Abstract
Objective
The purpose of this study was to evaluate the prognostic significance of tumor volume assessed by pretreatment MRI in stage IIB cervical cancer patients with concurrent chemoradiation therapy.
Methods
A retrospective chart review was performed on seventy five patients with cervical cancer who were treated with concurrent weekly cisplatin (40 mg/m2) and radiotherapy between January 2000 and April 2007. Potential prognostic factors were age, chemotherapy numbers, histology, tumor diameter and volume, lymph node (LN) involvement and pretreatment squamous cell carcinoma antigen (SCC-Ag) levels.
Results
The median follow-up time was 55 months (range, 8 to 104 months). The median tumor size and volume (range) were 4.5 cm (2 to 10) and 33.1 mL (4.2 to 392.7), respectively. Pelvic LN enlargement rate was 58.7%. Para-aortic LN enlargement rate was 14.7%. Using multivariate analysis, a tumor volume (>33 mL, p=0.025), pelvic LN enlargement (p=0.044) revealed a significantly unfavorable outcome on overall survival. PFS was influenced by tumor histology (p<0.001), pelvic LN enlargement (p=0.015) and pretreatment SCC-Ag levels (p=0.018). We found that 22 (29.3%) patients had recurrences and 14 (18.7%) patients died of disease. The 5-year overall survival rate was 80.6% (standard error, 4.9%) and 5-year PFS rate was 71.3% (standard error, 5.3%).
Conclusion
Tumor volume and pelvic LN involvement showed possibility to predict overall survival in patient with stage IIB cervical cancer. Optimal tumor volume and pelvic LN assessment by pretreatment MRI might be helpful to predict treatment outcome.
Keywords: Cervical neoplasms, Chemoradiation therapy, MRI, Tumor volume
INTRODUCTION
Cervical cancer is the only gynecological cancer staged clinically according to the International Federation of Gynecology and Obstetrics (FIGO) classification system. However, clinical staging has limitations in evaluation of several parameters including parametrial invasion, lymph node (LN) metastasis, pelvic wall invasion.1 Clinical evaluation of tumor size in cervical cancer remains inaccurate when compared with surgical staging. Additionally the FIGO clinical staging system has limited accuracy with staging errors increasing for more advanced disease. Since National Cancer Institute (NCI) issued clinical announcement that noted the improved survival with concurrent chemoradiation therapy (CCRT) compared to radiation alone among women with locally advanced cervical cancer in 1999, cisplatin-based combined chemotherarpy during external beam irradiation has been a standard treatment.2 It is known that increasing tumor size and volume affect overall survival and tumor recurrence.3,4 Pretreatment squamous cell carcinoma antigen (SCC-Ag) levels correlated with extent of disease, the response to treatment, and can be used to predict the tumor recurrence.5,6 In operable patients, accurate evaluations of tumor size, extension to surrounding tissue or LN metastasis are possible by pathologic report. However, prognostic factors assessment in inoperable patients who are planned for CCRT, should be evaluated by clinical examination and imaging studies.
Among imaging study modalities, magnetic resonance imaging (MRI) has been widely used to evaluate the size and volume of primary tumor, parametrial invasion and LN enlargement. Although computed tomography (CT) and MRI have a comparable accuracy in staging, MRI is regarded as the most reliable tool for the treatment planning of cervical cancer due to superior soft tissue contrast and multiplanar capability.7,8 Even though clinical stage is important prognostic factors, stage does not necessarily correlate with tumor size, volume, and LN involvement.9 And treatment outcome may vary according to tumor size, volume or other prognostic factors in patients with same stage IIB cervical cancer.
The aim of this study was to evaluate the prognostic significance of tumor size, volume and LN enlargement assessed by pretreatment MRI in presence of other prognostic factor such as age, histology, pretreatment SCC-Ag levels.
MATERIALS AND METHODS
1. Patient population
A retrospective chart review of patient with stage IIB cervical cancer who received an MRI scan before curative aimed CCRT was performed. Between January 2000 and April 2007, seventy five patients were treated with CCRT at the Yonsei University College of Medicine were diagnosed between. The cut-off date for follow-up was June 2008.
