Abstract
Over the last several decades, the relative contribution of early life events to individual disease susceptibility has been explored extensively. Only fairly recently, however, has it become evident that abnormal or excessive nociceptive activity experienced during the perinatal period may permanently alter the normal development of the CNS and influence future responses to somatosensory input. Given the significant rise in the number of premature infants receiving high-technology intensive care over the last twenty years, ex-preterm neonates may be exceedingly vulnerable to the long-term effects of repeated invasive interventions. The present review summarizes available clinical and laboratory findings on the lasting impact of exposure to noxious stimulation during early development, with a focus on the structural and functional alterations in nociceptive circuits, and its sexually dimorphic impact.
Keywords: neonate, inflammation, pain, nociception, development, infant
Premature Birth and Neonatal Intensive Care
Advances in perinatal medical care over the last two decades have substantially increased the survival of infants born premature [1]. As part of this life-saving care, however, preterm neonates are exposed to multiple invasive procedures in the neonatal intensive care unit (NICU) which are frequently accompanied by local inflammation and tissue damage lasting for several hours to days [2]. Growing clinical and basic science data suggests that exposure to repeated tissue damaging interventions in neonates may induce lasting changes in the CNS and have profound consequences for subsequent nociceptive processing [3; 4; 5; 6; 7; 8; 9; 10].
Premature birth, defined as birth prior to 37 weeks gestation, occurs at alarmingly high rates worldwide. According to the World Health Organization, 16.5% of all infants are born premature, with over 500,000 preterm babies born each year in the United States alone [11]. Indeed, the rates of premature births in the United States have been escalating steadily to nearly 35% of all live births within the last two decades [11]. While the underlying causes of prematurity are diverse and not completely understood, several factors are known to contribute to the increased prevalence of preterm births, including assisted reproductive techniques [11], tobacco, alcohol and illicit drug use during pregnancy [12], as well as high maternal blood pressure, diabetes and obesity [13].
Intrinsic to their care in the NICU, preterm infants undergo on average 14 noxious (painful and/or tissue damaging) procedures per day including repeated heel lances, endotracheal intubation, surgery, and respiratory and gastric suctioning [14; 15]. Mounting evidence indicates that nociceptive circuitry is both established and functional during late gestation, and that premature infants are indeed capable of mounting developmentally specific and distinct responses to noxious and non-noxious stimuli [16; 17]. Moreover, cortical activation in response to acute noxious stimulation in preterm neonates at 25 weeks has been reported, suggesting the potential for higher-level processing of pain [18; 19].
The Lasting Impact of Neonatal Noxious Stimulation
Pain is unique amongst sensory modalities. While olfactory, auditory, and tactile stimulation are plentiful after birth, the newborn mammalian CNS is rarely exposed to nociceptive input. During the last two decades, however, this situation has changed dramatically due to the wide application of intensive care interventions in high-risk preterm neonates [7; 20]. As the neonatal period is a sensitive window for experience-induced plasticity due to the ongoing maturation of nociceptive systems [21], accumulating evidence from clinical and animal research studies indicates that exposure to noxious stimulation, experienced early in life, can leave a legacy of altered somatosensory processing [8; 22; 23; 24; 25; 26; 27].
Long-Term Effects of Early Noxious Insult on Developing Nociceptive Systems-Clinical Studies
Early pioneering studies on the lasting impact of early life noxious stimulation in human infants reported that heel lance elicits decreased facial and enhanced cardiovascular responses, indicative of an increased threshold for pain, in preterm infants with prior NICU experience compared to age matched full-term infants [28]. Subsequent studies revealed that a higher frequency of invasive procedures in preterm infants is significantly associated with dampened nociceptive responses at 32 weeks of age compared to controls [29]. Moreover, decreased facial responsiveness to immunization at 4 and 8 months [30], and blunted nociceptive sensitivity have been reported in 18 month old former preterm neonates compared to full term peers [24]. Former NICU toddlers are also rated by parents as less pain sensitive compared to term-born controls, with a higher frequency of procedural pain exposure associated with more dampened nociceptive responsiveness to noxious stimulation at 18 months of age [24]. Furthermore, a recent study reported that former extremely preterm children display a generalized decrease in thermal but not mechanical nociceptive sensitivity during pre-adolescence, suggesting lasting centrally mediated alterations in nociceptive pathways [31].
In contrast to the aforementioned reduced nociceptive responsiveness following superficial (i.e. acute and inflammatory) types of neonatal noxious stimulation, deep somatic and visceral noxious stimulation (i.e. early surgery and tissue damage) in infancy leads to prolonged sensitization of nociceptive responses. Hypersensitivity to tissue damage is observed in human infants, in that a decrease in sensory thresholds is observed for days or weeks in the presence of local or deep visceral tissue injury [32; 33]. Furthermore in premature infants, the withdrawal reflex threshold in an area of local tissue damage following repeated heel lances is half the value of that on the intact contralateral heel for several months following the initial insult [34; 35]. Interestingly, this response is not restricted to the site of injury, as former NICU infants also display secondary hyperalgesia (sensitivity in surrounding areas of undamaged tissue) in the intact, contralateral limb [36]. Similarly, infants that experienced surgery within the first three months of life display enhanced hypersensitivity to subsequent surgery performed in the same dermatome that persists for more than one year [37]. This hypersensitivity is also not restricted to the site of tissue damage, as neonates demonstrate greater sensitivity to mechanical stimulation both in the area of incision and on the contralateral side of the body following corrective unilateral abdominal surgery [38]. Moreover, term-born males that experienced un-anesthetized neonatal circumcision respond more intensely to routine inoculation at 4-6 months in comparison to uncircumcised infants; this effect is partially attenuated by pre-treatment with a local anesthetic [39].
Interestingly, alterations in nociception do not appear to be transient in nature, whereby both full- and preterm infants with prior NICU experience display an increased threshold for acute thermal stimuli (i.e. decreased sensitivity), but enhanced perceptual sensitization to a prolonged heat stimulus (i.e. hyper-sensitivity) up to 14 years of age [31; 40]. Former preterm adolescents also display significantly greater tenderness in response to pressure [41], are more prone to lasting clinical somatization [40], and report earlier onset of pediatric migraine [42] compared to full-term peers. Indeed, 10-year old children with former NICU experience also rate pictures of medical events as more intense than pictures of psychosocial pain events, unlike term-born children [43]. (See Table 1)
Table 1. Neonatal Pain: Human Studies.
| Term | NICU | Age At Testing | Type Testing | Results | Citation |
|---|---|---|---|---|---|
| preterm <28 GA | yes | 32 wks PCA | CORT, facial & cardiac reactivity to blood collection | dec. CORT, dec. facial reacitivty | 4 |
| preterm & fullterm | yes | P1-P14 | painful procedures & anagesia administered | ave NICU infant 14 painful procedures/day & <35% receive anagesia | 15 |
| gestation | no | GW18-37 | plasma NE to intrahepatic vein insertion | inc. NE in response to vein insertion | 17 |
| preterm 28-36 GA | yes | 24-48 hrs old | Near Infrared Spectroscopy (NIRS) to venipuncture | inc. blood flow in SC, M>F | 18 |
| preterm 25-45 GA | yes | P1-P7 | NIRS to heel lance | inc. cerebral oxygenation in SC at 25 weeks GA | 19 |
| preterm | yes | >5 years old | cognitive and behavioral | preterm children reduced cognitive scores and inc. ADHD | 23 |
| preterm | yes | 18 mo CA | parental pain ratings | ELBW preterm toddlers dec pain sensitivity | 24 |
| preterm | yes | PW1-8 | facial and heart rate response to heel lance | dec. facial & HR response over 8 week period | 28 |
| preterm | yes | 32 wks PCA | facial and heart rate response to heel lance | inc. NICU pain results in dec facial & HR response | 29 |
| preterm | yes | 4 mo CA | facial and heart rate response to finger lance | ELBW infants display dec. para & inc. symp responses | 30 |
| preterm | yes | 28-49 wks PCA | cutaneous flexion reflex | inc. sensitivity with limb tissue damage | 31 |
| fullterm | no | <1 year old | mechanical abdominal skin reflex | dec. mechanical reflex thresold at wound site | 32 |
| preterm 25-34 GA | yes | 27-39 wks PCA | cutaneous flexion reflex | infants <29 wks PCA have low thresholds, inc. with PCA | 34 |
| preterm & fullterm | yes | 27-42 wks PCA | cutaneous flexion reflex | repeated mechanical stimulation lowers thresholds in infants <35 wks | 35 |
| fullterm | no | <3 years old | morphine & pain to surgery | inc. morphine, inc. pain following operations | 36 |
| fullterm | no | 30-95 wks PCA | mechanical abdominal skin reflex | dec. thresholds in abdomen of infants with UH | 37 |
| fullterm | no | 4-6 mo | pain ratings to routine innoculation | inc. pain in circumcized males to innoculation | 38 |
| preterm & fullterm | yes | 11-14 years old | withdrawl thresholds to thermal stimuli | infants with NICU exp show inc. thresholds for acute thermal stimuli | 39 |
| preterm & fullterm | yes | 12-18 years old | tenderness thresholds by dolorimeter | preterm children more tender points & lower tender thresholds, F>M | 40 |
| preterm & fullterm | yes | 3, 4.5 years old | somatization scores | ELBw preterm children higher clinical somatization | 41 |
| preterm & fullterm | yes | 8-15 years old | migraine suffering | former NICU children have earlier onset of pediatric migraines | 42 |
| preterm & fullterm | yes | 8-10 years old | Pediatric Pain Inventory-rating painful events | NICU children rate medical more intense than psychosocial pain | 43 |
| preterm <33 GA | yes | 3,6,8,18 mo | salivary cortisol | NICU infants low basal cortisol at 3 mo | 98 |
| fullterm | no | 45 mo | immunization pain | early surgical exp with analgesia does not affect iimmunization pain | 114 |
| preterm <26 GA | yes | 7-11 years old | sensory testing-thermal & mechanical | dec. sensitivity thermal not mechanical stimuli | 125 |
| preterm & fullterm | yes | 9-12 years old | sensory testing-thermal & mechanical | dec. sensitivity to thermal & mechanical stimuli | 126 |
KEY:
CORT-cortisol
GA-gestational age
PCA-post conceptional age
wks-weeks
ave-average
exp-experience
GW-gestational week
NE-norepinephrine
SC-somatosensory cortex
mo-months
CA-corrected age
P-postnatal day
PW-postnatal week
HR-heart rate
NICU-neontal intensive care unit
ELBW-extremely low birth weight
para-parasympathetic
symp-sympathetic
UH-unilateral hydronephrosis
Long-Term Effects of Early Noxious Insult on Developing Nociceptive Systems-Experimental Animal Studies
There is considerable parallel evidence in non-human animal models that neonatal noxious stimulation induces persistent alterations in somatosensory structure and function that last into adult life [8; 27; 44]. Data collected to date suggest, however, that the type of noxious stimulation (acute: lasting minutes to days, versus tonic: lasting weeks to months) is critical to the long-term impact. Early pioneering studies reported that chronic neonatal inflammation induced by unilateral intraplantar application of Complete Freund's adjuvant results in enhanced nociceptive sensitivity, as well as increased primary afferent nerve fiber innervation of the spinal cord that extend into adulthood [27]. Similarly, local hindpaw skin wounds induced during the first week of life result in long-lasting cutaneous hypersensitivity, expanded dorsal horn receptive fields, and profound sprouting of local sensory nerve terminals in adulthood [27; 44]. This hyperinnervation is associated with a long-lasting decrease in mechanical threshold in the wounded region, as well as a substantial up-regulation of growth factors including NGF and BDNF [45; 46; 47]. Moreover, repeated intraplantar carrageenan administration over the first three postnatal weeks results in enhanced nociceptive sensitivity [26]. Thermal hyperalgesia following exposure to repetitive needle pricks, and lasting visceral hyperalgesia associated with neonatal chronic chemical irritation of the colon have also been reported [22; 48]. Finally, a persistent neonatal lipopolysaccharide immune challenge produces long-lasting hypersensitivity to mechanical and thermal stimuli in adulthood [49].
