Abstract
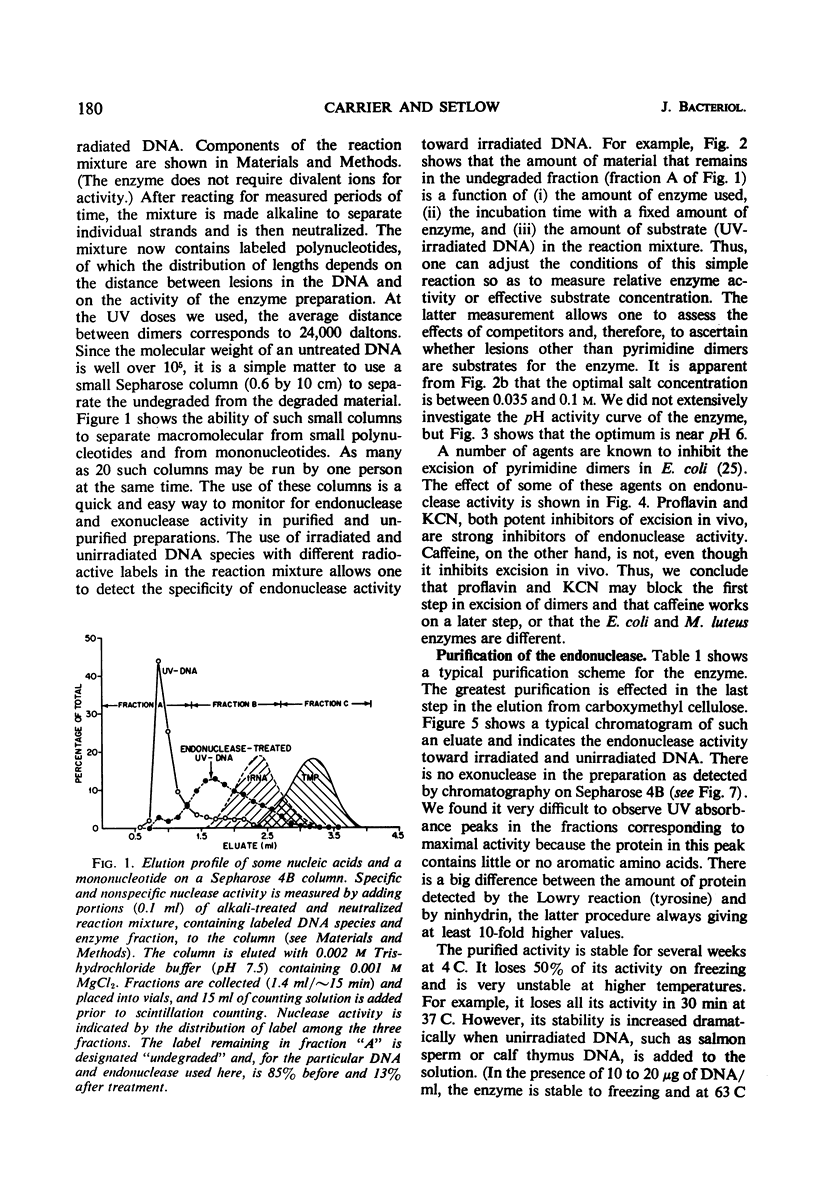
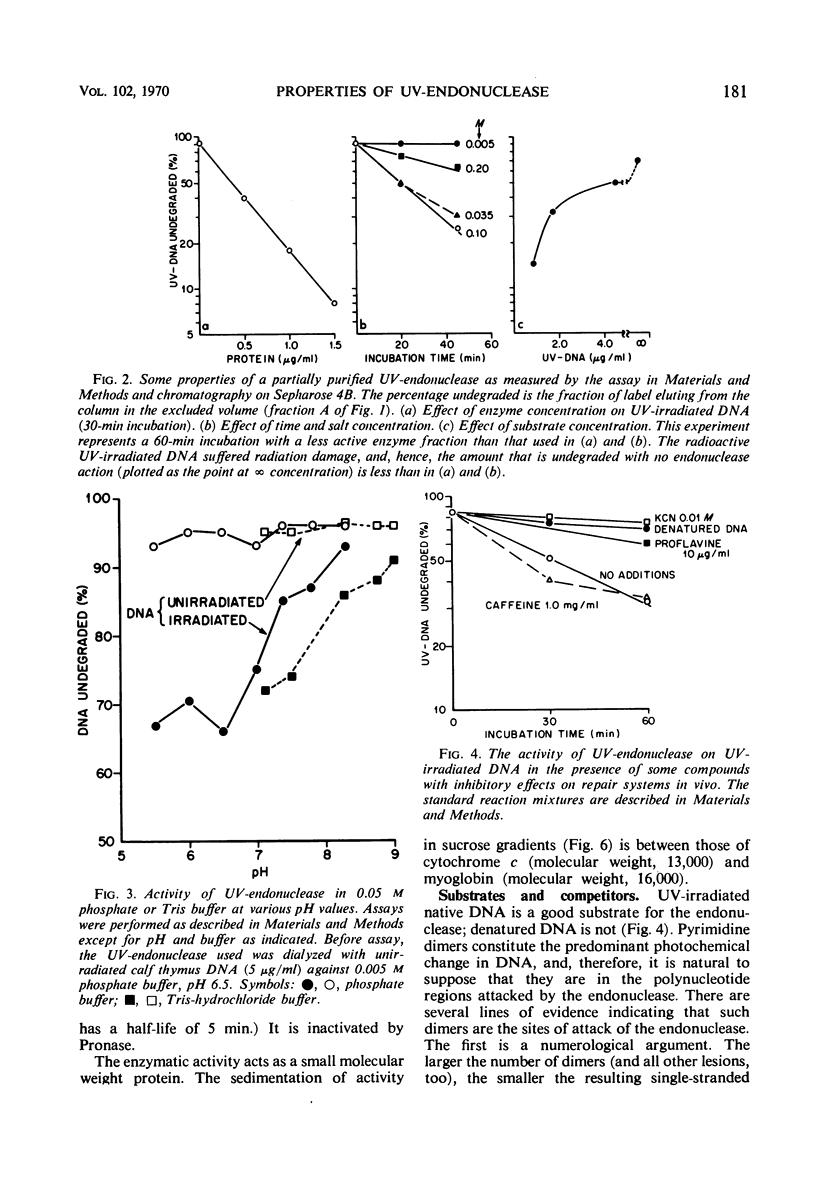
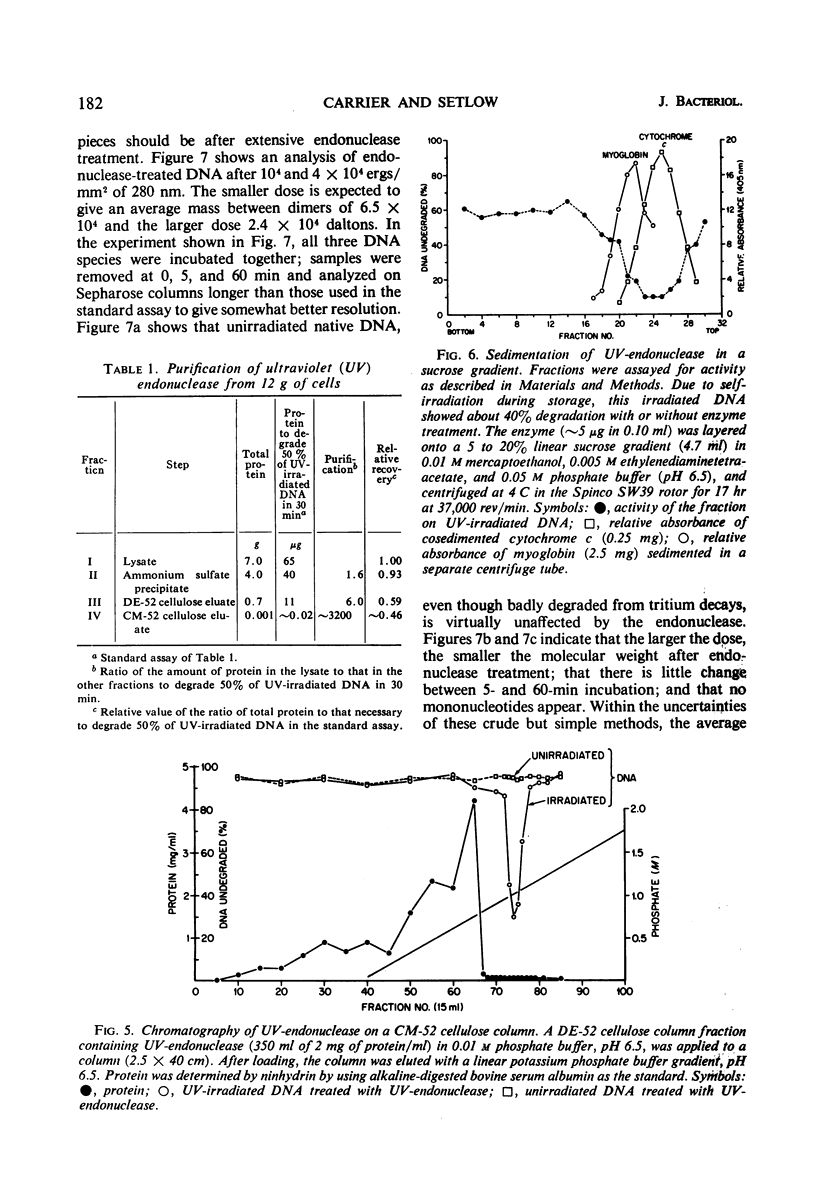
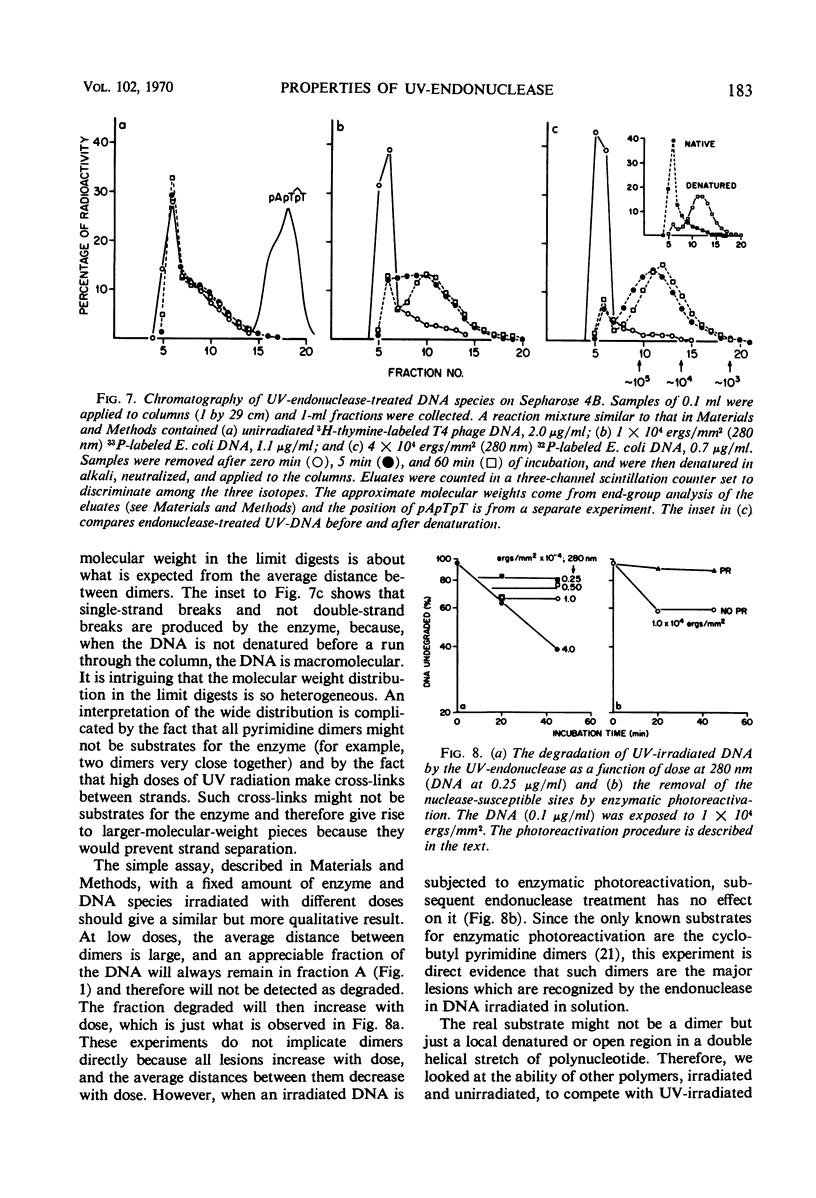
An endonuclease purified ∼3,200-fold from Micrococcus luteus is active on native ultraviolet-irradiated deoxyribonucleic acid (DNA), but is inactive on unirradiated native or denatured DNA and has no activity toward irradiated denatured DNA. The major type of lesion for the nucleolytic activity is the cyclobutane pyrimidine dimer. The enzyme makes a number of single-strand breaks approximately equal to the number of dimers, but dimers are not excised. This endonuclease—a small molecular weight protein—therefore has all the attributes hypothesized for the first enzyme in the sequential steps in repair of DNA in vivo. Another paper shows that the endonuclease is able to reactivate ultraviolet-irradiated transforming DNA.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BODMER W. F., GANESAN A. T. BIOCHEMICAL AND GENETIC STUDIES OF INTEGRATION AND RECOMBINATION IN BACILLUS SUBTILIS TRANSFORMATION. Genetics. 1964 Oct;50:717–738. doi: 10.1093/genetics/50.4.