Abstract
Several advances have recently expanded models of tumor growth and promoted the concept of tumor homeostasis, the hypothesis that primary tumors exert an anti-proliferative effect on both themselves and subclinical secondary metastases. Recent trials indicate that the characterization of tumor growth as uncontrolled is inconsistent with animal models, clinical models, and epidemiological models. There is a growing body of evidence which lends support to an updated concept of tumor growth: tumor homeostasis. In the case of breast cancer, if not all metastasizing tumors, these advances suggest an inconvenient truth. That is, if breast tumor cells metastasize to distant sites early in the tumorigenesis process, then removal of a breast tumor may hasten the development of its metastases. We explore the heretofore unappreciated notion that nucleotides generated by tumor cells following the secretion of an ADP-kinase can promote metastasis and support angiogenesis. Evidence is presented that blockade of the actions of nucleotides in the setting of newly diagnosed breast cancer may provide a useful adjunct to current anti-angiogenesis treatment.
Keywords: Secreted nucleoside diphosphate kinase-B (sNDPK-B), nucleotide receptors, angiogenesis, VEGFR2, transactivation
1. Introduction
Breast cancer is the most common cancer among women in the United States [1] and while it is the second deadliest cancer with a yearly mortality exceeded only by lung cancer, management of breast cancer in the US ranks among the best in the world [2]. The typical treatment regime under which this has been accomplished involves either mastectomy or lumpectomy plus postoperative (adjuvant) treatment. Nonetheless, more than forty-thousand women will die of the disease this year [1]. Clinical oncologists have known that breast cancer specific mortality is almost exclusively a function of a metastatic process [3]. Of all the types of breast cancer recurrence, the presence of distant metastases is associated with the least favorable outcome. Those whose breast cancer reoccurs at distant sites in the body have only a 9% chance of living an additional 10 years, compared to a 56% survival rate for women with a reoccurrence of breast cancer which is isolated to the breast [4]. Thus the imperative in breast cancer research is to understand the metastatic process and the philosophy of breast cancer care must be to utilize this understanding to prevent metastases.
Metastatic growth of tumors has been classically characterized as unlimited, yet several limiting factors do act as a brake on tumor growth. Adequate blood supply dictates that tumor cells must reside within 100 μm of a capillary blood vessel or risk necrosis. The rich vascularity of metastatic tumors has lead to the discovery that proliferating tumor cells have an intrinsic ability to generate the growth of new blood vessels; angiogenesis, that is a hallmark of cancer [5].
Excision of a primary tumor can lead to tumor metastases appearing shortly thereafter [6-8]. Assuming that cells weren’t “dislodged’ during surgery, an intellectually displeasing notion, it is reasonable to take this as evidence to suggest the presence of subclinical distant metastases at the time of loco-regional treatment. Indeed, although fine needle biopsy and large needle core biopsy have been shown to significantly impact the appearance of sentinel node metastases [9], tumor manipulation during excision is not thought to explain distant metastases [10]. Demichili and coauthors examined the hazard rate for recurrence of breast cancer in patients undergoing mastectomy, showing an early peak in recurrence, with the majority of recurrences around 18 months post-mastectomy [11]. This clearly indicates that the surgery is an aggravating event, with the result becoming clinically apparent approximately 18 months thereafter. This data strongly suggests that metastases present at the time of mastectomy have existed in a dormant state and have proliferated only once the antiangiogenic effects of the primary tumor have been removed. Animal studies confirmed this behavior of human tumor cells a decade ago [12-13]. Moreover, there is even compelling, if troubling evidence to indicate that the natural history of untreated breast cancer may rarely involve spontaneous regressions and even resolution rather than progression [14]. The notion of metastases as dormant, extant lesions awaiting events that trigger their conversion to rapidly growing tumors that attract a blood supply is supported by recent genomic studies that implicate nucleotide metabolism among other critical changes in gene transcription [15]. Efforts to maintain dormancy of metastases in patients when first seen by the oncologist might offer a new strategy for adjuvant treatments to prevent their growth.
Removal of a primary tumor and development of metastases is a likely clinical course even among the 10% of low rise (node negative) patients that will eventually develop distant metastases [1]. In consideration of these unique temporal features regarding metastasis development, a model of tumor homeostasis has been described in which the primary tumor exerts an anti-proliferative effect via an unknown mechanism upon subclinical micrometastases [14]. Furthermore, evidence indicates that this trend towards dormancy is a function of the primary tumor preventing the initiation to angiogenesis being made [16]. Thus, mechanisms that orchestrate primary tumor growth and development are disparate, although no doubt related, to those that encourage and support the growth of metastases.
Autopsy studies have sought to determine the prevalence of pre-clinical, presumably dormant breast cancers, and generally report a median prevalence of 8.9% of ductal carcinoma in situ (DCIS) [17]. When this data is contrasted with a median prevalence of DCIS of 1.3% of the female population, it suggests a large reservoir of clinically occult cancers which would never progress into metastatic disease. This is further supported by data showing that less than half of untreated low-grade DCIS will develop into metastatic disease over a 40 year period of time [18]. This may be due in part to a failure of the these occult tumor cells to induce angiogenesis. Thus, the balance of factors suppressing the growth of distant metastases as well as those that promote it is critical to the fact of metastatic disease in a given patient. Such a balance may be upset in favor of the growth of metastases in patients when primary tumors are removed and mechanisms such as those we discuss here can proceed to support angiogenesis. The notion that the extracellular actions of ATP contribute to dormancy of primary tumors or might stimulate their growth in situ cannot be clarified at preset. There is tantalizing evidence that either possibility might occur and is discussed below. The events that permitted intravasation and extravasation in the passage of tumor cells to distant sites may be proceed early on and unopposed by the primary tumor whose influence is that which suppresses angiogenesis. With the importance of angiogenesis increasingly apparent in clinical oncology, the formation and extracellular actions of nucleotides will be a valuable mechanism to exploit in decreasing the frequency of metastasis and subsequent deaths from cancer. If a role for purines can be firmly established in both intravasation/extravasation as well as angiogenesis and safe and effective antagonists of these mechanisms identified, a dual benefit could be realized with women at high risk for developing disease.
2. Extracellular ATP
Although originally viewed with much skepticism, the extracellular presence of ATP released from nerves as well as non-excitable cells [19] is well established and has been frequently reviewed in recent years [20]. From the first suggestion of the phenomenon by Burnstock in 1976 [21], to the first actual experimental evidence for the fact of ATP release by Westfall et al., in 1978 [22], there is now an extensive understanding of a near ubiquitous role for extracellular purines in most organ systems. Nucleotide release has been extensively studied in endothelial cells of blood vessels that are the object of interest in this review, the phenomenon has been described in epithelial cells by Schwiebert and colleagues who have offered a valuable discussion of the mechanisms of release that are beyond the scope of this discussion [23].
3. Tumor Cell Migration
Accepting that metastases in breast cancer are not the result of surgical manipulation, forces the inconvenient truth that in many cases, cells from a tumor in the breast have already spread to distant parts of the body at the time of diagnosis. Understanding the mechanisms of tumor invasion and migration is a central focus of current investigations. While a thorough review of these mechanisms is beyond the scope of this review, it is now possible to propose an important role for extracellular purines in the process of tumor cell invasion and metastasis.
As pointed out recently by Christofori [24], cells commonly move into and out of tissue spaces in the normal processes involved in embryonic development and immunity. It is reasonable to suspect therefore that tumor cells mimic many of these processes in their passage from primary tumor to metastatic site. Because it is known that breast cancer cells move in the body via both blood and lymph, we focus our discussion here to metastasis via blood simply because far more is known about purinergic mechanisms in blood vessels than in the lymphatics.
It is well known that leukocytes leave blood vessels to fight infection. The transendothelial migration of white cells out the blood stream and into tissue spaces requires that they move between capillary cells and through basement membrane of both the capillary and tissue. This process, diapedesis or transendothelial migration, is known to involve the interaction of leukocytes with the vessel wall [25]. Leukocytes must first bind to and then move along the capillary wall to the endothelial borders where they traverse the endothelium and the sub-endothelial basement membrane and migrate through the interstitial space. Intercellular adhesion molecule (ICAM)-1 and vascular cell adhesion molecule (VCAM)-1 cluster on the endothelial cell and appear to be responsible for leukocyte binding based on an interaction with specific leukocyte molecules (e.g., CD11, CD18, CD49 and CD29). The many steps that follow, including those that subserve transcellular versus paracellular passage of leukocytes [26], involve enzymatic steps and both leukocyte and endothelial cell deformation to permit transit to occur [27].
While little attention has been paid to a role for nucleotides or ATP receptors in this process, it is known that locally generated ATP may enhance leukocyte binding to endothelium [28] and that the expression of adhesion molecules by endothelium is enhanced by extracellular ATP acting at purine nucleotide receptors [29]. Moreover, polymorphonuclear leukocytes are known to elaborate ATP themselves [30], so that along with endothelial cells that are well known to elaborate ATP extracellularly [31], ATP receptors will be activated. Rapid metabolism of ATP locally would generate adenosine consistent with reversing the permeability of the endothelium [32] and dilating venous vessels to regulate flow down-stream [33]. Taken together with the known ability of ATP receptor stimulation to lead to endothelial cell contraction and thus, vascular permeability [34-36], it is reasonable to consider mechanisms that can maintain ATP levels extracellularly as consistent with promoting transendothelial migration.
Movement of breast tumor cells in the blood stream in a fashion that permits their targeting to particular distant sites is as yet not fully explained but is unlikely to be a mere mechanical consequence of where the relatively large tumor cell [37] becomes lodged in a capillary [38]. In fact, such trapping might reasonably result in regional infarcts rather than regulated extravasation were it not for the fact that tumor cells deform significantly in their passage in the blood stream and adhere specifically to endothelium in certain vascular beds [39-40]. In addition to the specific ability of tumor cells to elaborate surface factors in support of specific tumor–endothelial adhesive interactions in the microvasculature [41], is the knowledge that they release factors that ensure their passage to distant sites.
