Abstract
Epidemiological studies have raised the possibility of caffeine serving as a neuroprotective agent in Parkinson’s disease (PD). This possibility has gained support from findings that dopaminergic neuron toxicity induced by MPTP or other neurotoxins is attenuated by co-administration of caffeine in mice. Here we examined the time window of caffeine’s neuroprotection as well as the effects of caffeine’s metabolites (theophylline and paraxanthine) in the MPTP mouse model of PD. In the first experiment, caffeine pre-treatment (30mg/kg ip) significantly attenuated MPTP-induced striatal dopamine depletion when it was given 10min, 30min, 1hr, or 2hr but not 6hr before MPTP (40mg/kg ip) treatment. Meanwhile, caffeine post-treatment also significantly attenuated striatal dopamine loss when it was given 10min, 30min, 1hr or 2hr but not 4hr, 8hr or 24hr after MPTP injection. In the second experiment, both theophylline (10 or 20 mg/kg) and paraxanthine (10 or 30 mg/kg) administration (10min before MPTP) significantly attenuated MPTP-induced dopamine depletion in mice, as did caffeine (10mg/kg) treatment. Thus the metabolites of caffeine also provide neuroprotective effects in this mouse model of PD. The data suggest that if caffeine protects against putative toxin-induced dopaminergic neuron injury in humans, then precise temporal pairing between caffeine and toxin exposures may not be critical because the duration of neuroprotection by caffeine may be extended by protective effects of its major metabolites.
Keywords: dopamine, adenosine A2A receptor, theophylline, paraxanthine, striatum, neurotoxicity
Caffeine is the most widely used psychoactive substance in the world (Fredholm et al., 1999). Multiple epidemiological studies (both prospective and retrospective) have strongly linked caffeine intake to a reduced risk of developing Parkinson’s disease (PD, Benedetti et al., 2000; Ross et al., 2000; Ascherio et al., 2001), raising the possibility of neuroprotection by caffeine. This hypothesis has been strengthened by preclinical demonstrations of neuroprotection by caffeine in a variety of neurotoxin models of PD. Caffeine attenuated neurochemical and anatomical dopaminergic lesions induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) (Chen et al., 2001; Xu et al., 2002; Xu et al., 2006a; Chen et al., 2008; Singh et al 2009), 6-hydroxydopamine (Joghataie et al, 2004; Aguiar et al., 2006), or the pesticide combination of paraquat plus maneb (Kachroo et al., 2007). Moreover in contrast to the motor stimulant properties of caffeine, which show tolerance upon repeated exposure, the neuroprotective effect of caffeine was not reduced after repeated administration (Xu et al., 2002).
In the MPTP model of PD the neuroprotective effect of caffeine has been assessed by effectively co-administering it together with the toxicant, with caffeine preceding MPTP by 10 min. In humans, however, caffeine intake typically occurs once or a couple of times a day in a periodic fashion, and thus it may not necessarily pair together with the potential toxin exposure. Here we investigate caffeine pharmacokinetics and the temporal requirements for pairing caffeine and neurotoxin in mice in an effort to better understand the relationship between caffeine intake and reduced risk of PD. We employ a single-dose toxin regimen (MPTP•HCl 40 mg/kg, i.p.) because it affords simplicity in assessing the temporal features of protectant-toxicant relationships, and because it is the most common toxicant paradigm used in earlier studies of caffeine in PD models (Chen et al., 2001, Xu et al., 2002, Xu et al., 2006a). Despite its limitations (e.g., the lack of Lewy body-like inclusions or reliable behavioral deficits characteristic of PD) acute MPTP intoxication in mice remains one of the best-characterized animal models of PD and faithfully recapitulates core neurochemical and anatomical features of PD (Dawson et al., 2002).
To pursue the pharmacodynamics of neuroprotection by caffeine we considered the major routes of caffeine metabolism. In human adults caffeine is virtually completely metabolized, with less than 2% of the ingested compound being recoverable in urine unchanged (Arnaud, 1987; Somani and Gupta, 1988). In humans caffeine (i.e., 1,3,7-trimethylxanthine) is demethylated to its dimethyl metabolic intermediates, with over 80% of orally administered caffeine metabolized to paraxanthine (1,7-dimethylxanthine), and about 16% is converted to theobromine (3,7-dimethylxanthine) and theophylline (1,3-dimethylxanthine) (Lelo et al., 1986; Benowitz et al., 1995). In rodents, paraxanthine is the major metabolite, but levels of theophylline are also high (Bonati et al., 1984–1985; Fredholm et al., 1999). Paraxanthine can contribute to the pharmacological action of caffeine (Benowitz et al., 1995), especially during long-term caffeine consumption at higher doses when there is accumulation of paraxanthine in plasma due to its saturable metabolism (Denaro et al., 1990). These metabolites of caffeine share many of the pharmacological actions of caffeine (Denaro et al., 1990). Thus they should be taken into account when considering the biological actions of caffeine. Therefore, in the current study, we examined the effects of caffeine’s metabolites (theophylline and paraxanthine) as well as its time window of action in the MPTP model of PD.
