Abstract
This study investigated the relationships between trabecular microstructure and elastic modulus, compressive strength, and suture anchor pullout strength. Twelve fresh-frozen humeri underwent mechanical testing followed by micro-computed tomography (μCT). Either compression testing of cylindrical bone samples or pullout testing using an Arthrex 5 mm Corkscrew was performed in synthetic sawbone or at specific locations in the humerus such as the greater tuberosity, lesser tuberosity, and humeral head. Synthetic sawbone underwent identical mechanical testing and μCT analysis. Bone volume fraction (BVF), structural model index (SMI), trabecular thickness (TbTh), trabecular spacing (TbSp), trabecular number (TbN), and connectivity density were compared against modulus, compressive strength, and pullout strength in both materials. In cadaveric bone, modulus showed correlations to all of the microstructural properties, while compressive and pullout strength were only correlated to BVF, SMI, and TbSp. The microstructure of synthetic bone differed from cadaveric bone as SMI and TbTh showed little variation across the densities tested. Therefore, SMI and TbTh were the only microstructural properties that did not show correlations to the mechanical properties tested in synthetic bone. This study helps identify key microstructure-property relationships in cadaveric and synthetic bone as well as illustrate the similarities and differences between cadaveric and synthetic bone as biomechanical test materials.
Keywords: Suture Anchors, Pullout Strength, Micro-CT, Microstructure, Synthetic Bone
INTRODUCTION
Suture anchors are designed to provide soft-tissue fixation to bone. Rotator cuff repairs represent the most common shoulder-fixation surgery in which suture anchors are either tapped or screwed into the bone to create a point of fixation.(Tingart, Apreleva et al. 2003; Millenium Research Group 2008, 2007) Suture anchor failure can occur at several interfaces of the implanted device: the bone-anchor, anchor-suture, and suture-tissue interfaces.(Barber, Herbert et al. 1996; Barber and Herbert 1999; Tingart, Apreleva et al. 2004) Mechanical failure at the bone-anchor interface typically results in suture anchor loosening, migration, and pullout.(Djurasovic, Marra et al. 2001; McFarland, Park et al. 2005; Pietschmann, Fröhlich et al. 2009) The force required to pull the anchor from the bone is termed the pullout strength and is often used as a metric to compare the performance of different suture anchors.(Barber, Feder et al. 1997; Meyer, Fucentese et al. 2003; Tingart, Apreleva et al. 2003; Tingart, Apreleva et al. 2004; Barber, Herbert et al. 2006; Park, Keyurapan et al. 2006; Pietschmann, Fröhlich et al. 2008; Pietschmann, Fröhlich et al. 2009)
Previous studies investigating the correlation between pullout strength and bone mineral density (BMD) suggest that pullout strength increases with bone density.(Barber, Feder et al. 1997; Tingart, Apreleva et al. 2003; Meyer, Mayer et al. 2006) Other studies have investigated the relationships between BMD and mechanical properties such as compressive strength and modulus.(Pugh, Rose et al. 1973; Hvid, Bentzen et al. 1989; Ciarelli, Goldstein et al. 1991; Goulet, Goldstein et al. 1994; Mimar, Limb et al. 2008) Modulus has been correlated to BMD using computed tomography (CT) and compression testing of trabecular bone blocks from humeri, tibias, and femurs.(Ciarelli, Goldstein et al. 1991) Consequently, BMD has been proposed as a good predictor for trabecular bone mechanical integrity.(Ciarelli, Goldstein et al. 1991) Higher resolution imaging techniques, such as micro-CT (μCT), have led to investigations linking mechanical properties to trabecular microstructure. Bone volume fraction (BVF) has been correlated to modulus as well as other microstructural properties, such as trabecular number (TbN), thickness (TbTh), and spacing (TbSp), but has not been related to degree of anisotropy.(Goulet, Goldstein et al. 1994) μCT has also been combined with indentation, a minimally destructive mechanical testing method, to correlate modulus to BVF and map the ideal locations for mechanical fixation across the glenoid surface.(Mimar, Limb et al. 2008)
Few studies have investigated how the mechanical properties of bone affect pullout strength.(Chapman, Harrington et al. 1996; Murphy, Hill et al. 2001; Yakacki, Griffis et al. 2009) Predictive models of pullout strength have been developed based on the shear strength of the material or bearing area of the device after testing anchor pullout in both cadaveric and synthetic bone.(Chapman, Harrington et al. 1996; Yakacki, Griffis et al. 2009) The yield strength of the bone has also been proposed as a threshold for suture anchor pullout as stresses above this limit would create irreversible deformations and lead to anchor failure.(Murphy, Hill et al. 2001)
In a concurrent study, the influence of the microstructural properties of cadaveric bone on the pullout strength of suture anchors was investigated.(Yakacki, Poukalova et al.) It was found that three of six microstructural properties analyzed show a statistical correlation to pullout strength: BMD, structural model index (SMI), and TbTh. A model was developed using these three properties to explain the variances in the individual correlations. The study concluded that trabecular structures with higher BMD, thicker trabeculae, and greater plate-like structure (lower SMI) result in higher pullout values. The other microstructural properties studied, TbSp, TbN, and connectivity density, showed no significant correlation to pullout strength. Investigating these microstructural properties in relation to mechanical properties would provide another perspective on the factors affecting pullout strength of suture anchors in bone.
