1. Summary
The majority of human subjects who receive subcutaneous allergen immunotherapy (IT) develop decreased sensitivity to their allergens. Multiple factors may explain the efficacy of IT, some evidence supports a role for allergen specific IgG antibodies. There is controversy whether such antibodies act by blocking allergen binding to IgE or initiation of active inhibitory signaling through low affinity IgG receptors (FcγRIIB) on mast cells and basophils. In this study, we addressed this question using peripheral blood from cat non-allergic, cat allergic, and immunotherapy-treated cat allergic subjects. Blood from subjects who received IT contain IgG antibodies that mediate inhibition of basophil activation by a mechanism that is blocked by antibodies specific for the inhibitory IgG receptor FcγRIIB. Surprisingly, inhibition was also blocked by aglycosylated, putatively non-FcR binding, antibodies that are specific for the FcγRIIA, suggesting a contribution of this receptor to the observed effect. Consistent with a cooperative effect, ex vivo basophils were found to express both IgG receptors. In other studies we found that basophils from subjects who were both chronically exposed to allergen and were producing both cat allergen specific IgE and IgG, are hypo-responsive to allergen. These studies confirm that IgG antibodies produced during IT act primarily by stimulation of inhibitory signaling, and suggest that FcγRIIA and FcγRIIB function cooperatively in activation of inhibitory signaling circuit. We suggest that under normal physiologic conditions in which only a small proportion of FcεRI are occupied by IgE of a single allergen specificity, FcγRIIA co-aggregation may, by providing activated Lyn, be required to fuel activation of inhibitory FcγRIIB function.
Keywords: Human, Mast Cells/Basophils, Fc Receptors, Antibodies, Allergy
2. Introduction
Allergen immunotherapy (IT), first described by Noon in the early 1900’s, is one of the most effective treatments available for allergic rhinitis and allergic asthma [1-3]. This treatment typically involves subcutaneous injection of mixed allergen extracts weekly starting at a very low dose, increasing the dose weekly until a maintenance dose is reached. Shots are administered at least monthly to maintain desensitization once maintenance is reached. For appropriately selected patients, IT effectively decreases nasal symptom scores and medication requirements. It is the only therapy that can prevent sensitization to new allergens, decrease reactivity after allergen exposure and have lasting effects after treatment is discontinued [4]. In the last decade, our knowledge of dose requirements for effective monotherapy (immunization with a single allergen) and of adjuvant effects has increased significantly, however, many aspects of the mechanism by which allergen immunotherapy reduces sensitivity remain elusive.
Repeated subcutaneous injection of allergen (IT) has been shown to alter many facets of the immune system. Allergen immunotherapy causes decreased sensitivity of T cells to allergen-induced proliferation, increased frequency of inducible T regulatory cells, and a shift in the ratio of allergen-specific TH1 to TH2 cells. Such immunomodulation produces changes in the balance of cytokines with production toward reduced IL-3, IL-4, IL-5, IL-9 and IL-13, and increased TGF-β and IL-10. These modifications may favor production of allergen specific IgG and IgA, and reduced IgE production, modulating allergic inflammation mediated by mast cells, basophils and eosinophils [5].
Cellular mediators induced by IT may target mast cells and basophils, which have emerged as very important effector cells in allergic inflammation. Allergen cross-linking of IgE/FcεRI on mast cells and basophils trigger degranulation, releasing histamine, serine proteases and other inflammatory mediators. Intermediate and late phase mast cell responses include production and release of cytokines, e.g.IL-4 and IL-13, chemokines, prostaglandins and leukotrienes. Local cytokines may, in turn, regulate mast cell and basophil function by altering activity of proteins and changing gene expression [6].
Effects of IT may also be mediated by allergen specific IgG antibodies. Successful immunotherapy is usually correlated with an initial increase in allergen-specific IgG1, followed by an increase in IgG4 and, much later, a decrease in allergen-specific IgE antibodies. Allergen-specific IgG4 may act to block the interaction of IgE with allergen, decreasing CD23-mediated B cell allergen uptake and subsequent antigen processing and decreasing IgE mediated degranulation of mast cells and basophils [7, 8, Ejrnaes, 2006 #1685, 9]. Additionally, co-aggregation of the low affinity IgG receptor FcγRIIB with IgE / FcεRI by immune complexes may lead to active inhibition of mast cell and basophil degranulation [5, 10-13].
Basophils and mast cells express the immunoreceptor tyrosine-based activation motif (ITAM)-containing FcεRI complex (αβγ2) and FcγRIIB, an inhibitory immunoreceptor tyrosine-based inhibitory motif (ITIM)-containing low affinity IgG receptor. Mast cells derived from skin also express functional FcγRIIA, an ITAM containing low affinity IgG receptor [14]. In 2000, Kepley et al examined human basophils for expression of FcγRII using RT-PCR analysis. They reported the presence of FcγRIIA and FcγRIIB transcripts, but not FcγRIIC transcripts, in peripheral blood basophils [15]. These authors were unable to quantify expression levels of FcγRIIA and FcγRIIB on the surface because staining antibodies available at the time were not able to clearly distinguish FcγRIIA from FcγRIIB. The balance between stimulation of activating versus inhibitory FcR, may be central in determining the occurrence and severity of allergic, anaphylactic and Arthus reactions [16-18].
In this study we analyzed the production of IgG antibodies of various subclasses in subjects receiving immunotherapy for cat hair allergens. We then assessed the ability of these antibodies to modulate basophil response to allergen, and whether observed effects reflect blocking of allergen binding to IgE or engagement of inhibitory FcγR. To determine if IgG-mediated inhibition requires interaction with FcγRII, we assessed the blocking ability of FcγRIIA and FcγRIIB specific antibodies that were mutated to prevent glycosylation and therefore minimize or eliminate the ability of their Fc regions to bind FcγRIIB [19-22].
3. Materials and Methods
Subjects
Thirty-nine subjects, ranging in age from 19 to 64, were recruited through National Jewish Health General Clinical Research Center with IRB approval. Study groups included individuals who were non cat allergic by skin prick testing (n=16), cat allergic by skin prick testing (wheal > 3 mm, erythema > 10 mm) (n=17), and cat allergic, but on maintenance immunotherapy for > 6 months (>3 μg Fel d1 per monthly injection) (n=6). Subjects in the first two groups were naive to immunotherapy (Table I).
Table I.
Subject Demographics
| Non Cat Allergic (n=16) |
Cat Allergic (n=17) |
Cat Allergic on Immunotherapy (n=6) |
||
|---|---|---|---|---|
| Age (years) | 32.1 ± 10.7 | 32.8 ± 10.8 | 34.3 ± 9.99 | n.s. |
| Sex (M/F) | 3/13 | 6/11 | 1/5 | n.s. |
| Cat specific IgE (kU/dl)Ψ |
0 | 6.17 ± 6.8 | 19.4 ± 36.6 | p= 0.1* |
| Total IgE (kU/dl)Ψ |
88 ± 153 | 170 ± 120 | 742 ± 1083 | p = 0.005+ p= 0.014# |
| Asthma | 1/16 | 8/17 | 5/6 | p= 0.0007& |
| Atopic Dermatitis |
2/16 | 3/17 | 1/6 | n.s. |
| Any allergic symptomsω |
6/16 | 17/17 | 6/6 | p<0.0001& |
| Cat allergy symptomsω |
1/16 | 15/17 | 6/6 | |
| Cat Exposure | ||||
| none | 5 | 10 | 1 | |
| past | 3 | 3 | 4 | |
| current | 8 | 4 | 1 |
Unless otherwise specified, statistical analysis utilized Dunnett’s Method with a control.
