Abstract
Background
Orexin (hypocretin) signaling is implicated in drug addiction and reward, but its role in feeding and food-motivated behavior remains unclear.
Methods
We investigated orexin’s contribution to food-reinforced instrumental responding using an orexin 1 receptor (Ox1r) antagonist, orexin −/− (OKO) and littermate wild-type (WT) mice, and RNAi-mediated knockdown of orexin. C57BL/6J (n=76) and OKO (n=39) mice were trained to nose poke for food under a variable ratio (VR) schedule of reinforcement. Once responding stabilized, a progressive ratio (PR) schedule was initiated to evaluate motivation to obtain food reinforcement.
Results
Blockade of Ox1r in C57BL/6J mice impaired performance under both the VR and PR schedules of reinforcement, indicating impaired motivational processes. In contrast, OKO mice initially demonstrated a delay in acquisition, but eventually achieved levels of responding similar to those observed in WT animals. Moreover, OKO mice did not differ from WT mice under a PR schedule, indicating delayed learning processes but no motivational impairments. Considering the differences between pharmacological blockade of Ox1r and the OKO mice, animals with RNAi mediated knockdown of orexin were then generated and analyzed to eliminate possible developmental effects of missing orexin. Orexin gene knockdown in the lateral hypothalamus (LH) in C57BL/6J mice resulted in blunted performance under both the VR and PR schedules, resembling data obtained following Ox1r antagonism.
Conclusions
The behavior seen in OKO mice likely reflects developmental compensation often seen in mutant animals. These data suggest that activation of the Ox1r is a necessary component of food-reinforced responding and/or motivation in normal mice.
Keywords: Orexin, Hypocretin, Motivation, Instrumental learning, Food reward, SB-334867
INTRODUCTION
The orexins (or hypocretins) are neuropeptides implicated in multiple behavioral states ranging from sleep (1–3), general arousal, feeding and metabolism (4,5), to drug and natural reinforcement processes (6), as well as drug dependence/withdrawal states (7–9) The orexins are synthesized in neurons restricted to the lateral hypothalamus (LH) (1,10,11) that project throughout the brain (10,12,13). The orexin receptors, orexin 1 (Ox1r) and orexin 2 (Ox2r), are G-protein coupled receptors (11) located in numerous brain regions, including dopaminergic projections from the ventral tegmental area (VTA) to the nucleus accumbens (NAc) (14) that are known to regulate drug addiction and reward (15–18).
While many early studies focused on the role of orexins in narcolepsy and sleep (1–3), more recent work has begun to explore orexin’s role in reward and drug dependence. Evidence for the role of orexin in drug dependence comes from our laboratory where we have shown that a mutation of the orexin gene (7) and blockade of Ox1r (8) results in attenuated somatic withdrawal symptoms in morphine-dependent mice. The role of orexin in Pavlovian reward-related processes has been demonstrated by experiments in which the expression of morphine-, cocaine-, and food-induced conditioned place preference (CPP) was found to be positively correlated with activation of orexin-containing neurons (6). Furthermore, pharmacological blockade of Ox1r appears to attenuate CPP (6). Central orexin administration results in reinstatement of extinguished cocaine self-administration (19) and intra-VTA blockade of Ox1r prevents the development of cocaine sensitization (20).
While the potential involvement of orexin in mediating goal-directed behaviors, including food-reinforced responding, remains inconclusive, a role for orexin in feeding has been proposed. Intracerebroventricular administration of orexin (11,21–23) stimulates feeding and increases food intake. However, orexin’s role in food-motivated behavior, such as nose poking to obtain food reinforcement, remains unclear. Blockade of Ox1r attenuates operant responding for high fat food pellets in rats (24,25), but has no effect on lever-pressing for sucrose (26). Furthermore, while Ox1r antagonism has been reported to attenuate operant responding for nicotine and cocaine, it fails to effect operant responding for regular chow(24,27).
Here, we further investigate orexin’s contribution to food-reinforced instrumental responding using pharmacological blockade of the Ox1r, mice lacking the orexin precursor prepro-orexin (orexin −/−, OKO), and a novel RNAi mediated knockdown of the prepro-orexin gene in the LH. Together, these data demonstrate that Ox1r activation is an integral component of instrumental responding for food reinforcement.
MATERIALS AND METHODS
Subjects
Subjects for pharmacological experiments and viral studies were male C57BL/6J mice (n=76), between 8–12 weeks of age. Genetic studies were completed using prepro-orexin knock out (OKO) mice (n=20) (1) and wildtype (WT) littermates (N=19), that had been backcrossed more than five times to a C57BL/6J background, between the ages of 16–24 weeks. C57BL/6J mice were obtained from Jackson Laboratories and housed in groups of four or five. For the genetic experiments, mice supplied by Masashi Yanagisawa were bred and housed in groups of three to five. All mice were maintained on a 12:12 hour light:dark cycle. One week prior to experimental sessions, mice were placed on a mild food-restricted diet in which their weights were reduced to, and maintained at, 85% of their free-feeding weights by means of measured daily rations of Prolab RMH 3000 mouse chow with ad libitum access to water except during operant conditioning sessions. Home cage feeding occurred two hours following the conclusion of experimental sessions. On average, food restricted mice were given and consumed 9 to 10 kCal daily whereas non-restricted mice consume 14 to 19 kCal daily. No significant differences in weights were observed in any cohort prior to food restriction. All experimental sessions occurred during the light phase. All animal procedures were approved by the Yale Animal Care and Use Committee.
