Abstract
The need for non-invasive imaging of peripheral nerves that can reliably assess extent of nerve fiber degeneration and regeneration is increasingly realized. Availability of such a technology has several immediate clinical and preclinical applications. Diffusion tensor imaging (DTI) is an emerging magnetic resonance based technology that is particularly suited for imaging nerve fiber tracts. This review highlights immediate clinical and preclinical uses of non-invasive imaging of peripheral nerve regeneration and DTI as a potential technology that can fulfill these clinical and research needs.
Keywords: Diffusion, nerve, MRI, DTI, axon regeneration, axon degeneration, outcome measures, clinical trials, trauma, nerve injury
Introduction
The morbidity and health costs associated with neuropathic conditions are enormous because diseases of the peripheral nerves are among the commonest neurological disorders. It is estimated that 2.5% of the general population and 8% of those above age 55 have some form of neuropathic disorder (England and Asbury, 2004;Martyn and Hughes, 1997). The advances in non-invasive imaging technology have significantly improved diagnosis and management of various neurological diseases with most visible impact in the areas of stroke and multiple sclerosis. Magnetic resonance imaging (MRI) of the peripheral nerve (MRI neurography) is already in clinical use and it is most helpful in defining the anatomy of the nerves and in establishing continuity or discontinuity of the injured nerves patients with traumatic nerve injuries (Filler et al., 2004). In the context of available and evolving imaging technologies an emerging question is: whether there is a role for non-invasive imaging of nerve regeneration in clinical and preclinical settings, and if so, what are those needs and which imaging technologies can potentially fulfill these needs? In the clinical arena there is an immediate need for reliable measures to assess peripheral nerve regeneration in patients with traumatic nerve injuries. Another immediate and major clinical and preclinical need for non-invasive measurement of nerve regeneration is in the area of therapeutics aiming to enhance nerve regeneration/repair. For these applications, the technology with most promise and immediate availability, for clinical and preclinical use, is diffusion tensor imaging (DTI), an MR imaging technique, based on the movements of water molecules within biological tissues, particularly suitable for imaging nerve fiber tracts. This review outlines the clinical and preclinical needs to assess peripheral nerve regeneration and discusses DTI as a potential technology that can fulfill these needs.
Immediate clinical and preclinical needs
A) Traumatic nerve injuries
Civilian and combat trauma to limbs often results in serious injuries to the peripheral nerves that cause significant morbidity. Advances in trauma management including availability of dedicated centers have significantly reduced mortality, however, patients with severe and multiple injuries are left with significant disability and morbidity due to peripheral nerve injuries. It is estimated that up to 5% of all admissions to level I trauma centers have a peripheral nerve injury (Noble et al., 1998). In general, injuries to the upper extremity are more common than those to the lower extremity, accounting for two thirds of all peripheral nerve injuries (Noble et al., 1998). A significant proportion of nerve injuries leave the nerves in continuity and reliable predictors for recovery/regeneration in this group are not available (Midha and Kline, 1998). Conservative approach can save the patient a needless surgery but it requires following the patient for many months to 1–2 years for regeneration/recovery to occur. The major caveat of this approach is that “a window of opportunity” to repair the nerve can be missed or lost. Currently, noninvasive techniques that allow early assessment of regeneration in nerve lesions in continuity are not available in clinical practice (Stanisz et al., 2001).
Proximo-distal regeneration of severed axons is the most important mechanism of recovery for most severe nerve injuries. The crucial issues in the medical/surgical management of nerve injuries include: a) whether or not segments of nerve adjacent to injury are in physical continuity; b) extent of Wallerian-like degeneration in the nerve segment distal to the injury site; c) initiation of regeneration in injured axons proximal and across the injury site; and d) regeneration of injured axons in distal segment. Nerve discontinuity is often established during surgical exploration of patients with traumatic nerve injuries or MR neurography can identify this, thus confirming the need for surgical repair. However, majority of injuries do not disrupt the continuity of injured nerve (Sunderland Grade I–IV) (Noble et al., 1998). In Sunderland grade II–IV injuries, a fundamental dilemma is, whether or not the nerve needs repair and, if so, what is the appropriate time of repair and/or graft procedures? A substantial body of opinion indicates that after periods of 12–18 months (after nerve injury/transection) recovery of function following nerve repair is likely to be poor. The complete basis of this poor recovery after late repair is not well understood but most experts in the field would agree (also supported by experimental data) that decrease in neuronal capacity to regenerate, denervation-associated changes in the endoneurium (particularly Schwann cells) in the distal denervated segment of the injured nerve, and atrophic changes in denervated targets especially muscles all contribute to this poor recovery (Fu and Gordon, 1995a;Fu and Gordon, 1995b;Hoke, 2006). Protocols/algorithms about whether or not to operate at specific intervals after nerve injury are not widely/strictly followed largely due to lack of reliable noninvasive measures to assess ongoing regeneration of injured nerve fibers into the distal segment of the injured nerves (pathway).
