Abstract
The homing and migration of IgA antibody secreting cells (ASC) to the lactating mammary gland is essential to the passive transfer of immunity from mother to nursing neonate. Antibody secreting cells, located within the lactating mammary gland, produce high levels of antigen specific IgA antibodies. These antibodies, which are subsequently transferred to the nursing neonate via breast milk, provide passive immune protection against antigens previously encountered by the mother to the nursing infant. The efficient homing and accumulation of lymphocytes is highly dependent on cellular adhesion molecules expressed on the vascular endothelium and their integrin ligands. Vasculature within the lactating mammary gland is known to express several adhesion molecules, including VCAM-1 and MAdCAM-1. However, the role of these molecules in vivo has not been previously described. Here we show that α4 integrins and VCAM-1 play essential roles in mediating the accumulation of IgA ASCs to the lactating mammary gland. Conversely, neither MAdCAM-1 nor its major ligand α4β7 are required for efficient IgA ASC accumulation to this tissue.
Keywords: Mammary gland, IgA, VCAM-1, MAdCAM-1
1. Introduction
In mammals, milk from the mammary gland provides passive immune protection to immunologically naïve offspring via maternally secreted IgA antibody (Holmgren et al., 1976; Stoliar et al., 1976). This antibody is ingested by the neonate and serves to neutralize antigens previously encountered by the mother (Hanson et al., 2003). The presence of secretory IgA in milk has been shown to be chiefly due to the migration of IgA ASCs into the lactating mammary gland, as opposed to diffusion of serum IgA into the milk (Halsey et al., 1983). During pregnancy and lactation a large influx of T and B lymphocytes accumulate in the mammary gland (Hanson et al., 2003; Weisz-Carrington et al., 1977). This infiltration of lymphocytes to the mammary gland represents a unique model of induced lymphocyte migration in the absence of inflammation and provides a useful model to study the accumulation of IgA ASC to extraintestinal mucosal tissues.
Efficient pathogen clearance is highly dependent on the migration and accumulation of lymphocytes in specific tissues. Accordingly, it is essential that IgA ASCs accumulate at appropriate mucosal sites. The migration of leukocytes has been proposed to be mediated via a multi-step model in which circulating lymphocytes roll, tether, and firmly adhere to the vascular endothelium before extravasating into target tissues (Butcher and Picker, 1996). For this migratory process to occur, lymphocytes must express the appropriate adhesion molecule ligands as well as chemokine receptors. Interaction with the appropriate chemokines then upregulates integrin affinity, facilitating firm adhesion of the lymphocyte to the vessel wall as the lymphocyte-expressed integrins bind vascular-expressed cellular adhesion molecules. The successful completion of these steps ultimately results in the migration of the lymphocyte into the target tissue (Butcher and Picker, 1996; Jalkanen et al., 1986; Kunkel and Butcher, 2002; Kunkel and Butcher, 2003).
The adhesion molecule profile of a lymphocyte provides strong indications as to where it will localize. However, expression of a given adhesion molecule does not establish its role in trafficking to a specific tissue. A given lymphocyte may express a variety of chemokine receptors and adhesion molecule ligands. Definitively determining the adhesion associated molecules utilized in homing to a given tissue can only be accomplished through in vivo experimentation. Previous research has shown that IgA ASCs in the lactating mammary gland commonly express α4β7 and α4β1 integrins, and mammary gland vasculature expresses both VCAM-1 and MAdCAM-1 adhesion molecules (Bourges et al., 2008; Tanneau et al., 1999; van der Feltz et al., 2001). These findings suggest that one or both of these integrin pairs and vascular adhesion molecules are essential for efficient migration and accumulation of IgA ASC to this tissue. However, the in vivo role of these integrins and adhesion molecules in mediating the migration of IgA ASC into the lactating mammary gland has not been previously demonstrated.
