Abstract
Manganese (Mn) is an essential trace metal, however exposure to high Mn levels can result in neurodegenerative changes resembling Parkinson´s disease (PD). Information on Mn´s effects on endothelial cells of the blood-brain barrier (BBB) is lacking. Accordingly, we tested the hypothesis that BBB endothelial cells are a primary target for Mn-induced neurotoxicity. The studies were conducted in an in vitro BBB model of immortalized rat brain endothelial (RBE4) cells. ROS production was determined by F2-Isoprostane (F2-IsoPs) measurement. The relationship between Mn toxicity and redox status was investigated upon intracellular glutathione (GSH) depletion with diethylmaleate (DEM) or L-buthionine sulfoximine (BSO). Mn exposure (200 or 800 µM MnCl2 or MnSO4) for 4 or 24h led to significant decrease in cell viability vs. controls. DEM or BSO pre-treatment led to further enhancement in cytotoxicity vs. exposure to Mn alone, with more pronounced cell death after 24h DEM pre-treatment. F2-IsoPs levels in cells exposed to MnCl2 (200 or 800 µM), were significantly increased after 4h and remained elevated 24h after exposure compared with controls. Consistent with the effects on cell viability and F2-IsoPs, treatment with MnCl2 (200 or 800 µM) was also associated with a significant decrease in membrane potential. This effect was more pronounced in cells exposed to DEM plus MnCl2 vs. cells exposed to Mn alone. We conclude that Mn induces direct injury to mitochondria in RBE4 cells. The ensuing impairment in energy metabolism and redox status may modify the restrictive properties of the BBB compromising its function.
Keywords: RBE4 cells, manganese neurotoxicity, oxidative stress, glutathione, F2-Isoprostanes, mitochondria cytotoxicity
1 - Introduction
Manganese (Mn) is an essential trace metal required for normal lipid, protein and carbohydrate metabolism (Hurley and Keen, 1987). Mn plays an important role in numerous enzyme families including oxidoreductases, transferases, hydrolases, lyases, isomerases and ligases (Fitsanakis et al., 2005). Despite its essentiality, exposure to excessive Mn levels is associated with an irreversible brain disease characterized by psychiatric and motor disturbances (Chia et al., 1993; Pal et al., 1999; Stredrick et al., 2004). The disorder, referred to as manganism, is associated with elevated brain levels of Mn, especially the caudate-putamen, globus pallidus, substantia nigra, and subthalamic nuclei (Aschner et al., 1999; Dobson et al., 2004). Typical symptoms of chronic Mn exposure include tiredness, sleep disturbances, aggressiveness and behavioural disturbances (“manganese madness”). Delayed neurological disturbances encompass the extrapyramidal system, characterized by walking difficulties, dystonia and kinesia resembling many clinical features of PD (Mena et al., 1967). Mining, steel manufacturing and welding represent occupational exposures that are associated with Mn exposures to high Mn levels (Tanaka and Lieben, 1969; Cook et al., 1974; Myers et al., 2003). An organic Mn compound, methylcyclopentadienyl manganese tricarbonyl (MMT), used as an octane booster or anti-knock agent in gasoline, has also been shown to cause adverse health effects in humans in Canada and elsewhere (Abbott et al., 1987; Sierra et al., 1995; Dobson et al., 2004).
The BBB plays a major role in maintaining homeostasis in central nervous system (CNS), restricting passage of substances from the bloodstream. This is achieved by specialized endothelial cells and their interactions with glia (Davson and Segal, 1996; Rubin and Staddon, 1999; Fitsanakis et al., 2006; Moser et al., 2007). In order for blood-borne macro and micro-molecules to enter the brain parenchyma they must first cross the endothelial cells of the BBB. The high electrical resistance, reflecting upon the physical coupling of the tight junctions that link brain endothelial cells prevents any paracellular passage across the barrier in physiological conditions.
The BBB regulates the transport of essential trace metals including Mn (Bradbury, 1992) via facilitated diffusion (Rabin et al., 1993), active transport (Aschner and Gannon, 1994), divalent metal transport 1 (Erikson et al., 2002), ZIP8 and transferrin-dependent transport mechanisms (Aschner and Gannon, 1994; Au et al., 2008). Among different endothelial cell lines, the RBE4 line has shown great promise as an in vitro BBB model (Reichel et al., 2000; Aschner et al., 2002; Yang and Aschner, 2003; Fitsanakis et al., 2006). It is derived from immortalized (with the plasmid pEIA-neo) rat brain microvascular endothelium (Roux et al., 1994; Abbott et al., 1995). Immortalized RBE4 cells express enzymes and transporters localized in the BBB endothelium, with similar characteristics to those noted in vivo (Roux and Couraud, 2005). These cells were used in a wide range of studies including transferrin receptor-mediated (Huwyler et al., 1999), 3,4-dihydroxy-L-phenylalanine (Sampaio-Maia et al., 2001), choline (Friedrich et al., 2001) and serotonin transport (Brust et al., 2000), and P-glycoprotein activity (Bendayan et al., 2002). Using this model, Fitsanakis et al. (2006) characterized various aspects of Mn transport.
Brain tissue/cells are dependent on glucose-derived oxidative metabolism not only for energy needs, but also for the synthesis of tricarboxylic acid (TCA) cycle-related neurotransmitters, such as glutamate, aspartate, GABA, and acetylcholine (Clark and Lai, 1989). One proposed mechanism of Mn neurotoxicity is energy failure due to Mn inhibition of ATP synthesis. This Mn-induced impairment in energy metabolism could be attributed to decrease in the activities of enzymes of glucose oxidative metabolism. Interestingly, Mn is taken up by mitochondria via its high affinity for the calcium uniporter (Gavin et al., 1990; Gavin et al., 1999) and preferentially accumulates in these organelles. Enzymes located in the mitochondrial matrix are known to be vulnerable to Mn toxicity and inhibition of the TCA cycle enzyme, aconitase, has been suggested as one of the mechanisms underlying Mn neurotoxicity (Zheng et al., 1998). It was also demonstrated that Mn can directly inhibit ketoglutarate dehydrogenase complex activities in both liver and brain mitochondria and induce a dose-related lowering of the activities of two other TCA cycle enzymes, namely citrate synthase and malate dehydrogenase, further corroborating that mitochondria constitute a major subcellular target for Mn toxicity (Lai et al., 1999). Thus, Mn exerts inhibitory effects on TCA cycle (Lai et al., 1999; Malthankai et al., 2004) and on oxidative phosphorylation (Gavin et al., 1992).
Mitochondria are an important cellular source of ROS and are highly susceptible to oxidative damage. Under resting conditions between 2 and 5% of molecular oxygen consumed by mitochondria is partially reduced by the electron transport chain to form superoxide and subsequently hydrogen peroxide. Elevated mitochondrial ROS generation can inhibit one or more of the components of the respiratory chain, further accelerating the rate of superoxide formation (Turrens and Boveris, 1980), ultimately leading to cell death (Diemer et al., 2003). Interestingly, Mn preferentially localizes within these organelles (Liccione and Maines, 1988; Brown and Taylor, 1999), reflecting its high affinity for the calcium uniporter (Gavin et al., 1990; Gavin et al., 1999). Moreover, superoxide produced in the mitochondrial electron transport chain (ETC) catalyzes the transition shift of Mn2+ to Mn3+ through a set of reactions analogous to those mediated by SOD, resulting in increased oxidant capacity of Mn (HaMai and Bondy, 2004; Gunter et al., 2006).
Oxidative damage is a consequence of excessive oxidative stress, insufficient antioxidant potential or a combination of both (Chapple, 1997). High ROS levels produce tissue damage by a variety of mechanisms including DNA damage, lipid peroxidation, protein oxidation and depletion of cellular thiols (Halliwell and Gutteridge, 2005). Several cellular antioxidant systems afford protection against free radical damage including α-tocopherol, ascorbic acid, glutathione (GSH) as well as antioxidant enzymes, such as glutathione peroxidase (GPx) and superoxide dismutase (SOD). When ROS levels exceed the cellular redox capacity, cells are subjected to oxidative stress ensuing in cell damage. A delicate balance exists between cell death and survival, in that oxidative stress pushes the system toward damage while antioxidant defence mechanisms promote survival (Schulz et al., 2000; Sayre et al., 2008). GSH is the most abundant cellular antioxidant (Mytilineou et al., 2002) and the principal thiol for maintaining optimal redox balance.
Prostaglandin-F2–like compounds, termed F2–isoprostanes (F2-IsoPs), are a complex family of compounds produced from arachidonic acid via a free radical catalyzed mechanism (Morrow et al., 1990). The measurement of F2-IsoPs is the most reliable approach to assess oxidative stress status in vivo and can be used as authentic biomarkers of lipid peroxidation (Basu, 2004; Montuschi et al., 2004; Davies et al., 2007). Another consequence of increased oxidative stress is the induction of the mitochondrial permeability transition (MPT), a Ca2+ -dependent process characterized by the opening of the permeability transition pore in the inner mitochondrial membrane (Jacobson and Dunchen, 2002; Keil et al., 2006), which consequently leads to a collapse of the mitochondrial inner membrane potential and reduced ATP generation (Rama Rao and Norenberg, 2004).
While the precise mechanism of Mn-induced neurotoxicity remains to be fully defined, altered bioenergetics and oxidative stress are known critical factors in mediating its pathogenesis. Consistent with these effects Mn accelerates the oxidation of dopamine and other catecholamines and concurrently amplifies ROS generation (Ali et al., 1995; Sloot et al., 1996). Furthermore, divalent Mn catalyzes Fenton-like reactions generating hydroxyl radical, proteolytic degradation and protein turnover (Wedler, 1993; HaMai and Bondy, 2004).