Experimental procedures
Animals and drug treatments
Male C57BL/6 mice (~23–27 gm) from Charles River Laboratories (Wilmington, MA) were housed five in each cage in temperature- and humidity-controlled rooms with a 12-h dark: light cycle and had free access to food and water. In the first experiment, mice received saline or caffeine (30mg/kg) 10min, 30min, 1hr, 2hr or 6hr before (Experiment 1A), or 10min, 30min, 1hr, 2hr, 4hr, 8hr or 24hr after (Experiment 1B) MPTP (40mg/kg, n=7–20; note all doses for MPTP refer to that of its chloride salt, MPTP•HCl; Sigma-Aldrich, St. Louis, MO) or saline (n=3–6) treatment. In the second experiment, caffeine (10mg/kg), theophylline (10 or 20 mg/kg), paraxanthine (10 or 30 mg/kg) or saline was administered 10 min before MPTP (40mg/kg, n=7–20) or saline (n-3–7) treatment. In the third experiment, mice were treated with caffeine (10 or 30 mg/kg) and killed at 5, 10, 20, 30, 60, 120, 360min or 24hr (n=6 for each time point) after injection. All injections were administered intraperitoneally (ip) in a volume of 0.1ml/10 gm of body weight. All experiments were conducted in accordance with Massachusetts General Hospital and NIH Guidelines on the ethical use of animals.
Measurement of dopamine
In the first two experiments, one week after MPTP treatment, mice were killed by rapid cervical dislocation. The striatum was dissected out from the right cerebral hemisphere, frozen on dry ice and stored at −80°C until use. Each striatum was weighed, homogenized with 150 mM phosphoric acid and 0.2 mM EDTA and centrifuged at 12,000 g for 15 min at 4°C. Supernatants were analyzed for dopamine content using standard reverse-phase HPLC with electrochemical detection. Biogenic amines were separated on a C-18, 5- μm sphere column. The mobile phase consisted of 0.1 M sodium phosphate monobasic, 0.1 mM EDTA, 0.18 mM sodium octyl sulfate, and 8% methanol in filtered distilled water. The final pH of 3.3 was obtained with the addition of concentrated phosphoric acid, and the mobile phase was filtered and degassed before use.
Measurement of caffeine and its metabolites
In the third experiment, mice were killed at different time points by rapid cervical dislocation. Trunk blood was collected and centrifuged immediately at 12,000 × g for 30 min at 4°C. The right cerebral hemisphere was dissected out, frozen on dry ice, and stored at −80°C until use. Brain tissue was homogenized in 0.1 M monobasic sodium phosphate with a volume 10 times that of tissue weight (i.e., 10 μl/mg tissue assuming a tissue density of 1 μl/mg) and centrifuged at 12,000 × g for 15 min at 4°C. Serum and supernatant of brain homogenates were analyzed using liquid chromatography/mass spectrometry for determination of caffeine and its three dimethyl xanthine metabolites: paraxanthine, theophylline, and theobromine. The lower limit of quantitation was 0.030 μg/ml. The results of caffeine and metabolites concentrations in brain were calculated (i.e., μg/ml homogenate × 10 ml homogenate/g tissue) and presented as μg/g tissue. The analysis of caffeine and its metabolites was performed by Drs. R. L. Foltz and D. Andrenyak (Center for Human Toxicology, University of Utah, Salt Lake City, UT) as a service of the National Institute of Drug Abuse (NIDA Drug Supply & Analytical Services; Dr. H.H. Singh, program administrator).
Statistics
The dopamine content data from MPTP-treated mice were analyzed with one-way ANOVA followed by Fisher’s LSD tests.
Results
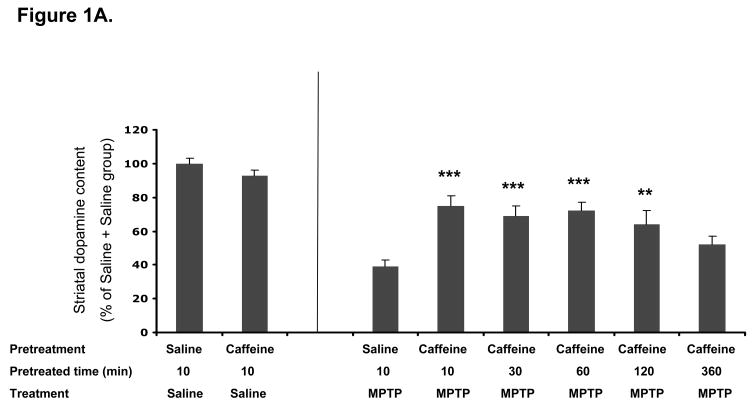
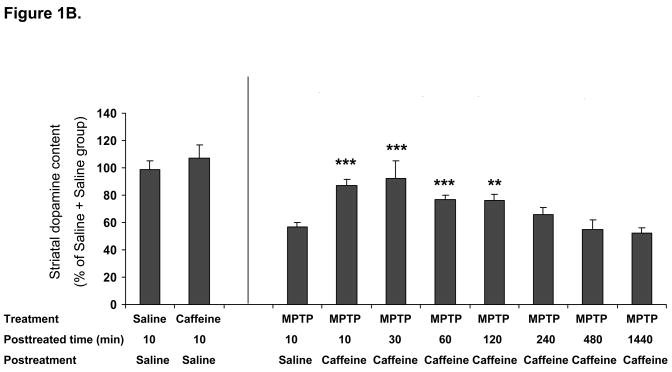
In the first experiment, we sought to determine the therapeutic window through which pre- or post-treatment with caffeine confers neuroprotection against MPTP-induced dopaminergic toxicity in mice (Figure 1). Caffeine (30 mg/kg, i.p.) significantly attenuated MPTP-induced dopamine depletion not only when it was effectively co-administered with (10min before) MPTP (p<0.001) as demonstrated previously (Chen et al., 2001), but also when it was given 30min (p<0.001), 1hr (p<0.001) or 2hr (p<0.01) before MPTP treatment. However when caffeine administration preceded that of MPTP by 6 hr, then any attenuation of dopamine loss did not reach statistical significance (Figure 1A). Similarly, when toxin exposure preceded caffeine treatment, striatal dopamine depletion was significantly reduced if caffeine was given 10min (p<0.001), 30min (p<0.001), 1hr (p<0.001) or 2hr (p<0.01) after MPTP treatment. However, MPTP-induced striatal dopamine depletion was not appreciably altered when caffeine was given 4, 8 or 24 hours after MPTP (Figure 1B).
