Abstract
The role of pathway-derived growth factors in the support of peripheral axon regeneration remains elusive. Few appropriate knock-out mice are available, and gene silencing techniques are rarely 100% effective. To overcome these difficulties, we have developed an in vitro organotypic co-culture system that accurately models peripheral nerve repair in the adult mammal. Spinal cord sections from P4 mice that express YFP in their neurons are used to innervate segments of P4 peripheral nerve. This reconstructed ventral root is then transected and joined to a nerve graft. Growth of axons across the nerve repair and into the graft can be imaged repeatedly with fluorescence microscopy to define regeneration speed, and parent neurons can be labeled in retrograde fashion to identify contributing neurons. Nerve graft harvested from adult mice remains viable in culture by both morphologic and functional criteria. Motoneurons are supported with GDNF for the first week in culture, after which they survive axotomy, and are thus functionally adult. This platform can be modified by using motoneurons from any genetically modified mouse that can be bred to express XFP, by harvesting nerve graft from any source, or by treating the culture systemically with antibodies, growth factors, or pathway inhibitors. The regeneration environment is controlled to a degree not possible in vivo, and the use of experimental animals is reduced substantially. The flexibility and control offered by this technique should thus make it a useful tool for the study of regeneration biology.
Keywords: axon, motoneuron, organotypic culture, coculture, regeneration, Schwann cell, nerve graft, GFP, mouse
Introduction
Schwann cells are often described as expressing one of only two possible phenotypes, myelinating or non-myelinating (Jessen and Mirsky, 2005). Recently, however, this view has been modified by the discovery that Schwann cell growth factor expression is regulated by both the modality of their axonal partners and by their central-peripheral location along the neuraxis (Brushart, et al., 2006, Hoke, et al.,2006). Predominately sensory and motor phenotypes have also been found to support regeneration on a modality-specific basis. What is not known, however, is which of the growth factors that we have shown to differ in expression are responsible for the modality-specific support of regeneration.
The role of pathway-derived growth factors in regeneration has remained elusive (Markus, et al., 2002). The ability to knockout the function of individual genes in mice offers the possibility of engrafting nerve that does not produce a factor or factors into normal mice to observe the subsequent regeneration deficit (English, et al., 2005). Unfortunately, however, many growth factors are required for development and can only be evaluated in conditional knockouts, few of which have been designed to study peripheral nerve. When growth factors are applied to a nerve repair with fibrin glue or miniosmotic pumps, the only certainty is the maximum dose that can be experienced by axons as they cross the repair; dilution within the wound and growth of axons beyond the surgical site obscure the actual dose of growth factor experienced by axons regenerating within the distal pathway. When growth factors are applied within an entubated nerve gap, they may promote distal stump reinnervation through effects on Schwann cell and fibroblast proliferation and / or migration, as well as on vascular ingrowth, making it difficult to isolate the effects on axonal growth itself.
A technique of nerve repair in vitro that permits absolute control of the regeneration environment could facilitate exploration of the role of pathway-derived growth factors in peripheral nerve regeneration. Early success in culturing intact mammalian nerve was limited to sensory and automomic fibers with their parent ganglia (Kanje, 1991, Tonge, et al., 1998). Regeneration speed after crush was determined by autoradiography, so that individual axons could not be visualized. A thorough Medline search disclosed no more recent reports of the repair of actual nerve segments in vitro. We now report the organotypic co-culture of spinal cord slices (Rothstein, et al., 1993) that express YFP in their motoneurons (Feng, et al., 2000) with grafts of peripheral nerve (Crang and Blakemore, 1986, Heumann, et al., 1987) to create the first in vitro model of adult mammalian nerve repair (Vyas et al., 2008).
Materials and Methods
Organotypic Co-culture of Spinal Cord and Peripheral Nerve
Experiments were performed on mice expressing a yellow variant of green fluorescent protein in sensory and motor neurons and their axons (Feng, et al., 2000). Transgenic B6.Cg-Tg(Thy1-YFP)16Jrs/J animals were obtained from Jackson Laboratories and maintained as heterozygous breeders (line thy1-YFP-H). All procedures were approved by the Animal Care and Use Committee of the Johns Hopkins Medical Institutions. Organotypic cultures were prepared from neonatal (P3-P4) animals using a modification of the technique described by Rothstein et al. (1993). Mouse pups were decapitated, their spinal cords were excised, and dura and root fragments were removed in dissection medium containing Hanks Balanced salt solution (HBSS, Gibco, Grand Island, NY), 4.3 mM sodium bicarbonate, 10mM HEPES (4-(2-Hydroxyethyl, piperazine-1-ethanesulfonic acid), 33.3 mM D-glucose, 5.8 mM magnesium sulfate, 0.03% BSA and penicillin/streptomycin (Gibco). Lumbar spinal cords were then cut into 350 µm transverse sections with a McIllwain tissue chopper. Six to ten usable slices of the lumbar enlargement can be obtained from a single pup. Slices were cultured on Transwell collagen-coated membrane inserts (Corning, Acton, MA.). The median and ulnar nerves of the sacrificed pup and those of a non- expressing littermate were harvested, bisected to double the number of available grafts, and one end of each graft was opposed to the ventral surface of the spinal cord to reconstruct ventral roots. The inserts were placed in a 6-well culture plate containing 1 mL of culture medium consisting of 50% minimal essential medium (Gibco), 25% HBSS, 25% heat-inactivated horse serum (Hyclone, Logan UT), 25 mM HEPES, 35mM D-glucose, 2 mM glutamine and penicillin/streptomycin. Slices were incubated at 37ºC in a humidified atmosphere containing 5% CO2, and culture medium was changed every other day. Spinal cord slices from wild type animals were prepared similarly for control experiments. In some experiments, glial cell line derived neurotrophic factor (GDNF, 70 ng/mL; R&D systems Inc, Minneapolis MN) or insulin like growth factor 1 (IGF-1, 50 ng/mL; Peprotech Inc, Rocky hill, NJ), or both were included in the medium to promote motoneuron survival. Addition of GDNF for 1 week is now part of the routine technique.
