Abstract
Cadmium (Cd) exposure causes glucosuria (glucose in the urine). Previously, it was shown that Cd exposure of primary cultures of mouse kidney cells (PMKC) decreased mRNA levels of the glucose transporters, SGLT1 and SGLT2 and that Sp1 from Cd-exposed cells displayed reduced binding to the GC boxes of the mouse SGLT1 promoter in vitro. Here, we identified a GC box upstream of mouse SGLT2 gene. ChIP assays on PMKC revealed that exposure to 5 μM Cd abolished Sp1 binding to SGLT1 GC box while it decreased Sp1 binding to SGLT2 GC sequence by 30% in vivo. The in vitro DNA binding assay, EMSA, demonstrated that binding of Sp1 from Cd (7.5 μM)-treated PMKC to the SGLT2 GC probe was 86% lower than in untreated cells. Sp1 is a zinc finger protein. Compared to PMKC exposed to 5 μM Cd alone, inclusion of 5 μM Zn restored SGLT1 and 2 mRNA levels by 15% and 30%, respectively. Cd (10 μM) decreased the binding of recombinant Sp1 (rhSp1) to SGLT1 and SGLT2 GC probes to 12% and 8% of untreated controls. Cd exerted no effect on GC-bound rhSp1. Co-treatment with Cd and Zn showed that added Zn significantly restored rhSp1 binding to the SGLT1 and SGLT2. Addition of Zn post Cd treatment was not stimulatory. We conclude Cd can replace Zn in Sp1 DNA binding domain to reduce its binding to GC sites in mouse SGLT1 and SGLT2 promoters.
Keywords: Cadmium, ChIP assay, Kidney, Sp1, SGLT, Zinc finger
Introduction
The xenobiotic heavy metal cadmium (Cd) has been recognized for its adverse effects on human health and, specifically, for causing damage to the kidney (Barbier et al., 2005). The kidney proximal tubule is the major site of Cd accumulation in the body (Thevenod, 2003) and Cd levels at or higher than 50 μg/g kidney wet weight have been associated with renal injury (Satarug and Moore, 2004). People who live in Cd-polluted regions or are occupationally exposed to this heavy metal can suffer from renal tubular dysfunction, which is manifested by the loss of glucose (glucosuria) and other essential nutrients such as phosphate and calcium in their urine (Kasuya et al., 1992; Thevenod, 2003).
Each day, about 180 grams of glucose in blood is filtered through the kidney glomerulus; almost all of it is retrieved by cells in the proximal tubule (Wright, 2001). The re-absorption of filtered glucose involves the secondary active sodium-dependent glucose transporters, SGLT1 and SGLT2, that are expressed on the apical side of the proximal tubule cells (Hediger and Rhoads, 1994). Both SGLT1 and SGLT2 reabsorb glucose from glomerular filtrate (Wood and Trayhurn, 2003). Therefore, changes in expression of these transporters can modulate glucose reabsorption. The roles of SGLT1 and SGLT2 in renal glucose reabsorption in human have been demonstrated by findings that the symptom of glucosuria can be the result of mutations in SGLT1 or SGLT2 genes (Online Mendelian Inheritance in Man, OMIM™, www.ncbi.nlm.nih.gov/omim/).
Transcriptional regulation of SGLT1 and SGLT2 has also been studied and the transcription factor HNF-1α has been identified as a positive regulator of both SGLT1 and SGLT2 genes in human, mouse, and ovine (Martin et al., 2000; Pontoglio et al., 2000; Vayro et al., 2001). In addition, transcription factor Sp1 has been shown to have two binding sites, known as GC boxes, in the promoter region of human SGLT1 gene and its binding to these GC boxes was shown to be necessary for SGLT1's basal gene expression (Martin et al., 2000). Our studies have shown that these Sp1 binding sites are conserved in the chromosomal sequences upstream of the mouse SGLT1 gene and that both a recombinant human Sp1 and nuclear Sp1 from cultured mouse kidney cells are able to bind to these sequences (Tabatabai et al., 2005).
Sp1 is considered a ubiquitous transcription factor that regulates the expression of many genes (Suske, 1999). It belongs to the family of zinc finger proteins which are characterized by three tandem Cys2His2 (C2H2) domains that are conserved in their DNA binding regions (Kaczynski et al., 2003). There are other zinc finger transcription factors with C2H2 domains, but the number of these domains or their organization differ from the Sp1 family of proteins. For instance, TFIIIA has 9 tandem C2H2 domains that are located at its N-terminus while MZF1's 13 C2H2 zinc fingers are organized in two domains that are separated by a proline- and glycine-rich sequence (Morris et al., 1994; Shastry, 1996). NMR structural studies of Sp1 have shown that the three C2H2 domains in the C-terminus participate in DNA binding of this transcription factor to the GC box sequences (Narayan et al., 1997; Oka et al., 2004).
The two Cys and the two His residues in each C2H2 domain of Sp1 coordinate one zinc (Zn) ion, which is required for Sp1 DNA binding activity (Kuwahara and Coleman, 1990; Razmiafshari et al., 2001). The DNA binding activity of Sp1 can be modulated by modifications in its phosphorylation or glycosylation states (Chu and Ferro, 2005; Li et al., 2004). However, whether its zinc finger DNA binding domain can be directly disrupted by cadmium has yielded ambiguous results (Kuwahara and Coleman, 1990; Thiesen and Bach, 1991; Razmiafshari and Zawia, 2000).
Our investigation of the molecular effect of cadmium on expression of kidney sodium-glucose cotransporters (SGLTs) had shown that in vitro exposure of primary cultures of mouse kidney cells (PMKC) to Cd resulted in inhibition of glucose uptake (Blumenthal et al., 1990; Tabatabai et al., 2001). This decline in glucose uptake accompanied decreases in mRNA levels of both SGLT1 and SGLT2 (Blumenthal et al., 1998; Tabatabai et al., 2001). The reduced mRNA level of SGLT1 in Cd-exposed kidney cells resulted from a transcriptional effect and did not involve enhanced mRNA degradation (Tabatabai et al., 2005). To explain the mechanism of this Cd-induced decrease in SGLT1 transcription, we showed that nuclear Sp1 from Cd-exposed cultured mouse kidney cells had reduced binding activity to the two GC boxes of SGLT1 promoter (Tabatabai et al., 2005).
In this study, we address the in vivo binding of Sp1 to SGLT1 and 2 promoter elements in the absence and presence of Cd and the in vitro direct effect of Cd on Sp1 DNA binding activity. Results show that Cd inhibits Sp1 association with these promoters and directly inhibits Sp1 binding to SGLT GC boxes. The latter is consistent with the replacement of zinc in Sp1 by Cd as a possible mechanism for Cd-induced decreases in SGLT1 and SGLT2 gene expression in kidney cells.
Methods
Materials
Collagenase type IV was purchased from Worthington Biochemical (Lakewood, NJ) and Soybean trypsin inhibitor from Invitrogen (Carlsbad, CA). All other cell culture media components were purchased from Sigma. Oligonucleotides were synthesized by Integrated DNA Technologies (Coralville, IA). The DIG Gel Shift Kit, 2nd Generation (Roche, Indianapolis, IN) was used to DIG-label oligonucleotides for EMSA. Infrared (IR) dye labeled oligonucleotides were synthesized by LI-COR (Lincoln, Nebraska). Oct2A protein and its DNA binding oligonucleotide were supplied in the DIG labeling kit. Recombinant human Sp1 (rhSp1) was supplied by Promega (Madison, WI). Rabbit polyclonal antibody to Sp1 (sc-59x, 2 mg/ml) and serum of un-immunized rabbits (SC-2345) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Protein quantification was performed with the DC Protein Assay (Bio-Rad Laboratories; Hercules, CA). All other reagents or chemicals were molecular biology grade and were obtained either from Thermo Fisher Scientific (Waltham, MA) or Sigma Co. (St. Louis, MO).
