Abstract
G protein-coupled metabotropic glutamate receptors (mGluRs) are expressed in widespread regions of the mammalian brain and are involved in the regulation of a variety of neuronal and synaptic activities. Group I mGluRs (mGluR1 and mGluR5 subtypes) are expressed in striatal medium spiny output neurons and are believed to play an important role in the modulation of cellular responses to dopamine stimulation with psychostimulants. In this study, we investigated the effect of a single dose of the psychostimulant amphetamine on mGluR1/5 protein expression in the rat forebrain in vivo. We found that acute systemic injection of amphetamine at a behaviorally active dose (5 mg/kg) was able to reduce mGluR5 protein levels in a confined biochemical fraction of synaptosomal plasma membranes enriched from the striatum. In contrast to the striatum, amphetamine increased mGluR5 protein levels in the medial prefrontal cortex. These changes in mGluR5 expression in both the striatum and the medial prefrontal cortex were transient and reversible. In addition, protein levels of mGluR1 in the enriched synaptosomal fraction from both the striatum and the medial prefrontal cortex remained stable in response to acute amphetamine. Similarly, Homer1b/c proteins, which are prominent anchoring proteins of mGluR1/5 and are highly expressed in the striatum and the medial prefrontal cortex, showed no change in their protein abundance in striatal and cortical synaptosomes after amphetamine administration. These data demonstrate differential sensitivity of mGluR1 and mGluR5 expression to amphetamine. Acute amphetamine injection is able to alter mGluR5 protein levels at synaptic sites in a subtype- and region-specific manner.
Keywords: mGluR1, mGluR5, dopamine, stimulant, cortex, caudate, nucleus accumbens, addiction, reward
1. Introduction
Metabotropic glutamate receptors (mGluRs) belong to G protein-coupled receptors. These receptors are densely expressed in mammalian brains and are involved in the modulation of a variety of neuronal and synaptic activities. Based on sequence homology, pharmacological profiles, and signaling mechanisms, eight subtypes of mGluRs so far cloned are organized into three functional groups (Conn and Pin, 1997). Group I mGluRs (mGluR1 and 5 subtypes) are positively coupled to membrane-bound phospholipase Cβ1 (PLCβ1) through Gαq proteins. Activation of group I mGluRs increases phosphoinositol hydrolysis, resulting in intracellular Ca2+ release and protein kinase C activation (Conn and Pin, 1997). Both group II (mGluR2 and 3 subtypes) and group III (mGluR4, 6, 7, and 8 subtypes) receptors are negatively coupled to adenylyl cyclase through Gαi/o proteins. Activation of these receptors reduces cAMP formation and inhibits protein kinase A (Conn and Pin, 1997). The linkages of mGluRs to those diverse intracellular signaling pathways enable mGluRs to dynamically modulate their activities in response to changing excitatory synaptic inputs.
The striatum represents a basal ganglia site highly innervated with glutamatergic afferents from widespread regions of the forebrain (McGeer et al., 1977; McGeorge and Faull, 1988). Similarly, glutamate receptors, including group I mGluRs, are densely expressed in striatal medium spiny neurons. A low to moderate level of mGluR1 and a high level of mGluR5 mRNAs are expressed in the vast majority of either striatonigral or striatopallidal projection neurons (Testa et al., 1994; 1995; Kerner et al., 1997). Immunostaining of the two receptor proteins is also found in neurons throughout the striatum (Tallaksen-Greene et al., 1998; Testa et al., 1998). Both mGluR1 and 5 are primarily postsynaptic on dendrites and spines and concentrated in an annulus surrounding the edge of the postsynaptic density as opposed to ionotropic glutamate receptors that are located centrally in the synapse (Takumi et al., 1999; Paquet and Smith, 2003; Mitrano and Smith, 2007). These characteristic subsynaptic distributions of mGluR1/5 imply a distinct role of these receptors in the modulation of excitatory synapses in the striatal region.
Amphetamine (AMPH) is a prototypic dopamine psychostimulant that stimulates dopamine release from the dopaminergic nerve terminals in the striatum (Hernandez et al., 1987; Butcher et al., 1988). In addition to the stimulating effect on dopamine, AMPH enhances extracellular glutamate levels in the striatum (Reid et al., 1997; Gray et al., 1999; Del Arco et al., 1998; 1999). Enhanced glutamate in concert with dopamine thereby increases output of the basal ganglia and induces a transient increase in peripheral motor activity. In response to altered synaptic levels of dopamine and glutamate, postsynaptic glutamate receptors such as group I mGluRs in medium spiny neurons may undergo dynamic alterations in expression. However, to date, the sensitivity of mGluR1/5 expression to AMPH exposure has not been thoroughly investigated.
In this study, we investigated the regulation of mGluR1 and 5 protein expression following AMPH administration. Alterations in mGluR1/5 protein abundance in the striatum and the medial prefrontal cortex (mPFC) were assessed in rats following a single intraperitoneal (i.p.) injection of the drug at a behaviorally active dose. In addition, key scaffold proteins for mGluR1/5, Homer1b/c, were detected in parallel with mGluR1/5 for its sensitivity to AMPH.
2. Results
2.1. Effects of AMPH on mGluR5 expression
To explore possible changes in mGluR5 expression in striatal and cortical neurons following AMPH administration, we subjected rats to a single dose of AMPH (5 mg/kg, i.p.). The rats were then sacrificed 1 h after AMPH injection for Western blot assessments of changes in mGluR5 protein levels in the striatum and mPFC. The selection of 1 h was based on preliminary studies. For Western blot analysis, we purified synaptic proteins by solubilizing membrane-bound proteins from synaptic plasma membrane preparations (Fig. 1A). In this form of protein extracts, functional mGluR5 was notably displayed as monomers (~130 kDa) and dimers (~250 kDa) at low and high levels (Fig. 1B), respectively, as described previously (Kuwajima et al., 2007; Mao et al., 2010). Following an acute injection of AMPH, basal mGluR5 protein levels in the striatum (both monomers and dimers) were markedly reduced as compared to those in the saline-treated animals (Fig. 1C). As shown in a quantitative graph in Fig. 1C, protein levels of mGluR5 monomers and dimers in AMPH-treated rats were reduced to 50.7 ± 5.0% of saline (p < 0.05) and 57.0 ± 5.7% of saline (p < 0.05), respectively. There was no change in striatal actin protein levels in AMPH-treated rats relative to saline-treated rats (Fig. 1C). These results demonstrate that synaptic mGluR5 (both monomers and dimers) is sensitive to AMPH. A single dose of AMPH reduces the synaptic level of the receptor in the striatum.
