Abstract
Oocyte-cumulus cell bidirectional communication is essential for normal development of the oocyte and cumulus cells (CCs) within the follicle. We showed recently that addition of recombinant growth differentiation factor 9 (GDF9), which signals through the SMAD2/3 pathway, during mouse oocyte in vitro maturation (IVM) increased fetal viability. This study thus aimed to observe the effects of disrupting oocyte-CC bidirectional communication during IVM on oocyte developmental competence and fetal outcomes. Cumulus-oocyte complexes (COCs) from equine chorionic gonadotropin-primed prepubertal (CBA/C57BL6) mice were cultured with or without 50 mIU/ml follicle-stimulating hormone (FSH) and 10 ng/ml epidermal growth factor (EGF) or 4 μM SMAD2/3 inhibitor SB-431542. Cumulus expansion and first polar body extrusion were then assessed, or COCs were fertilized and stained to evaluate sperm entry or cultured to the blastocyst stage. Embryo development and blastocyst quality were assessed, and Day 4.5 blastocysts were transferred to pseudopregnant recipients to analyze fetal outcomes. SMAD2/3 inhibition or FSH/EGF absence during IVM resulted in decreased cumulus expansion. First polar body extrusion and sperm entry were decreased in the absence of FSH/EGF, whereas only sperm entry was affected in SB-431542-matured COCs. Embryo development and blastocyst rates were unaffected; however, blastocyst quality was significantly altered, with reduced inner cell mass cell numbers in embryos derived from COCs matured in both treatments. When COCs were matured with SB-431542 in the absence of FSH/EGF, cumulus expansion was reduced, but fertilization, embryo development, and embryo quality were not. Inhibition of SMAD2/3 signaling in the presence of FSH/EGF significantly reduced fetal survival but had no effect on implantation or fetal and placental dimensions and morphology.
Keywords: blastocyst, cumulus cells, EGF, embryo, fetal, FSH, gamete biology, in vitro fertilization, in vitro maturation, oocyte-cumulus bidirectional loop, oocyte development, pregnancy, SMAD2/3
Disruption of oocyte-cumulus paracrine signaling during mouse oocyte in vitro maturation decreases subsequent blastocyst quality and fetal survival.
INTRODUCTION
In mammalian Graafian follicles, mural granulosa cells line the follicular wall, and a set of highly specialized somatic cells known as cumulus cells surround the oocyte, forming the cumulus-oocyte complex (COC). These layers of granulosa and cumulus cells mediate external endocrine signals to the oocyte, enabling oocyte growth and maturation. Prior to the ovulatory luteinizing hormone (LH) surge, cumulus cells are connected to the oocyte by transzonal cytoplasmic processes that facilitate intercellular gap junction communication [1]. Through these gap junctions, cumulus cells are able to transport amino acids, key metabolites, and signaling molecules that are essential for oocyte growth and development [2].
The dependency of oocytes on their surrounding cumulus cells has long been established. Oocytes do not metabolize glucose well [3, 4], and they are dependent on cumulus cells for pyruvate and other glycolytic products [5, 6] and amino acids [7], which are essential to their growth and development. However, research in the past decade has shown that the oocyte is not a passive recipient but a key regulator of its own development. Through both paracrine signaling, using oocyte-secreted factors (OSFs), such as growth differentiation factor 9 (GDF9), and gap junctional signaling, oocytes are able to influence surrounding cumulus cells and regulate their immediate microenvironment [2, 8]. Oocytes have been shown to be the rate-limiting factor of follicular growth [9] and have been shown to influence cumulus cell glycolytic activity [10] as well as amino acid transport [11]. Bovine cumulus-enclosed oocytes have been shown to have increased developmental competence when cocultured with denuded oocytes [12]. Furthermore, we have shown recently that addition of recombinant GDF9 during mouse oocyte in vitro maturation (IVM) significantly increased blastocyst quality and fetal survival [13]. This bidirectional communication between the oocytes and their cumulus cells thus appears vital for oocyte growth, development, and survival.
Growth differentiation factor 9 is a member of the transforming growth factor-β (TGF-β) superfamily, and hence it signals through the interaction of two membrane-bound serine/threonine kinase receptors. Growth differentiation factor 9 first binds to its bone morphogenetic protein (BMP) type 2 receptor (BMPR2) [14], and the activated BMPR2 then phosphorylates and activates the type 1 receptor, TGF-β 1 receptor (TGFBR1), also known as the activin A receptor type 2-like kinase 5 (ALK5) [15]. Once activated, TGFBR1 phosphorylates transcription factors SMADs 2 and 3 which, together with SMAD4, translocate to the nucleus and induce gene transcription through interaction with SMAD-response elements in gene promoter regions [16, 17].
Paracrine communication by the oocyte through SMAD2/3 signaling in cumulus cells is required for the process of cumulus expansion [18]. Cumulus expansion is the process where cumulus cells form a hyaluronan-rich viscoelastic matrix in response to gonadotrophin signals. Cumulus expansion facilitates ovulation and is necessary for female fertility in vivo, because mice with defective cumulus cell matrix formation are anovulatory and have compromised fertility [19]. In vitro, cumulus expansion can be stimulated through the use of follicle-stimulating hormone (FSH) and epidermal growth factor (EGF) ligands [20, 21]. However, these alone are insufficient to induce cumulus expansion without an oocyte-secreted, cumulus-enabling factor, because mouse COCs with surgically removed oocytes are unable to expand unless cocultured with denuded oocytes or with recombinant GDF9 [22–24]. Similarly, studies in our laboratory have shown that the oocyte-secreted, cumulus expansion-enabling factor is unable to induce cumulus expansion on its own without ligand (e.g., FSH) stimulation [22], reflecting the importance of bidirectional communication between the oocytes and their surrounding cumulus cells. Furthermore, upon gonadotropin stimulation, the oocyte has been suggested to permit mitogen-activated protein kinase (MAPK) activation in cumulus cells, an event which, in turn, is necessary for cumulus expansion and oocyte maturation [25].
Taken together, it appears that FSH/EGF and SMAD2/3 signaling have an interactive effect on cumulus expansion and meiotic maturation, and thus we hypothesized that these signaling pathways during IVM are also likely to have an impact on oocyte developmental competence. This study therefore sought to investigate the importance of oocyte and cumulus bidirectional communication through the SMAD2/3 and FSH/EGF signaling pathways during IVM on oocyte maturation, cumulus expansion, and subsequent embryonic and fetal development.
MATERIALS AND METHODS
All chemicals and reagents were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO) unless otherwise specified.
Isolation and Culture of COCs
All animals were purchased from Laboratory Animal Services (Adelaide, Australia) and treated in accordance with the “Australian Code of Practice for the Care and Use of Animals for Scientific Purposes.” Mice were housed in a 14L:10D cycle, and food and water were supplied ad libitum.