In contrast, a generalized decrease in nociceptive sensitivity as a consequence of acute or superficial stimulation such as foot shock and intraplantar formalin injections have been demonstrated [6; 50]. Likewise, a long-term global elevation of nociceptive thresholds in response to noxious thermal and mechanical stimulation following short-lasting local neonatal inflammation with intraplantar carrageenan has been reported [8]. Remarkably, this hypoalgesia is not only present in the neonatally injured hindpaw but is also present in the intact contralateral paw. The degree of hypoalgesia produced by the P0 insult is not trivial; paw withdrawal latencies increase by more than 40% in adult animals that were injured on the day of birth in comparison to control animals. Injury-induced hypoalgesia is observed in both adolescence (P40) and adulthood (P60), and is significantly greater in neonatally injured females compared to injured males [25]. Alternatively, a few studies using similar paradigms have failed to report any long-term effects on sensory thresholds [51; 52]. This may be due to variability in the concentration of the inflammatory agents used, as well as additional contributing factors that are still not well-understood. The abovementioned hypoalgesic response that we report is also associated with excessive hyperalgesia in the presence of on-going inflammation following a subsequent inflammatory insult in adulthood [8], with neonatally injured females again exhibiting significantly greater hyperalgesia in the inflamed paw than neonatally injured males. While an early study only reported this effect in the neonatally inflamed hindpaw [8], we observed enhanced hyperlagesia in both the neonatally injured and uninjured paws, which is consistent with previous studies reporting long-lasting sensitization of afferent neurons and hyperalgesia following neonatal insult [22; 46; 48]. These inconsistent results may be due to differences in the timing of neonatal injury, adult reinflammation and/or behavioral testing.
This increased hyperalgesia following re-inflammation in adulthood may appear disparate with the observed basal hypoalgesia. Anatomical studies, however, suggest that neonatal inflammatory insult results in alterations in primary afferent innervation of the dorsal horn [53], which may account for our observed hyperalgesia. In particular, neonatal inflammatory insult increases primary afferent innervation in the L3-L5 spinal cord, as reflected by increased expression of both CGRP and substance P immunoreactivity (unpublished observations). Parallel changes are not observed in CGRP expression in the thoracic spinal cord of injured animals, indicating that these changes are site-specific. Similar findings of an increase in substance P levels in laminae I and II of the dorsal horn have been reported following chronic inflammation in rodents [54]. As both CGRP and substance P are pro-nociceptive, enhanced dorsal horn release of these peptides due to increased primary afferent input would be associated with an enhanced response to noxious stimulation, and may provide the biological basis for the observed increased hyperalgesia following intraplantar CFA in adulthood. The dual findings of baseline hypoalgesia and enhanced hyperalgesia following a subsequent insult are also surprisingly consistent with previous reports in former premature children. Grunau and colleagues found that ex-preterm neonates are rated by parents as less reactive to everyday bumps and scrapes; however parents rate that these children experience medical procedural pain as more intense [24; 55]. Similarly, adolescents with prior NICU experience display an increased threshold for acute thermal stimuli (i.e. decreased sensitivity), but enhanced perceptual sensitization to a prolonged heat stimulus (i.e. hyper-sensitivity) [40]. Taken together, these data suggest that early life exposure to acute versus persistent noxious stimulation may differentially affect developing nociceptive circuitry, thereby producing distinct long-term effects. (See Table 2)
Table 2. Neonatal Pain: Experimental Animal Studies.
| Species | Model | Age At Injury | Age At Testing | Results | Citation |
|---|---|---|---|---|---|
| rat | repeated intaplantar formalin | P1-P7 | P74-P95 | inc. HPL, M>F | 6 |
| rat | intraplantar CGN | P3 | P41,P63,P125 | inc. PWL to mech & therm stimuli (hindpaws) | 8 |
| rat | intraplantar CFA | P1 | P56 | inc. nerve terminal fields lamina II in DH | 9 |
| rat | intraplantar CGN | P3 | P60 | inc. CRD thresholds | 10 |
| rat | repeated heelsticks | P0-P7 | P16,P22,P65 | dec. HPL & inc. preference for alcohol | 22 |
| rat | intraplantar CGN & Adult CFA | P0 | P40, P60 | bsln inc. PWL (hindpaws), inc. hyperalgesia to CFA; F>M | 25 |
| rat | intraplantar CGN & Adult CFA | P0 | P60 | bsln inc. PWL, inc. hyperalgesia to CFA | 26 |
| rat | intraplantar CFA | P0,P3 | P60 | unilateral inc. primary afferents in laminae I & II in DH | 27 |
| rat | hindpaw skin wounds | P1 | P19, P40 | inc. DH receptive fields | 44 |
| rat | hindpaw skin wounding | P1 | P1-P4 | inc. NGF in hindpaw skin | 45 |
| rat | hindpaw skin wounding | P0,P7 | P84 | hyperinnervation of hindpaw, dec PWL to mech stimuli | 46 |
| rat | chemical irritation-colon | P8-P21 | P60 | visceral hyperalgesia | 48 |
| rat | daily footshock | P1-P21 | P90 | inc. HPL, inc. morphine analgesia | 49 |
| mouse | laparotomy | P1 | P80 | inc. TFL & HPL, dec. pain response to acidic acid | 50 |
| rat | intraplantar CGN | P0 | P60 | inc. primary afferents in DH (L3-L5) spinal cord | 51 |
| rat | hot plate (2× daily) | P1-P15 | P90 | inc. acitve avoidance learning | 94 |
| mouse | repeated heelsticks | P8-P14 | P30 | inc. anxiety in EPM | 95 |
| rat | intraplantar CGN | P3 | P50 | dec. anxiety in EPM & inc. stress coping in FST | 97 |
| rat | morphine + intraplantar CGN | P0 | P60 | dec. morphine analgesia | 99 |
| rat | morphine + intraplantar formalin | P0-P7 | P1-P20 | dec. hyperlagesia | 116 |
| rat | intraperitoneal lipopolysaccharide | P14 | P56-P84 | dec. PWL to mech & therm stimuli | 127 |
| rat | intraplantar CGN | P0 | P60 | inc. BE & ENK, dec. MOR and DOR in PAG | 128 |
KEY:
TFL-tail flick latency
inc-increased
dec-decreased
CGN-carrageenan
PWL-paw withdrawal latency
mech-mechanical
therm-thermal
CFA-Complete Freund's Adjuvant
bsln-baseline
F-female
M-male
DR-dose response
CRD-colorectal distention
HPL-hotplate latency
NGF-nerve growth factor
EPM-elevated plus maze
FST-forced swim task
BE-beta-endorphin
ENK-enkephalin
MOR- mu opioid receptor
DOR-delta opiood receptor
PAG-periaqueductal gray
Interestingly, the aforementioned studies suggest that the long-term impact of neonatal noxious insult mirrors the developmental consequences of early life stress. Compelling evidence in experimental animal models has revealed that stressful experiences during the perinatal period result in profound and permanent consequences on the behavioral and neuroendocrine responses to stress stimuli in adulthood. Specifically, exposure to a potent stressor such as repeated maternal separation results in lasting hyperactivation of the hypothalamic pituitary adrenal (HPA) axis, while brief bouts of handling (i.e. mild stressor in rodents) during the perinatal period result in a stress hypo-responsive phenotype [56; 57; 58]. Thus, given that the lasting impact of early life stress on the HPA axis is dependent upon the degree (mild versus severe) of perinatal stress exposure, the type of noxious stimulation (acute versus tonic), similarly, appears to be critical to the long-term bivalent effects of neonatal insult on baseline nociceptive thresholds.
