Summary
Research on the actions of ethanol at the GABAergic synapse has traditionally focused on postsynaptic mechanisms, but recent data demonstrate that ethanol also increases both evoked and spontaneous GABA release in many brain regions. Using whole-cell voltage-clamp recordings, we previously showed that ethanol increases spontaneous GABA release at the rat interneuron-Purkinje cell synapse. This presynaptic ethanol effect is dependent on calcium release from internal stores, possibly through activation of inositol 1,4,5-trisphosphate receptors (IP3Rs). After confirming that ethanol targets vesicular GABA release, in the present study we used electron microscopic immunohistochemistry to demonstrate that IP3Rs are located in presynaptic terminals of cerebellar interneurons. Activation of IP3Rs requires binding of IP3, generated through activation of phospholipase C (PLC). We find that the PLC antagonist edelfosine prevents ethanol from increasing spontaneous GABA release. Diacylglycerol generated by PLC and calcium released by activation of the IP3R activate protein kinase C (PKC). Ethanol-enhanced GABA release was blocked by two PKC antagonists, chelerythrine and calphostin C. When a membrane impermeable PKC antagonist, PKC (19-36), was delivered intracellularly to the postsynaptic neuron, ethanol continued to increase spontaneous GABA release. Overall, these results suggest that activation of the PLC/IP3R/PKC pathway is necessary for ethanol to increase spontaneous GABA release from presynaptic terminals onto Purkinje cells.
Keywords: ethanol; γ-aminobutyric acid; miniature inhibitory postsynaptic currents; phospholipase C; protein kinase C; inositol 1,4,5-trisphosphate receptors
Introduction
The cerebellum plays a role in controlling sensory perception, motor coordination, motor learning and reflex adaptation (Baillieux et al., 2008). Acute effects of alcohol involving alterations in balance, speech, and motor coordination suggest disrupted cerebellar function; for this reason, the cerebellum is an excellent model to study the acute effects of alcohol on brain function. The cerebellar cortex has five main types of neurons: Golgi cells, stellate cells, basket cells, granule cells, and Purkinje cells (Palay and Chan-Palay, 1974). Purkinje cells, the sole output of the cerebellar cortex, are large GABAergic neurons oriented in a single row; their somata define the border between the molecular cell layer and the granule cell layer. The Golgi cells (interneurons located in the granule cell layer) and the stellate/basket cells (interneurons located in the molecular layer), release γ-aminobutyric acid (GABA) onto the granule cells and Purkinje cells, respectively.
Within the past few years, experiments in cerebellum have shown that besides a direct interaction with GABAA receptors, ethanol also increases GABA release from presynaptic terminals. Ethanol increases both the amount of GABA released onto cerebellar granule cells from Golgi cells (Carta et al., 2004) and the amount of GABA released onto Purkinje neurons from basket and stellate cells (Kelm et al., 2007, 2008a; Mameli et al., 2008; Ming et al., 2006). We have previously shown that internal calcium stores (Kelm et al., 2007) and the adenylate cyclase/protein kinase A (PKA) pathway (Kelm et al., 2008a) play an essential role in the ethanol-induced increase in GABA release at the interneuron-Purkinje cell synapse.
Calcium can be released from internal stores through activation of ryanodine receptors (RyRs) and IP3Rs. The RyR is activated by calcium alone, whereas the IP3R also requires inositol 1,4,5-trisphosphate (IP3) binding (Berridge et al., 2003). This IP3 is generated by phospholipase C (PLC), which catalyzes the conversion of phosphoinositol 4,5-bisphosphate into diacylglycerol (DAG) and IP3 (Kiselyov et al., 2003). DAG, together with the calcium released through the activated IP3R, can activate conventional PKC isoforms (α, βI, βII, γ) by inducing a conformational change that frees a regulatory pseudosubstrate from the catalytic domain (Boni and Rando, 1985; House and Kemp, 1987). Following exposure of the catalytic domain, PKC becomes phosphorylated and then phosphorylates a number of substrates, including the IP3R itself (Patterson et al., 2004). Phosphorylation of the IP3R can increase the amount of calcium released from internal stores (Bardo et al., 2006), forming a calcium-mediated positive feedback loop between activation of the IP3R and PKC.
There is no evidence that ethanol binds directly to either IP3Rs or RyRs. Suspecting that ethanol instead modulates calcium release from internal stores indirectly, we decided to investigate the role of the PLC/IP3R/PKC pathway in ethanol-enhanced spontaneous GABA release. Portions of the data were presented previously in abstract form (Kelm et al., 2008b).
Methods
Slice preparation for electrophysiology
Sprague-Dawley rats 13-20 days old were anesthetized with a 0.7-0.9 mL intraperitoneal injection of 75% urethane (Sigma, St. Louis, MO) and decapitated after disappearance of the plantar reflex. The brain was rapidly removed and placed in an ice cold 4-(2-hydroxyethyl)piperazine-1-ethanesulfonic acid (HEPES) buffered solution of the following composition (in mM): 145 NaCl, 5 KCl, 10 HEPES, 2 CaCl2, 1 MgCl2, 10 glucose and 5 sucrose (pH to 7.4 with NaOH). The cerebellum was dissected and parasagittal slices, 350 μm thick, were cut with a vibrating microtome (Leica VT1000S, Vashaw Scientific, Norcross, GA) in a low sodium solution of the following composition (in mM): 112.5 sucrose, 63 NaCl, 3 KCl, 1.25 NaH2PO4, 24 NaHCO3, 6 MgSO4, 0.5 CaCl2, 10 glucose, and gassed with 95% O2/5% CO2. The slices were placed in a chamber containing oxygenated artificial cerebrospinal fluid (ACSF) of the following composition (in mM): 124 NaCl, 3.25 KCl, 1.25 KH2PO4, 10 glucose, 2 MgSO4, 20 NaHCO3, 2 CaCl2, and gassed with 95% O2/5% CO2. The slices were equilibrated for at least one hour at room temperature before starting experiments.
Whole-cell voltage-clamp recordings
A slice was placed at the bottom of a chamber attached to the stage of a microscope (BX5OWI, Olympus, Japan), and perfused with oxygenated ACSF (21-24°C) at a flow rate of 0.5 ml/min. The cells were visualized using infrared illumination under differential interference contrast optics with a 40X LUMPlanFl water-immersion objective (Olympus) and displayed on a monitor via a video camera (C2400, Hamamatsu, Japan). Recording electrodes were pulled from borosilicate glass (Drummond Scientific Company, Broomall, PA) and had a resistance of 4-6 MΩ when filled with internal solution. The composition of the internal solution was the following (in mM):150 KCl, 3.1 MgCl2, 15 HEPES, 5 K-ATP, 5 EGTA, and 15 phosphocreatine (osmolarity approximately 310 mOsms). The internal solution pH was adjusted to 7.4 with KOH.