Figure 1.
Figure 1A. Caffeine pre-treatment significantly attenuate MPTP-induced dopaminergic toxicity. C57Bl/6 male mice received saline or caffeine (30mg/kg ip) at different time points before MPTP (40mg/kg, n=7–9) or saline (n=3–4) ip treatment. Striatal dopamine content was determined one week later. Bars represent striatal dopamine levels (mean ± SEM) calculated as percentage of their control (i.e., saline 10min before saline treatment group, in which dopamine content is 65±2.1 pmol/mg tissue). Data were analyzed with one-way ANOVA followed by Fisher’s LSD test. **-p<0.01, ***-p<0.001 when compared with saline 10min before MPTP treatment group.
Figure 1B. Caffeine post-treatment significantly attenuate MPTP-induced dopaminergic toxicity. C57Bl/6 male mice received saline or caffeine (30mg/kg ip) at different time points after MPTP (40mg/kg, n=7–20) or saline (n=5–6) ip treatment. Striatal dopamine content was determined one week after MPTP. Bars represent striatal dopamine levels (mean ± SEM) calculated as percentage of their control (i.e., saline 10min post saline treatment group in which dopamine content is 42±4.8 pmol/mg tissue). Data were analyzed with one-way ANOVA followed by Fisher’s LSD test. **-p<0.01, ***-p<0.001 when compared with Saline 10min post MPTP treatment group.
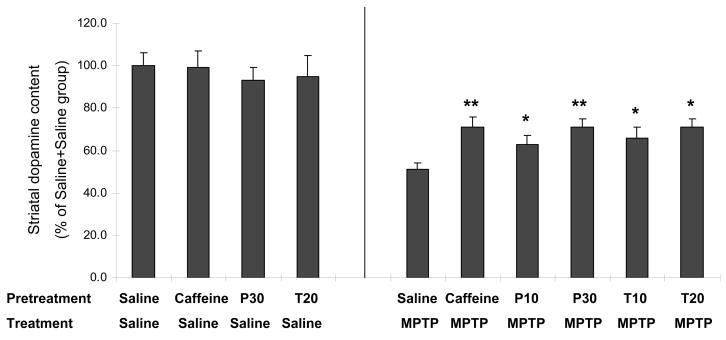
To further characterize the mechanism of caffeine’s neuroprotection in the MPTP mouse model of PD, in the second experiment we investigated the effects of caffeine metabolites theophylline and paraxanthine upon MPTP-induced striatal dopaminergic toxicity. Both theophylline (10mg/kg, p<0.05; 20mg/kg, p<0.05) and paraxanthine (10mg/kg, p<0.05; 30mg/kg, p<0.01) pre-treatments (10 min before MPTP) significantly attenuated MPTP-induced dopamine depletion in mice, as observed with the caffeine (10mg/kg, p<0.01) pre-treatment (Figure 2).
Figure 2. Caffeine’fs metabolites significantly attenuate MPTP-induced dopaminergic toxicity.
C57Bl/6 male mice received ip injection of caffeine (10mg/kg), theophylline (10 or 20 mg/kg), paraxanthine (10 or 30 mg/kg) or saline 10 min before MPTP (40mg/kg, n=7–20) or saline (n=3–7) ip treatment. Striatal dopamine content was determined one week later. Bars represent striatal dopamine levels (mean ± SEM) calculated as percentage of their control (i.e., saline 10min before saline treatment group in which dopamine content is 58±3.5 pmol/mg tissue). P10 or P30, paraxanthine 10 or 30 mg/kg, respectively; T10 or T20, theophylline 10 or 20 mg/kg, respectively. Data were analyzed with one-way ANOVA followed by Fisher’s LSD test. *-p<0.05, ***-p<0.01when compared with saline 10min before MPTP treatment group.
