Abstract
This article reviews the history and current status of vascular endothelial growth factor targeted therapy for the most common gynecologic malignancies - epithelial ovarian, endometrial and cervical cancers. The biologic rationale for targeting vascular endothelial growth factor (VEGF) for these disease sites is well-founded, and pre-clinical studies have supported the development of anti-VEGF agents. Their classification, known mechanisms of action, unique toxicities and clinical development are herein explored, the latter including issues related to study design, disease site and disease setting.
Keywords: Vascular endothelial growth factor, Ovarian neoplasms, Endometrial neoplasms, Cervical neoplasms, Angiogenesis, Bevacizumab
RATIONALE FOR MOLECULAR TARGETED THERAPY
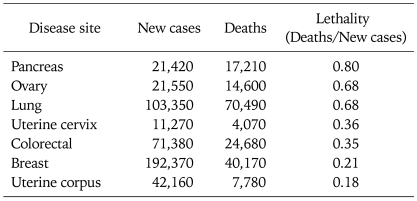
A plateau in the mortality statistics for the common gynecologic malignancies underscores limitations of surgical resection, cytotoxic therapy and regional radiation in the management of these diseases and suggests a role for continued research on novel therapeutics. Table 1 stratifies the 2009 American Cancer Society estimated incidence and mortality for major neoplastic sites in women in order of lethality. Ovarian cancers (the majority being epithelial in origin and including fallopian tube and primary peritoneal carcinomas), are tied with lung cancers as the second most lethal cancer site in women and represent the most lethal site of gynecologic malignancy in the United States, with an estimated 21,550 new cases and 14,600 deaths per year.1 Despite somewhat effective primary preventive approaches and advances in cytotoxic therapeutics, incidence and mortality rates have yet to decline convincingly. Endometrial carcinoma is the most common gynecologic malignancy, representing the vast majority of the 42,160 new cases of uterine cancer and most of the 7,780 uterine cancer deaths estimated in the US for 2009.1 Failure of preventive measures, such as weight control and management of chronic anovulation, is the most likely explanation for the continued rise in incidence for endometrial cancers. Although the majority of patients present with symptoms of abnormal vaginal bleeding and are found to have early stage disease that can be controlled with standard therapeutic modalities, according to 2006 estimates, at diagnosis 16% are diagnosed with locally advanced disease (including regional lymphatic spread) and 8% are found to have distant metastases; the corresponding 5-year survival rates for these two groups are 66% and 25% respectively.1 Carcinoma of the uterine cervix is the leading cause of cancer mortality in women world-wide, attributed mostly to the lack of broadly applied cytology screening programs in less industrialized regions. Despite the implementation of effective prevention, early detection and therapeutic methods in the US, however, the American Cancer Society estimates 4,070 cervix cancer deaths for 2009.1 According to 2006 estimates by the American Cancer Society, 32% of cancers are locally advanced or associated with regional nodal metastases, and 8% are associated with distant metastases. The corresponding 5-year survival rates are 55% and 17% respectively.2
Table 1.
US Incidence and mortality for female solid tumors: 20091
Recent studies have suggested that molecular targeted therapeutics may represent an important solution to the current barriers in gynecologic cancer control. Broadly speaking, in contrast to systemic cytotoxic drugs, molecular targeted agents are developed by first identifying key biological pathways driving tumor progression and potential targets therein.
VASCULAR ENDOTHELIAL GROWTH FACTOR SIGNALING IN PATHOGENESIS OF GYNECOLOGIC CANCERS
There is abundant evidence that vascular endothelial growth factor (VEGF) signal transduction plays a central role in disease progression and prognosis for carcinomas of the ovary, endometrium and cervix. From a molecular mechanistic standpoint, multiple cell types in the tumor microenvironment, including endothelial cells, stromal cells and tumor cells themselves may express functional VEGF receptors. The synthesis and release of VEGF by tumor cells may trigger these receptors and promote a phenotype conducive to tumor proliferation, invasion and metastasis. This is predominantly, though not exclusively, through induction of angiogenesis, characterized initially by the development of immature, abnormally permeable, micro-vascular networks from existing blood and lymphatic vessels. In epithelial ovarian cancer (EOC) and primary peritoneal cancer, VEGF expression is thought to be the key promoter of malignant ascites and pleural effusions.3-9
The degree of tumor angiogenesis within individual gynecologic tumors appears to have prognostic importance. Microvessel density (MVD) in primary epithelial carcinomas of the ovary,10-14 endometrium15-19 and cervix20-22 has correlated with extent of disease and has inversely correlated with overall survival (OS) or progression free survival (PFS) after initial therapy. Often this relationship to clinical outcome has been found to be independent of important clinical and pathologic prognostic factors.13,14,16-22 In addition VEGF has demonstrated prognostic value23-25 in accordance with its known functional relationship to angiogenesis.
