Abstract
The dorsal striatum is a large forebrain region involved in action initiation, timing, control, learning and memory. Learning and remembering skilled movement sequences requires the dorsal striatum, and striatal subregions participate in both goal-directed (action-outcome) and habitual (stimulus-response) learning. Modulation of synaptic transmission plays a large part in controlling input to as well as the output from striatal medium spiny projection neurons (MSNs). Synapses in this brain region are subject to short-term modulation, including allosteric alterations in ion channel function and prominent presynaptic inhibition. Two forms of long-term synaptic plasticity have also been observed in striatum, long-term potentiation (LTP) and long-term depression (LTD). LTP at glutamatergic synapses onto MSNs involves activation of NMDA-type glutamate receptors and D1 dopamine or A2A adenosine receptors. Expression of LTP appears to involve postsynaptic mechanisms. LTD at glutamatergic synapses involves retrograde endocannabinoid signaling stimulated by activation of metabotropic glutamate receptors (mGluRs) and D2 dopamine receptors. While postsynaptic mechanisms participate in LTD induction, maintained expression involves presynaptic mechanisms. A similar form of LTD has also been observed at GABAergic synapses onto MSNs. Studies have just begun to examine the roles of synaptic plasticity in striatal-based learning. Findings to date indicate that molecules implicated in induction of plasticity participate in these forms of learning. Neurotransmitter receptors involved in LTP induction are necessary for proper skill and goal-directed instrumental learning. Interestingly, receptors involved in LTP and LTD at glutamatergic synapses onto MSNs of the “indirect pathway” appear to have important roles in habit learning. More work is needed to reveal if and when synaptic plasticity occurs during learning and if so what molecules and cellular processes, both short- and long-term, contribute to this plasticity.
Keywords: Long-term plasticity, Dopamine, Glutamate, Endocannabinoid, Instrumental learning, Skill Learning
1. Neurotransmitters in Striatum: It's a jungle in there
The striatum is the major input nucleus of the basal ganglia, and as such it plays a crucial role in action control and action learning (Saint-Cyr et al., 1995; Graybiel, 1998; Lalonde and Botez-Marquard, 1997; Yin and Knowlton, 2006). These roles are accomplished via striatal generation of neuronal activity that initiates and terminates action sequences, processing of afferent inputs that influence this activity, and controlling the activity of downstream efferent target nuclei through inhibitory GABAergic projections. A rich interplay of the actions of numerous neurotransmitters is involved in the input, processing and output functions of the striatum (see Lovinger et al., 2003; Schmidt, 1995; Tepper et al., 2007 for review). As is the case for most large forebrain regions, the striatum contains a variety of small molecule and neuropeptide transmitters. The small molecules act both on ligand-gated ion channels (LGICs) to produce fast synaptic transmission, and on G-protein coupled receptors (GPCRs) to produce neuromodulation. Endocannabinoids, lipid metabolites that activate the CB1 GPCR, are an intriguing subclass of small molecules that modulate synaptic transmission in striatum (Lovinger et al., 2009), and more will be said about their emerging role in striatal function later in this review. Neuropeptides act predominantly, if not exclusive, through modulatory GPCRs. In this review, discussion of synaptic modulation and plasticity will be focused on the dorsal striatum (caudate and putamen nuclei in primates).
The striatum contains a single class of projection neurons known as medium spiny neurons (MSNs). These GABAergic neurons make up the vast majority of the total striatal neuronal complement (Tepper et al., 2007). Each MSN receives 10s of thousands of glutamatergic inputs from cortex and intralaminar thalamus, most of which synapse on the heads of the dendritic spines that stud the outer two-thirds of the MSN dendritic arbor (Tepper et al., 2007). MSNs also receive extensive GABAergic synaptic input from other MSNs as well as striatal interneurons (Tepper et al., 2004). Striatal interneurons are much less numerous than MSNs, but form extensive connections within the striatum. Neurons with a large, elongated soma (20-25 μm on the long axis) display tonic activity even in the brain slice preparation (the large aspiny neurons), and are known to be cholinergic (Bennett and Wilson, 1999; Zhou et al., 2002). The remainder of the interneurons in striatum are GABAergic, and most prominently include fast-spiking interneurons (FSNs) that express the calcium-binding protein parvalbumin, and low-threshold-spiking (LTSNs) that express neuropeptide Y (Tepper et al., 2004). These interneurons also receive glutamatergic synaptic input, with the balance between cortical and thalamic input varying with neuronal subtype. GABAergic connections among interneurons are likely to occur, but characterization of these connections is just beginning (Partridge et al., 2009).
Glutamatergic synapses act mainly through AMPA-type receptors to produce fast synaptic excitation, and NMDA-type receptors can also contribute to transmission and plasticity (Calabresi et al., 1992c; Calabresi et al., 2000a). GABAergic synapses produce fast inhibition exclusively through the GABAA-type receptors. There is also evidence for GABA-mediated excitation through this receptor type, but as yet there is not strong consensus as to when and where this occurs within the striatum (Bracci and Panzeri, 2006). Acetylcholine coming from the large aspiny neurons could potentially act via nicotinic ACh receptors, and these LGICs are abundant in striatum (Zhou et al., 2002). However, as yet there is little evidence for direct fast-acting ACh-nAChR-mediated synaptic responses in striatal neurons, with the exception of excitatory actions on fast spiking interneurons (Koos and Tepper, 2002).
2. Short-Term Modulation of Striatal Synaptic Transmission
Neuromodulation strongly impacts striatal function, and deficits in this more subtle type of synaptic communication play key roles in neurological disorders involving this brain region. A strong dopaminergic afferent input from the substantia nigra pars compacta innervates MSNs and striatal interneurons. The midbrain neurons that give rise to this input degenerate in Parkinson's disease, and it is clear that loss of dopaminergic input causes hypokinesia and other facets of this disorder. Dopamine can only act via GPCRs, and thus only modulates striatal neuronal function and synaptic transmission (Richfield et al., 1989; Surmeier et al., 2007). Glutamate, GABA and ACh can also act through GPCRs to influence the function of striatal neurons (Lovinger, 1991; Calabresi et al., 1991; Seabrook et al., 1991; Sugita et al., 1991). In addition, small molecule neurotransmitters such as adenosine, serotonin and endocannabinoids act predominantly, if not exclusively, via GPCRs to affect striatal neurons and synapses (Lovinger et al., 2009). Among the neuropeptides known to be expressed in striatum are opioid peptides, neurotensin, NPY, somatostatin and substance P. Many of the responses to these peptides are poorly characterized, but it is clear that GPCRs underlie the known actions. The gaseous small molecule neurotransmitter nitric oxide (NO) is also made by at least one subclass of striatal neuron, and appears to have important modulatory functions in striatum.
The full spectrum of striatal neuromodulation will not be discussed in the present review. The focus will be on synaptic modulation and plasticity, and thus GPCR effects on voltage-gated ion channels will not be discussed in any detail. While this topic would be a fitting and timely subject for another review, pertinent information can be found in a recent article (Surmeier et al., 2007). In addition, the intent of this paper is not to provide an exhaustive list of types of synaptic modulation produced by GPCR-acting receptors, but rather to highlight the dominant motifs in these modulatory actions within the striatum.
Before considering synaptic modulation known to occur in striatum, it is worth mentioning that certain modulatory responses that are common in other brain regions are not observed in striatal neurons, at least in MSNs. For example, GPCR activation of G-protein-activated inwardly rectifying postassium (GIRK) channels does not occur in MSNs. This is most likely due to the fact that GIRK channels are not expressed in these neurons. Thus, one prominent mechanism for slow inhibition of neurons does not operate in these cells. Modulation of neuronal excitability by altered function of the KCNQ-type potassium channel (also known as the m-channel), is another common response to GPCR activation in many forebrain neurons. The KCNQ2 and 3 subtypes are expressed MSNs (Shen et al. 2005), where they may reside on dendrites (Cooper et al., 2001). Shen et al. (2005) have shown that activation of muscarinic ACh receptors inhibits the current mediated by these channels, enhancing the excitability of MSNs.
Neuromodulatory GPCR-mediated effects on ligand-gated ion channels have been observed in striatum. For example, dopamine acting via the D1 class of receptors and the downstream DARPP-32 protein acts to maintain normal levels of function of AMPA-type glutamate receptors (Yan et al., 1999). This action appears to involve inhibition of phosphatases that, if unchecked, dephosphorylate AMPARs and cause a gradual decrease, or “rundown” of channel function. It should be noted, however, that this mechanism has not been shown to be activated by endogenous, synaptically-released dopamine. Dopamine also alters the function of GABAA receptors (Yan and Surmeier, 1997). Once again, there is no evidence as yet that this action is mimicked by endogenous dopamine.
Perhaps the most consistently observed form of synaptic modulation in striatum is presynaptic inhibition of neurotransmitter release. GPCRs that activate the Gi/o class of G-proteins are known to inhibit neurotransmitter release at synapses throughout the nervous system (Miller, 1998; Wu and Saggau, 1997). A variety of subtypes of these receptors have been identified at both glutamatergic and GABAergic synapses in striatum. For example, activation of adenosine A1, CB1 cannabinoid, GABAB, muscarinic ACh and group II and III metabotropic glutamate receptors has been shown to inhibit glutamatergic synaptic transmission in striatum (Lovinger, 1991; Lovinger and McCool, 1995; Calabresi et al., 1991, 1993; Gerdeman and Lovinger, 2001; Malenka and Kocsis, 1998; Pisani et al., 1997; Sugita et al., 1991). All of these effects appear to involve a presynaptic decrease in glutamate release. This form of synaptic inhibition can provide an effective brake on excitation of MSNs that prevents information flow into and through the basal ganglia. Less is known about which presynaptic receptors regulate GABA release in striatum. However, it is clear that CB1 cannabinoid receptors have such an action (Narushima et al., 2006, 2007; Szabo et al., 1998; Adermark et al., 2009; Adermark and Lovinger, 2009). In addition, activation of delta opiate, adenosine A1, GABAB and muscarinic ACh receptors reduces GABAergic transmission via an apparent presynaptic action (Centonze et al., 2001b; Jiang and North, 1991; Seabrook et al., 1991; Sugita et al., 1991; Waldmeier et al., 1998). Clearly, this disinhibitory modulation provides an important mechanism to produce a subtle increase in excitation of MSNs.
Dopamine produces modulation of neurotransmitter release at both GABAergic and glutamatergic striatal synapses (Bamford et al., 2004a; Centonze et al., 2004; Cepeda et al., 1993; Flores-Hernandez et al., 1997; Yin and Lovinger, 2006). However, it is not clear if these effects are due to dopamine receptors that reside directly on presynaptic terminals. Activation of D2 receptors inhibits glutamate release, but this effect is not readily observed when afferent inputs are activated at low stimulus frequencies (Bamford et al., 2004b; Centonze et al., 2004; Cepeda et al., 1993; Flores-Hernandez et al., 1997; Nicola and Malenka, 1998; Yin and Lovinger, 2006). This action contrasts with that of the other presynaptic Gi/o-coupled GPCRs mentioned above, which readily inhibit transmission regardless of stimulus frequency. Bamford and coworkers have suggested that this reflects preferential inhibition of particularly active synapses by presynaptic D2 receptors. (Bamford et al., 2004a,b). However, no specific molecular mechanism has been identified that mediates this selective inhibition. Alternatively, evidence from two recent studies suggests that D2-mediated inhibition of glutamatergic transmission results from production of an endocannabinoid retrograde signal leading to activation of presynaptic CB1 receptors and reduced glutamate release (Kreitzer and Malenka, 2005; Yin et al., 2006). It is not yet clear if this mechanism completely accounts for presynaptic D2 inhibition at glutamatergic synapses. Activation of D2 receptors has also been reported to inhibit GABA release at synapses onto striatal MSNs (Centonze et al., 2004). There is some evidence that endocannabinoids may also be involved in this disinhibitory synaptic modulation. However, more direct D2 actions may also occur.
