Abstract
Wnt signaling is essential for tooth formation. Dact proteins modulate Wnt signaling by binding to the intracellular protein Dishevelled (Dvl). Comparison of all known mouse Dact genes, Dact1-3, from the morphological initiation of mandibular first molar development after the onset of the root formation using sectional in situ hybridization showed distinct, complementary and overlapping expression patterns for the studied genes. While Dact2 expression was restricted to the dental epithelium including the enamel knot signaling centers and tooth specific preameloblasts, Dact1 and Dact3 showed developmentally regulated expression in the dental mesenchyme. Both mRNAs were first detected in the presumptive dental mesenchyme. After being downregulated from the condensed dental mesenchyme of the bud stage tooth germ, Dact1 was upregulated in the dental follicle masenchyme at the cap stage and subsequently also in the dental papilla at the bell stage where the expression persisted to the postnatal stages. In contrast, Dact3 transcripts persisted throughout the dental mesenchymal tissue components including the tooth-specific cells, preodontoblasts before transcripts were largely downregulated from the tooth germ postnatally. Collectively these results suggest that Dact1 and -3 may contribute to early tooth formation by modulation of Wnt signaling pathways in the mesenchyme, including preodontoblasts, whereas Dact2 may play important signal-modulating roles in the adjacent epithelial cells including the enamel knot signaling centers and preameloblasts. Future loss-of-function studies will help elucidate whether any of these functions are redundant, particularly for Dact1 and Dact3.
1. Results and Discussion
The tooth, in particular the mouse first molar, is an excellent model to analyze molecular signaling mechanisms of mammalian organogenesis. Tooth formation is controlled by signaling pathways conserved across species (for reviews see (Miletich and Sharpe, 2003; Thesleff, 2006), (Lesot and Brook, 2008; Luukko et al., 2008). Previous studies indicate that Wnt/ß-catenin signaling serves critical roles in odontogenesis. Recently, constitutive activation of the Wnt/ß-catenin pathway was shown to result in continuous supernumerary tooth formation, while inhibition of this pathway interferes with tooth formation by causing arrest at early developmental stages (van Genderen et al., 1994); (Andl et al., 2002; Jarvinen et al., 2006; Lammi et al., 2004; Liu et al., 2008; Wang et al., 2009). The functions of other, ß-catenin-independent Wnt signaling pathways, including the planar cell polarity (PCP) and Wnt/Ca2+ pathways, remains poorly understood in the tooth. Wnt signaling is modulated at many levels by multiple mechanisms, including the intracellular Dact (also known as Dapper or Frodo) proteins. Dact proteins regulate Wnt signaling at least in part by binding to Dishevelled (Dsh/Dvl) (Suriben et al., 2009) a cytoplasmic protein that is centrally placed in all Wnt signaling pathways (Veeman et al., 2003). Recently, Xenopus Dact protein (XDpr1a) was shown to inhibit Wnt/ß-catenin signaling when unphosphorylated, but to promote this signaling pathway when phosphorylated in an in vitro assay (Teran et al., 2009).
Three Dact paralogs, namely Dact1-3, have been characterized in mouse and they all show distinct expression domains during embryonic development suggesting differential signaling functions (Fisher et al., 2006). Dact1−/− mouse embryos show posterior malformations in the spine, genitourinary and distal digestive system that result from changes in ß-catenin independent signaling (Suriben et al., 2009) while targeted inactivation of Dact2 has been reported to lead to accelerated re-epithelialization during cutaneous wound healing through attenuating Tgfß signaling (Meng et al., 2008).
Epithelial expression of Dact2 in the tooth has been reported at the cap stage (Fisher et al., 2006) while the expression of other Dact genes in the tooth is not known. Given the regulatory functions of Dact proteins in Wnt signaling, which is essential for tooth formation, we systemically compared cellular mRNA expression of Dact1-3 during initiation and epithelial morphogenesis of the mandibular first molar tooth germ from embryonic day (E) 11.5 to day 14 postnatally (PN14) using sensitive radioactive in situ hybridization.