Virtually nothing is known about Mn´s effects on capillary endothelial cells and the potential for these cells to contribute to Mn-induced neurotoxicity. Accordingly, the principal aims of our study were to extend our previous observations and determine if endothelial cells of the BBB are directly targeted by Mn and to investigate the underlying mechanism of cytotoxicity using the following endpoints of toxicity: i) reduction of mitochondrial membrane potential as a predictive biomarker of oxidative stress; ii) increased F2-Isopts as indicators of lipid peroxidation, resulting from increased ROS generation and iii) redox (GSH) status as a mediator as cellular viability with the decrease of GSH as a potential indicator of the increase of ROS. A series of studies was carried out to address Mn´s ability to induce ROS, alter mitochondrial membrane potential and determine the role of GSH in modulating Mn-induced endothelial cells toxicity.
2 - Results
BSO and DEM potentiate Mn-induced RBE4 cell death
Fig. 1 and Fig. 2 depict the effects of MnCl2 or MnSO4 (200 or 800 µM) on RBE4 cell viability (measured by the MTT). A significant concentration-dependent decrease (p<0.05 for the 200 and p<0.001 for the 800 µM) in cell viability was noted for cells exposed to MnCl2 or MnSO4 for 4 or 24h compared with controls.
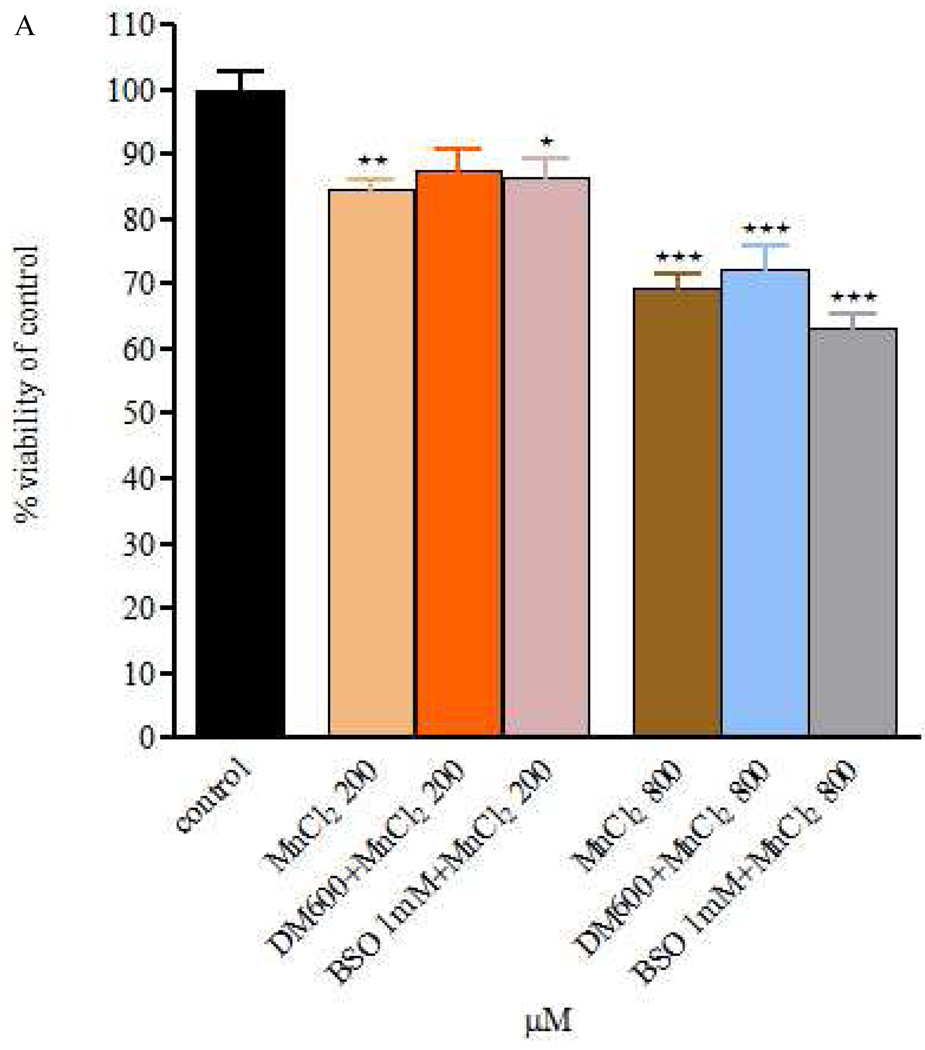
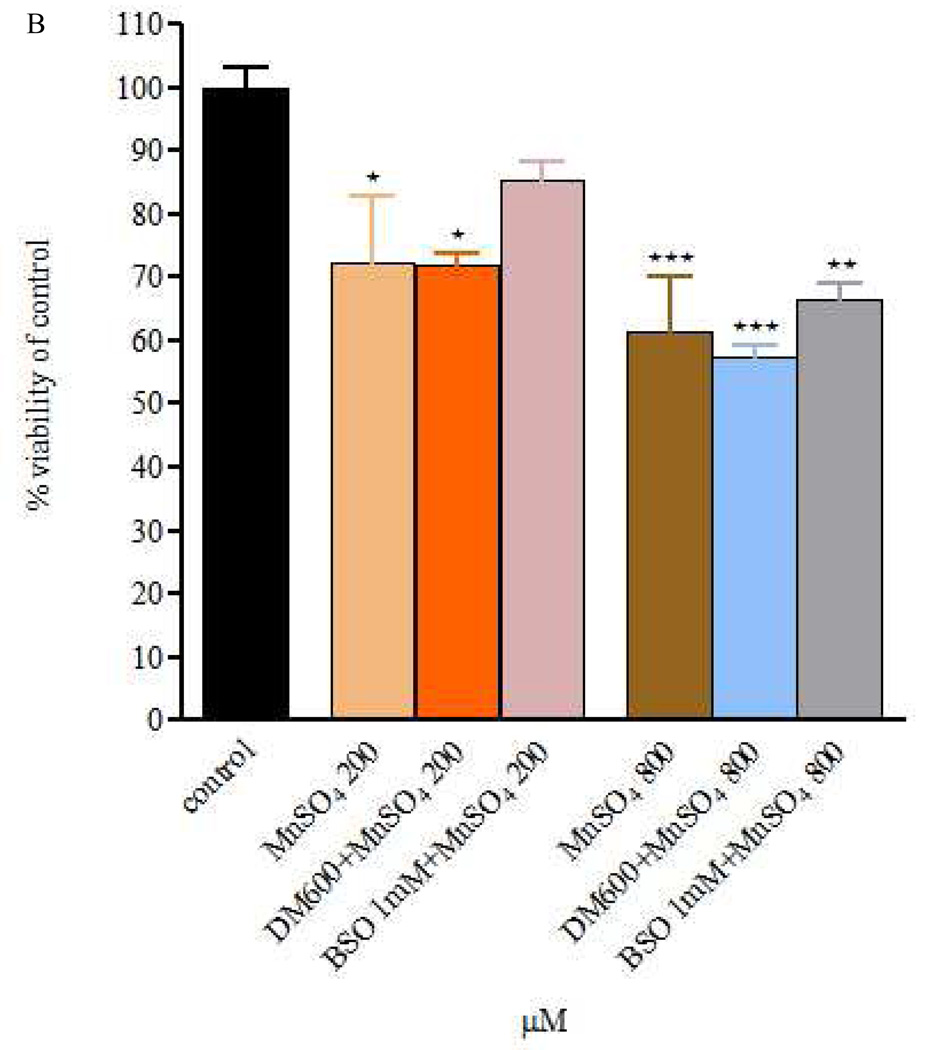
Figure 1.
Figure 1 A and B – Viability of RBE4 cells exposed to MnCl2 or MnSO4 during 4h (200 and 800 µM), after a 24h DEM 600 µM and BSO 1mM treatment. Values are expressed as means ± SEM from 3 independent experiments. * p< 0.05, ** p< 0.01, *** p< 0.001 compared to control; There are no statistical significant differences among the treatment groups.
