Abstract
Post-vaccinal encephalitis, although relatively uncommon, is a known adverse event associated with many live, attenuated smallpox vaccines. Although smallpox vaccination ceased globally in 1980, vaccine manufacture has resumed in response to concerns over the possible use of smallpox virus as an agent of bioterrorism. To better support the production of safer smallpox vaccines, we previously reported the development of a mouse model in which a relatively attenuated vaccine strain (Dryvax®) could be discerned from a more virulent laboratory strain (WR). Here we have further tested the performance of this assay by evaluating the neurovirulence of several vaccinia virus-based smallpox vaccines spanning a known range in neurovirulence for humans. Our data indicate that testing of 10 to 100 pfu of virus in mice following intracranial inoculation reliably assesses the virus’s neurovirulence potential for humans.
Introduction
Smallpox, a highly contagious disease caused by the variola virus, has a 30% mortality rate and is historically responsible for more deaths than all other infectious diseases combined. In 1979, the WHO declared that the disease had been eradicated following a decades-long worldwide vaccination campaign 13. Routine vaccination was discontinued in the U.S. in 1972 and worldwide in 1980, and, as a consequence, greater than half of the current global population is susceptible to infection by the virus 7,23. This has raised concerns over the possible weaponization of variola virus and its use as a bioterrorism agent 5,14, a concern given credence by the anthrax bioterrorism attacks in the U.S. shortly after 9/11 30. In response to this concern, some countries decided to resume vaccination of certain segments of their population, such as health care workers and members of the military. Since 2002, the U.S. vaccinated over 1.2 million military personnel and over 40,000 civilians with existing vaccine reserves 21. To address potential needs of the general population, vaccinia virus-based smallpox vaccine production was reinitiated by several manufacturers to produce stockpiles for mass vaccination in the event of an attack 4,15,27,31,32.
The potential resumption of mass vaccination against smallpox raises concerns with the safety of the vaccine strains themselves. Numerous serious adverse events have been reported following vaccination with vaccinia virus-based smallpox vaccines, both historically and in recent years, and, thus, efforts towards the development and manufacture of new generation vaccines of reduced reactogenicity have commenced. One of the most serious complications of smallpox vaccination is the development of encephalitis. Although rates of encephalitis following vaccination are rare, approximately 25% of cases are fatal and, in another 25% of cases, permanent neurological damage results 1–3,9–11,19,26.
The incidence of post-vaccinal encephalitis varies by vaccine strain, ranging from a conservative estimate of 46 cases per million doses of the Bern vaccine to no reported cases with the LC16m8 or MVA strains. The widely used Lister and Dryvax® strains are associated with 26 and 2.9 cases of post-vaccinal encephalitis per million doses, respectively 18,33. The differences in vaccine-specific rates of post-vaccinal encephalitis indicates differences in the level of attenuation of the parental viruses and highlights the necessity of a standardized, validated neurovirulence assay in which the virus’s human neurovirulence potential can be correctly assessed.
Through a review of the available data on neurovirulence following experimental infection of animals with vaccinia virus, we previously developed a prototype assay for vaccinia virus neurovirulence assessment 20. We have now furthered this work and this study provides data in support of the feasibility for validation of the model as a pre-clinical neurovirulence assay for the assessment of candidate vaccinia virus vaccines. Our data indicate that the relative human neurovirulence of vaccinia virus-based smallpox vaccine strains can be determined based on mouse mortality following intracerebral inoculation of 3-day old mice with 10 to 100 plaque forming units (pfu) of virus.
Materials and methods
Virus strains
The WR, Lister, and MVA vaccinia virus strains were purchased from The American Type Culture Collection (ATCC, Manassas, Virginia) and were used directly (without further passage in the laboratory). The Dryvax® vaccine was used directly from a commercial vaccine preparation (Wyeth Laboratories, Philadelphia, PA). The IHD-J and Copenhagan strain was kindly provided by Drs. Michael Merchlinsky, FDA/CBER and Dennis Hruby, Oregon State University, respectively. The IHD-J and Copenhagen strains were passaged once in Vero cells to generate working stocks. Based on a review of available published clinical studies, the presumed order of vaccine virus strain from high to low neurovirulence is as follows: Copenhagen, Lister, Dryvax®, and then MVA 18,33. The WR and IHD-J laboratory strains, having not been attenuated for human use, are expected to have the greatest neurovirulence potential.