Total and cat epithelium specific IgE were measured using Phadia CAP system
Cat allergic used as control; no significant difference between allergic and IT groups
Non allergic control in comparison to IT subjects
Cat allergic control in comparison with IT subjects
Chi-squared likelihood for difference between groups
Allergy symptoms (any vs. cat specific) were self-reported on questionnaire
Cat hair specific IgG measurement
ELISA plates were coated with allergen by incubation with ALK-Abello (Horsholm, Denmark) cat hair extract (1:10,000 BAU, Fel d1 content 31.4 μg/ml) overnight at 4° C, before subject’s serum was added, and plates were incubated at 4° C overnight. Binding of cat hair specific IgG was detected using either anti-human IgG-HRP (Biorad Laboratories, Hercules, CA), or a combination of IgG1-biotin, IgG2-biotin, IgG3-biotin or IgG4-biotin (Caltag, Burlingame, CA) and SA-HRP (BioSource, Nivelle, Belgium). To allow comparison among subjects and experiments, relative units (RU) of cat hair specific IgG were established by normalization of data to a control serum from a subject previously on immunotherapy containing moderate levels of anti-cat hair antibodies all IgG subclasses.
Flow cytometry-based CD203c assay of basophil activation
Whole blood was stimulated with cat hair extract, which is used for cat allergen immunotherapy (ALK-Abello, Horsholm, Denmark) and was normalized to Fel d1 concentration, anti-FcεR1α (Upstate, Lake Placid, NY), or f-met-leu-phe (fMLP) for 10 minutes at 37° C and then placed on ice. The Fel d1 concentration for the lot used was provided by ALK (31.4 μg Fel d1 per ml cat hair extract). Basophils were identified based on positive staining with antibodies specific for CD45 (BD Biosciences, San Jose, CA), IgE (Caltag, Burlingame, CA), and CD203c (Immunotech, Marseille, France). Relative CD203c staining also served as a marker of basophil activation. Red cell depleted samples were analyzed using a BD FacsCaliber flow cytometer [23]. In some cases human serum (20% v/v final concentration) was incubated with whole blood for 1 to 3 hours prior to stimulation and assay. Control serum lacking both cat hair specific IgG and IgE was used for the “no inhibitor” control. In some cases, antibodies specific for FcγRIIA (IV.3 N297Q) (MacroGenics, Rockville, MD) and FcγRIIB (2B6 N297Q) (MacroGenics, Rockville, MD) were added 30 minutes prior to addition of human sera and/or stimulation. BIAcore was used to demonstrate that, as previously shown by Minura et al, genetic elimination of the glycosylation effectively eliminated (within the limit of sensitivity of this assay) binding of the Fc region of these antibodies to FcγRII [22] (technique described in [24], personal communication Ezio Bonvini).
Cell surface analysis for FcγRIIA and FcγRIIB expression
Red cell depleted whole blood was fixed with 2% formaldehyde and stored at −80° C. Basophils identified as above were stained with anti-FcγRIIA (IV.3 N297Q), anti-FcγRIIB (2B6 N297Q) or an isotype control CH4420 N297Q (Fc region aglycosylated similar to IV.3 and 2B6) (MacroGenics, Rockville, MD). The cell line IIA1.6, transfected with human FcγRIIB (kindly provided by Mark Hogarth) was cultured in DMEM with 5% fetal bovine serum and stained with the aglycosylated antibodies as above to confirm the specificity of antibody interaction with FcγRII (Figure 2E).
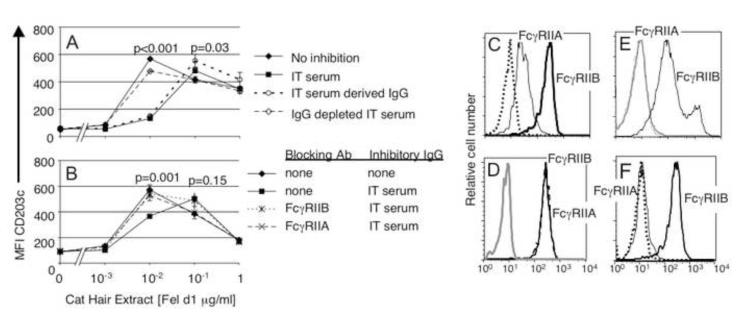
Figure 2. IgG antibodies produced during immunotherapy acutely inhibit basophil responses to allergen by FcγRIIA and FcγRIIB dependent mechanisms.
A) Serum (20% v/v) was added to whole blood from an allergic subject. “No inhibition” control serum containing no cat allergen specific IgG; “Immune serum” - serum from IT subject; “Purified IgG” and “IgG depleted serum” - see methods. Purified IgG mediated desensitization, and serum depleted of IgG lost this ability. B) Blocking with either anti-FcγRIIA (IV.3 N297Q) or anti-FcγRIIB (2B6 N297Q) reversed the effect. C) Immunofluorescence staining of ex vivo basophils with antibodies against FcγRIIA (IV.3 N297Q) (solid line) and FcγRIIB (2B6 N297Q) (heavy solid line) D) Ex vivo basophils were incubated with excess anti-FcγRIIA (IV.3 N297Q) (dashed black line) or no antibody (solid line) before addition of staining antibodies to identify basophils (CD45 and CD203) and for anti-FcγRIIB (2B6 N297Q) quantitation to assess blocking. The gray line represents isotype control. E) A murine B cell line (IIA1.6) expressing human FcγRIIB was stained by anti-FcγRIIB (2B6 N297Q) Alexa 488 (black line) but not by anti-FcγRIIA (IV.3 N297Q) Alexa 488 (gray line), or an isotype control antibody, CH4420 N297Q Alexa 488 (dotted line). F) Staining of CD20 positive peripheral blood B cell with anti-FcγRIIB (2B6 N297Q) (heavy solid line) but not anti-FcγRIIA (IV.3 N297Q) (dotted black line). The solid black line reflects isotype control staining.
IgG purification from human serum
IgG was isolated by protein G Sepharose chromatography with elution using 0.1 N acetic acid (pH 3.0) and concentrated using a centrifugal filter unit with a molecular weight limit of 100 kD (Millipore, Billerica, CA). Concentration of IgG was measured using nephelometry. IgG depleted sera were concentrated to original volume using a centrifugal filter unit with a molecular weight limit of 30 kD (Millipore, Billerica, MA), using serum albumin to standardize concentrations.
Statistics
Dunnett’s Method with control (non cat allergic control vs. immunotherapy treated, and cat allergic control vs. immunotherapy treated) was used for analysis of differences in levels of allergen-specific IgG antibody levels. Dunnett’s Method with control (no allergy symptoms) was also used for analysis of differences in expression of FcγRIIA and FcγRIIB. A linear regression model was used to evaluate the influence of high vs. low allergen-specific IgG and IgE on basophil sensitivity. Where present, error bars represent standard error of the mean.