Although previous studies have compared the trabecular microstructure to either mechanical properties or device performance, there have been no attempts to present all three collectively. Therefore, this paper aimed to simultaneously investigate the relationships between microstructure, mechanical properties, and suture anchor pullout strength. Furthermore, these relationships were examined in both cadaveric and synthetic bone to help aid the future selection and development of synthetic test materials commonly employed in fundamental scientific studies and regulatory approval testing. Osteoporotic bone was tested in this study to best represent the patient population with the greatest risk of suture anchor pullout.
MATERIALS AND METHODS
Materials
Twelve fresh-frozen human humeri with a mean age of 80.9 years (range of 74-85 years) were received and stored at −20°C (Table 1). The humeri were divided into two groups; Group 1 consisted of seven humeral heads, while the remaining five were placed into Group 2. If matched pairs of humeral heads were received, one humerus was placed in each group. Group 1 underwent compression testing and μCT scans while Group 2 underwent pullout testing and μCT scans. Each humerus was thawed at room temperature for 24 hours prior to any testing. In order to perform testing only in trabecular bone, the cortical layer on the humeral head and tuberosities was removed using a burring tool according to a previously developed technique.(Barber, Herbert et al. 1996; Barber, Herbert et al. 2006) It should be noted, portions of the cortical layer may be removed clinically when repairing a rotator cuff and is dependent on the surgeon’s preferred technique.
Table 1.
Donor information including age, gender, and cause of death.
| Donor# | Age | Gender | Cause of Death |
|---|---|---|---|
| S070496R | 85 | F | Senile dementia |
| S071015L | 81 | M | Intra Cranial Hemmorhage |
| S071015R | 81 | M | Massive stroke |
| S080062R | 78 | F | Sepsis |
| S080075L | 83 | F | Unspecified natural causes |
| S080075R | 83 | F | Unspecified natural causes |
| S080234L | 75 | F | Abdominal aortic aneurysm |
| S080234R | 75 | F | Abdominal aortic aneurysm |
| S080707L | 84 | M | Unspecified natural causes |
| S080707R | 84 | M | Unspecified natural causes |
| S080729L | 81 | M | End stage cardiac disease |
| S080729R | 81 | M | End stage cardiac disease |
Four densities of synthetic sawbone (5, 8, 10, and 15 pcf) were tested as cancellous bone representatives (Pacific Research Laboratories; Vashon, WA). These densities were selected because they are commonly used in biomechanical testing as osteoporotic cancellous bone substitutes.
Methods
Compression Testing (Group 1)
In preparation for sample extraction, each humerus was cut at the surgical neck below the greater and lesser tuberosities, and the anatomical neck, a narrow grove that separates the humeral head and greater tuberosity. A custom made punch, 8 mm diameter and 40 mm in length, was inserted into the designated sites (Figure 1). The sample was then extracted from the punch and cut to 12 mm in height. In total, 34 samples from seven humeri went through compression testing. Three samples were extracted from each density and cut to 12mm height, creating a total of 12 sawbone samples for compression testing.
Figure 1.
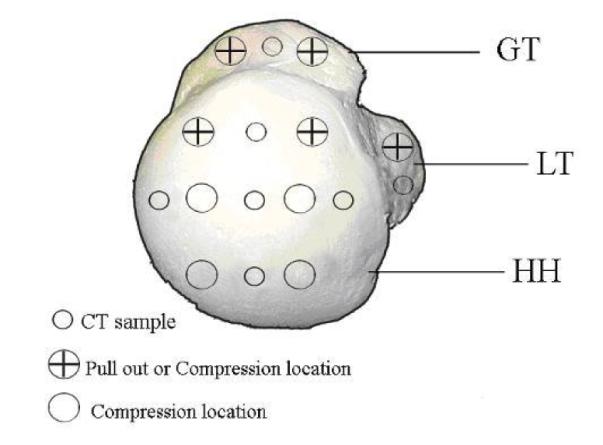
Superior view of the humeral head with marked test locations for μCT sampling, compression testing, and pullout testing.
Compression tests were performed on an Instron 5567 universal testing machine. Compression tests were performed using a crosshead speed of 0.1% strain per second and run to 60% strain. Data was calculated from each of the stress-strain curves (see online supplement). The modulus was taken as the maximum derivative of a best fit 3rd degree polynomial along the first 5% strain,(An 2000) while compressive strength was defined as the stress value at the first clear peak (yield point) along the stress-strain curve.
Pullout Testing (Group 2)
Pullout tests were performed using the same universal testing machine and a self-tapping 5mm Arthrex Corkscrew suture anchor. The anchor was threaded with #2 sutures (CP Medical; Portland, OR) and then inserted perpendicular and flush to the surface of the humerus at one of five testing sites in the greater tuberosity, lesser tuberosity, or humeral head (Figure 1). For synthetic bone, the anchor was inserted at the center of the machined block. The anchor was preloaded to 1N and pulled perpendicularly at a crosshead speed of 1 mm/sec. The failure load and failure method were recorded for each pullout test. 25 pullout tests were performed in cadaveric bone (n=5 per donor), while 12 pullout tests were performed in syntethic bone (n=3 per density).
Sample Extraction for μCT
Samples were extracted for μCT analysis after completion of the compression-sample extraction or pullout testing. Due to the size limitations of samples for μCT analysis, the compression samples extracted could not be scanned prior to mechanical testing. A custom made punch, 5 mm in diameter and 40 mm in length, was inserted into the designated sites to extract μCT sample (Figure 1). After extraction, the sample was cut to 10 mm in length and analyzed using μCT.