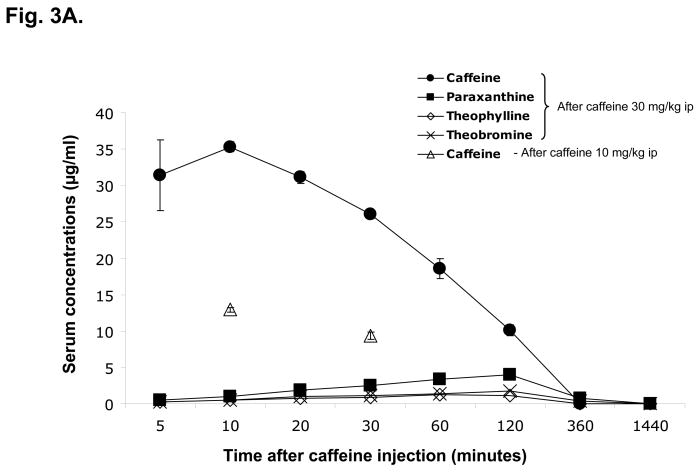
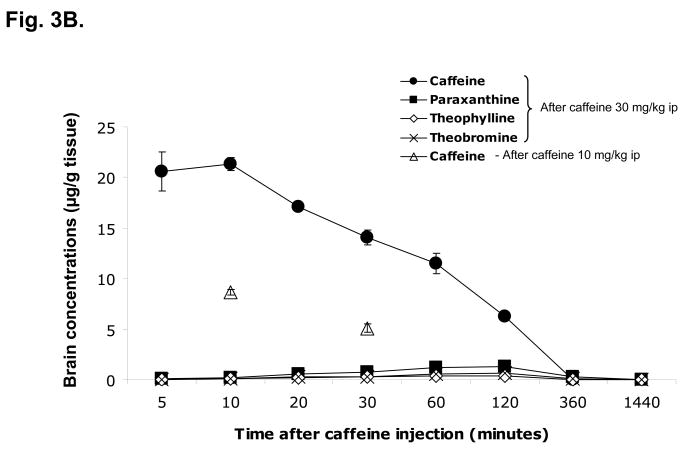
We also measured the concentrations of caffeine and its metabolites in serum and brain at different time points after injection (Figure 3). Caffeine concentrations in serum were higher than those in the brain at all time points. The brain levels of caffeine were about 54% – 65% of blood levels. Serum and brain concentrations of caffeine reached a peak at 10 min after injection (30 mg/kg ip). At 60 min after injection, the concentrations are about 50% of maximal values. Six hours after injection, caffeine levels were close to zero in both serum and brain. Caffeine could not be detected in either serum or brain 24 hours after injection. Meanwhile, the concentrations of caffeine’s metabolites (theophylline, paraxanthine and theobromine) increased steadily after injection (from 5 to 120 min), though they were much lower than those of caffeine for at least 2 hr after injection. The levels of metabolites were close to zero 6 hr and could not be detected 24 hours after injection. Paraxanthine was the major metabolite and its levels were higher than those of theophylline or theobromine at all time points. When administered at a 3-fold lower dose (10 mg/kg), caffeine showed a similar pharmacokinetic pattern with a proportionally smaller peak also seen 10 min after administration.
Figure 3. Serum and brain levels of caffeine and its metabolites after caffeine injection.
C57bl/6 male mice were treated with caffeine (10 or 30 mg/kg ip) and killed at 5, 10, 20, 30, 60, 120, 360min or 24hr (n=6 for each time point) after injection. Serum and brain concentrations of caffeine and its metabolites (paraxanthine, theophylline and theobromine) were determined.
Discussion
The results of current study demonstrated that caffeine’s neuroprotective effect in the MPTP mouse model of PD can be achieved when administered either preceding or following toxin exposure for up to 2 hrs. Moreover, caffeine’s neuroprotective effect was mimicked by comparable doses of its dimethyl metabolites theophylline and paraxanthine.
Interestingly, like caffeine, two of its dimethylxanthine metabolites displayed similar neuroprotective properties in this mouse model of PD. Both paraxanthine and theophylline, two major metabolites of caffeine in humans as well as rodents, attenuated the loss of striatal dopamine induced by acute MPTP intoxication. These metabolites share many features of caffeine’s actions including those affecting central nervous system. For example, theophylline and paraxanthine (using doses similar to those applied in this study) enhance motor (e.g., rotational or locomotor) behaviors in rodents as does caffeine (Wantanabe et al., 1982; Logan et al., 1986). Paraxanthine and theophylline also share pharmacologically properties with caffeine as all three methylxanthines are adenosine receptor antagonists, phosphodiesterase inhibitors and capable of releasing intracellular calcium stores. However relatively high concentrations of caffeine or its metabolites are required to exert their effects on phosphodiesterase and calcium release (Fredholm et al., 1999; Guerreiro et al., 2008). By contrast, lower dosages and concentrations are sufficient to achieve blockade of adenosine receptors. For example, serum caffeine concentrations produced by the ingestion of a single cup of caffeinated coffee are sufficient to significantly displace endogenous adenosine from A1 and A2A receptors, whereas near toxic exposures to caffeine are required to produce meaningfully inhibit phosphodiesterase activity. Furthermore, the behavioral stimulant potencies of caffeine and its metabolites correlate with their affinities for occupation of adenosine receptors (Kaplan et al., 1997). Theophylline is more potent than caffeine as an inhibitor of both adenosine A1 and A2A receptors, and paraxanthine is also at least as potent as caffeine (Mally and Stone, 1994). In the current experiments, the brain concentrations of caffeine (as shown in Fig. 3B) ranged from ~32 to 110 μM after 30 mg/kg ip, and ~26 to 44 μM after 10mg/kg ip. At these levels, caffeine mainly blocks adenosine A1 and A2A receptors, although other mechanisms cannot be excluded (Fredholm et al., 1999). We have demonstrated previously that the neuroprotective effect of caffeine probably involves its antagonism at the A2A adenosine receptor. For example, selective adenosine A2A, but not A1, antagonists (e.g., SCH58261 and KW6002) provided similar protection against MPTP toxicity in mice as those treated with caffeine (Chen et al., 2001). Moreover, caffeine’s neuroprotective effect was lost in A2A knockout mice that lack functional A2A receptors (Xu et al., 2006b). Thus, the neuroprotective effects of theophylline and paraxanthine may similarly rely on their actions as A2A receptor antagonists. Although paraxanthine has been found protect dopaminergic neurons in culture by an A2A receptor-independent mechanism, the concentrations employed were greater than those that bind adenosine receptors or that are achieved in vivo (Fredholm et al., 1999; Guerreiro et al., 2008). These findings are consistent with the hypothesis that caffeine or other methylxanthines confer protection on dopaminergic neurons in vivo by A2A receptors that are expressed on non-dopaminergic neurons (Xu et al, 2005).