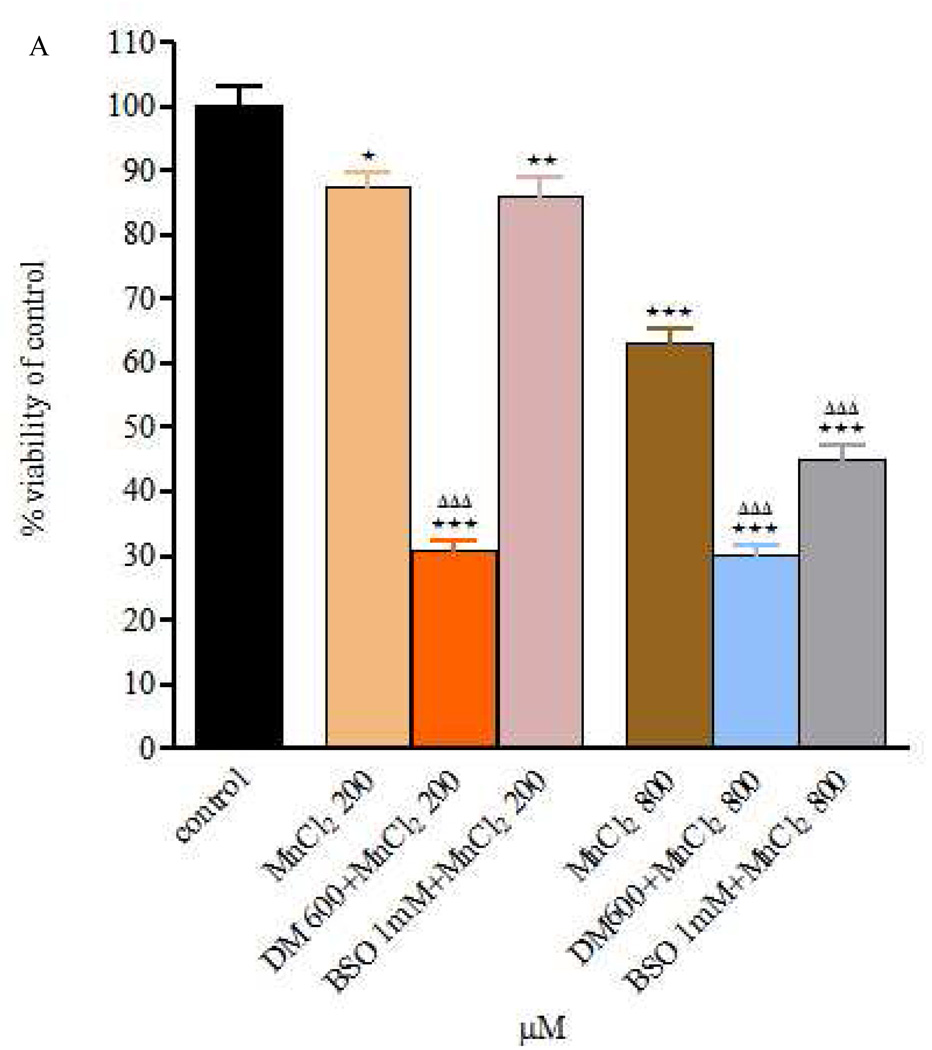
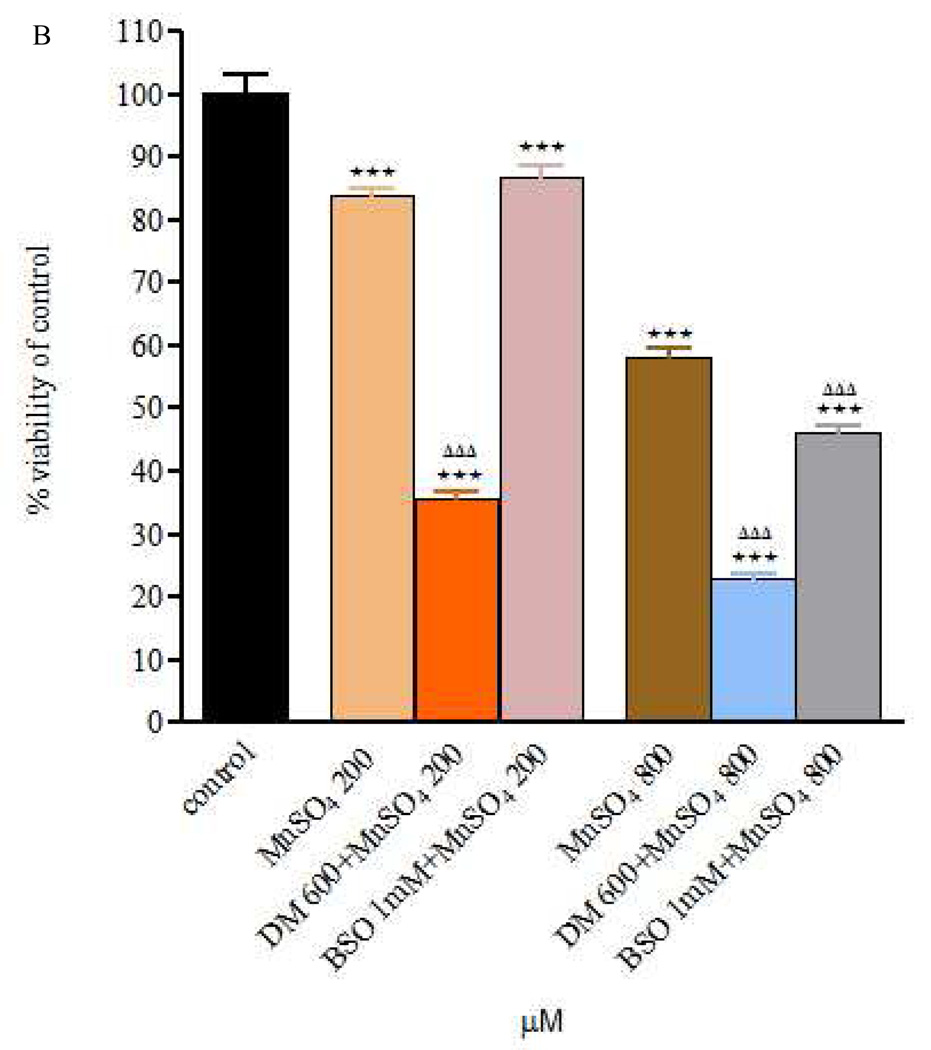
Figure 2.
Figure 2 A and B – Viability of RBE4 cells exposed to MnCl2 or MnSO4 during 24h (200 and 800 µM), after a 24h DEM 600 µM and BSO 1mM treatment. Values are expressed as means ± SEM from 3 independent experiments. * p< 0.05, ** p< 0.01, *** p< 0.001 compared to control; ΔΔΔ p< 0.001 compared to Mn 200 in Mn 200 µM group or Mn 800 in Mn 800 µM group, respectively.
The effect of MnCl2 or MnSO4 (200 and 800 µM) was also evaluated after 24h pre-treatment with DEM (600 µM) or BSO (1mM). As shown in Fig. 1A, in cells exposed to MnCl2 for 4 h immediately after DEM or BSO, cell viability was indistinguishable from cells exposed to MnCl2 (200 or 800 µM). Cells exposed under similar conditions to MnSO4 (Fig. 1B) responded in a manner analogous to cells exposed to MnCl2.
As shown in Fig. 2A, RBE4 cell viability was significantly reduced (p<0.001) when exposure to Mn (200 or 800µM) was extended to 24h pre-treatment with DEM (compared with controls and MnCl2 alone). Similarly, 24h pre-treatment with BSO prior to MnCl2 treatment (24h) was associated with a significant reduction in cell viability (p<0.05) compared with controls, but the effect was limited to the higher MnCl2 concentration (800 µM compared with MnCl2 alone). Similar trends and effects were noted for treatments of RBE4 cells with MnSO4 alone and after DEM or BSO pre-treatment (Fig. 2B).
As discussed above, in initial experiments we used 2 divalent Mn salts, the chloride and the sulphate in order to ascertain whether different physical-chemical properties might influence the extent of cytotoxicity in RBE4 cells (Fig. 1 and Fig. 2). As no significant differences between the two salts were noted in the viability assays, in further experiments we solely focused on the chloride salt. Notably, the chloride salt solution has higher water solubility (vs. the sulphate salt) and it has been more extensively used in experimental Mn models, both in vivo (Weber et al., 2002; Zhang et al., 2003) and in vitro (Rama Rao and Norenberg, 2004; Milatovic et al., 2007; Yin, et al., 2008).
Determination of F2-IsoPs in RBE4 cells exposed to MnCl2
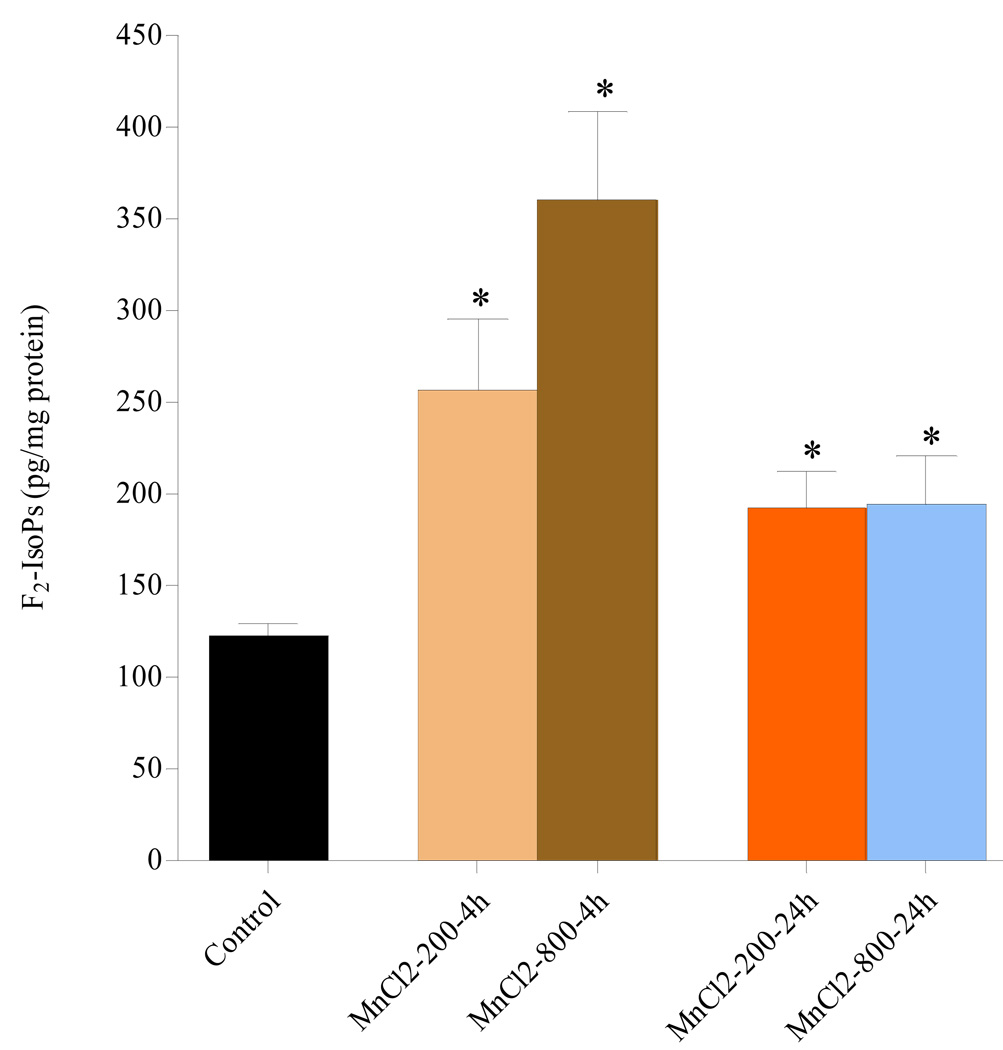
As shown in Fig. 3, in cells exposed for 4h to MnCl2 (200 or 800µM), there was a significant (p<0.05) and concentration-dependent increase in F2-IsoPs levels compared with controls. The effect of MnCl2 on F2-IsoPs was more pronounced after 4h exposure, yet remained elevated and statistically significant vs. controls (p<0.05) also after 24h exposure to MnCl2.