Primary mouse kidney cell culture
We have described in detail the preparation of primary cultures of mouse kidney cells (Tabatabai et al., 2003). Briefly, kidneys were isolated from 3 week old male C57BL/6 mice, minced, and then digested for 15 min at 37 °C with collagenase type IV in Hanks Balanced Salt Solution. Digested tissue was then plated into 35 mm culture plates in media containing 1:1 Dulbecco's Modified Eagles Media and Ham's F12 Media with supplements. Cultures were grown at 37 °C and 5% CO2 until about 80% confluent.
Effect of cadmium on in vivo binding of Sp1 to the Slc5a1 and Slc5a2 promoter sequences: ChIP assay
To examine the in vivo effect of Cd exposure on the binding of nuclear Sp1 from cultured mouse kidney cells to the sequences of GC-1 site in SGLT1 or to the GC box upstream of the SGLT2, we performed chromatin immunoprecipitation (IP) using a kit from Millipore (Billerica, MA). Primary cultures of mouse kidney cells were treated with 5μM CdCl2 and untreated cells were used as controls. After 24 hr, media was removed and cultures were treated for 10 min with 1% formaldehyde at 37 °C to cross-link the proteins to the chromatin. Cells were then scraped off the plates, pelleted by centrifugation at 500 xg for 4 min, and lysed. Cell lysates were sonicated to shear the DNA (Omni-Ruptor 250 sonicator), which was then used as the input sample. The input DNA was first pre-cleared for 30 min at 4 °C by incubation with protein A agarose beads blocked with salmon sperm DNA. The Sp1-chromatin DNA complexes were immunoprecipitated with overnight incubation of the pre-cleared samples with an antibody to Sp1 (12 μg) at 4 °C. Following IP, samples were incubated for 1 hr at 4 °C with fresh protein A agarose beads blocked with salmon sperm DNA. After several washes, the protein/DNA complexes were eluted, and the cross-linking was reversed by heating. Finally, chromatin DNA was purified using a QIAGEN kit in a total volume of 30 μl. The ChIP assay was also performed on input DNA incubated with un-immunized rabbit serum (SGLT1) or with no antibody (SGLT2) and samples were used as background controls.
To amplify the chromosomal sequences encompassing the GC1 box of the mouse SGLT1 promoter, forward and reverse PCR primers were designed based on our reported chromosomal DNA sequences (GenBank accession number AF208032). They, respectively, had the sequences 5′-CAGAGGACTTTTGTGCC-3′ and 5′-GCAAAGCTCAAGAGAGTCTG-3′. The GenBank sequences under accession number AJ292928 were used to design forward and reverse primers with the respective sequences of 5′-CTTCTGATCAGAGAGG-3′ and 5′-GCAATATATTTTGGGCAAAT-3′ to amplify the region encompassing the GC box upstream of the mouse SGLT2 gene. PCR was conducted with diluted input DNA or with 2 μl of the purified chromatin DNA, 0.2 μM of each forward and reverse PCR primers, and Platinum Taq DNA Polymerase (Invitrogen) in a total volume of 50 μl. After amplification, 10 μl aliquots were electrophoresed on a 2% agarose gel and the intensity of the ethidium bromide stained bands was determined using a Kodak EDAS 290 system. The PCR band intensities from the Sp1 IP or from background samples were normalized to that of the input DNA. The values for the background were subtracted from that of the IP with Sp1, and the resulting numbers were graphed using arbitrary unit.
Effect of zinc supplementation on mRNA expression of SGLT1 and SGLT2 in Cd-exposed cultured kidney cells
Primary cultures of mouse kidney cells were grown until about 80% confluent. Cells were treated with 5 μM CdCl2 alone, or were co-exposed to CdCl2 (5 μM) and either 5 or 10 μM ZnCl2 in culture media. Untreated cells were used as control. After 24 hr, total RNA was prepared and cDNA synthesized to serve as the template in PCR with SGLT1 or SGLT2 specific primers as we have previously described in detail (Tabatabai et al., 2005). Amplification of S15 encoding a small ribosomal subunit protein was used as PCR control as before. PCR products were electrophoresed on 2% agarose gels and the intensity of the ethidium bromide stained bands was determined using the Kodak EDAS 290 system.
Electrophoretic mobility shift assay (EMSA)
The DNA binding experiments were performed using DIG-labeled probes in EMSA assay as previously described in detail (Tabatabai et al., 2005). The 5′→3′ strands of SGLT1-GC1 (Tabatabai et al., 2005) and SGLT2-GC (Fig. 1) oligonucleotides had the sequences of CCCCTTAGCAGGCCCCTCCCTGCTCACAGAACAGACTTTACCTGCCG and TCTGATCAGAGAGGGGAGGGGATCTGGGAAAAGTTTGGGG, respectively. The underlined nucleotides are core sequences that are required for Sp1 binding. Double stranded SGLT GC probes were generated by heating equal molar amounts of each of the above with their respective complementary oligonucleotides at 95 °C for 10 min followed by cooling to room temperature for 1 hr. Double-stranded SGLT1-GC1 or SGLT2-GC were labeled with DIG-11-ddUTP using Terminal Transferase in a final 25μl volume according to the manufacturer's instructions (Roche; Indianapolis, IN). Oct2A binding oligonucleotide was also DIG labeled as above.
Fig. 1. Predicted transcription factor binding sites upstream of mouse SGLT2 gene.
Selected predicted transcription factor binding sequences are bold and underlined. Transcription factor names are indicated above their respective binding sequences. The sequence of the 5′-3′ strand for binding of Sp1 which was used as SGLT2-GC oligonucleotide probe is shaded in gray. M1, first methionine.
In EMSA, nuclear proteins from two independent primary cultures of mouse kidney cells were used. The preparation of nuclear protein extracts were previously described in detail (Tabatabai et al., 2005). To examine the effect of Cd, EMSA was performed with nuclear proteins from cultures that had been exposed to 7.5 μM CdCl2 for 24 hr. Nuclear proteins from cells in media alone were used as untreated controls. To examine Sp1 binding to the SGLT2-GC sequences, 0.1 μg rhSp1 or 1 μg nuclear protein extracts from either untreated or Cd-treated cells was added to 0.08 pmol DIG labeled SGLT2-GC probe (4 nM in the reaction mixture) in a final reaction volume of 20 μl. All binding reactions in this study were carried out in a modified DNA binding buffer [20mM Hepes (pH 7.6), 10mM (NH4)2SO4, 0.2% Tween-20, 30mM KCl]. [polyd(I-C)] (1 μg) and poly L-lysine (0.1 μg) were also added to reduce non-specific binding. Control binding reactions included the probe without any protein added.
For competition experiments, unlabeled double-stranded SGLT2-GC oligonucleotide at final concentrations of 0.08, 0.8, 8.0, or 80.0 pmol was added simultaneously with 0.08 pmol DIG-labeled SGLT2-GC probe to rhSp1 in binding reaction. For super-shift experiments, 6 μg antibody to Sp1 was added to the binding reactions. DNA binding was carried out at room temperature for 15 min; after that samples were subjected to electrophoresis on a 6% DNA Retardation Gel (Invitrogen). Blotting to a positively charged nylon membrane was followed by UV cross-linking. Blots were then incubated with alkaline phosphatase conjugated to anti-DIG antibody followed by addition of substrate CSPD (Tabatabai et al., 2005). The Kodak Image Station 2000R was used for chemiluminescence detection and quantification of bands.