Cumulus-oocyte complexes were obtained from 21- to 25-day-old CBA/C57BL6 female mice 46–48 h after administration of an intraperitoneal injection of 5 IU of equine chorionic gonadotropin (Folligon; Intervet, Bendigo, Australia). Germinal vesicle (GV)-stage COCs were aspirated using a 30.5-gauge needle from large antral follicles into Waymouth MB 752/1 medium supplemented with 5% fetal calf serum (Invitrogen, Carlsbad, CA) buffered with HEPES and sodium bicarbonate (handling medium). Cumulus-oocyte complexes were washed once in handling medium then cultured for 17.5–18 h at 37°C in 6% CO2, 5% O2, and 89% N2 in Waymouth MB 752/1 medium supplemented with 5% (v/v) fetal calf serum. In vitro maturation treatments included: 1) the positive control, 50 mIU/ml recombinant human FSH (Puregon; Organon, Oss, the Netherlands) and 10 ng/ml EGF; 2) absence of FSH/EGF; 3) 4 μM SB-431542 (generously donated by GlaxoSmithKline, Stevenage, U.K.); and 4) FSH/EGF plus SB-431542. The carrier control for SB-431542 was COCs treated with 0.04% (v/v) dimethyl sulfoxide (DMSO) plus FSH/EGF. SB-431542 has no toxicity to cells and acts as a competitive ATP-binding site kinase inhibitor with high specificity toward ACVR1B (ALK4), TGFBR1 (ALK5), and ACVR1C (ALK7) when used at <10 μM [26]. SB-431542 was used at 4 μM, because this concentration was shown previously to prevent cumulus expansion [18]. Only COCs with a uniform covering of compacted cumulus cells were used in this study.
Cumulus Expansion Assessment
Cumulus expansion was assessed blinded to treatments according to the 0–4 scale, and the cumulus expansion index (CEI) was calculated as described previously [27, 28]. Using this scale, score 0 indicates no detectable response characterized by the detachment of cumulus cells from the oocyte to assume a flattened monolayer of fibroblastic appearance. A score of +1 indicates the minimum observable response where COCs remain spherical, cumulus cells have a glistering appearance, and most cells remain compacted around the oocyte. For score +2 complexes, only the outermost layers of cumulus cells have expanded; score +3 complexes have all layers except the corona radiata (cells most proximal to the oocyte) prominently expanded; and a score of +4 indicates the maximum degree of expansion including the corona radiata [27].
Assessment of Meiotic Maturation and Sperm Penetration
After 18 h of IVM, oocytes were denuded by repeat gentle pipetting of COCs in MOPS-G1 [29] containing 1 mg/ml hyaluronidase. Oocytes then were washed in MOPS-G1 and then stained with 3 μM 4′-6-diamidino-2-phenylindole (DAPI) for 10 min to facilitate assessment of meiotic maturation status by the presence of nuclei-stained first polar bodies (PB1s). To assess the ability of sperm to bind to and penetrate the in vitro-matured oocytes, the presence of a decondensing sperm head was investigated. Oocytes were cultured with capacitated sperm as described below for 0.5 h, denuded, washed in MOPS-G1, and stained with DAPI for 10 min. The DAPI-stained oocytes/zygotes were mounted in modified MOPS-G1 (formulated without phenol red and albumin) and analyzed under ultraviolet light at 200× magnification for the presence of the PB1 or the sperm head in oocytes. Analysis of sperm penetration included all in vitro-matured oocytes, regardless of meiotic status.
In Vitro Fertilization and Embryo Culture
After 17.5–18 h of IVM, COCs were washed twice in fertilization medium (α-minimal essential media [Invitrogen] supplemented with 3 mg/ml bovine serum albumin [BSA], 75 mg/L penicillin G, and 50 mg/L streptomycin sulphate) and coincubated with capacitated sperm from CBA/C57BL6 mice with proven fertility for 4 h at 37°C in 6% CO2, 5% O2, and 89% N2. Presumptive zygotes were washed once in MOPS-G1, then again in G-1 v3 (Vitrolife, Kungsbacka, Sweden) and cultured in groups of approximately 10 in 20-μl G-1 v3 drops overlaid with mineral oil at 37°C in 6% CO2, 5% O2, and 89% N2. Fertilization rates were determined by the number of two-cell embryos, which was assessed 20 h after the 4-h gamete coincubation. Two-cell embryos were then transferred to fresh G-1 v3 drops for another 25–27 h, after which embryos were assessed for their rate of development as determined by the percentage of eight-cell and compacted embryos. All embryos were then transferred into G-2 v3 (Vitrolife) medium in 20-μl drops overlaid with mineral oil for 47–49 h at 37°C in 6% CO2, 5% O2, and 89% N2. Embryonic morphology was assessed at the end of the culture period (96–100 h of embryo culture) to determine blastocyst development.
Differential Staining
Blastocyst inner cell mass (ICM) and trophectoderm (TE) cell numbers were determined using a differential nuclei staining protocol described by Gardner et al. [30]. Briefly, 0.5% pronase at 37°C was used to dissolve the zona pellucida. Blastocysts were then incubated for 10 min in 10 mM 2,4,6-trinitrobenzenesulfonic acid at 4°C, and then in 0.1 mg/ml anti-dinitrophenyl-BSA for 10 min at 37°C with washes in HEPES-buffered modified G1 medium in between each step. Blastocysts were then placed in guinea pig serum with propidium iodide for 5 min at 37°C and then stained with bisbenzimide in ethanol at 4°C overnight. Prior to mounting in a glycerol drop on a siliconized slide, blastocysts were washed in 100% alcohol. Analysis was performed under a fluorescent microscope at 200× magnification, where ICM and TE cells appeared blue and pink, respectively, under ultraviolet light.
Embryo Transfer
Swiss female recipient mice were mated with vasectomized males and anesthetized on Day 3.5 of pseudopregnancy with 2% Avertin (0.015 ml/g body weight) prior to embryo transfer. Six blastocysts from one treatment were randomly assigned to each uterine horn. A total of 42 embryos were transferred per treatment. On Day 18 of pregnancy, the percentage of implantations, fetal development, fetal and placental weight, and fetal crown-to-rump length were assessed.
Statistical Analysis
All data represent three or more experimental replicates except when stated. Cumulus expansion and embryonic development were analyzed using a 2 × 2 ANOVA and univariate general linear analysis of variance using least-significant differences when treatments had equal error variance and a Dunnett T3 posthoc when treatments had unequal error variance. Meiotic maturation, sperm entry analysis, and pregnancy outcomes were assessed using a Fisher exact test. Treatments with a P value of ≤0.05 were taken to be significantly different. All statistical analyses were performed using SPSS version 13.0 for windows (SPSS, Chicago, IL) or GraphPad online software (GraphPad, La Jolla, CA).
RESULTS
Effect of FSH/EGF and SMAD2/3 Signaling During IVM on Cumulus Expansion
As expected, cumulus expansion did not occur in the absence of FSH/EGF (CEI, 0.3 ± 0.2 vs. 2.6 ± 0.2 with FSH/EGF). Addition of SB-431542 in the presence of FSH/EGF also significantly reduced cumulus expansion (CEI = 0.6 ± 0.2) to levels similar to those when FSH and EGF were absent. Interestingly, SB-431542 and absence of FSH/EGF did have an interactive combined negative impact on the morphology of the COCs observed at the end of the maturation period. Almost all complexes had total detachment of the cumulus cells from the oocytes to assume a flattened monolayer of fibroblastic appearance adhered to the bottom of the culture dish, rendering most oocytes completely denuded. As such, an observation that had not been described previously within the Vanderhyden scoring system [27], this treatment was excluded from cumulus expansion analysis. There was no significant difference in cumulus expansion between FSH/EGF (2.6 ± 0.2) and the carrier control (DMSO and FSH/EGF, 3.1 ± 0.3; P > 0.05).
