Abstract
Interferon gamma (IFNG) is a proinflammatory cytokine secreted in the uterus during early pregnancy. It is abundantly produced by uterine natural killer cells in maternal endometrium but also by trophoblasts in some species. In normal pregnancies of mice, IFNG plays critical roles that include initiation of endometrial vasculature remodeling, angiogenesis at implantation sites, and maintenance of the decidual (maternal) component of the placenta. In livestock and in humans, deviations in these processes are thought to contribute to serious gestational complications, such as fetal loss or preeclampsia. Interferon gamma has broader roles in activation of innate and adaptive immune responses to viruses and tumors, in part through upregulating transcription of genes involved in cell cycle regulation, apoptosis, and antigen processing/presentation. Despite this, rodent and human trophoblast cells show dampened responses to IFNG that reflect the resistance of these cells to IFNG-mediated activation of major histocompatibility complex (MHC) class II transplantation antigen expression. Lack of MHC class II antigens on trophoblasts is thought to facilitate survival of the semiallogeneic conceptus in the presence of maternal lymphocytes. This review describes the dynamic roles of IFNG in successful pregnancy and briefly summarizes data on IFNG in gestational pathologies.
Keywords: angiogenesis, cytokines, dendritic cells, female reproductive tract, immunology, interferon regulatory factor 1, interferon types 1 and 2, interleukin 12, interleukin 15, interleukin 18, major histocompatibility complex, mouse, pig, pregnancy, signal transducer and activator of transcription 1, trophoblast, uterine natural killer cell, uterus
Interferon gamma plays a dynamic role in pregnancy, and its dysregulation can lead to disease.
INTRODUCTION
IFNG was among the first cytokines identified [1]. It is well characterized genetically, structurally, and functionally in many species [2, 3]. IFNG plays important roles in diverse cellular processes, including activating innate and adaptive immune responses, inhibiting cell proliferation, and inducing apoptosis [4, 5]. It is also crucial in immune responses against pathogens and immunosurveillance of tumors [4, 6, 7]. These functions are achieved via receptor-mediated signals that lead to changes in the transcription of hundreds of genes [8]. Because the initial descriptions of distinct cytokine-producing lymphocyte subsets used IFNG as the hallmark molecule for type 1 immune responses, and because the type 1/type 2 cytokine paradigm was relevant to pregnancy both systemically [9] and at the maternal-fetal interface [10, 11], IFNG is routinely assayed in pregnancy research to establish deviations from normal, healthy gestation.
IFNG is produced primarily by natural killer cells (NK cells) and thymus-derived natural killer T cells (NKT cells), which are the major lymphoid effector cells of innate immunity. Natural killer T cells are distinguished from NK cells by somatic rearrangement of their T-cell receptor genes. The IFNG locus in NK cells has an undermethylated open reading configuration. This permits NK cells to rapidly respond to their external milieu and induce IFNG production independently of cell division or their stage of the cell cycle [12]. Activation of IFNG production in innate immune cells is usually preceded by signaling from type 1 (alpha/beta) IFN [13] and involves interactions with dendritic cells (DCs) [14]. Natural killer and NKT cells increase numerically at implantation sites early in gestation in mice, humans, and other species [15]. Other immune cells, such as T-helper 1 (Th1) cells, can be induced to synthesize and secrete IFNG. However, debate continues regarding whether myeloid lineage-derived cells (i.e., neutrophils, DCs, and macrophages) produce rather than take up and store IFNG in vivo [16]. Induction of IFNG production in T cells and activation of adaptive immune responses by IFNG require cell proliferation and are due, in part, to transcriptional induction of genes encoding proteins involved in antigen processing and presentation. These include major histocompatibility complex (MHC) class I and class II antigens [4]. IFNG-induced apoptosis and cell cycle arrest occur through activation of caspase (CASP) and cyclin-dependent kinase inhibitor 1A (CDKN1A) gene expression, respectively [4, 5].
In many species, trophoblast cells as well as endometrial cells synthesize one or more IFNs. In mice, type 1 IFNs are expressed from the two-cell stage and in the preimplantation trophoectoderm of the blastocyst. It is induced in the early decidua and remains cytoplasmic in the trophectodermal lineages throughout placental development [17]. By midpregnancy, IFNG is detected in trophoblast giant cells [18, 19]. IFNG receptor 1 (IFNGR1) and IFNGR2 are found on mouse oocytes and preimplantation embryos. At midgestation, Ifngr1 and Ifngr2 are expressed by labyrinthine and spongiotrophoblasts but are not expressed by trophoblast giant cells that appose maternal decidua [20, 21]. In pigs, IFNG is the major trophoblast-derived IFN. It is synthesized between Days 12 and 20 of the 114-day gestation period. Because the early porcine trophoblast has absent or weak IFNGR expression, IFNG is thought to primarily affect maternal cells and tissues. Indeed, porcine trophoblast IFNG modifies tight junctions in maternal epithelium and elevates uterine endothelial cell expression of MHC class II genes during blastocyst attachment [22]. The absence of IFNG production by horse and ruminant trophoblasts indicates that IFNG synthesis is not a universal feature in species having epitheliochorial placentation [23–25]. Ruminant trophoblast IFN-tau (IFNT) sustains ovarian corpora lutea, but porcine trophoblast IFNs (gamma and delta at 3:1) do not [24, 25]. Early in human pregnancy, trophoblast cells express IFNG intensely, but there is almost no expression by term [26]. In contrast, both known IFNG receptors, IFNGR1 and IFNGR2, are expressed by human trophoblast cells throughout pregnancy [27]. It is not yet known whether human trophoblast-derived IFN contributes to corpus luteum maintenance in vivo. Here, we briefly review the molecular signaling pathway of IFNG and its role in normal pregnancy, with emphasis on work conducted by the authors.
IFNG RECEPTORS AND SIGNALING PATHWAYS
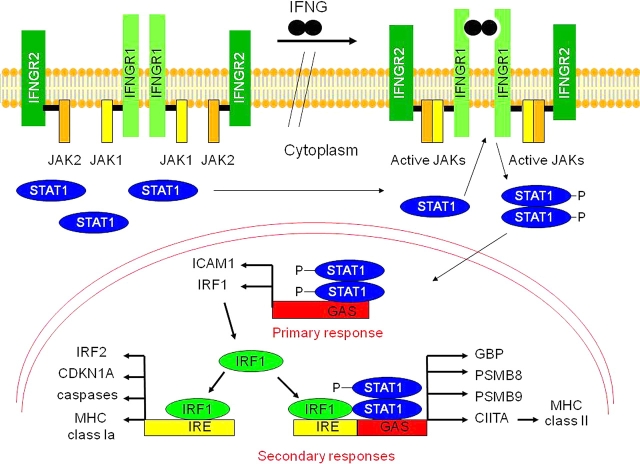
IFNG-mediated activation of gene transcription occurs primarily through the Janus kinase/signal transducer and activator of transcription-1 (JAK/STAT1) pathway (Fig. 1) [4, 5]. The IFNG receptor, IFNGR, is a cell surface receptor composed of two distinct chains, R1 (or alpha) and R2 (beta), encoded by different genes. Binding of IFNG to IFNGR activates receptor-associated JAK1 and JAK2, which subsequently phosphorylate the intracellular domain of IFNGR1 [4, 5]. The phosphorylated IFNGR1 provides a docking site for cytoplasmically localized monomers of the transcription factor STAT1 that subsequently are phosphorylated on tyrosine residue 701 by JAK1 and JAK2 [5]. Once STAT1 is phosphorylated, it homodimerizes, translocates to the nucleus, and activates the transcription of multiple genes containing an IFNG activating sequence (GAS) in their promoters. These genes include those encoding the transcription factor interferon regulatory factor 1 (IRF1) and the intracellular adhesion molecule-1 (ICAM1) [4, 5]. IRF1 directly activates transcription of the caspase genes involved in promoting apoptosis, the CDKN1A gene that inhibits cell growth, the genes encoding class Ia antigens of the MHC, transporters associated with antigen processing 1 (TAP1), TAP2, and the immunoproteasome subunits proteasome subunit beta type 8 (PSMB8), PSMB9, and PSMB10. The MHC and antigen-processing molecules are required for adaptive immune responses to pathogens and tumors [4]. Furthermore, STAT1 and IRF1 cooperate with the ubiquitously expressed transacting factor upstream stimulatory factor 1 (USF1) to activate transcription from the IFNG-inducible class II transactivator (CIITA) promoter IV [28]. CIITA subsequently activates transcription of the MHC class II genes [28].
FIG. 1.
Schematic of IFNG signaling through the JAK/STAT1 pathway. Binding of IFNG to its receptor results in activation of the JAKs, which phosphorylate cytoplasmic monomers of STAT1. Phosphorylated STAT1 dimerizes, translocates to the nucleus, and activates transcription from gene promoters containing GAS (primary responses), such as IRF1. In the secondary responses, IRF1 activates transcription of IRF2, CDKN1A, caspases, and the MHC class Ia genes by binding IFN response elements (IRE), whereas STAT1 and IRF1 cooperate to transactivate expression of other genes, such as guanylate binding protein (GBP), PSMB8, PSMB9, and CIITA. P, phosphorylated.
Cellular responses to IFNG are subject to negative control by protein tyrosine phosphatases (PTPs), suppressors of cytokine signaling-1 (SOCS1), and protein inhibitor of activated STAT (PIAS) [29]. SOCS1 downregulates IFNG-regulated responses either by inhibiting JAK2 kinase activity and/or by promoting JAK2 degradation [29]. PIAS1 directly inhibits STAT1 DNA-binding activity, and thereby blocks IFNG-inducible transcription. The mechanism by which PIAS inhibits STAT1 transactivation is not yet clear [29], but it is considered important in the downregulation of human uterine epithelial cell mucin 1 expression during blastocyst implantation [30]. The equilibrium between the activities of kinases, such as the JAKs and PTP family members, is important in regulating responsiveness to multiple cytokines, including IFNG, and to growth factors and hormones [29, 31]. In the absence of cytokines (i.e., IFNG), PTPs are constitutively active and are thought to prevent aberrant activation of JAK1 and JAK2, and thus generation of cytokine signals. Following exposure of responsive cells to IFNG, PTPs are transiently inactivated, JAK1 and JAK2 are activated, STATs are phosphorylated, and target gene expression is activated. SOCS1 is synthesized in response to IFNG, and the PTPs and SOCS1 subsequently attenuate cytokine responses by inactivating the JAK and/or STAT molecules [29, 31].
