Abstract
The autoimmune disease atherosclerosis contributes to several vascular complications. Besides vascular cells, inflammatory cells occur prominently in atherosclerotic lesions; lymphocytes play a detrimental role in the initiation and progression of this common vascular disease. Recent discoveries have led to the identification of several important lymphocyte types within the atherosclerotic lesions. However, peripheral lymphocytes and those in the lymphoid organs both figure critically in the regulation of atherosclerotic lesion growth. Although the concept of atherosclerosis as an autoimmune disease is well known, the ways in which autoantigens and autoantibodies contribute to atherogenesis in human or even in animal models remains largely unknown. For example, autoantigen immunization can either promote or attenuate atherogenesis in animals, depending on the antigen types and the routes and carriers of immunization. This essay summarizes recent findings regarding lesion inflammatory cell types, autoantigens, and autoantibody isotypes that can affect the initiation and progression of atherosclerosis from both human and animal studies.
Keywords: autoantigen, autoantibody, innate immunity, adaptive immunity, atherosclerosis
Introduction
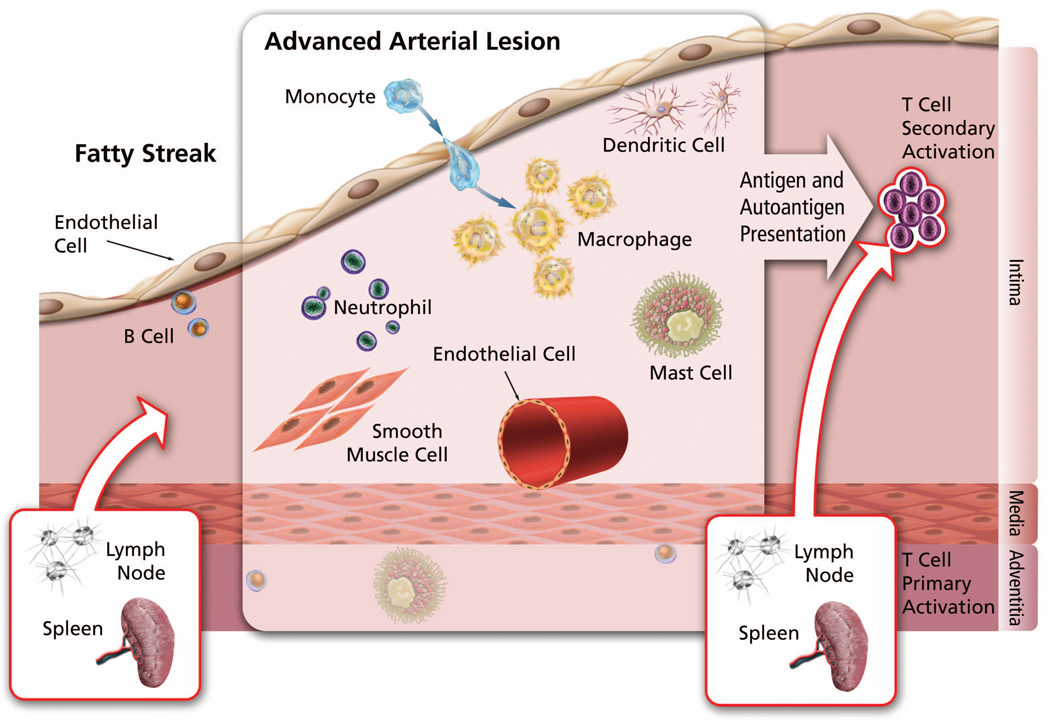
Atherosclerosis remains the most common cause of vascular complications, including stroke, myocardial infarction (MI), and aortic aneurysms. Data suggest that atherosclerosis also constitutes an autoimmune disease, in which autoantigens and autoantibodies affect this vasculature remodelling process. Processing and presentation of autoantigens and subsequent autoantibody production can occur in lymphoid organs as well as in nonlymphoid tissues such as aortas. In human atherosclerotic lesions, many cells participate in this process, including lymphocytes, macrophages, dendritic cells (DC), mast cells, and even vascular smooth muscle cells (SMC) or endothelial cells (EC) (Figure 1). Many of the inflammatory cells are recruited from the lymphoid organs or the circulation and participate in antigen or autoantigen presentation and T-cell activation. I will discuss our current understanding of how autoantigens and autoantibodies contribute to atherosclerosis in human and animal models.
Figure 1.
Atherosclerotic lesion professional and non-professional antigen presenting cells and (auto)antigen-mediated T cell activation.
Inflammatory cells in atherosclerotic lesions
The best-studied inflammatory cells in atherosclerotic lesions include T-cells, B-cells, DC, and macrophages. Few studies focus on other “minor” inflammatory cells such as mast cells and neutrophils. Besides T-cells, all other inflammatory cells can present antigens (antigen presenting cells, APC) to assist T-cell activation, essential for the early progression of atherosclerosis. In human or animal atherosclerotic lesions, T-cells include mainly CD4+ and CD8+ cells, although CD4+ cells dominate in number. It has been suggested that T-cell activation occurs initially in lymphoid organs such as spleen and lymph nodes and then migrates to the aorta for secondary activation by APC that present the same antigens (Figure 1). In turn, activated T-cells can stimulate other inflammatory cells such as macrophages to secrete inflammatory cytokines, proteases, and tissue factors.1
CD4+ T-cells contain two subsets: Th1 and Th2. In human atherosclerotic lesions, Th1 cells expressing the cytokines IFN-γ and IL2 prevail over IL4-, IL5-, and IL10- producing Th2 cells. T-cells isolated from human lesions produce a high amount of IFN-γ but a low amount of IL4.2 B-cells, which occur in many fewer numbers than T-cells, appear in the fatty streak or more external layer of the aortic wall (Figure 1).1 Both T- and B-cells play an essential role in the pathogenesis. A reduction of T- and B-cells can lead to decreased plaque development.1 With CD4+ T-cell transfer from immunocompetent to immunodeficient apolipoprotein E knockout Apoe−/− mice, disease increases dramatically, whereas atherosclerosis-prone but immunodeficient animals show reduced development of early lesions with the fatty streaks.3 In contrast, low-density lipoprotein receptor-deficient Ldlr−/− mice lacking B-cells had 30~40% increase in atherosclerosis, resulting in decreased serum anti-ox-LDL autoantibodies –suggesting that the B-cells that make autoantibodies are antiatherogenic. That explains why mice that go through splenectomy have enhanced atherosclerosis, a phenomenon that splenic B-cell reconstitution can reverse.4
In contrast to B-cells, DC occur in the subendothelial space with other immunocompetent cells. There, they capture autoantigens then migrate to lymphoid stations, where they present autoantigens to T-cells, provoking their responses.5 Thus, T-cells isolated from human atheroma can respond to specific autoantigens while DC and macrophages can present such autoantigens, giving rise to clonal expansion of their specific T-cells and autoantibodies.