μCT Analysis
μCT analysis was performed to assess the morphometric parameters of the trabecular bone including BVF, SMI, and average trabecular thickness, spacing, and number. A detailed explanation of μCT analysis can be seen in the online supplement.
Statistics
Linear regression was fit to each correlation, and residual plots showed no significant deviations from a normal distribution. Pearson coefficients (r) were calculated in order to evaluate the linear correlation of μCT variables against modulus, compressive strength, and pullout strength. Student’s t-test was calculated and tested against an alpha value of 0.01.
RESULTS
Compression tests were performed on 34 cadaveric bone samples and 12 sawbone samples. The compressive strength was typically between 5 and 12% strain in cadaveric bone and 7 and 10% strain in synthetic sawbone. By comparison, the stress-strain behavior of the cadaveric bone samples fell within the stress-strain behavior range of sawbone densities tested. The compression behavior of the samples is further detailed in the online supplemental material.
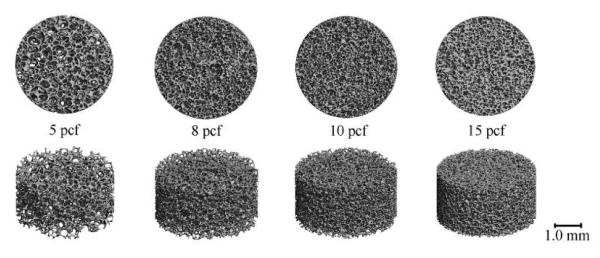
The mechanical properties and suture anchor performance were measured in conjunction with μCT analysis for both the cadaveric and synthetic bone. Representative μCT images for the four densities of sawbone studied are illustrated in Figure 2. It is visually apparent that the porosity of the sawbone matrix decreases with increasing density. The average values for microstructural, mechanical, and pullout properties of sawbone can be seen in Table 2. The range of microstructural properties of the cadaveric bone samples that underwent compression testing can be seen in Table 3. Unlike the synthetic material, all of the morphometric parameters varied from specimen to specimen. Average values for microstructural properties in cadaver bone specimens used for pullout testing have been shown in a concurrent study (See Appendix). (Yakacki, Poukalova et al.)
Figure 2.
μCT images from the four densities of sawbone tested.
Table 2.
Values of the microstructural parameters of the four sawbone samples represented in Figure 2.
| Specimen | 5 PCF | 8 PCF | 10 PCF | 15 PCF |
|---|---|---|---|---|
| BVF | 0.058 | 0.076 | 0.095 | 0.179 |
| SMI | 2.33 | 2.74 | 2.81 | 2.1 |
| Trabecular Thickness (mm) | 0.038 | 0.034 | 0.031 | 0.036 |
| Trabecular Spacing (mm) | 0.501 | 0.288 | 0.223 | 0.159 |
| Trabecular Number (1/mm) | 2.81 | 3.6 | 4.62 | 6.58 |
| Connectivity Density (1/mm3) | 72.7 | 269.5 | 490.5 | 856.7 |
| Average Modulus (MPa) | 14.1 | 29.5 | 51.5 | 87.6 |
| Average Strength (MPa) | 0.71 | 1.55 | 2.44 | 4.59 |
| Average Pullout (N) | 46.3 | 112.9 | 137.5 | 317 |
Table 3.
Average values for microstructural properties in cadavers that underwent compression testing
| Age | Sex | BVF | SMI | Tb.Th.(mm) | Tb.Sp.(mm) | Tb.N.(1/mm) | Conn. Den.(1/mm3) |
|---|---|---|---|---|---|---|---|
| 78 | F | 0.086 ± 0.016 | 2.21 ± 0.16 | 0.139 ± 0.005 | 0.909 ± 0.040 | 1.08 ± 0.06 | 3.84 ± 0.87 |
| 81 | M | 0.178 ± 0.036 | 0.94 ± 0.28 | 0.136 ± 0.008 | 0.741 ± 0.077 | 1.31 ± 0.16 | 6.28 ± 2.47 |
| 75 | F | 0.155 ± 0.017 | 1.95 ± 0.61 | 0.134 ± 0.008 | 0.738 ± 0.048 | 1.30 ± 0.08 | 4.73 ± 0.26 |
| 83 | F | 0.108 ± 0.020 | 1.91 ± 0.24 | 0.118 ± 0.011 | 0.672 ± 0.027 | 1.39 ± 0.04 | 6.16 ± 1.50 |
| 85 | F | 0.098 ± 0.006 | 2.36 ± 0.13 | 0.119 ± 0.005 | 0.739 ± 0.018 | 1.28 ± 0.03 | 4.31 ± 0.15 |
| 84 | M | 0.208 ± 0.067 | 1.09 ± 0.59 | 0.124 ± 0.018 | 0.506 ± 0.065 | 1.93 ± 0.26 | 14.14 ± 5.82 |
| 81 | M | 0.203 ± 0.065 | 1.46 ± 0.45 | 0.149 ± 0.028 | 0.651 ± 0.093 | 1.50 ± 0.18 | 8.93 ± 2.69 |
Six microstructural properties were compared against modulus, compressive strength, and pullout strength in both cadaveric and synthetic bone to identify statistically significant correlations. Tables 4 and 5 list the Pearson’s coefficient (r) along with the p-values of each microstructural property correlated to the mechanical properties and pullout strength for cadaveric and synthetic bone, respectively. In cadaveric bone, modulus was correlated to all of the microstructural properties. Trabecular thickness showed the weakest correlation (r = 0.48; p = 0.0041), while the remaining properties showed statistically significant correlations. The compressive strength and pullout strength of the cadaveric bone both show significant correlations to BVF, SMI, and trabecular thickness. In synthetic bone, modulus, compressive strength, and pullout strength were correlated to BVF, trabecular spacing, trabecular number, and connectivity density. SMI and trabecular thickness showed no correlations in sawbone due to the fact that these parameters showed little variability across the densities tested.