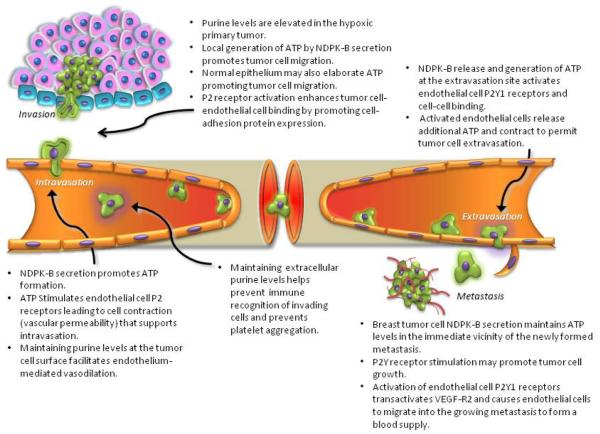
In the case of intravasation and extravasation events, cancer cells may mimic the normal events that leukocytes use to move through the endothelial barrier [42]. In addition to the proteolytic and cell-cell binding events that are critical in transendothelial passage and that are receiving much attention [43], is the release of factors in support of activation of purinergic receptors on endothelial cells. First, hypoxic tumors release purines the their immediate environment [44-45]. In this context, metabolism of such purine is proposed to be associated with tumor progression in breast cancer [46]. Human breast cancer cells in culture secrete a nucleoside diphosphate kinase (sNDPK-B) that supports the activation of ATP receptors (P2Y) on endothelial cells [47]. This kinase, secreted as a phosphoprotein capable of generating ATP from ADP locally [48], would support a cascade of events that would subserve vasodilation [49] and elicit additional vasoactive factor release from endothelial cells including ATP [31,50]. Although the many roles for NDPK that have been described in biology have recently been reviewed [51], the established role for sNDPK-B in breast cancer was ignored, and those seeking to describe cellular localization of NDPKs have not considered its extracellular role [52]. Nonetheless, the ability of metastasizing cells to harness purinergic mechanisms in blood vessels by extracellular elaboration of NDPK-B is well supported by invitro work [47-48,53-55]. The presence and subsequent metabolism of ATP in the blood stream will suppress platelet aggregation as both ATP [56-57] released by endothelium and/or regenerated by sNDPK-B at the site of intravasation/extravasation, as well as in the form of adenosine. Adenosine is a venous vasodilator [58] formed form ATP and ADP by endothelial ectonucleotidases [59]. These actions of purines are both useful if the invading cell is to enter past endothelium and passage to distant sites successfully (Fig 1).
Figure 1. Purinergic Mechanisms Supporting Breast Tumor Metastasis.
The role of purines is described in the context of the events associated with metastasis. Evidence is described in the text.
4. ATP and Cancer
In addition to a mechanism for generating ATP locally, many tumor cells express extracellularly-directed ATP receptors and there is evidence that ATP can suppress the growth of some tumors when carried in animals [60]. The role for purinergic receptor-mediated cancer growth regulation is, however unclear since ATP receptor activation on colorectal cancer cells in culture stimulates growth [61] while this is inhibitory in epithelial carcinoma cells [62]. In the breast cancer cell line MCF-7, the P2U receptor agonized by both ATP and UTP stimulates growth. The explanation for the disparate effects of ATP on growth likely lies in the subtypes of purinergic receptors expressed. Human breast cancer cell lines such as MDA-MB-231 do not express ATP receptors and as such, consideration of the role of ATP P2X or P2Y receptors in tumor suppression in this model is mute. We are not aware that purinergic receptor expression in tumors as they develop and metastasize in women has been addressed but it seems reasonable at this juncture in our understanding to include proliferation of tumor cells in the milieu of purinergic mechanisms that influence tumorigenesis (Fig. 1).
5. Angiogenesis
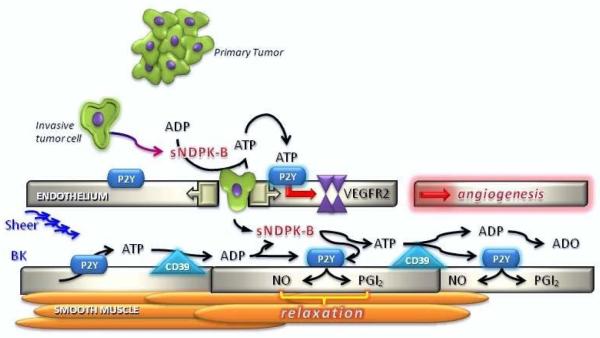
Tumor angiogenesis is the vital process of new blood vessel formation in support of tumor growth, development and metastasis [63-66]. Without access to oxygen and nutrients tumors cannot grow beyond 2-3 mm3 [67-68]. In normal endothelium, cell turnover occurs slowly despite the abundance of angiogenic factors present in the blood stream [69]. The maintenance of endothelial quiescence is thought to be due to the presence of a delicate balance of endogenous negative (anti-angiogenic) and positive (pro-angiogenic) regulators [70] [71]. Tumor formation is favored when these homeostatic regulators fail to function properly. The moment when this failure occurs, when a tumor begins to over-express pro-angiogenic factors and normal endothelial cells proliferate to form new blood vessels is called the angiogenic switch [65,72-75]. Cancer cells begin promoting angiogenesis early in tumorigenesis under mutagenic/hypoxic conditions that drive expression of pro-angiogenic proteins, such as vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), interleukin 8 (IL-8) and transforming growth factor β (TGF-β) [75-77]. Supported by in vitro data [47-48,54-55], we have proposed that under these mutagenic/hypoxic conditions breast cancer cells secrete nucleoside diphosphate kinase (sNDPK-B), which generates ATP from ADP locally and acts on the endothelial nucleotide receptor (P2Y receptor) to regulate blood flow and vascular permeability, which in turn enables metastasis (Fig. 2).
Fgure 2. Secreted NDPK Mediates P2YR/VEGFR-2 Activation in Tumor Angiogenesis.
We propose that breast cancer-secreted sNDPK-B is a significant angiogenesis promoter. sNDPK-B is secreted as a phosphoprotein [48] and elevates ATP levels in both arterioles and capillaries by re-phosphorylating ADP. Continued rounds of ATP production (enhanced normally from endothelium by sheer and agonists such as bradykinin (BK), are generated by sNDPK-B at the expense of nucleotides such as GTP released from necrotic cells in the metastatic environment. Extracellular ATP and ADP activates P2Y1 receptors on vascular ECs, which transactivates VEGFR2 to induce angiogenesis [55]. Additional events consistent with metastasis are supported by sNDPK-B; ATP inhibits platelet aggregation [167] maintaining blood flow despite the presence of foreign tumor cells; activation of P2Y receptors by ATP and ADP results in the release of nitric oxide (NO) and prostacyclin (PGI2) from endothelial cells leading to vasodilatation in arterioles. Breakdown of ADP to adenosine (ADO) inhibits platelet aggregation [168] and dilates venules [169] consistent with increasing the dissemination of tumor cells to distant sites. Dynamic regulation of ATP/ADP levels by sNDPK-B activates VEGFR2 even in the absence of VEGF [55]. At distant sites, P2Y receptor activation may be utilized by the metastatic cells to initiate angiogenesis early in metastasis when VEGF levels are low or absent [170] and will sum with VEGF levels to maximize angiogenic potential [55]. Thus we propose that dual inhibition of P2Y1R and VEGFR2 signaling may provide an effective mode of combination anti-angiogenic adjuvant therapy. P2Y1R-VEGFR2 signaling may be important in describing and understanding the VEGF signaling required for endothelial homeostasis in both tumor as well as normal vasculature.
6. Antiangiogenesis
Angiogenesis inhibitors which block development of new blood vessels, are one promising avenue of cancer research. Many antiangiogenic compounds have been described, including a number which, curiously, are found to be secreted by primary tumors. Angiostatin is a fragment of murine plasminogen generated by implanted primary human tumors and has proven to be a potent angiogenesis inhibitor. Angiostatin suppresses the formation of metastatic secondary tumors in the mouse model [12] and blocks the action of sNDPK-B in in vitro studies [78].
The mechanism by which anti-angiogenic factors work is unclear. Clinical success has been demonstrated with monoclonal antibodies to VEGF (bevacizumab, Avastin®) and epidermal growth factor receptors (EFGR) cetuximab (Erbitux®), gefitinib (Iressa®), and the related Her2 protein (trastuzumab/Herceptin®). Tyrosine kinase inhibitors have also demonstrated a clinical utility in inhibiting VEGF receptor, epidermal growth factor receptor, and platelet-derived growth factor receptor [79]. However, all of these anti-angiogenic therapies tend to exhibit low response rates when used as a monotherapy [80], are astonishingly expensive (annual treatment cost for Herceptin® is ~$80,000 USD and if given with Avastin®, as is often the case in advanced breast cancer, costs are well over $100,000 a year) [81-82] and resistance to these agents tends to build quickly [83]. This is likely due to the existence of multiple pathways available to stimulate angiogenesis, and the up-regulation of alternative pro-angiogenic signals in an evasive manner by the tumor [84]. Clearly clinicians are only able to partially prevent the angiogenic switch from being thrown.
Standard chemotherapy employs a varied arsenal of drugs that attack all dividing cells in the body leading to treatment-related toxicity [85]. Anti-angiogenic therapy, on the other hand targets only dividing endothelial cells with no measurable toxicity [86]. Genetic instability of tumor cells (high rate of mutation), which leads to the overgrowth of drug-resistant tumor cell populations, is a major problem with standard chemotherapeutic agents [87]. Tumor cells also over-express p-glycoprotein, a transport pump which pumps chemotherapeutics drugs out of the cells, reducing therapeutic effectiveness [87-89] and compounding treatment failure.
Anti-angiogenic drugs, such as Avastin® (Genentech), have proven useful in combination with therapies directly aimed at tumor cells, by normalizing the vasculature in the tumor, thereby allowing better drug access and perfusion [90-92]. Moreover, anti-angiogenic therapy is progressing toward the goal of terminating cancer growth completely by denying the tumor a blood supply. In addition to Avastin®, two more FDA-approved drugs sorafenib (Nexavar®, Onyx) and sunitinib (Sutent® [SU11248], Pfizer) are mixed tyrosine kinase inhibitors that inhibit several protein kinases, such as Raf kinase and VEGFR2 kinase [93].
