Abstract
The nucleus tractus solitarius (nTS) of the brainstem receives sensory afferent inputs, processes that information, and sends projections to a variety of brain regions responsible for influencing autonomic and respiratory output. The nTS sends direct projections to the rostral ventrolateral medulla (RVLM), an area important for cardiorespiratory reflexes and homeostasis. Since the net reflex effect of nTS processing ultimately depends on the properties of output neurons, we determined the characteristics of these RVLM-projecting nTS neurons using electrophysiological and immunohistochemical techniques. RVLM-projecting nTS neurons were identified by retrograde tracers. Patch clamp analysis in the horizontal brainstem nTS slice demonstrated that RVLM-projecting nTS cells exhibit constant latency solitary tract evoked EPSCs, suggesting they receive strong monosynaptic contacts from visceral afferents. Three distinct patterns of action potential firing, associated with different underlying potassium currents, were observed in RVLM-projecting cells. Following activation of the chemoreflex in conscious animals by three hours of acute hypoxia, 11.2 ± 1.9% of the RVLM-projecting nTS neurons were activated, as indicated by positive Fos-immunoreactivity. Very few RVLM-projecting nTS cells were catecholaminergic. Taken together, these data suggest that RVLM projecting nTS neurons receive strong monosynaptic inputs from sensory afferents and a subpopulation participates in the chemoreflex pathway.
Keywords: cardiovascular, respiration, chemoreflex, retrograde, Fos
Introduction
The nucleus tractus solitarius (nTS) is the primary site of termination for multiple visceral afferents including those involved in cardiorespiratory reflexes (Andresen and Kunze, 1994; Spyer, 1994; Kline, 2008). This vital brainstem nucleus processes and integrates information from sensory afferents such as the arterial baroreceptors and carotid body chemoreceptors. Projection neurons from the nTS then send this information to numerous brain regions involved in autonomic and respiratory regulation (Andresen and Kunze, 1994). The activity of nTS projection neurons depends on afferent input to the nTS, processing within the nucleus, and the intrinsic properties of the output neurons themselves. Ultimately, the activity of these projection neurons determines the net effect of the nTS on cardiorespiratory reflex responses.
The rostral ventrolateral medulla (RVLM) plays an essential role in basal and reflex regulation of the autonomic nervous system and breathing (Guyenet, 2006). This brain region contains neurons crucial for controlling sympathetic activity in both arterial baroreflex and chemoreflex pathways. Blockade of neuronal activity in the RVLM eliminates baroreflex-mediated changes in sympathetic nervous system activity in response to alterations in arterial pressure (Dampney, 1994a; Guyenet, 2006). Similarly, sympathoexcitatory responses to chemoreceptor stimulation require activation of neurons in the RVLM (Koshiya et al., 1993). Thus, the RVLM is critical in the pathway for cardiorespiratory reflex adjustments in response to changes in afferent input.
The nTS sends monosynaptic projections to the RVLM, including both excitatory and inhibitory inputs (Ross et al., 1985; Hancock, 1988; Van Bockstaele et al., 1989; Aicher et al., 1996; Koshiya and Guyenet, 1996). It has been suggested that excitatory synapses within the RVLM contribute to pressor responses to carotid chemoreflex activation and these may arise from direct nTS projections (Van Bockstaele et al., 1989; Aicher et al., 1996; Koshiya and Guyenet, 1996). However, the fundamental properties of these RVLM-projecting nTS neurons are not known. It is important to determine such characteristics because, in distinct populations of nTS projection neurons, the fundamental properties, including afferent processing, are target specific (Bailey et al., 2006). Moreover, the activity of these projection neurons directly influences cardiorespiratory reflex output.
This study was designed to examine the intrinsic characteristics of nTS neurons that project to the RVLM. We hypothesized that 1) given the importance of the RVLM in cardiorespiratory regulation, inputs from cranial visceral afferents to RVLM-projecting nTS neurons are monosynaptic and exhibit a high success rate of synaptic transmission; and 2) a subpopulation of RVLM-projecting nTS neurons is activated by acute hypoxia in vivo, as indicated by Fos expression. We used the in vitro brainstem slice preparation and Fos immunohistochemistry to test these hypotheses. Retrograde tracers were used to identify nTS neurons that project to the RVLM. Results of these studies indicate that RVLM-projecting nTS neurons exhibit heterogeneous firing properties that are influenced by their compliment of potassium currents. These cells receive strong monosynaptic contacts from visceral afferents and some of these contacts originate from the carotid body. In addition, acute hypoxia activates a population of RVLM-projecting nTS neurons that was not catecholaminergic.
Experimental Procedures
Animals
All procedures were conducted in accordance with the guidelines in the NIH “Guide for the Care and Use of Laboratory Animals” and were approved by the University of Missouri Animal Care and Use Committee. Adult male Sprague-Dawley rats (Harlan, Indianapolis, IN, n=30; 280 ± 37 grams) were maintained on a 12 hour light-dark cycle with food and water provided ad libitum. All rats were allowed a minimum of seven days to acclimate to the surroundings prior to any experimental procedure.
Surgical Procedures
Rats were anesthetized with Isoflurane [AErane, Baxter, Deerfield, IL (induction: 5% in 100% O2, 2L per minute; maintenance: 2-2.5%)]. Using aseptic technique, a catheter was inserted into the aorta via the femoral artery and arterial pressure was monitored. Rats then were placed in a stereotaxic apparatus (Kopf, Tujunga, CA), and the dorsal surface of the medulla exposed via a midline incision. Calamus scriptorius (CS, the caudal most portion of the area postrema) was identified and the head deflected downward so that inter-aural zero was 2.4 mm rostral to CS (Kiely and Gordon, 1993; Moffitt et al., 2002), placing the brainstem on the horizontal plane.
Microinjection Procedures
Double-barreled glass pipettes (OD 20-30 μm), with one barrel containing L-glutamate (Glu, 10 mM) and the second filled with retrograde tracer were inserted into the brainstem with the aid of a dissecting microscope. The pipette was advanced to the initial target stereotaxic coordinates for the RVLM (anterior-posterior +0.7-0.8 mm, lateral +1.6-1.8 mm, and ventral −3.6-4.2 mm relative to CS and the dorsal surface of the brain; brainstem positioned horizontally). The RVLM was identified functionally by pressor responses (≥ 10 mmHg) to microinjection of L-glutamate (10 mM, 30 nL). The retrograde tracer (30 nL) was then microinjected into the same site through the second barrel of the pipette over ~1 min. Microinjections were performed using a custom-built pressure microinjection system. Injection volumes were quantified by monitoring the movement of the meniscus within a pipette barrel of known diameter using a 150x microscope (Rolyn Optics, Corvina, CA) with a calibrated eyepiece micrometer. The retrograde tracer used varied depending upon the experiment. For immunohistochemistry experiments Fluoro-Gold (FG, 2% in dH2O, Fluorochrome Inc, Denver, CO) was injected. Because FG was not conducive to whole cell recording in the in vitro brainstem slice in our hands, brainstem slice electrophysiology experiments utilized fluorescent Retrobeads (LumaFluor, Naples, FL) which have been extensively used in electrophysiological studies and do not alter the physiological properties of recorded neurons (Katz et al., 1984; Dekin et al., 1987). For experiments in which nTS cell neuronal morphology was assessed, cholera toxin B (CtB, 1%) was used. Although not quantified, the distribution of Retrobead-, Fluoro-Gold-, and CtB-labeled cells did not appear different.
Following microinjection of retrograde tracer, the pipette remained in the medullary tissue for at least five minutes to minimize movement of tracer up the injection tract. The arterial catheter was withdrawn and wounds sutured closed. Rats were treated post operatively with subcutaneous fluids (3 ml, 0.9% saline) and administered Baytril (0.03 ml i.m., Bayer, Shawnee Mission, KS) and Buprenex (0.6 mg/mL s.c, Reckitt Benckiser Pharmaceuticals, Richmond, VA) to prevent infection and for pain management, respectively. Upon recovery from anesthesia animals were returned to their cages.
In a subset of animals, the carotid body was labeled with anterograde tracer to study RVLM-projecting nTS cells which receive contact from chemoreceptor sensory afferents. Briefly, rats were anesthetized with Isoflurane and a midline incision in the neck was made. The carotid body was located, separated from the surrounding tissue, and labeled with anterograde tracer. For electrophysiological studies (n = 3), the lipophilic dye DiI (Molecular Probes, Eugene OR) was placed on the carotid body and sealed in place by Kwik-Sil (WPI). For immunohistochemical studies (n = 3), CtB was microinjected directly into the carotid body. The neck wound was sutured closed. Microinjection of retrograde tracer in the RVLM was then performed.
Brainstem Slice Preparation and Electrophysiology
Seven to ten days after microinjection of retrograde tracer or 4 weeks following combined retrograde tracer and DiI anterograde tracer, brainstem slices containing the nTS were prepared (Kline et al., 2002). Animals were anesthetized with Isoflurane and decapitated. The brainstem was removed and placed in ice-cold low calcium-high magnesium artificial cerebral spinal fluid (aCSF) containing the following (in mM): 124 NaCl, 3 KCl, 1.2 NaH2PO4, 1.2 MgSO4, 25 NaHCO3, 11 D-glucose, 0.4 L-ascorbic acid, 2 MgCl2 and 1 CaCl2, saturated with 95% O2–5% CO2, pH 7.4 (300 mOsm). Horizontal slices (~300 μm) were cut using a vibrating microtome (VT 1000S; Leica, Germany). Tissue sections were placed in a superfusion chamber that contained inlet and outlet ports for aCSF flow. The submerged sections were secured with a nylon mesh and superfused at a flow rate of 3–4 ml/min with standard recording aCSF (in mM: 124 NaCl, 3 KCl, 1.2 NaH2PO4, 1.2 MgSO4, 25 NaHCO3, 11 D-glucose, 0.4 L-ascorbic acid, and 2 CaCl2, saturated with 95% O2–5% CO2, pH 7.4, 300 mOsm) at 31–33°C. All recordings were made from fluorescent Retrobead-identified RVLM projecting cells in the caudal nTS (medial and commissural subnuclei), which receives a high density of carotid body afferent and cardiorespiratory innervation (Andresen and Kunze, 1994; Kline et al., 2002). Caudal nTS neurons were visualized using an Olympus BX-51WI microscope (40x magnification, Tokyo, Japan) equipped with fluorescence, differential interface contrast, and an infrared-sensitive camera. The pipette was guided using a piezoelectric micromanipulator (PCS-6000; Burleigh, Victor, NY). Recording electrodes (8250 glass) were filled with a solution containing the following (in mM): 10 NaCl, 130 K-gluconate, 11 EGTA, 1 CaCl2, 10 HEPES, 1 MgCl2, 2 MgATP and 0.2 NaGTP, pH 7.3 (290-295 mOsm, 2.5–3.5 MΩ). For post-recording cell identification, in some experiments the pipette contained 1 mg/ml Alexa Fluor 594 Hydrazide (Molecular Probes, Carlsbad CA). Following recordings, sections were incubated in 4% paraformaldehyde (2 hr), washed in PBS and mounted on a gelatin-coated slide. Morphology was evaluated using the NeuronJ plugin (ver 1.4.1) in Image J.