Data were displayed on an oscilloscope (V-212, Hitachi, Japan), filtered and digitized at 5 kHz, and stored on a personal computer. The membrane potential was held at -70 mV using a patch-clamp amplifier (Axopatch 200B, Axon Instruments, Union City, CA), and data were collected with Clampex 8.1 software (Axon Instruments). The capacitance and access resistance were monitored continuously throughout the recordings; a change of 10% or more was sufficient to exclude the recording from analysis. To avoid contamination, only one protocol/recording was conducted per slice.
Drug preparation and drug delivery system
Tetrodotoxin (TTX, Sigma), 6-cyano-7-nitroquinoxaline-2,3,-dione (CNQX, Sigma), chelerythrine chloride (Tocris, Ellisville, MO), edelfosine (Tocris), calphostin C (Tocris) and protein kinase C inhibitor peptide [19-36] (PKC (19-36), Calbiochem, San Diego, CA) were made up as concentrated stock solutions (1000x) in distilled water and stored at -20°C (except for TTX, which was stored at 4°C). Bafilomycin A1 (Enzo Life Sciences, Farmingdale, NY), 1,2-bis(2-aminophenoxy) ethane-N,N, N′,N′-tetraacetic acid tetrakis acetoxymethyl ester (BAPTA-AM, Sigma), phorbol 12-myristate 13-acetate (PMA, Tocris) and 5-chloro-N-(6-phenylhexyl)-1-naphthalenesulfonamide (SC9, Tocris) were made up as concentrated stock solutions (1000x) in dimethyl sulfoxide and stored at -20°C. The final concentration of dimethyl sulfoxide used in the experiments was less than 0.1%, which does not alter properties of the miniature inhibitory postsynaptic currents (Kelm et al., 2007). The concentrated stock solutions were diluted in ACSF and inserted into sealed syringes on the day of use, except for PKC (19-36), which was added to the pipette internal solution on the day of use. TTX (1 μM) and CNQX (10 μM) were added to all solutions inserted into the sealed glass syringes. Slices were preincubated for a minimum of 30 minutes with edelfosine and BAPTA, two hours with calphostin C, and three hours with bafilomycin A1 before starting the recording. Calphostin C was exposed to cool-white fluorescent lighting during the pre-incubation period and experiment, as its ability to inhibit PKC is light-dependent (Bruns et al., 1991). Each sealed syringe was attached to Teflon tubing connected to a multi-barrel perfusion pencil (Automate Scientific, Inc.; Sarasota, FL; 250 μm tip diameter), which was positioned 150-250 μm from the cell being tested.
Protocol and analysis for mIPSC experiments
After the membrane of the cell was ruptured, the control solution was delivered through the multi-barrel perfusion pencil. Addition of 50 μM bicuculline methochloride, a GABAA antagonist, abolishes the mIPSCs (Kelm et al., 2007), confirming their GABAergic nature. Before starting an experiment, at least two repetitive mIPSC traces were recorded for 30 to 60 seconds to determine if there was a stable baseline mIPSC frequency. Once a stable baseline was obtained, a control recording was collected for at least 60 seconds. After delivering ethanol through the multi-barrel perfusion pencil, the ethanol recording was collected five minutes later; a washout recording was also collected.
Before the ability of the antagonist to decrease the ethanol-induced increase in mIPSC frequency was tested, a stable baseline mIPSC rate was established in the presence of the antagonist to avoid a summation of effects during the antagonist/ethanol recording. Thus, the baseline mIPSC frequency value in the presence of the antagonist served as the control value, and the antagonist was present throughout the remainder of the recording. For the majority of experiments, the antagonist inhibited the 100 mM ethanol effect on mIPSC frequency, so this was the only ethanol concentration tested. However, 100 mM ethanol still had an effect in the presence of an antagonist in Figure 4C, so an additional experiment was carried out with 50 mM ethanol- the lowest concentration that significantly increases mIPSC frequency at this synapse (Kelm et al., 2007, 2008a).
Figure 4.
Inhibition of PKC prevents ethanol from increasing mIPSC frequency. A: a trace from a representative neuron showing the effect of 100 mM ethanol on mIPSC frequency when 20 μM PKC (19-36) was included in the pipette internal solution (PKCint). B: chelerythrine (1 μM), calphostin C (1 μM), and SC9 (25 μM) blocked 100 mM ethanol from increasing mIPSC frequency compared to control (*, p<.05, one-way ANOVA, Dunnett's post hoc test). C: ethanol (50 and 100 mM) increased mIPSC frequency in the presence of 20 μM PKCint, (*, p<.05, one-way ANOVA, Dunnett's post hoc test).
The percent (%) change in mIPSC frequency, decay time and amplitude was calculated for each recording as 100 × (“ethanol response”/((“control”+“washout”)/2))-100. Washout was included in the calculation because there was no significant difference between control and washout values for any of the groups (data not shown). For the % change in mIPSC frequency calculation for the PKC agonists vs control experiments, the values were calculated as follows: 100 × (|“drug response” − “control”|)/”control”). All data were expressed as the mean ± SEM. Using miniAnalysis software, the mIPSC decay time was determined from a bi-exponential fit, and fast (τ-fast) and slow decay times (τ-slow) were analyzed separately.
Tissue preparation for electron microscopy
Male Sprague-Dawley rats were anesthetized with sodium pentobarbital (60 mg/kg, intraperitoneal). After inducing deep anesthesia, rats were intracardially perfused with heparinized saline followed by 500 ml of fixative, a mixture of 2% paraformaldehyde and 2% glutaraldehyde in phosphate buffer (0.1 M, pH 7.4). The brains were removed and postfixed with the same fixative for 2 hours at 4°C. Brains were sectioned with a Vibratome (40-60 μM) and collected in cold phosphate buffer.