Table 4.
r-values and p-values for mechanical properties against microstructural variables in cadaver testing
| BVF | SMI | Tb.Th. | Tb.Sp. | Tb.N. | Conn. Den. | ||
|---|---|---|---|---|---|---|---|
| Modulus | r-value | 0.77 | −0.58 | 0.48 | −0.64 | 0.70 | 0.76 |
| p-value | < 0.0001 | 0.0003 | 0.0041 | < 0.0001 | < 0.0001 | < 0.0001 | |
|
| |||||||
| Strength | r-value | 0.59 | −0.47 | 0.59 | −0.27 | 0.23 | 0.26 |
| p-value | 0.0002 | 0.005 | 0.0002 | 0.1225 | 0.1907 | 0.1375 | |
|
| |||||||
| Pullout | r-value | 0.60 | −0.75 | 0.61 | −0.12 | 0.15 | 0.37 |
| p-value | 0.0015 | < 0.0001 | 0.0012 | 0.5678 | 0.4742 | 0.0687 | |
Table 5.
r-values and p-values for mechanical properties against microstructural variables in sawbone testing. Note, the r-values for trabecular spacing increase to r = −0.995, −0.997, and −0.986 with a power fit.
| BVF | SMI | Tb.Th. | Tb.Sp. | Tb.N. | Conn. Den. | ||
|---|---|---|---|---|---|---|---|
| Modulus | r-value | 0.98 | −0.43 | −0.37 | −0.88 | 0.99 | 0.999 |
| p-value | < 0.0001 | 0.163 | 0.2365 | 0.0002 | < 0.0001 | < 0.0001 | |
|
| |||||||
| Strength | r-value | 0.99 | −0.47 | −0.32 | −0.87 | 0.99 | 0.995 |
| p-value | < 0.0001 | 0.1231 | 0.3106 | 0.0002 | < 0.0001 | < 0.0001 | |
|
| |||||||
| Pullout | r-value | 0.99 | −0.53 | −0.24 | −0.83 | 0.96 | 0.97 |
| p-value | < 0.0001 | 0.0763 | 0.4524 | 0.0008 | < 0.0001 | < 0.0001 | |
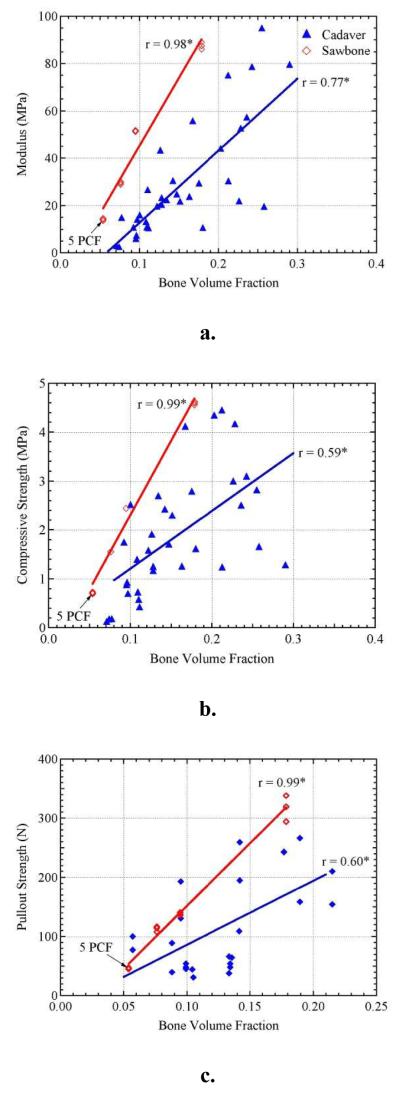
To better illustrate the relationships of the materials and microstructural parameters, the effect of BVF on modulus, compressive strength, and pullout strength can be seen in Figure 3. All three mechanical properties showed linear correlations to BVF in both cadaveric and synthetic bone. In synthetic sawbone, BVF showed significantly stronger correlations with r-values ≥ 0.98 due to the uniformity of the material. Furthermore, the sawbone curves tend to lie in the upper range of cadaveric bone data for all mechanical properties as a function of BVF.
Figure 3.
The effect of bone volume fraction on a). elastic modulus, b). compressive strength, and c). pullout strength. Sawbone densities increase from 5 to 15 pcf with the lowest density (5 pcf) labeled on the plots. *Significantly correlated at p < 0.01.