In vitro effect of cadmium on rhSp1 binding activity
EMSA was performed to determine the in vitro effect of Cd on binding of rhSp1 to SGLT1-GC1 and SGLT2-GC sequences. First, we examined the effect of the treatment of rhSp1 with Cd prior to the addition of the DNA probe to the binding reaction buffer. This was done by incubating rhSp1 (0.1 μg) with CdCl2 at the final concentrations of 0.5, 1, 2, 5, or 10 μM in a total volume of 17.5 μl in DNA binding buffer at room temperature. After 15 min, a 2.5 μl aliquot containing 0.08 pmol DIG-labeled probe, 1 μg [polyd(I-C)] and 0.1 μg poly L-lysine was added to untreated and Cd-treated rhSp1 samples, and binding was carried out at room temperature for 15 min. In control experiments, Oct2A protein (12-37 ng) was similarly treated with either 5 or 10 μM CdCl2 in binding buffer for 15 min at room temperature and then used in the EMSA with 0.08 pmol Oct2A DIG-labeled probe.
The second condition to examine was the effect of Cd treatment on rhSp1 that was already bound to the SGLT probe. First, binding reactions were carried out at room temperature with either SGLT1-GC1 or SGLT2-GC probe (0.08 pmol) and rhSp1 (0.1 μg) in binding buffer containing [polyd(I-C)] and poly L-lysine. After 15 min, CdCl2 was added to the binding reactions to the final concentrations of 0.5, 1, 2, 5, or 10 μM and allowed to react for additional 15 min at room temperature. Reactions without CdCl2 treatment were used as controls.
In vitro effect of zinc supplementation on cadmium-induced reduced rhSp1 binding activity
To determine the effect of extra Zn on the Cd-mediated inhibition of Sp1 DNA binding, 0.1 μg rhSp1 was treated for 15 min at room temperature in DNA binding buffer containing either 2 or 5 μM CdCl2 in a final volume of 17.5 μl. Simultaneously, ZnCl2 at molar ratios to CdCl2 ranging from 1:1 to 20:1 was added to the reaction mixture. Untreated rhSp1 and samples treated with CdCl2 alone were used as controls. The SGLT1-GC1 or SGLT2-GC probe (0.08 pmol) mixed with [polyd(I-C)] and poly L-lysine was then added to untreated and treated rhSp1 samples, and EMSA was performed as described above.
To investigate whether excess Zn can reverse the effect of Cd on binding activity of Sp1, rhSp1 was first incubated with CdCl2 at room temperature in DNA binding buffer. After 15 min, ZnCl2 was added at molar ratios to CdCl2 ranging from 1:1 to 20:1. Reactions were incubated for an additional 15 min at room temperature and then EMSA was performed with the addition of 0.08 pmol IR-labeled SGLT1-GC1 or SGLT2-GC probes. Infrared imaging and quantification of bands were made by direct scanning of the gels at 700 channel using Odyssey (LI-COR).
Statistical analysis
SigmaPlot (Systat Software, Inc.; San Jose, CA) was used to perform statistical analyses. Results obtained from the treatment group and the untreated controls were compared using the paired t-test. P values of ≤ 0.05 were designated as statistically significant.
Results
Sp1 binds to GC box sequence upstream of mouse SGLT2 gene
Since exposure of primary cultures of mouse kidney cells decreased mRNA expression of SGLT2 (Tabatabai et al., 2001), we examined the possible role of Sp1 in Cd-induced down-regulation of SGLT2 gene expression. The chromosomal sequences upstream of the mouse SGLT2 gene (GenBank accession number AJ292928) were used to predict transcription factor binding sites (www.genomatix.de). This analysis identified a potential binding site for Sp1 approximately 600 bases upstream of the first methionine codon of the mouse SGLT2 gene (Fig. 1). To examine whether Sp1 can bind to this GC sequence, we designed an oligonucleotide probe (SGLT2-GC) and used it in EMSA (Fig. 2).
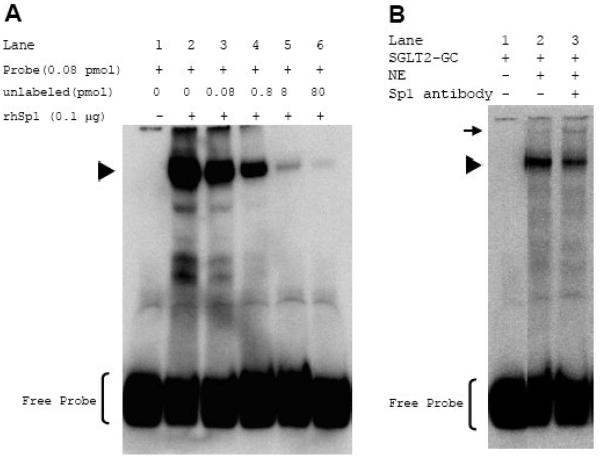
Fig. 2. Binding of Sp1 to SGLT2-GC sequence is specific.
A., EMSA was performed with DIG-labeled SGLT2-GC (0.08 pmol) as probe in binding reactions either without (lane 1) or with 0.1 μg rhSp1 (lanes 2-6). For competition assays, unlabeled double-stranded SGLT2-GC was added (lanes 3-6). B., EMSA was performed with DIG-labeled SGLT2-GC (0.08 pmol) without (lane 1) or with 1 μg nuclear protein extracts (NE) from cultured mouse kidney cells (lanes 2 & 3). Sp1 in the protein/probe complex was shown by addition of 6 μg polyclonal Sp1 antibody to the binding reaction and appearance of a super-shift band (lane 3). Positions of Sp1/probe complex and the super-shift band are indicated by ▶ and ➞, respectively.
When the binding assay was performed with a recombinant human Sp1 (rhSp1), a shifted probe corresponding to the Sp1/SGLT2-GC complex was observed (Fig. 2A, lanes 1 and 2). To show the specificity of this binding, a competition assay was performed by simultaneous addition of increasing concentrations of the unlabeled SGLT2-GC oligonucleotide to the binding reactions (lanes 3-6). The intensity of the rhSp1/probe complex decreased in a dose-dependent manner as the ratio of the concentration of the unlabeled to labeled oligonucleotide increased from 1:1 to 1,000:1. At 100 fold excess, the unlabeled SGLT2-GC almost completely out-competed the DIG-labeled DNA. The above result showed that rhSp1 could bind to the GC sequence upstream of the mouse SGLT2 promoter and that binding was sequence specific.
The nuclear proteins from primary cultures of mouse kidney cells were then used to determine whether renal nuclear Sp1 was also able to bind to the SGLT2-GC probe (Fig. 2B). EMSA showed that the addition of nuclear extract to this probe resulted in formation of a slow migrating band corresponding to a protein/probe complex (lane 2). The presence of Sp1 in this complex was shown with addition of Sp1 antibody to the binding reaction and the appearance of a super-shift band (lane 3). The data from the above experiments demonstrated that Sp1 was present in the nuclear extracts prepared from the cultured mouse kidney cells and that it could bind to the SGLT2-GC sequence.