Critical Period
The ability of early life experience to alter the organization of the CNS and subsequent behavior is a major focus of neuroscientific research. Previous research in both human and non-human animal models suggests that there are periods during nervous system development within which perturbations have long-lasting, if not permanent consequences. This is in contrast to the relatively transient effects associated with the same perturbations at times outside these periods [59; 60]. Work in our laboratory has indicated that there is indeed a critical period for the long-term consequences of neonatal inflammatory insult on adult sensory thresholds. Animals that experienced unilateral neonatal hindpaw inflammation on both postnatal days zero and eight (P0 and P8) display a significant decrease in sensitivity to noxious stimuli (hypoalgesia) in adulthood, compared to animals injured at two weeks of age (P14) [25]. Together, these results suggest that the impact of neonatal inflammation is dependent upon a sensitive period, and that noxious insult occurring outside of this critical window does not permanently alter thermal sensory thresholds. These results are consistent with previous animal studies that have also reported that neonatal injury permanently alters visceral and somatic sensory processing, however, only when induced during the first week of life [8; 10].
Sex Differences In Response to Neonatal Noxious Stimulation
Given the sizable body of literature that indicates that males and females experience pain differently [61; 62; 63], it is surprising that the majority of previous studies examining the impact of neonatal noxious insult have been conducted exclusively in male rodents [8; 26; 27]. Our laboratory has hypothesized that sexually dimorphic organizational hormones may contribute to significant sex differences in response to noxious inflammatory insult [64; 65; 66; 67]. Sex steroid hormones such as estrogens and androgens modulate prenatal and postnatal functional development and have potent influences on pain thresholds in male and female rats [68; 69]. Prenatally, males experience a significant surge of testicular testosterone that is centrally aromatized to estradiol and ultimately results in the masculinization of the male brain [64; 65; 66; 67]. In females, the ovaries are quiescent and intracerebral estradiol remains low at birth [64; 65; 66; 67]. Similar differences in hormone levels may also be present in peripheral tissues as well. Given that estrogens have been shown to exert neuromodulatory and neuroprotective effects following acute and chronic central injuries, increased perinatal central estradiol in males may contribute to lasting sexually dimorphic responses to early life noxious stimulation [64; 70; 71].
To our knowledge, we reported for the first time that neonatal inflammatory insult was indeed sexually dimorphic, with females displaying significantly greater basal hypoalgesia in adulthood in comparison to males. The paw withdrawal latency of females injured with 1% CGN was more than 3 seconds longer in both the inflamed and intact hindpaws compared to injured males [25]. Moreover, we showed that female rats injured at P14, when estradiol concentrations are comparable in males and females, displayed equivalent levels of baseline hypoalgesia as injured males. This further suggests that sex differences in the neonatal neuroendocrine environment contribute to the observed sexually dimorphic impact of neonatal inflammatory insult [25]. Estrogen also influences the expression of a number of pro-inflammatory as well as pro-nociceptive agents that may contribute to sex differences in nociceptive responses. For example, prostaglandins (which are pro-inflammatory) are released peripherally in response to injury, and estrogen has been shown to modulate both prostaglandin and COX-1 and COX-2 expression in peripheral tissues [72]. In adults, estrogens modulate vascular tone in a tissue specific manner (vasodilation, vasoconstriction), which may lead to differences in inflammation-induced edema [71]. Peripheral injury also results in increased BDNF that is thought to promote neuronal survival and healing. As estradiol increases BDNF expression centrally, this may also attenuate the adverse effect of peripheral injury [73].
In addition, activational gonadal hormones can alter the processing of nociceptive information. Sex-steroids influence endogenous opioid systems [74; 75], as well as the activity of other neuromodulators involved in nociceptive processing; including substance P, gamma-aminobutyric acid (GABA), glutamate, dopamine, serotonin and norepinephrine [76; 77]. Moreover, gonadal hormones have been shown to have a marked influence on estrous cycle effects on nociceptive and analgesic sensitivity in rodents [78; 79], as well as menstrual cycle variability in chronic pain conditions such as migraine headache [80], temporomandibular disorders [81], and fibromyalgia [82].
Thus, several mechanisms may contribute to a sexually dimorphic effect of neonatal noxious insult, including sex differences in the neuroendocrine environment at the time of injury and/or at the time of testing. While there are no reported sex differences in response to early life noxious stimulation in premature infants, primarily because of the small sample sizes that are unable detect sexual dimorphic effects, the aforementioned studies suggest that the lasting impact of procedural pain experienced in the NICU may indeed be sexually dimorphic. In addition, all of the previous experimental rodent studies that have examined the lasting consequences of neonatal noxious insult on developing nociceptive circuits have been conducted exclusively in males. Hence, the inclusion of female subjects in basic and clinical research studies examining this topic is warranted, as premature females may be at considerably increased risk for long-term consequences of early life trauma.
Increased Endogenous Opioid Tone: A Potential Mechanism for the Neonatal Injury-Induced Deficits in Nociceptive Responsiveness
While the impact of neonatal noxious stimulation on developing nociceptive circuitry and subsequent pain processing and perception has been the focus of a significant amount of research within the last decade, clinical and experimental studies have failed to elucidate the mechanisms underlying the reported lasting alterations in nociceptive responsiveness. The periaqueductal gray (PAG), and its descending projections to the rostral ventromedial medulla (RVM) and the spinal cord dorsal horn, constitute a primary anatomical circuit for the descending modulation of pain [83]. The PAG is rich in nerve terminals and fibers containing endogenous opioids [84], and opioid receptors are localized throughout the rostral-caudal axis of the PAG [85]. Interestingly, while in rats the anatomical connections for nociceptive modulation are present at birth, descending inhibitory controls are functionally immature throughout the first postnatal weeks [86; 87]. The delayed maturation of descending inhibition may therefore contribute to the increased vulnerability of the immature somatosensory system to excessive afferent input, whereby exposure to neonatal noxious stimulation during a critical window may alter the functional integrity of endogenous descending inhibitory systems. Indeed, our laboratory hypothesized that neonatal injury during this critical developmental period (P0-P8) [25] results in increased afferent drive to CNS sites responsive to noxious input (eg. PAG). This increased drive results in the activation of endogenous pain inhibitory circuits and the subsequent release of endogenous opioid peptides. As the inflammation associated with intraplantar carrageenan is persistent (lasting approximately 24-48 hours), the release of endogenous opioids is sustained, and this continuous opioid release, during a time of increased developmental plasticity, is subsequently maintained into adulthood. This is supported by mounting behavioral data [8; 25]. Specifically, the observable hypoalgesia following neonatal injury is limb non-specific (present in both the forepaws and hindpaws) and global in nature (somatic and visceral) [10; 25]. Therefore, it appears to involve multiple segmental levels of the spinal cord, various dermatomes, and occurs bilaterally. Indeed these results are not easily explained by the induced unilateral neonatal insult that impacts few ipsilateral spinal levels. Consequently, the hypoalgesia appears better explained by alterations in descending nociceptive circuitry, such as that arising from the PAG, that produce global, limb-non-specific analgesia along the entire axis when activated.
Parallel studies have reported that increased afferent drive during the developmental critical period results in the reorganization of somatosensory circuits in adulthood, and interestingly, changes in endogenous pain modulation in humans as a consequence of neonatal pain has been previously proposed to account for the long term changes in pain sensitivity observed in NICU infants exposed to frequent noxious interventions [88].
We have recently reported a significant increase in PAG opioid peptide expression (beta-endorphin and met/leu-enkephalin) as a consequence of neonatal injury [89]. It was notable that alterations in the endorphin and enkephalinergic systems were very well correlated across similar regions and levels of the PAG, suggesting that the two opioidergic systems act in parallel in response to neonatal inflammation and nociception. While comparable studies cannot be conducted in neonates, previous studies in adult rats have also reported that hindpaw inflammation results in upregulated biosynthesis of pro-dynorphin and pro-enkephalin in dorsal horn neurons [90; 91], and a significant increase in endogenous opioid peptide release within the PAG [92]. Noxious stimulation-induced changes in opioid peptide expression are also paralleled by an increase in mRNA expression [90]. As stated above, our working hypothesis is that neonatal inflammation results in the release of endogenous opioid peptides within the PAG as a mechanism of decreasing nociception. Surprisingly, a parallel decrease in opioid receptor expression was also noted. Previous studies have similarly reported a long-term increase in endogenous opioid peptide along with a concomitant decrease in mu and delta opioid receptor density in the lateral hypothalamus of offspring following gestational stress [93; 94].
Alternatively, however, opioid receptors are rapidly internalized following ligand binding, which would also result in a concomitant decrease in opioid receptor availability. Taken together, these studies suggest that early life stressors can confer long-lasting changes in supraspinal opioidergic circuits that are reflected by changes in peptide and receptor expression. Interestingly, we reported a significantly greater increase in met-enkephalin observed in neonatally injured females compared to males. This differential change in peptide expression may contribute to the increased hypoalgesia observed in females [25]. No significant sex difference in met-enkephalin immunoreactivity was present at baseline, indicating that the observed sex differences were injury-induced. In parallel behavioral studies, we found that intra-PAG administration of the opioid antagonist naloxone significantly reduced neonatal injury-induced hypoalgesia, further implicating the PAG as the primary site whereby neonatal injury permanently alters somatosensory processing [89].
While these results strongly suggest that persistent alterations in baseline nociceptive thresholds associated with neonatal inflammatory insult are mediated by a central increase in endogenous opioid tone, additional mechanisms may also contribute. Previous studies have demonstrated that early life insult also results in increased serotonergic receptor expression in the PAG [95], as well as upregulated GABA, serotonin, opioid, neuropeptide Y, tachykinin and interleukin systems at the spinal level [96]. Therefore, a multitude of alterations at the levels of the brain and spinal cord may contribute to the observable behavioral alterations in adulthood following neonatal noxious insult.