IFNG IN MOUSE GESTATIONAL ENDOMETRIUM
Decidualization of the mouse uterus is initiated antimesometrially, with implantation of hatched blastocysts at Gestation Day (GD) 4. Decidualization spreads laterally, then mesometrially. Coincident with mesometrial decidualization, a specialized lymphocyte subset with distinctive regional localization, the uterine NK (uNK) cell, appears in the decidua basalis [32]. This lineage is first distinguished at GD 5.5, when uNK cells begin to display carbohydrate modifications recognized by the lectin Dolichos biflorus agglutinin (DBA) [33] (Fig. 2, A and B). Uterine NK cells are highly proliferative, and from GDs 8–11 they divide in a lymphocyte-enriched myometrial region at the base of each placenta, the mesometrial lymphoid aggregate of pregnancy (MLAp; Fig. 2C). The uNK cells then move as postmitotic cells into the decidua basalis in very large numbers. Uterine NK cells in the MLAp surround the uterine artery branches entering into implantation sites. A pronounced vascular association (intravascular and intramural) is also seen for uNK cells in the decidua basalis (Fig. 2D). Uterine NK cells occur infrequently within the placenta [32], and in all locations their numbers decline after midgestation (i.e., GDs 10–12 in mice) [15].
FIG. 2.
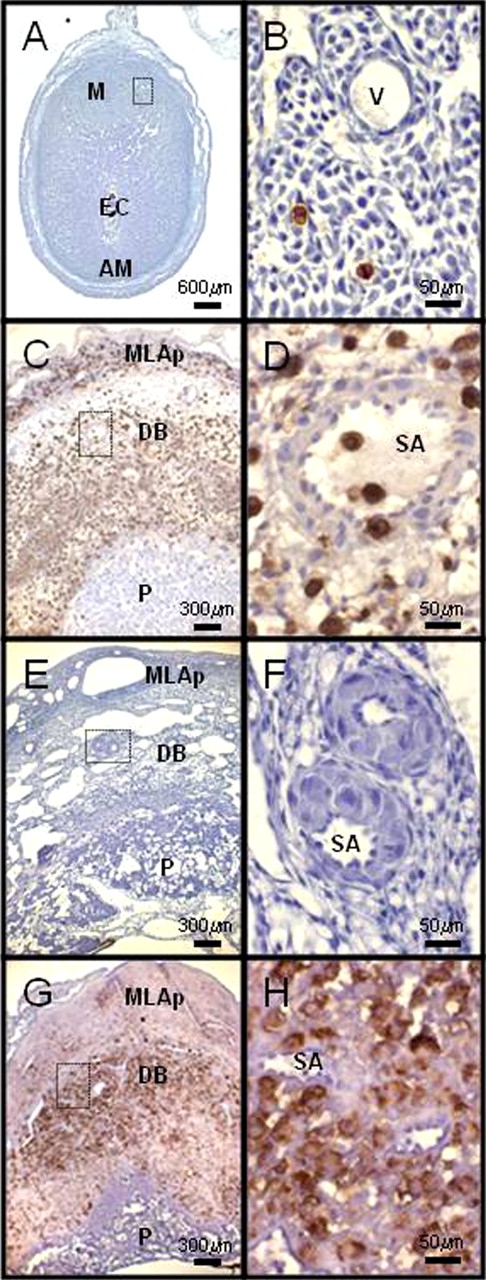
Photomicrographs of hematoxylin-stained, midsagittal sections of implantation sites from mice with different genetic mutations. DBA lectin reactivity was used to distinguish uNK cells (brown-red). All images have the uterine artery and mesometrium to the top. A, B) C57BL/6J mouse at GD 6. C, D) C57BL/6J mouse at GD 10. E, F) Alymphoid (NK−T−B−) mouse doubly knocked out for recombinase-activating gene-2 and common cytokine chain gamma, at GD 10. G, H) Interferon receptor 1 (binds IFNA and IFNB) knockout mouse at GD 10. The left column shows low-power images. The right column shows enlarged images from the areas boxed in the left panels. M, mesometrial region; AM, antimesometrial; EC, ectoplacental cone; and V, vessel refer to structures in the preplacental interval. MLAp, mesometrial lymphoid aggregate of pregnancy; DB, decidua basalis; P, placenta; and SA, spiral artery refer to structures after placental development.
Rodent uNK cells have a striking morphological appearance that was characterized by early histologists [32]. The cell's features are enormous size (40–100 μm in diameter), large numbers of membrane-bound cytoplasmic granules, an eccentric nucleus and, ultrastructurally, numerous protein-synthesizing organelles [32]. Between 1985 and 1995, immunohistochemical, gene knockout and bone marrow reconstitution approaches established that the transient, morphologically distinct, pregnancy-associated cells—then called “granulated metrial gland cells”—were NK lineage lymphocytes [15, 34]. Implantation sites in alymphoid mice do not show gestational adaptation of the spiral arteries that feed into each placenta and have an edematous, underdeveloped decidua (Fig. 2, E and F) [15, 34]. Specific progenitors of uNK cells are not yet described. It is known that endometrial decidualization rather than the presence of an embryo triggers mouse uNK cell differentiation and that uNK cell life spans are shorter in the absence of embryos [32, 35]. Uterine NK cell differentiation also occurs in genetically alymphoid mice after adoptive transfer of cells from any lymphoid organ of a nonpregnant, genetically normal donor mouse, or a T-lymphocyte and B-lymphocyte-deficient donor mouse. This ubiquitous presence of progenitor cells suggests there may not be a unique progenitor for NK cells of the uterus [36]. Eutopically transplanted uterine segments from similar donors decidualize when recipients are mated. Decidualized grafts in alymphoid recipients (NK−, T−, B−) do not contain uNK cells, whereas those in severe combined immunodeficient (NK+, T−, B−) and normal (NK+, T+, B+) hosts differentiate high numbers of uNK cells. This finding again suggests that most uNK progenitor cells do not reside in the uterus but home to decidua from the circulation [36].
Because NK cells in other sites are major sources of IFNG [12, 14], we measured IFNG in homogenates of freshly dissected endometrial subregions by ELISA and compared concentrations with those in homogenates of virgin mouse mesometrial tissue. Matched tissues from Ifng null mice were also used. IFNG was not detected in any specimen from Ifng null mice and was negligible in virgin uteri of normal outbred and inbred strains. Endometrial IFNG was detected in samples from normal mice at GD 6.5, and concentrations rose daily to achieve a 4- to 6-fold increase that peaked at GD 10 and then declined. This pattern matches the time course for expansion and decline in uNK cell numbers in pregnant mouse uterus. To establish whether uNK cells were the only mesometrial, IFNG-producing mouse cells in early to mid pregnancy, the study was repeated with a mouse strain having 1% of normal uNK cell numbers. In these mice, pregnancy induced a slight elevation in IFNG that was static from GD 6 to GD 16 [37]. We concluded that only a small proportion of endometrial IFNG came from sources other than uNK cells.
Implantation sites from Ifng null and Ifngr1 null mice differ from those in both normal and in uNK cell-deficient mice. Implantation sites of Ifng null and Ifngr1 null mice contain excessive numbers of uNK cells, most of which are very small (i.e., incompletely differentiated). Spiral artery modification did not occur, and widespread decidual necrosis was evident [37]. Lymphocyte-based production of IFNG was shown to be essential for induction of normal, pregnancy-associated structural changes in spiral arteries by adoptive transfer of bone marrow. Marrow transferred from Ifngr1 null mice (which produces IFNG) but not from Ifng null mice into alymphoid recipients before mating allowed normal arterial changes to occur during pregnancy [38]. Because IFNA and IFNB from macrophages or DCs are key regulators of NK activation and subsequent IFNG production in other tissues [39], we examined midgestation implantation sites from Ifnar1 null mice (Fig. 2, G and H). These implantation sites resemble those from Ifng null mice, suggesting that the type 1 IFN activation pathway is critical in early decidua and merits further study. A recent gene expression profile study that compared early decidua and deciduomata of pseudopregnancy in normal mice revealed that the presence of an embryo significantly altered expression of many type 1 IFN-regulated genes and elevated uNK cell numbers 7.3-fold [40]. From these findings, we expect future research will focus on physiological activation of antigen-presenting cells found in mouse MLAp and decidua basalis [41] and how their production of type 1 IFNs contributes to the downstream events of decidual maturation and uterine angiogenesis mediated through uNK cell-derived IFNG. This is consistent with previous in vitro studies that found cultures of cells from mouse deciduomata were more responsive to type 1 IFNs than to IFNG [42]. The observation that almost 1 in 5 of the 15 000 genes compared between mouse decidua and deciduoma were differentially expressed emphasizes the complexity of the maternal changes that accompany implantation success and the interdependence of the signaling networks to which IFNG contributes [40].
Treatment of pregnant alymphoid mice (GDs 6–11) with mouse recombinant IFNG induces normal morphology in their decidua and spiral arteries, supporting the centrality of physiological levels of IFNG in decidual development and gestational arterial remodeling in mice [38]. The proinflammatory cytokines interleukin 12 (IL12), IL15, and IL18 induce IFNG production by NK cells [43]. We addressed roles for these cytokines in pregnancy by examining implantation sites from mice genetically ablated for these Il genes. The NK cells were completely absent in Il15 null implantation sites [44], but neither IFNG synthesis nor uNK cell numbers were significantly reduced in Il12/Il18 double null implantation sites [45]. Thus, some but not all findings concerning uNK cells parallel findings for NK cells in extrauterine tissues. Other differences between mouse peripheral NK cells and uNK cells include the latter's use of Eomes (eomesodermin homolog) rather than Tbx21 (T-box 21) to regulate Ifng transcription [46] and the high levels of EFNB2 (Ephrin B2) expressed by uterine but not splenic NK cells. EFNB2 is a molecule that regulates cell migration and identifies arterial endothelium [47]. Expression of this molecule by uNK cells is thought to control their association with arteries rather than other vessel types within decidua.
IFNG AT THE PORCINE FETAL-MATERNAL INTERFACE
The discovery of abundant IFN production by trophoblast cells of livestock was of great interest and importance. Ruminant IFNT, the first to be described, had strong, classical antiviral activity. However, IFNT had another major action. It was hormonelike and participated in maternal recognition of pregnancy through its maintenance of corpora lutea. Thus, IFNT supported progesterone production and the continuation of pregnancy [24, 25]. Noninvasive porcine trophoblasts produce copious amounts of two IFNs over the interval of blastocyst attachment to the endometrium (i.e., GDs 12–20) but, in contrast to ruminants, IFNG is the predominant type. It is accompanied by trophoblast synthesis of a novel type 1 IFN, IFN-delta, at lower concentrations [22]. Both IFNs are localized to the apical side of porcine trophoblast cells. Porcine IFNG production was localized to the extraembryonic trophoectoderm. All other constituents of the fetal membranes and the embryo proper were unreactive [48]. IFN-delta was distributed evenly in all trophoblast cells, suggesting these two IFNs have different secretory pathways [49]. Although porcine lymphocytes produce only one IFNG mRNA (1.4 kb), porcine trophoblast cells synthesize 1.3- and 1.4-kb transcripts through use of both TATA box (Goldberg-Hogness box) transcription initiation sites in the porcine IFNG promoter [50].