Design and construction of shRNA and viral delivery
Please see Supplement 1.
Immunohistochemistry
Please see Supplement 1.
Operant conditioning apparatus
Please see Supplement 1.
Operant responding under a FR1(10)+VR2 schedule of reinforcement (“VR”)
Mice were habituated to the operant conditioning chambers and food pellets, during a single session, where they were placed in the chambers for 15 minutes and each magazine entry was reinforced with a single pellet (28). During subsequent sessions, animals were trained to nose poke for a food reinforcer during daily 25 minute sessions. Here, the position of the correct nose poke was counterbalanced between the left and right holes across mice. Insertions of the nose into the correct nose poke were reinforced with a single food pellet, while responding on the other two holes had no consequence. The first ten correct nose pokes were reinforced on a fixed ratio 1 (FR1) schedule, in which every entry into the correct nose poke was reinforced, after which correct nose pokes were reinforced on a variable ratio 2 (VR2) schedule, in which one, two, or three responses were reinforced as randomly determined by the operating computer [i.e., FR1(10)+VR2]. Since, to receive subsequent reinforcers, mice were required to retrieve each pellet, all pellets were consumed and none remained in the magazine. The acquisition phase continued until all mice displayed stable responding. This was defined as three consecutive sessions during which correct responses did not vary by more than ±10% averaged over three sessions.
Operant responding under a progressive ratio 4 schedule of reinforcement (“PR”)
When mice demonstrated acquisition of the nose poke response, a progressive ratio 4 (PR4) schedule of reinforcement was introduced. Under the PR4 schedule, the response requirement progressively increased on a linear scale, such that the response requirement was set to 1 for the first food reinforcer and increased by 4 for each subsequent reinforcer (i.e., 1, 5, 9… x + 4). Under this schedule, the ratio requirement ultimately becomes so high that mice reach a break point (BP) where they stop responding. BPs were operationally defined as the final ratio completed (which resulted in the delivery of a food pellet) before 5 minutes of no nose poking (28,29). Once animals reached their BPs, the house lights were turned off and the session automatically terminated. Mice were tested under the PR4 schedule of reinforcement for three consecutive sessions, held once per day.
Locomotor Activity and Free Feeding
Please see Supplement 1.
Drugs
SB-334867 (1-(2-methylbenzoxazol-6-yl)-3-[1,5]naphthyridin-4-yl urea hydrochloride) (Tocris, Ellisville, Missouri) was dissolved in 10% (w/v) (2-hyroxypropyl)-β-cyclodextrin/10% dimethyl sulfoxide (DMSO) in sterile water and was administrated at via i.p. injections 20 minutes prior to experimental sessions. The dose of SB-334867 (20 mg/kg) used for behavioral experiments is consistent with other recent studies (6,30), and is not associated with locomotor deficits often seen when higher doses are administered (31).
Data Analysis
For VR studies, mixed design two-way analyses of variance (ANOVAs) were conducted with either genotype, knockdown, or dose of SB-334867 as the between-subjects factor and session as the within-subjects factor. Within each experiment, separate ANOVAs were conducted to analyze reinforcers earned, active nose pokes, inactive nose pokes, and active nose pokes as percentage of total responses. Significant results (p<0.05) were followed by tests of simple main effects and Bonferroni pairwise comparisons. For PR studies, data obtained during three test sessions were averaged and separate independent samples t-tests were conducted to analyze active nose pokes, inactive nose pokes, and BPs. Separate ANOVAs were conducted to analyze locomotor activity with genotype or dose of SB-334867 as the between-subjects factor and time as the within-subject factor. For free feeding, separate independent samples t-tests were conducted to analyze total pellet consumption.
RESULTS
Ox1r blockade results in decreased responding for food
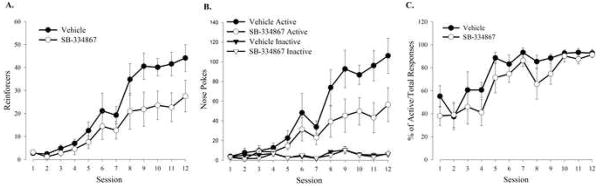
Based on previous findings implicating orexin in drug reward and motivational processes, we aimed to investigate orexin’s involvement in food-directed instrumental responding. To assess the role of Ox1rs, the selective Ox1r antagonist SB-334867 (20 mg/kg, i.p.) was administered to a naïve group of C57BL/6J mice prior to every VR conditioning session. Mice that received SB-334867 or vehicle displayed significant daily increases in reinforcements earned (Figure 1A; F(11, 132) = 31.76, P<0.0001) and active nose poke responses (Figure 1B; F(11, 132) = 23.35, P<0.0001), but no significant changes in inactive nose poke responses (Figure 1B) nor any session by treatment interactions, indicating successful acquisition of the task in both groups of animals. Expressing active nose pokes as a percentage of total responses further demonstrates successful, and similar, acquisition of the task by both vehicle and SB-334867 treated animals (Figure 1C). However, performance was significantly blunted and total pellet intake was significantly reduced compared to vehicle controls. Mice treated with vehicle reached asymptotic levels of reinforcements earned (Figure 1A; sessions 9–12: F(1, 12) = 4.85, P<0.048) and active nose poke responses (Figure 1B; sessions 9–12; F(1, 12) =4.89, P<0.04) that were significantly higher than mice treated with SB-334867, with no significant session by treatment interactions. No differences were observed in inactive nose poke responses made on the last four sessions (Figure 1B) suggesting that the reduction in nose-poking induced by SB-334867 is specific to food-motivated behavior rather than non-specific motoric effects. These data provide evidence that Ox1rs mediate food-directed behavior.