The staging was based on FIGO classification system. The procedure for clinical staging included a medical history, physical examination, routine laboratory tests, chest radiography, intravenous pyelography, cystoscopy, sigmoidoscopy and MRI scan. LN diameter greater than 1 cm in minimum diameter were considered positive node. SCC-Ag levels were measured before the start of CCRT and 1 month after completing treatment. Potential prognostic factors were age, numbers of chemotherapy cycle, tumor histology, tumor diameter and volume, LN involvement and pretreatment SCC-Ag levels.
2. Treatment policy
Radiotherapy was delivered with a combination of external irradiation and high-dose rate intracavitary radiation by a remote afterloading system using iridium192 sources (Gamma-Med II). External whole-pelvis irradiation was performed with a dose of 1.8 Gy per fraction 5 times per week to a midline dose of 27.0 to 36.0 Gy. This was followed by high-dose rate intracavitary radiation with 6 insertions (twice per week) with a fractional dose of 5.0 Gy to a total dose of 30.0 Gy at point A. After high-dose rate intracavitary radiation, patients received a second course of external irradiation with central shielding up to a total external dose of 45.0 to 50.4 Gy. In case of para-aortic LN enlargement on MRI, extended-field radiation was administered. During radiotherapy, cisplatin was given intravenously once a week at a dose of 40 mg/m2 of body surface area (BSA) with the total dose not exceeding 70 mg per week.
3. MRI imaging and tumor volume measurement
MRI was performed using a 1.5 Tesla scanner (INTERA ACHIEVA 1.5T; Philips Medical Systems, Best, Nederland) with a SENSE-body coil. Routine spin echo transverse, coronal, and sagittal plane T2 weighted images (TR/TE; 3632-4182/90 ms, number of excitation (NEX); 2, section thickness; 5 mm, intersection gap; 2 mm, field of view; 24 cm, matrix; 512×256) and transverse spin-echo T1 weighted images (TR/TE; 678.2/11 ms, NEX; 3, section thickness; 5 mm, intersection gap; 2 mm, field of view (FOV); 24 cm, matrix; 256×256) of the pelvis were acquired. Gradient echo precontrast fat suppressed sagittal T1 weighted images (THRIVE : TR/TE 3.1×1.9 ms; NEX; 1, echo-train length; 48, flip angle; 10°, section thickness; 4 mm, intersection gap; 2 mm, field of view; 37 cm, matrix; 336×307) were obtained. A gadolinium chelate (Dotarem; Guerbet, Aulnaysous-Bois, France) was administered intravenously at a dose of 0.2 mL per kilogram of body weight by hand injection. When contrast agent was seen at the pelvic aorta on the bolus tracking scan, 6 sequential sets of images were scanned during non-breath hold with a time interval of 20 seconds with parameters identical to the precontrast images. Contrast enhanced T1-weighted axial images (THRIVE: TR/TE=4.5/2.2 ms; NEX; 1, echo-train length; 60, flip angle; 15°, section thickness; 4 mm, intersection gap; 2 mm, field of view; 40 cm, matrix; 320×224) were obtained. The diameter-based calculation was computed by measuring the largest tumor diameter in each orthogonal plane on MRI scan. The longitudinal diameter (d1) along the long axis of the endometrial cavity on the sagittal images and the anteroposterior diameter (d2, orthogonal to the longitudinal diameter) were measured on the sagittal images. The largest lateral diameter (d3) was measured on the axial images. Diameter-based measurements were computed as an ellipsoid (V=d1×d2×d3×π/6) to calculate diameter-based volume (V).
4. Statistical analysis
Overall survival (OS) was assessed from the date of CCRT to death from any cause or the date of last contact. Progression free survival (PFS) was measured from the treatment start to either progression/relapse or to the date of last contact for patients who are alive and progression free. Survival curves were measured by the Kaplan-Meier method. Differences in survival were compared using the log-rank statistical test. Prognostic factor analyses for OS and PFS were performed using Cox regression method. Hazard ratio is given with 95% confidence intervals (95% CI). SPSS 12.0 (SPSS Inc., Chicago, IL, USA) was used for the statistical analysis. A p-value <0.05 was considered to be statistically significant.