Data were recorded using a Multiclamp700B amplifier, filtered at 2 kHz, and sampled at 10 kHz using pClamp10 software (Molecular Devices, Palo Alto, CA). Recordings were made in whole-cell voltage and current clamp modes. Neurons were rejected if resting membrane potential was more positive than −45 mV under current clamp mode during initial membrane rupture. No leak subtractions, liquid junction potential corrections, or series resistance compensations were performed. Input resistance was determined in voltage clamp mode from current induced by steps from −60 to −65 mV. All drugs in electrophysiology experiments were purchased from Tocris Bioscience (Ellisville, MO) and Sigma (St. Louis, MO).
Excitatory Postsynaptic Current (EPSC) Recording
Solitary tract (TS) evoked EPSCs were generated using a concentric bipolar stimulating electrode (FHC, Bowdoinham, ME) placed on the visible TS, which contains chemosensory and other visceral afferents (Andresen and Kunze, 1994). The TS was stimulated (0.1 ms) with an isolated programmable stimulator (A.M.P.I., Jerusalem, Israel). Although stimulation of the TS in this manner globally activates all sensory fibers, we limited our recordings to caudal, fluorescent Retrobead-labeled nTS neurons. The intensity of TS stimulation was progressively increased until an EPSC was evoked. Final stimulation intensity was set at 1.5x threshold. Recordings were made in voltage clamp mode (held at −60 mV) while TS was stimulated at 0.5 and 20 Hz. Spontaneous EPSCs were recorded in the absence of TS stimulation. In a subset of cells, the antagonist CNQX (10 μM, 2 min) was bath applied to determine whether non-NMDA glutamatergic receptors mediate neurotransmission at this synapse.
Analysis of Passive and Active Membrane Properties
Membrane voltage and action potential discharge (APD) were recorded in current (I) clamp mode. Resting membrane potential was recorded under I = 0 mode. Current clamp mode was used to record APD to stepwise current injection (500 ms duration, −20 to + 60 pA, 20 pA intervals). To determine the adaptation of APD during prolonged depolarizations (spike frequency adaptation, SFA), the duration of current injection was increased to 1500 ms. The SFA index was calculated by dividing the instantaneous firing frequency (FI) during the first interspike interval by the steady state firing frequency (FSS) measured during the last 200 ms of the current pulse (Tell and Bradley, 1994). Delayed excitation (DE), the delay in the onset of APD due to prior hyperpolarization of the membrane, was evaluated by negative current pulses (−10 to −80 pA, 750 ms) immediately prior to a positive current pulse (50 or 100 pA, 750 ms).
Potassium Current Measurements
Transient and steady state outward currents were determined in voltage clamp mode from step voltages between −100 and +40 mV (10 mV steps, 500 ms) from an initial potential of −90 mV. Transient current was determined by quantifying the difference between the peak and steady state current amplitude. No ion channels were blocked during potassium current recording.
Immunohistochemical Protocols
Acute Hypoxia
Five to seven days after the injection of retrograde tracer into the RVLM and CtB anterograde tracer into the carotid body, conscious rats in their home cage (n = 7) were placed into a hypoxic chamber (Biospherix Inc., Redfield, NY). Following a 30 minute acclimation period, the gas mixture was adjusted to bring the air in the chamber to a level of 10% O2 and maintained at that percentage for three hours. Gas levels and temperature were monitored throughout the protocol. Control animals in their home cage (n = 7) were placed next to the hypoxic chamber for the same time period, ensuring that they were exposed to the same sounds and other environmental stimuli as the hypoxic animals.
Immunohistochemistry
Immediately after the three hour period of hypoxia or normoxia, animals were deeply anesthetized with Isoflurane and transcardially perfused with 0.1M PBS (pH 7.4) followed by 4% paraformaldehyde (Sigma, 500 mL). Coronal brainstem sections (30 μm) from 3 hypoxic and 3 normoxic animals were cut on a vibrating microtome (VT 1000S; Leica, Germany) and immunohistochemistry was performed on every sixth section (1 in 6 series, separated by 180 μm). Similarly, in an additional group of rats (n = 4 hypoxic and 4 normoxic), horizontal sections (30 μm) were cut, and immunohistochemistry was performed on every third section (1 in 3 series, separated by 90 μm). Brains with prior CtB injections in the RVLM (n=2) were sectioned at 100 μm on a vibratome and used for evaluation of morphology of RVLM projecting nTS neurons.
Brainstem sections were treated with an immunohistochemical protocol to visualize Fos-immunoreactive (IR) cells within the nTS. Because catecholaminergic cells in the nTS have been reported to be important in cardiorespiratory reflexes and to project to the RVLM (Van Bockstaele et al., 1989), we also performed immunohistochemistry for tyrosine hydroxylase (TH), the rate limiting enzyme in catecholamine synthesis. To carefully evaluate both rostral-caudal and dorsal-ventral distributions, immunohistochemistry was performed in both coronal and horizontal sections. Previous experiments (Austgen et al., 2008) verified optimal primary and secondary antibody concentrations. Briefly, free floating sections were rinsed (3 × 10 min) in 0.1M PBS (pH 7.4). They were then blocked in 10% Normal Donkey Serum (NDS; Jackson ImmunoResearch, Inc.) in 0.3% Triton-0.1M PBS for 30 minutes. Tissue was then rinsed and incubated (room temperature, 16 hours) in 1% NDS and 0.3% Triton-0.1M PBS containing primary antibody against Fos (rabbit anti-Fos, 1:3000, Calbiochem, San Diego, CA) and TH (mouse anti-TH, 1:1000, Chemicon). Sections were rinsed and incubated (room temperature, 2 hours) in 1% NDS in 0.3% Triton-0.1M PBS including Cy3-conjugated donkey anti-rabbit IgG and Cy2-conjugated donkey anti-mouse IgG (1:200 Jackson ImmunoResearch, Inc., West Grove, PA). Individual immunohistochemistry protocols were conducted simultaneously on tissue from hypoxic and normoxic animals. In an additional series of sections from one hypoxic animal, FG was also visualized using an anti-FG antibody (guinea pig anti-FG, Protos Biotech, New York, NY, 1:500).
Sections from animals receiving CtB injections in the RVLM or carotid body underwent similar immunohistochemical protocols using a primary antibody against CtB (goat anti-cholera toxin B subunit; List Biological Laboratories, Inc., 1:2000). Following overnight incubation in primary antibody, tissue was rinsed and incubated in donkey anti-goat biotin (Jackson; 1:200) and streptavidin Cy3 (Jackson; 2 μg/ml), or Cy3-conjugated donkey anti-goat IgG.
Sections were mounted on gel-coated slides, air dried and coverslipped with ProLong Gold (Molecular Probes, Molecular Probes). All slides were then sealed with nail polish.
Antibody Specificity
In each protocol, primary antibodies were withheld from single sections to serve as controls. Antibody specificity for Fos (Calbiochem) was verified by the vendor and specificity of the TH antibody was verified in our laboratories by western blot (Austgen et al., 2008).
Verification of Injection Sites
In all animals, at the time of microinjection the RVLM was functionally verified as described above. Injection sites were also verified anatomically by comparing the injection site in a series of medullary slices to a standard brain atlas (Paxinos G and Watson C, 1997).
Microscopy and Image Analysis
Brainstem sections were examined using an Olympus epifluorescent microscope (BX51), with a 3-axis motorized stage (Ludl Electronic Products Ltd, Hawthorne, NY). Appropriate filter sets for Cy 2 [ex. λ480 nm; em. λ510 nm], Cy3 [ex. λ550 nm; em. λ570 nm], and Fluoro-Gold [ex. λ330 nm; em. λ515 nm] were used to visualize positive labeling. Images under each filter set in the same focal plane were captured using a cooled monochrome digital camera (ORCA-AG, Hamamatsu, Bridgewater, NJ). Images subsequently were combined and analyzed with Image J (ver. 1.41, NIH) using a custom made plugin (GAIA Group, Novato, CA, http://gaiag.net/index.html). Images obtained for colocalization of CtB-labeled afferents and RVLM-retrograde label were deconvolved using Image J (Parallel Spectral Deconvolution by Piotr Wendykier).
For immunohistochemical analysis of coronal sections, we evaluated the caudal nTS, which is the primary site for cardiorespiratory afferent innervation. The section containing the caudal pole of the area postrema was identified and designated as calamus scriptorius (CS, labeled as “0”). The nTS was then examined in rostral-caudal sections +900, through −720 μm relative to CS. For analysis of horizontal sections, the section containing the most ventral portion of the area postrema was identified as “0”. We then examined sections 180 μm dorsal through 450 μm ventral to that section. Briefly, the region of the nTS was outlined on the digital image of each section and cells were counted manually. Counting was performed by two individuals blinded to the experimental protocol and counts for each section averaged. Criteria used to identify positively labeled cells were as follows: Fos-IR labeling was identified as nuclear staining with a visible nucleolus. TH-IR cells were identified by cytosolic labeling with visible processes and a blank nuclear region. FG-positive cells displayed punctate cytosolic labeling surrounding a blank nuclear region or bright cytosolic labeling. For representative images, single photomicrographs were taken with the appropriate filters, imported into Photoshop (ver. 11.0 Adobe Systems, San Jose, CA) pseudocolored and combined. Image brightness and contrast only were adjusted for clarity.
Cells were considered to be colabeled if they exhibited these characteristics in more than one filter set in the same focal plane: a) for FG labeling and TH-IR, cytosolic labeling of the same shape was observed in both filter sets, or b) for Fos-IR and either FG labeling or TH-IR, the location of nuclear Fos staining corresponded to the blank region in cytosolic labeling of FG or TH-IR.
For evaluation of morphology in CtB-labeled RVLM-projecting neurons, 100 μm sections were used and captured using a microscope equipped with a confocal spinning disk (Olympus DSU, 2 μm slices). Only cells with the soma localized more than 20 μm from the top or bottom of the slice were evaluated. In addition, cells were counted only if the entire soma could be visualized and the processes were at least 15 μm long.