Additionally, noxious neonatal experiences lead not only to decreased nociceptive sensitivity in adulthood, but also to significant alterations in the behavioral and neuroendocrine responses to stress [6; 22; 97; 98; 99; 100]. Blunted emotionality, decreased anxiety, and reduced basal and stress induced plasma corticotropin releasing factor (CRF) and adrenocorticotropin hormone (ACTH) are displayed in adult rats following short-lasting, local inflammation experienced during the first week of life [6; 95]. Premature infants with extensive NICU care also exhibit low basal levels of stress hormones at 3 months of age compared to their full-term counterparts [101]. Thus, alterations in the developing hypothalamic-pituitary-adrenal axis may also contribute to the long-term basal hypoalgesia following neonatal hindpaw inflammation. Indeed, our preliminary data suggests that neonatal noxious stimulation produces a generalized reduction in reactivity to non-life threatening aversive environmental stimuli due to parallel alterations in supraspinal nociceptive and stress modulatory circuits [8; 95]. For example, neonatally injured male and female rats tested in adulthood display reduced anxiety in the open field and elevated plus maze, as indicated by an increase in time spent in the open areas. By contrast, in the forced swim test, injured animals display significantly shorter latencies to immobility suggesting a hyper-response to a strong physiological stressor (unpublished observations). Furthermore, corticosterone (CORT) levels were significantly blunted in injured females at baseline and following restraint stress. Anseloni et al [85] similarly reported that neonatally-injured animals displayed reduced anxiety behavior, however found increased latency to immobility to the forced swim test. The reasons for the discrepant results are not known, but may include differences in the type of injury paradigm employed, or in the age at the time of injury. Regardless, together these studies indicate that neonatal injury alters adulthood stress and anxiety-related behaviors in a sexually dimorphic manner, and contributes to mounting evidence that neonatal trauma in the absence of analgesics has long-term polysystemic adverse effects.
Clinical Implications for Changes in Endogenous Opioid Tone
Our studies have shown that persistent pain experienced early in life results in decreased mu and delta receptor availability, suggesting a persistent activation of opioid receptors due to enhanced release of endogenous opioid peptides. This decrease in receptor availability is supported by our previous findings demonstrating a significant rightward shift in the morphine dose-response curve in adult animals that were injured neonatally [102]. Similar results have been reported in children, in that the number of invasive procedures experienced in the neonatal intensive care unit are inversely correlated with morphine effectiveness [29]. Despite the current knowledge that neonates are responsive to noxious stimuli, the majority of routine procedures, including repeated heel-lances, endotracheal intubations and minor surgeries are performed in the absence of analgesics [15]. Indeed recent studies have reported that neonates receiving NICU treatment experience an average of 14 noxious procedures per day, with fewer than 35% receiving appropriate analgesic therapy [15]. The findings that neonatal injury results in long-term changes in opioid tone and hypoalgesia have serious implications for future pain management in neonates.
Effects Of Analgesia On Developing Nociceptive Circuitry
Despite the current knowledge that preterm infants are responsive to noxious stimulation [17; 103; 104] and the accumulating evidence that invasive procedures can have lasting effects on developing nociceptive circuitry, neonatal pain remains an under-recognized and under-treated condition in the NICU [15; 105; 106]. Indeed, many life-saving intensive care interventions are performed in the absence of analgesics [15; 105; 106; 107].
Conflicting evidence exists as to the clinical benefits of opioid analgesia in premature infants undergoing invasive procedures in the NICU [29; 108; 109; 110; 111]. For example, altered pain responses in former preterm neonates can be predicted by the number of previous painful procedures and are normalized by the early use of morphine as an analgesic [29]. In addition, post-operative morphine analgesia in preterm and full-term infants reduces behavioral and hormonal stress responses [112; 113; 114] and is associated with decreased mortality [115; 116]. Furthermore, 45 month-old children that experienced operations following pre-emptive analgesia during early life respond to immunization pain in a similar manner as non-operated age-matched controls [117]. Lastly, the long-term outcomes at 5-6 years of age of formerly preterm children who were exposed to morphine in the neonatal period indicate no adverse effects of morphine on intelligence, motor function, cognitive development or other behavioral outcomes [29; 30; 110; 118].
Alternatively, morphine is not recommended as a standard of care for acute pain resulting from invasive procedures in ventilated preterm newborns [119; 120]. Further conflicting evidence exists on the long-term effects of early opiate exposure on later cognitive and motor abilities in preterm infants. Specifically, higher morphine exposure is associated with poorer motor development at 8 months, but not 18 months of age [109]. Similarly, neonatal morphine analgesia contributes to subtle neurobehavioral differences, including altered motor function, in preterm infants at 36 weeks [111]. Parallel evidence in rodents suggests delays in motor development following early morphine exposure [121], and a recent study demonstrated marked learning impairments in passive avoidance and forced swim tasks in adulthood following neonatal exposure to opiates [122].
Clearly, the aforementioned studies imply the importance of additional research to evaluate both the short-term and long-term effects of morphine analgesia on the neurobehavioral outcomes of prematurity, specifically the impact of neonatal opiate exposure on motor and cognitive development. Further studies examining the effects of pre-emptive analgesics in the NICU are challenging, however, as the humane care of infants requires physicians to treat those perceived to be in distress. As such, the gaps in our knowledge of the long-term risks and benefits of analgesic therapy in newborns would greatly benefit from experimental animal models, as very few studies have examined whether opioid analgesics can be used to prevent the long-term sequelae associated with neonatal noxious insult [100].
We have previously demonstrated that pre-emptive morphine administration blocks neonatal injury induced thermal and mechanical hypoalgesia in both the injured and uninjured paws in adolescence (P40) and adulthood (P60) [99]. Moreover, morphine attenuation of the hypoalgesia was reported to be comparable in males and females [90]. These results are consistent with previous studies in rodents that report daily morphine administration prior to intraplantar formalin during the first week of life significantly reduces the long-term effects of repetitive pain [123]. Previous studies in humans have also reported that morphine therapy ameliorates the effects of early repetitive noxious stimuli in extremely low birth weight infants at 4 months of age [29]. Similarly, children who had minor neonatal operations and received pre-emptive analgesia responded to immunization pain in a similar manner as non-operated age-matched controls [117]. The ability of pre-emptive morphine to block the hypoalgesia may indeed occur through direct modulation of primary afferent drive into the spinal cord, thereby inhibiting the central relay of inflammatory pain and preventing the subsequent increase in descending endogenous opioid tone [99]. However, the effects of morphine in these studies may partly be a consequence of modification of the inflammatory response and/or the stress response to neonatal inflammatory insult [124; 125].
Neonatal morphine has also been shown to significantly attenuate CFA- induced hyperalgesia and increased the rate of recovery, such that both males and females recover 7 days faster than saline treated injured controls [99]. As previously stated, increased primary afferent innervation of the spinal cord dorsal horn following neonatal inflammatory insult may account for our observed hyperalgesia in adulthood [25]. Administration of morphine at the time of injury would be expected to inhibit this increase in primary afferent input, thereby preventing the entire cascade of behavioral, physiological and anatomical deficits associated with neonatal inflammation. In regard to recovery, clinical reports demonstrate that at 32 weeks of age, preterm infants experience a reduced rate of recovery to skin breaking procedures [126], and exhibit subtle differences in ability to recover from finger lance at 4 months compared to full term controls [30]. There are no reports on the impact of pre-emptive morphine on recovery rates in premature neonates; however, these data suggest that morphine analgesia may in fact significantly increase the rate of recovery following procedural pain in NICU infants.
While neonatal morphine administration does not significantly alter morphine's antinociceptive effects in adulthood in males or females (i.e. no significant shift in ED50 values), interestingly, a significant rightward shift in ED50 is noted in neonatally injured animals that do not receive neonatal morphine [99]. These results have serious clinical implications. Previous studies have reported that pre-term infants that experience surgery during the first three months of life have significantly higher peri- and post-operative analgesic requirements in response to surgery in the same or different dermatome compared to control infants [37; 114]. Similarly, mice exposed to chronic noxious stimulation display increased tail flick latencies compared to control animals, and a significant two-fold increase in the ED50 of morphine in response to abdominal constriction [127]. As noxious stimulation during the neonatal period leads to increased activation of opioid systems in a manner analogous to the repeated application of exogenous opiates, these studies suggest that neonatal injury produces cross-tolerance to the analgesic effects of morphine thereby decreasing the subsequent effectiveness of morphine [128; 129; 130]. Again interestingly, exposure to morphine neonatally does not result in a significant shift in ED50 values. Therefore, it appears that opioid cross tolerance may be associated with neonatal injury-induced chronic exposure to endogenous opioids resulting from a potentiation of the descending inhibitory circuit, and not a result of exposure to morphine on P0. Alternatively, neonatal stress associated with maternal separation and repeated handling has also been suggested to reduce opioid analgesia [99; 131]. This suggests that alterations in opioid analgesia may reflect a combined effect of neonatal nociceptive experience as well as early life stress, and may involve altered responsiveness of endogenous analgesia circuits as well as the hypothalamic-pituitary-adrenal axis [4].
Final Remarks
Although research into the long-term consequences of noxious stimulation during the neonatal period have spanned over two decades, our understanding of neonatal pain is literally still in its infancy. The studies presented in this review have established that exposure to neonatal noxious insult is associated with a lasting alteration in both basal nociceptive sensitivity and response to a subsequent injury in adulthood (See Figure 1). Moreover, the impact of neonatal injury appears to be significantly exacerbated in females in comparison to males. The clear presence of a sex difference in the response to early insult may indeed contribute to the higher prevalence, severity and duration of pain syndromes observed in women than men. Furthermore, the profound alterations of nociceptive thresholds following neonatal inflammation may be mediated by an experience-induced facilitated activation of descending nociceptive pathways, characterized by dynamic physiological and anatomical modification and modulation of opioidergic systems in the PAG. Finally, pre-emptive analgesia has been shown to ameliorate the long-term effects of neonatal injury on adult nociception, which provides compelling justification for the use of analgesics prior to the initiation of noxious procedures performed on neonates. Collectively, these studies present valuable information about the long-term consequences of neonatal noxious stimulation, which may ultimately lead to improved understanding and treatment of the lasting effects of repeated invasive interventions in premature infants in the NICU.
Figure 1.