3. Long-Term Synaptic Plasticity at Striatal Synapses
2.1. Striatal LTP
Studies from a number of laboratories over the last 15-20 years have revealed two predominant forms of long-lasting synaptic plasticity at glutamatergic striatal synapses: long-term potentiation (LTP) and long-term depression (LTD) (see Kreitzer and Malenka, 2008; Wickens, 2009 for review) (Figure 1). Striatal LTP is a long-lasting increase in the efficacy of glutamatergic synapses that is observed mainly at corticostriatal synapses (Charpier and Deniau, 1997; Calabresi et al., 1992c). Ideas about striatal-based learning have long included the postulate that LTP would be an important component of the strengthening of stimulus-response associations in this brain region (Mahon et al., 2004; Reynolds and Wickens, 2002). However, demonstrating LTP of synaptic responses in striatum proved difficult for early investigators working in brain slices and in vivo. In relatively simple brain slice experiments involving sharp electrode or whole-cell patch-clamp recording, LTP was not readily observed (Calabresi et al., 1992a,b; Lovinger et al., 1993; Partridge et al., 2000; Kerr and Wickens, 2001). The reasons for these difficulties in LTP induction are not clear, but appear to involve difficulties in activating receptors responsible for LTP induction combined with removal of some factor(s) needed for induction during whole-cell recordings (Calabresi et al., 1992b; Partridge et al., 2000). These problems have now been overcome to some extent through the use of techniques including recording in low extracellular magnesium, non-invasive field potential recording, and use of the “perforated-patch” technique that allows for whole-cell recording without removal of intracellular proteins and other important biomolecules (Calabresi et al., 1992b; Partridge et al., 2000; Dang et al., 2006; Shen et al., 2008). Realizing that long-term synaptic plasticity differs in different striatal subregions, with LTP predominating in dorsomedial and rostral striatum and LTD more prevalent in dorsolateral and caudal striatum (Figure 2), also allowed investigators to focus on areas where LTP was more easily induced (Partridge et al., 2000; Spencer and Murphy; 2000). Using these approaches it has been possible to determine the roles of different neurotransmitters in striatal LTP (Di Filippo et al., 2009; Lovinger et al., 2003; Wickens 2009).
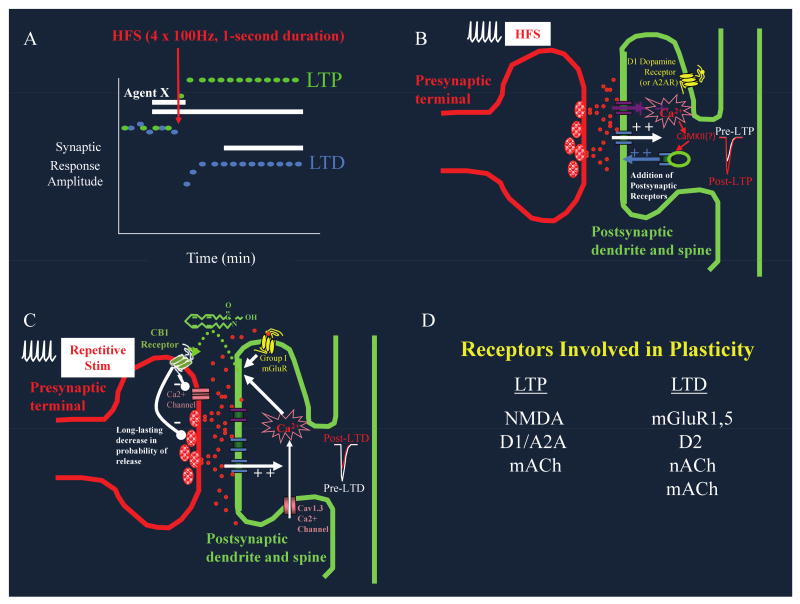
Figure 1.
Neurotransmitter and receptor roles in striatal LTP and LTD. A) Diagram showing types of long-term synaptic plasticity at glutamatergic striatal synapses, and the general paradigm for inducing plasticity and examining effects of pharmacological treatments. The white bar indicates time periods during which pharmacological agents can be given to modify induction or expression of LTP or LTD. B) Schematic diagram of mechanisms thought to be involved in striatal LTP. Induction of LTP involves activation of NMDARs, along with either D1 or A2A receptor activation in direct and indirect pathway neurons, respectively. Increases in intracellular calcium trigger biochemical changes, possibly including activation of the calmodulin-dependent protein kinase type II (CAMKIIa). The GPCRs stimulate adenylyl cyclase activity and phosphorylation of DARPP-32 (not shown), but the mechanisms of linking these signaling pathways to LTP expression are not yet known. Expression of LTP is thought to involve AMPAR insertion at the synapse. C) Schematic diagram of mechanisms involved in striatal LTD. Postsynaptic depolarization activates Cav1.3-type voltage-gated calcium channels, while glutamate activates group I mGluRs. The calcium and metabotropic signals converege to stimulate endocannabinoid (EC) synthesis and release. The EC acts on presynaptic CB1 receptors. Combined CB1 activation and presynaptic activity produces a long-lasting decrease in presynaptic release probability (possibly through decreased calcium channel function or more direct effects on vesicle fusion). Arrowheads indicate stimulation, while circular line endings indicate inhibition. D) Listing of neurotransmitter receptor subtypes implicated in LTP and LTD induction. Note that these receptors do not appear to be necessary for maintenance of LTP or LTD once plasticity has been induced.
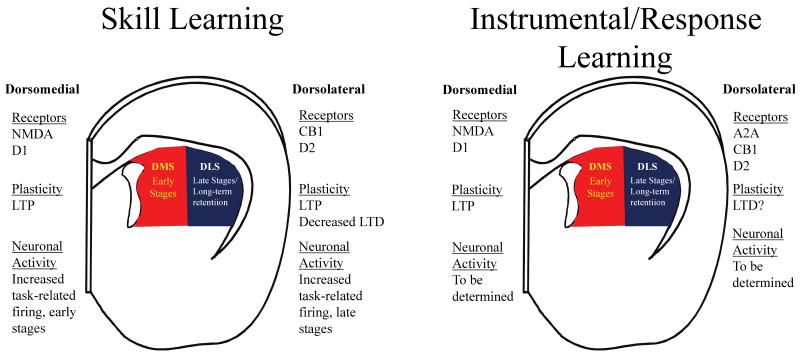
Figure 2.
Striatal-based skill and instrumental/response learning involve different mechanisms and different striatal subregions. Left) Schematic representation of a coronal hemislice of the brain containing the dorstal striatum. Receptors, types of synaptic plasticity and changes in firing of medium spiny neurons (MSNs) associated with skill learning in the dorsomedial striatum (DMS, red) and the dorsolateral striatum (DLS, blue). Involvement of the two subregions in different stages of skill learning are also highlighted. Right) Similar diagram with information about instrumental and response learning. Receptors, types of synaptic plasticity and changes in firing of medium spiny neurons (MSNs) associated with instrumental and response learning in the DMS (red) and the DLS (blue). Involvement of the two subregions in different stages of types of instrumental learning are also highlighted.
Glutamate and its receptors are critical for both LTP induction and expression. As with most forms of LTP observed in the CNS, striatal LTP requires activation of NMDA-type glutamate receptors (Calabresi et al., 1992b; Dang et al., 2006; Kerr and Wickens, 2001; Popescu et al., 2007; Shen et al., 2008) (Figure 1b). Calabresi and coworkers have examined LTP in striatal slices in nominally magnesium-free artificial cerebrospinal fluid (Calabresi et al., 1992b). Using this approach they have consistently observed LTP, probably due to removal of the magnesium block of the NMDAR intrinsic ion channel. Interestingly, activation of basolateral amygdala afferents that innervate ventral striatum enhances LTP at convergent cortical synapses onto MSNs (Popescu et al., 2007). Strong activation of NMDARs at this synapse appears to be important for this LTP-promoting effect. It is likely that AMPA-type glutamate receptors are involved in the expression of striatal LTP (Figure 1b). Recent work from Shen et al. (2008) provides evidence that LTP involves an increase in postsynaptic responsiveness of MSNs. In other brain regions, NMDAR-dependent, postsynaptically-expressed LTP involves insertion of additional AMPA receptors into synapses (see Kerchner and Nicoll, 2008 for review). While there is as yet no direct evidence for involvement of a similar mechanism in striatal LTP, it is reasonable to hypothesize that this will be the case in striatum as in other brain areas.
Dopamine activation of D1 receptors has also been implicated in striatal LTP (Figure 1b). Several investigators have observed that LTP is prevented in the presence of selective D1 antagonists and in gene-targeted mice lacking the receptor (Calabresi et al., 2000b; Kerr and Wickens, 2001; Lovinger et al., 2003). It is not yet clear how the D1 receptors participate in LTP induction. This receptor subtype is known to activate Golf-type G-proteins in striatum, generally resulting in increased adenylyl cyclase (AC) activity and production of cyclic AMP (cAMP). It is presumed that activation of this enzyme participates in LTP induction, although there is not yet much direct evidence supporting this idea. A recent report that the Golf-activating adenosine A2A receptor participates in LTP at synapses onto striatopallidal neurons also supports the idea of AC activation as an important step in LTP induction (Shen et al., 2008) (Figure 1b). Signaling pathways downstream of AC/cAMP include activation of protein kinase A by cAMP and phosphorylation of DARPP-32, a phosphatase inhibitor (Calabresi et al., 2000b). Gene-targeted knockout of DARPP-32 prevents LTP induction (Calabresi et al., 2000b). Presumably the inhibition of phosphatases involved in AMPAR trafficking may link this protein to receptor trafficking and postsynaptic LTP expression, although all of the steps in this pathway have not been thoroughly investigated. This enzyme has also been implicated in D1-mediated potentiation of NMDAR function (Flores-Hernandez et al., 2002). Thus, an alternative role for DARPP-32 might be in enhancement of NMDAR signaling needed for LTP induction.
The new finding that A2A antagonists block LTP in a subset of striatal neurons (Shen et al., 2008) indicates that adenosine has a role in striatal long-term synaptic plasticity. The parallel roles of dopamine/D1Rs and adenosine/A2ARs appear to reflect different receptor complements of the two major striatal efferent systems known as the direct and indirect pathways. Striatal MSNs that contribute to the direct pathway project mainly to the substantia nigra pars reticulata. These striatonigral neurons strongly express D1Rs and generally do not express A2A or D2Rs (Augood and Emson, 1994; Gerfen et al., 1990; Gong et al., 2003). Activation of the direct pathway has the net effect of increasing the output of neurons in the cortical areas that are the ultimate target of this circuit, allowing expression of actions. In contrast, the indirect pathway, involving striatal projections of the globus pallidus internal segment inhibits output of the ultimate cortical target neurons, presumably suppressing unwanted actions. The striatopallidal, indirect pathway MSNs express A2A and D2Rs, but exhibit little or no D1R expression (Augood and Emson, 1994; Gerfen et al., 1990). Thus, the segregation of dopamine and adenosine participation in striatal LTP may be related to the necessity for coordination between the direct and indirect pathways in production of proper action sequences.
