Abstract
Papillary thyroid cancer (PTC) is a common endocrine malignancy that frequently harbors the oncogenic T1799A BRAF mutation. As a novel prognostic molecular marker, this mutation has received considerable attention in recent years for its potential utility in the risk stratification and management of PTC. In PTC, BRAF mutation is closely associated with extrathyroidal extension, lymph node metastasis, advanced tumor stages, disease recurrence, and even patient mortality. Many of the responsible molecular derangements promoted by, or associated with, BRAF mutation have been identified, including over-expression of tumor-promoting genes, suppression of tumor-suppressor genes, and silencing of thyroid iodide-handling genes, resulting in impairment or loss of radioiodine avidity and hence the failure of radioiodine treatment of PTC. BRAF mutation can be readily tested for on thyroid fine needle aspiration biopsy specimens, with high preoperative predictive probabilities for clinicopathological outcomes of PTC. As such, knowledge of BRAF mutation status can facilitate more accurate risk stratification and better decision making at various steps in the management of PTC, from preoperative planning of initial surgical scale to postoperative decisions about appropriate radioiodine treatment and thyroid-stimulating hormone suppression, and to selections of appropriate surveillance modalities for PTC recurrence. The greatest utility of BRAF mutation status is in those cases where traditional clinicopathological criteria alone would otherwise be unreliable in the risk stratification and management of PTC. Use of this unique molecular marker, in conjunction with conventional clinicopathological risk factors, to assist the prognostication of PTC is likely to improve the efficiency of contemporary management of thyroid cancer.
Keywords: BRAF mutation, papillary thyroid cancer, risk stratification, prognosis, molecular marker
Introduction
Thyroid cancer is the most common endocrine malignancy and its incidence has seen a rapid global rise in recent decades.1, 2, 3, 4, 5 In the United States of America, this incidence rise is currently the fastest among all cancers, with an estimated incidence of 37,200 cases and a prevalence of > 360,000 cases for the year of 2009.5 The vast majority of thyroid cancers originate from follicular epithelial cells, which are histologically classified as papillary thyroid cancer (PTC), follicular thyroid cancer (FTC), and anaplastic thyroid cancer (ATC). Given the already large and still rising number of patients with thyroid cancer, vigorous efforts have been made to optimize the strategies for efficiently managing this cancer. The products of these efforts can be seen in the guidelines or consensuses on the management of thyroid cancer published by several organizations or experts in recent years.6,7,8,9,10 Such guidance based on evidence or expert opinion has contributed greatly to improving the standardization and efficiency of the management of thyroid cancer. However, there are still controversies in many areas over the optimal management of this cancer. In this context, effort from molecular thyroid cancer medicine has now shown great promises in helping improve the management of thyroid cancer. One of the best examples is the characterization of the T1799A BRAF mutation (termed BRAF mutation hereafter) as a novel and effective prognostic molecular marker,11,12 which has now started entering clinic for the management of PTC.
PTC and its risk management
PTC accounts for 80–90% of all thyroid cancers.1,2,3 The current rise in the incidence of thyroid cancer is virtually solely from an increased incidence of PTC.2,3,4 Consequently, the bulk of the effort in today’s thyroid cancer medicine is dedicated to managing PTC. In the USA and many European countries, the standard treatments for PTC consist of surgical thyroidectomy in virtually all patients, followed by radioiodine ablation in many patients.6,7,9,10,13 The latter treatment takes advantage of the unique radioiodine avidity of thyroid cells through their ability to take up and concentrate iodide as a substrate for thyroid hormone synthesis, a process that requires the normal function of several thyroid iodide-handling genes.14 Although with these treatments patients with PTC generally have a low mortality, the disease can recur and often progress into incurable disease that is surgically inoperable and lacks radioiodine avidity. Overall, the recurrence rate of PTC is high, around 20–30% at 15–20 years.15,16,17,18 Even in PTC that is regarded as low-risk based on conventional clinicopathological criteria, including papillary thyroid microcarcinoma (PTMC), recurrence is common and even patient death may occasionally occur.19,20 Recurrent PTC is associated with increased morbidity and mortality with significant psychoeconomic consequences. It has been well documented that a diagnosis of thyroid cancer has a significant impact on the lives of patients.21,22,23,24 Recurrent PTC conceivably has a similar significant impact on the lives of patients. Therefore, while trying to reduce the mortality of PTC, an important focus of contemporary thyroid cancer medicine is to effectively prevent and reduce recurrence of PTC. Appropriately designed initial treatments are the key to preventing the recurrence of PTC while at the same time balancing the risk of treatment-associated adverse effects. After the initial treatments, patients with PTC need to be appropriately followed with various surveillance measures for disease recurrence. In this process, challenges often arise with respect to how aggressive the initial surgical and radioiodine treatments should be and how to optimize the surveillance regimens during subsequent follow-up. Consequently, appropriate risk stratification with efficient prognostication is required to optimize the management of patients with PTC. This is traditionally performed using the conventional risk stratification system based on clinicopathological criteria, including patient age and gender, tumor size, status of extrathyroidal extension and metastasis, and stages of the tumor.6,7,9,10,13 Risk stratification based on this system, however, is often unreliable and incomplete, particularly when dealing with apparently low-risk patients and when making clinical decisions preoperatively when histopathological information is not available. This unreliability is a main cause of some of the major controversies over the optimal management of PTC. It is in this context that the BRAF mutation as a novel and effective prognostic molecular marker, to be reviewed here, has important utility in helping optimize the management of PTC.11,12
BRAF mutation and aggressive pathological and molecular derangements in PTC
The BRAF mutation is a common somatic mutation in thyroid cancer, occurring exclusively in about 45% of PTC and 25% of ATC; it does not occur in FTC, medullary thyroid cancer, and benign thyroid tumors.11 Through aberrantly and constitutively activating the Ras → Raf → MAP kinase/ERK pathway (MAP kinase pathway), the resultant mutant BRAF V600E kinase is potently oncogenic.25 Recent years have seen an explosion of literature on the role of this mutation in the tumorigenesis of PTC.11,12,26 Most of these studies, from various ethnic and geographical backgrounds, demonstrated a strong association of BRAF mutation with poor clinicopathological outcomes of PTC. In particular, as reviewed previously,12 many well-designed large studies demonstrated a close association of BRAF mutation with extrathyroidal extension, lymph node metastasis, and advanced TNM stages III/IV of PTC, which are all major clinicopathological risk factors conventionally associated with increased rates of recurrence and mortality of thyroid cancer.15,16,17,18 Among the several major subtypes of PTC, BRAF mutation is most commonly associated with the aggressive tall-cell variant of PTC and least commonly with the less aggressive follicular variant PTC.11,12 This relationship between BRAF mutation and poor clinicopathological outcomes of PTC has been widely confirmed in many more recent studies.27,28,29,30,31,32,33 Even in the case of PTMC, BRAF mutation was found to be associated with aggressive pathological outcomes of the tumor, such as extrathyroidal extension, lymph node metastasis, and high TNM stages.34,35,29,36 Interestingly, BRAF mutation in metastatic PTC in lymph nodes was associated with larger size of the metastases and extra-nodal extension.35 The aggressive pathogenic role of BRAF mutation in human PTC was completely reproduced in transgenic mice in which targeted over-expression or endogenous expression of the mutant BRAF in thyroid glands promoted the development of PTC with extensive invasion and metastasis.37,38
In contrast to BRAF mutation, Ras mutations and RET/PTC rearrangements, which are also common genetic alterations in PTC, were much less commonly associated with either aggressive pathogenesis of PTC in adult patients or relevant molecular derangements,39,40,41,42,43 suggesting that although these oncogenes are, like BRAF mutation, conventionally known to be coupled to the MAP kinase pathway, they are likely less potent than BRAF mutation in activating this pathway and promoting the aggressiveness of PTC. This is consistent with the finding in transgenic mice that targeted endogenous expression of mutant BRAF induced the development of aggressive PTC associated with aberrant silencing of important thyroid genes while a mutant H-Ras did not.38 BRAF mutation is uniquely associated with the over-expression of many classical tumor-promoting molecules in PTC. Examples include VEGF,44 c-MET,41 matrix metalloproteinase,29,42,45,46 nuclear factor kappa B,46 Ki-67,27 prohibitin,47 and vimentin.48 Aberrant promoter methylation of several tumor suppressor and DNA repair genes in association with BRAF mutation was also demonstrated and associated with aggressive pathological characteristics of PTC.49,50 When the tumor suppressor gene p27-Kip1 was examined in primary PTC and matched lymph node metastases, its expression was found to be decreased in both specimens in association with BRAF mutation.35 These serious molecular derangements associated with or promoted by BRAF mutation represent some of the molecular mechanisms underlying the unique role of BRAF mutation in promoting the aggressiveness of PTC.