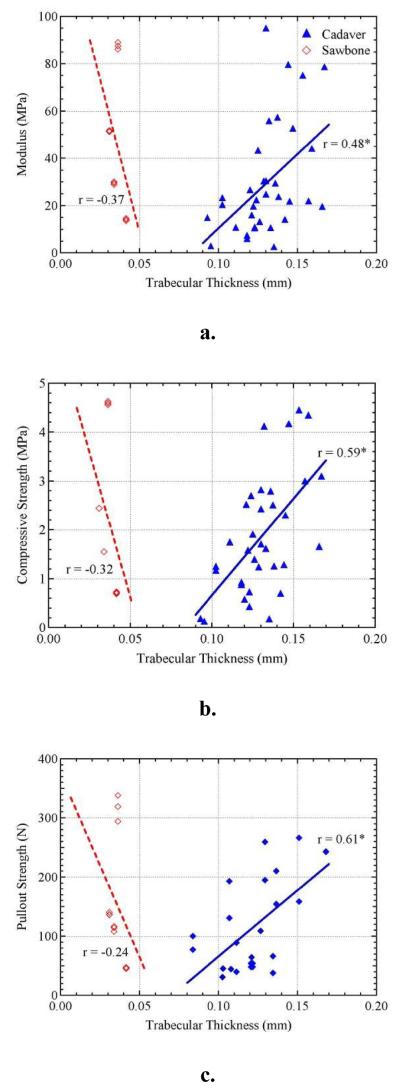
The relationship between trabecular thickness and modulus, compressive strength, and pullout strength can be seen in Figure 4. Compressive and pullout strength in cadaveric bone show significant positive linear correlations against trabecular thickness with r-values of 0.59 and 0.61 and p-values of 0.0002 and 0.0012, respectively. Modulus shows a weaker correlation against trabecular thickness (r = 0.48, p = 0.0041). However, the relationships differed in synthetic sawbone where there were no significant correlations to trabecular thickness. A near vertical line of data illustrates the limited variability of trabecular thickness in sawbone. In comparison to cadaveric bone, the trabecular thickness in sawbone is dramatically lower. The trabecular thickness ranged from 0.031 to 0.042 mm in sawbone and 0.095 to 0.167 mm in cadaveric bone.
Figure 4.
The effect of trabecular thickness on a). elastic modulus, b). compressive strength, and c). pullout strength. *Significantly correlated at p < 0.01.
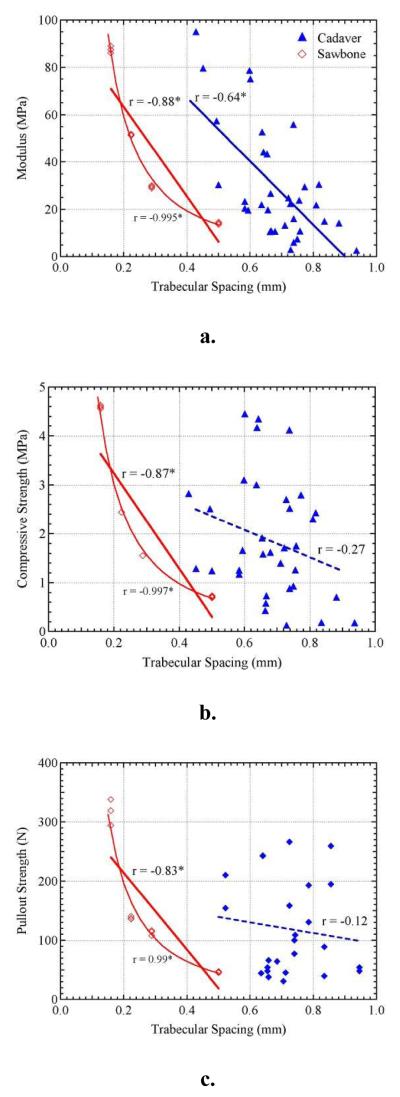
Figure 5 shows the relationship between the three mechanical properties and trabecular spacing. Cadaveric bone samples show a significant negative linear correlation for trabecular spacing against modulus (r = −0.64; p < 0.0001); however, do not show a significant correlation to compressive strength (r = −0.27; p = 0.1225) or pullout strength (r = −0.12; p = 0.5678). For sawbone, all three mechanical properties show significant correlations to trabecular spacing. It should be noted that the sawbone correlations to trabecular spacing were better represented by power fits (r = −0.995, −0.997, −0.986) rather than negative linear correlations (r = −0.88, −0.87, −0.83), respectively. Similar to trabecular thickness, the trabecular spacing of the synthetic sawbone material was lower than that of the cadaveric bone. Trabecular spacing ranged from 0.159 to 0.501 mm in sawbone and 0.656 to 0.835 mm in cadaveric bone.
Figure 5.
The effect of trabecular spacing on a). elastic modulus, b). compressive strength, and c). pullout strength. *Significantly correlated at p < 0.01.
DISCUSSION
To the best of the authors’ knowledge, no previous study has attempted to link mechanical properties, microstructural architecture, and suture anchor performance simultaneously. Furthermore, these characteristic properties were also investigated in both cadaveric and synthetic bone to help identify any key differences between the two biomechanical test materials. In total, 12 cadaver humeri were studied with 7 used in compression tests and 5 used in pullout tests. For synthetic bone, four different densities were used in compression and pullout tests.
In cadaveric bone, there is a linear relationship between BVF and all three properties tested with p-values ≤ 0.0015. These results support the notion that bone with a higher volume fraction, and consequently higher mineral density, will be stiffer, experience higher ultimate loads, and provide better fixation for bone anchoring devices. The correlation of BVF to modulus and strength is consistent with the results of previous studies.(Ciarelli, Goldstein et al. 1991; Goldstein, Goulet et al. 1993; Goulet, Goldstein et al. 1994; Giesen, Ding et al. 2004) Modulus and strength have been correlated to BVF with predictive models using the mean intercept length (MIL) and degree of anisotropy (DA) to explain some of the variances in the correlations.(Goulet, Goldstein et al. 1994) In this study, degree of anisotropy did not show any statistical differences in the three testing locations of the humerus (see online supplement) and were not included in the analysis.
