Abstract
The steroid hormone, progesterone (P), modulates neuroendocrine functions in the central nervous system resulting in alterations in physiology and reproductive behavior in female mammals. A wide body of evidence indicates that these neural effects of P are predominantly mediated via their intracellular progestin receptors (PRs) functioning as “ligand-dependent” transcription factors in the steroid-sensitive neurons regulating genes and genomic networks. In addition to P, intracellular PRs can be activated by neurotransmitters, growth factors and cyclic nucleotides in a ligand-independent manner via crosstalk and convergence of pathways. Furthermore, recent studies indicate that rapid signaling events associated with membrane PRs and/or extra-nuclear, cytoplasmic PRs converge with classical PR activated pathways in neuroendocrine regulation of female reproductive behavior. The molecular mechanisms, by which multiple signaling pathways converge on PRs to modulate PR-dependent female reproductive behavior, are discussed in this review.
Keywords: progesterone, progestin receptors, dopamine, signaling, cross talk, reproduction, behavior, brain, neurotransmitter, ligand-independent activation
1. Introduction
It has been recognized since the early 19th century that progesterone (P) is one of the most biologically active “progestins” of ovarian origin, which plays a major role in the reproduction of mammalian species. It was originally discovered as the mammalian pregnancy hormone, essential in the initiation and maintenance of pregnancy. In the decades following this discovery, the coordinating role of P in multiple interdependent reproductive functions, such as ovulation, mammary gland development and reproductive behavior has also been understood [40, 171, 210, 211]. In recent years, the non-reproductive functions of P have been expanded to include its effects on the central nervous system, i.e., neuroprotection, myelination, inflammation, cognition and mood [40, 44, 67, 171, 236]. These findings have stimulated extensive investigations into the molecular mechanisms by which P exerts its broad range of effects on the brain and behavior.
In this article, we will briefly review the current state of knowledge of the structural and functional aspects of progestin receptors (PRs), and discuss their functional role in the central nervous system. We will also discuss the neurotransmitter-PR interactions in the modulation of this behavioral response in rodents, and summarize our current knowledge of the cellular and molecular mechanisms involved in this regulation. The focus of this review is on PR-dependent effects on female reproductive behavior in rodent species. For further information on P effects on non-rodent species, the reader is directed to several excellent publications [14, 15, 17, 160, 80, 174].
2. Mechanisms of Progesterone Action
2.1 Classical mechanism
Progestins, including P, exert their biological effects primarily by binding to PRs [240]. Upon binding, PRs undergo conformational change, leading to their nuclear translocation, dimerization and DNA binding [171, 203–205, 259]. When bound directly or indirectly to deoxyribonucleic acid (DNA), PRs interact with basal transcriptional machinery, assisted by coactivator molecules, to initiate chromatin remodeling and transcription of genes [114, 135, 153, 177]. Phosphorylation of both PRs and their coactivators is thought to play a crucial role in the activation of PRs [229–230].
2.1.1 PR: Structural organization and function
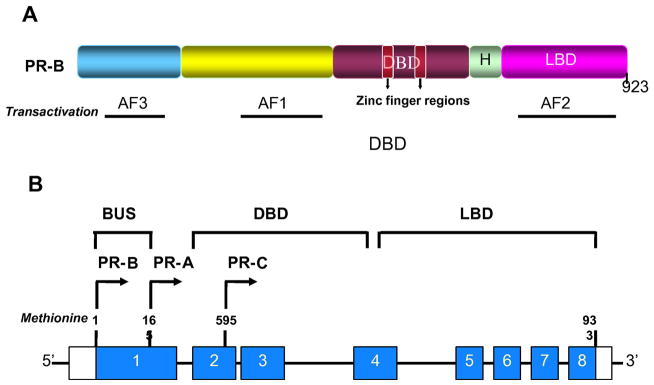
PRs have a modular protein structure consisting of distinct functional domains, capable of binding the ligand at the carboxyl (C)-terminal end (ligand binding domain; LBD), and a highly conserved DNA-binding domain (DBD), which is required for the transcriptional activity (Fig. 1A). The amino-terminal (N) region contains a transactivation function (AF1), which modulates the level and promoter specificity of target gene, by interacting with components of the core transcriptional complex and coregulator proteins [13]. A second activation function (AF2) is present in the LBD region, which contains sequences for dimerization, heat shock protein association, intermolecular silencing and intramolecular repression, in addition to P-binding function [114, 259]. A unique third activation function (AF3) is present in the N-terminal segment of human PR (B isoform), which can function autonomously or synergize with, the downstream activation functions (AF1 and AF2) to enhance their activity [128].
Fig. 1.
Schematic representation of structure and functional organization of progestin receptor (A) and isoforms (B). (A) Progestin receptors (PRs) have a highly conserved DNA binding domain (DBD) and a ligand-binding domain (LBD) connected by a variable Hinge region (H). The N-terminal region contains a transactivation function -1 (AF1). The LBD region contains the AF2 domain. The third activation function domain, AF3 (BUS), is present in the N-terminal segment, and is unique to PR-B. (B) Schematic representation of PR isoforms and splice variants. Nuclear PR gene is composed of 8 exons with 3100-bp coding region and 5′- and 3′-untranslated regions. PR-B and PR-A isoforms are transcribed from two alternate transcription initiation sites. PR-C isoform results from an in-frame initiation of translation and lacks exon 1.
In the absence of ligand, the inactive PRs are associated with a large complex of chaperone proteins in the cytoplasm of target cells [242]. Upon hormone binding, PRs dissociate from the chaperone proteins, dimerize, translocate to the nucleus and bind directly to progesterone-responsive elements (PRE) located in the regulatory regions of target gene DNA, or indirectly through tethering interactions with other transcription factors, i.e., Activator protein 1(AP1), Specificity protein 1(SP1), Signal transducers and activators of transcription proteins (STATs) [20, 68, 79]. Activated DNA-bound PRs, stimulate the rate of formation and/or stabilization of a preinitiation complex consisting of general transcription factors (GTFs), at enhancer-controlled promoters [132, 139, 149]. Whether the preinitiation complex is preformed or recruited sequentially, the rate of assembly of the complexes induced by PRs, in association with their coregulators, ultimately defines the transcriptionally permissive or non-permissive environment at the hormone-regulated promoters.
To date, ~300 nuclear receptor coregulators (coactivators and corepressors) have been biochemically and functionally identified [148]. Many of the coactivators have an L-X-X-L-L motif that permits the interaction of the coactivator with the receptor. In addition, coactivators also have other functional motifs, such as ribonucleic acid (RNA)-interacting domains, i.e., Lin11, Isl-1 and Mec-3 (LIM) domain and bromo domain, that interaction with transcriptional factors such as steroid receptor RNA activator [SRA; 147], small SRA binding protein (SLIRP) [116], and protein p53 [191]. Coactivators possess unique intrinsic enzyme activities including acetyl transferase, ubiquitin ligase, methyl transferase, small ubiquitin-like modifier (SUMO) ligase, phosphokinase, phosphatase, hydrolase, ribosylase, isomerase, helicase, and pseudouridylate synthetase activities [119, 130], which are required for nuclear receptor-mediated gene transcription [227]. The substrates of the enzymes associated with these coactivators involve histones and other coactivators present in the coactivator complex. The enzyme modulation properties of the coactivators afford a high level of regulatory flexibility in the control of PR-mediated gene expression. An excellent description of the coactivators involved in PR regulation can be found in other relevant publications [148, 154, 254]
2.1.2 Phosphorylation of PRs
PRs are phosphoproteins that undergo phosphorylation-dephosphorylation events and provide multi-functionality to P action. Phosphorylation events occur primarily on the serine residues within the N-terminus, and to a lesser extent throughout the PR. A total of 14 serine sites that are phosphorylated basally (in the absence of P), or in response to activation by P or various protein kinases, have been identified both in vitro and in vivo [117, 146]. Among these 14 residues, basal level phosphorylation has been identified on four serine residues (81, 162, 190 and 400). P-dependent phosphorylation has been demonstrated to occur on 3 serine residues within 60 min of treatment, (102, 294, 345). Other serine residues on PR are phosphorylated by specific protein kinases including mitogen-activated kinase (MAPK; on serine 294), casein kinase II (CKII; on serine 81) and cyclin-dependent kinase 2 (cdk2; on serines 25, 162, 190, 213, 400, 554, 676). While the role of PR phosphorylation is not fully understood, it is thought to influence the regulation of both P-dependent and -independent PR nuclear localization, receptor turnover, and coregulator interactions that occur during transcriptional regulation [195].
2.1.3 Multiple forms of PRs
Multiple PR isoforms are produced from a single gene, consisting of 8 exons [Fig. 1B], as a result of transcription from different translational sites [61, 133, 141]. PR-B is the full-length protein consisting of 933 amino acids (101–120 kDa), while PR-A (79–94 kDa) lacks 165 amino acids in the N-terminus, called the B-upstream sequence (BUS). This region encodes AF3 that is specific to the PR-B protein [96], which allows the binding of a subset of coactivators exclusively to PR-B, and not to PR-A. PR-A and PR-B proteins can dimerize as three species A: A and B: B homodimers and A: B heterodimers, which interact with PRE and bind to DNA, as well as GTFs, to regulate gene expression. Thus, PR-A and PR-B contain all the critical components for PR function, including the LBD, DBD and 2 of the three AF domains. The differential structure of the PR isoforms confers distinct tissue-specific responses to P through post-translational modifications, dimerization, and recruitment of cofactor proteins. This contributes to the differential transactivation properties of each isoform, leading to the regulation of distinct subsets of P-dependent target genes. Consistent with the distinct tissue- and promoter-specific activities of PR-A and PR-B in vitro, each individual isoform has been found to modulate distinct subset of reproductive functions by regulating diverse subset of target genes, as seen in their phenotypic response to P in the uterus, the ovary and the mammary gland [62,63, 192, 193].
A third isoform PR-C (60 kDa), resulting from an in-frame initiation of translation at Methionine-595, located within the DBD, was identified in T47D human breast cancer cells. This isoform (338 amino acids; 595–933) contains a large N-terminal truncation that lacks AF3, AF1, and the first zinc finger of DBD [266–267]. While PR-C cannot function as a transcription factor independently, it can interact with the other two isoforms to modulate their transcriptional activity [267]. Several novel truncated isoforms and splice variants have also been identified in vitro [234, 124, 125, 274], the functional relevance of which currently remain unknown. Expression analysis studies suggest that the latter were incapable of yielding translation products in vitro [233].
2.2 Non-classical mechanism
The classical view that PRs mediate P effects, acting as transcriptional factors to facilitate target gene expression, has undergone substantial modifications to incorporate recent discoveries of extra-nuclear, non-classical mechanisms of P regulation. These rapid signaling mechanisms are mediated by cytoplasmic protein kinase cascades [42, 145, 146, 169, 172] and are coupled to novel transmembrane G-protein coupled receptors [279], ion channels, adapter proteins and putative membrane receptors [42, 117, 235].
Rapid and transient activation of extranuclear PRs, independent of PR transcriptional activity, mediated by MAPK, has been demonstrated in mammalian cells in vitro [43, 186]. P signaling, mediated by G protein βγ subunits, has been shown to activate the downstream MAPK cascade during meiotic progression in xenopus oocytes, demonstrating a biologically important role for G proteins in non-classical signaling [28, 88, 89, 157]. Both an increase and a decrease in rapid Ca2+ influx by P has also been reported [115, 179]. In addition, Boonyaratanakornkit et al [42, 43] have demonstrated direct interactions between PRs and c-Src proteins, mediated by polyproline (PXXPXR) domains of PR, which lead to subsequent activation of downstream signaling kinases. Furthermore, a putative common-docking domain, which directly interacts with MEK1, a component of the MAPK cascade, has been reported in the N-terminal BUS of PR-B [117].
Recent evidence suggests the involvement of two types of novel membrane proteins unrelated to classical PRs, progesterone membrane receptor component 1 (PGMRC1; Mw~22 kDa) and progesterone membrane receptors (mPRs; Mw~40 kDa), in P signaling in several reproductive tissues and in the brain. PGMRC1, originally isolated from porcine liver membranes [84, 85, 95, 185], has also been identified in the rat (25-Dx, [203]) and in the human (Hpr6.6, [155]). PGMRC1 is thought to activate P450 proteins functioning as a component of multi-protein P-binding complex [223]. The mPRs, initially discovered in teleost ovaries, are G-protein coupled receptors (GPCRs) that belong to the seven-transmembrane progesterone adiponectin Q receptor (PAQR) family and comprise of at least three subtypes, α, β and γ. mPRs identified in the seatrout are localized to the plasma membrane, bind P with high affinity (Kd~5 nM), and have been shown to be involved in P-mediated induction of meiotic maturation [278, 279] and sperm motility [260]. mPRα receptors down-regulate adenylyl cyclase activity [257] by direct coupling to G proteins and activating pertussis-sensitive inhibitory proteins (Gi/o). Down-regulation of adenylyl cyclase activity has also been observed in the human breast cancer and myometrial cells, in vitro [131]. Zebrafish mPRs, when expressed in classical PR-deficient mammalian breast cancer cells, mediate a rapid and transient P-mediated activation of MAPK, and inhibition of 3′-5′-cyclic adenosine monophosphate (cAMP) production. In the human myometrial cells, mPR activation leads not only to a decline in cAMP levels, but also to the transactivation of classical PR-B. Transactivation of PR-B, not only involves Gi protein coupling, but also decreased steroid receptor coactivator-2 (SRC-2) levels, suggesting a crosstalk between the membrane and nuclear PRs at the transcriptional level [131].
2.3 Ligand-independent activation of PRs
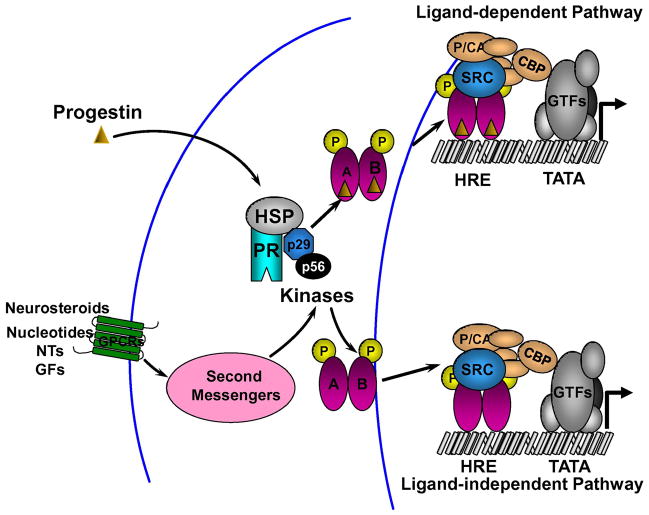
Although, the conventional model of P action assumes that PRs mediate P effects (ligand-dependent activation), a number of studies in the past couple of decades have shown that PRs can be activated by factors other than their cognate ligands (ligand-independent activation) (Fig. 2). Denner et al reported that P-dependent, PR-mediated transcription could be mimicked, in the absence of P, by 8-bromo-cAMP in vitro [68–70]. Protein kinase A (PKA) inhibitors blocked PR activation, suggesting that PR-mediated transcription could be modulated by phosphorylation of PR or other proteins in the transcription complex [70, 229–230]. These observations were soon followed by the studies of Power et al, who reported ligand-independent activation of PRs by neurotransmitter dopamine (DA) in vitro [214–215]. Subsequently, epidermal growth factor (EGF), heregulin, phorbol myristate acetate and insulin growth factors have also been shown to activate PRs, by increasing the phosphorylation of PRs in vitro [84–85, 212, 276].
Fig. 2.
Mechanisms of PR activation. Unliganded PR is present as an inactive complex associated with heat shock proteins (HSP) and chaperone proteins (p29, p56) in the cytoplasm. In the classical ligand dependent pathway of activation (LDA), progesterone and other progestins bind to the PR to induce conformational change, dissociation of HSPs and chaperone proteins. PRs undergo dimerization and bind to the hormone response element (HRE) in the target DNA. Ligand-induced conformational change facilitates the recruitment of cofactors and other general transcription factors (GTFs) to the promoter, producing a transcriptionally active complex that can direct gene transcription. Compounds such as cyclic nucleotides, neurotransmitters (NTs), growth factors (GFs) and neurosteroids can activate second messengers and protein kinase pathways to activate PR and/or coactivators in a ligand-independent manner.
While the precise mechanism of ligand-independent activation of PRs has remained elusive, several studies suggest the involvement of PR phosphorylation in this mechanism. For example, growth factor-initiated signaling pathways (EGF and heregulin) have been reported to enhance phosphorylation of PRs on distinct sites (see 2.2.1). MAPK-dependent signaling has been demonstrated to enhance PR phosphorylation on Ser294 [217–218]. This enhanced phosphorylation has been shown to result in the rapid nuclear translocation of unliganded PRs and nuclear export of liganded PRs, suggesting that MAPK signaling could regulate PR nuclear sequestration, by altering nucleo-cytoplasmic shuttling [144, 217–218]. Studies indicate that PR sequestration in the nucleus serves to protect the inactive and active PRs from degradation by the 26S proteosome pathway [217–218]. It is also tempting to speculate that distinct sets of genes could be activated by phosphorylated liganded PRs and unliganded phosphorylated PRs, via classical and non-classical mechanisms respectively [217–218].