Dact1 and -3 mRNAs were exclusively expressed in the mesenchymal tissue component of the molar tooth, but were differentially regulated. At the morphological onset of tooth formation, expression of both mRNAs was seen in the developing jaw mesenchyme including presumptive dental mesenchyme (Fig. 1B1, D1). However, Dact1 signal appeared to be weaker in the presumptive dental mesenchyme adjacent the epithelial thickening than in the mesenchyme surrounding this area. Two days later, at the bud stage (E13.5) differences in the expression of the Dact1 and Dact3 genes became evident (Fig. 1B2, D2). While Dact3 continued to be expressed in the condensed dental mesenchyme, Dact1 was downregulated there. However, Dact1 expression was observed in the jaw mesenchyme surrounding the condensed dental mesenchyme at this stage (arrows in Fig 1B2).
Figure 1.
Localization of Dact1-3 mRNAs during development of the mouse tooth. Frontal sections of the first mandibular molars. Arrows in B2 indicate jaw mesenchyme surrounding the epithelial bud and condensed dental mesenchyme, while arrows in B3 indicate the mesenchymal dental follicle. Buccal and lingual side is to the left and right, respectively. Basement membrane is marked with a dashed line in D3. Abbreviations: cm, condensed dental mesenchyme; de, dental epithelium; df, dental follicle; dp, dental papilla mesenchyme; E, embryonic age; eo, enamel organ; ide, inner dental epithelium; oe, oral epithelium; ode, outer dental epithelium; pek, primary enamel knot; pdm, presumptive dental mesenchyme; po, preodontoblasts; sek. secondary enamel knot. Scale bars: 100 μm.
Tooth-specific epithelial folding morphogenesis starts at the cap stage and continues throughout the bell stage (Kollar and Lumsden, 1979). During these stages, the mesenchymal dental follicle, surrounding the dental papilla and epithelial enamel organ is visible. The dental follicle gives rise to cemento-, fibro- and osteoblasts forming the tooth supporting periodontium. At the cap stage (E14.5) Dact1 expression was observed in the dental follicle and in the adjacent jaw mesenchyme (Fig. 1B3). In contrast, Dact3 was expressed both in the dental papilla and follicle (Fig. 1D3). At this stage Dact1 transcripts also appeared in the oral epithelium (Fig. 1B3).
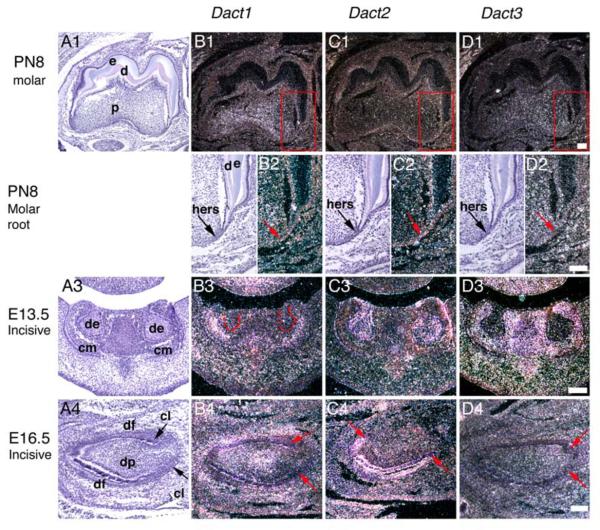
At the early bell stage (E16.5) Dact1 was upregulated in the cervical-middle part of the dental papilla mesenchyme (Fig. 1B4). In contrast, Dact3 transcripts were present throughout the dental papilla and follicle (Fig. 1D4). One day before birth at E18.5 the tooth-specific preodontoblasts are visible in the dental papilla mesenchyme adjacent to the inner dental epithelium at the most advanced cuspal areas (Lesot et al., 2001). At this stage, Dact1 continued to be expressed in the cervical-middle part of the dental papilla as well as in the dental follicle (Fig. 1B5). The similar expression pattern for Dact1 persisted after the enamel and dentin secretion and the onset of the root formation postnatally as shown for 8-day postnatal tooth germ (Figs. 2B1 and 2B2). Dact3 expression was apparent throughout the dental papilla, but the most prominent expression was detected in the coronal part of the papilla including the preodontoblasts (Fig. 1D5). Postnatally, at PN8 little if any specific Dact3 mRNA expression was seen in the dental pulp any longer (Figs. 2D1). No specific expression of Dact1 or -3 was observed in the Hertwig's epithelial root sheaths responsible for tooth formation (Fig. 2D2).