Antivirally active IFNG in flushings from a single porcine uterine horn peak at approximately GD 16 at 250 μg, but its physiological functions remain speculative. Porcine trophoblastic IFNs do not maintain corpora lutea. Because IFNGR is not or is only weakly expressed by porcine trophoblasts between GDs 12 and 20, the postulated major IFNG target is the uterus [51]. To address whether endometrium was the target for porcine trophoblast IFNG, immunohistochemistry was undertaken at periattachment GD 15. IFNG was localized to the luminal epithelium and to the stroma underlying areas adjacent to attaching trophoblasts. Blood vessels and uterine gland epithelia were relatively negative [22]. This suggested trophoblastic IFNG had a role in remodeling or depolarizing uterine epithelium during blastocyst attachment [22]. To strengthen this hypothesis, the inducible class II MHC antigen response of endometrial stroma to IFNG was assessed by immunostaining. In Day 15 cycling gilts (first-estrus pigs), endometrial stroma did not display MHC II expression whereas at GD 15, MHC II expression was intense in the endometrial stroma, particularly in vascular endothelial cells [22].
In vitro studies were conducted to determine whether porcine conceptus secretions acted on endometrial explants from pigs and cows. The secretions were collected from flushed blastocysts over several days of culture and were applied to endometrial explants, which were then evaluated for induced nucleotide uptake using radiolabeled ATP. No uptake of radiolabeled nucleotide was stimulated in the pig explants, although the porcine conceptus secretions enhanced isotope uptake in bovine endometrial explants at levels equivalent to IFNT or recombinant type 1 bovine IFN [52]. In vivo studies, however, support a paracrine hypothesis. Using continuous intrauterine minipump infusions of porcine conceptus secretions to ovariectomized, hormone-supported pigs, endometrial changes in expression of a number of genes known to be responsive to IFNs have been demonstrated. Of note, these trophoblast products, which were mixed but would be predominantly IFNG, induced IRF1 but not IRF2, STAT1, or class I MHC molecules in the stroma but not luminal epithelium of treated animals [53–55].
These data are consistent with a conclusion that despite the short biological half-life of IFNs, porcine trophoblastic IFNG crosses the endometrial epithelium without modification and acts on the endometrial stroma to promote successful conceptus attachment. Until recently, porcine endometrial IFNG has not been considered a product of endometrial lymphocytes [22]. However, as in other mammals, early porcine pregnancy enriches the endometrium in NK cells, posing an alternate or supplementary explanation to the hypothesis of paracrine conceptus secretion effects. A 2- to 3-fold enrichment of porcine uNK cells occurs between GDs 15 and 28, coincident with peak uterine IFNG secretion (GDs 15–16). Porcine uNK cell recruitment is distinct from that in humans and mice because it requires conceptus-mediated signals [56]. To define more precisely whether porcine endometrium and endometrial lymphocytes contribute to IFNG secretion during early pregnancy (GDs 15–23) and at midgestation (GD 50), we analyzed IFNG mRNA expression in endometrial or trophoblast biopsies and in endometrial lymphocytes (subsets have not yet been studied) or endothelial cells within attachment sites using cells isolated by laser-capture microdissection (Arcturus; MDS Analytical Technologies, Toronto, ON, Canada). Relative gene expression was analyzed by quantitative real-time PCR (Roche Applied Science) using beta-actin as the housekeeping gene. The important conclusions made from these studies of pure populations of cells harvested from their in situ environments are summarized in Figure 3A. First, IFNG mRNA expression is induced in porcine endometrium during early pregnancy in a nonuniform manner. The greatest induction occurred on the mesometrial side of the uterus at trophoblast attachment sites. Second, lymphocytes are a far more abundant source of IFNG transcripts than trophoblast cells from the same attachment site. Third, both endometrial and trophoblast mRNA expression of IFNG dropped significantly as pregnancy continued [57]. Whether porcine endometrial lymphocyte IFNG (GenBank accession no. 562551) [58] is identical structurally or functionally to porcine trophoblastic IFNG is not yet known.
FIG. 3.
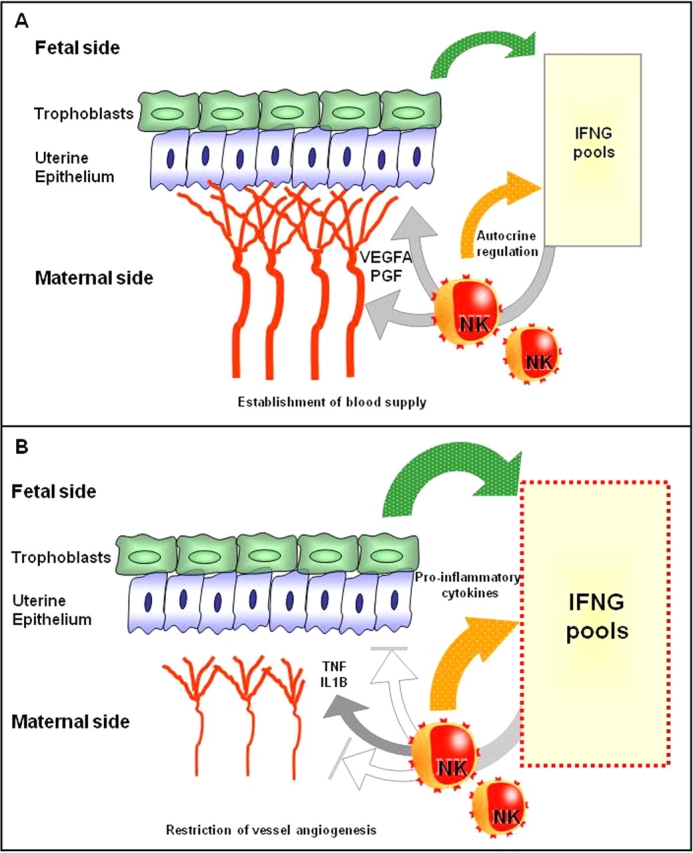
A) Between GDs 12 and 20, healthy porcine trophoblasts produce enormous amounts of IFNG. Because there is no or very weak trophoblast expression of IFNR at this time, the uterus is thought to be its target. Coincidently, uNK cells are enriched ∼3-fold, and conceptus-associated lymphocytes produce abundant IFNG transcripts. Both trophoblastic and endometrial IFNG could alter uterine epithelial cell polarity for successful conceptus attachment. Additionally, IFNG is likely to act on uNK cells (and, potentially, additional lymphocyte subtypes) to enhance their recognition of trophoblasts and their production of angiogenic factors. This would result in development of a robust, “non-hard-wired” vasculature supply for each placenta, regardless of litter size. B) In arresting early porcine attachment sites (GD 19–23), lymphocytes have stopped transcribing angiogenic genes. Lymphocytic, endometrial, and trophoblastic transcription of IFNG has been elevated to enlarge the IFNG pool at a localized conceptus attachment site. The elevation of transcripts for IFNG is greater than the accompanying elevation of transcripts for other proinflammatory cytokines (TNF and IL1B). We postulate the target of these elevated proinflammatory cytokines is the newly developed endometrial vasculature, which will be destroyed, resulting in growth arrest of the specific conceptus while adjacent littermates continue their development.
An important function of IFNG in successful mouse pregnancies is regulation of angiogenesis. This may also be an action of porcine endometrial IFNG. Endometrial angiogenesis begins in pigs at GD 15 [59], the time of peak IFNG secretion. We found porcine endometrial lymphocytes were highly angiogenic cells that transcribed and translated vascular endothelial cell and placenta growth factors (VEGFA and PGF, respectively) as well as their receptors fms-related tyrosine kinase 1 (FLT1) and kinase insert domain receptor (KDR) [57, 58]. These lymphocytes also appear to contribute to the regulation of angiogenesis during healthy porcine pregnancy through expression of hypoxia-inducible factor-1 alpha (HIF1A). Some of these genes, such as VEGFA and HIF1A, are IFNG regulated. The carefully controlled and coincident time course of induction of IFNG synthesis in porcine trophoblasts and in implantation site-localized endometrial lymphocytes supports physiological roles for IFNG during the periattachment and early postattachment phases of porcine pregnancy.
THE HUMAN MATERNAL-FETAL INTERFACE
The human maternal-fetal interface is considered to have two frontiers. One interface is in the uterus. This is a complex interface between the blood of the intervillous space and the spiral arteries that bathe floating trophoblastic villi and intravascular trophoblasts, respectively, and includes interactions between extravillous trophoblast cells and decidual immune and stromal cells. The second interface is the maternal circulation, where immune cells encounter circulating debris shed from the aging placenta. IFNG has been examined at both frontiers. Decidualization of the human uterus begins shortly after ovulation, in the mid to late secretory phases of the menstrual cycle just prior to the interval of uterine receptivity for transferred embryos. Specialized NK cells (called uterine, endometrial, or decidual) with intense expression of the surface marker CD56 appear with predecidualization in every menstrual cycle [60]. If pregnancy occurs, these cells expand rapidly in number. Estimating the duration of uNK cell enrichment in human decidua is difficult because of sampling ethics, but Bulmer and Lash [61] reported high uNK cell numbers to approximately Week 20 of gestation, with a rapid decline thereafter. Human uNK cells are highly analogous to those in mice and secrete IFNG [11]. IFNGR1 is expressed by human uterine epithelium [62], suggesting this cell population is an IFNG target prior to blastocyst implantation. Spiral arteries may also be targets, because IFNG binds to the extracellular proteoglycans of vascular smooth muscle cells, a process that concentrates IFNG and protects it from cleavage to an inactive form [63]. Experimental data support additional functions for human endometrial IFNG that include reduction of decidual renin, an angiogenic factor [64], and elevation of receptors on endothelium that promote selective leukocyte homing [65].
Microarray gene expression profiling studies consistently report no significant elevation of IFNG in human endometrium during the phase of embryo receptivity, although some IFN-regulated genes are elevated, including the NK cell-differentiating cytokine IL15 [66–69]. This is compatible with induction of an environment for uNK cell differentiation and subsequent IFNG synthesis. Microarray gene expression studies of early human decidua are less frequent but have been conducted. Chen et al. [70] found highly elevated expression of IFNA but did not report a change in expression of IFNG. Expression profiling of human uNK cells themselves has been done by Koopman et al. [71] and Hanna et al. [72]. Koopman et al. [71] did not detect elevated IFNG expression in uNK cells relative to levels of phenotypically analogous cells from blood. However, their gene expression comparisons were done between unmatched donors (i.e., blood NK cell and uterine uNK cell gene expression profiles from different women were compared). Hanna et al. [72] used a similar approach but used donor-matched comparisons between CD56brightCD16− uNK cells and CD56brightCD16− blood NK cells. These investigators found IFN-regulated genes and angiogenic genes to be the major gene categories elevated in CD56brightCD16− uNK cells relative to blood CD56brightCD16− NK cells of the same women [72].