Figure 1.
Ox1r blockade results in decreased responding for food. (A) Mean number of reinforcers earned. (B) Mean number of active and inactive nose pokes. (C) Mean number of active nose pokes represented as percentage of total responses. Vertical lines represent the standard error of the mean (SEM).
Ox1r blockade results in decreased progressive ratio responding
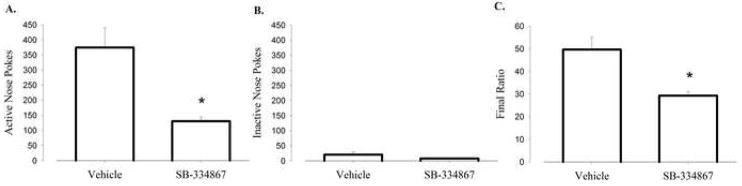
To assess further orexin’s role in food-motivated responding, a separate naïve cohort of C57BL/6J mice were tested for progressive ratio responding where each reinforcement earned requires more effort (nose-pokes) than the previous reinforcement. The mice were first trained on a VR schedule of food reinforcement until stable responding was seen, and then were switched to a PR4 schedule of reinforcement. Mice received SB-334867 (20 mg/kg, i.p) or vehicle prior to each PR4 operant conditioning session. To analyze PR responding, data obtained during three sessions were averaged and used for analysis. Mice treated with the 20 mg/kg dose of SB-334867 demonstrated a significant attenuation in BPs (Figure 2C; t(9) = 3.32, P<0.009), active nose poke responses (Figure 2A; t(9) = 3.30, P<0.009), but no change in inactive responses (Figure 2B) compared to vehicle treated animals. These data are consistent with a role for Ox1r in mediating food-motivated behavior.
Figure 2.
Ox1r blockade results in decreased progressive ratio responding. (A) Mean number of active and (B) inactive nose pokes averaged across three PR4 sessions. (C) Mean BP averaged across three PR4 sessions. Vertical lines represent the standard error of the mean (SEM).
Orexin −/− mice display a delayed instrumental acquisition
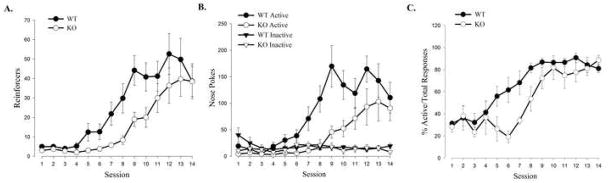
The use of SB-334867 established a role for Ox1r in instrumental responding to food. To complement the above data and explore non-Ox1r dependent behaviors, OKO mice were trained to nose poke for food reinforcement under a VR schedule of reinforcement. Interestingly, OKO mice displayed a delay in the acquisition of the instrumental task compared to WT controls. WT mice displayed significant daily increases in reinforcements earned (Figure 3A) and active nose poke responses (Figure 3B) from the fourth to the ninth operant conditioning sessions, and successfully acquired the task by the ninth session. OKO mice also displayed significant daily increases in reinforcements earned (Figure 3A) and active nose pokes responses (Figure 3B); however, reinforcements earned and active responses were less than WT and were maintained at significantly lower levels until the seventh operant conditioning session at which point animals began to demonstrate daily increases (Figure 3A–B; For reinforcements earned: genotype (F(1, 9) = 7.57, P<0.022), session (F(10, 90) = 30.98, P<0.0001), and interaction (F(10, 90) = 3.84, P<0.042); For active nose pokes: genotype (F(1, 9) = 7.14, P<0.03), session (F(10, 90) = 19.94, P<0.0001), and interaction (F(10, 90) = 4.12, P<0.025). Expressing active nose pokes as a percentage of total responses further reveals a significant daily increase in correct to incorrect response ratio in WT animals, which is significantly delayed in OKO mice (Figure 3C).
Figure 3. Orexin −/− mice display a delayed instrumental acquisition.
(A) Mean number of reinforcers earned. (B) Mean number of active and inactive nose pokes. (C) Mean number of active nose pokes represented as percentage of total responses. Vertical lines represent the standard error of the mean (SEM).
Although the rate of acquisition was similar between the genotypes, OKO mice were slower to initiate acquisition (Simple main effects of session for WT mice (F(10, 90) = 24.79, P<0.0001) and OKO mice (F(10, 90) = 14.71, P<0.001). For WT controls, reinforcements earned were stable during sessions 1–4, with session 5 showing the first significant increase, while OKO mice did not show a significant increase until session 9 (Bonferroni, P<.05).
As an assessment of general activity, responses made on the inactive nose pokes were recorded to determine whether acquisition differences seen between the genotypes may be a result of motoric deficits due to the orexin mutation. Responses on the inactive nose pokes were not significantly different between the genotypes (Figure 3B). As a measure of general exploratory behavior, entries into the magazine were also recorded, and no significant differences were found between the genotypes (data not shown).
