Abstract
HEPES has been widely employed as an organic buffer agent in cell culture medium as well as uptake and transport experiments in vitro. However, concentrations of HEPES used in such studies vary from one laboratory to another. In this study, we investigated the effect of HEPES on the uptake and bidirectional transport of P-gp substrates employing both Caco-2 and MDCK-MDR1 cells. ATP-dependent uptake of glutamic acid was also examined. ATP production was further quantified applying ATP Determination Kit. An addition of HEPES to the cellular washing and incubation media significantly altered the uptake and transport of P-gp substrates in both Caco-2 and MDCK-MDR1 cells. Uptake of P-gp substrates substantially diminished as the HEPES concentration was raised to 25 mM. Bidirectional (A-B and B-A) transport studies revealed that permeability ratio of PappB-A to PappA-B in the presence of 25 mM HEPES was significantly higher than control. The uptake of phenylalanine is an ATP-independent process, whereas the accumulation of glutamic acid is ATP-dependent. While phenylalanine uptake remained unchanged glutamic acid uptake was elevated with the addition of HEPES. Verapamil is an inhibitor of P-gp mediated uptake, elevation of cyclosporine uptake in the presence of 5 μM verapamil was compromised by the presence of 25 mM HEPES. The results of ATP assay indicated that HEPES stimulated the production of ATP. This study suggests that the addition of HEPES in the medium modulated the energy dependent efflux and uptake processes. The effect of HEPES on P-gp mediated drug efflux and transport may provide some mechanistic insight into possible reasons for inconsistencies in the results reported from various laboratories.
Keywords: HEPES, P-gp substrates, ATP dependent, uptake, transport, MDCK-MDR1
Introduction
HEPES, 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, has been widely employed as an organic buffer agent within a pH range of 6.0 to 8.5 in the culture medium and incubation buffer replacing bicarbonate.1
Several reports2–3 suggest that HEPES inhibited the uptake of taurine and β–amino acids by primary glial cells, but did not affect taurine uptake by neuronal cells using in vitro cell culture models. Two different mechanisms, fast and slow inhibition were raised. Fast inhibition, related to the presence of HEPES in the incubation medium, ensues within the first few minutes and then gradually accelerates. HEPES modifies the binding site specific for taurine, but not for β–amino acids, which may be partially explained by similarity in chemical structures of taurine and HEPES. Slow inhibition, due to the presence of HEPES in the culture medium, produced the maximal effect in 2–3 days and was reversible. It was hypothesized that HEPES modified the concentration of an intracellular modulator of the taurine transporter and thus altered the membrane structure and the kinetic parameters of taurine uptake. Another study4 reported the fluorescence-induced cytotoxicity of 25 mM HEPES in RPMI 1640 culture medium. This cytotoxicity was mainly caused by H2O2 generated in the irradiated medium. Yamamoto and Suzuki reported that HEPES buffer blocked chloride channels in neurons of Drosophila.5 Lund and Wiggins reported that HEPES and Tris competitively inhibited the binding of N-acetylglutamate with carbamoyl-phosphate synthase.6
P-glycoprotein (P-gp) is an apically polarized plasma membrane protein that belongs to the ATP-binding cassette (ABC) superfamily.7–8 This efflux pump has received considerable attention in both drug resistance and delivery since its discovery in 1976.9–10 It acts as an ATP-dependent efflux pump for a broad range of compounds such as cardiovascular and antifungal agents, HIV protease inhibitors (PI), steroids, calcium channel blockers, and cytotoxic drugs. P-gp expression in the intestine limits oral absorption by active secretion of absorbing drug molecules back into the lumen.11 Inhibition of the P-gp activity enhances oral absorption and bioavailability of the anti-cancer and anti-HIV drugs.12 Caco-2 cell monolayers are known to express high levels of P-gp and have been widely employed as an in vitro model for P-gp mediated drug efflux and transport studies.13 Another cell line, MDCK-MDR1, has been recommended as an alternative to Caco-2 for high throughput drug screening.14
There is a possibility that HEPES may alter efflux activity of P-gp since HEPES has been widely employed in culture media and used in incubation buffers. HEPES concentrations used in incubation buffers vary greatly from laboratory to laboratory such as 10 mM for the transport of cholic acid conjugated PI15, nevirapine16, ranitidine and famotidine17, rhodamin 12318, digoxin19 and saquinavir20–21, 15 mM for passively absorbed drugs22, Xa-inhibitors susceptible to efflux23, substrates for oligopeptide transporter24, and 25 mM for P-gp and MRP substrates25–27, beta-lactam antibiotic28, and taxol29. Some researchers like Wu et al.30 added different concentrations of HEPES for different transporters such as 5 mM for P-gp and 10 mM for monocarboxylic acid transporter. Several other investigators used Hanks’ balanced salt solutions (HBSS) without HEPES to study the transport of P-gp and MRP substrates.14, 31–32 As HEPES primarily functions as a buffer component, it is necessary to assess the effect of HEPES on P-gp mediated efflux process. In this investigation, we have first studied the effect of HEPES in the incubation buffer on the uptake of P-gp substrates ([3H]cyclosporine-A, [3H]ritonavir, and [3H]lopinavir) and L–amino acids ([3H]glutamic acid and [3H]phenylalanine) employing both Caco-2 and MDCK-MDR1 cell lines. Then, the bidirectional transepithelial transport rate of [3H]lopinavir was compared with addition of 25 mM HEPES to the incubation buffer with that of control without HEPES added in the buffer. Finally, we further investigated the influence of the addition of HEPES into cellular culture growth medium on the ATP production and uptake of lopinavir applying MDCK-MDR1 cells and ATP measurement assay.
Materials and Methods
[3H]cyclosporine-A (CsA) and [3H]phenylalanine were obtained from Amersham Biosciences (Piscataway, NJ). [3H]ritonavir and [3H]lopinavir were purchased from Moravek Biochemicals (Brea, CA). [3H]glutamic acid and [3H]diazepam were procured from PerkinElmer Life Science (Boston, MA).