Figure 3.
Quantitation of F2-IsoPs levels in RBE4 cells exposed to MnCl2 200 and 800 µM for either 4 or 24h. * p<0.05 compared to control.
Measurement of mitochondrial membrane potential
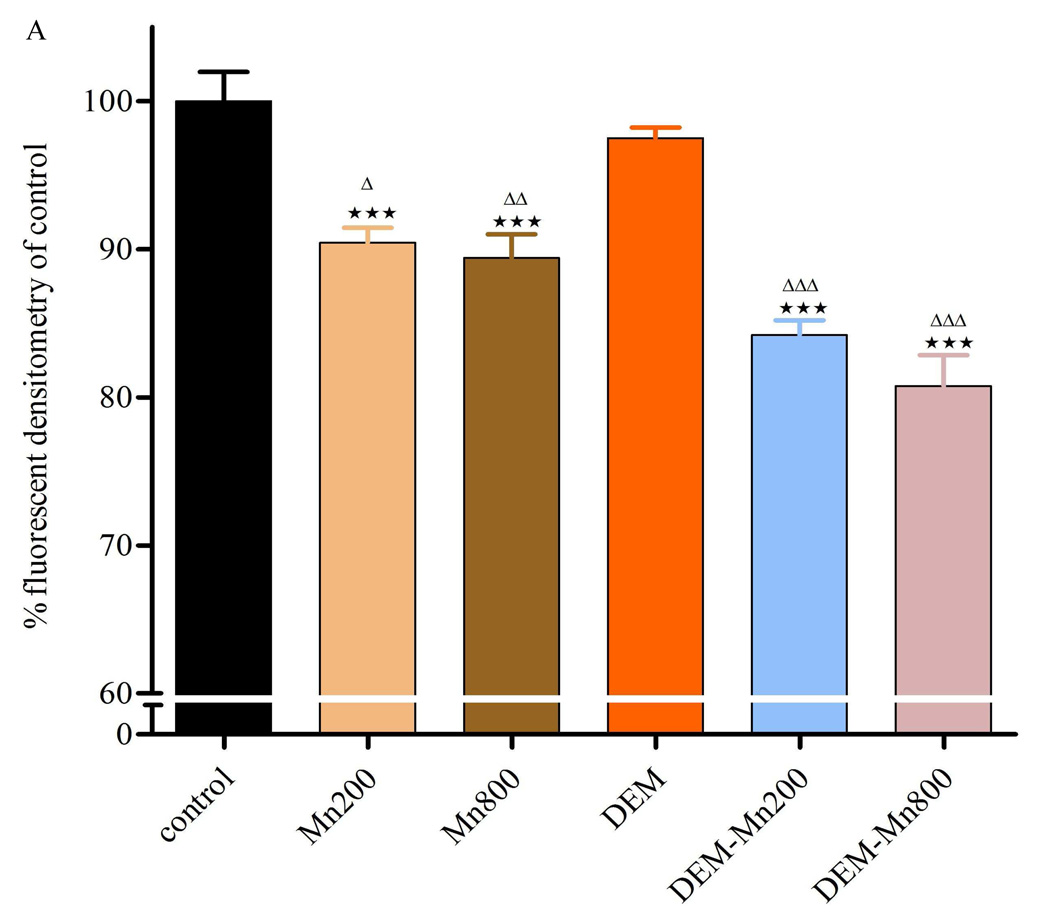
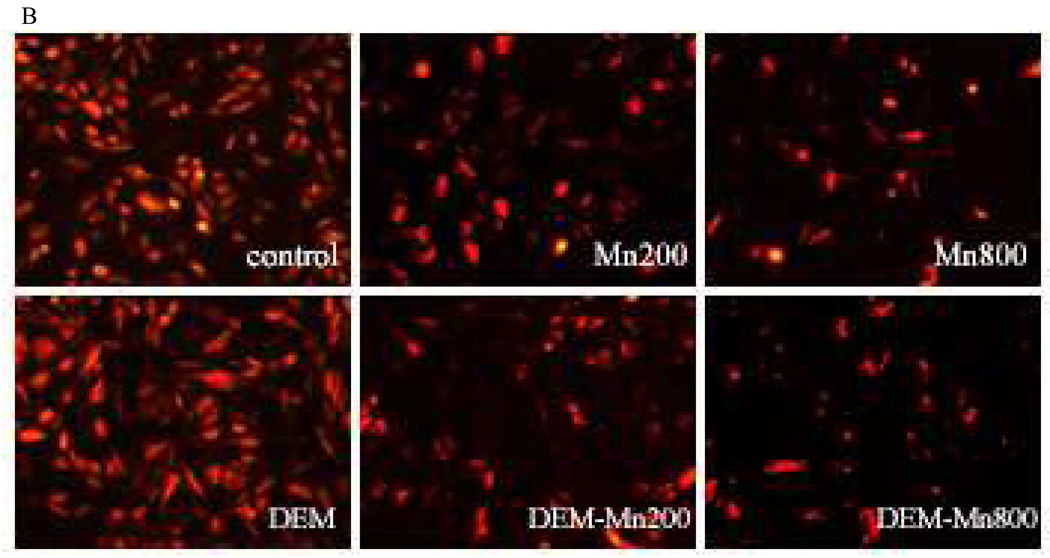
Exposure of RBE4s to MnCl2 (200 or 800µM) for 2h led to a significant decrease (p<0.001) in mitochondrial membrane potential compared with controls (Fig. 4A and B). These results are consistent with those obtained on cell viability and F2-IsoPs (Fig. 1,Fig. 3, and 5). Further decrease in fluorescence was noted in cells exposed to MnCl2 (p<0.001) plus DEM. As shown in Fig. 4A and B, DEM treatment alone had no effect on TMRE fluorescence in RBE4s when compared with controls. MnCl2, both at 200 or 800 µM led to a significant decrease in TMRE fluorescence when compared with controls. This effect was more pronounced and significant (p<0.001) in RBE4s treated for 2h with MnCl2 plus DEM (at both Mn concentrations).
Figure 4.
Figure 4 A and B – Quantitation of TMRE fluorescent intensities. Cultured RBE4 cells exposed to Mn at various concentrations (200 and 800 µM) with/without DEM for 2h. Values are expressed as mean±SEM of 12 random fields in each group from 3 independent experiments. *** p<0.001 vs. control; Δ p<0.05, ΔΔ p<0.01, ΔΔΔ p<0.001 vs. DEM; ▲▲p<0.01 vs. Mn 200 or Mn 800 µM.
3 - Discussion
Given its essentiality in multiple metabolic functions, Mn homeostasis is vital for the optimal CNS functioning. Accordingly, its transport across the BBB is tightly regulated. However, the potential for endothelially localized Mn to elicit neurotoxicity and modulate barrier function has yet to be reported. The present study extends upon our earlier report (Marreilha dos Santos et al., 2008) on the sensitivity of the BBB to Mn, providing evidence Mn affects endothelial cell redox status and mitochondria function.
The mechanism of Mn toxicity is not fully understood, yet altered bioenergetics and oxidative stress play a critical role in its neurotoxicity (Zheng et al., 1998; Malecki, 2001; Milatovic et al., 2007). Moreover, it was hypothesised that Mn toxicity is associated with mitochondrial dysfunction given its propensity to preferentially accumulate in the mitochondrial matrix (Liccione and Maines, 1988; Brown and Taylor, 1999), reflecting upon its high affinity for the calcium uniporter (Gavin et al., 1990; Gavin et al., 1999). Since cultured RBE4 cells preserve the characteristics of brain endothelial cells offering a unique in vitro BBB model for studying both solute transport and permeability (Rist et al., 1997; Aschner et al., 2002; Roux and Couraud, 2005), we specifically addressed whether Mn-induced endothelial toxicity is associated with compromised redox status.
As shown in Fig. 1 and Fig. 2, our studies establish that Mn chloride and Mn sulphate-induced ROS generation leads to loss of mitochondrial integrity and redox status in RBE4 cells. These effects are concentration-and time-dependent, and are further augmented when intracellular GSH levels are depleted (Fig. 1 and Fig. 2). After 24h of Mn exposure, a trend towards increased toxicity at the higher concentrations (800 µM) was observed for both the Mn chloride and sulphate salts. These results are consistent with earlier observations in other cell types, corroborating that Mn toxicity is dependent upon intracellular GSH levels and that impairment of mitochondrial function is a key mechanism for Mn toxicity (Desole et al., 1997; Stredrick et al., 2004; Dukhande et al., 2006). However, several other mechanisms can be invoked to explain Mn-induced mitochondrial dysfunction. For example, Mn2+ can substitute for Fe2+ in cytochromes of the cellular respiratory chain (with structure similar to haemoglobin; Missy et al., 2000), leading to incomplete reduction of O2 and the formation of free radicals and oxygenated compounds such as O2 − and H2O2. Mn was also shown to directly inhibit enzymes of the mitochondrial ETC (Husain et al., 1976; Singh et al., 1979). It might be expected that this inhibition would also enhance the rate of radical production, thereby aggravating the effects on ETC inhibition. The increase in radical production would be exacerbated by the Mn-induced reduction in GPx and SOD activities (Liccione and Maines, 1988).