In clinical practice, nerve conductions and EMG studies are most commonly used for the assessment of nerve injuries. This clinical tool is useful for confirming bedside localization of the site of nerve injuries and very effective in distinguishing between neurapraxia (Sunderland Grade I) and axonotmesis (Sunderland Grade II). However, electrodiagnostic testing is limited by its complete dependence on target innervation and virtual inability to provide information about regeneration in the pathway distal to the site of injury particularly when evoked sensory and motor responses are not elicited or in complete nerve injuries. Furthermore, electrodiagnostic testing is highly dependent on operative and interpretative skills of the electromyographer with wide variations between different centers. Sometime “Tinel’s” sign is used as a surrogate marker to determine the leading edge of the regenerating nerves, but this subjective test is very inaccurate bedside tool. Nerve biopsies although very sensitive and specific for detection of nerve repair, are not feasible particularly for injuries in continuity because this procedure permanently sacrifices a set of nerve fibers and this technique cannot be used serially.
B) Experimental therapeutics to enhance nerve regeneration/repair
The principal goal for researchers in this area is to devise strategies to enhance short and long distance nerve regeneration in various peripheral nerve disorders. Based on the current understanding of peripheral neurobiology it is increasingly realized that different interventions targeting specific elements of nerve repair such as growth state of neurons, axonal elongation in the nerve, and axonal reconnectivity with targets might be necessary to enhance successful regeneration. A fundamental issue for the evaluation of stage specific interventions to enhance nerve regeneration is the ability to follow growth state of the neuron and associated axonal elongation/regeneration in the nerve (pathway) prior to its reconnectivity with targets. Lack of non-invasive measures that can assess the growth state of neuron by monitoring regeneration in the nerve/pathway is a major hindrance in achieving this primary goal. Lack of reliable outcome measures that can monitor axon regeneration in the nerve/pathway has been considered as a contributor to the failure of several previous clinical trials (including those with neurotrophic factors) aimed at enhancing nerve regeneration in neuropathic conditions (Boulton, 2007). Availability of a technique that is based on the integrity of nerve fibers in nerve trunks and potential for detection and quantification of the extent of axon degeneration along the nerve tracts can facilitate patient recruitment and randomization process in the trials by inclusion of patients with spatially similar degeneration along the nerves in different treatment arms. Besides monitoring the efficacy of novel drugs in polyneuropathies non-invasive imaging of nerve regeneration can be used for examining the regeneration through existing or newer nerve conduits used for nerve repairs, which are anticipated to incorporate nanotechnology, gene/protein delivery, and stem cells and will go through studies for clinical use (Chew et al., 2007;Schlosshauer et al., 2006;Schlosshauer et al., 2003).
Use of animal models of nerve regeneration is an essential/indispensable step prior to advancing to clinical studies. Initial strategies for enhancing nerve regeneration will almost certainly be developed and tested in rodent models of nerve regeneration. Mouse models are particularly attractive because of the availability of transgenic strains, which allow investigation of the roles of specific genes and molecules in nerve regeneration. Moreover, technologies developed in mouse models are usually easily transferable/adaptable for application to larger animals and humans. Although morphological studies can provide definitive and quantitative measurement of regeneration in experimental animals, however, these studies are very labor intensive, cannot be used serially, and unsuitable for high throughput screening studies in animals.
Potential technologies
Development of validated noninvasive outcome measures that can quantitate axonal regeneration in the nerve/pathway and indirectly assess the growth state of the neuron and applied serially to patients and animals would address some of the clinical and research needs outlined above. Diffusion tensor imaging (DTI), molecular/ligand based imaging, and molecular labeling of the substrate and fluorescent imaging are the technologies that have the potential to measure axon regeneration in the nerve/pathway in the living subjects. The molecular/ligand based imaging is yet not at a translational stage largely because surface markers associated with growth state of neurons or axons and their growth cones are not well established. The molecular labeling of the substrate and fluorescent imaging is only applicable to transgenic mice/animals labeled with specific fluorescent proteins and not applicable for clinical use (Witzel et al., 2005). Other MR based technologies for evaluation of peripheral neuropathies include MR neurography (Filler et al., 2004) that provides anatomical and subjective and qualitative data that is not suitable to quantitatively monitor nerve fiber degeneration and regeneration. MRI techniques based on contrast agents such as iron particles or gadofluorine M (Stoll et al., 2006) are invasive and not based on nerve fiber integrity and/or content and their sensitivity and specificity in determining extent of axon degeneration and regeneration remains to be defined. This review is restricted to DTI as a potential technology to image nerve regeneration and other techniques are not subject of this review. Newer non-invasive technologies that are based on target connectivity and capability to assess distal nerve degeneration and regeneration have been recently reviewed elsewhere (Herrmann, 2008) and are not considered here. Attributes of imaging technologies that would make them ideally suitable for the clinical and preclinical needs discussed above are listed in Table 1.
