Abstract
Fgf10 is a critical component of mesenchymal-to-epithelial signaling during endodermal development. In the Fgf10 null pancreas, the embryonic progenitor population fails to expand, while ectopic Fgf10 expression forces progenitor arrest and organ hyperplasia. Using a conditional Fgf10 gain-of-function model, we observed that the timing of Fgf10 expression affected the cellular competence of the arrested pancreatic progenitors. We present evidence that the Fgf10-arrested progenitor state is reversible and that terminal differentiation resumes upon cessation of Fgf10 production. However, competence towards the individual pancreatic cell lineages depended upon the gestational time of when Fgf10 expression was attenuated. This revealed a competence window of endocrine and ductal cell formation that coincided with the pancreatic secondary transition between E13.5 and E15.5. We demonstrate that maintaining the Fgf10-arrested state during this period leads to permanent loss of competence for the endocrine and ductal cell fates. However, competence of the arrested progenitors towards the exocrine cell fate was retained throughout the secondary transition. Sustained Fgf10 expression caused irreversible loss of Ngn3 expression, which may underlie the loss of endocrine competence. Maintenance of exocrine competence may be attributable to continuous Ptf1a expression in the Fgf10-arrested progenitors. This may explain the rapid induction of Bhlhb8, a normally distalized cell intrinsic marker, following loss of ectopic Fgf10 expression. We conclude that the window for endocrine and ductal cell competence ceases during the secondary transition in pancreatic development.
Keywords: Fgf10, pancreas, endocrine, competence, exocrine, Ngn3, patterning
INTRODUCTION
The pancreas performs both endocrine and exocrine functions critical for proper glucose homeostasis and nutrient uptake. In the mouse, the pancreas originates as two separate dorsal and ventral evaginations of the posterior foregut around E8.5. At this time, the pancreatic primordia consist of progenitor cells competent to adopt endocrine, exocrine, and ductal fates (Gu et al., 2002). The buds will later fuse into one organ during the rotation of the gut at E12.5. At E9.5, glucagon-expressing alpha-like cells in the dorsal bud are detected, followed by similar cells appearing in the ventral bud one day later. Beta and epsilon cells are detectable at E11.5 and continue to form at higher rates during the secondary transition, which occurs between E13.5 and E15.5. During this time, delta cells also form, and exocrine and ductal differentiation begins. Following differentiation, endocrine cells become post-mitotic, delaminate, and organize into functional clusters visible at E18.5. These clusters will become the islets of Langerhans.
Cellular competence of the pancreatic progenitor cells controls which of the endocrine, ductal and exocrine cell fates emerge in the developing pancreas. Competence towards the individual endocrine cell sub-types capable of forming changes during pancreas development. Temporal gain-of-function studies of the pro-endocrine factor Ngn3 revealed that competence towards the alpha cell fate exists at the time of pancreas specification (E8.5) or earlier (Johansson et al., 2007). Competence for the β cell fate is reached after E11.5, and competence for the PP and γ cell subtypes occur after E14.5 (Johansson et al., 2007). Competence towards the ductal fate in the pancreas has also been investigated; the ductal precursors are present between E9.5 and E12.5 (Gu et al., 2002). The time period of when cellular competence towards the exocrine cell fate exists during pancreatic development remains unknown. Spatial organization of the pancreatic progenitor population also appears to influence cellular competence. Recent descriptions of regionalized gene expression patterns defined discrete domains that segregate the pancreatic progenitor population over time (Zhou et al., 2008). One particular domain located at the branching tips of the pancreatic epithelium was reported to contain multipotent pancreatic progenitor cells (Zhou et al., 2008). This domain is spatially unique as defined by its close proximity to the embryonic pancreatic mesenchyme.
Mesenchymal-to-epithelial signaling is critical during the development of most endodermally-derived organs, including the pancreas. The connection between mesenchymal signaling, epithelial morphogenesis, and cytodifferentiation in the pancreas was first described in the embryonic rat; experimental perturbation of the mesenchymal-epithelial ratio disrupted pancreatic cell differentiation, accelerating cytodifferentiation following pancreatic mesenchymal depletion (Wessells N.K. and Cohen J.H., 1967). Later explant studies revealed a skewed balance of endocrine versus exocrine cell development upon mesenchymal separation, implying that pancreatic progenitor cells are committed to endocrine lineages upon loss of mesenchymal signals (Gittes et al., 1996; Miralles et al., 1998). Comparable phenotypes have also been observed in Hes1, Dll1, and RBP-Jκ deficient mice, which are all deficient in Notch signaling; the pancreatic progenitor cells in these mutants undergo accelerated differentiation towards the earliest endocrine cell type capable of forming (Apelqvist et al., 1999; Jensen et al., 2000; Fujikura et al., 2006). These similar phenotypes suggest that activation of the Notch pathway during the proliferation phase of the early pancreatic epithelium is ultimately controlled by mesenchymal signals.
Fgf10 protein is normally secreted by the pancreatic mesenchyme. Fgf10 [GeneID: 14165] maintains and expands the pancreatic epithelial progenitor population, whereas its absence results in severe organ hypoplasia (Bhushan et al., 2001; Miralles et al., 1999). As this loss-of-function phenotype mimics that of the Notch signaling mutants, we and others generated Fgf10 gain-of-function models that allowed ectopic expression of Fgf10 during pancreatic development using the Pdx1 promoter (Hart et al., 2003; Norgaard et al., 2003). Fgf10 overexpression resulted in epithelial hyperplasia and an almost complete inhibition of terminal differentiation. The Fgf10-arrested progenitor cells remained active in Notch signaling as defined by the continued expression of Notch ligands, receptors, and the Notch target gene Hes1. Active Notch signaling may provide an explanation for the attenuation of the differentiation programs in the presence of elevated Fgf10. This hypothesis has been supported by explant studies that showed activation of the Notch pathway requires Fgf10 signaling during pancreatic progenitor cell renewal (Miralles et al., 2006). We concluded that Fgf10 mediates progenitor cell arrest in the developing pancreas.
In addition to its role in progenitor maintenance (Hart et al., 2003; Norgaard et al., 2003), Fgf10 influences the development of the exocrine compartment. Explant studies demonstrated that exocrine differentiation requires the presence of the pancreatic mesenchyme; acinar cells were not detected when the pancreatic epithelium was separated from the surrounding mesenchyme (Gittes et al., 1996; Miralles et al., 1998). Down-regulation of FGFR2IIIb, a receptor for Fgf10, in the pancreatic epithelium also reduced exocrine differentiation (Miralles et al., 1999). In contrast, the presence of Fgf10 significantly increased the number of acinar cells in pancreatic explants (Miralles et al., 2006). Expansion of the exocrine compartment in this study may reflect overall increased proliferation of the pancreatic progenitor population; however, a concomitant increase in the endocrine cell population was not observed (Miralles et al., 2006). Another explanation for the higher levels of exocrine differentiation is the loss of competence towards the endocrine cell lineages. This implies that Fgf10 alters the cellular competence of the undifferentiated pancreatic progenitors.
To address these functions of Fgf10, we generated a conditional Fgf10 gain-of-function model that allowed for temporal and spatial control of Fgf10 expression in the pancreatic epithelium. This model allowed us to determine whether cellular competence of the pancreatic progenitors towards any pancreatic fate is a function of time during organ formation. We report that the Fgf10-arrested progenitor state is reversible and that Fgf10-arrested cells undergo differentiation upon cessation of Fgf10 expression. We found a temporal window for endocrine and ductal cell competence, whereas exocrine competence was retained throughout the secondary transition. Progenitors arrested by Fgf10 during the secondary transition lose the ability to express Ngn3. In contrast, Ptf1a [GeneID: 19213] expression was maintained and unaffected during Fgf10-mediated arrest and subsequent release, which may explain the persistent competence for exocrine differentiation. In contrast to the irreversible loss of Ngn3, expression of the distalized exocrine marker, bhlhb8/Mist1 [GeneID: 17341] was rapidly restored upon cessation of Fgf10 expression
RESULTS
TetOFF control of Fgf10 in the pancreas leads to suppression of cell differentiation of all epithelial lineages
Three independent pTRE2-Fgf10FLAG transgenic mouse lines were evaluated. Double-transgenic (DTG) embryos from Pdx1-tTA; pTRE2-Fgf10 intercrosses in the absence of doxycycline (DOX) were analyzed at various gestational time points as outlined in Fig. 1A. Either single transgenic (STG, either Pdx1-tTA or pTRE2-Fgf10) or wild-type (WT) littermates were used as controls. All analyses were performed in triplicate. In the absence of DOX in the drinking water, continuous expression of the Fgf10FLAG transgene was detected within the pancreas of DTG embryos. All DTG embryos displayed a hyperplastic pancreas consisting of either very dense tissue (Fig. 2D) or cysts (Fig. 2B) compared to WT controls (Fig. 2A, C, and data not shown). Histological analysis of transgenic Fgf10 expression was performed using an Fgf10-specific antibody. Heterogeneous cytosolic and plasma membrane staining was observed (Fig. 2F, G, H). Although the individual lines displayed different levels of Fgf10 expression (Fig. 2F, G, H), comparable phenotypes were observed. Similar immunostaining patterns were obtained using an anti-FLAG antibody to detect transgenic Fgf10 (Supp. Fig. 1). Fgf10 protein was not observed in the epithelium of STG pTRE2-Fgf10 littermates, indicating that the transgene did not have leaky expression (Fig. 2E). Multiplex RT-PCR revealed an increase in Fgf10 mRNA levels at E16.5 in the DTG pancreas (Fig. 2I).