7. The Nucleotide Axis Hypothesis
Although once viewed as improbable if not even foolish to propose, it is now well established by many laboratories [94], including our own that cells release ATP as a local hormone [31,95]. Endothelial cells release ATP in response to sheer stress [96] as well as vasoactive mediators such as bradykinin and even ATP itself acting at the P2Y receptor [95]. Red blood cells are a rich source of ATP and release the nucleotide under hypoxic conditions [97]. ATP acting at its endothelial membrane receptor stimulates the release of nitric oxide and prostacyclin [98], both potent vasorelaxants that increase blood flow in local vascular beds. The endothelial cell expresses both P2Y1 as well as P2Y2 receptors [99] and both are stimulated by ATP and can be specifically antagonized by selective compounds [100-101]. We have proposed that nucleotides and adenosine in the blood stream constitute an hormonal axis that subserves the regulation of blood flow in vascular beds not primarily served by nervous regulation [31,49]. ATP acts immediately upon release as an autocrine and paracrine hormone by stimulating endothelial nucleotide receptors (P2Y1/2), and again following its metabolism to ADP (a more potent P2Y1 agonist than ATP) to dilate arterial resistance vessels since ADP is a nucleotide receptor agonist. The presence on endothelium of enzyme activities that degrade ATP to ADP [102] as well as an ecto-ADP-kinase that can regenerate ATP from ADP [95] suggests that nucleotide levels in the relatively acellular boundary layer next to endothelium [103] can be maintained to act in time and space in the blood stream at nucleotide receptors. The released purine can finally act again in the venous circulation as adenosine where it dilates the venous circulation to accommodate the increased flow signaled upstream and activates endothelial adenosine receptors [104] and inhibits platelet aggregation (Fig.2).
8. Intravasation/Extravasation of Tumor Cells
The extracellular actions of nucleotides also mediate changes in endothelial cell behavior consistent with events required for intravasation and extravasation of cancer cells to and from the blood stream [105]. Given then that nucleotides appear in the blood stream [106] and act to promote vasodilatation, antagonize platelet aggregation and stimulate endothelial cell migration, it is reasonable to wonder if tumor cells might exploit such mechanisms to metastasize (Fig. 1). In addition, the regulation of extracellular nucleotides is being recognized as important to the proliferation of various cancers [60,107]. The current clinical trials of the nucleotide receptor inhibitor drug suramin [108-109] which is known to inhibit growth factor signaling including that of nucleotide receptors involved in cancer growth and angiogenesis [110-111], emphasizes the role of nucleotides in tumor progression and legitimizes interest in nucleotide generating mechanisms in metastasis.
We have identified a cancer cell secreted molecule, nucleotide diphosphate kinase (sNDPK-B) that we propose can pathologically elevate extracellular trinucleotide levels at the expense of necrotic cells in the developing tumor, purines in the region of intravasation/extravasation from vessels and substrate in the blood stream. Inhibition of this nucleoside kinase and/or blockade of the actions of nucleotides at their receptors may constitute an additional therapeutic target in highly metastatic tumors such as breast cancer.
9. NDPK Inhibitors
Two NDPK-B inhibitors, epigallocatechin gallate (EGCG) and ellagic acid (EA), are both anticancer agents [112] and anti-angiogenic agents [113-114]. EGCG has been shown to inhibit cancer-associated enzymes such as matrix metalloproteinases (MMPs) [115] and the proteasome [116] and our laboratory has shown that EGCG and EA inhibit cancer cell-secreted NDPK-B transphosphorylase activity and inhibit in vitro angiogenesis [54]. We have shown that EA is a more potent sNDPK-B inhibitor based on the reduction of extracellular ATP levels [53] that, in part explains why EA is a more potent inhibitor of CD31+ endothelial cell angiogenesis (tubulogenesis) than is EGCG. Investigating the role for sNDPK-B inhibition as a way to manage metastasis in breast cancer and possibly, in other types of solid tumor cancers [48], is worthy of significant interest.
10. A Role for sNDPK-B
Nm23/NDPK enzymatic activity functions primarily as a nucleoside diphosphate kinase regenerating ATP levels for intracellular “housekeeping” enzymes by covalently transferring the γ-phosphate from a nucleoside triphosphate (NTP) such as GTP, to a nucleoside diphosphate acceptor (NDP; e.g., ADP) and has also been shown to act as a histidine kinase, a transcription activator and an exonuclease [117]. There are eight isoforms of this plurifunctional protein [118]. NDPK is distributed in the cytosol and plasma membrane, as well as the nucleus [119] where the NDPK-B isoform functions as PuF, a c-MYC transcription factor [117]. NM23 was originally described as non-metastatic 23 gene, which was found in mouse carcinoma cells as a homolog of the drosophila awd protein (altered wing disk) and was strictly thought to be inversely related to metastasis potential [120-121] although this has been shown to be less straightforward than originally proposed [117,122-124]. Indeed, less attention has been paid to NDPK-B’s enzymatic function in cancer and metastasis, and no attention paid other than our own findings to the secreted sNDPK-B isoform even though there is now evidence to support NM23’s putative role in promoting metastasis [117,125]. Nm23-h1 (NDPK-A) protein levels have been found in studies of patients with breast carcinoma [121,126-128] but our hypothesis regarding the secretion and extracellular actions of the sNDPK-B have not as yet been evaluated in vivo.
Ecto-NDPK (on the cell surface) is known to regulate extracellular nucleotide levels in various cell types [46,129-130]. Hamby et al. confirmed that the catalytically inactive H118Y mutant of NDPK-B significantly suppressed the lung metastasis of human melanoma cells in vivo [131]. NDPK (A or B) is secreted by, or present in the blood stream from various solid and hematological malignancies [48,132-135]. The disruption of CD39 (ecto-apyrase; EC 3.6.1.5) activity, the dominant vascular ectonucleotidases, and its regulation of nucleotide signaling has been observed to inhibit tumor angiogenesis and metastasis [136-137] consistent with, if not directly demonstrative of a role for sNDPK-B in promoting angiogenesis. We discovered that both MDA-MB-435S and MDA-MB-231 metastatic human breast carcinoma cells secrete sNDPK-B into their surrounding environment when cultured in vitro [48] while the non-metastatic breast cell line MCF-12 does not (Yokdang, unpublished observation).
Recently we have provided evidence for a nucleotide-dependent regulation of angiogenesis by breast cancer secreted sNDPK-B [47,54]. This can be mechanistically related to extracellular nucleotide elevation and subsequent activation of nucleotide receptors to regulate cancer growth [20,60] and tumor angiogenesis [136-137]. Our results show that pathologically-secreted sNDPK-B and its regulation of extracellular nucleotides utilize P2Y receptors to stimulate angiogenesis [55]. Based on our findings and published reports from other laboratories, we propose that secreted sNDPK-B regulates growth and development of metastases by stimulating angiogenesis and may facilitate intravasation, migration and extravasation early in the metastatic process (Fig. 1,2).
11. Vascular P2Y Nucleotide Receptors
Purinergic nucleotide (P2) receptors activated by ATP include both ligand-gated ion channels (P2X) and heterotrimeric G protein-coupled (P2Y) receptors [60]. P2Y receptors are recognized as important regulators of carcinogenesis and endothelial cell functions [138-139] and as described above, are integral modulators of platelet aggregation and blood flow regulation. Extracellular ATP activates P2Y1/2 receptors on vascular endothelial cells to release vasoactive mediators such as nitric oxide, prostacyclin, and additional ATP [31,95,107] which can elicit vasoactive and angiogenic effects and propagate these effects down-stream [140]. There are a myrid of possibilities presented by purinergic receptor expression [99] of both P2X, P2Y and adenosine receptor classes that may describe differences in vascular beds and thus particular targeting of metastasizing cells as well as disparate outcomes of receptor stimulation including apoptosis of tumor cells [141-143]. We focus on P2Y receptors and P2Y1 in particular as inhibitor studies suggest that these receptors are critical, if not exclusive, to understanding the mechanisms that support metastasis and angiogenesis.
In addition to our own work, only a handful of published reports provide evidence of the role of P2Y receptors in angiogenesis [34-35,136-137,144-147]. Therefore, information regarding this subject is limited. What is known is that endothelial P2Y receptors have been shown to stimulate angiogenic properties such as endothelial cell migration [144-145] and vascular permeability [34]. Activated P2Y2 receptors have also been observed to transactivate VEGFR-2, suggesting a direct link between extracellular nucleotide regulation and established growth factor signaling [148]. Recent evidence supports this cooperation and transactivation of a receptor tyrosine kinase (RTK; such as VEGFR2) by P2Y receptors (GPCR): Buvinic et al. observed that P2Y1R utilizes epithelial growth factor receptor (EGFR) signaling to stimulate cell proliferation [149] and we have provided in vitro evidence that endothelial P2Y1 receptor activation and subsequent VEGFR-2 signaling explains angiogenic signaling by nucleotides such as ATP [55].
12. Vascular Endothelial Growth Factor Receptor (VEGF-R)
VEGFR-2 (FLK-1) is thought to be the primary regulator of angiogenesis mediating the majority of the angiogenic and permeability-enhancing effects of VEGF [150]. Angiogenesis is generally thought to occur in capillary and post capillary beds [151] initiated by the production of proteases in stimulated endothelial cells, that degrade the surrounding basement membrane [152] and migrate into the tissue, where they proliferate and differentiate to form a new vessel. Endothelial cells produce specific growth factors, such as PDGF and TGF-β, that then attract supporting cells that produce basement membrane and mature the vessel [153-154]. Vessels surrounded by basement membrane and pericytes are regarded as mature.
Embryonic development, ovulation, wound healing and menstruation all require angiogenesis, however pathological angiogenesis occurs in a wide range of diseases, including tumors, chronic inflammatory diseases, and diabetic retinopathy, which results in hyper-permeable vessels with disorganized architecture and blind non-patent endings [155]. Tumor promoted vessels are unstable and immature and lack a complete basement membrane and pericytes [156]. Tumors may also establish circulation independent of angiogenesis, although the role of microvessel mimicry [157] that has been described in inflammatory breast cancer [158] and may be a feature of about 8% of surgically removed breast cancers [159] is not likely to subserve breast metastatic lesions as it seems to be a feature of non-metastasizing lesions [160].
In mammals, the VEGF pathway involves five secreted ligands (VEGF-A, VEGF-B, VEGF-C, VEGF-D and placental growth factor) and three primary receptors (VEGF-R1, VEGF-R2, VEGF-R3). The ligands are widely expressed and expression of VEGF-A is induced by hypoxia, which is a common feature of rapidly growing solid tumors and constitutes a key angiogenic signal. VEGF receptors show restricted cell-type expression. VEGF-R1 and VEGF-R2 are prominently expressed by vascular endothelial cells in addition to expression by other selected cell types. VEGF-A interacts with VEGF-R1 and VEGF-R2, whereas placental growth factor interacts strictly with VEGF-R1. The VEGF receptors are tyrosine kinases that autophosphorylate upon dimerization following ligand binding, which in turn activates various down-stream signaling pathways such as the p38 MAPK pathway. VEGF receptors signal diverse effects in endothelial cells including migration, proliferation, cell survival and regulation of gene expression [156].