Table 1.
Attributes of ideal imaging technology to detect degeneration and regeneration in peripheral nerves
| Preferred features |
| Non-invasive |
| No side effects |
| Serially applicable |
| Short evaluation/scan times |
| Sensitivity |
| Ability to qualitatively detect changes |
| Ability to quantitatively detect changes (low false negative rate) |
| Size threshold of nerves (<2–3 mm) |
| Wide dynamic range of parameter(s) affected |
| Specificity |
| Nerve fiber numbers/density |
| Nerve fiber size |
| Differentiate changes in nerve fiber numbers and size |
| Detect tips of injured nerve fibers |
| Distinguish between axon regeneration and collateral sprouting |
Diffusion tensor imaging (DTI)
Magnetic resonance imaging (MRI) is a rapidly evolving modality that allows two-dimensional (2D) or three-dimensional (3D) imaging of different tissues and organs in humans and small animals. Diffusion tensor imaging (DTI), an MR imaging technique, is based on the movements of water molecules within biological tissues (Basser et al., 1994). Diffusion-weighted imaging is based on detection of thermally driven random motion (diffusion) of water molecules in the direction of the field gradient (Stieltjes et al., 2001). Tissues have distinct structural properties that hinder diffusion in some directions and facilitate it in other directions and this feature is called anisotropy. Neural tissues are highly enriched in nerve fibers and tracts and water molecules tend to move along the nerve fibers and tracts. This motion of water molecules in a preferential orientation is called ‘anisotropic diffusion’ and DTI can create a new image contrast that shows locations of tissues with high anisotropy, namely nerve tracts. To determine all parameters of the diffusion tensor, at least six independent measurements (usually a much higher number is used) with diffusion gradients applied sequentially along six noncollinear directions are used (Bammer, 2003;Basser and Pierpaoli, 1996;Jellison et al., 2004). It has been shown that maximum diffusivity coincides with the fiber tract orientation (Basser et al., 1996;Jellison et al., 2004). Thus, DTI allows clear identification of nerve tracts and their anatomy (sizes and shapes). Typically three eigenvalues referred as λ1, λ2, and λ3 are used to derive quantitative DTI parameters. The primary eigenvalue λ1 (or λ‖) refers to the extent of water diffusion parallel to the direction of nerve fibers, whereas the average of secondary (λ2) and tertiary (λ3) eigenvalues measure the extent of water diffusion perpendicular to the direction of nerve fibers (λ⊥) and these eigenvalues are also used to derive fractional anisotropy (FA) values (reviewed in (Mori and Zhang, 2006)). Changes in diffusivities, particularly in parallel direction, and FA are considered a sensitive measure of nerve fiber integrity in different studies. Development and availability of special software allow reconstruction, tracing, and visualization of the specific neural tracts called tractography, which is based on DTI data particularly FA. Tractography has been extensively applied to reconstruction and tracing of white matter tracts in CNS, for example, corticospinal tracts in the brain and spinal cord (Conturo et al., 1999;Fujiyoshi et al., 2007;Mori and van Zijl, 2002;Mori et al., 2006;Tuch et al., 2001;Zhang et al., 2009). Overall, DTI is based on the physiological movements of water molecules and microstructures of the nerve tracts and it is quite likely that DTI parameters are not altered by normal or abnormal electrophysiological activity along intact and/or injured nerve fibers thus inability to detect physiological function of the nerve fibers; however, this issue has not been addressed experimentally.