Virus titration
The Dryvax®, Lister, Copenhagen, IHD-J, and WR virus strains were titrated on Vero cells by plaque assay as described previously 20. Because the MVA strain was adapted for growth in chick embryo fibroblast (CEF) cells and does not effectively replicate in most other cell lines, including Vero, this strain was titrated on CEF cells by standard immunofluorescence assay 6. Notably, the MVA strain does not form plaques, and thus cannot be titrated by plaque assay. Briefly, 0.1 mL of serially diluted MVA virus was incubated on CEF cell monolayers in 8-well chamber slides for 1 hr at 37°C in 5% CO2. The viral inocula were removed by aspiration and replaced with 500 uL E-MEM + 5% FBS per well and incubated for 1 day at 37°C in 5% CO2. Cells were then fixed in 4% paraformaldehyde in PBS for 30 min and then incubated with 0.2% Triton X-100 in PBS for 10 min at room temperature. The fixed, permeabilized cell monolayers were blocked with 3% normal goat serum in PBS (blocking buffer) followed by incubation with a 1:1000 dilution (in blocking buffer) of rabbit anti-vaccinia virus antiserum (Biodesign International, Saco, ME) at room temperature for 1 hr. Following three washes in PBS, cell monolayers were incubated with a 1:400 dilution (in blocking buffer) of Cy2 conjugated anti-rabbit IgG (Chemicon International, Temecula, CA.) for 1 hr at room temperature. The chamber slides were rinsed in PBS, cover-slipped, and the number of fluorescence focus units (ffu) in each chamber well was counted. The viral titer was quantified as number of ffu multiplied by the dilution factor and volume plated.
Working virus stocks were prepared by diluting the six viruses in E-MEM to final titers of 100 to 105 pfu or ffu per 10 uL. All virus stocks were stored at −800C and were re-titred at the time of inoculation to confirm dose administered to mice.
Animals
For survival studies, litters of 3-day old CD-1 mice (Harlan Sprague Dawley Inc., Indianapolis, IN) were inoculated intracranially (i.c.) in the left parietal lobe near anatomical midline, midway between the eye and the ear. Inocula consisted of 100-105 pfu or ffu of vaccinia virus in a 10 uL volume of E-MEM, or an equivalent volume of E-MEM alone as a negative control. The mice were observed twice daily over a 28 day period post-inoculation. Mice showing advanced signs of morbidity (dehydration and non-responsiveness to external stimuli) were humanely euthanized and included in mortality totals. All animal experiments were performed in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals 16.
