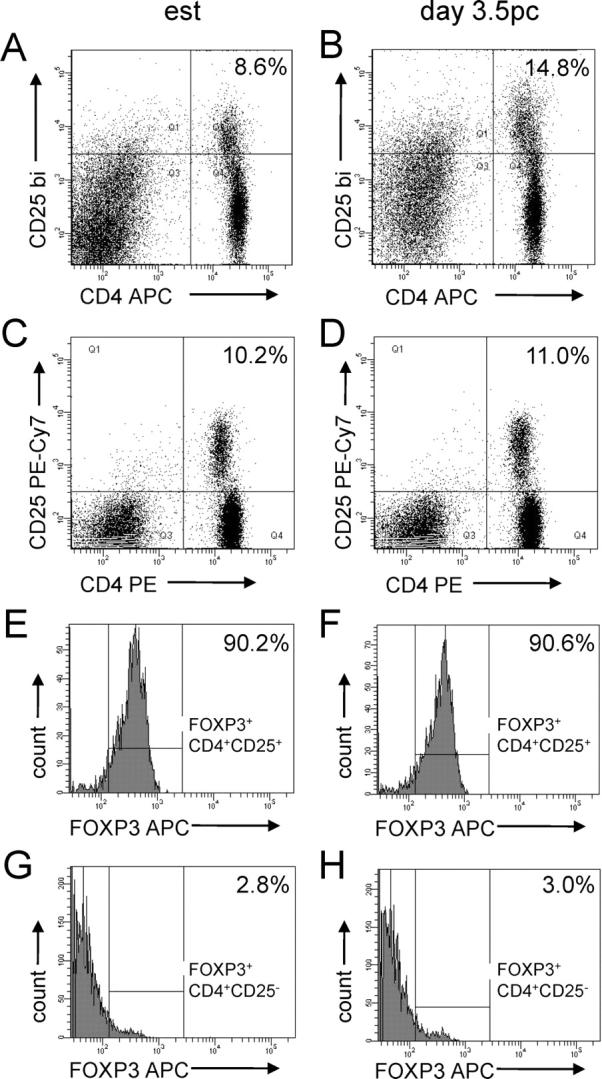
Flow cytometric analysis of CD4+CD25+ cells and FOXP3 expression in CD4+CD25+ cells and CD4+CD25− cells from para-aortic LN of virgin control B6 females (A, C, E, G), and Day 3.5 pc B6 females after mating with intact BALB/c males (B, D, F, H). Data shown are representative of n = 5–6 mice per group. The proportion of CD4+CD25+ cells as a percentage of CD4+ cells is shown in the top left corner of dot plots (A–D). Histograms show the expression of FOXP3 in CD4+CD25+ cells (E and F) and in CD4+CD25− cells (G and H), with the percentage of FOXP3+ cells within each population shown in the top left corner. APC, allophycocyanin; est, estrus.
Clear effects of the phase of estrous cycle on CD4+CD25+ cells in LNs, spleen, and blood were observed. In the para-aortic LNs, which drain the uterus, the CD4+CD25+ fraction (expressed as percent CD4+ cells) was significantly higher at the estrous stage (the time of ovulation and receptivity to mating) than at either the diestrous or proestrous stages of the cycle (P < 0.001 and P = 0.001, respectively) (Fig. 5A). In mesenteric LN, mice had fewer CD4+CD24+ cells at diestrus than at estrus (P = 0.006) (Fig. 5C). Similar fluctuations were seen in spleen and blood, but the variation between mice was higher in these compartments, and statistically significant differences were not observed (Fig. 5, B and D).
FIG. 5.
The effect of stage of estrous cycle and exposure to seminal vesicle fluid on CD4+CD25+ cell populations in female B6 mice. Para-aortic LN (PALN) (A), spleen (B), mesenteric LN (MLN) (C), and peripheral blood (D) cells were recovered at defined stages of the estrous cycle (di, diestrus; est, estrus; met, metestrus; pro, proestrus) or from Day 3.5 pc females after mating with intact, vasectomized (vas), or SVX BALB/c males, and the proportion of CD4+CD25+ cells as a percentage of CD4+ cells was evaluated by flow cytometry. Data are mean ± SEM, with the number of mice in each group shown in parentheses. The effect of treatment was analyzed by ANOVA and post hoc Sidak t-test (*P < 0.05 compared with estrus control group; #P < 0.05 compared with intact mated group).
Mating with intact males elicited a further 44% increase in CD4+CD25+ cells (expressed as percent CD4+ cells) by Day 3.5 pc in para-aortic LNs compared with estrous mice (P = 0.013) (Fig. 5A). In contrast, there was no increase in females mated with vasectomized or SVX males, where CD4+CD25+ cells were fewer than in females mated with intact males (both P < 0.001) (Fig. 5A), and, in the case of females mated with vasectomized males, less than in estrous females (P = 0.002). This shows that seminal plasma and sperm components of the seminal fluid are required to elicit the postmating increase in CD4+CD25+ cells in para-aortic LN.