Acetylcholine also appears to play a role in striatal LTP. Induction of LTP is prevented in the presence of muscarinic acetylcholine receptor (mAChR) antagonists (Calabresi et al., 1999; Lovinger et al., 2003) (Figure 1b). While it has been suggested that the mAChR1 receptor is the one responsible for these actions, the evidence for this assertion is not strong. However, blockade of M2-like mAChRs enhances rather than eliminates LTP (Calabresi et al., 1998), suggesting that the M1-like receptors may indeed participate in LTP induction. Cholinergic neurons are tonically active in brain slices, and can also be activated by glutamatergic inputs during afferent stimulation (Bennett and Wilson, 1999; Partridge et al., 2002; Pisani et al., 2002). Large cholinergic interneurons receive glutamatergic afferent input from thalamus and neocortex (Wilson et al., 1990). Thus, the cholinergic role in LTP might reflect a mechanism through which coactivation of thalamic, cortical and nigral afferents combines to enhance striatal throughput during learning. However, a well-known response of cholinergic interneurons is a pause in activity in response to reward-predictive stimuli in a pavlovian conditioning task (Aosaki et al., 1994; Apicella et al., 1996; Matsumoto et al., 2001). Thus, any potentiation involving cholinergic output could not be encoded at this time, and must be induced during periods of high activity. In this context it is interesting to note that some cholinergic neurons show increased activity before conditioned stimulus presentation, and the pause is often followed by an increase in activity (Aosaki et al., 1994). Thus, potentiation may be initiated during these periods prior to or just after the cholinergic pause, and might help to drive the increased activity seen during these time points. The resultant plasticity may help encode response timing during task performance.
2.2. Striatal LTD
Glutamatergic synapses in striatum also exhibit long-term decreases in synaptic efficacy, known as long-term depression (LTD) (Calabresi et al., 1992a,b; Lovinger et al., 1993; Walsh, 1993). This form of LTD can be induced by high frequency afferent stimulation, or as a result of timed pairing between activation of pre- and postsynaptic neuronal elements (spike-timing-dependent-plasticity, STDP) (Pawlak and Kerr, 2008; Shen et al., 2008; Fino et al., 2005). Striatal LTD involves a “retrograde signaling” mechanism in which postsynaptic induction mechanisms give rise to a presynaptic expression mechanism that appears to involve a decrease in probability of glutamate release (Choi and Lovinger, 1997a,b; Gerdeman et al., 2002) (Figure 1c). This form of long-term synaptic plasticity involves glutamate and dopamine (Figure 1c,d). Early studies by Calabresi and coworkers demonstrated that activation of D2 and D1-like receptors is necessary for induction of LTD by high frequency afferent activation (Calabresi et al., 1992a,b). The effect of D2 receptors on striatal LTD has been replicated numerous times using a variety of LTD induction paradigms including STDP (Tang et al., 2001; Kreitzer and Malenka, 2005; Wang et al., 2006; Shen et al., 2008). Deletion of the D2 receptor in mice, and depletion of striatal dopamine likewise prevent LTD induction using the high frequency stimulation paradigm (Calabresi et al., 1992a,b; Kreitzer and Malenka, 2007; Tang et al., 2001). Blockade of group I mGluRs also prevents LTD induction, and LTD can be induced by combined activation of group I mGluRs and mild postsynaptic depolarization (Figure 1c). Interestingly, when LTD is induced by a group I mGluR agonist it is resistant to D2 receptor blockade (Kreitzer and Malenka, 2005). This finding suggests that strong activation of glutamatergic mechanisms can induce LTD independent of dopaminergic signaling. Thus, the dopamine/D2 act as modulators of LTD, increasing the likelihood that it will occur. Pawlak and Kerr (2008) reached a similar conclusion that D2 receptor activation is modulatory for LTD induction. Presumably, under physiological conditions the dopamine/D2 signal is needed for LTD induction as synaptic activation of mGluRs would probably not be strong enough for dopamine-independent induction.
A variety of evidence indicates that LTD induction at gltuamatergic striatal synapses requires postsynaptic mechanisms. Preventing postsynaptic depolarization above ∼-60 mV prevents LTD even when strong high frequency stimulation is delivered at afferent fibers (Calabresi et al., 1992b; Calabresi et al., 1994; Choi and Lovinger, 1997a,b). Postsynaptic chelation of calcium by 10s of mM BAPTA or EGTA likewise disrupts LTD induction, and other postsynaptic manipulations have been shown to have a similar effect (Calabresi et al., 1994; Choi and Lovinger, 1997a,b). Several measures support a presynaptic site for LTD expression. The ratio of responses to paired afferent stimuli delivered at 50 ms intervals are sensitive to the probability of neurotransmitter release (Zucker et al., 1989), and an increase this paired-pulse ratio general signals a decrease in release probability. This ratio is indeed decreased in conjunction with LTD expression. An increase in the coefficient of variation of excitatory postsynaptic current (EPSC), another measure that indicates decreased neurotransmitter release, is also associated with LTD expression. Finally, one can measure the frequency and amplitude of miniature EPSCs (mEPSCs) with the frequency providing an index of the number of release events occurring per unit time and the amplitude providing a measure of postsynaptic responsiveness to each release event. Striatal LTD is associated with decreased mEPSC frequency, but not amplitude, also supporting a presynaptic site of LTD expression (Choi and Lovinger, 1997b).
The linkage between the postsynaptic induction and presynaptic expression of LTD involves endocannabinoid signaling (Gerdeman et al., 2002; Ronesi et al., 2004; Kreitzer and Malenka, 2005) (Figure 1c,d). Evidence collected to date indicates that endocannabinoids are produced by striatal MSNs as a result of glutamatergic and dopaminergic signaling combined with calcium entry into the neuron most likely via CaV1.3 L-type calcium channels (Kreitzer and Malenka, 2005; Wang et al., 2006; Adermark and Lovinger, 2007a). The endocannabinoid appears to be released from the postsynaptic neuron (perhaps through actions of a transporter or carrier molecule, Ronesi et al., 2004; Adermark and Lovinger, 2007b) to act on presynaptic CB1 receptors found on the corticostriatal afferent terminals. The CB1 receptor is expressed almost exclusively on presynaptic terminals in striatum and other brain regions (Katona et al., 2001; Katona et al., 2006; Matyas et al., 2006; Uchigashima et al., 2007), and is known to inhibit glutamate release when activated (Gerdeman and Lovinger, 2001; Huang et al., 2001; Kofalvi et al., 2005). The mechanisms by which a Gi/o-coupled receptor that would normally produce a transient inhibition of release comes to have a long-lasting effect are as yet unclear. Studies with antagonists for the CB1, D2 and group I mGlu receptors indicate that these receptors and their activating neurotransmitters only participate in LTD during the first few minutes after induction. Activation of CB1 receptors alone is only sufficient to induce LTD when combined with a threshold level of afferent activation (Singla et al., 2007; Adermark and Lovinger, 2007b). It is attractive to think that molecular mechanisms activated by CB1 receptors (e.g. inhibition of AC and voltage-gated calcium channels) need to combine or synergize with depolarization or calcium entry during afferent activation for LTD induction to occur. The early transmitter- and activity-driven events appear to set into motion subsequent molecular steps that maintain the prolonged decrease in neurotransmitter release probability (Choi and Lovinger, 1997a,b). Pharmacological studies have suggested roles for protein phosphorylation and protein translation in LTD expression and maintenance, but much remains to be learned about the LTD expression mechanisms (Calabresi et al., 1994; Yin et al., 2006).
Acetylcholine also has a modulatory role in striatal LTD (Figure 1c,d). Calabresi and coworkers found that mAChR antagonists enhance the magnitude of LTD at glutamatergic striatal synapses (Calabresi et al., 1992b; Bonsi et al., 2008). In a recent study, the Surmeier laboratory reported that these antagonists could rescue LTD in the presence of a D2R antagonist (Yang et al., 2006), and they may also rescue LTD that is lost in a mouse model of dystonia (Martella et al., 2009). The findings of Yang et al. (2006) have proven somewhat controversial (c.f. Kreitzer and Malenka, 2007), but seem to support the idea that D2 receptors modulate LTD induction rather than being absolutely necessary for LTD mechanisms. In contrast to the mACh antagonist effects, blockade of nicotinic ACh receptors (nAChRs) prevents LTD induction (Partridge et al., 2002). This blockade is reversed in the presence of dopamine reuptake inhibitors. It is well established that presynaptic nAChRs on dopaminergic terminals can stimulate dopamine release in striatum, and these LGICs have been implicated in stimulation-induced release of dopamine (Giorguieff et al., 1976; Kulak et al., 1997; Wonnacott et al., 2000; Zhou et al., 2001). Thus, it is likely that blockade of these receptors reduces dopamine release during high frequency stimulation, impairing the D2-signaling that normally promotes LTD.
Given that LTP and LTD at glutamatergic synapses are most often induced by similar patterns of afferent activation, a reasonable question is what factors determine which type of plasticity is induced under physiological conditions. Activation of different receptor subtypes is certainly one factor that we have already discussed. Activation of NMDA versus mGlu receptors may be biased by relative levels of the receptors at different synapses or by the ability of glutamate to reach extrasynaptic mGluRs. As yet, there is little information on how these factors influence induction of plasticity. There is reason to believe that tonic versus phasic firing of dopaminergic inputs can influence LTP and LTD induction. As discussed in Lovinger et al. (2003), tonic firing of dopaminergic inputs leading to a constant low level of DA may favor activation of higher affinity D2 receptors, biasing synapses toward LTD. Phasic dopaminergic neuron activity in conjunction with activation of cortical inputs would recruit lower affinity D1 receptors, favoring LTP. There is some experimental evidence for this idea from in vivo studies (Reynolds and Wickens, 2000; Reynolds et al., 2001). Differences in cholinergic neuronal activity may also be important, with strong firing of these neurons and mACh activation favoring LTP, while pauses in activity and cessation of mAChR activation favor LTD.
Induction of striatal LTP and LTD may also differ based on relative levels of postsynaptic depolarization during periods of strong afferent input. It will be important to explore the relationship between postsynaptic membrane potential and optimal NMDAR versus CaV1.3 mediated calcium signaling, as well as the thresholds necessary for LTP and LTD induction due to calcium entering via these two channel types.
In addition, the relative timing of pre and postsynaptic activity can influence LTP versus LTD induction, as evidenced by the STDP studies described above (Pawlak and Kerr, 2008; Shen et al., 2008; Fino et al., 2005). This may be due to differential timing of the receptor-induced intracellular signals needed for induction of the two types of plasticity. Activation of NMDAR-mediated calcium influx likely induces LTP regardless of whether or not there is coincident presynaptic activity, and thus a pre-before-post pairing would favor LTP. If LTD requires that CB1 activation occurs coincidentally with presynaptic activity (Singla et al. 2007), then post-before-pre pairing would favor this form of plasticity (as in Pawlak and Kerr, 2008; Shen et al., 2008) as the retrograde signal would presumably be arriving during periods of consistent presynaptic activation (but see Fino et al., 2005). The longer-lasting time window for induction of STDP-LTD versus STDP-LTP (Pawlak and Kerr, 2008) might also reflect the longer lasting effects of activation of GPCRs (mGluRs and CB1) versus ligand-gated ion channels (NMDARs). How the artificial STDP induction paradigms relate to conditions occurring in the striatum in vivo, where pre and postsynaptic activity are presumably occurring at multiple rates and patterns, remains to be determined. In this context, it will be interesting to examine interactions between the frequency and relative timing of pre and postsynaptic activity at different levels of afferent input. For example, the disinhibitory effect of LTD at GABAergic synapses (discussed in subsequent paragraphs) may favor LTP induction at glutamatergic synapses during particular patterns of pre and postsynaptic pairing.