Nerve Repair
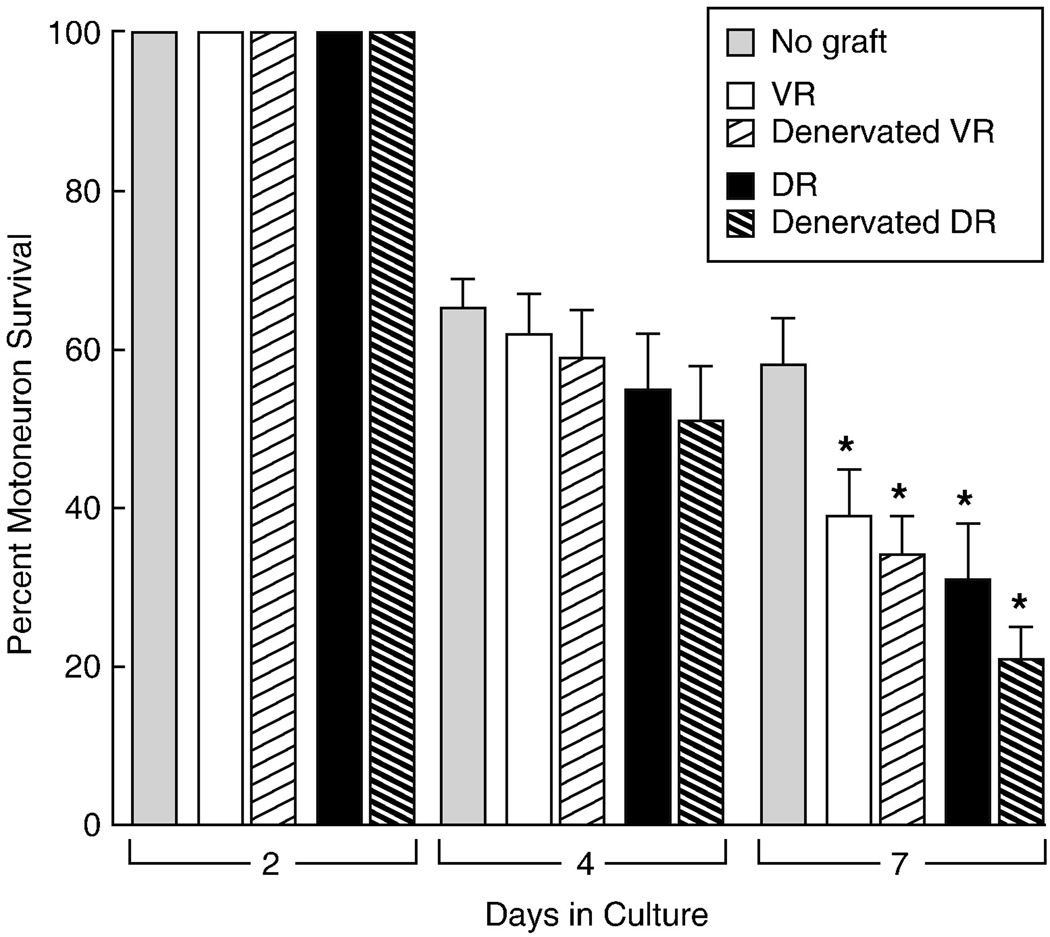
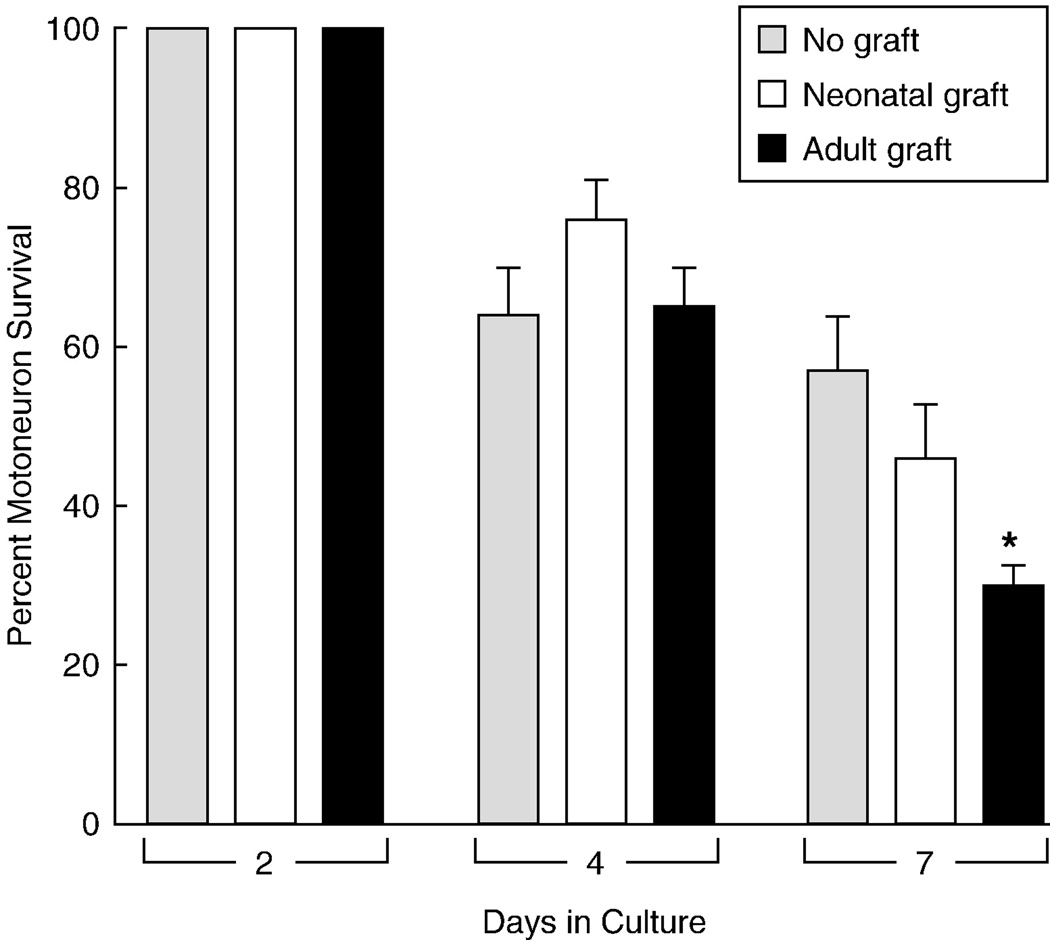
Five to seven days were allowed for fluorescent motor axons to reinnervate the reconstructed ventral roots. These were then transected 3–5mm from the spinal cord with microscissors and joined to nerve grafts by abbuting their ends on the culture membrane (Figure 1). The graft often retracted from the ventral root when the adjacent ends were cut transversely, but this tendency could be minimized by beveling the nerve ends to increase contact area. Grafts were obtained from a variety of sources. Age-matched graft was harvested from P3-P4 littermates that did not express YFP. The pups were decapitated, and unbranched segments of median and ulnar nerves were resected from the arms. Adult grafts were similarly harvested from the median and ulnar nerves of 1 month old C57Bl6 mice that had been anaesthetized by intraperitoneal injection of a mixture of xylazine (16 mg/ Kg) and ketamine (100 mg/Kg). Predegenerated dorsal and ventral roots were harvested from 250 gm Sprague Dawley rats after intraperitoneal injection of ketamine (87 mg/kg) and xylazine (13 mg/kg). Dorsal roots were denervated by excision of the L3, L4, L5, and L6 dorsal root ganglia; ventral roots were identified at their exit from the spinal cord and ligated with 10-0 nylon. Grafts were allowed to undergo Wallerian degeneration for 2 weeks before removal and immediate transfer to co-cultures that had been established previously.