RESULTS
1. Patient characteristics
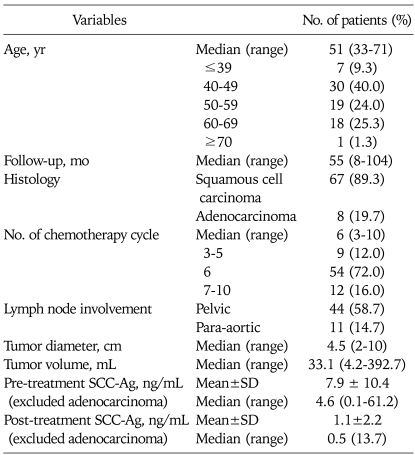
Seventy five patients were reviewed for this study. The patients and tumor-associated characteristics are summarized in Table 1. Fifty four patients (72.0%) received 6 cycles chemotherapy, but nine patients (12.0%) did not complete planned chemotherapy. Forty four patients (58.7%) showed pelvic LN enlargement on MRI scan. Eleven patients (14.7%) exhibited both pelvic and para-aortic LN enlargement. The median tumor diameter and volume were 4.5 cm (range, 2 to 10 cm) and 33.1 mL (range, 4.2 to 392.7 mL), respectively.
Table 1.
Patient characteristics (N=75)
SCC-Ag: squamous cell carcinoma antigen.
2. Progression free survival
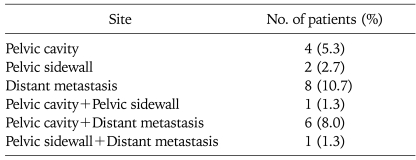
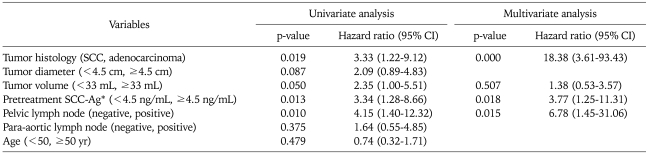
Twenty two patients (29.3%) had recurred. Eight patients had developed distant metastasis and 7 patients developed both local and distant metastasis (Table 2). The 5-year PFS rate was 71.3% (standard error, 5.3%). In univariate analysis, tumor histology, tumor volume, pretreatment SCC-Ag levels and pelvic LN involvement were statistically significant prognostic factor on PFS. By multivariate analysis of PFS according to these factors, tumor histology (squamous cell carcinoma), SCC-Ag levels (≥4.5 ng/mL) and pelvic LN involvement showed statistically significant factors on PFS (Table 3).
Table 2.
Site of recurrence
Table 3.
Univariate and multivariate analysis of prognostic factor for PFS (Cox proportional hazard)
PFS: progression free survival, SCC-Ag: squamous cell carcinoma antigen.
*Excluded adenocarcinoma.
3. Overall survival
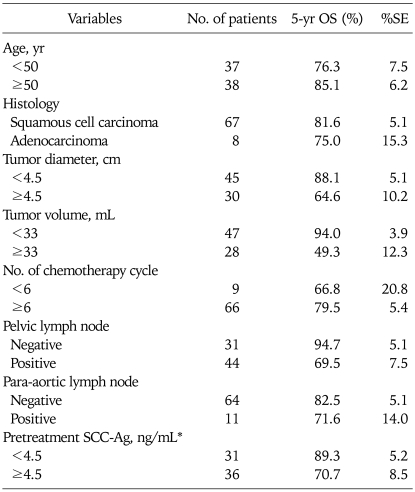
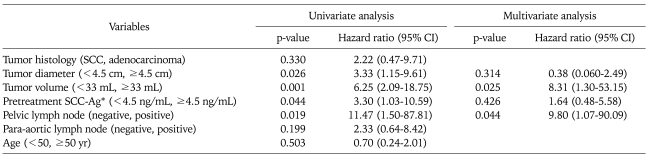
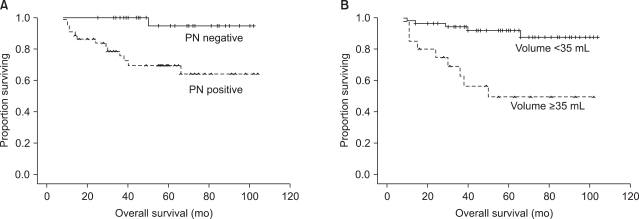
Among seventy five patients, 14 (18.7%) have died. The 5-year OS rate was 80.6% (standard error, 4.9%). Estimated (Kaplan-Meier) five-year survival rate according to patient subgroups were summarized in Table 4. The 5-year survival rate of patients with tumor volume of <33 mL was 94.0% compared with 49.3% in patients with tumor volume of ≥33 mL. In univariate analysis, large tumor diameter and volume, pelvic LN involvement and elevated pretreatment SCC-Ag levels were statistically significant relation to worse survival (Table 5). There was no significant relation between patient age, tumor histology or para-aortic LN involvement and OS. Using multivariate analysis, tumor volume of >33 mL (p=0.025) and positive pelvic LN involvement (p=0.044) revealed a significantly unfavorable outcome on overall survival. The hazard ratio for tumor volume of ≥33 mL was 8.31 (95% CI, 1.30 to 54.15) and for positive pelvic LN involvement was 9.80 (95% CI, 1.07 to 90.09). Overall survival curves for tumor volume and pelvic LN involvement were shown in Fig. 1.