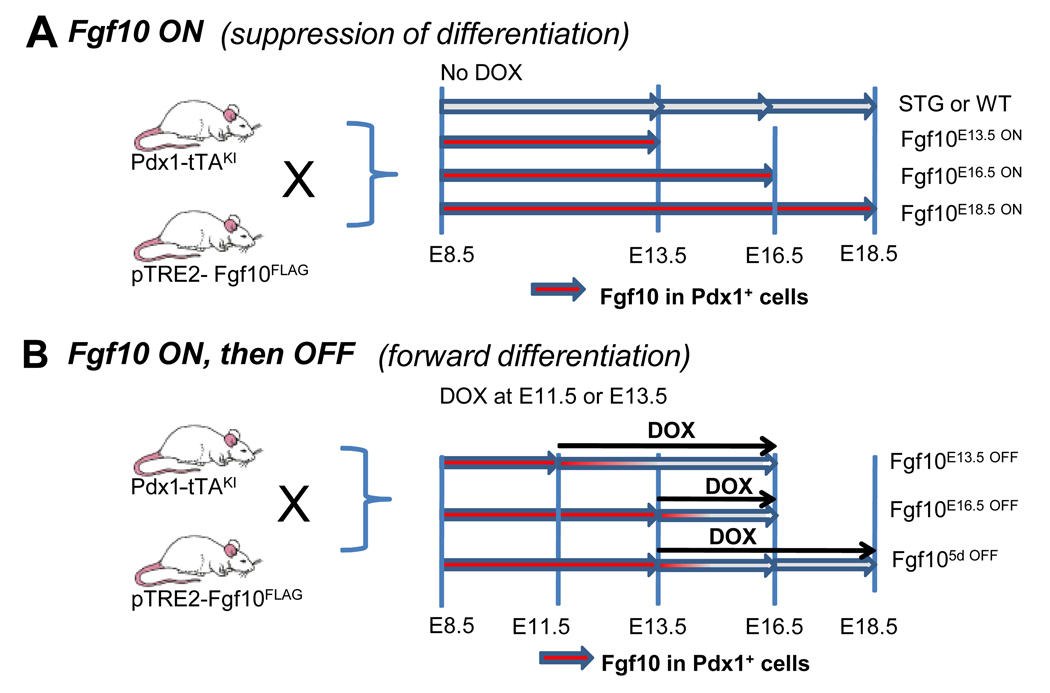
Figure 1. TetOFF control of Fgf10 in the developing pancreas.
Schematic representation of the TetOFF inducible system to control the timing of Fgf10 overexpression. Pdx-tTAKI mice were crossed with pTRE2-Fgf10FLAG mice to generate pTRE2-Fgf10; Pdx1-tTA DTG embryos. The endogenous Pdx1 promoter drives expression of the tTA transactivator protein throughout the embryonic pancreatic epithelium. The tTA protein transactivates Fgf10 expression in the absence of doxycycline (DOX). The phenotypic effects of constitutive and transient Fgf10 expression were examined by controlling the timing of its expression. (A) Ectopic Fgf10 expression is continuous in the absence of DOX. (B) Transgenic Fgf10 expression was shut-off at E11.5 or E13.5 by administering DOX to pregnant mothers. Embryos were harvested at E13.5, E16.5, and E18.5 where indicated in both A and B.
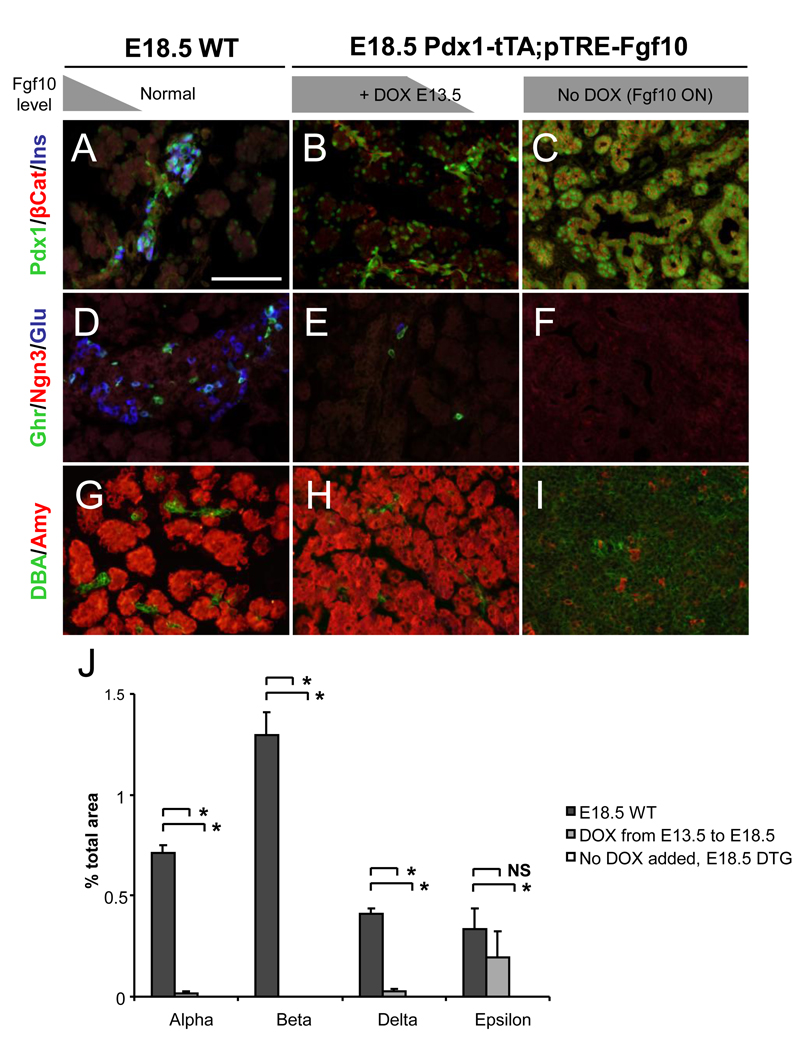
Figure 2. Pancreatic phenotype in DTG embryos with conditional Fgf10 overexpression.
Phenotype of the pTRE2-Fgf10; Pdx1-tTA DTG pancreas and expression of the transgene at E17.5. A–D: Exterior phenotype of WT and DTG (line 7572) in the absence of DOX. The DTG embryos display a cystic dorsal pancreas (B) and a highly condensed tissue in the ventral pancreatic region (D). E–H: Immunostaining of ectopic Fgf10 protein in WT and DTG pancreata (lines 7572, 5539, and 5510) at E16.5. Scale bar: E–H, 50 µm. I: Multiplex RT-PCR at 19 cycles confirming the expression of the Fgf10 mRNA (line 5539). Alpha-tubulin (A-Tubulin) used as internal control, E16.5. St; stomach, sp; spleen, duo; duodenum, dp; dorsal pancreas, vp; ventral pancreas.
Analysis of terminal differentiation and progenitor markers was performed at E13.5 when differentiation of insulin and exocrine cells recently commenced (Fig. 3A–F). At this time point, which corresponds to the onset of the secondary transition, the majority of pancreatic epithelial cells are undifferentiated progenitors expressing high levels of Pdx1 [GeneID: 18609] and β-catenin [GeneID: 12387] in absence of any mature endocrine hormone production (Fig. 3A, C). Insulin cell development occurs in a scattered fashion, driven by the activity of Ngn3 (Fig. 3E). Pdx1 is expressed at very high levels in the β-cells (arrow, Fig. 3A, C). Glucagon- and ghrelin-expressing cells were present (Fig. 3E), but somatostatin- and PP-type endocrine cells were not detected at this time point (data not shown). Terminal endocrine differentiation markers were mostly absent at E13.5 in DTG embryos (Fig. 3B, D, F). Ngn3 expression was absent, as was glucagon, insulin, and ghrelin expression. Instead, a hyperplastic pancreatic progenitor pool characterized by a low-level of Pdx1 immunoreactivity and a high-level of β-catenin expression was present (Fig 3B). Fgf10 was expressed more homogenously at E13.5 than at E16.5 in DTG embryos (Fig. 3G, H). Expression of Fgf10 in the pancreatic progenitors in the DTg pancreas was confirmed by the widespread and overlapping immunostaining pattern of FLAG-tagged Fgf10 and the progenitor markers Pdx1 and Sox9 during the secondary transition at E14.5 (Supp. Fig. 1).
Figure 3. Fgf10-mediated developmental arrest.
Immunostaining for pancreatic progenitor and differentiation markers at E13.5 (A–G) and E16.5 (H–P) in WT and DTG littermate pancreas. Primary antigens and their indicated secondary color detections are shown to the left. G and H: immunostaining of Fgf10. C, D, G, and H are confocal optical sections. O, P: whole-mount immunofluorescence staining for DBA and amylase and rendered Z-stack composite images of the ventral pancreatic regions. Duo; duodenum, vp; ventral pancreas. Scale bars: A, B, 200 µm; C, D and G, H, 20 µm; E, F and I–N, 100 µm; O, P, 500 µm.