A synergistic effect of P-dependent- and –independent activation of PRs has been reported in the upregulation of growth regulatory genes (cyclins D1 and E) in breast cancer cells in vitro [146, 217]. Cdk2, activated by P, indirectly facilitates PR function, by increasing the recruitment of SRC-1 to liganded PR. In addition, activated Cdk2 also mediates transcriptional activation of PR by phosphorylating PR Ser400 in a ligand-independent manner [212]. In contrast to the direct effects of Cdk2 on PR phosphorylation, cAMP-dependent activation of PR does not involve direct phosphorylation of PR, but affects phosphorylation of SRC-1, to bring about the functional cooperation of SRC-1 and CREB-binding protein [13, 229–230]. A detailed discussion of the studies on PR phosphorylation, using in vitro models, can be found in several excellent reviews [117, 145, 146].
3. Cellular function of P in brain and behavior
3.1 P in female reproductive behavior
In gonadally intact female rodents, the sequential release of ovarian estradiol (E2) and P, integrates the appearance of feminine reproductive behavior (heat, behavioral estrus) with ovulation [19, 41, 66]. In ovariectomized rats, guinea pigs, hamsters and mice, this behavior can be restored by timed sequential administration of E2 followed by P, or by high doses of E2 alone [18, 34, 39, 152, 210, 211, 275]. Such a sequential treatment with E2 and P maximizes the probability that the female will display “lordosis” response, a primary reflexive component of female reproductive behavior, upon mounting by a con-specific male [87, 210–211]. The sequential hormonal regimen also allows lower doses of each of the hormones to be used [59, 269], that results in a more predictable onset and termination of the period of sexual behavior [19, 41] and lordosis duration [262].
Sequential release of E2 and P, are also thought to be necessary for the display of species-typical proceptive or paracopulatory behaviors [19, 37, 39, 40]. These paracopulatory behaviors, exhibited by estrous females in the presence of a sexually active male, include hopping, darting, ear wiggling, approach towards and withdrawal from the male, and production of ultrasonic vocalizations [19, 173, 270]. While E2, by itself, is capable of inducing proceptive behaviors in female rats [81, 104], in most instances, adrenal P is essential for the display of proceptive behaviors [104, 251]. Paracopulatory behaviors have also been observed in ovariectomized and adrenalectomized E2-primed female rats [37, 40], and in DA- facilitation of reproductive behaviors in ovariectomized E2-primed female rats and mice [Mani and Reyna, unpublished observations], suggesting that these behaviors may not be totally dependent on P. For further discussion on the receptive and proceptive behaviors, the reader is referred to other excellent reviews [37, 40].
In addition to its facilitatory effects on female reproductive behavior in female rats, P also plays a role in the termination of sexual behavior during estrous cycle [244–246] and pregnancy [16]. Following exposure to P, rats, hamsters, guinea pigs and mice, become refractory to further stimulation of reproductive behavior, by the administration of P or by E2 and P [29–31, 49, 66, 74, 105, 190, 280–281]. This effect generally referred to as postestrous-refractoriness [190], sequential inhibition [30–31], or biphasic effect [280–281] of P, is believed to limit the duration of behavioral estrus to the periovulatory phase of the estrous cycle period, and is thought to occur as a result of P-dependent down-regulation of PRs [29–31].
Studies demonstrating the temporal concordance of E2-induced PRs with the expression of lordosis, suggest that P plays a significant role in the facilitation of female reproductive behavior [37 and references therein]. Both, nuclear receptor-mediated (classical or genomic)-, and extra-nuclear (membrane-initiated, non-classical or non-genomic)- mechanisms, have been identified in the PR activation of female reproductive behavior. In addition, others and we have also demonstrated ligand-independent activation of PRs, by neurotransmitter DA, in the facilitation of lordosis. Studies in the recent years suggest that these mechanisms are not mutually exclusive, but interact with each other to achieve the behavioral end-point. A detailed discussion on this topic is dealt with in sections 4.0 and 5.0.
3.2 Classical nuclear PR-dependent mechanisms in female reproductive behavior
3.2.1 Spatial and temporal correlation between PR induction and reproductive behavior
Although, diverse cellular mechanisms have been ascribed to P action in the brain, the primary mechanism (although not exclusive) involves its interaction with E2-induced nuclear PRs, which function as transcriptional factors, regulating the expression of genes and genomic neural networks, to initiate and/or sustain physiological response [40, 210–211]. The time course of activation and termination of female sexual behavior parallels E2-induced increase and decline in PRs in the ventrolateral region of the ventromedial hypothalamus (VMH) and the preoptic area (POA) of the brain [33, 46, 66, 206, 231]. Studies using PR antagonists [45, 75, 163, 231], protein and RNA synthesis inhibitors [183, 184, 220], antisense oligonucleotides to PR [162, 200, 213], and mutant mice with targeted deletion of PR gene (Fig. 3a), have provided substantial proof of involvement of PR-mediated genomic mechanism in mediation of P-facilitated female reproductive behavior [158, 165]. For an extensive discussion of these studies, the reader is referred to several excellent reviews [38, 39, 171, 210, 211].
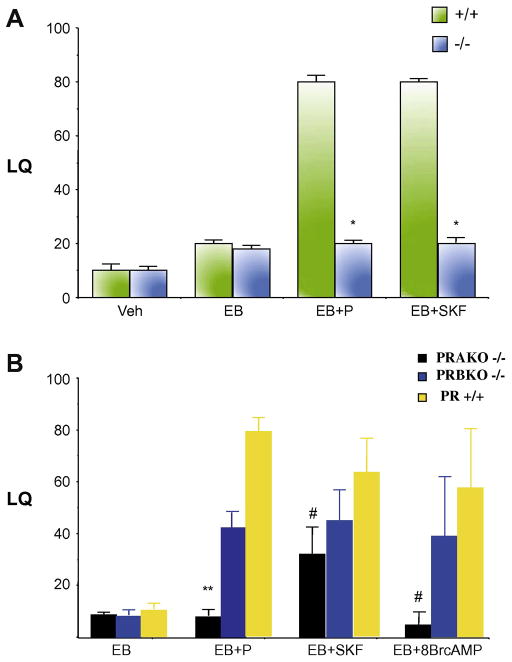
Fig. 3.
Ligand-dependent and –independent activation of nuclear PRs in female reproductive behavior in mice. (a) Ovariectomized, wild type (+/+) and homozygous (−/−) PR mutant mice were primed with estradiol benzoate (EB), followed by intracerebroventricular administration of progesterone (P) or D1 agonist, SKF38393 (SKF) 48 h later. Female receptive behavior in the presence of a male mouse was quantitated and represented as lordosis quotient (LQ). Statistically significant differences were seen in P- and SKF-facilitated lordosis responses of the −/− compared to their +/+ littermates (*P < 0.001). Adapted from Mani et al [165]. (b) Ligand dependent- and -independent induction of sexual receptivity in PR isoform-specific null mutant mice. Ovariectomized PRAKO−/−, PRBKO−/− and PR+/+ mice were primed with EB for 48 h, followed by icv administration of progesterone (P), dopamine D1 agonist SKF 81297 (SKF) or 8-Bromo-cAMP (8-Br-cAMP). P-facilitation of lordosis response was significantly lower in PRAKO−/− null mutants compared to the wild type animals (**P < 0.05). Statistically significant differences (#P < 0.01) were observed in SKF- and 8-Br-cAMP-treated animals compared with EB-treated controls. Adapted from Mani et al [168].
A temporal correlation between declining PRs and female reproductive behavior has been reported during the refractory period in guinea pigs [31, 32, 41]. During this period, the animals were hyposensitive to P and had low concentration of unoccupied hypothalamic PRs. Treatment with physiological levels of P, however, resulted in low levels of occupied nuclear PRs, suggesting that the hyposensitivity and the resulting heat termination could be attributable to the inadequate accumulation of occupied nuclear PRs, in response to P [33–34]. Interestingly, the animals regained P responsiveness upon administration of a high pharmacological dose of P. A large increase in P-occupied hypothalamic PRs accompanied the P responsiveness [29]. Furthermore, pharmacological agents that prevent degradation of the PRs by inhibiting 26S proteosome activity, not only stabilized the concentration of PRs within the hypothalamus and POA, but also prevented the P-induced refractoriness in female rats, confirming that the behavioral refractoriness is causally related to the down-regulation of PRs [78, 99, 102]. Detailed discussion and the interpretation of these studies can be found in several excellent articles [39, 40, 254].
3.2.2 PRs, Coactivators and Behavior
Following reports of the expression of several members of SRC family in the brain [51–55, 254 and references therein], several studies have examined the role of nuclear receptor coactivators in the PR-mediated female reproductive behaviors. Investigations into the role of the coactivators in P-facilitation of female reproductive behavior, using antisense oligonucleotides for SRC-1 and CBP, indicate the requirement of both the coactivators [187–189, 255–256]. Studies by Apostolakis et al [5] have extended the role of coactivators to include SRC-2 in the PR-mediated female reproductive behavior. Coactivators are also expressed in other regions of the brain, including the hippocampus and dentate gyrus [199], and have also been demonstrated to be involved in sexual differentiation and male sex behavior [51–55]. For a detailed discussion on the location and the role of coactivators in the brain the reader is directed to other publication [254].
3.2.3 PRs: non-inducible by Estrogens
Using the high affinity progestin, 3H-R5020 as a radioligand, two anatomically distinct classes of progestin binding sites were identified in the rat [159] and in the guinea pig [32]. In contrast to the E2-induced PRs present in the hypothalamus, POA and pituitary of the female rat [160], guinea pig [33] and the rabbit [60], another class of PRs, insensitive to E2-priming, was identified. These PRs were widely distributed in the cortex, hippocampus, amygdala, caudate-putamen and cerebellum [134]. Significant concentrations of PRs, non-inducible by E2, were also found in areas that contain inducible PRs [207]. However, it should be noted that neural P implants in areas like the midbrain reticular formation [228], habenula [252] and tegmentum [156, 252], some of which lack E2-induced PRs, also facilitate the expression of lordosis in E2-primed rats. While it is believed that these areas are components of the lordosis circuitry, it is not definitively known how the various regions are interconnected, or how they respond to hormones (in the absence of steroid receptors), to facilitate reproductive behavior. It is tempting to postulate that some of these effects could be mediated by non-genomic mechanisms of the steroid hormones, or interactions with neurotransmitters, neuropeptides or other sensory-motor components of the behavior. It is also possible that these non E2-inducible PRs could be mediating P effects that are not dependent upon prior exposure to E2: for example, alterations of cortical electroencephalograph patterns [6]. Whether P effects on negative changes in mood and anxiety seen during the late luteal phase of the menstrual cycle (when levels of P are high) in women could be mediated by the non E2-inducible PRs, remain to be examined [118].
3.2.4 PR isoforms in brain and behavior
As discussed in section 2.1.2, multiple forms of PR have been reported to date. However, with the exception of PR-A and PR-B isoforms, none of the other isoforms have been identified in the brain. In the rat brain, differential expression patterns and region- and hormone-specific regulation of the individual isoforms during development and in adulthood have been documented [48, 110–112, 249]. A detailed discussion on the spatiotemporal expression of neural PR isoforms and their region-specific regulation in the brain can be found in other reviews [170–171].
The development of mutant mice in which the expression of PR-A (PRAKO −/−) and PR-B (PRBKO −/−) isoforms have been selectively ablated, has facilitated the direct analyses of the individual contributions of PR isoforms in mediating neuronal responses to P [192–193]. Studies using the mutant mice, generated by the introduction of point mutations into the PR gene at the ATG codons encoding Methionine 1 (M1A) and Methionine 166 (M166A), have established a critical role for PR-A isoform in the P-facilitation of female receptive behavior in female mice. Ablation of PR-A significantly inhibited P-facilitated receptive behavior in PRAKO−/− mice, while the ablation of PR-B resulted in a decrease in the magnitude of lordosis response to P, compared to their wild type littermates [168]. This reduction of female receptive behavior in PRBKO−/− was not significant, suggesting that PR-A was necessary, but not sufficient, to mediate the full magnitude of the behavioral response in the PRBKO−/− mice (Fig. 3b).
In contrast to these observations in mice, studies in rats indicated that antisense oligonucleotides to PR-B isoform significantly inhibited P-facilitated female receptivity [cf.113], suggesting that PR-B was sufficient in mediating lordosis response in female rats. It is reasonable to assume that these conflicting observations of the requirement of PR-A in mice and PR-B in rats could reflect species-specific differences. However, deletion of PR (PR-A + PR-B), whether by targeted deletion (in mice [168]) or by the administration of a combination of PR-A + PR-B antisense oligonucleotides (in rats [113]), resulted in the inhibition of P-facilitated lordosis response, indicating similarities in PR requirement between the two species. This interesting conundrum needs further evaluation. Furthermore, intracerebroventricular (icv) administration of antisense oligonucleotides to PR-B or PR-A+PR-B combination inhibited not only P-, but also its ring A reduced metabolite (5α-pregnan-3, 20-dione (5α-DHP)-, and 5β, 3β-pregnan-20-one (5β, 3β-Pgl)- facilitated lordosis in EB -primed female rats [113]. Similar to the observations in response to P, the latter reports suggest the critical importance of PR in general, and PR-B isoform in specific, in P metabolite-facilitated female receptive behavior in rats. Further discussion on the role of P metabolites in female reproductive behavior can be found in section 3.3 below.
3.3 Membrane-initiated, non-classical actions of P
While genomic effects have been assumed to be the primary pathway for hormone action in the brain, there are numerous reports of short-latency effects of P suggesting the involvement of “non-classical” effects via putative cell surface receptors and other mechanisms coupled to second messenger signaling cascades. Rapid effects of P have been demonstrated in the release of gonadotropin releasing hormone (GnRH) [211], DA and acetylcholine [182] and excitatory amino acids [243], changes in neuronal activity [120, 136–137] and on facilitation of lordosis response in estrogen-primed female rats [142, 152, 181]. These rapid effects of P are not blocked by protein synthesis inhibitors, are mediated by their binding to putative cell surface membrane receptors [221–222], receptors that gate ion channels [94, 176], and are also coupled to certain second messenger systems [137, 147, 235].
In addition to P, several of its ring-A reduced metabolites, 5α-DHP and5β, 3β-Pgl, have been shown to facilitate lordosis response in ovariectomized, E2-primed female rats [23, 24, 91, 92, 98, 225]. This facilitation appears to involve the activation of MAPK pathway, since MAPK inhibitors decreased the display of proceptive and receptive responses in female rats [99–100, 103]. The behaviors were also inhibited by the administration of PR antagonist RU486, indicating functional interactions between the rapid, membrane-mediated pathways and nuclear PRs [102]. Such interactions between membrane-initiated P effects and intracellular PRs have been observed in the facilitation of sexual behavior in female hamsters, suggesting that both classical and non-classical mechanisms act in concert rather than independently [64–65].
Other studies have reported the involvement of cytoplasmic kinases, PKA, protein kinase C (PKC), Calcium and calmodulin kinase II (CaMKII) and protein kinase G (PKG) in mediating the rapid P effects in the VMH and POA in the female rat [11–12, 22, 56–57, 101, 140, 208, 209, 235]. Since the initiation of these non-classical effects occurs rapidly (in seconds or minutes) and is triggered at the membrane surface, the classical model of nuclear PR-mediation is inadequate to account for these effects. Numerous studies have identified crosstalk between various kinase-initiated pathways (by neurotransmitters, nucleotides and neuropeptides) and nuclear PRs in the brain, suggesting that both classical and non-classical mechanisms act in concert rather than independently. These interactions in the facilitation of female reproductive behavior will be discussed in section 4.0.
In a recent report, Sleiter et al [241] demonstrated the presence of mPRα and mPRβ message in the medial basal hypothalamus and their involvement in the negative feedback effects of P on GnRH secretion. Using the PR knockout mice and their wild type littermates in vivo and GT1-7 cells in vitro, the authors reported that P effects on cAMP inhibition (via Gi) were independent of classical nuclear PR isoforms, PR-A and PR-B, making a case for the involvement of mPRs in GnRH secretion. Whether mPRα and mPRβ play a role in P-facilitation of female reproductive behavior and could be involved in the crosstalk with nuclear PRs remains to be determined.
4. Neurotransmitters in Female Reproductive behavior
Facilitation of female reproductive behavior appears to include pathways other than those involving rapid non-genomic and slower genomic pathways activated by P. Steroid hormones have been shown to influence female reproductive behaviors by alteration in neurotransmitter biosynthesis and release [198], allosteric modulation of membrane receptors [73, 219], changes in neurotransmitter receptor densities, and interactions with G-protein coupling and subsequent intracellular signaling pathways in the hypothalamus and POA [76–77]. This steroid hormone-neurotransmitter interaction is not unidirectional. Not only do steroids affect neurotransmission, but changes in neurotransmission can also alter steroid activity. It has been recognized that in addition to steroid hormones, several neurotransmitters and neuropeptides influence female reproductive behavior in rodents. While the effects of acetylcholine, norepinephrine, serotonin (acting via 5-HT2, receptor subtype), DA (acting via receptor subtype D1) and the neuropeptides GnRH, TRH, prolactin, oxytocin, substance P, and GABAA are considered facilitatory, serotonin (acting receptor subtype 5-HT1A), DA (acting through receptor subtype D2), opioids, CRF, α-MSH, ACTH, β-endorphin, neuropeptide Y, cholecystokinin and glutamate are considered inhibitory on sexual behavior [26, 76, 93, 210, 250, 258].
Neuroanatomical studies have demonstrated the colocalization of neurotransmitters and neuropeptides in PR-containing neurons [210]. Dopamine-β-hydroxylase- and tyrosine hydroxylase- immunoreactive neurons have been reported to be present in close proximity with, and synapsing upon, PR-containing immunoreactive neurons in the POA and hypothalamus in guinea pig [35, 253], rat and monkey [126–127]. Autoradiographic studies in the rat brain demonstrate the presence of E2-concentrating neurons in the hypothalamus and POA that also have afferent input from catecholaminergic neurons [123]. Similarly, estrogen receptor immunoreactive neurons have been found to establish synaptic contact with dopamine-β-hydroxylase immunoreactive neurons in the hypothalamus of the guinea pig [253]. Although these studies do not definitively prove that genomic mechanism could be involved in neurotransmitter-related regulation of PRs involves, the feasibility cannot be ruled out.