Close appositions of terminals labeled from the carotid body on RVLM-labeled neurons activated by hypoxia were examined using spinning disk confocal microscopy (Olympus DSU, 0.1 - 0.5 μm slices) with a 40X or 60X oil objective. An RVLM-projecting nTS neuron was considered to have close appositions from the carotid body if a terminal (1-5 μm, (Massari et al., 1996; May et al., 2007) was observed within 0.5 μm of a soma that exhibited FG labeling.
Data Analysis
Electrophysiological data were analyzed via Molecular Devices Clampfit software. Each data point for a given trial was an average of 20 sweeps for evoked EPSCs at 0.5 Hz and 5 EPSC sweeps at 20 Hz. The time of EPSC initiation from stimulus artifact (i.e. latency) was determined as the first data point that exceeded noise. Jitter (S.D. of EPSC latency) was analyzed from 20 current sweeps as described previously (Doyle and Andresen, 2001; Kline et al., 2002).
Immunohistochemical data were analyzed at each rostral-caudal or dorsal-ventral level of the nTS and as the total of all sections counted within the nTS. Also, the percentage of double-labeled cells was determined relative to the total number of Fos-IR or the total number of FG labeled cells.
All data are presented as means ± SEM. Statistical analyses were performed with SigmaStat (3.5, Systat Software, San Jose, CA). Electrophysiological data were compared by Student's t-test and two-way repeated measures ANOVA. Changes in mean arterial pressure in response to glutamate injection into the RVLM were analyzed by Student's t-test. Total Fos-IR and FG labeled cells in normoxic and hypoxic animals and the Fos and FG colabeling also were compared by Student's t-test. The rostral-caudal or dorsal-ventral distribution of Fos, FG and colabeling after hypoxia was compared by one-way Repeated Measures ANOVA. When a significant interaction occurred, ANOVA's were followed by Student-Newman-Keuls post-hoc analysis. Statistical significance was accepted at p < 0.05.
Results
Verification of RVLM Injection Sites
In all animals, RVLM injection sites were identified functionally by pressor responses to microinjection of glutamate (10 mM, 30 nl) before injection of retrograde tracer. Responses for animals in the immunohistochemical and slice experiments were combined for analysis. Glutamate microinjection into the RVLM resulted in a significant increase in mean arterial pressure (11 ± 0.5 mmHg). Injection sites also were examined histologically. Figures 1A,B,D and E show representative injection sites from two individual animals in coronal and horizontal sections. The centers of the injection site for all animals are shown in Figures 1C and F. The midpoints of the injections were within 500 μm of the caudal pole of the facial nucleus in the ventrolateral medulla. This corresponds to the region of the RVLM containing presympathetic neurons (Dampney, 1994a; Dampney, 1994b; Guyenet, 2006). Injection sites were similar in animals from both the normoxic and hypoxic groups.
Figure 1. Injection sites for retrograde label into the RVLM.
Brightfield (A) and epifluorescent (B) photomicrographs of the same section showing an example, in the coronal plane, of an RVLM microinjection of Fluoro-Gold (FG). Arrow indicates the center of the injection site. C) Location of FG injection sites in animals with coronal tissue sections. Numbers indicate level (mm caudal) relative to Bregma. Brightfield (D) and epifluorescent (E) photomicrographs of the same section showing an example, in the horizontal plane, of an RVLM microinjection of FG. Arrow indicates the center of the injection site. F) Location of FG injection sites in animals with horizontal tissue sections. Upper right number indicates level (mm ventral) relative to Bregma. 7, Facial nucleus; IO, inferior olive; PY, pyramidal tract; nA, nucleus Ambiguus. Open circles, injection sites in normoxic animals; filled circles, injection sites in hypoxic animals.
Electrophysiological Characterization of RVLM-projecting nTS cells
Excitatory Postsynaptic Currents (EPSCs)
Bilateral microinjection of fluorescent Retrobeads into the RVLM resulted in nTS labeled cells that were distributed throughout the caudal nTS (medial and commissural subnuclei, Fig 2A left and middle, in the horizontal plane). Identification of fluorescent Retrobead-labeled cells allowed the placement of the recording pipette on the projecting cell for patch clamp recording (Fig 2A, right). The results of electrophysiological experiments are based on recordings from RVLM-projecting nTS neurons from 14 rats that were distributed in the medial and commissural subnuclei, an area of high carotid body afferent distribution. The mean values for membrane characteristics are as follows; resting membrane potential −54.2 ± 2.0 mV; input resistance, 439 ± 48 MΩ, and access resistance 13.7 ± 1 MΩ.
Figure 2. Synaptic properties of RVLM-projecting nTS cells.
A. Composite images of the horizontal nTS slice viewed under DIC (left) and DIC + epifluorescence (green, middle). mnTS, medial nTS; comnTS, commissural nTS. Right panel, identification of a Retrobead labeled cell in the nTS permitted placement of the patch electrode on the RVLM projecting cell. B. Representative example from a projecting cell of a TS evoked EPSC which was sensitive to CNQX (10 μM). C. TS evoked EPSCs were monosynaptically connected to afferent fibers. Left panel, superimposed current traces from a representative cell illustrating consistent EPSC latency (i.e., minimal jitter) which is indicative of a monosynaptic cell. Right panel, amplitude versus jitter distribution plot. Asterisk by symbol is likely a polysynaptic cell. D. Quantitative data for an EPSC train (20 Hz) are plotted as raw EPSC amplitude (left axis, closed diamonds) and change from the first EPSC (right axis, open squares) relative to EPSC within the train of events. TS-EPSCs reduced amplitude to 50% of maximum within the first three events. Inset, representative example of a train of TS-evoked EPSCs illustrating frequency dependent depression.
To characterize synaptic transmission in these cells, whole-cell voltage-clamp recordings were used to examine TS-evoked and spontaneous EPSCs in horizontal brainstem slices (Kline et al., 2002). Electrical shocks to the TS at 0.5 Hz evoked EPSCs that were abolished by the non-N-methyl-D-aspartate (non-NMDA) glutamate receptor antagonist CNQX (10 μM; n = 6). Figure 2B contains a representative trace of an EPSC which was blocked by CNQX. Of the neurons examined, the majority (21 of 22) were considered monosynaptic as determined by TS-EPSC characteristics. Monosynaptic EPSC amplitude averaged 208 ± 28 pA with a mean onset latency of 4.1 ± 0.4 ms. Jitter, the S.D. of onset latency and a primary indicator of a monosynaptic cell in the nTS, averaged 158 ± 13 μs (16 of 22 neurons had a jitter < 200 μs, and 21 of 22 neurons had jitter values < 300 μs, Fig 2C). Spontaneous EPSC amplitude for these monosynaptic cells averaged 23.8 ± 2.1 pA, and frequency averaged 7.3 ± 0.9 Hz (n=21 cells). Spontaneous events were also abolished by CNQX (data not shown). The remaining cell (n = 1, asterisk in Fig 2C) had a higher jitter of 355 μs, a TS-EPSC amplitude of 97.6 pA, and had an onset latency of 6.0 ms, suggesting it was polysynaptically connected (Doyle et al., 2004). sEPSC amplitude and event frequency for the polysynaptic cell was 24.4 pA and 14.3 Hz, respectively. Failure rate did not correlate with jitter and was comparable among all neurons, averaging 6 ± 2%.
To mimic the increase in afferent activity that would occur during an increase in chemosensory activity, the frequency and duration of TS stimulation were increased. Increasing the frequency of TS stimulation to 20 Hz produced depression in the EPSC train in monosynaptically connected RVLM-projecting nTS cells (Fig 2D). An example of EPSC depression in a cell is shown in the inset. This frequency dependent depression is a common feature in nTS synapses. In these projecting cells, EPSC amplitudes were decreased to 50% of their peak amplitude by the third event and remained at this level for the remainder of the stimulus protocol (Fig 2D, right axis).
Active Membrane Properties
The majority of the RVLM-projecting nTS neurons did not display spontaneous firing activity (20 of 26, 77%). Current induced action potential properties and firing characteristics of RVLM-projecting nTS neurons were recorded in 18 cells (11 rats). The excitability of identified monosynaptic RVLM-projecting cells (n = 17) was examined by current-induced step depolarization (+20 pA steps, 500 ms, interval of 2 sec) from a constant holding potential of −60 mV to evoke action potential discharge (APD). Three distinct firing patterns were observed in monosynaptic RVLM-projecting neurons (Fig 3): a) cells which spiked continuously during step depolarization (“tonic cells;” n=7, Fig 3A1), b) tonically firing cells which exhibited a delay in AP spiking (“delayed excitation cells;” n=6, Fig 3B1), and c) cells which fired 1-4 AP's at the beginning of the pulse then remained quiet during sustained depolarization (“phasic cells;” n = 4, Fig 3C1). The sole polysynaptic cell fired tonically. The characteristics of the first action potential in each firing type were not different (Table 1). In the seven cells which exhibited tonic firing properties, discharge began between +20 and +40 pA positive current injection (Fig 3D). At 60 pA, tonic cells fired 11.4 ± 3.8 action potentials in a 500 ms pulse. On the other hand, delayed excitation cells didn't discharge until +40 pA, and fired only 2.3 ± 1.6 AP's at 60 pA injection (500 ms duration, Fig 3D).
Figure 3. Active membrane properties of RVLM-projecting nTS cells.
Tonic (A), Delayed Excitation (DE, B) and Phasic (C) spiking neurons were observed in RVLM-projecting nTS cells. Representative examples are shown in A-C and insets illustrate recording protocol. Panels 1-3 for representative examples are from the same cell. Tonic cells immediately increased APD to a +60 pA current injection (A1) from a holding potential of −60 mV. Prior hyperpolarization did not alter firing properties of tonic spiking cells (A2). In the example shown, the initiation of discharge did not change whether the cell was held at −60 mV (grey line, arrow, number 1) or first hyperpolarized to −70 mV (black line, arrow, number 2). Delayed Excitation cells (B1) exhibited APD to a +60 pA current injection (−60 mV holding) only after an initial delay. Prior hyperpolarization to −70 mV (B2, black line, arrow, number 2) resulted in a greater delay in excitation. Phasic cells discharged 1-4 action potentials during sustained depolarization (+60 pA injection, from −60 mV, C1). Prior hyperpolarization to −70 mV produced variable effects on spike initiation. C2 illustrates the response in one cell. Potassium currents in the three cell spiking types are illustrated in A3-C3. Tonic cells did not exhibit prominent transient outward potassium currents (A3), whereas delayed excitation cells did (B3). Phasic cells had variable outward currents; C3 shows the currents in an individual phasic cell. D) Mean data illustrating number of action potentials evoked to incremental current injection. There was a progressive increase in APD with current injection in Tonic cells, but not DE cells. Tonic cells exhibited greater discharge compared to DE cells at 40 and 60 pA injection. E.) Summary of action potential delay to hyperpolarization in Tonic and DE cells. There was a progressive increase in delay to APD with hyperpolarization in DE cells, but not Tonic cells. F) Transient outward currents in DE and Tonic cells in response to depolarizing potentials. Note the larger transient currents in DE cells at more positive voltages. *, p < 0.05 between Tonic and DE cells.