Human colon carcinoma derived Caco-2 cell line was purchased from American Type Culture Collection (ATCC, Rockville, MD). MDCK-MDR1 cells were obtained as a gift from P. Borst (Netherlands Cancer Institute, Amsterdam, The Netherlands). Dulbecco modified Eagle medium (DMEM) and nonessential amino acids were obtained from Gibco (Invitrogen, Grand Island, NY). Penicillin, streptomycin, sodium bicarbonate, and HEPES were purchased from Sigma Chemical Company (St. Louis, MO). Fetal bovine serum (FBS), calf serum (CS), and trypsin/EDTA solution were supplied by Atlanta Biologic and Gibco, respectively.
Uptake studies were conducted with Dulbecco modified phosphate buffer saline (DPBS), containing 129 mM NaCl, 2.5 mM KCl, 7.4 mM Na2HPO4, 1.3 mM KH2PO4, 1 mM CaCl2, 0.7 mM MgSO4, 5.3 mM glucose at pH 7.4. DPBS also contained 0 mM, 15 mM, and 25 mM HEPES. These chemicals were of analytical grade and obtained from Sigma Chemical Co. (St. Louis, MO). Culture flasks (75-cm2 growth area) and 12-well tissue culture-treated plastic plates were purchased from MidSci (St. Louis, MO). Polyester Transwells® (pore size of 0.4 μm and 12 mm diameter) were procured from Costar (Cambridge, MA).
Cell culture
Caco-2 cells (passages 25–40) were cultured in DMEM supplemented with 10% FBS (heat inactivated), 1% nonessential amino acids, 100 U/mL penicillin, 100 mg/mL streptomycin, 25 mM HEPES, and 29 mM sodium bicarbonate at pH 7.4. Cells were grown at 37 °C in a tissue culture incubator with 5% CO2 and 95% air. The medium was changed every other day until the cells reached confluence. Cells were plated at a density of 66,000/cm2 in 12-well tissue culture-treated plastic plates, incubated at 37 °C in humidified atmosphere of 5% CO2 and 95% air, and allowed to grow for 21–24 days.
MDCK-MDR1 cells were grown under culture conditions similar to Caco-2 cells, with one exception. The culture medium was supplemented with 10% calf serum (heat inactivated) instead of FBS. Culture medium was replaced every other day. Cells were allowed to grow for 3 to 4 days to reach 80% confluence, and then were plated on to 12-well tissue culture plates and grown for 6–7 days to reach confluent before experiments were initiated.
Uptake experiments
Cell monolayers were washed 3 times with 2 mL DPBS buffer containing various concentrations of HEPES, i.e. 0, 15, and 25 mM. With each washing, plates were incubated at 37 °C for 10 minutes. Then, 1 mL drug solution ([3H]cyclosporine-A, [3H]ritonavir, [3H]lopinavir, [3H]glutamic acid, [3H]phenylalanine or [3H]diazepam, 0.5 μCi/mL) in DPBS containing HEPES (0, 15, or 25 mM) was added to each well and incubated at 37 °C for 30 minutes. Then cell monolayers were washed 3 times with ice-cold stop solution (200 mM KCl and 2 mM HEPES) to terminate uptake followed by overnight lysis with 1 mL 0.1% (v/v) Triton X-100 in 0.3 N sodium hydroxide at room temperature. Aliquots (500 μL) from each well were then transferred to scintillation vials containing 5 mL scintillation cocktail (Fisher Scientific, Fairlawn, NJ). Samples were analyzed by a liquid scintillation counter (Model LS-6500, Beckman Instruments, Inc., Fullerton, CA). Amount accumulated was normalized to the protein content of each well. Amount of protein in the cell lysate was measured by a BioRad protein estimation kit (BioRad, Hercules, CA).
Uptake of [3H]lopinavir into two sets of MDCK-MDR1 cells grown under culture medium with or without HEPES
Two sets of cells were seeded and grown on 12-well plates, one set was supplemented with culture medium without HEPES (defined as Set 1), and the other set was supplemented with culture medium containing 20 mM of HEPES (defined as Set 2). These 12-well plates were grown for 6–7 days before experiments were conducted.
Permeation solutions of [3H]lopinavir (0.5 μCi/mL) in DPBS containing various concentrations (0, 15, 25 mM) of HEPES were firstly made. To further study the influence of P-gp inhibitor, solutions of [3H]lopinavir (0.5 μCi/mL) containing different concentrations of unlabelled lopinavir (1 μM, 2 μM and 5 μM) in DPBS buffer with 25 mM HEPES were also prepared. Then, uptake of [3H]lopinavir was performed following the same procedure as above described.
Bidirectional transepithelial transport experiment
Permeability of [3H]lopinavir across MDCK-MDR1 cells was determined in 12-well Transwell® plates. Before each experiment, cell monolayers were washed 3 times with DPBS containing 0 or 25 mM of HEPES at pH 7.4 and 37 °C, respectively. [3H]lopinavir solution (0.5μci/mL) was prepared in DPBS containing various levels of HEPES. An aliquot (0.5 mL) was placed in the upper chamber of Transwell® plate for AB transport and 1.5 mL was added to the lower chamber for B-A transport. The receiver chamber was filled with DPBS (1.5 mL for A-B and 0.5 mL for B-A transport). Samples (100 μL) were withdrawn from the receiver chamber at predetermined time points and replaced with fresh DPBS buffer solution with or without HEPES to maintain sink conditions. Samples were then analyzed by scintillation counting. All experiments were performed at 37 °C.
Data analysis
Apparent permeability coefficient P (PA-B or PB-A) of lopinavir was calculated according to Eq. 1:
| Eq. 1 |
dQ/dt is the transport rate, C0 is the initial donor concentration, A is the surface area of the membrane. Flux rate (dQ/dt×1/A) was calculated by plotting amount of lopinavir transported per unit area as a function of time and determining the slope of the linear plot by linear regression.33
ATP assay
MDCK-MDR1 cells were grown on 96-well tissue culture plates and supplemented with culture medium containing no HEPES (Set 1) and 20 mM of HEPES (Set 2). These plates were grown for 6 to 7 days to reach full confluence. Lysis solution was added to each set and kept for 10 hours. The lysate from these sets was used for quantitative determination of ATP using ATP Determination Kit (A22066, Molecular Probes, Invitrogen). Luminescence was measured using a 96-well microtiter plate reader (SpectraFluor Plus; Tecan, Maennedorf, Switzerland). Protein content of each well was also measured using Bradford Protein dye and quantified using the same 96-well microtiter plate reader.