4. Results
Basophils from cat allergen sensitive individuals rapidly up regulate CD203c upon stimulation
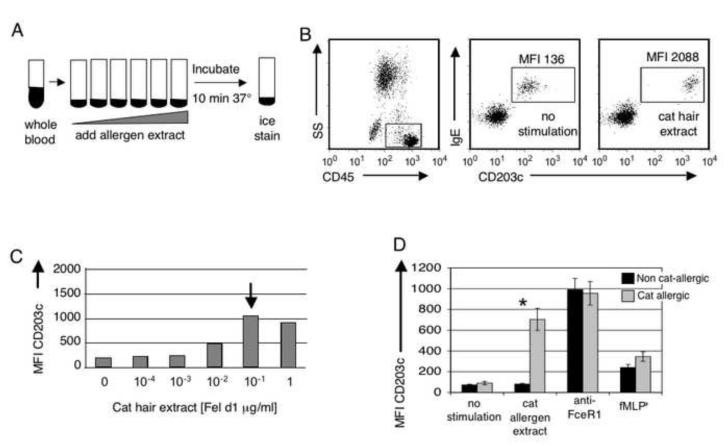
CD203c expression has been used to evaluate IgE-mediated basophil activation in applications ranging from evaluation of drug reactions to assessment of both food and stinging insect allergy [25]. In normal subjects, CD203c is weakly expressed on peripheral blood basophils, mast cells, and their CD34+ progenitors, but is rapidly up regulated after cell activation. To assess ex vivo basophil responsiveness to cat hair allergen, whole cat hair extract was added to sodium heparanized whole blood followed by incubation for 10 minutes at 37° C (Figure 1A). As shown in Figure 1B, and as previously reported [26], basophil CD203c expression increased up to 17 fold from baseline within 10 minutes of ex vivo stimulation with cat hair extract. We found this CD203c-based assay to be more sensitive than skin prick testing (one individual on IT was skin test negative) and cat hair specific IgE (4 skin test positive individuals with IgE anti-cat epithelial extract <0.35 kU/dl). Maximal activation occurred with Fel d1 exposure ranging from 0.001 μg/ml to greater than 1 μg/ml, the maximum dose used for stimulation. Figure 1C shows a representative titration curve. In contrast to other studies that have used histamine release as a measure of basophil activation, we found that basophils from all subjects tested responded to stimulation through their FcεRI (allergen and/or anti-FcεRIα antibody mediated cross-linking) (Figure 1D).
Figure 1. Basophils from cat allergic individuals are uniquely sensitive to cat allergen induced CD203c up-regulation.
A) Whole blood was stimulated with cat hair extract containing Fel d1 [0.0001 μg/ml to 1 μg/ml]. FcεRIα cross-linking antibodies and fMLP were used as positive controls. B) Cells from an allergic subject were stained with the pan-leukocyte marker CD45, IgE and CD203c to identify basophils and determine activation status. MFI of CD203c following a representative stimulation of ex vivo basophils is shown. C) A representative cat hair allergen dose response curve is shown here from a cat allergic subject. The arrow annotates the point of maximal basophil activation, or allergen “sensitivity” of the basophil. D) Average basophil CD203c expression for stimulated (as shown) non cat-allergic and cat allergic subjects. The basophil response for cat allergen extract shown was for the maximal activation; the dose required varied widely, from a fel d1 concentration of 0.001 μg/ml to the maximum does used 1 μg/ml. * p<0.001
IgG antibodies produced during IT reduce basophil sensitivity to allergen
Two previous reports have shown that sera collected from subjects post-immunotherapy are able to inhibit allergen induced donor basophil degranulation [27, 28]. The authors attributed the changes in histamine release profiles to increased IgG but did not exclude other serum factors. To address this question directly, we incubated basophils from a cat hair-allergic subject who had undetectable IgG anti-cat hair antibody with serum from a subject on immunotherapy. We then assessed responsiveness to allergen using CD203c as a marker for basophil activation. Sera containing cat hair-specific IgG decreased the sensitivity of basophils from a cat allergic subject (Figure 2A). IgG purified from these sera mediated the full effect. Sera depleted of IgG lost the ability to alter basophil sensitivity. Elevation of CD203c expression after addition of fMLP was not altered by serum or IgG from the IT subject (data not shown). These results confirmed that allergen-specific IgG is responsible for the observed alteration in basophil sensitivity, but do not differentiate IgG “blocking” effects from IgG/allergen immune-complex recruitment of FcγRIIB and inhibitory signaling.
IgG modulation of sensitivity depends upon function of both FcγRIIA and FcγRIIB
IgG Abs may modulate basophil responses by blocking allergen access to IgE that is associated with FcεRI, or by recruiting and activating inhibitory immunoglobulin Fc receptors. Inhibition of IgE-mediated mast cell activation via the low affinity IgG receptor FcγRIIB has been demonstrated in vivo in mice and in vitro using human and mouse cell lines [13, 29]. Proof that allergen specific IgG is capable of recruiting FcγRIIB in physiologic situations, i.e. in humans treated with immunotherapy, is lacking. To test for FcγRII involvement, we added blocking antibodies specific for either FcγRIIA (IV.3 N297Q) or FcγRIIB (2B6 N297Q) to whole blood one half hour before addition of serum containing cat-specific IgG. These antibodies were engineered to prevent glycosylation so that their own Fc regions do not engage Fc receptors detectably [19]. Loss of binding of the Fc portion of the N297Q antibodies to human CD32B and CD32A-131his was confirmed by BIAcore as described in [24] (personal communication Ezio Bonvini). One hour after addition of serum, cat hair allergen was added and responses measured (as described above). Blocking FcγRIIB abrogated the effects of the serum containing cat specific IgG Abs indicating that inhibitory signaling and not blocking is operative in inhibition. To our surprise however, FcγRIIA blocking Abs also reversed the effects of the immune serum (Figure 2B).
Based on these effects we wanted to first confirm the earlier finding of Keply which suggested that human basophils express both FcγRIIA and FcγRIIB [15]. As shown in Figure 2C ex vivo basophils were stained by both the aglycosylated FcγRIIA antibody IV.3 N297Q and aglycosylated anti-FcγRIIB antibody 2B4 N297Q.
The effects of aglycosylated anti-FcγRIIA could reflect bona fide function of FcγRIIA in inhibitory signaling, or it could result from either spurious crossreactivity of this antibody with FcγRIIB or binding of the Fc of this antibody to FcγRIIB. Either mechanism could block IgG-allergen immune complex co-aggregation of FcεR1 and FcγRIIB. To begin to address these possibilities we preincubated human basophils with excess anti-FcγRIIA (IV.3 N297Q) and assessed its effect on staining by 2B6 N297Q, the FcγRIIB specific antibody (Figure 2D). No blocking was seen. To further confirm that anti-FcγRIIA (IV.3 N297Q) did not bind FcγRIIB, the murine cell line IIA1.6 was transfected with human FcγRIIB and stained with anti-FcγRIIA IV.3 N297Q and anti-FcγRIIB 2B6 N297Q. Figure 2E shows anti-FcγRIIA IV.3 N297Q does not bind human FcγRIIB on these cells. Finally, as is shown in Figure 2F, this antibody does not stain ex vivo human B cells, which express FcγRIIB but not FcγRIIA. Taken together these findings argue strongly that the observed effects reflect a supporting role for FcγRIIA in promoting inhibitory signaling by FcγRIIB.