In the mesenteric LN, no significant increase was seen in mice mated with intact males compared with estrous mice, but fewer CD4+CD24+ cells were present after mating with vasectomized or SVX males compared with intact males (P < 0.001 and P = 0.001, respectively), and, in both cases, values were less than in estrous females (P < 0.001 and P = 0.009, respectively) (Fig. 5C). A similar effect was evident in the spleen, where fewer CD4+CD24+ cells were present after mating with vasectomized or SVX males compared with intact males (P = 0.051 and P = 0.043, respectively) (Fig. 5B). No significant effect of mating or seminal fluid composition on CD4+CD24+ cell number in the blood was apparent (Fig. 5D).
The Treg surface marker CD25 is also expressed by activated CD4+ effector cells, and T-cell activation is a characteristic of early pregnancy [23]. To confirm that the increase in CD4+CD25+ cells in the para-aortic LN reflects an increase in Treg cells, a different antibody cocktail was utilized to detect the Treg-specific marker FOXP3 in CD4+CD25+ cells from para-aortic LN of estrous mice and Day 3.5 pc females after mating with intact BALB/c males (Fig. 4, C and D). Consistent with previous reports [10], FOXP3 expression was largely restricted to CD4+CD25+ cells, and rarely detected in CD4+CD25− cells or CD4− cells. In both groups of mice (n = 5–6 mice/group), the percentage of CD4+CD25+ cells expressing FOXP3 was comparable (median [range] % FOXP3+ at estrus = 90.2% [83.5%–93.9%] and at Day 3.5 pc = 91.0% [83.2%–92.3%]) (Fig. 4, D and E). Small proportions (≤3%) of CD4+CD25− cells in both estrous and mated mice expressed the FOXP3 marker (Fig. 4, G and H). This indicates that the majority of CD4+CD25+ cells are indeed Treg cells. Since the proportion of FOXP3+ Treg cells among the expanded para-aortic LN CD4+CD25+ pool was unchanged by mating, the increases in CD4+CD25+ cells at Day 3.5 pc reflect an increase in the Treg cell population, rather than activation and proliferation of other FOXP3− CD4+ cells.
Hypertrophy of the para-aortic LN is characteristic of the immune response to early pregnancy, and depends on exposure to factors in the seminal plasma fraction of the seminal fluid [23]. To estimate the scale of effect of seminal fluid on the total size of the CD4+CD25+ cell pool, the absolute number of CD4+CD25+ cells in the para-aortic LN was calculated from flow cytometry data, factoring in the total number of cells recovered from LNs [23] (Fig. 6A) and the proportion of total cells expressing CD4 (Fig. 6B). Exposure to seminal fluid by mating with intact males increased the absolute number of CD4+CD25+ cells by 2.7-fold (P < 0.001), while absolute CD4+CD25+ cell numbers were unchanged after mating to vasectomized or SVX males compared with estrous values, and significantly less compared with the intact mated group (both P < 0.001) (Fig. 6C).
FIG. 6.
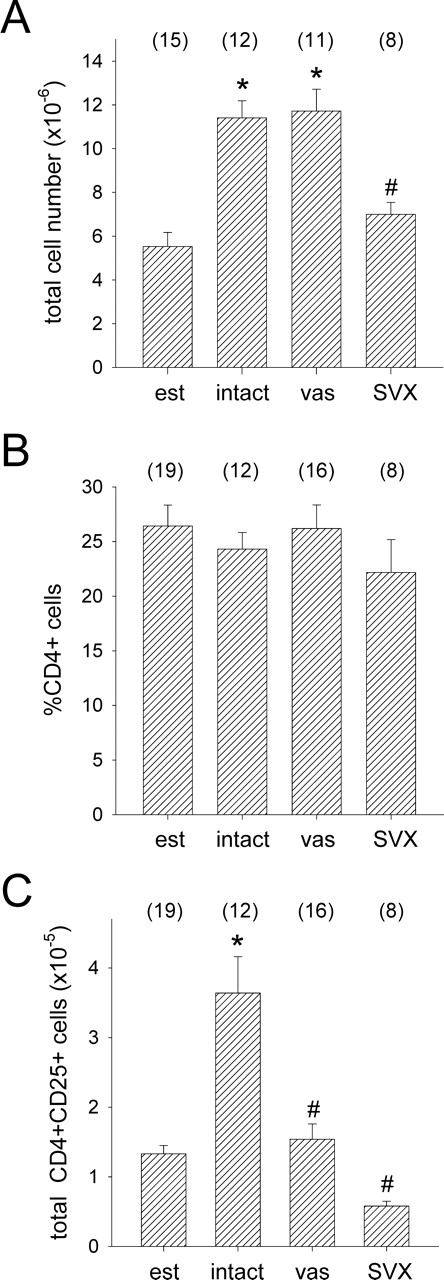
The effect of exposure to seminal fluid on absolute total cell numbers (A), percent CD4+ cells (B), and absolute CD4+CD25+ cells (C) in para-aortic LN of female B6 mice. Cells were recovered from unmated control mice at estrus (est), or from Day 3.5 pc females after mating with intact, vasectomized (vas) or SVX BALB/c males, and absolute numbers were calculated from the percent CD4+CD25+ data shown in Figure 5A. Data are mean ± SEM, with the number of mice in each group shown in parentheses. The effect of treatment was analyzed by ANOVA and post hoc Sidak t-test (*P < 0.01, compared with estrus control group; #P < 0.01 compared with intact mated group).