13. VEGF/ATP in Angiogenesis
In pathological angiogenesis induced by breast cancer cells, the levels of VEGF-R1 and VEGF-R2 mRNA are significantly increased by exposure to the tumor cells and the conditions that accrue such as hypoxia, as compared to the amounts observed in vessels of normal tissues surrounding the tumors [161]. This suggests that the ability of endothelial cells to respond to VEGF may lag behind that of ATP acting at P2Y receptors, whose expression is not delayed [162] and thus the sNDPK-B:ATP:P2Y pathway we describe may be effective in initiating angiogenesis and lowering the requirement of the angiogenic process for VEGF-R [55].
VEGF-R2 and P2Y receptors have been shown to localize in caveolar domains in the cell membrane [139,163-166] allowing the potential for substantial pro-angiogenic signaling with small amounts of agonist stimulation if these receptor systems were to interact in a spatially restricted region.
Conclusions
Our model proposes that sNDPK-B secretion by breast cancer cells both adjacent to (as primary tumors or micrometastases) and within capillaries (as intravasated tumor cells) elevates local ATP concentrations to permit entry of cells into the blood stream and to maintain their ability to move to distant sites once in the blood stream. Once at a distant site, breast tumor cells again benefit from local ATP production to extravasate and form a metastatic tumor. In the absence of suppressive factors such as those released from the primary tumor, distant tumor cells release sNDPK-B to induce endothelial cell tubulogenesis by generating ATP locally leading to activation of the endothelial P2Y receptor and transphosphorylation of VEGFR2 in the absence of VEGF (Fig. 1,2). Once the metastatic cells gain a blood supply and begin to proliferate, VEGF as well as ATP signal further angiogenesis as the tumor grows.
It is clear that elevated ATP levels from sNDPK-B and subsequent P2YR activation by ATP and ADP produce angiogenic signals in vitro. While the mechanisms by which P2Y receptors mediate vasodilatation and anti-platelet aggregation (advantageous to the transit of cancer cells to secondary sites) have been well worked out and are accepted, a role for these mechanisms in tumor angiogenesis remains to be defined in vivo. Demonstration of a role for P2Y signaling as orchestrated by the circulating cancer cell and subsequent micrometastases offers an exciting opportunity to add a one or more novel therapeutic targets to our adjuvant armamentarium.
Acknowledgements
This work was supported by US National Institutes of Health grant HD053028 to ILOB; fellowship stipend support to NY from NIH T32 CA09563, and a grant from the Clayton Foundation for Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- [1].Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer Statistics, 2009. CA Cancer J.Clin. 2009 doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- [2].Coleman MP, Quaresma M, Berrino F, Lutz JM, De AR, Capocaccia R, Baili P, Rachet B, Gatta G, Hakulinen T, Micheli A, Sant M, Weir HK, Elwood JM, Tsukuma H, Koifman S, Ga ES, Francisci S, Santaquilani M, Verdecchia A, Storm HH, Young JL. Cancer survival in five continents: a worldwide population-based study (CONCORD) Lancet Oncol. 2008;9:730–756. doi: 10.1016/S1470-2045(08)70179-7. [DOI] [PubMed] [Google Scholar]
- [3].Hagemeister FB, Jr., Buzdar AU, Luna MA, Blumenschein GR. Causes of death in breast cancer: a clinicopathologic study. Cancer. 1980;46:162–167. doi: 10.1002/1097-0142(19800701)46:1<162::aid-cncr2820460127>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- [4].Rugo HS. The importance of distant metastases in hormone-sensitive breast cancer. Breast. 2008;17(Suppl 1):S3–S8. doi: 10.1016/S0960-9776(08)70002-X. [DOI] [PubMed] [Google Scholar]
- [5].Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- [6].Retsky M, Demicheli R, Hrushesky W, Speer J, Swartzendruber D, Wardwell R. Recent translational research: computational studies of breast cancer. Breast Cancer Res. 2005;7:37–40. doi: 10.1186/bcr981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Demicheli R, Retsky MW, Hrushesky WJ, Baum M, Gukas ID. The effects of surgery on tumor growth: a century of investigations. Ann.Oncol. 2008;19:1821–1828. doi: 10.1093/annonc/mdn386. [DOI] [PubMed] [Google Scholar]
- [8].Retsky MW, Demicheli R, Hrushesky WJ, Baum M, Gukas ID. Dormancy and surgery-driven escape from dormancy help explain some clinical features of breast cancer. APMIS. 2008;116:730–741. doi: 10.1111/j.1600-0463.2008.00990.x. [DOI] [PubMed] [Google Scholar]
- [9].Hansen NM, Ye X, Grube BJ, Giuliano AE. Manipulation of the primary breast tumor and the incidence of sentinel node metastases from invasive breast cancer. Arch Surg. 2004;139:634–639. doi: 10.1001/archsurg.139.6.634. discussion 639-640. [DOI] [PubMed] [Google Scholar]
- [10].Peters-Engl C, Konstantiniuk P, Tausch C, Haid A, Hoffmann B, Jagoutz-Herzlinger M, Kugler F, Redtenbacher S, Roka S, Schrenk P, Steinmassl D. The impact of preoperative breast biopsy on the risk of sentinel lymph node metastases: analysis of 2502 cases from the Austrian Sentinel Node Biopsy Study Group. Br J Cancer. 2004;91:1782–1786. doi: 10.1038/sj.bjc.6602205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Demicheli R, Retsky MW, Hrushesky WJ, Baum M. Tumor dormancy and surgery-driven interruption of dormancy in breast cancer: learning from failures. Nat.Clin.Pract.Oncol. 2007;4:699–710. doi: 10.1038/ncponc0999. [DOI] [PubMed] [Google Scholar]
- [12].O’Reilly MS, Holmgren L, Shing Y, Chen C, Rosenthal RA, Cao Y, Moses M, Lane WS, Sage EH, Folkman J. Angiostatin: a circulating endothelial cell inhibitor that suppresses angiogenesis and tumor growth. Cold Spring Harb.Symp.Quant.Biol. 1994;59:471–482. doi: 10.1101/sqb.1994.059.01.052. [DOI] [PubMed] [Google Scholar]
- [13].O’Reilly MS, Holmgren L, Shing Y, Chen C, Rosenthal RA, Moses M, Lane WS, Cao Y, Sage EH, Folkman J. Angiostatin: a novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma. Cell. 1994;79:315–328. doi: 10.1016/0092-8674(94)90200-3. [DOI] [PubMed] [Google Scholar]
- [14].Zahl PH, Maehlen J, Welch HG. The natural history of invasive breast cancers detected by screening mammography. Arch.Intern.Med. 2008;168:2311–2316. doi: 10.1001/archinte.168.21.2311. [DOI] [PubMed] [Google Scholar]
- [15].Almog N, Ma L, Raychowdhury R, Schwager C, Erber R, Short S, Hlatky L, Vajkoczy P, Huber PE, Folkman J, Abdollahi A. Transcriptional switch of dormant tumors to fast-growing angiogenic phenotype. Cancer Research. 2009;69:836–844. doi: 10.1158/0008-5472.CAN-08-2590. [DOI] [PubMed] [Google Scholar]
- [16].Alix-Panabieres C, Muller V, Pantel K. Current status in human breast cancer micrometastasis. Curr.Opin.Oncol. 2007;19:558–563. doi: 10.1097/CCO.0b013e3282f0ad79. [DOI] [PubMed] [Google Scholar]
- [17].Erbas B, Provenzano E, Armes J, Gertig D. The natural history of ductal carcinoma in situ of the breast: a review. Breast Cancer Res.Treat. 2006;97:135–144. doi: 10.1007/s10549-005-9101-z. [DOI] [PubMed] [Google Scholar]
- [18].Sanders ME, Schuyler PA, Dupont WD, Page DL. The natural history of low-grade ductal carcinoma in situ of the breast in women treated by biopsy only revealed over 30 years of long-term follow-up. Cancer. 2005;103:2481–2484. doi: 10.1002/cncr.21069. [DOI] [PubMed] [Google Scholar]
- [19].Westfall DP, Sedaa KO, Shinozuka K, Bjur RA, Buxton IL. ATP as a cotransmitter. Ann.N.Y.Acad.Sci. 1990;603:300–310. doi: 10.1111/j.1749-6632.1990.tb37681.x. [DOI] [PubMed] [Google Scholar]
- [20].Burnstock G. Purinergic signalling. British Journal of Pharamcology. 2006;147(Suppl 1):S172–S181. doi: 10.1038/sj.bjp.0706429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Burnstock G. Do some nerve cells release more than one transmitter? Neuroscience. 1976;1:239–248. doi: 10.1016/0306-4522(76)90054-3. [DOI] [PubMed] [Google Scholar]
- [22].Westfall DP, Stitzel RE, Rowe JN. The postjunctional effects and neural release of purine compounds in the guinea-pig vas deferens. Eur J Pharmacol. 1978;50:27–38. doi: 10.1016/0014-2999(78)90250-9. [DOI] [PubMed] [Google Scholar]
- [23].Schwiebert EM, Zsembery A. Extracellular ATP as a signaling molecule for epithelial cells. Biochim Biophys Acta. 2003;1615:7–32. doi: 10.1016/s0005-2736(03)00210-4. [DOI] [PubMed] [Google Scholar]
- [24].Christofori G. New signals from the invasive front. Nature. 2006;441:444–450. doi: 10.1038/nature04872. [DOI] [PubMed] [Google Scholar]
- [25].Muller WA. Leukocyte-endothelial-cell interactions in leukocyte transmigration and the inflammatory response. Trends Immunol. 2003;24:327–334. doi: 10.1016/s1471-4906(03)00117-0. [DOI] [PubMed] [Google Scholar]