Effect of immunotherapy and environmental exposure to allergen on IgG antibody levels
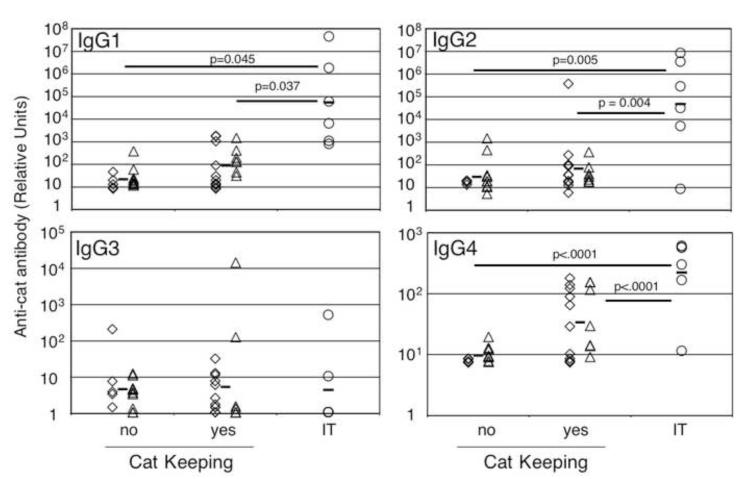
Chronic high dose natural exposure to cat hair allergens is associated with decreased allergic symptoms and increased expression of cat hair specific IgG1, IgG2 and IgG4 antibodies (Abs) [30, 31]. Likewise, immunotherapy leads to decreased sensitivity and increased levels of allergen specific IgG1, IgG4, and to a lesser extent IgG2 [5]. As a preliminary step in determining whether IgG Abs produced as a consequence of natural exposure differ from immunotherapy induced IgG Abs, we segregated patients who reported living with a cat for at least 3 months at some point during their lives (cat keepers) from non cat keepers and subjects receiving IT. Of the non-cat allergic individuals, 11 of 16 were past or current cat keepers. In the cat allergic group, 7 of 17 were current or past cat keepers. One IT subject was a current cat keeper (Table 1). IgG levels and isotype distribution within these groups is shown in Figure 3. Cat hair specific IgG1, IgG2, IgG3, IgG4 and total cat hair specific IgG Ab levels were measured by ELISA. Modest elevations of IgG1 and IgG2, and especially IgG4 Abs were associated with cat keeping. Most subjects on immunotherapy had significantly higher cat hair specific IgG1, IgG2 and IgG4 Abs than the naturally exposed group. However, some non-IT cat keeping individuals had levels of IgG4 Abs equivalent to subjects on IT. This direct comparison of allergen-specific IgG4 Ab levels in non-allergic and allergic subjects with or without IT demonstrates IgG4 may be elevated in non-allergic individuals and subjects naïve to immunotherapy. In our study, one individual on IT who reported improvement of symptoms made very little cat hair specific IgG4 (11 RU), but made a significant amount of cat hair specific IgG1 (60,627 RU).
Figure 3. Elevation in cat allergen specific IgG antibodies is associated with environmental exposure or immunotherapy.
Levels of allergen specific IgG Abs were determined by ELISA (see methods). Non cat allergic (◇) and cat allergic (△) individuals were separated into those with a cat in the home > 3 months (Cat Keeping) vs. those never living with a cat. Immunotherapy subjects are represented by circles (o). Dunnett’s method with control (either no cat keeping or yes cat keeping as indicated) was used for statistical analysis.
FcγRIIB expression is lower on IT subjects compared to non-allergic controls
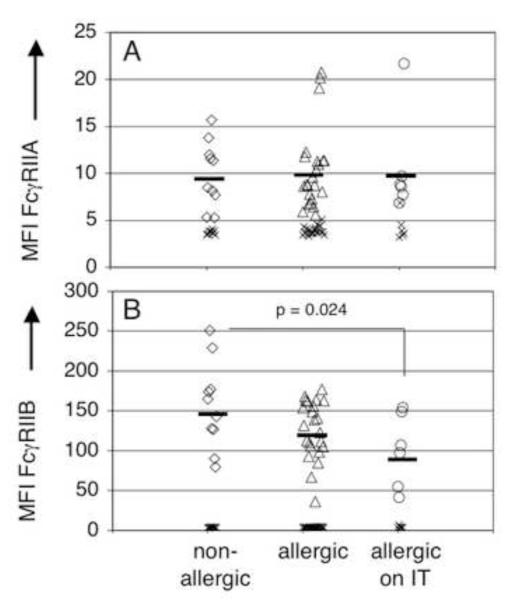
Results from blocking studies in Figure 2B and 2C confirm that human basophils express both FcγRIIA and FcγRIIB, and suggest that both are active. Shown in Figure 4 is immunofluorescence analysis of FcγR expression by basophils from subjects segregated into three cohorts; those who denied any allergy symptoms, those who reported any allergy symptoms, and those with allergies who are treated with monthly injections of allergen immunotherapy. As expected from experiments above demonstrating that FcγRIIA may play a role in IgG-mediated basophil regulation, basophils from the majority of subjects expressed low but significant levels of FcγRIIA (Figure 4A). Allergic/treatment status did not influence expression levels. FcγRIIB expression on basophils from subjects receiving allergen immunotherapy was significantly lower than that of non-allergic individuals (Figure 4B).
Figure 4. Both FcγRIIA and FcγRIIB are expressed on human basophils and immunotherapy modulates FcγRIIB expression.
Grouping was based on self-reported allergy symptoms and immunotherapy treatment. Diamonds (◇)represent subjects denying any allergy symptoms, triangles (△) represent subjects with allergies, circles (o) represent subjects on IT and x’s are the isotype control for each antibody. A) Basophils from all subjects expressed FcγRIIA (IV.3 N297Q), no difference was seen between groups B) The MFI of FcγRIIB (2B6 N297Q) was significantly decreased on basophils from subjects on IT compared to non allergic controls (MFI 96 vs. 152).