DISCUSSION
Preventing development of maternal type 1 immunity to conceptus transplantation antigens is essential for pregnancy. Maternal T cells recognize and can respond to paternal MHC antigens, indicating the necessity for mechanisms to limit their effector function [1, 3]. Treg cells are implicated in this protective role [6]; however, the factors regulating Treg cell abundance and function in pregnancy have not been elucidated. The current experiments demonstrate that generation of paternal antigen-specific tolerance in early pregnancy is initiated at the time of conception, when the female is first exposed to paternal alloantigens in the context of seminal fluid. Seminal fluid transmission activates a state of functional tolerance to paternal alloantigens, and this is associated with expansion of CD4+CD25+ cell populations confirmed to be Treg cells by virtue of their expression of the signature Treg cell marker, FOXP3. Both the seminal plasma and the sperm components of seminal fluid appear to be necessary for maximum expansion of the Treg cell pool and for full expression of functional tolerance, but conceptus tissue is not required for this initial phase of the response. Additionally, the data suggest that there is a degree of male partner specificity in the female reaction to seminal fluid.
The current findings concur with published studies showing that seminal fluid influences the female immune response. Insemination causes hypertrophy of LNs draining the uterus [25, 26]. This is associated with expression of lymphocyte activation markers and cytokine expression, and is completely dependent upon factors present in seminal plasma [23]. Mated females or females administered whole seminal fluid to the uterine cavity show partner-specific hyporesponsiveness in type 1 immunity to male MHC antigens, demonstrated by prolonged survival of male skin grafts [25, 27]. Consistent with this, we reported preliminary observations that seminal fluid inhibits rejection of paternal tumor cells, even when conception is prevented by surgical ligation of the uterine-oviductal junction [28]. The current study extends these observations by showing the following in two mouse strains: seminal fluid exposure in the absence of conception is sufficient to activate functional tolerance in females; consistent tolerance induction requires exposure to seminal plasma of the same male strain as the paternal challenge; two functional measures of type 1 immunity—tumor graft rejection and DTH response—are inhibited by seminal fluid exposure; and Treg cells are potentially involved in suppressing these type 1 responses.
Challenge with paternal skin grafts and tumor cell grafts have been previously utilized to demonstrate functional tolerance to paternal MHC and other antigens in pregnancy [3, 25, 29], but these assays can be variable in their specificity and sensitivity, depending on graft tissue expression of alloantigens and other tissue-restricted antigens. These factors presumably contributed to the quantitative differences in the results for the two strain combinations used in the tumor challenge experiments reported herein. Compelling evidence linking suppression of type 1 immunity with Treg-cell activity in pregnancy has now emerged [6, 30], but the antigen specificity and dependence of Treg cells is difficult to study without reliance on in vivo functional assays, since reagents to distinguish gestational tissue-reactive Treg cells among the systemic Treg pool are not available.
Previous studies have alluded to the likely importance of gestational tissues as the source of antigens and immune-deviating signals for activating tolerance mechanisms [3], including Treg cells [16]. However, the kinetics of expansion in CD4+CD25+ Treg cell populations within days of mating [6], and the necessity for Treg cell function prior to embryo implantation [19], argues against their dependence on fetal alloantigens. Furthermore, since the preimplantation embryo comprises, at most, a few hundred cells encapsulated in the zona pellucida, antigen associated with the embryo before implantation would be insufficient and inaccessible.
A role for seminal fluid in activating the female Treg response provides an explanation for why functional tolerance and Treg cell expansion are evident even before the time of embryo implantation. Since T cells generally take several days to generate a robust response after stimulation, a Treg response initiated at the time of conception would allow protective suppression to manifest by the time the embryo implants 4 days later. Several of the same antigens are present in seminal fluid and later expressed from paternal genes by the conceptus [31], including classical class Ia and nonclassical class Ib MHC antigens and minor antigens, such as H-Y [32, 33]. Therefore, Treg cells activated during the preimplantation period would exhibit protective suppression upon reencounter with MHC and other antigens expressed by fetal cells and placental trophoblast cells following embryo implantation [34, 35].
The male partner specificity evident in both the tumor challenge and DTH assays suggests that antigen specificity is conferred by alloantigen in seminal fluid. Both somatic cell- and spermatozoa-associated MHC class I, as well as soluble MHC class I, is present in seminal fluid [32, 36–38]. The growth of tumors after challenge in some BALB.K mice after mating with males expressing third-party MHC suggests that antigens other than alloantigens might also be involved, and H-Y antigen or other unique differentiation antigens on the surface of spermatozoa could fulfill this role [39]. These antigens are present on non-sperm cells, such as desquamated epithelial cells in the seminal plasma fraction, as well as expressed at lower levels by sperm, and this could explain why seminal plasma in the absence of sperm was sufficient to allow acceptance of BALB/c tumor cell challenges in BALB.K mice after mating with vasectomized males. A greater dependence on seminal plasma than on sperm for Treg cell generation is also evident in the qualitatively greater reduction in absolute Treg cell numbers seen after seminal plasma-deficient mating compared with sperm-deficient mating.
