Abstract
Background
In addicts drug cues attract attention, elicit approach and motivate drug-seeking and drug-taking behavior, and addicts find it difficult to resist such cues. In preclinical studies we have found, however, that food cues acquire incentive motivational properties only in a subset of individuals. For example, a food cue becomes attractive, eliciting approach and engagement with it, and acts as an effective conditional reinforcer in some rats, but not others. We asked, therefore, whether rats that have a propensity to attribute incentive salience to a food cue are the same ones that attribute incentive value to a drug (cocaine) cue.
Methods
We first used a Pavlovian conditioned approach procedure to determine which individual rats attributed incentive salience to a food cue. A second cue was then associated with the intravenous self-administration of cocaine. Later, the ability of the cocaine cue to maintain self-administration behavior and to reinstate self-administration following extinction was assessed.
Results
We report that in individuals that had a propensity to attribute incentive salience to a food cue, a cocaine cue spurred motivation to take drugs (its removal greatly diminished self-administration) and reinstated robust drug-seeking following extinction. However, in those individuals that failed to attribute incentive salience to a food cue the cocaine cue was relatively devoid of incentive motivational properties.
Conclusions
We conclude that it is possible to determine, prior to any drug experience, which individuals will most likely have difficulty resisting drug cues, a trait that may confer susceptibility to addiction.
Keywords: addiction, cocaine, incentive salience, sign-tracking, goal tracking, reinstatement
Introduction
Cues associated with rewards can themselves act as powerful incentives, arousing desire and instigating actions. For example, food-related cues can motivate eating even when one is sated (1,2), and in addicts, drug cues grab the attention more powerfully than other stimuli (3) and can produce craving and/or relapse (4,5). Even drug cues outside of conscious awareness activate brain motive circuits (6). The importance of drug-associated cues in motivating behavior has been confirmed using animal models of addiction. Drug cues are attractive and “wanted” - they are approached (7) and animals will work to get them (8–10); drug cues help maintain drug-seeking and -taking behavior - their omission greatly decreases seeking and self-administration (11–15); and drug cues are powerful instigators of reinstatement/relapse - promoting renewed drug seeking after extinction or prolonged abstinence (16,17). For reward-associated cues to motivate, however, they must acquire the ability to act as incentive stimuli - i.e., be attributed with incentive salience (18–22).
How do cues acquire the ability to motivate? It is often assumed that cues (conditional stimuli, CS) become incentive stimuli merely as a function of their conditional relationship with a reward (unconditional stimulus, US), but in fact, the ability of a CS to predict a US and evoke a conditional response (CR) is not sufficient to confer incentive value to the CS (23–26). When a localizable cue (a lever-CS) is associated with the receipt of food reward, only in some rats does the lever-CS itself become attractive, eliciting approach and engagement with it, and in these animals the lever-CS is also an effective conditional reinforcer (that is, animals will work to get it). But in other rats the lever-CS does not attract. These animals are fully capable of learning a Pavlovian CR, but the CR is not directed towards the CS, but rather, towards the place food will be delivered, and in these rats the lever-CS is relatively ineffective as a conditional reinforcer (23). Animals for which a localizable cue acts as an attractive incentive stimulus are called “sign-trackers” (STs - they approach the cue or “sign” (27)) and those for whom the cue does not have incentive properties are called “goal-trackers” (GTs - they learn to approach the location of reward delivery, or “goal” (28)). Note that the cue is predictive and acts as a fully effective CS, supporting Pavlovian learning, in both STs and GTs, and their respective CRs are learned at a comparable rate. But the cue itself serves as an attractive incentive stimulus only for STs (23,24).
Thus, only in susceptible individuals are localizable food cues attributed with incentive salience, acquiring the ability to attract and to reinforce new learning. It is important to know if there are similar individual differences in the propensity to attribute incentive salience to drug cues, because if drug cues act as incentives - “as a persistent goad to response generation” (20) in some individuals but not others - it is presumably the former who will have difficulty resisting them and thus be susceptible to continued drug use and addiction. We asked, therefore, whether individual differences in the propensity to attribute incentive salience to a food cue, determined prior to any drug experience, predicts individual differences in the ability of a cocaine cue to motivate drug-seeking and drug-taking behavior.
Material and Methods
Pavlovian training
Male Sprague Dawley rats were initially trained using a Pavlovian conditioning procedure described previously (29). Briefly, an illuminated retractable lever (the conditioned stimulus, CS) located to the left or right of a food magazine was inserted into the chamber for 8 s. As the lever was retracted, a single 45-mg banana-flavored food pellet (the unconditioned stimulus, US) was delivered into the magazine. This CS-US pairing occurred on a response-independent random-interval 90-s schedule 25 times per session for three sessions. The animals were then classified as sign-trackers or goal-trackers as described previously (29; see Supplementary Methods).
Surgery
After Pavlovian training rats were prepared with intravenous catheters (30; see Supplementary Methods).
Self-administration
Self-administration sessions began one week after surgery in chambers outfitted with two nose ports, but no levers or food magazine. A nose poke into the active port resulted in an intravenous injection of cocaine HCl (0.5 mg/kg/infusion in 25 μl delivered in 1.6 s) on a fixed ratio (FR) 1 schedule. After an infusion there was 20-s timeout period, during which time the active nose poke port light was illuminated. This light served as the CS signaling cocaine delivery. The training procedure we used guaranteed that all rats received exactly the same number of CS-US pairings by requiring all animals to take exactly the same number of infusions (i.e., session length was determined by how long it took to take a fixed number of infusions, which increased over days of training). (See Supplementary Methods).