Our study also established that BSO and DEM pre-treatment potentiate the effects of both Mn salts on RBE4 cells, with more pronounced effects in DEM pre-treated cells. While BSO inhibits the rate-limiting enzyme of GSH synthesis γ-glutamylcysteinyl synthetase, it does not eliminate the antioxidant activity of this thiol (Meister, 1995). In contrast, DEM is a GSH depletor both in vivo and in vitro, given its reaction with the thiol group of GSH (Plummer et al., 1981; Deneke and Fanburg, 1989; Meister 1991). Furthermore, BSO is more poorly transported across the BBB compared with DEM (Jain et al, 1991). Accordingly, the ability of DEM to exert a greater effect on Mn-induced cellular death is consistent with its increased potency in reducing intracellular GSH levels. Analogous to observations in other cell types, Mn toxicity in RBE4 cells is also dependent on the cellular redox status (Stredrick et al., 2004), corroborating the role of ROS generation as a primary mechanism for Mn-induced toxicity. Concerning the effects of BSO and DEM on cell viability of RBE4s, no significant difference was observed after comparing with control cells (data not shown).
We also directly evaluated the ability of MnCl2 to induce ROS formation in mitochondria of RBE4 cells by measuring lipid peroxidation rates and the collapse of the mitochondrial inner membrane potential. Major mitochondrial targets of ROS are the polyunsaturated fatty acids and protein components of the radical chain reaction (Cadenas and Davies, 2000). To our knowledge, this report is the first to establish increased oxidative stress/injury in RBE4 cells by measuring F2-IsoPs, the most sensitive biomarker of oxidative stress (Kadiiska et al., 2005). Measurements of F2-IsoPs have several advantages over other quantitative markers of oxidative stress. They are chemically stable, and substantially increase in various animal models of oxidant injury (Montuschi et al., 2004). Upon exposure of RBE4s to MnCl2, (200 or 800 µM) F2-IsoPs levels increased peaking at 4h post exposure (107% and 193% increase for 200 and 800 µM, respectively), and declining after 24h (yet remaining significantly elevated, with p<0.05, compared with controls, Fig 3). These results are consistent with observations in astrocytes (Milatovic et al., 2007), where following exposure to low MnCl2 concentration (100 µM), levels of F2-IsoPs were elevated at 2h, but indistinguishable from controls at 6h post-treatment. Corroborative evidence reflective of this pattern is also noted in a rat carbon tetrachloride in vivo model, with F2-IsoPs levels peaking 4h after treatment and remaining significantly elevated albeit lower at 24h (Morrow et al., 1992; Basu, 1999; Morrow and Roberts, 1999; Sodergren et al., 2001; Kadiiska et al., 2005).
The centrality of mitochondria in Mn-induced damage was further evaluated by direct measurements of the mitochondrial membrane potential, representing a common final pathway in many conditions associated with oxidative stress (Keil et al., 2006). Increased ROS formation and a subsequent change in mitochondrial permeability transition reflect a Ca2+-dependent process characterized by the opening of the permeability transition pore in the inner mitochondrial membrane. This process results in increased permeability to protons, ions and solutes (Zoratti and Szabo, 1995; Rama Rao and Norenberg, 2004), leading to collapse of the mitochondrial inner membrane potential. In turn, colloid osmotic swelling ensues with movement of metabolites across the inner membrane, defective oxidative phosphorylation, cessation of ATP synthesis and further generation of ROS, thus unabatedly feeding forward a vicious cycle. Fig. 4 A and B show a significant decrease in the mitochondrial membrane potential after exposure of RBE4s to MnCl2 (200 and 800 µM) for 2h vs. control cells. This effect is more pronounced in RBE4 cells treated with MnCl2 after GSH depletion (co-exposure with DEM at both concentrations).
Mn exposure also leads to a concentration-dependent decrease in TMRE fluorescence indicative of mitochondrial membrane potential loss. The mechanisms mediating Mn-induced opening of the mitochondrial permeability transition pore have yet to be defined. However, increased production of ROS and associated oxidative stress are generally considered major contributing factors. Other studies in astrocytes, cerebellar granule neurons and PC12 cells (Seyfried et al., 1999; Wullner et al., 1999; Rama Rao and Norenberg, 2004; Milatovic et al., 2007; Yin et al., 2008;) have shown that depletion of mitochondrial and cytoplasmic GSH result in increased generation of ROS, disruption of the mitochondrial membrane potential and rapid loss of mitochondrial function. In addition, Zhang et al. (2004), showed that high levels of MnCl2 (1mM) caused a significant dissipation of the mitochondrial membrane potential in isolated rat brain mitochondria consistent with alteration of the mitochondria permeability transition. Notably, an association between defects in mitochondrial energy production and neurodegenerative diseases is well established (Wallace et al., 1992; Blass and Gibson, 1999; Castellani et al., 2002; Ogawa et al., 2002) and these deficits are associated with ROS damage to the mitochondrial genome. Given that many metabolically active cell types, particularly tonically active motor neurons such as those in the substantia nigra and globus pallidus, two areas with propensity to accumulate Mn as well as endothelial cells require high levels of ATP to optimally function and survive (Klockgether et al., 1989) the effects of Mn would render these areas and cells, respectively, especially susceptible to ROS.
In summary, we extended our previous observations, showing that endothelial cells are a target of Mn-induced cytotoxicity and mitochondrial dysfunction mediates its toxic effect. In this study, we investigated oxidative stress as a possible mechanism for Mn toxicity, addressing the relationship between Mn cytotoxicity and biomarkers of oxidative stress namely GSH levels, F2-Isopts and mitochondrial membrane potential. Decreased cell viability in cells with compromised GSH status (induced by BSO and DEM) along with the increase in F2-Isopts (a marker of lipid peroxidation), corroborate the role of ROS generation as a key mediator of Mn toxicity in RBE4 cells. In addition, the centrality of mitochondria in Mn-induced damage was further evaluated by direct measurements of the mitochondrial membrane potential, representing a common final pathway in many conditions associated with oxidative stress. We suggest that direct Mn-induced injury to mitochondria and the ensuing impairment in their energy metabolism can modify the properties of the BBB and lead to changes in its permeability, which may facilitate the entrance into the brain parenchyma of endogenous and exogenous macro and micro-molecules resulting in brain toxicity. Future focus on direct measurements of BBB permeability upon Mn exposure should be pursued to determine whether injury to the BBB is associated with changes in nutrient and macromolecular transport from blood to brain.
4 - Experimental Procedure
Chemicals
Manganese chloride (MnCl2.H2O), manganese sulphate (MnSO4.H2O), buthionine sulfoximine (BSO), dimethyl maleate (DEM) and thyazolyl blue tetrazolium bromide (MTT) were purchased from Sigma (St. Louis, MO); heat inactivated fetal bovine serum (FBS), penicillin/streptomycin, alpha medium, F-10 Nutrient Mixture, penicillin/streptomycin and basic fibroblast growth factor from Gibco/Invitrogen, geneticin (G418) were purchased from Boehring Mannheim (Indianapolis, IN) and tetramethylrhodamineethylester (TMRE) was purchased from Invitrogen (Carlsbad, CA).
RBE4 cell culture and treatment
RBE4 cells were grown in T-75 collagen I-coated flasks until confluent. Cells were bathed in alpha-type medium containing 50% (v/v) alpha minimum essential medium (MEM), with 50% F-10 Nutrient Mixture supplemented with penicillin/streptomycin (100 U/ml), 10% heat-inactivated fetal bovine serum, 300 µg /ml geneticin (G418) and basic fibroblast growth factor (1 ng/ml). The cultures were maintained in a humidified 37°C, 5% CO2 air incubator. The medium was changed every 2 days. Upon confluence, the cells were transferred to 96-well collagen-I coated plates where they were assayed. The experiments were conducted at confluence with cell densities between 105 and 106 cells per well.
Exposure protocol of RBE4 cells to MnCl2 or MnSO4 and MTT assay
Confluent 96-well plates of RBE4s were treated with 1000 µM BSO or 600 µM DEM for 24h, and then exposed to MnCl2 or MnSO4 solutions (200 and 800 µM) for 4 or 24h (in fresh medium absent BSO or DEM). Other cells were treated with Mn salts for 4 or 24h in the absence of BSO or DEM treatments. After the exposures, viability was evaluated by the MTT assay according to the manufacturer´s protocol (Sigma). After the various experimental treatments, and at the end of the respective incubation periods, 100 µl of MTT solution was added to each well, followed by incubation of the plates for 4h at 37°C in a CO2 incubator. The reaction was terminated by removal of the media, and 20 minutes shaking at 70 rpm/min addition after 100 µl of HCl/isopropanol to each well. Levels of reduced MTT were determined by measuring the absorbance at 570 nm using a plate reader (Zenith 3100). Each experiment was independently repeated 3 times with a minimum of 3 plates/experiment.
Determination of F2-IsoPs
Cells were plated in 6-well collagen-I coated plates. At confluence, the cells were treated with MnCl2 and MnSO4 (200 and 800 µM), for 4 or 24h. Next, cells were resuspended in 0.5 ml of methanol containing 0.005% butylated hydroxytoluene, sonicated and then subjected to chemical saponification using 15% KOH to hydrolyze bound F2-IsoPs. The cell lysates were adjusted to a pH of 3, followed by the addition of 0.1 ng of 15-F2α-IsoPs-d4 internal standard. F2-IsoPs were subsequently purified by C18 and silica Sep-Pak extraction by thin layer chromatography. Next, pentafluorobenzyl ester, a trimethylsilyl derivative was added and the cells were analyzed by gas chromatography, negative ion chemical ionization-mass spectrometry. Gas chromatography (GC) was performed using a 15 m, 0.25 mm diameter, 0.25 µm film thickness, DB1701 fused silica capillary column. The column temperature was programmed from 200 to 300°C at 20°C/min after the first minute. Methane was used as the carrier gas at a flow rate of 1 ml/min. The ion source temperature was maintained at 250°C, electron energy at 70 eV, and filament current at 0.25 mA. Negative ion chemical ionization (NICI) mass spectrometry (MS) was performed with a Hewlett Packard HP5989A GC/MS instrument interfaced with an IBM Pentium III computer system (Morrow and Roberts, 1999). Protein content of RBE4 cells was measured by the method of Bradford (1976) with a Bio-Rad Protein Assay kit (Bio-Rad Lab, Richmond, CA). Bovine serum albumin was used as the standard. Each experiment was performed in duplicates and independently repeated three times.