Statistical analysis
All experiments were conducted at least in triplicate and results were expressed as mean ± SD. Statistical comparisons of mean values were evaluated by Student’s t-test using GraphPad InStat version 3.1 (GraphPad). Values of P < 0.05 were considered significant.
Results
Uptake study with P-gp substrates
To evaluate the effect of HEPES on drug uptake and transport, cellular accumulation of P-gp substrates e.g. cyclosporine-A, ritonavir, lopinavir, and amino acids e.g. glutamic acid, phenylalanine, was determined. We first examined the effect of various concentrations of HEPES on cyclosporine-A uptake employing both Caco-2 and MDCK-MDR1 cells. Uptake of cyclosporine-A was not statistically different between control and buffers containing 15 mM HEPES in Caco-2 cells but was significantly different in the presence of 25 mM HEPES (Fig. 1A). However, a significant difference in cyclosporine-A uptake between control and buffer containing 15 mM HEPES and 25 mM HEPES was evident in MDCK-MDR1 cells (Fig. 1B). A similar pattern was also observed in the accumulation of [3H]ritonavir and [3H]lopinavir into MDCK-MDR1 cells (Fig. 2). In general, addition of 15 or 25 mM HEPES in the uptake buffer resulted in significant reduction in the accumulation of all three tested P-gp substrates including cyclosporine-A, ritonavir, and lopinavir into MDCK-MDR1 cells. Overall relative uptake of these P-gp substrates was reduced to 85% – 92% and 75% – 80% as of the control with the addition of 15 mM and 25 mM HEPES, respectively.
Fig. 1.
Uptake of [3H]Cyclosporine-A in DPBS buffer containing 0 mM, 15 mM, and 25 mM HEPES into different cell lines. A: Caco-2 cells; B: MDCK-MDR1 cells. Results were expressed as mean ± SD, n = 4 – 6. * p < 0.05; ** p < 0.01 (from control, 0 mM HEPES).
Fig. 2.
Uptake of different P-gp substrates into MDCK-MDR1 cells in DPBS buffer containing 0 mM, 15 mM, and 25 mM HEPES. A: [3H]ritonavir; B: [3H]lopinavir. Results were expressed as mean ± SD, n = 4 – 8. * p < 0.05; ** p < 0.01 (from control, 0 mM HEPES).
Bidirectional transport study with P-gp substrate
Bidirectional transepithelial transport (A-B and B-A) of [3H]lopinavir was then examined across MDCK-MDR1 cells. Figure 3 depicts the cumulative amount of lopinavir transported with time. The transport rate of [3H]lopinavir from apical side to basal side (A-B) across MDCK-MDR1 cell monolayers was 0.10 pmol/min in control (no HEPES) and 0.076 pmol/min in buffer containing 25 mM HEPES over a 3-hour experiment. Therefore, it appears that addition of HEPES caused a significant decrease (24%) in absorptive direction (A-B). Conversely, the transport rate of [3H]lopinavir from basal to apical side (B-A) was 0.49 pmo/min for control (no HEPES) and 0.55 pmol/min in the presence of 25 mM HEPES, indicating an increased (11%) in the secretory direction (B-A). As summarized in Table 1, the permeability values of [3H]lopinavir in the A-B direction were 30.2 × 10−6 cm/sec for control and 22.8 × 10−6 cm/sec for the set with 25 mM HEPES. Such permeability values in the B-A direction were 148.5 × 10−6 cm/sec and 165.0 × 10−6 cm/sec for control and for the set with 25 mM HEPES, respectively. Permeability ratios (Papp(B-A)/Papp(A-B)) were 4.9 and 7.2 for control and for the set with 25 mM HEPES, respectively. Therefore permeability ratio was almost 50 percent higher with addition of 25 mM HEPES relative to control indicating an enhancement of P-gp mediated efflux which is an ATP-dependent process.
Fig. 3.
Cumulative amount of [3H]lopinavir transported with time (A-B and B-A) across MDCK-MDR1 cells in DPBS buffer containing 0 mM and 25 mM HEPES. Results were expressed as mean ± SD, n = 3 – 6.
Table 1.
Effect of HEPES on permeability of a P-gp substrate, lopinavir *
| P-gp substrate | Concentration of HEPES | PappA-B (× 10−6 cm/sec) | PappB-A (× 10−6 cm/sec) | PappB-A/PappA-B |
|---|---|---|---|---|
| 3[H] Lopinavir | ||||
| Control (0 mM) | 30.2 ± 2.56 | 148.5 ± 7.92 | 4.9 | |
| 25 mM | 22.8 ± 1.03 | 165.0 ± 11.09 | 7.2 |
Results were expressed as mean ± SD, n = 3–6.
Uptake of amino acids (glutamic acid and phenylalanine) in the presence of HEPES
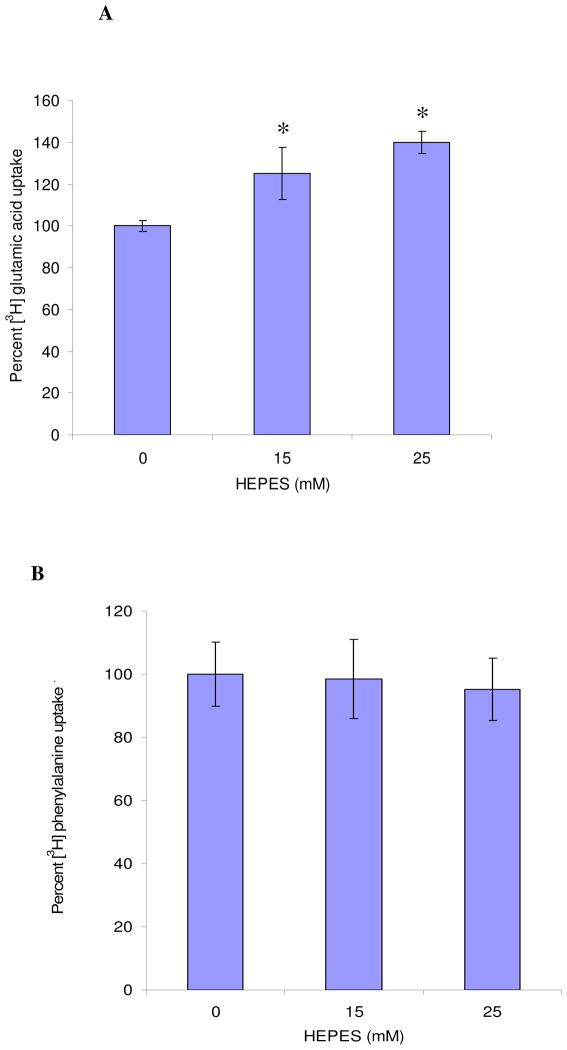
Effect of HEPES was further evaluated in relation to amino acid uptake considering both ATP-dependent and ATP-independent processes. Uptake of [3H]glutamic acid into MDCK-MDR1 cells was significantly elevated with the addition of HEPES. ATP-dependent uptake of [3H]glutamic acid was elevated by about 30% and 45% over the control in the presence of 15 mM and 25 mM of HEPES, respectively (Fig. 4A). In contrast, uptake of [3H]phenylalanine, an ATP-independent process showed no significant change in the presence of HEPES (Fig. 4B).