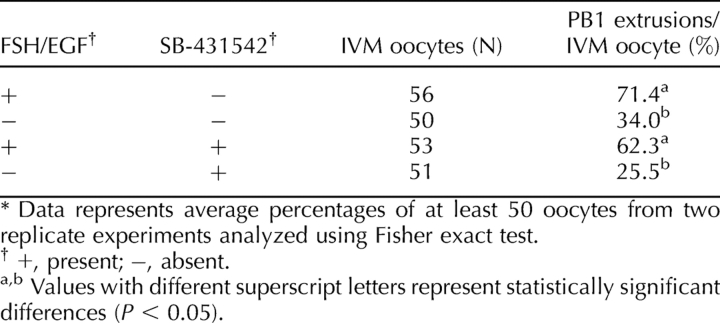
Effect of FSH/EGF and SMAD2/3 Signaling During IVM on Meiotic Maturation
Given the deficient cumulus expansion observed both in the absence of FSH/EGF and/or the presence of SB-431542, the need for SMAD2/3 and FSH/EGF signaling to complete meiosis 1 was investigated at the end of the 18-h maturation period. Lack of FSH and EGF in IVM significantly (P < 0.001) reduced PB1 extrusion to 34.0% at 18 h after maturation, compared with 71.4% when matured with FSH and EGF (Table 1). Inhibition of SMAD2/3 had no effect on PB1 extrusion (62.3%) in the presence of FSH/EGF, but only 25.5% of oocytes completed meiosis 1 after 18 h of culture without FSH/EGF in the presence of SB-431542. Two-way ANOVA analysis confirmed no interaction between SB-431542 and FSH/EGF; thus, inhibition of SMAD 2/3 had no additional consequences over the effects of lack of FSH/EGF on completion of meiotic maturation. There was no significant difference (P = 0.64) between the DMSO control and IVM with FSH/EGF on polar body extrusion (68% and 72%, respectively).
TABLE 1.
Effect of FSH/EGF and SMAD2/3 signaling during IVM on meiotic maturation.*
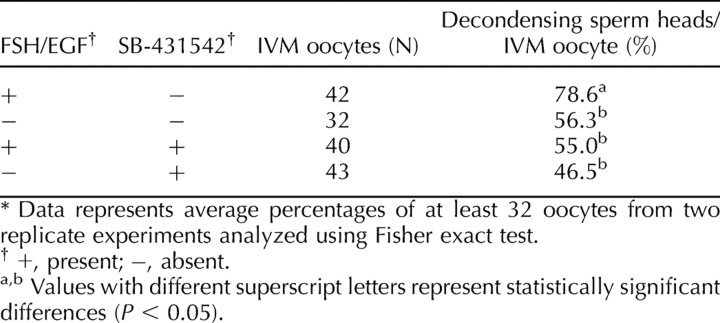
Effect of FSH/EGF and SMAD2/3 Signaling During IVM on Sperm Penetration
To investigate whether inhibition of oocyte-to-cumulus bidirectional communication and the resultant lack of cumulus expansion during IVM would have an effect on sperm penetration, oocytes were incubated with sperm from male mice with proven fertility for 30 min and stained to assess the presence of a sperm head within the oocytes. Both the absence of FSH/EGF and inhibition of SMAD2/3 significantly (P < 0.05) decreased sperm entry relative to the FSH/EGF control. Inhibition of SMAD2/3 in the absence of FSH/EGF did not further decrease sperm entry (Table 2). There was no significant difference (P = 0.50) in sperm penetration between the carrier (DMSO) control and positive (FSH/EGF) control (69% and 79%, respectively).
TABLE 2.
Effect of FSH/EGF and SMAD2/3 inhibition on sperm entry during IVM.*
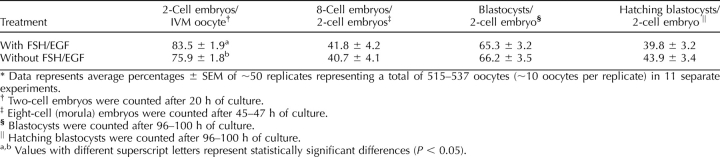
Effect of FSH/EGF and SMAD2/3 Signaling During IVM on Subsequent Embryo Development
The effects of either FSH/EGF and/or SB-431542 during IVM on subsequent embryo development were determined. The absence of FSH and EGF marginally but significantly decreased the percentage of two-cell embryos per IVM oocyte, compared with when the ligands were present (Table 3). Lack of FSH/EGF during IVM had no effect on the rate of development or the ability of resulting embryos to develop into blastocysts. The percentage of hatching blastocysts was also not significantly different from when FSH and EGF were used during IVM.
TABLE 3.
Effect of FSH/EGF exposure during IVM on subsequent embryo development.*
Inhibition of SMAD2/3 in the presence of FSH and EGF during IVM had no significant effect on two-cell embryo development, rate of embryo development, or blastocyst and hatching blastocyst formation when compared with its DMSO control (Table 4).
TABLE 4.
Effect of SMAD2/3 signaling during IVM on subsequent embryo development.*
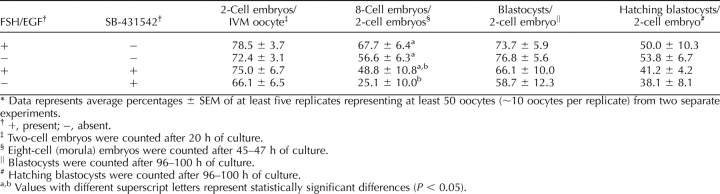
The combined effects of SMAD2/3 inhibition and FSH/EGF absence on oocyte maturation in vitro were then investigated and compared to IVM without FSH/EGF only and SMAD2/3 inhibition in the presence of FSH/EGF. In vitro maturation with FSH/EGF only was used as the positive control (Table 5). Oocytes matured under these combined conditions had no significant difference in their ability to form two-cell embryos. However, SB-431542 in the absence of FSH/EGF led to a significant decrease in embryo developmental rate, as shown in the lower percentages of eight-cell/compacted embryos compared with IVM without SB-431542, with or without FSH/EGF (P < 0.05). Two-way ANOVA analysis revealed that this decreased developmental rate was not due to an interaction between FSH/EGF and SB-431542. There were no differences between treatments in blastocyst and hatching blastocyst development.
TABLE 5.
Effect of FSH/EGF and SMAD2/3 signaling during IVM on subsequent embryo development.*
Effect of FSH/EGF and SMAD2/3 Signaling During IVM on Blastocyst Cell Numbers
Blastocyst quality was assessed by the determination of blastocyst cell numbers and cell allocation to the TE and ICM. In vitro maturation without FSH/EGF resulted in a significant decrease (P < 0.05) in total blastocyst cell numbers compared with when the ligands were present (48.3 ± 3.3 vs. 63.3 ± 3.5; P < 0.05). This could be attributed largely to a significant decrease (P < 0.001) in blastocyst ICM cell numbers: IVM without FSH/EGF produced blastocysts with approximately half the ICM size as IVM with FSH/EGF. There was no significant difference in TE cell numbers with the presence or absence of FSH/EGF (Table 6).
TABLE 6.