Experiment 1: Cue removal and resistance to extinction
After STs (final n = 14) and GTs (final n = 16) achieved an IC of 80, and showed stable levels of self-administration behavior, a cue removal test was conducted. For these two days all conditions were the same, and an active nose poke resulted in an infusion of cocaine, and the animals were allowed to take 80 injections – but the cue light (CS) was not illuminated. After two days of cue removal the CS was reintroduced and daily testing continued for four more days. The influence of cue removal on self-administration behavior was assessed by quantifying the rate of self-administration (injections/min).
To examine resistance to extinction in STs and GTs, animals were then tested under cued extinction conditions, during which time a nose poke resulted in the illumination of the CS, but no cocaine injection. Extinction sessions were fixed at 1 hr and responding was measured until both groups no longer showed a downward trend in responding for four consecutive sessions.
Experiment 2: Individual differences in cue-induced reinstatement
An independent group of rats received Pavlovian training as described above to identify STs (final n = 13) and GTs (final n = 12). These rats were then trained to self-administer cocaine, exactly as described above. Following the acquisition of self-administration all animals underwent extinction training for 7 daily 1-hr sessions. During these sessions, rats were not attached to the infusion pump swivel, and nose pokes had no programmed consequences (that is, animals received neither the drug nor the CS). Following extinction training (during which neither cues nor drug were available) all rats were left undisturbed in their home cages for a 30-day “incubation” period (31), after which they were returned to the self-administration chambers for a single cue-induced reinstatement test. During this test session animals were attached to the infusion pump swivel and a nose poke into the active port resulted in illumination of the nose port light (the CS) for 5 s, but no drug infusion.
Statistical Analysis
Linear mixed-effects models were used to analyze all repeated measures data (32). Two-way ANOVAs were used to compare responding on active and inactive nose pokes during reinstatement testing. Bonferroni corrected post hoc comparisons were conducted where appropriate. Statistical significance was set at P < 0.05.
Results
Group differences in the propensity to approach and engage a food-related cue
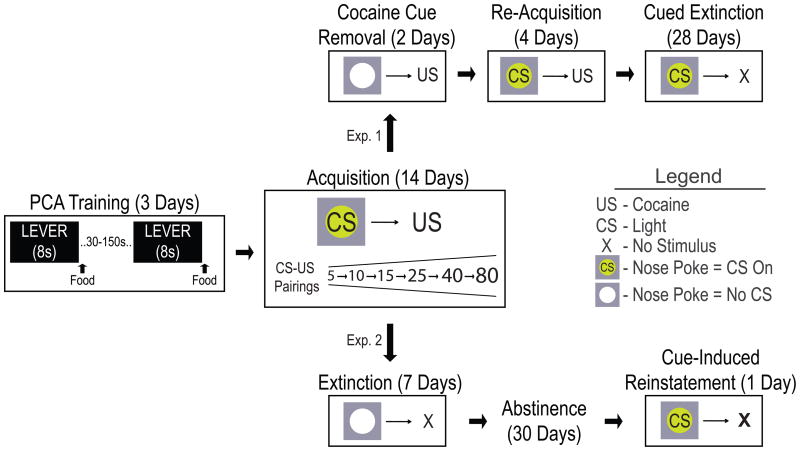
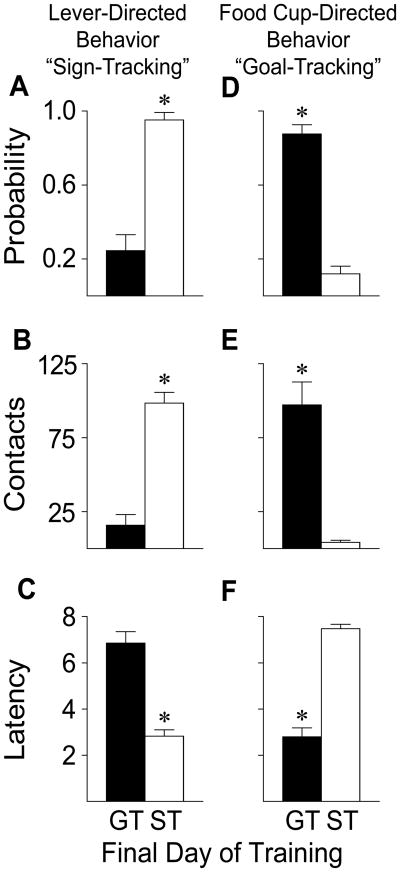
A summary of the experimental design is shown in Figure 1. In adult male rats presentation of a lever-CS for 8 s was followed immediately by the unconditional delivery of a food pellet on each of three days of training. As described previously (29), the one third of the rats showing the greatest number of lever deflections during the CS period were designated sign-trackers (STs) and the one third showing the fewest goal-trackers (GTs). The remaining rats were not used in any of these experiments. Rats designated as STs or GTs exhibited very different behavior on the last day of training (Figure 2). During the CS period STs had a high probability of rapidly approaching (Figure 2a, c) and vigorously engaging the lever-CS (Figure 2b). In contrast, during the CS period rats designated GTs seldom approached the lever-CS, but instead rapidly approached and engaged the food cup (Figure 2d–f). Thus, as in our previous studies (23), the lever was a predictive CS in both STs and GTs, and was effective in evoking a CR in both, but the food cue was attractive in STs but not GTs.
Figure 1.