Table 4.
Five-yr overall survival (OS) for patient subgroup
SCC-Ag: squamous cell carcinoma antigen.
*Excluded adenocarcinoma.
Table 5.
Univariate and multivariate analysis of prognostic factor for overall survival (Cox proportional hazard)
SCC-Ag: squamous cell carcinoma antigen.
*Excluded adenocarcinoma.
Fig. 1.
Overall survival curves for (A) pelvic LN involvement (left, p=0.044) and (B) tumor volume (right, p=0.025). PN: pelvic lymph node.
DISCUSSION
Tumor size and tumor infiltration into surrounding normal tissue can be accurately estimated in surgically treated patients. However it is usually not available in patients treated with CCRT. Previous studies have shown that MRI indicates the closest correlation to actual tumor volume that is currently performable in the clinical setting.10 In the evaluation of cervical cancer mass, MRI has several benefits with comparison to CT. The soft tissue contrast and multiplanar capability of MRI provides clear delineation of tumor. Tumor location including local invasion and diameter in T2 weighted MRI correlated well with that in surgical specimen.11,12 Therefore, more accurate tumor size measurement and assessment of invasion to surrounding normal tissue, the depth of stromal invasion and LN evaluation has been possible.
In this study, we evaluated the relationship of prognostic factors (tumor diameter, volume, LN involvement, age, histology and pretreatment SCC-Ag) to treatment outcome in 75 stage IIB cervical cancer patients treated with CCRT. Our results indicated that the tumor volume and pelvic LN involvement were significantly associated with OS. However, the data showed that tumor diameter, pretreatment SCC-Ag levels and para-aortic LN involvement had a poor relationship with OS. Burghardt et al.4 reported that OS was more closely related to tumor volume than to clinical or pathologic stage in early cervical cancer patients treated by radical hysterectomy and lymphadenectomy. In their series, 5-year survival rates of 91% for patients with tumor volume less than 2.5 mL, 79% for patients with tumor volume 2.5 to 10 mL, 70% for those with tumor volume 10 to 50 mL, and 48% for those with tumor volume more than 50 mL. In advanced FIGO stage I and II tumors treated with radical radiotherapy, the treatment outcome was dependent on clinically determined tumor size rather than stage.9 Our results revealed that the tumor diameter of ≥4.5 cm correlated with worse OS by univariate analysis, but there was no correlation with OS by multivariate analysis. In general, tumor mass of stage IIB cervical cancer is larger than early cervical cancer. We suppose that tumor volume is the appropriate parameter to predict treatment outcome than tumor diameter in large tumor mass.
FIGO criteria currently used to stage cervical cancer do not account for LN involvement, but the LN metastasis is major factor to determining prognosis and proper treatment. Increasing tumor size and increasing depth of stromal invasion correlated most strongly with LN metastasis and a shorter disease-free survival in previous studies of patients with FIGO stage 1B.3,13 The accuracy and sensitivity rates with MRI for detecting LN metastasis from cervical cancer are between 76 to 100% and 36 to 71%, respectively.14,15 However, the limitation of MRI is that it is not possible to differentiate metastatic LN from non-metastatic LN hyperplasia of similar size and shape. In our study, pelvic LN involvement correlated significantly with OS and PFS by multivariate analysis. Para-aortic LNs does not correlate with treatment outcome. The incidence of pelvic LN metastasis in stage II cervical cancer undergoing radical hysterectomy was approximately 35 to 45.8%. Our data revealed that pelvic LN metastasis and para-aortic LN metastasis were 58.7% and 14.7%, respectively. Also, patients with positive pelvic LNs have a 5-year survival rate of 69.5% whereas patients with positive para-aortic LNs were higher than patients with positive pelvic LNs. Para-aortic metastases were found in 4.5 to 7.2% of patients in this stage.16-18 Higher 5-year survival rates of >90% are reported among patients treated surgically with no evidence of LN metastasis, compared to patients with positive pelvic node (50 to 60%, 5-year survival) or para-aortic nodes (20 to 30%, 5-year survival).19 Although it is accepted commonly that patients with para-aortic LN metastases have lower OS and higher recurrence, the incidence of pelvic or para-aortic LN involvement and 5-year survival rates were relatively higher than previous studies. We can assume that a portion of LN involvement on MRI might be actually LN hyperplasia.