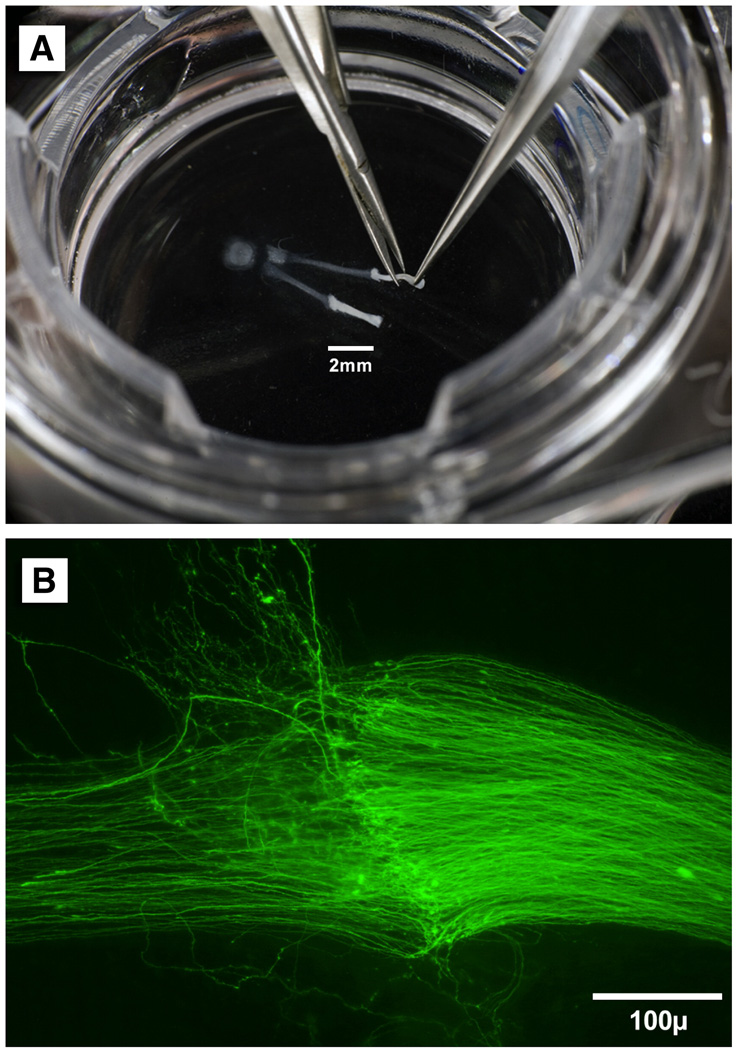
Figure 1.
A: Microinstruments are used to tailor nerve grafts and perform nerve repair on the surface of the Transwell membrane. B: A nerve repair viewed at 20x by fluorescence microscopy. The reconstruced ventral root (right) has been transected and joined to a nerve graft (left). Many axons have crossed the repair site to reinnervate the distal graft. Axons that are not constrained by direct nerve-to-nerve apposition grow away from the repair at right angles, suggesting the presence of soluble repulsive cues.
Tissue Imaging
A Zeiss Axiovert 200 inverted microscope was used for routine assessment of cultures, as well for ongoing counts of motoneurons and axons. In the grafting experiments, motoneuron counts were subjected to Poisson regression analysis to evaluate the ability of nerve grafts to promote motoneuron survival. The experiments in which growth factors were added to cultures without nerve grafts were with a longitudinal Poisson analysis, clustering for individual motoneuron pools. Final images were obtained by removing the culture membrane from the Transwell enclosure and maintaining it on a glass slide for photography with a Nikon Eclipse E600 fluorescent microscope equipped with a Spot CCD camera. Five to ten images were taken at successive focal plains and combined with Helicon Focus software. In some specimens, regenerating motoneurons were retrogradely labeled by transecting the distal tip of the graft, placing it on a 1mm square piece of pulled Parafilm, and covering the cut surface with crystals of Fluoro Ruby (Molecular Probes, Eugene Oregon) for 30 minutes. For electronmicroscopic evaluation, segments of fresh and cultured peripheral nerve were fixed in 4% glutaraldehyde in 0.1M Sorenson’s phosphate buffer, rinsed, impregnated with osmium, and embedded in Epon-Araldite. One-micron thick sections were stained with 1% toluidine blue. Thin sections (60–70 nm) were stained with uranyl acetate (2.5% in 50% ethanol) and lead citrate (3%), and photographed at 3000–40,000 magnifications with an Hitachi H-600 electron microscope. Negatives were scanned at 600 dpi and contrast and brightness levels were adjusted using Adobe Photoshop.