Previous reports have concluded that both antigen-dependent and antigen-independent mechanisms contribute to controlling expansion of the Treg cell pool in early pregnancy, suggesting that Treg cells might arise through both activation and proliferation, and conversion of phenotypically distinct precursor cells. Ovariectomy and steroid hormone replacement experiments demonstrate that estrogen is responsible for eliciting an increase in CD4+CD25+ Treg cell numbers and Foxp3 mRNA expression in the spleen, and in vitro experiments showed direct induction of Foxp3 in CD4+CD25− cells by 17-β-estradiol [15]. Fluctuating estrogen levels likely explain the elevated Foxp3 mRNA expression, indicating accumulation of Treg cells in the uterus at estrus, potentially in response to estrogen-induced expression of chemokines that target the CCR5 chemokine receptor controlling Treg cell recruitment [17]. Our data, showing significantly increased numbers of CD4+CD25+ cells in para-aortic LNs and mesenteric LNs at the estrous phase of the cycle, further support a role for estrogen, and suggest that these LNs, as well as the spleen, may contribute to generating an elevated circulating pool available for periodic Treg-cell recruitment into the uterus. Whether these cells are generated within peripheral lymphoid tissues or recruited via the circulation from the spleen, or perhaps the thymus, remains to be determined.
Similar to previous reports [6, 17, 40], our study shows a relative increase in Treg cell numbers in LNs during the first 4 days of pregnancy and, importantly, demonstrates that this increase is significant even relative to nonpregnant mice timed for the estrous stage of the cycle. Thus, while cycle-associated fluctuations partly explain the elevated CD4+CD25+ cell number in early pregnancy, the estrogen-regulated increase at the time of ovulation in not sufficient to explain the surge in Treg cell numbers during early pregnancy. This is consistent with other studies concluding a role for alloantigen-driven expansion in Treg cell numbers once pregnancy is initiated [16, 17]. Pregnancy-associated Treg cells appear to arise in peripheral tissues as opposed to the thymus, with several studies implicating the uterus draining para-aortic LNs as the predominant site of Treg pool expansion [6, 16].
Our data clearly identify seminal fluid as the missing signal of early pregnancy acting to stimulate Treg cell pool expansion in readiness for embryo implantation. Female exposure to both the sperm and seminal plasma components of seminal fluid appears to be necessary. Mating events deficient in either sperm or seminal plasma failed to sustain the high levels of Treg cells seen in LNs and spleen after mating with an intact male, and cell numbers instead were comparable to those seen in nonovulatory phases of the cycle. Since ovarian hormone levels are functionally comparable at Day 3.5 pc, regardless of the presence of sperm and seminal plasma in the ejaculate [41], these results discount the possibility that the ovarian hormone parameters of early pregnancy are sufficient to sustain high Treg cell numbers until the time of embryo implantation.
Collectively, these experiments suggest that the seminal fluid-driven expansion in Treg cell numbers is the mechanism underpinning the capacity of mated females to tolerate paternal tumor cell grafts. While this seems a likely proposal, we were not able to confirm a causal relationship by demonstrating any consistent reduction in tolerance of JR-5 cells after depletion of Treg cells by passive administration of anti-CD25 antibody to mated mice (data not shown). Thus, mechanisms in addition to Treg cells may contribute to suppression of type 1 immune responses after mating.
Like other antigen-specific immune responses, activation and proliferation of Treg cells requires an active process of antigen uptake, processing, and presentation of antigenic peptides by antigen-presenting cells (APCs). The female reproductive tract is well equipped to mount tolerogenic immune responses to seminal fluid antigens, since APCs are abundant in the endometrial tissue at the time of mating [42–44]. Indeed, seminal fluid has an active role in APC recruitment, with coitus eliciting a local inflammatory response in the female reproductive tissues [45]. Soluble factors in the plasma fraction of seminal fluid induce expression of proinflammatory cytokines and chemokines in epithelial cells lining the cervix and uterus, causing recruitment of macrophages, dendritic cells, and granulocytes, which accumulate in endometrial and decidual tissue [42–44]. These dendritic cells express markers indicative of a tolerogenic phenotype [46], which would be maintained by the type 2 cytokine-dominated environment of the decidual tissue [47].
Seminal plasma contains potent immune-regulatory molecules, including prostaglandins E (PGE) and transforming growth factor-β (TGFB) [48, 49], both of which have been linked with generation of immune suppression mediated by regulatory cells [7, 8]. Naïve CD4+CD25− T cells differentiate into a suppressor T-cell phenotype and express FOXP3 when TGFB is present [50]. TGFB is also implicated in proliferation of mature Treg cells through modifying the function and signaling capabilities of dendritic cells [51]. PGE2 may synergize with TGFB in this role, since in vitro experiments indicate that PGE2 can enhance the inhibitory capacity of human CD4+CD25+ Treg cells and induce a regulatory phenotype in CD4+CD25− T cells [52].