Compared with macrophages or lymphocytes, mast cells constitute minor inflammatory cells in human atherosclerotic lesions, though their low numbers do not indicate insignificance. Mast cell inactivation reduces atherosclerotic lesion development in Apoe−/− mice.6 Using mast cell-deficient KitW-sh/W-sh mice, we demonstrated that these minor inflammatory cells are indeed critical to diet-induced atherosclerosis in Ldlr−/− mice. KitW-sh/W-sh mice in the background of Ldlr−/− are protected from atherogenesis. Using mast cell reconstitution techniques, we found that mast cells release pro-inflammatory cytokines IL6 and IFN-γ to stimulate vascular cell release of matrix-degrading proteases.7 Therefore, different inflammatory cells serve different roles in atherogenesis.
Autoantigens
Autoantigens refer to those generated by structure modification on specific moieties of endogenous molecules. Although these antigens occur in human atherosclerotic lesions, they are not aorta-specific. In atherosclerotic lesions, oxidized LDL (ox-LDL), heat shock proteins (HSP), and β2-glycoprotein I (β2-GPI) are the most common autoantigens.
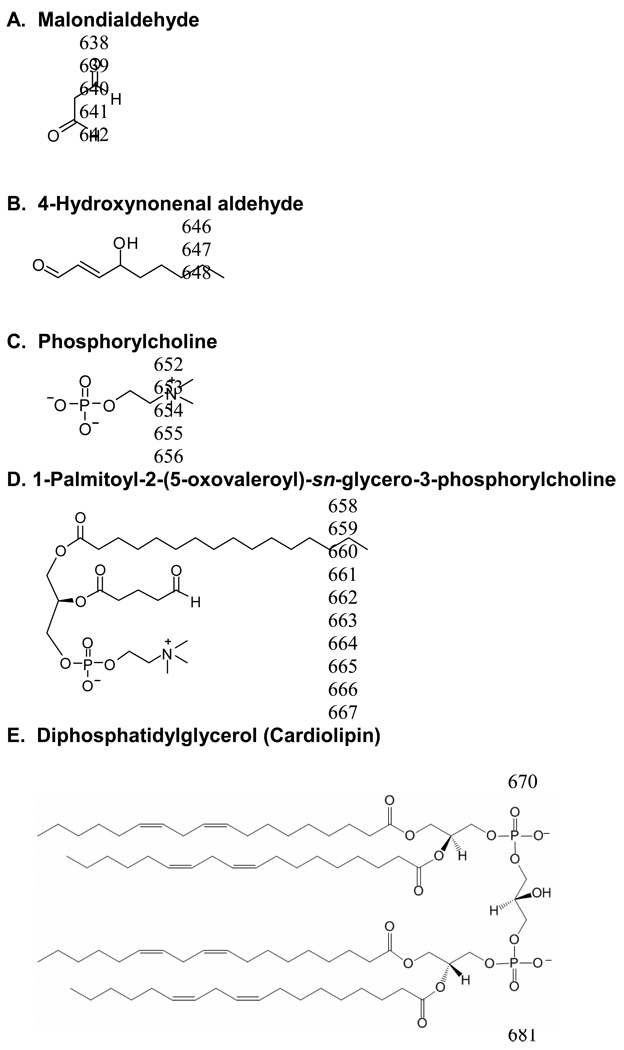
In early atherosclerotic lesions, LDL become trapped in the subendothelial space and subsequently oxidized.8 Two aldehydes with strong immunogenic properties develop: malondialdehyde (MDA) and 4-hydroxynonenal aldehyde (Figure 2A/2B) with anionic valence. This oxidation process associates with major structural modifications of LDL, including fragments of apo B-100 and generation of various aldehyde and phospholipids-adducts to apo B-derived peptides. DC and macrophages uptake ox-LDL and use MHC class II molecules to present ox-LDL for recognition by specific CD4+ T-cells and then lead to ox-LDL-specific T-cell expansion.9 Indeed, about 10% of lesional T-cells are ox-LDL-specific,9 which also appear in circulation. Transfer of ox-LDL-reactive T-cells to T-cell-deficient Apoe−/− scid/scid mice occurs more efficiently at lesion acceleration than transfer of T-cells without antigen specificity.10 Besides stimulating T-cells via APC, ox-LDL can promote inflammation by attracting monocytes and more T-cells to the lesions. Ox-LDL can also be cytotoxic to EC (causing a prothrombotic endothelial surface dysfunction) and stimulate the release of various soluble inflammatory and adhesion molecules. Macrophage scavenger receptors (SRs) remove ox-LDL, leading to ox-LDL macrophage intracellular accumulation and foam cell formation. Clinical studies showed the presence of IgM against MDA-modified apo B-100 peptide and association with plasma ox-LDL levels in humans.11
Figure 2.
Chemical structures of common autoantigen modification moieties or antigenic epitopes (A–D). E. Autoantigen cardiolinpin.
Autoantigen HSP shows high-sequence homology from bacteria to humans. For example, mycobacterial and chlamydial HSP65 show considerable mimicry with human HSP60.12 Most known risk factors or stressors evoke HSP expression on EC, SMC, and macrophages to maintain cell viability. In turn, HSP induces production of inflammatory cytokines and proteases from macrophages and mediates monocyte adhesion to the endothelium. In subjects with established cardiovascular disease (CVD), antibodies against HSP60/65 increase and predict further development of the disease.13 These antibodies specifically react with cells in the plaques and mediate lysis of stressed EC and macrophages. Chlamydial HSP65 isolated from human serum are cytotoxic to heat-stressed human EC, and antibodies to microbial HSP65 recognize specific epitopes on human HSP60 present in the arterial wall of healthy young people.12 Intralesional cells have significantly increased T-cell reactions against human HSP60 compared with peripheral T-cells, and T-cells isolated from human atherosclerotic lesions showed significant reaction against human HSP60 as well as chlamydial and mycobacterial HSP.14 On challenge with human HSP60, plaque T-cells express Th1 functions, including cytotoxicity and monocyte tissue factor production.14
High levels of autoantigen β2-GPI alongside CD4+ and CD8+ T-cells appear in atherosclerotic lesions. β2-GPI binds strongly to negatively charged molecules such as phospholipids and to activated platelets and apoptotic cell membranes, which mediate clearance of apoptotic cells from the circulation. In atherosclerotic lesions, β2-GPI colocalizes with ox-LDL and often forms covalent complexes that occur in autoimmune diseases. In patients with systemic lupus erythematosus (SLE) or antiphospholipid syndrome (APS), serum complexes and anti-β2-GPI-ox-LDL complex autoantibodies are elevated.15 Likewise, such complexes and antibodies present in the bloodstream of patients with vascular complications, such as MI and unstable angina, strongly associate with arterial thrombosis. These complexes inhibit ox-LDL uptake by macrophages through SRs, but are more rapidly internalized by macrophages as IgG immune complexes in the presence of anti-β2-GPI autoantibodies. These macrophages will then present β2-GPI antigenic peptides (on MHC class II) to specific T-cells. Therefore, circulating IgG immune complexes containing ox-LDL and β2-GPI can be proatherogenic. Indeed, adoptive transfer of β2-GPI-reactive lymphocytes enhances early atherosclerosis in Ldlr−/− mice.16
Autoimmune Diseases and Atherosclerosis
Atherosclerosis shares many similarities with other chronic autoimmune diseases such as SLE, rheumatoid arthritis (RA), APS, vasculitis, and type I diabetes. They all have evidence of activation of macrophages, lymphocytes, and EC; alteration in the Th1/Th2 ratio; and elevation of inflammatory cytokines. Vascular inflammation in autoimmune diseases may cause LDL oxidation and interaction of ox-LDL with various plasma proteins such as β2-GPI. These events may favour autoantibody production and accelerate arterial thrombosis.