Cadmium exposure inhibits the in vivo binding of Sp1 to the GC sequences upstream of SGLT1 and SGLT2 genes
A ChIP assay was performed on primary cultures of mouse kidney cells to examine the in vivo binding of Sp1 to the GC-1 box in the promoter of SGLT1 gene (Tabatabai et al., 2005) or to our identified GC site upstream of SGLT2 gene (Fig. 1). The Sp1 cross-linked to the chromatin was immunoprecipitated with antibody to Sp1; then PCR was performed on the purified chromatin. Fig. 3A shows that our designed PCR primers flanking the GC-1 Sp1 binding site of the SGLT1 promoter were able to amplify an expected 132 bp chromosomal DNA fragment that had been co-immunoprecipitated with Sp1. Similarly, Fig. 3C shows that we amplified an expected 72 bp DNA fragment containing the GC site upstream of the SGLT2 gene from the chromatin co-immunoprecipitated with Sp1. The above results show for the first time the in vivo binding of Sp1 to the GC-1 site of the SGLT1 promoter and to our identified GC site upstream of the SGLT2 gene in primary cultures of mouse kidney cells.
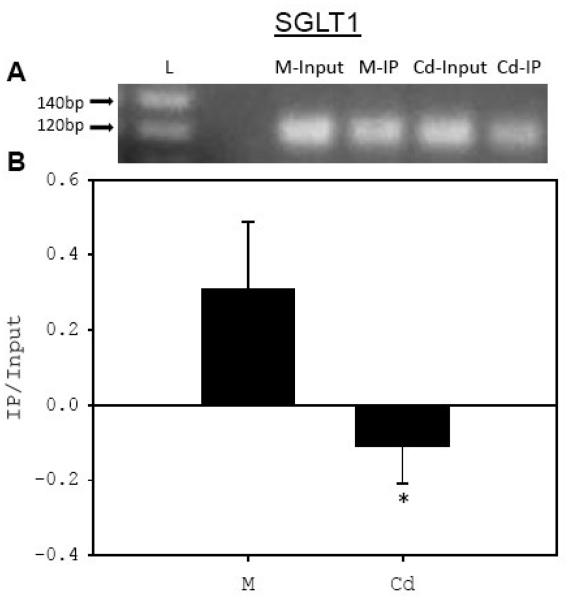
Fig. 3. Exposure of PMKC to Cd reduces in vivo binding of Sp1 to the GC boxes in the promoters of SGLT1 and SGLT2 genes.
PMKC were either left untreated (M) or treated for 24 hr with 5 μM CdCl2 (Cd). ChIP assay was performed using Sp1 antibody (12 μg) (IP) or with un-immunized rabbit serum for SGLT1 and without antibody for SGLT2 as background. PCR was performed on input DNA and on chromatin DNA isolated by ChIP using primers specific to either the GC-1 site of SGLT1 (A) or to the GC site of SGLT2 (C) genes. The intensity of the PCR bands from ChIP assays were normalized to the input DNA, and then background values were subtracted from that of the chromatin immunoprecipitated with Sp1 and graphed (B,D). L, 20 bp DNA ladder. (N = 5 for SGLT1; N = 3 for SGLT2).
The results of the ChIP assay with the SGLT1 GC-1 site showed that the intensity of the PCR product from Sp1 immunoprecipitated chromatin DNA isolated from the Cd-treated cells was below the background level (Fig. 3B). Similarly, compared to the PCR of the chromatin DNA from untreated controls, the intensity of the amplified chromosomal DNA of the SGLT2 GC site had decreased by 30% in reactions with the chromatin DNA from cells exposed to 5 μM Cd (Fig. 3D). These results show that the in vivo binding of Sp1 to the GC sites upstream of both SGLT1 and SGLT2 genes decreased significantly in Cd-exposed cultured mouse kidney cells.
Cadmium exposure reduces binding of kidney nuclear Sp1 to SGLT2-GC
We then examined whether binding of nuclear Sp1 from Cd-exposed mouse kidney cells to the SGLT2-GC sequence was altered. Nuclear protein extracts from cultured mouse kidney cells incubated for 24 hr in medium containing 7.5 μM CdCl2 or in Cd-free medium were used in EMSA experiments with the SGLT2-GC probe. Figure 4 shows that the binding of Sp1 from Cd-treated cells was 86% lower than that of nuclear Sp1 from control untreated cells. This finding confirms the ChIP result that exposure of cultured mouse kidney cells to Cd results in a decrease in binding activity of Sp1 to the GC box sequence upstream of the mouse SGLT2 gene.
Fig. 4. Exposure of PMKC to Cd reduces binding of nuclear Sp1 to SGLT2-GC.
EMSA was performed with DIG-labeled SGLT2-GC (0.08 pmol) and 1 μg nuclear proteins from untreated (NE-M) or 7.5 μM CdCl2 treated (NE-Cd) cultured mouse kidney cells. The intensities of the nuclear Sp1/probe bands were measured and the mean values ± SEM were graphed. (N = 2). A representative EMSA is shown above the graph.
Concurrent incubation of cultured kidney cells with Cd and Zn lessens the Cd-mediated inhibition of SGLT1 and SGLT2 mRNA expressions
We had shown that Cd exposure has inhibitory effects on the expression of SGLT1 and SGLT2 mRNA in primary cultures of mouse kidney cells (Tabatabai et al., 2001 and Fig. 5). Here, we performed RT-PCR to examine whether zinc supplementation has any effect on Cd-induced decreases in SGLT1 or SGLT2 mRNA levels. Fig. 5 shows that, compared to the untreated controls, 24 hr exposure of primary cultures of mouse kidney cells to 5 μM Cd resulted in 50% and 58% decreases in the SGLT1 and SGLT2 mRNA levels, respectively. Consistent with our previous data, Cd exposure did not affect S15 mRNA expression in these cells.
Fig. 5. Zn supplementation restores levels of SGLT1 and SGLT2 mRNA in Cd-exposed PMKC.
PMKC were either left untreated, treated with 5 μM Cd alone, or co-treated with 5 μM Cd and either 5 or 10 μM Zn for 24 hr. RNA was prepared, cDNA was synthesized, and PCR was performed with SGLT1, SGLT2, and S15 primers. The intensity of the PCR bands were measured and results were graphed as % intensity of the untreated controls (N = 4).
When cells were simultaneously exposed for 24 hr to 5 μM Cd and equimolar concentration of Zn, the mRNA level of SGLT1 increased to 65% and that of SGLT2 to 73% of their expression in control untreated cells (Fig. 5). Increasing the concentration of Zn to 10 μM, further elevated the mRNA levels of SGLT1 and SGLT2 to 68% and 79% of their control levels. However, increasing the Zn concentration to 4 fold molar excess over Cd did not have additional stimulatory effects on the mRNA levels of SGLT1 or SGLT2 (not shown). In addition, Zn supplementation had no effect on S15 expression.