DTI shows great potential as a non-invasive technology to detect axonal injury in CNS has been shown by a large number of studies. Both clinical and experimental studies indicate the utility of FA and DTI technology in detecting Wallerian-like/axonal degeneration in white matter tracts in the CNS to monitor degeneration/atrophy of these tracts (Agosta et al., 2007;Golay et al., 2002;Guleria et al., 2008;Gupta et al., 2006;Ito et al., 2001;Mori et al., 1999;Pierpaoli et al., 2001;Reich et al., 2005;Thomalla et al., 2004;Trip et al., 2006;Valsasina et al., 2007;Valsasina et al., 2005;Werring et al., 2000;Zhang et al., 2009). We were involved in a collaborative effort led by Drs. Zhang and Calabresi at Hopkins to evaluate this technology in dorsal column axon degeneration produced by dorsal root axotomy. These studies found that decreased FA and parallel diffusivity correlated with axon degeneration early on after injury that persisted up to 30 days after injury, the latest time point examined, suggesting that these DTI parameters are useful markers of axon degeneration. Cumulatively, the data in CNS studies indicate that DTI parameters are sensitive and specific imaging biomarkers for detection of myelinated axons/nerve fiber loss (Zhang et al., 2009).
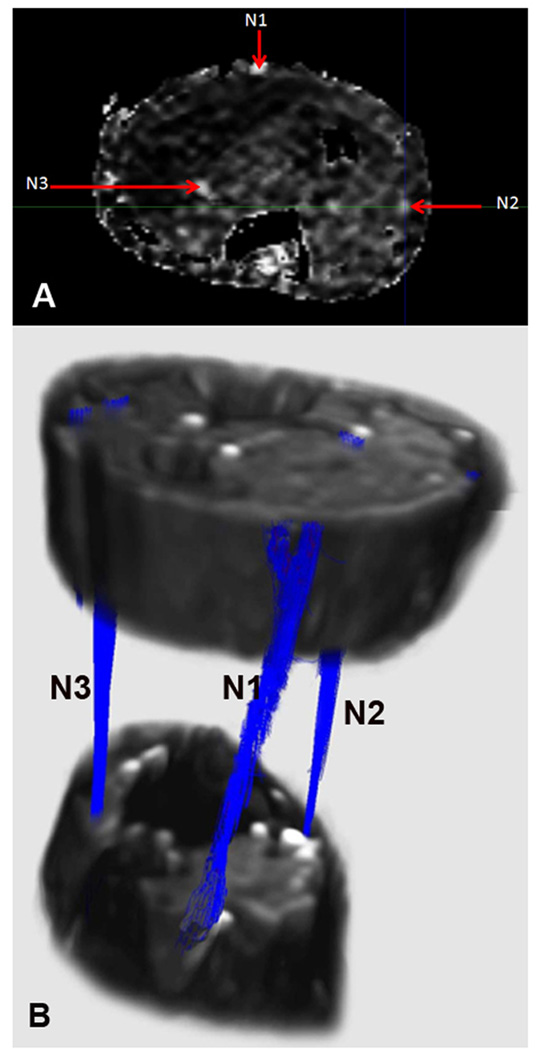
Can DTI technology be used for delineating normal peripheral nerves and to detect nerve fiber/axonal degeneration and axonal regeneration/repair to fulfill some of the needs highlighted above? It is now feasible to delineate peripheral nerves in human studies using DTI and associated tractography. Few published studies have focused on DTI of the normal human peripheral nerves including upper limb nerves particularly median nerves for which normal DTI parameters are already published (Hiltunen et al., 2005;Kabakci et al., 2007;Meek et al., 2006;Skorpil et al., 2004). ). In collaboration with Dr. Narayana’s MRI group at UTHSC-H, we have performed DTI of the normal human calf/leg and forearm regions. We found that nerve tractography allows imaging of the major nerves in the calf/leg region and their branches that include tibial, peroneal, and sural nerves. FA maps and tractography in a control subject are shown in Figure 1. The three eigenvalues λ1, λ2, and λ3 and FA for each individual nerve had a narrow range in the leg nerves. We also successfully imaged median, ulnar, and radial nerves in the forearm and branches of other nerves were also seen. Similar studies in small animals were performed in collaboration with Dr. Susumu Mori’s group at Hopkins. These ex vivo studies applied DTI and tractography to fixed mouse hind limbs and successfully delineated sciatic nerve and its branches from the surrounding tissues. This data is presented in this special issue in the manuscript authored by Lehmann et al. (Lehmann et al., 2009).These clinical and experimental findings are notable because in contrast to CNS white matter tracts peripheral nerves are intimately surrounded by other tissues particularly muscles, tendons, and fasciae that have their own diffusion tensor properties due to their specific microstructures/histology (Zhang et al., 2008). The studies showing successful application of DTI tractography to reconstruct peripheral nerves support the potential of this technology for detecting axon degeneration and regeneration.
Figure 1.
FA maps and tractography in the lower leg/ankle region. A) FA map. B) Tractography based on FA. N1= sural nerve; N2= superficial peroneal nerve; N3= Tibial nerve.