Patients with SLE, a chronic autoimmune inflammatory disease, are 5–6 times more likely to have coronary events than the normal population. In young women, the risk can increase to 50-fold.17 A large case-control study of nonhospitalized SLE patients with no signs of renal failure showed that the presence of plaques was much more common among SLE patients than controls. A recent case-control study indicated that coronary artery calcification was also more frequent in patients with lupus than controls.18 Anti-ox-LDL antibody titters associate with disease activity in SLE. Increased atherosclerosis was also found in lupus mice. Lupus-susceptible Ldlr−/− mice develop atherosclerosis under moderate dyslipidemia (chow diet), and overt accumulation of atherogenic lipoproteins can enhance SLE disease.19
RA, another prototypic autoimmune disease, also associates with accelerated vascular risk, resulting in early mortality and excess morbidity. RA patients can have up to double the risk of a cardiovascular event irrespective of the traditional CVD risk factors. Many of the same cells that comprise the inflammatory infiltrates in an RA joint lining are likewise found in the atherosclerotic plaques. Both aberrant cellular and humoral immune responses are integral to the pathogenesis of the two conditions. The immune dysregulation that characterizes RA seems to have a key role in the accelerated form of atherosclerosis present in this autoimmune disease.20 Examination of carotid intimal thickening (IMT) shows greater values of IMT in RA patients than controls. A recent study also showed an association between ox-LDL autoantibodies and CVD in RA.21
One in three APS patients develops arterial thrombosis (e.g., MI, stroke, and angina) during the disease evaluation.22 β2-GPI appears to constitute the major antigenic target for anti-phospholipid antibodies and plays a central role in the development of clinical complications of APS.
Vasculitis is a chronic inflammatory disease of the large (aorta, coronary, pulmonary, and cervical arteries), medium-sized (subclavian, mesenteric, iliac, and temporal arteries), and small (skin, intestine, kidney, respiratory tract, etc.) vessels. Like atherosclerotic lesions, vasculitic lesions also contain T-cells, monocytes, DC, and mast cells. These pro-inflammatory cells are critical to the pathogenesis. For examples, depletion of DC abrogates large vessel vasculitis whereas stimulation of these cells in normal temporal arteries induces vasculitis.23 Similar to atherosclerotic patients, those with large vessel vasculitis show high lesion CD4+CD28− Th1 cell contents while defect in regulator T-cells (Treg) and their serum MMP (matrix metalloproteinase) levels often increase, but anti-inflammatory IL10 decreased compared to control subjects.24 Like other autoimmune diseases, vasculitis often associates with increased IgG autoantibodies against ox-LDL, HSP, and β2GP-1.25–27 Besides those inflammatory signatures, vasculitic patients demonstrate endothelial cell injury, high IMT, arterial stiffness, or thoracic dilatation. Therefore, patients with vasculitis have high risk of developing atherosclerosis. Indeed, both populations share similar risk factors, such as diabetes, hypertension, and high serum levels of CRP and IgG autoantibodies against ox-LDL, HSP, and β2-GPI (Table 1).
Table 1.
Vasculitis and pathophysiology
| Group | Example | Lesion location | Preferred population | Vascular complication | Risk factor |
|---|---|---|---|---|---|
| Large vessel | Giant cell arteritis | Aorta and major branches |
Caucasian, >50 y/o | Accelerated atherosclerosis high aortic aneurysms but low IMT |
Diabetes, hypertension, high c-reactive protein (CRP) and autoantibody titters |
| Takayasu arteritis | Aorta, pulmonary, coronary, and cervical arteries |
Young women 15–45 y/o Asian Latin Americans |
Accelerated atherosclerosis vasa vasorum EC injury thrombus formation thoracic dilatation, rupture |
Viral infection and genetic factor | |
| Medium-sized vessel | Kawasaki disease | Subclavian, mesenteric, iliac, temporal arteries |
Infants and <5 y/o Asian Latin Americans |
Coronary artery dilatation cardiac failure high IMT and arterial stiffness |
Diabetes, hypertension, high CRP, autoantibody titters |
| Polyarteritis Nodosa | Temporal arteries and medium-to-small muscular arteries in kidney, gastrointestinal tract, skin, nerve, joint, and muscle |
Children and adults affected with hepatitis B or HIV or streptococcal virus |
Unknown relationship with atherosclerosis |
Viral infection | |
| Small vessel | Grouped according to whether they contain ANCA (anti-neutrophil cytoplasmic autoantibody) |
Small arteries in skin, intestine, kidney. respiratory tract, or any other organs |
Children and adults affected with hepatitis C |
CVD as a major cause of mortality in ANCA+ patients, arterial stiffness, and impaired vasodilatation |
Viral infection, diabetes, hypertension, impaired renal function, high CRP level and high autoantibody titters |
Autoantigen Immunization and Atherosclerosis Prevention
Autoantigen immunization-associated atherosclerosis is probably one of the most difficult phenomena to understand. The same autoantigens may yield controversial atherosclerosis phenotypes if different immunization protocols were used (Table 2). Atherosclerosis-prone mice receiving mucosal (oral or intranasal) immunization of the autoantigens ox-LDL, HSP, or β2-GPI without adjuvant demonstrated reduced atherosclerosis. Mouse lesions had lower numbers of macrophages and CD4+ cells and IFN-γ staining. Mechanistically, autoantigen mucosal immunization induces antigen-specific Treg and adaptive immune cells to produce anti-inflammatory IL4, IL10, and TGF-β to inhibit plaque inflammation and thus the progression of atherosclerosis and reduction in the reactivity of lymph node lymphocytes against autoantigens.28 Strong evidence indicates that Treg participate both in early and advanced stages of atherosclerosis, particularly in preventing and/or reversing plaque instability. Treg can be activated by rapamycin, anti-CD3 monoclonal antibodies, or indirectly via DC activation. Naïve CD4+ T cells express CD25 and forkhead boxP3 (FoxP3) after incubation with rapamycin-treated and HSP60-loaded DC, and display antigen specificity. Adoptive transfer of these HSP60-specific CD4+CD25high T-cells inhibited plaque formation in Apoe−/− mice.29 Treg cell activation requires CD28-CD80/CD86 co-stimulation. Deficiency of these co-stimulatory molecules or depletion of CD25 using CD25-neutralizing antibodies leads to increased atherosclerosis.30
Table 2.