Effect of FSH/EGF exposure during oocyte IVM on blastocyst quality.*
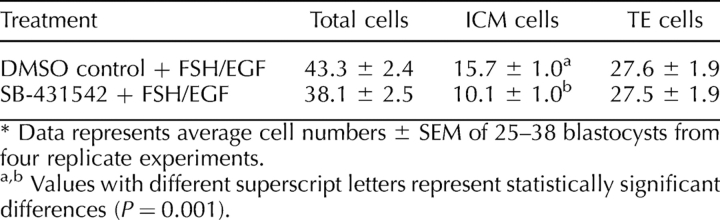
Despite no decrease in blastocyst development rates, SMAD2/3 inhibition in the presence of FSH and EGF also had a similar effect, with a tendency for lower total cell numbers (38.1 ± 2.5 vs. 43.3 ± 2.4) and a significant decrease in ICM cell numbers (10.1 ± 1.0 vs. 15.7 ± 1.0; P < 0.001), whereas TE cell numbers were unaffected (Table 7).
TABLE 7.
Effect of SMAD2/3 signaling during oocyte IVM on blastocyst quality.*
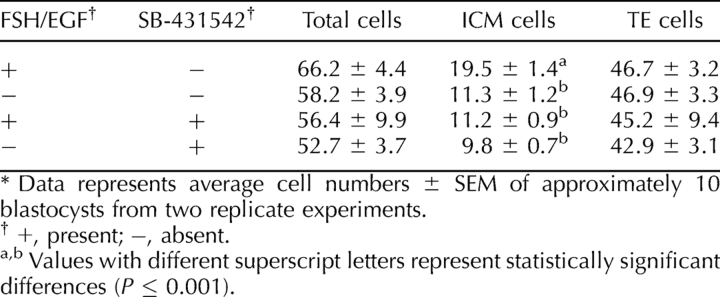
Absence of FSH/EGF and inhibition of SMAD2/3 during IVM did not have a combined effect on resulting blastocyst quality. Aside from reduced blastocyst ICM cell numbers when compared with embryos derived from oocytes matured with FSH/EGF only, blastocysts resulting from oocytes that had been matured with both FSH/EGF absence and SMAD2/3 inhibition did not have total, ICM, or trophectoderm cell numbers significantly different from that of the other treatments, as shown in Table 8.
TABLE 8.
Effect of FSH/EGF and SMAD2/3 signaling during oocyte IVM on blastocyst quality.*
Effect of SMAD2/3 Inhibition During IVM on Pregnancy Outcomes
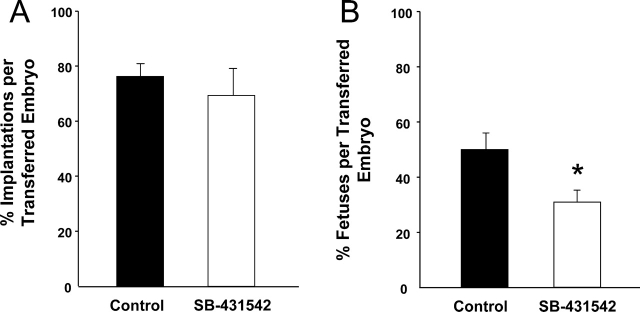
Inhibition of SMAD2/3 did not have any effect on the capacity of the blastocyst to implant but significantly reduced fetal survival per embryo transferred (50.0 ± 6.3 control vs. 30.1 ± 4.4; P < 0.05; Fig. 1). Fetuses that developed from oocytes matured with SMAD2/3 inhibition were not significantly different in weight, placental:fetal weight ratio, or crown-to-rump length compared with the DMSO control (Fig. 2). There were also no morphological abnormalities in fetuses developed from oocytes matured with SMAD2/3 inhibition.
FIG. 1.
Effect of SMAD2/3 inhibition in the presence of FSH/EGF during IVM on pregnancy outcomes. Day 4.5 blastocysts developed from COCs matured in the DMSO (0.04% [v/v]) control or with 4 μM SB-431542 in the presence of FSH (50 mIU) and EGF (10 ng/ml) were transferred to pseudopregnant recipients and outcomes were analyzed on Day 18 of pregnancy. Black and white bars represent embryos derived from oocytes matured with the DMSO carrier control and SB-431542 treatment, respectively. Data represent percentage of mean number of implantation sites (A) or fetuses (B) per embryo transferred ± SEM of seven replicate experiments. *Significant difference with P < 0.05. N = 42 embryos transferred per treatment.
FIG. 2.
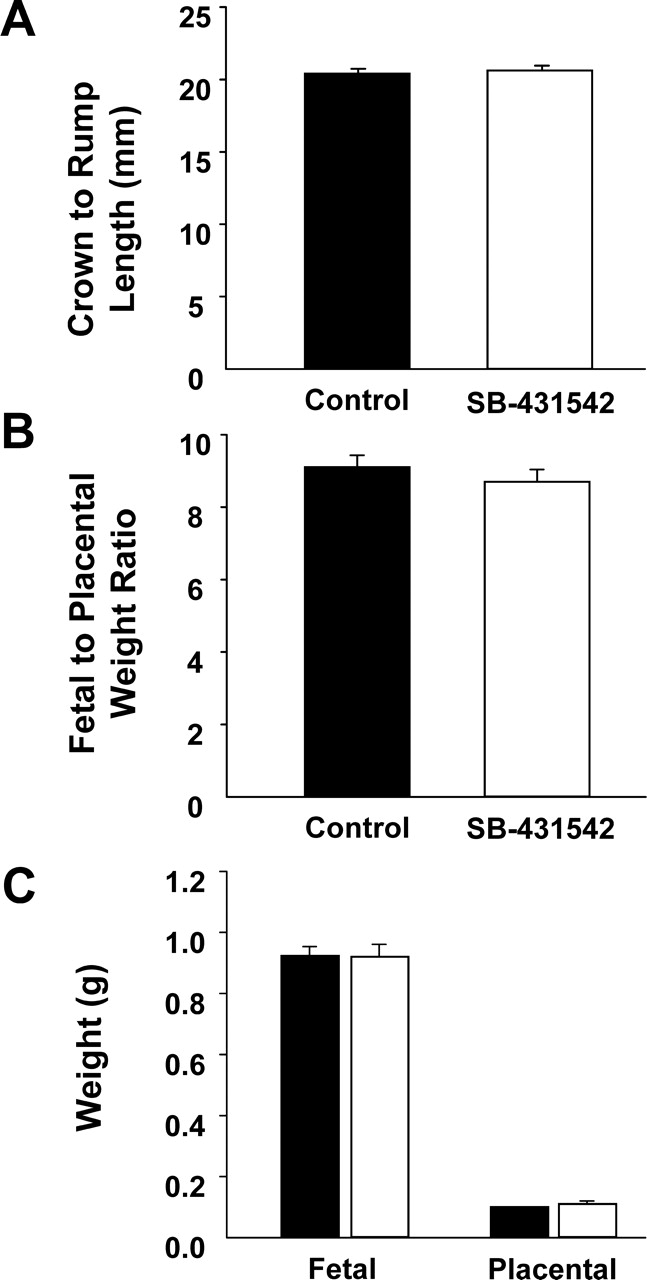
Effect of SMAD2/3 inhibition in the presence of FSH/EGF during IVM on fetal and placental outcomes. Day 4.5 blastocysts developed from COCs matured in the DMSO (0.04% [v/v]) control or with 4 μM SB-431542 in the presence of FSH (50 mIU) and EGF (10 ng/ml) were transferred to pseudopregnant recipients, and outcomes were analyzed on Day 18 of pregnancy. Black and white bars represent embryos derived from oocytes matured with the DMSO carrier control and SB-431542 treatment, respectively. Data represent percentage of mean fetal crown-to-rump length (A), fetal:placental weight ratio (B), and fetal and placental weight (C) ± SEM of 12–21 fetuses/placentas in seven replicate experiments. N = 42 embryos transferred per treatment.