Fig. 1.
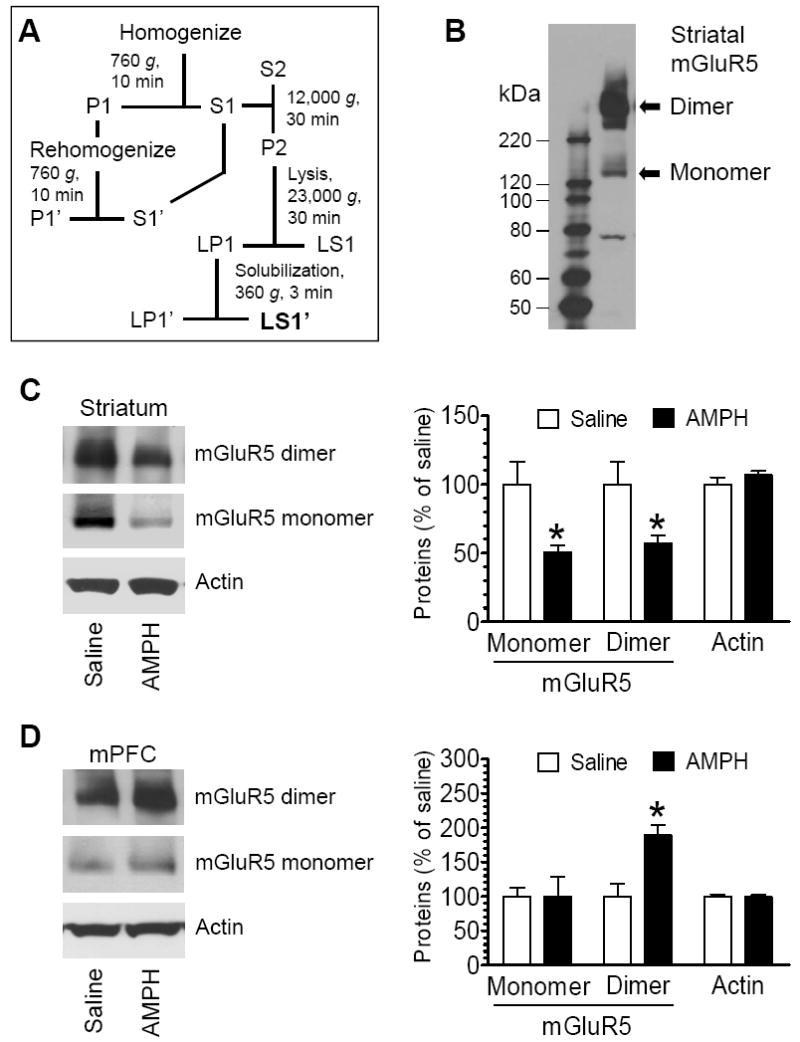
Effects of acute AMPH administration on mGluR5 expression. (A) Schematic illustration of biochemical fractionation procedures for enriching synaptic membrane-bound proteins. (B) A representative immunoblot showing mGluR5 monomers and dimers in the striatum. (C and D) Effects of acute AMPH administration on mGluR5 protein levels in the striatum (C) and mPFC (D). Representative immunoblots of mGluR5 and actin are shown left to the quantification of immunoblot results. Arrows indicate the bands quantified. Rats were injected with a single dose of saline (1 ml/kg, i.p.) or AMPH (5 mg/kg, i.p.). They were sacrificed 1 h after the drug injection. Data are expressed as means ± S.E.M. (n = 4-5 per group). *p < 0.05 versus saline.
The mPFC is also enriched with mGluR5 and is implicated in processing drug effects (Berke and Hyman, 2000; Wise, 2002). We therefore investigated whether mGluR5 expression in the mPFC is subject to the modulation by AMPH. Interestingly, in contrast to the reduction of mGluR5 in the striatum, the mGluR5 protein level in the mPFC was elevated in rats treated with AMPH relative to rats treated with saline. As shown in Fig. 1D, mGluR5 dimers were elevated to 188.8 ± 5.8% of saline (p < 0.05), even though mGluR5 monomers remained unchanged 99.1 ± 29.3% of saline, p > 0.05). Thus, mGluR5 in the mPFC, primarily in the form of dimmers, is sensitive to AMPH, and can be enhanced in its expression in response to AMPH.
2.2. Effects of AMPH on mGluR1 expression
Using the same protein samples, protein levels of mGluR1 in AMPH- and saline-treated rats were assessed using Western blots. Like mGluR5, functional mGluR1 receptors exist in monomer and dimer forms in a synaptic protein-enriched fraction from the striatum (Kuwajima et al., 2007). In gel electrophoresis, mGluR1 proteins migrated into a very light monomer band (~130 kDa) and a predominant dimer band (~250 kDa) (Fig. 2A). Due to the dominant nature of dimers, we focused our quantification analysis of mGluR1 protein expression on the form of dimers. In rats treated with a single dose of AMPH, mGluR1 showed a minimal change in its protein levels in the striatum. As can be seen in Fig. 2B, the amount of mGluR1 dimers in AMPH-treated rats was 89.7 ± 12.8% (p > 0.05) of that in saline-treated rats. In the mPFC, mGluR1 dimer levels were seemingly lowered in AMPH-treated rats compared to saline-treated rats (80.2 ± 19.6% of saline), but it did not reach statistical significance (p > 0.05; Fig. 2C). These data indicate that acute AMPH administration had a minimal impact on mGluR1 expression in the striatum and mPFC.
Fig. 2.