For determination of virus growth in brain, litters of 3-day old CD-1 mice were inoculated i.c. with 10 uL of E-MEM containing 101 or 102 pfu or ffu of virus. At various times post-inoculation, four to five mice in each virus-inoculated group were randomly selected then euthanized and brains were removed. Brains were homogenized (separately) in E-MEM +2% FBS (20% w/v) by mechanical disruption followed by ultrasonication. Brain homogenates were clarified by centrifugation and the virus concentration in supernatants was determined as described above (immunostaining of CEF inoculated cultures for brains inoculated with the MVA virus and Vero cell-based plaque assay for all others).
A subset of brains were assessed for virus antigen distribution on day 2 post inoculation as previously described 20. Briefly, whole brains were flash frozen in isobutene and cut into 8 um sagittal sections using a cryostat. Brain sections selected at standard distances from anatomical midline were then placed onto glass slides and fixed in acetone and stained immunohistochemically for virus antigen as described above (polyclonal rabbit anti-vaccinia virus followed by Cy2-conjugated anti-species secondary IgG). Sections were assessed using a fluorescence microscope.
Results
Mouse mortality
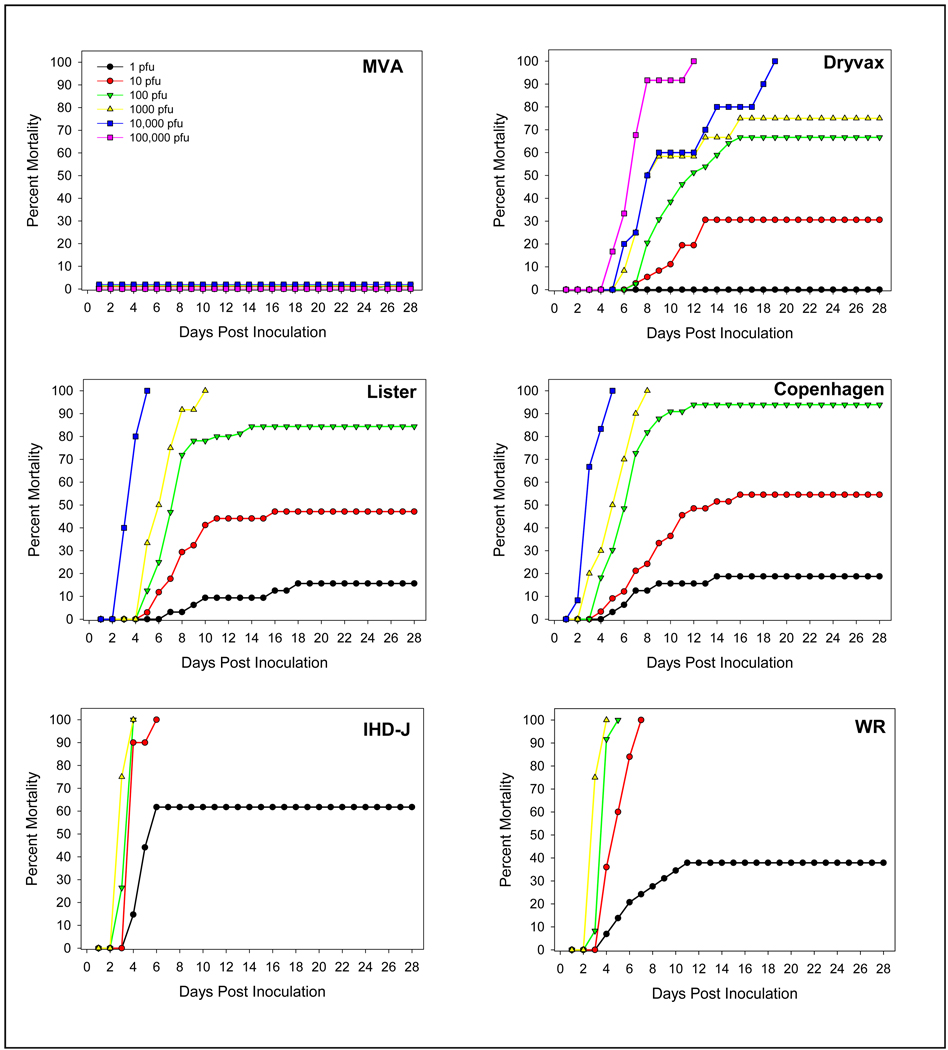
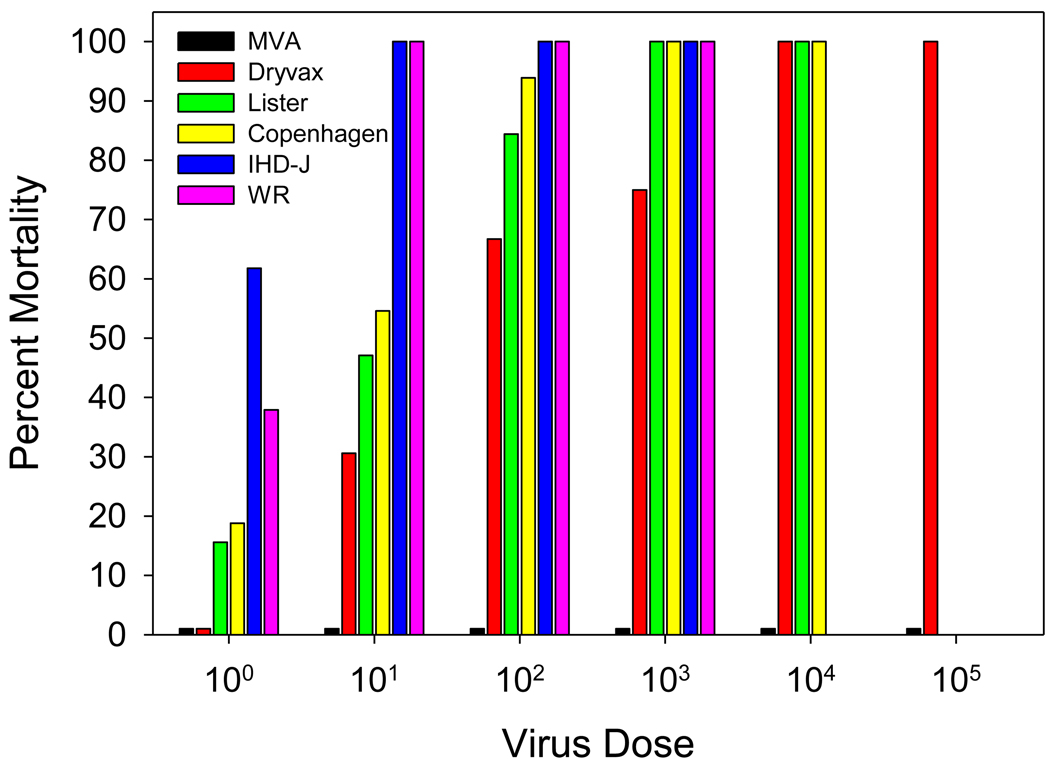
Virus dose dependant mortality is shown in Figure 1. None of the mice inoculated with the MVA strain (at any dose), nor mice inoculated with negative control material (E-MEM) died or showed any signs of disease. For all other virus strains, signs of disease (which included dehydration, lethargy, hind limb paralysis, seizures, lack of response to external stimuli and death) were virus strain dependant and developed within 2 to 6 days of inoculation. Although disease onset and severity was virus strain and virus dose dependant (as reflected in Figure 1), the clinical picture was similar. During the first week post inoculation, mice developed seizures, partial paralysis and dehydration, followed by, in some mice, a lack of response to external stimuli. Mice not responding to external stimuli or were otherwise in a moribund state were euthanized for humane reasons and were considered as cases of mortality. After the second week, some of the surviving mice improved clinically, although not completely, as evidenced by residual paralysis, ruffled fur, and dehydration. There appeared to be a correlation between increasing virus dose and increased disease onset and frequency. Virus-specific LD50 values are shown in Table 1. The lowest LD50 values (most virulent) were observed with the WR (1.5 pfu) and IHD-J (1.0 pfu) strains, reflecting the relatively low attenuation history of these viruses. Of the human vaccines strains, the Copenhagen strain was the most virulent, having an LD50 of 6.6 pfu, followed by Lister (11.2 pfu), Dryvax® (44.7 pfu) and MVA (>105 ffu). This LD50 rank order parallels the known rates of post-vaccinal encephalitis associated with these vaccine strains.