PCR of Fresh And Cultured Nerve
Total RNA was extracted from the nerves using Trizol (Invitrogen). The cDNA was synthesized using 2 µg of total RNA in the presence of Ready-to GoTM You Prime First Strand beads (Biosciences, Arlington Heights, Il) and random primers (Invitrogen/Life Technologies). Measurements of mRNA levels were performed by real-time RT-PCR using two-color DNA Engine Opticon System (M.J. Research Inc.) and the relative amount of gene of interest was normalized to the amount of GAPDH mRNA in the same PCR reaction. To avoid the possibility of amplifying contaminating DNA, all of the primers for real-time RT-PCR were designed with an intron sequence inside the cDNA to be amplified; reactions were performed with appropriate negative control samples (template-free control samples); a uniform amplification of the products was rechecked by analyzing the melting curves of the amplified products (dissociation graphs); the melting temperature (Tm) was 57°C to 60°C; the probe Tm was at least 10°C higher than primer Tm; and gel electrophoresis was performed to confirm the correct size of the amplification and the absence of nonspecific bands. Protein products were sequenced and the sequences were validated at the time each probe was constructed. The RT-PCR experiments were conducted by a technician who was blinded to the nature of groups. The Real-time PCR parameters and the primer and probe sequences have been published (Hoke, et al., 2006). The data was analyzed using analysis of variance with Bonferroni post-hoc correction for multiple comparisons (The critical alpha level was set at p=0.005).
Results
Peripheral nerve and spinal cord cultures were optimized separately before combining them to form a co-culture system. The viability of cultured P4 and adult peripheral nerve segments was assessed by both morphologic and functional criteria. Electronmicroscopic evaluation (Figure 2) revealed that, in comparison with adult nerve, P4 nerve had fewer, more thinly-myelinated axon profiles and prominent clusters of unmyelinated axons. After 5 days in culture, Wallerian degeneration was relatively complete in the P4 nerve, but had barely begun in the adult nerve. Most axons had normal-appearing myelin, though the axoplasm itself was clumped in many profiles. Schwann cells were morphologically normal in both P4 and adult cultured nerve. To assess Schwann cell function, competitive real-time RT-PCR was used to quantify upregulation of NGF, BDNF, GDNF, and PTN by both fresh and cultured adult ventral and dorsal roots (Figure 3). With the exception of BDNF, these factors were all upregulated in the relative proportions established for dorsal and ventral root in previous experiments (Brushart, et al., 2006, Hoke, et al., 2006). Upregulation of BDNF by cultured ventral root ( 3-fold) was quite weak in comparison with that seen in cutaneous nerve in vivo (60-fold), and would have escaped notice on the scale used to analyze the in vivo experiments. The culture environment was thus sufficient to support the continued viability and function of peripheral nerve segments from both P4 and adult animals.
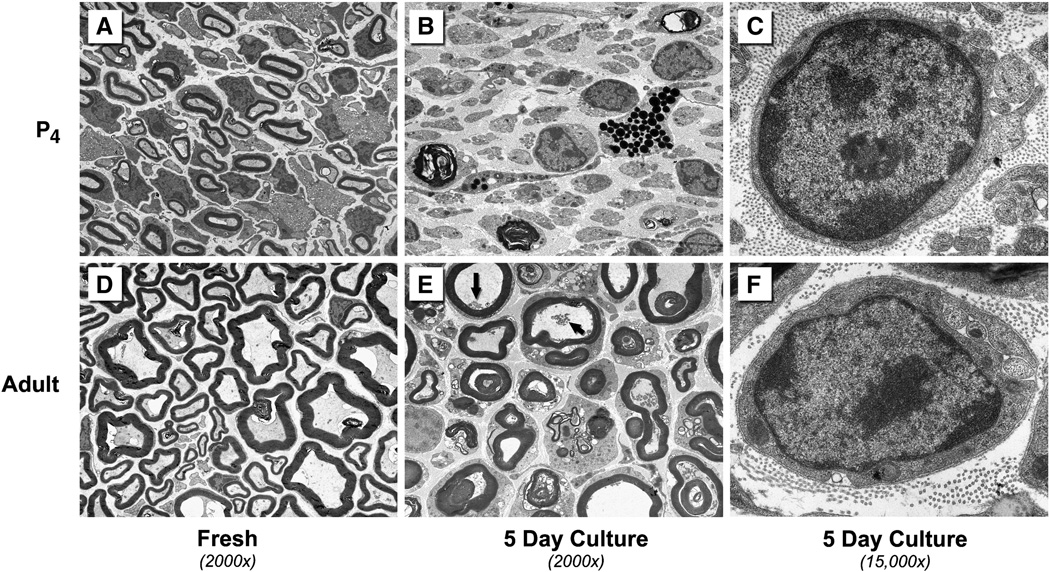
Figure 2.
Electronmicroscopic evaluation of P4 and adult mouse median nerves. A: Normal P4 median nerve. The majority of cross-sectional area is occupied by Schwann cells and myelination is underway. B: Wallerian degeneration proceeds rapidly in P4 nerve and is nearly complete after 5 days. Clumps of black myelin debris remain. C: Schwann cells are morphologically normal after 5 days in culture. D: Normal adult median nerve. E: After 5 days in culture there is clumping of axoplasm (arrows), but most myelin profiles appear normal. Wallerian degeneration is delayed when blood-borne inflammatory cells are not available. F: Adult Schwann cells are morphologically normal after 5 days in culture.
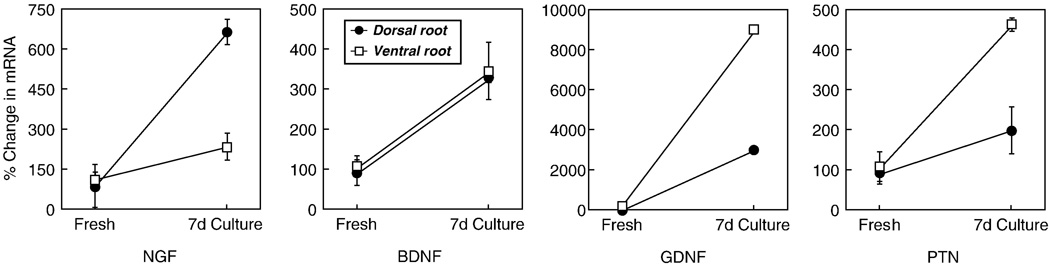
Figure 3.
Competitive real-time RT-PCR was used to evaluate Schwann cell function in adult ventral and dorsal root grafts that had been cultured for 1 week. NGF, BDNF, and PTN were all upregulated to minor degrees, consistent with the relative lack of Schwann cell reactivity. GDNF, in contrast, was upregulated substantially, suggesting that GDNF expression is a more sensitive indicator of early Wallerian degeneration.