Fig. 4.
Uptake of different L- amino acids into MDCK-MDR1 cells in DPBS buffer containing 0 mM, 15 mM, and 25 mM HEPES. A: [3H]glutamic acid; B: [3H]phenylalanine. Results were expressed as mean ± SD, n = 4 – 6. * p < 0.05 (from control, 0 mM HEPES).
Effect of HEPES on passive diffusion
We also examined if HEPES exert any influence on membrane permeability by altering membrane properties. Uptake of [3H]diazepam by MDCK-MDR1 cells in the absence and presence of HEPES was performed to evaluate the effect of HEPES on passive diffusion. Accumulation amount of diazepam in buffers containing 0, 15, and 25 mM HEPES are 4.41 ± 0.35, 4.54 ± 0.19 and 4.26 ± 0.10 fmol/min/mg protein, respectively, indicating no significant difference.
Effect of verapamil
A substantial lowering in cyclosporine-A uptake in the presence of 25 mM HEPES may be the result of enhanced P-gp mediated efflux through ATP production. To prove our hypothesis, further experiments were conducted. A 5 μM verapamil was included to verify ATP involvement. The results (Fig. 5) demonstrate that [3H] cyclosporine-A uptake in the presence of 5 μM verapamil was much higher than its respective control without verapamil. It is interesting to note that addition of 5 μM verapamil in the buffer containing no HEPES caused complete inhibition of P-gp ATPase activity leading to enhanced uptake of cyclosporine-A compared to control. This 5 μM verapamil mediated inhibition of P-gp ATPase activity was incomplete when buffer contained 25 mM HEPES.
Fig. 5.
Uptake of [3H]cyclosporine-A into Caco-2 cells in DPBS buffer containing 0 mM, 25 mM HEPES and with addition of 5 μM of verapamil, respectively. Results were expressed as mean ± SD, n = 4 – 6. * p < 0.05 (from control, 0 mM HEPES).
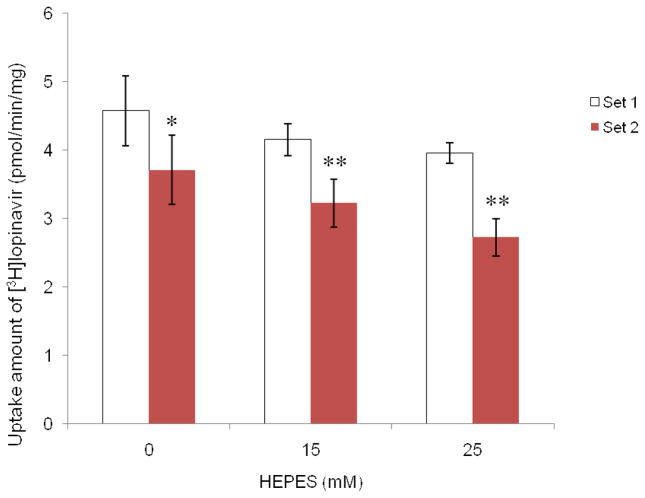
Uptake of lopinavir into MDCK-MDR1 cells grown under culture medium with or without HEPES
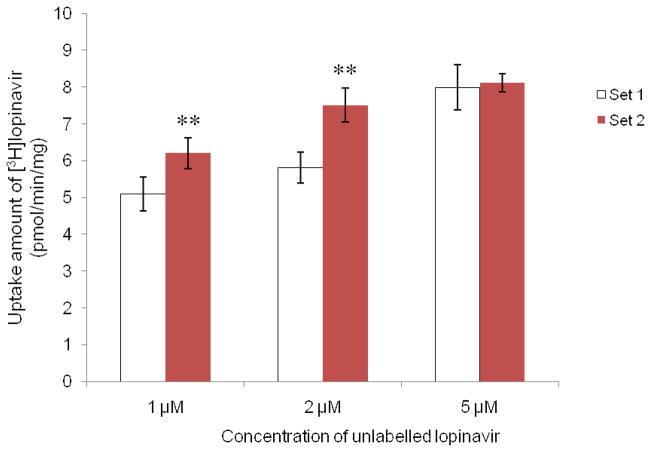
To provide further insights of the addition of HEPES in cellular growth medium on the uptake of P-gp substrate, cells were grown under different media with no HEPES (Set 1) or with 20 mM HEPES (Set 2), and then the uptake of [3H]lopinavir was measured. Figure 6 demonstrated the significant effect of HEPES in growth medium. Accumulation of [3H]lopinavir by Set 2 cells was significantly less than that by Set 1 cells at all concentrations of HEPES tested. The uptake of lopinavir decreased as HEPES concentration increased. The difference in lopinavir accumulation in Set 2 cells was more pronounced than that in Set 1. Figure 7 depicted the uptake of [3H]lopinavir in the presence of different concentrations of unlabelled lopinavir (inhibitor) by the two sets of cells. For each set of cells, cellular accumulation of [3H]lopnavir was elevated as the concentration of inhibitor increased. In the presence of lower concentrations (1 μM or 2 μM) of unlabelled lopinavir, higher accumulation of [3H]lopnavir was observed for Set 2 cells compared to Set 1 cells. In the presence of higher concentrations (5 μM) of unlabelled lopinavir, such elevation was not statistically different between these two sets of cells since P-gp was saturated.
Fig. 6.