The pattern of terminal differentiation and progenitor markers at E16.5 in DTG embryos was similar to the observations at E13.5, but revealed a more pronounced disruption of tissue morphology of the pancreas in DTG embryos. At this time point, the DTG pancreas consists almost entirely of a cytoplasmic-sparse, cuboidal epithelial-cell network that expressed β-catenin and Pdx1 (Fig 3J). The Pdx1-expressing cells were not β cells, as they did not co-express insulin (Fig. 3J). In addition, these cells displayed an epithelial morphology inconsistent with a typical endocrine cell. Compared to WT littermates (Fig. 3I), insulin expression was almost completely absent in the DTG pancreas (Fig. 3J). Expression of glucagon and ghrelin was also absent in the DTG pancreas (Fig. 3N). Using the ductal-specific lectin, Dolichos biflorus agglutinin (DBA), no evidence of ductal differentiation in the DTG embryos was observed (Fig. 3L, P). However, low levels of reactivity to DBA were observed (Fig. 3L, P), which is a characteristic of normal pancreatic progenitor cells (Kobayashi et al., 2002). Exocrine cell development, signified by amylase expression, occurred predominantly in the peripheral regions of the DTG pancreas where ectopic Fgf10 expression was found to be lowest (Fig. 3L, P, and data not shown). Whole-mount immunofluorescence for amylase and DBA of the intact distal foregut/midgut region, followed by confocal scanning and 3D rendering, revealed that exocrine cell development occurred only in the distal-most regions of the E16.5 DTG pancreas (Fig. 3P) in contrast to the extensive development of exocrine tissue in WT littermates (Fig. 3O).
Ectopic Fgf10 expression maintains the undifferentiated state of the pancreatic epithelium
To further characterize the Fgf10-mediated arrested state, markers for the pancreatic progenitor cells were analyzed by immunohistochemistry. To ensure that Fgf10 signaling was ongoing, we performed immunohistochemistry for Etv4/PEA3 [GeneID: 18612], a known target of Fgf10 signaling in both the developing pancreas and lung (Kobberup et al., 2007; Liu et al., 2003). Widespread high expression of Etv4 was observed in the DTG pancreas compared to the control (Fig. 4A and B). Next, markers expressed in the pancreatic progenitor population were evaluated. Glut2/Slc2a2 [GeneID: 20526] is normally expressed in the plasma membrane of pancreatic epithelial progenitor cells prior to the secondary transition, after which its expression becomes confined to the β cells (Fig. 4C) (Pang et al., 1994). We observed that the arrested organ in DTG mice consists almost entirely of Glut2-expressing cells (Fig. 4D). As increased Notch signaling may explain the developmental arrest resulting from ectopic Fgf10 expression, we examined the expression of the Notch target gene Hes1 [GeneID: 15205]. Hes1 is normally expressed at variable levels in the remaining pancreatic progenitors at E16.5 and within the pancreatic interstitial cells (Fig. 4E). In contrast, the developmentally arrested DTG pancreas expressed Hes1 at various levels throughout the epithelium (Fig. 4F). Sox9 [GeneID: 20682] was also widely expressed throughout the arrested epithelium (Fig. 4H). Sox9 is a progenitor marker normally restricted to remnant progenitor cells at E16.5 (Fig. 4G) (Seymour et al., 2007). We also performed immunostaining for Hnf6/Onecut1 [GeneID: 15379], which is normally expressed in progenitor cells and is later restricted to the developing ductal compartment (Fig. 4I) (Jacquemin et al., 2000). Hnf6 was widely expressed within the arrested epithelium (Fig. 4J). Nkx6.1 [GeneID: 18096], which is normally co-expressed with Pdx1 in the progenitor cells prior to E13.5 and subsequently in mature beta cells (Oster et al., 1998), was also expressed broadly in the developmentally arrested DTG pancreas at E16.5 (Fig. 4L). As we only observed exocrine development in the peripheral regions of the DTG pancreas (Fig. 3P), we were curious if this could be explained by expression of either Ptf1a or bhlhb8, which encodes MIST1. Ptf1a was expressed at increased levels in the arrested DTG pancreas (Fig. 4N) compared to the normal expression of Ptf1a in mature exocrine cells of WT controls (Fig. 4M). MIST1, which is required for exocrine cell function and is restricted to mature exocrine cells where it is expressed (Pin et al., 2001), was detected in the DTG pancreas, albeit at much reduced levels as compared to WT (Fig. 4O and P). In contrast to the WT pancreas, in which segregation of Nkx6.1/Pdx1 and Ptf1a immunoreactivity has occurred at E16.5 (Fig. 4Q), the DTG pancreatic epithelium consists of a mixture of Pdx1/Ptf1a/Nkx6.1-triple-positive cells, as well as cells co-expressing Pdx1 and Ptf1a, but not Nkx6.1, (Fig. 4R).
Figure 4. Progenitor cell markers are upregulated in the Fgf10-arrested epithelium.
Immunostaining for markers of progenitors at E16.5 in control and DTG pancreata. A, B: Etv4, C, D: Glut2, E, F: Hes1, G, H: Sox9, I, J: Hnf6, K, L: Nkx6.1. M, N: Ptf1a, O, P: Mist1. R, S: triple immunofluorescence analysis of Pdx1, Ptf1a and Nkx6.1. Secondary color detections of Pdx1, Ptf1a, and Nkx6.1 are indicated. Scale bar: 50 µm.
Doxycycline allows rapid clearing of exogenous Fgf10 protein
In order to determine the kinetics of Fgf10 loss upon attenuation of transgenic Fgf10 expression in DTG embryos, DOX was administered at E13.5 for one, two, or three consecutive days. At E13.5, Fgf10 protein was widely expressed throughout the arrested DTG pancreatic epithelium (Fig. 5A). One day after DOX administration, a significant reduction of Fgf10 was observed (Fig. 5B), and this reduction was relatively homogenous throughout the pancreas. At two days following DOX treatment, an almost complete loss of Fgf10 staining was observed (Fig. 5C), while three days after DOX administration, Fgf10 expression was undetectable (Fig. 5D). The reduction of Fgf10 mRNA levels following DOX administration was verified by multiplex RT-PCR (MPX-RT-PCR) (Fig. 5E). The levels of Fgf10 mRNA in embryos receiving DOX at E13.5, followed by gene expression analysis at E14.5, E15.5, and E16.5, were compared to embryos in which DOX was not administered at the same time points. After one day of DOX treatment, Fgf10 expression levels were dramatically decreased compared to the untreated control. We found an almost complete elimination of exogenous Fgf10 mRNA after 2 days of DOX treatment.
Figure 5. Attenuation of transgenic Fgf10 expression by administration of DOX.
A–D: Immunostaining for Fgf10 expression in the DTG pancreas. DOX was administered at E13.5 for one, two, or three consecutive days and then analyzed at E14.5 (B), E15.5 (C), or E16.5 (D), respectively. Scale bar: A–D, 100 µm. E: Multiplex RT-PCR at 19 cycles confirming the reduction of expression of the Fgf10 mRNA (line 5539) after DOX administration. Control littermates were included for comparison. Alpha-tubulin was used as internal standard. Asterisks indicate comparisons that have statistical significance (p < 0.05). NS: not statistically significant.
Conditional control of Fgf10 during pancreatic development reveals windows of ductal and endocrine competence
The competence of Fgf10-arrested progenitor cells to differentiate into the different cell types of the mature pancreas upon cessation of ectopic Fgf10 expression was assessed. We performed a series of individual experiments outlined in Fig. 1B. We eliminated ectopic Fgf10 expression at E11.5 (Fgf10E11.5 OFF) or E13.5 (Fgf10E13.5 OFF) through administration of DOX at the indicated time points. Embryos were collected at E16.5 for histological examination and for gene expression analysis using MPX-RT-PCR. The arrested pancreas, due to constitutive Fgf10 expression (Fgf1016.5 ON) in the absence of DOX, was included for comparative purposes (Fig. 6D, H, L). At E16.5, Fgf10E11.5 OFF DTG embryos were remarkably similar to E16.5 WT littermates. The expression patterns of Pdx1, β-catenin, and insulin in the Fgf10E11.5 OFF DTG pancreas revealed the presence of a centrally located islet cell mass (Fig. 6B) similar to the WT pancreas (Fig. 6A). β-catenin staining was observed with the highest expression confined to cells with an apparent ductal morphology (Fig. 6B). Expression patterns of glucagon, ghrelin, and Ngn3 in the Fgf10E11.5 OFF DTG pancreas were also similar to WT embryos (Fig. 6E, F). The presence of amylase- and DBA-positive cells was observed with a similar spatial organization as WT littermates (Fig. 6I, J). However, morphometric analysis of the endocrine cell populations and MPX-RT-PCR analysis of the expression of endocrine cell marker genes showed a significant reduction in the endocrine cell compartment (Fig. 6M and N).
Figure 6. Cellular competence after release of Fgf10 suppression.