- [26].Wittchen ES. Endothelial signaling in paracellular and transcellular leukocyte transmigration. Front Biosci. 2009;14:2522–2545. doi: 10.2741/3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Rowe RG, Weiss SJ. Breaching the basement membrane: who, when and how? Trends Cell Biol. 2008;18:560–574. doi: 10.1016/j.tcb.2008.08.007. [DOI] [PubMed] [Google Scholar]
- [28].Sud’ina GF, Mirzoeva OK, Galkina SI, Pushkareva MA, Ullrich V. Involvement of ecto-ATPase and extracellular ATP in polymorphonuclear granulocyte-endothelial interactions. FEBS Lett. 1998;423:243–248. doi: 10.1016/s0014-5793(98)00102-1. [DOI] [PubMed] [Google Scholar]
- [29].Ferrero ME. A new approach to the inflammatory/autoimmune diseases. Recent Pat Antiinfect Drug Discov. 2009;4:108–113. doi: 10.2174/157489109788490343. [DOI] [PubMed] [Google Scholar]
- [30].Eltzschig HK, Eckle T, Mager A, Kuper N, Karcher C, Weissmuller T, Boengler K, Schulz R, Robson SC, Colgan SP. ATP release from activated neutrophils occurs via connexin 43 and modulates adenosine-dependent endothelial cell function. Circ Res. 2006;99:1100–1108. doi: 10.1161/01.RES.0000250174.31269.70. [DOI] [PubMed] [Google Scholar]
- [31].Yang S, Cheek DJ, Westfall DP, Buxton IL. Purinergic axis in cardiac blood vessels. Agonist-mediated release of ATP from cardiac endothelial cells. Circ Res. 1994;74:401–407. doi: 10.1161/01.res.74.3.401. [DOI] [PubMed] [Google Scholar]
- [32].Eltzschig HK, Weissmuller T, Mager A, Eckle T. Nucleotide metabolism and cell-cell interactions. Methods Mol Biol. 2006;341:73–87. doi: 10.1385/1-59745-113-4:73. [DOI] [PubMed] [Google Scholar]
- [33].Berne RM. The role of adenosine in the regulation of coronary blood flow. Circ Res. 1980;47:807–813. doi: 10.1161/01.res.47.6.807. [DOI] [PubMed] [Google Scholar]
- [34].Tanaka N, Nejime N, Kagota S, Kubota Y, Yudo K, Nakamura K, Kunitomo M, Takahashi K, Hashimoto M, Shinozuka K. ATP participates in the regulation of microvessel permeability. J.Pharm.Pharmacol. 2006;58:481–487. doi: 10.1211/jpp.58.4.0007. [DOI] [PubMed] [Google Scholar]
- [35].Tanaka N, Kawasaki K, Nejime N, Kubota Y, Takahashi K, Hashimoto M, Kunitomo M, Shinozuka K. P2Y receptor-mediated enhancement of permeation requires Ca2+ signalling in vascular endothelial cells. Clin.Exp.Pharmacol.Physiol. 2003;30:649–652. doi: 10.1046/j.1440-1681.2003.03893.x. [DOI] [PubMed] [Google Scholar]
- [36].McClenahan D, Hillenbrand K, Kapur A, Carlton D, Czuprynski C. Effects of extracellular ATP on bovine lung endothelial and epithelial cell monolayer morphologies, apoptoses, and permeabilities. Clin Vaccine Immunol. 2009;16:43–48. doi: 10.1128/CVI.00282-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Chambers AF, MacDonald IC, Schmidt EE, Morris VL, Groom AC. Clinical targets for anti-metastasis therapy. Adv Cancer Res. 2000;79:91–121. doi: 10.1016/s0065-230x(00)79003-8. [DOI] [PubMed] [Google Scholar]
- [38].Steinbauer M, Guba M, Cernaianu G, Kohl G, Cetto M, Kunz-Schughart LA, Geissler EK, Falk W, Jauch KW. GFP-transfected tumor cells are useful in examining early metastasis in vivo, but immune reaction precludes long-term tumor development studies in immunocompetent mice. Clin Exp Metastasis. 2003;20:135–141. doi: 10.1023/a:1022618909921. [DOI] [PubMed] [Google Scholar]
- [39].Schluter K, Gassmann P, Enns A, Korb T, Hemping-Bovenkerk A, Holzen J, Haier J. Organ-specific metastatic tumor cell adhesion and extravasation of colon carcinoma cells with different metastatic potential. Am J Pathol. 2006;169:1064–1073. doi: 10.2353/ajpath.2006.050566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Glinsky VV, Glinsky GV, Rittenhouse-Olson K, Huflejt ME, Glinskii OV, Deutscher SL, Quinn TP. The role of Thomsen-Friedenreich antigen in adhesion of human breast and prostate cancer cells to the endothelium. Cancer Res. 2001;61:4851–4857. [PubMed] [Google Scholar]
- [41].Glinskii OV, Huxley VH, Glinsky GV, Pienta KJ, Raz A, Glinsky VV. Mechanical entrapment is insufficient and intercellular adhesion is essential for metastatic cell arrest in distant organs. Neoplasia. 2005;7:522–527. doi: 10.1593/neo.04646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Chambers AF, Naumov GN, Varghese HJ, Nadkarni KV, MacDonald IC, Groom AC. Critical steps in hematogenous metastasis: an overview. Surg Oncol Clin N Am. 2001;10:243–255. vii. [PubMed] [Google Scholar]
- [43].Miles FL, Pruitt FL, van Golen KL, Cooper CR. Stepping out of the flow: capillary extravasation in cancer metastasis. Clin Exp Metastasis. 2008;25:305–324. doi: 10.1007/s10585-007-9098-2. [DOI] [PubMed] [Google Scholar]
- [44].Blay J, White TD, Hoskin DW. The extracellular fluid of solid carcinomas contains immunosuppressive concentrations of adenosine. Cancer Res. 1997;57:2602–2605. [PubMed] [Google Scholar]
- [45].Spychala J. Tumor-promoting functions of adenosine. Pharmacol Ther. 2000;87:161–173. doi: 10.1016/s0163-7258(00)00053-x. [DOI] [PubMed] [Google Scholar]
- [46].Spychala J, Lazarowski E, Ostapkowicz A, Ayscue LH, Jin A, Mitchell BS. Role of estrogen receptor in the regulation of ecto-5′-nucleotidase and adenosine in breast cancer. Clin.Cancer Res. 2004;10:708–717. doi: 10.1158/1078-0432.ccr-0811-03. [DOI] [PubMed] [Google Scholar]
- [47].Rumjahn SM, Javed MA, Wong N, Law WE, Buxton IL. Purinergic regulation of angiogenesis by human breast carcinoma-secreted nucleoside diphosphate kinase. British Journal of Cancer. 2007;97:1372–1380. doi: 10.1038/sj.bjc.6604019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Anzinger J, Malmquist NA, Gould J, Buxton IL. Secretion of a nucleoside diphosphate kinase (Nm23-H2) by cells from human breast, colon, pancreas and lung tumors. Proc.West Pharmacol.Soc. 2001;44:61–63. [PubMed] [Google Scholar]
- [49].Oxhorn BC, Cheek DJ, Buxton IL. Role of nucleotides and nucleosides in the regulation of cardiac blood flow. AACN.Clin.Issues. 2000;11:241–251. doi: 10.1097/00044067-200005000-00007. [DOI] [PubMed] [Google Scholar]
- [50].Bogle RG, Coade SB, Moncada S, Pearson JD, Mann GE. Bradykinin and ATP stimulate l-arginine uptake and nitric oxide release in vascular endothelial cells. Biochemical and Biophysical Research Communications. 1991;180(2):926–932. doi: 10.1016/s0006-291x(05)81154-4. [DOI] [PubMed] [Google Scholar]
- [51].Mehta A, Orchard S. Nucleoside diphosphate kinase (NDPK, NM23, AWD): recent regulatory advances in endocytosis, metastasis, psoriasis, insulin release, fetal erythroid lineage and heart failure; translational medicine exemplified. Mol Cell Biochem. 2009;329:3–15. doi: 10.1007/s11010-009-0114-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Bosnar MH, Bago R, Cetkovic H. Subcellular localization of Nm23/NDPK A and B isoforms: a reflection of their biological function? Mol Cell Biochem. 2009;329:63–71. doi: 10.1007/s11010-009-0107-4. [DOI] [PubMed] [Google Scholar]
- [53].Malmquist NA, Anzinger JJ, Hirzel D, Buxton IL. Ellagic acid inhibits nucleoside diphosphate kinase-B activity. Proc.West Pharmacol.Soc. 2001;44:57–59. [PubMed] [Google Scholar]
- [54].Rumjahn SM, Baldwin KA, Buxton IL. P2y receptor-mediated angiogenesis via vascular endothelial growth factor receptor 2 signaling. Proc.West Pharmacol.Soc. 2007;50:58–60. [PMC free article] [PubMed] [Google Scholar]
- [55].Rumjahn SM, Yokdang N, Baldwin KA, Thai J, Buxton IL. Purinergic regulation of vascular endothelial growth factor signaling in angiogenesis. British Journal of Cancer. 2009;100:1465–1470. doi: 10.1038/sj.bjc.6604998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Packham MA, Ardlie NG, Mustard JF. Effect of adenine compounds on platelet aggregation. American Journal of Physiology. 1969;217(4):1009–1017. doi: 10.1152/ajplegacy.1969.217.4.1009. [DOI] [PubMed] [Google Scholar]
- [57].Soslau G, Brodsky I, Parker J. Occupancy of P 2 purinoceptors with unique properties modulates the function of human platelets. Biochim.Biophys.Acta Mol.Cell Res. 1993;1177:199–207. doi: 10.1016/0167-4889(93)90041-m. [DOI] [PubMed] [Google Scholar]
- [58].Rubio R, Wiedmeier VT, Berne RM. Relationship Between Coronary Flow and Adenosine Production and Release. J.Mol.and Cell Cardiol. 1974;6:561–566. doi: 10.1016/0022-2828(74)90036-4. [DOI] [PubMed] [Google Scholar]
- [59].Gordon EL, Pearson JD, Slakey LL. The hydrolysis of extracellular adenine nucleotides by cultured endothelial cells from pig aorta. Feed-forward inhibition of adenosine production at the cell surface. J Biol.Chem. 1986;261:15496–15507. [PubMed] [Google Scholar]
- [60].White N, Burnstock G. P2 receptors and cancer. Trends in Pharmacological Sciences. 2006;27:211–217. doi: 10.1016/j.tips.2006.02.004. [DOI] [PubMed] [Google Scholar]
- [61].Hopfner M, Lemmer K, Jansen A, Hanski C, Riecken EO, Gavish M, Mann B, Buhr H, Glassmeier G, Scherubl H. Expression of functional P2-purinergic receptors in primary cultures of human colorectal carcinoma cells. Biochem Biophys Res Commun. 1998;251:811–817. doi: 10.1006/bbrc.1998.9555. [DOI] [PubMed] [Google Scholar]