The OKO mice demonstrated a significant delay in the acquisition of food-reinforced instrumental responding, but achieved maximal levels of responding similar to WT animals once the task was acquired (Figure 3A–C). Analysis of reinforcers earned, active nose pokes, and inactive nose pokes during the last three sessions failed to reveal significant genotype or session main effects or interactions. Together, these data show that mice lacking the orexin gene have attenuated acquisition of a food-directed operant response, but normal performance once the task has been learned.
Orexin −/− mice display normal responding under a progressive ratio schedule of reinforcement
Since OKO mice have a learning impairment but normal VR performance, we investigated behavioral responding under a PR4 schedule of food reinforcement. To analyze PR responding, data obtained during three sessions were averaged and used for analysis. The OKO mice failed to display reduced BPs (Figure S1C in Supplement 1), active nose poke responses (Figure S1A in Supplement 1) or inactive nose poke responses (Figure S1B in Supplement 1). These data suggest that, unlike mice with pharmacological antagonism of Ox1r, mice lacking the orexin gene show normal food-directed motivation.
Pharmacological or genetic disruption of orexin signaling fails to effect locomotor activity or free feeding
To rule out the possibility that our behavioral effects are due to SB-334867 induced motoric effects instead of motivational deficits, locomotor activity was assessed. SB-334867 treated mice displayed general locomotor activity that is comparable to vehicle treated animals. Similarly, OKO and WT mice did not differ in general locomotor activity (Figure S2A in Supplement 1). Furthermore, to rule out the possibility that SB-334867 may have induced satiation, rather than motivational effects, a separate group of C57BL/6J mice were presented with 5 g of the food pellets and consumption was recorded. SB-334867 treated mice consumed as many pellets as vehicle treated mice. Similarly, pellet consumption by OKO mice was comparable to consumption seen by WT mice (Figure S2B in Supplement 1).
Conditional orexin knockdown in the LH results in decreased responding for food
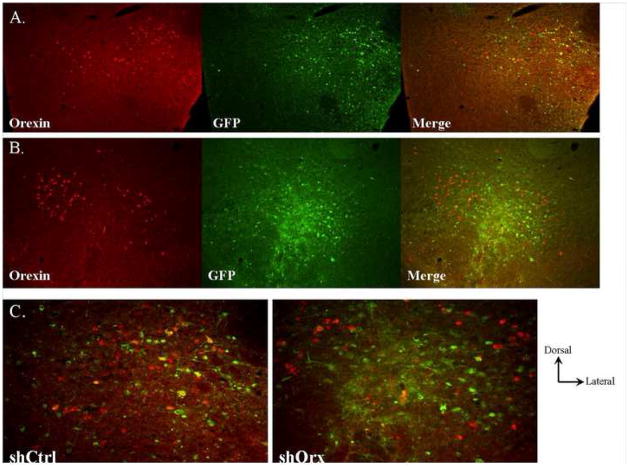
Pharmacological blockade of Ox1r results in reduced performance and food motivation that is in contrast with normal motivation seen in animals with genetic mutation of the orexin gene. This could be due to developmental adaptations in OKO animals missing the orexin gene product during development. To test this, we generated a viral vector (32,33) to reduce orexin gene expression in adult mice. Adenoassociated viral (AAV) vectors were engineered to express either a short hairpin RNA (shRNA) targeting orexin (shOrx) or a shRNA that does not have an endogenous mRNA target to serve as a control (shCtrl). Incorporation of an EGFP marker into the viral construct allowed the regions of infection to be accurately identified. Fluorescent microscopy analysis demonstrated that the AAV efficiently infected the LH (Figure 4A+B, GFP). The shCtrl control virus does not alter the field of orexin neuron expression (Figure 4A), and infected neurons are seen to maintain orexin expression (Figure 4A right panel; Figure 4C, left panel). In contrast, infusion of shOrx virus disrupts the field of orexin expression (Figure 4B) and infected neurons do not co-express orexin, consistent with efficient knockdown of the target protein. Animals were considered to have significant orexin gene knockdown if they were targeted correctly in the LH with a substantial region of the perifornical area infected bilaterally.
Figure 4.
Example of lateral hypothalamic infection of shCtrl (A) and shOrx (B) virus. Sections were processed for detection of orexin neuropeptide (red) and GFP from viral infection (green). Right panels in (A) and (B) show merged images. (C) Higher power analysis of infection sites showing persistent orexin expression in neurons infected with shCtrl in contrast to no orexin expression seen in neurons infected with shOrx.
To assess viral knockdown, orexin-labeled cells were counted for ShCtrl (n=3) and ShOrx (n=3) mice. ShCtrl mice shown a significantly larger number of orexin-labeled cells (366.33±28.14) compared to ShOrx mice (209.67±30.33) (t(4)=3.79, P<0.019). Overall, the conditional orexin knockdown resulted in a 43% decrease of orexin-ir cells.
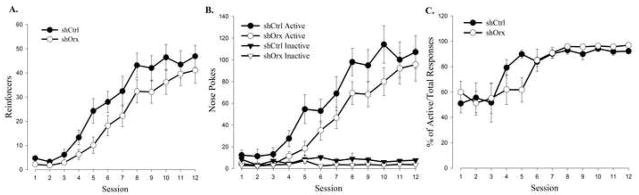
Conditional orexin knockdown resulted in significant daily increases in reinforcements earned (Figure 5A; F(10, 200) = 81.76, P<0.0001) and active nose poke responses (Figure 5B; F(10,200) = 59.62, P<0.0001) with no significant session by virus interactions indicating successful acquisition of the task. Expressing active nose pokes as a percentage of total responses further demonstrates successful, and similar, acquisition of the task by both shCtrl and shOrx animals (Figure 5C).