Chronic in vivo exposure of allergic individuals to cat hair allergen-specific IgG antibodies is associated with basophil unresponsiveness to allergen
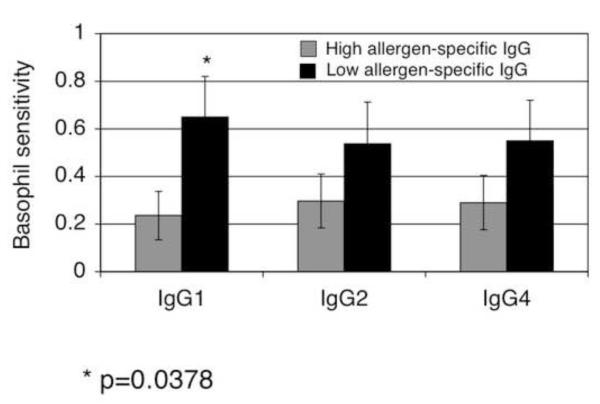
Studies described above demonstrate that IgG Abs produced following IT are capable of reducing basophil responsiveness to allergen by a mechanism dependent on FcγRIIA and FcγRIIB. To assess physiologic relevance, we examined whether basophils from subjects with high vs. low IgG anti-cat hair Ab levels exhibited decreased responsiveness to allergen. Individuals with elevated cat-allergen specific IgG1 (>400 RU) (p=0.04) in fact were less sensitive to cat allergen (Figure 5). Significance was not reached for elevated levels of either cat allergen-specific IgG2 (>100 RU) or IgG4 (>50 RU). The increased sensitivity of basophils in subjects who did not have elevated cat hair specific IgG could not attributed to increased cat allergen specific IgE levels as subjects with lower allergen specific IgG also had lower allergen specific IgE (see Figure 5 legend). One subject enrolled in the study donated blood before and 7 months after reaching maintenance immunotherapy. A ten-fold decrease in basophil sensitivity was accompanied by a significant increase in cat hair allergen specific IgG (Figure 6A). In this subject, IT did not result in a significant change in either FcγRIIA or FcγRIIB expression (FcγRIIA MFI 4.72 pre to 4.07 post; FcγRIIB MFI 109 pre to 92.6 post) (Figure 6B).
Figure 5. Reduced basophil sensitivity to allergen is observed in subjects with elevated cat hair allergen specific IgG1.
To assess the association of between basophil sensitivity and allergen-specific IgG, we analyzed the relationship between cat allergen specific IgG levels and basophil sensitivity (y-axis), dividing subjects into those whose sera contained high vs. low cat specific IgG. The analysis was done for cat hair allergen specific IgG1 (high > 400 relative units (RU)), IgG2 (high > 100 RU), and IgG4 (high > 50 RU). The relationship between high vs. low allergen-specific IgG and basophil sensitivity reached significance for IgG1 (*p=0.04), but not IgG2 (p=0.24) or IgG4 (p=0.21). Mean cat allergen specific IgE were as follows: IgG1 low and high, 5.3 and 6.45 kU/dl respectively, IgG2 low and high 5.2 and 6.6 kU/dl respectively and IgG4 low and high 5.7 and 5.5 kU/dl respectively.
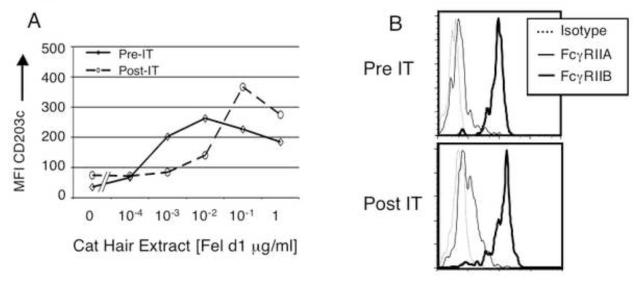
Figure 6. IT reduces basophil sensitivity to cat hair allergen.
Data was collected for one individual pre- and post-immunotherapy. A). After seven months of maintenance IT, basophil sensitivity had decreased 10 fold. Pre-IT cat hair allergen specific IgG was 0.2 RU, post-IT cat hair allergen specific IgG was 5.5 × 103 RU. Expression levels of low affinity IgG receptors were minimally changed (B). FcγRIIA: pre IT △ MFI 4.7; post △ MFI 4.1; FcγRIIB: pre IT △ MFI 109; post △ MFI 93.
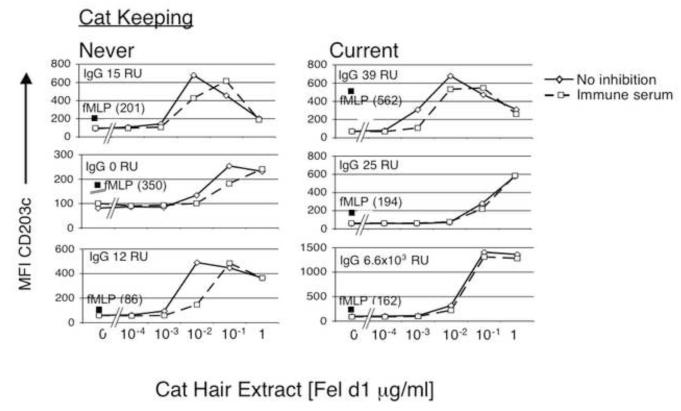
Basophil desensitization in allergic individuals that express allergen-specific IgG and chronically exposed to cat hair allergen
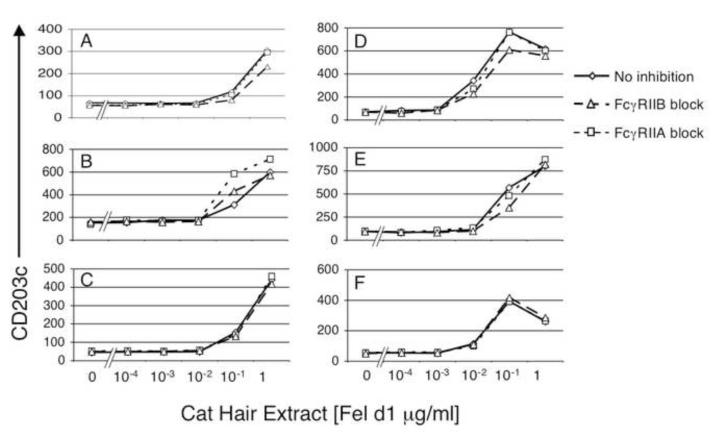
Interesting and important insight was provided by observations made in cases of allergic individuals who were current cat keepers. When serum from an IT subject (20% v/v) containing high levels of cat allergen specific IgG1 (6503 RU), IgG2 (5118 RU) and IgG4 (302 RU) were added to whole blood from cat keeping allergic individuals, the effect on basophil sensitivity to cat allergen was minimal. Figure 7 shows the effect of serum containing high levels of cat allergen specific IgG on responses of basophils from subjects who were never keepers of cats (left column) or were current cat keepers (right column). Hyporesponsiveness to allergen specific IgG occurred even in cat keeping subjects with low levels of baseline cat hair allergen specific IgG (top two plots in right column, cat allergen specific IgG RU is < 40). Whether this is indicative of intrinsic changes in basophil responsiveness due to chronic exposure to cat allergen/IgG/IgE complexes or reflects ongoing inhibitory function of IgG and FcγR was examined next. To distinguish these two possibilities in vitro, FcγRIIA and FcγRIIB were blocked prior to the addition of cat hair allergen in subjects receiving immunotherapy. Acutely blocking FcγRII had a minimal effect on allergen responsiveness of basophils from all but one of the immunotherapy subjects tested, suggesting that decreased sensitivity was not dependent on ongoing interaction of IgG with FcγRII (Figure 8). All patients on IT have elevated cat hair allergen specific IgG and are exposed to high dose cat allergen in monthly subcutaneous injections promoting circulating IgG/cat allergen complexes. These experiments support the possibility that chronic in vivo exposure of IgG-containing allergen immune complexes cause an intrinsic change in basophil reactivity. We are currently exploring whether this effect is antigen specific. Our findings also suggest that high levels of allergen specific IgG are not sufficient to block allergen binding to IgE on basophils, a mechanism suggested for the modulatory effects of immunotherapy-induced IgG on B cells [7].