Maintenance of a representative population of motoneurons throughout the course of regeneration is a prerequisite for a realistic model of nerve repair. In routine organotypic spinal cord culture, however, substantial numbers of motoneurons are lost in the first week after the proximal axotomy that is inherent in slice preparation (Figure 4). We explored the possibility that providing nerve graft as a source of Schwann-cell derived growth factors would promote motoneuron survival. Grafts of fresh or predegenerated adult ventral or dorsal root (Figure 5) and grafts of adult or P4 median nerve (Figure 6) not only failed to promote motoneuron survival, but reduced it significantly (p < 0.01 for root grafts, p < 0.001 for adult/ P4 grafts). Morphologic evaluation revealed that motoneurons were dying before they could extend axons into the grafts. GDNF and IGF-1 have been found previously to support motoneurons in spinal cord slice culture (Bilak, et al., 2001, Rakowicz, et al., 2002). On the basis of these observations, GDNF and IGF-1, individually and together, were added to the culture (Figure 4). IGF-1 alone failed to enhance motoneuron survival. GDNF, in contrast, dramatically enhanced survival during the first week, with subsequent loss of 1/3 of these neurons during the next three weeks. GDNF and IGF-1 together prolonged the survival of nearly all rescued motoneurons for the duration of the experiment Survival in GDNF and GDNF + IGF-1 groups was always significantly better than that in IGF-1 and no treatment groups (p < 0.01). Increase in the number of motoneurons expressing YFP during the first week of culture is consistent with increased expression of the Thy-1 promoter (that drives YFP expression in the B6.Cg-Tg(Thy1-YFP)16Jrs/J mouse) in the early post-natal period (Dickson, et al., 1986, Caroni, 1997), and may be influenced by progressive flattening of the slice and improved ability to resolve individual motoneurons.
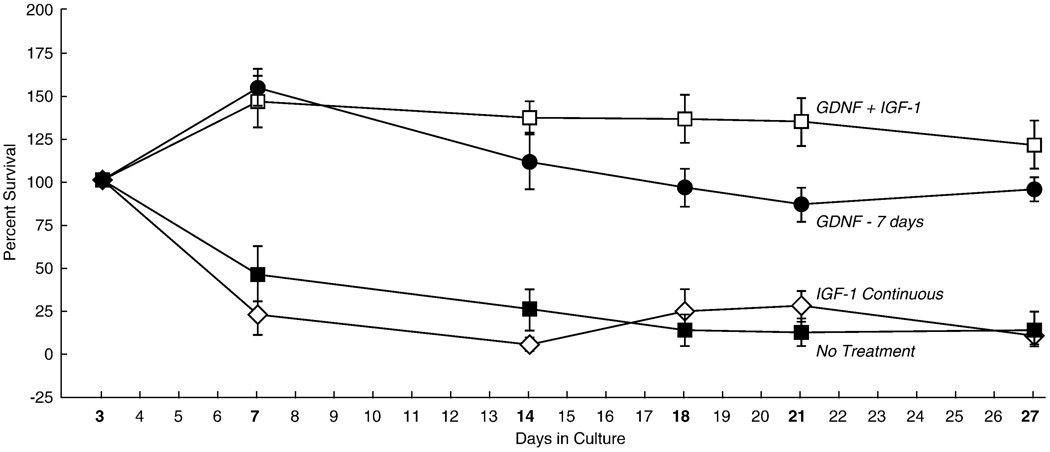
Figure 4.
Survival of motoneurons expressing YFP in untreated cultures, and in cultures treated with growth factors. Substantial numbers of motoneurons are lost in the first week in culture, a deficit not improved by treatment with IGF-1. Treatment with GDNF not only rescues the motoneurons that express YFP initially, but supports the addition of additional YFP-expressing neurons, an effect that is potentiated by IGF-1. Survival in GDNF and GDNF + IGF-1 groups was always significantly better than that in IGF-1 and no treatment groups (p < 0.01).
Figure 5.
Motoneuron survival after ventral root reconstruction with grafts of fresh or pre-degenerated ventral or dorsal root. When compared with cultures set up without grafts, all graft types significantly decreased motoneuron survival after one week in culture (*: p < 0.01). Morphologic evaluation revealed that the majority of motoneurons did not extend axons to the grafts, and were thus never able to derive growth factors from graft Schwann cells.
Figure 6.
Motoneuron survival after ventral root reconstruction with adult or neonatal median nerve graft. After 7 days in culture, survival in cultures with adult grafts was significantly less than that in cultures without any graft (*: p= 0.001). As in Figure 5, few motoneurons survived to extend axons to the grafts.
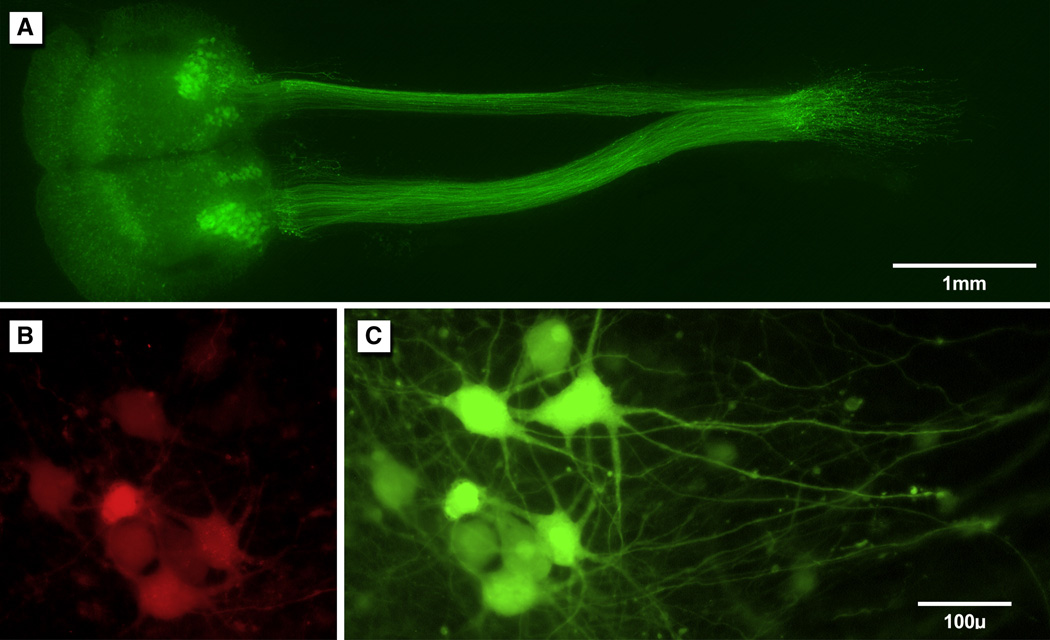
Once spinal cord and peripheral nerve culture were optimized, co-culture was established by using segments of median and ulnar nerve from non-expressing littermates of spinal cord donor pups to reconstruct ventral roots (Figure 7). Direct contact of motor axons exiting the ventral surface of the spinal cord slice with Schwann cell tubes in the nerve graft was sufficient to promote reinnervation of the substitute ventral root. Axons that escaped onto the culture membrane could not be recaptured. Once a co-culture had been established, it was possible to retrogradely label neurons that projected their axons through the graft by applying crystals of Fluoro- Ruby to their cut ends (Figure 7). Grafts were found to be reinnervated exclusively by YFP-expressing neurons that resembled motoneurons in both their form and location.