- [62].Coutinho-Silva R, Stahl L, Cheung KK, de Campos NE, de Oliveira Souza C, Ojcius DM, Burnstock G. P2X and P2Y purinergic receptors on human intestinal epithelial carcinoma cells: effects of extracellular nucleotides on apoptosis and cell proliferation. Am J Physiol Gastrointest Liver Physiol. 2005;288:G1024–1035. doi: 10.1152/ajpgi.00211.2004. [DOI] [PubMed] [Google Scholar]
- [63].Bautch VL, Ambler CA. Assembly and patterning of vertebrate blood vessels. Trends Cardiovasc.Med. 2004;14:138–143. doi: 10.1016/j.tcm.2004.02.002. [DOI] [PubMed] [Google Scholar]
- [64].Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocrine Reviews. 2004;25:581–611. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- [65].Jain RK. Molecular regulation of vessel maturation. Nat.Med. 2003;9:685–693. doi: 10.1038/nm0603-685. [DOI] [PubMed] [Google Scholar]
- [66].Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J.Clin.Oncol. 2005;23:1011–1027. doi: 10.1200/JCO.2005.06.081. [DOI] [PubMed] [Google Scholar]
- [67].Folkman J. Tumor angiogenesis: therapeutic implications. New England Journal of Medicine. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- [68].Holmgren L, O’Reilly MS, Folkman J. Dormancy of micrometastases: balanced proliferation and apoptosis in the presence of angiogenesis suppression. Nat.Med. 1995;1:149–153. doi: 10.1038/nm0295-149. [DOI] [PubMed] [Google Scholar]
- [69].Duncker DJ, Bache RJ. Regulation of coronary blood flow during exercise. Physiol Rev. 2008;88:1009–1086. doi: 10.1152/physrev.00045.2006. [DOI] [PubMed] [Google Scholar]
- [70].Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat.Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- [71].Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- [72].Nyberg P, Xie L, Kalluri R. Endogenous inhibitors of angiogenesis. Cancer Research. 2005;65:3967–3979. doi: 10.1158/0008-5472.CAN-04-2427. [DOI] [PubMed] [Google Scholar]
- [73].Rastinejad F, Polverini PJ, Bouck NP. Regulation of the activity of a new inhibitor of angiogenesis by a cancer suppressor gene. Cell. 1989;56:345–355. doi: 10.1016/0092-8674(89)90238-9. [DOI] [PubMed] [Google Scholar]
- [74].Udagawa T, Fernandez A, Achilles EG, Folkman J, D’Amato RJ. Persistence of microscopic human cancers in mice: alterations in the angiogenic balance accompanies loss of tumor dormancy. FASEB J. 2002;16:1361–1370. doi: 10.1096/fj.01-0813com. [DOI] [PubMed] [Google Scholar]
- [75].Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat.Rev.Cancer. 2003;3:401–410. doi: 10.1038/nrc1093. [DOI] [PubMed] [Google Scholar]
- [76].Kesisis G, Broxterman H, Giaccone G. Angiogenesis inhibitors. Drug selectivity and target specificity. Curr.Pharm.Des. 2007;13:2795–2809. doi: 10.2174/138161207781757033. [DOI] [PubMed] [Google Scholar]
- [77].Jain RK, Duda DG, Clark JW, Loeffler JS. Lessons from phase III clinical trials on anti-VEGF therapy for cancer. Nat.Clin.Pract.Oncol. 2006;3:24–40. doi: 10.1038/ncponc0403. [DOI] [PubMed] [Google Scholar]
- [78].Buxton ILO. Inhibition of nm23 gene product (NDPK-B) by angiostatin, polyphenols and nucleoside analogs. Proceedings of the Western Pharmacology Society. 2008;51:30–34. [PMC free article] [PubMed] [Google Scholar]
- [79].Brown AP, Citrin DE, Camphausen KA. Clinical biomarkers of angiogenesis inhibition. Cancer Metastasis Rev. 2008;27:415–434. doi: 10.1007/s10555-008-9143-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Gonzalez-Angulo AM, Morales-Vasquez F, Hortobagyi GN. Overview of resistance to systemic therapy in patients with breast cancer. Advances in Experimental Medicine and Biology. 2007;608:1–22. doi: 10.1007/978-0-387-74039-3_1. [DOI] [PubMed] [Google Scholar]
- [81].Poncet B, Bachelot T, Colin C, Ganne C, Jaisson-Hot I, Orfeuvre H, Peaud PY, Jacquin JP, Salles B, Tigaud JD, Mechin-Cretinon I, Marechal F, Fournel C, Trillet-Lenoir V. Use of the monoclonal antibody anti-HER2 trastuzumab in the treatment of metastatic breast cancer: a cost-effectiveness analysis. Am.J.Clin.Oncol. 2008;31:363–368. doi: 10.1097/COC.0b013e3181637356. [DOI] [PubMed] [Google Scholar]
- [82].Kolesar JM. Bevacizumab: improved survival at what cost? Am.J.Health Syst.Pharm. 2005;62:1017. doi: 10.1093/ajhp/62.10.1017. [DOI] [PubMed] [Google Scholar]
- [83].Viloria-Petit A, Crombet T, Jothy S, Hicklin D, Bohlen P, Schlaeppi JM, Rak J, Kerbel RS. Acquired resistance to the antitumor effect of epidermal growth factor receptor-blocking antibodies in vivo: a role for altered tumor angiogenesis. Cancer Research. 2001;61:5090–5101. [PubMed] [Google Scholar]
- [84].Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat.Rev.Cancer. 2008;8:592–603. doi: 10.1038/nrc2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].I. National Cancer. Angiogenesis Inhibitors in Cancer Research. 1999.
- [86].I. National Cancer. SEER Cancer Statistics Review 1975-2005. 2008.
- [87].Gottesman MM. Mechanisms of cancer drug resistance. Annu.Rev.Med. 2002;53:615–627. doi: 10.1146/annurev.med.53.082901.103929. [DOI] [PubMed] [Google Scholar]
- [88].Juliano RL, Ling V. A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochemica et Biophysica Acta. 1976;455:152–162. doi: 10.1016/0005-2736(76)90160-7. [DOI] [PubMed] [Google Scholar]
- [89].Ueda K, Cardarelli C, Gottesman MM, Pastan I. Expression of a full-length cDNA for the human “MDR1” gene confers resistance to colchicine, doxorubicin, and vinblastine. Proc.Natl.Acad.Sci.U.S.A. 1987;84:3004–3008. doi: 10.1073/pnas.84.9.3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Ansiaux R, Baudelet C, Jordan BF, Beghein N, Sonveaux P, De WJ, Martinive P, Gregoire V, Feron O, Gallez B. Thalidomide radiosensitizes tumors through early changes in the tumor microenvironment. Clin.Cancer Res. 2005;11:743–750. [PubMed] [Google Scholar]
- [91].Jain RK. Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Nat.Med. 2001;7:987–989. doi: 10.1038/nm0901-987. [DOI] [PubMed] [Google Scholar]
- [92].Tong RT, Boucher Y, Kozin SV, Winkler F, Hicklin DJ, Jain RK. Vascular normalization by vascular endothelial growth factor receptor 2 blockade induces a pressure gradient across the vasculature and improves drug penetration in tumors. Cancer Research. 2004;64:3731–3736. doi: 10.1158/0008-5472.CAN-04-0074. [DOI] [PubMed] [Google Scholar]
- [93].Kruck S, Kuczyk MA, Gakis G, Kramer MW, Stenzl A, Merseburger AS. Novel therapeutic options in metastatic renal cancer - review and post ASCO 2007 update. Rev.Recent Clin.Trials. 2008;3:212–216. doi: 10.2174/157488708785700311. [DOI] [PubMed] [Google Scholar]
- [94].Burnstock G. Vascular control of purines with emphasis on the coronary system. European Heart Journal. 1989;10:15–21. doi: 10.1093/eurheartj/10.suppl_f.15. [DOI] [PubMed] [Google Scholar]
- [95].Buxton IL, Kaiser RA, Oxhorn BC, Cheek DJ. Evidence supporting the Nucleotide Axis Hypothesis: ATP release and metabolism by coronary endothelium. Am.J.Physiol Heart Circ.Physiol. 2001;281:H1657–H1666. doi: 10.1152/ajpheart.2001.281.4.H1657. [DOI] [PubMed] [Google Scholar]
- [96].Bodin P, Burnstock G. ATP-Stimulated Release of ATP by Human Endothelial Cells. J.Cardiovasc.Pharmac. 1996;27:872–875. doi: 10.1097/00005344-199606000-00015. [DOI] [PubMed] [Google Scholar]
- [97].Ellsworth ML, Ellis CG, Goldman D, Stephenson AH, Dietrich HH, Sprague RS. Erythrocytes: oxygen sensors and modulators of vascular tone. Physiology.(Bethesda.) 2009;24:107–116. doi: 10.1152/physiol.00038.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacological Reviews. 1998;50:413–492. [PubMed] [Google Scholar]
- [99].von Kugelgen I, Wetter A. Molecular pharmacology of P2Y-receptors. Naunyn Schmiedebergs Arch Pharmacol. 2000;362:310–323. doi: 10.1007/s002100000310. [DOI] [PubMed] [Google Scholar]
- [100].Ivanov AA, Ko H, Cosyn L, Maddileti S, Besada P, Fricks I, Costanzi S, Harden TK, Calenbergh SV, Jacobson KA. Molecular modeling of the human P2Y2 receptor and design of a selective agonist, 2′-amino-2′-deoxy-2-thiouridine 5′-triphosphate. J.Med.Chem. 2007;50:1166–1176. doi: 10.1021/jm060903o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Jacobson KA, Costanzi S, Ohno M, Joshi BV, Besada P, Xu B, Tchilibon S. Molecular recognition at purine and pyrimidine nucleotide (P2) receptors. Curr Top Med Chem. 2004;4:805–819. doi: 10.2174/1568026043450961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Kaczmarek E, Koziak K, Sevigny J, Siegel JB, Anrather J, Beaudoin AR, Bach FH, Robson SC. Identification and characterization of CD39/vascular ATP diphosphohydrolase. Journal of Biological Chemistry. 1996;271:33116–33122. doi: 10.1074/jbc.271.51.33116. [DOI] [PubMed] [Google Scholar]
- [103].Rugonyi S. Effect of blood flow on near-the-wall mass transport of drugs and other bioactive agents: a simple formula to estimate boundary layer concentrations. J.Biomech.Eng. 2008;130:021010. doi: 10.1115/1.2899571. [DOI] [PubMed] [Google Scholar]