Immunization-mediated anti- and pro-atherogenesis in animal models.
| Antigen | Immunization Protocol | Carrier/Adjuvant | Animal (sex) | Induction of atherosclerosis | Reference |
|---|---|---|---|---|---|
| Antiatherogenic immunization: | |||||
| Human ox-LDL | Oral immunization | none | Ldlr−/− Mice (unknown gender) | Atherogenic diet | 33 |
| Mycobacterium HSP65 | Oral or Nasal immunization | none | Ldlr−/− Mice (female) |
M. tuberculosis immunization in IFA or atherogenic diet |
34, 35 |
| Humaṇ oṛ bovinẹ β2-GPI | Oral immunization | none | Ldlr−/− Mice (female) | Atherogenic diet | 36 |
| WHHL rabbit MDA-LDL | Subcutaneous immunization | CFA and IFA | WHHL rabbit (both genders) | Atherogenic diet | 31 |
| Murine MDA-LDL | Subcutaneous immunization | CFA and IFA | Ldlr−/− Mice (female) | Atherogenic diet | 32 |
| Human apo B-100 peptide-45 -74, -143, -210, or -240 |
Subcutaneous immunization | cationized BSA/aluminium | Apoe−/− mice (male) | Atherogenic diet | 37, 38 |
| Human ox-LDL | Intraperitoneal injection | none | neonatal Apoe−/− mice (both genders) | Atherogenic diet | 30 |
| S. pneumoniae | Subcutaneous immunization | CFA and IFA or none | Ldlr−/− mice (both genders) | Atherogenic diet | 40 |
| Phosphorylcholine-KLH | Intraperitoneal immunization | Oligonucleotide CpG | Apoe−/− (female) | Chow diet | 41 |
| None | Subcutaneous immunization | Aluminium hydroxide | Apoe−/− (male) | Chow diet | 42 |
| Proatherogenic immunization: | |||||
| Human β2-GPI | Subcutaneous immunization | CFA | Ldlr−/− or Apoe−/− mice (female) | Chow or atherogenic diet | 43, 44 |
| Recombinant HSP65 or M. tuberculosis |
Subcutaneous immunization | IFA | C57BL/6J mice (female) | Atherogenic diet | 45 |
| Recombinant HSP 65 or M. tuberculosis |
Subcutaneous immunization | IFA | Ldlr−/− mice (female) | Chow diet | 46 |
| Mycobacterium HSP65 | Intracutaneous injections | CFA and IFA | NZW rabbits (male) | Chow or atherogenic diet | 47 |
Abbreviations: WHHL: Watanabe heritable hyperlipidemic; NZW: New Zealand White; KLH: keyhole limpet hemocyanin; CFA: complete Freund’s adjuvant, IFA: incomplete Freund’s adjuvant; BSA: bovine serum albumin.
Complete Freund’s adjuvant (CFA)-mediated immunization of autoantigen MDA-LDL (primary subcutaneous immunization in CFA followed by subsequent immunization in incomplete Freund’s adjuvant, IFA) also yielded decreased atherosclerotic lesion sizes in Watanabe heritable hyperlipidemic rabbits and in Ldlr−/− mice,31,32 but through different mechanisms from those of mucosal immunization (Table 2). CFA-mediated immunizations of MDA-LDL or its fragments of human apo B-100 produce a T-cell-dependent increase in the titters of autoantibodies against MDA-LDL and great reduction of atherosclerosis in Apoe−/− mice with increased IgG1 (Th2-specific). Such atheroprotective effects of autoantigen immunization occur independently of adjuvant, regardless of whether using Freund’s adjuvant (FA) or cationized bovine serum albumin (BSA) and aluminum.31–47 Injection of high-dose ox-LDL in neonatal Apoe−/− mice without adjuvant also reduced atherosclerosis and resulted in suppression of the T-cell response against ox-LDL.39 These findings concur with the observations that patients with MI have lower levels of MDA-LDL autoantibodies, supporting the notion that antibodies to autoantigens are atheroprotective. This conclusion is not only true in ox-LDL-immunized animals, but also in animals receiving pathogenic bacteria, which also act through increased ox-LDL autoantibodies. Binder et al. demonstrated that Streptococcus pneumoniae immunization resulted in increased ox-LDL autoantibody T15 titter and concomitant reduction in atherosclerotic lesion size in Ldlr−/− mice after a similar immunization protocol to those of MDA-LDL discussed above.31,32,40 In addition to increased T15 titter, pneumococci immunized mice also displayed increased in vivo formation of circulating IgM immune complexes with apo B-containing particles. Treatment with anti-phosphorylcholine IgM isolated from a T15 idiotype hybridoma reduced vein graft atherosclerosis in Apoe−/− mice, suggesting the importance of phosphorylcholine moiety (Figure 2C) on autoimmunogens. In supporting this hypothesis, immunization with phosphorylcholine-coupled KLH (keyhole limpet hemocyanin) associates both with a 3-fold increase in specific antibodies and a 40% reduction in atherosclerosis at the aortic root, even using oligonucleotide CpG as adjuvant,41 which does not possess intrinsic atheromodulating properties. Therefore, stimulation of the immune response against oxidized phospholipids represents one possible approach for the development of an immunomodulatory therapy for atherosclerosis.
Importantly, not all autoantigen immunizations yield atheroprotective phenotypes. Immunization of Apoe−/− or Ldlr−/− mice with β2-GPI induced an increase of atherosclerosis.43,44 Similarly, parental immunization of Ldlr−/− mice or normocholesterolemic rabbits with mycobacterial HSP-65 in IFA results in the development of atherosclerosis, and T-cells isolated from these plaques respond specifically to HSP-65 in vitro (Table 2).48 Transfer of HSP65-reactive cells also accelerates disease in hypercholesterolemic animal models. Of importance, when CFA (containing mineral oil and heat-killed mycobacterial HSP65) is injected into non-hypercholesterolemic animals, atherosclerotic lesions develop in the aorta, principally in sites exposed to an elevated hemodynamic stress.49 Such adjuvant-initiated atherosclerosis did not occur in IFA-immunized animals, likely due to the lack of mycobacterial HSP65 contaminations in IFA. Therefore, selection of the adjuvant in autoantigen immunization can prove critical to atherosclerosis outcomes.