In vitro pre-treatment of rhSp1 with cadmium reduces its binding to SGLT1 and SGLT2 GC boxes
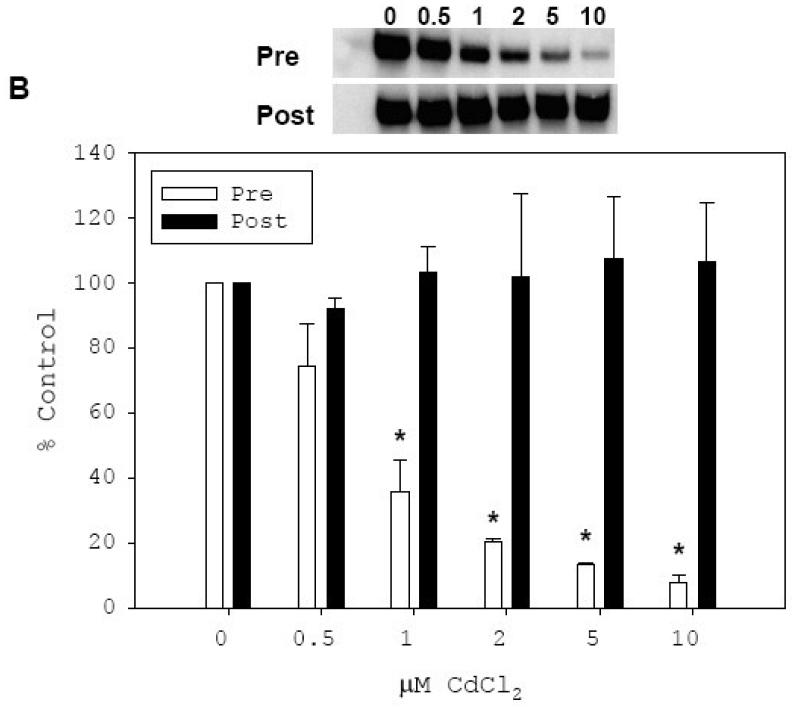
Exposure of cultured mouse kidney cells to 7.5 μM Cd resulted in significant decreases in in vitro binding of nuclear Sp1 to the GC boxes in SGLT1 (Tabatabai et al., 2005) and SGLT2 (Fig. 4) promoters. We further investigated the in vitro direct effect of Cd on binding of rhSp1 to these sequences. RhSp1 was treated with CdCl2 at final concentrations of 0.5, 1, 2, 5 and 10 μM and then allowed to bind to either SGLT1-GC1 or SGLT2-GC probe (Fig. 6). EMSA analysis with SGLT1-GC1 probe showed that pre-treatment of rhSp1 with Cd for 15 min reduced its binding to the SGLT1-GC1, and that increasing the concentration of Cd from 0.5 to 10 μM caused a dose-dependent decline in binding of the rhSp1 to SGLT1-GC1 from 72% to 12% of untreated control (Fig. 6A, open bars). Similarly, data presented in Fig. 6B revealed that treatment of rhSp1 with 0.5 and 1 μM Cd reduced its binding to SGLT2-GC to 74% and 36% of the untreated control, respectively (open bars). Further increases of Cd concentration to 2 and 5 μM, resulted in additional reductions in Sp1 binding to 20% and 13% of control, respectively. Exposure to 10 μM Cd almost abolished its binding to SGLT2-GC. Overall, incubation of rhSp1 with 0.5 to 10 μM concentrations of CdCl2 dose-dependently inhibited its subsequent binding to either SGLT1-GC1 or SGLT2-GC probe.
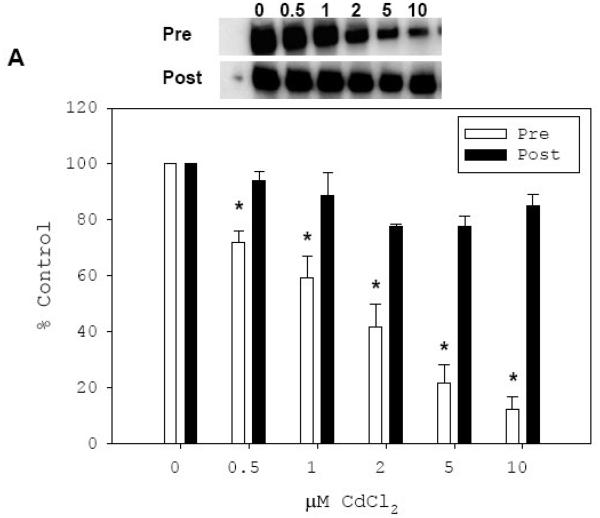
Fig. 6. In vitro inhibitory effect of Cd on binding of rhSp1 to SGLT1-GC1 and SGLT2-GC.
Effect of Cd treatment on binding of rhSp1 to SGLT1-GC1 (A) and SGLT2-GC (B) probes were determined. Effect of pre-treatment with Cd was determined by incubating rhSp1 (0.1 μg) for 15 min at room temperature with CdCl2 at concentrations of 0.5, 1, 2, 5, or 10 μM. Binding reactions were then carried out for 15 min at room temperature by addition of 0.08 pmol DIG-labeled SGLT1-GC1 (A) or SGLT2-GC (B) probe. These reactions are designated as “Pre” and the representative EMSAs are shown above the graphs. The intensity of each Sp1/probe complex was measured and mean values ± SEM of percent untreated controls were graphed (open bars). Also, the effect of Cd treatment on DNA-bound rhSp1 was determined. Binding reactions were carried out for 15 min at room temperature with 0.1 μg rhSp1 and 0.08 pmol DIG-labeled SGLT1-GC1 (A) or SGLT2-GC (B) probe. After binding, reactions were treated with CdCl2 at concentrations of 0.5, 1, 2, 5, or 10 μM for 15 min at room temperature. These reactions are designated as “Post” and the representative EMSAs are shown above the graphs. The intensity of each Sp1/probe complex was measured and mean values ± SEM of percent untreated controls were graphed (filled bars). N = 3-5 and P ≤ 0.03 between respective treated and untreated control is indicated by *.
To investigate whether Cd can similarly inhibit the binding activity of rhSp1 already associated with either SGLT probe, we first allowed rhSp1 to bind to SGLT1-GC1 (Fig. 6A) or SGLT2-GC (Fig. 6B) for 15 min and then treated these reactions for additional 15 min with Cd at final concentrations of 0.5, 1, 2, 5, and 10 μM. The result of the above EMSA experiments demonstrated that rhSp1 bound to SGLT1-GC1 or SGLT2-GC probe was protected against the inhibitory effect of Cd. Even at a concentration of 10 μM, Cd could not reverse the binding of rhSp1 to either SGLT-GC probe and its binding remained at 100% of untreated control (filled bars).
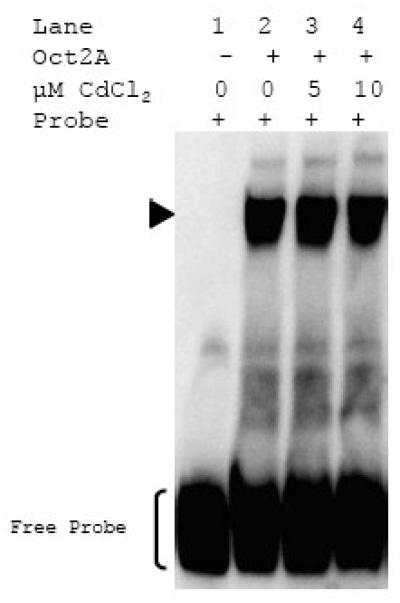
The specificity of Cd-induced inhibition of Sp1 binding to either SGLT1-GC1 or SGLT2-GC sequences was investigated by treating the non-zinc finger transcription factor Oct2A (Hatzopoulos et al., 1990) with 5 and 10 μM Cd prior to DNA binding reaction with Oct2A specific oligonucleotide (Fig. 7). This EMSA experiment showed that exposure to Cd did not alter Oct2A binding activity. We concluded that Cd-induced inhibition of Sp1 binding to the SGLT-GC sequences was due to its effect on the zinc finger DNA binding domain of this transcription factor and not due to non-specific impact on protein or DNA structure.
Fig. 7. Cd does not affect binding of Oct2A.
Non-zinc finger transcription factor, Oct2A, was either untreated or treated with 5 and 10 μM CdCl2 for 15 min at room temperature. Binding reactions were carried out for 15 min at room temperature with the DIG-labeled Oct2A probe (0.08 pmol) and either without Oct2A (lane 1), with untreated Oct2A (lane 2), or Cd-treated Oct2A (lanes 3 and 4). Representative EMSA from 3 independent experiments is shown.