There is some experimental evidence that Wallerian degeneration in peripheral nerves can be detected by this technology. Two previous ex vivo experimental studies have reported decreased anisotropy in rat and frog segments of the nerves undergoing Wallerian degeneration (Beaulieu et al., 1996;Stanisz et al., 2001). First study reporting application of DTI and tractography to axonal degeneration and regeneration with histological validation was reported earlier this year (Takagi et al., 2009). This ex vivo study on excised (isolated) rat nerves showed that FA and parallel diffusivity decreased with nerve fiber degeneration and recovered with nerve fiber regeneration in sciatic nerve crush model. Tractography based on FA values (determined arbitrarily) showed loss of fiber tracking distal to the crush site at earlier time points and recovery of fiber tracking with regeneration at later time points.
We have examined the same issue in mouse sciatic nerve crush model using DTI and tractography. Our ex vivo studies show that among DTI parameters changes in FA value most closely correlate with nerve fiber degeneration and regeneration and recovery as determined by functional (electrophysiological) studies. FA and parallel diffusivity also correlated with number of myelinated fibers in the nerves. We also examined nerves after transection in which all axons have degenerated and determined DTI parameters in these nerve trunks lacking nerve fibers. Using FA values obtained from transected nerves as threshold for tractography we could clearly determine the longitudinal extent of regeneration in sciatic nerve crush model distal to the crush site (Lehmann et al., 2009). Overall our studies support the potential of this non-invasive imaging technology to measure nerve fiber degeneration and regeneration in peripheral nerves.
In my opinion, translational studies in humans to test the potential of DTI technology for the issues outlined above would best be done initially in patients with complete traumatic nerve transections undergoing nerve repairs. The fundamental reason to focus on this group in initial studies is the fact that this group is expected to be very uniform in that there will be complete degeneration in the distal segments of the injured/severed nerves undergoing repair, which will allow determination of both the dynamic (range of changes in different parameters) and kinetic (change over time in different parameters) range of DTI parameters in segments of the human nerves undergoing complete Wallerian degeneration and subsequent regeneration. This information will not only be critical to follow up regeneration in patients with complete traumatic nerve injuries but it would also be extremely important to establish the so-called ‘floor (or ceiling) effect’ or maximum decrease (or increase) of individual DTI parameters for application to set ‘thresholds’ to detect nerve fiber degeneration and regeneration in clinical situations in which the injury and repair responses are not ‘all or none’ but intermediate in nature. Lack of values which allow setting thresholds of individual DTI parameters is the major reason which in our opinion justifies examination of complete nerve injuries as a first test condition for this technology before wider application to other clinical neuropathies in which assessment of nerve fiber degeneration and regeneration could improve management decisions.
The scope and breadth of applicability of this technology to peripheral nerve disorders will be determined by the dynamic range of DTI parameters in normal and diseased nerves, DTI signal of the tissues surrounding nerves and dynamic range of change in this signal with neuropathies, and the resolution of the imaging hardware and software. The imaging technology is likely to continue to improve with improvements in hardware such as MRI magnet size and appropriate coils to image different peripheral nerves and with development of newer acquisition and analysis software. Current DTI technology allows imaging of small terminal nerves such as median nerve at the wrist or sural nerve at the ankle but with the technological advances the resolution is likely to improve to fascicular or subfascicular level. This assertion is supported by recent endeavors to successfully image mouse sciatic nerve and its terminal branches with high strength magnets (Lehmann et al., 2009). For wider application of this technology, another issue that requires attention includes determination of the course of peripheral nerves, which is often tortous particularly around joints. Use of bony landmarks, 3D reconstructions, DTI tractography, and special coils around joints will likely allow accurate delinination of the course of peripheral nerves. It is anticipated that increasing interest in DTI will prompt more clinical and preclinical studies that in next 3–5 years will allow us to determine the real potential of this technology to quantitatively monitor peripheral nerve injury and repair.