DISCUSSION
We have shown previously that the addition of recombinant GDF9 during IVM increases blastocyst development [12] and subsequent fetal viability [13]. The purpose of this study was thus to investigate the effects of disrupting this oocyte-cumulus bidirectional communication during IVM on subsequent embryonic and fetal development.
The recognition of the mammalian oocyte's ability to control follicular cell development and function has only emerged recently, in this decade [2, 8]. Oocytes have been shown to orchestrate follicular development [9], secrete mitogens eliciting cumulus and granulosa cell proliferation [31–33], prevent cumulus cell luteinization [34], lower the apoptotic index of cumulus cells [35], and thereby maintain the distinct cumulus cell phenotype [33]. Although it has long been established that preovulatory oocytes can only use oxidative phosphorylation for energy production and are dependent on cumulus cells to metabolize glucose and provide the carboxylic acid substrates [3], it has only been discovered recently that oocytes regulate cumulus cell metabolism, enabling transport of glycolytic products to themselves [10]. Oocytes have also been discovered to be poor synthesizers of cholesterol and manipulate cumulus cell cholesterol synthesis for provision of products of the cholesterol biosynthetic pathway [36].
Oocytes exert these effects through the use of paracrine factors, such as BMP15 and GDF9 [2]. Growth differentiation factor 9 mediates its effects through activation of the SMAD2/3 signaling pathway in cumulus cells. SMAD2/3 signaling has been shown to be the mechanistic pathway that oocytes use to regulate cumulus cell cholesterol synthesis [36], gene transcription, expansion [18], proliferation [31], and differentiation of granulosa cells during follicle antrum formation to the cumulus cell lineage [37]. Given the dependency of cumulus cell functions on oocyte paracrine signaling, we hypothesized that inhibiting SMAD2/3 signaling would be detrimental to oocyte developmental competence. The SMAD2/3 pathway was therefore targeted for disruption of oocyte-to-cumulus communication.
This was achieved through the use of SB-431542, a small molecule that acts as a competitive ATP-binding site kinase inhibitor highly specific for ACVR1B (ALK4), TGFBR1 (ALK5), and ACVR1C (ALK7), the kinases that activate SMAD2/3 signaling [16], When used at concentrations under 10 μM, SB-431542 was found to have no effect on ACVRL1 (ALK1) and ACVR1 (ALK2) , nor did it affect MAPK (ERK), MAPK8 (JNK), or MAPK signaling pathways [26]. The concentration used in this study was 4 μM, because SB-431542 was found to be nontoxic and have the greatest, but specific, inhibitory effect on expression of cumulus expansion gene transcripts at 4 μM [18].
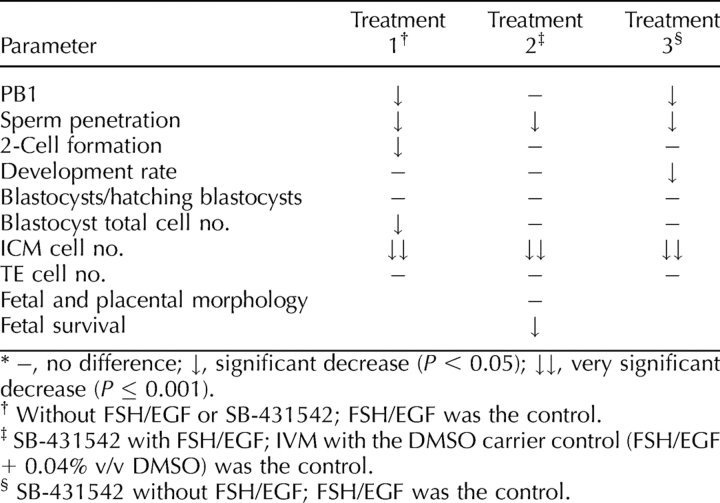
As summarized in Table 9, SMAD2/3 inhibition during IVM had no effect on meiotic maturation or two-cell embryo formation, but it significantly decreased the ability of sperm to penetrate these oocytes. This is most likely attributed to the lack of an expanded cumulus matrix, resulting in a temporary delay in sperm penetration, which would have been neutralized to that of the controls by the end of the 4-h gamete coincubation period, given that there were no significant differences in two-cell embryo formation. These results are consistent with previous findings, where SMAD2/3 inhibition during bovine IVM had no effect on two-cell embryo development [12], and where GDF9 had no effect on meiotic resumption, despite inducing MAPK activity [25]. Although blastocyst formation was unaffected, blastocyst quality was severely reduced, with significant reductions in ICM cell numbers in blastocysts derived from oocytes matured with SMAD2/3 inhibition compared with controls. Fetal survival rates were also subsequently reduced following embryo transfer of these blastocysts. These effects of SMAD2/3 inhibition are directly opposite those observed when recombinant GDF9, an activator of the SMAD2/3 signaling pathway [31], was added during oocyte IVM, which resulted in increased fertilization rates, blastocyst ICM cell numbers, and fetal survival rates [13]. As such, oocyte-to-cumulus signaling via the SMAD2/3 pathway and the consequential effects on cumulus cell functions have a direct and significant impact on oocyte developmental competence, illustrating the importance of the oocyte-cumulus cell regulatory loop to oocyte development.
TABLE 9.
Summary of results of treatments with respect to IVM.*
It is well established that FSH and EGF have positive effects on meiotic maturation [38], cumulus expansion, oocyte fertilization, and subsequent embryo development and fetal outcomes when used during oocyte IVM [39–43]. These effects are mediated through the cumulus cells to the oocyte, because oocytes do not have FSH receptors [44], and these ligands only improve oocyte developmental competence in the presence of cumulus cells [21, 45–47]. Hence, the FSH and EGF signaling pathways were targeted as the mode for cumulus-to-oocyte communication in this study.
Polar body one extrusion was significantly reduced in oocytes matured in the absence of FSH/EGF. Follicle-stimulating hormone and EGF are both known to have meiotic-inducing effects and override the inhibitory effects of hypoxanthine to induce meiotic maturation in cumulus-enclosed oocytes [38]. Sperm penetration was also lower in oocytes matured in the absence of FSH/EGF compared with the FSH/EGF IVM control. A decrease in two-cell embryo formation was also observed and was most likely due to the decreased meiotic maturation rates.
Contrary to another study [40], we did not observe any effects of FSH and EGF during oocyte IVM on the ability of oocytes to form blastocysts, but we did observe an effect on blastocyst quality, as depicted by the substantial decrease in total and ICM numbers when FSH and EGF were not present. This is most likely due to differing culture conditions and mouse strains. C57BL6/CBA hybrid mice have been shown to produce blastocysts with a low apoptotic index [48]; IVM in Waymouth media produces maximal rates of blastocyst formation [49]; and IVM with serum has been shown to increase blastocyst formation [50] and blastocyst cell numbers [51]. Similarly, SMAD2/3 inhibition in the presence of FSH/EGF only affected blastocyst quality, with a significant reduction in ICM cell numbers compared with the control.