Tissue processing for electron microscopy
The basic approach has been described previously (Phend et al., 1992; Racz and Weinberg, 2006). Cerebellar areas of interest were excised, cryoprotected in 30% glycerol, quickly frozen, freeze-substituted in a Leica Automatic Freeze Substitution system, and embedded in Lowicryl HM-20. Sections were cut at ∼90 nm with an ultramicrotome and collected on nickel grids. The sections were incubated with normal rabbit serum for 20 minutes to suppress nonspecific binding and then incubated overnight on a shaker at room temperature with an affinity-purified IP3R primary antibody (goat, 1:30000), kindly provided by A.H. Sharp. This antibody was characterized previously, showing a single band migrating at a rate corresponding to 260 kDa in a Western blot of rat cerebellar homogenates. The regional distribution of IP3R throughout the brain as probed by this antibody corresponds well with data from other antibodies and from in situ hybridization studies (Sharp et al., 1993a). For the experiments that involved both GABA and IP3R primary antibody, the GABA antibody (guinea pig, Abcam, Cambridge, MA) was diluted to 1:30000, and the IP3R (goat) antibody was diluted 1:50000. Following overnight incubation, the grids were rinsed and incubated for 20 minutes in normal rabbit serum, then with rabbit anti-goat immunoglobulin G conjugated to 10 nm gold (1:15, Ted Pella, Redding, CA) for two hours at room temperature. After rinsing, sections were counterstained with 1% uranyl acetate followed by Sato's lead and examined with a Philips Tecnai electron microscope (Hillsboro, OR) at 80 kV. Images were collected with a Gatan 12-bit 1024 × 1024 CCD camera (Pleasanton, CA).
Quantitative analysis for electron microscopy
Attention was restricted to Purkinje cell and molecular layers of the cerebellar cortex. Once a suitable area was found, images of the field were acquired at ×6500 magnification. For analysis, profiles representing GABAergic presynaptic terminals, glutamatergic presynaptic terminals, parallel fibers, and Purkinje cell dendrites were identified. For each identified profile, gold particles coding for IP3R were counted; profiles that could not be clearly identified were ignored. Profile areas were measured using Image J software, and the number of particles per square μm was computed; data were expressed as means ± SEM. We found that images of EM fields containing plastic resin without tissue express 0.1 ± 0.1 particles per square μm2 (n = 4), providing a lower bound for nonspecific label. To account for this nonspecific label, 0.1 has been subtracted from values in the Results section that are expressed in particles per square μm2. Gold particles associated with mitochondria or the plasma membrane were ignored as putative nonspecific background. Parallel fibers were analyzed as bundles instead of individual fibers. However, it is hard to control for nonspecific staining in the plasma membranes, so the plasma membrane area was excluded from analysis. To subtract the area of the plasma membranes from the parallel fiber bundle, the number of individual fibers within a bundle was determined, and this number was multiplied by the average area of the plasma membrane surrounding an individual fiber for that bundle. This value–the total estimated plasma membrane area for the bundle–was subtracted from the total area of the parallel fiber bundle to determine the area of the individual parallel fibers without their plasma membranes.
In experiments that did not include a GABA antibody, visual assessments were used to differentiate between GABAergic terminals and glutamatergic terminals. Glutamatergic synapses are typically asymmetric with a thick postsynaptic density, whereas GABAergic synapses are symmetric with a less pronounced postsynaptic density, and contain a mixture of flattened and spherical vesicles, in contrast to glutamatergic terminals (Palay and Chan-Palay, 1974). In the region examined, GABA presynaptic terminals originate predominantly from basket cells and stellate cells. Golgi cells are also GABAergic, but terminate in the granule cell layer. The glutamatergic presynaptic terminals were predominantly from parallel fibers, though a few may have been from climbing fibers.
Statistics
One-way analysis of variance (ANOVA), repeated measures ANOVA, Dunnett's post hoc test, t-test, and regression analysis were performed as indicated. A two-tailed p value less than 0.05 was accepted as statistically significant.
Results
Ethanol increases vesicular release of GABA
Whole-cell recordings from Purkinje cells in cerebellar slices with TTX and CNQX in the bath were used to determine the effect of ethanol on mIPSC properties. Ethanol (100 mM) increased mIPSC frequency (3.6 ± 0.3 Hz, n = 22) compared to control (2.9 ± 0.3 Hz) and washout (2.8 ± 0.3 Hz, Fig. 1B); there was no significant difference between the control and washout values. The ethanol-induced increase in mIPSC frequency was not correlated with baseline mIPSC frequency [R2 = 0.065; F(1,20) = 1.4; p = 0.25], suggesting that ethanol increases mIPSC frequency to the same extent, regardless of the basal level of GABA release.
Figure 1.
Ethanol increases vesicular release of GABA. A: a trace from a representative neuron illustrating the effect of 100 mM ethanol on mIPSC frequency. B: ethanol significantly increased mIPSC frequency compared to control and washout [*, p<.05; repeated measures ANOVA; F(2,42) = 18.3]. The mIPSC frequency values of the control and washout groups were not significantly different (p = 0.6). C: incubating the slice with bafilomycin A1 (baf A1) greatly reduced mIPSC frequency. In the presence of bafilomycin A1, 100 mM ethanol did not significantly increase mIPSC frequency compared to control (p = 2.6). D: the data shown in B, represented as a % change (see methods for explanation). 100 mM ethanol significantly increased mIPSC frequency (see B) in contrast to weak and non-significant effects on mIPSC fast decay time (τ-fast), slow decay time (τ-slow) and amplitude.
To determine whether the ethanol-induced increase in mIPSC frequency was due to increased vesicular release of GABA, we used a vacuolar proton pump inhibitor, bafilomycin A1 (Bowman et al., 1988), which eliminates the pH and electrical gradients necessary for GABA transporters to fill the vesicles (Maycox et al., 1990). In the presence of 2 μM bafilomycin A1, mIPSC frequency declined more than a hundred-fold in both control (0.022 ± 0.008 Hz, Fig. 1C) and ethanol conditions (0.022 ± 0.01 Hz, n = 6), and ethanol did not significantly increase mIPSC frequency compared to control. This suggests that ethanol's action to increase mIPSC frequency selectively targets the GABA vesicles that were functionally eliminated by bafilomycin A1. Overall, these results support that the ethanol-induced increase in mIPSC frequency should be interpreted as an increase in spontaneous GABA release from the presynaptic terminal.
From the data represented in Figure 1A, we calculated the % change in mIPSC frequency induced by ethanol (see methods for details). While ethanol significantly increased mIPSC frequency (30.8 ± 6.0%, n = 22, Fig. 1D), there was no significant effect of 100 mM ethanol on mIPSC fast decay time (5.6 ± 2.6%), slow decay time (0.9 ± 3.4%) or amplitude (4.0 ± 2.1%). We conclude that ethanol increases spontaneous GABA release from vesicles in the presynaptic terminal at the interneuron-Purkinje cell synapse, while having little or no effect postsynaptically.