Acknowledgments
Dr. Sheikh is supported by Dr. Dr. Miriam and Sheldon G. Adelson Medical Research Foundation, NIH/NINDS (NS42888 and NS54962), and GBS Foundation. The author thanks Dr. Ponnada Narayana (Department of Radiology, UTHSC-H) and Ms. Anuh George (Department of Neurology UTHSC-H) for providing DTI tractography image and Drs. Susumu Mori and Jiangyang Zhang for providing helpful suggestions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Agosta F, Absinta M, Sormani MP, Ghezzi A, Bertolotto A, Montanari E, Comi G, Filippi M. In vivo assessment of cervical cord damage in MS patients: a longitudinal diffusion tensor MRI study. Brain. 2007;130:2211–2219. doi: 10.1093/brain/awm110. [DOI] [PubMed] [Google Scholar]
- 2.Bammer R. Basic principles of diffusion-weighted imaging. Eur. J. Radiol. 2003;45:169–184. doi: 10.1016/s0720-048x(02)00303-0. [DOI] [PubMed] [Google Scholar]
- 3.Basser PJ, Mattiello J, Lebihan D. Mr Diffusion Tensor Spectroscopy and Imaging. Biophysical Journal. 1994;66:259–267. doi: 10.1016/S0006-3495(94)80775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. Journal of Magnetic Resonance Series B. 1996;111:209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- 5.Beaulieu C, Does MD, Snyder RE, Allen PS. Changes in water diffusion due to Wallerian degeneration in peripheral nerve. Magn Reson. Med. 1996;36:627–631. doi: 10.1002/mrm.1910360419. [DOI] [PubMed] [Google Scholar]
- 6.Boulton AJ. Whither clinical research in diabetic sensorimotor peripheral neuropathy? Problems of end point selection for clinical trials. Diabetes Care. 2007;30:2752–2753. doi: 10.2337/dc07-1374. [DOI] [PubMed] [Google Scholar]
- 7.Chew SY, Mi R, Hoke A, Leong KW. Aligned Protein-Polymer Composite Fibers Enhance Nerve Regeneration: A Potential Tissue-Engineering Platform. Adv. Funct. Mater. 2007;17:1288–1296. doi: 10.1002/adfm.200600441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conturo TE, Lori NF, Cull TS, Akbudak E, Snyder AZ, Shimony JS, McKinstry RC, Burton H, Raichle ME. Tracking neuronal fiber pathways in the living human brain. Proc. Natl. Acad. Sci. U. S. A. 1999;96:10422–10427. doi: 10.1073/pnas.96.18.10422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.England JD, Asbury AK. Peripheral neuropathy. The Lancet. 2004;363:2151–2161. doi: 10.1016/S0140-6736(04)16508-2. [DOI] [PubMed] [Google Scholar]
- 10.Filler AG, Maravilla KR, Tsuruda JS. MR neurography and muscle MR imaging for image diagnosis of disorders affecting the peripheral nerves and musculature. Neurologic Clinics. 2004;22:643. doi: 10.1016/j.ncl.2004.03.005. vii. [DOI] [PubMed] [Google Scholar]
- 11.Fu SY, Gordon T. Contributing factors to poor functional recovery after delayed nerve repair: prolonged axotomy. Journal of Neuroscience. 1995a;15:3876–3885. doi: 10.1523/JNEUROSCI.15-05-03876.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fu SY, Gordon T. Contributing factors to poor functional recovery after delayed nerve repair: prolonged denervation. Journal of Neuroscience. 1995b;15:3886–3895. doi: 10.1523/JNEUROSCI.15-05-03886.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fujiyoshi K, Yamada M, Nakamura M, Yamane J, Katoh H, Kitamura K, Kawai K, Okada S, Momoshima S, Toyama Y, Okano H. In vivo tracing of neural tracts in the intact and injured spinal cord of marmosets by diffusion tensor tractography. Journal of Neuroscience. 2007;27:11991–11998. doi: 10.1523/JNEUROSCI.3354-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Golay X, Jiang H, van Zijl PCM, Mori S. High-resolution isotropic 3D diffusion tensor Imaging of the human brain. Magnetic Resonance in Medicine. 2002;47:837–843. doi: 10.1002/mrm.10143. [DOI] [PubMed] [Google Scholar]
- 15.Guleria S, Gupta RK, Saksena S, Chandra A, Srivastava RN, Husain M, Rathore R, Narayana PA. Retrograde Wallerian degeneration of cranial corticospinal tracts in cervical spinal cord injury patients using diffusion tensor imaging. Journal of Neuroscience Research. 2008;86:2271–2280. doi: 10.1002/jnr.21664. [DOI] [PubMed] [Google Scholar]