Given that striatal LTP and LTD involve different expression mechanisms, it is possible that both types of plasticity could occur simultaneously at the same synapse. While combined plasticity of this type would appear to be futile, this is not necessarily the case. Postsynaptically-expressed LTP leads to an increase in the response to every afferent input. The decrease in probability of glutamate release during LTD expression has its largest effect on responses to single afferent inputs and in the first response during a burst of presynaptic activity. However, relative synaptic facilitation (e.g. paired-pulse facilitation) is greater when release probability is decreased. Thus, LTD might serve as a high-pass frequency filter for glutamatergic synaptic input. In the case of simultaneous LTP and LTD, responses to single low frequency synaptic inputs could be diminished even as responses to bursts were enhanced. As yet there is no evidence that LTP and LTD can occur simultaneously in the same neuron, let alone the same synapse, but this is an interesting situation to contemplate.
Endocannabinoid-dependent striatal LTD is not restricted to glutamatergic synapses, but also appears to be readily inducible at GABAergic synapses (Adermark et al., 2009; Adermark and Lovinger, 2009). Stimulation of afferents to striatal MSNs at 1 Hz for 1 min induces a long-lasting decrease in GABAergic synaptic transmission onto the MSNs. Blockade of mGluRs, CB1 and L-type voltage-gated calcium channels prevents induction of this GABAergic LTD. As with LTD at glutamatergic synapses, the LTD at GABAergic synapses becomes resistant to CB1 blockade several minutes after induction (Adermark and Lovinger, 2009). The role of mGluRs in this form of LTD indicates that induction is heterosynaptic, involving the influence of glutamatergic transmission on GABAergic synaptic efficacy. The role of other neurotransmitters, including dopamine and GABA itself, in induction and/or expression of LTD at striatal GABAergic synapses has not been explored in any detail.
Induction of LTD at striatal GABAergic and glutamatergic synapses is dependent on the frequency and duration of glutamatergic afferent activation, with lower frequencies and shorter durations inducing LTD at GABAergic synapses, while higher frequencies/longer durations favor glutamatergic LTD. This sets up a situation in which opposing long-term changes in MSN output can be produced in a manner that depends on the patterns of afferent input, with lower frequencies favoring long-lasting disinhibition and higher frequencies promoting inhibition (Adermark and Lovinger, 2009).
Less is known about the roles of other striatal neurotransmitters and receptors in the induction and modulation of LTP and LTD. Activation of presynaptic group II mGluRs has been shown to induce a form of LTD that may share presynaptic expression mechanisms with the LTD discussed above (Kahn et al., 2001). Blockade of adenosine receptors does not alter LTD induction or expression (Lovinger and Choi, 1995), suggesting that this transmitter may only participate in LTP. It will be interesting to determine if other small molecules present in striatum, such as serotonin, participate in plasticity. The role of neuropeptides in striatal long-term synaptic plasticity has not received any attention as of yet.
Theories of learning involving synaptic efficacy changes suggest that mechanisms should exist to reverse plasticity so as to avoid driving synapses to extreme efficacies. Thus, one might predict that mechanisms for de-potentiation and de-depression exist in striatum to reverse LTP and LTD respectively. Depotentiation at glutamatergic synapses has been reported (Picconi et al., 2003, 2006; Centonze et al., 2006). Low frequency afferent activation produces this plasticity, and activation of NMDA receptors appears to be required for induction of this de-potentiation. Theoretically, this type of plasticity can counteract LTP, preventing destabilization of circuitry due to positive feedback. Pharmacological studies indicate involvement of muscarinic ACh receptors in the induction of depotentiation (Picconi et al., 2006). Activation of D1 dopamine receptors or chronic treatment with L-Dopa prevent depotentiation (Picconi et al., 2003). In addition, loss of depotentiation and impaired flexibility of response learning are both observed in mice treatment with 3-nitropropionic acid in a pharmaracological model or early-stage Huntington's disease (Picconi et al., 2006). No report of de-depression has yet appeared. These mechanisms would seem to be needed to produce flexible memory storage of the type that appears to be involved in learning of goal-direct actions. However, the learning of skills is relatively irreversible. Thus, it is not clear that erasure of plasticity is necessarily involved in this type of inflexible learning. Some limit on the amount of potentiation or depression that can occur would be needed to ensure that synaptic transmission does not become too efficacious or that responses do not disappear altogether. However, these limitations may arise from the properties of the expression mechanisms themselves (e.g. limits on the extent to which probability can be decreased or limits of the number of receptors that can be incorporated postsynaptically). Experiments attempting to “saturate” striatal LTD indicate that synaptic efficacy can only be decreased to a certain point even with strong repetitive stimulation (Yin et al., 2009). Thus, there is some sort of limit on the expression level of LTD that helps to determine where the efficacy values can be set for irreversible learning.
Before moving to the topic of striatal plasticity in learning and memory, it is worth considering how LTP and LTD differ across striatal projection pathways, subregions and subcompartments. The predominance of LTP in dorsomedial striatum and LTD in dorsolateral striatum, at least in the slice preparation, has already been mentioned. This segregation may be related to the relative abundance of key neurotransmitter receptors in different striatal subregions. The striatum can also be subdivided into the “patch” and “matrix” compartments based on the relative expression of different molecular markers and patterns of afferent and efferent connections (Gerfen, 1992; Joel and Weiner, 2000; Canales, 2005). At present there is little information as to whether or not there are subcompartmental differences in plasticity, and this is certainly an area that needs exploration. The idea that LTP and LTD differ at synapses onto direct and indirect pathway MSNs has also been discussed (Kreitzer and Malenka, 2007; Shen et al., 2008; Wang et al., 2007). The neurons contributing to these two pathways clearly differ in many respects. Whether or not there is segregation of plasticity at synapses onto these different MSN subtypes in vivo is an open question. Given that synapses onto both direct and indirect pathway MSNs contain NMDA and CB1 receptors (Kreitzer and Malenka, 2007; Shen et al., 2008), it would seem most likely that LTP and LTD occur at synapses onto both types of neurons.
3. Synaptic Plasticity in Striatal-Based Learning and Memory
It has long been known that the striatum, including the dorsal striatum, plays critical roles in learning and memory. Early studies implicated the dorsal striatum in response-based learning and instrumental conditioning (Divac et al., 1967; Konorski, 1967). More recent studies using excitotoxic lesions, neurochemical approaches, and direct measurement of striatal neuronal activity in vivo have revealed roles for the dorsal striatum in skill learning, response-based learning, and instrumental conditioning involving both goal-directed and habitual responding (Yin and Knowlton, 2006; Yin et al., 2008,2009; Grahn et al. 2008; Wickens et al., 2007) (Figure 2). Studies of the ventral striatum/nucleus accumbens indicate roles for this brain region in spatial learning, pavlovian conditioning, and pavlovian-instrumental transfer, among other types of learning (Corbit et al., 2001; Ito et al., 2008; Parkinson et al., 2000; Roitman et al., 2005). The striatal subregions participate in these types of learning as part of at least 3 distinct circuits: an “associative” circuit involving associative cortical regions (e.g. medial prefrontal cortex) connectons to the dorsomedial striatum/caudate; a “sensorimotor” circuit involving sensory and motor cortex connections to the dorsolateral striatum/putamen; and a “limbic” circuit involving limbic cortex, basolateral amygdala and hippocampal connections to the ventral striatum/nucleus accumbens (Yin and Knowlton, 2006; Yin et al., 2008). Indeed, it can be argued that the striatum as a whole participates in most types of learning through participation in the actions of these circuits. Despite this vigorous renewal of interest in striatal-based learning and memory, little is known about the contributions of striatal synaptic plasticity to learning and memory.
Neurotransmitters and receptors implicated in striatal synaptic plasticity have been shown to participate in striatal-based learning and memory (Figure 2). Packard and coworkers used posttraining injection of antagonists into the dorsal striatum to show that activation of NMDA receptors is crucial for memory in a cued version of the Morris Water Maze task (Packard and Teather, 1997). This was an elegant experimental design, as the drugs were not present during training or testing. Thus, the antagonist actions could not be ascribed to deficits in the ability to physically perform the task. Infusion of glutamate itself increased response-based performance on a cross-maze task, another learning paradigm involving striatum (Packard, 1999). A similar postraining intracaudate injection paradigm was used to examine effects of D2R activation on memory in a Win-Stay variant of the radial arm maze task. Posttraining injection of a D2 agonist improved performance in this task, but not in a Win-Shift task that has a stronger spatial/hippocampal contribution (Packard and White, 1991). It is also notable that intrastriatal injection of a local anesthetic, which should inactivate the structure, just prior to a retention test did not impair the ability of the animal to perform a cross maze task, but just altered the strategy used by the animal from a response-based to a place-based approach (Packard and McGaugh, 1996). This finding indicates that the striatum, is not functioning merely as a motor control system in the performance of this task.
Activation of both NMDA and D1 receptors is involved in learning to press a lever for rewarding stimulation in the substantia nigra (i.e. the intracranial self-stimulation or ICSS paradigm) (Reynolds et al., 2001). Simultaneous activation of cortical glutamatergic afferents and ICSS-pattern stimulation of nigral dopaminergic neurons produces LTP of the glutamatergic activation of MSNs. This plastic change is tightly associated with acquisition of behaviors that elicit ICSS.
Studies of habitual instrumental learning also indicate roles for neurotransmitters and receptrors involved in striatal synaptic plasticity (Figure 2). For example, lesioning nigrostriatal dopaminergic inputs prevents habit learning while preserving goal-directed learned actions (Faure et al., 2005). Using an instrumental conditioning paradigm, Yin and coworkers demonstrated that direct injection of an NMDAR antagonist into dorsomedial striatum prevented action-outcome learning (Yin et al., 2005). This experiment also involved post-training injection, so effects of receptor blockade on task performance was not an issue in this study either. Furthermore, these experiments were designed to differentiate between action-outcome and habitual learning based on the effects of reward devaluation. Thus, the animals still demonstrated instrumental responding in the task, but responding was insensitive to devaluation. This experimental design thus provides an intrinsic control for activity and ability to perform the actions required for lever pressing. Using a similar experimental design, Hilario et al. (2007) demonstrated a role for endocannabinoids and the CB1 receptor in habit learning. This study revealed that gene-targeted mice lacking CB1 do not learn the habit-based strategy for instrumental learning, but show normal action-outcome learning. When mice are treated with CB1 antagonist during training but not testing, they also show impaired habit learning and intact action-outcome learning (Hilario et al., 2007). Excitotoxic lesions of dorsolateral striatum disrupted habit learning in this study, as previously reported in rat (Hilario et al., 2007; Yin et al., 2004). While these studies do not directly assess if striatal CB1 receptors are the ones responsible for the habit learning deficit, it is certainly worth considering this possibility for future studies. It will also be interesting to determine if CB1-mediated striatal LTD is involved in habit learning.
Another recent study indicates that CB1 receptor activation is necessary for extinction of response-based learning in the plus-maze paradigm (Rueda-Orozco et al., 2008). In this case, investigators injected a CB1 antagonist directly into the dorsolateral striatum, and this treatment prevented extinction. Changes in extinction learning were associated with changes in c-Fos immediate-early gene expression in dorsolateral striatum, supporting the idea that activation of neurons in this brain region is important for this type of learning. These findings are interesting as they directly implicate striatal CB1 receptors in response learning.
Studies have begun to explore the role of LTP and LTD in skill learning more explicitly (summarized in Figure 2). Costa and coworkers observed that striatal neurons, presumably mostly MSNs, develop task-related firing patterns during the acquisition and retention of improved performance on the rotarod rotating-dowel task (Costa et al., 2004). The patterns of task-related firing that developed included both increases and decreases in activity that related to task performance. Further studies indicated that task-related firing develops in the dorsomedial striatum during early phases of training as performance markedly improves (Yin et al., 2009). Once the animal reaches asymptotic, non-decremental performance, task-related firing is more prominent in dorsolateral striatum and the changes in dorsomedial striatum diminish. These findings indicate that the locus of task control may shift from the associative to the sensorimotor circuit as task performance becomes automatized and essentially immutable. Findings from studies in a maze paradigm also revealed striatal neuronal activity related to response learning (Jog et al., 1999).