BRAF mutation and poor clinical outcomes of PTC
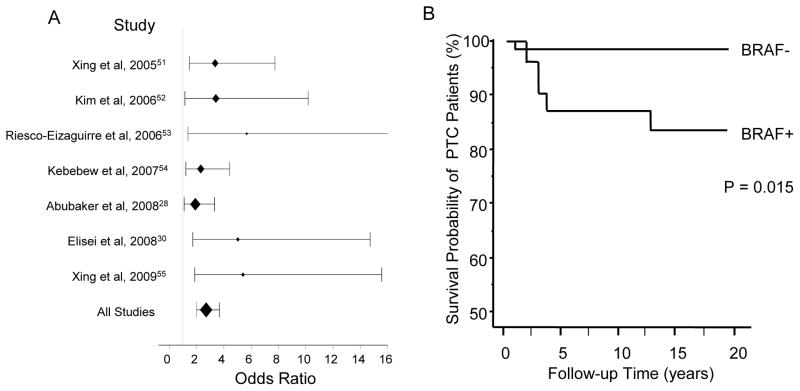
Consistent with the association of BRAF mutation with aggressive pathological and molecular derangements of PTC is the close association of BRAF mutation with PTC recurrence initially demonstrated in 2005.51 Many subsequent studies from various ethnic and geographical backgrounds from around the world confirmed this finding.28,30,52,53,54,55 These studies, with documented follow-up procedures for the patients investigated, widely showed high odds ratios for disease persistence/recurrence of PTC with BRAF mutation (Table 1 and Fig 1A), thus demonstrating the strong predictive power of BRAF mutation for the recurrence of PTC. Some of these studies also performed multivariate analyses to justify for the confounding effects of the conventional clinicopathological factors and demonstrated a strong and incremental predictive power of BRAF mutation for the recurrence of PTC.30,51,54 Interestingly, in one of these studies, it was shown that the predictive power of BRAF mutation for PTC recurrence was even higher in conventionally low-risk patients, such as those with stage I and II disease, than it was in the overall analysis on all PTC patients.51 The association of BRAF mutation in primary PTC tumors with disease recurrence is consistent with the recently reported finding of an extremely high prevalence of BRAF mutation, around 80–85%, in recurrent PTC tumors.27,31 A recent study with a long-term (15-year median) follow-up of patients investigated the role of BRAF mutation in PTC-associated mortality and demonstrated a significant association of BRAF mutation with increased mortality of PTC,30 (Fig 1B). Interestingly, PTC developed from mutant BRAF in transgenic mice was associated with a high and rapid mortality rate of animals.37
Table 1.
High predictive power of BRAF mutation for recurrence of papillary thyroid cancer [recurrent cases/total cases (%)]
| Patient Groups | BRAF Mutation (+) | BRAF Mutation (−) | Clinical Follow-up Months (median) | Odds Ratio (95% CI) | P value | References |
|---|---|---|---|---|---|---|
| American | 23/92 (25) | 9/96 (9) | 15 | 3.37 (1.47–7.74) | 0.006 | Xing et al, 200551 |
| Korean | 32/149 (21) | 4/54 (7) | 88 | 3.42 (1.15–10.18) | 0.022 | Kim et al, 200652 |
| Spanish | 9/28 (32) | 3/39 (8) | 36 | 5.68 (1.37–23.52) | 0.021 | Riesco- Eizaguirre et al, 200653 |
| American | 38/111 (34) | 18/98 (18) | 72 | 2.31 (1.21–4.41) | 0.012 | Kebebew et al, 200754 |
| Middle Eastern | 44/153 (29) | 25/143 (18) | 66 | 1.91 (1.09–3.32) | 0.027 | Abubaker et al, 200828 |
| Italian | 13/38 (28) | 6/64 (9) | 180 | 5.03 (1.72–14.73) | 0.003 | Elisei et al, 200830 |
| American | 15/40 (38) | 6/60 (10) | 24 | 5.40 (1.87–15.57) | 0.002 | Xing et al, 200955 |
| Overall | 174/611 (28) | 71/554 (13) | - | 2.71 (2.00–3.67) | < 0.001 | - |
| Predictive Probabilities | Positive: 28.5% | Negative: 87.2% | - | - | - | - |
The P values were calculated using Fisher’s exact test. The raw data were provided by Dr. Electron Kebebew and Dr. Khawla S. Al-Kuraya, through a personal communication, for references 54 and 28, respectively. The data from reference 55 included in this table has no overlap with the data in reference 51. This table is updated from reference 12.
Figure 1.
A. Association of BRAF mutation with PTC recurrence -- Odds ratios for PTC recurrence with BRAF mutation in various studies. The line of odds ratio for Riesco-Ezaguirre et al data is truncated at the value of 16. The data from reference 55 included here has no overlap with the data in reference 51. B. Association of BRAF mutation with decreased survival probability of PTC patients. Fig 1B is adapted from Elisei et al30 with permission.