Elucidating the molecular mechanisms mediating lymphocyte migration to mucosal sites is fundamental to targeting vaccine responses to appropriate tissues. In this study we sought to assess the functional importance of integrins and vascular adhesion molecules in mediating the accumulation of IgA ASC to the lactating mammary gland. This was done through the administration of anti-adhesion molecule, function-blocking antibodies and then assessing changes in the accumulation of IgA ASC in the lactating mammary gland. Here we show that α4 integrins and VCAM-1, but not α4β7 or MAdCAM-1, play pivotal roles in the migration of IgA ASC to the lactating murine mammary gland.
2. Materials and methods
2.1. RNA Isolation
Mammary gland samples were taken from the fourth abdominal mammary glands of BALB/c mice with the subiliac lymph node removed. Tissues were then stored at -80°C for further analysis. Approximately 0.1 mg of tissue was added to 1mL of TRIzol reagent (Invitrogen) and the tissue then homogenized. After 5 minutes, 200μL chloroform was added to each tube, shaken vigorously, and allowed to stand 2-3 minutes. Samples were then centrifuged at 12,000×g for 15 minutes and the aqueous phase removed. The aqueous phase was mixed with 500μL isopropyl alcohol and incubated at -20°C for 10 minutes with 5μL of GlycoBlue (Ambion) added for visualization purposes. Samples were centrifuged at 12,000×g for 10 minutes at 4°C, washed with 1mL 75% ethanol, and incubated at 60°C with 50μL RNAse free water for 10 minutes. RNA concentration was determined and samples diluted in dH20 to 400 ng/μL.
2.2. Quantitative RT-PCR
Relative concentrations of IgA, MAdCAM-1, and VCAM-1 mRNA were assessed by relative quantification using a Verso 1-step QRT-PCR ROX kit (Thermo Fisher Scientific). FAM-labeled probe and primers for the IgA constant region and the vascular adhesion molecule MAdCAM-1 were designed using the Applied Biosystems service Assay by Design with IgA forward primers consisting of the sequence (5-ACTCTAACCCCGTCCAAGAATTG-3) and reverse primer (5-GCTGGCAGGAAGGAATAGTAATAGG-3). FAM-labeled probe and primers for MAdCAM-1 were also obtained from Applied Biosystems with a forward primer sequence of (GCTGACCCATAGAAAGGAGATTCC) and reverse primer sequence (GCTCAGCAGAGGTCGTGTT). Primer and probe for mouse VCAM-1 (FAM-labeled) and GAPDH (VIC-labeled) were obtained from Applied Biosystems as inventoried assays. Samples were run on the 7300 Real-Time PCR System from Applied Biosystems and analyzed with Relative Quantification software from Applied Biosystems. Statistics were generated via comparisons with an endogenous control (GAPDH), with all samples standardized to a virgin mouse mammary gland sample, arbitrarily set to a value of one.
2.3. Blocking Antibodies
All antibodies were generously provided by Dr. Eugene Butcher at the Stanford School of Medicine (Palo Alto, California). Function-blocking antibodies used included: anti-α4 (clone PS/2) anti-β7 (clone Fib 504) anti-α4β7 (clone DATK-32) anti-MAdCAM-1 (clone MECA367) and anti-VCAM-1 (clone MK2.7). All antibodies were diluted in PBS and 100μg of antibody injected intraperitoneally on days 1, 3, 5, and 7 postpartum. Negative controls included injection of PBS or injection of an anti-PNAd antibody (clone MECA 79). All antibodies used were rat IgG2 antibodies with the exception of MECA 79 which was a rat IgM antibody.
2.4. Serum and Milk Sample Preparation
The Institutional Animal Care and Use Committee (IACUC) at Brigham Young University approved all studies involving mice. In all experiments, female BALB/c mice in their first pregnancy were used. On day 9 postpartum, mice were separated from their pups for 2-4 hours before being anesthetized with ketamine-xylazine solution. Mice were then injected with 2 IUs of oxytocin (Sigma-Aldrich) and milk was extracted as previously described (Parr et al., 1995). Serum was also extracted at this time from the retro-orbital sinus. Milk and serum samples were then centrifuged at 16,100×g for 5 minutes at RT and refrigerated for 10 minutes. Milk fat was then removed and the upper fraction of the milk (whey) was collected. All samples were then stored at -80°C for further analysis.