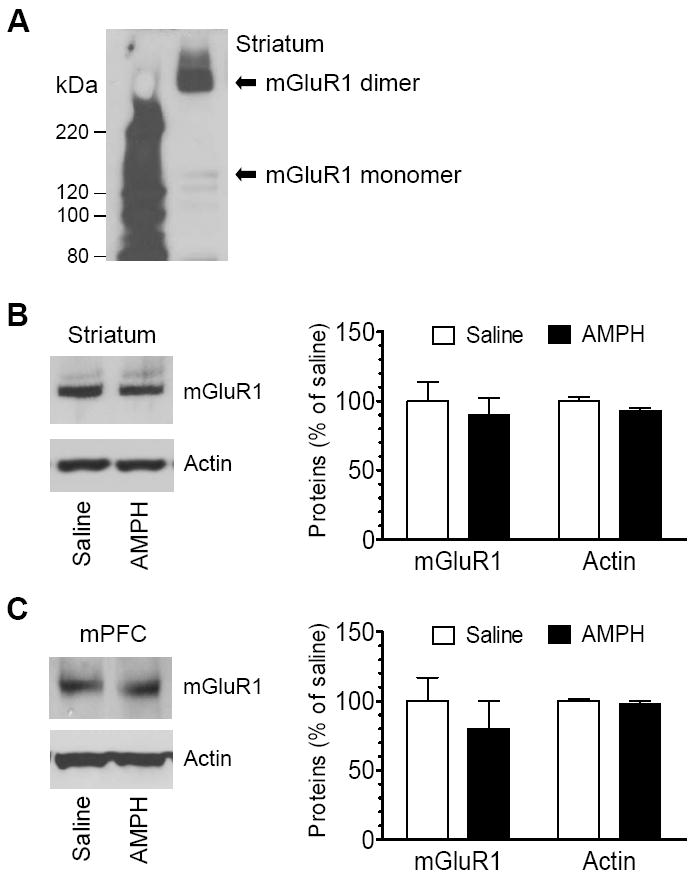
Effects of acute AMPH administration on mGluR1 expression. (A) A representative immunoblot showing mGluR1 monomers and dimers in the striatum. (B and C) Effects of acute AMPH administration on mGluR1 protein levels in the striatum (B) and mPFC (C). Representative immunoblots of mGluR1 and actin are shown left to the quantification of dimers in immunoblot results. Rats were injected with a single dose of saline (1 ml/kg, i.p.) or AMPH (5 mg/kg, i.p.). They were sacrificed 1 h after the drug injection. Data are expressed as means ± S.E.M. (n = 4-5 per group).
2.3. Effects of AMPH on Homer1b/c expression
Homer is a prominent scaffold protein for mGluR1/5 (Brakeman et al., 1997). Through its C-terminal coiled-coil structure and leucine zipper motifs, Homer possesses a capability for self-assembly (Xiao et al., 1998). Moreover, the N-terminal EVH1 (Enabled/vasodilator-stimulated phosphoprotein homology 1) domain of Homer binds to C-terminal intracellular tails of mGluR1/5 (Brakeman et al., 1997; Kato et al., 1998). Thus, Homer plays important roles in controlling stability, expression, and post-receptor signaling efficacy of mGluR1/5 (Xiao et al., 2000). Among three subfamilies of Homer proteins that all bind mGluR5 (Tu et al., 1998), Homer1b/c and Homer2a/b are present at a high and low level, respectively, whereas Homer3 is lacking in striatal neurons in vivo (Shiraishi et al., 2004). To determine whether Homer expression could be altered in parallel with those changes in mGluR5, we tested changes in Homer1b/c protein levels in the striatum and cortex in response to AMPH. A clear band of Homer1b/c proteins (~45-48 kDa) was exhibited in synaptic fractions from striatal tissue (Fig. 3A). An acute injection of AMPH appeared to slightly reduce Homer1b/c expression, although this reduction did not reach a statistically significant level (82.7 ± 2.7% of saline, P > 0.05; Fig. 3B). In the mPFC, AMPH did not show a significant effect on Homer1b/c expression since Homer1b/c protein levels were not significantly altered in AMPH-treated rats relative to saline-treated rats (112.6 ± 12.3% of saline, P > 0.05; Fig. 3C). These data reveal an insensitive nature of Homer1b/c proteins in their expression in response to acute AMPH administration.
Fig. 3.
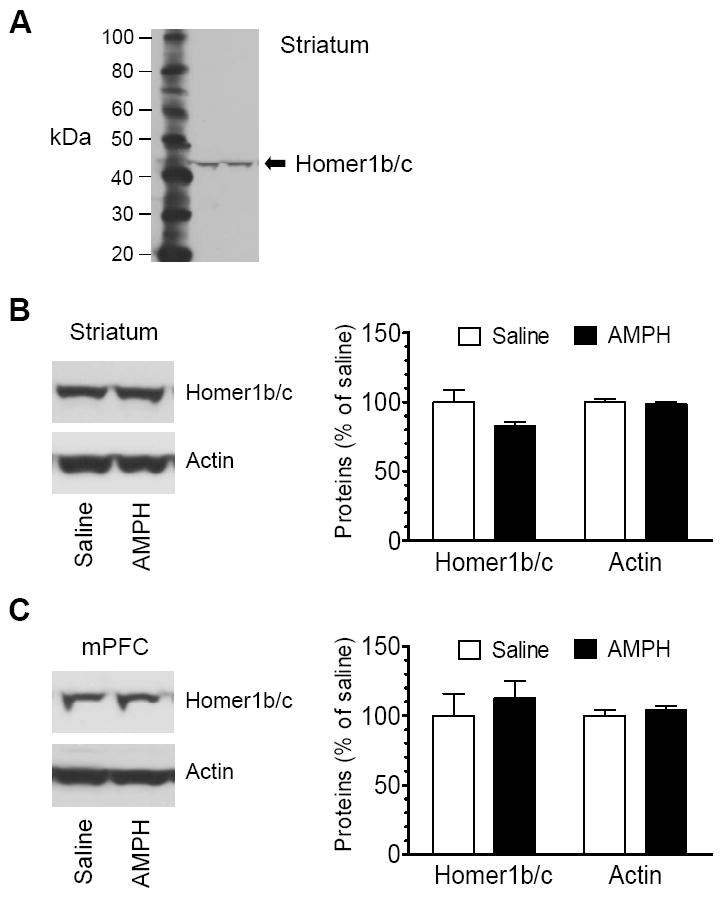
Effects of acute AMPH administration on Homer1b/c expression. (A) A representative immunoblot showing Homer1b/c in the striatum. (B and C) Effects of acute AMPH administration on Homer1b/c protein levels in the striatum (B) and mPFC (C). Representative immunoblots of Homer1b/c and actin are shown left to the quantification of immunoblot results. Rats were injected with a single dose of saline (1 ml/kg, i.p.) or AMPH (5 mg/kg, i.p.). They were sacrificed 1 h after the drug injection. Data are expressed as means ± S.E.M. (n = 4-5 per group).
2.4. Time-dependent effects of AMPH on mGluR5 expression
A time-course study was carried out to characterize the temporal property of alterations in mGluR5 expression in rats treated with acute injection of saline or AMPH. The three time points (15 min, 1 h, and 5 h after drug injection) were chosen. At an early time point (15 min), AMPH did not alter basal levels of mGluR5 in the striatum (82.0 ± 7.0% of saline, P > 0.05; Fig. 4A) and mPFC (118.4 ± 15.1% of saline, P > 0.05; Fig. 4B). Only at 1 h, AMPH induced a significant decrease and increase in mGluR5 levels in the striatum (69.7 ± 7.0% of saline, P < 0.05; Fig. 4A) and mPFC (208. ± 15.9% of saline, P < 0.05; Fig. 4B), respectively. The changes (either the down- or the upregulation) were reversible as they returned to the basal level 5 h after AMPH injection (Fig. 4A and 4B). These data demonstrate that AMPH induces a transient change in mGluR5 expression in both the striatum and cortex.