Presynaptic terminals contain IP3R
While our electrophysiological data suggest that calcium release from IP3Rs plays a direct role in ethanol-enhanced spontaneous GABA release (Kelm et al., 2007), a potential nonspecific effect with the IP3R antagonist on calcium signaling could not be ruled out (Missiaen et al., 2001). Therefore, we used double-label immunogold electron microscopy to determine whether IP3Rs are expressed in the presynaptic terminals of GABAergic interneurons. IP3R and GABA immunogold labeling was colocalized in 18 out of 30 (60%) presynaptic GABAergic terminals. However, double labeling often leads to increased background noise. To control for this, we repeated the experiment in the absence of the IP3R primary antibody. In this case, GABA immunogold labeling colocalized with the secondary gold corresponding to IP3R in only 4 out of 30 (13%) presynaptic GABAergic terminals. On this basis, we tentatively conclude that at least 45% of presynaptic GABAergic terminals in the molecular layer contain IP3R protein (60-13%=47%). While not every GABAergic terminal was immunopositive for IP3R, it is unclear whether this variability is biological or reflects technical limitations because of the stochastic nature of immunogold labeling.
The technical complexities of immunogold double labeling may compromise the sensitivity and specificity of the procedure; therefore, we used sections single-labeled for IP3R to quantify the amount of IP3R label in morphologically-identified GABA terminals, finding that 29 out of 35 (85%) terminals contained at least one gold particle coding for IP3R. The higher value of this estimate than the 47% cited above may arise from the technical difficulties associated with the double label procedure, though we cannot exclude that a fraction of the most weakly-labeled terminals might reflect background. To provide further evidence for the authenticity of the IP3R signal, we systematically quantified the amount of IP3R label in morphologically-identified GABA terminals, glutamate terminals, parallel fibers and Purkinje cell dendrites. By far the densest label for IP3R was found in Purkinje cell dendrites (49.4 ± 6.4 particles/μm2, n = 47, Fig. 2C). GABA terminals (10.8 ± 2 particles/μm2, n = 35), glutamate terminals (9.7 ± 1.8 particles/μm2, n = 39), and parallel fibers (4.4 ± 1 particles/μm2, n = 29, Fig. 2C) exhibited markedly weaker labeling for IP3R. IP3R expression was significantly greater in presynaptic terminals than in parallel fibers (a conservative reference for possible background staining). We conclude that the observed staining in presynaptic terminals is unlikely to reflect nonspecific background. Overall, these results suggest that IP3R is expressed in a large fraction of the presynaptic terminals of cerebellar interneurons.
Figure 2.
GABAergic presynaptic terminals are immunopositive for IP3R. A: micrograph shows a GABAergic presynaptic terminal immunopositive for IP3R making a symmetric synaptic contact with the dendritic shaft of a Purkinje cell. Immediately above the terminal is a bundle of parallel fibers. B: micrograph shows two glutamatergic presynaptic terminals making asymmetric synaptic contacts with Purkinje cell spines (arrows). Note IP3R labeling associated with endomembranes within the spines. The top terminal is immunopositive for the IP3R. A bundle of parallel fibers is visible in the top right corner. C: presynaptic terminals likely, for their morphology, to be GABAergic, probable glutamatergic presynaptic terminals, and Purkinje cell spines had more IP3R staining than parallel fibers (*, p<.05, t test).
Inhibition of PLC prevents ethanol from increasing mIPSC frequency
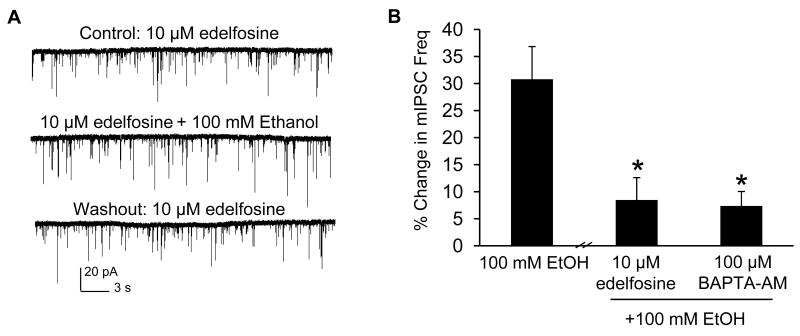
To provide further support for previously-published electrophysiological data suggesting that calcium release mediated by IP3Rs plays a role in ethanol-enhanced spontaneous GABA release (Kelm et al., 2007), we asked whether intracellular mediators upstream of IP3R activation are involved in this ethanol mechanism. Since IP3 is generated by the action of PLC, we tested the effect of the phosphoinositol-specific PLC antagonist edelfosine (10 μM), finding that the ability of ethanol to increase mIPSC frequency was blocked (8.4 ± 4.1%, n = 9, Fig. 3B) compared to control. Because activation of the IP3R also requires calcium, we tested the effect of calcium chelation, finding that the ability of ethanol to increase mIPSC frequency was also prevented in the presence of 100 μM BAPTA-AM (7.4 ± 2.7%, n = 8, Fig. 3B), a membrane-permeable calcium chelator, compared to control. These results indicate that both PLC and calcium are involved in ethanol-enhanced spontaneous GABA release.
Figure 3.
Inhibition of PLC blocks ethanol from increasing mIPSC frequency. A: a trace from a representative neuron demonstrating the effect of 100 mM ethanol on mIPSC frequency in the presence of edelfosine (10 μM). B: Edelfosine (10 μM) and BAPTA-AM (100 μM) prevented 100 mM ethanol from increasing mIPSC frequency compared to control (*, p<.05, one-way ANOVA, Dunnett's post hoc test).
PKC activation is necessary for ethanol to increase mIPSC frequency
IP3R activation increases calcium release from internal stores, and the released calcium acts synergistically with DAG to activate protein kinase C (PKC). To determine whether PKC might be involved in ethanol-enhanced GABA release, we tested two general PKC antagonists—chelerythrine, which acts at the PKC ATP binding site (Herbert et al., 1990), and calphostin C, which acts at the C1 domain (Rotenberg et al., 1995; Tamaoki, 1991)—finding that both antagonists prevented ethanol from increasing mIPSC frequency (chelerythrine (1 μM): 7.2 ± 4.8%, n = 8; calphostin C (1 μM): 11.1 ± 4.7%, n = 14, Fig. 4B). To determine whether the PKC antagonists were acting specifically at the presynaptic terminal, a membrane impermeable PKC antagonist, PKC (19-36), was included in the pipette internal solution, thus limiting exposure of the PKC antagonist to the postsynaptic neuron. With 20 μM PKC (19-36) in the pipette internal solution, both 50 mM (18.9 ± 4.4%, n = 6) and 100 mM (26.3 ± 3.4%, n = 6) ethanol significantly increased mIPSC frequency compared to control (0.7 ± 2.0%, n = 6, Fig. 4C). There was an increase in mIPSC amplitude (22.8 ± 1.8 pA) when recording from neurons with 20 μM PKC (19-36) in the pipette internal solution compared to neurons recorded in control conditions (18.5 ± 1.3 pA, p<.05, t test). These results are consistent with the inhibitory effect PKC has on GABAA receptor function (Kumar et al., 2005) and suggest that PKC (19-36) was reaching the postsynaptic neuron.