- [104].Gorman MW, Ogimoto K, Savage MV, Jacobson KA, Feigl EO. Nucleotide coronary vasodilation in guinea pig hearts. Am.J.Physiol Heart Circ.Physiol. 2003;285:H1040–H1047. doi: 10.1152/ajpheart.00981.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Erlinge D, Burnstock G. P2 receptors in cardiovascular regulation and disease. Purinergic.Signal. 2008;4:1–20. doi: 10.1007/s11302-007-9078-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Gorman MW, Marble DR, Ogimoto K, Feigl EO. Measurement of adenine nucleotides in plasma. Luminescence. 2003;18:173–181. doi: 10.1002/bio.721. [DOI] [PubMed] [Google Scholar]
- [107].Burnstock G. Pathophysiology and therapeutic potential of purinergic signaling. Pharmacological Reviews. 2006;58:58–86. doi: 10.1124/pr.58.1.5. [DOI] [PubMed] [Google Scholar]
- [108].George S, Dreicer R, Au JJ, Shen T, Rini BI, Roman S, Cooney MM, Mekhail T, Elson P, Wientjes GM, Ganapathi R, Bukowski RM. Phase I/II trial of 5-fluorouracil and a noncytotoxic dose level of suramin in patients with metastatic renal cell carcinoma. Clin.Genitourin.Cancer. 2008;6:79–85. doi: 10.3816/CGC.2008.n.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Smaldone MC, Gayed BA, Tomaszewski JJ, Gingrich JR. Strategies to enhance the efficacy of intravescical therapy for non-muscle invasive bladder cancer. Minerva Urol.Nefrol. 2009;61:71–89. [PubMed] [Google Scholar]
- [110].Hotz B, Buhr HJ, Hotz HG. Intravital microscopic characterization of suramin effects in an orthotopic immunocompetent rat model of pancreatic cancer. J.Gastrointest.Surg. 2008;12:900–906. doi: 10.1007/s11605-008-0507-x. [DOI] [PubMed] [Google Scholar]
- [111].Bhargava S, Hotz B, Hines OJ, Reber HA, Buhr HJ, Hotz HG. Suramin inhibits not only tumor growth and metastasis but also angiogenesis in experimental pancreatic cancer. J.Gastrointest.Surg. 2007;11:171–178. doi: 10.1007/s11605-006-0081-z. [DOI] [PubMed] [Google Scholar]
- [112].Suganuma M, Okabe S, Kai Y, Sueoka N, Sueoka E, Fujiki H. Synergistic effects of (--)-epigallocatechin gallate with (--)-epicatechin, sulindac, or tamoxifen on cancer-preventive activity in the human lung cancer cell line PC-9. Cancer Research. 1999;59:44–47. [PubMed] [Google Scholar]
- [113].Cao Y, Cao R. Angiogenesis inhibited by drinking tea. Nature. 1999;398:381. doi: 10.1038/18793. [DOI] [PubMed] [Google Scholar]
- [114].Singh AK, Seth P, Anthony P, Husain MM, Madhavan S, Mukhtar H, Maheshwari RK. Green tea constituent epigallocatechin-3-gallate inhibits angiogenic differentiation of human endothelial cells. Archives of Biochemistry and Biophysics. 2002;401:29–37. doi: 10.1016/S0003-9861(02)00013-9. [DOI] [PubMed] [Google Scholar]
- [115].Yamakawa S, Asai T, Uchida T, Matsukawa M, Akizawa T, Oku N. (-)-Epigallocatechin gallate inhibits membrane-type 1 matrix metalloproteinase, MT1-MMP, and tumor angiogenesis. Cancer Letters. 2004;210:47–55. doi: 10.1016/j.canlet.2004.03.008. [DOI] [PubMed] [Google Scholar]
- [116].Kazi A, Wang Z, Kumar N, Falsetti SC, Chan TH, Dou QP. Structure-activity relationships of synthetic analogs of (-)-epigallocatechin-3-gallate as proteasome inhibitors. Anticancer Research. 2004;24:943–954. [PubMed] [Google Scholar]
- [117].Postel EH. Multiple biochemical activities of NM23/NDP kinase in gene regulation. J.Bioenerg.Biomembr. 2003;35:31–40. doi: 10.1023/a:1023485505621. [DOI] [PubMed] [Google Scholar]
- [118].McDermott WG, Boissan M, Lacombe ML, Steeg PS, Horak CE. Nm23-H1 homologs suppress tumor cell motility and anchorage independent growth. Clin.Exp.Metastasis. 2008;25:131–138. doi: 10.1007/s10585-007-9128-0. [DOI] [PubMed] [Google Scholar]
- [119].Bertheua P, Merino M, Steeg P, DeLaRosa A. NM23 protein in neoplastic and nonneoplastic thyroid tissues. American Journal of Pathology. 1994;145:26–32. [PMC free article] [PubMed] [Google Scholar]
- [120].Steeg PS, Bevilacqua G, Kopper L, Thorgeirsson UP, Talmadge JE, Liotta LA, Sobel ME. Evidence for a novel gene associated with low tumor metastatic potential. Journal of the National Cancer Institute. 1988;80(3):200–204. doi: 10.1093/jnci/80.3.200. [DOI] [PubMed] [Google Scholar]
- [121].Heimann R, Ferguson DJ, Hellman S. The relationship between nm23, angiogenesis, and the metastatic proclivity of node-negative breast cancer. Cancer Research. 1998;58:2766–2771. [PubMed] [Google Scholar]
- [122].Palmieri D, Horak CE, Lee JH, Halverson DO, Steeg PS. Translational approaches using metastasis suppressor genes. J.Bioenerg.Biomembr. 2006;38:151–161. doi: 10.1007/s10863-006-9039-9. [DOI] [PubMed] [Google Scholar]
- [123].Okabe-Kado J, Kasukabe T, Honma Y, Hanada R, Nakagawara A, Kaneko Y. Clinical significance of serum NM23-H1 protein in neuroblastoma. Cancer Sci. 2005;96:653–660. doi: 10.1111/j.1349-7006.2005.00091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Niitsu N, Nakamine H, Okamoto M, Tamaru JI, Hirano M. A clinicopathological study of nm23-H1 expression in classical Hodgkin’s lymphoma. Ann.Oncol. 2008;19:1941–1946. doi: 10.1093/annonc/mdn413. [DOI] [PubMed] [Google Scholar]
- [125].Roymans D, Willems R, Van Blockstaele DR, Slegers H. Nucleoside diphosphate kinase (NDPK/NM23) and the waltz with multiple partners: possible consequences in tumor metastasis. Clin.Exp.Metastasis. 2002;19:465–476. doi: 10.1023/a:1020396722860. [DOI] [PubMed] [Google Scholar]
- [126].Sauer T, Furu I, Beraki K, Jebsen PW, Ormerod E, Naess O. nm23 protein expression in fine-needle aspirates from breast carcinoma: inverse correlation with cytologic grading, lymph node status, and ploidy. Cancer. 1998;84:109–114. [PubMed] [Google Scholar]
- [127].Russell RL, Geisinger KR, Mehta RR, White WL, Shelton B, Kute TE. nm23--relationship to the metastatic potential of breast carcinoma cell lines, primary human xenografts, and lymph node negative breast carcinoma patients. Cancer. 1997;79:1158–1165. doi: 10.1002/(sici)1097-0142(19970315)79:6<1158::aid-cncr14>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- [128].Sawan A, Lascu I, Veron M, Anderson JJ, Wright C, Horne CHW, Angus B. NDP-K/nm23 expression in human breast cancer in relation to relapse, survival, and other prognostic factors: An immunohistochemical study. J.Pathol. 1994;172:27–34. doi: 10.1002/path.1711720107. [DOI] [PubMed] [Google Scholar]
- [129].Lazarowski ER, Boucher RC, Harden TK. Constitutive release of ATP and evidence for major contribution of ecto-nucleotide pyrophosphatase and nucleoside diphosphokinase to extracellular nucleotide concentrations. Journal of Biological Chemistry. 2000;275:31061–31068. doi: 10.1074/jbc.M003255200. [DOI] [PubMed] [Google Scholar]
- [130].Lazarowski ER, Homolya L, Boucher RC, Harden TK. Identification of an ecto-nucleoside diphosphokinase and its contribution to interconversion of P2 receptor agonists. J Biol Chem. 1997;272:20402–20407. doi: 10.1074/jbc.272.33.20402. [DOI] [PubMed] [Google Scholar]
- [131].Hamby CV, Abbi R, Prasad N, Stauffer C, Thomson J, Mendola CE, Sidorov V, Backer JM. Expression of a catalytically inactive H118Y mutant of nm23-H2 suppresses the metastatic potential of line IV Cl 1 human melanoma cells. International Journal of Cancer. 2000;88:547–553. doi: 10.1002/1097-0215(20001115)88:4<547::aid-ijc5>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- [132].Huwer H, Kalweit G, Engel M, Welter C, Dooley S, Gams E. Expression of the candidate tumor suppressor gene nm23 in the bronchial system of patients with squamous cell lung cancer. Eur.J.Cardiothorac.Surg. 1997;11:206–209. doi: 10.1016/s1010-7940(97)86704-8. [DOI] [PubMed] [Google Scholar]
- [133].Niitsu N, Okabe-Kado J, Okamoto M, Takagi T, Yoshida T, Aoki S, Hirano M, Honma Y. Serum nm23-H1 protein as a prognostic factor in aggressive non-Hodgkin lymphoma. Blood. 2001;97:1202–1210. doi: 10.1182/blood.v97.5.1202. [DOI] [PubMed] [Google Scholar]
- [134].Okabe-Kado J, Kasukabe T, Honma Y. Differentiation inhibitory factor Nm23 as a prognostic factor for acute myeloid leukemia. Leuk.Lymphoma. 1998;32:19–28. doi: 10.3109/10428199809059243. [DOI] [PubMed] [Google Scholar]
- [135].Okabe-Kado J, Kasukabe T, Honma Y. Expression of cell surface NM23 proteins of human leukemia cell lines of various cellular lineage and differentiation stages. Leuk.Res. 2002;26:569–576. doi: 10.1016/s0145-2126(01)00171-0. [DOI] [PubMed] [Google Scholar]
- [136].Jackson SW, Hoshi T, Wu Y, Sun X, Enjyoji K, Cszimadia E, Sundberg C, Robson SC. Disordered purinergic signaling inhibits pathological angiogenesis in cd39/Entpd1-null mice. American Journal of Pathology. 2007;171:1395–1404. doi: 10.2353/ajpath.2007.070190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Goepfert C, Sundberg C, Sevigny J, Enjyoji K, Hoshi T, Csizmadia E, Robson S. Disordered cellular migration and angiogenesis in cd39-null mice. Circulation. 2001;104:3109–3115. doi: 10.1161/hc5001.100663. [DOI] [PubMed] [Google Scholar]