Compared with BRAF mutation-negative cases, recurrent PTC with BRAF mutation required more aggressive treatments, such as surgical re-operation and external beam radiation, and showed a higher rate of incurability.51 An important cause is the BRAF mutation-associated loss of radioiodine avidity and consequent failure of PTC to respond to radioiodine ablation therapy.51,53,56 The direct underlying molecular basis is the BRAF mutation-promoted loss of the expression of thyroid iodide-handling genes in PTC, including the genes for sodium/iodide symporter (NIS), thyroid-stimulating hormone (TSH) receptor, thyroperoxidase, thyroglobulin (Tg), and pendrin.33,43,53,56,57,58 In contrast, RET/PTC and Ras mutations had little impact on the expression of thyroid iodide-handling genes in PTC.41,43 Silencing of these thyroid genes upon induced expression of the mutant BRAF was also demonstrated in in vitro thyroid cell line studies53,59 and in PTC tumors generated in transgenic mice.37,38 Mutant BRAF also caused defective transportation of NIS to the cell membrane, resulting in mislocalization of NIS in the cytoplasm, thus impairing the ability of thyroid cells to take up radioiodine.53 These are some of the important molecular mechanisms underlying BRAF mutation-promoted loss of radioiodine avidity in PTC and, hence, failure of radioiodine treatment and consequent disease recurrence.
High predictive power of BRAF mutation status for the prognosis of PTC
Given its strong association with aggressive clinicopathological outcomes and serious molecular derangements in PTC, the BRAF mutation has become a unique and valuable prognostic molecular marker in the management of PTC. As discussed above, it is the disease persistence/recurrence that is practically a primary concern in managing PTC in most patients. Conceivably, when PTC recurs, it represents the existence of cancer, which, in terms of the impact on the life of the patient, is similar to, or perhaps even more profound, than a new diagnosis of thyroid cancer.21,22,23,24 Based on the overall data in Table 1, the positive and negative predictive probabilities for BRAF mutation to predict recurrence of PTC are 28% and 87%, respectively. This positive probability of BRAF mutation to predict PTC recurrence is, in a practical sense, a significant one. In terms of the significance and potential consequence of cancer risk, this can be better appreciated if one considers the case of cytologically “indeterminate” thyroid nodules which are associated with a probability of about 15–20% for malignancy and are thus virtually always recommended for thyroidectomy.6,60 Another analogous case to consider is general thyroid nodules that have a probability of about 5% for malignancy but, even with this much lower probability, invasive fine needle aspiration biopsy (FNAB) is currently recommended for virtually all patients with thyroid nodules of a size (e.g., > 1.0 cm) suitable for biopsy.6,60 Therefore, a positive predictive probability of around 30% for BRAF mutation to predict PTC recurrence is highly relevant clinically. Similarly, a high negative predictive value of around 90% to exclude PTC recurrence is also highly relevant clinically. As will be discussed in the following sections, given these high predictive powers, BRAF mutation status may have a place particularly in some challenging areas related to optimization of the management of PTC.
BRAF mutation-assisted decision making on initial surgical management of PTC
A major, and perhaps the most commonly encountered, challenge related to initial surgical decision making for PTC is the often preoperative uncertainty about the appropriate surgical extent for a particular patient.61 For example, it is often not straightforward to decide whether total thyroidectomy (as opposed to lobectomy) and neck dissection (as opposed to no neck dissection) should be pursued. Such surgical extents are generally associated with significantly decreased recurrence and mortality rates but also pose increased risk for surgical complications, such as injuries to the recurrent laryngeal nerve and parathyroid glands.62,63,64,65,66 Recurrence of PTC occurs most commonly in neck lymph nodes, particularly in the central neck compartment, and re-operation of recurrent PTC is associated with an even further increased surgical risk.65,67,68,69 Therefore, in recent years, compartment-based neck dissection, particularly central neck dissection, usually assisted by preoperative neck ultrasonogrphy evaluation and intra-operative examination, has been increasingly performed during the primary surgery for PTC. Preoperative ultrasonography is, however, often insensitive and non-specific in identifying extrathyroidal extension of the tumor and metastasized lymph nodes in deep locations of the neck, particularly in the central neck.70,71,72,73,74 Moreover, preoperative ultrasonography has no documented role in predicting the clinical outcomes of PTC, such as recurrence. With intra-operative examination for lymph node metastases, even with careful experienced surgeons the sensitivity and specificity of this maneuver for identifying metastasized lymph nodes can be poor.75 It is therefore often a significant challenge to decide who should receive lymph node dissection, particularly when prophylactically. Given its unique prognostic power discussed above, BRAF mutation may have a special utility in helping minimize this clinical dilemma if it can be tested for preoperatively. Indeed, a recent study has provided direct evidence that BRAF mutation detected preoperatively on FNAB specimens predicted well pathological aggressiveness of PTC, such as extrathyroidal extension, lymph node metastasis, advanced TNM stages, and disease recurrence.55 In this study, the positive and negative predictive probabilities of BRAF mutation to predict PTC recurrence were tested on preoperative FNAB specimens and shown to be around 30% and 90%, respectively. This is similar to the findings with BRAF mutation analyzed in primary tumors discussed above. Therefore, use of BRAF mutation can be a novel and useful prognostic strategy to assist preoperative risk stratification and tailored surgical management of PTC.
As recently discussed,76 this BRAF mutation-assisted preoperative prognostication may be particularly useful for the management of conventionally low-risk PTC, such as PTMC or PTC with low TNM stages. It has been consistently shown that a subgroup of such apparently low-risk patients can progress with recurrence or even mortality in some cases, albeit with a lower rate than conventionally high-risk patients.19,20,77 Preoperative BRAF mutation testing may help identify this group of patients for relatively more aggressive initial surgical treatments, such as total thyroidectomy instead of lobectomy. With a positive preoperative BRAF mutation testing, prophylactic central neck dissection in the absence of apparent pathologic lymph nodes may also be favored in a right clinical setting given the highly probable mutant BRAF-driven microscopic lymph node metastasis of PTC which, as discussed above, may be insensitive to radioiodine ablation due to impaired radioiodine avidity and hence has an increased risk for future recurrence if not removed surgically. On the other hand, given the high negative predictive probability of around 90%, a negative preoperative BRAF mutation test in these patients may greatly favor less aggressive surgery, perhaps sparing prophylactic neck dissection and supporting only lobectomy in an appropriate clinical setting. Even without neck dissection, microscopic PTC metastases in this BRAF mutation-negative group of patients is expected to be highly sensitive to radioiodine and can therefore be effectively cured with radioiodine ablation after thyroidectomy. This BRAF mutation-assisted approach for preoperative surgical decision making will likely significantly reduce the recurrence rate of PTC since compartment-based removal of neck lymph nodes in appropriate patients is effective in preventing the recurrence of PTC. At the same time, this approach will likely also reduce surgical complications since fewer patients would need to be aggressively surgically treated. This is because only about one third of cases of PTMC or low-stage PTC harbor BRAF mutation12,76 and, in some series, as low as 18–24% of PTMC harbored BRAF mutation.29,78 Thus, the prevalence of BRAF mutation is much lower in the low-risk PTC patients compared with the overall prevalence of 45% of this mutation in PTC in general and the majority of low-risk patients could consequently be spared from “promiscuous” prophylactic neck dissection. Conventional clinicopathological factors and the technical quality of the surgical service are also important to consider in this BRAF mutation-guided surgical decision making to optimize the balance of the risk of PTC recurrence and the risk of surgical complications.