We also determined the effect of two PKC agonists on baseline spontaneous GABA release, finding that both PMA (which acts at the C1 domain) and SC9 (which acts at the C2 domain) significantly increased spontaneous GABA release by 72.5 ± 19.7% (n = 7) and 17.7 ± 5.5% (n = 8), respectively (p<. 05, t test). Additionally, SC9 prevented 100 mM ethanol from increasing spontaneous GABA release (0.8 ± 3.8%, n = 7, Fig. 4B). Overall, we conclude that the mechanism of ethanol-enhanced GABA release involves presynaptic activation of PKC.
Discussion
Elucidating the mechanism of ethanol-enhanced GABA release will deepen our understanding of how GABAergic neurotransmission contributes to the intoxicating effects of alcohol. Because people who are less sensitive to alcohol intoxication are at increased risk of alcoholism (Schuckit, 2009), understanding the molecular mechanisms that underlie alcohol intoxication is directly relevant to our understanding of alcoholism. The lowest ethanol concentration at which we detected a significant effect on spontaneous GABA release is 50 mM (Kelm et al., 2007); an intraperitoneal injection of an equivalent alcohol dose (∼2 g/kg) induces sedation and ataxia in rats (Chuck et al., 2006). Therefore, the effect of ethanol on GABA release would only be relevant to behaviors seen at higher blood alcohol concentrations.
After confirming that ethanol increases mIPSC frequency at the cerebellar interneuron-Purkinje cell synapse (Kelm et al., 2007, 2008a; Ming et al., 2006), we performed experiments to establish the source of this increase in mIPSC frequency. We have previously shown that ethanol increases mIPSC frequency at the interneuron-Purkinje cell synapse in the mechanically dissociated neuron preparation (Kelm et al. 2007), where communication from nearby neurons and glia is eliminated. To further characterize this ability of ethanol to increase GABA release, we used bafilomycin A1, which blocks vesicles from filling with GABA (Bowman et al., 1988; Maycox et al,. 1990). In the presence of bafilomycin A1, mIPSC frequency was vastly reduced both in control and ethanol conditions, demonstrating that the ethanol-induced increase in mIPSC frequency in the absence of bafilomycin A1 reflects ethanol-enhancement of spontaneous vesicular GABA release from the presynaptic terminal. Our data suggest that ethanol has little or no postsynaptic effect at this synapse; however, a postsynaptic effect cannot be ruled out completely because the intracellular milieu of the postsynaptic neuron is altered during whole-cell voltage-clamp recordings (Sarantopoulos et al. 2004).
We showed previously that release of calcium from internal stores plays an important role in ethanol-enhanced spontaneous GABA release (Kelm et al., 2007), but whether both IP3Rs and RyRs are involved remained unclear. An elegant study provided evidence from electrophysiology, calcium imaging and immunohistochemistry that RyRs play a functional role in the generation of spontaneous GABA release at the interneuron-Purkinje cell synapse (Llano et al., 2000). Other studies have confirmed a role for RyRs in spontaneous neurotransmitter release and have defined a role for RyRs in evoked transmitter release and long-term synaptic plasticity (Collin et al., 2005).
Unlike the RyRs, there is little evidence that IP3Rs are expressed in presynaptic terminals. Summarizing available data, inhibition of IP3Rs in rat barrel cortex reduces spontaneous glutamate release, suggesting that IP3Rs are present in the presynaptic terminals (Simkus and Stricker, 2002). However, these experiments could not exclude potential nonspecific effects seen on calcium signaling with 2-aminoethoxydiphenylborate, the IP3R antagonist used (Missiaen et al., 2001). In retinal amacrine cells, which release transmitter from dendrites, immunohistochemistry, electrophysiology and calcium imaging studies found that only IP3Rs contribute to transmitter release, despite the presence of both IP3Rs and RyRs in the dendrites (Warrier et al., 2005). Electron microscopic studies in the bed nucleus of the stria terminalis, deep cerebellar nuclei, and substantia nigra showed IP3R staining in synaptic terminals, but no quantification was performed to further characterize this observation (Sharp et al., 1993b; Sharp et al., 1999). Light microscopic immunohistochemistry found IP3R staining in the molecular layer of the cerebellum described as fine ‘granular staining’, suggesting that IP3Rs are expressed in the neurophil (Sharp et al., 1999); however, this finding is difficult to interpret because of the extraordinarily high levels of IP3R in dendritic spines of Purkinje cells.
In the present work, IP3R staining was found in the presynaptic terminals of cerebellar interneurons using postembedding immunogold electron microscopy. The cerebellar Purkinje cells exhibited much higher levels of IP3R labeling than presynaptic terminals, consistent with previous evidence that IP3R expression in Purkinje cells is the highest in the entire brain (Worley et al., 1989). Because IP3R staining is found in all three layers (molecular, Purkinje, and granule cell layers) of the cerebellum (Sharp et al. 1999), no entirely satisfactory negative control was available. Using staining in parallel fibers as an upper bound of nonspecific staining, we found that IP3R expression was significantly higher in both GABAergic terminals and glutamatergic terminals than in parallel fibers. It is unknown whether parallel fibers express IP3R; moreover, we cannot exclude that an unknown protein in parallel fibers cross-reacts with our IP3R antibody. Nevertheless, we consider this result strong evidence that IP3R is indeed present in these terminals because the level of IP3R label in the terminals is significantly higher than the level of label in the parallel fibers. Therefore, the preponderance of evidence argues that the IP3R staining in the GABAergic presynaptic terminals reflected authentic IP3R protein. Considering published evidence for RYRs, we suggest that both receptor classes may play a role in ethanol-enhanced spontaneous GABA release from the terminals of cerebellar interneurons.