Schematic illustrating the experimental design. All animals received identical Pavlovian conditioned approach (PCA) and cocaine self-administration acquisition training. Following acquisition, Experiment 1 (Exp. 1), and Experiment 2 (Exp. 2) diverged as shown. During self administration, the conditional stimulus (CS) was a nose poke port cue light and the unconditional stimulus (US) was an intravenous infusion of cocaine. Depending on the experimental phase, an active nose poke produced the CS and US (Acquisition/Re-Acquisition), the CS but no US (Cued Extinction), no CS but the US (Cue Removal), or nothing (X – Extinction).
Figure 2.
Behavior directed towards the lever-CS (“sign-tracking”) or the food cup (the location of US delivery; “goal-tracking”) during the 8 s CS period on the final day of Pavlovian training. The topography of the conditional response (CR) is different in rats designated sign-trackers (STs, n=14) vs. goal-trackers (GTs, n=16). The mean ± SEM for (a) probability of approaching the lever [# trials with a lever contact/#trials per session] (CS) during the 8 s CS period, (b) number of lever contacts (c) latency to the first lever contact after CS presentation, (d) probability of approach to the food tray during the 8 s CS period, (e) number of food tray contacts during the CS period, and (f) latency to the first food tray entry after CS presentation. *, indicates significant group differences, p < 0.001.
No group differences in the acquisition of cocaine self-administration
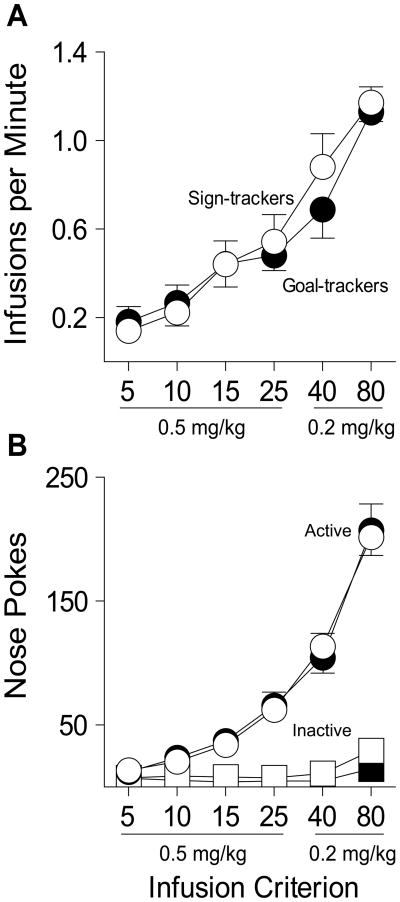
Following Pavlovian training all rats were equipped with an intravenous (i.v.) catheter and trained to make an instrumental response (nose poke) for an i.v. injection of cocaine. In this situation the injection of cocaine (the US) was paired with illumination of the nose port, and therefore this light served as the cocaine-paired cue (drug CS). The training procedure was designed to eliminate any potential group differences in the number of CS (light) - US (cocaine) pairings and to mitigate possible differences in acquisition of self-administration behavior. Thus, rats were allowed to take a fixed number of injections each day (i.e., session length was determined by how long it took to reach the criterion number of injections), and the number allowed increased over days of training (Figure 1). With this procedure group differences in acquisition would be evident by differences in the rate of self-administration, but there were no group differences for this measure at any infusion criterion (Figure 3a; F(1,73) = 1.108, p = .296). Both groups also made the same number of active (F(1,83) = .107, p = .744) and inactive nose pokes (F(1,43) = 2.429, p = .126), and discriminated between the active and inactive nose ports (Figure 3b).
Figure 3.
Acquisition of cocaine self-administration behavior in sign-trackers (n=14) and goal-trackers (n=16). (a) The mean ± SEM number of cocaine infusions per minute for infusion criteria 5, 10, 15, and 25 (0.5 mg/kg/inf) and 40 and 80 (0.2 mg/kg/inf). (b) The mean ± SEM number of active and inactive nose poke responses at each infusion criterion.
Removal of the cocaine cue decreases self-administration in STs but not GTs
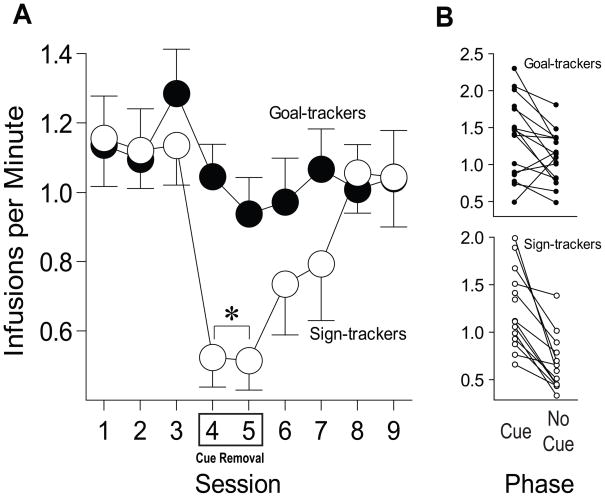
By the end of training at the final infusion criterion (80 injections of 0.2 mg/kg per test session) all rats showed stable levels of cocaine self-administration (first 3 sessions shown in Figure 4A). During these baseline sessions each cocaine injection was accompanied by illumination of the nose poke port. On sessions 4 and 5 a nose poke into the active port still produced an i.v. injection of cocaine, but the light did not illuminate. Removal of the drug cue sharply decreased the rate of self-administration in STs by approximately half, and the magnitude of this effect was significantly correlated with how vigorously animals engaged the lever-CS on the first day of Pavlovian training (r = .545, p = .022). In GTs cue removal produced a small decrease in self-administration, but this was not statistically significant. Fig. 4b shows the data averaged over the 3 baseline sessions and the 2 cue removal sessions (effect of group, F(1,36) = 4.954, p = .03; effect of phase, F(1,50) = 19.332, p < .0001; group × phase interaction, F(1,50) = 4.668, p = .01; posthoc tests showed no effect of cue removal in GTs, p > .05, but a large effect in STs, p < .001). Reintroduction of the cocaine cue reinstated baseline levels of self-administration in STs within a few days (sessions 6–9 in Figure 4a). All rats continued to discriminate between the active and inactive nose poke during cue removal (Active M = 224.88, SEM = 13.57; Inactive M = 24.43, SEM = 6.95), and there were no group differences in the total number of active or inactive responses (data not shown).