Figure 1.
Virus-induced mortality in mice assessed over a 28 day period following intracerebral inoculation with different vaccinia virus strains. Due to high mortality following administration of 104 pfu of the Lister and Copenhagen strains and following administration of 103 pfu of the IHD-J and WR strains, higher doses of these viruses were not tested to avoid unnecessary use of animals. In addition, for humane purposes, moribund mice were euthanized and included in mortality counts.
Table 1.
Vaccinia virus strain specific mouse LD50
| Virus | Dose (pfu or ffu) |
Mortality* | LD50 (pfu or ffu) |
|---|---|---|---|
| MVA | 100 | 0/12 | ≥ 100,000 |
| 101 | 0/12 | ||
| 102 | 0/12 | ||
| 103 | 0/12 | ||
| 104 | 0/12 | ||
| 105 | 0/21 | ||
| Dryvax® | 100 | 0/22 | 44.7 |
| 101 | 11/36 | ||
| 102 | 26/39 | ||
| 103 | 9/12 | ||
| 104 | 10/10 | ||
| 105 | 12/12 | ||
| Lister | 100 | 5/32 | 11.2 |
| 101 | 16/34 | ||
| 102 | 27/32 | ||
| 103 | 12/12 | ||
| 104 | 10/10 | ||
| Copenhagen | 100 | 6/32 | 6.6 |
| 101 | 18/33 | ||
| 102 | 31/33 | ||
| 103 | 10/10 | ||
| 104 | 12/12 | ||
| WR | 100 | 11/29 | 1.5 |
| 101 | 25/25 | ||
| 102 | 20/20 | ||
| 103 | 12/12 | ||
| IHD-J | 100 | 21/34 | 1.0 |
| 101 | 20/20 | ||
| 102 | 34/34 | ||
| 103 | 12/12 | ||
| Medium | - | 0/20 | - |
For humane purposes, moribund mice were euthanized and included in mortality counts.
Virus strain dependant mortality by dose is shown in Figure 2. At virus doses of ≥103 pfu, there was little to no difference in mouse mortality among the replication-competent virus strains, suggesting the inappropriateness of use of a virus dose ≥103 pfu in the pre-clinical neurovirulence assay. A similar conclusion was suggested by the data obtained using a dose of 1 pfu, in that no differences were seen between the neurovirulence of MVA and Dryvax®. Discrimination between the degree of neurovirulence of the strains was best using a dose of 10 or 100 pfu, with some indication of a slight sensitivity advantage using a dose of 10 pfu, although this was not statistically significant.
Figure 2.
Dose dependant comparison of virus-induced mortality in mice assessed over a day period following intracerebral inoculation with different vaccinia virus strains Due to high mortality following administration of 104 pfu of the Lister and Copenhagen strains and following administration of 103 pfu of the IHD-J and WR strains, higher doses of these viruses were not tested to avoid unnecessary use of animals. In addition, for humane purposes, moribund mice were euthanized and included in mortality counts. Of note, data for this figure was derived from Figure 1.