Our evidence that IP3Rs are expressed in both GABAergic and glutamatergic presynaptic terminals seems inconsistent with ethanol's lack of effect on glutamate release onto Purkinje cells (Belmeguenai et al., 2008; Carta et al., 2006; Mameli et al., 2008). Interestingly, a cannabinoid receptor agonist decreases spontaneous GABA release onto cerebellar Purkinje cells, but has no effect on spontaneous glutamate release unless extracellular calcium concentrations are increased (Yamasaki et al., 2006). The authors conclude that cannabinoids selectively affect “calcium-enhanced” neurotransmitter release onto Purkinje cells, and this calcium-enhanced neurotransmitter release only occurs at GABAergic presynaptic terminals at normal extracellular calcium levels. We speculate that a similar mechanism might regulate ethanol-enhanced GABA release at the interneuron-Purkinje cell synapse, which would explain the lack of ethanol effect on glutamate release.
The present study also investigated the role of the PLC/PKC pathway in ethanol-enhanced spontaneous GABA release. The phosphoinositol-specific PLC antagonist edelfosine blocked ethanol enhancement of spontaneous GABA release, suggesting that PLC contributes to the ability of ethanol to increase GABA release. These data must be treated cautiously, since edelfosine can have varying effects on intracellular calcium levels based on the cell type, proliferation state, and the edelfosine concentration used (Bergmann et al., 1994). However, one would expect to see a change in spontaneous GABA release if there was a change in intracellular calcium after exposure to edelfosine, whereas a between-cell comparison found no effect of edelfosine on spontaneous GABA release compared to neurons exposed to control conditions (data not shown). Thus, it is unlikely that edelfosine is affecting calcium levels in our system.
Calcium is required for IP3R activation and is released from the IP3R following activation, leading us to test the effects of the calcium chelator BAPTA-AM. Consistent with our hypothesis, we found that BAPTA-AM prevented the ethanol enhancement of spontaneous GABA release. Together with our previous work showing that ethanol increases spontaneous GABA release when BAPTA is included in the internal solution (Kelm et al., 2007), these data imply that ethanol acts via a calcium-dependent presynaptic mechanism to increase spontaneous GABA release.
We further found that two general PKC antagonists with different mechanisms of action inhibited ethanol-enhanced GABA release, suggesting that PKC plays a role in ethanol-enhanced GABA release. Limiting exposure of a membrane impermeable PKC antagonist to the postsynaptic neuron did not block the ethanol effect on GABA release but did decrease baseline mIPSC amplitude compared to when neurons are exposed to control conditions. These data demonstrate that the PKC antagonist is reaching the postsynaptic neuron, and a postsynaptic, PKC-dependent mechanism is not involved in the ability of ethanol to increase GABA release. Overall, these results suggest that the PKC antagonists were acting through a presynaptic mechanism to block ethanol-enhanced spontaneous GABA release.
Two PKC agonists increased baseline spontaneous GABA release, which is consistent with the ability of ethanol to increase GABA release involving activation of the PLC/IP3R/PKC pathway. The PKC domains where these agonists act are conserved in other proteins (Lou et al., 2008; Rizo and Sudhof, 1998), raising the possibility that their effect on spontaneous GABA release is independent of PKC. However, because these two unrelated agonists that act at different domains both modified baseline spontaneous GABA release, we consider it likely that this was a PKC-dependent effect. Additionally, we found that a PKC agonist blocked ethanol-enhanced GABA release. One explanation for these results is the PKC agonist elicited a level of PKC activation that resulted in a “ceiling effect”, which prevented ethanol from further increasing the activation of PKC, and the ability of ethanol to increase GABA release requires an increase in PKC activation. It remains unclear whether ethanol affects PKC activation in other model systems (Gordon et al., 2001; Stubbs and Slater, 1999), but the ability of ethanol to increase GABA release onto neurons in the ventral tegmental area is dependent on activation of the 5-hydroxytryptamine 2C G protein-coupled receptor, which activates the PLC/IP3R/PKC pathway (Theile et al., 2009).
It remains unclear how ethanol activates the PLC/IP3R/PKC pathway at the interneuron-Purkinje cell synapse. In addition to directly affecting PLC, ethanol could be activating a Gαq-linked GPCR, but we are unaware of any Gαq-linked GPCRs that are located on the presynaptic terminals of the cerebellar interneurons. Future research should examine whether such a receptor exists. We have shown previously that activation of the adenylate cyclase/protein kinase A (PKA) pathway is also necessary for ethanol to increase GABA release (Kelm et. al., 2008), so the PLC/PKC pathway could be activated through cross-talk with the adenylate cyclase/PKA pathway, as certain ethanol mechanisms involve cross-talk between the two pathways (Tabakoff et al., 2001; Yao et al., 2008).
In summary, we have shown that ethanol increases GABA release at the interneuron-Purkinje cell synapse, and that this ethanol effect is dependent upon activation of the PLC/IP3R/PKC pathway. With electron microscopy we determined that IP3Rs are expressed in the presynaptic terminals of cerebellar interneurons, consistent with our previous data finding that calcium release from internal stores is necessary for ethanol to increase spontaneous GABA release (Kelm et al., 2007). A PLC antagonist, a calcium chelator, PKC antagonists, and a PKC agonist blocked the ethanol-induced increase in spontaneous GABA release, and the effect of the PKC antagonists was presynaptic. In view of previous evidence that the adenylate cyclase/PKA pathway is also involved in this ethanol mechanism (Kelm et al., 2008), we conclude that activation of both the PLC/PKC pathway and the adenylate cyclase/PKA pathway is necessary for ethanol to increase spontaneous GABA release at the interneuron-Purkinje cell synapse.
Acknowledgments
The authors thank A.H. Sharp for providing the IP3R antibody and K.D. Phend for histological assistance. This work was supported by a National Research Service Award Predoctoral Fellowship AA 17025; the National Institute on Alcohol Abuse and Alcoholism Grants AA 11605, AA 14284, and AA 14949; the National Institute of Neurological Disorders and Stroke Grant NS 35527; and the Bowles Center for Alcohol Studies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
M. Katherine Kelm, Department of Pharmacology, School of Medicine, University of North Carolina, Chapel Hill, North Carolina; Bowles Center for Alcohol Studies, School of Medicine, University of North Carolina, Chapel Hill, North Carolina.