Figure 5.
RNAi mediated knockdown of the orexin gene results in decreased responding for food. (A) Mean number of reinforcers earned. (B) Mean number of active and inactive nose pokes. (C) Mean number of active nose pokes represented as percentage of total responses. Vertical lines represent the standard error of the mean (SEM).
However, performance was blunted and total pellet intake was reduced compared to shCtrl controls. There was a significant knockdown effect when reinforcements earned (F(1,20) = 4.99, P<0.037) and active nose poke responses (F(1,20) = 5.9, P<0.025) were used as the dependent measure, but no significant interactions were seen. These data are similar to those obtained following blockade of Ox1r and are consistent with a role for orexin acting via Ox1r to influence food-directed behavior.
Conditional orexin knockdown in the LH results in decreased progressive ratio responding
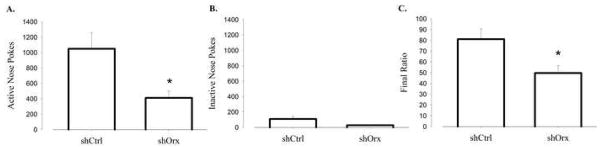
To characterize further the effects of a conditional orexin knockdown in motivation, shOrx mice were tested under the PR4 schedule of reinforcement. ShOrx mice demonstrated a attenuation in BPs (Figure 6C; t(20) = 2.75, P<0.012), active nose poke responses (Figure 6A; t(20) = 2.97, P<0.007), but no change in inactive responses (Figure 6B) compared to shCtrl controls. These data are also similar to those obtained following blockade of Ox1r in C57BL6/J mice.
Figure 6.
RNAi mediated knockdown of the orexin gene results in decreased progressive ratio responding. (A) Mean number of active and (B) inactive nose pokes averaged across three PR4 sessions. (C) Mean BP averaged across three PR4 sessions. Vertical lines represent the standard error of the mean (SEM).
DISCUSSION
The present study tested the hypothesis that orexin signaling plays an integral role in operant responding for food-reinforcement by using pharmacological as well as genetic manipulations of the orexin system. Here, we report that pharmacological blockade of Ox1r via administration of SB-334867 or viral knockdown of the orexin precursor peptide impairs operant responding for food reinforcement under the VR as well as the PR schedules of reinforcement. These data suggest a role for orexin, and more specifically for Ox1r action, in food motivation. Conversely, OKO mice demonstrated impaired initial acquisition of food reinforced responding and subsequent normal performance and motivation as measured by PR. The novel orexin knockdown animals were behaviorally similar to the SB-334867 treated animals, suggesting that orexin action mediates performance of a food motivated task whereas developmental compensation in the OKO mice results in normal behavior.
It is unlikely that the present findings were due to SB-334867 induced satiation or motoric effects instead of motivational deficits. We have demonstrated here that administration of SB-334867 prior to free-feeding sessions where food pellets are freely-available does not affect pellet consumption. Furthermore, administration of SB-334867 does not alter general activity as measured by locomotor activity chambers. Furthermore, OKO mice do not differ in locomotor activity or free feeding when compared with WT controls.
Orexin’s role in feeding has received some attention since the original description of the effects of exogenous orexin on food intake (11). These results are generally viewed as secondary to arousal and motor activation (34,35), but studies with the Ox1r antagonists have yielded results consistent with a role for endogenous orexin in aspects of food intake. For instance, home cage food consumption is increased by intracerebroventricular administration of orexin A (11,31,36) and is decreased by SB-334867 administration (36–38). However, little is known about orexin’s role in food motivation. Here, we report that SB-334867 treatment reduces food-directed motivation, suggesting that Ox1r activity does play a role in this behavior.
To complement the pharmacological data, we also investigated the performance of mice lacking the orexin precursor peptide during a food-directed instrumental response. Surprisingly, OKO mice displayed a significant delay in the acquisition of this task; however, no differences were observed between OKO and WT mice in the expression of the instrumental response once the task was acquired; all mice reached similar maximal asymptotic levels of responding. This pattern suggests that orexin function may be necessary for instrumental learning, but has little effect in mediating performance of response-contingent goal-directed behaviors. Specifically, the orexin mutation failed to affect motivational processes when effort to respond is increased, as under the PR schedule. Together, data obtained with the OKO mice suggest that orexin is not required for normal food motivation.
Although only a few investigations of orexin’s role in learning have been reported, orexin appears to play a role. Intracerebroventricular administration of orexin A has been shown to enhance acquisition, consolidation and retrieval of a passive avoidance task (39,40), whereas intra-hippocampal blockade of Ox1r impairs the acquisition of a passive avoidance task (41) and the Morris water maze (42,43). Reward-related learning also appears to be modulated by orexin as blocking orexin signaling via a unilateral lesion of the lateral hypothalamus in conjunction with Ox1r antagonism on the contralateral side blocks the development of morphine CPP (44). The absence of orexin function may mediate learning deficits seen in OKO mice.