Evaluation of mitochondrial membrane potential
The mitochondrial membrane potential was measured with the fluorescent dye TMRE (Patenaude et al., 2004). At confluence, RBE4 cells grown in 6-well collagen coated plates were treated with MnCl2 (200 and 800 µM) alone and MnCl2 (same concentrations) plus DEM, for 2 h. Next, the culture medium was removed and the cells were loaded for 30 min with TMRE at a final concentration of 50 nM (in HEPES buffer at 37°C in a 5% CO2 incubator). At the end of treatment, cells were rinsed with PBS and examined with a Zeiss inverted fluorescent microscope (Zeiss Axiovert S100, Carl Zeiss MicroImaging Inc.) equipped with a cooled digital camera (Photometrics CoolSNAP, Roper Scientific Photometrics, Tucson, AZ). Images were captured at 10× magnification with a digital camera. Fluorescent intensities were calculated in 8 randomly selected fields per experiment and were analysed with the NIH software (Scion Incorporation, Frederick, MD). In each image field, the total number of pixels was quantified on a gray scale (0255 counts) and the mean pixel value in each image field was obtained and expressed as means ± SEM. The fluorescent intensities were expressed as percent fluorescence change over control. Each experiment was performed in duplicate plates and independently repeated three times.
Statistical Analysis
All data from the experiments were presented as mean ± SEM of three to four independent experiments. The data were analysed by one-way analysis of variance (ANOVA), followed by Bonferroni multiple comparison test with the statistical significance set at p<0.05. The Bonferroni test was used to correct for multiple comparisons and assure the strictest analyses. All analyses were carried out using Graph Pad Prism 4.02 for Windows (Graph Pad Software, San Diego, CA, USA).
Acknowledgements
The authors gratefully acknowledge support by National Institute of Environmental Health and Safety (NIEHS) 10563 (MA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott PJ. Methylcyclopentadienyl manganese tricarbonyl (MMT) in petrol: the toxicological issues. Sci. Total Environ. 1987;67:247–255. doi: 10.1016/0048-9697(87)90215-4. [DOI] [PubMed] [Google Scholar]
- Abbott NJ, Couraud PO, Roux F. Studies on an immortalized brain endothelial cell line: characterization permeability and transport. In: Greenwood J, Begley DJ, Segal MB, editors. New concepts of a blood-brain barrier. New York: Plenum Press; 1995. pp. 239–249. [Google Scholar]
- Ali SF, Duhart HM, Newport GD, Lipe GW, Slikker W. Manganese-induced reactive oxygen species: comparison between Mn+2 and Mn+3. Neurodegeneration. 1995;4(3):329–334. doi: 10.1016/1055-8330(95)90023-3. [DOI] [PubMed] [Google Scholar]
- Aschner M, Gannon M. Manganese (Mn) transport across the rat blood-brain barrier: Saturable and transferring-dependent transport mechanisms. Brain Res. Bull. 1994;33:345–349. doi: 10.1016/0361-9230(94)90204-6. [DOI] [PubMed] [Google Scholar]
- Aschner M, Vrana KE, Zheng W. Manganese uptake and distribution in the central nervous system (CNS) Neurotoxicology. 1999;20:173–180. [PubMed] [Google Scholar]
- Aschner M, Shanker G, Erikson K, Yang J, Mutkus L. The uptake of manganese in brain endothelial cultures. Neurotoxicology. 2002;23:165–168. doi: 10.1016/s0161-813x(02)00056-6. [DOI] [PubMed] [Google Scholar]
- Au C, Benedetto A, Aschner M. Manganese transport in eukaryotes: The role of DMT-1. Neurotoxicology. 2008;29:569–576. doi: 10.1016/j.neuro.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu S. Oxidative injury induced cyclooxygenase activation in experimental hepatotoxicity. Biochem. Biophys. Res. Commun. 1999;254(3):764–767. doi: 10.1006/bbrc.1998.9956. [DOI] [PubMed] [Google Scholar]
- Basu S. Isoprostanes: Novel bioactive products of lipid peroxidation. Free Rad. Res. 2004;38(2):105–122. doi: 10.1080/10715760310001646895. [DOI] [PubMed] [Google Scholar]
- Bendayan R, Lee G, Bendayan M. Functional expression and localization of p-glycoprotein at the blood-brain barrier. Microscopic Res. Tech. 2002;57:365–380. doi: 10.1002/jemt.10090. [DOI] [PubMed] [Google Scholar]
- Blass GP, Gibson GE. Cerebrometabolic aspects of delirium in relationship to dementia. Dement. Geriatr. Cogn. Disord. 1999;10:335–338. doi: 10.1159/000017165. [DOI] [PubMed] [Google Scholar]
- Bradbury MWB. Trace metal transport at the blood brain barrier. In: Brad MWB, editor. Physiology and Pharmacology of the blood brain barrier. Handbook for Experimental Pharmacology. vol. 103. Berlin: Springer; 1992. pp. 263–278. [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brown S, Taylor NL. Could mitochondrial dysfunction play a role in manganese toxicity? Environ. Toxicol. Pharmacol. 1999;7:49–57. doi: 10.1016/s1382-6689(98)00054-4. [DOI] [PubMed] [Google Scholar]
- Brust P, Friedrich A, Krizbai IA, Bergmann R, Roux F, Ganapathy V, Johansen B. Functional expression of the serotonin transporter in immortalized rat brain microvessel endothelial cells. J. Neurochem. 2000;74(3):1241–1248. doi: 10.1046/j.1471-4159.2000.741241.x. [DOI] [PubMed] [Google Scholar]
- Cadenas E, Davies KJA. Mitochondrial free radical generation, oxidative stress and aging. Free Radic. Biol. Med. 2000;29:222–230. doi: 10.1016/s0891-5849(00)00317-8. [DOI] [PubMed] [Google Scholar]
- Castellani R, Hirai K, Aliev G, Drew KL, Nunomura A, Takeda A. Role of mitochondrial dysfunction in Alzheimer´s disease. J. Neurosci. Res. 2002;70:357–360. doi: 10.1002/jnr.10389. [DOI] [PubMed] [Google Scholar]
- Chapple ILC. Reactive oxygen species and antioxidants in inflammatory diseases. J. Clin. Period. 1997;24:287–296. doi: 10.1111/j.1600-051x.1997.tb00760.x. [DOI] [PubMed] [Google Scholar]
- Chia SE, Foo SC, Gan SL, Jeyaratnam J, Tian CS. Neurobehavioural functions among workers exposed to manganese ore. Scand. J. Work. 1993;19:264–270. doi: 10.5271/sjweh.1475. [DOI] [PubMed] [Google Scholar]
- Clark JB, Lai JCK. Glycolytic, tricarboxylic acid cycle and related enzymes in brain. In: Boulton AA, Baker GB, Butterworth RF, editors. NeuroMethods. Vol. 11. Clifton, NY: Humana; 1989. pp. 233–281. [Google Scholar]
- Cook DG, Fhan S, Brait KA. Chronic manganese intoxication. Arch. Neurol. 1974;30:59–74. doi: 10.1001/archneur.1974.00490310061010. [DOI] [PubMed] [Google Scholar]
- Davies SS, Amarnath V, Brame CJ, Boutaud O, Roberts LJ. Measurement of chronic oxidative and inflammatory stress by quantification of isoketal/levuglandin gamma-ketoaldehyde protein adducts using liquid chromatography tandem mass spectrometry. Nat. Protoc. 2007;2(9):2079–2091. doi: 10.1038/nprot.2007.298. [DOI] [PubMed] [Google Scholar]
- Davson H, Segal MB. Physiology of the CSF and blood-brain barriers. New York: CRC Press; 1996. [Google Scholar]
- Deneke SM, Fanburg BL. Regulation of cellular glutathione. Am. J. Physiol. Lung Cell. Mol. Physiol. 1989;257:L163–L173. doi: 10.1152/ajplung.1989.257.4.L163. [DOI] [PubMed] [Google Scholar]
- Desole MS, Sciola L, Delogu MR, Sircana S, Migheli R, Miele E. Role of oxidative stress in the manganese and 1-methyl-4-(2´-ethylphenyl)-1,2,3,6-tetrahydropyridine-induced apoptosis in PC12 cells. J. Neurochem. 1997;31:169–176. doi: 10.1016/s0197-0186(96)00146-5. [DOI] [PubMed] [Google Scholar]
- Diemer T, Allen JA, Hales KH, Hales DB. Reactive oxygen disrupts mitochondria in MA-10 tumor Leydig cells and inhibits steroidogenic acute regulatory (StAR) protein and steroidogenesis. Endocrinology. 2003;144:2882–2891. doi: 10.1210/en.2002-0090. [DOI] [PubMed] [Google Scholar]
- Dobson AW, Erikson KM, Aschner M. Manganese neurotoxicity. Ann. N. Y. Acad. Sci. 2004;1012:115–128. doi: 10.1196/annals.1306.009. [DOI] [PubMed] [Google Scholar]
- Dukhande VV, Malthankar-Phatak GH, Hugus JJ, Daniels CK, Lai JCK. Manganese-induced neurotoxicity is differentially enhanced by glutathione depletion in astrocytoma and neuroblastoma cells. Neurochem. Res. 2006;31:1349–1357. doi: 10.1007/s11064-006-9179-7. [DOI] [PubMed] [Google Scholar]