Stimulation of the MAPK pathway through increased cAMP levels in cumulus cells leads to cumulus expansion, and both GDF9 and FSH/EGF have been shown to activate MAPK through independent pathways [25, 52, 53], increasing cumulus expansion gene transcripts, such as prostaglandin-endoperoxide synthase 2 (Ptgs2) and hyaluronan synthase 2 (Has2) [18, 19]. However, FSH and/or EGF, together with an oocyte-secreted cumulus expansion-enabling factor, such as GDF9, must be present for mouse cumulus expansion [22, 24], and it has been suggested that oocyte paracrine factors “license” MAPK activation by cAMP in cumulus cells [25]. Although the importance of MAPK to oocyte developmental competence remains to be elucidated, the interactive effect of FSH/EGF and SMAD2/3 signaling could explain why the absence of FSH/EGF and SMAD2/3 signaling produced similar adverse outcomes on oocyte developmental competence.
Aside from maintaining the cumulus cell phenotype for the provision of substrates and metabolites crucial for oocyte development, there are other probable factors and mechanisms for how and why oocyte-cumulus bidirectional communication prior to and during oocyte maturation regulates cumulus cell function to affect oocyte developmental competence. Oocytes denuded from their cumulus cells prior to IVM were found to undergo GVBD at a higher rate than cumulus-enclosed oocytes, but had a lower incidence of PB1 extrusion [54] and could not develop into blastocysts [46]. Ge et al. [54] also showed that removal of cumulus cells prior to IVM altered cortical granule redistribution, spindle assembly, mitochondria distribution and function, and meiosis-promoting factor activity, all of which were significantly rescued to levels comparable to cumulus-enclosed oocytes, when the denuded oocytes were matured on a cumulus cell monolayer derived from GV-stage COCs. Meiotic spindle morphology was also more reflective of in vivo-ovulated oocytes in COCs matured with EGF [55]. Although the presence of cumulus cells is important to the acquisition of oocyte developmental competence, recent studies suggest that the act of expansion itself or the number of cumulus cells left attached to the oocyte at the end of the maturation period is not [46, 47, 56]. Our findings further support this, given that PB1 extrusion in oocytes matured without FSH/EGF but with the SMAD2/3 inhibitor was not significantly different from without FSH/EGF only (25.5% vs. 34.0%; Table 1), nor was there any significant difference in sperm penetration or two-cell embryo formation in all the treatments—IVM without FSH/EGF, SB-431542 with FSH/EGF, and SB-431542 without FSH/EGF—where cumulus expansion was negatively affected, despite the differences in morphology and the number of actual cumulus cells left attached to the oocyte at the end of the maturation period. It is also important to highlight that all subsequent developmental data were expressed as a percentage of two-cell embryos, not the number of IVM oocytes; hence, the lack of differences in blastocyst and hatching blastocyst formation was independent of any events perturbing meiotic maturation and fertilization in COCs matured with both SMAD2/3 inhibition and without FSH/EGF in comparison with when only one inhibitory mechanism was present. This implies that expansion itself is not reflective of oocyte developmental competence in vitro but is an indication of the signaling capability of the oocyte and the cumulus cells.
Increased oocyte maturation rates and developmental competence have been linked to prolonged persistence of oocyte-cumulus cell gap junctional communication associated with high levels of cAMP but independent of cumulus expansion [57, 58]. This is supported by the findings that cumulus cells translate ligand stimulatory signals in a paracrine manner [38, 59], maintaining dictyate arrest and inducing GVBD in a timely, coordinated fashion [60]. Because FSH increases cAMP levels [45], it is possible that bidirectional communication between the oocyte and cumulus via SMAD2/3 and FSH and EGF signaling prolongs gap junction communication, allowing the exchange of factors necessary for optimal oocyte developmental competence. However, this remains for further investigation.
Oocyte-cumulus bidirectional communication is thus an intricate relationship and essential for the development and function of both cell types. The findings of this study show for the first time the interdependency of both directions of oocyte and cumulus communication and demonstrate the importance of their persistence during oocyte maturation to the acquisition of oocyte developmental competence and subsequent embryonic and fetal development.
Acknowledgments
The authors wish to thank Associate Professor Jeremy Thompson and Dr. Megan Mitchell for their support and useful discussions, and Alicia Stump, Kara Cashman, Samantha Schulz, and Lesley Ritter for their technical assistance.
Footnotes
1Supported by Australian National Health and Medical Research Council (NHMRC) project grants. R.B.G. is a recipient of an R.D. Wright Fellowship from the NHMRC, and M.L. is a recipient of an NHMRC Senior Research Fellowship.
REFERENCES
- Albertini DF, Combelles CM, Benecchi E, Carabatsos MJ.Cellular basis for paracrine regulation of ovarian follicle development. Reproduction 2001; 121: 647–653. [DOI] [PubMed] [Google Scholar]
- Gilchrist RB, Lane M, Thompson JG.Oocyte-secreted factors: regulators of cumulus cell function and oocyte quality. Hum Reprod Update 2008; 14: 159–177. [DOI] [PubMed] [Google Scholar]
- Biggers JD, Whittingham DG, Donahue RP.The pattern of energy metabolism in the mouse oocyte and zygote. Proc Natl Acad Sci U S A 1967; 58: 560–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leese HJ, Barton AM.Pyruvate and glucose uptake by mouse ova and preimplantation embryos. J Reprod Fertil 1984; 72: 9–13. [DOI] [PubMed] [Google Scholar]
- Donahue RP, Stern S.Follicular cell support of oocyte maturation: production of pyruvate in vitro. J Reprod Fertil 1968; 17: 395–398. [DOI] [PubMed] [Google Scholar]
- Leese HJ, Barton AM.Production of pyruvate by isolated mouse cumulus cells. J Exp Zool 1985; 234: 231–236. [DOI] [PubMed] [Google Scholar]
- Colonna R, Mangia F.Mechanisms of amino acid uptake in cumulus-enclosed mouse oocytes. Biol Reprod 1983; 28: 797–803. [DOI] [PubMed] [Google Scholar]