Figure 7.
A. Organotypic co-culture of spinal cord (left) and peripheral nerve (right); reconstruction of fluorescent images obtained at 10x. Motor axons expressing YFP have reinnervated the ulnar (above) and median (below) nerves of a non- expressing littermate, so that all YFP within the grafts must be derived from motor axons. B: Motoneurons labeled with Fluoro Ruby. The dye is applied to the distal end of the nerve graft and transported centrally to the reinnervating motoneurons. Only motoneurons are labeled, indicating that they are the only neurons that projected axons into the graft. C: The same neurons shown in B, demonstrating their native YFG expression.
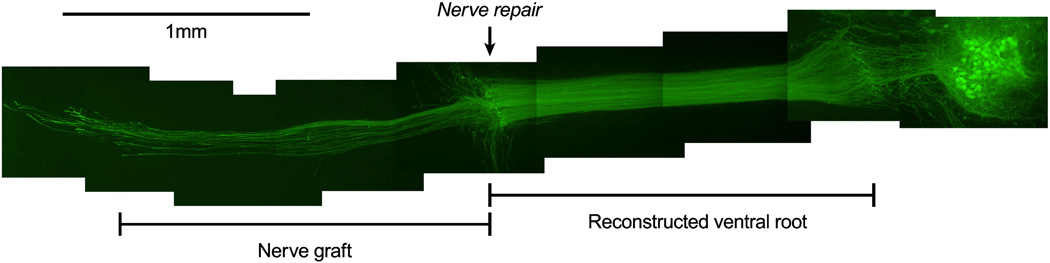
In the early stages of developing this model we attempted the culture of actual adult spinal cord tissue, using a variety of strategies to promote motoneuron survival. We cultured tissue from Bax- knockout mice to block apoptosis and from C57Bl6/wlds mice to minimize long tract degeneration, supported tissue with a variety of growth factor combinations, and applied Riluzole to minimize excitotoxicity, all to no avail. As a result of these failures, we switched to a strategy of beginning with neonatal motoneurons and maturing them till they behaved like adult neurons. Spinal cord and P4 median nerve were co-cultured with GDNF for 1 week to preserve motoneurons and fully reinnervate the new ventral root. Although IGF-1 enhanced long-term motoneuron survival (Figure 4), it was not used routinely because of its cost. One week after plating, GDNF was withdrawn and nerve repair was performed (Figure 1). The reconstructed ventral root was transected and its cut surface apposed to that of a second nerve graft that contained no YFP. This graft was then reinnervated by motor axons that remained within the three-dimensional structure of the nerve and eventually grew out the other end of the graft onto the culture surface (Figure 8). In no instance has the second axotomy reduced the number of surviving motoneurons.
Figure 8.
Photographic reconstruction of a successful nerve repair; original magnification 20x. Motoneurons were supported with GDNF. One week after the co-culture was established, the reconstructed ventral root was transected sharply and joined to a fresh segment of non-expressing P4 median nerve. Axons crossed the repair to reinnervate the distal graft within 48 hours.
Discussion
Our technique of nerve repair in vitro unites several lines of investigation to provide a new tool for the study of nerve repair and regeneration. Peripheral nerve was first maintained in vitro nearly a century ago to investigate the process of Wallerian degeneration (Ingebrigsten, 1916, Abercrombie and Johnson, 1942). More recent studies of cultured nerve have focused on Schwann cell proliferation and growth factor production (Heumann, et al., 1987, Svenningsen and Kanje, 1998). Similarly, organotypic culture of motoneurons within slices of spinal cord is a well-established procedure that has been used routinely for the assessment of motoneuron biology and growth factor effects (Rothstein, et al., 1993, Bilak, et al., 2001, Rakowicz, et al., 2002). Replacement of the cumbersome roller tube technique by culture on a porous membrane greatly facilitated the accessibility of spinal cord tissue for manipulation and observation (Delfs, et al., 1989, Stoppini, et al., 1991). Recently, membrane-based co-culture of organotypic spinal cord with additional spinal cord or cortex has been used to study spinal cord regeneration, establishing the ability of axons to pass from one tissue slice to another (Lee, et al., 2002, Oishi, et al., 2004).
The sine qua non of our model was the development of mice expressing fluorescent protein in their neurons (Feng et al., 2000). These mice initially provided a convenient technique for visualizing the behavior of individual axons in fixed specimens of routine nerve repairs (Witzel, et al., 2005). More importantly, however, they could be used to study the process of regeneration as it occurred in vivo. Motor endplate reinnervation could be observed on the surface of muscle (Nguyen, et al., 2002) and individual axons could be followed as they regenerated on the surface of the spinal cord (Kerschensteiner, et al., 2005). Superficial axons could be imaged through the skin and their regeneration front observed as it progressed down a limb, though fine axonal detail could be obtained only by removing the nerves for further study (Pan, et al., 2003). Repeated attempts in our laboratory to follow the regeneration of individual axons after nerve repair in living animals were confounded by scar formation, the ability to image only the most superficial axons (those that had often escaped from the repair to regenerate aberrantly), and difficulty working with a background of intense fluorescence. This failure, and the availability of the tools described above, stimulated us to use spinal cord sections expressing YFP in their motoneurons to reinnervate co-cultured peripheral nerve grafts.