Table 1.
Action Potential Properties of RVLM-projecting nTS neurons
| Tonic Spiking |
DE Spiking |
Phasic Spiking |
|
|---|---|---|---|
| Threshold (mV) | −29.11 ± 3 06 | −2511 ± 1.89 | −23.46 ± 3.47 |
| Peak amplitude (mV, Threshold to Peak) | 67.57 ± 3.37 | 69.96 ± 4.10 | 58.54 ± 2.58 |
| Peak amplitude (mV, from −60 mV) | 98.46 ± 4.55 | 104.86 ± 3.51 | 95.08 ± 5.38 |
| Overshoot (mV, over 0 mV) | 38.46 ± 4.55 | 44.86 ± 3.51 | 35.08 ± 5.38 |
| Half Width (ms) | 0.84 ± 0.07 | 0.99 ± 0.07 | 1.00 ± 0.07 |
| Rise time (ms, 10-90) | 0.47 ± 0.05 | 0.50 ± 0.11 | 0.62 ± 0.03 |
| Rise Slope (mV/ms, 10-90) | 127.99 ± 16.15 | 145.18 ± 25.42 | 79.57 ± 7.36 |
| Decay time (ms, 90-10) | 0.65 ± 0.06 | 0.82 ± 0.06 | 0.70 ± 0.06 |
| Decay Slope (mV/ms, 90-10) | −89.87 ± 9.30 | −70.75 ± 6.27 | −70.69 ± 6.75 |
Values are means ± SEM. Tonic cells, n=7; DE cells, n = 6; Phasic cells, n = 4
Subsequently, we extended the current duration from 500 to 1500 ms to examine the spike frequency adaptation index (SFA index, defined as the ratio of FI to FSS) of RVLM-projecting nTS cells. SFA was quantified for each cell based on steady state frequencies [5-10, 11-20, 21-30, 31-40 Hz, (Vincent and Tell, 1997)]. In general, RVLM-projecting cells reduced instantaneous frequency (SFA > 1) over a 1500 ms depolarizing pulse (Tonic: 1.42 ± 0.58; Delayed excitation cells: 1.86 ± 0.63 at 5 – 10 Hz). The SFA index was not different between tonic and delayed excitation cells at any given steady state frequency between 5 and 40 Hz. SFA was not determined in phasic cells.
Hyperpolarizing the membrane prior to depolarizing the cell to threshold differentially affected firing in the three identified cells types (Fig 3, A2, B2, C2). In tonic cells, firing properties were not altered due to initial hyperpolarization (Fig 3A2 and Fig 3E). In contrast, increasing prior hyperpolarization in delayed excitation cells significantly increased the delay in the onset of APD (n= 6, Fig 3B2 and Figure 3E). In 3 of 6 delayed excitation cells, strong hyperpolarization (> −60 pA injection) completely eliminated APD. In 3 phasic cells, prior hyperpolarization either did not alter firing (n = 1; Fig 3C2) or eliminated spiking (n = 2).
Potassium Currents (Ik) in RVLM-Projecting Neurons
Outward potassium currents were investigated to determine their potential contribution to the firing characteristics of RVLM-projecting nTS neurons. Step depolarization from −100 to +40 mV elicited outward potassium currents in RVLM-projecting neurons (Fig 3A3-C3). As observed with action potential firing properties, there was heterogeneity of transient outward current in projecting neurons. The transient K+ current was defined as the difference between transient (peak) and steady state (ss) current. Transient K+ currents were significantly larger in delayed excitation (DE) cells compared to tonically firing cells over a voltage range of 0 to 40 mV (Figure 3F). Transient K+ currents in phasic cells were variable; one example is shown in Fig 3C3.
Morphology
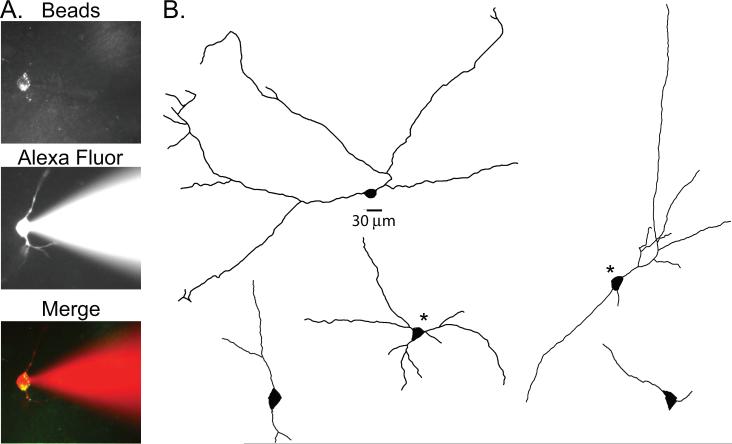
Five RVLM projecting nTS neurons were successfully filled with Alexa Fluor 594 during recording (Figure 4A) and were processed for morphology. Neurons were observed as either bipolar or multipolar cells based on soma shape and origin of the dendrites (Figure 4B). In separate experiments, CtB was injected into the RVLM to evaluate the prevalence of the different morphological types of RVLM-projecting nTS cells. Of the total of 244 CtB-labeled nTS neurons counted from 2 animals, 87% of RVLM-projecting cells exhibited bipolar morphology (n=212) whereas 13% were multipolar (n=32).
Figure 4. Morphology of RVLM-projecting nTS neurons.
Left: Example of an identified RVLM-projecting nTS neuron containing Retrobeads from the RVLM (top panel), with a patch pipette containing Alexa Fluor 594 Hydrazide dye attached (middle panel); Alexa dye can be seen filling the cell; overlay of the images showing that the cell being recorded contained Retrobeads (bottom panel). Right: Morphology of five Alexa Fluor filled RVLM-projecting neurons that were bipolar and multipolar. * indicates multipolar cells.
Immunohistochemical Experiments on RVLM-projecting cells
As shown in the electrophysiological experiments, RVLM-projecting cells were distributed throughout the caudal nTS, and a portion of them were activated by hypoxia as indicated by Fos-IR. Figure 5 contains photomicrographs from representative animals showing FG labeling in the coronal (Fig 5A) and horizontal (Fig 5E) planes. Consistent with Retrobead labeling in our slice experiments, RVLM-projecting neurons were present in the commissural and medial nTS, in the region of termination of cardiorespiratory afferents.
Figure 5. RVLM-projecting nTS neurons exhibit Fos-IR in response to hypoxia.
A. Example of FG retrograde labeling (grey scale) from the RVLM in a coronal section of the dorsal medulla in an individual rat. B. Merged photomicrographs from the same coronal section as in A showing FG (pseudocolored blue), Fos-IR (pseudocolored red) and TH-IR (pseudocolored green). C. Higher magnification of boxed region in B. Note colabeling of FG and Fos-IR (arrowhead) and Fos-IR and TH-IR (double arrowhead) but not FG and TH-IR. D. Single 1.0 μm confocal section demonstrating Fos (left panel) and FG-labeling (middle panel) in an individual cell. Right panel is a merged image demonstrating the presence of Fos colabeled with the FG cell. E. Example of FG retrograde labeling (grey scale) from the RVLM in a horizontal section through the dorsal medulla in an individual rat. F. Merged photomicrographs from the same horizontal section as in E showing FG (pseudocolored blue), Fos-IR (pseudocolored red) and TH-IR (pseudocolored green). G. Higher magnification of boxed region in F. Note colabeling of FG and Fos-IR (arrowhead) and Fos-IR and TH-IR (double arrowhead) but not FG and TH-IR. H. Single 1.0 μm confocal section illustrating Fos (left panel) and TH (middle panel) in an individual cell. Right panel is a merged image demonstrating Fos colabeled with TH. 4V, Fourth ventricle; AP, area postrema; CC, central canal; TS, tractus solitarius.
Figure 6 depicts the rostral-caudal distribution of FG-labeled, Fos-IR and cells colabeled with FG or Fos-IR as determined from coronal sections. Figure 6A includes representative examples, Figure 6B shows the distribution of FG-labeled neurons and colabeling as a percentage of the number of FG-labeled cells, and Figure 6C shows the distribution of Fos-IR cells and colabeling as a percentage of the number of Fos-IR cells.
Figure 6. Distribution of RVLM projecting neurons and hypoxia-induced Fos immunolabeling in nTS in the coronal plane.
Diagrammatic representation of the distribution in an individual hypoxia-exposed rat of RVLM-projecting (FG, top row), Fos-IR (middle row) and RVLM-projecting + Fos-IR neurons (bottom row) at three different caudal to rostral levels within the nTS. Caudal-rostral levels shown relative to calamus scriptorius (0 in A). B) Caudal-rostral distribution of labeling; Left axis: Number of FG-labeled neurons in the nTS (open squares); Right axis: % of FG labeled nTS neurons in acutely hypoxic rats that are colabeled with Fos-IR (black squares) or TH-IR (grey squares). a, p < 0.05 from −720 μm; †, sustained increase from −720 μm; ††, further increase from −360 μm. C) Caudal-rostral distribution of labeling in acutely hypoxic rats; Left axis: Number of Fos-IR neurons in the nTS (open circles). Right axis: % of Fos-IR nTS neurons that are colabeled with FG (black circles) or TH-IR (grey circles). a, p < 0.05 from −720, −360 and −180 μm; †, sustained increase rostrally.
Figure 7 depicts the dorsal-ventral distribution of FG-labeled, Fos-IR and cells colabeled with FG or Fos-IR. Figure 7A includes representative examples, Figure 7B shows the distribution of FG-labeled neurons and colabeling as a percentage of the number of FG-labeled cells, and Figure 7C shows the distribution of Fos-IR cells and colabeling as a percentage of the number of Fos-IR cells.
Figure 7. Distribution of RVLM projecting neurons and hypoxia-induced Fos immunolabeling in nTS in the horizontal plane.