A number of second messenger molecules, including cAMP, 3′-5′-cyclic guanosine monophosphate (cGMP) and nitric oxide (NO) can also substitute for P in the facilitation of reproductive behavior in female rats [56–57, 103]. NO has been demonstrated to be a mediator of GnRH release and a facilitator of female reproductive behavior [164]. It has also been shown to be a signal transducer in the facilitatory effects of norepinephrine (mediated by α1-adrenoceptor), dibutyryl-cAMP (db-cAMP), GnRH, prostaglandin E2 (PGE2), ring-A metabolites of P, or vaginocervical stimulation (VCS)[58, 101–103]. The involvement of PKG in the facilitation of lordosis by db-cAMP and PGE2, but not by GnRH, has also been reported [103]. Furthermore, MAPK inhibitor also blocked db-cAMP-, PGE2-, or GnRH-facilitated lordosis. These studies suggest the involvement of multiple signal transduction pathways in female reproductive behavior. The messenger molecules and their activated pathways that have been reported to interact with PRs, in the facilitation of rodent female reproductive behavior, are listed in Table 1. The crosstalk between these signaling pathways and PRs will be discussed further in section 4.2.
Table 1.
Factors and pathways linking PR effects on reproductive behaviors
| Factors | Pathway(s) | References |
|---|---|---|
| Progesterone | PKA/MAPK | [166, 100] |
| Dopamine D1 agonist (SKF28293) | cAMP/PKA/DARP-32 | [166] |
| δ opioids | MAPK | [2] |
| cAMP | PKA | [166 |
| cGMP | NO | [56, 57] |
| cGMP | PKG/MAPK | [100] |
| PGE2 | PKA | [223] |
| GnRH | cAMP/PKA/MAPK | [25, 103] |
| 5α-pregnan-3, 20-dione (5α-DHP) | MAPK | [100] |
| 5α, 3α-pregnanolone (5α, 3α-Pgl) | MAPK | [100] |
| Vaginocervical stimulation (VCS) | PKA/MAPK | [102] |
4.1 Neurotransmitter and PR interactions in female reproductive behavior
As described in section 2.3, ligand-independent activation of PRs by DA was first described in vitro [214]. Following these observations, the physiological relevance of ligand-independent activation of PRs was demonstrated in DA-facilitated female reproductive behavior in rats and mice [163, 165]. Icv administration of apomorphine, a DA receptor stimulant, or the D1 agonist, SKF 38393 (SKF), mimicked P effects in the facilitation of lordosis response in female rats [163]. The facilitatory effect of SKF was found to be specific to the D1 receptor subtype confirming and extending an earlier report in which DA agonists, infused into the hypothalamus and POA facilitated lordosis response in female rats [90]. The effect of SKF on lordosis response was blocked by the administration of PR antagonists, D1 receptor antagonist or antisense oligonucleotides to PR mRNA [163], demonstrating the requirement of intracellular PRs in DA-facilitation of female reproductive behavior. Furthermore, PR knockout mice were unable to exhibit SKF-facilitated female reproductive behavior, while their wild type littermates responded to SKF [165]. These studies provided definitive evidence for the obligatory role of PRs as transcriptional mediators in DA-facilitation of female reproductive behavior.
Studies on the involvement of the PR isoforms on ligand independent activation by DA have revealed an interesting dichotomy. Using PR isoform-specific knockout mice Mani et al [168] demonstrated that both PR-A and PR-B isoforms are essential for the expression of the full complement of SKF-facilitated female reproductive behavior. The studies also demonstrated that the effects of cyclic nucleotide, 8-bromo-cAMP (8-br-cAMP), were primarily mediated by PR-A [168]. PRAKO−/− mice failed to display 8-br-cAMP-facilitated lordosis while PRBKO−/− mice displayed reduced levels, suggesting that SKF and 8bBr-cAMP are perhaps recruiting distinct intracellular signaling pathways to activate distinct PR isoforms and downstream genes (Fig. 3b). It is possible that SKF-and 8-br-cAMP- activation of murine PRs could involve altered phosphorylation of distinct coactivators, diverse sets of coactivators, dissimilar phosphorylation sites on the PRs and/or coactivators, in the multicomponent steroid receptor complexes that serve as a sensor(s) for modulatory signals.
Studies using antisense oligonucleotides to DA receptor subtypes indicate that DA-facilitated, PR-mediated behavioral effects occur via D1B (D5) subtype, and not the D1A subtype (167). In situ hybridization and immunohistochemical studies confirm the coexpression of D1A/D1B and PRs in the medial POA, lateral ventromedial nucleus of the hypothalamus and the arcuate nucleus of female rats [36, 154]. Collectively, the data suggest crosstalk between the P- and DA-initiated pathways in the mediation of female reproductive behavior in rats and mice. As will be discussed in section 4.2, rapid membrane-initiated signaling cascades can also interact with the genomic pathways at the level of intracellular PRs to enhance or initiate facilitation of female reproductive behavior.
Ligand-independent mechanism activation of PRs has also been observed in other behaviors. Behaviorally relevant stimulus, such as the VCS, has been shown to activate neural PRs in the absence of P [9–10]. PR antagonists have been reported to inhibit GnRH- and PGE2- facilitated reproductive behavior in female rats [25]. PR antagonist RU486 [2], also inhibited δ opioid agonist, [D-Pen2, D-Pen5]-enkephalin (DPDPE),-facilitation of lordosis in EB-primed female rats [1]. The involvement of PRs in the α1-adrenergic receptor-facilitated female reproductive behavior has also been reported [58]. Thus, indirect activation of PRs, by each of these compounds, appears to be a common mechanism mediating female reproductive behavior in rats and mice. Furthermore, the mechanisms by which many of these neurotransmitters and neuropeptides activate PRs appear to involve second messenger cascades as discussed below in sections 4.2 and 5.0.
In addition to the female reproductive behavior, ligand-independent activation of PRs has also been observed in other physiological processes. Ligand-independent activation of PR has been demonstrated in GnRH self-priming and preovulatory gonadotropin surges [4, 50, 150, 221–222, 261]. Antisense oligonucleotides to PR inhibited GnRH surges in female rats, suggesting the requirement of PR in GnRH regulation [50]. Oleson et al have demonstrated a role for ligand-independent activation of estrogen receptors (ERs) by DA agonist during development, and in juvenile play behavior [201–202], suggesting that ligand-independent activation is not exclusive to PRs and female reproductive behavior.
4.2 Intracellular signaling pathways in female reproductive behavior
A variety of compounds that activate several second and third messenger systems and stimulate protein kinases within the neurons, also facilitate female reproductive behavior in EB-primed rats. These compounds include adenosine and guanosine nucleotides, which can substitute for P, and cause an elevation in their second messengers, cAMP and cGMP respectively [21, 56, 151, 269]. GnRH- and PGE2-permissive effects over female receptivity also involve an increase in levels of cAMP and PKA activity [223]. Recently, Gonzalez-Flores et al [103] have reported the involvement of downstream kinase, MAPK, in the facilitation of female reproductive behavior in ovariectomized, EB-primed female rats, not only by P, but also by GnRH, PGE2 or di-bromo-cAMP. MAPK inhibitor, PD98059, was able to effectively inhibit the facilitation of reproductive behavior, induced by these compounds [100, 103]. The involvement of MAPK pathway has also been shown in δ opioid-facilitation of lordosis [2]. Interestingly, not only does MAPK activated pathway facilitate lordosis, but it also subsequently promotes termination of behavioral estrous by targeting the PR for degradation by the 26S proteasome [47, 99].
Phosphodiesterase inhibitors that increase cAMP, by interfering with the hydrolysis of cAMP, have been shown to potentiate the effects of sub-threshold doses of GnRH and P in the facilitation of lordosis [22]. Studies on cGMP effects on lordosis have identified a role for protein kinase G (PKG) in the signaling cascade. In addition, the cGMP-mediated response also required the presence of nuclear PRs, suggesting the existence of cross-talk between the two pathways [56–57]. Inhibitors to PKG were effective in reducing the lordosis response induced by P and its ring-A reduced progestins in ovariectomized, EB-primed female rats [100] or by VCS [101]. Studies on P-mediated signal transduction pathways also indicate rapid elevations in hypothalamic cAMP levels and PKA activity, by P, in EB-primed rats and mice [166]. Inhibitors of PKA reduced not only P-, but also GnRH- and PGE2-facilitated female reproductive behavior, in EB-primed rats [166, 223]. Signaling cascades mediating the P- and DA agonist-facilitated female reproductive behavior have also been shown to include a third messenger, DARPP-32 (dopamine and cAMP regulated phosphoprotein-32), the absence of which rendered both rats and mice incapable of expressing P- or SKF-facilitated lordosis response [166].
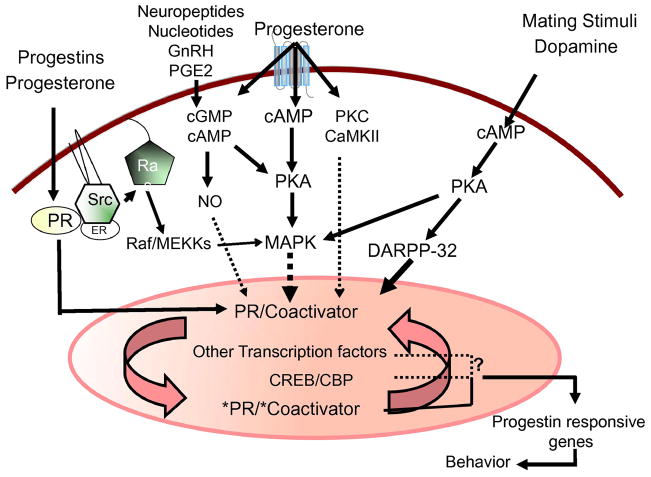
There is no doubt that the expression of female reproductive behavior involves substantial interplay between the rapid membrane-initiated extra-nuclear mechanisms, the slower genomic actions of P, and ligand-independent mechanisms that modulate signaling kinases (Fig. 4). However, a larger question that remains unanswered is the mechanism by which these pathways are coordinated to bring about transcriptional regulation of PRs, at the genomic level. It is reasonable to speculate that P-stimulated, rapid non-classical activation of cytoplasmic signaling pathways, can regulate gene expression independent of PRE binding [42, 43, 82, 83, 216], by increasing the phosphorylation of CREB (Ca 2+/cAMP response element binding protein) or other transcription factors, i.e., ATF-1, enabling them to become transcriptional regulators [7]. In several regions of the rat brain lacking the classical PRs, E2 causes a rapid increase in p–CREB with no concomitant increases in protein or mRNA levels [109, 277]. P, on the other hand, appears to have a bimodal effect on the phosphorylation of CREB, bringing about a rapid decrease followed by an increase [109]. These rapid effects on CREB phosphorylation also appear to be nuclear receptor-mediated, since PR antagonists block the hormonal effects on CREB phosphorylation, suggesting a cross talk between the two signaling pathways.
Fig. 4.
Crosstalk between intracellular signaling pathways in female reproductive behavior. The schematic representation of potential signaling pathways operating in the hypothalamus and preoptic areas. (1) Classical genomic mechanism of action by progesterone- and ring-A class of progestins, mediated by nuclear PRs, plays a predominant role. The ligands allosterically bind to their cognate nuclear receptors and activate PRs to promote interactions with coactivator proteins (2) Progesterone effects mediated by second messengers (cAMP, cGMP) and extra-nuclear signaling kinases (PKA, PKC, CaMKII), activates MAPK signal transduction cascade, leading to plausible phosphorylation of nuclear TFs, PRs/PR coactivators, CREB, and/or its associated protein CBP. (3) Progesterone and progestins, act via the Src kinase, to interact with extranuclear PRs and activate MAPK cascade. (4) Progesterone acting via the extra-nuclear PKA/MAPK/DARPP-32 pathway can cause a decrease in phosphatase activity and an increase in phosphorylation of PR and/or its coactivators. (5) Mating stimuli (VCS) and dopamine D1 agonist can stimulate PKA activation. D1 agonist-stimulated PKA-mediated pathway phosphorylates DARPP-32, which inhibits PP1, leading to the activation of CREB/PR/coactivators. VCS-stimulated PKA activation can also interact with MAPK cascade. (6) Neuropeptides, nucleotides, GnRH and PGE2 can act through various receptor- and/or second messengers (cAMP, cGMP, NO) and transmit signals to the nuclear PRs or other TFs. Interactions between the signal transduction pathways may serve as an amplification mechanism to converge on nuclear TFs and/or coactivators to regulate gene transcription and translation to facilitate female reproductive behavior.
It is possible that protein kinases can also have effects on ion channels [180]. P has been shown to induce transcription of immediate early genes (IEGs) containing CRE-sequences such as c-fos and c-jun [178]. These genes encode the transcription factors, Fos and Jun that can form hetero- or homodimers and regulate downstream gene expression, by acting on target AP-1 DNA recognition sequences near promoter elements. AP-1 elements can substitute for hormone response elements in the steroid regulation of gene transcription [143]. Evidence from in vitro experiments indicates that ER and PR can also mediate transcription of genes controlled by an AP-1 enhancer element [262, 265]. Furthermore, it has also been demonstrated that the two distinct classes of transcription factors, steroid hormone receptors and AP-1 complexes, interact to modulate each other’s activity [3, 238]. In addition, PR coregulators could also integrate steroid hormone signaling through CBP [161, 258, 271]. Functional cooperation between MAPK cascade-mediated phosphorylation of coactivator SRC-1 and CBP has been demonstrated in the activation nuclear PRs in vitro [229–230]. Thus, second messenger systems can potentially modulate gene expression via multiple transcription factors or coactivators, providing an alternative pathway to the genome [248, 263, 264]. The molecular mechanisms recruited by PRs to differentiate between various stimuli to activate female reproductive behavior remain to be determined.
5. Dopamine signaling pathway convergence with PRs
Protein phosphorylation is common to the pathways and molecular mechanisms through which neurotransmitters and steroid hormones produce their biological effects. The regulatory mechanisms governing a variety of cellular processes in target cells are dependent not only on the state of intracellular phosphorylation of the receptor, but also on the dynamic balance between cellular protein kinases and phosphatases. This has also been found to be true to PRs, where the equilibrium between transcriptionally active and inactive forms of the receptor is under the regulation of kinases and phosphatases [70, 239].
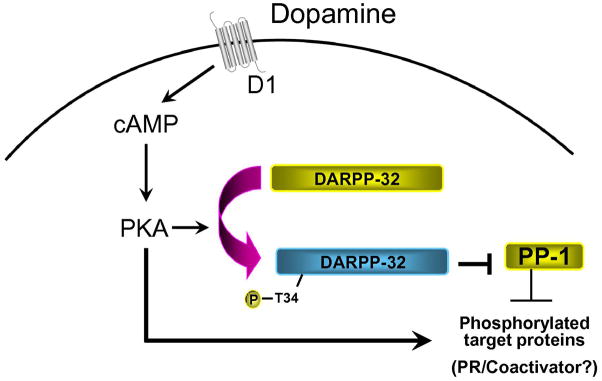
In the mammalian brain, a tissue having an abundance of kinases and phosphatases, protein kinases and protein phosphatases play an important role in phosphorylation of signaling molecules involved in signal transduction mechanisms [194, 239]. Neuronal phosphoproteins are components of the signal transduction pathway, initiated by neurotransmitters and cyclic nucleotides [106–108], and can be phosphorylated/dephosphorylated in response to extracellular stimuli. Signal transduction cascades initiated by P and SKF significantly increase hypothalamic intracellular cAMP levels and PKA activities, resulting in enhanced phosphorylation of the neuronal phosphoprotein DARPP-32 on Threonine 34 (Thr34) [166, 260–261]. Phosphorylation of DARPP-32 on Thr34 by PKA converts it into a potent inhibitor of serine/threonine protein phosphatase1 (PP1) [121–122, 261]. PP1 has broad substrate specificity and controls the state of phosphorylation and activity of numerous physiologically important substrates, including transcription factors, ion pumps, voltage-gated ion channels and neurotransmitter receptors [108, 122]. Thus compounds that increase or decrease phospho-Thr34 of DARPP-32 inhibit or activate PP1 respectively, thereby increasing or decreasing the state of phosphorylation and activity of large array of downstream physiological effectors [108]. PR and/or its coregulators could be the potential substrate proteins indirectly activated by DARPP-32 (Fig. 5).
Fig. 5.
Mechanism of dopamine action in the hypothalamus. Dopamine acting via D1 receptor subtype stimulates an increase in cAMP levels and PKA activity, leading to enhanced phosphorylation of the neuronal phosphoprotein DARPP-32 on Thr34. Phosphorylation of DARPP-32 converts the phosphoprotein to a potent inhibitor of protein phosphatase 1 (PP-1). Phosphatase inhibition leads to increased kinase activity and increased phosphorylation of target proteins including PR and/or coactivators.