Fig. 4.
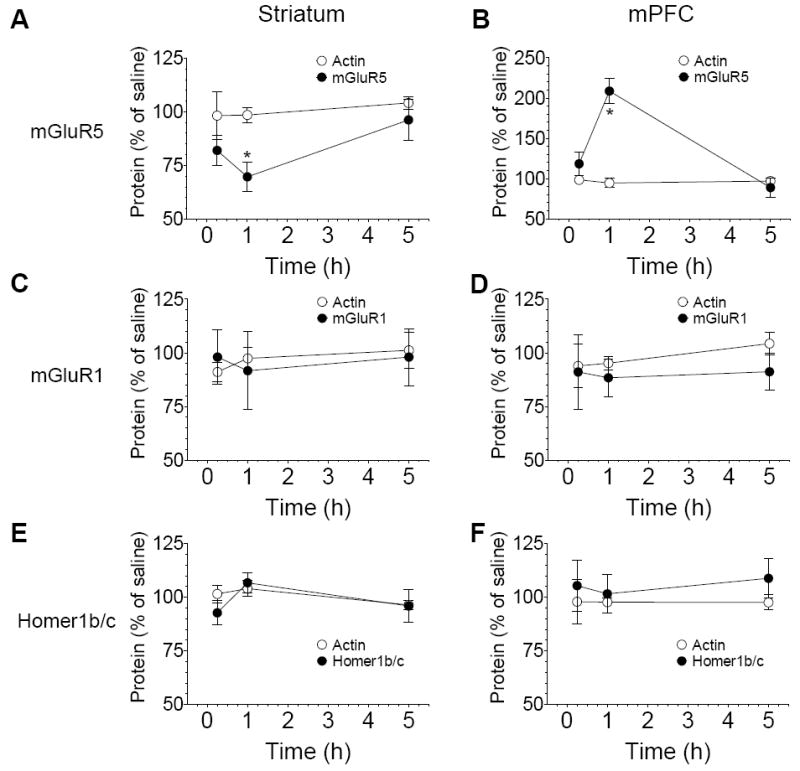
The time-dependent effect of AMPH on mGluR1, mGluR5, and Homer1b/c expression. (A and B) Time-dependent changes in mGluR5 protein levels in the striatum (A) and mPFC (B) following acute injection of AMPH. (C and D) Effects of AMPH on mGluR1 expression in the striatum (C) and mPFC (D). (E and F) Effects of AMPH on Homer1b/c expression in the striatum (E) and mPFC (F). Rats were subject to a single dose of AMPH (5mg/kg) or saline (1 ml/kg, i.p.). They were sacrificed at different time points (15 min, 1 h, or 5 h after drug injection). Data are expressed as means ± S.E.M. (n = 4–5 per group). *P < 0.05 versus saline at the same time point.
Early results have shown that mGluR1 protein levels were not altered by AMPH at 1 h time point (see Fig. 2). In this time course study, mGluR1 expression in the striatum and mPFC was not changed in AMPH-treated rats at an early (15 min) and a later (5 h) time point (Fig. 4C and 4D). At all three time points surveyed, protein levels of Homer1b/c were not altered significantly in AMPH-treated rats compared to saline-treated rats (Fig. 4E and 4F). These data suggest a minimal impact of AMPH on mGluR1 and Homer1b/c expression in striatal and cortical neurons.
2.5. Effects of amphetamine on motor activity
To correlate neurochemical changes with behavioral responses to AMPH, we monitored changes in motor activity in response to AMPH. Both ambulatory (horizontal activity) and stereotypic activities were monitored following an acute injection of AMPH (5 mg/kg, i.p.). As expected, AMPH at this dose caused typical motor responses (Wang and McGinty, 1995; Parelkar et al., 2009). A marked increase in ambulatory activity was seen in AMPH-treated rats (Fig. 5A), accompanied by an increase in stereotypic activity (Fig. 5B).
Fig. 5.
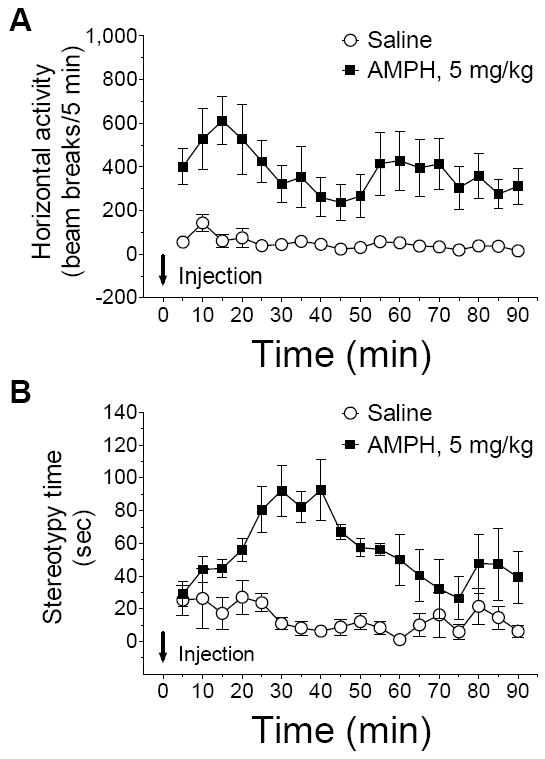
Effects of acute injection of amphetamine on behavioral activity. Rats were given a single dose of amphetamine (5 mg/kg, i.p.) or saline (1 ml/kg, i.p). The data are expressed as means ± S.E.M. (n = 4-8 per group).
3. Discussion
This study investigated the effect of acute AMPH administration on mGluR1, mGluR5, and Homer1b/c expression in the rat forebrain in vivo. The data obtained indicate the region- and time-specific regulation of mGluR5 protein levels in striatal and cortical neurons in response to AMPH. While AMPH downregulated mGluR5 in the striatum, it upregulated mGluR5 in the mPFC. The regulation of mGluR5 was transient and reversible. Unlike mGluR5, mGluR1 expression in both the striatum and mPFC was resistant to AMPH. Homer1b/c expression also remained stable in the two regions throughout a time course surveyed. These data demonstrate that acute AMPH injection can induce a significant change in mGluR5 expression in a region-and time-dependent fashion.