Diagrammatic representation of the distribution in an individual hypoxia-exposed rat of RVLM-projecting (top row), Fos-IR (middle row) and RVLM-projecting + Fos-IR neurons (bottom row) at three different dorsal-ventral levels within the nTS. Dorsal-ventral levels shown relative to the ventral aspect of the area postrema (0 in A). AP, area postrema; 4V, Fourth ventricle; TS, tractus solitarius. B) Dorsal-ventral distribution; Left axis: Number of FG-labeled neurons in the nTS (open squares); Right axis: % of FG labeled nTS neurons in acutely hypoxic rats that are colabeled with Fos-IR (black squares) or TH-IR (grey squares). †, sustained increase from +180 μm. C) Dorsal-ventral distribution of labeling in acutely hypoxic rats; Left axis: Number of Fos-IR neurons in the nTS (open circles); Right axis: % of Fos-IR nTS neurons after acute hypoxia that are colabeled with FG (black circles) or TH-IR (grey circles). †, different from +180 μm with no further change ventrally.
RVLM-Projecting (FG-labeled) Neurons
An example of the distribution of FG labeling in three rostral-caudal coronal sections from a representative animal is shown in Figure 6A, top panels. The average rostral-caudal distribution, from all coronal sections counted, of RVLM-projecting (FG) nTS neurons is shown in Figure 6B. The number of FG-labeled nTS cells (open squares) increased from the more caudal sections of nTS through CS (defined as “0”, Fig 6B, left axis). The greatest number of RVLM-projecting nTS neurons was observed in the region of CS and through the most rostral extent of the area postrema (0 to +900 μm rostral to CS).
The dorsal-ventral distribution of RVLM-projecting cells was evaluated using horizontal brainstem sections. The distribution of FG labeling in three dorsal–ventral horizontal sections from a representative animal is shown in Figure 7A, top panels. Data from examination of FG labeling in horizontal sections are shown in Figure 7B. The number of FG-labeled cells (left axis, open squares) was relatively low in more dorsal regions of the nTS and increased ventrally. The number of RVLM-projecting cells remained constant from −90 through −450 μm relative to the ventral pole of the area postrema (0).
Fos Immunoreactivity
Fos-immunoreactivity (IR) was used to examine nTS cells, particularly RVLM-projecting nTS neurons, that were activated by hypoxia. Figure 8 shows examples of Fos-IR in coronal (A and B) or horizontal (D and E) brainstem sections from rats exposed to normoxia (A and D) or 3-hr hypoxia (B and E). Note the increased number of Fos-IR cells in the nTS of the hypoxic animals. The number of Fos-IR cells within the nTS was significantly increased by 3 hr hypoxia when evaluated in either coronal (n = 3, normoxia, and 3, hypoxia, Fig 8C) or horizontal (n = 4, normoxia, and 4, hypoxia, Fig 8F) sections.
Figure 8. Acute hypoxia increases Fos-IR in the nTS.
Representative photomicrographs, in the coronal (A and B) or horizontal (D and E) plane, from individual animals exposed to three hours of normoxia or hypoxia (10% O2). C & F) The number of Fos-IR nTS neurons per section following exposure to three hours of normoxia or hypoxia (C; coronal: n = 3 each; F: horizontal: n = 4 each). 4V, Fourth ventricle; AP, area postrema; cc, central canal; nTS, nucleus tractus solitarius; TS, tractus solitarius.
The relative rostral-caudal distribution of Fos-IR cells in coronal sections from a hypoxic animal is shown in Figure 6A, middle row. The distribution of Fos-IR cells was quantified in Figure 6C, left axis. The number of Fos-IR cells (open circles) was relatively low from −720 through −360 μm caudal to CS and increased progressively to CS (0). The number of nTS cells exhibiting Fos-IR then remained high throughout the level of the area postrema.
The dorsal-ventral distribution of Fos-IR was examined using horizontal brainstem sections. Figure 7A, middle row, depicts this distribution in a representative hypoxic animal. As shown in Figure 7C, left axis, the number of Fos-IR cells (open circles) increased progressively to −180 μm relative to the ventral pole of the area postrema (0) and remained high throughout the more ventral regions of the nTS.
Colocalization of Fos in RVLM-Projecting nTS Neurons
Colocalization of Fos-IR with RVLM-projecting (FG labeled) neurons was observed in the nTS of animals exposed to 3 hr hypoxia. Examples of Fos-IR and FG colabeling in coronal and horizontal sections from individual hypoxic rats are shown in Figure 5B, C, F and G. Figure 5D shows spinning disk confocal images of Fos- and FG colabeling in a hypoxic animal in which an antibody was used to visualize FG. Colabeling was observed throughout the caudal nTS. The number of nTS cells exhibiting both Fos-IR and FG was significantly greater in tissue from hypoxic (68 ± 12, n = 7) compared to normoxic (5 ± 3, n = 7) rats. The overall percentage of RVLM-projecting cells that was also Fos-IR in all hypoxic animals (n = 7) was 11.2 ± 1.9%.
The rostral-caudal distribution of Fos-IR and FG colabeled cells in hypoxic rats was examined using coronal brainstem sections (n = 3). An example of rostral-caudal distribution of FG and Fos-IR colabeling in an individual animal is shown in Figure 6A, bottom panels. We evaluated the number of FG and Fos-IR colabeled cells relative to the number of FG labeled cells (Fig 6B, right axis). The percentage of RVLM-projecting nTS cells that expressed Fos in response to acute hypoxia was relatively low in the more caudal regions of the nTS although the data were variable at −540 μm relative to CS. This percentage increased in the more rostral regions of the nTS evaluated, so that ~20-25% of the RVLM-projecting cells at the level of the area postrema from +360 to +900 μm rostral to CS were activated by hypoxia. The percentage was higher even though the absolute number of FG-labeled cells was actually greater at these more rostral levels.
We also examined FG and Fos-IR colabeling relative to the number of nTS cells activated (exhibiting Fos-IR) in response to acute hypoxia. Of the Fos-IR cells in the nTS, overall (n = 7) 8 ± 3% also projected to the RVLM. As shown in Figure 6C, right axis, the percentage of Fos-IR nTS cells that were also labeled with FG was relatively low in the more caudal regions of the nTS but was variable at −540 μm caudal. This percentage increased to 10-15% of Fos-IR cells in the most rostral sections examined (Fig 6C, right axis).
The dorsal-ventral distribution of Fos-IR and FG colabeled cells following hypoxia was examined using horizontal brainstem sections (n = 4). An example of the distribution of this colabeling in a single animal is shown in Figure 7A, bottom panels. The percentage of RVLM-projecting (FG-labeled) nTS cells that also exhibited Fos-IR following acute hypoxia was relatively constant throughout the dorsal–ventral extent of the nTS (Figure 7B, right axis). Similarly, when Fos-IR nTS cells were examined, the percent that were also labeled with FG was relatively consistent throughout the dorsal-ventral extent of the nTS (Fig 7C, right axis).
Colocalization in Catecholaminergic nTS Neurons
TH-IR cells were distributed throughout the nTS. However, there were very few RVLM-projecting neurons that were also TH-IR (0.9 ± 0.4%, n = 7). Therefore, the percentage of FG-labeled cells that was also TH-IR was very low throughout the rostral-caudal (Fig 6B) and the dorsal–ventral (Fig 7B) extent of the nTS (right axes).
Following exposure to 3 hr acute hypoxia, colocalization of Fos-IR and TH-IR was observed in the nTS. Examples of Fos- and TH-IR colabeling in coronal and horizontal sections from individual hypoxic rats are shown in Figure 5C and G. Figure 5H shows spinning disk confocal images of Fos- and TH colabeling in a hypoxic animal.
The number of cells colabeled with Fos- and TH-IR was significantly greater in the nTS of hypoxic (129 ± 19, n=7) compared to normoxic (10 ± 6, n = 7) rats. For all hypoxic rats (n = 7), 15 ± 1% of nTS cells exhibiting Fos-IR in response to acute hypoxia were catecholaminergic (TH-IR). Examination of the rostral-caudal distribution of Fos- and TH-IR colabeling (Fig 6C, right axis) indicated that the relative amount of colabeling was least in the caudal nTS. The percentage of Fos-IR cells also exhibiting positive TH-IR progressively increased more rostrally, and was greatest between +360 to +900 μm rostral to CS. Fos- and TH-IR colabeling also was observed throughout the dorsal-ventral extent of the nTS examined (Fig 7C). The percentage of Fos-IR nTS cells exhibiting colabeling with TH-IR was greatest (reaching approximately 25% of the number of Fos-IR cells) in the region dorsal to the ventral pole of the area postrema (0), decreasing to low levels more ventrally (−360 to −450 μm, Figure 7C, right axis).
Carotid body afferents are in close apposition to RVLM-projecting nTS cells and colabeled cells are activated by hypoxia
Our initial electrophysiological data indicated that a substantial majority of RVLM-projecting nTS neurons receive monosynaptic connections from visceral afferents. Therefore, in a subset of animals, we labeled the carotid body with either DiI or CtB in combination with RVLM retrograde labeling (Retrobeads or FG) to determine if carotid body afferents terminate near RVLM-projecting nTS cells. A representative example of close apposition of DiI-labeled terminals to a RVLM-projecting nTS cell in the horizontal brainstem slice is shown in Figure 9A. TS stimulation elicited EPSC's in caudal nTS cells which possessed DiI-labeled terminals and Retrobeads (RVLM-projecting). In three cells from two animals, EPSC latency was 3.62 ± 0.65 ms and jitter was 125 ± 6 μs. Such jitter values suggest a monosynaptic connection.
Figure 9. RVLM-projecting cells which are activated by hypoxia receive input from carotid body afferents.

Representative photomicrographs from anterograde carotid body labeled and RVLM-projecting nTS cells. A) Image of RVLM-projecting nTS cell observed in the live in vitro brainstem slice that has closely apposed DiI-labeled carotid body terminals (arrow). Inset, image demonstrating placement of patch pipette on dual labeled cells for recording of EPSCs. B. & C) Spinning disk confocal images of RVLM-projecting nTS cells which exhibit Fos-IR in response to hypoxia and also possess closely apposed CtB-labeled carotid body terminals (arrows). B) Z-projection of 7 separate 0.1 μm slices taken with a confocal spinning disk microscope (final image is 0.7 μm). C) Single 0.5 μm confocal section. Inset in C, zoomed single slice image of asterisk labeled terminal demonstrating close apposition of terminal to FG cell. Scale in inset, 1 μm. Fos-IR is pseudocolored blue, Retrobeads (panel A) and FG (panel B and C) are pseudocolored green, DiI (panel A) and CtB (Panels B & C) are pseudocolored red.