A critical requirement of DARPP-32 in the P-and SKF-facilitation of sexual receptivity has been demonstrated in rats and mice [166]. Antisense oligonucleotides to DARPP-32, but not sense oligonucleotides, administered icv into the third cerebral ventricle, inhibited SKF- and P-facilitated sexual receptivity in EB-primed female rats [166]. EB-primed female mice carrying a null mutation for the gene encoding DARPP-32 exhibited significantly lower levels of P- or SKF-facilitated sexual receptivity compared to their wild type littermates. Similar to DA effects in the neostriatum, SKF, as well as P, significantly increased hypothalamic cAMP levels and PKA activities, and enhanced phosphorylation of DARPP-32 on Thr34 [166]. SKF-induced increases were inhibited by the D1 receptor subclass DA antagonist, SCH 23390, indicating that the increases were due to the effects of SKF, initiated at its membrane receptor [166]. P-induced increases, however, were not inhibited by SCH 23390, suggesting that the observed increases were due to the direct effects of P and not secondary to modulation of DA receptors by P [166]. Rp-cAMPS, a compound that blocks cAMP signal transduction cascade by inhibiting PKA, inhibited SKF-and P-facilitated sexual receptivity in EB-primed female rats [166]. These observations indicate that DARPP-32 activation is an obligatory step in the regulation of sexual receptivity. It is likely that the mechanisms include both modulation of DARPP-32, making it an efficient inhibitor of PP1 [108, 121] and/or DARPP-32’s indirect effects on phosphorylation (activation) of PRs and/or PR-associated coactivators [229–230].
DARPP-32, a 205 amino acid protein is highly conserved in mammals. Studies on DARPP-32 regulation in dopaminoceptive neurons have identified multiple phosphorylation sites on DARPP-32 at Threonine75 (Thr75), Serine102 (Ser102) and Serine 137(Ser137), in addition to Thr34 [64]. This multi-site phosphorylation involves enzyme-directed and substrate-directed complex feedback loops and amplifies the effects of DARPP-32, by converting it into a better substrate for phosphorylation at Thr34, by PKA, contributing to its inhibitory effects on the downstream PP1 cascade [108]. A detailed discussion on the regulation of these feedback loops can be found in several publications [172, 27, 97, 138, 196, 197, 260]. While the involvement of the Thr34/Thr375 feedback loop on DA-mediated DARPP-32 regulation has been extensively studied in the neostriatum and nucleus accumbens, its role in P- and SKF-regulation of PR- sensitive areas of the hypothalamus is unknown. Interestingly, Casein kinase enzymes I and II, known to phosphorylate DARPP-32 on Ser137 and Ser102 respectively [71, 72, 97], also regulate PR phosphorylation [276]. Thus, phosphorylation of serine residues on DARPP-32 could also be critical for P- and SKF-facilitated signals in female reproductive behavior. It is reasonable to speculate that such an intricate regulation of DARPP-32 at the multiple sites could provide a mechanism by which signal amplification is achieved to selectively alter P- and SKF- effects on behavior and physiology.
Interestingly, DARPP-32 also has a critical integrative role mediating the actions of various biogenic amines (DA and serotonin), aminoacids (glutamate and GABA), neuromodulators (adenosine and NO) neuropeptides (opioids, cholecystokinin and neurotensin), steroids (E2 and P), therapeutic agents (antipsychotics, antidepressants), and drugs of abuse (ethanol, caffeine, cocaine and amphetamine) [247]. Whether DARPP-32 could be involved in all or any of the modulatory effects of neurotransmitters and neuropeptides, on female reproductive behavior in rodents, remains to be examined.
Summary and Conclusions
It is becoming abundantly clear that the integration of the ligand-dependent and ligand-independent mechanisms of PR activation is essential for neuroendocrine regulation of female reproductive behaviors. In addition, multiple intra- and intercellular mechanisms coexist and share signaling components to ensure that the female is in behavioral estrus at the right time. While the functional role of multiple signaling pathways can be explained by their ability to relay, amplify and integrate signals from a variety of extracellular stimuli, the molecular mechanisms by which this synchronization occurs remains unclear. It will be critical to understand how neuronal kinases and phosphatases, activated by neurotransmitters, regulate the equilibrium between transcriptionally active and inactive states of PRs and their coregulators, in regulating female reproductive behavior. Furthermore, the molecular mechanisms by which this equilibrium could be fine-tuned by neuronal phosphoproteins, such as DARPP-32, functioning perhaps as signal amplifier remain to be established. Future studies will likely reveal further insights into the mechanisms by which the multiple signals converge and reinforce, neuronal responses to environmental and behavioral events, to alter steroid hormone effects on female reproductive behavior.
Acknowledgments
This work was supported by the United States Public Health Service grants MH57442 and MH63954 (SKM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Acosta-Martinez M, Etgen AM. The role of delta-opioid receptors in the facilitation of lordosis behavior. Behav Brain Res. 2002;136:93–102. doi: 10.1016/s0166-4328(02)00103-1. [DOI] [PubMed] [Google Scholar]
- 2.Acosta-Martínez M, Gonzalez-Flores O, Etgen AM. The role of progestin receptors and the mitogen-activated protein kinase pathway in delta opioid receptor facilitation of female reproductive behaviors. Horm Behav. 2006;49:458–462. doi: 10.1016/j.yhbeh.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 3.Alkhalaf M, Murphy LC. Regulation of c-jun and jun-B by progestins in T47-D human breast cancer cells. Mol Endo. 1992;6:1625–1633. doi: 10.1210/mend.6.10.1448115. [DOI] [PubMed] [Google Scholar]
- 4.An BS, Selva DM, Hammond GL, Rivero-Muller A, Rahman N, Leung PCK. Steroid receptor coactivator-3 is required for progesterone receptor transactivation of target genes in response to gonadotropin-releasing hormone treatment of pituitary cells. J Biol Chem. 2006;281:20817–20824. doi: 10.1074/jbc.M600743200. [DOI] [PubMed] [Google Scholar]
- 5.Apostolakis EM, Ramamurphy M, Zhou D, Oñate S, O’Malley BW. Acute disruption of select steroid receptor coactivators prevents reproductive behavior in rats and unmasks genetic adaptation in knockout mice. Mol Endocrinol. 2002;16:1511–1523. doi: 10.1210/mend.16.7.0877. [DOI] [PubMed] [Google Scholar]
- 6.Arai Y, Gorski RA. Effect of anti-estrogen on steroid-induced sexual receptivity in ovariectomized rats. Physiol Behav. 1968;3:351–353. [Google Scholar]
- 7.Armstrong RC, Montminy MR. Transsynaptic control of gene expression. Annu Rev Neurosci. 1993;16:17–29. doi: 10.1146/annurev.ne.16.030193.000313. [DOI] [PubMed] [Google Scholar]
- 8.Auger AP, Tetel MJ, McCarthy MM. Steroid receptor coactivator-1 (SRC-1) mediates the development of sex-specific brain morphology and behavior. Proc Natl Acad Sci U S A. 2000;97:7551–7555. doi: 10.1073/pnas.97.13.7551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Auger AP, Moffatt CA, Blaustein JD. Progesterone-independent activation of rat brain progestin receptors by reproductive stimuli. Endocrinol. 1997;138:511–514. doi: 10.1210/endo.138.1.4986. [DOI] [PubMed] [Google Scholar]
- 10.Auger AP, LaRiccia LM, Moffatt CA, Blaustein JD. Progesterone, but not progesterone-independent activation of progestin receptors by a mating stimulus, rapidly decreases progestin receptor immunoreactivity in female rat brain. Horm Behav. 2000;37:135–144. doi: 10.1006/hbeh.1999.1565. [DOI] [PubMed] [Google Scholar]
- 11.Balasubramanian B, Portillo W, Reyna A, Chen JZ, Moore AN, Dash PK, Mani SK. Nonclassical mechanisms of progesterone action in the brain. I PKC activation in the hypothalamus of female rats. 2008;149:5509–5517. doi: 10.1210/en.2008-0712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Balasubramanian B, Portillo W, Reyna A, Chen JZ, Moore AN, Dash PK, Mani SK. Non-classical mechanisms of progesterone action in the brain: II. Role calcium and calmodulin-dependent protein kinase II in progesterone-mediated signaling in the hypothalamus of female rats. Endocrinol. 2008;149:5518–5526. doi: 10.1210/en.2008-0713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bai W, Weigel NL. Phosphorylation and steroid hormone action. Vitam Horm. 1995;51:289–313. doi: 10.1016/s0083-6729(08)61042-0. [DOI] [PubMed] [Google Scholar]
- 14.Baum MJ, Keverne EB, Everitt BJ, Herbert J, de Greef WJ. Effects of progesterone and estradiol on sexual attractivity of female rhesus monkeys. Physiol Behav. 1977;18:659–670. doi: 10.1016/0031-9384(77)90064-6. [DOI] [PubMed] [Google Scholar]
- 15.Baum MJ, Everitt BJ, Herbert J, Keverne EB. Hormonal basis of proceptivity and receptivity in female primates. Arch Sex Behav. 1977;6:173–192. doi: 10.1007/BF01541126. [DOI] [PubMed] [Google Scholar]
- 16.Baum MJ, deGreef WJ, Kloet GA, Schretlen PJ. Evidence that a factor besides progesterone, prolactin, or plasma-estradiol-binding protein inhibits estrogen-induced sexual receptivity in pregnant rats. J Comp Physiol Psychol. 1979;93:278–294. doi: 10.1037/h0077558. [DOI] [PubMed] [Google Scholar]
- 17.Baum MJ, Gerlach JL, Krey LC, McEwen BS. Biochemical and autoradiographic analysis of estrogen-inducible progestin receptors in female ferret brain and pituitary: Correlations with effects of progesterone on sexual behavior and gonadotropin-releasing hormone-stimulated secretion of luteinizing hormone. Brain Res. 1986;368:296–309. doi: 10.1016/0006-8993(86)90574-3. [DOI] [PubMed] [Google Scholar]
- 18.Barfield RJ, Chen JJ. Activation of estrous behavior in ovariectomized rats by intracerebral implants of estradiol benzoate. Endocrinol. 1977;101:1716–1725. doi: 10.1210/endo-101-6-1716. [DOI] [PubMed] [Google Scholar]
- 19.Beach FA. Importance of progesterone to induction of sexual receptivity in spayed female rats. Proc Soc Exp Biol Med. 1942;51:369–371. [Google Scholar]
- 20.Beato M, Arnemann G, Chalepakis ES, Willman T. Gene regulation by steroid hormones. J Steroid Biochem. 1987;27:9–14. doi: 10.1016/0022-4731(87)90288-3. [DOI] [PubMed] [Google Scholar]
- 21.Beyer C, Canchola E, Larsson K. Facilitation of lordosis behavior in the ovariectomized estrogen primed rat by dibutyryl cAMP. Physiol Behav. 1981;26:249–251. doi: 10.1016/0031-9384(81)90019-6. [DOI] [PubMed] [Google Scholar]
- 22.Beyer C, Gonzalez-Mariscal G. Elevation in hypothalamic cAMP as a common factor in the facilitation of 37 ordosis in rodents: A working hypothesis. Ann N Y Acad Sci. 1986;474:270–281. doi: 10.1111/j.1749-6632.1986.tb28018.x. [DOI] [PubMed] [Google Scholar]
- 23.Beyer C, González-Mariscal G, Eguíbar JR, Gómora P. Lordosis facilitation in estrogen primed rats by intrabrain injection of pregnanes. Pharmacol Biochem Behav. 1988;31:919–926. doi: 10.1016/0091-3057(88)90405-4. [DOI] [PubMed] [Google Scholar]
- 24.Beyer C, Gonzalez-Flores O, Gonzalez-Mariscal G. Ring A reduced progestins potently stimulate estrous behavior in rats: paradoxical effect through the progesterone receptor. Physiol Behav. 1995;58:985–993. doi: 10.1016/0031-9384(95)00141-5. [DOI] [PubMed] [Google Scholar]
- 25.Beyer C, González-Flores O, González-Mariscal G. Progesterone receptor participates in the stimulatory effect of LHRH, prostaglandin E2, and cyclic AMP on lordosis and proceptive behaviours in rats. J Neuroendocrinol. 1997;9:609–614. doi: 10.1046/j.1365-2826.1997.00617.x. [DOI] [PubMed] [Google Scholar]
- 26.Beyer C, González-Flores O, García-Juárez M, González-Mariscal G. Non-ligand activation of estrous behavior in rodents: cross-talk at the progesterone receptor. Scand J Psychol. 2003;44:221–229. doi: 10.1111/1467-9450.00339. [DOI] [PubMed] [Google Scholar]
- 27.Bibb JA, Snyder GL, Nishi A, Meijer L, Fienberg AA, Tsai LH, Kwon YT, Girault JA, Czernik AJ, Huganir RL, Hemmings HC, Nairn AC, Greengard P. Phosphorylation of DARPP-32 by Cdk5 modulates dopamine signaling in neurons. Nature. 1999;402:669–671. doi: 10.1038/45251. [DOI] [PubMed] [Google Scholar]
- 28.Blackmore PF. Extragenomic actions of progesterone in human sperm and progesterone metabolites in human platelets. Steroids. 1999;64:149–156. doi: 10.1016/s0039-128x(98)00109-3. [DOI] [PubMed] [Google Scholar]
- 29.Blaustein JD. Progesterone in high doses may overcome progesterone’s desensitization effect on lordosis by translocation of hypothalamic progestin receptors. Horm Behav. 1982;16:175–190. doi: 10.1016/0018-506x(82)90017-4. [DOI] [PubMed] [Google Scholar]
- 30.Blaustein JD, Wade GN. Sequential inhibition of sexual behavior by progesterone in female rats: Comparison with a synthetic antiestrogen. J Compar Physiol Psychol. 1977;91:752–760. doi: 10.1037/h0077365. [DOI] [PubMed] [Google Scholar]
- 31.Blaustein JD, Feder HH. Cytoplasmic progestin receptors in female guinea pig brain and their relationship to refractoriness in expression of female sexual behavior. Brain Res. 1977;177:489–498. doi: 10.1016/0006-8993(79)90466-9. [DOI] [PubMed] [Google Scholar]
- 32.Blaustein JD, Feder HH. Cytoplasmic progestin receptors in guinea pig brain: Characteristics and relationship to the induction of sexual behavior. Brain Res. 1979;169:481–497. doi: 10.1016/0006-8993(79)90398-6. [DOI] [PubMed] [Google Scholar]
- 33.Blaustein JD, Feder HH. Nuclear progestin receptors in guinea pig brain measured by an in vitro exchange assay after hormonal treatments after lordosis. Endocrinol. 1980;106:1061–1069. doi: 10.1210/endo-106-4-1061. [DOI] [PubMed] [Google Scholar]
- 34.Blaustein JD, Olster DH. Gonadal steroid hormone receptors and social behaviors. In: Balthazart J, editor. Advances in Comparative and Environmental Physiology. Springer-Verlag; Berlin: 1989. pp. 31–104. [Google Scholar]
- 35.Blaustein JD, Turcotte J. A small population of tyrosine hydroxylase immunoreactive neurons in the guinea pig arcuate nucleus contain progesterone receptor immunoreactivity. J Neuroendocrinol. 1989;1:333–338. doi: 10.1111/j.1365-2826.1989.tb00125.x. [DOI] [PubMed] [Google Scholar]
- 36.Blaustein JD, Lubbers LS, Meredith J, Wade GN. Dopamine receptor subtypes in progestin receptor (PR)-rich regions of the hypothalamus in female rats. Abstr Sac. Neurosci. 1999;748(14):1881. [Google Scholar]
- 37.Blaustein JD, Erskine MS. Feminine sexual behavior: Cellular integration of hormonal and afferent information in the rodent forebrain. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain and Behavior. 1. Vol. 1. Academic Press; New York: 2002. pp. 139–214. [Google Scholar]
- 38.Blaustein JD. Neuronal steroid hormone receptors: they’re not just for hormones anymore. Endocrinol. 2004;145:1075–1081. doi: 10.1210/en.2003-1485. [DOI] [PubMed] [Google Scholar]
- 39.Blaustein JD, Mani SK. Feminine sexual behavior from the neuroendocrine and molecular neurobiological perspectives. In: Blaustein JD, Lajtha A, editors. Handbook of Neurochemistry and Molecular Neurobiology: Behavioral Neurochemistry and Neuroendocrinology; Berlin, Heidelberg: Springer-Verlag; 2007. pp. 95–150. [Google Scholar]
- 40.Blaustein JD. Feminine reproductive behavior and physiology in rodents: integration of hormonal, behavioral and environmental influences. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain and Behavior. 2. Vol. 3. Academic Press; San Diego: 2009. pp. 67–107. [Google Scholar]