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder R. L., Beers R. F. Nonphotoreactivating Repair of Ultraviolet Light-Damaged Transforming Deoxyribonucleic Acid by Micrococcus lysodeikticus Extracts. J Bacteriol. 1965 Sep;90(3):681–686. doi: 10.1128/jb.90.3.681-686.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman L., Kaplan J. C., Kushner S. R., Mahler I. Enzymes involved in the early stages of repair of ultraviolet-irradiated DNA. Cold Spring Harb Symp Quant Biol. 1968;33:229–234. doi: 10.1101/sqb.1968.033.01.026. [DOI] [PubMed] [Google Scholar]
- HIRS C. H., MOORE S., STEIN W. H. Peptides obtained by tryptic hydrolysis of performic acid-oxidized ribonuclease. J Biol Chem. 1956 Apr;219(2):623–642. [PubMed] [Google Scholar]
- Howard-Flanders P. DNA repair. Annu Rev Biochem. 1968;37:175–200. doi: 10.1146/annurev.bi.37.070168.001135. [DOI] [PubMed] [Google Scholar]
- Kaplan J. C., Kushner S. R., Grossman L. Enzymatic repair of DNA, 1. Purification of two enzymes involved in the excision of thymine dimers from ultraviolet-irradiated DNA. Proc Natl Acad Sci U S A. 1969 May;63(1):144–151. doi: 10.1073/pnas.63.