2.5. IgA ELISAs
Flat bottom ELISA plates were coated with 50μL of rat anti-mouse IgA (clone C10-3) (BD Biosciences) diluted to 2μg/ml in PBS, and incubated overnight at 4°C. Plates were washed with PBS, and blocked with 200μL of ELISA blocking buffer for 30 minutes at RT. Plates were washed again and serum and milk samples added, serially diluted and incubated for one hour at room temperature. Plates were then washed, and 50μL of phosphatase labeled goat-anti-mouse IgA (KPL, Gaithersburg, Maryland) diluted at 1:1000 was added and incubated at RT for 1 hour. Plates were washed, and incubated with 50μL of PNPP substrate for 15 minutes. OD readings were taken at 405nm. Levels of IgA antibody were calculated through the use of a standard curve and a Student's T-test was performed to determine statistical significance.
2.6. Immunohistology
Mammary gland tissue was collected from mice 9 days postpartum and frozen in Tissue-Tek OCT mounting medium. Frozen sections (12 m) were fixed in cold acetone for 10 min. After drying, slides were stained with FITC-labeled anti-IgA (BD Biosciences). Counter staining of nuclei was performed using the DNA binding stain TO-PRO-3 (Invitrogen). Staining was visualized using confocal microscopy.
2.7. Statistical analysis
All statistical analyses were performed using a 2 tailed, homoscedastic, Student's T test. A value of p<0.01 was considered significant.
3. Results
3.1. IgA mRNA levels increase dramatically in the murine mammary gland during lactation
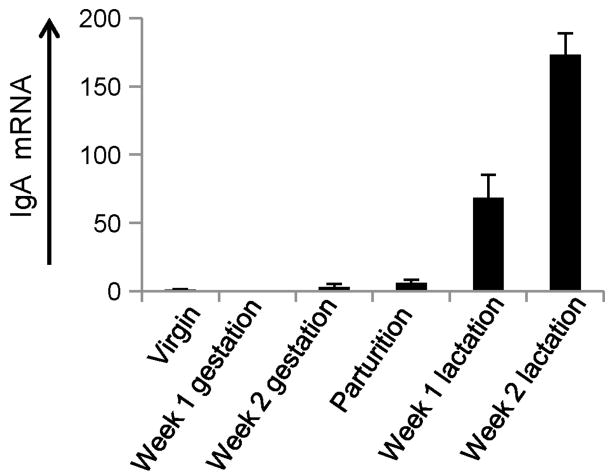
During gestation and lactation dramatic immunological changes occur in the mammary gland, including changes in chemokine and adhesion molecule expression which ultimately result in a large influx of IgA ASC (Bourges et al., 2008; Lamm et al., 1978; Morteau et al., 2008; Tanneau et al., 1999; van der Feltz et al., 2001; Weisz-Carrington et al., 1977; Wilson and Butcher, 2004). This highly regulated influx of cells represents a unique model to study induced lymphocyte recruitment in the absence of inflammation. The predictable and rapid influx of IgA ASC also provides a convenient model to study the recruitment of IgA ASC to mucosal tissues in the absence of large numbers of resident plasma cells. To confirm and quantify this highly regulated increase of IgA ASC into the mammary gland, we assayed IgA mRNA levels from murine mammary gland samples obtained at different stages of pregnancy and lactation. Samples were obtained from mice at the following time points: virgin, one week gestation, two weeks gestation, parturition, one week lactation, and two weeks lactation. Results showed that levels of IgA mRNA gradually increase by the end of pregnancy (increasing ∼6 fold compared to virgin mouse tissues). One week following the start of lactation IgA mRNA levels increased ∼70 fold and by two weeks post partum IgA mRNA levels had increased >170 fold compared to pre-pregnancy levels (Fig. 1). These findings support previous immunohistologic data reported in mice and other animal models (Bourges et al., 2008; Chabaudie et al., 1993; Tanneau et al., 1999; Weisz-Carrington et al., 1977).
Figure 1.