Virus replication in mouse brain
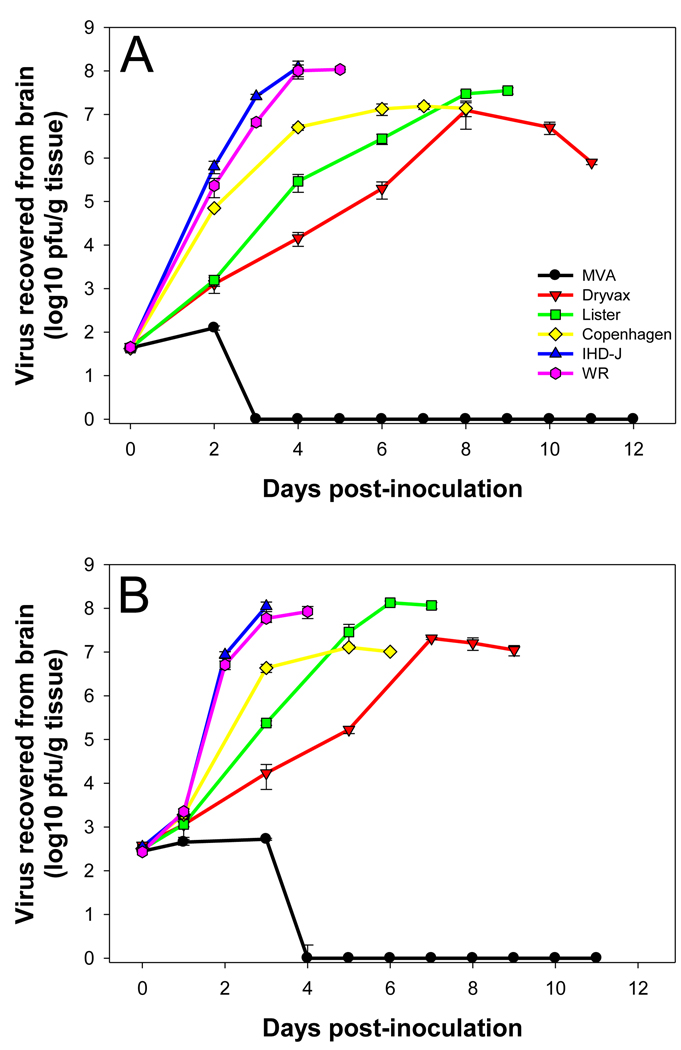
The kinetics of virus growth in mouse brain following inoculation of 10 and 100 pfu of virus is shown in Figures 3A and 3B, respectively. Virus was measured in mouse brain up until the mean day of mortality (calculated from the data shown in Figure 1). At a dose of 10 pfu, MVA could be recovered in low amounts from mouse brain over a two-day period following inoculation, likely reflecting the inoculum itself as opposed to productive replication of the virus. MVA could not be recovered from brains after day 2. A similar finding was seen using a dose of 100 pfu, where a titer approximating that of the inoculum was measured over a three-day period following inoculation, but no virus could be recovered thereafter. In contrast, the other five viruses (IHD-J, WR, Copenhagen, Lister, Dryvax®), at both doses (10 pfu and 100 pfu), productively replicated in mouse brain. Interestingly, at a dose of 10 pfu Dryvax®, Lister, and Copenhagen all replicated to similar peak titers (approximately 1×107 pfu/g tissues), yet mortality rates differed significantly (30% to 55%). Likewise, at a dose of 100 pfu, IHD-J, WR and Lister replicated to similar peak titers (approximately 1×108 pfu/g tissues) and Copenhagen and Dryvax replicated to similar peak titers (approximately 1×107 pfu/g tissues), yet mortality rates differed significantly (84% for Lister vs. 100% for IHD-J and WR; 67% for Dryvax vs. 94% for Copenhagen). The only parameter consistently correlating with the LD50 data was speed of replication at early time points. At a dose of 10 pfu, by day 4 post inoculation, the rank order of the viruses in terms of concentration in the brain was IHD-J/WR > Copenhagen > Lister > Dryvax, all separated from each other by at least 10-fold (Figure 3A). The same pattern was seen by day 3 post inoculation at a dose of 100 pfu (Figure 3B).
Figure 3.
Infectious virus recovered from mouse brain measured following intracerebral inoculation with (A) 101 pfu or ffu and (B) 102 pfu or ffu of different vaccinia virus strains.
An assessment of virus antigen distribution in mouse brain (data not shown) confirmed our earlier findings comparing mice injected with the Dryvax versus WR strains. In both the current and previous study 20, at early time points (days 2 and 4 in the previous study and day 2 in the present study) vaccinia virus antigen expression in mice inoculated with the Dryvax strain was limited to periventricular areas whereas antigen expression in mice inoculated with the WR strain was more widely distributed, seen throughout the ventricular system as well as in the cortex, hippocampus and brain stem. A staining pattern similar to that of WR was seen in mice inoculated with the IHD-J strain. The distribution of virus in brains of mice inoculated with the Copenhagen and Lister strains at day 2 post inoculation appeared somewhat more extensive than that seen with Dryvax but not as widespread and intense as that seen with IHD-J and WR; however, this was highly variable between animals and was difficult to determine to a high degree of confidence. We did not examine antigen distribution at other time points.