Immunostaining of terminal differentiation markers in the E16.5 pancreas after turning off ectopic Fgf10 expression by administering DOX from either E11.5 (B, F, J) or E13.5 (C, G, K). E16.5 pancreas from DTG embryos not administered DOX (D, H, L) and WT embryos (A, E, I) are shown for comparison. Secondary color detections of the primary antigens is shown to the left. Scale bar: A–L, 100 µm. (M): Multiplex RT-PCR analysis of hormone expression levels relative to alpha-tubulin. (N) Morphometric analysis of immunostained frozen sections in A–L. Percent of total pancreatic area are shown as means +/− S.D. Asterisks indicate comparisons that have statistical significance (p < 0.05). NS: not statistically significant.
As ectopic Fgf10 expression until E11.5 significantly reduced the competence of the arrested progenitor cells to differentiate into endocrine cells, the effect of maintaining ectopic Fgf10 expression immediately prior to the secondary transition at E13.5 was explored. At E16.5, the pancreas of Fgf10E13.5 OFF DTG embryos displayed striking differences compared to WT littermates and Fgf10E11.5 OFF DTG embryos. Upon macroscopic evaluation, Fgf10E13.5 OFF DTG pancreata were notably larger than their WT counterparts, although Fgf10E13.5 OFF pancreata were notably smaller than the Fgf1016.5 ON DTG pancreata (data not shown). In the DTG Fgf10E13.5 OFF pancreas, glucagon, insulin, and somatostatin cells were almost completely absent, except for very few glucagon clusters (Fig. 6C, G, and data not shown). This was paralleled by a general increase of Pdx1 expression compared to WT embryos (Fig. 6A, C). Most pancreatic epithelial cells remained β-catenin positive (Fig. 6C); however, they displayed a morphology similar to exocrine cells. Accordingly, the majority of the cells were exocrine as evidenced by amylase staining (Fig. 6K). DBA-positive duct cells in the DTG Fgf10E13.5 OFF pancreas were detected at reduced levels compared to WT (Fig. 6I, K). The endothelial compartment appeared normal in the DTG Fgf10E13.5 OFF pancreas (data not shown). Morphometric and MPX-RT-PCR analyses of the endocrine cell complement showed a reduced number of endocrine cells, which correlated with the immunostaining results (Fig. 6M, N). In support of this observation, we also observed a significantly reduced number of Ngn3+ cells in the DTG Fgf10E13.5 OFF pancreas at E16.5. Interestingly, the numbers of somatostatin and ghrelin cells were least affected (Fig. 6N). As very few PP cells were detected in control embryos at the gestational time points analyzed following DOX administration, statistical analysis of PP cell formation using morphometry was not possible. Although rare in the WT pancreas, a reduction in the number of PP cells compared to WT was observed in the Fgf10E11.5 OFF and Fgf10E13.5 OFF pancreata. (Supp. Fig. 2).
To determine if the effects observed at E16.5 in the Fgf10E11.5 OFF and Fgf10E13.5 OFF experiments were attributable to the developmental time period (E11.5 versus E13.5) rather than the duration following DOX administration (3 versus 5 days), E18.5 embryos were analyzed in which DOX was administered for 5 days beginning at E13.5 (Fgf105d OFF). If the loss of endocrine competence was restricted to a certain time period in development, we would expect to observe a similar phenotype as the Fgf10E13.5 OFF pancreas. If endocrine competence was dependent upon the duration of time following DOX administration (i.e. controlled by a remaining Fgf10 threshold), the observed phenotype would resemble the phenotype of the Fgf10E11.5 OFF pancreas. Histological analysis at E18.5 revealed that the WT pancreas consisted of mature acini, ducts, and centrally located endocrine cells (Fig. 7A, D, G). As a second control, we analyzed the arrested state of constitutive Fgf10 expression (Fgf10E18.5 ON) at E18.5 to assess if Fgf10 would still be able to keep the progenitor cell population arrested at this time point. The staining pattern of certain markers in the Fgf10E18.5 ON pancreas reflected the expression patterns in the Fgf10E16.5 ON pancreas (Fig. 6D, H, L); an almost complete loss of endocrine and ductal cells, as well as a pronounced reduction of exocrine cells, was observed at E18.5 (Fig. 7C, F, I). The development of a ductular network of Pdx1/β-catenin-expressing cells was more prominent at E18.5 (Fig. 7G) than at E16.5 (Fig. 6I) in the pancreata of WT embryos. In comparison, Fgf105d OFF pancreata at E18.5 displayed an almost complete loss of endocrine cell development (Fig 7B, E), except for the development of few ghrelin- and somatostatin-positive cells (Fig 7E and data not shown). These observations were corroborated by morphometric analysis (Fig. 7J). We noted a strong reduction in ductal cell formation, whereas widespread exocrine cell formation had occurred (Fig. 7H). This phenotype strongly resembles that of the Fgf10E13.5 OFF pancreas, not the Fgf10E11.5 OFF pancreas. We conclude that endocrine cell competence is restricted in time and does not re-establish even after 5 days in the absence of ectopic Fgf10 expression.
Figure 7. The endocrine competence window is irreversibly lost.
Immunostaining of terminal differentiation markers in the DTG pancreas after turning off ectopic Fgf10 expression with DOX from E13.5 (B, E, H). E18.5 pancreas from DTG embryos not administered DOX (C, F, I) and WT embryos (A, D, G) are shown for comparison. Primary antigen combinations and respective secondary color detections are shown to the left. Scale bar: A–I, 100 µm. J: Morphometric analysis of alpha, beta, and delta cells in stained frozen sections in A–I. Percentages of individual endocrine subtypes per total area of the pancreas are shown as means +/− S.D. Asterisks indicate comparisons that have statistical significance (p < 0.05). NS: not statistically significant.
Development of pancreatic exocrine cells at the expense of ductal and endocrine cell types
The loss of DBA+-ductal type cells in the Fgf10 arrested pancreas indicated a similar inhibitory effect on ductal competence as was observed for the endocrine compartment. DBA is a marker for adult ductal cells, but lower levels of reactivity are also observed in pancreatic progenitors (Kobayashi et al., 2002). Therefore, additional ductal-specific markers were used to define the effects on ductal development in the presence of elevated Fgf10. Acetylated tubulin, which identifies ciliated duct cells, was detected in WT E16.5 embryos throughout the ductal system (Supp. Fig. 4A). The number of ciliated duct cells was significantly reduced in E16.5 DTG embryos (Supp. Fig. 4B). TROMA1, an antibody that binds to cytokeratin 8/Krt8 [GeneID: 16691], only detected ductal cells in the control E16.5 embryo and did not stain cells in the E16.5 DTG pancreas. Altogether, these markers reveal a general deficiency in duct cell formation in the continued presence of ectopic Fgf10 expression.
Given that we observed a reduction of endocrine and ductal cells in the developmentally arrested DTG pancreas, we were curious whether the exocrine fate was adopted at the expense of these other cell fates. Based on amylase immunostaining and DBA reactivity, we performed a morphometric analysis of ductal and exocrine areas of the Fgf10E11.5 OFF and Fgf10E13.5 OFF pancreas at E16.5 (Fig. 8A, B). Similar to the complete loss of endocrine cells (Fig. 6N, 7J), ductal cells were also completely eliminated in the E16.5 DTG transgenic pancreas in the absence of DOX. However, DOX administration at E11.5 allowed for almost normal ductal development (18% reduction, p=0.07), whereas administration of DOX at E13.5 resulted in an 84% reduction in the total ductal cell area (p=0.006) (Fig. 8A). This reduction in ductal cell development is proportionate to the loss of endocrine cells observed in the same organ, suggesting that ductal and endocrine competence is equally affected by Fgf10. Quantification of the exocrine area revealed a general reduction in the Fgf1016.5 ON DTG pancreas, whereas early administration of DOX (Fgf1011.5 OFF) showed no difference with the WT pancreas (1.02-fold, p=0.61) (Fig. 8B). The small reduction in ductal and endocrine cells detected in this experiment may not be sufficient to allow detection of an increased exocrine cell population. However, upon DOX administration at E13.5 (Fgf1013.5 OFF), the loss of endocrine and ductal cells resulted in a significant increase in the total exocrine area at E16.5 (1.41 fold, p=0.013) (Fig. 8A, B). To validate that the increased area of exocrine cells was due to exocrine differentiation rather than increased proliferation in this compartment, the mitotic index of the amylase-expressing population was assessed using phosphorylated Histone H3 (pHH3) (Supp. Fig. 3). Three different time points (E14.5, E15.5 and E16.5) analyzed following DOX administration at E13.5 revealed no statistically different index of proliferation between WT controls and the DTG embryos (Supp. Fig. 3A). However, in both DTG and control littermates, the mitotic index of the exocrine cell population decreased by approximately 80% between E15.5 to E16.5, indicating that normal exocrine cells reduce their proliferative activity upon maturation.
Figure 8. Morphometric assessment of ductal and exocrine cell development upon release from Fgf10-induced progenitor arrest.