An important finding in this work is the sensitivity of mGluR5 expression to the psychostimulant AMPH. This sensitivity has the following characteristics. First, the response of mGluR5 to AMPH was subtype-selective. Another group I mGluR subtype, mGluR1, did not show a parallel change in response to AMPH. The two subtypes (mGluR1 and mGluR5) share many neurochemical and physiological properties. They both are expressed in striatal medium spiny neurons. However, mGluR5 is expressed at a higher level whereas mGluR1 at a lower level (Mao and Wang, 2001). This may permit greater sensitivity of mGluR5 to AMPH stimulation. Second, mGluR5 expression was regulated in a region-specific manner. While mGluR5 protein levels were downregulated in the striatum, its levels were upregulated in the mPFC. Apparently, mGluR5 plays different roles in the two regions (see below), and is thus subject to differential regulation of its expression. Third, altered mGluR5 expression in either the striatum or the mPFC was time-dependent. It became evident 1 h after drug administration and returned to the normal level by 5 h. The dynamic changes in mGluR5 expression seemed to correspond well with the behavioral response to AMPH. These transient changes in gene expression and behaviors indicate a reversible nature of neurochemical and behavioral responses to an acute injection of AMPH. Finally, while acute AMPH induced a transient reduction of mGluR5, chronic AMPH (4 mg/kg, i.p., once daily for 7 days) produced a long-lasting loss of mGluR5 in striatal neurons (Mao et al., 2010). Similarly, repeated cocaine administration reduced mGluR5 and Homer1b/c protein levels in the medial nucleus accumbens after a 3 week withdrawal period (Swanson et al., 2001). These persistent changes are considered to be essential elements in the remodeling of excitatory transmission in reward circuits in response to chronic drug exposure and are important for addictive properties of drugs of abuse.
Downregulation of a number of receptors could be a feedback mechanism underlying desensitization of receptor activity in response to ligand/agonist stimulation. AMPH has been shown to markedly enhance extracellular glutamate levels in the striatum (Reid et al., 1997; Gray et al., 1999; Del Arco et al., 1999). This enhanced glutamate level can then activate mGluR5 and subsequently triggers a ligand-initiated homologous desensitization through a mechanism involving the downregulation of synaptic mGluR5. Indeed, a great deal of evidence from heterologous cells and neurons has demonstrated the existence of the agonist-induced desensitization of mGluR5 (reviewed in Dhami and Ferguson, 2006; Mao et al., 2008). In response to a brief activation by glutamate, mGluR5 underwent pronounced and rapid desensitization (Gereau and Heinemann, 1998). This desensitization seems to require protein kinase C or G protein-coupled receptor kinases (GRK2 family) to phosphorylate specific serine/threonine sites in intracellular domains of the receptor (Gereau and Heinemann, 1998; Sorensen and Conn, 2003). Such phosphorylation could disrupt the calmodulin binding to mGluR5, thereby reducing mGluR5 surface expression (Lee et al., 2008). In addition to a phosphorylation-dependent mechanism, phosphorylation-independent desensitization and internalization of mGluR5 could be induced in striatal neurons by GRK2 (Ribeiro et al., 2009). Noticeably, in contrast to the downregulation of mGluR5 expression in the striatum, mGluR5 expression in the mPFC was upregulated after AMPH. The similar region-specific regulation was also seen in rats treated with repeated cocaine administration. In response to repeated cocaine, protein levels of mGluR1a, Homer2a/b, and NMDA receptor NR2B subunits were downregulated in the nucleus accumbens shell and upregulated in the prefrontal cortex (Ary and Szumlinski, 2007). AMPH has been shown to induce differential responses in extracellular glutamate levels in the two regions. In an in vivo microdialysis study comparing the region-specific effects of AMPH, AMPH (2 mg/kg, i.p.) induced a robust increase in glutamate levels in the nucleus accumbens, while it did not in the prefrontal cortex (Shoblock et al., 2003; but Reid et al., 1997). Thus, the glutamatergic transmission in the two regions may differentially respond to stimulants. This difference, along with other unknown mechanisms, may determine the direction of the regulation of mGluR5 expression in these areas and distinct functional implications of changed mGluR5 expression in the two areas.
This study also investigated the effect of AMPH on Homer expression. As a tethering protein, Homer1b/c link mGluR1/5 to multiple downstream signaling pathways (Brakeman et al., 1997; Tu et al., 1998; Xiao et al., 1998; Mao et al., 2005; Yang et al., 2006). Moreover, a number of studies have shown that constitutively expressed long form Homer, including Homer1b/c, regulates surface expression, clustering, and dendritic/synaptic targeting of mGluR1/5, although results were inconclusive (Roche et al., 1999; Ciruela et al., 2000; Ango et al., 2002; Kammermeier, 2006). In HEK293 cells, Homer1c elevated surface expression of mGluR1α by increasing surface trafficking and reducing the rate of loss from cell surface of the receptor (Ciruela et al., 2000). However, in both heterologous cells and cultured cerebellar granule neurons, Homer1b reduced surface expression of mGluR5 by increasing intracellular retention of mGluR5 in the endoplasmic reticulum (Roche et al., 1999; Ango et al., 2002). In this study, in striatal neurons that express a high level of Homer1b/c (Shiraishi et al., 2004), we found that AMPH did not induce a significant change in Homer1b/c expression, while the drug induced a marked change in mGluR5. The lack of parallel changes in Homer1b/c abundance narrows the likelihood that AMPH alters mGluR5 expression through the regulation of its anchoring Homer1b/c proteins. Thus, a Homer1b/c-independent mechanism is more likely to underlie the effect of AMPH on mGluR5 expression.
4. Experimental procedures
4.1. Animals
Adult male Wistar rats weighing 225-275 g (Charles River, New York, NY) were individually housed in clear plastic cages. Animals were maintained on a 12/12 h light dark cycle; lights were turned on at 7:00 am. The housing environment was maintained at 23°C and humidity at 50 ± 10% with food and water available ad libitum. An at least 7-day accommodation period was allowed prior to the commencement of the experiment. All animal use procedures were in strict accordance with the US National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee.
4.2. Systemic drug injection
Rats received an i.p. injection of saline or D-amphetamine sulfate (Sigma-Aldrich, St. Louis, MO). The dose of AMPH was calculated as the salt. Rats were treated with a single i.p. injection of AMPH (5 mg/kg) in home cages. The dose of AMPH was chosen based on the fact that this dose of the drug caused typical motor stimulation (Wang and McGinty, 1995; Parelkar et al., 2009). Age-matched rats received an acute injection of saline (1 ml/kg) and served as controls.