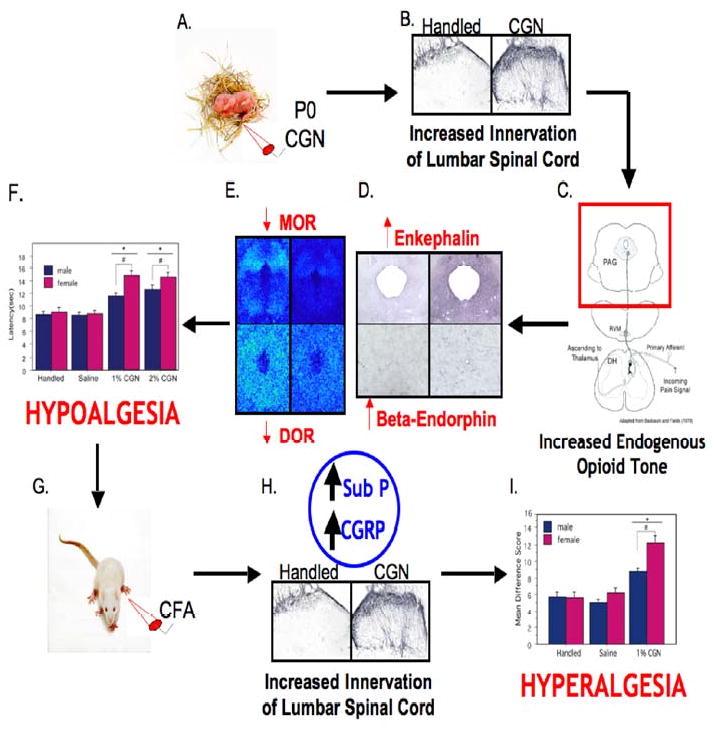
The Lasting Impact of Neonatal Inflammatory Insult: A Summary. (A) Intraplantar carrageenan (CGN) on the day of birth (P0) results in (B: left-handled; right-1% CGN) a lasting increase in primary afferent innervation of the dorsal horn of the lumbar spinal cord, ultimately leading to (C) an increase in endogenous opioid tone which is characterized by (D: top left-met enkephalin handled; top right-met enkephalin 1% CGN; bottom right-beta endorphin 1% CGN; bottom left-beta endorphin handled) a significant increase in enkephalin and beta endorphin immunoreactivity and (E: top left-MOR handled; top right-MOR 1% CGN; bottom right-DOR 1% CGN; bottom left-DOR handled) a significant decrease in mu and delta opioid receptor binding in the PAG. This increase in opioid tone contributes to the (F) observed hypoalgesia at baseline testing. (G) In the presence of a subsequent major noxious insult in adulthood, (H: left-handled; right-1% CGN) neonatally injured animals have increased release of pro-nociceptive peptides (i.e. CGRP and substance P) compared to handled animals, resulting in (I) enhanced hyperalgesia following intraplantar CFA.
Acknowledgments
This work was supported by National Institute of Health grants DA16272 and AR49555 awarded to Anne Z. Murphy, the Center for Behavioral Neuroscience NSF: IBN 9876754, and the Georgia State University Brains and Behavior Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- 1.Grunau RE, Holsti L, Peters JW. Long-term consequences of pain in human neonates. Semin Fetal Neonatal Med. 2006;11:268–75. doi: 10.1016/j.siny.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 2.Anand KJ. Clinical importance of pain and stress in preterm neonates. Biol Neonate. 1998;73:1–9. doi: 10.1159/000013953. [DOI] [PubMed] [Google Scholar]
- 3.Anand KJ. Pain, plasticity, and premature birth: a prescription for permanent suffering? Nat Med. 2000;6:971–3. doi: 10.1038/79658. [DOI] [PubMed] [Google Scholar]
- 4.Grunau RE, Holsti L, Haley DW, Oberlander T, Weinberg J, Solimano A, Whitfield MF, Fitzgerald C, Yu W. Neonatal procedural pain exposure predicts lower cortisol and behavioral reactivity in preterm infants in the NICU. Pain. 2005;113:293–300. doi: 10.1016/j.pain.2004.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whitfield MF, Grunau RE. Behavior, pain perception, and the extremely low-birth weight survivor. Clin Perinatol. 2000;27:363–79. doi: 10.1016/s0095-5108(05)70026-9. [DOI] [PubMed] [Google Scholar]
- 6.Bhutta AT, Rovnaghi C, Simpson PM, Gossett JM, Scalzo FM, Anand KJ. Interactions of inflammatory pain and morphine in infant rats: long-term behavioral effects. Physiol Behav. 2001;73:51–8. doi: 10.1016/s0031-9384(01)00432-2. [DOI] [PubMed] [Google Scholar]
- 7.Lidow MS. Long-term effects of neonatal pain on nociceptive systems. Pain. 2002;99:377–83. doi: 10.1016/S0304-3959(02)00258-0. [DOI] [PubMed] [Google Scholar]
- 8.Ren K, Anseloni V, Zou SP, Wade EB, Novikova SI, Ennis M, Traub RJ, Gold MS, Dubner R, Lidow MS. Characterization of basal and re-inflammation-associated long-term alteration in pain responsivity following short-lasting neonatal local inflammatory insult. Pain. 2004;110:588–96. doi: 10.1016/j.pain.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 9.Walker SM, Meredith-Middleton J, Cooke-Yarborough C, Fitzgerald M. Neonatal inflammation and primary afferent terminal plasticity in the rat dorsal horn. Pain. 2003;105:185–95. doi: 10.1016/s0304-3959(03)00201-x. [DOI] [PubMed] [Google Scholar]
- 10.Wang G, Ji Y, Lidow MS, Traub RJ. Neonatal hind paw injury alters processing of visceral and somatic nociceptive stimuli in the adult rat. J Pain. 2004;5:440–9. doi: 10.1016/j.jpain.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 11.Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Menacker F, Kirmeyer S. Births: final data for 2004. Natl Vital Stat Rep. 2006;55:1–101. [PubMed] [Google Scholar]
- 12.Shiono PH, Klebanoff MA, Nugent RP, Cotch MF, Wilkins DG, Rollins DE, Carey JC, Behrman RE. The impact of cocaine and marijuana use on low birth weight and preterm birth: a multicenter study. Am J Obstet Gynecol. 1995;172:19–27. doi: 10.1016/0002-9378(95)90078-0. [DOI] [PubMed] [Google Scholar]
- 13.Mathews TJ, MacDorman MF. Infant mortality statistics from the 2003 period linked birth/infant death data set. Natl Vital Stat Rep. 2006;54:1–29. [PubMed] [Google Scholar]
- 14.Barker DP, Rutter N. Exposure to invasive procedures in neonatal intensive care unit admissions. Arch Dis Child Fetal Neonatal Ed. 1995;72:F47–8. doi: 10.1136/fn.72.1.f47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simons SH, van Dijk M, Anand KS, Roofthooft D, van Lingen RA, Tibboel D. Do we still hurt newborn babies? A prospective study of procedural pain and analgesia in neonates. Arch Pediatr Adolesc Med. 2003;157:1058–64. doi: 10.1001/archpedi.157.11.1058. [DOI] [PubMed] [Google Scholar]
- 16.Anand KJ, Carr DB. The neuroanatomy, neurophysiology, and neurochemistry of pain, stress, and analgesia in newborns and children. Pediatr Clin North Am. 1989;36:795–822. doi: 10.1016/s0031-3955(16)36722-0. [DOI] [PubMed] [Google Scholar]
- 17.Giannakoulopoulos X, Teixeira J, Fisk N, Glover V. Human fetal and maternal noradrenaline responses to invasive procedures. Pediatr Res. 1999;45:494–9. doi: 10.1203/00006450-199904010-00007. [DOI] [PubMed] [Google Scholar]
- 18.Bartocci M, Bergqvist LL, Lagercrantz H, Anand KJ. Pain activates cortical areas in the preterm newborn brain. Pain. 2006;122:109–17. doi: 10.1016/j.pain.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 19.Slater R, Cantarella A, Gallella S, Worley A, Boyd S, Meek J, Fitzgerald M. Cortical pain responses in human infants. J Neurosci. 2006;26:3662–6. doi: 10.1523/JNEUROSCI.0348-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fitzgerald M. Painful beginnings. Pain. 2004;110:508–9. doi: 10.1016/j.pain.2004.05.022. [DOI] [PubMed] [Google Scholar]
- 21.Fitzgerald M, Jennings E. The postnatal development of spinal sensory processing. Proc Natl Acad Sci U S A. 1999;96:7719–22. doi: 10.1073/pnas.96.14.7719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anand KJ, Coskun V, Thrivikraman KV, Nemeroff CB, Plotsky PM. Long-term behavioral effects of repetitive pain in neonatal rat pups. Physiol Behav. 1999;66:627–37. doi: 10.1016/s0031-9384(98)00338-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhutta AT, Cleves MA, Casey PH, Cradock MM, Anand KJ. Cognitive and behavioral outcomes of school-aged children who were born preterm: a meta-analysis. Jama. 2002;288:728–37. doi: 10.1001/jama.288.6.728. [DOI] [PubMed] [Google Scholar]
- 24.Grunau RV, Whitfield MF, Petrie JH. Pain sensitivity and temperament in extremely low-birth-weight premature toddlers and preterm and full-term controls. Pain. 1994;58:341–6. doi: 10.1016/0304-3959(94)90128-7. [DOI] [PubMed] [Google Scholar]
- 25.LaPrairie JL, Murphy AZ. Female rats are more vulnerable to the long-term consequences of neonatal inflammatory injury. Pain. 2007;132 1:S124–33. doi: 10.1016/j.pain.2007.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lidow MS, Song ZM, Ren K. Long-term effects of short-lasting early local inflammatory insult. Neuroreport. 2001;12:399–403. doi: 10.1097/00001756-200102120-00042. [DOI] [PubMed] [Google Scholar]
- 27.Ruda MA, Ling QD, Hohmann AG, Peng YB, Tachibana T. Altered nociceptive neuronal circuits after neonatal peripheral inflammation. Science. 2000;289:628–31. doi: 10.1126/science.289.5479.628. [DOI] [PubMed] [Google Scholar]
- 28.Johnston CC, Stevens B, Yang F, Horton L. Developmental changes in response to heelstick in preterm infants: a prospective cohort study. Dev Med Child Neurol. 1996;38:438–45. doi: 10.1111/j.1469-8749.1996.tb15101.x. [DOI] [PubMed] [Google Scholar]
- 29.Grunau RE, Oberlander TF, Whitfield MF, Fitzgerald C, Lee SK. Demographic and therapeutic determinants of pain reactivity in very low birth weight neonates at 32 Weeks' postconceptional Age. Pediatrics. 2001;107:105–12. doi: 10.1542/peds.107.1.105. [DOI] [PubMed] [Google Scholar]
- 30.Oberlander TF, Grunau RE, Whitfield MF, Fitzgerald C, Pitfield S, Saul JP. Biobehavioral pain responses in former extremely low birth weight infants at four months' corrected age. Pediatrics. 2000;105:e6. doi: 10.1542/peds.105.1.e6. [DOI] [PubMed] [Google Scholar]