- Eppig J.Oocyte control of ovarian follicular development and function in mammals. Reproduction 2001; 122: 829–838. [DOI] [PubMed] [Google Scholar]
- Eppig JJ, Wigglesworth K, Pendola FL.The mammalian oocyte orchestrates the rate of ovarian follicular development. Proc Natl Acad Sci U S A 2002; 99: 2890–2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura K, Pendola FL, Eppig JJ.Oocyte control of metabolic cooperativity between oocytes and companion granulosa cells: energy metabolism. Dev Biol 2005; 279: 20–30. [DOI] [PubMed] [Google Scholar]
- Eppig JJ, Pendola FL, Wigglesworth K, Pendola JK.Mouse oocytes regulate metabolic cooperativity between granulosa cells and oocytes: amino acid transport. Biol Reprod 2005; 73: 351–357. [DOI] [PubMed] [Google Scholar]
- Hussein TS, Thompson JG, Gilchrist RB.Oocyte-secreted factors enhance oocyte developmental competence. Dev Biol 2006; 296: 514–521. [DOI] [PubMed] [Google Scholar]
- Yeo CX, Gilchrist RB, Thompson JG, Lane M.Exogenous growth differentiation factor 9 in oocyte maturation media enhances subsequent embryo development and fetal viability in mice. Hum Reprod 2008; 23: 67–73. [DOI] [PubMed] [Google Scholar]
- Vitt UA, Mazerbourg S, Klein C, Hsueh AJ.Bone morphogenetic protein receptor type II is a receptor for growth differentiation factor-9. Biol Reprod 2002; 67: 473–480. [DOI] [PubMed] [Google Scholar]
- Mazerbourg S, Klein C, Roh J, Kaivo-Oja N, Mottershead DG, Korchynskyi O, Ritvos O, Hsueh AJW.Growth differentiation factor-9 signaling is mediated by the type I receptor, activin receptor-like kinase 5. Mol Endocrinol 2004; 18: 653–665. [DOI] [PubMed] [Google Scholar]
- Piek E, Heldin CH, Ten Dijke P.Specificity, diversity, and regulation in TGF-beta superfamily signaling. FASEB J 1999; 13: 2105–2124. [PubMed] [Google Scholar]
- Kaivo-Oja N, Bondestam J, Kamarainen M, Koskimies J, Vitt U, Cranfield M, Vuojolainen K, Kallio JP, Olkkonen VM, Hayashi M, Moustakas A, Groome NP, et al. Growth differentiation factor-9 induces Smad2 activation and inhibin B production in cultured human granulosa-luteal cells. J Clin Endocrinol Metab 2003; 88: 755–762. [DOI] [PubMed] [Google Scholar]
- Dragovic RA, Ritter LJ, Schulz SJ, Amato F, Thompson JG, Armstrong DT, Gilchrist RB.Oocyte-secreted factor activation of SMAD 2/3 signaling enables initiation of mouse cumulus cell expansion. Biol Reprod 2007; 76: 848–857. [DOI] [PubMed] [Google Scholar]
- Russell DL, Robker RL.Molecular mechanisms of ovulation: co-ordination through the cumulus complex. Hum Reprod Update 2007; 13: 289–312. [DOI] [PubMed] [Google Scholar]
- Diaz FJ, O'Brien MJ, Wigglesworth K, Eppig JJ.The preantral granulosa cell to cumulus cell transition in the mouse ovary: development of competence to undergo expansion. Dev Biol 2006; 299: 91–104. [DOI] [PubMed] [Google Scholar]
- Downs SM, Daniel SA, Eppig JJ.Induction of maturation in cumulus cell-enclosed mouse oocytes by follicle-stimulating hormone and epidermal growth factor: evidence for a positive stimulus of somatic cell origin. J Exp Zool 1988; 245: 86–96. [DOI] [PubMed] [Google Scholar]
- Dragovic RA, Ritter LJ, Schulz SJ, Amato F, Armstrong DT, Gilchrist RB.Role of oocyte-secreted growth differentiation factor 9 in the regulation of mouse cumulus expansion. Endocrinology 2005; 146: 2798–2806. [DOI] [PubMed] [Google Scholar]
- Elvin JA, Clark AT, Wang P, Wolfman NM, Matzuk MM.Paracrine actions of growth differentiation factor-9 in the mammalian ovary. Mol Endocrinol 1999; 13: 1035–1048. [DOI] [PubMed] [Google Scholar]
- Buccione R, Vanderhyden BC, Caron PJ, Eppig JJ.FSH-induced expansion of the mouse cumulus oophorus in vitro is dependent upon a specific factor(s) secreted by the oocyte. Dev Biol 1990; 138: 16–25. [DOI] [PubMed] [Google Scholar]
- Su YQ, Denegre JM, Wigglesworth K, Pendola FL, O'Brien MJ, Eppig JJ.Oocyte-dependent activation of mitogen-activated protein kinase (ERK1/2) in cumulus cells is required for the maturation of the mouse oocyte-cumulus cell complex. Dev Biol 2003; 263: 126–138. [DOI] [PubMed] [Google Scholar]
- Inman GJ, Nicolas FJ, Callahan JF, Harling JD, Gaster LM, Reith AD, Laping NJ, Hill CS.SB-431542 is a potent and specific inhibitor of transforming growth factor-beta superfamily type I activin receptor-like kinase (ALK) receptors ALK4, ALK5, and ALK7. Mol Pharmacol 2002; 62: 65–74. [DOI] [PubMed] [Google Scholar]
- Vanderhyden BC, Caron PJ, Buccione R, Eppig JJ.Developmental pattern of the secretion of cumulus expansion-enabling factor by mouse oocytes and the role of oocytes in promoting granulosa cell differentiation. Dev Biol 1990; 140: 307–317. [DOI] [PubMed] [Google Scholar]
- Fagbohun C, Downs S.Maturation of the mouse oocyte-cumulus cell complex: stimulation by lectins. Biol Reprod 1990; 42: 413–423. [DOI] [PubMed] [Google Scholar]
- Gardner DK, Lane M.Culture of the mammalian preimplantation embryo. Gardner DK, Lane M, Watson AJ.A Laboratory Guide to the Mammalian Embryo New York:Oxford University Press;2004: 41–61. [Google Scholar]
- Gardner DK, Lane MW, Lane M.EDTA stimulates cleavage stage bovine embryo development in culture but inhibits blastocyst development and differentiation. Mol Reprod Dev 2000; 57: 256–261. [DOI] [PubMed] [Google Scholar]
- Gilchrist RB, Ritter LJ, Myllymaa S, Kaivo-Oja N, Dragovic RA, Hickey TE, Ritvos O, Mottershead DG.Molecular basis of oocyte-paracrine signalling that promotes granulosa cell proliferation. J Cell Sci 2006; 119: 3811–3821. [DOI] [PubMed] [Google Scholar]
- Hickey TE, Marrocco DL, Amato F, Ritter LJ, Norman RJ, Gilchrist RB, Armstrong DT.Androgens augment the mitogenic effects of oocyte-secreted factors and growth differentiation factor 9 on porcine granulosa cells. Biol Reprod 2005; 73: 825–832. [DOI] [PubMed] [Google Scholar]
- Li R, Norman RJ, Armstrong DT, Gilchrist RB.Oocyte-secreted factor(s) determine functional differences between bovine mural granulosa cells and cumulus cells. Biol Reprod 2000; 63: 839–845. [DOI] [PubMed] [Google Scholar]
- Vanderhyden BC, Tonary AM.Differential regulation of progesterone and estradiol production by mouse cumulus and mural granulosa cells by A factor(s) secreted by the oocyte. Biol Reprod 1995; 53: 1243–1250. [DOI] [PubMed] [Google Scholar]