It has been suggested that only a measure of BVF would be needed for estimation of mechanical properties.(Goldstein, Goulet et al. 1993) BVF is highly correlated to the other microstructural properties (TbSp, TbTh, TbN, and connectivity density), which would explain why modulus was correlated to all of the microstructural properties investigated in this study. However, SMI was not usually taken into account when suggesting BVF alone would be a sufficient predictor of performance. Using finite-element and digital topological analysis, plate-like trabecular structures has been shown to play a more influential role in determining modulus than rod-like trabeculae.(Liu, Sajda et al. 2006) This would suggest the need to take into account more than just bone density when assessing mechanical properties of bone. For example, two samples of bone with equal BVF but different SMI would experience different properties.
The compressive and pullout strengths both showed correlations to BVF, SMI, and trabecular thickness in cadaveric bone. It is important to emphasize that these two measures of mechanical performance are influenced by the same morphometric parameters, which can be explained due to the fact that yielding is a precursor to suture anchor pullout (i.e. yielding will occur before the bone exhibits failure). Yielding results in irrecoverable deformations and leads up to a yield point, which defines the compressive strength and is the first peak maximum on a stress-strain curve. Once a yield point (compressive strength) is reached, large-scale deformations are free to occur, which will ultimately cause suture anchor pullout. These data suggest that the compressive strength of the material may be correlated to pullout strength. However, yield strength and pullout strength were measured in two separate cadaveric specimen groups and thus could not be related.
Synthetic sawbone showed significant correlations between BVF, trabecular spacing, trabecular number, and connectivity density against all three properties tested with p-values ≤ 0.0008. The correlations in the synthetic bone have smaller variances compared to cadaveric bone and can be attributed to the uniformity of the synthetic bone matrix. Microstructural analysis of the sawbone material showed that trabecular strut thickness and SMI of the sawbone material did not vary consistently across the different densities tested. Therefore, correlations could not be made between the mechanical properties tested and trabecular thickness and SMI. The synthetic sawbone allowed for compression and pullout testing to be performed on the same sample population and thus allowed a correlation between compressive strength and pullout strength (r = 0.99, p ≤ 0.0001). These results help validate a previous study’s hypothesis that a material’s strength, either yield or compressive strength, can be used as a predictor for bone anchor pullout.(Murphy, Hill et al. 2001)
In a review of sawbone materials, it is recommended that an ideal biomechanical test include both synthetic and cadaveric test models, as synthetic bone will help offer a more uniform test matrix.(Hausmann 2006) However, to the best of the authors’ knowledge, there is no recommended way to choose a specific sawbone density for a given application. One can always try to match the stiffness and strength of the synthetic bone to the real bone, but this requires the destructive means of a mechanical test. Therefore, Table 6 was designed as an aid in the selection process for synthetic sawbone by non-destructive CT analysis. Table 6 matches the performance of the sawbone to the linear regression of the mechanical properties compared to BVF as shown in Figure 3. Ergo, if one can obtain a reasonable estimate for the BVF of the bone to be tested, an appropriate sawbone can be selected. Table 6 can also be used to match specific mechanical properties, such as the modulus or pullout strength, or for overall performance for the three mechanical properties tested in this study.
Table 6.
A selection aid in determining the appropriate synthetic sawbone density for biomechanical testing based on a measure of BVF. First, a measure of BVF must be obtained or estimated. Next, choose a specific mechanical property, or an average of the mechanical properties, to match between sawbone and human bone. Lastly, determine the recommended density of sawbone to use by selecting the column in which the BVF measurement falls within.
| Property to Match | Measured Bone Volume Fraction | |||
|---|---|---|---|---|
| Pullout | <0.10 | 0.10 - 0.13 | 0.14 - 0.20 | >0.20 |
| Strength | <0.12 | 0.12 - 0.17 | 0.18 - 0.25 | >0.25 |
| Modulus | <0.14 | 0.14 - 0.20 | 0.21 - 0.29 | >0.29 |
| Average | <0.12 | 0.12 - 0.17 | 0.18 - 0.25 | >0.25 |
|
| ||||
| Recommended Density (pcf) | 5 | 8 | 10 | ≥15 |
This study helps progress the fundamental relationships between mechanical properties, microstructural properties, and device performance in cadaveric bone. It should be noted that the donor age for this study ranged from 74 to 85 years old and exhibited deficient bone quality of osteopenic bone. Younger donors would have produced higher quality bone (i.e. increased BVF, SMI, TbTh), which would have resulted in enhanced mechanical properties and fixation strength; however, patients with healthy bone quality are less susceptible to suture anchor pullout and are more prone to other modes of failure such as suture breakage. The intent of this study was to focus on a sample population that is more subject to suture anchor pullout. This study is intended to illustrate the key microstructural properties of trabecular bone that affect the behavior of bone under loads and ultimately fixation devices, such as suture anchors. Furthermore, these property-performance relationships were compared in synthetic bone since sawbone is often used as an alternative test material to cadaveric bone. This will in turn aid future studies in describing the similarities between results using the two materials as well as help choose an appropriate representative synthetic material. When using synthetic bone, the differences in trabecular microstructure, such as TbSp and TbTh, should be taken into account with the method used and device tested to ensure the validity of the study.