- 31.Walker SM, Franck LS, Fitzgerald M, Myles J, Stocks J, Marlow N. Long-term impact of neonatal intensive care and surgery on somatosensory perception in children born extremely preterm. Pain. 2009;141:79–87. doi: 10.1016/j.pain.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 32.Andrews K, Fitzgerald M. Cutaneous flexion reflex in human neonates: a quantitative study of threshold and stimulus-response characteristics after single and repeated stimuli. Dev Med Child Neurol. 1999;41:696–703. doi: 10.1017/s0012162299001425. [DOI] [PubMed] [Google Scholar]
- 33.Andrews K, Fitzgerald M. Wound sensitivity as a measure of analgesic effects following surgery in human neonates and infants. Pain. 2002;99:185–95. doi: 10.1016/s0304-3959(02)00100-8. [DOI] [PubMed] [Google Scholar]
- 34.Fitzgerald M, Millard C, MacIntosh N. Hyperalgesia in premature infants. Lancet. 1988;1:292. doi: 10.1016/s0140-6736(88)90365-0. [DOI] [PubMed] [Google Scholar]
- 35.Fitzgerald M, Shaw A, MacIntosh N. Postnatal development of the cutaneous flexor reflex: comparative study of preterm infants and newborn rat pups. Dev Med Child Neurol. 1988;30:520–6. doi: 10.1111/j.1469-8749.1988.tb04779.x. [DOI] [PubMed] [Google Scholar]
- 36.Andrews K, Fitzgerald M. The cutaneous withdrawal reflex in human neonates: sensitization, receptive fields, and the effects of contralateral stimulation. Pain. 1994;56:95–101. doi: 10.1016/0304-3959(94)90154-6. [DOI] [PubMed] [Google Scholar]
- 37.Peters JW, Schouw R, Anand KJ, van Dijk M, Duivenvoorden HJ, Tibboel D. Does neonatal surgery lead to increased pain sensitivity in later childhood? Pain. 2005;114:444–54. doi: 10.1016/j.pain.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 38.Andrews KA, Desai D, Dhillon HK, Wilcox DT, Fitzgerald M. Abdominal sensitivity in the first year of life: comparison of infants with and without prenatally diagnosed unilateral hydronephrosis. Pain. 2002;100:35–46. doi: 10.1016/s0304-3959(02)00288-9. [DOI] [PubMed] [Google Scholar]
- 39.Taddio A, Goldbach M, Ipp M, Stevens B, Koren G. Effect of neonatal circumcision on pain responses during vaccination in boys. Lancet. 1995;345:291–2. doi: 10.1016/s0140-6736(95)90278-3. [DOI] [PubMed] [Google Scholar]
- 40.Hermann C, Hohmeister J, Demirakca S, Zohsel K, Flor H. Long-term alteration of pain sensitivity in school-aged children with early pain experiences. Pain. 2006;125:278–85. doi: 10.1016/j.pain.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 41.Buskila D, Neumann L, Zmora E, Feldman M, Bolotin A, Press J. Pain sensitivity in prematurely born adolescents. Arch Pediatr Adolesc Med. 2003;157:1079–82. doi: 10.1001/archpedi.157.11.1079. [DOI] [PubMed] [Google Scholar]
- 42.Maneyapanda SB, Venkatasubramanian A. Relationship between significant perinatal events and migraine severity. Pediatrics. 2005;116:e555–8. doi: 10.1542/peds.2005-0454. [DOI] [PubMed] [Google Scholar]
- 43.Grunau RE, Whitfield MF, Petrie J. Children's judgements about pain at age 8-10 years: do extremely low birthweight (< or = 1000 g) children differ from full birthweight peers? J Child Psychol Psychiatry. 1998;39:587–94. [PubMed] [Google Scholar]
- 44.Torsney C, Fitzgerald M. Spinal dorsal horn cell receptive field size is increased in adult rats following neonatal hindpaw skin injury. J Physiol. 2003;550:255–61. doi: 10.1113/jphysiol.2003.043661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Constantinou J, Reynolds ML, Woolf CJ, Safieh-Garabedian B, Fitzgerald M. Nerve growth factor levels in developing rat skin: upregulation following skin wounding. Neuroreport. 1994;5:2281–4. doi: 10.1097/00001756-199411000-00019. [DOI] [PubMed] [Google Scholar]
- 46.Reynolds ML, Fitzgerald M. Long-term sensory hyperinnervation following neonatal skin wounds. J Comp Neurol. 1995;358:487–98. doi: 10.1002/cne.903580403. [DOI] [PubMed] [Google Scholar]
- 47.Whitby DJ, Ferguson MW. Immunohistochemical localization of growth factors in fetal wound healing. Dev Biol. 1991;147:207–15. doi: 10.1016/s0012-1606(05)80018-1. [DOI] [PubMed] [Google Scholar]
- 48.Al-Chaer ED, Kawasaki M, Pasricha PJ. A new model of chronic visceral hypersensitivity in adult rats induced by colon irritation during postnatal development. Gastroenterology. 2000;119:1276–85. doi: 10.1053/gast.2000.19576. [DOI] [PubMed] [Google Scholar]
- 49.Boisse L, Spencer SJ, Mouihate A, Vergnolle N, Pittman QJ. Neonatal immune challenge alters nociception in the adult rat. Pain. 2005;119:133–41. doi: 10.1016/j.pain.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 50.Shimada C, Kurumiya S, Noguchi Y, Umemoto M. The effect of neonatal exposure to chronic footshock on pain-responsiveness and sensitivity to morphine after maturation in the rat. Behav Brain Res. 1990;36:105–11. doi: 10.1016/0166-4328(90)90165-b. [DOI] [PubMed] [Google Scholar]
- 51.Hohmann AG, Neely MH, Pina J, Nackley AG. Neonatal chronic hind paw inflammation alters sensitization to intradermal capsaicin in adult rats: a behavioral and immunocytochemical study. J Pain. 2005;6:798–808. doi: 10.1016/j.jpain.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 52.Walker CD, Kudreikis K, Sherrard A, Johnston CC. Repeated neonatal pain influences maternal behavior, but not stress responsiveness in rat offspring. Brain Res Dev Brain Res. 2003;140:253–61. doi: 10.1016/s0165-3806(02)00611-9. [DOI] [PubMed] [Google Scholar]
- 53.LaPrairie J, Murphy A. Neonatal injury diffrentially affects male and female sensory thresholds and response to re-injury in adulthood. Soc Neuroscience Abst. 2005 [Google Scholar]
- 54.Honor P, Menning PM, Rogers SD, Nichols ML, Basbaum AI, Besson JM, Mantyh PW. Spinal substance P receptor expression and internalization in acute, short-term, and long-term inflammatory pain states. J Neurosci. 1999;19:7670–8. doi: 10.1523/JNEUROSCI.19-17-07670.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grunau RE, Oberlander T, Holsti L, Whitfield MF. Bedside application of the Neonatal Facial Coding System in pain assessment of premature neonates. Pain. 1998;76:277–86. doi: 10.1016/S0304-3959(98)00046-3. [DOI] [PubMed] [Google Scholar]
- 56.Caldji C, Tannenbaum B, Sharma S, Francis D, Plotsky PM, Meaney MJ. Maternal care during infancy regulates the development of neural systems mediating the expression of fearfulness in the rat. Proc Natl Acad Sci U S A. 1998;95:5335–40. doi: 10.1073/pnas.95.9.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, Pearson D, Plotsky PM, Meaney MJ. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277:1659–62. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- 58.Plotsky PM, Meaney MJ. Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Brain Res Mol Brain Res. 1993;18:195–200. doi: 10.1016/0169-328x(93)90189-v. [DOI] [PubMed] [Google Scholar]
- 59.Bishop B. Neural plasticity: Part 2. Postnatal maturation and function-induced plasticity. Phys Ther. 1982;62:1132–43. doi: 10.1093/ptj/62.8.1132. [DOI] [PubMed] [Google Scholar]
- 60.Rabinowicz T, de Courten-Myers GM, Petetot JM, Xi G, de los Reyes E. Human cortex development: estimates of neuronal numbers indicate major loss late during gestation. J Neuropathol Exp Neurol. 1996;55:320–8. [PubMed] [Google Scholar]
- 61.Berkley KJ. Sex differences in pain. Behav Brain Sci. 1997;20:371–80. doi: 10.1017/s0140525x97221485. discussion 435-513. [DOI] [PubMed] [Google Scholar]
- 62.Berkley KJ, Robbins A, Sato Y. Afferent fibers supplying the uterus in the rat. J Neurophysiol. 1988;59:142–63. doi: 10.1152/jn.1988.59.1.142. [DOI] [PubMed] [Google Scholar]
- 63.Unruh AM. Gender variations in clinical pain experience. Pain. 1996;65:123–67. doi: 10.1016/0304-3959(95)00214-6. [DOI] [PubMed] [Google Scholar]
- 64.Amateau SK, Alt JJ, Stamps CL, McCarthy MM. Brain estradiol content in newborn rats: sex differences, regional heterogeneity, and possible de novo synthesis by the female telencephalon. Endocrinology. 2004;145:2906–17. doi: 10.1210/en.2003-1363. [DOI] [PubMed] [Google Scholar]
- 65.Balthazart J, Ball GF. Is brain estradiol a hormone or a neurotransmitter? Trends Neurosci. 2006;29:241–9. doi: 10.1016/j.tins.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 66.Cornil CA, Ball GF, Balthazart J. Functional significance of the rapid regulation of brain estrogen action: where do the estrogens come from? Brain Res. 2006;1126:2–26. doi: 10.1016/j.brainres.2006.07.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Weisz J, Ward IL. Plasma testosterone and progesterone titers of pregnant rats, their male and female fetuses, and neonatal offspring. Endocrinology. 1980;106:306–16. doi: 10.1210/endo-106-1-306. [DOI] [PubMed] [Google Scholar]