Figure 2.
Localization of Dact1-3 mRNAs during development of the mouse tooth. Sagittal (A1-D2, A4-D4) and frontal (A3-D3) sections of the first mandibular molars (A1-D2) and mandibular incisors (A3-D4). Figures B2-D2 are higher magnifications from the areas in figures B1-D1 marked by red boxes. In frontal sections, buccal and lingual side is to the left and right, respectively. In sagittal sections rostral and dorsal side is to left and right, respectively. Basement membrane is marked with a dashed line in B3. Dact3 does not show specific expression in tooth in D1. Abbreviations: cl, cervical loop; cm, condensed dental mesenchyme; d, dentin; de, dental epithelium; df, dental follicle; dp, dental papilla mesenchyme; e, enamel; hers, Hertwig's epithelial root sheath. Scale bars: 100 μm.
Dact1 and Dact3 expression in the lower jaw incisor tooth germ correlated to that observed in the molars. At the epithelial thickening stage Dact1 transcripts were present in the dental and jaw mesenchyme. Later at the bud and cap stage, expression continued in the jaw mesenchyme next to the dental mesenchyme as shown for the E13.5 bud stage incisor in figure 2B3, and subsequently the expression also appeared in the dental follicle. During the bell stage Dact1 transcripts become upregulated in the middle part of the dental papilla and pulp (see Fig. 2B4 for a E16.5 incisors). Dact3 expression was present in the mesenchymal tissue components during E11.5-E14.5 (Fig. 2D3). At E16.5, however, only a few, if any transcripts were observed in the incisor mesenchyme any longer (see Figs. 2D4). Postnatally, a prominent Dact3 hybridization signal was confirmed to muscle tissue while little if any specific Dact3 expression was seen in the incisor pulp (not shown).
In contrast to Dact1 and -3, Dact2 expression was solely restricted to the dental epithelium. At the epithelial thickening stage (E11.5), Dact2 showed prominent expression in the dental epithelium (Fig. 1C1). The expression continued in the epithelial dental bud at E13.5 and in the epithelial enamel organ of the E14.5 cap stage tooth germ. This included cells of primary enamel knot, a signaling center that regulates tooth shape (Figs. 1C2 and 1C3) (Jernvall et al., 2000; Jernvall et al., 1994). Later during the bell stage when the final shape of the tooth is established, Dact2 expression continued in cells of the epithelial enamel organ including the inner and outer dental epithelium, stellate reticulum, stratum intermedium and cervical loops, which later form Hertwig's epithelial root sheath responsible for root formation (Figs. 1C4 and 1C5). Molar tooth crown morphogenesis is characterized by formation of the cusps and histodifferentiation of the inner dental epithelium cells to ameloblasts, which produce enamel. Of note, Dact2 was expressed in cells of the inner dental epithelium, which are precursors of the ameloblasts, and in the cervical loops. The secondary and tertiary enamel knots present in the bell stage molar regulate individual cusp formation and the final shape of the molar crown (Jernvall et al., 1994; Luukko et al., 2003). Of special interest is the finding that Dact2 expression also included both the upper and lower compartments (Luukko et al., 2003) of the secondary enamel knots (SEKs) as shown for E16 molar (arrows in Fig. 1C4) and the tertiary enamel knot (TEK) (not shown). The expression of Dact2 was downregulated from ameloblasts secreting enamel and by PN8 no specific expression of Dact2 was seen in the tooth germ. In addition, the Hertwig's epithelial root sheaths were devoid of transcripts (Figs. 2C1 and 2C2).