To determine if RVLM-projecting nTS cells that express Fos in response to hypoxia receive close appositions from carotid body terminals, immunohistochemistry was performed in three animals. Five to seven days following FG retrograde and CtB anterograde tracer placement, rats were exposed to 10% O2 for three hours. Spinning disk confocal microscopy indicated that CtB labeled terminals could be observed in close apposition to nTS cells colabeled with FG and Fos-IR (Figure 9B and C). Similar staining was observed in all three animals.
Discussion
The nTS is a critical region of the medulla which receives visceral afferent inputs, processes that information, and sends projections to a variety of brain regions which then influence autonomic and respiratory output, neuroendocrine and behavioral responses. The intrinsic characteristics of projection neurons are critically important because they determine neuronal responses to afferent input, its modulation, and ultimately efferent output. Separate populations of nTS neurons appear to project to different brain regions (Hermes et al., 2006), and the properties of nTS output neurons vary depending upon their target within the CNS (Bailey et al., 2006). The nTS sends monosynaptic projections to the RVLM, but little is known concerning the intrinsic properties of these neurons. Using a combination of labeling techniques, in vitro electrophysiology and functional immunohistochemistry we determined the intrinsic characteristics of labeled nTS neurons that project to the RVLM. The data indicate that RVLM-projecting nTS neurons discharge with at least three different patterns, with their firing properties influenced, in part, by the potassium currents they exhibit. Each of these subtypes receives primarily strong monosynaptic contacts from visceral afferents. A population of non-catecholaminergic RVLM-projecting nTS neurons is activated (as indicated by Fos-IR) by hypoxia. In addition, a portion of these RVLM-projecting hypoxia sensitive cells possesses closely apposed labeled carotid body terminals, suggesting monosynaptic contacts. Taken together, our electrophysiological and immunohistochemical data suggest that RVLM projecting nTS neurons are a heterogeneous population that receives strong monosynaptic inputs from visceral afferents. A subpopulation of these neurons receives afferent input from the arterial chemoreceptors and likely participates in the chemoreflex pathway.
The distribution of RVLM-projecting neurons within the nTS in the current study was similar to that reported previously (Ross et al., 1985; Aicher et al., 1996; Chan and Sawchenko, 1998b; Kantzides et al., 2005; Kantzides and Badoer, 2006). Most of the retrograde labeling appeared to be localized in the caudal two-thirds of the nTS, and ventral to the area postrema. Spontaneous EPSCs were observed in all RVLM-projecting cells recorded, confirming that these cells were connected to the nTS neural network. Examination of synaptic transmission in these neurons indicated that more than 95% make monosynaptic contacts with visceral afferents. TS stimulation produced large, low jitter evoked EPSCs with low failure rates. Prolonging stimulation to 20 events at 20 Hz produced an EPSC train that exhibited frequency dependent depression. These properties are characteristics of nTS neurons receiving monosynaptic sensory input (Chen et al., 1999; Doyle and Andresen, 2001; Kline et al., 2002). At least some of these RVLM-projecting nTS neurons appear to receive monosynaptic inputs from the arterial chemoreceptors because they possess closely apposed terminals labeled from the carotid body and exhibit low jitter TS-EPSCs.
The properties of RVLM-projecting nTS cells are consistent with a simple reflex pathway through the nTS, in which sensory afferents directly excite output neurons. Because carotid body afferents appear to synapse on RVLM-projecting nTS neurons activated by hypoxia, the carotid chemoreflex may involve a monosynaptic pathway through the nTS. This is similar to what has been proposed for nTS neurons that project to another region of the brain, the caudal ventrolateral medulla (CVLM) (Bailey et al., 2006; Li and Yang, 2007). In contrast, nTS cells which project to the paraventricular nucleus (PVN) of the hypothalamus exhibit small amplitude EPSCs, little frequency dependent depression and higher failure rates, and appear to utilize a polysynaptic pathway within the nTS (Bailey et al., 2006). Of interest, the distribution of CVLM- and PVN-projecting cells spanned the medial nTS and was similar to that of the RVLM-projecting cells in the current study. Additionally, rostral nTS cells likely involved in gustatory reflexes that project to the parabrachial nucleus (PBN) are monosynaptically connected to visceral afferents (Suwabe and Bradley, 2009). These differences in connections between sensory afferents and output cells suggest that within the nTS the functional properties of a given reflex are determined by the sensory afferent activated, the intrinsic properties of the postsynaptic cell, and the synapse between the two.
Although second-order nTS neurons primarily utilize non-NMDA type glutamate receptors (Kline et al., 2002;Doyle and Andresen, 2001), these neurons also appear to express functional NMDA receptors (Aylwin et al., 1997; Bonham and Chen, 2002). We specifically tested monosynaptic RVLM-projecting cells, and found that bath application of the non-NMDA receptor antagonist CNQX abolished TS evoked EPSCs and sEPSCs. Thus, similar to CVLM-projecting nTS neurons (Bailey et al., 2006; Li and Yang, 2007) and other second-order nTS cells (Chen et al., 1999; Doyle and Andresen, 2001; Kline et al., 2002; Laaris and Weinreich, 2007), non-NMDA receptors appear to mediate the majority of excitatory inputs to RVLM-projecting nTS cells.
To examine the postsynaptic properties of RVLM-projecting nTS neurons, positive current was injected in RVLM-projecting cells. These experiments revealed a heterogeneous group of neurons in terms of action potential discharge. We identified neurons that exhibited tonic spiking, delayed excitation spiking, and phasic spiking in response to sustained depolarization. While responses in phasic cells were variable, both tonic and delayed excitation cells exhibited pronounced spike frequency adaptation. Prior hyperpolarization increased the delay in spiking in DE cells, but did not appreciably alter firing properties of tonic cells. Similar diversity of firing properties has been identified in other populations of nTS neurons, including ventral lateral nTS neurons (Dekin et al., 1987) and in rostral PBN-projecting nTS cells (Suwabe and Bradley, 2009).
The firing properties of a neuron are determined by the background ionic currents and a delay in excitation has been demonstrated to be due to the activation of the transient potassium current (Dekin et al., 1987; Dekin and Getting, 1987; Suwabe and Bradley, 2009). Consistent with their firing properties, tonic cells did not express prominent transient outward current whereas delayed excitation cells expressed large outward currents. However, other ionic currents including calcium, calcium-activated potassium, and hyperpolarization-activated currents may prominently modify cell spiking, membrane potential and synaptic integration. Thus differences in these currents may also contribute to observed differences in the firing characteristics of the RVLM projecting nTS neurons evaluated in the current experiments. Future experiments will be required to examine these additional possibilities and more fully identify the ionic currents that could contribute to heterogeneous discharge characteristics.
A population of medial and commissural nTS neurons receiving chemoreceptor afferent input has been shown electrophysiologically to project to the RVLM (Koshiya and Guyenet, 1996). Therefore, we evaluated functionally the extent to which RVLM-projecting nTS neurons are activated in response to chemoreceptor stimulation by using Fos immunohistochemistry following an acute bout of hypoxia. We restricted our analysis to the more caudal regions of the nTS, which have been shown to be involved in cardiorespiratory function (Dampney, 1994a; Guyenet, 2006). The use of coronal and horizontal nTS tissue sections for immunohistochemistry permitted analysis of the rostral-caudal and dorsal-ventral extent of Fos-IR and RVLM-projecting cells. Furthermore, examination of horizontal sections allowed comparison of immunohistochemical data to our electrophysiological studies as well as those from other investigators.
As shown previously (Erickson and Millhorn, 1994; Hirooka et al., 1997; Teppema et al., 1997; Gozal et al., 1999; Berquin et al., 2000), exposure to acute hypoxia increased Fos-IR throughout the rostral-caudal and dorsal-ventral extent of the nTS region examined. Expression of Fos was not due to nonspecific stress associated with the experimental protocol because animals that remained normoxic displayed very little Fos-IR despite exposure to similar environmental stimuli. Overall, throughout the caudal nTS ~11% of the RVLM-projecting neurons were activated by hypoxia (exhibited Fos-IR). At the level of the area postrema, a higher proportion (~20-25%) of the RVLM-projecting neurons also displayed Fos-IR. Furthermore, approximately 10-15% of the nTS cells in this region that displayed Fos-IR in response to hypoxia also projected to the RVLM. A previous study in rabbits (Hirooka et al., 1997) reported that, following exposure to 10% O2 for 60 min, approximately 50% of Fos-IR nTS neurons also projected to the RVLM. The reasons for the difference in the percentage of Fos-IR cells projecting to the RVLM between these two studies are unclear, but could relate to species differences, the difference in the duration of hypoxic exposure (3h in the current study compared with 1h in the previous work), or the fact that in the other study animals were sacrificed 90 min after being returned to normoxia. In the current experiments rats were sacrificed immediately after the period of hypoxia and therefore results may be more specific to the hypoxic stimulus compared to other studies. Regardless of the difference in magnitude, together these data suggest that a population of nTS neurons with projections to the RVLM participates in the reflex response to hypoxia.
It is interesting that only a minority of nTS cells projecting to the RVLM exhibited Fos-IR in response to hypoxia. The nature of the RVLM-projecting nTS cells that did not express Fos is unknown. Some of these neurons may be involved in chemoreflex function but did not exhibit Fos-IR, because Fos is not expressed by all neurons in response to activation. Also, neurons that are inhibited by a given stimulus would not be expected to express Fos (Dragunow and Faull, 1989). Alternatively, the remaining Fos-negative RVLM-projecting neurons may be involved in other reflex pathways such as cardiac mechanoreceptor (Kantzides et al., 2005; Kantzides and Badoer, 2006) or baroreceptor (Chan and Sawchenko, 1998a) reflexes. The proportion of RVLM-projecting nTS cells exhibiting Fos-IR following hypoxia is consistent with observations in other reflex pathways. For example, only ~12-13% of CVLM-projecting nTS neurons express Fos in response to baroreceptor activation (Weston et al., 2003). Similarly, a relatively small number of RVLM-projecting neurons is activated by cardiac mechanoreceptor stimulation (Kantzides et al., 2005; Kantzides and Badoer, 2006). Taken together, these data suggest that, overall, nTS neurons projecting to a given brain region likely are a heterogeneous population with small subpopulations of these projection neurons subserving different specific reflexes.