- Erikson KM, Shihabi ZK, Aschner JL, Aschner M. Manganese accumulates in iron deficient rat brain regions in a heterogeneous fashion and is associated with neurochemical alterations. Biol. Trace Elem. Res. 2002;87:143–156. doi: 10.1385/BTER:87:1-3:143. [DOI] [PubMed] [Google Scholar]
- Fitsanakis V, Garcia S, Aschner M. Manganese dynamics, distribution, and neurotoxicity. In: Aschner M, Kimelberg H, editors. The role of glia in neurotoxicity. New York: CRC Press; 2005. [Google Scholar]
- Fitsanakis V, Piccola G, Aschner JL, Aschner M. Characteristics of manganese (Mn) transport in rat brain endothelial (RBE4) cells, an in vitro model of the blood-brain barrier. Neurotoxicology. 2006;27:60–70. doi: 10.1016/j.neuro.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Friedrich A, George R, Bridges C, Prasad P, Ganapathy V. Transport of choline and its relationship to the expression of the organic cation transporters in a rat brain microvessel endothelial cell line (RBE4) Biochim. Biophys. Acta. 2001;1512:299–307. doi: 10.1016/s0005-2736(01)00333-9. [DOI] [PubMed] [Google Scholar]
- Gavin CE, Gunter KK, Gunter TE. Manganese and calcium efflux kinetics in brain mitochondria. Relevance to manganese toxicity. Biochem. J. 1990;266:329–334. doi: 10.1042/bj2660329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavin CE, Gunter KK, Gunter TE. Manganese sequestration by mitochondria and inhibition of oxidative phosphorylation. Toxicol. Appl. Pharmacol. 1992;115:1–5. doi: 10.1016/0041-008x(92)90360-5. [DOI] [PubMed] [Google Scholar]
- Gavin CE, Gunter KK, Gunter TE. Manganese and calcium transport in mitochondria: implications for manganese toxicity. Neurotoxicology. 1999;20(2–3):445–453. [PubMed] [Google Scholar]
- Gunter TE, Gavin CE, Aschner M, Gunter KK. Speciation of manganese in cells and mitochondria: a search for the proximal cause of manganese neurotoxicity. Neurotoxicology. 2006;27(5):765–776. doi: 10.1016/j.neuro.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Haliwell B, Gutteridge JMC. Free radicals in biology and medicine. 3rd ed. Oxford University Press; 2005. [Google Scholar]
- HaMai D, Bondy SC. Oxidative basis of manganese neurotoxicity. Ann. N.Y. Acad. Sci. 2004;1012:129–141. doi: 10.1196/annals.1306.010. [DOI] [PubMed] [Google Scholar]
- Hurley LS, Keen CL. Manganese. In: Mertz W, editor. Trace elements in human and animal nutrition. 5th ed. Vol. I. New York, NY: Academic Press; 1987. pp. 185–223. [Google Scholar]
- Husain R, Seth PK, Chandra SV. Early inhibition of succinic dehydrogenase by manganese in rat gonads. Bull Environ. Contam. Toxicol. 1976;16(1):118–121. doi: 10.1007/BF01753116. [DOI] [PubMed] [Google Scholar]
- Huwyler J, Froidevaux S, Roux F, Eberle AN. Characterization of transferring receptor in an immortalized cell line of rat brain endothelial cells, RBE4. J. Recep. Sig. Transd. Res. 1999;19:729–739. doi: 10.3109/10799899909036683. [DOI] [PubMed] [Google Scholar]
- Jacobson J, Duchen MR. Mitochondrial oxidative stress and cell death in astrocytes – requirement for stored Ca2+ and sustained opening of the permeability transition pore. J. Cell Sci. 2002;115:1175–1188. doi: 10.1242/jcs.115.6.1175. [DOI] [PubMed] [Google Scholar]
- Jain A, Martensson J, Stole E, Auld PAM, Meister A. Glutathione deficiency leads to mitochondrial damage in brain. Proc. Natl. Acad. Sci. USA. 1991;88:1913–1917. doi: 10.1073/pnas.88.5.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadiiska MB, Gladen BC, Baird DD, Germolec D, Graham LB, Parker CE, Nyska A, Wachsman JT, Ames BN, Basu S, Brot N, FitzGerald GA, Floyd RA, George M, Heinecke JW, Hatch GE, Hensley K, Lawson JA, Marnett LJ, Morrow JD, Murray DM, Plastaras J, Roberts LJ, II, Rokach J, Shigenaga MK, Sohal RS, Sun J, Tice RR, Van Thiel DH, Wellner D, Walter PB, Tomer KB, Mason RP, Barrett JC. Biomarkers of oxidative stress, study II. Are oxidation products of lipids, proteins, and DNA markers of CCl4 poisoning? Free Radical Biol. Med. 2005;38:698–710. doi: 10.1016/j.freeradbiomed.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Keil U, Scherping I, Hauptmann S, Schuessel K, Eckert A, Muller WE. Piracetam improves mitochondrial dysfunction following oxidative stress. Br. J. Pharmacol. 2006;147(2):199–208. doi: 10.1038/sj.bjp.0706459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klockgether T, Turski L. Excitatory amino acids and the basal ganglia: implications for the therapy of Parkinson´s disease. Trends Neurosci. 1989;12:285–286. doi: 10.1016/0166-2236(89)90007-6. [DOI] [PubMed] [Google Scholar]
- Lai JCK, Minski MJ, Chan AWK, Leung TKC, Lim L. Manganese mineral interactions in brain. Neurotoxicology. 1999;20:433–444. [PubMed] [Google Scholar]
- Liccione JJ, Maines MD. Selective vulnerability of glutathione metabolism and cellular defense mechanisms in rat striatum to manganese. J. Pharm. Exp.Ther. 1988;247:156–161. [PubMed] [Google Scholar]
- Malecki E. Manganese toxicity is associated with mitochondrial dysfunction and DNA fragmentation in rat primary striatal neurons. Brain Res. Bull. 2001;55:225–228. doi: 10.1016/s0361-9230(01)00456-7. [DOI] [PubMed] [Google Scholar]
- Malthankar GV, White B, Bhushan A, Daniels CK, Rodnick KJ, James CK, Lai JCK. Differential lowering by Manganese treatment of activities of glycolytic and tricarboxylic acid (TCA) cycle enzymes investigated in neuroblastoma and astrocytoma cells is associated with Manganese-induced cell death. Neurochem Res. 2004;29:709–717. doi: 10.1023/b:nere.0000018841.98399.ce. [DOI] [PubMed] [Google Scholar]
- Marreilha dos Santos AP, Santos D, Au C, Milatovic D, Aschner M, Batoreu MCC. Antioxidants prevent the cytotoxicity of manganese in RBE4 cells. Brain Res. 2008;1236:200–205. doi: 10.1016/j.brainres.2008.07.125. [DOI] [PubMed] [Google Scholar]
- Meister A. Glutathione deficiency produced by inhibition of its synthesis, and its reversal; applications in research and therapy. Pharmacol. Therap. 1991;51:155–194. doi: 10.1016/0163-7258(91)90076-x. [DOI] [PubMed] [Google Scholar]
- Meister A. Mitochondrial changes associated with glutathione deficiency. Biochim. Biophys. Acta. 1995;1271:35–42. doi: 10.1016/0925-4439(95)00007-q. [DOI] [PubMed] [Google Scholar]
- Mena I, Marin O, Fuenzalida S, Cotzias GC. Chronic manganese poisoning, clinical picture and manganese turnover. Neurology. 1967;17:128–136. doi: 10.1212/wnl.17.2.128. [DOI] [PubMed] [Google Scholar]
- Milatovic D, Yin Z, Gupta RC, Sidoryk M, Albrecht J, Aschner JL, Aschner M. Manganese induces oxidative impairment in cultured rat astrocytes. Toxicol. Sci. 2007;98(1):198–205. doi: 10.1093/toxsci/kfm095. [DOI] [PubMed] [Google Scholar]
- Missy P, Joyeux M, Lanhers MC, Cunat L, Burnel D. Effects of subchronic exposure to manganese chloride on tissue distribution of three essential elements in rats. Int. J. Toxicol. 2000;19:313–321. [Google Scholar]
- Montuschi P, Barnes PJ, Roberts LJ. Isoprostanes: markers and mediators of oxidative stress. FASEB J. 2004;18:1791–1800. doi: 10.1096/fj.04-2330rev. [DOI] [PubMed] [Google Scholar]
- Morrow JD, Hill KE, Burk RF, Nammour TM, Badr KF, Roberts LJ. A series of prostaglandin F2-like compounds are produced in vivo in humans by a non-cyclooxygenase, free radical-catalyzed mechanism. Proc. Natl. Acad. Sci.USA. 1990;87:9383–9387. doi: 10.1073/pnas.87.23.9383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow JD, Awad JA, Boss HJ, Blair IA, Roberts LJ. Non-cyclooxygenase-derived prostanoids (F2-isoprostanes) are formed in situ on phospholipids. Proc. Natl. Acad. Sci. USA. 1992;89:10721–10725. doi: 10.1073/pnas.89.22.10721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow JD, Roberts LJ. Mass spectrometric quantification of F2-isoprostanes in biological fluids and tissues as measure of oxidant stress. Methods Enzymology. 1999;300:3–12. doi: 10.1016/s0076-6879(99)00106-8. [DOI] [PubMed] [Google Scholar]
- Moser VC, Aschner M, Richardson RJ, Philbert MA. Toxic Responses of the Nervous System. Casarett and Doull´s, Toxicology, The Basic Science of Poisons. 2007. 2007:631–664. [Google Scholar]