- 41.Boling JL, Blandau RJ. The estrogen-progesterone induction of mating responses in the spayed female rat. Endocrinol. 1939;25:359–364. [Google Scholar]
- 42.Boonyaratanakornkit V, Edwards DP. Receptor mechanisms mediating non- genomic actions of sex steroid. Semin Reprod Med. 2007;25:139–153. doi: 10.1055/s-2007-973427. [DOI] [PubMed] [Google Scholar]
- 43.Boonyaratanakornkit V, Bi Y, Rudd M, Edwards DP. The role and mechanism of progesterone receptor activation of extra-nuclear signaling pathways in regulating gene transcription and cell cycle progression. Steroids. 2008;73:922–928. doi: 10.1016/j.steroids.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 44.Brinton RD, Thompson RF, Foy MR, Baudry M, Wang JM, Finch CE, Morgan TE, Pike CJ, Mack WJ, Stanczyk FZ, Nilsen J. Progesterone receptors: form and function in brain. Front Neuroendocrinol. 2008;29:313–339. doi: 10.1016/j.yfrne.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brown TJ, Blaustein JD. Abbreviation of the period of sexual behavior in female guinea pigs by the progesterone antagonist, RU 486. Brain Res. 1986;373:103–113. doi: 10.1016/0006-8993(86)90320-3. [DOI] [PubMed] [Google Scholar]
- 46.Brown TJ, Moore MJ, Blaustein JD. Maintenance of progesterone-facilitated sexual behavior in female rats requires continued hypothalamic protein synthesis and nuclear progestin receptor occupation. Endocrinol. 1987;121:298–304. doi: 10.1210/endo-121-1-298. [DOI] [PubMed] [Google Scholar]
- 47.Camacho-Arroyo I, Villamar-Cruz O, Gonzalez-Arenas A, Guerra-Araiza C. Participation of the 26S proteasome in the regulation of progesterone receptor concentrations in the rat brain. Neuroendocrinol. 2002;76:267–271. doi: 10.1159/000066623. [DOI] [PubMed] [Google Scholar]
- 48.Camacho-Arroyo I, Guerra-Araiza C, Cerbon MA. Progesterone receptor isoforms are differentially regulated by sex steroids in the rat forebrain. Neuroreport. 1998;9:3993–3996. doi: 10.1097/00001756-199812210-00001. [DOI] [PubMed] [Google Scholar]
- 49.Carter CS, Landauer MR, Tierney BM, Jones T. Regulation of female sexual behavior in the golden hamster: Behavioral effects of mating and ovarian hormones. J Compar Physiol Psychol. 1976;90:839–850. doi: 10.1037/h0077274. [DOI] [PubMed] [Google Scholar]
- 50.Chappell PE, Levine JE. Stimulation of gonadotropin-releasing hormone surges by estrogen. I. Role of hypothalamic progesterone receptors. Endocrinol. 2000;141:1477–1485. doi: 10.1210/endo.141.4.7428. [DOI] [PubMed] [Google Scholar]
- 51.Charlier TD, Lakaye B, Ball GF, Balthazart J. Steroid receptor coactivator SRC-1 exhibits high expression in steroid-sensitive brain areas regulating reproductive behaviors in the quail brain. Neuroendocrinol. 2002;76:297–315. doi: 10.1159/000066624. [DOI] [PubMed] [Google Scholar]
- 52.Charlier TD, Ball GF, Balthazart J. Inhibition of steroid receptor coactivator-1 blocks estrogen and androgen action on male sex behavior and associated brain plasticity. J Neurosci. 2005;25:906–913. doi: 10.1523/JNEUROSCI.3533-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Charlier TD, Ball GF, Balthazart J. Plasticity in the expression of the steroid receptor coactivator-1 in the Japanese quail brain: Effect of sex, testosterone, stress and time of the day. Neurosci. 2006;172:333–343. doi: 10.1016/j.neuroscience.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 54.Charlier TD, Harada N, Ball GF, Balthazart J. Targeting steroid receptor coactivator-1 expression with locked nucleic acids antisense reveals different thresholds for the hormonal regulation of male sexual behavior in relation to aromatase activity and protein expression. Behav Brain Res. 2006;172:333–343. doi: 10.1016/j.bbr.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 55.Charlier TD. Importance of steroid receptor coactivators in the modulation of steroid action on brain and behavior. Psychoneuroendocrinol. 2009 Jun 11; doi: 10.1016/j.psyneuen.2009.05.004. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 56.Chu HP, Etgen AM. A potential role of cyclic GMP in the regulation of lordosis behavior of female rats. Horm Behav. 1997;32:125–132. doi: 10.1006/hbeh.1997.1413. [DOI] [PubMed] [Google Scholar]
- 57.Chu HP, Morales JC, Etgen AM. Cyclic GMP may potentiate lordosis behaviour by progesterone receptor activation. J Neuroendocrinol. 1999;11:107–113. doi: 10.1046/j.1365-2826.1999.00298.x. [DOI] [PubMed] [Google Scholar]
- 58.Chu HP, Etgen AM. Ovarian hormone dependence of alpha (1)-adrenoceptor activation of the nitric oxide-cGMP pathway: relevance for hormonal facilitation of lordosis behavior. J Neurosci. 1999;19:7191–7197. doi: 10.1523/JNEUROSCI.19-16-07191.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Collins VJ, Boling JI, Dempsey EW, Young WC. Quantitative studies of experimentally induced sexual receptivity in the spayed guinea pig. Endocrinol. 1938;23:181–196. [Google Scholar]
- 60.Comacho-Arroyo I, Guerra-Araiza C, Cerbon MA. Progesterone receptor isoforms are differentially regulated by sex steroids in the rat forebrain. Neuroreport. 1998;9:3993–3996. doi: 10.1097/00001756-199812210-00001. [DOI] [PubMed] [Google Scholar]
- 61.Conneely OM, Kettleberger DM, Tsai MJ, Schrader WT, O’Malley BW. The chicken progesterone receptor A and B isoforms are products of an alternate translation initiation event. J Biol Chem. 1989;264:14062–14064. [PubMed] [Google Scholar]
- 62.Conneely OM, Mulac-Jericevic B, DeMayo FJ, Lydon JP, O’Malley BW. Reproductive functions of progesterone receptors. Recent Prog Horm Res. 2002;57:339–355. doi: 10.1210/rp.57.1.339. [DOI] [PubMed] [Google Scholar]
- 63.Conneely OM, Mulac-Jericevic B, Lydon JP. Progesterone-dependent regulation of female reproductive activity by two distinct progesterone receptor isoforms. Steroids. 2003;68:771–778. doi: 10.1016/s0039-128x(03)00126-0. [DOI] [PubMed] [Google Scholar]
- 64.DeBold JF, Frye CA. Genomic and nongenomic actions of progesterone in the control of female hamster sexual behavior. Horm Behav. 1994;28:445–453. doi: 10.1006/hbeh.1994.1042. [DOI] [PubMed] [Google Scholar]
- 65.DeBold JF, Frye CA. Progesterone and neural mechanisms of hamster sexual behavior. Psychoneuroendocrinol. 1994;19:563–579. doi: 10.1016/0306-4530(94)90041-8. [DOI] [PubMed] [Google Scholar]
- 66.Dempsey EW, Hertz R, Young WC. The experimental induction of oestrus (sexual receptivity) in the normal and ovariectomized guinea pig. Am J Physiol. 1936;116:201–209. [Google Scholar]
- 67.deNicola AF, Labombarda F, Gonzalez Denisele MC, Gonzalez SL, Garay L, Meyer M, Gargiulo G, Guennoun R, Schumacher M. Progesterone neuroprotection in traumatic CNS injury and motoneuron degeneration. Front Neuroendocrinol. 2009;30:173–187. doi: 10.1016/j.yfrne.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 68.Denner LA, Weigel NL, Schrader WT, O’Malley BW. Hormone-dependent regulation of chicken progesterone receptor deoxyribonucleic acid binding and phosphorylation. Endocrinol. 1989;125:3051–3058. doi: 10.1210/endo-125-6-3051. [DOI] [PubMed] [Google Scholar]
- 69.Denner LA, Schrader WT, O’Malley BW, Weigel NL. Hormonal regulation in identification of chicken progesterone receptor phosphorylation sites. J Biol Chem. 1990;265:16548–16555. [PubMed] [Google Scholar]
- 70.Denner LA, Weigel NL, Maxwell BL, Schrader WT, O’Malley BW. Regulation of progesterone receptor-mediated transcription by phosphorylation. Science. 1990;250:1740–1743. doi: 10.1126/science.2176746. [DOI] [PubMed] [Google Scholar]
- 71.Desdouits F, Cohen D, Nairn AC, Greengard P, Girault JA. Phosphorylation of DARPP-32, a dopamine and cAMP-regulated phosphoprotein, by casein kinase I in vitro and in vivo. J Biol Chem. 1995;270:8772–8778. doi: 10.1074/jbc.270.15.8772. [DOI] [PubMed] [Google Scholar]
- 72.Desdouits F, Siciliano JC, Greengard P, Girault JA. Dopamine and cAMP-regulated phosphoprotein DARPP-32: phosphorylation of Ser-137 by casein kinase I inhibits dephosphorylation of Thr-34 by calcineurin. Proc Natl Acad Sci USA. 1995;92:2682–2685. doi: 10.1073/pnas.92.7.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dohanich GP, Witcher JA, Weaver D, Clemens L. Alteration of muscarinic binding in specific brain areas following estrogen treatment. Brain Res. 1982;241:347–350. doi: 10.1016/0006-8993(82)91075-7. [DOI] [PubMed] [Google Scholar]
- 74.Edwards DA, Whalen RE, Nadler RD. Induction of estrus: Estrogen- progesterone interactions. Physiol Behav. 1968;3:29–33. [Google Scholar]
- 75.Etgen AM, Barfield RJ. Antagonism of female sexual behavior with intracerebral implants of antiprogestin RU 38486: correlation with binding to neural progestin receptors. Endocrinol. 1986;119:1610–1617. doi: 10.1210/endo-119-4-1610. [DOI] [PubMed] [Google Scholar]
- 76.Etgen AM, Ungar S, Petitti N. Estradiol and progesterone modulation of norepinephrine neurotransmission: Implications for the regulation of female reproductive behavior. J Neuroendocrinol. 1992;4:255–271. doi: 10.1111/j.1365-2826.1992.tb00167.x. [DOI] [PubMed] [Google Scholar]
- 77.Etgen AM. Ovarian steroid and growth factor regulation of female reproductive function involves modification of hypothalamic alpha 1-adrenoceptor signaling. Ann N Y Acad Sci. 2003;1007:153–161. doi: 10.1196/annals.1286.015. [DOI] [PubMed] [Google Scholar]
- 78.Etgen AM, Gonzalez-Flores O, Todd BJ. The role of insulin-like growth factor-I and growth factor-associated signal transduction pathways in estradiol and progesterone facilitation of female reproductive behaviors. Front Neuroendocrinol. 2006;27:363–375. doi: 10.1016/j.yfrne.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 79.Evans RM. The steroid and thyroid hormone receptor superfamily. Science. 1988;240:885–889. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fabre-Nys C, Gelez H. Sexual behavior in ewes and other domestic ruminants. Horm Behav. 2007;52:18–25. doi: 10.1016/j.yhbeh.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 81.Fadem BH, Barfield RJ, Whalen RE. Dose-response and time-response relationships between progesterone and the display of patterns of receptive behavior in the female rat. Horm Behav. 1979;13:40–48. doi: 10.1016/0018-506x(79)90033-3. [DOI] [PubMed] [Google Scholar]
- 82.Faivre E, Skildum A, Pierson-Mullany L, Lange CA. Integration of progesterone receptor mediated rapid signaling and nuclear actions in breast cancer cell models: role of mitogen-activated protein kinases and cell cycle regulators. Steroids. 2005;70:418–426. doi: 10.1016/j.steroids.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 83.Faivre EJ, Lange CA. Progesterone receptors upregulate Wnt-1 to induce epidermal growth factor receptor transactivation and c-Src-dependent sustained activation of Erk1/2 mitogen-activated protein kinase in breast cancer cells. Mol Cell Biol. 2007;27:466–80. doi: 10.1128/MCB.01539-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Faivre EJ, Daniel AR, Hillard CJ, Lange CA. Progesterone receptor rapid signaling mediates serine 345 phosphorylation and tethering to specificity protein 1 transcription factors. Mol Endocrinol. 2008;22:823–837. doi: 10.1210/me.2007-0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Falkenstein E, Meyer C, Eisen C, Scriba PC, Wehling M. Full length cDNA sequence of a progesterone membrane-binding protein from porcine vascular smooth muscle cells. Biochem Biophy Res Commun. 1996;229:86–89. doi: 10.1006/bbrc.1996.1761. [DOI] [PubMed] [Google Scholar]
- 86.Falkenstein E, Schmeiding K, Lange A, Meyer C, Gerdes D, Welsch U, Wehling M. Localization of a putative progesterone membrane binding protein in porcine hepatocytes. Cell Mol Biol. 1998;44:571–578. [PubMed] [Google Scholar]
- 87.Feder HH. Hormones and sexual behavior. Annu Rev Psychol. 1984;35:165–200. doi: 10.1146/annurev.ps.35.020184.001121. [DOI] [PubMed] [Google Scholar]
- 88.Ferrell JE, Jr, Machleder EM. The biochemical basis of an all-or-none cell fate switch in Xenopus oocytes. Science. 1998;280:895–898. doi: 10.1126/science.280.5365.895. [DOI] [PubMed] [Google Scholar]
- 89.Ferrell JE., Jr Xenopus oocyte maturation: new lessons from a good egg. Bioessays. 1999;21:833–842. doi: 10.1002/(SICI)1521-1878(199910)21:10<833::AID-BIES5>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 90.Foreman MM, Moss RL. Role of hypothalamic dopaminergic receptors in the control of lordosis behavior in the female rat. Physiol Behav. 1979;22:282–289. doi: 10.1016/0031-9384(79)90088-x. [DOI] [PubMed] [Google Scholar]
- 91.Frye CA, Van Keuren KR, Rao PN, Erskine MS. Progesterone and 3 alpha-androstanediol conjugated to bovine serum albumin affects estrous behavior when applied to the MBH and POA. Behav Neurosci. 1996;110:603–612. doi: 10.1037//0735-7044.110.3.603. [DOI] [PubMed] [Google Scholar]
- 92.Frye CA, Vongher JM. Progestins’ rapid facilitation of lordosis when applied to the ventral tegmentum corresponds to efficacy at enhancing GABA (A) receptor activity. J Neuroendocrinol. 1999;11:829–837. doi: 10.1046/j.1365-2826.1999.00367.x. [DOI] [PubMed] [Google Scholar]
- 93.Frye CA, Walf AA. Activity of protein kinase C is important for 3alpha, 5alpha-THP’s actions at dopamine type 1-like and/or GABAA receptors in the ventral tegmental area for lordosis of rats. Brain Res Bull. 2008;77:91–97. doi: 10.1016/j.brainresbull.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gee KW, Chang WC, Brinton RE, McEwen BS. GABA-dependent modulation of the Cl- ionophore by steroids in rat brain. Eur J Pharmacol. 1987;136:419–423. doi: 10.1016/0014-2999(87)90317-7. [DOI] [PubMed] [Google Scholar]
- 95.Gerdes D, Wehling M, Leube B, Falkenstein E. Cloning and tissue expression of two putative steroid membrane receptors. Biol Chem. 1998;379:907–911. doi: 10.1515/bchm.1998.379.7.907. [DOI] [PubMed] [Google Scholar]
- 96.Giangrande PH, McDonnell DP. The A and B isoforms of the human progesterone receptor: two functionally different transcription factors encoded by a single gene. Recent Prog Horm Res. 1999;54:291–313. [PubMed] [Google Scholar]
- 97.Girault JA, Hemmings HC, William KR, Nairn AC, Greengard P. Phosphorylation of DARPP-32, a dopamine and cAMP-regulated phosphoprotein, by casein kinase II. J Biol Chem. 1989;264:21748–21759. [PubMed] [Google Scholar]
- 98.Glaser JH, Etgen AM, Barfield RJ. Intrahypothalamic effects of progestin agonists on estrous behavior and progestin receptor binding. Physiol Behav. 1985;34:871–878. doi: 10.1016/0031-9384(85)90006-x. [DOI] [PubMed] [Google Scholar]
- 99.González-Flores O, Guerra-Araiza C, Cerbón M, Camacho-Arroyo I, Etgen AM. The 26S proteasome participates in the sequential inhibition of estrous behavior induced by progesterone in rats. Endocrinol. 2004;145:2328–2336. doi: 10.1210/en.2003-1162. [DOI] [PubMed] [Google Scholar]
- 100.González-Flores O, Shu J, Camacho-Arroyo I, Etgen AM. Regulation of 53 ordosis by cyclic 3′, 5′-guanosine monophosphate, progesterone, and its 5alpha-reduced metabolites involves mitogen-activated protein kinase. Endocrinol. 2004;145:5560–5567. doi: 10.1210/en.2004-0823. [DOI] [PubMed] [Google Scholar]
- 101.González-Flores O, Beyer C, Lima-Hernández FJ, Gómora-Arrati P, Gómez-Camarillo MA, Hoffman K, Etgen AM. Facilitation of estrous behavior by vaginal cervical stimulation in female rats involves alpha1-adrenergic receptor activation of the nitric oxide pathway. Behav Brain Res. 2007;176:237–243. doi: 10.1016/j.bbr.2006.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.González-Flores O, Etgen AM, Komisaruk BK, Gómora-Arrati P, Macias-Jimenez A, Lima-Hernández FJ, Garcia-Juárez M, Beyer C. Antagonists of the protein kinase A and mitogen-activated protein kinase systems and of the progestin receptor block the ability of vaginocervical/flank-perineal stimulation to induce female rat sexual behaviour. J Neuroendocrinol. 2008;20:1361–1367. doi: 10.1111/j.1365-2826.2008.01794.x. [DOI] [PubMed] [Google Scholar]