4.3. Protein extraction
Rats were anesthetized with Equithesin (9 ml/kg, i.p.) and decapitated at time points indicated. Brains were quickly removed and cut into coronal sections. The entire striatum, including the dorsal caudate putamen and ventral nucleus accumbens, and the mPFC were separately dissected into a 1.5 ml microtube containing ice-cold homogenization buffer: 0.32 M sucrose, 4 mM HEPES, pH 7.4, and a protease inhibitor cocktail (Completetm, Roche). The sample was homogenized by sonication. The homogenate was centrifuged at 760 g for 10 min at 4°C to generate the supernatant 1 (S1) and the pellet 1 (P1). P1 was resuspended with 1 volume of the homogenization buffer, re-homogenized by sonication, and centrifuged at 760 g for 10 min at 4°C to generate the S1’ and P1’ fractions. The P1’ fraction containing unbroken cells, nuclei, and large debris was discarded. The S1’ fraction was added to the S1 fraction to generate the final S1 supernatant. S1 was then centrifuged at 12,000 g for 30 min at 4°C to generate the supernatant 2 (S2) and the pellet 2 (P2) containing crude synaptosomal membranes. P2 was washed once with 1 volume of homogenization buffer and centrifuged at 12,000 g for 30 min at 4°C to produce the final P2 pellet. Washed P2 was resuspended in a small volume of homogenization buffer and lysed hypo-osmotically by adding 9 volumes of ice-cold water containing a cocktail of protease inhibitors and homogenization in a 5-sec sonication. The P2 lysate was brought to 4 mM HEPES, pH 7.4, by adding a small volume of a concentrated solution, and was centrifuged at 23,000 g for 20 min at 4°C to yield the lysate pellet 1 (LP1) which was enriched with synaptic plasma membranes. The LP1 pellet was resuspended and solubilized in the RIPA buffer containing 1% NP-40 and 1% sodium deoxycholate. Insolubilized particulates were removed by centrifugation at 360 g for 3 min at 4°C. Protein concentrations in the supernatant fraction were determined and adjusted for Western blot analysis.
4.4. Western blot
The equal amount of protein (usually 20 μg/20 μl/lane) was separated on SDS NuPAGE Novex 4-12% gels (Invitrogen, Carsbad, CA). Proteins were transferred to the polyvinylidene fluoride membrane (Millipore, Bedford, MA) and blocked in blocking buffer (5% nonfat dry milk in phosphate-buffered saline and 0.1% Tween 20) for 1 h. The blots were washed and incubated in the blocking buffer containing a primary rabbit antibody against mGluR1 (Upstate/Millipore, Billerica, MA), mGluR5 (Upstate/Millipore), or actin (Santa Cruz Biotechnology, Santa Cruz, CA), or a goat antibody against Homer1b/c (Santa Cruz) usually at 1:1000 overnight at 4°C. This was followed by 1 h incubation in a horseradish peroxidase-linked secondary antibody against rabbit or goat (Jackson Immunoresearch Laboratory, West Grove, PA) at 1:5000. Immunoblots were developed with the enhanced chemiluminescence reagents (ECL; Amersham Pharmacia Biotech, Piscataway, NJ), and captured into Kodak Image Station 2000R. Kaleidoscope-prestained standards (Bio-Rad, Hercules, CA) and MagicMark XP Western protein standards (Invitrogen) were used for protein size determination. The density of immunoblots was measured using the Kodak 1D Image Analysis software (Mao et al., 2005; Liu et al., 2006).
4.5. Behavioral assessment
Behavioral activity was assessed with an infrared photo-cell-based, automated Opto-Varimex-Micro apparatus (Columbus Instruments, Columbus, OH) in a sound-attenuated room as described previously (Liu et al., 2009; Mao et al., 2009). Briefly, rats in standard transparent rectangular rodent cage (42 × 24 × 20 cm high) were habituated to the environment for 2 h. Three sensor pairs positioned in x, y (horizontal), and z (vertical, above the animal’s normal height) directions were assigned to each cage to provide measurements about horizontal and vertical activities. Motor activity was recorded at 5 min intervals before and after drug injection. Status about infrared beam interruptions by presence of animals was transferred from all sensors to a computer with operating VersaMax software. Stereotypy was detected using computer-generated stereotypy time recorded by VersaMax monitors, which refers to the total time that stereotypic behaviors (repetitive breaks of a given beam or beams with an interval less than 1 s) were observed.
4.6. Statistics
The results are presented as means ± S.E.M., and were evaluated using a one-way analysis of variance followed by a Bonferroni (Dunn) comparison of groups using least squares-adjusted means or two-tailed unpaired Student’s t-test. Probability levels of < 0.05 were considered statistically significant.