The expression of Dact2 in the incisors correlated to that of in molars. Dact2 transcripts were confirmed to the dental epithelia including the cervical loops as shown for E13.5 and E16.5 tooth germs (Figs. 2C3 and C4). However, at PN2 no specific Dact2 expression appeared in the tooth germ any longer (not shown).
In conclusion, our results here show that the three Dact genes exhibit distinct, overlapping and complementary expression domains during tooth development. While Dact1 and -3 showed differentially, developmentally regulated expression in the dental mesenchymal tissue components, the expression of Dact2 was restricted to the dental epithelium. These results suggest that regulation of Wnt signaling by Dact proteins may serve important functions in tooth initiation, crown morphogenesis and differentiation of tooth-specific cells. Moreover, the overlapping expression of Dact1 and -3 suggest that developmental functions of their protein products may be either redundant and/or complementary during tooth formation. Loss-of-function studies in the future will help elucidate the in vivo functions of the Dact proteins.
2. Experimental Procedures
The animal use was approved by the Animal Welfare Committee of the Preclinical Institutes, University of Bergen. Mice (CBA & NMRI) were mated overnight and the appearance of vaginal plug was taken as day 0 of embryogenesis (E0.5). The developmental stages of the tooth germs were judged from the tissue sections according to morphological criteria. Tissue processing, sectional in situ hybridization, photomicrography and processing of the images were performed as described (Kettunen et al., 1998; Luukko et al., 1996; Kettunen et al., 2005). Plasmids containing cDNA fragments of Dact1-3 have been described earlier in (Fisher et al., 2006). Sections were exposed for 3 weeks. No specific hybridization signal was detected in the control sections hybridized with corresponding sense probes (data not shown).
3. Acknowledgements
Ms Kjellfrid Haukanes is acknowledged for skillful technical assistance. We thank the staff of the animal facility for careful mouse husbandry. This study has been supported by a grant from the Norwegian Cancer Society.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
4. References
- Andl T, Reddy ST, Gaddapara T, Millar SE. WNT signals are required for the initiation of hair follicle development. Dev Cell. 2002;2:643–53. doi: 10.1016/s1534-5807(02)00167-3. [DOI] [PubMed] [Google Scholar]
- Fisher DA, Kivimae S, Hoshino J, Suriben R, Martin PM, Baxter N, Cheyette BN. Three Dact gene family members are expressed during embryonic development and in the adult brains of mice. Dev Dyn. 2006;235:2620–30. doi: 10.1002/dvdy.20917. [DOI] [PubMed] [Google Scholar]
- Jarvinen E, Salazar-Ciudad I, Birchmeier W, Taketo MM, Jernvall J, Thesleff I. Continuous tooth generation in mouse is induced by activated epithelial Wnt/beta-catenin signaling. Proc Natl Acad Sci U S A. 2006;103:18627–32. doi: 10.1073/pnas.0607289103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernvall J, Keranen SV, Thesleff I. Evolutionary modification of development in mammalian teeth: quantifying gene expression patterns and topography. Proc Natl Acad Sci U S A. 2000;97:14444–8. doi: 10.1073/pnas.97.26.14444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernvall J, Kettunen P, Karavanova I, Martin LB, Thesleff I. Evidence for the role of the enamel knot as a control center in mammalian tooth cusp formation: non-dividing cells express growth stimulating Fgf-4 gene. Int J Dev Biol. 1994;38:463–9. [PubMed] [Google Scholar]