Figure 4.
Effects of removal of a cocaine-associated cue on self-administration behavior in sign-trackers (n=14) and goal-trackers (n=16). (a) The mean ± SEM number of cocaine infusions (0.2 mg/kg/infusion) per minute in sign-trackers and goal-trackers in the presence of the cocaine cue (sessions 1–3, 6–9) and when the light cue was not presented along with the injection of cocaine (sessions 4–5). (b) The rate of cocaine self-administration (infusions/min) for individual animals averaged over the baseline period (cocaine cue present, sessions 1–3) and the cue removal sessions (sessions 4–5). *, indicates a significant group difference, p < 0.05.
STs showed resistance to extinction relative to GTs
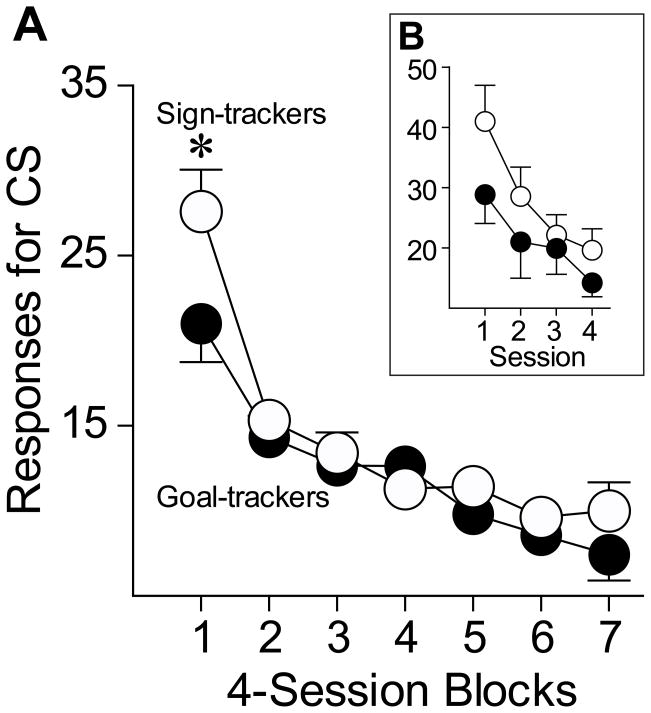
After the resumption of baseline levels of self-administration (session 9 in Figure 4) all animals underwent 28 days of extinction training, during which time nose pokes no longer produced cocaine, but they did result in illumination of the light CS. Over this period of time both STs and GTs slowly decreased responding for the CS (Figure 5, effect of session block, F(6,125) = 15.459, p <.0001), and there was a small but significant group difference in responses during this cued extinction phase (effect of group, F(1,321) = 4.806, p = .029; interaction non-significant). Inspection of these data indicates that STs made more responses for the CS than GTs, primarily during the first four sessions (Figure 5b-inset, one-tailed t test, t(54) = −2.02, p = 0.024).
Figure 5.
Extinction of responding for cocaine in sign-trackers (n=14) and goal-trackers (n=16), when a response continued to produce the cocaine-associated CS. (a) The mean ± SEM number of active responses for the CS, expressed in 4-session blocks. (b) The mean ± SEM number of active responses for the CS during the first 4 extinction sessions (effect of group, F(1,85) = 4.57, p = 0.035). *, indicates a significant group difference, p < 0.05.
A cocaine-associated cue produces more robust reinstatement in STs than GTs
An independent group of rats underwent Pavlovian training to identify STs and GTs, exactly as above (data not shown - they are similar to those in Figure 2). The rats were then trained to self-administer cocaine exactly as described above (data not shown – they exhibited the same pattern of acquisition as illustrated in Figure 3). After stable responding all animals underwent 7 days of extinction training, except in this experiment nose pokes had no consequence (that is, nose pokes produced neither cocaine nor the CS). Under these conditions there were no group differences in responding during extinction (data not shown). After extinction all animals were tested for cue-induced reinstatement of responding similar to previous studies (30). In this test nose pokes again resulted in presentation of the cocaine CS (illumination of the nose-poke port), but no cocaine was delivered.