The mechanism by which caffeine and in turn blockade of adenosine A2A receptors protect against MPTP-induced dopaminergic toxicity remains uncertain, although various hypotheses have been suggested (Chen et al., 2008; Yu et al., 2008; Carta et al., 2009). MPTP is metabolized to MPDP+ and then oxidized to active metabolite MPP+. Previously, we have shown that caffeine does not change MPTP’s entry into the brain or its metabolizism to MPDP+ and MPP+ in the C57Bl/6 mice (Chen et al., 2001, 2002). MPP+ level peaks at 90min after single injection (Giovanni et al, 1991; Chen et al., 2002). Therefore, our current demonstration that caffeine produces its full protective effect even when administered 2 hr after MPTP injection further substantiates that caffeine’s action occurs downstream of MPTP entry into the CNS and of its conversion to the active dopaminergic neuron toxin MPP+.
The pharmacokinetic profile of caffeine and its metabolites corresponded closely with the functional time course of neuroprotection by caffeine in the present study. Six hours after caffeine injection, caffeine cannot be detected, and only very low levels of paraxanthine or theobromine can be detected in the blood and brain. Similarly, we did not see caffeine’s protection when it was given six hours before MPTP. Thus the physical presence of caffeine or related methylxanthine and their direct action (for example, blockade of A2A receptors) might be required in order for caffeine to disrupt a critical early pathophysiological step in the injury of dopaminergic neurons.
We found that caffeine’s half-life was about 60 min in both blood and brain in C57Bl/6 male mice agreeing with that found in CD1 and CD-COBS mice (Bonati 1984–85; Kaplan et al., 1989, 1990), accounting in part for the relatively narrow time window of protection by caffeine. In addition, although paraxanthine was found to protective and to be the major dimethylxanthine metabolite of caffeine in C57Bl/6 mice, its levels (as well as the levels of the other two metabolites) are much lower than those of caffeine at all time points studied. Similar differences between levels of caffeine and its metabolites were found in CD-COBS (Bonati 1984–85) and DBA/2J (Kuzmin 2000) mice. However, caffeine’s metabolism varies considerably among different species of animals and humans. For example, in humans, caffeine’s half life was found to be around 2.5–4.5 hours (Bonati 1984–85; Arnaud 1987) instead of ~1 hour in rodents. Moreover, paraxanthine levels were found to be more than one-third of caffeine levels in humans while paraxanthine level was a much smaller fraction (~one-tenth to one-fifth) of that of caffeine in rodents (Bonati 1984–85 and current study). Thus the demonstration of neuroprotection by paraxanthine and theophylline may contribute only modestly to caffeine’s neuroprotective effect in C57Bl/6 mice employed here. Of practical significance, the relative brevity of the 4 hr period straddling MPTP exposure in which protection was demonstrated suggests that more continuous caffeine exposure may be required to test its protective potential in chronic toxin or genetic mouse models of PD.
In humans however, the relatively prolonged half-life of caffeine, which results in elevated levels of caffeine and paraxanthine after bolus intake (Bruce et al., 1986), together with the newly identified neuroprotective potential of its primary metabolite paraxanthine, raise the possibility that any true protective effect of individual caffeine ingestions could be relatively long-lasting. Epidemiological studies linking higher rates of caffeine consumption to a reduced risk of PD showed the reduced risk was greatest for those consuming multiple cups of coffee per day (Benedetti et al., 2000; Ross et al., 2000; Ascherio et al., 2001), in whom significant blockade of adenosine receptors may have been achieved by dimethylxanthine metabolites as much as by caffeine throughout much of the day. The current findings demonstrate that caffeine must be paired with the dopaminergic neuron toxin MPTP (i.e., with co-administration or a stagger of no more than a few hours) in order for its neuroprotective effect to be realized. By contrast in humans, if a neuroprotective effect of caffeine were in fact the basis of the reduced risk amongst caffeine consumers, then close pairing between caffeine and episodic exposures to putative environmental neurotoxins contributing to PD may not be necessary. This temporal relationship between caffeine and toxin exposures in the MPTP model and its extrapolation to humans has implications for possible neuroprotection trials of caffeine in PD, which have been suggested based on available epidemiological and laboratory data (Richardson et al., 1997; Kanda et al., 1998; Grondin et al., 1999; Ravina et al., 2003) despite a lack of demonstrated association between caffeine use and clinical progression in PD (Schwarzschild et al., 2003; Simon et al, 2008) For example, a regimen of twice or thrice daily caffeine dosing could produce effectively continuous blockade of adenosine receptors and allow for a reliable pharmacological design for such a trial.