Autoantibodies and Atherosclerosis
Immunization of atherosclerosis-prone animals with ox-LDL, regardless of adjuvant selection, demonstrated the importance of autoantibodies in atherosclerosis animal models.31–39 The most extensively characterized anti-ox-LDL autoantibody is an IgM called EO6, which reacts against an oxidized phospholipid in modified LDL that has been identified as 1-palmitoyl-2-(5-oxovaleroyl)-sn-glycero-3-phosphorylcholine (Figure 2D). Palinski et al.50 first cloned this ox-LDL monoclonal antibody that recognizes the phosphorylcholine epitope in oxidized phospholipids and those on apoptotic cell surfaces from B-cell hybridoma from Apoe−/− mice. This autoantibody is indistinguishable from the natural antibody T15, which is secreted by a specialized subclass of B lymphocytes termed B-1 cells in the peritoneal cavity and spleen. Functionally, these autoantibodies block the selective binding to ox-LDL and apoptotic cells by SRs in a dose-dependent manner and thereby prevent macrophage lipid accumulation. In addition to IgM, IgG also inhibits ox-LDL uptake by macrophages. In MDA-LDL-immunized Apoe−/− mice, the IgG titters against MDA-LDL correlate inversely with plaque size and serum cholesterol levels.51 These antibodies neutralize the many proatherogenic effects of ox-LDL and minimally oxidized LDL and inhibit apoptotic blebs to induce monocyte adhesion by EC. In humans, antibodies present in the plasma target a series of MDA-modified peptide sequences in apo B-100. Clinical studies indicated that IgM against MDA-LDL and ox-LDL associated inversely with carotid or femoral IMT, and IgG against ox-LDL also correlated inversely with carotid IMT. In a nested case-control study, these IgM predicted the risk of developing acute MI and causing cardiac death.11
In contrast, anti-bacterial HSP65 antibody levels correlate positively with the presence of sonographically visible atherosclerotic lesions in the carotid arteries and established coronary atherosclerosis, and associate with elevated levels of coronary calcification independent of established coronary artery disease (CAD) risk factors. A subsequent follow-up study confirmed that such correlation held true in a 5-year period of observations, especially in those with progressive carotid atherosclerosis.13 Human serum HSP65 antibodies react with recombinant form of human HSP60 and those present in EC, macrophages, and SMC in atherosclerotic lesions. Purified anti-HSP65 antibodies are indeed cytotoxic to EC. Similarly, antibodies targeting human HSP60 also associate with severity of CAD. Increased levels of soluble HSP60 and antibodies to HSP60 predict increased risk of CAD. A large population-based study showed the presence of serum soluble HSP60 in patients with carotid atherosclerosis, a correlation that held independent of age, sex, and other risk factors.52
Bacterial infection-associated atherosclerosis has been linked to HSP60/65. Anti-HSP60 antibody correlates strongly with human IgA to Chlamydia pneumoniae and with IgG to Helicobacter pylori. Infection with C. pneumoniae showed proatherogenic effect. Research suggests that bacterial HSP60 product exerts a direct proatherogenic effect by stimulating TNF-α and MMP production53 and IgA anti-HSP60 and anti-C pneumoniae increases the risk of coronary events 7 times.54 Serum IgG antibody against particular sequences of the H. pylori-derived HSP60 appeared predominantly in CVD patients. H. pylori infection induces atherosclerosis in mice by causing autoimmunity to endogenous HSP60 due to molecular mimicry and enhancing HSP-specific Th1 immune responses. Antibiotics or anti-HSP60 antibodies reduce atherosclerosis in these mice.55
Similar to antibodies to HSP60/65, those against autoantigens β2-GPI or cardiolipin (Figure 2E) also positively correlate with cardiovascular events. Case-control studies demonstrated the association of anti-cardiolipin antibodies (aCL) with stroke and acute MI,56 and IgG/IgM/IgA aCL and IgA for anti-β2-GPI associate with increased risk of ischemic stroke, arterial thrombosis, atherosclerotic immune process, acute MI, and peripheral vascular diseases. Although the exact mechanisms remain unknown, anti-β2-GPI was thought to interact with β2-GPI on EC membrane and induce pro-inflammatory and procoagulant endothelial phenotype that is proatherognic.57
Autoantigen-Associated Clinical Trials
As discussed in Table 2, several promising observations were made from animal studies using autoantigen mucosal immunization or parental immunization with oxidization-modified antigens. These data suggest the possibility of using the same approaches in human atherosclerosis prevention. Although direct application of the same immunization protocol in treating human atherosclerosis is waiting to be developed, autoantigen HSP mucosal immunization or vaccination did prove effective in patients with other autoimmune diseases (e.g., RA and type I diabetes) or in different cancer patients (Table 3). As we discussed above (Table 2), animals receiving ox-LDL immunization demonstrated antiatherogenic phenotype mainly by increasing anti-ox-LDL antibodies, suggesting the possibility of using anti-ox-LDL as a therapy for human atherosclerosis. Indeed, a phase II clinical trial using this strategy has been initiated by Genentech, Inc., South San Francisco, California.
Table 3.
Few selected atherosclerosis autoantigen-related clinical trials.
| Antigen and protocol | Status | Reference or institution information |
|---|---|---|
| HSP oral immunization, once a day, 6 month | Safe, developed tolerogenic T cell response, showed reduced TNFα, increased IL10, and improved RA |
58 |
| dnaJP1 (HSP peptide), oral, once a day | Phase II trial for RA, achieved >40% ACR20 response * and 80% reduction of in vitro T cell TNFα production and increase in tolerogenic cytokines IL10 and FoxP3; ready for phase III trial |
Adeona Pharmaceuticals, Inc. Ann Arbor, MI |
| DiaPep277 (HSP peptide), subcutaneous injection | Phase II trial for type I diabetes, preserved endogenous insulin production |
59 |
| HSP peptide p27 and p35 | Phase I clinical trial for type I diabetes | Hadassah University Hospital, Jerusalem, Israel |
| HSP96 vaccine | Phase III on melanoma, showed 29% survival extension | 60 |
| HSP70 vaccine | Phase I trial on genital herpes | 60 |
| HSP65 vaccine | Phase II trial on intraepithelial neoplasia, showed >50% reduction |
60 |
| HSP65 vaccine | Phase II trial on cervical intraepithelial neoplasia | 60 |
| Anti-ox-LDL | Phase II clinical trial on cardiovascular diseases | Genentech, Inc. South San Francisco, CA |
ACR20: a composite endpoint developed by the American College of Rheumatology and accepted as an US Food and Drug Administration approvable scoring criteria.
It is intriguing that Apoe−/− mice receiving aluminium hydroxide subcutaneous immunization without any antigen had reduced atherosclerosis compared with those that received saline alone, presumably via down-regulated T-cell activation but increased Treg activity.42 Indeed, aluminium hydroxide is the most common vaccine adjuvant in both humans and animals.61 It is primarily used in tetanus, diphtheria, pertussis, and poliomyelitis due to its confirmed safety. Therefore, it is possible that aluminium hydroxide vaccination without any antigen may be used in treating human atherosclerosis.
Together, different autoantigens, autoantibodies, and bacterial infections can either promote or protect against atherogenesis — depending on the type of antigens, antibodies, bacterial strains, and even immunization protocols with mechanisms more complicated than we currently know.
Acknowledgements
This study is supported by an Established Investigator Award from the American Heart Association (0840118N), and grants from the National Institute of Health (HL60942, HL81090, HL88547).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No conflicts of interest are declared.