BRAF mutation-assisted decision making on initial radioiodine treatment of PTC
Radioiodine ablation following total or near-total thyroidectomy is a standard medical treatment for PTC in many patients in the USA and many other countries.6,7,9,10,13 The benefit of radioiodine treatment in preventing recurrence and mortality of thyroid cancer has been generally demonstrated for conventionally high-risk patients but inconsistently in low-risk patients79,80,81 Radioiodine treatment may be associated with adverse effects, including the risk for the development of a second primary cancer.82,83 While the overall therapeutic benefit of radioiodine treatment in the conventionally low-risk patients, is still debatable, it may be helpful to use BRAF mutation status to assist the decision making in choosing this treatment. As discussed above, the apparently low-risk initial status of a subgroup of PTC patients can give way to aggressive progression with recurrence and even mortality. Since patients who fall into this group usually harbor BRAF mutation, use of BRAF mutation may well help identify them for special management, such as radioiodine treatment. This may particularly apply to the case of PTMC. There has been no general agreement on whether and how to treat PTMC with radioiodine.19,84 This is because, as large meta analyses showed,20,77 it is not possible to discriminate on the basis of clinicopathological criteria between the aggressive and indolent cases of PTMC. Given the strong association of BRAF mutation with the poorer clinicopathological outcomes in PTMC or low-stage PTC,29,34,35,36,51 it is reasonable to propose that at least BRAF mutation-positive PTMC be considered for radioiodine treatment. Since this group of patients account for the minority of the low-risk patients, it is practically feasible to treat them. Although whether radioiodine treatment can reduce disease recurrence in these patients would need studies to directly test, at this time it may be at least advisable not to spare this group of patients from radioiodine treatment given the known aggressive role of BRAF mutation. Moreover, radioiodine ablation of residual thyroid tissues will improve the specificity of surveillance testing for recurrence using serum thyroglobulin and radioiodine body scan that is likely to be more commonly needed for these BRAF mutation-positive patients who may require a more vigilant follow-up surveillance for their increased risk of recurrence.
Given the impairment of radioiodine avidity of PTC associated with BRAF mutation, a relatively high dose of radioiodine, perhaps 75 mCi or higher, may be reasonable for the conventionally apparently low-risk but BRAF mutation-positive patients. For the conventionally low-risk and BRAF mutation-negative patients, there is not enough data at this time to support the use of BRAF mutation status in deciding whether to treat them with radioiodine. For this group of patients, it is advisable that radioiodine treatment continue to be guided by the conventional risk factors as done in current practice, with perhaps a higher threshold in initiating the treatment in a right clinical setting. If radioiodine treatment is determined to be the option for these patients, it may be reasonable to use a relatively low dose of radioiodine, perhaps 30 mCi, since BRAF mutation-negative PTC in these patients is expected to be highly sensitive to radioiodine ablation and this dose is generally effective in ablating residual normal and cancerous thyroid tissues in low-risk patients.
For PTC patients with conventionally high risk, such as stage III or IV disease, BRAF mutation status may not have a significant role at this time in altering the current approach of determining the need for radioiodine treatment as its benefit in reducing PTC recurrence and mortality in these patients has been well demonstrated.79,80 However, it remains to be investigated whether BRAF mutation-positive and -negative patients in this high-risk group benefit differentially from radioiodine treatment and whether different doses of radioiodine should be administered to them since BRAF mutation status affects radioiodine sensitivity. The answer to this important question will help optimize the balance between the benefits and harm of radioiodine treatments. Empirically, perhaps a dose of 100 mCi can be generally considered for BRAF mutation-negative cases and a dose of 150 mCi or higher for BRAF mutation-positive cases in this conventionally high-risk group of PTC patients.
BRAF mutation-assisted decision making in follow-up of patients with PTC
After the initial surgical and radioiodine treatments, patients with PTC are clinically observed and managed with various standard procedures. Among these, TSH suppression therapy is commonly used as an effort to reduce recurrence and mortality rates.6,7,9,10,13 Like radioiodine therapy for thyroid cancer, the benefit of this practice has been generally shown in high-risk patients but inconclusive in low-risk patients.79,85,86 Low TSH level is achieved by administering a supraphysiologic dose of thyroxine to the patient. It is a general practice to maintain a degree of TSH suppression commensurate with the risk level of the disease in a specific patient. For example, per the 2006 American Thyroid Association guideline,6 TSH should be generally maintained at undetectable levels for high-risk patients and low subnormal range for low-risk patients. The intent of this approach is to optimize the balance between the benefit of improving clinical outcomes of thyroid cancer and minimizing the adverse effects of iatrogenic hyperthyroidism. In this approach, the inherent deficiency of the clinicopathological criteria used in risk stratification, as discussed above, can be problematic, particularly in apparently low-risk patients. Here again, given the BRAF mutation as a strong risk factor for aggressiveness and progression of PTC, even in conventionally low-risk patients, use of BRAF mutation status may help more appropriately target the TSH levels. For instance, one consideration could be that, for PTMC or low-risk PTC, BRAF mutation-negative patients be generally maintained at TSH levels in the low normal range and BRAF mutation-positive patients be generally maintained at TSH levels in low subnormal ranges. The TSH level in high-risk patients, such as those with stage III or IV disease, may be targeted at the undetectable level regardless of the BRAF mutation status as conventionally recommended until further studies become available.
The level of surveillance for PTC recurrence during clinical follow-up in the current practice may also be tailored based on the BRAF mutation status. Principally, BRAF mutation-positive patients may generally need to be more frequently followed and more vigilantly surveyed, with a lower threshold, for instance, in choosing to use testing modalities, such as TSH-stimulated serum Tg testing, radioiodine body scan, and positron emission tomography (PET) scan. Given the high rate of loss of radioiodine avidity in recurrent PTC with BRAF mutation, it may be reasonable to weigh more heavily toward PET scans than toward radioiodine body scans in deciding the diagnostic imaging modalities in a BRAF mutation-positive patient in a right clinical setting. In contrast, in a BRAF mutation-negative PTC patient, after the initial total thyroidectomy and radioiodine ablation, less aggressive testing modalities can be generally pursued. For example, neck ultrasonography and TSH-stimulated serum Tg testing, or perhaps just a Tg testing alone, may be sufficient for most patients and negative results of these tests can be more reliably used to demonstrate a cure of the disease. Again, it should be noted that BRAF mutation may have the best prognostic utility in the management of PTC when applied in conjunction with the use of conventional clinicopathological risk factors.