In addition, actual parametrial involvement was pathologically confirmed in approximately 21 to 55% in the clinical stage IIB cervical cancer.16,20,21 This suggests that almost one half of the patients are overstaged due to difficulty in distinguishing parametrial extension from inflammatory change, endometriosis, adhesion, and irregular shape of large cervical tumor.20,21 The 5-year survival rate of stage stage IIB cervical cancer patients with radical hysterectomy ranged between 55 to 77%.22 In comparison with our 5-year survival rates, a part of our patients might be overstaged. Exact evaluations of LN status in the staging of cervical cancer are essential to direct treatment and reduce morbidity. More recently, positron emission tomography is more sensitive than MRI or CT for detecting LN metastases in patients with cervical cancer.23,24 Therefore, other diagnostic modalities such as positron emission tomography or sentinel node biopsy may have a potential role for the exact assessment of LN metastases.
For imaging-based tumor size assessment, simple diameter measurement of three orthogonal diameters or three-dimensional (3D) volumetry with region-of-interest (ROI) quantitative image analysis are commonly used. The measurement of orthogonal tumor diameters is the simplest, fastest, and most practical methods extensively used in the radiology department. Simple diameter-based tumor measurement appears to be equivalent to contour tracing/ROI analysis 3D tumor volumetry for tumor size assessment for predicting outcome.25,26 Diameter-based measurement can be easily computed as an ellipsoid-shaped tumor volume, but does not consider irregularities in tumor border and shape, which can be accounted for by 3D ROI volumetry. Thus it may be less accurate because cervical cancers have irregular contour that deviate from the idealized ellipsoid volume.
Tumor histology (adenocarcinoma) and pretreatment SCC-Ag levels of ≥4.5 ng/mL revealed poor prognosis in PFS, but no correlation with OS. Generally, adenocarcinoma has a poor prognosis and relatively resistant response to radiation therapy.27 SCC-Ag levels correlate with the extent of the disease, the response to treatment, survival and recurrence.28 There were only 9 patients with adenocarcinoma histology in our study. The results may not have been large enough to be able to make a clear declaration. Thus, we suggest that a prospective study involving a large cohort of patients will be required to confirm this possibility.
We conclude that in patient with stage IIB cervical cancer, tumor volume and pelvic LN involvement provided possibility to predict OS. Therefore, optimal tumor volume and pelvic LN assessment by pretreatment MRI might be helpful to predict treatment outcome.
ACKNOWLEDGEMENTS
This study was supported by the Brain Korea (BK) 21 Project for Medical Sciences, Yonsei University and a grant from the Korean Health 21 R&D Project, Ministry of Health & Welfare and Family, Republic of Korea (0412-CR01-0704-0001).