The direct antecedents of nerve repair in vitro have been limited in their scope. The first innervated mammalian nerves to be grown in culture were autonomic and sensory, and their regeneration after crush was measured by autoradiography (Kanje, 1991, Tonge, et al., 1998). More recently, in vitro regeneration of sensory axons across a collagen-filled peripheral nerve gap was followed by light microscopy using special software (Ozturk and Erdogan, 2004). Motoneurons have been grown within a three-dimensional sponge and their regeneration observed immunohistochemically, though peripheral nerve was not included in the construct (Gingras, et al., 2008).
Several steps were taken to validate our system as an appropriate model for the regeneration of adult mammalian motor axons. Adult median nerve was cultured and found to undergo Wallerian degeneration slowly, yet Schwann cells were normal morphologically and upregulated the expression of several growth factors. Spinal cord sections were harvested at P4, and, as expected, the majority of motoneurons succumbed to the axotomy inherent in slice preparation. Support with GDNF for one week, however, allowed the majority of these to survive. Survival after axotomy has been used as an operational definition of an adult motoneuron, and usually occurs in the mouse by P10 (Li, et al., 1994, Brunet, et al., 2007). Motoneurons in our system were subject to a second axotomy at P11 when the reconstructed ventral roots were transected and joined to grafts; persistent survival of nearly all motoneurons in spite of this insult indicates that they had become matured physiologically as well as temporally while in culture. Our model is thus truly representative of nerve repair in an adult mammal.
The in vitro model of repair and grafting of adult mammalian nerve described above is a basic platform that can be used to explore a wide variety of regeneration questions. Motoneurons expressing YFP can be derived from any mouse mutant that can be bred on a C57Bl6 background, permitting evaluation of regeneration when specific neuronal components or products are over- or underexpressed. Similarly, the nerve graft that is used to convey regenerating motor axons can be harvested not only from any genetically engineered mouse, but from other species as well; in the absence of blood-born inflammatory cells, immunologic reaction within the culture is negligible . We have used rat nerve successfully; it is reasonable to expect that human nerve could also be used. Nerve graft can be further specialized by taking it from a portion of the nervous system with a desired Schwann cell phenotype (Brushart, et al., 2006, Hoke, et al., 2006), or by predegenerating it for a specific period of time in vivo before harvesting it for culture. Schwann cell gene expression can be modified further by electroporation or viral gene transfer (Aspalter, et al., 2008, Haastert and Grothe, 2007). In addition to the above means of varying individually the properties of Schwann cell and motoneuron, it is also possible to add specific pathway inhibitors or growth factors to the entire culture to evaluate the contributions of that pathway to the regeneration process. These experiments can be performed with precise control of the regeneration environment to a level not possible in vivo, and require less expense and the sacrifice of fewer animals than would a similar in vivo experiment. The flexibility and control offered by this technique should thus make it a useful tool for the study of regeneration biology.
Acknowledgments
This work was supported by the Robert Packard Foundation and by NIH RO1 NS034484. The authors thank Catherine Kiefe for preparation of illustrations.
References
- Abercrombie M, Johnson ML. The outwandering of cells in tissue cultures of nerves undergoing Wallerian degeneration. Journal of Experimental Biology. 1942;19:266–286. [Google Scholar]
- Aspalter M, Vyas A, Feiner J, Griffin J, Brushart T, Redett R. Modification of Schwann cell gene expression by electroporation in vivo. J Neurosci Methods. 2008 doi: 10.1016/j.jneumeth.2008.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilak MM, Corse AM, Kuncl RW. Additivity and potentiation of IGF-I and GDNF in the complete rescue of postnatal motor neurons. Amyotroph Lateral Scler Other Motor Neuron Disord. 2001;2:83–91. doi: 10.1080/146608201316949523. [DOI] [PubMed] [Google Scholar]
- Brunet N, Tarabal O, Portero-Otin M, Oppenheim RW, Esquerda JE, Caldero J. Survival and death of mature avian motoneurons in organotypic slice culture: trophic requirements for survival and different types of degeneration. J.Comp.Neurol. 2007;501:669–690. doi: 10.1002/cne.21157. [DOI] [PubMed] [Google Scholar]
- Brushart TM, Redett R, Hameed H, Zhou C, Hoke A. Neuroscience Meeting Planner. Washington, DC: Society for Neuroscience; 2006. Differential growth factor expression by subsets of Schwann cells Presentation 787.3. online. [Google Scholar]
- Caroni P. Overexpression of growth-associated proteins in the neurons of adult transgenic mice. J Neurosci Methods. 1997;71:3–9. doi: 10.1016/s0165-0270(96)00121-5. [DOI] [PubMed] [Google Scholar]
- Crang AJ, Blakemore WF. Observations on Wallerian degeneration in explant cultures of cat sciatic nerve. J Neurocytol. 1986;15:471–482. doi: 10.1007/BF01611730. [DOI] [PubMed] [Google Scholar]
- Delfs J, Friend J, Ishimoto S, Saroff D. Ventral and dorsal horn acetylcholinesterase neurons are maintained in organotypic cultures of postnatal rat spinal cord explants. Brain Res. 1989;488:31–42. doi: 10.1016/0006-8993(89)90690-2. [DOI] [PubMed] [Google Scholar]