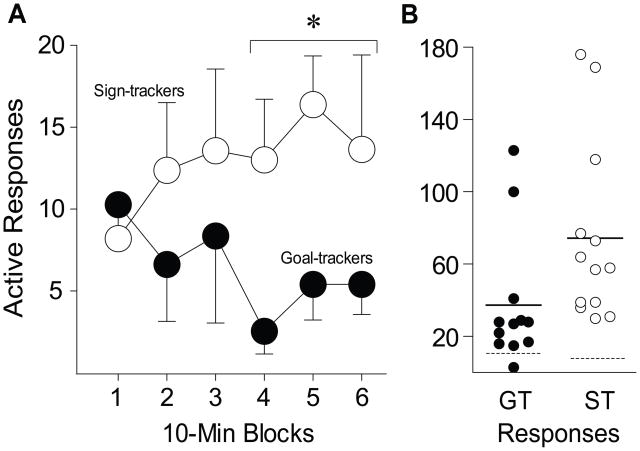
Sign trackers showed more robust reinstatement of cocaine seeking than GTs (Figure 6, the group × time [block] interaction was significant, F(5,45) = 2.871, p = .025). This was because STs increased their number of active nose pokes over the session (F(1,61) = 5.867, p = .018), but GTs did not (Figure 6a). A comparison of active and inactive port responses (Figure 6b, the dashed line indicates the average inactive port responses) indicated that both groups made more active nose pokes than inactive nose pokes, but STs reinstated responding to a significantly greater degree than GTs, as indicated by a significant group by port interaction (F(1,46) = 6.489, p = .014; effect of group, F(1,46) = 5.539, p = .023; effect of port, F(1,46) = 93.463, p <.0001).
Figure 6.
Cue-induced reinstatement of responding in sign-trackers (n=12) and goal-trackers (n=13). Following extinction, all animals were given a single, 60-min cue reinstatement test session, in which active responses resulted in presentation of the cocaine cue. (a) Mean ± SEM number of active port nose pokes in 10-min blocks over the 60-min session. (b) The total number of active port responses in individual animals (the solid horizontal line indicates the group mean and the dashed line the average number of responses at the inactive port). *, indicates a significant group difference, p < 0.05.
Discussion
If cues associated with rewards are attributed with incentive salience they acquire the ability to act as incentive stimuli. Incentive stimuli have three fundamental properties: (1) they attract, eliciting approach towards them; (2) they are “wanted”, in the sense that animals will work to get them; and (3) they can spur ongoing instrumental actions to obtain the associated reward, as in the Pavlovian to instrumental transfer effect (21,22). We have reported, however, that if a localizable cue is associated with presentation of a food reward at a different location the cue acquires incentive motivational properties in some individuals, but not others - even though all individuals learn the CS-US association as indicated by the development and vigor of their respective CRs (23,24). This suggests that the ability of a cue to act as an incentive stimulus is dissociable from its ability to act as a conditional stimulus (CS) that evokes a conditional response (CR, 23). In the present study we asked whether rats that have a propensity to attribute incentive salience to a food cue are the same ones for whom a cocaine cue would come to motivate cocaine self-administration behavior - maintain drug taking and instigate reinstatement after extinction. They are. The cocaine cue was much more effective in motivating drug-taking behavior and instigating reinstatement in STs (who approached the food cue) than in GTs (who did not).
It is well known that drug cues are important for maintaining and reinstating drug-seeking behavior, presumably because they spur incentive motivation for drugs (8,13,14,17). In most drug self-administration studies drug delivery is paired with presentation of a cue for good reason. This is because omitting the cue greatly attenuates self-administration behavior (12,13,15,34). Indeed, cues are capable of maintaining responding for long periods of time even in the absence of the drug, on second-order schedules of reinforcement, for example (11,35). Of course, there is a large literature on both human and non-human animals concerning the importance of drug cues in instigating reinstatement/relapse (17). In addicts, many drugs cues (e.g., people, places and paraphernalia) evoke craving and/or relapse (4,5), and their attention is biased towards such cues (3). In rats, even stimuli associated with a single session of cocaine self-administration can motivate renewed drug-seeking a year later (36). In such studies there is, however, considerable individual variation in the effectiveness of cues in motivating behavior. The data reported here suggest this variation is not just due to experimental error, but much of it may be due to real individual variation in the propensity for cues to acquire incentive motivational properties. In the present study we considered only the effects of discrete localizable stimuli (a lever CS and a light CS), but many other manipulations influence self-administration behavior and reinstatement/relapse - including contextual stimuli, stress, and a “prime” with the drug itself (17,37,38). It remains to be determined whether variation in the propensity to attribute incentive salience to a discrete localizable cue also predicts the ability of these other classes of stimuli to produce desire and instigate actions. Furthermore, in the present experiment the cocaine cue was located at the same place where a response produced an injection of cocaine, a situation that may produce especially “compulsive” behavior (25). Further studies will be required to explore the influence of different arrangements between the CS and manipulandum, and whether similar individual differences are evident if a cue is paired with cocaine in a response-independent manner (7).
It is important to emphasize that the differences between STs and GTs reported here are not a function of differential exposure to either the drug itself, the number of CS-US pairings, or learning per se. We used a training procedure that held these variables constant across animals. Rather than using sessions of a fixed duration we allowed each animal to take the same number of injections each day, insuring that all animals would be exposed to the same amount of drug and the same number of CS-drug pairings. Indeed, there were no group differences in learning cocaine self-administration behavior. Furthermore, the difference between STs and GTs in the Pavlovian task is not attributable to differences in learning, as both groups learn their respective CRs at a comparable rate (23).
What might account for the large difference between STs and GTs in their propensity to attribute incentive value to either the food cue or the cocaine cue? Presumably this is due to variation in the operation of brain systems that attribute incentive salience to reward cues, or brain systems that moderate that process. Of course, there is a wealth of evidence implicating ascending mesotelencephalic dopamine (DA) systems in Pavlovian conditioned motivational processes (21,39,40), which prompts questions about differences in DA function between STs and GTs. Unfortunately, there has been very little research on this question, although the little that has been done suggests such differences exist (25,41). For example, a recent report (29) found higher levels of nucleus accumbens dopamine D1 receptor mRNA and lower levels of D2 mRNA in STs compared to GTs, and another found STs are more prone to cocaine sensitization (42).