Neurotransmitters and receptors involved in striatal LTP have been implicated in learning the rotarod skill (Figure 2). For example, gene-targeted mice with greatly decreased levels of the essential NMDA receptor subunit NR1 specifically in striatal MSNs lack functional synaptic NMDARs at MSN synapses (Dang et al., 2006). These mice show no improvement in the accelerating rotarod task and also lack LTP at excitatory synapses in striatum. Antagonists of the D1 dopamine receptor also disrupt rotarod performance when given within the first day or two of training (Yin et al., 2009). However, this antagonist effect is not present if animals are trained for several days prior to D1 antagonist administration. This finding suggests two intriguing possibilities. First, that striatal LTP may no longer be necessary once animals have mastered a particular skill. Second, that D1 receptors are no longer needed for task-related LTP once a skill is well learned. Related to the latter idea, it is possible that A2A receptors in the indirect pathway may substitute for D1 receptors during the latter phases of skill learning. These two ideas can be tested by examining effects of NMDAR antagonists in well-trained animals. If LTP is no longer necessary at this point in training then the drugs may have no effect.
The role of the A2A receptor in striatal-based instrumental conditioning has also been examined using a gene targeted mouse in which expression of A2A is eliminated in striatum (Yu et al., 2009). When trained on an instrumental conditioning task that promotes habitual learning, the A2A knockout mice showed only goal-directed responding. Acquisition of the instrumental task and performance of the lever pressing operant were normal in these mice. Thus, the effects are not due to motor impairment.
Ex vivo recordings from striatal slices also revealed training-related changes in synaptic efficacy that resembled LTP (Yin et al., 2009). After the early stages of rotarod training, the efficacy of AMPAR-mediated glutamatergic transmission was increased in dorsomedial striatum. At later stages, the efficacy changes shifted to the dorsolateral striatum, and subsided in the dorsomedial subregion. This pattern fits well with the shift from associative to sensorimotor circuitry observed in the in vivo electrophysiological studies. Surprisingly, the efficacy of NMDAR-mediated glutamatergic transmission also increased with training (Yin et al., 2009).
The link between striatal synaptic plasticity and learning has been strengthened by recent studies examining the role of adenylate cyclase type 5 (AC5) (Kheirbek et al., 2009). This AC subtype is abundantly expressed in striatal MSNs, with expression lower in cortex and hippocampus. Gene-targeted mice lacking this enzyme exhibit a deficiency in the induction of LTD by physiological stimulation that appears to result from a lack of the D2R modulation needed for induction. LTD can be induced by activation of group I mGluRs or CB1 receptors in striatal slices from these mice. Furthermore, direct postsynaptic intracellular application of cyclic AMP into MSNs fosters LTP induction during high frequency stimulation in wild-type mouse slices, but no such plasticity is observed in the AC5 knockout mice. These mice show deficiencies in several striatal-based skill, response-based, appetitive pavlovian and pavlovian-intrumental transfer learning (Kheirbek et al., 2008, 2009). Interestingly, aversive pavlovian learning and spatial learning in the water maze were unimpaired in these mice (Kheirbek et al., 2008). In addition, the AC5 knockout mice learned an instrumental task and appeared to be using a goal-directed strategy in this learning (Kheirbek et al., 2008). These findings lead to a number of intriguing hypotheses about the role of striatal synaptic plasticity in learning and memory. The most obvious inference is that not only plasticity itself, but also dopamine modulation of plasticity, is necessary for the types of learning that are impaired in these mice. Second, the AC5 role in learning probably involves all striatal subregions, as impairments in skill and response learning likely involve the dorsal striatum, while pavlovian conditioning is thought to involve nucleus accumbens (Yin et al., 2008). Of course, additional evidence using alternative in vivo experimental models will be helpful in evaluating the hypotheses generated from this study.
The observation that some forms of learning are resistant to AC5 loss is intriguing. Goal-directed instrumental conditioning, spatial learning and aversive pavlovian conditioning all involve circuits that connect to striatal subregions. However, it is not yet clear what role the striatum plays in these forms of learning. Furthermore, it might be the case that these forms of learning still involve the striatum, but are less dependent on dopaminergic modulation of striatal plasticity. Habit learning was not explicitly examined in these mice, although the response-based learning measured in a water-filled cross maze by these investigators may well have a strong habitual component. Clearly, more work of this type is needed to determine the mechanisms linking plasticity to learning.
Popescu and coworkers have also elucidated mechanisms through which the basolateral amygdala and ventral caudate nucleus communicate in the induction of plasticity and in appetitive pavlovian conditioning. Using striatal slices, these investigators found that LTP of synapses onto MSNs was enabled by coactivation of BLA and cortical afferent inputs (Popescu et al., 2009). Strong activation of NMDA receptors by the BLA synapses provides a crucial signal for the induction of this plasticity. Coherent local field potential activity occurs in BLA and ventral striatal MSNs, and increases during learning of the pavlovian task, and this activity may drive the plasticity needed for this type of learning (Popescu et al., 2009).
Relatively little research has examined the contributions of striatal short-term synaptic plasticity to learning and memory. However, it is possible that transient changes in synaptic function help to code the current value of a particular stimulus or outcome, and therefore control moment-to-moment goal-directed responding. Short-term synaptic plasticity might also be necessary for proper action selection in a particular context through activation and suppression of different direct and indirect pathway components. Clearly, more work is needed to separate the molecular mechanisms of short- and long-term synaptic plasticity in striatum, and determine the roles of these different plastic changes in skill, response, goal directed, and habitual learning.
4. Summary
Modulation of synaptic transmission either over the short- or long-term is a prominent mechanism for controlling striatal output, as activity of the MSN projection neurons is strongly influenced by their synaptic inputs. Electrophysiological studies have now established two prominent forms of long-lasting synaptic plasticity in striatum, LTP and LTD, as well as several forms of short-lasting synaptic modulation. The molecular mechanisms of these forms of plasticity are still being determined. However, there is already a growing body of evidence implicating striatal LTP and LTD in learning and memory involving this brain region. Mechanisms involved in LTP of synapses onto direct pathway neurons appear to facilitate skill learning and goal-directed actions. Interestingly, molecules implicated in LTP at synapses onto indirect pathway neurons as well as those involved in LTD at synapses onto both neuronal subtypes have been implicated in habit learning. Plasticity at synapses onto MSNs could sculpt output of the two different pathways to favor precise timing of action sequences while suppressing unnecessary sequences. These studies are intriguing, but the molecules implicated in LTP and LTD could also participate in other forms of synaptic modulation important for learning and memory. There is also emerging evidence that plasticity in the dorsomedial and dorsolateral striatal subregions may contribute to early and late phases of skill and instrumental learning, respectively. Additional information about plasticity mechanisms within particular striatal subregions, output pathways and the patch/matrix compartments in vivo is needed to fully evaluate the processing capabilities of the striatum. Furthermore, almost all of the studies to date have focused on plasticity at glutamatergic synapses, and much work remains to determine what forms of plasticity occur at GABAergic and other types of striatal synapses. Finally, the work to date has largely compared molecular manipulations in brain slices to behavior of the whole animal. More studies examining learning-related physiological changes in vivo and in ex vivo slice studies are certainly needed.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adermark L, Lovinger DM. Combined activation of L-type Ca2+ channels and synaptic transmission is sufficient to induced striatal long-term depression. J Neurosci. 2007a;27:6781–6787. doi: 10.1523/JNEUROSCI.0280-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adermark L, Lovinger DM. Retrograde endocannabinoid signaling at striatal synapses requires a regulated postsynaptic release step. Proc Natl Acad Sci USA. 2007b;104:20564–20569. doi: 10.1073/pnas.0706873104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adermark L, Lovinger DM. Frequency-dependent inversion of net striatal output by endocannabinoid-dependent plasticity at different synaptic inputs. J Neurosci. 2009;29(5):1375–1380. doi: 10.1523/JNEUROSCI.3842-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adermark L, Talani G, Lovinger DM. Endocannabinoid-dependent plasticity at GABAergic and glutamatergic synapses in the striatum is regulated by synaptic activity. Eur J Neurosci. 2009;29:32–41. doi: 10.1111/j.1460-9568.2008.06551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aosaki T, Tsubokawa H, Ishida A, Watanabe K, Graybiel AM, Kimura M. Responses of tonically active neurons in the primate's striatum undergo systematic changes during behavioral sensorimotor conditioning. J Neurosci. 1994;14(6):3969–3984. doi: 10.1523/JNEUROSCI.14-06-03969.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apicella P, Legallet E, Trouche E. Responses of tonically discharging neurons in monkey striatum to visual stimuli presented under passive conditions and during task performance. Neurosci Lett. 1996;203(3):147–150. doi: 10.1016/0304-3940(96)12328-4. [DOI] [PubMed] [Google Scholar]
- Augood SJ, Emson PC. Adenosine A2a receptor mRNA is expressed by enkephalin cells but not by somatostatin cells in rat striatum: a co-expression study. Brain Res Mol Brain Res. 1994;22(14):204–210. doi: 10.1016/0169-328x(94)90048-5. [DOI] [PubMed] [Google Scholar]
- Bamford NS, Robinson S, Palmiter RD, Joyce JA, Moore C, Meshul C. Dopamine modulates release from corticostriatal terminals. J Neurosci. 2004a;24(43):9541–9552. doi: 10.1523/JNEUROSCI.2891-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamford NS, Shang H, Schmitz Y, Wu NP, Cepeda C, Levine MS, Schmauss C, Zakharenko SS, Zablow L, Sulzer D. Heterosynaptic dopamine neurotransmission selects sets of corticostriatal terminals. Neuron. 2004b;42(4):653–663. doi: 10.1016/s0896-6273(04)00265-x. [DOI] [PubMed] [Google Scholar]