Paracrine influences of secreted human trophoblast products have been assessed by examining global gene expression profiles of treated, decidualized endometrial cell cultures. More than 20 IFN-induced and regulated genes were among the most highly upregulated genes. These included IFNGR1, JAK2, IRF1, GAS1, and GAS7 [73]. Overall, upregulation of genes involved in activation of immune responses was the major finding. The authors suggest that responses of decidual cells reflect amplification of trophoblast gene expression, which is an important new hypothesis. However, despite the parallels between this study and those in the endometria of pigs infused with conceptus secretory products, the authors of the human study could not identify IFN or antiviral activity in their trophoblasts or trophoblast-conditioned medium. The cultured, decidualized endometrial cells exposed to trophoblast-conditioned medium also did not show upregulation of IFNA, IFNB, or IFNG. Thus, it is unclear which trophoblast products are inducing the human IFN-like response. Given the dominance of IFN-stimulated genes within the rapidly upregulated genes in human decidual cultures, there is merit in recalling that removal of uNK cell-derived IFNG in mice limits the differentiation and viability of decidual stromal cells [74].
Cytokines in plasma or serum from healthy, nonpregnant adults are predominantly proinflammatory (type 1). Gradually, across the duration of normal pregnancy, women invert this ratio and become type 2 (regulatory) cytokine dominant in the third trimester [75]. Women showing type 1-dominant cytokine profiles in the third trimester are considered to have threatened pregnancies [75]. There are multiple sources for plasma cytokines in pregnant women, including trophoblasts, maternal endothelial cells, and circulating leukocytes. By the third trimester, circulating leukocytes have acquired an activated phenotype, with gains in expression of CD11B, CD14, CD64, and intracellular reactive oxygen species. This has provided the concept that pregnancy is a proinflammatory state [76]. Normally in late pregnancy, MHC class II expression on leukocytes is decreased, and IFNG levels are low [75]. Studies using intracellular cytokine staining to compare women with normal pregnancies to nonpregnant controls indicate that TNF production is elevated throughout normal pregnancy, IL12p70 is elevated in the first and second trimesters, and IL18 is elevated only in the first trimester. The modifications in cytokine production occur in many immune cell types, including CD4+ and CD8+ T cells, myeloid DCs, and NK cells [75, 76]. In a small, gestational time course study, the shift to type 2 cytokine production in circulating cells occurred first in NK cells during the first trimester. The authors postulated that NK cells or their products might promote skewing of cytokine production toward type 2 in the other circulating immune cell subsets [76].
IFNG AND GENE EXPRESSION IN HUMAN AND MOUSE TROPHOBLASTS
Although IFNG clearly plays important roles in normal murine pregnancy through maintenance of the decidual layer and remodeling of the uterine vasculature, the impact of IFNG on the conceptus is less well defined. Trophoblast cells are the only blastocyst-derived cells in direct contact with maternal tissues, and they play multiple roles in successful pregnancy. In species with hemochorial placentation, including humans and mice, trophoblast cells invade deeply into the uterine wall during implantation and subsequent placental development. In vitro studies have found that IFNG inhibits the migration and invasion of first-trimester human trophoblast cells and of trophoblast-derived choriocarcinoma cells [77–79]. Inhibition of trophoblast invasion by IFNG correlates with downregulation of expression of matrix metalloproteinase 2 (MMP2) and MMP9 [77–79]. From these studies, the authors proposed that IFNG secreted by uNK cells plays a role in preventing excessive invasion of trophoblast cells into the uterine wall during implantation. Analyses of implantation sites of mice lacking uNK cells or IFNG suggest that this is not the case, at least in mice. Studies of early mouse ectoplacental cone-stage trophoblasts cultured with IFNG support the suggestion that IFNG promotes phagocytosis by trophoblast cells as a mechanism for fetal acquisition of iron and other nutritional substrates prior to placental development and function [80].
The fact that IFNG is crucial for activation of adaptive immune responses to pathogens and for immune surveillance of tumors raises an interesting immunological conundrum: How do genetically disparate trophoblast cells evade the deleterious effects of this proinflammatory cytokine? Primary human cytotrophoblast cells from term placentae, human trophoblast-derived choriocarcinoma cells, and mouse trophoblast cells are resistant to IFNG-activated apoptosis and to IFNG-induced release of nitric oxide and other reactive oxygen intermediates [81–83]. Despite the fact that IFNG is present in early placenta and gestational endometrium, neither human nor rodent trophoblast cells express polymorphic MHC class II genes. This inertness is attributed to silencing of expression of CIITA, the master regulator of constitutive and IFNG-inducible MHC class II gene transcription [84–86]. The inability of trophoblast cells to express MHC class II antigens is thought to be important for preventing transplant rejection reactions directed against the conceptus [87]. Although first-trimester trophoblast cells do not express CIITA in response to IFNG [86], differing results were reported for their sensitivity to IFNG-mediated apoptosis [78, 79]. Taken together, these observations suggest that trophoblast cells respond selectively to IFNG.
Based on these collective results, we examined IFNG signal transduction and inducible gene expression in IFNGR+ human choriocarcinoma cells and in term cytotrophoblast cells. Activation of JAK1 and JAK2 in response to IFNG treatment was compromised in these cells relative to fibroblast or epithelial cells [88]. This correlated with significantly reduced levels of activated STAT1 and lower levels of IFNG-induced expression of multiple genes, including IRF1 [88]. Importantly, treatment of choriocarcinoma cells with the protein tyrosine phosphatase inhibitor pervanadate promoted JAK and STAT1 activation and upregulation of IFNG-responsive gene expression [88]. These results strongly suggest that IFNG signal transduction is inhibited in human trophoblast cells by PTPs. To date, the specific PTP(s) responsible for this phenomenon have not been identified. Constitutive expression of SOCS1 has been detected in syncytiotrophoblasts during normal pregnancy [89] but its potential role in inhibiting trophoblastic IFNG responses has not yet been investigated.
JAK1 and JAK2 are key components of the signal transduction pathways used by a wide array of cytokines, growth factors, and hormones, including interleukins, leukemia inhibitory factor, leptin, and insulin. Protein tyrosine phosphatases attenuate responses to all of these soluble factors [31, 90]. Many of these cytokines and growth factors are present in the placenta and play crucial roles in regulating trophoblast functions. Protein tyrosine phosphatase non-receptor type 1 (PTPN1), type 2 (PTPN2), and type 11 (PTPN11) are ubiquitously expressed (including trophoblast cells; J. Choi and S. Murphy, unpublished data), and they regulate multiple signaling pathways, including IFNG [29, 31]. Thus, it will be important and clinically relevant to determine the precise mechanism(s) responsible for the ability of human trophoblast cells to differentially respond to ligands that use JAKs and PTPs for signal transduction.
As mentioned above, IFNG inhibits invasion of both first-trimester human trophoblast cells and JEG-3 choriocarcinoma cells in extracellular matrix (Matrigel) invasion assays [77–79] via mechanisms correlated with decreasing expression of MMP2 and MMP9. In fibrosarcoma cells, IFNG downregulates transcription of MMP2 and MMP9, effects mediated by both activated STAT1 and CIITA [91, 92]. Importantly, because activated (phosphorylated) STAT1 is present only very transiently and CIITA expression is completely silenced in human trophoblast cells, these are unlikely factors downregulating MMP expression in trophoblasts. Thus, novel mechanism(s) likely account for IFNG-mediated inhibition of trophoblast MMP expression.
Recently, we found that the dampening of IFNG-inducible gene expression in trophoblasts is conserved between human and mouse trophoblast cells. However, this is achieved in mice by a mechanism distinct from that used by humans. In mice, STAT1 tyrosine phosphorylation and DNA-binding ability are comparable in IFNG-treated trophoblast and fibroblast cells (J. Choi, R. Holtz, and S. Murphy, unpublished data). Treatment of mouse trophoblast cells with histone deacetylase (HDAC) inhibitors, such as trichostatin A, alleviated the dampening of IFNG-inducible expression of such genes as IRF1, CIITA, and MHC class II genes (J. Choi, R. Holtz, and S. Murphy, unpublished data) [93]. The specific HDAC(s) involved are not yet identified. Moreover, it is not yet clear whether the observed effects of HDACs are mediated through direct effects on STAT1 function, through inhibition of histone acetylation at target promoters, or through a combination of these mechanisms.
A wide variety of viruses and other pathogens inhibit cellular responses to IFNG and IFNA/IFNB by repressing the JAK/STAT pathway to evade host immune responses [94, 95]. These pathogens use multiple different mechanisms to block IFN signaling, including degradation of the IFNGRs, JAKs, or STAT1; blocking JAK and/or STAT1 activation; and inhibition of transcriptional activation by STAT1 [94, 95]. Mutations or deletions of viral genes responsible for inhibiting the JAK/STAT pathway result in attenuation of viral infection, demonstrating directly the importance of this pathway in mediating antiviral immune responses [94]. Disruption of the JAK/STAT pathway is also an important immunoevasion mechanism used by some tumors [7]. Our studies have now identified trophoblast cells as one of the only normal cell types that repress IFNG signaling. Collectively, these results suggest that inhibition of the JAK-STAT pathway in response to IFNG in trophoblast cells contributes to successful human and mouse pregnancy by preventing responses, such as apoptosis and activation of immune responses, that could result in placental damage.
IFNG AND GESTATIONAL COMPLICATIONS
Fetal Loss in Mice
A widely used model of spontaneous, midgestation fetal loss in mice is the CBA female mated by the DBA/2 male. Gestational outcomes are usually compared with those in CBA females mated by BALB/c males to preserve a common, paternally inherited MHC haplotype. Losses in the abortion-prone mating vary from 25% to 40% of each litter and are usually <10% in the control mating. The abortion-prone phenotype is only seen when mice are maintained by conventional, not ultraclean, husbandry, implicating preactivation of pathogen-sensing regulatory pathways in fetal loss. IFNG has been associated with these pregnancy losses but not as the direct agent of fetal death. Postulated effectors also include tumor necrosis factor (TNF), macrophages, oxidative stress, and procoagulants [96, 97]. A recent study addressed roles for the complement cascade in the fetal growth restriction and abortions seen in DBA-mated CBA pregnancies. The activated, smaller fragment of complement component C5 (C5a) was identified as the underlying trigger to conceptus death. C5a induced soluble FLT1, an antagonist of angiogenesis, whereas blocking C5a reduced TNF and promoted angiogenesis and fetal survival [98]. C5a receptor 1 (C5AR1 or CD88) is expressed by endothelial cells and macrophages and can be upregulated by IFNG but not by TNF [99]. Synthesis of C1 inhibitor and other complement members is also regulated by IFNG [100]. Studies of this model of spontaneous fetal loss are continuing and will lead to a fuller understanding of roles of IFNG and other molecules.