Morphometric analysis of immunostained E16.5 WT and DTG pancreas for ductal and exocrine differentiation. DOX was administered at indicated time points. A: Morphometric analysis of the ductal differentiation measured by percentage total DBA positive total area. B: Morphometric analysis of exocrine differentiation measured by percentage of amylase total area. Values representing the percentage of total ductal and exocrine area of the pancreas are shown as means +/− S.D. is shown. Asterisks indicate comparisons that have statistical significance (p < 0.05). NS: not statistically significant.
Genes involved in defining the competence window of pancreatic fate decisions
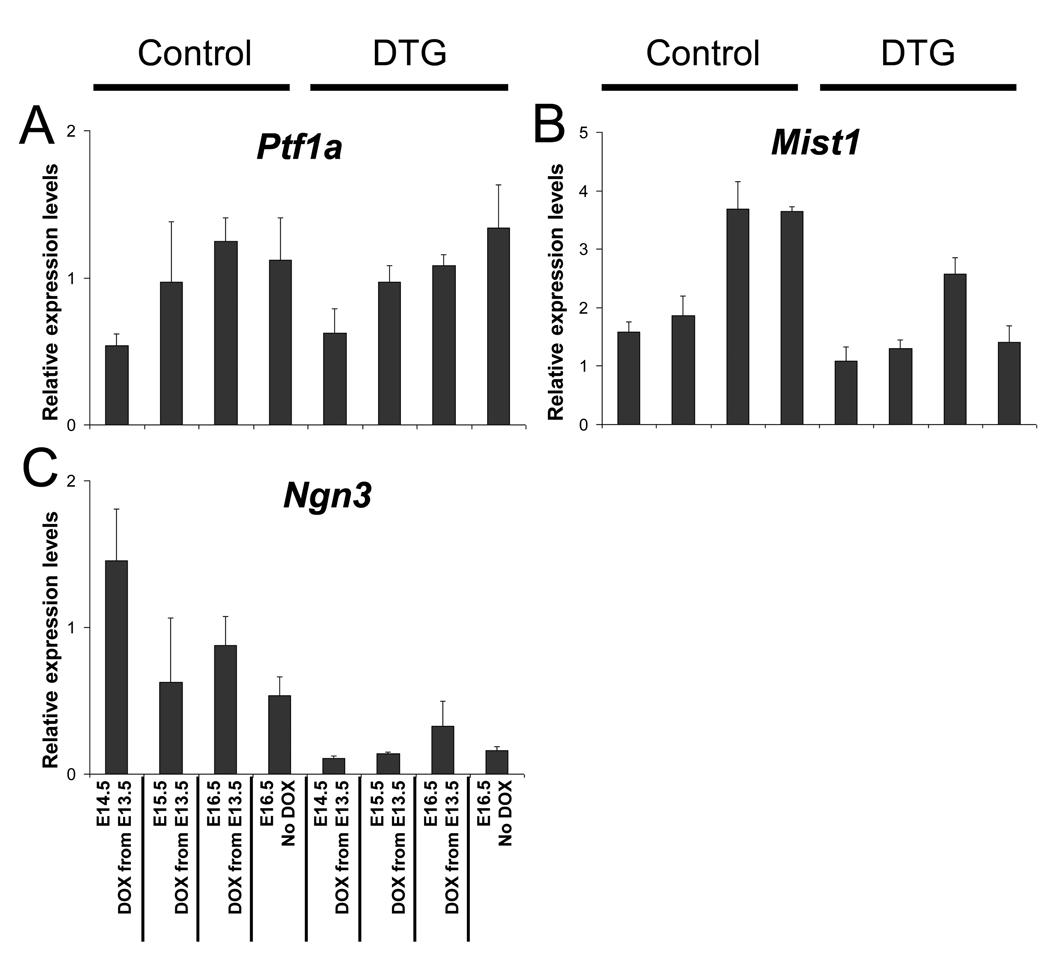
Our results could be explained by alterations in the expression pattern of determinants for the individual pancreatic lineages. Therefore, we determined if expression of some of the genes encoding the determination factors normally involved in the exocrine (Ptf1a and MIST1) and endocrine (Ngn3) cell fates were differentially affected when the organ was released from Fgf10-induced arrest. To accomplish this task, the same pancreatic samples that were used to determine the kinetics of Fgf10 down-regulation (Fig. 5E) were used. Interestingly, expression of Ptf1a, which is both a pancreatic progenitor marker and required for exocrine development (Kawaguchi et al., 2002), was continuously expressed in the presence of Fgf10 (Fig. 9A). Endogenous Ptf1a expression steadily increases as exocrine differentiation commences during the secondary transition. Fgf10 overexpression did not alter the pattern of Ptf1a expression in DTG embryos. Normal MIST1 expression levels rapidly increased after E15.5 at the end of the secondary transition. A general reduction in MIST1/bhlhb8 mRNA was noted in the Fgf10-arrested organ, but nearly normal levels of MIST1 were observed at time points following DOX administration at E13.5 (Fig. 9B). Consequently, MIST1-expression was rapidly restored to normal levels upon removal of Fgf10, possibly as a result of the persistent presence of Ptf1a. In contrast, Ngn3 mRNA levels remained low at all time points evaluated following DOX administration in DTG embryos. Ngn3 expression remained completely suppressed by Fgf10 overexpression up to 2 days following administration of DOX. When Fgf10 levels have declined to basal levels after three days of administering DOX, Ngn3 expression was only minimally restored in DTG embryos (Fig. 9C). Endogenous Ngn3 expression levels decline throughout the secondary transition (Fig. 9C). Suppression of Ngn3 expression may explain the overall reduction in endocrine cell differentiation in the organ while Fgf10 expression was maintained.
Figure 9. Pro-exocrine and pro-endocrine differential expression explains a pro-exocrine patterning event of the arrested epithelium.
Multiplexed RT-PCR analysis of Ptf1a (A), bhlhb8/MIST1 (B), and Ngn3 (C) expression levels relative to the internal standard TBP. DOX was administered to DTG embryos at E13.5. Embryos were collected at E14.5, E15.5, and E16.5 as indicated. E16.5 DTG embryos not treated with DOX are shown for comparison.
DISCUSSION
Through a conditional Fgf10 gain-of-function model, we present evidence of a competence window of differentiation during pancreatic development. Endocrine, ductal, and exocrine cell terminal differentiation normally occur during the secondary transition between E13.5 and E15.5 in the mouse. Our study reveals that prolonged exposure to Fgf10 differentially affects these terminal differentiation events. We found that the competence to form the endocrine and ductal cell lineages depended upon the gestational timing, not the duration, of Fgf10 exposure. We propose a model of this observation (Fig. 10). Based on this model, we predict that sustained Fgf10 expression throughout the secondary transition causes an irreversible loss of endocrine competence through inhibition of Ngn3 activation. Sustained Fgf10 expression during this period also negatively regulates the competence towards the ductal cells fate, as evidenced by the lack of several ductal-specific markers (Supp. Fig. 4). We conclude that the competence window for ductal and endocrine cell formation is irreversibly lost as a result of ectopic Fgf10 expression during the secondary transition. We also attribute the shift towards the exocrine cell fate in our conditional Fgf10 model as a result of the maintenance of Ptf1a in the Fgf10-arrested progenitors and the rapid restoration of bhlh8/MIST1 upon Fgf10 withdrawal.
Figure 10. An endocrine competence window in pancreatic development.
During pancreagenesis, differentiation to endocrine, ductal and exocrine cell lineages occurs during the secondary transition between E13.5 and E15.5. The normal levels of Fgf10 decline following the primary transition at E8.5–E10.5 (A). Increasing the duration 10 and level of Fgf10 until E11.5 reduces endocrine and ductal cell development, but allows exocrine differentiation to proceed (B). Further extending Fgf10 expression to E13.5 results in an almost complete loss of endocrine and ductal differentiation, while exocrine differentiation occurs unhindered (C). Expression of Fgf10 throughout pancreatic development blocks all differentiation upon attenuation of Fgf10 expression prior to birth (D). Long-term removal of ectopic Fgf10 does not restore endocrine or ductal cell competence. This suggests the existence of a temporal endocrine and ductal competence window (illustrated in A and B) that is regulated by the presence of Fgf10.