References
- 1.Hansson GK, Libby P. The immune response in atherosclerosis: a double-edged sword. Nat Rev Immunol. 2006;6:508–519. doi: 10.1038/nri1882. [DOI] [PubMed] [Google Scholar]
- 2.Benagiano M, Azzurri A, Ciervo A, Amedei A, Tamburini C, Ferrari M, Telford JL, Baldari CT, Romagnani S, Cassone A, D'Elios MM, Del Prete G. T helper type 1 lymphocytes drive inflammation in human atherosclerotic lesions. Proc Natl Acad Sci USA. 2003;100:6658–6663. doi: 10.1073/pnas.1135726100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou X, Nicoletti A, Elhage R, Hansson GK. Transfer of CD4(+) T cells aggravates atherosclerosis in immunodeficient apolipoprotein E knockout mice. Circulation. 2000;102:2919–2922. doi: 10.1161/01.cir.102.24.2919. [DOI] [PubMed] [Google Scholar]
- 4.Caligiuri G, Nicoletti A, Poirier B, Hansson GK. Protective immunity against atherosclerosis carried by B cells of hypercholesterolemic mice. J Clin Invest. 2002;109:745–753. doi: 10.1172/JCI07272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bobryshev YV. Dendritic cells in atherosclerosis: current status of the problem and clinical relevance. Eur Heart J. 2005;26:1700–1704. doi: 10.1093/eurheartj/ehi282. [DOI] [PubMed] [Google Scholar]
- 6.Bot I, de Jager SC, Zernecke A, Lindstedt KA, van Berkel TJ, Weber C, Biessen EA. Perivascular mast cells promote atherogenesis and induce plaque destabilization in apolipoprotein E-deficient mice. Circulation. 2007;115:2516–2525. doi: 10.1161/CIRCULATIONAHA.106.660472. [DOI] [PubMed] [Google Scholar]
- 7.Sun J, Sukhova GK, Wolters PJ, Yang M, Kitamoto S, Libby P, MacFarlane LA, Mallen-St Clair J, Shi GP. Mast cells promote atherosclerosis by releasing proinflammatory cytokines. Nat Med. 2007;13:719–724. doi: 10.1038/nm1601. [DOI] [PubMed] [Google Scholar]
- 8.Shoenfeld Y, Sherer Y, George J, Harats D. Autoantibodies associated with atherosclerosis. Ann Med. 2000;32 Suppl 1:37–40. [PubMed] [Google Scholar]
- 9.Stemme S, Faber B, Holm J, Wiklund O, Witztum JL, Hansson GK. T lymphocytes from human atherosclerotic plaques recognize oxidized low density lipoprotein. Proc Natl Acad Sci USA. 1995;92:3893–3897. doi: 10.1073/pnas.92.9.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou X, Robertson AK, Hjerpe C, Hansson GK. Adoptive transfer of CD4+ T cells reactive to modified low-density lipoprotein aggravates atherosclerosis. Arterioscler Thromb Vasc Biol. 2006;26:864–870. doi: 10.1161/01.ATV.0000206122.61591.ff. [DOI] [PubMed] [Google Scholar]
- 11.Fredrikson GN, Hedblad B, Berglund G, Alm R, Ares M, Cercek B, Chyu KY, Shah PK, Nilsson J. Identification of immune responses against aldehyde-modified peptide sequences in apoB associated with cardiovascular disease. Arterioscler Thromb Vasc Biol. 2003;23:872–878. doi: 10.1161/01.ATV.0000067935.02679.B0. [DOI] [PubMed] [Google Scholar]
- 12.Perschinka H, Mayr M, Millonig G, Mayerl C, van der Zee R, Morrison SG, Morrison RP, Xu Q, Wick G. Cross-reactive B-cell epitopes of microbial and human heat shock protein 60/65 in atherosclerosis. Arterioscler Thromb Vasc Biol. 2003;23:1060–1065. doi: 10.1161/01.ATV.0000071701.62486.49. [DOI] [PubMed] [Google Scholar]
- 13.Xu Q, Kiechl S, Mayr M, Metzler B, Egger G, Oberhollenzer F, Willeit J, Wick G. Association of serum antibodies to heat-shock protein 65 with carotid atherosclerosis: clinical significance determined in a follow-up study. Circulation. 1999;100:1169–1174. doi: 10.1161/01.cir.100.11.1169. [DOI] [PubMed] [Google Scholar]
- 14.Benagiano M, D'Elios MM, Amedei A, Azzurri A, van der Zee R, Ciervo A, Rombolà G, Romagnani S, Cassone A, Del Prete G. Human 60-kDa heat shock protein is a target autoantigen of T cells derived from atherosclerotic plaques. J Immunol. 2005;174:6509–6517. doi: 10.4049/jimmunol.174.10.6509. [DOI] [PubMed] [Google Scholar]
- 15.McNeil HP, Simpson RJ, Chesterman CN, Krilis SA. Anti-phospholipid antibodies are directed against a complex antigen that includes a lipid-binding inhibitor of coagulation: beta 2-glycoprotein I (apolipoprotein H) Proc Natl Acad Sci USA. 1990;87:4120–4124. doi: 10.1073/pnas.87.11.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.George J, Harats D, Gilburd B, Afek A, Shaish A, Kopolovic J, Shoenfeld Y. Adoptive transfer of beta(2)-glycoprotein I-reactive lymphocytes enhances early atherosclerosis in LDL receptor-deficient mice. Circulation. 2000;102:1822–1827. doi: 10.1161/01.cir.102.15.1822. [DOI] [PubMed] [Google Scholar]
- 17.Manzi S, Meilahn EN, Rairie JE, Conte CG, Medsger TA, Jr, Jansen-McWilliams L, D'Agostino RB, Kuller LH. Age-specific incidence rates of myocardial infarction and angina in women with systemic lupus erythematosus: comparison with the Framingham Study. Am J Epidemiol. 1997;145:408–415. doi: 10.1093/oxfordjournals.aje.a009122. [DOI] [PubMed] [Google Scholar]
- 18.Asanuma Y, Oeser A, Shintani AK, Turner E, Olsen N, Fazio S, Linton MF, Raggi P, Stein CM. Premature coronary-artery atherosclerosis in systemic lupus erythematosus. N Engl J Med. 2003;349:2407–2415. doi: 10.1056/NEJMoa035611. [DOI] [PubMed] [Google Scholar]
- 19.Braun N, Wade NS, Wakeland EK, Major AS. Accelerated atherosclerosis is independent of feeding high fat diet in systemic lupus erythematosus-susceptible LDLr(−/−) mice. Lupus. 2008;17:1070–1078. doi: 10.1177/0961203308093551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sherer Y, Shoenfeld Y. Mechanisms of disease: atherosclerosis in autoimmune diseases. Nat Clin Pract Rheumatol. 2006;2:99–106. doi: 10.1038/ncprheum0092. [DOI] [PubMed] [Google Scholar]
- 21.Peters MJ, van Halm VP, Nurmohamed MT, Damoiseaux J, Tervaert JW, Twisk JW, Dijkmans BA, Voskuyl AE. Relations between autoantibodies against oxidized low-density lipoprotein, inflammation, subclinical atherosclerosis, and cardiovascular disease in rheumatoid arthritis. J Rheumatol. 2008;35:1495–1499. [PubMed] [Google Scholar]