Fetal Loss in Commercial Pigs
North American commercial meat pigs also provide an excellent model to study spontaneous loss of genetically normal conceptuses. Two waves of unexplained fetal loss occur during the 114-day porcine gestation interval. The first losses occur during conceptus attachment to the endometrium and reduce litter size approximately 30%. The second wave of losses occurs between GDs 50 and 70 and reduces litter size a further 10%–15% [101, 102]. The reasons fetuses die are poorly understood, because embryo transfer studies suggest most are potentially viable. Long-term studies on uterine capacity, placental efficiency, genetics, and nutrition have failed to identify factors accounting for these losses. In addition, genetic breeding selection pressure for these traits has not significantly improved neonatal litter sizes [57].
Endometrial biopsies collected from GD 15–23 attachment sites holding retarded but viable porcine conceptuses had highly elevated expression of IFNG, TNF, IL1B, and IL1R compared with biopsies from neighboring healthy littermate attachment sites [58]. Endometrial lymphocytes collected by laser-capture microdissection and trophoblast biopsies from the same GD 20 arresting conceptus attachment sites had significantly more IFNG transcripts than the same samples from a healthy littermate site. There was less gain in TNF mRNA expression. However, at GD 50, IFNG transcripts were not elevated in lymphocytes (not subclass identified) dissected from arresting attachment sites, whereas TNF transcripts were highly elevated [57]. This suggests highly specific localization of the mechanisms regulating endometrial cytokine expression, perhaps mediated by the fetal-placental unit. Further, there appear to be distinct phases of pregnancy when a specific cytokine mechanism has the potential to contribute to fetal loss. Decreased expression of genes promoting angiogenesis accompanied the shifts in cytokine gene expression in endometria associated with both GD 20 and GD 50 arresting fetuses (Fig. 3B) [57, 58].
Human Gestational Syndromes
IFNG has been widely assessed as a potential mediator of many complications of human pregnancy. Almost half of the publications in PubMed in late 2008 on “IFNG and pregnancy” addressed infectious diseases, particularly parasitic diseases, such as malaria (Croy and Hatta, unpublished data). Neither literature on infectious diseases nor literature on efficacy and safety of vaccination during pregnancy (assessed by induction of IFNG) is covered in this review. The three human complications we briefly include are autoimmune disease, preterm labor, and preeclampsia.
Autoimmune disease.
Prepregnancy disease states, such as autoimmunity, allergy, and asthma, are associated with activation of the immune system and type 1 cytokine production that is promoted by estrogens [103]. Pregnancy can relieve or exacerbate symptoms for many of these diseases, which occur more often in women. Studies of cytokines in plasma from patients with rheumatoid arthritis, a disease that improves clinically during pregnancy, showed type 1 dominance, with IFNG elevation above that seen in healthy controls only during the first trimester. Similar data are reported for patients with systemic lupus erythematosus. Plasma from the pregnant rheumatoid arthritis patients had elevated decoy receptor signaling for TNF and IL1 [104], but no mechanism has been reported for the reduction in IFNG. Reduced cytokine circulation is likely to explain the observed clinical improvements.
Multiple sclerosis is another autoimmune disease in which remission during pregnancy is frequent, followed by postpartum relapse [105]. In a study of type 1:type 2 cytokine ratios across pregnancy in eight patients and controls with IFNG as the type 1 marker, a shift to type 2 dominance was seen in six patients, who all entered remission. The remaining two patients had increasing dominance of type 1 immunity in each successive trimester and no gestational relief from symptoms [106]. This again implicates IFNG among the cytokines inducing clinical signs. Improved understanding of the differences between patient responses to pregnancy and the alterations occurring in immune cells and cytokines within the implantation sites of autoimmune women would have significant clinical impact.
Pregnancy in diabetic women has a high potential for serious fetal consequences, including malformations and death, and for preeclampsia. Polymorphisms in the IFNG gene have been associated with type 1 diabetes [107], as has strong IFNG production accompanied by loss of IL4 secretion from blood T cells [108]. A study of more than 200 pregnant type 1 diabetic women considered whether the type 2 cytokine dominance of late pregnancy would enhance autoantibody production in the mothers and promote transfer of these antibodies to their fetuses. The cytokine shifts of pregnancy did not appear to do this, although the cytokine levels were assumed rather than measured [109]. There is no literature on lymphocyte subsets or IFNG production within the decidua of diabetic women, but this has been addressed in mouse models. At midgestation in spontaneously type 1 diabetic mice of the strain nonobese diabetic, elevated decidual IFNG was found, although uNK cell numbers were below normal [110]. It has also been reported that IFNG treatment of pregnant mice with acute, chemically induced diabetes reduced fetal birth defects [111]. Further study of pregnancies in autoimmune animals will be valuable for defining the importance, time course relationships, and regulation of cells affecting innate and adaptive immunity and the shifts in their cytokine profiles in relation to fetal risk.
Preterm labor.
Spontaneous preterm labor is another leading cause of infant morbidity and mortality. It is frequently associated with fetal growth restriction and serious developmental disorders. Spontaneous preterm labor has numerous etiologies ranging from stress to fetal membrane infection. In normal pregnancy at term, IFNG is not detectable in amniotic fluid and is found at low concentrations in plasma and at significant levels in placenta, amnion, and choriodecidua [112]. Wilke et al. [113] reported that women delivering preterm had lower plasma IFNG between Weeks 20 and 25 of gestation than women delivering at term, and that these levels rose rather than declined between midgestation and birth. This was confirmed in a very large cohort study [114] that, however, reached the conclusion that there was limited value in measurement of midpregnancy cytokines for prediction of preterm delivery. Not all studies find cytokine differences between these groups of women or use common assay techniques [115]. Even in women with active malarial infection within their placentae, IFNG levels could not be correlated with preterm delivery [116]. Others have associated TNF, IL1, IL6, and IL8 with preterm labor [117]. Hanna et al. [118] measured IFNG in placental and decidual tissues from normal and preterm deliveries and found more decidual than placental IFNG in both groups. They proposed that withdrawal of IFNG may be involved in the onset of preterm or term labor by upregulation of cyclooxygenase expression and prostaglandin E2 production [118]. Proinflammatory cytokine dysregulation can also occur in fetal as well as placental tissue and is thought to complicate pregnancies, participating, for example, in neonatal brain injury and cerebral palsy [119]. Elevation of fetal IFNG has been associated with neonatal damage to white matter, whereas elevated IL6 and IL8 were associated with intraventricular hemorrhages [120].
Preeclampsia.
Preeclampsia is a life-threatening human syndrome with sudden onset of hypertension and renal failure after midpregnancy. About 5% of all pregnancies are affected, but predictive diagnostic criteria and a full understanding of its causes remain elusive [121, 122]. Preeclampsia may arise from maternal, fetal, or shared etiologies [123], and it culminates in systemic endothelial cell inflammation [124]. IFNG concentrations are elevated in plasma, circulating leukocytes, and decidual tissue from women with preeclampsia compared with gestation stage-matched pregnant control women [76]. This has been proposed to be the key cytokine disturbance promoting vascular dysregulation and disease progression [76, 125]. There is current enthusiasm for the hypothesis that suboptimal concentrations of angiogenic growth factors underlie preeclampsia [126]. IFNG decreases production of VEGF by human endometrial stromal cells [127] and contributes in complex ways to the expression of genes in endometrial endothelial cells [128]. Because human uNK cells are a source of VEGF, PGF, and IFNG, there is elevated interest internationally in gaining improved understanding of the genetics [123], regulation, activation, and angiogenic functions of human uNK cells [128].
CONCLUSIONS AND PERSPECTIVE
Studies of maternal and trophoblast-derived IFNG during pregnancy have provided critical insights into the surprisingly diverse and dynamic roles of this proinflammatory cytokine at the maternal-fetal interface. IFNG clearly plays critical roles in establishing and maintaining this interface in mice and pigs in concert with other cytokines. Studies using microarray, proteomics, and laser-capture microdissection approaches are enhancing our understanding of the cytokine-endocrine relationships of pregnancy and challenge us to explore them more deeply for the promotion of human and animal health. IFNG appears to have the special role in pregnancy of promoting angiogenesis that is not “hard wired,” but develops in conceptus-triggered locations to promote the success of pregnancy.
In normal pregnancies, semiallogeneic trophoblast cells are not subject to transplant rejection reactions by maternal lymphocytes. This may be due in part to intrinsic regulatory mechanisms that prevent IFNG-induced expression of MHC molecules, a pathway of immunoevasion known for tumors and cells infected by certain viruses. However, gestational complications that include fetal loss have been linked to elevation in IFNG. In pigs, IFNG elevation was more strongly implicated in periattachment losses than at midgestation, whereas in autoimmune diabetic mice, IFNG elevation and induction of congenital anomalies were at midgestation. This reminds us that change is rapid in the conceptus and in the maternal uterine and systemic environments throughout the course of pregnancy and that the roles for cytokines and/or their relative timing of expression can be species specific. In the early postimplantation period when the endometrial neovasculature is being formed, IFNG-linked, immune-mediated compromise of angiogenesis must be considered as a mechanism contributing to fetal stress and subsequent complications.
Footnotes
1Supported by the Natural Sciences and Engineering Research Council Canada, the Canadian Institutes of Health Research, Ontario Pork, the Ontario Ministry of Agriculture, Food and Rural Affairs, AgriFood Canada, the Canada Research Chairs Program, and the National Institute of Child Health and Development.