Richard J. Weinberg, Department of Cell & Developmental Biology, School of Medicine, University of North Carolina, Chapel Hill, North Carolina; Neuroscience Center; School of Medicine, University of North Carolina, Chapel Hill, North Carolina
Hugh E. Criswell, Department of Psychiatry, School of Medicine, University of North Carolina, Chapel Hill, North Carolina; Bowles Center for Alcohol Studies, School of Medicine, University of North Carolina, Chapel Hill, North Carolina
George R. Breese, Department of Pharmacology School of Medicine, University of North Carolina, Chapel Hill, North Carolina; Department of Psychiatry, School of Medicine, University of North Carolina, Chapel Hill, North Carolina; Neuroscience Center; School of Medicine, University of North Carolina, Chapel Hill, North Carolina; Bowles Center for Alcohol Studies, School of Medicine, University of North Carolina, Chapel Hill, North Carolina
References
- Baillieux H, De Smet HJ, Paquier PF, De Deyn PP, Marien P. Cerebellar neurocognition: insights into the bottom of the brain. Clin Neurol Neurosurg. 2008;110:763–773. doi: 10.1016/j.clineuro.2008.05.013. [DOI] [PubMed] [Google Scholar]
- Bardo S, Cavazzini MG, Emptage N. The role of the endoplasmic reticulum Ca2+ store in the plasticity of central neurons. Trends Pharmacol Sci. 2006;27:78–84. doi: 10.1016/j.tips.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Belmeguenai A, Botta P, Weber JT, Carta M, De Ruiter M, De Zeeuw CI, Valenzuela CF, Hansel C. Alcohol impairs long-term depression at the cerebellar parallel fiber-Purkinje cell synapse. J Neurophysiol. 2008;100:3167–3174. doi: 10.1152/jn.90384.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann J, Junghahn I, Brachwitz H, Langen P. Multiple effects of antitumor alkyl-lysophospholipid analogs on the cytosolic free Ca2+ concentration in a normal and a breast cancer cell line. Anticancer Res. 1994;14:1549–1556. [PubMed] [Google Scholar]
- Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- Boni LT, Rando RR. The nature of protein kinase C activation by physically defined phospholipid vesicles and diacylglycerols. J Biol Chem. 1985;260:10819–10825. [PubMed] [Google Scholar]
- Bowman EJ, Siebers A, Altendorf K. Bafilomycins: a class of inhibitors of membrane ATPases from microorganisms, animal cells, and plant cells. Proc Natl Acad Sci U S A. 1988;85:7972–7976. doi: 10.1073/pnas.85.21.7972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruns RF, Miller FD, Merriman RL, Howbert JJ, Heath WF, Kobayashi E, Takahashi I, Tamaoki T, Nakano H. Inhibition of protein kinase C by calphostin C is light-dependent. Biochem Biophys Res Commun. 1991;176:288–293. doi: 10.1016/0006-291x(91)90922-t. [DOI] [PubMed] [Google Scholar]
- Carta M, Mameli M, Valenzuela CF. Alcohol enhances GABAergic transmission to cerebellar granule cells via an increase in Golgi cell excitability. J Neurosci. 2004;24:3746–3751. doi: 10.1523/JNEUROSCI.0067-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carta M, Mameli M, Valenzuela CF. Alcohol potently modulates climbing fiber-->Purkinje neuron synapses: role of metabotropic glutamate receptors. J Neurosci. 2006;26:1906–1912. doi: 10.1523/JNEUROSCI.4430-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuck TL, McLaughlin PJ, Arizzi-LaFrance MN, Salamone JD, Correa M. Comparison between multiple behavioral effects of peripheral ethanol administration in rats: sedation, ataxia, and bradykinesia. Life Sci. 2006;79:154–161. doi: 10.1016/j.lfs.2005.12.045. [DOI] [PubMed] [Google Scholar]
- Collin T, Marty A, Llano I. Presynaptic calcium stores and synaptic transmission. Curr Opin Neurobiol. 2005;15:275–281. doi: 10.1016/j.conb.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Gordon AS, Yao L, Jiang Z, Fishburn CS, Fuchs S, Diamond I. Ethanol acts synergistically with a D2 dopamine agonist to cause translocation of protein kinase C. Mol Pharmacol. 2001;59:153–160. doi: 10.1124/mol.59.1.153. [DOI] [PubMed] [Google Scholar]
- Herbert JM, Augereau JM, Gleye J, Maffrand JP. Chelerythrine is a potent and specific inhibitor of protein kinase C. Biochem Biophys Res Commun. 1990;172:993–999. doi: 10.1016/0006-291x(90)91544-3. [DOI] [PubMed] [Google Scholar]
- House C, Kemp BE. Protein kinase C contains a pseudosubstrate prototope in its regulatory domain. Science. 1987;238:1726–1728. doi: 10.1126/science.3686012. [DOI] [PubMed] [Google Scholar]
- Kelm MK, Criswell HE, Breese GR. Calcium release from presynaptic internal stores is required for ethanol to increase spontaneous gamma-aminobutyric acid release onto cerebellum Purkinje neurons. J Pharmacol Exp Ther. 2007;323:356–364. doi: 10.1124/jpet.107.126144. [DOI] [PubMed] [Google Scholar]
- Kelm MK, Criswell HE, Breese GR. The Role of Protein Kinase A in the Ethanol-Induced Increase in Spontaneous GABA Release Onto Cerebellar Purkinje Neurons. J Neurophysiol. 2008a;100:3417–3428. doi: 10.1152/jn.90970.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelm MK, Criswell HE, Weinberg RJ, Breese GR. The Role of Phospholipase C, Protein Kinase C and Inositol 1,4,5-trisphosphate Receptors in Ethanol-enhanced Spontaneous GABA Release. Society for Neuroscience Annual Meeting; Washington D.C.: 2008b. [Google Scholar]
- Kiselyov K, Shin DM, Muallem S. Signalling specificity in GPCR-dependent Ca2+ signalling. Cell Signal. 2003;15:243–253. doi: 10.1016/s0898-6568(02)00074-8. [DOI] [PubMed] [Google Scholar]
- Kumar S, Khisti RT, Morrow AL. Regulation of native GABAA receptors by PKC and protein phosphatase activity. Psychopharm. 2005;183:241–247. doi: 10.1007/s00213-005-0161-x. [DOI] [PubMed] [Google Scholar]
- Llano I, Gonzalez J, Caputo C, Lai FA, Blayney LM, Tan YP, Marty A. Presynaptic calcium stores underlie large-amplitude miniature IPSCs and spontaneous calcium transients. Nat Neurosci. 2000;3:1256–1265. doi: 10.1038/81781. [DOI] [PubMed] [Google Scholar]