When the population of nTS neurons that expressed Fos-IR in response to acute hypoxia was examined, only a relatively small percentage was labeled with FG, indicating that a small proportion of nTS neurons activated by hypoxia provides direct inputs to the RVLM. The role of the remaining neurons was not addressed in the current study but they may be interneurons within the chemoreflex circuit, or relay chemoreceptor-related information to other brain regions. This is consistent with the response of nTS neurons in other reflex pathways. For example, only ~15% of nTS neurons exhibiting Fos-IR in response to baroactivation project to the CVLM (Weston et al., 2003), and less than 5% of nTS neurons activated by cardiac mechanoreceptor stimulation project to the RVLM (Kantzides et al., 2005).
Catecholaminergic neurons within the nTS are activated by hypoxia (Erickson and Millhorn, 1994; Teppema et al., 1997). Therefore, we examined whether a population of FG-labeled nTS neurons that was activated by hypoxia might also exhibit TH-IR. The current data indicate that almost no RVLM-projecting nTS cells also contained TH. While this result is in conflict with a previous study (Van Bockstaele et al., 1989), it is in agreement with other work suggesting that few RVLM-projecting nTS neurons are catecholaminergic (Blessing et al., 1987; Hirooka et al., 1997). Although TH neurons apparently did not project to the RVLM, a substantial number of Fos-IR cells in the nTS was co-labeled with TH, indicating that hypoxia activated catecholaminergic neurons in the nTS. Other CNS targets of these catecholaminergic neurons that were activated by hypoxia were not a primary focus of this study. However, neurons in the hypothalamus, including the paraventricular nucleus (PVN), exhibit Fos-IR in response to hypoxia (Berquin et al., 2000) and TH-IR has been observed in nTS neurons retrogradely labeled from the PVN (Hermes et al., 2006). Thus, it is possible that at least a portion of catecholaminergic nTS neurons that exhibit Fos-IR following hypoxia project to the PVN.
Methodological Considerations
In the present study, we functionally identified the RVLM by microinjection of glutamate within 500 μm of the caudal pole of the facial nucleus and recorded the resulting increase in blood pressure. We did not measure respiration. However, both presympathetic neurons and respiratory neurons are intermingled within the area of the RVLM. Previous studies microinjected the excitatory amino acid DL-homocysteic acid (DLH) into similar coordinates as the present study and observed a comparable increase in mean arterial pressure (+13 ± 2 mmHg vs. 11 ± 0.5 mmHg in the current study) as well as bradypnea (Monnier et al., 2003). This is consistent with the notion that this area also corresponds to the respiratory Bőtzinger complex. Also, since anatomical connections have been identified from the commissural nTS to the Bőtzinger complex (Otake et al., 1992) and retrotrapezoid nucleus (Takakura et al., 2006), a portion of the recorded RVLM-projecting neurons in the nTS may constitute cells projecting to respiratory neurons. Similarly, the spread of retrograde tracer may have incorporated the nucleus ambiguus so that a portion of the nTS cells examined may be involved in controlling parasympathetic responses. However, microinjection of glutamate into the same site in the RVLM as FG injection did not appreciably decrease heart rate, reducing the likelihood that we labeled the nucleus ambiguus projecting neurons in the nTS. Lastly, we may not have labeled all of the RVLM-projecting nTS cells with our retrograde label so that we may have underestimated the number of these neurons.
Another consideration is that we did not quantitatively assess whether our Fos-IR, RVLM-projecting nTS neurons were monosynaptically activated. However, in our electrophysiological experiments a high proportion (>95%) of RVLM-projecting nTS neurons were monosynaptically connected. Therefore it is likely that at least some of the RVLM-projecting neurons that expressed Fos-IR in response to hypoxia receive direct afferent inputs. We did not measure arterial blood pressure in these experiments. Arterial pressure may have been altered during hypoxia, and therefore we cannot completely eliminate the possibility that some Fos expression due to the hypoxic stimulus could have been mediated by arterial baroreceptors. Similarly, we cannot eliminate an effect of direct hypoxic stimulation on Fos expression in nTS neurons. Nevertheless, it is reasonable to assume that at least a portion of Fos expression is due to input from the chemoreceptors. This concept is supported by our data in three animals indicating the presence of terminals labeled from the carotid body in close apposition to RVLM-projecting Fos-IR cells.
Physiological Relevance
The RVLM is critical for the maintenance of sympathetic tone as well as reflex responses to baroreceptor and chemoreceptor activation. Hypoxic exposure in vivo results in tachypnea and changes in blood pressure, which is due in part to activation of neurons in the ventrolateral medulla. In the present study, we show that RVLM-projecting nTS neurons are activated in the conscious rat by acute hypoxic episodes. RVLM-projecting cells recorded in the brainstem slice exhibited several different firing patterns. Although we did not determine if responsiveness to hypoxia was cell type specific, one can speculate that such heterogeneous firing properties may differentially modulate the respiratory or cardiovascular system in the RVLM, or mediate responses to different afferent inputs. Alternatively, these various firing properties may govern the pattern of neurotransmitter and neuromodulator release within the RVLM region. Because the firing characteristics of the different classes of nTS neurons vary, the overall cardiovascular and respiratory reflex response to a given afferent input would likely differ based on which type of neuron is/are involved in the pathway.
Regardless of the firing types, RVLM-projecting nTS cells activated by hypoxia likely receive direct monosynaptic afferent inputs. Although we did not quantitatively determine the specific afferent source, presumably the inputs arise, at least in part, from the carotid body chemoreceptors and our labeling studies support this concept. Direct activation of RVLM-projection neurons by chemoafferents would provide for rapid, high fidelity transmission of chemoreceptor information through the nTS to the RVLM and robust reflex responses to hypoxia. RVLM-projecting nTS output neurons also could synapse on interneurons within the RVLM that, in turn, may influence respiratory or autonomic output in response to chemoreceptor activation. This would allow for local processing within the RVLM and possible recruitment of different sympathetic beds and/or alterations in autonomic or respiratory pattern due to the coupling of the cardiorespiratory control systems in this area.
In summary, the results of the present study demonstrate RVLM-projecting cells in the caudal nTS receive strong monosynaptic contacts from visceral afferents and exhibit diverse firing properties. At least some of these monosynaptic inputs arise from the carotid body. In addition, a portion of these RVLM-projecting nTS neurons is activated by hypoxia but appears not to be catecholaminergic. Taken together, these data suggest RVLM-projecting nTS neurons form several functional units, some of which would relay chemoafferent information faithfully to the RVLM to increase cardiorespiratory activity during acute and chronic bouts of hypoxia.
Acknowledgements & grants
We thank Sarah A. Friskey and Heather A. Dantzler for their outstanding technical expertise. Supported by HL-085108 (DDK) and HL-55306 (EMH).
Comprehensive list of abbreviations
- AP
Action Potential
- CNQX
6-cyano-7-nitroquinoxaline-2,3-dione
- CS
calamus scriptorius
- CtB
cholera toxin B
- DE
Delayed excitation
- EPSC
Excitatory Postsynaptic Current
- FG
Fluoro-Gold
- IK
Potassium current
- IR
Immunoreactive
- nTS
nucleus tractus solitarius
- NMDA
N-methyl-D-aspartate
- RVLM
Rostral Ventrolateral Medulla
- SFA
Spike Frequency Adaptation
- TH
Tyrosine Hydroxylase
- TS
Tractus Solitarius
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Aicher SA, Saravay RH, Cravo SL, Jeske I, Morrison SF, Reis DJ, Milner TA. Monosynaptic projections from the nucleus tractus solitarii to C1 adrenergic neurons in the rostral ventrolateral medulla: comparison with input from the caudal ventrolateral medulla. J Comp Neurol. 1996;373:62–75. doi: 10.1002/(SICI)1096-9861(19960909)373:1<62::AID-CNE6>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Andresen MC, Kunze DL. Nucleus tractus solitarius - gateway to neural circulatory control. Annu Rev Physiol. 1994;56:93–116. doi: 10.1146/annurev.ph.56.030194.000521. [DOI] [PubMed] [Google Scholar]
- Austgen JR, Fong AY, Foley CM, Mueller PJ, Kline DD, Heesch CM, Hasser EM. Expression of group I metabotropic glutamate receptors on phenotypically different cells within the nucleus of the solitary tract in the rat. Neuroscience. 2008;159:701–716. doi: 10.1016/j.neuroscience.2008.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aylwin ML, Horowitz JM, Bonham AC. NMDA receptors contribute to primary visceral afferent transmission in the nucleus of the solitary tract. J Neurophysiol. 1997;77:2539–2547. doi: 10.1152/jn.1997.77.5.2539. [DOI] [PubMed] [Google Scholar]
- Bailey TW, Hermes SM, Andresen MC, Aicher SA. Cranial visceral afferent pathways through the nucleus of the solitary tract to caudal ventrolateral medulla or paraventricular hypothalamus: target-specific synaptic reliability and convergence patterns. J Neurosci. 2006;26:11893–1902. doi: 10.1523/JNEUROSCI.2044-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berquin P, Bodineau L, Gros F, Larnicol N. Brainstem and hypothalamic areas involved in respiratory chemoreflexes: a Fos study in adult rats. Brain Res. 2000;857:30–40. doi: 10.1016/s0006-8993(99)02304-5. [DOI] [PubMed] [Google Scholar]
- Blessing WW, Hedger SC, Joh TH, Willoughby JO. Neurons in the area postrema are the only catecholamine-synthesizing cells in the medulla or pons with projections to the rostral ventrolateral medulla (C1-area) in the rabbit. Brain Res. 1987;419:336–340. doi: 10.1016/0006-8993(87)90604-4. [DOI] [PubMed] [Google Scholar]