Limitations in this study include the inability to perform compression tests, pullout tests, and μCT in the same location. This allows for error due to variations by location. Previous studies have shown that significant variations can occur in trabecular structure in elderly patients by location.(Chen, Shoumura et al. 2008; Lancianese, Kwok et al. 2008) Future studies should investigate the extent of these variations in the humerus. Due to the high resolution used for μCT the entire head of the humerus could not be scanned, thus creating the need to take small samples adjacent to the testing locations. Furthermore, future work should attempt to correlate compressive strength and pullout strength in cadaveric bone. The cortical layer was removed prior to all testing, which likely decreased the values of all three mechanical properties. However, the removal of the cortical layer allowed the study to focus on specifically the trabecular structure. In addition, the trabecular discontinuity at the edge of the samples caused by extraction could cause variation in the mechanical properties and CT analysis. The surface effects of machined bone have been shown to increase the compliance of samples at the testing interface and results in a decrease of modulus with decreasing sample size.(Zhu, Keller et al. 1994)
CONCLUSIONS
BVF showed significant correlations to modulus, compressive strength, and pullout strength in both cadaveric and synthetic bone. In cadaveric bone, compressive and pullout strength were also correlated to SMI and trabecular thickness. In synthetic bone, SMI and trabecular spacing did not consistently vary across the densities tested and thus did not show any significant correlations to the mechanical properties. Lastly, an appropriate synthetic bone density can be recommended for biomechanical testing to match specific properties such as modulus and pullout strength via a measurement of a patient’s BVF.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank Kurt Jacobus PhD and Parth Brahmbhatt for their help and support of this study. This study was funded in part by the NIH/NIAMS (1R43AR056154-01).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- An Y, Robert D. Mechanical Testing of Bone and the Bone-Implant Interface. CRC Press; Boca Raton: 2000. [Google Scholar]
- Barber FA, Feder SM, et al. The relationship of suture anchor failure and bone density to proximal humerus location: A cadaveric study. Arthroscopy: The Journal of Arthroscopic & Related Surgery. 1997;13(3):340–345. doi: 10.1016/s0749-8063(97)90031-1. [DOI] [PubMed] [Google Scholar]
- Barber FA, Herbert MA. Suture anchors--update 1999. Arthroscopy: The Journal Of Arthroscopic & Related Surgery: Official Publication Of The Arthroscopy Association Of North America And The International Arthroscopy Association. 1999;15(7):719–725. doi: 10.1016/s0749-8063(99)70003-4. [DOI] [PubMed] [Google Scholar]
- Barber FA, Herbert MA, et al. Suture anchor strength revisited. Arthroscopy: The Journal Of Arthroscopic & Related Surgery: Official Publication Of The Arthroscopy Association Of North America And The International Arthroscopy Association. 1996;12(1):32–38. doi: 10.1016/s0749-8063(96)90216-9. [DOI] [PubMed] [Google Scholar]
- Barber FA, Herbert MA, et al. Sutures and suture anchors--update 2006. Arthroscopy: The Journal Of Arthroscopic & Related Surgery: Official Publication Of The Arthroscopy Association Of North America And The International Arthroscopy Association. 2006;22(10):1063.e1061–1069. doi: 10.1016/j.arthro.2006.04.106. [DOI] [PubMed] [Google Scholar]
- Chapman JR, Harrington RM, et al. Factors affecting the pullout strength of cancellous bone screws. J Biomech Eng. 1996;118(3):391–398. doi: 10.1115/1.2796022. [DOI] [PubMed] [Google Scholar]
- Chen H, Shoumura S, et al. Regional variations of vertebral trabecular bone microstructure with age and gender. Osteoporosis International: A Journal Established As Result Of Cooperation Between The European Foundation For Osteoporosis And The National Osteoporosis Foundation Of The USA. 2008;19(10):1473–1483. doi: 10.1007/s00198-008-0593-3. [DOI] [PubMed] [Google Scholar]
- Ciarelli MJ, Goldstein SA, et al. Evaluation of orthogonal mechanical properties and density of human trabecular bone from the major metaphyseal regions with materials testing and computed tomography. J Orthop Res. 1991;9(5):674–682. doi: 10.1002/jor.1100090507. [DOI] [PubMed] [Google Scholar]
- Djurasovic M, Marra G, et al. Revision rotator cuff repair: factors influencing results. The Journal Of Bone And Joint Surgery. American Volume. 2001;83-A(12):1849–1855. doi: 10.2106/00004623-200112000-00013. [DOI] [PubMed] [Google Scholar]
- Giesen EB, Ding M, et al. Changed morphology and mechanical properties of cancellous bone in the mandibular condyles of edentate people. J Dent Res. 2004;83(3):255–259. doi: 10.1177/154405910408300314. [DOI] [PubMed] [Google Scholar]