Uptake of [3H]lopinavir in DPBS buffer containing 0 mM, 15 mM, and 25 mM HEPES into different sets of MDCK-MDR1 cells. Set 1: cells grown in culture medium without HEPES; Set 2: cells grown in culture medium with addition of 20 mM HEPES. Results were expressed as mean ± SD, n = 4 – 6. * p < 0.05; ** p < 0.01 (Set 2 compared with Set 1).
Fig. 7.
Uptake of [3H]lopinavir in the presence of different concentrations of unlabelled lopinavir (1 μM, 2 μM and 5 μM) into different sets of MDCK-MDR1 cells. Set 1: cells grown in culture medium without HEPES; Set 2: cells grown in culture medium with addition of 20 mM HEPES. Results were expressed as mean ± SD, n = 4 – 6. ** p < 0.01 (Set 2 compared with Set 1).
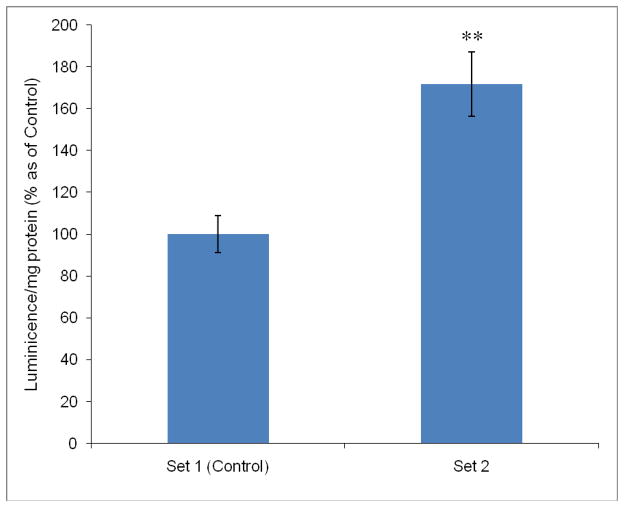
Effect of HEPES on ATP production
To further expedite the effect of HEPES on ATP production, MDCK-MDR1 cells were exposed under different culture media containing no HEPES (Set 1) or 20 mM of HEPES (Set 2). The production of ATP was quantitatively detected using ATP Determination Kit. The results depicted in Fig. 8 demonstrated that ATP production was 70% elevated with addition of 20 mM of HEPES in Set 2 cells compared to Set 1.
Fig. 8.
Effect of HEPES on ATP production in MDCK-MDR1 cells. Set 1: cells grown in culture medium without HEPES; Set 2: cells grown in culture medium with addition of 20 mM HEPES. Results were expressed as mean ± SD, n = 4 – 6. ** p < 0.01 (Set 2 compared with Set 1, Set 1 as control).
Discussion
Inconsistent or even contradictory results in the literature on uptake and transport of P-gp substrates in the same cell types have led to our investigation on the influence of buffer compositions on drug permeation. For example, different values of permeability ratio (PappB-A/PappA-B) of Digoxin, such as 1414 and 43.419, were reported by different research groups, even though the same cell line was used. It is difficult to compare the different results caused by various concentrations of HEPES used in the growth medium and transport buffer, because investigators have used different P-gp substrates and cell lines. Culture conditions of these cell lines also varied. It is our hypothesis that HEPES may alter P-gp mediated drug transport activity. Our results suggest that HEPES can modulate ATP-dependent transport activity. Interference of buffer components on drug uptake and transport studies can cause significant variation in experimental results.
Bicarbonate or CO2 is an important component in the buffer medium and is required for a number of biochemical reactions. A bicarbonate – CO2 buffer provides excellent pH balance as long as cultures remain in the incubator. But as soon as the culture is removed from incubator, the bicarbonate containing media turns alkaline very rapidly due to loss of CO2. HEPES at concentration around 10 mM to 25 mM is one of the most commonly added ingredients in biological buffer systems to maintain pH stability.
In order to systematically investigate the biological effect of HEPES, the interference of the buffer component on uptake and transport can be classified into three categories: interference by altering influx, efflux, or both. Effect of buffer components on drug influx has been already reported in the literature. In this report, we describe for the first time the effect of HEPES on the ATP-dependent carrier mediated drug uptake and transport. Results presented in this article indicate that HEPES can alter uptake and transport of P-gp substrates in both Caco-2 and MDCK-MDR1 cells. Uptake of cyclosporine-A was not significantly altered in buffers containing 10 mM HEPES compared to the control. However, significant differences in the uptake of cyclosporine-A, ritonavir and lopinavir between control and buffers containing 15 and 25 mM HEPES were evident in MDCK-MDR1 cells. Uptake experiments with P-gp substrates clearly demonstrate that addition of HEPES in the uptake buffer solution results in reduced influx of P-gp substrates (cyclosporine-A, ritonavir and lopinavir) in a similar fashion (Figs. 1–2). These results on uptake of P-gp substrates were consistent in MDCK-MDR1 cells. MDR1 gene transfected MDCK cell line expresses high levels of P-gp relative to Caco-2 cells in which expression of P-gp depends on culture conditions.14, 34 Uptake of P-gp substrates diminished as HEPES concentration in buffer ascended. P-gp is an ATP-dependent efflux transporter with a broad range of substrate specificity.35–36 Higher HEPES concentrations in buffer solution caused higher efflux resulting in lower uptake. Such reduced uptake of various P-gp substrates in MDCK-MDR1 cells may suggest an enhanced activity of efflux pump resulting in diminished drug transport. The elevation in permeability ratio from 4.9 to 7.2 in the presence of 25 mM HEPES may indicate stimulation of efflux system (Fig. 3 and Table 1). Since uptake experiments were carried out only for 30 min, this time duration may not be sufficient to cause higher intracellular P-gp accumulation. Moreover, the activation of efflux pump requires ATP, so it is not unreasonable to hypothesize that HEPES may stimulate ATP production/ATP hydrolysis. To test our hypothesis of ATP involvement in this process, carrier mediated uptake experiments of glutamic acid and phenylalanine were performed. Uptake of glutamic acid is energy dependent,37–39 whereas phenylalanine uptake is energy independent.40 Uptake of glutamic acid was facilitated with the addition of HEPES in the uptake medium (Fig. 4A), whereas there was no change in phenylalanine uptake – an energy-independent process under similar conditions (Fig. 4B). Higher ATP level enhanced the energy-dependent influx function of amino acid transporter for glutamic acid. Uptake of [3H]phenylalanine in both apical and basolateral membranes was inhibited by unlabeled phenylalanine indicating the presence of neutral amino acid transporter in MDCK cells.41 Gomes and Soares-da-Silva have reported the expression of Na+-independent LAT2 (large neutral amino acid transporter) in transfected renal cell line.42 Transport of phenylalanine by LAT2 is proton coupled and energy independent40, which explains why no change in phenylalanine uptake occurred in the presence of HEPES in MDCK-MDR1 cells. HEPES may be involved, in someway, with ATP dependent process and thus cause enhanced uptake of glutamic acid and reduced uptakes of cyclosporine-A, ritonavir and lopinavir.