Relative IgA mRNA levels in the murine mammary gland during peripartum period and lactation. Relative IgA mRNA levels are displayed in the Y-axis with mRNA levels from virgin mice set to a value of one. RNA was collected and analyzed from mammary gland samples from the following groups, listed in the X-axis: virgin mouse (six animals), one week gestation (five animals), two weeks gestation (four animals), parturition (four animals), one week lactation (three animals), and two weeks lactation (two animals). Relative IgA mRNA levels increased dramatically beginning at birth and continued to increase through the lactation period. Data represent the average value of all animals tested in each experimental group.
3.2. Kinetics of VCAM-1 & MAdCAM-1 mRNA levels in the developing mammary gland
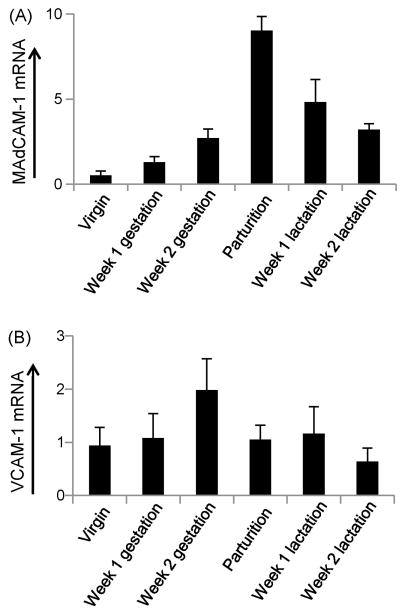
In previous research it has been shown that the mRNA expression level of the adhesion associated molecule CCL28 is tightly regulated during pregnancy and lactation (Wilson and Butcher, 2004). Based on this observation we next sought to better understand the mRNA expression kinetics of vascular-expressed adhesion molecules in the mammary gland. As shown previously through immunohistologic analysis (Bourges et al., 2008; Tanneau et al., 1999), our quantitative RT-PCR data confirmed that MAdCAM-1 mRNA levels significantly increased in the mammary gland during pregnancy and peaked at birth. The level of MAdCAM-1 mRNA then decreased following parturition (Fig. 2a). We found VCAM-1 mRNA to be expressed in the mammary gland throughout the perinatal period. However, unlike IgA and MAdCAM-1, no significant changes in mammary gland VCAM-1 mRNA levels were observed during this period (Fig. 2b).
Figure 2.
Relative MAdCAM-1 and VCAM-1 mRNA levels in the murine mammary gland during the peripartum period. (a) Relative MAdCAM-1 mRNA levels in the murine mammary gland during peripartum period and lactation. (b) Relative VCAM-1 mRNA levels in the murine mammary gland during the peripartum period and lactation. MAdCAM-1 and VCAM-1 mRNA levels are displayed in the Y-axis with mRNA from virgin mouse set to a value of one. Mammary tissues were collected and analyzed from the following groups, listed in the X-axis: virgin mouse (six animals), one week gestation (five animals), two weeks gestation (four animals), parturition (four animals), one week lactation (four animals), and two weeks lactation (four animals). Data represent the average value of all animals tested in each experimental group.