- Bennett BD, Wilson CJ. Spontaneous activity of neostriatal cholinergic interneurons in vitro. J Neurosci. 1999;19:5586–5596. doi: 10.1523/JNEUROSCI.19-13-05586.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonsi P, Martella G, Cuomo D, Platania P, Sciamanna G, Bernardi G, Wess J, Pisani A. Loss of muscarinic autoreceptor function impairs long-term depression but not long-term potentiation in the striatum. J Neurosci. 2008;28:6258–6263. doi: 10.1523/JNEUROSCI.1678-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracci E, Panzeri S. Excitatory GABAergic effects in striatal projection neurons. J Neurophysiol. 2006;95(2):1285–1290. doi: 10.1152/jn.00598.2005. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Mercuri NB, De Murtas M, Bernardi G. Involvement of GABA systems in feedback regulation of glutamate-and GABA-mediated synaptic potentials in rat neostriatum. J Physiol. 1991;440:581–599. doi: 10.1113/jphysiol.1991.sp018726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabresi P, Maj R, Mercuri NB, Bernardi G. Coactivation of D1 and D2 dopamine receptors is required for long-term synaptic depression in the striatum. Neurosci Lett. 1992a;142:95–99. doi: 10.1016/0304-3940(92)90628-k. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Maj R, Pisani A, Mercuri NB, Bernardi G. Long-term synaptic depression in the striatum: physiological and pharmacological characterization. J Neurosci. 1992b;12:4224–4233. doi: 10.1523/JNEUROSCI.12-11-04224.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabresi P, Pisani A, Mercuri NB, Bernardi G. Long-term potentiation in the striatum is unmasked by removing the voltage-dependent magnesium block of NMDA receptor channels. Eur J Neuroscience. 1992c;4:929–935. doi: 10.1111/j.1460-9568.1992.tb00119.x. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Pisani A, Mercuri NB, Bernardi G. Heterogeneity of metabotropic glutamate receptors in the striatum: electrophysiological evidence. Eur J Neurosci. 1993;5(10):1370–1377. doi: 10.1111/j.1460-9568.1993.tb00923.x. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Pisani A, Mercuri NB, Bernardi G. Post-receptor mechanisms underlying striatal long-term depression. J Neurosci. 1994;14(8):4871–4881. doi: 10.1523/JNEUROSCI.14-08-04871.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabresi P, Centonze D, Gubellini P, Pisani A, Bernardi G. Blockade of M2-like muscarinic receptors enhances long-term potentiation at corticostriatal synapses. Eur J Neurosci. 1998;10(9):3020–3023. doi: 10.1111/j.1460-9568.1998.00348.x. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Centonze D, Gubellini P, Bernardi G. Activation of M1-like muscarinic receptors is required for the induction of corticostriatal LTP. Neuropharmacology. 1999;38(2):323–326. doi: 10.1016/s0028-3908(98)00199-3. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Centonze D, Gubellini P, Marfia GA, Pisani A, Sancesario G, Bernardi G. Synaptic transmission in the striatum: from plasticity to neurodegeneration. Prog Neurobiol. 2000a;61(3):231–265. doi: 10.1016/s0301-0082(99)00030-1. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Gubellini P, Centonze D, Picconi B, Bernardi G, Chergui K, Svenningsson P, Fienberg AA, Greengard P. Dopamine and cyclic adenosine 3′,5′ monophosphate-regulated phosphoprotein 32 kDa controls both striatal long-term depression and long-term potentiation, opposing forms of synaptic plasticity. J Neurosci. 2000b;20:8443–8451. doi: 10.1523/JNEUROSCI.20-22-08443.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canales JJ. Stimulant-induced adaptations in neostriatal matrix and striosome systems: transiting from instrumental responding to habitual behavior in drug addiction. Neurobiol Learn Mem. 2005;83(2):93–103. doi: 10.1016/j.nlm.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Centonze D, Saulle E, Pisani A, Bernardi G, Calabresi P. Adenosine-mediated inhibition of striatal GABAergic synaptic transmission during in vitro ischaemia. Brain. 2001;124(Pt 9):1855–1865. doi: 10.1093/brain/124.9.1855. [DOI] [PubMed] [Google Scholar]
- Centonze D, Battista N, Rossi S, Mercuri NB, Finazzi-Agro A, Bernardi G, Calabresi P, Maccarrone M. A critical interaction between dopamine D2 receptors and endocannabinoids mediates the effects of cocaine on striatal gabaergic transmission. Neuropsychopharmacology. 2004;29:1488–1497. doi: 10.1038/sj.npp.1300458. [DOI] [PubMed] [Google Scholar]
- Centonze D, Costa C, Rossi S, Prosperetti C, Pisani A, Usiello A, Bernardi G, Mercuri NB, Calabresi P. Chronic cocaine prevents depotentiation at corticostriatal synapses. Biol Psychiatry. 2006;60(5):436–443. doi: 10.1016/j.biopsych.2005.11.018. [DOI] [PubMed] [Google Scholar]
- Cepeda C, Buchwald NA, Levine MS. Neuromodulatory actions of dopamine in the neostriatum are dependent upon the excitatory amino acid receptor subtypes activated. Proc Natl Acad Sci USA. 1993;90:9576–9580. doi: 10.1073/pnas.90.20.9576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charpier S, Deniau JM. In vivo activity-dependent plasticity at cortico-striatal connections: evidence for physiological long-term potentiation. Proc Natl Acad Sci USA. 1997;94:7036–7040. doi: 10.1073/pnas.94.13.7036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S, Lovinger DM. Decreased probability of neurotransmitter release underlies striatal long-term depression and postnatal development of corticostriatal synapses. Proc Natl Acad Sci USA. 1997a;94:2665–2670. doi: 10.1073/pnas.94.6.2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S, Lovinger DM. Decreased frequency but not amplitude of quantal synaptic responses associated with expression of corticostriatal long-term depression. J Neurosci. 1997b;17:8613–8620. doi: 10.1523/JNEUROSCI.17-21-08613.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper EC, Harrington E, Jan YN, Jan LY. M channel KCNQ2 subunits are localized to key sites for control of neuronal network oscillations and synchronization in mouse brain. J Neurosci. 2001;21(24):9529–9540. doi: 10.1523/JNEUROSCI.21-24-09529.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit LH, Muir JL, Balleine BW. The role of the nucleus accumbens in instrumental conditioning: Evidence of a functional dissociation between accumbens core and shell. J Neurosci. 2001;21:3251–3260. doi: 10.1523/JNEUROSCI.21-09-03251.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa RM, Cohen D, Nicolelis MA. Differential corticostriatal plasticity during fast and slow motor skill learning in mice. Curr Biol. 2004;14:1124–1134. doi: 10.1016/j.cub.2004.06.053. [DOI] [PubMed] [Google Scholar]
- Dang M, Yin HH, Lovinger DM, Li Y. Disrupted motor learning and long-term synaptic plasticity in mice lacking NMDAR1 in the striatum. Proc Natl Acad Sci USA. 2006;103(41):15254–15259. doi: 10.1073/pnas.0601758103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Filippo M, Picconi B, Tantucci M, Ghiglieri V, Bagetta V, Sgobio C, Tozzi A, Parnetti L, Calabresi P. Short-term and long-term plasticity at corticostriatal synapses: implications for learning and memory. Behav Brain Res. 2009;199(1):108–118. doi: 10.1016/j.bbr.2008.09.025. [DOI] [PubMed] [Google Scholar]
- Divac I, Rosvold HE, Szwarcbart MK. Behavioral effects of selective ablation of the caudate nucleus. J Comp Physiol Psychol. 1967;63:184–190. doi: 10.1037/h0024348. [DOI] [PubMed] [Google Scholar]
- Faure A, Haberland U, Conde F, El Massioui N. Lesion to the nigrostriatal dopamine system disrupts stimulus-response habit formation. J Neurosci. 2005;25:2771–2780. doi: 10.1523/JNEUROSCI.3894-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fino E, Glowinski J, Venance L. Bidirectional activity-dependent plasticity at corticostriatal synapses. J Neurosci. 2005;25(49):11279–11287. doi: 10.1523/JNEUROSCI.4476-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores-Hernandez J, Galarraga E, Bargas J. Dopamine selects glutamatergic inputs to neostriatal neurons. Synapse. 1997;25:185–195. doi: 10.1002/(SICI)1098-2396(199702)25:2<185::AID-SYN9>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Flores-Hernandez J, Cepeda C, Hernandez-Echeagaray E, Calvert CR, Jokel ES, Fienberg AA, Greengard P, Levine MS. Dopamine enhancement of NMDA currents in dissociated medium-sized striatal neurons: role of D1 receptors and DARPP-32. J Neurophysiol. 2002;30:10–20. doi: 10.1152/jn.00361.2002. [DOI] [PubMed] [Google Scholar]
- Gerdeman G, Lovinger DM. CB1 cannabinoid receptor inhibits synaptic release of glutamate in rat dorsolateral striatum. J Neurophysiol. 2001;85:468–471. doi: 10.1152/jn.2001.85.1.468. [DOI] [PubMed] [Google Scholar]
- Gerdeman GL, Ronesi J, Lovinger DM. Postsynaptic endocannabinoid release is critical to long-term depression in the striatum. Nat Neurosci. 2002;5:446–451. doi: 10.1038/nn832. [DOI] [PubMed] [Google Scholar]
- Gerdeman GL, Partridge JG, Lupica CR, Lovinger DM. It could be habit forming: drugs of abuse and striatal synaptic plasticity. Trends Neurosci. 2003;26:184–192. doi: 10.1016/S0166-2236(03)00065-1. [DOI] [PubMed] [Google Scholar]
- Gerfen CR. The neostriatal mosaic: multiple levels of compartmental organization. Trends Neurosci. 1992;15(4):133–139. doi: 10.1016/0166-2236(92)90355-c. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, Monsma FJ, Jr, Sibley DR. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science. 1990;250:1429–1432. doi: 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- Giorguieff MF, Le Floc'h ML, Westfall TC, Glowinski J, Besson MJ. Nicotinic effect of acetylcholine on the release of newly synthesized (3H)dopamine in rat striatal slices and cat caudate nucleus. Brain Res. 1976;106:117–131. doi: 10.1016/0006-8993(76)90077-9. [DOI] [PubMed] [Google Scholar]
- Gong S, Zheng C, Doughty ML, Losos K, Didkovsky N, Schambra UB, Nowak NJ, Joyner A, Leblanc G, Hatten ME, Heintz N. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature. 2003;425(6961):917–925. doi: 10.1038/nature02033. [DOI] [PubMed] [Google Scholar]
- Grahn JA, Parkinson JA, Owen AM. The cognitive functions of the caudate nucleus. Prog Neurobiol. 2008;86(3):141–155. doi: 10.1016/j.pneurobio.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Graybiel AM. The Basal Ganglia and Chunking of Action Repertoires. Neurobiol Learn Mem. 1998;70:119–136. doi: 10.1006/nlme.1998.3843. [DOI] [PubMed] [Google Scholar]
- Gubellini P, Saulle E, Centonze D, Bonsi P, Pisani A, Bernardi G, Conquet F, Calabresi P. Selective involvement of mGlu1 receptors in corticostriatal LTD. Neuropharmacology. 2001;40:839–846. doi: 10.1016/s0028-3908(01)00021-1. [DOI] [PubMed] [Google Scholar]