This hypothesis was further supported by verapamil data (Fig. 5). Addition of verapamil in the buffer containing no HEPES caused enhanced uptake of cyclosporine-A due to inhibition of P-gp but returned to control value once 25 mM HEPES was incorporated in the buffer. The primary structure of P-gp displays two nucleotide binding sites in the cytoplasmic domain. The mechanism of P-gp function is incompletely understood. ATPase activity leading to ATP hydrolysis is the main driving force of P-gp activity. When a xenobiotics agent binds P-gp, it stimulates ATPase activity and verapamil inhibits such modulator-stimulated P-gp ATPase activity. Such inhibitory activity of verapamil is also concentration dependent.43 As we mentioned that addition of HEPES in the medium leads to higher ATP production. Addition of 5 μM verapamil in the buffer containing no HEPES caused complete inhibition of P-gp ATPase activity leading to enhanced uptake of cyclosporine-A compared to control. This inhibition of P-gp ATPase activity was insufficient with 5 μM verapamil when 25 mM HEPES incorporated in the buffer. As a result, cyclosporine-A uptake, though higher than that in buffer with 25 mM HEPES alone was less compared to the respective control with 0 mM HEPES with 5 μM verapamil. The effect of HEPES in the growth medium on the uptake of [3H]lopinavir in the absence and presence of different concentrations of unlabelled lopinavir provided further evidence that HEPES stimulated the function of P-gp (Fig. 6 and Fig. 7).
The mechanism of HEPES involved in this energy dependent process is further elucidated by the quantitative determination of ATP in the cell growth phase. The luminescence produced is proportional to the amount of ATP present in the sample. Luminescence production by luciferase varies depending on buffer composition.44 Phosphate buffer produced 70% inhibition on light output. Light production was highest when reaction mixture contained Tricine buffer followed by Tris and Glycylglycine. HEPES produced moderate effect on this light output by luciferase. Several mechanisms of ATP-dependent luminescence production by luciferase have been postulated including structural conformation of luciferase binding domains45 and two nucleotide triphosphate binding sites46. Kinetics of luciferase47 has also been reported. HEPES along with magnesium and calcium present in the buffer system may influence a cascade of cellular kinetic events associated with ATP dependent processes. ATP hydrolysis by ATPase is required for P-gp mediated efflux. Enhancement of ATP production by HEPES may result in higher transport of P-gp substrates. The results of our ATP assay, based on the luminescent property of firefly luciferase, indicated that ATP production was significantly elevated with the addition of HEPES (Fig. 8).
Recently, inhibitory effect of HEPES on the taurine uptake in cultured rat and chicken neural cells has been implicated due to the structural similarity between taurine and HEPES. Effect of HEPES on the uptake of β–alanine and leucine by chicken and rat neuronal cells was also reported. Slow inhibition was involved in the uptake of both taurine and β–alanine possibly due to modulation of membrane fluidity by HEPES.2–3 In our present study, no change in the passive diffusion of diazepam was observed. Therefore, enhancement of membrane fluidity by HEPES is not a plausible mechanism for reduced uptake of cyclosporine-A, ritonavir and lopinavir across MDCK-MDR1 monolayers.
Conclusion
Addition of HEPES in the growth medium, uptake and transport buffer can affect ATP dependent cellular processes. Results presented in this report suggest that HEPES can alter ATP dependent influx and efflux processes. Addition of HEPES enhanced the production of ATP. These results may also explain the inconsistencies in data available in literature which may have been caused by differences in the concentration of HEPES used in uptake/transport buffer or media.
Acknowledgments
This study was supported by NIH grants R01 GM 64320 and R01 A1 071199.
References
- 1.Williamson JD, Cox P. Use of a new buffer in the culture of animal cells. J Gen Virol. 1968;2:309–312. doi: 10.1099/0022-1317-2-2-309. [DOI] [PubMed] [Google Scholar]
- 2.Lleu PL, Rebel G. Effect of HEPES on the taurine uptake by cultured glial cells. J Neurosci Res. 1989;23:78–86. doi: 10.1002/jnr.490230111. [DOI] [PubMed] [Google Scholar]
- 3.Rebel G, Lleu PL, Petegnief V, Frauli-Meischner M, Guerin P, Lelong IH. Effect of HEPES on the uptake of taurine by cultured nervous cells. Adv Exp Med Bio. 1992;315:277–285. doi: 10.1007/978-1-4615-3436-5_33. [DOI] [PubMed] [Google Scholar]
- 4.Zigger JS, Jr, Lepe-Zuniga JL, Vistica B, Gery I. Analysis of the cytotoxic effects of light-exposed HEPES–containing culture medium. In vitro Cell Dev Biol. 1985;21:282–287. doi: 10.1007/BF02620943. [DOI] [PubMed] [Google Scholar]
- 5.Yamamoto D, Suzuki N. Blockage of chloride channels by HEPES buffer. Proc Roy Soc London, Ser B. 1987;230:93–100. doi: 10.1098/rspb.1987.0011. [DOI] [PubMed] [Google Scholar]