- 103.González-Flores O, Gómora-Arrati P, Garcia-Juárez M, Gómez-Camarillo MA, Lima-Hernández FJ, Beyer C, Etgen AM. Nitric oxide and ERK/MAPK mediation of estrous behavior induced by GnRH, PGE2 and db-cAMP in rats. Physiol Behav. 2009;96:606–612. doi: 10.1016/j.physbeh.2008.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gorzalka BB, Moe IV. Adrenal role in proceptivity and receptivity induced by two modes and estradiol treatment. Physiol Behav. 1994;55:29–34. doi: 10.1016/0031-9384(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 105.Goy RW, Phoenix CH, Young WC. Inhibitory action of the corpus luteum on the hormonal induction of estrous behavior in the guinea pig. Gen Comp Endocrinol. 1966;6:267–275. doi: 10.1016/s0016-6480(66)80014-x. [DOI] [PubMed] [Google Scholar]
- 106.Greengard P, Nairn AC, Girault JA, Ouimet CC, Snyder GL, Fisone G, Allen PB, Fienberg A, Nishi A. The DARPP-32/protein phosphatase-l cascade: A model for Signal integration. Brain Res Brain Res Rev. 1998;26:274–284. doi: 10.1016/s0165-0173(97)00057-x. [DOI] [PubMed] [Google Scholar]
- 107.Greengard P, Allen PB, Nairn AC. Beyond the dopamine receptor: the DARPP-32/protein phosphatase-1 cascade. Neuron. 1999;23:435–447. doi: 10.1016/s0896-6273(00)80798-9. [DOI] [PubMed] [Google Scholar]
- 108.Greengard P. The neurobiology of slow synaptic transmission. Science. 2001;294:1024–1030. doi: 10.1126/science.294.5544.1024. [DOI] [PubMed] [Google Scholar]
- 109.Gu G, Rojo AA, Zee MC, Yu J, Simerly RB. Hormonal regulation of CREB phosphorylation in the anteroventral periventricular nucleus. J Neurosci. 1996;16:3035–3044. doi: 10.1523/JNEUROSCI.16-09-03035.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Guerra-Araiza C, Cerbon MA, Morimoto S, Camacho-Arroyo I. Progesterone receptor isoforms expression pattern in the rat brain during the estrous cycle. Life Sci. 2000;66:1743–1752. doi: 10.1016/s0024-3205(00)00497-5. [DOI] [PubMed] [Google Scholar]
- 111.Guerra-Araiza C, Coyoy-Salgado A, Camacho-Arroyo I. Sex differences in the regulation of progesterone receptor isoforms expression in the rat brain. Brain Res Bull. 2002;59:105–109. doi: 10.1016/s0361-9230(02)00845-6. [DOI] [PubMed] [Google Scholar]
- 112.Guerra-Araiza C, Villamar-Cruz O, González-Arenas A, Chavira R, Camacho-Arroyo I. Changes in progesterone receptor isoforms content in the rat brain during the oestrous cycle and after oestradiol and progesterone treatments. J Neuroendocrinol. 2003;15:984–990. doi: 10.1046/j.1365-2826.2003.01088.x. [DOI] [PubMed] [Google Scholar]
- 113.Guerra-Araiza C, Gómora-Arrati P, García-Juárez M, Armengual-Villegas A, Miranda-Martínez A, Lima-Hernández FJ, Camacho-Arroyo I, González-Flores O. Role of progesterone receptor isoforms in female sexual behavior induced by progestins in rats. Neuroendocrinol. 2009;90:73–81. doi: 10.1159/000224406. [DOI] [PubMed] [Google Scholar]
- 114.Guiochon-Mantel A, Loosefelt H, Lescop P, Sar S, Atger M, Perrot-Applanat M, Milgrom E. Mechanisms of nuclear localization of the progesterone receptor: evidence for interaction between monomers. Cell. 1989;57:1147–1154. doi: 10.1016/0092-8674(89)90052-4. [DOI] [PubMed] [Google Scholar]
- 115.Grosse B, Kachkache M, Le Mellay V, Lieberherr M. Membrane signalling and progesterone in female and male osteoblasts. I. Involvement of intracellular Ca(2+), inositol trisphosphate, and diacylglycerol, but not cAMP. J Cell Biochem. 2000;79:334–345. [PubMed] [Google Scholar]
- 116.Hatchell EC, Colley SM, Beveridge DJ, Epis MR, Stuart LM, Giles KM, Redfern AD, Miles LE, Barker A, MacDonald LM, Arthur PG, Lui JC, Golding JL, McCulloch RK, Metcalf CB, Wilce JA, Wilce MC, Lanz RB, O’Malley BW, Leedman PJ. SLIRP, a small SRA binding protein, is a nuclear receptor corepressor. Mol Cell. 2006;22:657–668. doi: 10.1016/j.molcel.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 117.Hagan CR, Faivre EJ, Lange CA. Scaffolding actions of membrane-associated progesterone receptors. Steroids. 2009;74:568–572. doi: 10.1016/j.steroids.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Halbreich U, Endicott J, Goldstein S, Nee J. Premenstrual changes and changes in gonadal hormones. Acta Psychiat Scand. 1986;74:576–586. doi: 10.1111/j.1600-0447.1986.tb06287.x. [DOI] [PubMed] [Google Scholar]
- 119.Han SJ, Jung SY, Malovannaya A, Kim T, Lanz RB, Qin J, O’Malley BW. A scoring system for the follow up study of nuclear receptor coactivator complexes. Nucl Recept Signal. 2006;4:E014. doi: 10.1621/nrs.04014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Havens MD, Rose JD. Estrogen-dependent and estrogen-independent effects of progesterone on the electrophysiological excitability of dorsal midbrain neurons in golden hamsters. Neuroendocrinol. 1988;48:120–129. doi: 10.1159/000124999. [DOI] [PubMed] [Google Scholar]
- 121.Hemmings HC, Jr, Nairn AC, Greengard P. DARPP-32, a dopamine- and adenosine 3′:5′-monophosphate-regulated neuronal phosphoprotein. II. Comparison of the kinetics of phosphorylation of DARPP-32 and phosphatase inhibitor 1. J Biol Chem. 1984;259:14491–14497. [PubMed] [Google Scholar]
- 122.Hemmings HC, Jr, Nairn AC, Greengard P. Protein kinases and phosphoproteins in the nervous system. Res Publ Assoc Res Nerv Ment Dis. 1986;64:47–69. [PubMed] [Google Scholar]
- 123.Heritage AS, Stumpf EE, Sar M, Grant LD. Brainstem catecholamine neurons are target sites for sex steroid hormones. Science. 1980;207:1377–1379. doi: 10.1126/science.7355296. [DOI] [PubMed] [Google Scholar]
- 124.Hirata S, Shoda T, Kato J, Hoshi K. The novel isoform of the progesterone receptor cDNA in the human testis and detection of its mRNA in the human uterine endometrium. Oncology. 2000;59:39–44. doi: 10.1159/000055286. [DOI] [PubMed] [Google Scholar]
- 125.Hirata S, Shoda T, Kato J, Hoshi K. The novel exon, exon T, of the human progesterone receptor gene and the genomic organization of the gene. J Steroid Biochem Mol Biol. 2002;80:365–367. doi: 10.1016/s0960-0760(02)00019-5. [DOI] [PubMed] [Google Scholar]
- 126.Horvath T, Naftolin F, Leranth C. Presence of calbindin and lack of parvalbumin in progesterone receptor-containing neurons of the monkey mediobasal hypothalamus. Neuroscience. 1992;50:309–314. doi: 10.1016/0306-4522(92)90425-2. [DOI] [PubMed] [Google Scholar]
- 127.Horvath T, Naftolin F, Leranth C. Beta-endorphin innervation of dopamine neurons in the rat hypothalamus: a light and electron microscopic double immunostaining study. Endocrinol. 1992;131:1547–1555. doi: 10.1210/endo.131.3.1354605. [DOI] [PubMed] [Google Scholar]
- 128.Horwitz KB, Tung L, Takimoto GS. Novel mechanisms of antiprogestin action. J Steroid Biochem Mol Biol. 1995;53:9–17. doi: 10.1016/0960-0760(95)00035-x. [DOI] [PubMed] [Google Scholar]
- 129.Horwitz KB, Jackson TA, Bain DL, Richer JK, Takimoto GS, Tung L. Nuclear receptor coactivators and corepressors. Mol Endocrinol. 1996;10:1167–1177. doi: 10.1210/mend.10.10.9121485. [DOI] [PubMed] [Google Scholar]
- 130.Jordan VC, O’Malley BW. Selective estrogen-receptor modulators and antihormonal resistance in breast cancer. J Clin Oncol. 2007;25:5815–5824. doi: 10.1200/JCO.2007.11.3886. [DOI] [PubMed] [Google Scholar]
- 131.Karteris E, Zervou S, Pang Y, Dong J, Hillhouse EW, Randeva HS, Thomas P. Progesterone signaling in human myometrium through two novel membrane G protein-coupled receptors: Potential function in functional progesterone withdrawal at term. Mol Endocrionol. 2006;20:1519–1534. doi: 10.1210/me.2005-0243. [DOI] [PubMed] [Google Scholar]
- 132.Kastner P, Krust A, Mendelsohn C, Garnier JM, Zelent A, Leroy P, Staub A, Chambon P. Murine isoforms of retinoic acid receptor gamma with specific patterns of expression. Proc Natl Acad Sci USA. 1990;87:2700–2704. doi: 10.1073/pnas.87.7.2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kastner P, Krust A, Turcotte B, Stropp U, Tora L, Gronemeyer H, Chambon P. Two distinct estrogen-regulated promoters generate transcripts encoding the two functionally different human progesterone receptor isoforms A and B. EMBO J. 1990;9:1603–1614. doi: 10.1002/j.1460-2075.1990.tb08280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kato J, Grouch T. Specific progesterone receptors in the hypothalamus and anterior hypophysis of rat. Endocrinol. 1977;101:912–928. doi: 10.1210/endo-101-3-920. [DOI] [PubMed] [Google Scholar]
- 135.Katzenellenbogen JA, O’Malley BW, Katzenellenbogen BS. Tripartite steroid hormone receptor pharmacology: interaction with multiple effector sites as a basis for the cell- and promoter-specific action of these hormones. Mol Endocrinol. 1996;10:119–131. doi: 10.1210/mend.10.2.8825552. [DOI] [PubMed] [Google Scholar]
- 136.Kelly MJ, Moss RL, Dudley CA. The effects of microelectrophoretically applied estrogen, cortisol and acetylcholine on medial preoptic-septal unit activity throughout the estrous cycle of the female rat. Exp Brain Res. 1977;30:53–64. doi: 10.1007/BF00237858. [DOI] [PubMed] [Google Scholar]
- 137.Kelly MJ, Lagrange AH, Wagner EJ, Ronnekleiv O. Rapid effects of estrogens to modulate G protein coupled receptors via activation of protein kinase A and protein kinase C pathways. Steroids. 1999;64:64–75. doi: 10.1016/s0039-128x(98)00095-6. [DOI] [PubMed] [Google Scholar]
- 138.King MM, Huang CY, Chock P, Nairn AC, Hemmings HC, Chan KF, Greengard P. Mammalian brain phosphoproteins as substrates for calcineurin. J Biol Chem. 1984;259:8080–8083. [PubMed] [Google Scholar]
- 139.Klein-Hitpass L, Tsai SY, Weigel NL, Allan GF, Riley D, Rodriguez R, Schrader WT, Tsai MJ, O’Malley BW. The progesterone receptor stimulates cell-free transcription by enhancing the formation of a stable preinitiation complex. Cell. 1990;60:247–257. doi: 10.1016/0092-8674(90)90740-6. [DOI] [PubMed] [Google Scholar]
- 140.Kow L, Mobbs CV, Pfaff DW. Role of second messenger systems and neuronal activity in the regulation of lordosis by neurotransmitters, neuropeptides, and estrogen: A review. Neurosci Biobehav Rev. 1994;18:1–18. doi: 10.1016/0149-7634(94)90028-0. [DOI] [PubMed] [Google Scholar]
- 141.Kraus WL, Montano MM, Katzenellenbogen BS. Cloning of the rat progesterone receptor gene 5′-region and identification of two functionally distinct promoters. Mol Endocrinol. 1993;7:1603–1616. doi: 10.1210/mend.7.12.8145766. [DOI] [PubMed] [Google Scholar]
- 142.Kubli-Garfias C, Whalen RE. Induction of lordosis behavior in female rats by intravenous administration of progesterone. Horm Behav. 1977;93:5925–5930. doi: 10.1016/0018-506x(77)90073-3. [DOI] [PubMed] [Google Scholar]
- 143.Kushner PJ, Baxter JD, Duncan KG, et al. Eukaryotic regulatory elements lurking in plasmid DNA: The activator protein-1 site in pUC. Mol Endo. 1994;8:405–407. doi: 10.1210/mend.8.4.8052261. [DOI] [PubMed] [Google Scholar]
- 144.Labriola L, Salatino M, Proietti CJ. Heregulin induces transcriptional activation of progesterone receptor by mechanism that requires functional ErbB-2 and mitogen-activated protein kinase in breast cancer cells. Mol Cell Biol. 2003;23:1095–1111. doi: 10.1128/MCB.23.3.1095-1111.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lange CA. Making sense of cross talk between steroid hormone receptors and intracellular signaling pathways: who will have the last word? Mol Endocrinol. 2004;18:269–278. doi: 10.1210/me.2003-0331. [DOI] [PubMed] [Google Scholar]
- 146.Lange CA, Gioeli D, Hammes SR, Marker PC. Integration of rapid signaling events with steroid hormone receptor action in breast and prostate cancer. Ann Rev Physiol. 2007;69:171–199. doi: 10.1146/annurev.physiol.69.031905.160319. [DOI] [PubMed] [Google Scholar]
- 147.Lanz RB, McKenna NJ, Onate SA, Albrecht U, Wong J, Tsai SY, Tsai MJ, O’Malley BW. A steroid receptor coactivator, SRA, functions as an RNA and is present in an SRC-1 complex. Cell. 1999;97:17–27. doi: 10.1016/s0092-8674(00)80711-4. [DOI] [PubMed] [Google Scholar]
- 148.Lanz RB, Lonard DM, O’Malley BW. Nuclear receptor coregulators in human diseases. In: Kumar R, O’Malley BW, editors. Nuclear receptor coregulators and human diseases. World Scientific publishing; New Jersey: 2008. pp. 1–134. [Google Scholar]
- 149.Leroy P, Krust A, Zelent A, Mendelsohn C, Garnier JM, Kastner P, Dierich A, Chambon P. Multiple isoforms of the mouse retinoic acid receptor alpha are generated by alternative splicing and differential induction by retinoic acid. EMBO J. 1991;10:59–69. doi: 10.1002/j.1460-2075.1991.tb07921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Levine JE, Chappell PE, Schneider JS, Sleiter NC, Szabo M. Progesterone receptors as neuroendocrine integrators. Front Neuroendocrinol. 2001;22:69–106. doi: 10.1006/frne.2001.0210. [DOI] [PubMed] [Google Scholar]
- 151.Limbird LE. Activation and attenuation of adenylate cyclase. The role of GTP-binding proteins as macromolecular messengers in receptor–cyclase coupling. Biochem J. 1981;195:1–13. doi: 10.1042/bj1950001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lisk RD. Diencephalic placement of estradiol and sexual receptivity in the female rat. Amer J Physiol. 1962;203:493–496. doi: 10.1152/ajplegacy.1962.203.3.493. [DOI] [PubMed] [Google Scholar]
- 153.Lonard DM, O’Malley BW. Nuclear receptor coregulators: Judges, juries and executioners of cellular regulation. Mol Cell. 2007;27:691–700. doi: 10.1016/j.molcel.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 154.Lonstein JS, Blaustein JD. Immunocytochemical investigation of nuclear progestin receptor expression within dopaminergic neurons of the female rat brains. J Neuroendocrinology. 2004;16:534–543. doi: 10.1111/j.1365-2826.2004.01198.x. [DOI] [PubMed] [Google Scholar]
- 155.Lösel R, Wehling M. Nongenomic actions of steroid hormones. Nat Rev Mol Cell Biol. 2003;4:46–56. doi: 10.1038/nrm1009. [DOI] [PubMed] [Google Scholar]
- 156.Luttge WG, Hughes JR. Intracerebral implantation of progesterone: Reexamination of the brain sites responsible for facilitation of sexual receptivity in estrogen-primed ovariectomized rats. Physiol Behav. 1976;17:771–775. doi: 10.1016/0031-9384(76)90038-x. [DOI] [PubMed] [Google Scholar]
- 157.Lutz LB, Kim B, Jahani D, Hammes SR. G protein βγ subunits inhibit nongenomic progesterone-induced signaling and maturation in Xenopus laevis oocytes. J Biol Chem. 2000;275:41512–41520. doi: 10.1074/jbc.M006757200. [DOI] [PubMed] [Google Scholar]
- 158.Lydon JP, DeMayo FJ, Funk CR, Mani SK, Hughes AR, Montgomery CA, Jr, Shyamala G, Conneely OM, O’Malley BW. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev. 1995;9:2266–2278. doi: 10.1101/gad.9.18.2266. [DOI] [PubMed] [Google Scholar]
- 159.MacLusky NJ, McEwen BS. Oestrogen modulates progestin receptor concentration in some brain areas and not in others. Nature. 1978;274:276–277. doi: 10.1038/274276a0. [DOI] [PubMed] [Google Scholar]
- 160.MacLusky NJ, Lieberburg I, Krey LC, McEwen BS. Progestin receptors in the brain and pituitary of bonnet monkey (Macaca radiata): Differences between the monkey and the rat in the distribution of progestin receptors. Endocrinol. 1980;106:185–191. doi: 10.1210/endo-106-1-185. [DOI] [PubMed] [Google Scholar]
- 161.Mahajan MA, Samuels HH. A new family of nuclear receptor coregulators that integrate nuclear receptor signaling through CREB-binding protein. Mol Cell Biol. 2000;20:5048–5063. doi: 10.1128/mcb.20.14.5048-5063.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Mani SK, Blaustein JD, Allen JMC, Law SW, O’Malley BW, Clark JH. Inhibition of rat sexual behavior by antisense oligonucleotides to the progesterone receptor. Endocrinol. 1994;135:1409–1414. doi: 10.1210/endo.135.4.7925102. [DOI] [PubMed] [Google Scholar]