What causes the loss of endocrine and ductal cell competences when Fgf10 is overexpressed? We observed that Ngn3 expression is negatively influenced by the continued presence of Fgf10 (Fig. 3F, N). This inhibition of Ngn3 expression by Fgf10 may explain the general loss of endocrine cell formation; Ngn3 activity is required for endocrine cell fate determination (Gradwohl et al., 2000). We also found that when the arrested organ is released from Fgf10 suppression, the endocrine cell types normally forming late (somatostatin and ghrelin) were preferentially formed (Fig. 6N, 7J), possibly reflecting delayed Ngn3 activation. In support of this observation, Johansson et al. (2007) reported that the timing of Ngn3 activation significantly affected which endocrine cell sub-types were capable of forming; competence towards the γ cell subtype occurs after E14.5 (Johansson et al., 2007). A possible explanation for suppression of Ngn3 expression is active Notch signaling as evidenced by the increased levels of Hes1 (Fig. 4F). Hes1 maintains the progenitor state and represses Ngn3 target genes, such as NeuroD (Jensen et al., 2000). Increased levels of Sox9 expression in the Fgf10-arrested epithelium (Fig. 4H) may also contribute to the high levels of Hes1 as Sox9 has been shown to regulate Hes1 (Seymour et al., 2007). Furthermore, Fgf10 signaling has also been shown to stimulate the Notch pathway in the pancreatic epithelium (Miralles et al., 2006). However, Fgf10-mediated Notch signaling cannot explain why Ngn3 expression was not restored upon later attenuation of ectopic Fgf10 expression (Fig. 9C). This implies that other factors necessary for Ngn3 expression are only present prior to and during the secondary transition. We did observe several Ngn3+ cells after the secondary transition in WT and DTG embryos (Fig. 3M, 6E, 6F, 6G), although far fewer of these cells were observed at E16.5 than during the secondary transition. This suggests that endocrine progenitors persist beyond the secondary transition. In support of this observation, others have reported the existence of Ngn3+ cells in the adult pancreas, although the presence of these cells is extremely rare (Gu et al., 2002; Xu et al., 2008). The concomitant loss of ductal cell competence implies that the instructional cues for this particular cell fate are active in a common ductal/endocrine precursor. However, as the necessary factors for duct cell fate determination are currently unknown, it is difficult to speculate on why ductal competence is lost; we cannot determine whether the extended duration of Fgf10 expression affected a common ductal/endocrine precursor, the individual ductal cell lineage, or both. Identification of the factors that underlie ductal cell competence will be necessary to distinguish between these possibilities.
Why is exocrine competence unaffected by Fgf10 overexpression throughout the secondary transition? Widespread Fgf10 expression may commit the pancreatic progenitors towards this cell fate. This notion was suggested by pancreatic explant studies that showed increased exocrine differentiation, at the expense of endocrine cell development, occurred in the presence of Fgf10 (Miralles et al., 1999). However, it is clear that Fgf10 does not control exocrine fate determination because exocrine differentiation proceeds in Fgf10 null mice (Bhushan et al., 2001). Widespread Hes1 staining in the Fgf10-arrested epithelium (Fig. 4F) suggests that Notch signaling may be involved in regulating exocrine competence. Yet, Notch gain-of-function models showed that activation of the Notch pathway potently suppresses terminal differentiation of both endocrine and exocrine lineages (Murtaugh et al., 2003; Hald et al., 2003). Also, loss-of-function of Hes1 demonstrated that exocrine differentiation proceeds in that model (Jensen et al., 2000). Consequently, active Notch signaling cannot solely be used to explain the persistent exocrine competence in the Fgf10-arrested progenitors. Competence for the exocrine lineage throughout the secondary transition may be attributable to sustained Ptf1a expression in the Fgf10-arrested epithelium (Fig. 4R, 9A). Expression of Ptf1a is necessary for development of the exocrine compartment (Dong et al., 2008). Ptf1a is unlikely a direct transcriptional target of Fgf10 because Fgf10 would otherwise have an instructive role in exocrine development; we demonstrated that exocrine differentiation is also suppressed in the presence of Fgf10 (Fig. 3P, 4P, 8B). Furthermore, we would have expected a strong induction of Ptf1a in the presence of Fgf10, which was not observed (Fig. 9A). In zebrafish, Ptf1a expression remains in the Fgf10 mutant pancreas, indicating its expression is independent of Fgf10 (Dong et al., 2007). Therefore, other downstream effectors of Fgf10 signaling potentially influence Ptf1a activity in establishing the exocrine compartment. Notably, we have identified two targets of Fgf10 signaling, Etv4 and Etv5, with similar expression patterns to Ptf1a (Fig. 4A and (Kobberup et al., 2007)). Further studies using gene ablation of Etv4 and Etv5 specifically in pancreas will address their role in regulating the activation of the pro-exocrine factors, such as Ptf1a.
Segregation of the epithelial progenitor population into distal and central domains potentially patterns the developing pancreas. Zhou et al. (2007) described the distal domain using a “tip and trunk” analogy of the epithelial progenitor pool prior to differentiation (Zhou et al., 2008). These authors demonstrated that the distal “tips” of the branching pancreatic epithelium contain the pancreatic progenitors that become committed to the exocrine cell fate, while cells in the “trunk” are committed to endocrine and ductal cell lineages (Zhou et al., 2008). In support of this hypothesis, Ptf1a expression is clearly distalized and significantly down-regulated in the centralized region as the mouse organ approaches the secondary transition (Hald et al., 2008). In the Fgf10 overexpressing pancreas, the distal segregation of Ptf1a expression is not observed (Fig. 4N, R). Ptf1a remains expressed throughout the Fgf10-arrested cells. MIST1, a marker of fully differentiated exocrine cells (Johnson et al., 2004), is only expressed at the distal tips of the wild-type pancreas (Fig. 4O). However, in the Fgf10-arrested organ, MIST1 appears widely distributed at low levels (Fig. 4P). The loss of Ngn3, which is normally only observed in centralized progenitors, in combination with the widened expression pattern of Ptf1a and MIST1, suggest a loss of the central domain in response to prolonged Fgf10 expression and a possible replacement by a more distalized one. Such a result is in agreement with the observed loss of the endocrine and ductal cells and would argue that the time of Fgf10 exposure gradually shifts competency zones within the organ.
The absence of instructional cues for particular fates to emerge in the presence of elevated Fgf10 expression likely underlies the loss of endocrine and ductal competence. Our results indicate that spatial segregation of certain cell intrinsic components is required for establishing the patterning domains. For example, Hnf6 is maintained in presence of Fgf10 (Fig. 3J). Although Hnf6 was reported to be an upstream activator of Ngn3 gene expression (Jacquemin et al., 2000), it fails to activate Ngn3 in the Fgf10-arrested pancreas upon attenuation of Fgf10 expression. Moreover, the increased levels of Hnf6 in the Fgf10-arrested progenitors (Fig. 4L) could not specify the ductal cell fate (Fig. 8A). Thus, we conclude that Hnf6 expression alone is insufficient in generating a pro-endocrine/pro-ductal field. It is currently unknown to what extent loss of Ngn3 may be causal to loss of central patterning. Given that distal/central patterning of the developing pancreas is likely to involve a large battery of cell signaling components, some of these are likely to have been impacted by ectopic Fgf10 expression. Rescue experiments using Ngn3 gain-of-function should be useful to address to what extent endocrine and possibly ductal competence relies on this factor.
Although our observations pertain to the effects of Fgf10 protein on patterning during later pancreatic development, it is of interest to compare our results to the role of Fgf10 during early endoderm formation. The initial study of Fgf10 deficient mice concluded that Fgf10 is required for pancreatic growth (Bhushan et al., 2001), although studies on endodermal patterning were not performed. Analyses of Fgf10 function in zebrafish by Dong et al. (2007) have provided a more detailed picture of the role of Fgf10 in establishing cell fates in the hepatopancreatic ductal system (HPD) (Dong et al., 2007). At time of pancreatic budding, Fgf10 is expressed in Isl1-positive mesenchymal cells adjacent to the core structure of the HPD, and its absence leads to a generally failed formation and thinning of these ducts. These regions become populated by ectopically forming endocrine and liver cells. In mice, Hes1 mutants display ectopic formation of endocrine cells in developing pancreas (Jensen et al., 2000) and in the bile duct epithelium (Sumazaki et al., 2004). As the bile duct is part of the HPD system, this argues that Fgf10 controls Notch signaling in the early endoderm. The observation that Fgf10 is involved in suppressing differentiation in the proximal pancreas and liver adjacent to the HPD in zebrafish and our observation that overexpression of Fgf10 suppresses the centralized pancreatic cell fates in mice (i.e. endocrine and ductal cell types) later in pancreatic development suggest of the timing and location of Fgf10 expression is crucial for organ patterning.
Our observations have clinical implications as Fgf10 is currently used in cell-based therapeutic approaches for diabetes. Fgf10 has already been implemented in the successful conversion of human embryonic stem cells towards the beta-cell fate through a directed differentiation strategy (D'Amour et al., 2006). The D’Amour study convincingly suggests that hES cells are highly suitable for a cellular replacement therapy (Madsen and Serup, 2006). However, the current methodology fails to efficiently generate clean fractions of insulin-producing cells. The competence window for endocrine cell formation described herein should not be overlooked. Our findings illustrate that comprehension of cellular competence during pancreatic development may provide an additional basis of understanding endocrine differentiation. This highlights a current need to characterize the local morphogen environment preceding and during the secondary transition and how this is controlled by Fgf10. Knowledge of such an environment should be highly beneficial to optimize the current directed differentiation protocols.
EXPERIMENTAL PROCEDURES
Cloning of pTRE2-Fgf10FLAG
The Fgf10FLAG fragment was amplified from the Pdx1-Fgf10FLAG plasmid (Norgaard et al., 2003) to include a 5’ BamH1 and a 3’ Xba1 site. This fragment was digested with the respective enzymes and subcloned into the linearized pTRE2 plasmid (Clontech, Palo Alto, CA). The pTRE2 promoter consists of multiple modified tetracycline response elements in the proximal promoter.