Protection against the inhibitory effect of cadmium by zinc
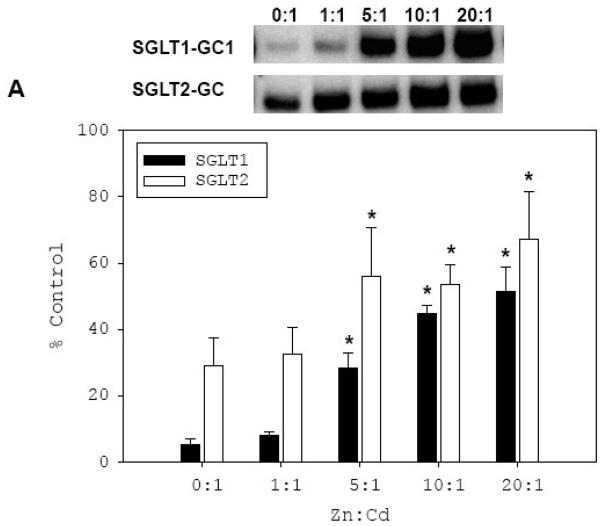
We investigated if the reduced binding of Cd-treated rhSp1 to the GC sequences of SGLT1 and SGLT2 could be blocked by the availability of extra Zn as observed in vivo. Therefore, we first co-treated rhSp1 with a constant inhibitory concentration of Cd while increasing the concentration of Zn, and then performed the DNA binding assay with either SGLT1-GC1 or SGLT2-GC probe (Fig. 8A). The results with SGLT1-GC1 (filled bars) demonstrated that, as expected, treatment of rhSp1 with 5 μM Cd alone prior to the DNA binding assay reduced its binding to SGLT1-GC1. Co-treatment with an equal molar concentration of Zn could not block this inhibition. However, 5 fold molar excess of Zn during Cd treatment exerted a protective effect and increased binding of rhSp1 to SGLT1-GC1 5.4 fold. Further increase of Zn concentration to 10 and 20 fold that of Cd resulted in dose-dependent improvement in rhSp1 binding to SGLT1-GC1 to 45% and 52% of untreated control, respectively.
Fig. 8. Effect of Zn supplementation on inhibitory effect of Cd on binding of rhSp1 to SGLT1-GC1 and SGLT2-GC probes.
A, Effect of co-exposure to Cd and Zn on rhSp1. rhSp1 (0.1 μg) was simultaneously treated for 15 min at room temperature with CdCl2 (2-5 μM) and ZnCl2 at indicated molar ratios to Cd. Binding reactions were then carried out for 15 min at room temperature with 0.08 pmol of DIG-labeled SGLT1-GC1 (filled bars) or SGLT2-GC (open bars) probe. Binding reactions with untreated rhSp1 were used as controls. The intensity of each Sp1/probe complex was measured and mean values ± SEM of percent untreated controls were graphed. Representative EMSAs are shown above the graph. N = 3-5 and P ≤ 0.03 between respective treated and untreated control is indicated by * B, Effect of Zn on Cd-treated rhSp1. rhSp1 (0.1 μg) was pre-treated with CdCl2 (2-5 μM) for 15 min at room temperature. Then, ZnCl2 was added at indicated molar ratios to Cd and reactions were incubated for additional 15 min. Binding reactions were then carried out for 15 min at room temperature by addition of 0.08 pmol of IR-labeled SGLT1-GC1 (filled bars) or SGLT2-GC (open bars) probe. The intensity of each Sp1/probe complex was measured and mean values ± SEM of percent untreated controls were graphed. Representative EMSAs are shown above the graph. N = 3-5 and P ≤ 0.03 between respective treated and untreated control is indicated by *.
When the above co-treatment experiment was repeated with SGLT2-GC (open bars), qualitatively similar results were obtained. Co-treatment of rhSp1 with an equal molar concentration of Zn did not alter the inhibitory effect of Cd. However, 10 fold molar excess Zn over Cd resulted in enhancement in binding from 33% to 54% of the untreated control. The Zn-induced enhanced binding of Cd-treated rhSp1 to SGLT2-GC reached 67% of control with 20 fold excess Zn. The data from both experiments demonstrated that Zn can protect against the inhibitory effect of Cd.
We then investigated whether Zn can reverse the inhibitory effect of Cd on binding of Sp1 to SGLT1 and SGLT2 GC sequences. First, rhSp1 was treated with Cd for 15 min after which Zn was added at molar ratios to Cd ranging from 1 to 20 (Fig. 8B). Addition of equal molar concentration of Zn had a slight stimulatory effect on binding of Cd-treated rhSp1 to SGLT1-GC1 (filled bars). However, this increase was found not to be statistically significant (P=0.2 between 0:1 and 1:1). Similarly, an equal molar concentration of Zn did not affect binding to SGLT2-GC (open bars). Treatment of Cd-exposed rhSp1 with 5 to 10 fold Zn dose-dependently decreased the binding and 20 fold excess almost completely abolished Sp1 binding to both SGLT1 and SGLT2 GC sequences.
Discussion
Cadmium preferentially accumulates in the kidney cortex and causes toxicity to the proximal tubule, and induces glucosuria (Bernard, 2004; Savolainen, 1995). Cadmium-mediated inhibition of renal glucose uptake can be studied in a mouse kidney cell culture system (Blumenthal et al., 1990). We showed that Cd exposure caused a decrease in the sodium-dependent uptake of glucose in primary cultures of mouse kidney cells (PMKC), and that this decline in uptake paralleled decreases in the mRNA levels of glucose transporters SGLT1 and SGLT2 (Blumenthal et al., 1998; Tabatabai et al., 2001). We also demonstrated that the promoter of mouse SGLT1 gene contains two GC boxes for the binding of the transcription factor Sp1 (Tabatabai et al., 2005). In this paper, we also identified a GC box upstream of the SGLT2 gene (Fig. 1). Using the ChIP assay on primary cultures of mouse kidney cells, this study revealed for the first time that Sp1 binds to these GC sites in vivo (Fig. 3). In addition, exposure to 5 μM Cd abolished the in vivo binding of Sp1 to the SGLT1 GC-1 and decreased its binding to the SGLT2 GC site by 30% in PMKC (Fig. 3) while the Sp1 protein expression levels remained unchanged (Tabatabai et al., 2005). EMSA analysis consistently showed that the in vitro binding activity of the nuclear Sp1 from Cd-exposed PMKC to the SGLT1 GC-1 or to the SGLT2 GC oligonucleotide probes was 80% to 90% less than that of Sp1 from untreated controls (Tabatabai et al., 2005 and Fig. 4).
Transcription factor Sp1 is a member of the Sp1- and Kruppel-Like family of proteins (Sp1/KLF) with conserved C-terminal DNA binding region consisting of three zinc finger C2H2 domains, in which association with zinc is essential in binding activity of the protein (Kaczynski et al., 2003). To determine whether Cd could directly affect the zinc finger and, therefore, the binding of Sp1 to the GC-rich sequences of SGLT1 or SGLT2 promoters, in vitro DNA binding activity to SGLT1 and SGLT2 GC probes was compared by using a commercial recombinant human Sp1 (rhSp1). We found that the in vitro exposure of rhSp1 to 0.5 to 10 μM concentrations of Cd resulted in concentration-dependent decreases in its subsequent binding activity to both SGLT1 and SGLT2 probes, and that 10 μM Cd was sufficient to almost abolish rhSp1 binding to either probe (Fig. 6). Although these probes contained chromosomal sequences flanking the core Sp1 binding site, we suggest that these surrounding DNA regions did not play roles in the Cd-induced decreases in binding of Sp1 to the SGLT GC box probes used in this study.