Recent studies on two selectively-bred lines of rats also suggest a relationship between the propensity to attribute incentive salience to reward-related cues and DA (43). Rats selectively-bred for up to 18 generations for a high or low locomotor response to a novel environment (bHRs and bLRs, respectively) differ dramatically in their propensity to attribute incentive salience to reward cues. Essentially all bHR animals approach a cue associated with food or with cocaine (they are STs) and no bLRs do so (they are GTs). Furthermore, a food cue is a more effective conditional reinforcer in bHR/STs than bLR/GTs. There are a number of differences in the DA systems of these animals. The bHR/ST rats are more sensitive to the psychomotor activating effects of the DA D2/3 agonist quinpirole, and this behavioral supersensitivity is associated with a much greater proportion of DA D2high receptors in the dorsal striatum in these animals (despite lower D2 mRNA and no difference in total D2 receptor binding). Also, studies using fast-scan cyclic voltammetry revealed that bHR/ST animals have more frequent spontaneous DA “release events” in the accumbens core. Thus, the available data suggest that animals prone to attribute incentive salience to reward cues (STs) have a more active DA system than those who do not (GTs) (43). In addition, with Pavlovian training there is a transfer of the phasic DA signal (assessed with fast-scan cyclic voltammetry in the accumbens core) from a food US to a lever-CS in STs, but this does not occur in GTs (44). It appears that during Pavlovian conditioning the transfer of a phasic DA signal from the US to the CS is not due to a gain in predictive strength, but requires that the CS be attributed with incentive salience - as it is in STs but not GTs. This may be why acquisition of a ST CR is DA-dependent whereas learning a GT CR is not (S. Flagel, unpublished data). Taken together, these data suggest that STs and GTs differ in dopaminergic signaling in ways that may be related to differences in their tendency to attribute incentive salience to stimuli.
The extent to which the ST/GT and HR/LR traits are genetically (or epigenetically) related remains to be determined (43). In outbred animals these two traits are not correlated (24). Nevertheless, there are other traits that may contribute to addiction vulnerability and are associated with the tendency to show a ST or GT phenotype. Relative to GTs, both bHR/ST animals (43) and outbred STs (unpublished studies) have difficulty withholding an action in order to receive a reward, as assessed using a differential reinforcement of low rates (DRL) task, and STs show more premature (“impulsive”) responses on a 2-choice serial reaction time task (V. Lovic, unpublished data). These studies suggest that STs tend to show “impulsive actions” relative to GTs. Of course, individuals that combine both a propensity to make “impulsive actions” and to be motivationally aroused by drug cues may be especially susceptible to addiction.
Many of the behaviors characteristic of STs are similar to those seen after a lesion of the subthalamic nucleus (STN). Animals with a STN lesion more readily acquire cocaine self-administration, more readily approach (“sign-track”) cues associated with both food and cocaine reward, and show impulsive actions on a DRL task (45–47). These symptoms may be due to an increased tendency to attribute incentive salience to reward cues in animals with a STN lesion and, therefore, the STN may be part of a neural system that normally moderates the degree to which incentive salience is attributed to reward cues (47). It should be adaptive to attribute incentive value to cues that predict the location of natural rewards, because approaching such cues would often lead one to the reward itself. It may also be important, however, to moderate this process, because the excessive attribution of incentive salience to cues may be maladaptive and contribute to psychopathologies, including over-eating (1,2) and addiction (48,49). Thus, the differences between STs and GTs may be due to intrinsic differences in DA systems that actively attribute incentive salience to reward cues, to differences in systems that oppose this process, such as the STN, or both.
In conclusion, we report that it is possible to predict, prior to rats having any experience with cocaine, which individuals will most likely show cue-induced reinstatement of cocaine-seeking following extinction, and for which ones a cue will motivate drug-taking behavior. It appears rats that have a propensity to attribute incentive salience to a food cue also attribute incentive salience to a cocaine cue. The neural mechanism(s) responsible for these differences are unknown, although preliminary data implicates DA systems. Of course, individuals for whom drug cues become attractive will have more difficulty resisting them than individuals for whom cues are mere predictors of reward. Thus, cues may continually goad some individuals to action (20,48), and this trait may render these individuals most susceptible to addiction. It will be important to determine the psychological and neurobiological mechanisms responsible for variation in the propensity to attribute incentive salience to reward cues, as this may not only increase susceptibility to addiction, but to other compulsive behavioral disorders as well, including eating disorders and compulsive gambling.
Supplementary Material
Acknowledgments
This research was supported by a grant from the National Institute on Drug Abuse to TER (Grant No. R37 DA04294). We thank Adam Dziuba for technical assistance, and Kent Berridge, Shelly Flagel, Vedran Lovic, and Jason Uslaner for very helpful comments on an earlier version of the manuscript.