Clinical relevance of the present findings also arises given the routine long-term use of theophylline to treat asthma. A protective effect of theophylline in a mouse model of PD raises the possibility that asthmatics chronically treated with theophylline may be at reduced risk for developing PD. Although theophylline use is routine its prevalence (e.g., relative to caffeine exposure) is modest and unlikely to be informative in prospective epidemiological studies of PD. Nevertheless, in light of its established safety record for chronic pharmacotherapy in clinical practice our findings suggest it may also be considered as a candidate disease-modifying treatment for PD. Indeed based on its known adenosine receptor antagonism and preclinical benefits for motor control, theophylline has been tested in small-scale clinical trials for patients with PD resulting in both positive (Mally and Stone, 1994) and negative (Magnussen et al., 1977; Kulisevsky et al., 2002) results. However, like selective A2A receptor antagonists in clinical trials for PD (currently developed by multiple pharmaceutical companies such as Kyowa-Kirin, Schering-Plough, Biogen-Idec, etc.), neither theophylline nor caffeine has yet been investigated in a trial designed to assess long-term outcomes in PD. Our findings support the practicality of considering natural methylxanthines with adenosine antagonist properties as candidate neuroprotectants in PD.
Acknowledgments
Source of support: This work is supported by NIH grants ES10804, DA13508 and NS60991, USAMRAA W81XWH-04-1-0881, American Parkinson Disease Association and National Parkinson Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguiar LM, Nobre HV, Jr, Macêdo DS, Oliveira AA, Freitas RM, Vasconcelos SM, Cunha GM, Sousa FC, Viana GS. Neuroprotective effects of caffeine in the model of 6-hydroxydopamine lesion in rats. Pharmacol Biochem Behav. 2006;84:415–419. doi: 10.1016/j.pbb.2006.05.027. [DOI] [PubMed] [Google Scholar]
- Arnaud MJ. The pharmacology of caffeine. Prog Drug Res. 1987;31:273–313. doi: 10.1007/978-3-0348-9289-6_9. [DOI] [PubMed] [Google Scholar]
- Ascherio A, Zhang SM, Hernan MA, Kawachi I, Colditz GA, Speizer FE, Willett WC. Prospective study of caffeine consumption and risk of Parkinson’s disease in men and women. Ann Neurol. 2001;50:56–63. doi: 10.1002/ana.1052. [DOI] [PubMed] [Google Scholar]
- Benedetti MD, Bower JH, Maqraganore DM, McDonnell SK, Peterson BJ, Ahlskog JE, Schaid DJ, Rocca WA. Smoking, alcohol, and coffee consumption preceding Parkinson’s disease: a case-control study. Neurology. 2000;55:1350–1358. doi: 10.1212/wnl.55.9.1350. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Jacob P, III, Mayan H, Denaro C. Sympathomimetic effects of paraxanthine and caffeine in humans. Clin Pharmacol Ther. 1995;58:684–691. doi: 10.1016/0009-9236(95)90025-X. [DOI] [PubMed] [Google Scholar]
- Bonati M, Latini R, Tognoni G, Young JF, Garattini S. Interspecies comparison of in vivo caffeine pharmacokinetics in man, monkey, rabbit, rat, and mouse. Drug Metab Rev. 1984–1985;15(7):1355–83. doi: 10.3109/03602538409029964. [DOI] [PubMed] [Google Scholar]
- Bruce M, Scott N, Lader M, Marks V. The psychopharmacological and electrophysiological effects of single doses of caffeine in healthy human subjects. Br J Clin Pharmacol. 1986;22(1):81–7. doi: 10.1111/j.1365-2125.1986.tb02883.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carta AR, Kachroo A, Schintu N, Xu K, Schwarzschild MA, Wardas J, Morelli M. Inactivation of neuronal forebrain A receptors protects dopaminergic neurons in a mouse model of Parkinson’s disease. J Neurochem. 2009;111(6):1478–89. doi: 10.1111/j.1471-4159.2009.06425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JF, Xu K, Jacques Petz J, Staal R, Xu YH, Beilstein M, Sonsalla PK, Castagnoli K, Castagnoli N, Jr, Schwarzschild MA. Neuroprotection by caffeine and A(2A) adenosine receptor inactivation in a model of Parkinson’s disease. J Neurosci. 2001;21:RC143, 1–6. doi: 10.1523/JNEUROSCI.21-10-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JF, Steyn S, Staal R, Petzer JP, Xu K, Van Der Schyf CJ, Castagnoli K, Sonsalla PK, Castagnoli N, Jr, Schwarzschild MA. 8-(3-Chlorostyryl)caffeine may attenuate MPTP neurotoxicity through dual actions of monoamine oxidase inhibition and A2A receptor antagonism. J Biol Chem. 2002;277(39):36040–4. doi: 10.1074/jbc.M206830200. [DOI] [PubMed] [Google Scholar]