- 68.Aloisi AM, Ceccarelli I, Fiorenzani P. Gonadectomy affects hormonal and behavioral responses to repetitive nociceptive stimulation in male rats. Ann N Y Acad Sci. 2003;1007:232–7. doi: 10.1196/annals.1286.022. [DOI] [PubMed] [Google Scholar]
- 69.Liu NJ, Gintzler AR. Prolonged ovarian sex steroid treatment of male rats produces antinociception: identification of sex-based divergent analgesic mechanisms. Pain. 2000;85:273–81. doi: 10.1016/s0304-3959(99)00278-x. [DOI] [PubMed] [Google Scholar]
- 70.Garcia-Segura LM, Azcoitia I, DonCarlos LL. Neuroprotection by estradiol. Prog Neurobiol. 2001;63:29–60. doi: 10.1016/s0301-0082(00)00025-3. [DOI] [PubMed] [Google Scholar]
- 71.Maggi A, Ciana P, Belcredito S, Vegeto E. Estrogens in the nervous system: mechanisms and nonreproductive functions. Annu Rev Physiol. 2004;66:291–313. doi: 10.1146/annurev.physiol.66.032802.154945. [DOI] [PubMed] [Google Scholar]
- 72.Zhang Y, Shaffer A, Portanova J, Seibert K, Isakson PC. Inhibition of cyclooxygenase-2 rapidly reverses inflammatory hyperalgesia and prostaglandin E2 production. J Pharmacol Exp Ther. 1997;283:1069–75. [PubMed] [Google Scholar]
- 73.Allen AL, McCarson KE. Estrogen increases nociception-evoked brain-derived neurotrophic factor gene expression in the female rat. Neuroendocrinology. 2005;81:193–9. doi: 10.1159/000087002. [DOI] [PubMed] [Google Scholar]
- 74.Berglund LA, Derendorf H, Simpkins JW. Desensitization of brain opiate receptor mechanisms by gonadal steroid treatments that stimulate luteinizing hormone secretion. Endocrinology. 1988;122:2718–26. doi: 10.1210/endo-122-6-2718. [DOI] [PubMed] [Google Scholar]
- 75.Smith YR, Zubieta JK, del Carmen MG, Dannals RF, Ravert HT, Zacur HA, Frost JJ. Brain opioid receptor measurements by positron emission tomography in normal cycling women: relationship to luteinizing hormone pulsatility and gonadal steroid hormones. J Clin Endocrinol Metab. 1998;83:4498–505. doi: 10.1210/jcem.83.12.5351. [DOI] [PubMed] [Google Scholar]
- 76.Duval P, Lenoir V, Moussaoui S, Garret C, Kerdelhue B. Substance P and neurokinin A variations throughout the rat estrous cycle; comparison with ovariectomized and male rats: I. Plasma, hypothalamus, anterior and posterior pituitary. J Neurosci Res. 1996;45:598–609. doi: 10.1002/(SICI)1097-4547(19960901)45:5<598::AID-JNR9>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 77.Smith SS. Female sex steroid hormones: from receptors to networks to performance--actions on the sensorimotor system. Prog Neurobiol. 1994;44:55–86. doi: 10.1016/0301-0082(94)90057-4. [DOI] [PubMed] [Google Scholar]
- 78.Craft RM, Mogil JS, Aloisi AM. Sex differences in pain and analgesia: the role of gonadal hormones. Eur J Pain. 2004;8:397–411. doi: 10.1016/j.ejpain.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 79.Fillingim RB, Ness TJ. Sex-related hormonal influences on pain and analgesic responses. Neurosci Biobehav Rev. 2000;24:485–501. doi: 10.1016/s0149-7634(00)00017-8. [DOI] [PubMed] [Google Scholar]
- 80.MacGregor EA. Menstruation, sex hormones, and migraine. Neurol Clin. 1997;15:125–41. doi: 10.1016/s0733-8619(05)70299-1. [DOI] [PubMed] [Google Scholar]
- 81.Warren MP, Fried JL. Temporomandibular disorders and hormones in women. Cells Tissues Organs. 2001;169:187–92. doi: 10.1159/000047881. [DOI] [PubMed] [Google Scholar]
- 82.Anderberg UM, Liu Z, Berglund L, Nyberg F. Plasma levels on nociceptin in female fibromyalgia syndrome patients. Z Rheumatol. 1998;57 2:77–80. doi: 10.1007/s003930050241. [DOI] [PubMed] [Google Scholar]
- 83.Basbaum AI, Fields HL. Endogenous pain control systems: brainstem spinal pathways and endorphin circuitry. Annu Rev Neurosci. 1984;7:309–38. doi: 10.1146/annurev.ne.07.030184.001521. [DOI] [PubMed] [Google Scholar]
- 84.Reichling DB, Kwiat GC, Basbaum AI. Anatomy, physiology and pharmacology of the periaqueductal gray contribution to antinociceptive controls. Prog Brain Res. 1988;77:31–46. doi: 10.1016/s0079-6123(08)62777-6. [DOI] [PubMed] [Google Scholar]
- 85.Mansour A, Fox CA, Burke S, Akil H, Watson SJ. Immunohistochemical localization of the cloned mu opioid receptor in the rat CNS. J Chem Neuroanat. 1995;8:283–305. doi: 10.1016/0891-0618(95)00055-c. [DOI] [PubMed] [Google Scholar]
- 86.Boucher T, Jennings E, Fitzgerald M. The onset of diffuse noxious inhibitory controls in postnatal rat pups: a C-Fos study. Neurosci Lett. 1998;257:9–12. doi: 10.1016/s0304-3940(98)00779-4. [DOI] [PubMed] [Google Scholar]
- 87.Fitzgerald M, Koltzenburg M. The functional development of descending inhibitory pathways in the dorsolateral funiculus of the newborn rat spinal cord. Brain Res. 1986;389:261–70. doi: 10.1016/0165-3806(86)90194-x. [DOI] [PubMed] [Google Scholar]
- 88.Goffaux P, Lafrenaye S, Morin M, Patural H, Demers G, Marchand S. Preterm births: Can neonatal pain alter the development of endogenous gating systems? Eur J Pain. 2008 doi: 10.1016/j.ejpain.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 89.Laprairie JL, Murphy AZ. Neonatal injury alters adult pain sensitivity by increasing opioid tone in the periaqueductal gray. Front Behav Neurosci. 2009;3:31. doi: 10.3389/neuro.08.031.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Iadarola MJ, Douglass J, Civelli O, Naranjo JR. Differential activation of spinal cord dynorphin and enkephalin neurons during hyperalgesia: evidence using cDNA hybridization. Brain Res. 1988;455:205–12. doi: 10.1016/0006-8993(88)90078-9. [DOI] [PubMed] [Google Scholar]
- 91.Noguchi K, Morita Y, Kiyama H, Sato M, Ono K, Tohyama M. Preproenkephalin gene expression in the rat spinal cord after noxious stimuli. Brain Res Mol Brain Res. 1989;5:227–34. doi: 10.1016/0169-328x(89)90039-9. [DOI] [PubMed] [Google Scholar]
- 92.Williams FG, Mullet MA, Beitz AJ. Basal release of Met-enkephalin and neurotensin in the ventrolateral periaqueductal gray matter of the rat: a microdialysis study of antinociceptive circuits. Brain Res. 1995;690:207–16. doi: 10.1016/0006-8993(95)00554-4. [DOI] [PubMed] [Google Scholar]
- 93.Sanchez MD, Milanes MV, Pazos A, Diaz A, Laorden ML. Autoradiographic evidence of mu-opioid receptors down-regulation after prenatal stress in offspring rat brain. Brain Res Dev Brain Res. 1996;94:14–21. doi: 10.1016/0165-3806(96)00032-6. [DOI] [PubMed] [Google Scholar]
- 94.Sanchez MD, Milanes MV, Pazos A, Diaz A, Laorden ML. Autoradiographic evidence of delta-opioid receptor downregulation after prenatal stress in offspring rat brain. Pharmacology. 2000;60:13–8. doi: 10.1159/000028341. [DOI] [PubMed] [Google Scholar]
- 95.Anseloni VC, He F, Novikova SI, Turnbach Robbins M, Lidow IA, Ennis M, Lidow MS. Alterations in stress-associated behaviors and neurochemical markers in adult rats after neonatal short-lasting local inflammatory insult. Neuroscience. 2005;131:635–45. doi: 10.1016/j.neuroscience.2004.11.039. [DOI] [PubMed] [Google Scholar]
- 96.Ren K, Novikova SI, He F, Dubner R, Lidow MS. Neonatal local noxious insult affects gene expression in the spinal dorsal horn of adult rats. Mol Pain. 2005;1:27. doi: 10.1186/1744-8069-1-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bernardi M, Genedani S, Bertolini A. Behavioral activity and active avoidance learning and retention in rats neonatally exposed to painful stimuli. Physiol Behav. 1986;36:553–5. doi: 10.1016/0031-9384(86)90330-6. [DOI] [PubMed] [Google Scholar]
- 98.Schellinck HM, Stanford L, Darrah M. Repetitive acute pain in infancy increases anxiety but does not alter spatial learning ability in juvenile mice. Behav Brain Res. 2003;142:157–65. doi: 10.1016/s0166-4328(02)00406-0. [DOI] [PubMed] [Google Scholar]
- 99.Sternberg WF, Ridgway CG. Effects of gestational stress and neonatal handling on pain, analgesia, and stress behavior of adult mice. Physiol Behav. 2003;78:375–83. doi: 10.1016/s0031-9384(03)00015-5. [DOI] [PubMed] [Google Scholar]