- 6.Lund P, Wiggins D. Inhibition of carbamoyl-phosphate synthase (ammonia) by Tris and HEPES. Effect on Ka for N-acetylglutamate. Biochem J. 1987;243:273–276. doi: 10.1042/bj2430273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Juranka PF, Zastawny RL, Ling V. P-glycoprotein: multidrug-resistance and a superfamily of membrane-associated transport proteins. FASEB J. 1989;3:2583–2592. doi: 10.1096/fasebj.3.14.2574119. [DOI] [PubMed] [Google Scholar]
- 8.Fromm MF. The influence of MDR1 polymorphisms on P-glycoprotein expression and function in humans. Adv Drug Del Rev. 2002;54:1295–1310. doi: 10.1016/s0169-409x(02)00064-9. [DOI] [PubMed] [Google Scholar]
- 9.Hochman JH, Yamazaki M, Ohe T, Lin JH. Evaluation of drug interactions with P-glycoprotein in drug discovery: in vitro assessment of the potential for drug-drug interactions with P-glycoprotein. Curr Drug Metab. 2002;3:257–273. doi: 10.2174/1389200023337559. [DOI] [PubMed] [Google Scholar]
- 10.Juliano RL, Ling V. A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochim Biophys Acta. 1976;455:152–162. doi: 10.1016/0005-2736(76)90160-7. [DOI] [PubMed] [Google Scholar]
- 11.Aungst BJ. P-glycoprotein, secretory transport, and other barriers to the oral delivery of anti-HIV drugs. Adv Drug Del Rev. 1999;39:105–116. doi: 10.1016/s0169-409x(99)00022-8. [DOI] [PubMed] [Google Scholar]
- 12.Choo EF, Leake B, Wandel C, Imamura H, Wood AJ, Wilkinson GR, Kim RB. Pharmacological inhibition of P-glycoprotein transport enhances the distribution of HIV-1 protease inhibitors into brain and testes. Drug Metab Disp. 2000;28:655–660. [PubMed] [Google Scholar]
- 13.Bailey CA, Bryla P, Malick AW. The use of the intestinal epithelial cell culture model, Caco-2, in pharmaceutical development. Adv Drug Del Rev. 1996;22:85–103. [Google Scholar]
- 14.Tang F, Horie K, Borchardt RT. Are MDCK cells transfected with the human MDR1 gene a good model of the human intestinal mucosa? Pharm Res. 2002a;19:765–772. doi: 10.1023/a:1016140429238. [DOI] [PubMed] [Google Scholar]
- 15.Kagedahl M, Swaan PW, Redemann CT, Tang M, Craik CS, Szoka FC, Jr, Oie S. Use of the intestinal bile acid transporter for the uptake of cholic acid conjugates with HIV-1 protease inhibitory activity. Pharm Res. 1997;14:176–180. doi: 10.1023/a:1012044526054. [DOI] [PubMed] [Google Scholar]
- 16.Glynn SL, Yazdanian M. In vitro blood-brain barrier permeability of nevirapine compared to other HIV antiretroviral agents. J Pharm Sci. 1998;87:306–310. doi: 10.1021/js970291i. [DOI] [PubMed] [Google Scholar]
- 17.Lee K, Ng C, Brouwer KL, Thakker DR. Secretory transport of ranitidine and famotidine across Caco-2 cell monolayers. J Pharmacol Exp Therap. 2002;303:574–580. doi: 10.1124/jpet.102.038521. [DOI] [PubMed] [Google Scholar]
- 18.Troutman MD, Thakker DR. Rhodamine 123 requires Carrier-mediated influx for its activity as a P-glycoprotein substrate in Caco-2 cells. Pharm Res. 2003;20:1192–1199. doi: 10.1023/a:1025096930604. [DOI] [PubMed] [Google Scholar]
- 19.Zhang S, Morris ME. Effect of the flavonoids A and silymarin on the P-glycoprotein-mediated transport of digoxin and vinblastine in human intestinal Caco-2 cells. Pharm Res. 2003;20:1184–1191. doi: 10.1023/a:1025044913766. [DOI] [PubMed] [Google Scholar]
- 20.Eagling VA, Profit L, Back DJ. Inhibition of the CYP3A4-mediated metabolism and P-glycoprotein-mediated transport of the HIV-1 protease inhibitor saquinavir by grapefruit juice components. Br J Clin Pharmacol. 1999;48:543–552. doi: 10.1046/j.1365-2125.1999.00052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Profit L, Eagling VA, Back DJ. Modulation of P-glycoprotein function in human lymphocytes and Caco-2 cell monolayers by HIV-1 protease inhibitors. AIDS. 1999;13:1623–1627. doi: 10.1097/00002030-199909100-00004. [DOI] [PubMed] [Google Scholar]
- 22.Pade V, Stavchansky S. Estimation of the relative contribution of the transcellular and paracellular pathway to the transport of passively absorbed drugs in the Caco-2 cell culture model. Pharm Res. 1997;14:1210–1215. doi: 10.1023/a:1012111008617. [DOI] [PubMed] [Google Scholar]
- 23.Schipper NG, Osterberg T, Wrange U, Westberg C, Sokolowski A, Rai R, Young W, Sjostrom B. In vitro intestinal permeability of factor Xa inhibitors: influence of chemical structure on passive transport and susceptibility to efflux. Pharm Res. 2001;18:1735–1741. doi: 10.1023/a:1013378731183. [DOI] [PubMed] [Google Scholar]
- 24.Ocheltree SM, Keep RF, Shen H, Yang D, Hughes BA, Smith DE. Preliminary investigation of proton-coupled oligopeptide transporters in neural retina pigment epithelium (RPE): lack of functional activity in RPE plasma membranes. Pharm Res. 2003;20:1364–1372. doi: 10.1023/a:1025741723724. [DOI] [PubMed] [Google Scholar]
- 25.Cummins CL, Mangravite LM, Benet LZ. Characterizing the expression of CYP3A4 and efflux transporters (P-gp, MRP1, and MRP2) in CYP3A4-transfected Caco-2 cells after induction with sodium butyrate and the phorbol ester 12-o-tetradecanoylphorbol-13-acetate. Pharm Res. 2001;18:1102–1109. doi: 10.1023/a:1010914624111. [DOI] [PubMed] [Google Scholar]
- 26.Hosoya KI, Kim KJ, Lee VH. Age-dependent expression of P-glycoprotein gp170 in Caco-2 cell monolayers. Pharm Res. 1996;13:885–890. doi: 10.1023/a:1016005212640. [DOI] [PubMed] [Google Scholar]