- Bonham AC, Chen C-Y. Glutamatergic neural transmission in the nucleus tractus solitarius: N-Methyl-D-Aspartate receptors. Clin Exp Pharmacol Physiol. 2002;29:497–502. doi: 10.1046/j.1440-1681.2002.03662.x. [DOI] [PubMed] [Google Scholar]
- Chan RKW, Sawchenko PE. Organization and transmitter specificity of medullary neurons activated by sustained hypertension: implications for understanding baroreceptor reflex circuitry. J Neurosci. 1998a;18:371–387. doi: 10.1523/JNEUROSCI.18-01-00371.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan RKW, Sawchenko PE. Organization and transmitter specificity of medullary neurons activated by sustained hypertension: implications for understanding baroreceptor reflex circuitry. J Neurol Sci. 1998b;18:371–387. doi: 10.1523/JNEUROSCI.18-01-00371.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CY, Horowitz JM, Bonham AC. A presynaptic mechanism contributes to depression of autonomic signal transmission in NTS. Am J Physiol. 1999;277:H1350–H1360. doi: 10.1152/ajpheart.1999.277.4.H1350. Heart Circ Physiol 46. [DOI] [PubMed] [Google Scholar]
- Dampney RAL. Functional organization of central pathways regulating the cardiovascular system. Physiol Rev. 1994a;74:323–364. doi: 10.1152/physrev.1994.74.2.323. [DOI] [PubMed] [Google Scholar]
- Dampney RAL. The subretrofacial vasomotor nucleus: anatomical, chemical and pharmacological properties and role in cardiovascular regulation. Prog Neurobiol. 1994b;42:197–227. doi: 10.1016/0301-0082(94)90064-7. [DOI] [PubMed] [Google Scholar]
- Dekin MS, Getting PA. In vitro characterization of neurons in the ventral part of the nucleus tractus solitarius. II. Ionic basis for repetitive firing patterns. J Neurophysiol. 1987;58:215–229. doi: 10.1152/jn.1987.58.1.215. [DOI] [PubMed] [Google Scholar]
- Dekin MS, Getting PA, Johnson SM. In vitro characterization of neurons in the ventral part of the nucleus tractus solitarius. I. Identification of neuronal types and repetitive firing properties. J Neurophysiol. 1987;58:195–214. doi: 10.1152/jn.1987.58.1.195. [DOI] [PubMed] [Google Scholar]
- Doyle MW, Andresen MC. Reliability of monosynaptic sensory transmission in brain stem neurons in vitro. J Neurophysiol. 2001;85:2213–2223. doi: 10.1152/jn.2001.85.5.2213. [DOI] [PubMed] [Google Scholar]
- Doyle MW, Bailey TW, Young-Ho J, Appleyard SM, Low MJ, Andresen MC. Strategies for cellular indentification in nucleus tractus solitarius slices. J Neurosci Methods. 2004;137:37–48. doi: 10.1016/j.jneumeth.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Dragunow M, Faull R. The use of c-fos as a metabolic marker in neuronal pathway tracing. J Neurosci Meth. 1989;29:261–265. doi: 10.1016/0165-0270(89)90150-7. [DOI] [PubMed] [Google Scholar]
- Erickson JT, Millhorn DE. Hypoxia and electrical stimulation of the carotid sinus nerve induce Fos-like immunoreactivity within catecholaminergic and serotoninergic neurons of the rat brainstem. J Comp Neurol. 1994;348:161–182. doi: 10.1002/cne.903480202. [DOI] [PubMed] [Google Scholar]
- Gozal D, Xue YD, Simakajornboon N. Hypoxia induces c-Fos protein expression in NMDA but not AMPA glutamate receptor labeled neurons within the nucleus tractus solitarii of the conscious rat. Neurosci Lett. 1999;262:93–96. doi: 10.1016/s0304-3940(99)00065-8. [DOI] [PubMed] [Google Scholar]
- Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci. 2006;7:335–346. doi: 10.1038/nrn1902. [DOI] [PubMed] [Google Scholar]
- Hancock MB. Evidence for direct projections from the nucleus of the solitary tract onto medullary adrenaline cells. J Comp Neurol. 1988;276:460–467. doi: 10.1002/cne.902760310. [DOI] [PubMed] [Google Scholar]
- Hermes SM, Mitchell JL, Aicher SA. Most neurons in the nucleus tractus solitarii do not send collateral projections to multiple autonoic targets in the rat brain. Exp Neurol. 2006;198:539–551. doi: 10.1016/j.expneurol.2005.12.028. [DOI] [PubMed] [Google Scholar]
- Hirooka Y, Polson JW, Potts PD, Dampney RAL. Hypoxia-induced Fos expression in neurons projecting to the pressor region in the rostral ventrolateral medulla. Neuroscience. 1997;80:1209–1224. doi: 10.1016/s0306-4522(97)00111-5. [DOI] [PubMed] [Google Scholar]
- Kantzides A, Badoer E. Activation of NADPH-diaphorase-positive projections to the rostral ventrolateral medulla following cardiac mechanoreceptor stimulation in the conscious rat. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1626–R1638. doi: 10.1152/ajpregu.00532.2005. [DOI] [PubMed] [Google Scholar]
- Kantzides A, Owens NC, de Matteo R, Badoer E. Right atrial stretch activates neurons in autonomic brain regions that project to the rostral ventrolateral medulla in the rat. Neuroscience. 2005;133:775–786. doi: 10.1016/j.neuroscience.2005.02.038. [DOI] [PubMed] [Google Scholar]
- Katz LC, Burkhalter A, Dreyer WJ. Fluorescent latex microspheres as a retrograde neuronal marker for in vivo and in vitro studies of visual cortex. Nature. 1984;310:498–500. doi: 10.1038/310498a0. [DOI] [PubMed] [Google Scholar]
- Kiely JM, Gordon FJ. Non-NMDA receptors in the rostral ventrolateral medulla mediate somatosympathetic pressor responses. J Auton Nerv Syst. 1993;43:231–240. doi: 10.1016/0165-1838(93)90329-s. [DOI] [PubMed] [Google Scholar]
- Kline DD. Plasticity in glutamatergic NTS neurotransmission. Respir Physiol Neurobiol. 2008 doi: 10.1016/j.resp.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline DD, Takacs KN, Ficker E, Kunze DL. Dopamine modulates synaptic transmission in the nucleus of the solitary tract. J Neurophysiol. 2002;88:2736–2744. doi: 10.1152/jn.00224.2002. [DOI] [PubMed] [Google Scholar]
- Koshiya N, Guyenet PG. NTS neurons with carotid chemoreceptor inputs arborize in the rostral ventrolateral medulla. Am J Physiol. 1996;270:R1273–R1278. doi: 10.1152/ajpregu.1996.270.6.R1273. [DOI] [PubMed] [Google Scholar]
- Koshiya N, Huangfu D, Guyenet PG. Ventrolateral medulla and sympathetic chemoreflex in the rat. Brain Res. 1993;609:174–184. doi: 10.1016/0006-8993(93)90871-j. [DOI] [PubMed] [Google Scholar]
- Laaris N, Weinreich D. Prostaglandin E2 depresses solitary tract-mediated synaptic transmission in the nucleus tractus solitarius. Neuroscience. 2007;146:792–801. doi: 10.1016/j.neuroscience.2007.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li DP, Yang Q. Membrane and synaptic properties of nucleus tractus solitarius neurons projecting to the caudal ventrolateral medulla. Auton Neurosci. 2007;136:69–81. doi: 10.1016/j.autneu.2007.04.006. [DOI] [PubMed] [Google Scholar]
- Massari VJ, Shirahata M, Johnson TA, Gatti PJ. Carotid sinus nerve terminals which are tyrosine hydroxylase immunoreactive are found in the commissural nucleus of the tractus solitarius. J Neurocytol. 1996;25:197–208. doi: 10.1007/BF02284796. [DOI] [PubMed] [Google Scholar]
- May OL, Erisir A, Hill DL. Ultrastructure of primary afferent terminals and synapses in the rat nucleus of the solitary tract: comparison among the greater superficial petrosal, chorda tympani, and glossopharyngeal nerves. J Comp Neurol. 2007;502:1066–1078. doi: 10.1002/cne.21371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffitt JA, Heesch CM, Hasser EM. Increased GABAA inhibition of the RVLM following hindlimb unloading in rats. Am J Physiol Regul Integr Comp Physiol. 2002;283:R604–R614. doi: 10.1152/ajpregu.00341.2001. [DOI] [PubMed] [Google Scholar]
- Monnier A, Alheid GF, McCrimmon DR. Defining ventral medullary respiratory compartments with a glutamate receptor agonist in the rat. J Physiol. 2003;548:859–874. doi: 10.1113/jphysiol.2002.038141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otake K, Ezure K, Lipski J, Wong She RB. Projections from the commissural subnucleus of the nucleus of the solitary tract: an anterograde tracing study in the cat. J Comp Neurol. 1992;324:365–378. doi: 10.1002/cne.903240307. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates: Academic Press. 1997 doi: 10.1016/0165-0270(80)90021-7. [DOI] [PubMed] [Google Scholar]
- Ross CA, Ruggiero DA, Reis DJ. Projections from the nucleus tractus solitarii to the rostral ventrolateral medulla. J Comp Neurol. 1985;242:511–534. doi: 10.1002/cne.902420405. [DOI] [PubMed] [Google Scholar]
- Spyer KM. Central nervous mechanisms contributing to cardiovascular control. J Physiol. 1994;474:1–19. doi: 10.1113/jphysiol.1994.sp019997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suwabe T, Bradley RM. Characteristics of rostral solitary tract nucleus neurons with identified afferent connections that project to the parabrachial nucleus in rats. J Neurophysiol. 2009 doi: 10.1152/jn.91182.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takakura AC, Moreira TS, Colombari E, West GH, Stornetta RL, Guyenet PG. Peripheral chemoreceptor inputs to retrotrapezoid nucleus (RTN) CO2-sensitive neurons in rats. J Physiol. 2006;572:503–523. doi: 10.1113/jphysiol.2005.103788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tell F, Bradley RM. Whole-cell analysis of ionic currents underlying the firing pattern of neurons in the gustatory zone of the nucleus tractus solitarii. J Neurophysiol. 1994;71:479–492. doi: 10.1152/jn.1994.71.2.479. [DOI] [PubMed] [Google Scholar]
- Teppema LJ, Veening JG, Kranenburg A, Dahan A, Berkenbosch A, Olievier C. Expression of c-fos in the rat brainstem after exposure to hypoxia and to normoxic and hyperoxic hypercapnia. J Comp Neurol. 1997;388:169–190. doi: 10.1002/(sici)1096-9861(19971117)388:2<169::aid-cne1>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Pieribone VA, Aston-Jones G. Diverse afferents converge on the nucleus paragigantocellularis in the rat ventrolateral medulla: retrograde and anterograde tracing studies. J Comp Neurol. 1989;290:561–584. doi: 10.1002/cne.902900410. [DOI] [PubMed] [Google Scholar]
- Vincent A, Tell F. Postnatal changes in electrophysiological properties of rat nucleus tractus solitarii neurons. Eur J Neurosci. 1997;9:1612–1624. doi: 10.1111/j.1460-9568.1997.tb01519.x. [DOI] [PubMed] [Google Scholar]
- Weston M, Wang H, Stornetta RL, Sevigny CP, Guyenet PG. Fos expression by glutamatergic neurons of the solitary tract nucleus after phenylephrine-induced hypertension in rats. J Comp Neurol. 2003;460:525–541. doi: 10.1002/cne.10663. [DOI] [PubMed] [Google Scholar]