- 22.Ginsburg KS, Liang MH, Newcomer L, Goldhaber SZ, Schur PH, Hennekens CH, Stampfer MJ. Anticardiolipin antibodies and the risk for ischemic stroke and venous thrombosis. Ann Intern Med. 1992;117:997–1002. doi: 10.7326/0003-4819-117-12-997. [DOI] [PubMed] [Google Scholar]
- 23.Ma-Krupa W, Jeon MS, Spoerl S, Tedder TF, Goronzy JJ, Weyand CM. Activation of arterial wall dendritic cells and breakdown of self-tolerance in giant cell arteritis. J Exp Med. 2004;199:173–183. doi: 10.1084/jem.20030850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cohen Tervaert JW. Translational mini-review series on immunology of vascular disease: accelerated atherosclerosis in vasculitis. Clin Exp Immunol. 2009;156:377–385. doi: 10.1111/j.1365-2249.2009.03885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Slot MC, Theunissen R, van Paassen P, Damoiseaux JG, Cohen Tervaert JW. Limburg Nephrology Working Group. Anti-oxidized low-density lipoprotein antibodies in myeloperoxidase-positive vasculitis patients preferentially recognize hypochlorite-modified low density lipoproteins. Clin Exp Immunol. 2007;149:257–264. doi: 10.1111/j.1365-2249.2007.03420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gruber R, Lederer S, Bechtel U, Lob S, Riethmüller G, Feucht HE. Increased antibody titers against mycobacterial heat-shock protein 65 in patients with vasculitis and arteriosclerosis. Int Arch Allergy Immunol. 1996;110:95–98. doi: 10.1159/000237318. [DOI] [PubMed] [Google Scholar]
- 27.Espinosa G, Tàssies D, Font J, Muñoz-Rodríguez FJ, Cervera R, Ordinas A, Reverter JC, Ingelmo M. Antiphospholipid antibodies and thrombophilic factors in giant cell arteritis. Semin Arthritis Rheum. 2001;31:12–20. doi: 10.1053/sarh.2001.23499. [DOI] [PubMed] [Google Scholar]
- 28.George J. Mechanisms of disease: the evolving role of regulatory T cells in atherosclerosis. Nat Clin Pract Cardiovasc Med. 2008;5:531–540. doi: 10.1038/ncpcardio1279. [DOI] [PubMed] [Google Scholar]
- 29.Yang K, Li D, Luo M, Hu Y. Generation of HSP60-specific regulatory T cell and effect on atherosclerosis. Cell Immunol. 2006;243:90–95. doi: 10.1016/j.cellimm.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 30.Ait-Oufella H, Salomon BL, Potteaux S, Robertson AK, Gourdy P, Zoll J, Merval R, Esposito B, Cohen JL, Fisson S, Flavell RA, Hansson GK, Klatzmann D, Tedgui A, Mallat Z. Natural regulatory T cells control the development of atherosclerosis in mice. Nat Med. 2006;12:178–180. doi: 10.1038/nm1343. [DOI] [PubMed] [Google Scholar]
- 31.Palinski W, Miller E, Witztum JL. Immunization of low density lipoprotein (LDL) receptor-deficient rabbits with homologous malondialdehyde-modified LDL reduces atherogenesis. Proc Natl Acad Sci USA. 1995;92:821–825. doi: 10.1073/pnas.92.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Binder CJ, Hartvigsen K, Chang MK, Miller M, Broide D, Palinski W, Curtiss LK, Corr M, Witztum JL. IL-5 links adaptive and natural immunity specific for epitopes of oxidized LDL and protects from atherosclerosis. J Clin Invest. 2004;114:427–437. doi: 10.1172/JCI20479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Puijvelde GH, Hauer AD, de Vos P, van den Heuvel R, van Herwijnen MJ, van der Zee R, van Eden W, van Berkel TJ, Kuiper J. Induction of oral tolerance to oxidized low-density lipoprotein ameliorates atherosclerosis. Circulation. 2006;114:1968–1976. doi: 10.1161/CIRCULATIONAHA.106.615609. [DOI] [PubMed] [Google Scholar]
- 34.Harats D, Yacov N, Gilburd B, Shoenfeld Y, George J. Oral tolerance with heat shock protein 65 attenuates Mycobacterium tuberculosis-induced and high-fat-diet-driven atherosclerotic lesions. J Am Coll Cardiol. 2002;40:1333–1338. doi: 10.1016/s0735-1097(02)02135-6. [DOI] [PubMed] [Google Scholar]
- 35.Maron R, Sukhova G, Faria AM, Hoffmann E, Mach F, Libby P, Weiner HL. Mucosal administration of heat shock protein-65 decreases atherosclerosis and inflammation in aortic arch of low-density lipoprotein receptor-deficient mice. Circulation. 2002;106:1708–1715. doi: 10.1161/01.cir.0000029750.99462.30. [DOI] [PubMed] [Google Scholar]
- 36.George J, Yacov N, Breitbart E, Bangio L, Shaish A, Gilburd B, Shoenfeld Y, Harats D. Suppression of early atherosclerosis in LDL-receptor deficient mice by oral tolerance with beta 2-glycoprotein I. Cardiovasc Res. 2004;62:603–609. doi: 10.1016/j.cardiores.2004.01.028. [DOI] [PubMed] [Google Scholar]
- 37.Fredrikson GN, Söderberg I, Lindholm M, Dimayuga P, Chyu KY, Shah PK, Nilsson J. Inhibition of atherosclerosis in apoE-null mice by immunization with apoB-100 peptide sequences. Arterioscler Thromb Vasc Biol. 2003;23:879–884. doi: 10.1161/01.ATV.0000067937.93716.DB. [DOI] [PubMed] [Google Scholar]
- 38.Fredrikson GN, Andersson L, Söderberg I, Dimayuga P, Chyu KY, Shah PK, Nilsson J. Atheroprotective immunization with MDA-modified apo B-100 peptide sequences is associated with activation of Th2 specific antibody expression. Autoimmunity. 2005;38:171–179. doi: 10.1080/08916930500050525. [DOI] [PubMed] [Google Scholar]
- 39.Nicoletti A, Paulsson G, Caligiuri G, Zhou X, Hansson GK. Induction of neonatal tolerance to oxidized lipoprotein reduces atherosclerosis in ApoE knockout mice. Mol Med. 2006;6:283–290. [PMC free article] [PubMed] [Google Scholar]
- 40.Binder CJ, Hörkkö S, Dewan A, Chang MK, Kieu EP, Goodyear CS, Shaw PX, Palinski W, Witztum JL, Silverman GJ. Pneumococcal vaccination decreases atherosclerotic lesion formation: molecular mimicry between Streptococcus pneumoniae and oxidized LDL. Nat Med. 2003;9:736–743. doi: 10.1038/nm876. [DOI] [PubMed] [Google Scholar]