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Moriguchi E., Suzuki K. Enzymatic breakdown of UV-irradiated DNA by the extract from Micrococcus lysodeicticus. Biochem Biophys Res Commun. 1966 Jul 20;24(2):195–202. doi: 10.1016/0006-291x(66)90719-4. [DOI] [PubMed] [Google Scholar]
- Muhammed A. Studies on the yeast photoreactivating enzyme. I. A method for the large scale purification and some properties of the enzyme. J Biol Chem. 1966 Jan 25;241(2):516–523. [PubMed] [Google Scholar]
- Nakayama H., Okubo S., Sekiguchi M., Takagi Y. A deoxyribonuclease activity specific for ultraviolet-irradiated DNA: a chromatographic analysis. Biochem Biophys Res Commun. 1967 Apr 20;27(2):217–223. doi: 10.1016/s0006-291x(67)80064-0. [DOI] [PubMed] [Google Scholar]
- Notani N., Goodgal S. H. On the nature of recombinants formed during transformation in Hemophilus influenzae. J Gen Physiol. 1966 Jul;49(6):197–209. doi: 10.1085/jgp.49.6.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okubo S., Nakayama H., Sekiguchi M., Takagi Y. A mutant of Micrococcus lyosodeikticus defective in a deoxyribonuclease activity specific for ultraviolet-irradiated DNA. Biochem Biophys Res Commun. 