- Hilario MRF, Clouse E, Yin HH, Costa RM. Endocannabinoid signaling is critical for habit formation. Frontiers in Integrative Neuroscience. 2007;1:6. doi: 10.3389/neuro.07/006.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CC, Lo SW, Hsu KS. Presynaptic mechanisms underlying cannabinoid inhibition of excitatory synaptic transmission in rat striatal neurons. J Physiol. 2001;532:731–748. doi: 10.1111/j.1469-7793.2001.0731e.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito R, Robbins TW, Pennartz CM, Everitt BJ. Functional interaction between the hippocampus and nucleus accumbens shell is necessary for the acquisition of appetitive spatial context conditioning. J Neurosci. 2008;28(27):6950–6959. doi: 10.1523/JNEUROSCI.1615-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang ZG, North RA. Membrane properties and synaptic responses of rat striatal neurones in vitro. J Physiol. 1991;443:533–553. doi: 10.1113/jphysiol.1991.sp018850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joel D, Weiner I. The connections of the dopaminergic system with the striatum in rats and primates: an analysis with respect to the functional and compartmental organization of the striatum. Neuroscience. 2000;96(3):451–474. doi: 10.1016/s0306-4522(99)00575-8. [DOI] [PubMed] [Google Scholar]
- Jog MS, Kubota Y, Connolly CI, Hillegaart V, Graybiel AM. Building neural representations of habits. Science. 1999;286:1745–1749. doi: 10.1126/science.286.5445.1745. [DOI] [PubMed] [Google Scholar]
- Kahn L, Alonso G, Robbe D, Bockaert J, Manzoni OJ. Group 2 metabotropic glutamate receptors induced long term depression in mouse striatal slices. Neurosci Lett. 2001;316(3):178–182. doi: 10.1016/s0304-3940(01)02397-7. [DOI] [PubMed] [Google Scholar]
- Katona I, Rancz EA, Acsady L, Ledent C, Mackie K, Hajos N, Freund TF. Distribution of CB1 cannabinoid receptors in the amygdala and their role in the control of GABAergic transmission. J Neurosci. 2001;21:9506–9518. doi: 10.1523/JNEUROSCI.21-23-09506.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katona I, Urban GM, Wallace M, Ledent C, Jung KM, Piomelli D, Mackie K, Freund TF. Molecular composition of the endocannabinoid system at glutamatergic synapses. J Neurosci. 2006;26:5628–5637. doi: 10.1523/JNEUROSCI.0309-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerchner GA, Nicoll RA. Silent synapses and the emergence of a postsynaptic mechanism for LTP. Nat Rev Neurosci. 2008;9(11):813–825. doi: 10.1038/nrn2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr JN, Wickens JR. Dopamine D-1/D-5 Receptor activation is required for long-term potentiation in the rat neostriatum in vitro. J Neurophysiol. 2001;85:117–124. doi: 10.1152/jn.2001.85.1.117. [DOI] [PubMed] [Google Scholar]
- Kheirbek MA, Beeler JA, Ishikawa Y, Zhuang X. A cAMP pathway underlying reward prediction in associative learning. J Neurosci. 2008;28(44):11401–11408. doi: 10.1523/JNEUROSCI.4115-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kheirbek MA, Britt JP, Beeler JA, Ishikawa Y, McGehee DS, Zhuang X. Adenylyl cyclase type 5 contributes to corticostriatal plasticity and striatum-dependent learning. J Neurosci. 2009;29(39):12115–12124. doi: 10.1523/JNEUROSCI.3343-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofalvi A, Rodrigues RJ, Ledent C, Mackie K, Vizi ES, Cunha RA, Sperlagh B. Involvement of cannabinoid receptors in the regulation of neurotransmitter release in the rodent striatum: a combined immunochemical and pharmacological analysis. J Neurosci. 2005;25:2874–2884. doi: 10.1523/JNEUROSCI.4232-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konorski J. Integrative Activity of the Brain. University of Chicago Press; Chicago: 1967. [Google Scholar]
- Koos T, Tepper JM. Dual cholinergic control of fast-spiking interneurons in the neostriatum. J Neurosci. 2002;22:529–535. doi: 10.1523/JNEUROSCI.22-02-00529.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC, Malenka RC. Dopamine modulation of state-dependent endocannabinoid release and long-term depression in the striatum. J Neurosci. 2005;25:10537–10545. doi: 10.1523/JNEUROSCI.2959-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC, Malenka RC. Endocannabinoid-mediated rescue of striatal LTD and motor deficits in Parkinson's disease models. Nature. 2007;445:643–647. doi: 10.1038/nature05506. [DOI] [PubMed] [Google Scholar]
- Kreitzer AC, Malenka RC. Striatal plasticity and basal ganglia circuit function. Neuron. 2008;60(4):543–554. doi: 10.1016/j.neuron.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulak JM, Nguyen TA, Olivera BM, McIntosh JM. α-Conotoxin MII blocks nicotine-stimulated dopamine release in rat striatal synaptosomes. J Neurosci. 1997;17:5263–5270. doi: 10.1523/JNEUROSCI.17-14-05263.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalonde R, Botez-Marquard T. The neurobiological basis of movement initiation. Rev Neurosci. 1997;8(1):35–54. doi: 10.1515/revneuro.1997.8.1.35. [DOI] [PubMed] [Google Scholar]
- Lovinger DM. Trans-1-aminocyclopentane-1,3-dicarboxylic acid (t-ACPD) decreases synaptic excitation in rat striatal slices through a presynaptic action. Neurosci Lett. 1991;129(1):17–21. doi: 10.1016/0304-3940(91)90710-b. [DOI] [PubMed] [Google Scholar]
- Lovinger DM, Tyler EC, Merritt A. Short- and long-term synaptic depression in rat neostriatum. J Neurophysiol. 1993;70:1937–1949. doi: 10.1152/jn.1993.70.5.1937. [DOI] [PubMed] [Google Scholar]
- Lovinger DM, McCool BA. Metabotropic glutamate receptor-mediated presynaptic depression at corticostriatal synapses involves mGLuR2 or 3. J Neurophysiol. 1995;73:1076–1083. doi: 10.1152/jn.1995.73.3.1076. [DOI] [PubMed] [Google Scholar]
- Lovinger DM, Choi S. Activation of adenosine A1 receptors initiates short-term synaptic depression in rat striatum. Neurosci Lett. 1995;199(1):9–12. doi: 10.1016/0304-3940(95)12024-x. [DOI] [PubMed] [Google Scholar]
- Lovinger DM, Partridge JG, Tang KC. Plastic control of striatal glutamatergic transmission by ensemble actions of several neurotransmitters and targets for drugs of abuse. Ann NY Acad Sci. 2003;1003:226–240. doi: 10.1196/annals.1300.014. [DOI] [PubMed] [Google Scholar]
- Lovinger DM, Davis MI, Costa RM. Endocannabinoid Signaling in the Striatum. In: Steiner H, Tseng KY, editors. Handbook of Basal Ganglia Structure and Function. Elsevier; 2009. in press. [Google Scholar]
- Mahon S, Deniau JM, Charpier S. Corticostriatal plasticity: life after the depression. Trends Neurosci. 2005;27(8):460–467. doi: 10.1016/j.tins.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Kocsis JD. Presynaptic actions of carbachol and adenosine on corticostriatal synaptic transmission studied in vitro. J Neurosci. 1998;8:3750–3756. doi: 10.1523/JNEUROSCI.08-10-03750.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martella G, Tassone A, Sciamanna G, Platania P, Cuomo D, Viscomi MT, Bonsi P, Cacci E, Biagioni S, Usiello A, Bernardi G, Sharma N, Standaert DG, Pisani A. Impairment of bidirectional synaptic plasticity in the striatum of a mouse model of DYT1 dystonia: role of endogenous acetylcholine. Brain. 2009;132(Pt 9):2336–2349. doi: 10.1093/brain/awp194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto N, Minamimoto T, Graybiel AM, Kimura M. Neurons in the thalamic CM-Pf complex supply striatal neurons with information about behaviorally significant sensory events. J Neurophysiol. 2001;85(2):960–976. doi: 10.1152/jn.2001.85.2.960. [DOI] [PubMed] [Google Scholar]
- Matyas F, Yanovsky Y, Mackie K, Kelsch W, Misgeld U, Freund TF. Subcellular localization of type 1 cannabinoid receptors in the rat basal ganglia. Neuroscience. 2006;137:337–361. doi: 10.1016/j.neuroscience.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Miller RJ. Presynaptic Receptors. Ann Rev Pharmacool Toxicol. 1998;38:201–207. doi: 10.1146/annurev.pharmtox.38.1.201. [DOI] [PubMed] [Google Scholar]
- Narushima M, Uchigashima M, Hashimoto K, Watanabe M, Kano M. Depolarization-induced suppression of inhibition mediated by endocannabinoids at synapses from fast-spiking interneurons to medium spiny neurons in the striatum. Eur J Neurosci. 2006;24(8):2246–2252. doi: 10.1111/j.1460-9568.2006.05119.x. [DOI] [PubMed] [Google Scholar]
- Narushima M, Uchigashima M, Fukaya M, Matsui M, Manabe T, Hashimoto K, Watanabe M, Kano M. Tonic enhancement of endocannabinoid-mediated retrograde suppression of inhibition by cholinergic interneuron activity in the striatum. J Neurosci. 2007;27:496–506. doi: 10.1523/JNEUROSCI.4644-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicola SM, Malenka RC. Modulation of synaptic transmission by dopamine and norepinephrine in ventral but not dorsal striatum. J Neurophysiol. 1998;79:1768–1776. doi: 10.1152/jn.1998.79.4.1768. [DOI] [PubMed] [Google Scholar]
- Packard MG, White NM. Dissociation of hippocampus and caudate nucleus memory systems by posttraining intracerebral injection of dopamine agonists. Behav Neurosci. 1991;105(2):295–306. doi: 10.1037//0735-7044.105.2.295. [DOI] [PubMed] [Google Scholar]
- Packard MG, McGaugh JL. Inactivation of hippocampus or caudate nucleus with lidocaine differentially affects expression of place and response learning. Neurobiol Learn Mem. 1996;65(1):65–72. doi: 10.1006/nlme.1996.0007. [DOI] [PubMed] [Google Scholar]
- Packard MG, Teather LA. Double dissociation of hippocampal and dorsal-striatal memory systems by posttraining intracerebral injections of 2-amino-5-phosphonopentanoic acid. Behav Neurosci. 1997;111(3):543–551. doi: 10.1037//0735-7044.111.3.543. [DOI] [PubMed] [Google Scholar]
- Packard MG. Glutamate infused posttraining into the hippocampus or caudate-putamen differentially strengthens place and response learning. Proc Natl Acad Sci USA. 1999;96(22):12881–12886. doi: 10.1073/pnas.96.22.12881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson JA, Willoughby PJ, Robbins TW, Everitt BJ. Disconnection of the anterior cingulate cortex and nucleus accumbens core impairs Pavlovian approach behavior: further evidence for limbic cortical-ventral striatopallidal systems. Behav Neurosci. 2000;114:42–63. [PubMed] [Google Scholar]
- Partridge JG, Tang KC, Lovinger DM. Regional and postnatal heterogeneity of activity-dependent long-term changes in synaptic efficacy in the dorsal striatum. J Neurophysiol. 2000;84:1422–1429. doi: 10.1152/jn.2000.84.3.1422. [DOI] [PubMed] [Google Scholar]
- Partridge JG, Apparsundaram S, Gerhardt GA, Ronesi J, Lovinger DM. Nicotinic acetylcholine receptors interact with dopamine in induction of striatal long-term depression. J Neurosci. 2002;22:2541–2549. doi: 10.1523/JNEUROSCI.22-07-02541.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge JG, Janssen MJ, Chou DY, Abe K, Zukowska Z, Vicini S. Excitatory and Inhibitory Synapses in Neuropeptide Y-expressing Striatal Interneurons. J Neurophysiol. 2009;102(5):3038–3045. doi: 10.1152/jn.00272.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlak V, Kerr JN. Dopamine receptor activation is required for corticostriatal spike-timing-dependent plasticity. J Neurosci. 2008;28:2435–2446. doi: 10.1523/JNEUROSCI.4402-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picconi B, Centonze D, Håkansson K, Bernardi G, Greengard P, Fisone G, Cenci MA, Calabresi P. Loss of bidirectional striatal synaptic plasticity in L-DOPA-induced dyskinesia. Nat Neurosci. 2003;6(5):501–506. doi: 10.1038/nn1040. [DOI] [PubMed] [Google Scholar]