- [138].Kaiser RA, Buxton IL. Nucleotide-mediated relaxation in guinea-pig aorta: selective inhibition by MRS2179. British Journal of Pharamcology. 2002;135:537–545. doi: 10.1038/sj.bjp.0704476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Kaiser RA, Oxhorn BC, Andrews G, Buxton IL. Functional compartmentation of endothelial P2Y receptor signaling. Circulation Research. 2002;91:292–299. doi: 10.1161/01.res.0000030711.21521.ac. [DOI] [PubMed] [Google Scholar]
- [140].Kashiwagi S, Izumi Y, Gohongi T, Demou ZN, Xu L, Huang PL, Buerk DG, Munn LL, Jain RK, Fukumura D. NO mediates mural cell recruitment and vessel morphogenesis in murine melanomas and tissue-engineered blood vessels. J.Clin.Invest. 2005;115:1816–1827. doi: 10.1172/JCI24015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Fishman P, Bar-Yehuda S, Synowitz M, Powell JD, Klotz KN, Gessi S, Borea PA. Adenosine receptors and cancer. Handb Exp Pharmacol. 2009:399–441. doi: 10.1007/978-3-540-89615-9_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Deli T, Csernoch L. Extracellular ATP and cancer: an overview with special reference to P2 purinergic receptors. Pathol Oncol Res. 2008;14:219–231. doi: 10.1007/s12253-008-9071-7. [DOI] [PubMed] [Google Scholar]
- [143].Shabbir M, Burnstock G. Purinergic receptor-mediated effects of adenosine 5′-triphosphate in urological malignant diseases. Int J Urol. 2009;16:143–150. doi: 10.1111/j.1442-2042.2008.02207.x. [DOI] [PubMed] [Google Scholar]
- [144].Kaczmarek E, Erb L, Koziak K, Jarzyna R, Wink MR, Guckelberger O, Blusztajn JK, Trinkaus-Randall V, Weisman GA, Robson SC. Modulation of endothelial cell migration by extracellular nucleotides: involvement of focal adhesion kinase and phosphatidylinositol 3-kinase-mediated pathways. Thrombosis Haemostasis. 2005;93:735–742. doi: 10.1267/THRO05040735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Satterwhite CM, Farrelly AM, Bradley ME. Chemotactic, mitogenic, and angiogenic actions of UTP on vascular endothelial cells. Am.J Physiol. 1999;276:H1091–H1097. doi: 10.1152/ajpheart.1999.276.3.H1091. [DOI] [PubMed] [Google Scholar]
- [146].Tanaka N, Kawasaki K, Kubota Y, Nakamura K, Hashimoto M, Kunitomo M, Shinozuka K. P2Y-receptor regulates size of endothelial cells in an intracellular Ca2+ dependent manner. Life Sci. 2003;72:1445–1453. doi: 10.1016/s0024-3205(02)02416-5. [DOI] [PubMed] [Google Scholar]
- [147].Tanaka N, Kawasaki K, Nejime N, Kubota Y, Nakamura K, Kunitomo M, Takahashi K, Hashimoto M, Shinozuka K. P2Y receptor-mediated Ca(2+) signaling increases human vascular endothelial cell permeability. J.Pharmacol.Sci. 2004;95:174–180. doi: 10.1254/jphs.fpj03036x. [DOI] [PubMed] [Google Scholar]
- [148].Seye CI, Yu N, Gonzalez FA, Erb L, Weisman GA. The P2Y2 nucleotide receptor mediates vascular cell adhesion molecule-1 expression through interaction with VEGF receptor-2 (KDR/Flk-1) Journal of Biological Chemistry. 2004;279:35679–35686. doi: 10.1074/jbc.M401799200. [DOI] [PubMed] [Google Scholar]
- [149].Buvinic S, Bravo-Zehnder M, Boyer JL, Huidobro-Toro JP, Gonzalez A. Nucleotide P2Y1 receptor regulates EGF receptor mitogenic signaling and expression in epithelial cells. Journal of Cell Sciences. 2007;120:4289–4301. doi: 10.1242/jcs.03490. [DOI] [PubMed] [Google Scholar]
- [150].Shibuya M. Differential roles of vascular endothelial growth factor receptor-1 and receptor-2 in angiogenesis. J.Biochem.Mol.Biol. 2006;39:469–478. doi: 10.5483/bmbrep.2006.39.5.469. [DOI] [PubMed] [Google Scholar]
- [151].Dvorak HF, Nagy JA, Feng D, Brown LF, Dvorak AM. Vascular permeability factor/vascular endothelial growth factor and the significance of microvascular hyperpermeability in angiogenesis. Curr.Top.Microbiol.Immunol. 1999;237:97–132. doi: 10.1007/978-3-642-59953-8_6. [DOI] [PubMed] [Google Scholar]
- [152].Moses MA. The regulation of neovascularization of matrix metalloproteinases and their inhibitors. Stem Cells. 1997;15:180–189. doi: 10.1002/stem.150180. [DOI] [PubMed] [Google Scholar]
- [153].Hirschi KK, D’Amore PA. Pericytes in the microvasculature. Cardiovascular Research. 1996;32:687–698. [PubMed] [Google Scholar]
- [154].Grant DS, Kleinman HK. Regulation of capillary formation by laminin and other components of the extracellular matrix. EXS. 1997;79:317–333. doi: 10.1007/978-3-0348-9006-9_13. [DOI] [PubMed] [Google Scholar]
- [155].Gerwins P, Skoldenberg E, Claesson-Welsh L. Function of fibroblast growth factors and vascular endothelial growth factors and their receptors in angiogenesis. Crit Rev.Oncol.Hematol. 2000;34:185–194. doi: 10.1016/s1040-8428(00)00062-7. [DOI] [PubMed] [Google Scholar]
- [156].Matsumoto T, Claesson-Welsh L. VEGF receptor signal transduction. Sci.STKE. 2001;2001:RE21. doi: 10.1126/stke.2001.112.re21. [DOI] [PubMed] [Google Scholar]
- [157].Folberg R, Hendrix MJ, Maniotis AJ. Vasculogenic mimicry and tumor angiogenesis. American Journal of Pathology. 2000;156:361–381. doi: 10.1016/S0002-9440(10)64739-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Shirakawa K, Wakasugi H, Heike Y, Watanabe I, Yamada S, Saito K, Konishi F. Vasculogenic mimicry and pseudo-comedo formation in breast cancer. International Journal of Cancer. 2002;99:821–828. doi: 10.1002/ijc.10423. [DOI] [PubMed] [Google Scholar]
- [159].Shirakawa K, Shibuya M, Heike Y, Takashima S, Watanabe I, Konishi F, Kasumi F, Goldman CK, Thomas KA, Bett A, Terada M, Wakasugi H. Tumor-infiltrating endothelial cells and endothelial precursor cells in inflammatory breast cancer. International Journal of Cancer. 2002;99:344–351. doi: 10.1002/ijc.10336. [DOI] [PubMed] [Google Scholar]
- [160].Yue WY, Chen ZP. Does vasculogenic mimicry exist in astrocytoma? Journal of Histochemistry and Cytochemistry. 2005;53:997–1002. doi: 10.1369/jhc.4A6521.2005. [DOI] [PubMed] [Google Scholar]
- [161].Boocock CA, Charnock-Jones DS, Sharkey AM, McLaren J, Barker PJ, Wright KA, Twentyman PR, Smith SK. Expression of vascular endothelial growth factor and its receptors flt and KDR in ovarian carcinoma. Journal of the National Cancer Institute. 1995;87:506–516. doi: 10.1093/jnci/87.7.506. [DOI] [PubMed] [Google Scholar]
- [162].Wang L, Karlsson L, Moses S, Hultgardh-Nilsson A, Andersson M, Borna C, Gudbjartsson T, Jern S, Erlinge D. P2 receptor expression profiles in human vascular smooth muscle and endothelial cells. Journal of Cardiovascular Pharmacology. 2002;40:841–853. doi: 10.1097/00005344-200212000-00005. [DOI] [PubMed] [Google Scholar]
- [163].Cho CH, Lee CS, Chang M, Jang IH, Kim SJ, Hwang I, Ryu SH, Lee CO, Koh GY. Localization of VEGFR-2 and PLD2 in endothelial caveolae is involved in VEGF-induced phosphorylation of MEK and ERK. Am.J.Physiol Heart Circ.Physiol. 2004;286:H1881–H1888. doi: 10.1152/ajpheart.00786.2003. [DOI] [PubMed] [Google Scholar]
- [164].Labrecque L, Royal I, Surprenant DS, Patterson C, Gingras D, Beliveau R. Regulation of vascular endothelial growth factor receptor-2 activity by caveolin-1 and plasma membrane cholesterol. Mol.Biol.Cell. 2003;14:334–347. doi: 10.1091/mbc.E02-07-0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Ikeda S, Ushio-Fukai M, Zuo L, Tojo T, Dikalov S, Patrushev NA, Alexander RW. Novel role of ARF6 in vascular endothelial growth factor-induced signaling and angiogenesis. Circulation Research. 2005;96:467–475. doi: 10.1161/01.RES.0000158286.51045.16. [DOI] [PubMed] [Google Scholar]
- [166].Massimino ML, Griffoni C, Spisni E, Toni M, Tomasi V. Involvement of caveolae and caveolae-like domains in signalling, cell survival and angiogenesis. Cell Signal. 2002;14:93–98. doi: 10.1016/s0898-6568(01)00232-7. [DOI] [PubMed] [Google Scholar]
- [167].Soslau G, McKenzie RJ, Brodsky I, Devlin TM. Extracellular ATP inhibits agonist-induced mobilization of internal calcium in human platelets. Biochemica et Biophysica Acta. 1995;1268:73–80. doi: 10.1016/0167-4889(95)00051-s. [DOI] [PubMed] [Google Scholar]
- [168].Cristalli G, Vittori S, Thompson RD, Padgett WL, Shi D, Daly JW, Olsson RA. Inhibition of platelet aggregation by adenosine receptor agonists. Naunyn Schmiedebergs Arch.Pharmacol. 1994;349:644–650. doi: 10.1007/pl00004904. [DOI] [PubMed] [Google Scholar]
- [169].Berne RM. Cardiac nucleotides in hypoxia: possbile role in regulation of coronary blood flow. American Journal of Physiology. 1963;204(2):317–322. doi: 10.1152/ajplegacy.1963.204.2.317. [DOI] [PubMed] [Google Scholar]
- [170].Lee S, Chen TT, Barber CL, Jordan MC, Murdock J, Desai S, Ferrara N, Nagy A, Roos KP, Iruela-Arispe ML. Autocrine VEGF signaling is required for vascular homeostasis. Cell. 2007;130:691–703. doi: 10.1016/j.cell.2007.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]