- 27.Maeng H, Yoo H, Kim I, Song I, Chung S, Shim C. P-glycoprotein- mediated transport of berberine across Caco-2 cell monolayers. J Pharm Sci. 2002;91:2614–2621. doi: 10.1002/jps.10268. [DOI] [PubMed] [Google Scholar]
- 28.Hu M, Chen J, Zhu Y, Dantzig AH, Stratford RE, Jr, Kuhfeld MT. Mechanism and kinetics of transcellular transport of a new beta–lactam antibiotic loracarbef across an intestinal epithelial membrane model system (Caco-2) Pharm Res. 1994;11:1405–1413. doi: 10.1023/a:1018935704693. [DOI] [PubMed] [Google Scholar]
- 29.Walle UK, Walle T. Taxol transport by human intestinal epithelial Caco-2 cells. Drug Metab Disp. 1998;26:343–346. [PubMed] [Google Scholar]
- 30.Wu X, Whitfield LR, Stewart BH. Atorvastatin transport in the Caco-2 cell model: contributions of P-glycoprotein and the proton-monocarboxylic acid co-transporter. Pharm Res. 2000;17:209–215. doi: 10.1023/a:1007525616017. [DOI] [PubMed] [Google Scholar]
- 31.Imamura Y, Shimizu K, Yamashita M, Yamaoka K, Takakura Y, Hashida M. Transport characteristics of ebastine and its metabolites across human intestinal epithelial Caco-2 cell monolayers. Bio Pharmacol Bull. 2001;24:930–934. doi: 10.1248/bpb.24.930. [DOI] [PubMed] [Google Scholar]
- 32.Tang F, Horie K, Borchardt RT. Are MDCK cells transfected with the human MRP2 gene a good model of the human intestinal mucosa? Pharm Res. 2002b;19:773–779. doi: 10.1023/a:1016192413308. [DOI] [PubMed] [Google Scholar]
- 33.Sikri V, Pal D, Jain R, Kalyani D, Mitra AK. Cotransport of macrolide and fluoroquinolones, a beneficial interaction reversing P-glycoprotein efflux. Amer J Therap. 2004;11:433–442. doi: 10.1097/01.mjt.0000132643.69143.64. [DOI] [PubMed] [Google Scholar]
- 34.Anderle P, Niederer E, Rubas W, Hilgendorf C, Spahn-Langguth H, Wunderli-Allenspach H, Merkle HP, Langguth P. P-Glycoprotein (P-gp) mediated efflux in Caco-2 cell monolayers: the influence of culturing conditions and drug exposure on P-gp expression levels. J Pharm Sci. 1998;87:757–762. doi: 10.1021/js970372e. [DOI] [PubMed] [Google Scholar]
- 35.Sauna ZE, Ambudkar SV. Evidence for a requirement for ATP hydrolysis at two distinct steps during a single turnover of the catalytic cycle of human P-glycoprotein. Proc Natl Acad Sci USA. 2000;97:2515–2520. doi: 10.1073/pnas.97.6.2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shimabuku AM, Nishimoto T, Ueda K, Komano T. P-glycoprotein. ATP hydrolysis by the N-terminal nucleotide-binding domain. J Bio Chem. 1992;267:4308–4311. [PubMed] [Google Scholar]
- 37.Atlante A, Passarella S, Pierro P, Di Martino C, Quagliariello E. The mechanism of proline/glutamate antiport in rat kidney mitochondria. Energy dependence and glutamate-carrier involvement. Eur J Biochem. 1996;241:171–177. doi: 10.1111/j.1432-1033.1996.0171t.x. [DOI] [PubMed] [Google Scholar]
- 38.Maycox PR, Deckwerth T, Hell JW, Jahn R. Glutamate uptake by brain synaptic vesicles. Energy dependence of transport and functional reconstitution in proteoliposomes. J Bio Chem. 1988;263:15423–15428. [PubMed] [Google Scholar]
- 39.Karppinen A, Lahdesmaki P. Uptake of glutamate into synaptic vesicles: dependence on vesicle treatment, ions, temperature and energy supply. Cell Mol Bio Incl Cyto Enzymol. 1979;25:195–202. [PubMed] [Google Scholar]
- 40.Gandhi MD, Pal D, Mitra AK. Identification and functional characterization of a Na(+)-independent large neutral amino acid transporter (LAT2) on ARPE-19 cells. Int J Pharm. 2004;275:189–200. doi: 10.1016/j.ijpharm.2004.01.035. [DOI] [PubMed] [Google Scholar]
- 41.Putnam WS, Ramanathan S, Pan L, Takahashi LH, Benet LZ. Functional characterization of monocarboxylic acid, large neutral amino acid, bile acid and peptide transporters, and P-glycoprotein in MDCK and Caco-2 cells. J Pharm Sci. 2002;91:2622–2635. doi: 10.1002/jps.10264. [DOI] [PubMed] [Google Scholar]
- 42.Gomes P, Soares-da-Silva P. Na(+)/H(+) exchanger activity and dopamine D(1)-like receptor function in two opossum kidney cell clonal sublines. Cell Physiol Biochem. 2002;12:259–268. doi: 10.1159/000067896. [DOI] [PubMed] [Google Scholar]
- 43.Orlowski S, Mir LM, Belehradek J, Jr, Garrigos M. Effects of steroids and verapamil on P-glycoprotein ATPase activity: progesterone, desoxycorticosterone, corticosterone and verapamil are mutually non-exclusive modulators. Biochem J. 1996;317:515–522. doi: 10.1042/bj3170515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Webster JJ, Chang JC, Manley ER, Spivey HO, Leach FR. Buffer effects on ATP analysis by firefly luciferase. Anal Biochem. 1980;106:7–11. doi: 10.1016/0003-2697(80)90111-6. [DOI] [PubMed] [Google Scholar]
- 45.Denburg JL, McElroy WD. Anion inhibition of firefly luciferase. Arch Biochem Biophys. 1970;141:668–675. doi: 10.1016/0003-9861(70)90187-6. [DOI] [PubMed] [Google Scholar]
- 46.Steghens JP, Min KL, Bernengo JC. Firefly luciferase has two nucleotide binding sites: effect of nucleoside monophosphate and CoA on the light-emission spectra. Biochem J. 1998;336:109–113. doi: 10.1042/bj3360109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lembert N, Idahl LA. Regulatory effects of ATP and luciferin on firefly luciferase activity. Biochem J. 1995;305:929–933. doi: 10.1042/bj3050929. [DOI] [PMC free article] [PubMed] [Google Scholar]