Discussion
Several different vaccinia virus-based smallpox vaccines were used in the eradication effort and nearly all were associated with complications, ranging from general side effects such as regional lymphadenopathy, myalgia, fever, nausea, and malaise to more severe complications such as post-vaccinal encephalitis, encephalomyelitis, encephalopathy, myopericarditis, vaccinia necrosum, and eczema vaccinatum. Following smallpox eradication, routine vaccination was discontinued, a move supported by the risk benefit ratio of use of reactogenic vaccines against a disease no longer in existence. However, in 2002 the U.S. and British governments called for the resumption of the manufacture of smallpox vaccine to create national stockpiles as a precautionary measure in the event of a bioterrorist attack using smallpox 32. Following similar concerns voiced by several other countries, in 2004 the WHO proposed the establishment of a global smallpox vaccine reserve 31. In response to this need, the manufacture of “new generation” smallpox vaccines commenced. It is hoped that the many improvements in vaccine manufacture and control since the production of early traditional smallpox vaccines will result in the production of safer, less reactogenic, vaccines 33.
One of the most serious adverse events following vaccination is post-vaccinal encephalitis. Rates of post-vaccinal encephalitis vary by vaccine strain, ranging from 44.9 cases per million vaccinations using the Bern strain (used in Germany and Austria in the 1950’s and 1960’s) to zero cases using the replication deficient MVA strain 8,18,24,33.
National regulatory authorities require neurovirulence safety testing of live vaccines derived from viruses that target the central or peripheral nervous system 17, although the methods have not been defined. To address this need, we previously developed a prototype assay for vaccinia virus neurovirulence assessment 20. Here, we have furthered this work, providing data in support of use of the assay as a pre-clinical tool in which the neurovirulence of candidate vaccinia virus strains can be assessed. In our studies, four vaccinia virus strains previously used in smallpox vaccine campaigns were tested: the Copenhagen strain, reportedly associated with 33.3 cases of post-vaccinal encephalitis per million vaccinations; the Lister strain, associated with 26.2 cases of post-vaccinal encephalitis per million vaccinations; and Dryvax® strain, associated with 2.9 cases of post-vaccinal encephalitis per million vaccinations; and the MVA strain, which has not been causally associated with neurology adverse events 18,33. Our data show that based on an assessment of mouse morbidity and mortality following intracerebral inoculation with 10 or 100 pfu of virus, the rank order of vaccine virus neurovirulence in humans, namely Copenhagen > Lister > Dryvax® > MVA, was identical to the rank order of vaccine virus neurovirulence in our mouse model. Also included in the assessment were the WR and IHD-J laboratory strains 12,20,25,28,29, which, as expected, were more neurotoxic in mice than any of the vaccine strains. Of the two doses, neurovirulence discrimination among the virus strains appeared slightly better when 10 pfu of virus was used.
Based on virus replication kinetics in mouse brain, speed of replication, not peak titers, appeared to be the key factor responsible for virus strain-specific differences in mortality. Replication speed advantage at early stages of infection may allow the virus to briefly evade the host antiviral response, resulting in more rapid virus spread from the site of injection to other brain regions, resulting in increased virulence. While we saw clear evidence of increased early (day 2 post inoculation) virus spread in mouse brain upon comparing viruses at the more extreme ends of the virulence spectrum (e.g., IHD-J and WR vs. Dryvax), such differences were not as clear for more intermediate comparisons (e.g., Copenhagen vs. Lister or Lister vs. Dryvax), although the data did trend towards intermediate levels of spread by Copenhagen and Lister. In earlier comparative studies of vaccinia virus neurovirulence in intrathalamically inoculated monkeys, vaccinia virus strain-specific LD50’s correlated with the spread of virus in brain at early time points 22. Whether or not our hypothesis that speed of replication and early virus dissemination are key factors in neurovirulence remains to be proven. Nonetheless, for the purpose of developing and using a pre-clinical neurovirulence safety assay, the use of virus growth kinetics in the brain or virus spread in the brain do not appear to be practical endpoints.
In conclusion, our data support the use of mice to assess the human neurovirulence potential of vaccinia virus-based smallpox vaccines. We propose for this assay that mortality be assessed following intracerebral inoculation of 3-day old CD-1 mice with 10 to 100 pfu of virus. The neurovirulence of a candidate vaccine strain should be assessed based on its performance in the assay relative to a “low” virulence replication competent reference virus, such as Dryvax®, using an appropriate confidence interval for the comparison, e.g., in a “no worse than” scenario. A “high” virulence replication competent reference virus, such as WR or IHD-J should be used as a positive assay control. Validation of this assay by independent laboratories would provide the basis for use of this model to support the development, licensure and use of safer new-generation vaccinia virus-based smallpox vaccines.