Our comparative analysis of the in vitro effect of Cd on Sp1 binding to SGLT1 and SGLT2 GC box sequences indicate that in vivo Cd may directly interact with Sp1 protein to cause a decrease in transcriptional expression of SGLT1 and SGLT2 genes. We were also able to show the specificity of the Cd effect on zinc fingers of Sp1 by the lack of inhibitory effect of this metal on DNA binding activity of non-zinc finger transcription factor Oct2A (Fig. 7). This result supports the view that Cd ion does not independently affect DNA structural properties and, thereby, alter Sp1 DNA binding.
Cadmium ion is chemically similar to zinc. Our data are consistent with the hypothesis that Cd replaces Zn in the zinc finger DNA binding domain of Sp1, alters its conformation, and, thereby, inhibits its DNA binding affinity. The reaction of Cd with Sp1 may be written as
| (1) |
According to this equilibrium reaction, addition of Zn should favor Zn-Sp1. In fact, simultaneous incubation of rhSp1 with Cd and increasing amounts of Zn elevated the level of subsequent DNA binding to the SGLT1 and 2 DNA probes (Fig 8A). This protection by Zn was not observed until the molar ratio of Zn to Cd was increased to 5 fold. From this result, we infer that Cd binds stronger to Sp1 than Zn does. Consistent with the above in vitro results, the levels of SGLT1 and SGLT2 mRNA in PMKC co-exposed to equimolar concentrations of Cd and Zn were 15% to 30% higher than in their levels in cells exposed to Cd alone (Fig. 5). Increasing the Zn concentration to a two-fold molar excess over Cd, marginally elevated the SGLT mRNA levels. However, Zn at 4 times molar excess to Cd did not further enhance SGLT1 or SGLT2 mRNA levels (data not shown).
Addition of Zn to the mixture of Cd and Zn-Sp1 failed to reverse the observed Cd inhibition of Sp1 binding to SGLT1 and 2 GC probes (Fig. 8B). Indeed, excess Zn resulted in increased inhibition. Clearly, Zn behaved differently in this experiment than in the previous one even though the final concentration of all the reactants was the same in both versions of the Cd-Zn competition experiments. Only the order of addition changed. Thus, equilibrium reaction 1 is not sufficient to explain the interaction of Cd with Zn-Sp1. Instead, because the sequence of addition of reactants determines the outcome of the experiment, other reactions must come into play as well. We propose that Cd-Sp1 in reaction 1 retains normal C2H2 metal ion binding but its usual peptide conformation is perturbed. However, in the presence of Cd, which has a stronger preference for sulfhydryl group coordination, the structure undergoes rearrangement to form CdSp1′.
| (2) |
In the new conformation, Cd is hypothetically bound to 4 cysteinyl sulfhydryl groups from adjacent finger domains. Because this complex has a larger equilibrium constant for binding Cd than Zn; Zn no longer competes to restore the native Zn-Sp1 conformation:
| (3) |
The findings of this study follow a history of experiments inquiring into the reaction of metal ions with Sp1 and other Zn-finger structures. Earlier studies were contradictory, some supporting an inhibitory effect of Cd, other finding no such effects (Kuwahara and Coleman, 1990; Thiesen and Bach, 1991; Razmiafshari and Zawia, 2000). The current experiments strongly support concentration dependent Cd displacement of Zn from Zn-Sp1.
The results also parallel those for the reaction of transcription factor IIIA (Zn-TFIIIA) with Cd (Huang et al., 2004). Indeed, in that study it was proposed that this reaction leads to inter-finger binding of Cd to 4 cysteinyl sulfhydryl groups. Finally, chemical and NMR structural studies of Zn- and Cd-finger 3 of TFIIIA showed that Cd binding reduces affinity for cognate DNA binding as a result of minor changes in finger peptide conformation (Krepkiy et al., 2004). A significant difference between the behavior of the two proteins is that Zn-TFIIIA bound to its cognate DNA still undergoes reaction with Cd. The basic observation of Cd inhibition of Zn-finger protein association with specific DNA sequences was also seen in the study with Tramtrack (Roesijadi et al., 1998
Another important conclusion of this study is that Cd fails to dissociate rhSp1 from its preformed protein/DNA complexes with either SGLT1 or SGLT2 probe (Fig. 6). These data support the idea that the zinc finger domains of free Sp1 are the direct targets of Cd and that the prior contact of these domains with nucleotides protects Sp1 against subsequent reaction with cadmium. The sequences surrounding the Sp1 contact site may enhance DNA-dependent protection against Cd. Such DNA protection may have some generality. For example, treatment of SGLT1-GC1-bound rhSp1 with the Zn chelator, ethylendiaminetetraacetic acid (EDTA) does not reduce DNA binding, whereas incubation of rhSp1 with EDTA prior to the addition of the DNA probe abolishes its subsequent binding to SGLT1-GC1 (manuscript in preparation).
The results of this study support the hypothesis that the down-regulation of renal expression of SGLT1 and SGLT2 mRNA in response to Cd may result from decreases in the pool of Zn-containing Sp1 and parallel increases in Cd-bound Sp1.
Acknowledgements
This research was supported by National Institutes of Health grants ES-04016 and ES-04184.
Abbreviations
- Cd
cadmium
- ChIP
chromatin immunoprecipitation
- Cys
cysteine
- EMSA
electrophoretic mobility shift assay
- His
histidine
- IR
infrared
- rhSp1
recombinant human Sp1
- SGLT
sodium-glucose cotransporter
- Zn
zinc
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
None of the authors of this manuscript has any conflict of interest to report.