- Myers JE, Thompson ML, Ramushu S, Young T, Jeebhay MF, London L, et al. The nervous system effects of occupational exposure on workers in a South African manganese smelter. Neurotoxicology. 2003;24:885–894. doi: 10.1016/S0161-813X(03)00081-0. [DOI] [PubMed] [Google Scholar]
- Mytilineou C. Glutathione depletion and oxidative stress. Parkinsonism & Related Disorders. 2002;8:385–387. doi: 10.1016/s1353-8020(02)00018-4. [DOI] [PubMed] [Google Scholar]
- Ogawa O, Zhu X, Perry G, Smith MA. Mitochondrial abnormalities and oxidative imbalance in neurodegenerative diseases. Sci. Aging Knowl. Environ. 2002;41:16. doi: 10.1126/sageke.2002.41.pe16. [DOI] [PubMed] [Google Scholar]
- Pal KPA, Samii A, Calne DB. Manganese neurotoxicity: A review of clinical features, imaging and pathology. Neurotoxicology. 1999;20:227–238. [PubMed] [Google Scholar]
- Patenaude A, Ven Murthy MR, Mirault ME. Mitochondrial thioredoxin system: effects of TrxR2 overexpression on redox balance, cell growth, and apoptosis. J. Biol. Chem. 2004;279(26):27302–27314. doi: 10.1074/jbc.M402496200. [DOI] [PubMed] [Google Scholar]
- Plummer JL, Smith BR, Sies H, Bend JR. Chemical depletion of glutathione in vivo. Methods Enzymol. 1981;77:50–59. doi: 10.1016/s0076-6879(81)77010-1. [DOI] [PubMed] [Google Scholar]
- Rabin O, Hegedus L, Bourre JM, Smith QR. Rapid brain uptake of manganese(II) across the blood-brain barrier. J. Neurochem. 1993;61:509–517. doi: 10.1111/j.1471-4159.1993.tb02153.x. [DOI] [PubMed] [Google Scholar]
- Rama Rao KV, Norenberg MD. Manganese induces the mitochondrial permeability transition in cultured astrocytes. J. Biol. Chem. 2004;279:32333–32338. doi: 10.1074/jbc.M402096200. [DOI] [PubMed] [Google Scholar]
- Reichel A, Begley DJ, Abbott NJ. Carrier-mediated delivery of metabothropic glutamate receptor ligands to the central nervous system: Structural tolerance and potential of the L-system amino acid transporter at the blood-brain barrier. J. Cereb. Blood Metab. 2000;20:168–174. doi: 10.1097/00004647-200001000-00021. [DOI] [PubMed] [Google Scholar]
- Rist RJ, Romero IA, Chan MWK, Couraud F, Abbott NJ. F-actin cytoskeleton and sucrose permeability of immortalized rat brain microvascular endothelial cell monolayers: effects of cyclic AMP and astrocytic factors. Brain Res. 1997;768:10–18. doi: 10.1016/s0006-8993(97)00586-6. [DOI] [PubMed] [Google Scholar]
- Roux F, Durieu-Trautmann O, Chaverot N, Claire M, Mailly P, Bourre JM. Regulation of gamma-glutamyl transpeptidase and alkaline phosphatise activities in immortalized rat brain microvessel endothelial cells. J. Cell Physiol. 1994;159:101–113. doi: 10.1002/jcp.1041590114. [DOI] [PubMed] [Google Scholar]
- Roux F, Couraud PO. Rat brain endothelial cell lines for the study of blood-brain barrier permeability and transport functions. Cell. Mol. Neurobiol. 2005;25:41–58. doi: 10.1007/s10571-004-1376-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin LL, Staddon JM. The cell biology of the blood-brain barrier. Annu. Rev. Neurosci. 1999;22:11–28. doi: 10.1146/annurev.neuro.22.1.11. [DOI] [PubMed] [Google Scholar]
- Sampaio-Maia B, Serrao M, Soares da Silva P. Regulatory pathways and uptake of 1-DOPA by capillary cerebral endothelial cells, astrocytes, and neuronal cells. Am. J. Physiol. Cell Physiol. 2001;280:C333–C342. doi: 10.1152/ajpcell.2001.280.2.C333. [DOI] [PubMed] [Google Scholar]
- Sayre LM, Perry G, Smith MA. Oxidative stress and neurotoxicity. Chem. Res. Toxicol. 2008;21:172–188. doi: 10.1021/tx700210j. [DOI] [PubMed] [Google Scholar]
- Schulz JB, Lindenau J, Seyfried J, Dichgans J. Glutathione, oxidative stress and neurodegeneration. Eur. J. Biochem. 2000;267:4904–4811. doi: 10.1046/j.1432-1327.2000.01595.x. [DOI] [PubMed] [Google Scholar]
- Seyfried J, Soldner F, Schulz JB, Klockgether T, Kovar KA, Wullner U. Differential effects of L-buthionine sulfoximine and ethacrynic acid on glutathione levels and mitochondrial function in PC12 cells. Neurosci. Letters. 1999;264:1–4. doi: 10.1016/s0304-3940(99)00107-x. [DOI] [PubMed] [Google Scholar]
- Sierra P, Loranger S, Kennedy G, Zayed J. Occupational and environmental exposure of automobile mechanics and nonautomotive workers to airborne manganese arising from the combustion of methyl cyclopentadienyl manganese tricarbonyl (MMT) Am. Ind. Hyg. Assoc.J. 1995;56:713–716. doi: 10.1080/15428119591016746. [DOI] [PubMed] [Google Scholar]
- Singh S, Shukla GS, Srivastava RS, Chandra SV. The interaction between ethanol and manganese in rat brain. Arch. Toxicol. 1979;41:307–316. doi: 10.1007/BF00296901. [DOI] [PubMed] [Google Scholar]
- Sloot WN, Korf J, Koster JF, DeWitt LEA, Gramsbergen JBP. Manganese-induced hydroxyl radical formation in rat striatum is not attenuated by dopamine depletion or iron chelation in vivo. Exp. Neurol. 1996;138:236–245. doi: 10.1006/exnr.1996.0062. [DOI] [PubMed] [Google Scholar]
- Stredrick DL, Stokes AH, Travis JW, Willard MF, Johnson EA, Lash LH, Aschner M, Vrana KE. Manganese-induced cytotoxicity in dopamine-producing cells. Neurotoxicology. 2004;25:543–553. doi: 10.1016/j.neuro.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Sodergren E, Cederberg J, Vessby B, Basu S. Vitamin E reduces lipid peroxidation in experimental hepatotoxicity in rats. Eur. J. Nutr. 2001;40:10–16. doi: 10.1007/pl00007381. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Lieben J. Manganese poisoning and exposure in Pennsylvania. Arch. Environ. Health. 1969;19:674–684. doi: 10.1080/00039896.1969.10666909. [DOI] [PubMed] [Google Scholar]
- Turrens JF, Boveris A. Generation of superoxide anion by the NADH dehydrogenase of bovine heart mitochondria. Biochem. J. 1980;191:421–430. doi: 10.1042/bj1910421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DC, Shoffner JM, Watts RL, Juncos JL, Toroni A. Mitochondrial oxidative phosphorylation defects in Parkinson´s disease. Ann. Neurol. 1992;32:113–114. doi: 10.1002/ana.410320123. [DOI] [PubMed] [Google Scholar]
- Weber S, Dorman DC, Lash LH, Erikson K, Vrana KE, Aschner M. Effects of manganese (Mn) on the developing rat brain: Oxidative-stress related endpoints. Neurotoxicology. 2002;23:169–175. doi: 10.1016/s0161-813x(02)00014-1. [DOI] [PubMed] [Google Scholar]
- Wedler FC. Biological significance of manganese in mammalian systems. In: Ellis PG, Luscombe DK, editors. Progress in Medicinal Chemistry. Vol. 30. 1993. pp. 89–133. [DOI] [PubMed] [Google Scholar]
- Wullner U, Seyfried J, Groscurth J, Beinroth S, Winter S, Gleichmann M, Heneka M, Loschmann P, Schulz JB, Weller M, Klockgether T. Glutathione depletion and neuronal cell death: the role of reactive oxygen intermediates and mitochondrial function. Brain Res. 1999;826:53–62. doi: 10.1016/s0006-8993(99)01228-7. [DOI] [PubMed] [Google Scholar]
- Yang J, Aschner M. Developmental aspects of blood-brain barrier (BBB) and rat brain endothelial (RBE4) cells as in vitro model for studies on chloropyrifos transport. Neurotoxicol. 2003;24:741–745. doi: 10.1016/S0161-813X(03)00025-1. [DOI] [PubMed] [Google Scholar]
- Yin Z, Aschner JL, dos Santos AP, Aschner M. Mitochondrial-dependent manganese neurotoxicity in rat astrocyte cultures. Brain Res. 2008;1203:1–11. doi: 10.1016/j.brainres.2008.01.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Zhou Z, Fu J. Effect of manganese chloride exposure on liver and brain mitochondria function in rats. Environ. Res. 2003;93:149–157. doi: 10.1016/s0013-9351(03)00109-9. [DOI] [PubMed] [Google Scholar]
- Zhang S, Fu J, Zhou Z. In vitro effect of manganese chloride exposure on reactive oxygen species generation and respiratory chain complexes activities of mitochondria isolated from rat brain. Toxicol in Vitro. 2004;18:71–77. doi: 10.1016/j.tiv.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Zheng W, Ren S, Graziano JH. Manganese inhibits mitochondrial aconitase: a mechanism of manganese neurotoxicity. Brain Res. 1998;799:334–342. doi: 10.1016/s0006-8993(98)00481-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoratti M, Szabo I. The mitochondrial permeability transition. Biochim. Biophys. Acta. 1995;1241:139–176. doi: 10.1016/0304-4157(95)00003-a. [DOI] [PubMed] [Google Scholar]