It is possible that while OKO mice demonstrate normal motivation for regular food pellets, motivation to obtain a high fat reinforcer may be altered. Unlike WT controls, OKO mice fail to increase corn-oil consumption following intra-NAc DAMGO administration (45). Surprisingly, orexin over-expressors are resistant to high fat diet-induced obesity (46). Together, these findings suggest that in genetic models, orexin may mediate metabolic and/or behavioral processes of high fat food consumption.
It is imperative to note that while OKO mice are valuable in the investigation of orexin functions, behavioral differences may not necessarily reflect direct orexin function. Conditional knockdown of the orexin gene resulted in a behavioral pattern that was similar to mice treated with the Ox1r antagonist. The differences between PR data obtained from the OKO and SB-334867 treated mice suggests that some developmental compensatory mechanism may exist in OKO mice, such that the relevant function served by Ox1r stimulation is acquired by another neural and/or molecular mechanism in mice lacking orexin. Alternatively, the apparent learning deficiencies seen in OKO mice may reflect function of the Ox2r receptor since SB-334867 has a higher binding affinity and selectivity for the Ox1r over the Ox2r (47). To test the role of orexin without developmental compensation, we constructed an RNAi knockdown vector targeting the orexin precursor peptide in order to create a conditional orexin knockdown in an adult animal. ShOrx mice displayed significant reductions in responding for food reinforcement. Furthermore, when presented with the PR task, shOrx mice reached significantly lower BPs compared to shCtrl mice. These data suggest that orexin mediates the motivation to obtain food reinforcement and serves as an example of the importance of conditional (adult-restricted) genetic manipulations to assess the role of gene products in behavior. Together, data obtained from the pharmacological, OKO, and orexin knockdown experiments suggest developmental compensatory mechanisms or secondary effects in OKO mice. Overall, these findings demonstrate a positive role for orexin function, via Ox1r, in food-mediated instrumental responding.
A role for orexin in reward has been suggested by demonstration that Fos expression in orexin cells is increased following place preference to morphine-, cocaine-, and food-paired environments (6,44). Additionally, orexin has been implicated self-administration of nicotine (27) and relapse to palatable food seeking (25). More recently, Ox1r antagonism was reported to affect self-administration of cocaine and high fat food with no effects on responding for regular chow in rats (24), suggesting a role for orexin in hedonic processing. Data presented here demonstrates that orexin contributes to the motivation to obtain food reinforcement in mice, and that these effects are not restricted to high fat palatable foods.
Although orexin activity has been linked to food intake, supporting evidence is complex as orexin stimulates feeding only when administered during the light cycle (11,21–23) and decreases feeding when administered at the onset of the dark cycle (22). It has therefore been suggested that orexin’s role in feeding involves a more generalized arousal component (4,5). It is notable that arousal is a necessary component of instrumental responding. During instrumental tasks, arousal is generated by the presentation of reinforcement (48) and can become conditioned to the context where reinforcement has occurred (49). Levels of arousal are also related to asymptotic response rates and are regulated by the schedule of reinforcement. Increased arousal results in more efficient, goal-directed responding (49). Thus, we propose that orexin’s effects on food-motivated tasks may occur via modulating general behavioral arousal that is essential for maximal performance in instrumental behavior.
Supplementary Material
Acknowledgments
We thank Jennifer Quinn, Shannon Gourley, and Peter Olausson for suggestions about the experimental design, and members of the DiLeone lab for suggestions on the manuscript. We also thank Masashi Yanagisawa for supplying the orexin knockout mice. This work was supported by National Institutes of Health grants RO1DA017676 (R.J.D), F32DA023739 (R.S.) and RL1AA017537 (R.J.D. and J.R.T.) as well as support of State of Connecticut, Department of Mental Health and Addiction Services (R.J.D. and J.R.T.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, et al. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–451. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- 2.Lin L, Faraco J, Li R, Kadotani H, Rogers W, Lin X, et al. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98:365–376. doi: 10.1016/s0092-8674(00)81965-0. [DOI] [PubMed] [Google Scholar]
- 3.Peyron C, Faraco J, Rogers W, Ripley B, Overeem S, Charnay Y, et al. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat Med. 2000;6:991–997. doi: 10.1038/79690. [DOI] [PubMed] [Google Scholar]
- 4.Sutcliffe JG, De Lecea L. The hypocretins: setting the arousal threshold. Nat Rev Neurosci. 2002;3:339–349. doi: 10.1038/nrn808. [DOI] [PubMed] [Google Scholar]