Acknowledgements
This work was supported by CBER/FDA - NIAID/NIH Interagency Agreement Y1-AI-6153-01 and in part by an appointment to the Research Participation Program at the Center for Biologics Evaluation and Research administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and the U.S. Food and Drug Administration. The findings and conclusions in this article have not been formally disseminated by the Food and Drug Administration and should not be construed to represent any Agency determination or policy.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference list
- 1.Update: adverse events following civilian smallpox vaccination--United States, 2003. MMWR Morb. Mortal. Wkly. Rep. 2004;53:106–107. [PubMed] [Google Scholar]
- 2.Abrahams BC, Kaufman DM. Anticipating smallpox and monkeypox outbreaks: complications of the smallpox vaccine. Neurologist. 2004;10:265–274. doi: 10.1097/01.nrl.0000138998.11209.88. [DOI] [PubMed] [Google Scholar]
- 3.Belongia EA, Naleway AL. Smallpox vaccine: the good, the bad, and the ugly. Clin. Med Res. 2003;1:87–92. doi: 10.3121/cmr.1.2.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bradbury J. USA to increase smallpox vaccine stockpile. Lancet Infect Dis. 2001;1:290. doi: 10.1016/S1473-3099(01)00132-3. [DOI] [PubMed] [Google Scholar]
- 5.Breman JG, Henderson DA. Poxvirus dilemmas--monkeypox, smallpox, and biologic terrorism. N. Engl. J Med. 1998;339:556–559. doi: 10.1056/NEJM199808203390811. [DOI] [PubMed] [Google Scholar]
- 6.Carroll MW, Moss B. Host range and cytopathogenicity of the highly attenuated MVA strain of vaccinia virus: propagation and generation of recombinant viruses in a nonhuman mammalian cell line. Virology. 1997;238:198–211. doi: 10.1006/viro.1997.8845. [DOI] [PubMed] [Google Scholar]
- 7.Cohen J. Bioterrorism. Smallpox vaccinations: how much protection remains? Science. 2001;294:985. doi: 10.1126/science.294.5544.985. [DOI] [PubMed] [Google Scholar]
- 8.Frey SE, Newman FK, Kennedy JS, Sobek V, Ennis FA, Hill H, Yan LK, Chaplin P, Vollmar J, Chaitman BR, Belshe RB. Clinical and immunologic responses to multiple doses of IMVAMUNE (Modified Vaccinia Ankara) followed by Dryvax challenge. Vaccine. 2007;25:8562–8573. doi: 10.1016/j.vaccine.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fulginiti VA. Risks of smallpox vaccination. JAMA. 2003;290:1452. doi: 10.1001/jama.290.11.1452-a. [DOI] [PubMed] [Google Scholar]
- 10.Fulginiti VA, Papier A, Lane JM, Neff JM, Henderson DA. Smallpox vaccination: a review, part II. Adverse events. Clin. Infect Dis. 2003;37:251–271. doi: 10.1086/375825. [DOI] [PubMed] [Google Scholar]
- 11.Gurvich EB, Vilesova IS. Vaccinia virus in postvaccinal encephalitis. Acta Virol. 1983;27:154–159. [PubMed] [Google Scholar]
- 12.Hayasaka D, Ennis FA, Terajima M. Pathogeneses of respiratory infections with virulent and attenuated vaccinia viruses. Virol. J. 2007;4:22. doi: 10.1186/1743-422X-4-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henderson DA. Smallpox eradication. Public Health Rep. 1980;95:422–426. [PMC free article] [PubMed] [Google Scholar]
- 14.Henderson DA, Inglesby TV, Bartlett JG, Ascher MS, Eitzen E, Jahrling PB, Hauer J, Layton M, McDade J, Osterholm MT, O'Toole T, Parker G, Perl T, Russell PK, Tonat K. Smallpox as a biological weapon: medical and public health management. Working Group on Civilian Biodefense. JAMA. 1999;281:2127–2137. doi: 10.1001/jama.281.22.2127. [DOI] [PubMed] [Google Scholar]
- 15.Hwang HS. [The strategic plan for preparedness and response to bioterrorism in Korea] J Prev. Med Public Health. 2008;41:209–213. doi: 10.3961/jpmph.2008.41.4.209. [DOI] [PubMed] [Google Scholar]
- 16.Institute of Laboratory Animal Resources, Commission on Life Sciences National Research Council. National Academy Press; Guide for the Care and Use of Laboratory Animals. 1996:1–140. http://oacu.od.nih.gov/regs/index.htm.