Figure 7. Current exposure to environmental cat allergen decreases acute IgG mediated effects on basophil sensitivity.
Serum from the IT subject used in Figure 2 (20% v/v), containing high amounts of cat specific IgG, was added to whole blood of allergic subjects prior to cat allergen stimulation. The immune serum mediated a greater change in basophil responsiveness in subjects not exposed to cat in the home (left panel). Baseline cat hair allergen specific IgG is documented in each of the plots by “IgG”. The filled square is the geometric mean fluorescence intensity of CD203c expression following 10 minutes of stimulation with fMLP (see methods).
Figure 8. Hyporesponsiveness of “IT” basophils does not reflect acute FcγR signaling.
Blocking antibodies against either FcγRIIA (IV.3 N297Q) or FcγRIIB (2B6 N297Q) were added to samples 30 minutes before allergen was added. Subject B recorded a negative skin prick test to cat allergen and reported efficacy of his treatment (he currently keeps a cat). In this individual, blocking FcγRIIA had a greater effect than blocking FcγRIIB, but both resulted in enhanced basophil sensitivity. Despite high levels of cat hair allergen specific IgG Abs in all other subjects, blocking FcγR did not decrease basophil sensitivity. Cat hair allergen specific IgG levels: A - 4.0 × 103, B - 6.1 × 105, C - 5.0 × 103, D - 5.8 × 104, E - 1.5 × 103 and F - 5.5 × 103.
5. Discussion
Previous studies have led to suggestions that the beneficial effects of immunotherapy may be mediated by regulatory T cells, altered Th cytokine milieu, blocking antibodies and/or active IgG immune complex induced inhibitory signaling. Studies described here test the effects of blood born agents produced during IT on the activation of basophils by allergen. Results demonstrate that cat hair allergen-specific IgG antibodies are produced in the course of IT and inhibit basophil responses to allergen via a mechanism that requires their binding to both FcγRIIA and FcγRIIB immunoglobulin Fc receptors. The ability of FcγR blockade to prevent inhibition indicates that IgG antibodies produced in response to IT do not act primarily by preventing allergen access to IgE-liganded FcεRI, and reveal a previously unrecognized cooperative role for FcγRIIA in inhibitory signaling. Consistent with requirements for both IgG receptors, we found that both FcγRIIA and FcγRIIB are expressed on ex vivo human basophils. Finally, it should be noted that our identification of peripheral blood basophils with the markers CD45, IgE and CD203C would not exclude mast cell progenitors.
A caveat to the interpretation that FcγRIIA functions in promotion of inhibitory signaling in this situation comes from the following. It is remotely possible that when bound to FcγRIIA, the Fc of aglycosylated IV.3 associates with and blocks neighboring FcγRIIB despite undetectable affinity for the receptor. Such blocking might not be detected by staining with higher affinity anti-FcγRIIB antibody (Figure 2C). This possibility could be excluded by showing that either F(ab’)2 or F(ab) fragments of IV.3 also block inhibitory signaling. Unfortunately, despite repeated attempts we were unable to produce IV.3 fragments that retained FcγRIIA binding activity, and thus could not undertake this experiment. Such instability is often seen in monoclonal antibodies.
Interestingly, FcγRIIB expression on basophils from subjects receiving cat allergen immunotherapy was present at significantly lower levels than on basophils from subjects without any allergic symptoms (p=0.024). The observed differential expression of FcγRIIB in these subjects is reminiscent of IL-4 down-regulation of FcγRIIB expression by activated B cells. This effect opposes the influence of IL-4, IL-10 or TGF-β which up-regulate FcγRIIB expression on dendritic cells and monocytes [10]. Thus FcγRIIB expression on basophils could be affected by the cytokine milieu in these subjects.
The reported effects, while demonstrating an active inhibitory role for IgG antibodies in IT, do not exclude functions of regulatory T cells or Th cytokines whose functions would not be manifest at the level of basophil activation. Furthermore, we cannot exclude the possibility that IT with other allergens or using other regimens may lead to production of blocking antibodies.
Perhaps the most surprising findings in these studies were the expression and requisite role of FcγRIIA in inhibitory signaling by basophil FcγR. Until description of the FcγRIIA and FcγRIIB specific antibodies IV.3 and 2B6, respectively, it was not possible to distinguish these receptors by cell staining. The low frequency of basophils in peripheral blood limited analysis using immunochemical and genetic approaches. Previous studies examining FcγRIIB modulation of IgE-mediated activation have been done either in the murine system, which lacks FcγRIIA, or in systems that have not excluded the involvement of FcγRIIA [13].
The observed role of FcγRIIA in inhibitory signaling is logical when one considers that FcγRIIB phosphorylation and consequent recruitment and stimulation of effector phosphatases (SHIP-1 and SHP-1) requires activation of the Lyn tyrosine kinase by a co-aggregated ITAM-containing receptor such as FcγRIIA or FcεR1. Of further conceptual importance is the fact once activated the primary FcγRIIB effector pathway, i.e. the SHIP-1, Dok-1 pathway, is able to function in trans to inhibit remotely stimulated receptors [32, 33]. In the system utilized here Lyn activating function theoretically could be provided by FcεRI or FcγRIIA co-aggregated with FcγRIIB. However, since on ex vivo basophils FcεRI are occupied by IgE with many alternate specificities, the effective concentration of FcεRI that could co-aggregate with FcγRIIB is very low relative to FcγRIIA. Since FcγRIIA and B are low affinity receptors, they are not preoccupied and thus are available for high stoichiometry co-aggregation by IgG-containing immune complexes. Thus we suggest that IgG-allergen immune complexes co-aggregate these receptors leading to FcγRIIA activation of Lyn, which phosphorylates tyrosines within the ITIM of FcγRIIB, resulting in the recruitment and activation of the SHIP-1 circuit. Phosphorylated SHIP-1 acting in concert with its adaptor downstream of kinase, Dok-1, then act in trans to inhibit FcεRI signaling. The ability of IgG immune complexes to trigger SHIP-1 activation in the absence of association with IgE receptors, could explain the reported nonspecific desensitization of mediator cells [34].
It is noteworthy that under certain circumstances FcγRIIA is able to activate SHIP-1 signaling independent of FcγRIIB [35]. If this mode of SHIP-1 recruitment were playing an independent role in modulating IT, the IgG effects seen should not have been sensitive to FcγRIIB blockade (Figure 2B). Instead, it appears allergen/immune complex clustering of FcγRIIA and FcγRIIB serves to regulate IgE mediated activation.
This concept may relate to another unexpected finding reported here. Basophils from subjects that were chronically exposed to allergen via cat keeping or allergen immunotherapy were found to display reduced sensitivity to cat allergen. This hyporesponsiveness was associated with occurrence, in these subjects, of IgG anti-allergen antibodies. We hypothesize that this hyporesponsiveness is caused by chronic in vivo stimulation of basophils by allergen-IgG immune complexes. Consistent with such a mechanism, basophils from these subjects were also refractile to inhibitory stimuli ex vivo. FcγRII receptors on these cells may be desensitized by chronic immune complex stimulation in vivo. This possibility is currently under study.