- 100.Sternberg WF, Scorr L, Smith LD, Ridgway CG, Stout M. Long-term effects of neonatal surgery on adulthood pain behavior. Pain. 2005;113:347–53. doi: 10.1016/j.pain.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 101.Grunau RE, Haley DW, Whitfield MF, Weinberg J, Yu W, Thiessen P. Altered basal cortisol levels at 3, 6, 8 and 18 months in infants born at extremely low gestational age. J Pediatr. 2007;150:151–6. doi: 10.1016/j.jpeds.2006.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Laprairie JL, Johns ME, Murphy AZ. Preemptive morphine analgesia attenuates the long-term consequences of neonatal inflammation in male and female rats. Pediatr Res. 2008;64:625–30. doi: 10.1203/PDR.0b013e31818702d4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Smith RP, Gitau R, Glover V, Fisk NM. Pain and stress in the human fetus. Eur J Obstet Gynecol Reprod Biol. 2000;92:161–5. doi: 10.1016/s0301-2115(00)00441-3. [DOI] [PubMed] [Google Scholar]
- 104.Vanhatalo S, van Nieuwenhuizen O. Fetal pain? Brain Dev. 2000;22:145–50. doi: 10.1016/s0387-7604(00)00089-9. [DOI] [PubMed] [Google Scholar]
- 105.Kahn DJ, Richardson DK, Gray JE, Bednarek F, Rubin LP, Shah B, Frantz ID, 3rd, Pursley DM. Variation among neonatal intensive care units in narcotic administration. Arch Pediatr Adolesc Med. 1998;152:844–51. doi: 10.1001/archpedi.152.9.844. [DOI] [PubMed] [Google Scholar]
- 106.Porter FL, Wolf CM, Gold J, Lotsoff D, Miller JP. Pain and pain management in newborn infants: a survey of physicians and nurses. Pediatrics. 1997;100:626–32. doi: 10.1542/peds.100.4.626. [DOI] [PubMed] [Google Scholar]
- 107.Johnston CC, Collinge JM, Henderson SJ, Anand KJ. A cross-sectional survey of pain and pharmacological analgesia in Canadian neonatal intensive care units. Clin J Pain. 1997;13:308–12. doi: 10.1097/00002508-199712000-00008. [DOI] [PubMed] [Google Scholar]
- 108.Anand KJ, Hall RW, Desai N, Shephard B, Bergqvist LL, Young TE, Boyle EM, Carbajal R, Bhutani VK, Moore MB, Kronsberg SS, Barton BA. Effects of morphine analgesia in ventilated preterm neonates: primary outcomes from the NEOPAIN randomised trial. Lancet. 2004;363:1673–82. doi: 10.1016/S0140-6736(04)16251-X. [DOI] [PubMed] [Google Scholar]
- 109.Grunau RE, Whitfield MF, Petrie-Thomas J, Synnes AR, Cepeda IL, Keidar A, Rogers M, Mackay M, Hubber-Richard P, Johannesen D. Neonatal pain, parenting stress and interaction, in relation to cognitive and motor development at 8 and 18 months in preterm infants. Pain. 2009;143:138–46. doi: 10.1016/j.pain.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.MacGregor R, Evans D, Sugden D, Gaussen T, Levene M. Outcome at 5-6 years of prematurely born children who received morphine as neonates. Arch Dis Child Fetal Neonatal Ed. 1998;79:F40–3. doi: 10.1136/fn.79.1.f40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rao R, Sampers JS, Kronsberg SS, Brown JV, Desai NS, Anand KJ. Neurobehavior of preterm infants at 36 weeks postconception as a function of morphine analgesia. Am J Perinatol. 2007;24:511–7. doi: 10.1055/s-2007-986675. [DOI] [PubMed] [Google Scholar]
- 112.Farrington EA, McGuinness GA, Johnson GF, Erenberg A, Leff RD. Continuous intravenous morphine infusion in postoperative newborn infants. Am J Perinatol. 1993;10:84–7. doi: 10.1055/s-2007-994711. [DOI] [PubMed] [Google Scholar]
- 113.Bouwmeester NJ, Anand KJ, van Dijk M, Hop WC, Boomsma F, Tibboel D. Hormonal and metabolic stress responses after major surgery in children aged 0-3 years: a double-blind, randomized trial comparing the effects of continuous versus intermittent morphine. Br J Anaesth. 2001;87:390–9. doi: 10.1093/bja/87.3.390. [DOI] [PubMed] [Google Scholar]
- 114.Bouwmeester NJ, Hop WC, van Dijk M, Anand KJ, van den Anker JN, Tibboel D. Postoperative pain in the neonate: age-related differences in morphine requirements and metabolism. Intensive Care Med. 2003;29:2009–15. doi: 10.1007/s00134-003-1899-4. [DOI] [PubMed] [Google Scholar]
- 115.Anand KJ, Hickey PR. Halothane-morphine compared with high-dose sufentanil for anesthesia and postoperative analgesia in neonatal cardiac surgery. N Engl J Med. 1992;326:1–9. doi: 10.1056/NEJM199201023260101. [DOI] [PubMed] [Google Scholar]
- 116.Anand KJ, Sippell WG, Aynsley-Green A. Randomised trial of fentanyl anaesthesia in preterm babies undergoing surgery: effects on the stress response. Lancet. 1987;1:62–6. doi: 10.1016/s0140-6736(87)91907-6. [DOI] [PubMed] [Google Scholar]
- 117.Peters JW, Koot HM, de Boer JB, Passchier J, Bueno-de-Mesquita JM, de Jong FH, Duivenvoorden HJ, Tibboel D. Major surgery within the first 3 months of life and subsequent biobehavioral pain responses to immunization at later age: a case comparison study. Pediatrics. 2003;111:129–35. doi: 10.1542/peds.111.1.129. [DOI] [PubMed] [Google Scholar]
- 118.Roze JC, Denizot S, Carbajal R, Ancel PY, Kaminski M, Arnaud C, Truffert P, Marret S, Matis J, Thiriez G, Cambonie G, Andre M, Larroque B, Breart G. Prolonged sedation and/or analgesia and 5-year neurodevelopment outcome in very preterm infants: results from the EPIPAGE cohort. Arch Pediatr Adolesc Med. 2008;162:728–33. doi: 10.1001/archpedi.162.8.728. [DOI] [PubMed] [Google Scholar]
- 119.Bellu R, de Waal KA, Zanini R. Opioids for neonates receiving mechanical ventilation. Cochrane Database Syst Rev. 2008:CD004212. doi: 10.1002/14651858.CD004212.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Simons SH, van Dijk M, van Lingen RA, Roofthooft D, Duivenvoorden HJ, Jongeneel N, Bunkers C, Smink E, Anand KJ, van den Anker JN, Tibboel D. Routine morphine infusion in preterm newborns who received ventilatory support: a randomized controlled trial. JAMA. 2003;290:2419–27. doi: 10.1001/jama.290.18.2419. [DOI] [PubMed] [Google Scholar]
- 121.Handelmann GE, Dow-Edwards D. Modulation of brain development by morphine: effects on central motor systems and behavior. Peptides. 1985;6 2:29–34. doi: 10.1016/0196-9781(85)90131-7. [DOI] [PubMed] [Google Scholar]
- 122.McPherson RJ, Gleason C, Mascher-Denen M, Chan M, Kellert B, Juul SE. A new model of neonatal stress which produces lasting neurobehavioral effects in adult rats. Neonatology. 2007;92:33–41. doi: 10.1159/000100084. [DOI] [PubMed] [Google Scholar]
- 123.Abbott FV, Guy ER. Effects of morphine, pentobarbital and amphetamine on formalin-induced behaviours in infant rats: sedation versus specific suppression of pain. Pain. 1995;62:303–12. doi: 10.1016/0304-3959(94)00277-L. [DOI] [PubMed] [Google Scholar]
- 124.Adamson WT, Windh RT, Blackford S, Kuhn CM. Ontogeny of mu- and kappa-opiate receptor control of the hypothalamo-pituitary-adrenal axis in rats. Endocrinology. 1991;129:959–64. doi: 10.1210/endo-129-2-959. [DOI] [PubMed] [Google Scholar]
- 125.Stein C, Millan MJ, Shippenberg TS, Peter K, Herz A. Peripheral opioid receptors mediating antinociception in inflammation. Evidence for involvement of mu, delta and kappa receptors. J Pharmacol Exp Ther. 1989;248:1269–75. [PubMed] [Google Scholar]
- 126.Morison SJ, Holsti L, Grunau RE, Whitfield MF, Oberlander TF, Chan HW, Williams L. Are there developmentally distinct motor indicators of pain in preterm infants? Early Hum Dev. 2003;72:131–46. doi: 10.1016/s0378-3782(03)00044-6. [DOI] [PubMed] [Google Scholar]
- 127.Christie MJ, Trisdikoon P, Chesher GB. Tolerance and cross tolerance with morphine resulting from physiological release of endogenous opiates. Life Sci. 1982;31:839–45. doi: 10.1016/0024-3205(82)90538-0. [DOI] [PubMed] [Google Scholar]
- 128.Lett BT, Grant VL, Koh MT, Flynn G. Prior experience with wheel running produces cross-tolerance to the rewarding effect of morphine. Pharmacol Biochem Behav. 2002;72:101–5. doi: 10.1016/s0091-3057(01)00722-5. [DOI] [PubMed] [Google Scholar]
- 129.Stoller DC, Sim-Selley LJ, Smith FL. Role of kappa and delta opioid receptors in mediating morphine-induced antinociception in morphine-tolerant infant rats. Brain Res. 2007;1142:28–36. doi: 10.1016/j.brainres.2007.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Stoller DC, Thornton SR, Smith FL. Loss of antinociceptive efficacy in rat pups infused with morphine from osmotic minipumps. Pharmacology. 2002;66:11–8. doi: 10.1159/000063250. [DOI] [PubMed] [Google Scholar]
- 131.Coutinho SV, Plotsky PM, Sablad M, Miller JC, Zhou H, Bayati AI, McRoberts JA, Mayer EA. Neonatal maternal separation alters stress-induced responses to viscerosomatic nociceptive stimuli in rat. Am J Physiol Gastrointest Liver Physiol. 2002;282:G307–16. doi: 10.1152/ajpgi.00240.2001. [DOI] [PubMed] [Google Scholar]