REFERENCES
- Wheelock EF.Interferon-like virus-inhibitor induced in human leukocytes by phytohemagglutinin. Science 1965; 149: 310–311. [PubMed] [Google Scholar]
- Schoenborn JR, Wilson CB.Regulation of interferon-gamma during innate and adaptive immune responses. Adv Immunol 2007; 96: 41–101. [DOI] [PubMed] [Google Scholar]
- Pestka S, Krause CD, Walter MR.Interferons, interferon-like cytokines, and their receptors. Immunol Rev 2004; 202: 8–32. [DOI] [PubMed] [Google Scholar]
- Boehm U, Klamp T, Groot M, Howard JC.Cellular responses to interferon-gamma. Annu Rev Immunol 1997; 15: 749–795. [DOI] [PubMed] [Google Scholar]
- Stark GR, Kerr IM, Williams BR, Silverman RH, Schreiber RD.How cells respond to interferons. Annu Rev Biochem 1998; 67: 227–264. [DOI] [PubMed] [Google Scholar]
- Szabo SJ, Sullivan BM, Peng SL, Glimcher LH.Molecular mechanisms regulating Th1 immune responses. Annu Rev Immunol 2003; 21: 713–758. [DOI] [PubMed] [Google Scholar]
- Dunn GP, Koebel CM, Schreiber RD.Interferons, immunity and cancer immunoediting. Nat Rev Immunol 2006; 6: 836–848. [DOI] [PubMed] [Google Scholar]
- Boehm U, Guethlein L, Klamp T, Ozbek K, Schaub A, Fütterer A, Pfeffer K, Howard JC.Two families of GTPases dominate the complex cellular response to IFN-gamma. J Immunol 1998; 161: 6715–6723. [PubMed] [Google Scholar]
- Saito S, Sakai M, Sasaki Y, Tanebe K, Tsuda H, Michimata T.Quantitative analysis of peripheral blood Th0, Th1, Th2 and the Th1:Th2 cell ratio during normal human pregnancy and preeclampsia. Clin Exp Immunol 1999; 117: 550–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H, Mosmann TR, Guilbert L, Tuntipopipat S, Wegmann TG.Synthesis of T helper 2-type cytokines at the maternal-fetal interface. J Immunol 1993; 151: 4562–4573. [PubMed] [Google Scholar]
- Saito S, Nishikawa K, Morii T, Enomoto M, Narita N, Motoyoshi K, Ichijo M.Cytokine production by CD16-CD56bright natural killer cells in the human early pregnancy decidua. Int Immunol 1993; 5: 559–563. [DOI] [PubMed] [Google Scholar]
- Tato CM, Martins GA, High FA, DiCioccio CB, Reiner SL, Hunter CA.Innate production of IFN-gamma by NK cells is independent of epigenetic modification of the IFN-gamma promoter. J Immunol 2004; 173: 1514–1517. [DOI] [PubMed] [Google Scholar]
- Takaoka A, Yanai H.Interferon signalling network in innate defence. Cell Microbiol 2006; 8: 907–922. [DOI] [PubMed] [Google Scholar]
- Della Chiesa M, Sivori S, Castriconi R, Marcenaro E, Moretta A.Pathogen-induced private conversations between natural killer and dendritic cells. Trends Microbiol 2005; 138: 128–136. [DOI] [PubMed] [Google Scholar]
- Croy BA, van den Heuvel MJ, Borzychowski AM, Tayade C.Uterine natural killer cells: a specialized differentiation regulated by ovarian hormones. Immunol Rev 2006; 214: 161–185. [DOI] [PubMed] [Google Scholar]
- Bogdan C, Schleicher U.Production of interferon-gamma by myeloid cells–fact or fancy? Trends Immunol 2006; 27: 282–290. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Kurohmaru M, Hayashi Y.Localization of type I interferon in murine trophoblast and decidua during decidual formation. J Reprod Fertil 1992; 95: 559–565. [DOI] [PubMed] [Google Scholar]
- La Bonnardiere C.No detection of interferon-gamma activity during early pregnancy in the mouse. Am J Reprod Immunol 1993; 30: 26–31. [DOI] [PubMed] [Google Scholar]
- Platt JS, Hunt JS.Interferon-gamma gene expression in cycling and pregnant mouse uterus: temporal aspects and cellular localization. J Leukoc Biol 1998; 64: 393–400. [DOI] [PubMed] [Google Scholar]
- Truchet S, Wietzerbin J, Debey P.Mouse oocytes and preimplantation embryos bear the two sub-units of interferon-gamma receptor. Mol Reprod Dev 2001; 60: 319–330. [DOI] [PubMed] [Google Scholar]
- Chen H, Kamath LR, Pace JL, Russell SW, Hunt JS.Expression of the interferon-gamma receptor gene in mouse placentas is related to stage of gestation and is restricted to specific subpopulations of trophoblast cells. Placenta 1994; 15: 109–121. [DOI] [PubMed] [Google Scholar]
- Cencic A, Guillomot M, Koren S, La Bonnardière C.Trophoblastic interferons: do they modulate uterine cellular markers at the time of conceptus attachment in the pig? Placenta 2003; 24: 862–869. [DOI] [PubMed] [Google Scholar]
- Grunig G, Antczak DF.Horse trophoblasts produce tumor necrosis factor alpha but not interleukin 2, interleukin 4, or interferon gamma. Biol Reprod 1995; 52: 531–539. [DOI] [PubMed] [Google Scholar]
- Roberts RM.Interferon-tau, a type 1 interferon involved in maternal recognition of pregnancy. Cytokine Growth Factor Rev 2007; 18: 403–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer TE, Johnson GA, Bazer FW, Burghardt RC.Fetal-maternal interactions during the establishment of pregnancy in ruminants. Soc Reprod Fertil Suppl 2007; 64: 379–396. [DOI] [PubMed] [Google Scholar]
- Paulesu L, Romagnoli R, Cintorino M, Ricci MG, Garotta G.First trimester human trophoblast expresses both interferon-gamma and interferon-gamma-receptor. J Reprod Immunol 1994; 27: 37–48. [DOI] [PubMed] [Google Scholar]
- Banerjee S, Smallwood A, Moorhead J, Chambers AE, Papageorghiou A, Campbell S, Nicolaides K.Placental expression of interferon-gamma (IFN-gamma) and its receptor IFN-gamma R2 fail to switch from early hypoxic to late normotensive development in preeclampsia. J Clin Endocrinol Metab 2005; 90: 944–952. [DOI] [PubMed] [Google Scholar]
- Harton JA, Ting JP.Class II transactivator: mastering the art of major histocompatibility complex expression. Mol Cell Biol 2000; 20: 6185–6194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuai K, Liu B.Regulation of JAK-STAT signalling in the immune system. Nat Rev Immunol 2003; 3: 900–911. [DOI] [PubMed] [Google Scholar]
- Brayman MJ, Dharmaraj N, Lagow E, Carson DD.MUC1 expression is repressed by protein inhibitor of activated signal transducer and activator of transcription-y. Mol Endocrinol 2007; 21: 2725–2737. [DOI] [PubMed] [Google Scholar]
- Rane SG, Reddy EP.Janus kinases: components of multiple signaling pathways. Oncogene 2000; 19: 5662–5679. [DOI] [PubMed] [Google Scholar]
- Peel S.Granulated metrial gland cells. Adv Anat Embryol Cell Biol 1989; 115: 1–112. [DOI] [PubMed] [Google Scholar]
- Paffaro VA, Jr, Bizinotto MC, Joazeiro PP, Yamada AT.Subset classification of mouse uterine natural killer cells by DBA lectin reactivity. Placenta 2003; 24: 479–488. [DOI] [PubMed] [Google Scholar]
- Guimond MJ, Wang B, Croy BA.Engraftment of bone marrow from severe combined immunodeficient (SCID) mice reverses the reproductive deficits in natural killer cell-deficient tg epsilon 26 mice. J Exp Med 1998; 187: 217–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herington JL, Bany BM.Effect of the conceptus on uterine natural killer cell numbers and function in the mouse uterus during decidualization. Biol Reprod 2007; 76: 579–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chantakru S, Miller C, Roach LE, Kuziel WA, Maeda N, Wang WC, Evans SS, Croy BA.Contributions from self-renewal and trafficking to the uterine NK cell population of early pregnancy. J Immunol 2002; 168: 22–28. [DOI] [PubMed] [Google Scholar]
- Ashkar AA, Croy BA.Interferon-gamma contributes to the normalcy of murine pregnancy. Biol Reprod 1999; 61: 493–502. [DOI] [PubMed] [Google Scholar]
- Ashkar AA, Di Santo JP, Croy BA.Interferon gamma contributes to initiation of uterine vascular modification, decidual integrity, and uterine natural killer cell maturation during normal murine pregnancy. J Exp Med 2000; 192: 259–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matikainen S, Paananen A, Miettinen M, Kurimoto M, Timonen T, Julkunen I, Sareneva T.IFN-alpha and IL-18 synergistically enhance IFN-gamma production in human NK cells: differential regulation of Stat4 activation and IFN-gamma gene expression by IFN-alpha and IL-12. Eur J Immunol 2001; 31: 2236–2245. [PubMed] [Google Scholar]
- Kashiwagi A, DiGirolamo CM, Kanda Y, Niikura Y, Esmon CT, Hansen TR, Shioda T, Pru JK.The postimplantation embryo differentially regulates endometrial gene expression and decidualization. Endocrinology 2007; 148: 4173–4184. [DOI] [PubMed] [Google Scholar]
- Zarnani AH, Moazzeni SM, Shokri F, Salehnia M, Jeddi-Tehrani M.Kinetics of murine decidual dendritic cells. Reproduction 2007; 133: 275–283. [DOI] [PubMed] [Google Scholar]
- Austin KJ, Bany BM, Belden EL, Rempel LA, Cross JC, Hansen TR.Interferon-stimulated gene-15 (Isg15) expression is up-regulated in the mouse uterus in response to the implanting conceptus. Endocrinology 2003; 144: 3107–3113. [DOI] [PubMed] [Google Scholar]
- Yu J, Wei M, Becknell B, Trotta R, Liu S, Boyd Z, Jaung MS, Blaser BW, Sun J, Benson DM, Jr, Mao H, Yokohama A, et al. Pro- and antiinflammatory cytokine signaling: reciprocal antagonism regulates interferon-gamma production by human natural killer cells. Immunity 2006; 24: 575–590. [DOI] [PubMed] [Google Scholar]
- Ashkar AA, Black GP, Wei Q, He H, Liang L, Head JR, Croy BA.Assessment of requirements for IL-15 and IFN regulatory factors in uterine NK cell differentiation and function during pregnancy. J Immunol 2003; 171: 2937–2944. [DOI] [PubMed] [Google Scholar]
- Zhang JH, He H, Borzychowski AM, Takeda K, Akira S, Croy BA.Analysis of cytokine regulators inducing interferon production by mouse uterine natural killer cells. Biol Reprod 2003; 69: 404–411. [DOI] [PubMed] [Google Scholar]