- Picconi B, Passino E, Sgobio C, Bonsi P, Barone I, Ghiglieri V, Pisani A, Bernardi G, Ammassari-Teule M, Calabresi P. Plastic and behavioral abnormalities in experimental Huntington's disease: a crucial role for cholinergic interneurons. Neurobiol Dis. 2006;22(1):143–152. doi: 10.1016/j.nbd.2005.10.009. [DOI] [PubMed] [Google Scholar]
- Pisani A, Calabresi P, Centonze D, Bernardi G. Activation of group III metabotropic glutamate receptors depresses glutamatergic transmission at corticostriatal synapse. Neuropharmacology. 1997;36(6):845–851. doi: 10.1016/s0028-3908(96)00177-3. [DOI] [PubMed] [Google Scholar]
- Pisani A, Bonsi P, Catania MV, Giuffrida R, Morari M, Marti M, Centonze D, Bernardi G, Kingston AE, Calabresi P. Metabotropic glutamate 2 receptors modulate synaptic inputs and calcium signals in striatal cholinergic interneurons. J Neurosci. 2002;22(14):6176–6185. doi: 10.1523/JNEUROSCI.22-14-06176.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popescu AT, Saghyan AA, Paré D. NMDA-dependent facilitation of corticostriatal plasticity by the amygdala. Proc Natl Acad Sci USA. 2007;104(1):341–346. doi: 10.1073/pnas.0609831104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popescu AT, Popa D, Paré D. Coherent gamma oscillations couple the amygdala and striatum during learning. Nat Neurosci. 2009;12(6):801–807. doi: 10.1038/nn.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds JN, Hyland BI, Wickens JR. A cellular mechanism of reward-related learning. Nature. 2001;413(6851):67–70. doi: 10.1038/35092560. [DOI] [PubMed] [Google Scholar]
- Reynolds JN, Wickens JR. Dopamine-dependent plasticity of corticostriatal synapses. Neural Netw. 2002;15:507–521. doi: 10.1016/s0893-6080(02)00045-x. [DOI] [PubMed] [Google Scholar]
- Reynolds JN, Wickens JR. Substantia nigra dopamine regulates synaptic plasticity and membrane potential fluctuations in the rat neostriatum, in vivo. Neuroscience. 2000;99(2):199–203. doi: 10.1016/s0306-4522(00)00273-6. [DOI] [PubMed] [Google Scholar]
- Richfield EK, Penney JB, Young AB. Anatomical and affinity state comparisons between dopamine D1 and D2 receptors in the rat central nervous system. Neuroscience. 1989;30:767–777. doi: 10.1016/0306-4522(89)90168-1. [DOI] [PubMed] [Google Scholar]
- Ronesi J, Gerdeman GL, Lovinger DM. Disruption of endocannabinoid release and striatal long-term depression by postsynaptic blockade of endocannabinoid membrane transport. J Neurosci. 2004;24:1673–1679. doi: 10.1523/JNEUROSCI.5214-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roitman MF, Wheeler RA, Carelli RM. Nucleus accumbens neurons are innately tuned for rewarding and aversive taste stimuli, encode their predictors, and are linked to motor output. Neuron. 2005;45:587–597. doi: 10.1016/j.neuron.2004.12.055. [DOI] [PubMed] [Google Scholar]
- Rueda-Orozco PE, Montes-Rodriguez CJ, Soria-Gomez E, Méndez-Díaz M, Prospéro-García O. Impairment of endocannabinoids activity in the dorsolateral striatum delays extinction of behavior in a procedural memory task in rats. Neuropharmacology. 2008;55(1):55–62. doi: 10.1016/j.neuropharm.2008.04.013. [DOI] [PubMed] [Google Scholar]
- Saint-Cyr JA, Taylor AE, Nicholson K. Behavior and the basal ganglia. Adv Neurol. 1995;65:1–28. [PubMed] [Google Scholar]
- Schmidt WJ. Balance of transmitter activities in the basal ganglia loops. J Neural Transm Suppl. 1995;46:67–76. [PubMed] [Google Scholar]
- Seabrook GR, Howson W, Lacey MG. Subpopulations of GABA-mediated synaptic potentials in slices of rat dorsal striatum are differentially modulated by presynaptic GABAB receptors. Brain Res. 1991;562(2):332–334. doi: 10.1016/0006-8993(91)90642-9. [DOI] [PubMed] [Google Scholar]
- Shen W, Hamilton SE, Nathanson NM, Surmeier DJ. Cholinergic suppression of KCNQ channel currents enhances excitability of striatal medium spiny neurons. J Neurosci. 2005;25(32):7449–7458. doi: 10.1523/JNEUROSCI.1381-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W, Flajolet M, Greengard P, Surmeier DJ. Dichotomous dopaminergic control of striatal synaptic plasticity. Science. 2008;321:848–851. doi: 10.1126/science.1160575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singla S, Kreitzer AC, Malenka RC. Mechanisms for synapse specificity during striatal long-term depression. J Neurosci. 2007;27(19):5260–5264. doi: 10.1523/JNEUROSCI.0018-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer JP, Murphy KP. Bi-directional changes in synaptic plasticity induced at corticostriatal synapses in vitro. Exp Brain Res. 2000;135(4):497–503. doi: 10.1007/s002210000523. [DOI] [PubMed] [Google Scholar]
- Sugita S, Uchimura N, Jiang ZG, North RA. Distinct muscarinic receptors inhibit release of gamma-aminobutyric acid and excitatory amino acids in mammalian brain. Proc Natl Acad Sci USA. 1991;88(6):2608–2611. doi: 10.1073/pnas.88.6.2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung KW, Choi S, Lovinger DM. Activation of group I mGluRs is necessary for induction of long-term depression at striatal synapses. J Neurophysiol. 86:2405–2412. doi: 10.1152/jn.2001.86.5.2405. [DOI] [PubMed] [Google Scholar]
- Surmeier DJ, Ding J, Day M, Wang Z, Shen W. D1 and D2 dopamine-receptor modulation of striatal glutamatergic signaling in striatal medium spiny neurons. Trends Neurosci. 2007;30(5):228–235. doi: 10.1016/j.tins.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Szabo B, Dorner L, Pfreundtner C, Norenberg W, Starke K. Inhibition of GABAergic inhibitory postsynaptic currents by cannabinoids in rat corpus striatum. Neuroscience. 1998;85:395–403. doi: 10.1016/s0306-4522(97)00597-6. [DOI] [PubMed] [Google Scholar]
- Tang K, Low MJ, Grandy DK, Lovinger DM. Dopamine-dependent synaptic plasticity in striatum during in vivo development. Proc Natl Acad Sci USA. 2001;98(2001):1255–1260. doi: 10.1073/pnas.031374698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepper JM, Koós T, Wilson CJ. GABAergic microcircuits in the neostriatum. Trends Neurosci. 2004;27(11):662–669. doi: 10.1016/j.tins.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Tepper JM, Abercrombie ED, Bolam JP. Basal ganglia macrocircuits. Prog Brain Res. 2007;160:3–7. doi: 10.1016/S0079-6123(06)60001-0. [DOI] [PubMed] [Google Scholar]
- Uchigashima M, Narushima M, Fukaya M, Katona I, Kano M, Watanabe M. Subcellular arrangement of molecules for 2-arachidonoyl-glycerol-mediated retrograde signaling and its physiological contribution to synaptic modulation in the striatum. J Neurosci. 2007;27:3663–3676. doi: 10.1523/JNEUROSCI.0448-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh JP. Depression of excitatory synaptic input in rat striatal neurons. Brain Res. 1993;1608(1):123–128. doi: 10.1016/0006-8993(93)90782-i. [DOI] [PubMed] [Google Scholar]
- Wang Z, Kai L, Day M, Ronesi J, Yin HH, Ding J, Tkatch T, Lovinger DM, Surmeier DJ. Dopaminergic control of corticostriatal long-term synaptic depression in medium spiny neurons is mediated by cholinergic interneurons. Neuron. 2006;50:443–452. doi: 10.1016/j.neuron.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Wickens JR, Horvitz JC, Costa RM, Killcross S. Dopaminergic mechanisms in actions and habits. J Neurosci. 2007;27(31):8181–8183. doi: 10.1523/JNEUROSCI.1671-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickens JR. Synaptic plasticity in the basal ganglia. Behav Brain Res. 2009;199(1):119–128. doi: 10.1016/j.bbr.2008.10.030. [DOI] [PubMed] [Google Scholar]
- Wilson CJ, Chang HT, Kitai ST. Firing patterns and synaptic potentials of identified giant aspiny interneurons in the rat neostriatum. J Neurosci. 1990;10(2):508–519. doi: 10.1523/JNEUROSCI.10-02-00508.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wonnacott S, Kaiser S, Mogg A, Soliakov L, Jones IW. Presynaptic nicotinic receptors modulating dopamine release in the rat striatum. Eur J Pharmacol. 2000;393:51–58. doi: 10.1016/s0014-2999(00)00005-4. [DOI] [PubMed] [Google Scholar]
- Wu LG, Saggau P. Presynaptic inhibition of elicited neurotransmitter release. Trends Neurosci. 1997;20(5):204–212. doi: 10.1016/s0166-2236(96)01015-6. [DOI] [PubMed] [Google Scholar]
- Yan Z, Surmeier DJ. D5 dopamine receptors enhance Zn2+-sensitive GABA(A) currents in striatal cholinergic interneurons through a PKA/PP1 cascade. Neuron. 1997;19(5):1115–26. doi: 10.1016/s0896-6273(00)80402-x. [DOI] [PubMed] [Google Scholar]
- Yan Z, Hsieh-Wilson L, Feng J, Tomizawa K, Allen PB, Fienberg AA, Nairn AC, Greengard P. Protein phosphatase 1 modulation of neostriatal AMPA channels: regulation by DARPP-32 and spinophilin. Nat Neurosci. 1999;2(1):13–17. doi: 10.1038/4516. [DOI] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ, Balleine BW. Lesions of dorsolateral striatum preserve outcome expectancy but disrupt habit formation in instrumental learning. Eur J Neurosci. 2004;19:181–189. doi: 10.1111/j.1460-9568.2004.03095.x. [DOI] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ, Balleine BW. Blockade of NMDA receptors in the dorsomedial striatum prevents action-outcome learning in instrumental conditioning. Eur J Neurosci. 2005;22:505–512. doi: 10.1111/j.1460-9568.2005.04219.x. [DOI] [PubMed] [Google Scholar]
- Yin HH, Davis MI, Ronesi JA, Lovinger DM. The role of protein synthesis in striatal long-term depression. J Neurosci. 2006;26:11811–11820. doi: 10.1523/JNEUROSCI.3196-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin HH, Lovinger DM. Frequency-specific and D2 receptor-mediated inhibition of glutamate release by retrograde endocannabinoid signaling. Proc Natl Acad Sci USA. 2006;103:8251–8256. doi: 10.1073/pnas.0510797103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ. The role of the basal ganglia in habit formation. Nat Rev Neurosci. 2006;7(6):464–476. doi: 10.1038/nrn1919. [DOI] [PubMed] [Google Scholar]
- Yin HH, Ostlund SB, Balleine BW. Reward-guided learning beyond dopamine in the nucleus accumbens: the integrative functions of cortico-basal ganglia networks. Eur J Neurosci. 2008;28(8):1437–1448. doi: 10.1111/j.1460-9568.2008.06422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin HH, Prasad-Mulcare S, Hilario MRF, Clouse E, Davis MI, Hansson AC, Lovinger DM, Costa RM. Dynamic reorganization of striatal circuits during the acquisition and consolidation of a skill. Nature Neuroscience. 2009;12(3):333–341. doi: 10.1038/nn.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C, Gupta J, Chen JF, Yin HH. Genetic deletion of A2A adenosine receptors in the striatum selectively impairs habit formation. J Neurosci. 2009;29(48):15100–15103. doi: 10.1523/JNEUROSCI.4215-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou FM, Liang Y, Dani JA. Endogenous nicotinic cholinergic activity regulates dopamine release in the striatum. Nat Neurosci. 2001;4:1224–1229. doi: 10.1038/nn769. [DOI] [PubMed] [Google Scholar]
- Zhou FM, Wilson CJ, Dani JA. Cholinergic interneuron characteristics and nicotinic properties in the striatum. J Neurobiol. 2002;53(4):590–605. doi: 10.1002/neu.10150. [DOI] [PubMed] [Google Scholar]
- Zucker RS. Short-term Synaptic Plasticity. Ann Rev Neurosci. 1989;12:13–31. doi: 10.1146/annurev.ne.12.030189.000305. [DOI] [PubMed] [Google Scholar]