- 5.Willie JT, Chemelli RM, Sinton CM, Yanagisawa M. To eat or to sleep? Orexin in the regulation of feeding and wakefulness. Annu Rev Neurosci. 2001;24:429–458. doi: 10.1146/annurev.neuro.24.1.429. [DOI] [PubMed] [Google Scholar]
- 6.Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437:556–559. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- 7.Georgescu D, Zachariou V, Barrot M, Mieda M, Willie JT, Eisch AJ, et al. Involvement of the lateral hypothalamic peptide orexin in morphine dependence and withdrawal. J Neurosci. 2003;23:3106–3111. doi: 10.1523/JNEUROSCI.23-08-03106.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharf R, Sarhan M, DiLeone RJ. Orexin mediates the expression of precipitated morphine withdrawal and concurrent activation of the nucleus accumbens shell. Biol Psychiatry. 2008;64:175–183. doi: 10.1016/j.biopsych.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou Y, Bendor J, Hofmann L, Randesi M, Ho A, Kreek MJ. Mu opioid receptor and orexin/hypocretin mRNA levels in the lateral hypothalamus and striatum are enhanced by morphine withdrawal. J Endocrinol. 2006;191:137–145. doi: 10.1677/joe.1.06960. [DOI] [PubMed] [Google Scholar]
- 10.Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, et al. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- 12.Date Y, Ueta Y, Yamashita H, Yamaguchi H, Matsukura S, Kangawa K, et al. Orexins, orexigenic hypothalamic peptides, interact with autonomic, neuroendocrine and neuroregulatory systems. Proc Natl Acad Sci U S A. 1999;96:748–753. doi: 10.1073/pnas.96.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, et al. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, et al. Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol. 2001;435:6–25. doi: 10.1002/cne.1190. [DOI] [PubMed] [Google Scholar]
- 15.Bardo MT. Neuropharmacological mechanisms of drug reward: beyond dopamine in the nucleus accumbens. Crit Rev Neurobiol. 1998;12:37–67. doi: 10.1615/critrevneurobiol.v12.i1-2.30. [DOI] [PubMed] [Google Scholar]
- 16.Beninger RJ. The role of dopamine in locomotor activity and learning. Brain Res. 1983;287:173–196. doi: 10.1016/0165-0173(83)90038-3. [DOI] [PubMed] [Google Scholar]
- 17.Wise RA. Catecholamine theories of reward: a critical review. Brain Res. 1978;152:215–247. doi: 10.1016/0006-8993(78)90253-6. [DOI] [PubMed] [Google Scholar]
- 18.Wise RA, Rompre PP. Brain dopamine and reward. Annu Rev Psychol. 1989;40:191–225. doi: 10.1146/annurev.ps.40.020189.001203. [DOI] [PubMed] [Google Scholar]
- 19.Boutrel B, Kenny PJ, Specio SE, Martin-Fardon R, Markou A, Koob GF, et al. Role for hypocretin in mediating stress-induced reinstatement of cocaine-seeking behavior. Proc Natl Acad Sci U S A. 2005;102:19168–19173. doi: 10.1073/pnas.0507480102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Borgland SL, Taha SA, Sarti F, Fields HL, Bonci A. Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron. 2006;49:589–601. doi: 10.1016/j.neuron.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 21.Kunii K, Yamanaka A, Nambu T, Matsuzaki I, Goto K, Sakurai T. Orexins/hypocretins regulate drinking behaviour. Brain Res. 1999;842:256–261. doi: 10.1016/s0006-8993(99)01884-3. [DOI] [PubMed] [Google Scholar]
- 22.Lubkin M, Stricker-Krongrad A. Independent feeding and metabolic actions of orexins in mice. Biochem Biophys Res Commun. 1998;253:241–245. doi: 10.1006/bbrc.1998.9750. [DOI] [PubMed] [Google Scholar]
- 23.Sweet DC, Levine AS, Billington CJ, Kotz CM. Feeding response to central orexins. Brain Res. 1999;821:535–538. doi: 10.1016/s0006-8993(99)01136-1. [DOI] [PubMed] [Google Scholar]
- 24.Borgland SL, Chang SJ, Bowers MS, Thompson JL, Vittoz N, Floresco SB, et al. Orexin A/hypocretin-1 selectively promotes motivation for positive reinforcers. J Neurosci. 2009;29:11215–11225. doi: 10.1523/JNEUROSCI.6096-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nair SG, Golden SA, Shaham Y. Differential effects of the hypocretin 1 receptor antagonist SB 334867 on high-fat food self-administration and reinstatement of food seeking in rats. British Journal of Pharmacology. 2008;154:406–416. doi: 10.1038/bjp.2008.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Richards JK, Simms JA, Steensland P, Taha SA, Borgland SL, Bonci A, et al. Inhibition of orexin-1/hypocretin-1 receptors inhibits yohimbine-induced reinstatement of ethanol and sucrose seeking in Long-Evans rats. Psychopharmacology. 2008;199:109–117. doi: 10.1007/s00213-008-1136-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hollander JA, Lu Q, Cameron MD, Kamenecka TM, Kenny PJ. Insular hypocretin transmission regulates nicotine reward. Proc Natl Acad Sci U S A. 2008;105:19480–19485. doi: 10.1073/pnas.0808023105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gourley SL, Wu FJ, Kiraly DD, Ploski JE, Kedves AT, Duman RS, et al. Regionally specific regulation of ERK MAP kinase in a model of antidepressant-sensitive chronic depression. Biol Psychiatry. 2008;63:353–359. doi: 10.1016/j.biopsych.2007.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Skjoldager P, Pierre PJ, Mittleman G. Reinforcer magnitude and progressive ratio responding in the rat: effects of increased effort, prefeeding, and extinction. Learn Motiv. 1993;24:303–343. [Google Scholar]