Transgenic mice derivation
The pTRE2-Fgf10FLAG plasmid was linearized for pronuclear injection by restriction digestion with BsrB1. Fertilized oocytes were injected with the linearized fragments (3 ng/µl) and transferred to pseudopregnant recipients. Genotyping was performed on paw tissue from embryos and tail clips from adults using transgene-specific primers. Three out of seven transgenic founders transmitted the construct to the next generation (pTRE2-Fgf10). Animals were housed at UCDHSC Center for Comparative Medicine under IACUC approved conditions in accordance with institutional guidelines.
Conditional expression using doxycycline
Functional testing of the three transmitting pTRE2-Fgf10FLAG founder lines was performed through intercrosses with Pdx1-tTA knockin mice (Pdx1-tTAKI), allowing transgene induction in absence of DOX (Holland et al., 2005). The Pdx1 promoter restricts tTA expression to the developing pancreas, duodenum, and posterior stomach. Attenuation of pTRE2-driven Fgf10 expression was accomplished by administering 2 µg/ml of DOX (Calbiochem, CA, USA) and 5% sucrose in the drinking water. Transgenic Fgf10 expression was shut off by adding DOX to the drinking water at the indicated time points. The transgenic line D5539 was used throughout this study except for additional lines described in Fig. 2. Phenotypic results obtained using the lines D5510, 7572, and D5510 were similar to the D5539 line.
Tissue isolation and histology
Embryo and tissue isolation were performed in ice-cold PBS under a stereomicroscope (Olympus SZX12, Olympus, Center Valley, PA). All stainings were performed on 7 µm frozen sections as previously described (Norgaard et al., 2003). For whole-mount (WM) immunofluorescence, dissected guts were fixed overnight (O/N) in 4% PFA at 4°C. Embryos were equilibrated in MeOH for 4 hours at −20°C. Next, the embryos were incubated in 4:1:1 MeOH:DMSO:H2O2 for 2 hours. Embryos were then transferred to MeOH and equilibration to PBS was performed from 100% MeOH via gradual transfers between 75%, 50%, and 25% MeOH. The embryos were blocked in 0.5% TNB blocking solution (Perkin Elmer, Waltham, MA) for 3 hours at room temperature. Primary antibodies were applied O/N at 4°C with gentle nutation. Embryos were washed 10 times in PBS over a period of 3 hours. Secondary antibodies were applied at 1:500 dilution O/N at 4°C with gentle nutation. Embryos were finally washed 10 times in PBS over a period of 2 hours. Embryos were then equilibrated in MeOH and were immersed in 1:2 benzyl alcohol:benzyl benzoate (BABB) for clearing. Embryos were mounted in BABB in depression slides and subsequently sealed and analyzed. Antibodies and lectin dye used are listed in Supplementary Table 1B. Immunofluorescense imaging was performed on an Olympus BX51 microscope using a Pixera CL600 camera (Pixera, San Jose, CA) or on Leica SP5 confocal microscope (Leica, Bannockburn, IL) for whole mount imaging.
Multiplex RT-PCR
Multiplex RT–PCR was performed at 19 cycles as previously described (Jensen et al., 1996). Amplicons were co-amplified together with alpha-tubulin as an internal standard. Expression levels of various pancreatic marker genes were normalized with the levels of alpha-tubulin where indicated. The total number of multiplexed amplicons per reaction varied from four to six. Multiplex combinations were selected based on homology and amplicon size. Primer sequences are listed in Supplementary Table IA.
Morphometry
Cryosections were stained using antibodies described in Supplementary Table IB. The total area of stained cells and pancreas from five equally spaced sections were counted using ImagePro Software (Media Cybernetics, Bethesda, MD). Students t-test (two-tailed, equal variance) was used to determine statistical significance. The proliferative index of exocrine cells was calculated based on co-immunofluorescence of pHH3 and amylase. The numbers of pHH3/amylase double-positive cells were expressed as a function of total count of the amylase-expressing cells.
Supplementary Material
Triple immunofluorescence analysis of pancreatic progenitor markers (Pdx1 and Sox9) and FLAG-tagged Fgf10 in WT and DTG pancreas at E14.5. Immunostaining with these markers was performed with the transgenic line D5539. A, B: Sox9, C, D: Pdx1, E, F: FLAG, G, H: merged image. Scale bar: 50 µm.
Immunostaining for markers of beta-, glucagon-, and PP-cells in the pancreas at E16.5 and E18.5. A, B, C: Control littermates were included for comparison. Ectopic Fgf10 expression was turned of by administering DOX from either E11.5 (A, D) or E13.5 (B, C, E, F). The presence of beta-, glucagon-, and PP-cells were analyzed at E16.5 (A, B, D, E) or E18.5 (C, F) after attenuating Fgf10 expression. Secondary color detections of the primary antigens are indicated. Scale bar: 100 µm.
Morphometric analysis of immunostained frozen sections to determine the proliferative index of amylase-positive cells in control and DTG embryos (A). Immunostaining for amylase and pHH3 of control (B, C, D) and DTG pancreata (E, F, G) from embryos collected at E14.5 (B, E), E15.5 (C, F), and E16.5 (D, G) after DOX administration at E13.5. Secondary color detections of the primary antigens are indicated. Scale bar: 100 µm.
Immunostaining for the ductal markers, DBA, acetylated tubulin (Ace Tubulin), and keratin 8 (TROMA) in E16.5 control pancreas (A, C) and DTG pancreas (B, D). Secondary color detections of primary antigens are shown to the left. Scale bar: 100 µm. Asterisk denotes a neuronal bundle. Insert in A shows a magnification of ciliated ductal cells.
ACKNOWLEDGEMENTS
We are grateful for reagents generously provided by various investigators as outlined in Supplementary Table 1. We also would like to thank Drs. John Hutton, Yong-Sik Kim, and Solomon Afelik for discussions and/or critically reading the manuscript. This work was supported by the NIH R01 DK070636 (J.J), and the Juvenile Diabetes Research Foundation (J.J, Award 1-2007-109). Additional support was also provided by the Barbara Davis Center DERC NIH P30 DK57516 and the Chicago Diabetes Project.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Apelqvist A, Li H, Sommer L, Beatus P, Anderson DJ, Honjo T, Hrabe dA, Lendahl U, Edlund H. Notch signalling controls pancreatic cell differentiation. Nature. 1999;400:877–881. doi: 10.1038/23716. [DOI] [PubMed] [Google Scholar]
- Bhushan A, Itoh N, Kato S, Thiery JP, Czernichow P, Bellusci S, Scharfmann R. Fgf10 is essential for maintaining the proliferative capacity of epithelial progenitor cells during early pancreatic organogenesis. Development. 2001;128:5109–5117. doi: 10.1242/dev.128.24.5109. [DOI] [PubMed] [Google Scholar]
- D'Amour KA, Bang AG, Eliazer S, Kelly OG, Agulnick AD, Smart NG, Moorman MA, Kroon E, Carpenter MK, Baetge EE. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat.Biotechnol. 2006;24:1392–1401. doi: 10.1038/nbt1259. [DOI] [PubMed] [Google Scholar]
- Dong PD, Munson CA, Norton W, Crosnier C, Pan X, Gong Z, Neumann CJ, Stainier DY. Fgf10 regulates hepatopancreatic ductal system patterning and differentiation. Nat.Genet. 2007;39:397–402. doi: 10.1038/ng1961. [DOI] [PubMed] [Google Scholar]
- Dong PD, Provost E, Leach SD, Stainier DY. Graded levels of Ptf1a differentially regulate endocrine and exocrine fates in the developing pancreas. Genes Dev. 2008;22:1445–1450. doi: 10.1101/gad.1663208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujikura J, Hosoda K, Iwakura H, Tomita T, Noguchi M, Masuzaki H, Tanigaki K, Yabe D, Honjo T, Nakao K. Notch/Rbp-j signaling prevents premature endocrine and ductal cell differentiation in the pancreas. Cell Metab. 2006;3:59–65. doi: 10.1016/j.cmet.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Gittes GK, Galante PE, Hanahan D, Rutter WJ, Debase HT. Lineage-specific morphogenesis in the developing pancreas: role of mesenchymal factors. Development. 1996;122:439–447. doi: 10.1242/dev.122.2.439. [DOI] [PubMed] [Google Scholar]
- Gradwohl G, Dierich A, LeMeur M, Guillemot F. neurogenin3 is required for the development of the four endocrine cell lineages of the pancreas. Proc.Natl.Acad.Sci.U.S.A. 2000;97:1607–1611. doi: 10.1073/pnas.97.4.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu G, Dubauskaite J, Melton DA. Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development. 2002;129:2447–2457. doi: 10.1242/dev.129.10.2447. [DOI] [PubMed] [Google Scholar]