- Goldstein SA, Goulet R, et al. Measurement and significance of three-dimensional architecture to the mechanical integrity of trabecular bone. Calcified Tissue International. 1993;53(Suppl 1):S127. doi: 10.1007/BF01673421. [DOI] [PubMed] [Google Scholar]
- Goulet RW, Goldstein SA, et al. The relationship between the structural and orthogonal compressive properties of trabecular bone. Journal Of Biomechanics. 1994;27(4):375–389. doi: 10.1016/0021-9290(94)90014-0. [DOI] [PubMed] [Google Scholar]
- Hausmann JT. Sawbones in Biomechanical Settings - a Review. Osteo Trauma Care. 2006;14:259–264. [Google Scholar]
- Hvid I, Bentzen SM, et al. X-ray quantitative computed tomography: the relations to physical properties of proximal tibial trabecular bone specimens. J Biomech. 1989;22(89):837–844. doi: 10.1016/0021-9290(89)90067-5. [DOI] [PubMed] [Google Scholar]
- Lancianese SL, Kwok E, et al. Predicting regional variations in trabecular bone mechanical properties within the human proximal tibia using MR imaging. Bone. 2008;43(6):1039–1046. doi: 10.1016/j.bone.2008.07.247. [DOI] [PubMed] [Google Scholar]
- Liu XS, Sajda P, et al. Quantification of the roles of trabecular microarchitecture and trabecular type in determining the elastic modulus of human trabecular bone. J Bone Miner Res. 2006;21(10):1608–1617. doi: 10.1359/jbmr.060716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland EG, Park HB, et al. Suture anchors and tacks for shoulder surgery, part 1: biology and biomechanics. The American Journal Of Sports Medicine. 2005;33(12):1918–1923. doi: 10.1177/0363546505282621. [DOI] [PubMed] [Google Scholar]
- Meyer DC, Fucentese SF, et al. Mechanical testing of absorbable suture anchors. Arthroscopy: The Journal Of Arthroscopic & Related Surgery: Official Publication Of The Arthroscopy Association Of North America And The International Arthroscopy Association. 2003;19(2):188–193. doi: 10.1053/jars.2003.50015. [DOI] [PubMed] [Google Scholar]
- Meyer DC, Mayer J, et al. Ultrasonically implanted PLA suture anchors are stable in osteopenic bone. Clinical Orthopaedics and Related Research. 2006;(442):143–148. doi: 10.1097/01.blo.0000185033.32220.57. [DOI] [PubMed] [Google Scholar]
- Millenium Research Group US Markets for Orthopedic Soft Tissue Solutions. 2008 2007.
- Mimar R, Limb D, et al. Evaluation of the mechanical and architectural properties of glenoid bone. Journal of Shoulder and Elbow Surgery. 2008;17(2):336–341. doi: 10.1016/j.jse.2007.07.024. [DOI] [PubMed] [Google Scholar]
- Murphy TP, Hill CM, et al. Pullout properties of 3.5-mm AO/ASIF self-tapping and cortex screws in a uniform synthetic material and in canine bone. Veterinary Surgery: VS: The Official Journal Of The American College Of Veterinary Surgeons. 2001;30(3):253–260. doi: 10.1053/jvet.2001.23344. [DOI] [PubMed] [Google Scholar]
- Park HB, Keyurapan E, et al. Suture anchors and tacks for shoulder surgery, part II: the prevention and treatment of complications. The American Journal Of Sports Medicine. 2006;34(1):136–144. doi: 10.1177/0363546505284240. [DOI] [PubMed] [Google Scholar]
- Pietschmann MF, Fröhlich V, et al. Suture anchor fixation strength in osteopenic versus non-osteopenic bone for rotator cuff repair. Archives Of Orthopaedic And Trauma Surgery. 2009;129(3):373–379. doi: 10.1007/s00402-008-0689-4. [DOI] [PubMed] [Google Scholar]
- Pietschmann MF, Fröhlich V, et al. Pullout strength of suture anchors in comparison with transosseous sutures for rotator cuff repair. Knee Surgery, Sports Traumatology, Arthroscopy: Official Journal Of The ESSKA. 2008;16(5):504–510. doi: 10.1007/s00167-007-0460-3. [DOI] [PubMed] [Google Scholar]
- Pugh JW, Rose RM, et al. Elastic and viscoelastic properties of trabecular bone: dependence on structure. J Biomech. 1973;6(5):475–485. doi: 10.1016/0021-9290(73)90006-7. [DOI] [PubMed] [Google Scholar]
- Tingart MJ, Apreleva M, et al. Anchor design and bone mineral density affect the pull-out strength of suture anchors in rotator cuff repair: which anchors are best to use in patients with low bone quality? The American Journal Of Sports Medicine. 2004;32(6):1466–1473. doi: 10.1177/0363546503262644. [DOI] [PubMed] [Google Scholar]
- Tingart MJ, Apreleva M, et al. Pullout strength of suture anchors used in rotator cuff repair. The Journal Of Bone And Joint Surgery. American Volume. 2003;85-A(11):2190–2198. doi: 10.2106/00004623-200311000-00021. [DOI] [PubMed] [Google Scholar]
- Yakacki CM, Griffis J, et al. Bearing area: A new indication for suture anchor pullout strength? Journal of Orthopaedic Research. 2009;27(8):1048–1054. doi: 10.1002/jor.20856. [DOI] [PubMed] [Google Scholar]
- Yakacki CM, Poukalova M, et al. Pullout Strength of Suture Anchors - Part I: Effect of Trabecular Microstructure using Micro-CT. (In Review)
- Zhu M, Keller TS, et al. Effects of specimen load-bearing and free surface layers on the compressive mechanical properties of cellular materials. J Biomech. 1994;27(1):57–66. doi: 10.1016/0021-9290(94)90032-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.