- Kollar EJ, Lumsden AG. Tooth morphogenesis: the role of the innervation during induction and pattern formation. J Biol Buccale. 1979;7:49–60. [PubMed] [Google Scholar]
- Lammi L, Arte S, Somer M, Jarvinen H, Lahermo P, Thesleff I, Pirinen S, Nieminen P. Mutations in AXIN2 cause familial tooth agenesis and predispose to colorectal cancer. Am J Hum Genet. 2004;74:1043–50. doi: 10.1086/386293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesot H, Brook AH. Epithelial histogenesis during tooth development. Arch Oral Biol. 2008 doi: 10.1016/j.archoralbio.2008.05.019. [DOI] [PubMed] [Google Scholar]
- Lesot H, Lisi S, Peterkova R, Peterka M, Mitolo V, Ruch JV. Epigenetic signals during odontoblast differentiation. Adv Dent Res. 2001;15:8–13. doi: 10.1177/08959374010150012001. [DOI] [PubMed] [Google Scholar]
- Liu F, Chu EY, Watt B, Zhang Y, Gallant NM, Andl T, Yang SH, Lu MM, Piccolo S, Schmidt-Ullrich R, Taketo MM, Morrisey EE, Atit R, Dlugosz AA, Millar SE. Wnt/beta-catenin signaling directs multiple stages of tooth morphogenesis. Dev Biol. 2008;313:210–24. doi: 10.1016/j.ydbio.2007.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luukko K, Loes S, Furmanek T, Fjeld K, Kvinnsland IH, Kettunen P. Identification of a novel putative signaling center, the tertiary enamel knot in the postnatal mouse molar tooth. Mech Dev. 2003;120:270–6. doi: 10.1016/s0925-4773(02)00458-6. [DOI] [PubMed] [Google Scholar]
- Luukko K, Moe K, Sijaona A, Furmanek T, Hals Kvinnsland I, Midtbo M, Kettunen P. Secondary induction and the development of tooth nerve supply. Ann Anat. 2008;190:178–87. doi: 10.1016/j.aanat.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Meng F, Cheng X, Yang L, Hou N, Yang X, Meng A. Accelerated re-epithelialization in Dpr2-deficient mice is associated with enhanced response to TGFbeta signaling. J Cell Sci. 2008;121:2904–12. doi: 10.1242/jcs.032417. [DOI] [PubMed] [Google Scholar]
- Miletich I, Sharpe PT. Normal and abnormal dental development. Hum Mol Genet. 2003;12(Spec No 1):R69–73. doi: 10.1093/hmg/ddg085. [DOI] [PubMed] [Google Scholar]
- Suriben R, Kivimae S, Fisher DA, Moon RT, Cheyette BN. Posterior malformations in Dact1 mutant mice arise through misregulated Vangl2 at the primitive streak. Nat Genet. 2009 doi: 10.1038/ng.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teran E, Branscomb AD, Seeling JM. Dpr Acts as a molecular switch, inhibiting Wnt signaling when unphosphorylated, but promoting Wnt signaling when phosphorylated by casein kinase Idelta/epsilon. PLoS One. 2009;4:e5522. doi: 10.1371/journal.pone.0005522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thesleff I. The genetic basis of tooth development and dental defects. Am J Med Genet A. 2006;140:2530–5. doi: 10.1002/ajmg.a.31360. [DOI] [PubMed] [Google Scholar]
- van Genderen C, Okamura RM, Farinas I, Quo RG, Parslow TG, Bruhn L, Grosschedl R. Development of several organs that require inductive epithelial-mesenchymal interactions is impaired in LEF-1-deficient mice. Genes Dev. 1994;8:2691–703. doi: 10.1101/gad.8.22.2691. [DOI] [PubMed] [Google Scholar]
- Wang XP, O'Connell DJ, Lund JJ, Saadi I, Kuraguchi M, Turbe-Doan A, Cavallesco R, Kim H, Park PJ, Harada H, Kucherlapati R, Maas RL. Apc inhibition of Wnt signaling regulates supernumerary tooth formation during embryogenesis and throughout adulthood. Development. 2009;136:1939–49. doi: 10.1242/dev.033803. [DOI] [PMC free article] [PubMed] [Google Scholar]