Acknowledgments
This work was supported by grants from the NIH (R01DA010355 and R01MH061469) and a grant from Saint Luke’s Hospital Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ango F, Robbe D, Tu JC, Xiao B, Worley PF, Pin JP, Bockaert J, Fagni L. Homer-dependent cell surface expression of metabotropic glutamate receptor type 5 in neurons. Mol Cell Neurosci. 2002;20:323–329. doi: 10.1006/mcne.2002.1100. [DOI] [PubMed] [Google Scholar]
- Ary AW, Szumlinski KK. Regional differences in the effects of withdrawal from repeated cocaine upon Homer and glutamate receptor expression: a two-species comparison. Brain Res. 2007;1184:295–305. doi: 10.1016/j.brainres.2007.09.035. [DOI] [PubMed] [Google Scholar]
- Berke JD, Hyman SE. Addiction, dopamine, and the molecular mechanisms of memory. Neuron. 2000;25:515–532. doi: 10.1016/s0896-6273(00)81056-9. [DOI] [PubMed] [Google Scholar]
- Brakeman PR, Lanahan AA, O’Brien R, Roche K, Barnes CA, Huganir RL, Worley PF. Homer: a protein that selectively binds metabotropic glutamate receptors. Nature. 1997;386:284–288. doi: 10.1038/386284a0. [DOI] [PubMed] [Google Scholar]
- Butcher SP, Fairbrother IS, Kelly JS, Arbuthnott GW. Amphetamine-induced dopamine release in the rat striatum: an in vivo microdialysis study. J Neurochem. 1988;50:346–355. doi: 10.1111/j.1471-4159.1988.tb02919.x. [DOI] [PubMed] [Google Scholar]
- Ciruela F, Soloviev MM, Chan WY, McIIhinney RA. Homer-1c/Vesl-1L modulates the cell surface targeting of metabotropic glutamate receptor type 1alpha: evidence for an anchoring function. Mol Cell Neurosci. 2000;15:36–50. doi: 10.1006/mcne.1999.0808. [DOI] [PubMed] [Google Scholar]
- Conn PJ, Pin JP. Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- Del Arco A, Gonzalez-Mora JL, Armas VR, Mora F. Amphetamine increases the extracellular concentration of glutamate in striatum of the awake rat: involvement of high affinity transporter mechanisms. Neuropharmacology. 1999;38:943–954. doi: 10.1016/s0028-3908(99)00043-x. [DOI] [PubMed] [Google Scholar]
- Del Arco A, Martinez R, Mora F. Amphetamine increases extracellular concentrations of glutamate in the prefrontal cortex of the awake rat: a microdialysis study. Neurochem Res. 1998;23:1153–1158. doi: 10.1023/a:1020769816332. [DOI] [PubMed] [Google Scholar]
- Dhami GK, Ferguson SS. Regulation of metabotropic glutamate receptor signaling, desensitization and endocytosis. Pharmacol Ther. 2006;111:260–271. doi: 10.1016/j.pharmthera.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Gereau RW, 4th, Heinemann SF. Role of protein kinase C phosphorylation in rapid desensitization of metabotropic glutamate receptor 5. Neuron. 1998;20:143–151. doi: 10.1016/s0896-6273(00)80442-0. [DOI] [PubMed] [Google Scholar]
- Gray AM, Rawls SM, Shippenberg TS, McGinty JF. The kappa-opioid agonist, U-69593, decreases acute amphetamine-evoked behaviors and calcium-dependent dialysate levels of dopamine and glutamate in the ventral striatum. J Neurochem. 1999;73:1066–1074. doi: 10.1046/j.1471-4159.1999.0731066.x. [DOI] [PubMed] [Google Scholar]
- Hernandez L, Lee F, Hoebel BG. Simultaneous microdialysis and amphetamine infusion in the nucleus accumbens and striatum of freely moving rats: increase in extracellular dopamine and serotonin. Brain Res Bull. 1987;19:623–628. doi: 10.1016/0361-9230(87)90047-5. [DOI] [PubMed] [Google Scholar]
- Kammermeier PJ. Surface clustering of metabotropic glutamate receptor I induced by long Homer proteins. BMC Neuroscience. 2006;7:1. doi: 10.1186/1471-2202-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato A, Ozawa F, Saitoh Y, Fukazawa Y, Sugiyama H, Inoduchi K. Novel members of the Vesl/Homer family of PDZ proteins that bind metabotropic glutamate receptors. J Biol Chem. 1998;273:23969–23975. doi: 10.1074/jbc.273.37.23969. [DOI] [PubMed] [Google Scholar]
- Kerner JA, Standaert DG, Penney JB, Young AB, Jr, Landwehrmeyer GB. Expression of group one metabotropic receptor subunit mRNAs in neurochemically identified neurons in the rat neostriatum, neocortex, and hippocampus. Mol Brain Res. 1997;48:259–269. doi: 10.1016/s0169-328x(97)00102-2. [DOI] [PubMed] [Google Scholar]
- Kuwajima M, Dehoff MH, Furunichi T, Worley PF, Hall RA, Smith Y. Localization and expression of group I metabotropic glutamate receptors in the mouse striatum, globus pallidus, and subthalamic nucleus: regulatory effects of MPTP treatment and constitutive Homer deletion. J Neurosci. 2007;27:6249–6260. doi: 10.1523/JNEUROSCI.3819-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Lee J, Choi KY, Hepp R, Lee JY, Lim MK, Chatani-Hinze M, Roche PA, Kim DG, Ahn YS, Kim CH, Roche KW. Calmodulin dynamically regulates the trafficking of the metabotropic glutamate receptor mGluR5. Proc Natl Acad Sci USA. 2008;105:12575–12580. doi: 10.1073/pnas.0712033105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XY, Chu XP, Mao LM, Wang M, Lan HX, Li MH, Zhang GC, Parelkar NK, Fibuch EE, Haines M, Neve KA, Liu F, Xiong ZG, Wang JQ. Modulation of D2R-NR2B interactions in response to cocaine. Neuron. 2006;52:897–909. doi: 10.1016/j.neuron.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Liu XY, Mao LM, Zhang GC, Papasian CJ, Fibuch EE, Lan HX, Zhou HF, Xu M, Wang JQ. Activity-dependent modulation of limbic dopamine D3 receptors by CaMKII. Neuron. 2009;61:425–438. doi: 10.1016/j.neuron.2008.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao LM, Guo ML, Wang JQ. Repeated amphetamine administration reduces group I metabotropic glutamate receptor protein levels in the rat striatum in vivo. Neurosci Lett. 2010 in press. [Google Scholar]
- Mao LM, Liu XY, Zhang GC, Chu XP, Fibuch EE, Wang LS, Liu Z, Wang JQ. Phosphorylation of group I metabotropic glutamate receptors (mGluR1/5) in vitro and in vivo. Neuropharmacology. 2008;55:403–408. doi: 10.1016/j.neuropharm.2008.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L, Wang JQ. Differentially altered mGluR1 and mGluR5 mRNA expression in rat caudate nucleus and nucleus accumbens in the development and expression of behavioral sensitization to repeated amphetamine administration. Synapse. 2001;41:230–240. doi: 10.1002/syn.1080. [DOI] [PubMed] [Google Scholar]