- 16.Gupta RK, Saksena S, Hasan KM, Agarwal A, Haris M, Pandey CM, Narayana PA. Focal Wallerian degeneration of the corpus callosum in large middle cerebral artery stroke: serial diffusion tensor imaging. J. Magn Reson. Imaging. 2006;24:549–555. doi: 10.1002/jmri.20677. [DOI] [PubMed] [Google Scholar]
- 17.Herrmann DN. Noninvasive and minimally invasive detection and monitoring of peripheral neuropathies. Expert. Rev. Neurother. 2008;8:1807–1816. doi: 10.1586/14737175.8.12.1807. [DOI] [PubMed] [Google Scholar]
- 18.Hiltunen J, Suortti T, Arvela S, Seppa M, Joensuu R, Hari R. Diffusion tensor imaging and tractography of distal peripheral nerves at 3 T. Clin. Neurophysiol. 2005;116:2315–2323. doi: 10.1016/j.clinph.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 19.Hoke A. Mechanisms of Disease: what factors limit the success of peripheral nerve regeneration in humans? Nat. Clin. Pract. Neurol. 2006;2:448–454. doi: 10.1038/ncpneuro0262. [DOI] [PubMed] [Google Scholar]
- 20.Ito R, Melhem ER, Mori S, Eichler FS, Raymond GV, Moser HW. Diffusion tensor brain MR imaging in X-linked cerebral adrenoleukodystrophy. Neurology. 2001;56:544–547. doi: 10.1212/wnl.56.4.544. [DOI] [PubMed] [Google Scholar]
- 21.Jellison BJ, Field AS, Medow J, Lazar M, Salamat MS, Alexander AL. Diffusion tensor imaging of cerebral white matter: a pictorial review of physics, fiber tract anatomy, and tumor imaging patterns. AJNR Am. J. Neuroradiol. 2004;25:356–369. [PMC free article] [PubMed] [Google Scholar]
- 22.Kabakci N, Gurses B, Firat Z, Bayram A, Ulug AM, Kovanlikaya A, Kovanlikaya I. Diffusion tensor imaging and tractography of median nerve: normative diffusion values. AJR Am. J. Roentgenol. 2007;189:923–927. doi: 10.2214/AJR.07.2423. [DOI] [PubMed] [Google Scholar]
- 23.Lehmann HC, Zhang J, Mori S, Sheikh KA. Diffusion tensor imaging to assess axonal regeneration in peripheral nerves. 2009 doi: 10.1016/j.expneurol.2009.10.012. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martyn CN, Hughes RA. Epidemiology of peripheral neuropathy. Journal of Neurology,Neurosurgery,and Psychiatry. 1997;62:310–318. doi: 10.1136/jnnp.62.4.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meek MF, Stenekes MW, Hoogduin HM, Nicolai JP. In vivo three-dimensional reconstruction of human median nerves by diffusion tensor imaging. Experimental Neurology. 2006;198:479–482. doi: 10.1016/j.expneurol.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 26.Midha R, Kline DG. Evaluation of the neuroma in continuity. Philadelphia, PA: W.B.Saunders Comapany; 1998. [Google Scholar]
- 27.Mori S, Crain BJ, Chacko VP, van Zijl PCM. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Annals of Neurology. 1999;45:265–269. doi: 10.1002/1531-8249(199902)45:2<265::aid-ana21>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 28.Mori S, van Zijl PC. Fiber tracking: principles and strategies - a technical review. NMR Biomed. 2002;15:468–480. doi: 10.1002/nbm.781. [DOI] [PubMed] [Google Scholar]
- 29.Mori S, Zhang JY. Principles of diffusion tensor imaging and its applications to basic neuroscience research. Neuron. 2006;51:527–539. doi: 10.1016/j.neuron.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 30.Noble J, Munro CA, Prasad VS, Midha R. Analysis of upper and lower extremity peripheral nerve injuries in a population of patients with multiple injuries. Journal of Trauma. 1998;45:116–122. doi: 10.1097/00005373-199807000-00025. [DOI] [PubMed] [Google Scholar]
- 31.Pierpaoli C, Barnett A, Pajevic S, Chen R, Penix LR, Virta A, Basser P. Water diffusion changes in Wallerian degeneration and their dependence on white matter architecture. Neuroimage. 2001;13:1174–1185. doi: 10.1006/nimg.2001.0765. [DOI] [PubMed] [Google Scholar]
- 32.Reich DS, Smith S, Jones CK, Dubey P, Zackowski KM, van Zijl PC, Mori S, Calabresi PA. Abnormalities in white matter fiber tracts in MS revealed by diffusion tensor imaging: Effects at and distant from demyelinating lesions. Neurology. 2005;64:A242. [Google Scholar]
- 33.Schlosshauer B, Dreesmann L, Schaller HE, Sinis N. Synthetic nerve guide implants in humans: a comprehensive survey. Neurosurgery. 2006;59:740–747. doi: 10.1227/01.NEU.0000235197.36789.42. [DOI] [PubMed] [Google Scholar]