- 17.International Association for Biologicals. IABs scientific workshop on neurovirulence tests for live virus vaccines, January 31-February 1, 2005, Geneva. Biologicals. 2006;34:233–236. doi: 10.1016/j.biologicals.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 18.Kretzschmar M, Wallinga J, Teunis P, Xing S, Mikolajczyk R. Frequency of adverse events after vaccination with different vaccinia strains. PLoS. Med. 2006;3:e272. doi: 10.1371/journal.pmed.0030272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lane JM, Ruben FL, Abrutyn E, Millar JD. Deaths attributable to smallpox vaccination, 1959 to 1966, and 1968. JAMA. 1970;212:441–444. [PubMed] [Google Scholar]
- 20.Li Z, Rubin SA, Taffs RE, Merchlinsky M, Ye Z, Carbone KM. Mouse neurotoxicity test for vaccinia-based smallpox vaccines. Vaccine. 2004;22:1486–1493. doi: 10.1016/j.vaccine.2003.10.022. [DOI] [PubMed] [Google Scholar]
- 21.Mora LF, Khan AH, Sperling LS. Cardiac Complications after Smallpox Vaccination. South. Med J. 2009 doi: 10.1097/SMJ.0b013e31819fe55b. [DOI] [PubMed] [Google Scholar]
- 22.Morita M, Aoyama Y, Arita M, Amona H, Yoshizawa H, Hashizume S, Komatsu T, Tagaya I. Comparative studies of several vaccinia virus strains by intrathalamic inoculation into cynomolgus monkeys. Arch Virol. 1977;53:197–208. doi: 10.1007/BF01314664. [DOI] [PubMed] [Google Scholar]
- 23.Mortimer PP. The new cell culture smallpox vaccine should not be offered to the general population. Rev. Med Virol. 2003;13:17–20. doi: 10.1002/rmv.383. [DOI] [PubMed] [Google Scholar]
- 24.Parrino J, McCurdy LH, Larkin BD, Gordon IJ, Rucker SE, Enama ME, Koup RA, Roederer M, Bailer RT, Moodie Z, Gu L, Yan L, Graham BS. Safety, immunogenicity and efficacy of modified vaccinia Ankara (MVA) against Dryvax challenge in vaccinia-naive and vaccinia-immune individuals. Vaccine. 2007;25:1513–1525. doi: 10.1016/j.vaccine.2006.10.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Payne LG. Significance of extracellular enveloped virus in the in vitro and in vivo dissemination of vaccinia. J Gen. Virol. 1980;50:89–100. doi: 10.1099/0022-1317-50-1-89. [DOI] [PubMed] [Google Scholar]
- 26.Rockoff A, Spigland I, Lorenstein B, Rose AL. Postvaccinal encephalomyelitis without cutaneous vaccination reaction. Ann. Neurol. 1979;5:99–101. doi: 10.1002/ana.410050116. [DOI] [PubMed] [Google Scholar]
- 27.Saito T, Fujii T, Kanatani Y, Saijo M, Morikawa S, Yokote H, Takeuchi T, Kuwabara N. Clinical and immunological response to attenuated tissue-cultured smallpox vaccine LC16m8. JAMA. 2009;301:1025–1033. doi: 10.1001/jama.2009.289. [DOI] [PubMed] [Google Scholar]
- 28.Smee DF, Bailey KW, Wong MH, Sidwell RW. Effects of cidofovir on the pathogenesis of a lethal vaccinia virus respiratory infection in mice. Antiviral Res. 2001;52:55–62. doi: 10.1016/s0166-3542(01)00159-0. [DOI] [PubMed] [Google Scholar]
- 29.Smee DF, Hurst BL, Wong MH, Glazer RI, Rahman A, Sidwell RW. Efficacy of N-methanocarbathymidine in treating mice infected intranasally with the IHD and WR strains of vaccinia virus. Antiviral Res. 2007;76:124–129. doi: 10.1016/j.antiviral.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Varkey P, Poland GA, Cockerill FR, III, Smith TF, Hagen PT. Confronting bioterrorism: physicians on the front line. Mayo Clin. Proc. 2002;77:661–672. doi: 10.4065/77.7.661. [DOI] [PubMed] [Google Scholar]
- 31.WHO. Global smallpox vaccine reserve: Report by the Secretariat. 2004;EB115/36:1–3. [Google Scholar]
- 32.WHO. SMALLPOX, BIOTERRORISM AND THE WORLD HEALTH ORGANIZATION. 2006:1–9. [Google Scholar]
- 33.Wiser I, Balicer RD, Cohen D. An update on smallpox vaccine candidates and their role in bioterrorism related vaccination strategies. Vaccine. 2007;25:976–984. doi: 10.1016/j.vaccine.2006.09.046. [DOI] [PubMed] [Google Scholar]