Concluding Remarks
Compelling data are now available that demonstrate the high and specific prognostic power of BRAF mutation in PTC. This is well reflected by its strong predictive value for the pathological aggressiveness, recurrence, and even mortality of PTC. There are well-documented molecular bases to support the unique aggressive role of BRAF mutation in PTC tumorigenesis and hence its prognostic value, including the mutation-promoted over-expression of tumor-promoting molecules, suppression of tumor suppressor genes, and silencing of iodide-handling genes with impaired radioiodine avidity in PTC. The prognostic value of BRAF mutation may have great utility in many clinical areas of PTC, such as tailoring appropriate surgical and radioiodine treatments, particularly for conventionally low-risk patients, and determining appropriate management during patient follow-up. The ability to use BRAF-mutation testing on thyroid FNAB specimens to preoperatively predict clinicopathological outcomes of PTC makes it possible and ideal to use this molecular marker at an early stage to assist decision making for PTC. Although specific indications and strategies using BRAF mutation for the management of PTC need to be defined, it is expected that the prognostic use of this remarkable molecular marker will add a new and effective dimension to the current risk stratification system of PTC and, hence, may have a significant impact on the practice of contemporary thyroid cancer medicine.
Acknowledgments
Funding Support: This work is supported by NIH grant RO1-CA113507 to the author.
Footnotes
Interest of Conflict: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hundahl SA, Fleming ID, Fremgen AM, Menck HR. A national cancer data base report on 53,856 cases of thyroid carcinoma treated in the U.S. 1985–1995. Cancer. 1998;83:2638–48. doi: 10.1002/(sici)1097-0142(19981215)83:12<2638::aid-cncr31>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 2.Leenhardt L, Grosclaude P, Cherie-Challine L. Increased incidence of thyroid carcinoma in France: a true epidemic or thyroid nodule management effects? Report from the French thyroid cancer committee. Thyroid. 2004;14:1056–60. doi: 10.1089/thy.2004.14.1056. [DOI] [PubMed] [Google Scholar]
- 3.Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA. 2006;295:2164–7. doi: 10.1001/jama.295.18.2164. [DOI] [PubMed] [Google Scholar]
- 4.Sprague BL, Warren Andersen S, Trentham-Dietz A. Thyroid cancer incidence and socioeconomic indicators of health care access. Cancer Causes Control. 2008;19:585–93. doi: 10.1007/s10552-008-9122-0. [DOI] [PubMed] [Google Scholar]
- 5.Horner MJ, Ries LAG, Krapcho M, Neyman N, Aminou R, Howlader N, Altekruse SF, Feuer EJ, Huang L, Mariotto A, Miller BA, Lewis DR, Eisner MP, Stinchcomb DG, Edwards BK. SEER Cancer Statistics Review. National Cancer Institute; Bethesda, MD: 1975–2006. [Accessed September 16, 2009]. http://seer.cancer.gov/csr/1975_2006/, based on November 2008 SEER data submission, posted to the SEER web site, 2009. at http://seer.cancer.gov/csr/1975_2006/) [Google Scholar]
- 6.Cooper DS, Doherty GM, Haugen BR, et al. The American Thyroid Association Guidelines Taskforce. Management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2006;16:109–42. doi: 10.1089/thy.2006.16.109. [DOI] [PubMed] [Google Scholar]
- 7.Pacini F, Schlumberger M, Dralle H, Elisei R, Smit JW, Wiersinga W. European Consensus for the management of patients with differentiated thyroid carcinoma of the follicular epithelium. European Journal of Endocrinology. 2006;154:787–803. doi: 10.1530/eje.1.02158. [DOI] [PubMed] [Google Scholar]
- 8.Sundram F, Robinson BG, Kung A, et al. Well-differentiated epithelial thyroid cancer management in the Asia Pacific region: a report and clinical practice guideline. Thyroid. 2006;16:461–9. doi: 10.1089/thy.2006.16.461. [DOI] [PubMed] [Google Scholar]
- 9.Sherman SI, Angelos P, Ball DW, et al. National Comprehensive Cancer Network Thyroid Carcinoma Panel. Thyroid carcinoma. J Natl Compr Canc Netw. 2007;5:568–621. doi: 10.6004/jnccn.2007.0052. [DOI] [PubMed] [Google Scholar]
- 10.Pacini F, Castagna MG, Brilli L, Jost L ESMO Guidelines Working Group. Ann Oncol. Suppl 2. Vol. 19. 2008. Differentiated thyroid cancer: ESMO clinical recommendations for diagnosis, treatment and follow-up; pp. ii99–101. [DOI] [PubMed] [Google Scholar]
- 11.Xing M. BRAF mutation in thyroid cancer. Endocr Relat Cancer. 2005;12:245–62. doi: 10.1677/erc.1.0978. [DOI] [PubMed] [Google Scholar]
- 12.Xing M. BRAF mutation in papillary thyroid cancer: pathogenic role, molecular bases, and clinical implications. Endocr Rev. 2007;28:742–62. doi: 10.1210/er.2007-0007. [DOI] [PubMed] [Google Scholar]
- 13.Mazzaferri EL. An overview of the management of thyroid cancer. In: Mazzaferri EL, Harmer C, Mallick UK, Kendall-Taylor P, editors. Practical Management of Thyroid Cancer: A Multidisciplinary Approach. London, England: Springer-Verlag; 2006. pp. 1–28. [Google Scholar]
- 14.Nilsson M. Iodide handling by the thyroid epithelial cell. Exp Clin Endocrinol Diabetes. 2001;109:13–17. doi: 10.1055/s-2001-11014. [DOI] [PubMed] [Google Scholar]
- 15.Hay ID, Bergstralh EJ, Goellner JR, Ebersold JR, Grant CS. Predicting outcome in papillary thyroid carcinoma: development of a reliable prognostic scoring system in a cohort of 1779 patients surgically treated at one institution during 1940 through 1989. Surgery. 1993;114:1050–57. [PubMed] [Google Scholar]
- 16.Mazzaferri EL, Jhiang SM. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med. 1994;97:418–28. doi: 10.1016/0002-9343(94)90321-2. [DOI] [PubMed] [Google Scholar]
- 17.Fonseca E, Soares P, Rossi S, Sobrinho-Simoes M. Prognostic factors in thyroid carcinomas. Verh Dtsch Ges Pathol. 1997;81:82–96. [PubMed] [Google Scholar]