- Chen X, Lan X, Roche I, Liu R, Geiger JD. Caffeine protects against MPTP-induced blood-brain barrier dysfunction in mouse striatum. J Neurochem. 2008 Nov;107(4):1147–57. doi: 10.1111/j.1471-4159.2008.05697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson TM, Mandir AS, Lee MK. Animal models of PD: pieces of the same puzzle? Neuron. 2002;35:219–222. doi: 10.1016/s0896-6273(02)00780-8. [DOI] [PubMed] [Google Scholar]
- Delle Donne KT, Sonsalla PK. Protection against methamphetamine-induced neurotoxicity to neostriatal dopaminergic neurons by adenosine receptor activation. J Pharmacol Exp Ther. 1994;271:1320–1325. [PubMed] [Google Scholar]
- Denaro CP, Brown CR, Wilson M, Jacob P, 3rd, Benowitz NL. Dose-dependency of caffeine metabolism with repeated dosing. Clin Pharmacol Ther. 1990;48:277–285. doi: 10.1038/clpt.1990.150. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Bättig K, Holmén J, Nehlig A, Zvartau EE. Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol Rev. 1999 Mar;51(1):83–133. [PubMed] [Google Scholar]
- Giovanni A, Sieber BA, Heikkila RE, Sonsalla PK. Correlation between the neostriatal content of the 1-methyl-4-phenylpyridinium species and dopaminergic neurotoxicity following 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine administration to several strains of mice. J Pharmacol Exp Ther. 1991;257(2):691–7. [PubMed] [Google Scholar]
- Grondin R, Bedard PJ, Hadj Tahar A, Grégoire L, Mori A, Kase H. Antiparkinsonian effect of a new selective adenosine A2A receptor antagonist in MPTP-treated monkeys. Neurology. 1999;52:1673–1677. doi: 10.1212/wnl.52.8.1673. [DOI] [PubMed] [Google Scholar]
- Guerreiro S, Toulorge D, Hirsch E, Marien M, Sokoloff P, Michel PP. Paraxanthine, the primary metabolite of caffeine, provides protection against dopaminergic cell death via stimulation of ryanodine receptor channels. Mol Pharmacol. 2008 Oct;74(4):980–9. doi: 10.1124/mol.108.048207. [DOI] [PubMed] [Google Scholar]
- Joghataie MT, Roghani M, Negahdar F, Hashemi L. Protective effect of caffeine against neurodegeneration in a model of Parkinson’s disease in rat: behavioral and histochemical evidence. Parkinsonism Relat Disord. 2004;10:465–468. doi: 10.1016/j.parkreldis.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Kachroo A, Prasad K, Irizarry MC, Richfield EK, Schwarzschild MA. Caffeine protects against combined paraquat and maneb-induced neurotoxicity of dopaminergic nigral neurons. Program #265.20. 2007 Society for Neuroscience Annual Meeting; San Diego, CA. 2007. [Google Scholar]
- Kanda T, Jackson MJ, Smith LA, Pearce RK, Nakamura J, Kase H, Kuwana Y, Jenner P. Adenosine A2A antagonist: a novel antiparkinsonian agent that does not provoke dyskinesia in parkinsonian monkeys. Ann Neurol. 1998;43:507–513. doi: 10.1002/ana.410430415. [DOI] [PubMed] [Google Scholar]
- Kaplan GB, Greenblatt DJ, Leduc BW, Thompson ML, Shader RI. Relationship of plasma and brain concentrations of caffeine and metabolites to benzodiazepine receptor binding and locomotor activity. J Pharmacol Exp Ther. 1989;248(3):1078–83. [PubMed] [Google Scholar]
- Kaplan GB, Tai NT, Greenblatt DJ, Shader RI. Caffeine-induced behavioural stimulation is dose- and concentration-dependent. Br J Pharmacol. 1990;100(3):435–40. doi: 10.1111/j.1476-5381.1990.tb15824.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan GB, Greenblatt DJ, Ehrenberg BL, Goddard JE, Cotreau MM, Harmatz JS, Shader RI. Dose-dependent pharmacokinetics and psychomotor effects of caffeine in humans. J Clin Pharmacol. 1997;37:693–703. doi: 10.1002/j.1552-4604.1997.tb04356.x. [DOI] [PubMed] [Google Scholar]
- Kulisevsky J, Barbanoj M, Gironell A, Antonijoan R, Casas M, Pascual-Sedano B. A double-blind crossover, placebo-controlled study of the adenosine A2A antagonist theophylline in Parkinson’s disease. Clin Neuropharmacol. 2002;25:25–31. doi: 10.1097/00002826-200201000-00005. [DOI] [PubMed] [Google Scholar]
- Kuzmin A, Johansson B, Semenova S, Fredholm BB. Differences in the effect of chronic and acute caffeine on self-administration of cocaine in mice. Eur J Neurosci. 2000;12:3026–32. doi: 10.1046/j.1460-9568.2000.00179.x. [DOI] [PubMed] [Google Scholar]