- Tayade C, Fang Y, Black GP, Paffaro VA, Jr, Erlebacher A, Croy BA.Differential transcription of Eomes and T-bet during maturation of mouse uterine natural killer cells. J Leukoc Biol 2005; 78: 1347–1355. [DOI] [PubMed] [Google Scholar]
- Zhang J, Dong H, Wang B, Zhu S, Croy BA.Dynamic changes occur in patterns of endometrial EFNB2/EPHB4 expression during the period of spiral arterial modification in mice. Biol Reprod 2008; 79: 450–458. [DOI] [PubMed] [Google Scholar]
- Lefèvre F, Martinat-Botté F, Guillomot M, Zouari K, Charley B, La Bonnardière C.Interferon-gamma gene and protein are spontaneously expressed by the porcine trophectoderm early in gestation. Eur J Immunol 1990; 20: 2485–2490. [DOI] [PubMed] [Google Scholar]
- Lefèvre F, Boulay V.A novel and atypical type one interferon gene expressed by trophoblast during early pregnancy. J Biol Chem 1993; 268: 19760–19768. [PubMed] [Google Scholar]
- Cencic A, La Bonnardière C.Trophoblastic interferon-gamma: current knowledge and possible role(s) in early pig pregnancy. Vet Res 2002; 33: 139–157. [DOI] [PubMed] [Google Scholar]
- D'Andréa S, La Bonnardière C.Cloning of the porcine interferon-gamma receptor and its foeto-endometrial expression in early pregnancy. Mol Reprod Dev 1998; 51: 225–234. [DOI] [PubMed] [Google Scholar]
- Short EC, Jr, Geisert RD, Groothuis PG, Blair RM, Schmitt RA, Fulton RW.Porcine conceptus proteins: antiviral activity and effect on 2′,5′-oligoadenylate synthetase. Biol Reprod 1992; 46: 464–469. [DOI] [PubMed] [Google Scholar]
- Joyce MM, Burghardt JR, Burghardt RC, Hooper RN, Jaeger LA, Spencer TE, Bazer FW, Johnson GA.Pig conceptuses increase uterine interferon-regulatory factor 1 (IRF1), but restrict expression to stroma through estrogen-induced IRF2 in luminal epithelium. Biol Reprod 2007; 77: 292–302. [DOI] [PubMed] [Google Scholar]
- Joyce MM, Burghardt RC, Geisert RD, Burghardt JR, Hooper RN, Ross JW, Ashworth MD, Johnson GA.Pig conceptuses secrete estrogen and interferons to differentially regulate uterine STAT1 in a temporal and cell type-specific manner. Endocrinology 2007; 148: 4420–4431. [DOI] [PubMed] [Google Scholar]
- Joyce MM, Burghardt JR, Burghardt RC, Hooper RN, Bazer FW, Johnson GA.Uterine MHC class I molecules and beta 2-microglobulin are regulated by progesterone and conceptus interferons during pig pregnancy. J Immunol 2008; 181: 2494–2505. [DOI] [PubMed] [Google Scholar]
- Engelhardt H, Croy BA, King GJ.Conceptus influences the distribution of uterine leukocytes during early porcine pregnancy. Biol Reprod 2002; 66: 1875–1880. [DOI] [PubMed] [Google Scholar]
- Tayade C, Fang Y, Hilchie D, Croy BA.Lymphocyte contributions to altered endometrial angiogenesis during early and midgestation fetal loss. J Leukoc Biol 2007; 82: 877–886. [DOI] [PubMed] [Google Scholar]
- Tayade C, Black GP, Fang Y, Croy BA.Differential gene expression in endometrium, endometrial lymphocytes, and trophoblasts during successful and abortive embryo implantation. J Immunol 2006; 176: 48–56. [DOI] [PubMed] [Google Scholar]
- Dantzer V, Leiser R.Initial vascularisation in the pig placenta: I. Demonstration of nonglandular areas by histology and corrosion casts. Anat Rec 1994; 238: 177–190. [DOI] [PubMed] [Google Scholar]
- Manaster I, Mizrahi S, Goldman-Wohl D, Sela HY, Stern-Ginossar N, Lankry D, Gruda R, Hurwitz A, Bdolah Y, Haimov-Kochman R, Yagel S, Mandelboim O.Endometrial NK cells are special immature cells that await pregnancy. J Immunol 2008; 181: 1869–1876. [DOI] [PubMed] [Google Scholar]
- Bulmer JN, Lash GE.Human uterine natural killer cells: a reappraisal. Mol Immunol 2005; 42: 511–521. [DOI] [PubMed] [Google Scholar]
- Tabibzadeh S.Evidence of T-cell activation and potential cytokine action in human endometrium. J Clin Endocrinol Metab 1990; 71: 645–659. [DOI] [PubMed] [Google Scholar]
- Hurt-Camejo E, Rosengren B, Sartipy P, Elfsberg K, Camejo G, Svensson L.CD44, a cell surface chondroitin sulfate proteoglycan, mediates binding of interferon-gamma and some of its biological effects on human vascular smooth muscle cells. J Biol Chem 1999; 274: 18957–18964. [DOI] [PubMed] [Google Scholar]
- Jikihara H, Poisner AM, Handwerger S.Interferon-gamma inhibits the synthesis and release of renin from human decidual cells. Biol Reprod 1996; 54: 1311–1316. [DOI] [PubMed] [Google Scholar]
- Thomson AJ, Greer MR, Young A, Boswell F, Telfer JF, Cameron IT, Norman JE, Campbell S.Expression of intercellular adhesion molecules ICAM-1 and ICAM-2 in human endometrium: regulation by interferon-gamma. Mol Hum Reprod 1999; 5: 64–70. [DOI] [PubMed] [Google Scholar]
- Kao LC, Tulac S, Lobo S, Imani B, Yang JP, Germeyer A, Osteen K, Taylor RN, Lessey BA, Giudice LC.Global gene profiling in human endometrium during the window of implantation. Endocrinology 2002; 143: 2119–2138. [DOI] [PubMed] [Google Scholar]
- Carson DD, Lagow E, Thathiah A, Al-Shami R, Farach-Carson MC, Vernon M, Yuan L, Fritz MA, Lessey B.Changes in gene expression during the early to mid-luteal (receptive phase) transition in human endometrium detected by high-density microarray screening. Mol Hum Reprod 2002; 8: 871–879. [DOI] [PubMed] [Google Scholar]
- Ponnampalam AP, Weston GC, Trajstman AC, Susil B, Rogers PA.Molecular classification of human endometrial cycle stages by transcriptional profiling. Mol Hum Reprod 2004; 10: 879–893. [DOI] [PubMed] [Google Scholar]
- Mirkin S, Arslan M, Churikov D, Corica A, Diaz JI, Williams S, Bocca S, Oehninger S.In search of candidate genes critically expressed in the human endometrium during the window of implantation. Hum Reprod 2005; 20: 2104–2117. [DOI] [PubMed] [Google Scholar]
- Chen JW, Chen JJ, Tzeng CR, Li HN, Chang SJ, Cheng YF, Chang CW, Wang RS, Yang PC, Lee YT.Global analysis of differentially expressed genes in early gestational decidua and chorionic villi using a 9600 human cDNA microarray. Mol Hum Reprod 2002; 8: 475–484. [DOI] [PubMed] [Google Scholar]
- Koopman LA, Kopcow HD, Rybalov B, Boyson JE, Orange JS, Schatz F, Masch R, Lockwood CJ, Schachter AD, Park PJ, Strominger JL.Human decidual natural killer cells are a unique NK cell subset with immunomodulatory potential. J Exp Med 2003; 198: 1201–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna J, Goldman-Wohl D, Hamani Y, Avraham I, Greenfield C, Natanson-Yaron S, Prus D, Cohen-Daniel L, Arnon TI, Manaster I, Gazit R, Yutkin V, et al. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat Med 2006; 12: 1065–1074. [DOI] [PubMed] [Google Scholar]
- Hess AP, Hamilton AE, Talbi S, Dosiou C, Nyegaard M, Nayak N, Genbecev-Krtolica O, Mavrogianis P, Ferrer K, Kruessel J, Fazleabas AT, Fisher SJ, et al. Decidual stromal cell response to paracrine signals from the trophoblast: amplification of immune and angiogenic modulators. Biol Reprod 2007; 76: 102–117. [DOI] [PubMed] [Google Scholar]
- Greenwood JD, Minhas K, di Santo JP, Makita M, Kiso Y, Croy BA.Ultrastructural studies of implantation sites from mice deficient in uterine natural killer cells. Placenta 2000; 21: 693–702. [DOI] [PubMed] [Google Scholar]
- Germain SJ, Sacks GP, Soorana SR, Sargent IL, Redman CW.Systemic inflammatory priming in normal pregnancy and preeclampsia: the role of circulating syncytiotrophoblast microparticles. J Immunol 2007; 178: 5949–5956. [DOI] [PubMed] [Google Scholar]
- Sargent IL, Borzychowski AM, Redman CWG.NK cells and human pregnancy—an inflammatory view. Trends Immunol 2006; 27: 399–404. [DOI] [PubMed] [Google Scholar]
- Karmakar S, Dhar R, Das C.Inhibition of cytotrophoblastic (JEG-3) cell invasion by interleukin 12 involves an interferon gamma-mediated pathway. J Biol Chem 2004; 279: 55297–55307. [DOI] [PubMed] [Google Scholar]
- Hu Y, Dutz JP, MacClaman CD, Yong P, Tan R, von Dadelszen P.Decidual NK cells alter in vitro first trimester extravillous cytotrophoblast migration: a role for IFN-gamma. J Immunol 2006; 177: 8522–8530. [DOI] [PubMed] [Google Scholar]
- Lash GE, Otun HA, Innes BA, Kirkley M, de Oliveira L, Searle RF, Robson SC, Bulmer JN.Interferon-gamma inhibits extravillous trophoblast cell invasión by a mechanism that involves both changes in apoptosis and protease levels. FASEB J 2006; 20: 2512–2518. [DOI] [PubMed] [Google Scholar]
- Hoshida MS, Gorjão R, Lima C, Daher S, Curi R, Bevilacqua E.Regulation of gene expression in mouse trophoblast cells by interferon-gamma. Placenta 2007; 28: 1059–1072. [DOI] [PubMed] [Google Scholar]
- Yui J, Garcia-Lloret M, Wegmann TG, Guilbert LJ.Cytotoxicity of tumour necrosis factor-alpha and gamma-interferon against primary human placental trophoblasts. Placenta 1994; 15: 819–835. [DOI] [PubMed] [Google Scholar]
- Sun QH, Peng JP, Xia HF.IFN gamma pretreatment sensitizes human choriocarcinoma cells to etoposide-induced apoptosis. Mol Hum Reprod 2006; 12: 99–105. [DOI] [PubMed] [Google Scholar]
- Smith SC, Guilbert LJ, Yui J, Baker PN, Davidge ST.The role of reactive nitrogen/oxygen intermediates in cytokine-induced trophoblast apoptosis. Placenta 1999; 20: 309–315. [DOI] [PubMed] [Google Scholar]
- Murphy SP, Tomasi TB.Absence of MHC class II antigen expression in trophoblast cells results from a lack of class II transactivator (CIITA) gene expression. Mol Reprod Dev 1998; 51: 1–12. [DOI] [PubMed] [Google Scholar]
- Morris AC, Riley JL, Fleming WH, Boss JM.MHC class II gene silencing in trophoblast cells is caused by inhibition of CIITA expression. Am J Reprod Immunol 1998; 40: 385–394. [DOI] [PubMed] [Google Scholar]