- 18.Gilliland FD, Hunt WC, Morris DM, Key CR. Prognostic factors for thyroid carcinoma. A population-based study of 15,698 cases from the Surveillance, Epidemiology and End Results (SEER) program 1973–1991. Cancer. 1997;79:564–73. doi: 10.1002/(sici)1097-0142(19970201)79:3<564::aid-cncr20>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 19.Mazzaferri EL. Management of low-risk differentiated thyroid cancer. Endocr Pract. 2007;13:498–512. doi: 10.4158/EP.13.5.498. [DOI] [PubMed] [Google Scholar]
- 20.Roti E, degli Uberti EC, Bondanelli M, Braverman LE. Thyroid papillary microcarcinoma: a descriptive and meta-analysis study. Eur J Endocrinol. 2008;159:659–73. doi: 10.1530/EJE-07-0896. [DOI] [PubMed] [Google Scholar]
- 21.Giusti M, Sibilla F, Cappi C, et al. A case-controlled study on the quality of life in a cohort of patients with a history of differentiated thyroid carcinoma. J Endocrinol Invest. 2005;28:599–608. doi: 10.1007/BF03347258. [DOI] [PubMed] [Google Scholar]
- 22.Tan LG, Nan L, Thumboo J, Sundram F, Tan LK. Health-related quality of life in thyroid cancer survivors. Laryngoscope. 2007;117:507–10. doi: 10.1097/MLG.0b013e31802e3739. [DOI] [PubMed] [Google Scholar]
- 23.Hoftijzer HC, Heemstra KA, Corssmit EP, van der Klaauw AA, Romijn JA, Smit JW. Quality of life in cured patients with differentiated thyroid carcinoma. J Clin Endocrinol Metab. 2008;93:200–3. doi: 10.1210/jc.2007-1203. [DOI] [PubMed] [Google Scholar]
- 24.Sawka AM, Goldstein DP, Brierley JD, et al. The impact of thyroid cancer and post-surgical radioactive iodine treatment on the lives of thyroid cancer survivors: a qualitative study. PLoS ONE. 2009;4:e4191. doi: 10.1371/journal.pone.0004191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–54. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 26.Knauf JA, Fagin JA. Role of MAPK pathway oncoproteins in thyroid cancer pathogenesis and as drug targets. Curr Opin Cell Biol. 2009 Feb 19; doi: 10.1016/j.ceb.2009.01.013. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 27.Nakayama H, Yoshida A, Nakamura Y, et al. Clinical significance of BRAF (V600E) mutation and Ki-67 labeling index in papillary thyroid carcinomas. Anticancer Res. 2007;27:3645–9. [PubMed] [Google Scholar]
- 28.Abubaker J, Jehan Z, Bavi P, et al. Clinicopathological analysis of papillary thyroid cancer with PIK3CA alterations in a Middle Eastern population. J Clin Endocrinol Metab. 2008;93:611–8. doi: 10.1210/jc.2007-1717. [DOI] [PubMed] [Google Scholar]
- 29.Frasca F, Nucera C, Pellegriti G, et al. BRAF(V600E) mutation and the biology of papillary thyroid cancer. Endocr Relat Cancer. 2008;15:191–205. doi: 10.1677/ERC-07-0212. [DOI] [PubMed] [Google Scholar]
- 30.Elisei R, Ugolini C, Viola D, et al. BRAFV600E mutation and outcome of patients with papillary thyroid carcinoma: A 15-year median follow-up study. J Clin Endocrinol Metab. 2008;93:3943–9. doi: 10.1210/jc.2008-0607. [DOI] [PubMed] [Google Scholar]
- 31.Henderson YC, Shellenberger TD, Williams MD, et al. High rate of BRAF and RET/PTC dual mutations associated with recurrent papillary thyroid carcinoma. Clin Cancer Res. 2009;15:485–91. doi: 10.1158/1078-0432.CCR-08-0933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim SK, Song KH, Lim SD, et al. Clinical and pathological features and the BRAF(V600E) mutation in patients with papillary thyroid carcinoma with and without concurrent hashimoto thyroiditis. Thyroid. 2009;19:137–41. doi: 10.1089/thy.2008.0144. [DOI] [PubMed] [Google Scholar]
- 33.Oler G, Cerutti JM. High prevalence of BRAF mutation in a Brazilian cohort of patients with sporadic papillary thyroid carcinomas: correlation with more aggressive phenotype and decreased expression of iodide-metabolizing genes. Cancer. 2009;115:972–80. doi: 10.1002/cncr.24118. [DOI] [PubMed] [Google Scholar]
- 34.Lupi C, Giannini R, Ugolini C, et al. Association of BRAF V600E mutation with poor clinicopathological outcomes in 500 consecutive cases of papillary thyroid carcinoma. J Clin Endocrinol Metab. 2007;92:4085–90. doi: 10.1210/jc.2007-1179. [DOI] [PubMed] [Google Scholar]
- 35.Rodolico V, Cabibi D, Pizzolanti G, et al. BRAF V600E mutation and p27 kip1 expression in papillary carcinomas of the thyroid <or=1 cm and their paired lymph node metastases. Cancer. 2007;110:1218–26. doi: 10.1002/cncr.22912. [DOI] [PubMed] [Google Scholar]
- 36.Lee X, Gao M, Ji Y, et al. Analysis of differential BRAF(V600E) mutational status in high aggressive papillary thyroid microcarcinoma. Ann Surg Oncol. 2009;16:240–5. doi: 10.1245/s10434-008-0233-3. [DOI] [PubMed] [Google Scholar]
- 37.Knauf JA, Ma X, Smith EP, et al. Targeted expression of BRAFV600E in thyroid cells of transgenic mice results in papillary thyroid cancers that undergo dedifferentiation. Cancer Res. 2005;65:4238–45. doi: 10.1158/0008-5472.CAN-05-0047. [DOI] [PubMed] [Google Scholar]
- 38.Malguarnera R, Chakravarty D, Franco A, et al. Modeling BRAF-induced thyroid tumorigenesis in mice: evidence of a causal role of oncogenic BRAF activation on loss of thyroid differentiated function in vivo. Thyroid. 2008;18(s1):S–71. [Google Scholar]
- 39.Adeniran AJ, Zhu Z, Gandhi M, et al. Correlation between genetic alterations and microscopic features, clinical manifestations, and prognostic characteristics of thyroid papillary carcinomas. Am J Surg Pathol. 2006;30:216–22. doi: 10.1097/01.pas.0000176432.73455.1b. [DOI] [PubMed] [Google Scholar]
- 40.Mitsutake N, Knauf JA, Mitsutake S, Mesa C, Jr, Zhang L, Fagin JA. Conditional BRAFV600E expression induces DNA synthesis, apoptosis, dedifferentiation, and chromosomal instability in thyroid PCCL3 cells. Cancer Res. 2005;65:2465–73. doi: 10.1158/0008-5472.CAN-04-3314. [DOI] [PubMed] [Google Scholar]