Footnotes
Financial Disclosures
The authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schachter S. Obesity and eating. Internal and external cues differentially affect the eating behavior of obese and normal humans. Science. 1968;161:751–756. doi: 10.1126/science.161.3843.751. [DOI] [PubMed] [Google Scholar]
- 2.Weingarten HP. Conditioned cues elicit eating in sated rats: A role for learning in meal initiation. Science. 1983;220:431–433. doi: 10.1126/science.6836286. [DOI] [PubMed] [Google Scholar]
- 3.Field M, Cox WM. Attentional bias in addictive behaviors: A review of its development, causes, and consequences. Drug Alcoh Depend. 2008;97:1–20. doi: 10.1016/j.drugalcdep.2008.03.030. [DOI] [PubMed] [Google Scholar]
- 4.Ehrman RN, Robbins SJ, Childress AR, O’Brien CP. Conditioned responses to cocaine-related stimuli in cocaine abuse patients. Psychopharmacology (Berl) 1992;107:523–529. doi: 10.1007/BF02245266. [DOI] [PubMed] [Google Scholar]
- 5.O’Brien CP, Childress AR, Ehrman R, Robbins SJ. Conditioning factors in drug abuse: can they explain compulsion? J Psychopharmacol. 1998;12:15–22. doi: 10.1177/026988119801200103. [DOI] [PubMed] [Google Scholar]
- 6.Childress AR, et al. Prelude to passion: limbic activation by “unseen” drug and sexual cues. PLoS One. 2008;3:e1506. doi: 10.1371/journal.pone.0001506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uslaner JM, Acerbo MJ, Jones SA, Robinson TE. The attribution of incentive salience to a stimulus that signals an intravenous injection of cocaine. Beh Brain Res. 2006;169:320–324. doi: 10.1016/j.bbr.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 8.Davis WM, Smith SG. Role of conditioned reinforcers in the initiation, maintenance and extinction of drug-seeking behavior. Integr Physiol Behav Sci. 1976;11:222–236. doi: 10.1007/BF03000316. [DOI] [PubMed] [Google Scholar]
- 9.Goddard B, Leri F. Reinstatement of conditioned reinforcing properties of cocaine-conditioned stimuli. Pharmacol Biochem Behav. 2006;83:540–546. doi: 10.1016/j.pbb.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 10.Di Ciano P, Everitt BJ. Conditioned reinforcing properties of stimuli paired with self-administered cocaine, heroin or sucrose: implications for the persistence of addictive behaviour. Neuropharmacology. 2004;47:202–213. doi: 10.1016/j.neuropharm.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 11.Schindler CW, Panlilio LV, Goldberg SR. Second-order schedules of drug self-administration in animals. Psychopharmacology (Berl) 2002;163:327–344. doi: 10.1007/s00213-002-1157-4. [DOI] [PubMed] [Google Scholar]
- 12.Caggiula AR, Donny EC, White AR, Chaudrhi N, Booth S, Gharib MA, et al. Cue dependency of nicotine self-administration and smoking. Pharmacol Biochem Behav. 2001;70:515–530. doi: 10.1016/s0091-3057(01)00676-1. [DOI] [PubMed] [Google Scholar]
- 13.Schenk S, Partridge B. Influence of a conditioned light stimulus on cocaine self-administration in rats. Psychopharmacology (Berl) 2001;154:390–396. doi: 10.1007/s002130000608. [DOI] [PubMed] [Google Scholar]
- 14.Arroyo M, Markou A, Robbins TW, Everitt BJ. Acquisition, maintenance and reinstatement of intravenous cocaine self-administration under a second-order schedule of reinforcement in rats: effects of conditioned cues and continuous access to cocaine. Psychopharmacology (Berl) 1999;140:331–344. doi: 10.1007/s002130050774. [DOI] [PubMed] [Google Scholar]
- 15.Panlilio LV, Weiss SJ, Schindler CW. Cocaine self-administration increased by compounding discriminative stimuli. Psychopharmacology (Berl) 1996;125:202–208. doi: 10.1007/BF02247329. [DOI] [PubMed] [Google Scholar]
- 16.De Wit H, Stewart J. Reinstatement of cocaine-reinforced responding in the rat. Psychopharmacology (Berl) 1981;75:134–143. doi: 10.1007/BF00432175. [DOI] [PubMed] [Google Scholar]
- 17.Shaham Y, Shalev U, Lu L, de Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology, and major findings. Psychopharmacology (Berl) 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- 18.Bindra D. How adaptive behavior is produced: A perceptual-motivation alternative to response reinforcement. Behav Brain Sci. 1978;1:41–91. [Google Scholar]
- 19.Bolles RC. Reinforcement, expectancy and learning. Psychol Rev. 1972;79:394–409. [Google Scholar]
- 20.Stewart J, de Wit H, Eikelboom R. Role of unconditioned and conditioned drug effects in the self-administration of opiates and stimulants. Psychol Rev. 1984;91:251–268. [PubMed] [Google Scholar]
- 21.Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev. 2002;26:321–352. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- 22.Berridge KC. Reward learning: reinforcement, incentives, and expectations. In: Medlin DL, editor. The Psychology of Learning and Motivation. Academic Press; Oxford: 2001. pp. 223–278. [Google Scholar]
- 23.Robinson TE, Flagel SB. Dissociating the predictive and incentive motivational properties of reward-related cues through the study of individual differences. Biol Psychiatry. 2009;65:869–873. doi: 10.1016/j.biopsych.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flagel SB, Akil H, Robinson TE. Individual differences in the attribution of incentive salience to reward-related cues: Implications for addiction. Neuropharmacology. 2009;56:139–148. doi: 10.1016/j.neuropharm.2008.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tomie A, Grimes KL, Pohorecky LA. Behavioral characteristics and neurobiological substrates shared by Pavlovian sign-tracking and drug abuse. Brain Res Rev. 2008;58:121–135. doi: 10.1016/j.brainresrev.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zener K. The significance of behavior accompanying conditioned salivary secretion for theories of the conditioned response. Am J Psychol. 1937;50:384–403. [Google Scholar]