- 163.Mani SK, Allen JMC, Clark JH, Blaustein JD, O’Malley BW. Convergent pathways for steroid hormone- and neurotransmitter-induced rat sexual behavior. Science. 1994;265:1246–1249. doi: 10.1126/science.7915049. [DOI] [PubMed] [Google Scholar]
- 164.Mani SK, Allen JMC, Rettori V, McCann SM, O’Malley BW, Clark JH. Nitric oxide mediates sexual behavior in female rats. Proc Natl Acad Sci U S A. 1994;91:6468–6472. doi: 10.1073/pnas.91.14.6468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Mani SK, Allen JMC, Lydon JP, Mulac-Jericevic B, Blaustein JD, DeMayo FJ, Conneely OM, O’Malley BW. Dopamine requires the unoccupied progesterone receptor to induce sexual behavior in mice. Mol Endocrinol. 1996;10:1728–1737. doi: 10.1210/mend.10.12.8961281. [DOI] [PubMed] [Google Scholar]
- 166.Mani SK, Fienberg AA, O’Callaghan JP, Snyder GL, Allen PB, Dash PK, Moore AN, Mitchell AJ, Bibb J, Greengard P, O’Malley BW. Requirement for DARPP-32 in progesterone-facilitated sexual receptivity in female rats and mice. Science. 2000;287:1053–1056. doi: 10.1126/science.287.5455.1053. [DOI] [PubMed] [Google Scholar]
- 167.Mani SK, Mitchell AM, O’Malley BW. Progesterone receptors and dopamine receptors are required in Δ9 –tetrahydrocannabinal-modulation of sexual receptivity in female rats. Proc Natl Acad Sci USA. 2001;98:1249–1254. doi: 10.1073/pnas.031563998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Mani SK, Reyna AM, Chen JZ, Mulac-Jericevic B, Conneely OM. Differential response of progesterone receptor isoforms in hormone-dependent and –independent facilitation of female sexual receptivity. Mol Endocrinol. 2006;20:1322–1332. doi: 10.1210/me.2005-0466. [DOI] [PubMed] [Google Scholar]
- 169.Mani SK. Signaling mechanisms in progesterone-neurotransmitter interactions. Neuroscience. 2006;138:773–781. doi: 10.1016/j.neuroscience.2005.07.034. [DOI] [PubMed] [Google Scholar]
- 170.Mani S. Progestin receptor subtypes in the brain: the known and the unknown. Endocrinol. 2008;149:2750–2756. doi: 10.1210/en.2008-0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Mani SK, O’Malley BW. Mechanism of progesterone receptor action in the brain. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain and Behavior. 2. Vol. 3. Academic Press; San Diego: 2009. pp. 1467–1503. [Google Scholar]
- 172.Mani SK, Portillo W, Reyna A. Steroid hormone action in the brain: Cross talk between signalling pathways. J Neuroendocrinol. 2009;21:243–247. doi: 10.1111/j.1365-2826.2009.01844.x. [DOI] [PubMed] [Google Scholar]
- 173.McClintock MK, Adler NT. The role of female during copulation in wild and domestic Norway rats (Rattus norvegicus) Behav. 1978;67:67–96. [Google Scholar]
- 174.McEwen BS. Neural gonadal actions. Science. 1981;211:1303–1311. doi: 10.1126/science.6259728. [DOI] [PubMed] [Google Scholar]
- 175.McEwen BS. Non-genomic and genomic effects of steroids on neural activity. Trends Pharmacol Sci. 1991;12:141–146. doi: 10.1016/0165-6147(91)90531-v. [DOI] [PubMed] [Google Scholar]
- 176.McEwen BS. Steroid hormone actions in the brain: When is genome involved? Horm Behav. 1994;28:396–405. doi: 10.1006/hbeh.1994.1036. [DOI] [PubMed] [Google Scholar]
- 177.McKenna NJ, Lanz RB, O’Malley BW. Nuclear receptor coregulators: Cellular and molecular biology. Endocr Rev. 1999;20:321–344. doi: 10.1210/edrv.20.3.0366. [DOI] [PubMed] [Google Scholar]
- 178.Meredith JM, Auger AP, Blaustein JD. D1 dopamine receptor agonist (SKF-38393) induction of Fos immunoreactivity in progestin receptor-containing areas of female rat brain. J Neuroendocrinol. 1997;9:383–394. doi: 10.1046/j.1365-2826.1997.00594.x. [DOI] [PubMed] [Google Scholar]
- 179.Mendoza C, Soler A, Tesarik J. Nongenomic steroid action: independent targeting of a plasma membrane calcium channel and a tyrosine kinase. Biochem Biophys Res Commun. 1995;210:518–523. doi: 10.1006/bbrc.1995.1690. [DOI] [PubMed] [Google Scholar]
- 180.Mermelstein PG, Becker JB, Surmeier DJ. Estradiol reduces calcium currents in rat neostriatal neurons via a membrane receptor. J Neurosci. 1996;16:595–604. doi: 10.1523/JNEUROSCI.16-02-00595.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Meyerson B. Latency between intravenous injection of progestins and the appearance of oestrous behavior in estrogen-treated ovariectomized rats. Horm Behav. 1972;3:1–10. doi: 10.1016/0018-506x(72)90001-3. [DOI] [PubMed] [Google Scholar]
- 182.Meiri H. Is synaptic transmission modulated by progesterone? Brain Res. 1986;385:193–196. doi: 10.1016/0006-8993(86)91566-0. [DOI] [PubMed] [Google Scholar]
- 183.Meisel RL, Pfaff DW. RNA and protein synthesis inhibitors: Effects on sexual behavior in female rats. Brain Res Bull. 1984;12:187–193. doi: 10.1016/0361-9230(84)90188-6. [DOI] [PubMed] [Google Scholar]
- 184.Meisel RL, Pfaff DW. Specificity and neural sites of action of anisomycin in the reduction of facilitation of female sexual behavior in rats. Horm Behav. 1985;19:237–251. doi: 10.1016/0018-506x(85)90024-8. [DOI] [PubMed] [Google Scholar]
- 185.Meyer C, Schmid R, Scriba PC, Wehling M. Purification and partial sequencing of high affinity progesterone binding site(s) from porcine liver membranes. Eur J Biochem. 1996;239:726–731. doi: 10.1111/j.1432-1033.1996.0726u.x. [DOI] [PubMed] [Google Scholar]
- 186.Migliaccio A, Piccolo D, Castoria G, Di Domenico M, Bilancio A, Lombardi M, Gong W, Beato M, Auricchio F. Activation of the Src/p21ras/Erk pathway by progesterone receptor via cross-talk with estrogen receptor. EMBO J. 1998;17:2008–2018. doi: 10.1093/emboj/17.7.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Molenda HA, Griffin AL, Auger AP, McCarthy MM, Tetel MJ. Nuclear receptor coactivators modulate hormone-dependent gene expression in brain and female reproductive behavior in rats. Endocrinol. 2002;143:436–444. doi: 10.1210/endo.143.2.8659. [DOI] [PubMed] [Google Scholar]
- 188.Molenda-Figueira HA, Williams CA, Griffin AL, Rutledge EM, Blaustein JD, Tetel MJ. Nuclear receptor coactivators function in estrogen receptor-and progestin receptor-dependent aspects of sexual behavior in female rats. Horm Behav. 2006;50:383–392. doi: 10.1016/j.yhbeh.2006.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Molenda-Figueira HA, Murphy SD, Shea KL, Siegal NK, Zhao Y, Chadwic JG, Jr, Denner LA, Tetel MJ. Steroid receptor coactivator-1 from brain physically interacts differentially with steroid receptor subtypes. Endocrinol. 2008;149:5272–5279. doi: 10.1210/en.2008-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Morin LP. Progesterone: Inhibition of rodent sexual behavior. Physiol Behav. 1977;18:701–715. doi: 10.1016/0031-9384(77)90069-5. [DOI] [PubMed] [Google Scholar]
- 191.Mujtaba S, He Y, Zeng L, Yan S, Plotnikova O, Sachchidanand, Sanchez R, Zeleznik-Le NJ, Ronai Z, Zhou MM. Structural mechanism of the bromodomain of the coactivator CBP in p53 transcriptional activation. Mol Cell. 2004;13:251–263. doi: 10.1016/s1097-2765(03)00528-8. [DOI] [PubMed] [Google Scholar]
- 192.Mulac-Jericevic B, Mullinax RA, DeMayo FJ, Lydon JP, Conneely OM. Subgroup of reproductive functions of progesterone mediated by progesterone receptor-B isoform. Science. 2000;289:1751–1754. doi: 10.1126/science.289.5485.1751. [DOI] [PubMed] [Google Scholar]
- 193.Mulac-Jericevic B, Lydon JP, DeMayo FJ, Conneely OM. Defective mammary gland morphogenesis in mice lacking the progesterone receptor B isoform. Proc Natl Acad Sci USA. 2003;100:9744–9749. doi: 10.1073/pnas.1732707100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Nairn AC, Hemmings HC, Jr, Greengard P. Protein kinases in the brain. Ann Rev Biochem. 1985;54:931–976. doi: 10.1146/annurev.bi.54.070185.004435. [DOI] [PubMed] [Google Scholar]
- 195.Narayanan R, Adigun AA, Edwards DP, Weigel NL. Cyclin-dependent kinase activity is required for progesterone receptor function: Novel role for cyclin A/Cdk2 as a progesterone receptor coactivator. Mol Cell Biol. 2005;25:264–277. doi: 10.1128/MCB.25.1.264-277.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Nishi A, Snyder GL, Greengard P. Bidirectional regulation of DARPP-32 phosphorylation by dopamine. J Neurosci. 1997;17:8147–8155. doi: 10.1523/JNEUROSCI.17-21-08147.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Nishi A, Bibb JA, Snyder GL, Higashi H, Nairn AC, Greengard P. Amplification of dopaminergic signaling by a positive feedback loop. Proc Natl Acad Sci USA. 2000;97:12840–12845. doi: 10.1073/pnas.220410397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Nock B, Feder HH. Neurotransmitter modulation of steroid action in target cells that mediate reproduction and reproductive behavior. Neurosci Biobehav Rev. 1981;5:437–447. doi: 10.1016/0149-7634(81)90014-2. [DOI] [PubMed] [Google Scholar]
- 199.Ogawa H, Nishi M, Kawat M. Localization of nuclear coactivators p300 and steroid receptor coactivator 1 in the rat hippocampus. Brain Res. 2001;890:197–202. doi: 10.1016/s0006-8993(00)03158-9. [DOI] [PubMed] [Google Scholar]
- 200.Ogawa S, Olazabal S, Pfaff DW. Effects of intrahypothalamic administration of antisense DNA for progesterone receptor mRNA on reproductive behavior and progesterone receptor immunoreactivity. J Neurosci. 1994;14:1766–1774. doi: 10.1523/JNEUROSCI.14-03-01766.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Olesen KM, Jessen HM, Auger CJ, Auger AP. Dopaminergic activation of estrogen receptors in neonatal brain alters progestin receptor expression and juvenile social play behavior. Endocrinol. 2005;146:3705–3712. doi: 10.1210/en.2005-0498. [DOI] [PubMed] [Google Scholar]
- 202.Olesen KM, Auger CJ, Auger AP. Regulation of progestin receptor expression in the developing rat brain by a dopamine D1 receptor antagonist. J Neuroendocrinol. 2007;19:481–488. doi: 10.1111/j.1365-2826.2007.01554.x. [DOI] [PubMed] [Google Scholar]
- 203.O’Malley BW, Means AR. Female steroid hormones and target cell nuclei. Science. 1974;183:610–620. doi: 10.1126/science.183.4125.610. [DOI] [PubMed] [Google Scholar]
- 204.O’Malley BW, Tsai SY, Bagchi M, Weigel NL, Schrader WT, Tsai MJ. Molecular mechanism of action of a steroid hormone receptor. Recent Prog Horm Res. 1991;47:1–24. doi: 10.1016/b978-0-12-571147-0.50005-6. [DOI] [PubMed] [Google Scholar]
- 205.O’Malley BW, Schrader WT, Mani S, Smith CL, Weigel NL, Conneely OM, Clark JH. An alternative ligand-independent pathway for activation of steroid receptors. Recent Prog Horm Res. 1995;50:333–347. doi: 10.1016/b978-0-12-571150-0.50020-2. [DOI] [PubMed] [Google Scholar]
- 206.Parsons B, MacLusky NJ, Krey LC, Pfaff DW, McEwen BS. Temporal relationship between estrogeninducible progestin receptors in the female rat brain and the time course of estrogen activation of mating behavior. Endocrinol. 1980;107:774–779. doi: 10.1210/endo-107-3-774. [DOI] [PubMed] [Google Scholar]
- 207.Parsons B, Rainbow TC, MacLusky NJ, McEwen BS. Progestin receptor levels in rat hypothalamic and limbic nuclei. J Neurosci. 1982;2:1446–1452. doi: 10.1523/JNEUROSCI.02-10-01446.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Petitti N, Etgen AM. Progesterone depression of norepinephrine-stimulated cAMP accumulation in hypothalamic slices. Mol Brain Res. 1989;5:109–119. doi: 10.1016/0169-328x(89)90002-8. [DOI] [PubMed] [Google Scholar]
- 209.Pettiti N, Etgen AM. α1-adrenoreceptor augmentation of β-stimulated cAMP formation is enhanced by estrogen and reduced by progesterone in rat hypothalamic slices. J Neurosci. 1990;10:2842–2849. doi: 10.1523/JNEUROSCI.10-08-02842.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Pfaff DW, Schwartz-Giblin S, McCarthy MM, Kow L. Cellular and molecular mechanisms of female reproductive behavior. In: Knobil E, Neill JD, editors. Physiology of Reproduction. Raven; New York: 1994. pp. 107–220. [Google Scholar]
- 211.Pfaff DW, Schwanzel-Fukuda M, Parhar IS, Lauber AH, McCarthy MM, Kow LM. GnRH neurons and other cellular and molecular mechanisms for simple mammalian reproductive behaviors. Recent Prog Horm Res. 1994;49:1–25. doi: 10.1016/b978-0-12-571149-4.50005-2. [DOI] [PubMed] [Google Scholar]
- 212.Pierson-Mullany LK, Lange CA. Phosphorylation of progesterone receptor serine 400 mediates ligand-dependent transcriptional activity in response to activation of cyclin-dependent protein kinase 2. Mol Cell Biol. 2004;24:10542–10547. doi: 10.1128/MCB.24.24.10542-10557.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Pollio G, Xue P, Zanisi A, Nicolin A, Maggi A. Antisense oligonucleotide blocks progesterone-induced lordosis behavior in ovariectomized rats. Mol Brain Res. 1993;19:135–139. doi: 10.1016/0169-328x(93)90158-l. [DOI] [PubMed] [Google Scholar]
- 214.Power RF, Mani SK, Codina J, Conneely OM, O’Malley BW. Dopaminergic and ligand independent activation of steroid hormone receptors. Science. 1991;254:1636–1639. doi: 10.1126/science.1749936. [DOI] [PubMed] [Google Scholar]
- 215.Power RF, Lydon JP, Conneely OM, O’Malley BW. Dopamine activation of an orphan of the steroid receptor superfamily. Science. 1991;252:1546–1548. doi: 10.1126/science.2047861. [DOI] [PubMed] [Google Scholar]
- 216.Proietti L, Salatino M, Rosemblit C, Carnevale R, Pecci A, Kornblihtt AR, Molinolo AA, Frahm I, Charreau EH, Schillaci R, Elizalde PV. Progestins induce transcriptional activation of signal transducer and activator of transcription 3 (Stat3) via a Jak- and Src-dependent mechanism in breast cancer cells. Mol Cell Biol. 2005;25:4826–40. doi: 10.1128/MCB.25.12.4826-4840.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Qui M, Olsen A, Faivre E, Horwitz KB, Lange CA. Mitogen-activated protein kinase regulates nuclear association of human progesterone receptors. Mol Endo. 2003;17:628–642. doi: 10.1210/me.2002-0378. [DOI] [PubMed] [Google Scholar]
- 218.Qui M, Lange CA. MAP kinases couple multiple functions of human progesterone recptors: Degradation, transcriptional synergy, and nuclear association. J Steroid Biochem Mol Biol. 2003;85:147–157. doi: 10.1016/s0960-0760(03)00221-8. [DOI] [PubMed] [Google Scholar]
- 219.Rainbow TC, Degroff V, Luine VN, McEwen BS. Estradiol 17 beta increases the number of muscarinic receptors in hypothalamic nuclei. Brain Res. 1980;198:239–243. doi: 10.1016/0006-8993(80)90362-5. [DOI] [PubMed] [Google Scholar]
- 220.Rainbow TC, McGinnis MY, Davis PG, McEwen BS. Application of anisomysin to the lateral ventromedial nucleus of the hypothalamus inhibits the activation of sexual behavior by estradiol and progesterone. Brain Res. 1982;223:417–423. doi: 10.1016/0006-8993(82)91217-3. [DOI] [PubMed] [Google Scholar]
- 221.Ramirez VD, Dluzen DE, Ke FC. Effects of progesterone and its metabolites on neuronal membranes. Ciba Found Symp. 1990;153:125–144. doi: 10.1002/9780470513989.ch7. [DOI] [PubMed] [Google Scholar]
- 222.Ramirez VD, Zheng J, Siddique KM. Membrane progesterone receptors for estrogen, progesterone and testosterone in the rat brain: Fantasy or reality. Cell Mol Neurobiol. 1996;16:175–198. doi: 10.1007/BF02088175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Ramírez-Orduña JM, Lima-Hernández FJ, García-Juárez M, González-Flores O, Beyer C. Lordosis facilitation by LHRH, PGE2 or db-cAMP requires activation of the kinase A signaling pathway in estrogen primed rats. Pharmacol Biochem Behav. 2007;86:169–175. doi: 10.1016/j.pbb.2006.12.022. [DOI] [PubMed] [Google Scholar]