- 41.Giordano TJ, Kuick R, Thomas DG, et al. Molecular classification of papillary thyroid carcinoma: distinct BRAF, RAS, and RET/PTC mutation-specific gene expression profiles discovered by DNA microarray analysis. Oncogene. 2005;24:6646–56. doi: 10.1038/sj.onc.1208822. [DOI] [PubMed] [Google Scholar]
- 42.Mesa C, Jr, Mirza M, Mitsutake N, et al. Conditional activation of RET/PTC3 and BRAFV600E in thyroid cells is associated with gene expression profiles that predict a preferential role of BRAF in extracellular matrix remodeling. Cancer Res. 2006;66:6521–9. doi: 10.1158/0008-5472.CAN-06-0739. [DOI] [PubMed] [Google Scholar]
- 43.Romei C, Ciampi R, Faviana P, Agate, et al. BRAFV600E mutation, but not RET/PTC rearrangements, is correlated with a lower expression of both thyroperoxidase and sodium iodide symporter genes in papillary thyroid cancer. Endocr Relat Cancer. 2008;15:511–20. doi: 10.1677/ERC-07-0130. [DOI] [PubMed] [Google Scholar]
- 44.Jo YS, Li S, Song JH, et al. Influence of the BRAF V600E mutation on expression of vascular endothelial growth factor in papillary thyroid cancer. J Clin Endocrinol Metab. 2006;91:3667–70. doi: 10.1210/jc.2005-2836. [DOI] [PubMed] [Google Scholar]
- 45.Melillo RM, Castellone MD, Guarino V, et al. The RET/PTC-RAS-BRAF linear signaling cascade mediates the motile and mitogenic phenotype of thyroid cancer cells. J Clin Invest. 2005;115:1068–81. doi: 10.1172/JCI22758. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 46.Palona I, Namba H, Mitsutake N, et al. BRAFV600E promotes invasiveness of thyroid cancer cells through nuclear factor kappaB activation. Endocrinology. 2006;147:5699–707. doi: 10.1210/en.2006-0400. [DOI] [PubMed] [Google Scholar]
- 47.Franzoni A, Dima M, D’Agostino M, et al. Prohibitin Is Overexpressed in Papillary Thyroid Carcinomas Bearing the BRAF(V600E) Mutation. Thyroid. 2009 Feb 10; doi: 10.1089/thy.2008.0235. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 48.Watanabe R, Hayashi Y, Sassa M, et al. Possible Involvement of BRAFV600E in Altered Gene Expression in Papillary Thyroid Cancer. Endocr J. 2009 Feb 4; doi: 10.1507/endocrj.k08e-329. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 49.Hu S, Liu D, Tufano RP, et al. Association of aberrant methylation of tumor suppressor genes with tumor aggressiveness and BRAF mutation in papillary thyroid cancer. Int J Cancer. 2006;119:2322–9. doi: 10.1002/ijc.22110. [DOI] [PubMed] [Google Scholar]
- 50.Guan H, Ji M, Hou P, et al. Hypermethylation of the DNA mismatch repair gene hMLH1 and its association with lymph node metastasis and T1799A BRAF mutation in patients with papillary thyroid cancer. Cancer. 2008;113:247–55. doi: 10.1002/cncr.23548. [DOI] [PubMed] [Google Scholar]
- 51.Xing M, Westra WH, Tufano RP, et al. BRAF mutation predicts a poorer clinical prognosis for papillary thyroid cancer. J Clin Endocrinol Metab. 2005;90:6373–9. doi: 10.1210/jc.2005-0987. [DOI] [PubMed] [Google Scholar]
- 52.Kim TY, Kim WB, Rhee YS, et al. The BRAF mutation is useful for prediction of clinical recurrence in low-risk patients with conventional papillary thyroid carcinoma. Clin Endocrinol (Oxf) 2006;65:364–8. doi: 10.1111/j.1365-2265.2006.02605.x. [DOI] [PubMed] [Google Scholar]
- 53.Riesco-Eizaguirre G, Gutierrez-Martinez P, Garcia-Cabezas MA, Nistal M, Santisteban P. The oncogene BRAF V600E is associated with a high risk of recurrence and less differentiated papillary thyroid carcinoma due to the impairment of Na+/I- targeting to the membrane. Endocr Relat Cancer. 2006;131:257–69. doi: 10.1677/erc.1.01119. [DOI] [PubMed] [Google Scholar]
- 54.Kebebew E, Weng J, Bauer J, et al. The prevalence and prognostic value of BRAF mutation in thyroid cancer. Ann Surg. 2007;246:466–70. doi: 10.1097/SLA.0b013e318148563d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xing M, Clark DP, Guan H, et al. BRAF Mutation Testing of Thyroid Fine Needle Aspiration Biopsy Specimens for Preoperative Risk Stratification in Papillary Thyroid Cancer. J Clin Oncol. 2009 doi: 10.1200/JCO.2008.20.1426. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mian C, Barollo S, Pennelli G, et al. Molecular characteristics in papillary thyroid cancers (PTCs) with no (131) I uptake. Clin Endocrinol (Oxf) 2008;681:108–16. doi: 10.1111/j.1365-2265.2007.03008.x. [DOI] [PubMed] [Google Scholar]
- 57.Durante C, Puxeddu E, Ferretti E, et al. BRAF mutations in papillary thyroid carcinomas inhibit genes involved in iodine metabolism. J Clin Endocrinol Metab. 2007;927:2840–43. doi: 10.1210/jc.2006-2707. [DOI] [PubMed] [Google Scholar]
- 58.Espadinha C, Santos JR, Sobrinho LG, Bugalho MJ. Expression of iodine metabolism genes in human thyroid tissues: evidence for age and BRAF(V600E) mutation dependency. Clin Endocrinol (Oxf) 2009;70:629–34. doi: 10.1111/j.1365-2265.2008.03376.x. [DOI] [PubMed] [Google Scholar]
- 59.Liu D, Hu S, Hou P, Jiang D, Condouris S, Xing M. Suppression of BRAF/MEK/MAP kinase pathway restores expression of iodide-metabolizing genes in thyroid cells expressing the V600E BRAF mutant. Clin Cancer Res. 2007;13:1341–9. doi: 10.1158/1078-0432.CCR-06-1753. [DOI] [PubMed] [Google Scholar]
- 60.Gharib H, Papini E. Thyroid nodules: clinical importance, assessment, and treatment. Endocrinol Metab Clin North Am. 2007;36:707–35. doi: 10.1016/j.ecl.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 61.Witt RL. Initial surgical management of thyroid cancer. Surg Oncol Clin N Am. 2008;17:71–91. doi: 10.1016/j.soc.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 62.Hay ID, Grant CS, Taylor WF, McConahey WM. Ipsilateral lobectomy versus bilateral lobar resection in papillary thyroid carcinoma: a retrospective analysis of surgical outcome using a novel prognostic scoring system. Surgery. 1987;102:1088–95. [PubMed] [Google Scholar]