- 30.Lawrence AJ, Cowen MS, Yang HJ, Chen F, Oldfield B. The orexin system regulates alcohol-seeking in rats. Br J Pharmacol. 2006;148:752–759. doi: 10.1038/sj.bjp.0706789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rodgers RJ, Halford JC, Nunes de Souza RL, Canto de Souza AL, Piper DC, Arch JR, et al. SB-334867, a selective orexin-1 receptor antagonist, enhances behavioural satiety and blocks the hyperphagic effect of orexin-A in rats. Eur J Neurosci. 2001;13:1444–1452. doi: 10.1046/j.0953-816x.2001.01518.x. [DOI] [PubMed] [Google Scholar]
- 32.Hommel JD, Sears RM, Georgescu D, Simmons DL, DiLeone RJ. Local gene knockdown in the brain using viral-mediated RNA interference. Nat Med. 2003;9:1539–1544. doi: 10.1038/nm964. [DOI] [PubMed] [Google Scholar]
- 33.Hommel JD, Trinko R, Sears RM, Georgescu D, Liu ZW, Gao XB, et al. Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron. 2006;51:801–810. doi: 10.1016/j.neuron.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 34.Hagan JJ, Leslie RA, Patel S, Evans ML, Wattam TA, Holmes S, et al. Orexin A activates locus coeruleus cell firing and increases arousal in the rat. Proc Natl Acad Sci U S A. 1999;96:10911–10916. doi: 10.1073/pnas.96.19.10911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kilduff TS, Peyron C. The hypocretin/orexin ligand-receptor system: Implications for sleep and sleep disorders. Trends Neurosci. 2000;23:359–365. doi: 10.1016/s0166-2236(00)01594-0. [DOI] [PubMed] [Google Scholar]
- 36.Haynes AC, Jackson B, Overend P, Buckingham RE, Wilson S, Tadayyon M, et al. Effects of single and chronic intracerebroventricular administration of the orexins on feeding in the rat. Peptides. 1999;20:1099–1105. doi: 10.1016/s0196-9781(99)00105-9. [DOI] [PubMed] [Google Scholar]
- 37.Haynes AC, Jackson B, Chapman H, Tadayyon M, Johns A, Porter RA, et al. A selective orexin-1 receptor antagonist reduces food consumption in male and female rats. Regul Pept. 2000;96:45–51. doi: 10.1016/s0167-0115(00)00199-3. [DOI] [PubMed] [Google Scholar]
- 38.White CL, Ishii Y, Mendoza T, Upton N, Stasi LP, Bray GA. Effect of a selective OX1R antagonist on food intake and body weight in two strains of rats that differ in susceptibility to dietary-induced obesity. Peptides. 2005;26:2331–2338. doi: 10.1016/j.peptides.2005.03.042. [DOI] [PubMed] [Google Scholar]
- 39.Telegdy G, Adamik A. The action of orexin A on passive avoidance learning. Involvement of transmitters. Regul Pept. 2002;104:105–110. doi: 10.1016/s0167-0115(01)00341-x. [DOI] [PubMed] [Google Scholar]
- 40.Jaeger LB, Farr SA, Banks WA, Morley JE. Effects of orexin-A on memory processing. Peptides. 2002;23:1683–1688. doi: 10.1016/s0196-9781(02)00110-9. [DOI] [PubMed] [Google Scholar]
- 41.Akbari E, Motamedi F, Naghdi N, Noorbakhshnia M. The effect of antagonization of orexin 1 receptors in CA1 and dentate gyrus regions on memory processing in passive avoidance task. Behav Brain Res. 2008;187:172–177. doi: 10.1016/j.bbr.2007.09.019. [DOI] [PubMed] [Google Scholar]
- 42.Akbari E, Naghdi N, Motamedi F. Functional inactivation of orexin 1 receptors in CA1 region impairs acquisition, consolidation and retrieval in Morris water maze task. Behav Brain Res. 2006;173:47–52. doi: 10.1016/j.bbr.2006.05.028. [DOI] [PubMed] [Google Scholar]
- 43.Akbari E, Naghdi N, Motamedi F. The selective orexin 1 receptor antagonist SB-334867-A impairs acquisition and consolidation but not retrieval of spatial memory in Morris water maze. Peptides. 2007;28:650–656. doi: 10.1016/j.peptides.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 44.Harris GC, Wimmer M, Randall-Thompson JF, Aston-Jones G. Lateral hypothalamic orexin neurons are critically involved in learning to associate an environment with morphine reward. Behavioural Brain Research. 2007;183:43–51. doi: 10.1016/j.bbr.2007.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zheng H, Patterson LM, Berthoud HR. Orexin signaling in the ventral tegmental area is required for high-fat appetite induced by opioid stimulation of the nucleus accumbens. J Neurosci. 2007;27:11075–11082. doi: 10.1523/JNEUROSCI.3542-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Funato H, Tsai AL, Willie JT, Kisanuki YY, Williams SC, Sakurai T, et al. Enhanced orexin receptor-2 signaling prevents diet-induced obesity and improves leptin sensitivity. Cell Metab. 2009;9:64–76. doi: 10.1016/j.cmet.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Duxon MS, Stretton J, Starr K, Jones DN, Holland V, Riley G, et al. Evidence that orexin-A-evoked grooming in the rat is mediated by orexin-1 (OX1) receptors, with downstream 5-HT2C receptor involvement. Psychopharmacology (Berl) 2001;153:203–209. doi: 10.1007/s002130000550. [DOI] [PubMed] [Google Scholar]
- 48.Killeen PR, Hanson SJ, Osborne SR. Arousal: its genesis and manifestation as response rate. Psychol Rev. 1978;85:571–581. [PubMed] [Google Scholar]
- 49.Killeen PR, Sitomer MT. MPR. Behav Processes. 2003;62:49–64. doi: 10.1016/S0376-6357(03)00017-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.