- 224.Richmond G, Clemens LG. Cholinergic mediation of feminine sexual receptivity: Demonstration of progesterone independence using a progestin receptor antagonist. Brain Res. 1986;373:159–163. doi: 10.1016/0006-8993(86)90326-4. [DOI] [PubMed] [Google Scholar]
- 225.Rodriguez-Manzo G, Cruz ML, Beyer C. Facilitation of lordosis behavior in ovariectomized estrogen-primed rats by medial preoptic implantation of 5 beta, 3 beta, pregnenolone: a ring A reduced progesterone metabolite. Physiol Behav. 1986;36:277–281. doi: 10.1016/0031-9384(86)90016-8. [DOI] [PubMed] [Google Scholar]
- 226.Rohe HJ, Ahmed IS, Twist KE, Craven RJ. PGRMC1 (progesterone receptor membrane component 1): a targetable protein with multiple functions in steroid signaling, P450 activation and drug binding. Pharmacol Ther. 2009;121:14–19. doi: 10.1016/j.pharmthera.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Rosenfeld MG, Lunyak VV, Glass CK. Sensors and signals: a coactivator/corepressor/epigenetic code for integrating signal-dependent programs of transcriptional response. Genes Dev. 2006;20:1405–1428. doi: 10.1101/gad.1424806. [DOI] [PubMed] [Google Scholar]
- 228.Ross J, Claybough C, Clemens LG, Gorski RA. Short latency of estrous behavior with intracerebral gonadal hormones in ovariectomized rats. Endocrinology. 1971;89:32–38. doi: 10.1210/endo-89-1-32. [DOI] [PubMed] [Google Scholar]
- 229.Rowan BG, Weigel NL, O’Malley BW. Phosphorylation of steroid receptor coactivator-l. Identification of the phosphorylation sites and phosphorylation through the mitogen-activated protein kinase pathway. J Biol Chem. 2000;275:4475–4483. doi: 10.1074/jbc.275.6.4475. [DOI] [PubMed] [Google Scholar]
- 230.Rowan BG, Garrison N, Weigel NL, O’Malley BW. 8-Bromo-cyclic AMP induces phosphorylation of two sites in SRC-l that facilitate ligand-independent activation of the chicken progesterone receptor and are critical for functional cooperation between SRC-l and CREB binding protein. Mol Cell Biol. 2000;20:8720–8730. doi: 10.1128/mcb.20.23.8720-8730.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Rubin BS, Barfield RJ. Priming of estrous responsiveness by implants of 17/3-estradiol in the ventromedial hypothalamic nucleus of female rats. Endocrinol. 1983;106:504–509. doi: 10.1210/endo-106-2-504. [DOI] [PubMed] [Google Scholar]
- 232.Rupprecht R, Holsboer F. Neuroactive steroids: mechanisms of action and neuropsychopharmacological perspectives. Trends Neurosci. 1999;22:410–416. doi: 10.1016/s0166-2236(99)01399-5. [DOI] [PubMed] [Google Scholar]
- 233.Samalecos A, Gellerson B. Systematic expression analysis and antibody screening do not support the existence of naturally occurring progesterone receptor (PR)-C, PR-M, or other truncated PR isoforms. Endocrinol. 2008;149:5872–5887. doi: 10.1210/en.2008-0602. [DOI] [PubMed] [Google Scholar]
- 234.Saner KJ, Welter BH, Zhang F, Hansen E, Dupont B, Wei Y, Price TM. Cloning and expression of a novel, truncated, progesterone receptor. Mol Cell Endocrinol. 2003;200:155–163. doi: 10.1016/s0303-7207(02)00380-5. [DOI] [PubMed] [Google Scholar]
- 235.Schumacher M, Coirini H, Robert E, Guennoun R, El-Etr M. Genomic and membrane actions of progesterone: Implications for reproductive physiology and behavior. Behav Brain Res. 1999;105:37–52. doi: 10.1016/s0166-4328(99)00081-9. [DOI] [PubMed] [Google Scholar]
- 236.Schumacher M, Sitruk-Ware R, De Nicola AF. Progesterone and progestins: neuroprotection and myelin repair. Curr Opin Pharmacol. 2008;8:740–746. doi: 10.1016/j.coph.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 237.Selmin O, Lucier GW, Clark GC, Tritscher AM, Vanden Heuvel JP, Gastel JA, Walker NJ, Sutter TR, Bell DA. Isolation and characterization of a novel gene induced by 2,3,7,8-tetrachlorodibenzo-p-dioxin in rat liver. Carcinogenesis. 1996;17:2609–2615. doi: 10.1093/carcin/17.12.2609. [DOI] [PubMed] [Google Scholar]
- 238.Shemshedini L, Knauthe R, Sassone-Corsi P, Pornon A, Gronemeyer H. Cell-specific inhibitory and stimulatory effects of fos and jun on transcription activation by nuclear receptors. EMBO J. 1991;10:3839–3849. doi: 10.1002/j.1460-2075.1991.tb04953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Shenolikar S, Nairn AC. Protein phosphatases: recent progress. Adv Second Messenger Phosphoprotein Res. 1991;23:1–121. [PubMed] [Google Scholar]
- 240.Sherman MR, Corvol PL, O’Malley BW. Progesterone-binding components of chick oviduct I. Preliminary characterization of cytoplasmic components. J Biol Chem. 1970;245:6085–6096. [PubMed] [Google Scholar]
- 241.Sleiter N, Pang Y, Park C, Horton TH, Dong J, Thomas P, Levine JE. Progesterone receptor A (PRA) and PRB-independent effects of progesterone on gonadotropin-releasing hormone release. Endocrinol. 2009;150:3833–3844. doi: 10.1210/en.2008-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Smith DF, Faber LE, DO Purification of unactivated progesterone receptor and identification of novel receptor-associated proteins. J Biol Chem. 1990;265:3996–4003. [PubMed] [Google Scholar]
- 243.Smith SS, Waterhouse BD, Woodward DJ. Sex steroid effects on extrahypothalamic CNS. II. Progesterone, alone and in combination with estrogen, modulates cerebellar responses to amino acid neurotransmitters. Brain Res. 1987;422:52–62. doi: 10.1016/0006-8993(87)90539-7. [DOI] [PubMed] [Google Scholar]
- 244.Sodersten P, Eneroth P. Evidence that progesterone does not inhibit the induction of sexual receptivity by oestradiol-17β in the rat. J Endocrinol. 1981;89:63–69. doi: 10.1677/joe.0.0890063. [DOI] [PubMed] [Google Scholar]
- 245.Sodersten P, Hansen S. Effects of oestradiol and progesterone on the induction and duration of sexual receptivity in cyclic female rats. J Endocrinol. 1977;74:477–485. doi: 10.1677/joe.0.0740477. [DOI] [PubMed] [Google Scholar]
- 246.Sodersten P, Hansen S. Induction of sexual receptivity by oestradiol benzoate in cyclic female rats: Influence of ovarian secretion before injection of oestradiol benzoate. J Endocrinol. 1979;80:398–395. doi: 10.1677/joe.0.0800389. [DOI] [PubMed] [Google Scholar]
- 247.Svenningsson P, Nishi A, Fisone G, Girault JA, Nairn AC, Greengard P. DARPP-32: an integrator of neurotransmission. Annu Rev Pharmacol Toxicol. 2004;44:269–296. doi: 10.1146/annurev.pharmtox.44.101802.121415. [DOI] [PubMed] [Google Scholar]
- 248.Sweatt JD. Mitogen-activated protein kinases in synaptic plasticity and memory. Curr Opin Neurobiol. 2004;14:311–317. doi: 10.1016/j.conb.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 249.Szabo M, Kilen SM, Nho SJ, Schwartz NB. Progesterone receptor A and B messenger ribonucleic acid levels in the anterior pituitary of rats are regulated by estrogen. Biol Reprod. 2000;62:95–102. doi: 10.1095/biolreprod62.1.95. [DOI] [PubMed] [Google Scholar]
- 250.Taleisnik S, Sawyer CH. Activation of the CNS noradrenergic system may inhibit as well as facilitate pituitary luteinizing hormone release. Neuroendocrinol. 1986;44:265–268. doi: 10.1159/000124655. [DOI] [PubMed] [Google Scholar]
- 251.Tennent BJ, Smith ER, Davidson JM. The effects of estrogen and progesterone on female rat proceptive behavior. Horm Behav. 1980;14:65–75. doi: 10.1016/0018-506x(80)90016-1. [DOI] [PubMed] [Google Scholar]
- 252.Tennent BJ, Smith ER, Davidson JM. Effects of progesterone implants in the habenula and midbrain on proceptive and receptive behavior in the female rat. Horm Behav. 1982;16:352–373. doi: 10.1016/0018-506x(82)90033-2. [DOI] [PubMed] [Google Scholar]
- 253.Tetel MJ, Blaustein JD. Immunocytochemical evidence for noradrenergic regulation of estrogen receptor concentrations in the guinea pig hypothalamus. Brain Res. 1991;565:321–329. doi: 10.1016/0006-8993(91)91664-m. [DOI] [PubMed] [Google Scholar]
- 254.Tetel MJ, Lange CA. Molecular genomics of progestin actions. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain and Behavior. 2. Vol. 3. Academic Press; San Diego: 2009. pp. 1439–1465. [Google Scholar]
- 255.Tetel MJ. Nuclear receptor coactivators: essential players for steroid hormone action in the brain and in behaviour. J Neuroendocrinol. 2009;21:229–237. doi: 10.1111/j.1365-2826.2009.01827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Tetel MJ, Auger AP, Charlier TD. Who’s in charge? Nuclear receptor coactivator and corepressor function in brain and behavior. Front Neuroendocrinol. 2009;30:328–342. doi: 10.1016/j.yfrne.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Thomas P, Pang Y, Dong J, Groenen J, Kelder J, de Vlieg J, Zhu Y, Tubbs C. Steroid and G protein binding characteristics of the seatrout and human progestin membrane receptor α subtypes and their evolutionary origins. Endocrinol. 2007;148:705–718. doi: 10.1210/en.2006-0974. [DOI] [PubMed] [Google Scholar]
- 258.Torchia J, Rose DW, Inostroza J, Kamei Y, Westin S, Glass CK, Rosenfeld MG. The transcriptional co-activator p/CIP binds CBP and mediates nuclear-receptor function. Nature. 1997;387:677–684. doi: 10.1038/42652. [DOI] [PubMed] [Google Scholar]
- 259.Tsai MJ, O’Malley BW. Molecular mechanism of action of steroid/thyroid receptor superfamily members. Annu Rev Biochem. 1994;63:451–486. doi: 10.1146/annurev.bi.63.070194.002315. [DOI] [PubMed] [Google Scholar]
- 260.Tubbs C, Thomas P. Functional characteristics of membrane progestin receptor alpha (mPRα) subtypes: A review with new data showing mPRα expression in seatrout sperm and its association with sperm motility. Steroids. 2008;73:935–941. doi: 10.1016/j.steroids.2007.12.022. [DOI] [PubMed] [Google Scholar]
- 261.Turgeon JL, Waring DW. Activation of the progesterone receptor by the gonadotropin-releasing hormone self-priming signaling pathway. Mol Endocrinol. 1994;8:860–869. doi: 10.1210/mend.8.7.7984148. [DOI] [PubMed] [Google Scholar]
- 262.Umayahara Y, Kawamori R, Yamasaki Y, Kajimoto Y, Kamada T, Watada H, et al. Estrogen regulation of the insulin-like growth factor I gene transcription involves an AP-1 enhancer. J Biol Chem. 1994;269:16433–16442. [PubMed] [Google Scholar]
- 263.Uphouse L, Hiegel C, Guptarak J, Maswood N. Progesterone reduces the effect of the serotonin 1B/1D receptor antagonist, GR 127935, on lordosis behavior. Horm Behav. 2009;55:169–174. doi: 10.1016/j.yhbeh.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Vegeto E, Allan GF, Schrader WT, Tsai MJ, McDonnell DP, O’Malley BW. The mechanism of RU486 antagonism is dependent on the conformation of the carboxy-terminal tail of the human progesterone receptor. Cell. 1992;69:703–713. doi: 10.1016/0092-8674(92)90234-4. [DOI] [PubMed] [Google Scholar]
- 265.Walaas SI, Aswad DW, Greengard P. A dopamine- and cyclic AMP-regulated phosphoprotein enriched in dopamine-innervated brain regions. Nature. 1983;301:69–71. doi: 10.1038/301069a0. [DOI] [PubMed] [Google Scholar]
- 266.Walaas SI, Greengard P. DARPP-32, a dopamine- and adenosine 3′: 5′-monophosphate-regulated phosphoprotein enriched in dopamine-innervated brain regions. I. Regional and cellular distribution in the rat brain. J Neurosci. 1984;4:84–98. doi: 10.1523/JNEUROSCI.04-01-00084.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Wallen K, Thonton JE. Progesterone and duration of heat in estrogen treated, ovariectomized guinea pigs. Physiol Behav. 1979;22:95–97. doi: 10.1016/0031-9384(79)90409-8. [DOI] [PubMed] [Google Scholar]
- 268.Watters JJ, Campbell JS, Cunningham MJ, Krebs EG, Dorsa DM. Rapid membrane effects of steroids in neuroblastoma cells: effects of estrogen on mitogen activated protein kinase signalling cascade and c-fos immediate early gene transcription. Endocrinol. 1997;138:4030–4033. doi: 10.1210/endo.138.9.5489. [DOI] [PubMed] [Google Scholar]
- 269.Watters JJ, Dorsa DM. Transcriptional effects on neuronal neurotensin gene expression involve cAMP/protein kinase A-dependent signaling mechanisms. J Neurosci. 1998;18:6672–6680. doi: 10.1523/JNEUROSCI.18-17-06672.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Webb P, Lopez GN, Uht RM, Kushner PJ. Tamoxifen activation of the estrogen receptor/AP-1 pathway: potential origin for the cell-specific estrogen-like effects of antiestrogens. Mol Endocrinol. 1995;9:443–456. doi: 10.1210/mend.9.4.7659088. [DOI] [PubMed] [Google Scholar]
- 271.Wei LL, Gonzalez-Aller C, Wood WM, Miller LA, Horwitz KB. 5′-Heterogeneity in human progesterone receptor transcripts predicts a new amino-terminal truncated “C”-receptor and unique A-receptor messages. Mol Endocrinol. 1990;4:1833–1840. doi: 10.1210/mend-4-12-1833. [DOI] [PubMed] [Google Scholar]
- 272.Wei LL, Hawkins P, Baker C, Norris B, Sheridan PL, Quinn PG. An amino-terminal truncated progesterone receptor isoform, PRc, enhances progestin-induced transcriptional activity. Mol Endocrinol. 1996;10:1379–1387. doi: 10.1210/mend.10.11.8923464. [DOI] [PubMed] [Google Scholar]
- 273.Whalen RE, Gorzalka BB, DeBold JF, Quadagno DM, Ho GK, Hough JC., Jr Studies on the effect of intracerebral actinimycin D implants on estrogen-induced receptivity in rats. Horm Behav. 1974;5:337–343. doi: 10.1016/0018-506x(74)90019-1. [DOI] [PubMed] [Google Scholar]
- 274.Whalen RE, Lauber AH. Progesterone substitutes: cGMP mediation. Neurosci Biobehav Rev. 1986;10:47–53. doi: 10.1016/0149-7634(86)90032-1. [DOI] [PubMed] [Google Scholar]
- 275.White NR, Barfield RJ. Playback of female rat ultrasonic vocalizartions during sexual behavior. Physiol Behav. 1989;45:229–233. doi: 10.1016/0031-9384(89)90123-6. [DOI] [PubMed] [Google Scholar]
- 276.Xu Y, Klein-Hitpass L, Bagchi MK. E1A-mediated repression of progesterone receptor-dependent transactivation involves inhibition of the assembly of a multisubunit coactivation complex. Mol Cell Biol. 2000;20:2138–2146. doi: 10.1128/mcb.20.6.2138-2146.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Yamanaka T, Hirata S, Shoda T, Hoshi K. Progesterone receptor mRNA variant containing novel exon insertions between exon 4 and exon 5 in human uterine endometrium. Endocrinol J. 2002;49:473–482. doi: 10.1507/endocrj.49.473. [DOI] [PubMed] [Google Scholar]
- 278.Young WC. Psychobiology of sexual behavior in the guinea pig. In: Lehrman DS, Hinde RA, Shaw E, editors. Advances in the Study of Behavior. Academic Press; New York: 1960. pp. 1–110. [Google Scholar]
- 279.Zhang X, Jeyakumar M, Petukhov S, Bagchi M. A nuclear receptor corepressor modulates transcriptional activity of antagonist-occupied steroid hormone receptor. Mol Endocrinol. 1998;12:513–524. doi: 10.1210/mend.12.4.0089. [DOI] [PubMed] [Google Scholar]
- 280.Zhou Y, Watters JJ, Dorsa DM. Estrogen rapidly induces the phosphorylation of the cAMP response element binding protein in rat brain. Endocrinol. 1996;137:2163–2166. doi: 10.1210/endo.137.5.8612562. [DOI] [PubMed] [Google Scholar]
- 281.Zhu Y, Rice CD, Pang Y, Pace M, Thomas P. Cloning, expression, and characterization of a membrane progestin receptor and evidence it is an intermediary in meiotic maturation of fish oocytes. Proc Natl Acad Sci U S A. 2003;100:2231–2236. doi: 10.1073/pnas.0336132100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Zhu Y, Bond J, Thomas JP. Identification, classification and partial characterization of genes in humans and other vertebrates homologous to a fish membrane progestin receptor. Proc Natl Acad Sci USA. 2003;100:2237–2242. doi: 10.1073/pnas.0436133100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Zucker I. Facilitatory and inhibitory effects of progesterone on sexual responses of spayed guinea pigs. J Comp Physiol Psych. 1966;3:376–381. [Google Scholar]
- 284.Zucker I. Biphasic effects of progesterone on sexual receptivity in the female guinea pig. J Comp Physiol Psychol. 1968;63:472–478. doi: 10.1037/h0025819. [DOI] [PubMed] [Google Scholar]