- Hussein TS, Froiland DA, Amato F, Thompson JG, Gilchrist RB.Oocytes prevent cumulus cell apoptosis by maintaining a morphogenic paracrine gradient of bone morphogenetic proteins. J Cell Sci 2005; 118: 5257–5268. [DOI] [PubMed] [Google Scholar]
- Su YQ, Sugiura K, Wigglesworth K, O'Brien MJ, Affourtit JP, Pangas SA, Matzuk MM, Eppig JJ.Oocyte regulation of metabolic cooperativity between mouse cumulus cells and oocytes: BMP15 and GDF9 control cholesterol biosynthesis in cumulus cells. Development 2008; 135: 111–121. 18045843 [Google Scholar]
- Diaz FJ, Wigglesworth K, Eppig JJ.Oocytes determine cumulus cell lineage in mouse ovarian follicles. J Cell Sci 2007; 120: 1330–1340. [DOI] [PubMed] [Google Scholar]
- Coticchio G, Rossi G, Borini A, Grondahl C, Macchiarelli G, Flamigni C, Fleming S, Cecconi S.Mouse oocyte meiotic resumption and polar body extrusion in vitro are differentially influenced by FSH, epidermal growth factor and meiosis-activating sterol. Hum Reprod 2004; 19: 2913–2918. [DOI] [PubMed] [Google Scholar]
- Ali A, Sirard MA.Protein kinases influence bovine oocyte competence during short-term treatment with recombinant human follicle stimulating hormone. Reproduction 2005; 130: 303–310. [DOI] [PubMed] [Google Scholar]
- De La Fuente R, O'Brien MJ, Eppig JJ.Epidermal growth factor enhances preimplantation developmental competence of maturing mouse oocytes. Hum Reprod 1999; 14: 3060–3068. [DOI] [PubMed] [Google Scholar]
- Eppig JJ, Hosoe M, O'Brien MJ, Pendola FM, Requena A, Watanabe S.Conditions that affect acquisition of developmental competence by mouse oocytes in vitro: FSH, insulin, glucose and ascorbic acid. Mol Cell Endocrinol 2000; 163: 109–116. [DOI] [PubMed] [Google Scholar]
- Izadyar F, Zeinstra E, Bevers MM.Follicle-stimulating hormone and growth hormone act differently on nuclear maturation while both enhance developmental competence of in vitro matured bovine oocytes. Mol Reprod Dev 1998; 51: 339–345. [DOI] [PubMed] [Google Scholar]
- Merriman JA, Whittingham DG, Carroll J.The effect of follicle stimulating hormone and epidermal growth factor on the developmental capacity of in-vitro matured mouse oocytes. Hum Reprod 1998; 13: 690–695. [DOI] [PubMed] [Google Scholar]
- van Tol HT, van Eijk MJ, Mummery CL, van den Hurk R, Bevers MM.Influence of FSH and hCG on the resumption of meiosis of bovine oocytes surrounded by cumulus cells connected to membrana granulosa. Mol Reprod Dev 1996; 45: 218–224. [DOI] [PubMed] [Google Scholar]
- Sirard MA, Desrosier S, Assidi M.In vivo and in vitro effects of FSH on oocyte maturation and developmental competence. Theriogenology 2007; 68(suppl 1):S71–S76. [DOI] [PubMed] [Google Scholar]
- Ge L, Han D, Lan GC, Zhou P, Liu Y, Zhang X, Sui HS, Tan JH.Factors affecting the in vitro action of cumulus cells on the maturing mouse oocytes. Mol Reprod Dev 2008; 75: 136–142. [DOI] [PubMed] [Google Scholar]
- Atef A, François P, Christian V, Marc-André S.The potential role of gap junction communication between cumulus cells and bovine oocytes during in vitro maturation. Mol Reprod Dev 2005; 71: 358–367. [DOI] [PubMed] [Google Scholar]
- Kamjoo M, Brison DR, Kimber SJ.Apoptosis in the preimplantation mouse embryo: effect of strain difference and in vitro culture. Mol Reprod Dev 2002; 61: 67–77. [DOI] [PubMed] [Google Scholar]
- van de Sandt JJ, Schroeder AC, Eppig JJ.Culture media for mouse oocyte maturation affect subsequent embryonic development. Mol Reprod Dev 1990; 25: 164–171. [DOI] [PubMed] [Google Scholar]
- Eppig JJ, O'Brien MJ.Comparison of preimplantation developmental competence after mouse oocyte growth and development in vitro and in vivo. Theriogenology 1998; 49: 415–422. [DOI] [PubMed] [Google Scholar]
- Sagirkaya H, Misirlioglu M, Kaya A, First NL, Parrish JJ, Memili E.Developmental potential of bovine oocytes cultured in different maturation and culture conditions. Anim Reprod Sci 2007; 101: 225–240. [DOI] [PubMed] [Google Scholar]
- Su YQ, Wigglesworth K, Pendola FL, O'Brien MJ, Eppig JJ.Mitogen-activated protein kinase activity in cumulus cells is essential for gonadotropin-induced oocyte meiotic resumption and cumulus expansion in the mouse. Endocrinology 2002; 143: 2221–2232. [DOI] [PubMed] [Google Scholar]
- Shimada M, Hernandez-Gonzalez I, Gonzalez-Robayna I, Richards JS.Paracrine and autocrine regulation of epidermal growth factor-like factors in cumulus oocyte complexes and granulosa cells: key roles for prostaglandin synthase 2 and progesterone receptor. Mol Endocrinol 2006; 20: 1352–1365. [DOI] [PubMed] [Google Scholar]
- Ge L, Sui HS, Lan GC, Liu N, Wang JZ, Tan JH.Coculture with cumulus cells improves maturation of mouse oocytes denuded of the cumulus oophorus: observations of nuclear and cytoplasmic events. Fertil Steril 2008; 90: 2376–2388. [DOI] [PubMed] [Google Scholar]
- Rossi G, Macchiarelli G, Palmerini MG, Canipari R, Cecconi S.Meiotic spindle configuration is differentially influenced by FSH and epidermal growth factor during in vitro maturation of mouse oocytes. Hum Reprod 2006; 21: 1765–1770. [DOI] [PubMed] [Google Scholar]
- Gutnisky C, Dalvit GC, Pintos LN, Thompson JG, Beconi MT, Cetica PD.Influence of hyaluronic acid synthesis and cumulus mucification on bovine oocyte in vitro maturation, fertilisation and embryo development. Reprod Fertil Dev 2007; 19: 488–497. [DOI] [PubMed] [Google Scholar]
- Modina S, Luciano AM, Vassena R, Baraldi-Scesi L, Lauria A, Gandolfi F.Oocyte developmental competence after in vitro maturation depends on the persistence of cumulus-oocyte comunications which are linked to the intracellular concentration of cAMP. Ital J Anat Embryol 2001; 106: 241–248. [PubMed] [Google Scholar]
- Thomas RE, Armstrong DT, Gilchrist RB.Bovine cumulus cell-oocyte gap junctional communication during in vitro maturation in response to manipulation of cell-specific cyclic adenosine 3′,5′-monophosophate levels. Biol Reprod 2004; 70: 548–556. [DOI] [PubMed] [Google Scholar]
- Byskov AG, Yding Andersen C, Hossaini A, Guoliang X.Cumulus cells of oocyte-cumulus complexes secrete a meiosis-activating substance when stimulated with FSH. Mol Reprod Dev 1997; 46: 296–305. [DOI] [PubMed] [Google Scholar]
- Farin CE, Rodriguez KF, Alexander JE, Hockney JE, Herrick JR, Kennedy-Stoskopf S.The role of transcription in EGF- and FSH-mediated oocyte maturation in vitro. Anim Reprod Sci 2007; 98: 97–112. [DOI] [PMC free article] [PubMed] [Google Scholar]