We have utilized a T-cell receptor-transgenic model with OVA as a model paternal antigen to provide direct evidence that male antigens delivered in seminal fluid can activate an antigen-specific T-cell response in female mice. OVA delivered in the ejaculate was found to activate and elicit proliferation of adoptively transferred CD4+ and CD8+ OVA-specific T cells specifically within para-aortic LNs draining the uterus, and female dendritic cells were required to activate the response [53]. This demonstrates the feasibility of uptake of seminal fluid antigens by uterine dendritic cells and cross-presentation to T cells in para-aortic LNs after mating.
The extent to which Treg cells arise from differentiation of naïve CD4+ T cells, or, alternatively, from proliferation of existing Treg cells, and the role of antigen in either pathway, remains to be determined. The effect of successive exposures to paternal antigens through multiple matings, or previous pregnancy, warrants examination to investigate whether these events progressively enlarge the paternal antigen-reactive Treg pool. Another unanswered question is the physiological significance of the seminal fluid-driven expansion in the Treg pool for successful pregnancy. Embryo transfer studies will be required to evaluate whether it is essential or simply a facilitating mechanism to boost suppressive activity prior to the sustained immune-regulating function of placental tissue taking effect. The success of artificial insemination with diluted semen or washed sperm, as well as embryo transfer in mice and other species, clearly shows that successful pregnancy can occur without a natural mating at the outset, although there is mounting evidence that assisted reproductive technologies are associated with suboptimal reproductive outcomes [54], and lack of seminal fluid conditioning of the female immune response may partly account for this [55]. In mice, embryo transfer generally employs recipients mated to vasectomized males, but fetal loss and abnormality is considerably greater when embryos are transferred without recipient exposure to male fluids [56]. When recipient females are mated with seminal vesicle-deficient males, transferred embryos give rise to fetuses with impaired growth trajectories and placental development [57]. In rats, implantation rates and fetal growth are similarly impaired unless females are inseminated prior to embryo transfer [58].
The prospect of Treg cell regulation by seminal fluid has implications for human reproductive health. In women, seminal fluid elicits inflammatory cytokine expression in cervical epithelial cells [59] and, in vivo, causes recruitment of APCs into the ectocervical epithelium (Sharkey and Robertson, unpublished results). Although, to date, there are no studies on the effects of intercourse on Treg cells in women, clinical studies indicate that there may be both acute and cumulative effects on fertility of exposure to seminal constituents, and implicate a partner-specific mode of action. Live birth rates in couples undergoing in vitro fertilization treatments are significantly improved when intercourse occurs around the time of embryo transfer [60, 61], and administration of seminal plasma may increase pregnancy rate in women experiencing recurrent spontaneous abortion [62]. In the condition of pre-eclampsia, which results from a type 1-dominated immune response linked with insufficient Treg cell activity [63, 64], there is a protective benefit of exposure to semen over time [65, 66]. Markedly increased rates of pre-eclampsia are evident in pregnancies with donor oocytes or semen [67], when prior exposure to the donor sperm or conceptus antigens has not occurred. Primary unexplained infertility is also associated with reduced expression of FOXP3 mRNA [68], but whether seminal fluid exposure can alter endometrial Treg cell populations is unknown.
In summary, this study shows that exposure to seminal fluid at mating is necessary and sufficient for conferring maximal immune tolerance at the time of embryo implantation in mice. Seminal fluid appears to act through providing antigen and cytokine signals to further expand populations of Treg cells potentiated by estrogen at the time of ovulation. This finding reinforces a function for seminal fluid beyond simply providing a vehicle to deliver sperm for fertilization, and adds further complexity to its emerging role in signaling physiological changes that prepare the female reproductive tract for embryo implantation [45]. A better understanding of the key antigens and immune-deviating components of seminal fluid and their mode of action in the female will be of utility in designing new therapeutic agents for treating infertility and subfertility in women, and for promoting reproductive competence in animals.
Acknowledgments
We thank Vicki Mau, Leonie Hicks, Leanne Srpek, and Camilla Dorian for their technical assistance.
Footnotes
1Supported by the National Health and Medical Research Council Australia project and fellowship grants.