Under certain conditions IgG immune complexes cause type III hypersensitivity responses known as Arthus reactions. Cell participation in Arthus reactions may depend upon the balance of their expression of FcγRIIA and FcγRIIB. Previous studies have shown that a 10% contamination of ITAM-containing receptor aggregates with ITIM-containing receptors, e.g. FcγRIIB, leads to inhibition of signaling [36]. Although the precise ratio cannot be concluded due to requisite use of different antibodies, it is possible to compare basophil expression of the FcγRIIB to B cell expression of the FcγRIIB (they are approximately the same, Figure 2C and F). One can also compare expression levels of ITAM containing FcγRIIA on basophils to levels of FcγRIIA expressed on monocytes or neutrophils. When this comparison is made, basophil expression of FcγRIIA is one full log lower (data not shown). Therefore the net effect of immune complex binding to basophils is likely to be cis inhibition of FcγRIIA mediated cell activation, and trans inhibition of FcεRI mediated signaling. This ratio of expression of inhibitory and activating receptors likely predisposes basophils to be resistant to participation in Arthus reactions [17]. It is noteworthy that FcγR expression can be regulated by the cytokine milieu. In mice, FcγRIIB expression by dendritic cells and monocytes is upregulated by IL-4, IL-10 and TGF-β. On activated B cells, IL-4 down regulates FcγRIIB expression [10]. Thus cytokine milieu may modulate cellular participation in Arthus reactions. An example of this modulation may exist in the reduced expression of FcγRIIB on basophils from allergic subjects.
It is well established that FcγR binding is influenced by both IgG isotype and FcγR polymorphisms [37]. Addition of cat hair allergen-specific IgG antibodies from subjects on immunotherapy was sufficient to mediate a shift in allergen sensitivity of basophils. In subjects lacking chronic high dose exposure to cat allergen, the presence of cat allergen-specific IgG antibodies in whole blood was also associated with a shift in basophil sensitivity (Figures 5 and 6). Levels of allergen specific IgG1 Abs were inversely associated with basophil sensitivity, independent of cat hair allergen-specific IgE levels. Although the IgG1 subclass was particularly associated with decreased basophil sensitivity, most (9/12) individuals we studied had elevated cat hair specific IgG Abs from multiple IgG subclasses. Because of limiting material, we did not analyze the contribution of allergen specific IgG subclass representation on efficacy of immunotherapy. Such studies are ongoing.
Acknowledgements
We thank Trudi Madigan, Janice Herrell, Gwendolyn Marsh, Kimberly Usuda, Deborah Corliss, Brianna Quinn and Pat Kittleson, who are current or former members of the National Jewish General Clinical Research Center for their assistance with many facets of this project. We thank Teri Lebo for technical assistance with flow cytometry, Tom Galaway for assistance developing the ELISAs and Sandy Duran for her helpful assistance in preparation of this manuscript.
Footnotes
Disclosures: S Johnson, E Bonvini and S Koenig are employed by MacroGenics, Inc., whose potential product was studied in the present work. These three authors have filed patent applications related to the work that is described in the present study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Noon LCB. Prophylactic inoculation against hayfever. Lancet. 1911;177:1572–3. [Google Scholar]
- [2].Calderon MA, Alves B, Jacobson M, Hurwitz B, Sheikh A, Durham S. Allergen injection immunotherapy for seasonal allergic rhinitis. Cochrane Database Syst Rev. 2007:CD001936. doi: 10.1002/14651858.CD001936.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Abramson MJ, Puy RM, Weiner JM. Allergen immunotherapy for asthma. Cochrane Database Syst Rev. 2003:CD001186. doi: 10.1002/14651858.CD001186. [DOI] [PubMed] [Google Scholar]
- [4].Nelson HS. Allergen immunotherapy: where is it now? J Allergy Clin Immunol. 2007;119:769–79. doi: 10.1016/j.jaci.2007.01.036. [DOI] [PubMed] [Google Scholar]
- [5].Akdis M, Akdis CA. Mechanisms of allergen-specific immunotherapy. J Allergy Clin Immunol. 2007;119:780–91. doi: 10.1016/j.jaci.2007.01.022. [DOI] [PubMed] [Google Scholar]
- [6].Prussin C, Metcalfe DD. 5. IgE, mast cells, basophils, and eosinophils. J Allergy Clin Immunol. 2006;117:S450–6. doi: 10.1016/j.jaci.2005.11.016. [DOI] [PubMed] [Google Scholar]
- [7].Wachholz PA, Durham SR. Mechanisms of immunotherapy: IgG revisited. Curr Opin Allergy Clin Immunol. 2004;4:313–8. doi: 10.1097/01.all.0000136753.35948.c0. [DOI] [PubMed] [Google Scholar]
- [8].Wachholz PA, Soni NK, Till SJ, Durham SR. Inhibition of allergen-IgE binding to B cells by IgG antibodies after grass pollen immunotherapy. J Allergy Clin Immunol. 2003;112:915–22. doi: 10.1016/s0091-6749(03)02022-0. [DOI] [PubMed] [Google Scholar]
- [9].van Neerven RJ, Knol EF, Ejrnaes A, Wurtzen PA. IgE-mediated allergen presentation and blocking antibodies: regulation of T-cell activation in allergy. Int Arch Allergy Immunol. 2006;141:119–29. doi: 10.1159/000094714. [DOI] [PubMed] [Google Scholar]
- [10].Nimmerjahn F, Ravetch JV. Fcgamma receptors: old friends and new family members. Immunity. 2006;24:19–28. doi: 10.1016/j.immuni.2005.11.010. [DOI] [PubMed] [Google Scholar]
- [11].Till SJ, Francis JN, Nouri-Aria K, Durham SR. Mechanisms of immunotherapy. J Allergy Clin Immunol. 2004;113:1025–34. doi: 10.1016/j.jaci.2004.03.024. [DOI] [PubMed] [Google Scholar]
- [12].Kraft S, Kinet JP. New developments in FcepsilonRI regulation, function and inhibition. Nat Rev Immunol. 2007;7:365–78. doi: 10.1038/nri2072. [DOI] [PubMed] [Google Scholar]
- [13].Zhang K, Zhu D, Kepley C, Terada T, Saxon A. Chimeric human fcgamma-allergen fusion proteins in the prevention of allergy. Immunol Allergy Clin North Am. 2007;27:93–103. doi: 10.1016/j.iac.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Zhao W, Kepley CL, Morel PA, Okumoto LM, Fukuoka Y, Schwartz LB. Fc gamma RIIa, not Fc gamma RIIb, is constitutively and functionally expressed on skin-derived human mast cells. J Immunol. 2006;177:694–701. doi: 10.4049/jimmunol.177.1.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kepley CL, Cambier JC, Morel PA, Lujan D, Ortega E, Wilson BS, et al. Negative regulation of FcepsilonRI signaling by FcgammaRII costimulation in human blood basophils. J Allergy Clin Immunol. 2000;106:337–48. doi: 10.1067/mai.2000.107931. [DOI] [PubMed] [Google Scholar]