- Mao LM, Wang W, Chu XP, Zhang GC, Liu XY, Yang YJ, Haines M, Papasian CJ, Fibuch EE, Buch S, Chen JG, Wang JQ. Stability of surface NMDA receptors controls synaptic and behavioral adaptations to amphetamine. Nat Neurosci. 2009;12:602–610. doi: 10.1038/nn.2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L, Yang L, Tang Q, Samdani G, Zhang G, Wang JQ. The scaffold protein Homer1b/c links metabotropic glutamate receptor 5 to extracellular signal-regulated protein kinase cascades in neurons. J Neurosci. 2005;25:2741–2752. doi: 10.1523/JNEUROSCI.4360-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeer PL, McGeer EG, Scherer U, Singh R. A glutamatergic corticostriatal path. Brain Res. 1977;128:369–373. doi: 10.1016/0006-8993(77)91003-4. [DOI] [PubMed] [Google Scholar]
- McGeorge AJ, Faull RLM. The organization of the projection from the cerebral cortex to the striatum in the rat. Neuroscience. 1988;29:503–537. doi: 10.1016/0306-4522(89)90128-0. [DOI] [PubMed] [Google Scholar]
- Mitrano DA, Smith Y. Comparative analysis of the subcellular and subsynaptic localization of mGluR1a and mGluR5 metabotropic glutamate receptors in the shell and core of the nucleus accumbens in rat and monkey. J Comp Neurol. 2007;500:788–806. doi: 10.1002/cne.21214. [DOI] [PubMed] [Google Scholar]
- Paquet M, Smith Y. Group I metabotropic glutamate receptors in the monkey striatum: subsynaptic association with glutamatergic and dopaminergic afferents. J Neurosci. 2003;23:7659–7669. doi: 10.1523/JNEUROSCI.23-20-07659.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parelkar NK, Jiang Q, Chu XP, Guo ML, Mao LM, Wang JQ. Amphetamine alters Ras-quanine nucleotide-releasing factor expression in the rat striatum in vivo. Eur J Pharmacol. 2009;619:50–56. doi: 10.1016/j.ejphar.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid MS, Hsu K, Jr, Berger SP. Cocaine and amphetamine preferentially stimulate glutamate release in the limbic system: studies on the involvement of dopamine. Synapse. 1997;27:95–105. doi: 10.1002/(SICI)1098-2396(199710)27:2<95::AID-SYN1>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Ribeiro FM, Ferreira LT, Paquet M, Cregan T, Ding Q, Gros R, Ferguson SS. Phosphorylation-independent regulation of metabotropic glutamate receptor 5 desensitization and internalization by G protein-coupled receptor kinase 2 in neurons. J Biol Chem. 2009;284:23444–23453. doi: 10.1074/jbc.M109.000778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche KW, Tu JC, Petralia RS, Xiao B, Wenthold RJ, Worley PF. Homer 1b regulates the trafficking of group I metabotropic glutamate receptors. J Biol Chem. 1999;274:25953–25957. doi: 10.1074/jbc.274.36.25953. [DOI] [PubMed] [Google Scholar]
- Shiraishi Y, Mizutani A, Yuasa S, Mikoshiba K, Furuichi T. Differential expression of Homer family proteins in the developing mouse brain. J Comp Neurol. 2004;473:582–599. doi: 10.1002/cne.20116. [DOI] [PubMed] [Google Scholar]
- Shoblock JR, Sullivan EB, Maisonneuve IM, Glick SD. Neurochemical and behavioral differences between d-methamphetamine and d-amphetamine in rats. Psychopharmacology (Berl) 2003;165:359–369. doi: 10.1007/s00213-002-1288-7. [DOI] [PubMed] [Google Scholar]
- Sorensen SD, Conn PJ. G protein-coupled receptor kinases regulate metabotropic glutamate receptor 5 function and expression. Neuropharmacology. 2003;44:699–706. doi: 10.1016/s0028-3908(03)00053-4. [DOI] [PubMed] [Google Scholar]
- Swanson DJ, Baker DA, Carson D, Worley PF, Kalivas PW. Repeated cocaine administration attenuates group I metabotropic glutamate receptor-mediated glutamate release and behavioral activation: a potential role for Homer. J Neurosci. 2001;21:9043–9052. doi: 10.1523/JNEUROSCI.21-22-09043.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takumi Y, Matsubara A, Rinvik E, Ottersen OP. The arrangement of glutamate receptors in excitatory synapses. Annu NY Acad Sci. 1999;868:474–482. doi: 10.1111/j.1749-6632.1999.tb11316.x. [DOI] [PubMed] [Google Scholar]
- Tallaksen-Greene SJ, Kaatz KW, Romano C, Albin RL. Localization of mGluR1a-like immunoreactivity and mGluR5-like immunoreactivity in identified populations of striatal neurons. Brain Res. 1998;780:210–217. doi: 10.1016/s0006-8993(97)01141-4. [DOI] [PubMed] [Google Scholar]
- Testa CM, Friberg IK, Weiss SW, Standaert DG. Immunohistochemical localization of metabotropic glutamate receptors mGluR1a and mGluR2/3 in the rat basal ganglia. J Comp Neurol. 1998;390:5–19. [PubMed] [Google Scholar]
- Testa CM, Standaert DG, Landwehrmeyer GB, Penney JB, Jr, Young AB. Differential expression of mGluR5 metabotropic glutamate receptor mRNA by rat striatal neurons. J Comp Neurol. 1995;354:241–252. doi: 10.1002/cne.903540207. [DOI] [PubMed] [Google Scholar]
- Testa CM, Standaert DG, Young AB, Penney JB., Jr Metabotropic glutamate receptor mRNA expression in the basal ganglia of the rat. J Neurosci. 1994;14:3005–3018. doi: 10.1523/JNEUROSCI.14-05-03005.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu JC, Xiao B, Yuan JP, Lanahan AA, Leoffert K, Li M, Linden DJ, Worley PF. Homer binds a novel proline-rich motif and links group 1 metabotropic glutamate receptors with IP3 receptors. Neuron. 1998;21:717–726. doi: 10.1016/s0896-6273(00)80589-9. [DOI] [PubMed] [Google Scholar]
- Wang JQ, McGinty JF. Dose-dependent alteration in zif/268 and preprodynorphin mRNA expression induced by amphetamine and methamphetamine in rat forebrain. J Pharmacol Exp Ther. 1995;273:909–917. [PubMed] [Google Scholar]
- Wise RA. Brain reward circuitry: insights from unsensed incentives. Neuron. 2002;36:229–240. doi: 10.1016/s0896-6273(02)00965-0. [DOI] [PubMed] [Google Scholar]
- Xiao B, Tu JC, Petralia RS, Yuan JP, Doan A, Breder CD, Ruggiero A, Lanahan AA, Wenthold RJ, Worley PF. Homer regulates the association of group I metabotropic glutamate receptors with multivalent complexes of homer-related, synaptic proteins. Neuron. 1998;21:707–716. doi: 10.1016/s0896-6273(00)80588-7. [DOI] [PubMed] [Google Scholar]
- Xiao B, Tu JC, Worley PF. Homer: a link between neural activity and glutamate receptors. Curr Opin Neurobiol. 2000;10:370–374. doi: 10.1016/s0959-4388(00)00087-8. [DOI] [PubMed] [Google Scholar]
- Yang L, Mao L, Chen H, Catavsan M, Kozinn J, Arora A, Liu X, Wang JQ. A signaling mechanism from Gaq-protein-coupled metabotropic glutamate receptors to gene expression: role of the c-Jun N-terminal kinase pathway. J Neurosci. 2006;26:971–980. doi: 10.1523/JNEUROSCI.4423-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]