- Hald J, Hjorth JP, German MS, Madsen OD, Serup P, Jensen J. Activated Notch1 prevents differentiation of pancreatic acinar cells and attenuate endocrine development. Dev.Biol. 2003;260:426–437. doi: 10.1016/s0012-1606(03)00326-9. [DOI] [PubMed] [Google Scholar]
- Hald J, Sprinkel AE, Ray M, Serup P, Wright C, Madsen OD. Generation and characterization of Ptf1a antiserum and localization of Ptf1a in relation to Nkx6.1 and Pdx1 during the earliest stages of mouse pancreas development. J.Histochem.Cytochem. 2008;56:587–595. doi: 10.1369/jhc.2008.950675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart A, Papadopoulou S, Edlund H. Fgf10 maintains notch activation, stimulates proliferation, and blocks differentiation of pancreatic epithelial cells. Dev.Dyn. 2003;228:185–193. doi: 10.1002/dvdy.10368. [DOI] [PubMed] [Google Scholar]
- Holland AM, Gonez LJ, Naselli G, MacDonald RJ, Harrison LC. Conditional expression demonstrates the role of the homeodomain transcription factor Pdx1 in maintenance and regeneration of beta-cells in the adult pancreas. Diabetes. 2005;54:2586–2595. doi: 10.2337/diabetes.54.9.2586. [DOI] [PubMed] [Google Scholar]
- Jacquemin P, Durviaux SM, Jensen J, Godfraind C, Gradwohl G, Guillemot F, Madsen OD, Carmeliet P, Dewerchin M, Collen D, Rousseau GG, Lemaigre FP. Transcription factor hepatocyte nuclear factor 6 regulates pancreatic endocrine cell differentiation and controls expression of the proendocrine gene ngn3. Mol.Cell Biol. 2000;20:4445–4454. doi: 10.1128/mcb.20.12.4445-4454.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen J, Pedersen EE, Galante P, Hald J, Heller RS, Ishibashi M, Kageyama R, Guillemot F, Serup P, Madsen OD. Control of endodermal endocrine development by Hes-1. Nat.Genet. 2000;24:36–44. doi: 10.1038/71657. [DOI] [PubMed] [Google Scholar]
- Jensen J, Serup P, Karlsen C, Nielsen TF, Madsen OD. mRNA profiling of rat islet tumors reveals nkx 6.1 as a beta-cell-specific homeodomain transcription factor. J.Biol.Chem. 1996;271:18749–18758. doi: 10.1074/jbc.271.31.18749. [DOI] [PubMed] [Google Scholar]
- Johansson KA, Dursun U, Jordan N, Gu G, Beermann F, Gradwohl G, Grapin-Botton A. Temporal control of neurogenin3 activity in pancreas progenitors reveals competence windows for the generation of different endocrine cell types. Dev.Cell. 2007;12:457–465. doi: 10.1016/j.devcel.2007.02.010. [DOI] [PubMed] [Google Scholar]
- Johnson CL, Kowalik AS, Rajakumar N, Pin CL. Mist1 is necessary for the establishment of granule organization in serous exocrine cells of the gastrointestinal tract. Mech.Dev. 2004;121:261–272. doi: 10.1016/j.mod.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Cooper B, Gannon M, Ray M, MacDonald RJ, Wright CV. The role of the transcriptional regulator Ptf1a in converting intestinal to pancreatic progenitors. Nat.Genet. 2002;32:128–134. doi: 10.1038/ng959. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Spilde TL, Li Z, Marosky JK, Bhatia AM, Hembree MJ, Prasadan K, Preuett BL, Gittes GK. Lectin as a marker for staining and purification of embryonic pancreatic epithelium. Biochem.Biophys.Res.Commun. 2002;293:691–697. doi: 10.1016/S0006-291X(02)00278-4. [DOI] [PubMed] [Google Scholar]
- Kobberup S, Nyeng P, Juhl K, Hutton J, Jensen J. ETS-family genes in pancreatic development. Dev.Dyn. 2007;236:3100–3110. doi: 10.1002/dvdy.21292. [DOI] [PubMed] [Google Scholar]
- Liu Y, Jiang H, Crawford HC, Hogan BL. Role for ETS domain transcription factors Pea3/Erm in mouse lung development. Dev.Biol. 2003;261:10–24. doi: 10.1016/s0012-1606(03)00359-2. [DOI] [PubMed] [Google Scholar]
- Madsen OD, Serup P. Towards cell therapy for diabetes. Nat.Biotechnol. 2006;24:1481–1483. doi: 10.1038/nbt1206-1481. [DOI] [PubMed] [Google Scholar]
- Miralles F, Czernichow P, Ozaki K, Itoh N, Scharfmann R. Signaling through fibroblast growth factor receptor 2b plays a key role in the development of the exocrine pancreas. Proc.Natl.Acad.Sci.U.S.A. 1999;96:6267–6272. doi: 10.1073/pnas.96.11.6267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miralles F, Czernichow P, Scharfmann R. Follistatin regulates the relative proportions of endocrine versus exocrine tissue during pancreatic development. Development. 1998;125:1017–1024. doi: 10.1242/dev.125.6.1017. [DOI] [PubMed] [Google Scholar]
- Miralles F, Lamotte L, Couton D, Joshi RL. Interplay between FGF10 and Notch signalling is required for the self-renewal of pancreatic progenitors. Int.J.Dev.Biol. 2006;50:17–26. doi: 10.1387/ijdb.052080fm. [DOI] [PubMed] [Google Scholar]
- Murtaugh LC, Stanger BZ, Kwan KM, Melton DA. Notch signaling controls multiple steps of pancreatic differentiation. Proc.Natl.Acad.Sci.U.S.A. 2003;100:14920–14925. doi: 10.1073/pnas.2436557100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norgaard GA, Jensen JN, Jensen J. FGF10 signaling maintains the pancreatic progenitor cell state revealing a novel role of Notch in organ development. Dev.Biol. 2003;264:323–338. doi: 10.1016/j.ydbio.2003.08.013. [DOI] [PubMed] [Google Scholar]
- Oster A, Jensen J, Edlund H, Larsson LI. Homeobox gene product Nkx 6.1 immunoreactivity in nuclei of endocrine cells of rat and mouse stomach. J.Histochem.Cytochem. 1998;46:717–721. doi: 10.1177/002215549804600603. [DOI] [PubMed] [Google Scholar]
- Pang K, Mukonoweshuro C, Wong GG. Beta cells arise from glucose transporter type 2 (Glut2)-expressing epithelial cells of the developing rat pancreas. Proc.Natl.Acad.Sci.U.S.A. 1994;91:9559–9563. doi: 10.1073/pnas.91.20.9559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pin CL, Rukstalis JM, Johnson C, Konieczny SF. The bHLH transcription factor Mist1 is required to maintain exocrine pancreas cell organization and acinar cell identity. J.Cell Biol. 2001;155:519–530. doi: 10.1083/jcb.200105060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour PA, Freude KK, Tran MN, Mayes EE, Jensen J, Kist R, Scherer G, Sander M. SOX9 is required for maintenance of the pancreatic progenitor cell pool. Proc.Natl.Acad.Sci.U.S.A. 2007;104:1865–1870. doi: 10.1073/pnas.0609217104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumazaki R, Shiojiri N, Isoyama S, Masu M, Keino-Masu K, Osawa M, Nakauchi H, Kageyama R, Matsui A. Conversion of biliary system to pancreatic tissue in Hes1-deficient mice. Nat.Genet. 2004;36:83–87. doi: 10.1038/ng1273. [DOI] [PubMed] [Google Scholar]
- Wessells NK, Cohen JH. Early pancreas morphogenesis: morphogenesis, tissue interations, and mass effects. Dev.Biol. 1967;15:237–270. doi: 10.1016/0012-1606(67)90042-5. [DOI] [PubMed] [Google Scholar]
- Xu X, D'Hoker J, Stange G, Bonne S, De LN, Xiao X, Van de CM, Mellitzer G, Ling Z, Pipeleers D, Bouwens L, Scharfmann R, Gradwohl G, Heimberg H. Beta cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell. 2008;132:197–207. doi: 10.1016/j.cell.2007.12.015. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature. 2008;455:627–632. doi: 10.1038/nature07314. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Triple immunofluorescence analysis of pancreatic progenitor markers (Pdx1 and Sox9) and FLAG-tagged Fgf10 in WT and DTG pancreas at E14.5. Immunostaining with these markers was performed with the transgenic line D5539. A, B: Sox9, C, D: Pdx1, E, F: FLAG, G, H: merged image. Scale bar: 50 µm.
Immunostaining for markers of beta-, glucagon-, and PP-cells in the pancreas at E16.5 and E18.5. A, B, C: Control littermates were included for comparison. Ectopic Fgf10 expression was turned of by administering DOX from either E11.5 (A, D) or E13.5 (B, C, E, F). The presence of beta-, glucagon-, and PP-cells were analyzed at E16.5 (A, B, D, E) or E18.5 (C, F) after attenuating Fgf10 expression. Secondary color detections of the primary antigens are indicated. Scale bar: 100 µm.
Morphometric analysis of immunostained frozen sections to determine the proliferative index of amylase-positive cells in control and DTG embryos (A). Immunostaining for amylase and pHH3 of control (B, C, D) and DTG pancreata (E, F, G) from embryos collected at E14.5 (B, E), E15.5 (C, F), and E16.5 (D, G) after DOX administration at E13.5. Secondary color detections of the primary antigens are indicated. Scale bar: 100 µm.
Immunostaining for the ductal markers, DBA, acetylated tubulin (Ace Tubulin), and keratin 8 (TROMA) in E16.5 control pancreas (A, C) and DTG pancreas (B, D). Secondary color detections of primary antigens are shown to the left. Scale bar: 100 µm. Asterisk denotes a neuronal bundle. Insert in A shows a magnification of ciliated ductal cells.