3.3. The vascular adhesion molecule VCAM-1 and α4 integrins are essential to efficient IgA ASC accumulation to the lactating mammary gland
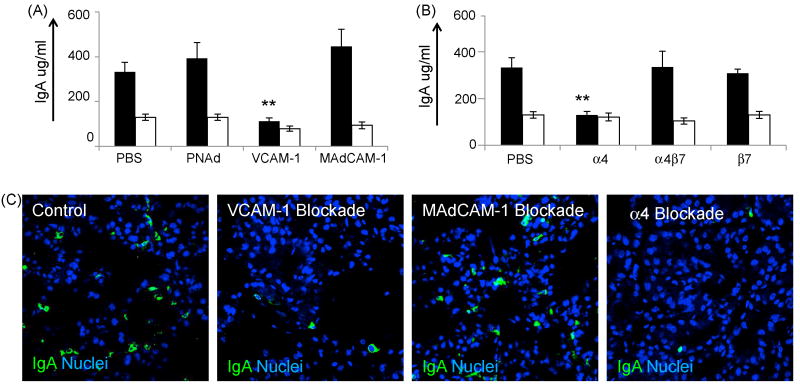
Having confirmed the presence of the vascular addressins VCAM-1 and MAdCAM-1 in the murine mammary gland we next tested the hypothesis that one or both of these molecules was essential to the homing and accumulation of IgA ASC to the lactating mammary gland. Based on the kinetics of IgA ASC accumulation into the lactating mammary gland, in which a large influx of ASC began accumulating soon after birth, we designed experiments in which function-blocking antibodies to MAdCAM-1 and VCAM-1 were administered, as described in Materials and methods. On day 9 postpartum, milk, serum and tissues were collected and IgA antibody levels determined by ELISA. Results show that milk IgA levels did not decrease following the injection of the MAdCAM-1 function-blocking antibody MECA 367, suggesting that MAdCAM-1 mediated homing was not utilized in the homing and accumulation of IgA ASCs to this tissue. Conversely, injection of the VCAM-1 blocking antibody MK2.7 resulted in a significant reduction of IgA antibody accumulation in the milk (p=0.008). These results suggest that the functional blockade of the vascular adhesion molecule VCAM-1 prevented the efficient accumulation of IgA ASCs to the lactating mammary gland. As a control, total IgA levels were also determined in serum samples of all mice. Analysis of serum IgA levels demonstrated that mice treated with anti-MAdCAM-1 and anti-VCAM-1 contained similar levels of IgA as compared to control mice, demonstrating that the use of these function-blocking antibodies did not decrease systemic IgA antibody levels (Fig. 3a).
Figure 3.
Functional blockade of VCAM-1 and α4 integrins inhibits IgA ASC accumulation to the lactating mammary gland. (a) IgA levels in milk (black bars) and serum (white bars) following functional blockade of VCAM-1 (three animas), MAdCAM-1 (five animals), and PNAd (four animals) and diluent control as described in Materials and methods. (b) IgA protein levels in milk (black bars) and serum (white bars) following functional blockade of lymphocyte-expressed integrins α4 (six animals), α4β7 (five animals), β7(seven animals), and diluent control (ten animals), as listed in the X-axis. (c) Immunohistology of mammary tissue following blockade of VCAM-1, MAdCAM-1, α4 integrins and diluent control. IgA staining is shown in green, nuclei are shown in blue. Quantitative data in figures 3a and 3b represents the average value of all animals tested in each experimental group. Error bars represent the standard error of the mean. ** indicates statistical significance at p<0.01 when compared to IgA levels in animals injected with PBS.
In an effort to determine the lymphocyte-expressed ligands utilized in IgA ASC accumulation to the mammary gland, function-blocking antibodies against various lymphocyte-expressed integrins were employed. Results of these experiments showed that milk IgA levels were dramatically reduced following injection of the function-blocking anti-α4 integrin antibody PS/2 (p=0.003). No significant differences in milk IgA levels were seen following treatment with function-blocking antibodies against β7 or α4β7 integrins. Analysis of serum IgA levels demonstrated that mice treated with anti-integrin antibodies contained similar levels of IgA as compared to control mice, suggesting that the use of function-blocking antibodies did not result in cytodepletion of integrin expressing lymphocytes (Fig. 3b).
To visualize IgA ASC recruitment to the lactating mammary gland, immunohistologic staining was performed on mammary gland tissues from mice treated with function-blocking antibodies. Immunohistologic inspection of mammary gland tissue (Fig. 3c.) supports the quantitative ELISA data illustrated in figures 3a and 3b.
3. Discussion
The efficient homing of lymphocytes depends on highly regulated molecular interactions between tissue-expressed chemokines and lymphocyte-expressed chemokine receptors, as well as interactions involving lymphocyte-expressed adhesion molecules and their cognate vascular ligands. Identifying and characterizing the molecular interactions which mediate lymphocyte homing to mucosal tissues has important implications for the potentiation of antigen-specific immune protection at specific mucosal sites.