1967 Apr 20;27(2):224–229. doi: 10.1016/s0006-291x(67)80065-2. [DOI] [PubMed] [Google Scholar]
- ROERSCH A., VAN DER KAM P. C., ADEMA J. DARK REACTIVATION OF ULTRAVIOLET IRRADIATED BACTERIOPHAGE DEOXYRIBONUCLEIC ACID IN VITRO. Biochim Biophys Acta. 1964 Feb 17;80:346–348. [PubMed] [Google Scholar]
- STRAUSS B. S. Differential destruction of the transforming activity of damaged deoxyribonucleic acid by a bacterial enzyme. Proc Natl Acad Sci U S A. 1962 Sep 15;48:1670–1675. doi: 10.1073/pnas.48.9.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow J. K., Boling M. E., Bollum F. J. The chemical nature of photoreactivable lesions in DNA. Proc Natl Acad Sci U S A. 1965 Jun;53(6):1430–1436. doi: 10.1073/pnas.53.6.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow R. B., Carrier W. L. Pyrimidine dimers in ultraviolet-irradiated DNA's. J Mol Biol. 1966 May;17(1):237–254. doi: 10.1016/s0022-2836(66)80105-5. [DOI] [PubMed] [Google Scholar]
- Setlow R. B. Cyclobutane-type pyrimidine dimers in polynucleotides. Science. 1966 Jul 22;153(3734):379–386. doi: 10.1126/science.153.3734.379. [DOI] [PubMed] [Google Scholar]