REFERENCES
- Trowsdale J, Betz AG.Mother's little helpers: mechanisms of maternal-fetal tolerance. Nat Immunol 2006; 7: 241–246. [DOI] [PubMed] [Google Scholar]
- Guleria I, Sayegh MH.Maternal acceptance of the fetus: true human tolerance. J Immunol 2007; 178: 3345–3351. [DOI] [PubMed] [Google Scholar]
- Tafuri A, Alferink J, Moller P, Hammerling GJ, Arnold B.T cell awareness of paternal alloantigens during pregnancy. Science 1995; 270: 630–633. [DOI] [PubMed] [Google Scholar]
- Jiang SP, Vacchio MS.Multiple mechanisms of peripheral T cell tolerance to the fetal “allograft.” J Immunol 1998; 160: 3086–3090. [PubMed] [Google Scholar]
- Zhou M, Mellor AL.Expanded cohorts of maternal CD8+ T-cells specific for paternal MHC class I accumulate during pregnancy. J Reprod Immunol 1998; 40: 47–62. [DOI] [PubMed] [Google Scholar]
- Aluvihare VR, Kallikourdis M, Betz AG.Regulatory T cells mediate maternal tolerance to the fetus. Nat Immunol 2004; 5: 266–271. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S.Regulatory T cells: key controllers of immunologic self-tolerance. Cell 2000; 101: 455–458. [DOI] [PubMed] [Google Scholar]
- Shevach EM.CD4+ CD25+ suppressor T cells: more questions than answers. Nat Rev Immunol 2002; 2: 389–400. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M.Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol 1995; 155: 1151–1164. [PubMed] [Google Scholar]
- Fontenot JD, Gavin MA, Rudensky AY.Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol 2003; 4: 330–336. [DOI] [PubMed] [Google Scholar]
- Samy ET, Setiady YY, Ohno K, Pramoonjago P, Sharp C, Tung KS.The role of physiological self-antigen in the acquisition and maintenance of regulatory T-cell function. Immunol Rev 2006; 212: 170–184. [DOI] [PubMed] [Google Scholar]
- Aluvihare VR, Betz AG.The role of regulatory T cells in alloantigen tolerance. Immunol Rev 2006; 212: 330–343. [DOI] [PubMed] [Google Scholar]
- Guerin LR, Prins JR, Robertson SA.Regulatory T cells and immune tolerance in pregnancy: a new target for infertility treatment? Hum Reprod Update 2009; (in press). published online ahead of print 11 March 2009; doi 10.1093/humupd/dmp004. [DOI] [PMC free article] [PubMed]
- Erlebacher A, Vencato D, Price KA, Zhang D, Glimcher LH.Constraints in antigen presentation severely restrict T cell recognition of the allogeneic fetus. J Clin Invest 2007; 117: 1399–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polanczyk MJ, Carson BD, Subramanian S, Afentoulis M, Vandenbark AA, Ziegler SF, Offner H.Cutting edge: estrogen drives expansion of the CD4+CD25+ regulatory T cell compartment. J Immunol 2004; 173: 2227–2230. [DOI] [PubMed] [Google Scholar]
- Zhao JX, Zeng YY, Liu Y.Fetal alloantigen is responsible for the expansion of the CD4(+)CD25(+) regulatory T cell pool during pregnancy. J Reprod Immunol 2007; 75: 71–81. [DOI] [PubMed] [Google Scholar]
- Kallikourdis M, Andersen KG, Welch KA, Betz AG.Alloantigen-enhanced accumulation of CCR5+ ‘effector' regulatory T cells in the gravid uterus. Proc Natl Acad Sci U S A 2007; 104: 594–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson SA, Sharkey DJ.The role of semen in induction of maternal immune tolerance to pregnancy. Semin Immunol 2001; 13: 243–254. [DOI] [PubMed] [Google Scholar]
- Zenclussen AC, Gerlof K, Zenclussen ML, Sollwedel A, Bertoja AZ, Ritter T, Kotsch K, Leber J, Volk HD.Abnormal T-cell reactivity against paternal antigens in spontaneous abortion: adoptive transfer of pregnancy-induced CD4+CD25+ T regulatory cells prevents fetal rejection in a murine abortion model. Am J Pathol 2005; 166: 811–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson SA, Mau VJ, Tremellen KP, Seamark RF.Role of high molecular weight seminal vesicle proteins in eliciting the uterine inflammatory response to semen in mice. J Reprod Fertil 1996; 107: 265–277. [DOI] [PubMed] [Google Scholar]
- Dent LA. Immune responses to a metastasizing murine fibrosarcoma. Adelaide, Australia:: Flinders University of South Australia;; 1987. PhD thesis. [Google Scholar]
- Sugiura K, Stock CC.Studies in a tumor spectrum. III. The effect of phosphoramides on the growth of a variety of mouse and rat tumors. Cancer Res 1955; 15: 38–51. [PubMed] [Google Scholar]
- Johansson M, Bromfield JJ, Jasper MJ, Robertson SA.Semen activates the female immune response during early pregnancy in mice. Immunology 2004; 112: 290–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang X, Gao JX, Jiang Q, Wen J, Seifers N, Su L, Godfrey VL, Zuo T, Zheng P, Liu Y.The Scurfy mutation of FoxP3 in the thymus stroma leads to defective thymopoiesis. J Exp Med 2005; 202: 1141–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beer AE, Billingham RE.Host responses to intra-uterine tissue, cellular and fetal allografts. J Reprod Fertil Suppl 1974; 21: 59–88. [Google Scholar]