References
- Barbier O, Jacquillet G, Tauc M, Cougnon M, Poujeol P. Effect of heavy metals on, and handling by, the kidney. Nephron Physiol. 2005;99:105–110. doi: 10.1159/000083981. [DOI] [PubMed] [Google Scholar]
- Bernard A. Renal dysfunction induced by cadmium: biomarkers of critical effects. Biometals. 2004;17:519–523. doi: 10.1023/b:biom.0000045731.75602.b9. [DOI] [PubMed] [Google Scholar]
- Blumenthal SS, Lewand DL, Buday MA, Kleinman JG, Krezoski SK, Petering DH. Cadmium inhibits glucose uptake in primary cultures of mouse cortical tubule cells. Am.J.Physiol. 1990;258:F1625–F1633. doi: 10.1152/ajprenal.1990.258.6.F1625. [DOI] [PubMed] [Google Scholar]
- Blumenthal SS, Ren LF, Lewand DL, Krezoski SK, Petering DH. Cadmium decreases SGLT1 messenger RNA in mouse kidney cells. Toxicology and Applied Pharmacology. 1998;149:49–54. doi: 10.1006/taap.1997.8353. [DOI] [PubMed] [Google Scholar]
- Chu S, Ferro TJ. Sp1: regulation of gene expression by phosphorylation. Gene. 2005;348:1–11. doi: 10.1016/j.gene.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Hatzopoulos AK, Stoykova AS, Erselius JR, Goulding M, Neuman T, Gruss P. Structure and expression of the mouse Oct2a and Oct2b, two differentially spliced products of the same gene. Development. 1990;109:349–362. doi: 10.1242/dev.109.2.349. [DOI] [PubMed] [Google Scholar]
- Hediger MA, Rhoads DB. Molecular physiology of sodium-glucose cotransporters. Physiol Rev. 1994;74:993–1026. doi: 10.1152/physrev.1994.74.4.993. [DOI] [PubMed] [Google Scholar]
- Huang ML, Krepkiy D, Hu WN, Petering DH. Zn-, Cd-, and Pb-transcription factor IIIA: properties, DNA binding, and comparison with TFIIIA-finger 3 metal complexes. Journal of Inorganic Biochemistry. 2004;98:775–785. doi: 10.1016/j.jinorgbio.2004.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczynski J, Cook T, Urrutia R. Sp1- and Kruppel-like transcription factors. Genome Biol. 2003;4:206. doi: 10.1186/gb-2003-4-2-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadonaga JT, Carner KR, Masiarz FR, Tjian R. Isolation of cDNA encoding transcription factor Sp1 and functional analysis of the DNA binding domain. Cell. 1987;51:1079–1090. doi: 10.1016/0092-8674(87)90594-0. [DOI] [PubMed] [Google Scholar]
- Kasuya M, Teranishi H, Aoshima K, Katoh T, Horiguchi H, Morikawa Y, Nishijo M, Iwata K. Water pollution by cadmium and the onset of itai-itai disease. Water Science Technology. 1992;25:149–156. [Google Scholar]
- Krepkiy D, Forsterling FH, Petering DH. Interaction of Cd2+ with Zn finger 3 of transcription factor IIIA: structures and binding to cognate DNA. Chem.Res.Toxicol. 2004;17:863–870. doi: 10.1021/tx030057+. [DOI] [PubMed] [Google Scholar]
- Kuwahara J, Coleman JE. Role of the zinc(II) ions in the structure of the three-finger DNA binding domain of the Sp1 transcription factor. Biochemistry. 1990;29:8627–8631. doi: 10.1021/bi00489a019. [DOI] [PubMed] [Google Scholar]
- Li L, He S, Sun JM, Davie JR. Gene regulation by Sp1 and Sp3. Biochem.Cell Biol. 2004;82:460–471. doi: 10.1139/o04-045. [DOI] [PubMed] [Google Scholar]
- Martin MG, Wang J, Solorzano-Vargas RS, Lam JT, Turk E, Wright EM. Regulation of the human Na(+)-glucose cotransporter gene, SGLT1, by HNF-1 and Sp1. Am.J.Physiol Gastrointest.Liver Physiol. 2000;278:G591–G603. doi: 10.1152/ajpgi.2000.278.4.G591. [DOI] [PubMed] [Google Scholar]
- Morris JF, Hromas R, Rauscher FJ., III Characterization of the DNA-binding properties of the myeloid zinc finger protein MZF1: two independent DNA-binding domains recognize two DNA consensus sequences with a common G-rich core. Mol.Cell Biol. 1994;14:1786–1795. doi: 10.1128/mcb.14.3.1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan VA, Kriwacki RW, Caradonna JP. Structures of zinc finger domains from transcription factor Sp1. Insights into sequence-specific protein-DNA recognition. J.Biol.Chem. 1997;272:7801–7809. doi: 10.1074/jbc.272.12.7801. [DOI] [PubMed] [Google Scholar]
- Oka S, Shiraishi Y, Yoshida T, Ohkubo T, Sugiura Y, Kobayashi Y. NMR structure of transcription factor Sp1 DNA binding domain. Biochemistry. 2004;43:16027–16035. doi: 10.1021/bi048438p. [DOI] [PubMed] [Google Scholar]
- Pontoglio M. Hepatocyte nuclear factor 1, a transcription factor at the crossroads of glucose homeostasis. J.Am.Soc.Nephrol. 2000;11(Suppl 16):S140–S143. [PubMed] [Google Scholar]
- Razmiafshari M, Zawia NH. Utilization of a synthetic peptide as a tool to study the interaction of heavy metals with the zinc finger domain of proteins critical for gene expression in the developing brain. Toxicol.Appl.Pharmacol. 2000;166:1–12. doi: 10.1006/taap.2000.8950. [DOI] [PubMed] [Google Scholar]
- Razmiafshari M, Kao J, d'Avignon A, Zawia NH. NMR identification of heavy metal-binding sites in a synthetic zinc finger peptide: toxicological implications for the interactions of xenobiotic metals with zinc finger proteins. Toxicol.Appl.Pharmacol. 2001;172:1–10. doi: 10.1006/taap.2001.9132. [DOI] [PubMed] [Google Scholar]
- Roesijadi G, Bogumil R, Vasak M, Kagi JH. Modulation of DNA binding of a tramtrack zinc finger peptide by the metallothionein-thionein conjugate pair. J.Biol.Chem. 1998;273:17425–17432. doi: 10.1074/jbc.273.28.17425. [DOI] [PubMed] [Google Scholar]
- Satarug S, Moore MR. Adverse health effects of chronic exposure to low-level cadmium in foodstuffs and cigarette smoke. Environ.Health Perspect. 2004;112:1099–1103. doi: 10.1289/ehp.6751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savolainen H. Cadmium-associated renal disease. Ren Fail. 1995;17:483–487. doi: 10.3109/08860229509037613. [DOI] [PubMed] [Google Scholar]
- Shastry BS. Transcription factor IIIA (TFIIIA) in the second decade. J.Cell Sci. 1996;109(Pt 3):535–539. doi: 10.1242/jcs.109.3.535. [DOI] [PubMed] [Google Scholar]
- Suske G. The Sp-family of transcription factors. Gene. 1999;238:291–300. doi: 10.1016/s0378-1119(99)00357-1. [DOI] [PubMed] [Google Scholar]
- Tabatabai NM, Blumenthal SS, Lewand DL, Petering DH. Differential regulation of mouse kidney sodium-dependent transporters mRNA by cadmium. Toxicol.Appl.Pharmacol. 2001;177:163–173. doi: 10.1006/taap.2001.9321. [DOI] [PubMed] [Google Scholar]
- Tabatabai NM, Blumenthal SS, Petering DH. Adverse effect of cadmium on binding of transcription factor Sp1 to the GC-rich regions of the mouse sodium-glucose cotransporter 1, SGLT1, promoter. Toxicology. 2005;207:369–382. doi: 10.1016/j.tox.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Thevenod F. Nephrotoxicity and the proximal tubule. Insights from cadmium. Nephron Physiol. 2003;93:87–93. doi: 10.1159/000070241. [DOI] [PubMed] [Google Scholar]
- Thiesen HJ, Bach C. Transition metals modulate DNA-protein interactions of SP1 zinc finger domains with its cognate target site. Biochem.Biophys.Res.Commun. 1991;176:551–557. doi: 10.1016/s0006-291x(05)80219-0. [DOI] [PubMed] [Google Scholar]
- Vayro S, Wood IS, Dyer J, Shirazi-Beechey SP. Transcriptional regulation of the ovine intestinal Na plus /glucose cotransporter SGLT1 gene - Role of HNF-1 in glucose activation of promoter function. European Journal of Biochemistry. 2001;268:5460–5470. doi: 10.1046/j.0014-2956.2001.02488.x. [DOI] [PubMed] [Google Scholar]
- Wood IS, Trayhurn P. Glucose transporters (GLUT and SGLT): expanded families of sugar transport proteins. Br.J.Nutr. 2003;89:3–9. doi: 10.1079/BJN2002763. [DOI] [PubMed] [Google Scholar]
- Wright EM. Renal Na(+)-glucose cotransporters. Am.J.Physiol Renal Physiol. 2001;280:F10–F18. doi: 10.1152/ajprenal.2001.280.1.F10. [DOI] [PubMed] [Google Scholar]