- 34.Schlosshauer B, Muller E, Schroder B, Planck H, Muller HW. Rat Schwann cells in bioresorbable nerve guides to promote and accelerate axonal regeneration. Brain Research. 2003;963:321–326. doi: 10.1016/s0006-8993(02)03930-6. [DOI] [PubMed] [Google Scholar]
- 35.Skorpil M, Karlsson M, Nordell A. Peripheral nerve diffusion tensor imaging. Magn Reson. Imaging. 2004;22:743–745. doi: 10.1016/j.mri.2004.01.073. [DOI] [PubMed] [Google Scholar]
- 36.Stanisz GJ, Midha R, Munro CA, Henkelman RM. MR properties of rat sciatic nerve following trauma. Magn Reson. Med. 2001;45:415–420. doi: 10.1002/1522-2594(200103)45:3<415::aid-mrm1054>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 37.Stieltjes B, Kaufmann WE, van Zijl PCM, Fredericksen K, Pearlson GD, Solaiyappan M, Mori S. Diffusion tensor imaging and axonal tracking in the human brainstem. Neuroimage. 2001;14:723–735. doi: 10.1006/nimg.2001.0861. [DOI] [PubMed] [Google Scholar]
- 38.Stoll G, Wessig C, Gold R, Bendszus M. Assessment of lesion evolution in experimental autoimmune neuritis by gadofluorine M-enhanced MR neurography. Experimental Neurology. 2006;197:150–156. doi: 10.1016/j.expneurol.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 39.Takagi T, Nakamura M, Yamada M, Hikishima K, Momoshima S, Fujiyoshi K, Shibata S, Okano HJ, Toyama Y, Okano H. Visualization of peripheral nerve degeneration and regeneration: monitoring with diffusion tensor tractography. Neuroimage. 2009;44:884–892. doi: 10.1016/j.neuroimage.2008.09.022. [DOI] [PubMed] [Google Scholar]
- 40.Thomalla G, Glauche V, Koch MA, Beaulieu C, Weiller C, Rother J. Diffusion tensor imaging detects early Wallerian degeneration of the pyramidal tract after ischemic stroke. Neuroimage. 2004;22:1767–1774. doi: 10.1016/j.neuroimage.2004.03.041. [DOI] [PubMed] [Google Scholar]
- 41.Trip SA, Wheeler-Kingshott C, Jones SJ, Li WY, Barker GJ, Thompson AJ, Plant GT, Miller DH. Optic nerve diffusion tensor imaging in optic neuritis. Neuroimage. 2006;30:498–505. doi: 10.1016/j.neuroimage.2005.09.024. [DOI] [PubMed] [Google Scholar]
- 42.Tuch DS, Wedeen VJ, Dale AM, George JS, Belliveau JW. Conductivity tensor mapping of the human brain using diffusion tensor MRI. Proc. Natl. Acad. Sci. U. S. A. 2001;98:11697–11701. doi: 10.1073/pnas.171473898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Valsasina P, Agosta F, Benedetti B, Caputo D, Perini M, Salvi F, Prelle A, Filippi M. Diffusion anisotropy of the cervical cord is strictly associated with disability in amyotrophic lateral sclerosis. Journal of Neurology,Neurosurgery,and Psychiatry. 2007;78:480–484. doi: 10.1136/jnnp.2006.100032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Valsasina P, Rocca MA, Agosta F, Benedetti B, Horsfield MA, Gallo A, Rovaris M, Comi G, Filippi M. Mean diffusivity and fractional anisotropy histogram analysis of the cervical cord in MS patients. Neuroimage. 2005;26:822–828. doi: 10.1016/j.neuroimage.2005.02.033. [DOI] [PubMed] [Google Scholar]
- 45.Werring DJ, Toosy AT, Clark CA, Parker GJ, Barker GJ, Miller DH, Thompson AJ. Diffusion tensor imaging can detect and quantify corticospinal tract degeneration after stroke. Journal of Neurology,Neurosurgery,and Psychiatry. 2000;69:269–272. doi: 10.1136/jnnp.69.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Witzel C, Rohde C, Brushart TM. Pathway sampling by regenerating peripheral axons. Journal of Comparative Neurology. 2005;485:183–190. doi: 10.1002/cne.20436. [DOI] [PubMed] [Google Scholar]
- 47.Zhang J, Jones M, DeBoy CA, Reich DS, Farrell JA, Hoffman PN, Griffin JW, Sheikh KA, Miller MI, Mori S, Calabresi PA. Diffusion tensor magnetic resonance imaging of Wallerian degeneration in rat spinal cord after dorsal root axotomy. Journal of Neuroscience. 2009;29:3160–3171. doi: 10.1523/JNEUROSCI.3941-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang J, Zhang G, Morrison B, Mori S, Sheikh KA. Magnetic resonance imaging of mouse skeletal muscle to measure denervation atrophy. Experimental Neurology. 2008;212:448–457. doi: 10.1016/j.expneurol.2008.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]