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Hricak H, Yu KK. Radiology in invasive cervical cancer. AJR Am J Roentgenol. 1996;167:1101–1108. doi: 10.2214/ajr.167.5.8911159. [DOI] [PubMed] [Google Scholar]
- 2.National Cancer Institute. NCI issues clinical announcement on cervical cancer: chemotherapy plus radiation improves survival. Bethesda: National Cancer Institute; 1999. [Google Scholar]
- 3.Eifel PJ, Morris M, Wharton JT, Oswald MJ. The influence of tumor size and morphology on the outcome of patients with FIGO stage IB squamous cell carcinoma of the uterine cervix. Int J Radiat Oncol Biol Phys. 1994;29:9–16. doi: 10.1016/0360-3016(94)90220-8. [DOI] [PubMed] [Google Scholar]
- 4.Burghardt E, Baltzer J, Tulusan AH, Haas J. Results of surgical treatment of 1028 cervical cancers studied with volumetry. Cancer. 1992;70:648–655. doi: 10.1002/1097-0142(19920801)70:3<648::aid-cncr2820700318>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 5.Micke O, Bruns F, Schafer U, Prott FJ, Willich N. The impact of squamous cell carcinoma (SCC) antigen in patients with advanced cancer of uterine cervix treated with (chemo-)radiotherapy. Anticancer Res. 2005;25:1663–1666. [PubMed] [Google Scholar]
- 6.Ogino I, Nakayama H, Okamoto N, Kitamura T, Inoue T. The role of pretreatment squamous cell carcinoma antigen level in locally advanced squamous cell carcinoma of the uterine cervix treated by radiotherapy. Int J Gynecol Cancer. 2006;16:1094–1100. doi: 10.1111/j.1525-1438.2006.00449.x. [DOI] [PubMed] [Google Scholar]
- 7.Scheidler J, Heuck AF. Imaging of cancer of the cervix. Radiol Clin North Am. 2002;40:577–590. doi: 10.1016/s0033-8389(01)00007-0. [DOI] [PubMed] [Google Scholar]
- 8.Amendola MA, Hricak H, Mitchell DG, Snyder B, Chi DS, Long HJ, 3rd, et al. Utilization of diagnostic studies in the pretreatment evaluation of invasive cervical cancer in the United States: results of intergroup protocol ACRIN 6651/GOG 183. J Clin Oncol. 2005;23:7454–7459. doi: 10.1200/JCO.2004.00.5397. [DOI] [PubMed] [Google Scholar]
- 9.Thoms WW, Jr, Eifel PJ, Smith TL, Morris M, Delclos L, Wharton JT, et al. Bulky endocervical carcinoma: a 23-year experience. Int J Radiat Oncol Biol Phys. 1992;23:491–499. doi: 10.1016/0360-3016(92)90003-z. [DOI] [PubMed] [Google Scholar]
- 10.Ebner F, Tamussino K, Kressel HY. Magnetic resonance imaging in cervical carcinoma: diagnosis, staging, and follow-up. Magn Reson Q. 1994;10:22–42. [PubMed] [Google Scholar]
- 11.Narayan K, McKenzie A, Fisher R, Susil B, Jobling T, Bernshaw D. Estimation of tumor volume in cervical cancer by magnetic resonance imaging. Am J Clin Oncol. 2003;26:e163–e168. doi: 10.1097/01.coc.0000091358.78047.b5. [DOI] [PubMed] [Google Scholar]
- 12.Oellinger JJ, Blohmer JU, Michniewicz K, Siewert C, Wust P, Gutberlet M, et al. Pre-operative staging of cervical cancer: comparison of magnetic resonance imaging (MRI) and computed tomography (CT) with histologic results. Zentralbl Gynakol. 2000;122:82–91. [PubMed] [Google Scholar]
- 13.Zaino RJ, Ward S, Delgado G, Bundy B, Gore H, Fetter G, et al. Histopathologic predictors of the behavior of surgically treated stage IB squamous cell carcinoma of the cervix: a Gynecologic Oncology Group study. Cancer. 1992;69:1750–1758. doi: 10.1002/1097-0142(19920401)69:7<1750::aid-cncr2820690717>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 14.Choi SH, Kim SH, Choi HJ, Park BK, Lee HJ. Preoperative magnetic resonance imaging staging of uterine cervical carcinoma: results of prospective study. J Comput Assist Tomogr. 2004;28:620–627. doi: 10.1097/01.rct.0000138007.77725.0a. [DOI] [PubMed] [Google Scholar]
- 15.Yu KK, Hricak H, Subak LL, Zaloudek CJ, Powell CB. Preoperative staging of cervical carcinoma: phased array coil fast spin-echo versus body coil spin-echo T2-weighted MR imaging. AJR Am J Roentgenol. 1998;171:707–711. doi: 10.2214/ajr.171.3.9725301. [DOI] [PubMed] [Google Scholar]