Here we demonstrate that the vascular adhesion molecule VCAM-1 and the lymphocyte-expressed integrin α4 are essential to the efficient migration of IgA ASC to the lactating mammary gland. These findings are consistent with previous hypotheses and extend earlier studies demonstrating essential roles for CCR10 and CCL28 in this process (Bourges et al., 2008; Morteau et al., 2008; Wilson and Butcher, 2004). Importantly, these data suggest that accumulation of IgA ASC to the lactating mammary gland is accomplished through a distinctly different pathway than homing to intestinal tissues which is thought to depend primarily on CCR9/CCL25 and MAdCAM-1/α4β7 interactions (Feng et al., 2006; Hieshima et al., 2004; Kuklin et al., 2001; Pabst et al., 2004; Schippers et al., 2009). These results suggest that efficient IgA mediated immune protection of extraintestinal sites may require immunization strategies which result in high expression of VCAM-1 ligands and CCR10 on immunization induced IgA ASC. Conversely, efficient immunization of intestinal sites would require induction of CCR9 and α4β7 on responding IgA ASC.
These results clearly demonstrate that neither MAdCAM-1 nor its principal ligand, α4β7, are essential for efficient accumulation of IgA ASCs to the lactating mammary gland, as functional blockade of either of these molecules does not diminish IgA accumulation in the milk. However, many IgA ASCs localizing to the mammary gland do express α4β7 (Wilson unpublished results; Bourges et al., 2008), and MAdCAM-1 is clearly expressed in mammary tissue. Furthermore, it has been shown that CCL28 binding to CCR10 efficiently upregulates α4β7 integrin binding to MAdCAM-1 in vitro (Hieshima et al., 2004; Miles et al., 2008). It is unclear why IgA ASC in the presence of CCL28 and MAdCAM-1 cannot efficiently utilize these molecules to accumulate to the lactating mammary gland in vivo. It is possible that although α4β7/MAdCAM-1 interactions can mediate both the rolling and firm adhesion of lymphocytes in vitro, efficient in vivo diapedesis and accumulation may require interactions with additional homing associated molecules. It has previously been hypothesized that MAdCAM-1 is utilized in the trafficking of T cells into the mammary gland (Tanneau et al., 1999); however, the role of T cells in the developing mammary gland is itself unclear.
It has previously been shown that the expression of CCL28 was tightly correlated with the increase in IgA ASC accumulating in mammary tissue. In this study we found that although VCAM-1 is essential for efficient IgA ASC accumulation to the mammary gland, increased expression of this molecule does not appear to precede the influx of IgA ASC. Although VCAM-1 mRNA levels remain constant throughout the peripartum period the specific microenvironments where this molecule is expressed may change during pregnancy and lactation. Immunohistological studies in pigs suggest that vascular expression of VCAM-1 occurs mainly following parturition, whereas during pregnancy this adhesion molecule is expressed primarily by mammary gland fibroblasts (Bourges et al., 2008). Conversely, strong expression of VCAM-1 has been demonstrated in the mammary gland of non-lactating mice, and VCAM-1 plays a vital role in the migration of MMTV infected lymphocytes to the mammary gland in non-lactating animals (Finke and Acha-Orbea, 2001). These findings indicate that sufficient levels of VCAM-1 are constitutively expressed on mammary endothelial cells to support VCAM-1 mediated homing in lactating and non-lactating animals.
Immunohistologic studies using a porcine model suggest that only a fraction of IgA ASC accumulating in the lactating mammary gland express α4 integrins (Bourges et al., 2008). Data presented here clearly demonstrate that in the mouse, functional blockade of α4 integrins results in a dramatic decrease in the number of plasmablasts localizing to the lactating mammary gland. This suggests that either distinct differences exist in the molecular mechanisms of IgA localization to the lactating mammary gland in some species, or that integrins, necessary for lymphocyte accumulation, are sometimes downregulated following successful entry into the target tissue, as has been hypothesized previously (Bourges et al., 2008).
In summary, we show that the vascular addressin VCAM-1 and α4 integrins are essential to efficient IgA ASC migration to the lactating mammary gland; as α4β1 has been shown to bind to VCAM-1, it appears that interactions between this integrin pair and vascular adhesion molecule are crucial for the effective migration of IgA ASCs into the lactating mammary gland and efficient passive transfer of IgA mediated immunity to the nursing neonate.