- Dickson G, Prentice H, Julien JP, Ferrari G, Leon A, Walsh FS. Nerve growth factor activates Thy-1 and neurofilament gene transcription in rat PC12 cells. Embo J. 1986;5:3449–3453. doi: 10.1002/j.1460-2075.1986.tb04668.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English AW, Meador W, Carrasco DI. Neurotrophin-4/5 is required for the early growth of regenerating axons in peripheral nerves. Eur.J.Neurosci. 2005;21:2624–2634. doi: 10.1111/j.1460-9568.2005.04124.x. [DOI] [PubMed] [Google Scholar]
- Feng G, Mellor R, Bernstein M, Keller-Peck C, Nguyen QT, Wallace M, Nerbonne J, Lichtman J, Sanes J. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of YFP. Neuron. 2000;28:41–51. doi: 10.1016/s0896-6273(00)00084-2. [DOI] [PubMed] [Google Scholar]
- Gingras M, Beaulieu MM, Gagnon V, Durham HD, Berthod F. In vitro study of axonal migration and myelination of motor neurons in a three -dimensional tissue-engineered model. Glia. 2008;56:354–364. doi: 10.1002/glia.20617. [DOI] [PubMed] [Google Scholar]
- Haastert K, Grothe C. Gene therapy in peripheral nerve reconstruction approaches. Current gene therapy. 2007;7:221–228. doi: 10.2174/156652307780859035. [DOI] [PubMed] [Google Scholar]
- Heumann R, Lindholm D, Bandtlow C, Meyer M, Radeke MJ, Misko TP, Shooter E, Thoenen H. Diferential regulation of mRNA encoding nerve growth factor and its receptor in rat sciatic nerve during development, degeneration, and regeneration: role of macrophages. PNAS. 1987;84:8735–8739. doi: 10.1073/pnas.84.23.8735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoke A, Redett R, Hameed H, Jari R, Li JB, Griffin JW, Brushart TM. Schwann cells express motor and sensory phenotypes that regulate axon regeneration. J.Neurosci. 2006;26:9646–9655. doi: 10.1523/JNEUROSCI.1620-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingebrigsten R. A contribution to the biology of peripheral nerves in transplantation. Journal of Experimental Medicine. 1916;23:251–264. doi: 10.1084/jem.23.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen KR, Mirsky R. The origin and development of glial cells in peripheral nerves. Nat.Rev.Neurosci. 2005;6:671–682. doi: 10.1038/nrn1746. [DOI] [PubMed] [Google Scholar]
- Kanje M. Survival and regeneration of the adult rat vagus nerve in culture. Brain Res. 1991;550:340–342. doi: 10.1016/0006-8993(91)91338-2. [DOI] [PubMed] [Google Scholar]
- Kerschensteiner M, Schwab ME, Lichtman JW, Misgeld T. In vivo imaging of axonal degeneration and regeneration in the injured spinal cord. Nat.Med. 2005;11:572–577. doi: 10.1038/nm1229. [DOI] [PubMed] [Google Scholar]
- Lee YS, Baratta J, Yu J, Lin VW, Robertson RT. AFGF promotes axonal growth in rat spinal cord organotypic slice co-cultures. J Neurotrauma. 2002;19:357–367. doi: 10.1089/089771502753594927. [DOI] [PubMed] [Google Scholar]
- Li L, Oppenheim RW, Lei M, Houenou LJ. Neurotrophic agents prevent motoneuron death following sciatic nerve section in the neonatal mouse. J.Neurobiol. 1994;25:759–766. doi: 10.1002/neu.480250702. [DOI] [PubMed] [Google Scholar]
- Markus A, Patel TD, Snider WD. Neurotrophic factors and axonall growth. Curr.Opin.Neurobiol. 2002;12:523–531. doi: 10.1016/s0959-4388(02)00372-0. [DOI] [PubMed] [Google Scholar]
- Nguyen QT, Sanes JR, Lichtman JW. Pre-existing pathways promote precise projection patterns. Nat.Neurosci. 2002;5:861–867. doi: 10.1038/nn905. [DOI] [PubMed] [Google Scholar]
- Oishi Y, Baratta J, Robertson RT, Steward O. Assessment of factors regulating axon growth between the cortex and spinal cord in organotypic co-cultures: effects of age and neurotrophic factors. J Neurotrauma. 2004;21:339–356. doi: 10.1089/089771504322972121. [DOI] [PubMed] [Google Scholar]
- Ozturk G, Erdogan E. Multidimensional long-term time-lapse microscopy of in vitro peripheral nerve regeneration. Microsc Res Tech. 2004;64:228–242. doi: 10.1002/jemt.20075. [DOI] [PubMed] [Google Scholar]
- Pan YA, Misgeld T, Lichtman JW, Sanes JR. Effects of neurotoxic and neuroprotective agents on peripheral nerve regeneration assayed by time-lapse imaging in vivo. J.Neurosci. 2003;23:11479–11488. doi: 10.1523/JNEUROSCI.23-36-11479.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakowicz WP, Staples CS, Milbrandt J, Brunstrom JE, Johnson EM., Jr Glial cell line-derived neurotrophic factor promotes the survival of early postnatal spinal motor neurons in the lateral and medial motor columns in slice culture. J.Neurosci. 2002;22:3953–3962. doi: 10.1523/JNEUROSCI.22-10-03953.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein JD, Jin L, Dykes-Hoberg M, Kuncl RW. Chronic inhibition of glutamate uptake produces a model of slow neurotoxicity. Proc Natl Acad Sci U S A. 1993;90:6591–6595. doi: 10.1073/pnas.90.14.6591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoppini L, Buchs PA, Muller D. A simple method for organotypic cultures of nervous tissue. J Neurosci Methods. 1991;37:173–182. doi: 10.1016/0165-0270(91)90128-m. [DOI] [PubMed] [Google Scholar]
- Svenningsen èF, Kanje M. Regulation of Schwann cell proliferation in cultured segments of the adult rat sciatic nerve. J.Neurosci.Res. 1998;52:530–537. doi: 10.1002/(SICI)1097-4547(19980601)52:5<530::AID-JNR5>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Tonge D, Edstrom A, Ekstrom P. Use of explant cultures of peripheral nerves of adult vertebrates to study axonal regeneration in vitro. Prog Neurobiol. 1998;54:459–480. doi: 10.1016/s0301-0082(97)00072-5. [DOI] [PubMed] [Google Scholar]
- Vyas AA, Feiner J, Aspalter M, Hoke A, Brushart T. Neuroscience Meeting Planner. Washington, DC: Society for Neuroscience; 2008. Development of a spinal cord- peripheral nerve coculture system to study neuronal regeneration. Program No. 327.20. online. [Google Scholar]
- Witzel C, Rohde C, Brushart TM. Pathway sampling by regenerating peripheral axons. J.Comp.Neurol. 2005;485:183–190. doi: 10.1002/cne.20436. [DOI] [PubMed] [Google Scholar]