- Piazzon I, Matusevich M, Deroche A, Nepomnaschy I, Pasqualini CD.Early increase in graft-versus-host reactivity during pregnancy in the mouse. J Reprod Immunol 1985; 8: 129–137. [DOI] [PubMed] [Google Scholar]
- Hancock RJ, Faruki S.Assessment of immune responses to H-Y antigen in naturally inseminated and sperm-injected mice using cell-mediated cytotoxicity assays. J Reprod Immunol 1986; 9: 187–194. [DOI] [PubMed] [Google Scholar]
- Robertson SA, Mau VJ, Hudson SA, Tremellen KP.Cytokine-leukocyte networks and the establishment of pregnancy. Am J Reprod Immunol 1997; 37: 438–442. [DOI] [PubMed] [Google Scholar]
- Smith RN, Powell AE.The adoptive transfer of pregnancy-induced unresponsiveness to male skin grafts with thymus-dependent cells. J Exp Med 1977; 146: 899–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darrasse-Jeze G, Klatzmann D, Charlotte F, Salomon BL, Cohen JL.CD4+CD25+ regulatory/suppressor T cells prevent allogeneic fetus rejection in mice. Immunol Lett 2006; 102: 106–109. [DOI] [PubMed] [Google Scholar]
- Thaler CJ.Immunological role for seminal plasma in insemination and pregnancy. Am J Reprod Immunol 1989; 21: 147–150. [DOI] [PubMed] [Google Scholar]
- Hutter H, Dohr G.HLA expression on immature and mature human germ cells. J Reprod Immunol 1998; 38: 101–122. [DOI] [PubMed] [Google Scholar]
- Fernandez N, Cooper J, Sprinks M, AbdElrahman M, Fiszer D, Kurpisz M, Dealtry G.A critical review of the role of the major histocompatibility complex in fertilization, preimplantation development and feto-maternal interactions. Hum Reprod Update 1999; 5: 234–248. [DOI] [PubMed] [Google Scholar]
- Redline RW, Lu CY.Localization of fetal major histocompatibility complex antigens and maternal leukocytes in murine placenta. Implications for maternal-fetal immunological relationship. Lab Invest 1989; 61: 27–36. [PubMed] [Google Scholar]
- Jaffe L, Robertson EJ, Bikoff EK.Distinct patterns of expression of MHC class I and beta 2-microglobulin transcripts at early stages of mouse development. J Immunol 1991; 147: 2740–2749. [PubMed] [Google Scholar]
- Alexander NJ, Anderson DJ.Immunology of semen. Fertil Steril 1987; 47: 192–204. [DOI] [PubMed] [Google Scholar]
- Martin-Villa JM, Longas J, Arnaiz-Villena A.Cyclic expression of HLA class I and II molecules on the surface of purified human spermatozoa and their control by serum inhibin B levels. Biol Reprod 1999; 61: 1381–1386. [DOI] [PubMed] [Google Scholar]
- Koelman CA, Coumans ABC, Nijman HW, Doxiadis IIN, Dekker GA, Claas FHJ.Correlation between oral sex and a low incidence of preeclampsia: a role for soluble HLA in seminal fluid? J Reprod Immunol 2000; 46: 155–166. [DOI] [PubMed] [Google Scholar]
- Shetty J, Bronson RA, Herr JC.Human sperm protein encyclopedia and alloantigen index: mining novel allo-antigens using sera from ASA-positive infertile patients and vasectomized men. J Reprod Immunol 2007; 77: 23–31. [DOI] [PubMed] [Google Scholar]
- Tai P, Wang J, Jin H, Song X, Yan J, Kang Y, Zhao L, An X, Du X, Chen X, Wang S, Xia G, et al. Induction of regulatory T cells by physiological level estrogen. J Cell Physiol 2008; 214: 456–464. [DOI] [PubMed] [Google Scholar]
- Gangnuss S, Sutton-McDowall ML, Robertson SA, Armstrong DT.Seminal plasma regulates corpora lutea macrophage populations during early pregnancy in mice. Biol Reprod 2004; 71: 1135–1141. [DOI] [PubMed] [Google Scholar]
- McMaster MT, Newton RC, Dey SK, Andrews GK.Activation and distribution of inflammatory cells in the mouse uterus during the preimplantation period. J Immunol 1992; 148: 1699–1705. [PubMed] [Google Scholar]
- Robertson SA, Allanson M, Mau VJ.Molecular regulation of uterine leukocyte recruitment during early pregnancy in the mouse. Troph Research 1998; 11: 101–120. [Google Scholar]
- Blois SM, Alba Soto CD, Tometten M, Klapp BF, Margni RA, Arck PC.Lineage, maturity, and phenotype of uterine murine dendritic cells throughout gestation indicate a protective role in maintaining pregnancy. Biol Reprod 2004; 70: 1018–1023. [DOI] [PubMed] [Google Scholar]
- Robertson SA.Seminal plasma and male factor signalling in the female reproductive tract. Cell Tissue Res 2005; 322: 43–52. [DOI] [PubMed] [Google Scholar]
- Blois SM, Kammerer U, Alba Soto C, Tometten MC, Shaikly V, Barrientos G, Jurd R, Rukavina D, Thomson AW, Klapp BF, Fernandez N, Arck PC.Dendritic cells: key to fetal tolerance? Biol Reprod 2007; 77: 590–598. [DOI] [PubMed] [Google Scholar]
- Robertson SA.Control of the immunological environment of the uterus. Rev Reprod 2000; 5: 164–174. [DOI] [PubMed] [Google Scholar]