Acknowledgments
We wish to acknowledge the editorial assistance of Susanne Linderman in preparing this manuscript. This work was supported by a National Institutes of Health Grant RAI072769A (to E.W.).
Footnotes
Fax: +1 801 422 0519
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bourges D, Meurens F, Berri M, Chevaleyre C, Zanello G, Levast B, Melo S, Gerdts V, Salmon H. New insights into the dual recruitment of IgA+ B cells in the developing mammary gland. Mol Immunol. 2008;45:3354–62. doi: 10.1016/j.molimm.2008.04.017. [DOI] [PubMed] [Google Scholar]
- Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science. 1996;272:60–6. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- Chabaudie N, Le Jan C, Olivier M, Salmon H. Lymphocyte subsets in the mammary gland of sows. Res Vet Sci. 1993;55:351–5. doi: 10.1016/0034-5288(93)90106-p. [DOI] [PubMed] [Google Scholar]
- Feng N, Jaimes MC, Lazarus NH, Monak D, Zhang C, Butcher EC, Greenberg HB. Redundant role of chemokines CCL25/TECK and CCL28/MEC in IgA+ plasmablast recruitment to the intestinal lamina propria after rotavirus infection. J Immunol. 2006;176:5749–59. doi: 10.4049/jimmunol.176.10.5749. [DOI] [PubMed] [Google Scholar]
- Finke D, Acha-Orbea H. Differential migration of in vivo primed B and T lymphocytes to lymphoid and non-lymphoid organs. Eur J Immunol. 2001;31:2603–11. doi: 10.1002/1521-4141(200109)31:9<2603::aid-immu2603>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Halsey JF, Mitchell CS, McKenzie SJ. The origins of secretory IgA in milk: a shift during lactation from a serum origin to local synthesis in the mammary gland. Ann N Y Acad Sci. 1983;409:452–60. doi: 10.1111/j.1749-6632.1983.tb26889.x. [DOI] [PubMed] [Google Scholar]
- Hanson LA, Korotkova M, Lundin S, Haversen L, Silfverdal SA, Mattsby-Baltzer I, Strandvik B, Telemo E. The transfer of immunity from mother to child. Ann N Y Acad Sci. 2003;987:199–206. doi: 10.1111/j.1749-6632.2003.tb06049.x. [DOI] [PubMed] [Google Scholar]
- Hieshima K, Kawasaki Y, Hanamoto H, Nakayama T, Nagakubo D, Kanamaru A, Yoshie O. CC chemokine ligands 25 and 28 play essential roles in intestinal extravasation of IgA antibody-secreting cells. J Immunol. 2004;173:3668–75. doi: 10.4049/jimmunol.173.6.3668. [DOI] [PubMed] [Google Scholar]
- Holmgren J, Hanson LA, Carlson B, Lindblad BS, Rahimtoola J. Neutralizing antibodies against Escherichia coli and Vibrio cholerae enterotoxins in human milk from a developing country. Scand J Immunol. 1976;5:867–71. doi: 10.1111/j.1365-3083.1976.tb03036.x. [DOI] [PubMed] [Google Scholar]
- Jalkanen S, Reichert RA, Gallatin WM, Bargatze RF, Weissman IL, Butcher EC. Homing receptors and the control of lymphocyte migration. Immunol Rev. 1986;91:39–60. doi: 10.1111/j.1600-065x.1986.tb01483.x. [DOI] [PubMed] [Google Scholar]
- Kuklin NA, Rott L, Feng N, Conner ME, Wagner N, Muller W, Greenberg HB. Protective intestinal anti-rotavirus B cell immunity is dependent on alpha 4 beta 7 integrin expression but does not require IgA antibody production. J Immunol. 2001;166:1894–902. doi: 10.4049/jimmunol.166.3.1894. [DOI] [PubMed] [Google Scholar]