- Lou X, Korogod N, Brose N, Schneggenburger R. Phorbol esters modulate spontaneous and Ca2+-evoked transmitter release via acting on both Munc13 and protein kinase C. J Neurosci. 2008;28:8257–8267. doi: 10.1523/JNEUROSCI.0550-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mameli M, Botta P, Zamudio PA, Zucca S, Valenzuela CF. Ethanol decreases Purkinje neuron excitability by increasing GABA release in rat cerebellar slices. J Pharmacol Exp Ther. 2008;327:910–917. doi: 10.1124/jpet.108.144865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maycox PR, Hell JW, Jahn R. Amino acid neurotransmission: spotlight on synaptic vesicles. Trends Neurosci. 1990;13:83–87. doi: 10.1016/0166-2236(90)90178-d. [DOI] [PubMed] [Google Scholar]
- Ming Z, Criswell HE, Yu G, Breese GR. Competing presynaptic and postsynaptic effects of ethanol on cerebellar purkinje neurons. Alcohol Clin Exp Res. 2006;30:1400–1407. doi: 10.1111/j.1530-0277.2006.00167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missiaen L, Callewaert G, De Smedt H, Parys JB. 2-Aminoethoxydiphenyl borate affects the inositol 1,4,5-trisphosphate receptor, the intracellular Ca2+ pump and the non-specific Ca2+ leak from the non-mitochondrial Ca2+ stores in permeabilized A7r5 cells. Cell Calcium. 2001;29:111–116. doi: 10.1054/ceca.2000.0163. [DOI] [PubMed] [Google Scholar]
- Palay SL, Chan-Palay V. Cerebellar Cortex:Cytology and Organization. Springer-Verlag; New York: 1974. [Google Scholar]
- Patterson RL, Boehning D, Snyder SH. Inositol 1,4,5-trisphosphate receptors as signal integrators. Annu Rev Biochem. 2004;73:437–465. doi: 10.1146/annurev.biochem.73.071403.161303. [DOI] [PubMed] [Google Scholar]
- Phend KD, Weinberg RJ, Rustioni A. Techniques to optimize post-embedding single and double staining for amino acid neurotransmitters. J Histochem Cytochem. 1992;40:1011–1020. doi: 10.1177/40.7.1376741. [DOI] [PubMed] [Google Scholar]
- Racz B, Weinberg RJ. Spatial organization of cofilin in dendritic spines. Neuroscience. 2006;138:447–456. doi: 10.1016/j.neuroscience.2005.11.025. [DOI] [PubMed] [Google Scholar]
- Rizo J, Sudhof TC. C2-domains, structure and function of a universal Ca2+-binding domain. J Biol Chem. 1998;273:15879–15882. doi: 10.1074/jbc.273.26.15879. [DOI] [PubMed] [Google Scholar]
- Rotenberg SA, Huang MH, Zhu J, Su L, Riedel H. Deletion analysis of protein kinase C inactivation by calphostin C. Mol Carcinog. 1995;12:42–49. doi: 10.1002/mc.2940120107. [DOI] [PubMed] [Google Scholar]
- Schuckit MA. An overview of genetic influences in alcoholism. J Subst Abuse Treat. 2009;36:S5–14. [PubMed] [Google Scholar]
- Sharp AH, Dawson TM, Ross CA, Fotuhi M, Mourey RJ, Snyder SH. Inositol 1,4,5-trisphosphate receptors: immunohistochemical localization to discrete areas of rat central nervous system. Neuroscience. 1993a;53:927–942. doi: 10.1016/0306-4522(93)90478-x. [DOI] [PubMed] [Google Scholar]
- Sharp AH, McPherson PS, Dawson TM, Aoki C, Campbell KP, Snyder SH. Differential immunohistochemical localization of inositol 1,4,5-trisphosphate- and ryanodine-sensitive Ca2+ release channels in rat brain. J Neurosci. 1993b;13:3051–3063. doi: 10.1523/JNEUROSCI.13-07-03051.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp AH, Nucifora FC, Jr, Blondel O, Sheppard CA, Zhang C, Snyder SH, Russell JT, Ryugo DK, Ross CA. Differential cellular expression of isoforms of inositol 1,4,5-triphosphate receptors in neurons and glia in brain. J Comp Neurol. 1999;406:207–220. [PubMed] [Google Scholar]
- Simkus CR, Stricker C. The contribution of intracellular calcium stores to mEPSCs recorded in layer II neurones of rat barrel cortex. J Physiol. 2002;545:521–535. doi: 10.1113/jphysiol.2002.022103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarantopoulos C, McCallum JB, Kwok WM, Hogan Q. Beta-escin diminishes voltage-gated calcium current rundown in perforated patch-clamp recordings from rat primary afferent neurons. J Neurosci Methods. 2004;139:61–68. doi: 10.1016/j.jneumeth.2004.04.015. [DOI] [PubMed] [Google Scholar]
- Stubbs CD, Slater SJ. Ethanol and protein kinase C. Alcohol Clin Exp Res. 1999;23:1552–1560. [PubMed] [Google Scholar]
- Tabakoff B, Nelson E, Yoshimura M, Hellevuo K, Hoffman PL. Phosphorylation cascades control the actions of ethanol on cell cAMP signalling. J Biomed Sci. 2001;8:44–51. doi: 10.1007/BF02255970. [DOI] [PubMed] [Google Scholar]
- Tamaoki T. Use and specificity of staurosporine, UCN-01, and calphostin C as protein kinase inhibitors. Methods Enzymol. 1991;201:340–347. doi: 10.1016/0076-6879(91)01030-6. [DOI] [PubMed] [Google Scholar]
- Theile JW, Morikawa H, Gonzales RA, Morrisett RA. Role of 5-hydroxytryptamine2C receptors in Ca2+-dependent ethanol potentiation of GABA release onto ventral tegmental area dopamine neurons. J Pharmacol Exp Ther. 2009;329:625–633. doi: 10.1124/jpet.108.147793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrier A, Borges S, Dalcino D, Walters C, Wilson M. Calcium from internal stores triggers GABA release from retinal amacrine cells. J Neurophysiol. 2005;94:4196–4208. doi: 10.1152/jn.00604.2005. [DOI] [PubMed] [Google Scholar]
- Worley PF, Baraban JM, Snyder SH. Inositol 1,4,5-trisphosphate receptor binding: autoradiographic localization in rat brain. J Neurosci. 1989;9:339–346. doi: 10.1523/JNEUROSCI.09-01-00339.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki M, Hashimoto K, Kano M. Miniature synaptic events elicited by presynaptic Ca2+ rise are selectively suppressed by cannabinoid receptor activation in cerebellar Purkinje cells. J Neurosci. 2006;26:86–95. doi: 10.1523/JNEUROSCI.2258-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao L, Fan P, Jiang Z, Gordon A, Mochly-Rosen D, Diamond I. Dopamine and ethanol cause translocation of epsilonPKC associated with epsilonRACK: cross-talk between cAMP-dependent protein kinase A and protein kinase C signaling pathways. Mol Pharmacol. 2008;73:1105–1112. doi: 10.1124/mol.107.042580. [DOI] [PMC free article] [PubMed] [Google Scholar]