- 16.Kawagoe T, Kashimura M, Matsuura Y, Sugihara K, Toki N, Aoki T. Clinical significance of tumor size in stage IB and II carcinoma of the uterine cervix. Int J Gynecol Cancer. 1999;9:421–426. doi: 10.1046/j.1525-1438.1999.99059.x. [DOI] [PubMed] [Google Scholar]
- 17.Sakuragi N, Satoh C, Takeda N, Hareyama H, Takeda M, Yamamoto R, et al. Incidence and distribution pattern of pelvic and paraaortic lymph node metastasis in patients with Stages IB, IIA, and IIB cervical carcinoma treated with radical hysterectomy. Cancer. 1999;85:1547–1554. doi: 10.1002/(sici)1097-0142(19990401)85:7<1547::aid-cncr16>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 18.Takeda N, Sakuragi N, Takeda M, Okamoto K, Kuwabara M, Negishi H, et al. Multivariate analysis of histopathologic prognostic factors for invasive cervical cancer treated with radical hysterectomy and systematic retroperitoneal lymphadenectomy. Acta Obstet Gynecol Scand. 2002;81:1144–1151. doi: 10.1034/j.1600-0412.2002.811208.x. [DOI] [PubMed] [Google Scholar]
- 19.Tinga DJ, Timmer PR, Bouma J, Aalders JG. Prognostic significance of single versus multiple lymph node metastases in cervical carcinoma stage IB. Gynecol Oncol. 1990;39:175–180. doi: 10.1016/0090-8258(90)90428-n. [DOI] [PubMed] [Google Scholar]
- 20.Inoue T, Morita K. The prognostic significance of number of positive nodes in cervical carcinoma stages IB, IIA, and IIB. Cancer. 1990;65:1923–1927. doi: 10.1002/1097-0142(19900501)65:9<1923::aid-cncr2820650909>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 21.Kamura T, Tsukamoto N, Tsuruchi N, Kaku T, Saito T, To N, et al. Histopathologic prognostic factors in stage IIb cervical carcinoma treated with radical hysterectomy and pelvic-node dissection: an analysis with mathematical statistics. Int J Gynecol Cancer. 1993;3:219–225. doi: 10.1046/j.1525-1438.1993.03040219.x. [DOI] [PubMed] [Google Scholar]
- 22.Suprasert P, Srisomboon J, Kasamatsu T. Radical hysterectomy for stage IIB cervical cancer: a review. Int J Gynecol Cancer. 2005;15:995–1001. doi: 10.1111/j.1525-1438.2005.00259.x. [DOI] [PubMed] [Google Scholar]
- 23.Rose PG, Adler LP, Rodriguez M, Faulhaber PF, Abdul-Karim FW, Miraldi F. Positron emission tomography for evaluating para-aortic nodal metastasis in locally advanced cervical cancer before surgical staging: a surgicopathologic study. J Clin Oncol. 1999;17:41–45. doi: 10.1200/JCO.1999.17.1.41. [DOI] [PubMed] [Google Scholar]
- 24.Narayan K, Hicks RJ, Jobling T, Bernshaw D, McKenzie AF. A comparison of MRI and PET scanning in surgically staged loco-regionally advanced cervical cancer: potential impact on treatment. Int J Gynecol Cancer. 2001;11:263–271. doi: 10.1046/j.1525-1438.2001.011004263.x. [DOI] [PubMed] [Google Scholar]
- 25.Mayr NA, Taoka T, Yuh WT, Denning LM, Zhen WK, Paulino AC, et al. Method and timing of tumor volume measurement for outcome prediction in cervical cancer using magnetic resonance imaging. Int J Radiat Oncol Biol Phys. 2002;52:14–22. doi: 10.1016/s0360-3016(01)01808-9. [DOI] [PubMed] [Google Scholar]
- 26.Hatano K, Sekiya Y, Araki H, Sakai M, Togawa T, Narita Y, et al. Evaluation of the therapeutic effect of radiotherapy on cervical cancer using magnetic resonance imaging. Int J Radiat Oncol Biol Phys. 1999;45:639–644. doi: 10.1016/s0360-3016(99)00228-x. [DOI] [PubMed] [Google Scholar]
- 27.Lea JS, Sheets EE, Wenham RM, Duska LR, Coleman RL, Miller DS, et al. Stage IIB-IVB cervical adenocarcinoma: prognostic factors and survival. Gynecol Oncol. 2002;84:115–119. doi: 10.1006/gyno.2001.6473. [DOI] [PubMed] [Google Scholar]
- 28.Molina R, Filella X, Lejarcegui JA, Pahisa J, Torne A, Rovirosa A, et al. Prospective evaluation of squamous cell carcinoma and carcinoembryonic antigen as prognostic factors in patients with cervical cancer. Tumour Biol. 2003;24:156–164. doi: 10.1159/000073846. [DOI] [PubMed] [Google Scholar]