- van den Elsen PJ, van der Stoep N, Vietor HE, Wilson L, van Zutphen M, Gobin SJ.Lack of CIITA expression is central to the absence of antigen presentation functions of trophoblast cells and is caused by methylation of the IFN-gamma inducible promoter (PIV) of CIITA. Hum Immunol 2000; 61: 850–862. [DOI] [PubMed] [Google Scholar]
- Hunt JS.Stranger in a strange land. Immunol Rev 2006; 21: 36–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JC, Petroff MG, Holtz R, Alfaidy N, Murphy SP.Dampening of IFN-gamma-inducible gene expression in human choriocarcinoma cells is due to phosphatase-mediated inhibition of the JAK/STAT-1 pathway. J Immunol 2007; 178: 1598–1607. [DOI] [PubMed] [Google Scholar]
- Blumenstein M, Keelan JA, Bowen-Shauver JM, Mitchell MD.Suppressors of cytokine signaling proteins in human preterm placental tissues. J Mol Endocrinology 2005; 35: 165–175. [DOI] [PubMed] [Google Scholar]
- Greenhalgh CJ, Hilton DJ.Negative regulation of cytokine signaling. J Leukoc Biol 2001; 70: 348–356. [PubMed] [Google Scholar]
- Ma Z, Qin H, Benveniste EN.Transcriptional suppression of matrix metalloproteinase-9 gene expression by IFN-gamma and IFN-beta: critical role of STAT-1alpha. J Immunol 2001; 167: 5150–5159. [DOI] [PubMed] [Google Scholar]
- Nozelli S, Ma Z, Wilson C, Shah R, Benveniste EN.Class II major histocompatibility complex transactivator (CIITA) inhibits matrix metalloproteinase-9 gene expression. J Biol Chem 2004; 279: 38577–38589. [DOI] [PubMed] [Google Scholar]
- Holtz R, Choi JC, Petroff MG, Piskurich JF, Murphy SP.Class II transactivator (CIITA) promoter methylation does not correlate with silencing of CIITA transcription in trophoblasts. Biol Reprod 2003; 69: 915–924. [DOI] [PubMed] [Google Scholar]
- Goodbourn S, Didcock L, Randall RE.Interferons: cell signalling, immune modulation, antiviral response and virus countermeasures. J Gen Virol 2000; 81: 2341–2364. [DOI] [PubMed] [Google Scholar]
- Zhu H, Nelson DR, Crawford JM, Liu C.Defective Jak-Stat activation in hepatoma cells is associated with hepatitis C viral IFN-alpha resistance. J Interferon Cytokine Res 2005; 25: 528–539. [DOI] [PubMed] [Google Scholar]
- Clark DA, Chaouat G, Arck PC, Mittruecker HW, Levy GA.Cytokine-dependent abortion in CBA x DBA/2 mice is mediated by the procoagulant fgl2 prothrombinase. J Immunol 1998; 160: 545–549. [PubMed] [Google Scholar]
- Haddad EK, Duclos AJ, Antecka E, Lapp WS, Baines MG.Role of interferon-gamma in the priming of decidual macrophages for nitric oxide production and early pregnancy loss. Cell Immunol 1997; 181: 68–75. [DOI] [PubMed] [Google Scholar]
- Girardi G, Yarilin D, Thurman JM, Holers VM, Salmon JE.Complement activation induces dysregulation of angiogenic factors and causes fetal rejection and growth restriction. J Exp Med 2006; 203: 2165–2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg M, Martin U, Rheinheimer C, Köhl J, Bautsch W, Böttger EC, Klos A.IFN-gamma up-regulates the human C5a receptor (CD88) in myeloblastic U937 cells and related cell lines. J Immunol 1995; 155: 4419–4426. [PubMed] [Google Scholar]
- Lappin DF, Guc D, Hill A, McShane T, Whaley K.Effect of interferon-gamma on complement gene expression in different cell types. Biochem J 1992; 281(pt 2):437–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu MC, Hentzel MD, Dziuk PJ.Effect of stage of gestation, litter size and uterine space on the incidence of mummified fetuses in pigs. J Anim Sci 1988; 66: 3202–3207. [DOI] [PubMed] [Google Scholar]
- Geisert RD, Schmitt RAM.Early embryonic survival in the pig: can it be improved? J Anim Sci 2002; 80: E54–E65. [Google Scholar]
- Correale J, Arias M, Gilmore W.Steroid hormone regulation of cytokine secretion by proteolipid protein-specific CD4+ T cell clones isolated from multiple sclerosis patients and normal control subjects. J Immunol 1998; 161: 3365–3374. [PubMed] [Google Scholar]
- Ostensen M, Forger F, Villiger PM.Cytokines and pregnancy in rheumatic disease. Ann N Y Acad Sci 2006; 1069: 353–363. [DOI] [PubMed] [Google Scholar]
- Vukusic S, Confavreux C.Pregnancy and multiple sclerosis: the children of PRIMS. Clin Neurol Neurosurg 2006; 108: 266–270. [DOI] [PubMed] [Google Scholar]
- Al-Shammri S, Rawoot P, Azizieh F, AbuQoora A, Hanna M, Saminathan TR, Raghupathy R.Th1/Th2 cytokine patterns and clinical profiles during and after pregnancy in women with multiple sclerosis. J Neurol Sci 2004; 222: 21–27. [DOI] [PubMed] [Google Scholar]
- Jahromi M, Millward A, Demaine A.A CA repeat polymorphism of the IFN-gamma gene is associated with susceptibility to type 1 diabetes. J Interferon Cytokine Res 2000; 20: 187–190. [DOI] [PubMed] [Google Scholar]
- Wilson SB, Kent SC, Patton KT, Orban T, Jackson RA, Exley M, Porcelli S, Schatz DA, Atkinson MA, Balk SP, Strominger JL, Hafler DA.Extreme Th1 bias of invariant Valpha24JalphaQ T cells in type 1 diabetes. Nature 1998; 391: 177–181. [DOI] [PubMed] [Google Scholar]
- Hämäläinen AM, Savola K, Kulmala PK, Koskela P, Akerblom HK, Knip M;, Finnish TRIGR Study Group. Disease-associated autoantibodies during pregnancy and at birth in families affected by type 1 diabetes. Clin Exp Immunol 2001; 126: 230–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke SD, Dong H, Hazan AD, Croy BA.Aberrant endometrial features of pregnancy in diabetic NOD mice. Diabetes 2007; 56: 2919–2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punareewattana K, Holladay SD.Immunostimulation by complete Freund's adjuvant, granulocyte macrophage colony-stimulating factor, or interferon-gamma reduces severity of diabetic embryopathy in ICR mice. Birth Defects Res A Clin Mol Teratol 2004; 70: 20–27. [DOI] [PubMed] [Google Scholar]
- Veith GL, Rice GE.Interferon gamma expression during human pregnancy and in association with labour. Gynecol Obstet Invest 1999; 48: 163–167. [DOI] [PubMed] [Google Scholar]
- Wilke C, Renz H, Tekesin I, Hellmeyer L, Herz U, Schmidt S.Suppression of IL-2 and IFN-gamma production in women with spontaneous preterm labor. J Perinat Med 2006; 34: 20–27. [DOI] [PubMed] [Google Scholar]
- Curry AE, Vogel I, Drews C, Schendel D, Skogstrand K, Flanders WD, Hougaard D, Olsen J, Thorsen P.Mid-pregnancy maternal plasma levels of interleukin 2, 6, and 12, tumor necrosis factor-alpha, interferon-gamma, and granulocyte-macrophage colony-stimulating factor and spontaneous preterm delivery. Acta Obstet Gynecol Scand 2007; 86: 1103–1110. [DOI] [PubMed] [Google Scholar]
- Bahar AM, Ghalib HW, Moosa RA, Zaki ZMS, Thomas C, Nabri OA.Maternal serum interleukin-6, interleukin-8, tumor necrosis factor-alpha and interferon-gamma in preterm labor. Acta Obstet Gynecol Scand 2003; 82: 543–549. [PubMed] [Google Scholar]
- Suguitan AL, Jr, Cadigan TJ, Nguyen TA, Zhou A, Leke RJ, Metenou S, Thuita L, Megnekou R, Fogako J, Leke RG, Taylor DW.Malaria-associated cytokine changes in the placenta of women with pre-term deliveries in Yaounde, Cameroon. Am J Trop Med Hyg 2003; 69: 574–581. [PubMed] [Google Scholar]
- Park JS, Park CW, Lockwood CJ, Norwitz ER.Role of cytokines in preterm labor and birth. Minerva Ginecol 2005; 57: 349–366. [PubMed] [Google Scholar]
- Hanna N, Bonifacio L, Reddy P, Hanna I, Weinberger B, Murphy S, Laskin D, Sharma S.IFN-gamma-mediated inhibition of COX-2 expression in the placenta from term and preterm labor pregnancies. Am J Reprod Immunol 2004; 51: 311–318. [DOI] [PubMed] [Google Scholar]
- Patrick LA, Smith GN.Proinflammatory cytokines: a link between chorioamnionitis and fetal brain injury. J Obstet Gynaecol Can 2002; 24: 705–709. [DOI] [PubMed] [Google Scholar]
- Hansen-Pupp I, Harling S, Berg AC, Cilio C, Hellström-Westas L, Ley D.Circulating interferon-gamma and white matter brain damage in preterm infants. Pediatr Res 2005; 58: 946–952. [DOI] [PubMed] [Google Scholar]
- Sibai B, Dekker G, Kupferminc M.Pre-eclampsia. Lancet 2005; 365: 785–799. [DOI] [PubMed] [Google Scholar]
- Lyall F, Belfort M.Pre-eclampsia Etiology Clinical Practice. Cambridge, UK:Cambridge University Press;2007: 1–534. [Google Scholar]
- Hiby SE, Walker JJ, O'shaughnessy KM, Redman CW, Carrington M, Trowsdale J, Moffett A.Combinations of maternal KIR and fetal HLA-C genes influence the risk of preeclampsia and reproductive success. J Exp Med 2004; 200: 957–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JM.Endothelial dysfunction in preeclampsia. Semin Reprod Endocrinol 1998; 16: 5–15. [DOI] [PubMed] [Google Scholar]
- Redman CWG, Sargent IL.Immunological factors and placentation: implications for pre-eclampsia. Lyall F, Belfort M.Pre-eclampsia Etiology Clinical Practice Cambridge, UK:Cambridge University Press;2007: 103–120. [Google Scholar]
- Widmer M, Villar J, Benigni A, Conde-Agudelo A, Karumanchi SA, Lindheimer M.Mapping the theories of preeclampsia and the role of angiogenic factors: a systematic review. Obstet Gynecol 2007; 109: 168–180. [DOI] [PubMed] [Google Scholar]
- Kawano Y, Matsui N, Kamihigashi S, Narahara H, Miyakawa I.Effects of interferon-gamma on secretion of vascular endothelial growth factor by endometrial stromal cells. Am J Reprod Immunol 2000; 43: 47–52. [DOI] [PubMed] [Google Scholar]
- Kitaya K, Yasuo T, Yamaguchi T, Fushiki S, Honjo H.Genes regulated by interferon-gamma in human uterine microvascular endothelial cells. Int J Mol Med 2007; 20: 689–697. [PubMed] [Google Scholar]