- Lelo A, Miners JO, Robson R, Birkett DJ. Assessment of caffeine exposure: caffeine content of beverages, caffeine intake, and plasma concentrations of methylxanthines. Clin Pharmacol Ther. 1986;39:54–59. doi: 10.1038/clpt.1986.10. [DOI] [PubMed] [Google Scholar]
- Logan L, Seale TW, Carney JM. Inherent differences in sensitivity to methylxanthines among inbred mice. Pharmacol Biochem Behav. 1986;24(5):1281–6. doi: 10.1016/0091-3057(86)90185-1. [DOI] [PubMed] [Google Scholar]
- Magnussen I, Dupont E, Jakobsen P. Theophyllamine and the antiparkinsonian response to levodopa treatment. Acta Neurol Scand. 1977;56:29–36. doi: 10.1111/j.1600-0404.1977.tb01406.x. [DOI] [PubMed] [Google Scholar]
- Mally J, Stone TW. The effect of theophylline on parkinsonian symptoms. J Pharm Pharmacol. 1994;46:515–517. doi: 10.1111/j.2042-7158.1994.tb03840.x. [DOI] [PubMed] [Google Scholar]
- McCormack AL, Thiruchelvam M, Manning-Bog AB, Thiffault C, Langston JW, Cory-Slechta DA, Di Monte DA. Environmental risk factors and Parkinson’s disease: selective degeneration of nigral dopaminergic neurons caused by the herbicide paraquat. Neurobiolo Dis. 2002;10:119–127. doi: 10.1006/nbdi.2002.0507. [DOI] [PubMed] [Google Scholar]
- Ravina BM, Fagan SC, Hart RG, Hovinga CA, Murphy DD, Dawson TM, Marler JR. Neuroprotective agents for clinical trials in Parkinson’s disease: a systematic assessment. Neurology. 2003;60:1234–1240. doi: 10.1212/01.wnl.0000058760.13152.1a. [DOI] [PubMed] [Google Scholar]
- Richardson PJ, Kase H, Jenner PG. Adenosine A2A receptor antagonists as new agents for the treatment of Parkinson’s disease. Trends Pharmacol Sci. 1997;18:338–344. doi: 10.1016/s0165-6147(97)01096-1. [DOI] [PubMed] [Google Scholar]
- Ross GW, Abbott RD, Petrovitch H, White LR, Tanner CM. Relationship between caffeine intake and parkinson disease. JAMA. 2000;283:2674–2679. [PubMed] [Google Scholar]
- Schwarzschild MA, Chen JF, Tennis M, Messing S, Kamp C, Ascherio A, et al. Relating caffeine consumption to Parkinson’s disease progression and dyskinesias development. Mov Disord. 2003;18(9):1982–1083. [Google Scholar]
- Singh S, Singh K, Gupta SP, Patel DK, Singh VK, Singh RK, Singh MP. Effect of caffeine on the expression of cytochrome P450 1A2, adenosine A2A receptor and dopamine transporter in control and 1-methyl 4-phenyl 1, 2, 3, 6-tetrahydropyridine treated mouse striatum. Brain Res. 2009;1283:115–126. doi: 10.1016/j.brainres.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Somani SM, Gupta P. Caffeine: a new look at an age-old drug. Intl J Clin Pharmacol Ther Toxicol. 1988;26:521–533. [PubMed] [Google Scholar]
- Watanabe H, Ikeda M, Watanabe K. Development of tolerance to dopaminergic stimulating effect of theophylline in mice with unilateral striatal 6-hydroxydopamine lesions. Eur J Pharmacol. 1982;79:125–8. doi: 10.1016/0014-2999(82)90583-0. [DOI] [PubMed] [Google Scholar]
- Xu K, Xu YH, Chen JF, Schwarzschild MA. Caffeine’s neuroprotection against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine toxicity shows no tolerance to chronic caffeine administration in mice. Neurosci Lett. 2002;322:13–16. doi: 10.1016/s0304-3940(02)00069-1. [DOI] [PubMed] [Google Scholar]
- Xu K, Bastia E, Schwarzschild M. Therapeutic potential of adenosine A(2A) receptor antagonists in Parkinson’s disease. Pharmacol Ther. 2005;105(3):267–310. doi: 10.1016/j.pharmthera.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Xu K, Xu Y, Brown-Jermyn D, Chen JF, Ascherio A, Dluzen DE, Schwarzschild MA. Estrogen prevents neuroprotection by caffeine in the mouse 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson’s disease. J Neurosci. 2006a;26(2):535–41. doi: 10.1523/JNEUROSCI.3008-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K, Xu YH, Chen JF, Schwarzschild MA. Neuroprotection by caffeine in the MPTP model of Parkinson’s disease: the role of adenosine A2A receptor. Program No. 470.7. Neuroscience Meeting Planner; 2006; Atlanta, GA: Society for Neuroscience; 2006b. Online. [Google Scholar]
- Yu L, Shen HY, Coelho JE, Araújo IM, Huang QY, Day YJ, Rebola N, Canas PM, Rapp EK, Ferrara J, Taylor D, Müller CE, Linden J, Cunha RA, Chen JF. Adenosine A2A receptor antagonists exert motor and neuroprotective effects by distinct cellular mechanisms. Ann Neurol. 2008;63(3):338–46. doi: 10.1002/ana.21313. [DOI] [PubMed] [Google Scholar]