- 41.Caligiuri G, Khallou-Laschet J, Vandaele M, Gaston AT, Delignat S, Mandet C, Kohler HV, Kaveri SV, Nicoletti A. Phosphorylcholine-targeting immunization reduces atherosclerosis. J Am Coll Cardiol. 2007;50:540–546. doi: 10.1016/j.jacc.2006.11.054. [DOI] [PubMed] [Google Scholar]
- 42.Wigren M, Bengtsson D, Dunér P, Olofsson K, Björkbacka H, Bengtsson E, Fredrikson GN, Nilsson J. Atheroprotective effects of Alum are associated with capture of oxidized LDL antigens and activation of regulatory T cells. Circ Res. 2009;104:e62–e70. doi: 10.1161/CIRCRESAHA.109.196667. [DOI] [PubMed] [Google Scholar]
- 43.George J, Afek A, Gilburd B, Blank M, Levy Y, Aron-Maor A, Levkovitz H, Shaish A, Goldberg I, Kopolovic J, Harats D, Shoenfeld Y. Induction of early atherosclerosis in LDL-receptor-deficient mice immunized with beta2-glycoprotein I. Circulation. 1998;98:1108–1115. doi: 10.1161/01.cir.98.11.1108. [DOI] [PubMed] [Google Scholar]
- 44.Afek A, George J, Shoenfeld Y, Gilburd B, Levy Y, Shaish A, Keren P, Janackovic Z, Goldberg I, Kopolovic J, Harats D. Enhancement of atherosclerosis in beta-2-glycoprotein I-immunized apolipoprotein E-deficient mice. Pathobiology. 1999;67:19–25. doi: 10.1159/000028046. [DOI] [PubMed] [Google Scholar]
- 45.George J, Shoenfeld Y, Afek A, Gilburd B, Keren P, Shaish A, Kopolovic J, Wick G, Harats D. Enhanced fatty streak formation in C57BL/6J mice by immunization with heat shock protein-65. Arterioscler Thromb Vasc Biol. 1999;19:505–510. doi: 10.1161/01.atv.19.3.505. [DOI] [PubMed] [Google Scholar]
- 46.Afek A, George J, Gilburd B, Rauova L, Goldberg I, Kopolovic J, Harats D, Shoenfeld Y. Immunization of low-density lipoprotein receptor deficient (LDL-RD) mice with heat shock protein 65 (HSP-65) promotes early atherosclerosis. J Autoimmun. 2000;14:115–121. doi: 10.1006/jaut.1999.0351. [DOI] [PubMed] [Google Scholar]
- 47.Xu Q, Dietrich H, Steiner HJ, Gown AM, Schoel B, Mikuz G, Kaufmann SH, Wick G. Induction of arteriosclerosis in normocholesterolemic rabbits by immunization with heat shock protein 65. Arterioscler Thromb. 1992;12:789–799. doi: 10.1161/01.atv.12.7.789. [DOI] [PubMed] [Google Scholar]
- 48.Afek A, George J, Gilburd B, Rauova L, Goldberg I, Kopolovic J, Harats D, Shoenfeld Y. Immunization of low-density lipoprotein receptor deficient (LDL-RD) mice with heat shock protein 65 (HSP-65) promotes early atherosclerosis. J Autoimmun. 2000;14:115–121. doi: 10.1006/jaut.1999.0351. [DOI] [PubMed] [Google Scholar]
- 49.Mandal K, Jahangiri M, Xu Q. Autoimmunity to heat shock proteins in atherosclerosis. Autoimmun Rev. 2004;3:31–37. doi: 10.1016/S1568-9972(03)00088-0. [DOI] [PubMed] [Google Scholar]
- 50.Palinski W, Hörkkö S, Miller E, Steinbrecher UP, Powell HC, Curtiss LK, Witztum JL. Cloning of monoclonal autoantibodies to epitopes of oxidized lipoproteins from apolipoprotein E-deficient mice. Demonstration of epitopes of oxidized low density lipoprotein in human plasma. J Clin Invest. 1996;98:800–814. doi: 10.1172/JCI118853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou X, Caligiuri G, Hamsten A, Lefvert AK, Hansson GK. LDL immunization induces T-cell-dependent antibody formation and protection against atherosclerosis. Arterioscler Thromb Vasc Biol. 2001;21:108–114. doi: 10.1161/01.atv.21.1.108. [DOI] [PubMed] [Google Scholar]
- 52.Xu Q, Schett G, Perschinka H, Mayr M, Egger G, Oberhollenzer F, Willeit J, Kiechl S, Wick G. Serum soluble heat shock protein 60 is elevated in subjects with atherosclerosis in a general population. Circulation. 2000;102:14–20. doi: 10.1161/01.cir.102.1.14. [DOI] [PubMed] [Google Scholar]
- 53.Kol A, Sukhova GK, Lichtman AH, Libby P. Chlamydial heat shock protein 60 localizes in human atheroma and regulates macrophage tumor necrosis factor-alpha and matrix metalloproteinase expression. Circulation. 1998;98:300–307. doi: 10.1161/01.cir.98.4.300. [DOI] [PubMed] [Google Scholar]
- 54.Xu Q, Schett G, Seitz CS, Hu Y, Gupta RS, Wick G. Surface staining and cytotoxic activity of heat-shock protein 60 antibody in stressed aortic endothelial cells. Circ Res. 1994;75:1078–1085. doi: 10.1161/01.res.75.6.1078. [DOI] [PubMed] [Google Scholar]
- 55.Ayada K, Yokota K, Kobayashi K, Shoenfeld Y, Matsuura E, Oguma K. Chronic infections and atherosclerosis. Ann N Y Acad Sci. 2007;1108:594–602. doi: 10.1196/annals.1422.062. [DOI] [PubMed] [Google Scholar]
- 56.Brey RL, Abbott RD, Curb JD, Sharp DS, Ross GW, Stallworth CL, Kittner SJ. beta(2)-Glycoprotein 1-dependent anticardiolipin antibodies and risk of ischemic stroke and myocardial infarction: the honolulu heart program. Stroke. 2001;32:1701–1706. doi: 10.1161/01.str.32.8.1701. [DOI] [PubMed] [Google Scholar]
- 57.Kobayashi K, Kishi M, Atsumi T, Bertolaccini ML, Makino H, Sakairi N, Yamamoto I, Yasuda T, Khamashta MA, Hughes GR, Koike T, Voelker DR, Matsuura E. Circulating oxidized LDL forms complexes with beta2-glycoprotein I: implication as an atherogenic autoantigen. J Lipid Res. 2003;44:716–726. doi: 10.1194/jlr.M200329-JLR200. [DOI] [PubMed] [Google Scholar]
- 58.van de Ven A, Prakken B, Albani S. Immunological tolerance in the therapy of rheumatoid arthritis. Discov Med. 2007;7:46–50. [PubMed] [Google Scholar]
- 59.Raz I, Elias D, Avron A, Tamir M, Metzger M, Cohen IR. Beta-cell function in new-onset type 1 diabetes and immunomodulation with a heat-shock protein peptide (DiaPep277): a randomised, double-blind, phase II trial. Lancet. 2001;358:1749–1753. doi: 10.1016/S0140-6736(01)06801-5. [DOI] [PubMed] [Google Scholar]
- 60.Holzer AM, Martiniuk F, Levis WR. Heat-shock proteins as drugs: potential applications in cancer, infections, and autoimmune and atopic diseases. J Drugs Dermatol. 2007;6:393–399. [PubMed] [Google Scholar]
- 61.Lindblad EB. Aluminium compounds for use in vaccines. Immunol Cell Biol. 2004;82:497–505. doi: 10.1111/j.0818-9641.2004.01286.x. [DOI] [PubMed] [Google Scholar]