- 63.Scheumann GF, Gimm O, Wegener G, Hundeshagen H, Dralle H. Prognostic significance and surgical management of locoregional lymph node metastases in papillary thyroid cancer. World J Surg. 1994;18:559–67. doi: 10.1007/BF00353765. [DOI] [PubMed] [Google Scholar]
- 64.Sywak M, Cornford L, Roach P, Stalberg P, Sidhu S, Delbridge L. Routine ipsilateral level VI lymphadenectomy reduces postoperative thyroglobulin levels in papillary thyroid cancer. Surgery. 2006;140:1000–5. doi: 10.1016/j.surg.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 65.White ML, Gauger PG, Doherty GM. Central lymph node dissection in differentiated thyroid cancer. World J Surg. 2007;31:895–904. doi: 10.1007/s00268-006-0907-6. [DOI] [PubMed] [Google Scholar]
- 66.Bilimoria KY, Bentrem DJ, Ko CY, et al. Extent of surgery affects survival for papillary thyroid cancer. Ann Surg. 2007;246:375–81. doi: 10.1097/SLA.0b013e31814697d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wilson DB, Staren ED, Prinz RA. Thyroid reoperations: indications and risks. Am Surg. 1998;64:674–678. [PubMed] [Google Scholar]
- 68.Reddy RM, Grigsby PW, Moley JF, Hall BL. Lymph node metastases in differentiated thyroid cancer under 2 cm. Surgery. 2006;140:1050–4. doi: 10.1016/j.surg.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 69.Schuff KG, Weber SM, Givi B, Samuels MH, Andersen PE, Cohen JI. Efficacy of nodal dissection for treatment of persistent/recurrent papillary thyroid cancer. Laryngoscope. 2008;118:768–75. doi: 10.1097/MLG.0b013e318162cae9. [DOI] [PubMed] [Google Scholar]
- 70.Shimamoto K, Satake H, Sawaki A, Ishigaki T, Funahashi H, Imai T. Preoperative staging of thyroid papillary carcinoma with ultrasonography. Eur J Radiol. 1998;29:4–10. doi: 10.1016/s0720-048x(97)00184-8. [DOI] [PubMed] [Google Scholar]
- 71.Kouvaraki MA, Shapiro SE, Fornage BD, et al. Role of preoperative ultrasonography in the surgical management of patients with thyroid cancer. Surgery. 2003;134:946–54. doi: 10.1016/s0039-6060(03)00424-0. [DOI] [PubMed] [Google Scholar]
- 72.Stulak JM, Grant CS, Farley DR, et al. Value of preoperative ultrasonography in the surgical management of initial and reoperative papillary thyroid cancer. Arch Surg. 2006;141:489–94. doi: 10.1001/archsurg.141.5.489. [DOI] [PubMed] [Google Scholar]
- 73.Ito Y, Tomoda C, Uruno T, et al. Clinical significance of metastasis to the central compartment from papillarymicrocarcinoma of the thyroid. World J Surg. 2006;30:91–9. doi: 10.1007/s00268-005-0113-y. [DOI] [PubMed] [Google Scholar]
- 74.Bonnet S, Hartl D, Leboulleux S, et al. Prophylactic Lymph Node Dissection for Papillary Thyroid Cancer Less than 2 cm: Implications for Radioiodine Treatment. J Clin Endocrinol Metab. 2008 Dec 30; doi: 10.1210/jc.2008-1931. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 75.Moley JF, DeBenedetti MK. Patterns of nodal metastases in palpable medullary thyroid carcinoma: recommendations for extent of node dissection. Ann Surg. 1999;229:880–7. doi: 10.1097/00000658-199906000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xing M. BRAF Mutation in Papillary Thyroid Microcarcinoma: The Promise of Better Risk Management. Ann Surg Oncol. 2009;16:801–03. doi: 10.1245/s10434-008-0298-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pazaitou-Panayiotou K, Capezzone M, Pacini F. Clinical features and therapeutic implication of papillary thyroid microcarcinoma. Thyroid. 2007;17:1085–92. doi: 10.1089/thy.2007.0005. [DOI] [PubMed] [Google Scholar]
- 78.Ugolini C, Giannini R, Lupi C, et al. Presence of BRAF V600E in very early stages of papillary thyroid carcinoma. Thyroid. 2007;17:381–8. doi: 10.1089/thy.2006.0305. [DOI] [PubMed] [Google Scholar]
- 79.Chow S-M, Law SKW, Chan JKC, Au S-K, Yau S, Lau W-H. Papillary microcarcinoma of the thyroid: prognostic significance of lymph node metastases and multifocality. Cancer. 2003;98:31–40. doi: 10.1002/cncr.11442. [DOI] [PubMed] [Google Scholar]
- 80.Jonklaas J, Sarlis NJ, Litofsky D, et al. Outcomes of patients with differentiated thyroid carcinoma following initial therapy. Thyroid. 2006;16:1229–42. doi: 10.1089/thy.2006.16.1229. [DOI] [PubMed] [Google Scholar]
- 81.Sawka AM, Brierley JD, Tsang RW, et al. An updated systematic review and commentary examining the effectiveness of radioactive iodine remnant ablation in well-differentiated thyroid cancer. Endocrinol Metab Clin North Am. 2008;37:457–80. doi: 10.1016/j.ecl.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 82.Rubino C, de Vathaire F, Dottorini ME, et al. Second primary malignancies in thyroid cancer patients. Br J Cancer. 2003;89:1638–44. doi: 10.1038/sj.bjc.6601319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Brown AP, Chen J, Hitchcock YJ, Szabo A, Shrieve DC, Tward JD. The risk of second primary malignancies up to three decades after the treatment of differentiated thyroid cancer. J Clin Endocrinol Metab. 2008;93:504–15. doi: 10.1210/jc.2007-1154. [DOI] [PubMed] [Google Scholar]
- 84.Hay ID. Management of patients with low-risk papillary thyroid carcinoma. Endocr Pract. 2007;13:521–33. doi: 10.4158/EP.13.5.521. [DOI] [PubMed] [Google Scholar]
- 85.Cooper DS, Specker B, Ho M, et al. Thyrotropin suppression and disease progression in patients with differentiated thyroid cancer: results from the National Thyroid Cancer Treatment Cooperative Registry. Thyroid. 1998;8:737–44. doi: 10.1089/thy.1998.8.737. [DOI] [PubMed] [Google Scholar]
- 86.McGriff NJ, Csako G, Gourgiotis L, Lori CG, Pucino F, Sarlis NJ. Effects of thyroid hormone suppression therapy on adverse clinical outcomes in thyroid cancer. Ann Med. 2002;34:554–64. doi: 10.1080/078538902321117760. [DOI] [PubMed] [Google Scholar]