- 27.Hearst E, Jenkins H. Monograph of the Psychonomic Society. Austin: 1974. Sign-tracking: the stimulus-reinforcer relation and directed action. [Google Scholar]
- 28.Boakes R. Performance on learning to associate a stimulus with positive reinforcement. In: Davis H, Hurwitz H, editors. Operant-Pavlovian Interactions. Erlbaum; Hillsdale: 1977. pp. 67–97. [Google Scholar]
- 29.Flagel SB, Watson SJ, Robinson TE, Akil H. Individual differences in the propensity to approach signals vs goals promote different adaptations in the dopamine system of rats. Psychopharmacology (Berl) 2007;191:599–607. doi: 10.1007/s00213-006-0535-8. [DOI] [PubMed] [Google Scholar]
- 30.Crombag HS, Badiani A, Maren S, Robinson TE. The role of contextual versus discrete drug-associated cues in promoting the induction of psychomotor sensitization to intravenous amphetamine. Behav Brain Res. 2000;116:1–22. doi: 10.1016/s0166-4328(00)00243-6. [DOI] [PubMed] [Google Scholar]
- 31.Grimm JW, Hope BT, Wise RA, Shaham Y. Incubation of cocaine craving after withdrawal. Nature. 2000;412:141–142. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Verbeke G, Molenberghs G. Linear Mixed Models for Longitudinal Data. Springer; New York: 2000. [Google Scholar]
- 33.Kippin TE, Fuchs RA, See RE. Contributions of prolonged contingent and noncontingent cocaine exposure to enhanced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2006;187:60–67. doi: 10.1007/s00213-006-0386-3. [DOI] [PubMed] [Google Scholar]
- 34.Chaudhri N, Caggiula AR, Donny EC, Booth S, Gharib MA, Craven LA, et al. Sex differences in the contribution of nicotine and nonpharmacological stimuli to nicotine self-administration in rats. Psychopharmacology (Berl) 2005;180:258–266. doi: 10.1007/s00213-005-2152-3. [DOI] [PubMed] [Google Scholar]
- 35.Everitt BJ, Robbins TW. Second-order schedules of drug reinforcement in rats and monkeys: measurement of reinforcing efficacy and drug-seeking behaviour. Psychopharmacology (Berl) 2000;153:17–30. doi: 10.1007/s002130000566. [DOI] [PubMed] [Google Scholar]
- 36.Ciccocioppo R, Marin-Fardon R, Weiss F. Stimuli associated with a single cocaine experience elicit long-lasting cocaine-seeking. Nat Neurosci. 2004;7:495–496. doi: 10.1038/nn1219. [DOI] [PubMed] [Google Scholar]
- 37.Crombag HS, Bossert JM, Koya E, Shaham Y. Context-induced relapse to drug seeking: a review. Philos Trans R Soc Lond B Biol Sci. 2008;363:3233–3243. doi: 10.1098/rstb.2008.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De Wit H. Priming effect with drugs and other reinforcers. Exp Clin Psychopharmacol. 1996;4:5–10. [Google Scholar]
- 39.Berridge KC, Robinson TE. What is the role of dopamine in reward: Hedonic impact, reward learning, or incentive salience? Brain Res Rev. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- 40.Di Chiara G. A motivational learning hypothesis of the role of mesolimbic dopamine in compulsive drug use. J Psychopharmacol. 1998;12:54–67. doi: 10.1177/026988119801200108. [DOI] [PubMed] [Google Scholar]
- 41.Tomie A, Aquado AS, Pohorecky LA, Benjamin D. Individual differences in Pavlovian autoshaping of lever pressing in rats predict stress-induced corticosterone release and mesolimbic levels of monoamines. Pharmacol Biochem Behav. 2000;65:509–517. doi: 10.1016/s0091-3057(99)00241-5. [DOI] [PubMed] [Google Scholar]
- 42.Flagel SB, Watson SJ, Akil H, Robinson TE. Individual differences in the attribution of incentive salience to a reward-related cue: influence on cocaine sensitization. Behav Brain Res. 2008;186:48–56. doi: 10.1016/j.bbr.2007.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Flagel SB, Robinson TE, Clark JJ, Clinton SM, Watson SJ, Seeman P, et al. An animal model of genetic vulnerability to behavioral disinhibition and responsiveness to reward-related cues: Implications for addiction. Neuropsychopharmacology. 2009 doi: 10.1038/npp.2009.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Phillip PEM, Clark JJ, Flagel SB, Clinton SM, Robinson TE, Akil H. Transfer of phasic dopamine release from unconditioned to conditioned stimuli requires the attribution of incentive salience. Soc Neurosci Abstracts. 2008:876.2. [Google Scholar]
- 45.Uslaner JM, Yang P, Robinson TE. Subthalamic nucleus lesions enhance the psychomotor-activating, incentive motivational and neurobiological effects of cocaine. J Neurosci. 2005;25:8407–8415. doi: 10.1523/JNEUROSCI.1910-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Uslaner JM, Robinson TE. Subthalamic nucleus lesions increase impulsive action and decrease impulsive choice – mediation by enhanced incentive motivation? Eur J Neurosci. 2006;24:2345–2354. doi: 10.1111/j.1460-9568.2006.05117.x. [DOI] [PubMed] [Google Scholar]
- 47.Uslaner JM, Dell’Orco JM, Pevzner A, Robinson TE. The influence of subthalamic nucleus lesions on sign-tracking to stimuli paired with food and drug rewards: facilitation of incentive salience attribution? Neuropsychopharmacology. 2008;33:2352–2361. doi: 10.1038/sj.npp.1301653. [DOI] [PubMed] [Google Scholar]
- 48.Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- 49.Kapur S. Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. Am J Psychiatry. 2003;160:13–23. doi: 10.1176/appi.ajp.160.1.13. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.