- [16].Daeron M, Lesourne R. Negative signaling in Fc receptor complexes. Adv Immunol. 2006;89:39–86. doi: 10.1016/S0065-2776(05)89002-9. [DOI] [PubMed] [Google Scholar]
- [17].Malbec O, Daeron M. The mast cell IgG receptors and their roles in tissue inflammation. Immunol Rev. 2007;217:206–21. doi: 10.1111/j.1600-065X.2007.00510.x. [DOI] [PubMed] [Google Scholar]
- [18].Ott VL, Cambier JC. Activating and inhibitory signaling in mast cells: new opportunities for therapeutic intervention? J Allergy Clin Immunol. 2000;106:429–40. doi: 10.1067/mai.2000.109428. [DOI] [PubMed] [Google Scholar]
- [19].Veri MC, Gorlatov S, Li H, Burke S, Johnson S, Stavenhagen J, et al. Monoclonal antibodies capable of discriminating the human inhibitory Fcgamma-receptor IIB (CD32B) from the activating Fcgamma-receptor IIA (CD32A): biochemical, biological and functional characterization. Immunology. 2007;121:392–404. doi: 10.1111/j.1365-2567.2007.02588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Mackay M, Stanevsky A, Wang T, Aranow C, Li M, Koenig S, et al. Selective dysregulation of the FcgammaIIB receptor on memory B cells in SLE. J Exp Med. 2006;203:2157–64. doi: 10.1084/jem.20051503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Rankin CT, Veri MC, Gorlatov S, Tuaillon N, Burke S, Huang L, et al. CD32B, the human inhibitory Fc-gamma receptor IIB, as a target for monoclonal antibody therapy of B-cell lymphoma. Blood. 2006;108:2384–91. doi: 10.1182/blood-2006-05-020602. [DOI] [PubMed] [Google Scholar]
- [22].Mimura Y, Sondermann P, Ghirlando R, Lund J, Young SP, Goodall M, et al. Role of oligosaccharide residues of IgG1-Fc in Fc gamma RIIb binding. J Biol Chem. 2001;276:45539–47. doi: 10.1074/jbc.M107478200. [DOI] [PubMed] [Google Scholar]
- [23].Yasnowsky KM, Dreskin SC, Efaw B, Schoen D, Vedanthan PK, Alam R, et al. Chronic urticaria sera increase basophil CD203c expression. J Allergy Clin Immunol. 2006;117:1430–4. doi: 10.1016/j.jaci.2006.02.016. [DOI] [PubMed] [Google Scholar]
- [24].Stavenhagen JB, Gorlatov S, Tuaillon N, Rankin CT, Li H, Burke S, et al. Fc optimization of therapeutic antibodies enhances their ability to kill tumor cells in vitro and controls tumor expansion in vivo via low-affinity activating Fcgamma receptors. Cancer Res. 2007;67:8882–90. doi: 10.1158/0008-5472.CAN-07-0696. [DOI] [PubMed] [Google Scholar]
- [25].Kleine-Tebbe J, Erdmann S, Knol EF, MacGlashan DW, Jr., Poulsen LK, Gibbs BF. Diagnostic tests based on human basophils: potentials, pitfalls and perspectives. Int Arch Allergy Immunol. 2006;141:79–90. doi: 10.1159/000094495. [DOI] [PubMed] [Google Scholar]
- [26].Ocmant A, Peignois Y, Mulier S, Hanssens L, Michils A, Schandene L. Flow cytometry for basophil activation markers: the measurement of CD203c up-regulation is as reliable as CD63 expression in the diagnosis of cat allergy. J Immunol Methods. 2007;320:40–8. doi: 10.1016/j.jim.2006.12.002. [DOI] [PubMed] [Google Scholar]
- [27].Ball T, Sperr WR, Valent P, Lidholm J, Spitzauer S, Ebner C, et al. Induction of antibody responses to new B cell epitopes indicates vaccination character of allergen immunotherapy. Eur J Immunol. 1999;29:2026–36. doi: 10.1002/(SICI)1521-4141(199906)29:06<2026::AID-IMMU2026>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- [28].Mothes N, Heinzkill M, Drachenberg KJ, Sperr WR, Krauth MT, Majlesi Y, et al. Allergen-specific immunotherapy with a monophosphoryl lipid A-adjuvanted vaccine: reduced seasonally boosted immunoglobulin E production and inhibition of basophil histamine release by therapy-induced blocking antibodies. Clin Exp Allergy. 2003;33:1198–208. doi: 10.1046/j.1365-2222.2003.01699.x. [DOI] [PubMed] [Google Scholar]
- [29].Daeron M, Malbec O, Latour S, Arock M, Fridman WH. Regulation of high-affinity IgE receptor-mediated mast cell activation by murine low-affinity IgG receptors. J Clin Invest. 1995;95:577–85. doi: 10.1172/JCI117701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Woodfolk JA. High-dose allergen exposure leads to tolerance. Clin Rev Allergy Immunol. 2005;28:43–58. doi: 10.1385/CRIAI:28:1:043. [DOI] [PubMed] [Google Scholar]
- [31].Platts-Mills T, Vaughan J, Squillace S, Woodfolk J, Sporik R. Sensitisation, asthma, and a modified Th2 response in children exposed to cat allergen: a population-based cross-sectional study. Lancet. 2001;357:752–6. doi: 10.1016/S0140-6736(00)04168-4. [DOI] [PubMed] [Google Scholar]
- [32].Brauweiler A, Merrell K, Gauld SB, Cambier JC. Cutting Edge: Acute and chronic exposure of immature B cells to antigen leads to impaired homing and SHIP1-dependent reduction in stromal cell-derived factor-1 responsiveness. J Immunol. 2007;178:3353–7. doi: 10.4049/jimmunol.178.6.3353. [DOI] [PubMed] [Google Scholar]
- [33].Blery M, Delon J, Trautmann A, Cambiaggi A, Olcese L, Biassoni R, et al. Reconstituted killer cell inhibitory receptors for major histocompatibility complex class I molecules control mast cell activation induced via immunoreceptor tyrosine-based activation motifs. J Biol Chem. 1997;272:8989–96. doi: 10.1074/jbc.272.14.8989. [DOI] [PubMed] [Google Scholar]
- [34].Lichtenstein LM, Levy DA. Is desensitization’ for ragweed hay fever immunologically specific? Int Arch Allergy Appl Immunol. 1972;42:615–26. doi: 10.1159/000230642. [DOI] [PubMed] [Google Scholar]
- [35].Nakamura K, Malykhin A, Coggeshall KM. The Src homology 2 domain-containing inositol 5-phosphatase negatively regulates Fcgamma receptor-mediated phagocytosis through immunoreceptor tyrosine-based activation motif-bearing phagocytic receptors. Blood. 2002;100:3374–82. doi: 10.1182/blood-2002-03-0787. [DOI] [PubMed] [Google Scholar]
- [36].Sarmay G, Koncz G, Gergely J. Integration of activatory and inhibitory signals in human B-cells. Immunol Lett. 1996;54:93–100. doi: 10.1016/s0165-2478(96)02655-7. [DOI] [PubMed] [Google Scholar]
- [37].van Sorge NM, van der Pol WL, van de Winkel JG. FcgammaR polymorphisms: Implications for function, disease susceptibility and immunotherapy. Tissue Antigens. 2003;61:189–202. doi: 10.1034/j.1399-0039.2003.00037.x. [DOI] [PubMed] [Google Scholar]