- Kelly RW, Critchley HO.Immunomodulation by human seminal plasma: a benefit for spermatozoon and pathogen? Hum Reprod 1997; 12: 2200–2207. [DOI] [PubMed] [Google Scholar]
- Robertson SA, Ingman WV, O'Leary S, Sharkey DJ, Tremellen KP.Transforming growth factor beta-a mediator of immune deviation in seminal plasma. J Reprod Immunol 2002; 57: 109–128. [DOI] [PubMed] [Google Scholar]
- Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM.Conversion of peripheral CD4+CD25-naïve T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med 2003; 198: 1875–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiringhelli F, Puig PE, Roux S, Parcellier A, Schmitt E, Solary E, Kroemer G, Martin F, Chauffert B, Zitvogel L.Tumor cells convert immature myeloid dendritic cells into TGF-beta-secreting cells inducing CD4+CD25+ regulatory T cell proliferation. J Exp Med 2005; 202: 919–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baratelli F, Lin Y, Zhu L, Yang SC, Heuze-Vourc'h N, Zeng G, Reckamp K, Dohadwala M, Sharma S, Dubinett SM.Prostaglandin E2 induces FOXP3 gene expression and T regulatory cell function in human CD4+ T cells. J Immunol 2005; 175: 1483–1490. [DOI] [PubMed] [Google Scholar]
- Moldenhauer LM, Hayball JD, Robertson SA.Conceptus antigens activate the maternal immune response in pregnancy utilising maternal antigen presenting cells. J Reprod Immunol 2006; 71: 148.(abstract). [Google Scholar]
- Thompson JG, Kind KL, Roberts CT, Robertson SA, Robinson JS.Epigenetic risks related to assisted reproductive technologies: short- and long-term consequences for the health of children conceived through assisted reproduction technology: more reason for caution? Hum Reprod 2002; 17: 2783–2786. [DOI] [PubMed] [Google Scholar]
- Robertson SA.Seminal fluid signaling in the female reproductive tract: lessons from rodents and pigs. J Anim Sci 2007; 85: E36–E44. [DOI] [PubMed] [Google Scholar]
- Watson JG, Carroll J, Chaykin S.Reproduction in mice: the fate of spermatozoa not involved in fertilization. Gamete Res 1983; 7: 75–84. [Google Scholar]
- Bromfield JJ, Roberts CT, Robertson SA.Seminal plasma programs uterine receptivity and pregnancy outcome. Abstracts of the 37th Annual Meeting of the Society for the Study of Reproduction, August 1–4, 2004, Vancouver, BC, Canada.Biol Reprod 2004; special issue:Abstract 7. [Google Scholar]
- Carp HJ, Serr DM, Mashiach S, Nebel L.Influence of insemination on the implantation of transferred rat blastocysts. Gynecol Obstet Invest 1984; 18: 194–198. [DOI] [PubMed] [Google Scholar]
- Sharkey DJ, Macpherson AM, Tremellen KP, Robertson SA.Seminal plasma differentially regulates inflammatory cytokine gene expression in human cervical and vaginal epithelial cells. Mol Hum Reprod 2007; 13: 491–501. [DOI] [PubMed] [Google Scholar]
- Bellinge BS, Copeland CM, Thomas TD, Mazzucchelli RE, O'Neil G, Cohen MJ.The influence of patient insemination on the implantation rate in an in vitro fertilization and embryo transfer program. Fertil Steril 1986; 46: 2523–2526. [DOI] [PubMed] [Google Scholar]
- Tremellen KP, Valbuena D, Landeras J, Ballesteros A, Martinez J, Mendoza S, Norman RJ, Robertson SA, Simon C.The effect of intercourse on pregnancy rates during assisted human reproduction. Hum Reprod 2000; 15: 2653–2658. [DOI] [PubMed] [Google Scholar]
- Coulam CB, Stern JJ.Effect of seminal plasma on implantation rates. Early Pregnancy 1995; 1: 33–36. [PubMed] [Google Scholar]
- Saito S, Sakai M.Th1/Th2 balance in preeclampsia. J Reprod Immunol 2003; 59: 161–173. [DOI] [PubMed] [Google Scholar]
- Sasaki Y, Darmochwal-Kolarz D, Suzuki D, Sakai M, Ito M, Shima T, Shiozaki A, Rolinski J, Saito S.Proportion of peripheral blood and decidual CD4(+) CD25(bright) regulatory T cells in pre-eclampsia. Clin Exp Immunol 2007; 149: 139–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klonoff-Cohen HS, Savitz DA, Celafo RC, McCann MF.An epidemiologic study of contraception and preeclampsia. JAMA 1989; 262: 3143–3147. [PubMed] [Google Scholar]
- Robillard PY, Hulsey TC, Perianin J, Janky E, Miri EH, Papiernik E.Association of pregnancy-induced hypertension with duration of sexual cohabitation before conception. Lancet 1995; 344: 973–975. [DOI] [PubMed] [Google Scholar]
- Salha O, Sharma V, Dada T, Nugent D, Rutherford AJ, Tomlinson AJ, Philips S, Allgar V, Walker JJ.The influence of donated gametes on the incidence of hypertensive disorders of pregnancy. Hum Reprod 1999; 14: 2268–2273. [DOI] [PubMed] [Google Scholar]
- Jasper MJ, Tremellen KP, Robertson SA.Primary unexplained infertility is associated with reduced expression of the T-regulatory cell transcription factor Foxp3 in endometrial tissue. Mol Hum Reprod 2006; 12: 301–308. [DOI] [PubMed] [Google Scholar]