- Kunkel EJ, Butcher EC. Chemokines and the tissue-specific migration of lymphocytes. Immunity. 2002;16:1–4. doi: 10.1016/s1074-7613(01)00261-8. [DOI] [PubMed] [Google Scholar]
- Kunkel EJ, Butcher EC. Plasma-cell homing. Nat Rev Immunol. 2003;3:822–9. doi: 10.1038/nri1203. [DOI] [PubMed] [Google Scholar]
- Lamm ME, Weisz-Carrington P, Roux ME, McWilliams M, Phillips-Quagliata JM. Development of the IgA system in the mammary gland. Adv Exp Med Biol. 1978;107:35–42. doi: 10.1007/978-1-4684-3369-2_5. [DOI] [PubMed] [Google Scholar]
- Miles A, Liaskou E, Eksteen B, Lalor PF, Adams DH. CCL25 and CCL28 promote alpha4 beta7-integrin-dependent adhesion of lymphocytes to MAdCAM-1 under shear flow. Am J Physiol Gastrointest Liver Physiol. 2008;294:G1257–67. doi: 10.1152/ajpgi.00266.2007. [DOI] [PubMed] [Google Scholar]
- Morteau O, Gerard C, Lu B, Ghiran S, Rits M, Fujiwara Y, Law Y, Distelhorst K, Nielsen EM, Hill ED, Kwan R, Lazarus NH, Butcher EC, Wilson E. An indispensable role for the chemokine receptor CCR10 in IgA antibody-secreting cell accumulation. J Immunol. 2008;181:6309–15. doi: 10.4049/jimmunol.181.9.6309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabst O, Ohl L, Wendland M, Wurbel MA, Kremmer E, Malissen B, Forster R. Chemokine receptor CCR9 contributes to the localization of plasma cells to the small intestine. J Exp Med. 2004;199:411–6. doi: 10.1084/jem.20030996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr EL, Bozzola JJ, Parr MB. Purification and measurement of secretory IgA in mouse milk. J Immunol Methods. 1995;180:147–57. doi: 10.1016/0022-1759(94)00310-s. [DOI] [PubMed] [Google Scholar]
- Schippers A, Leuker C, Pabst O, Kochut A, Prochnow B, Gruber AD, Leung E, Krissansen GW, Wagner N, Muller W. Mucosal addressin cell-adhesion molecule-1 controls plasma-cell migration and function in the small intestine of mice. Gastroenterology. 2009;137:924–33. doi: 10.1053/j.gastro.2009.05.039. [DOI] [PubMed] [Google Scholar]
- Stoliar OA, Pelley RP, Kaniecki-Green E, Kkaus MH, Carpenter CC. Secretory IgA against enterotoxins in breast-milk. Lancet. 1976;1:1258–61. doi: 10.1016/s0140-6736(76)91735-9. [DOI] [PubMed] [Google Scholar]
- Tanneau GM, Hibrand-Saint Oyant L, Chevaleyre CC, Salmon HP. Differential recruitment of T- and IgA B-lymphocytes in the developing mammary gland in relation to homing receptors and vascular addressins. J Histochem Cytochem. 1999;47:1581–92. doi: 10.1177/002215549904701210. [DOI] [PubMed] [Google Scholar]
- van der Feltz MJ, de Groot N, Bayley JP, Lee SH, Verbeet MP, de Boer HA. Lymphocyte homing and Ig secretion in the murine mammary gland. Scand J Immunol. 2001;54:292–300. doi: 10.1046/j.1365-3083.2001.00933.x. [DOI] [PubMed] [Google Scholar]
- Weisz-Carrington P, Roux ME, Lamm ME. Plasma cells and epithelial immunoglobulins in the mouse mammary gland during pregnancy and lactation. J Immunol. 1977;119:1306–7. [PubMed] [Google Scholar]
- Wilson E, Butcher EC. CCL28 controls immunoglobulin (Ig)A plasma cell accumulation in the lactating mammary gland and IgA antibody transfer to the neonate. J Exp Med. 2004;200:805–9. doi: 10.1084/jem.20041069. [DOI] [PMC free article] [PubMed] [Google Scholar]