- Setlow R. B. The photochemistry, photobiology, and repair of polynucleotides. Prog Nucleic Acid Res Mol Biol. 1968;8:257–295. doi: 10.1016/s0079-6603(08)60548-6. [DOI] [PubMed] [Google Scholar]
- Shimada K., Nakayama H., Okubo S., Sekiguchi M., Takagi Y. An endonucleolytic activity specific for ultraviolet-irradiated DNA in wild type and mutant strains of Micrococcus lysodeikticus. Biochem Biophys Res Commun. 1967 Jun 9;27(5):539–545. doi: 10.1016/s0006-291x(67)80021-4. [DOI] [PubMed] [Google Scholar]
- Strauss B., Searashi T., Robbins M. Repair of DNA studied with a nuclease specific for UV-induced lesions. Proc Natl Acad Sci U S A. 1966 Sep;56(3):932–939. doi: 10.1073/pnas.56.3.932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi Y., Sekiguchi M., Okubo S., Nakayama H., Shimada K., Yasuda S., Nishimoto T., Yoshihara H. Nucleases specific for ultraviolet light-irradiated DNA and their possible role in dark repair. Cold Spring Harb Symp Quant Biol. 1968;33:219–227. doi: 10.1101/sqb.1968.033.01.025. [DOI] [PubMed] [Google Scholar]