In addition to correlative clinical studies cited above, pre-clinical investigations have provided a strong rationale for clinical trials of anti-VEGF therapeutics in gynecologic malignancies. There is ample evidence in human ovarian cancer xenograft models that direct blockade of VEGF activity alone can result in decreased tumor growth, metastasis and malignant ascites formation.3,7,26-29 Multiple mechanisms may explain the observed "anti-tumor" effect in these pre-clinical models of VEGF inhibition. In the most widely accepted model, the anti-angiogenic effect involves both blockade in the formation of new vessels and maturation of existing immature vessels. The latter process, initially described by Jain et al.30 involves normalization of the primitive tumor microvasculature through endothelial cell maturation, restoration of pericyte continuity, decrease in micro-vascular permeability and interstitial pressure, and re-establishment of normal flow. Vascular normalization is thought to result in decreased metastatic potential and enhanced delivery of other systemic anti-tumor agents such as cytotoxic drugs. Indirect evidence of an anti-neoplastic effect independent of tumor angiogenesis has also been described for some solid tumors. Functional VEGF receptors are expressed on tumor cells in multiple solid tumor types, including colon, breast and ovarian carcinomas.31-35 Studies of breast carcinoma cells in vitro have demonstrated that stimulation with exogenous VEGF may increase invasive potential and stimulate growth factor signaling.31
CLINICAL TRIALS OF VEGF INHIBITORS FOR GYNECOLOGIC MALIGNANCIES
1. General considerations
Two broad classes of anti-VEGF agents have been developed for clinical application - those which directly neutralize VEGF (ligand-specific) and those which bind to and inactivate functional VEGF receptors. The first class is comprised of large molecules which tend to be administered systemically. These include bevacizumab, a humanized monoclonal antibody (mAb) which neutralizes VEGF-A, the predominantly active species of VEGF;36 and VEGF-TrapR1R2 (aflibercept, AVE 0005), a soluble decoy receptor generated by fusing the constant region of IgG1 with the ligand binding domains of two principle anti-VEGF receptors, then optimized for VEGF binding affinity and pharmacokinetics.37 The second class is comprised mostly (but not exclusively) of orally administered small molecules which tend to block tyrosine kinase activity located in the cytoplasmic domain of VEGF receptors. The exception to this is the drug ramucirumab, a monoclonal antibody which specifically recognizes the predominantly active VEGF receptor, VEGFR-2.38
It is worth discussing relative advantages and disadvantages of small molecular inhibitors (Ibs) and monoclonal antibodies (mAbs). Ibs are orally bio-available, but often require daily administration due to their relatively short half-lives. In addition, based on the route of administration, systemic levels may be more highly variable than for mAbs. While some of the Ibs (e.g. cediranib,39 pazopanib40) are almost exclusively specific for VEGFR, others target multiple signal transduction pathways in addition to that for VEGF. Examples of the latter agents include sorafenib41 and sunitinib,42 which target both VEGFR and the platelet-derived growth factor receptor (PDGFR). PDGF signal transduction appears to stimulate later phases of tumor angiogenesis involving vessel maturation (see discussion of PDGF inhibition below). MAb therapy is systemic in nature but requires less frequent administration due to longer clearance times; they are in principle more uniformly bio-distributed than Ibs. Due to affinity for single targets, they are associated with a more limited range of potential anti-tumor effects. Recent strategies have involved combinations with both mAbs and Ibs, for example the phase I/II trial of bevacizumab and sorafenib discussed later in this article.
VEGF inhibitors have demonstrated unique toxicities, some which appear to be related to interference with known physiologic effects of VEGF, and others whose mechanisms have yet to be elucidated. The largest database comes from studies of bevacizumab36,43 in clinical trials utilizing the US National Cancer Institute (NCI) Common Toxicity Criteria (CTC),44 though the spectrum of adverse effects appears to be consistent across most VEGF inhibitors. Proteinuria is common but fortunately mild and self-limited in the vast majority of patients; nephrotic range proteinuria has been observed in only 0.5% of the treated population. Hypertension is also common, and though 8% to 18% develop grade 3 or higher blood pressure elevation, the majority of such patients can be stabilized with single agent anti-hypertensive therapy. Mucosal hemorrhage can be seen, most commonly in the form of low grade epistaxis; however, patients with central bronchogenic non-small cell lung cancers are at risk for high grade hemoptysis. Interference with wound healing has been a source of concern in the treatment of patients in the peri-operative period. Fortunately, wound dehiscense has been limited to 1% patients at potential risk. In large-scale randomized control trials of bevacizumab, the incidence of arterial thrombotic events (ATE) has been approximately 4.4% in treated subjects, compared with 1.9% in controls; risk factors include advanced age and pre-existing arterial vascular disease. Other rare but unique toxicities include reversible posterior leukoencephalopathy syndrome (RPLS), occurring in fewer than 0.1% of patients and characterized by a variety of central nervous system manifestations such as mental status changes, seizures, visual disturbance, usually with hypertension. Perhaps the most concerning adverse effect is the development of gastrointestinal perforation (GIP) or fistula. This complication has been reported in approximately 2.4% overall, and in 5% of patients with epithelial ovarian cancer. The mechanisms of GIP have not yet been elucidated; however, proposed risk factors include intestinal obstruction, ischemia, trans-mural tumor infiltration, infectious or non-infectious inflammatory bowel diseases - unlikely dose or duration of therapy.
While it is important to recognize the contribution of published historical experience, for succinctness, and in order to concentrate on the highest levels of evidence, the current article reviews only prospective clinical research. Needless to say, the integration of VEGF targeted therapeutics into clinical trials for gynecologic malignancies has been pursued enthusiastically, yet the knowledgebase generated from gynecologic cancer trials pales in comparison to that produced through research on non-gynecologic tumors. A review of the current US NCI database45 identified 51 registered trials in at least phase II development, 37 examining VEGF neutralizing agents and 15 investigating VEGFR inhibitors (one trial evaluates a combination of both drug classes). Of these 51, only 20 have been closed and published final results are available for only four studies. When one takes into account the epidemiologic aspects of gynecologic malignancies discussed earlier, it is not surprising that the 41 of these 51 clinical trials utilizing VEGF inhibitors have been focused on the treatment of EOC (including primary peritoneal and fallopian tube carcinomas).
The remainder of this article is focused on the assessment of this group of clinical trials. The information is organized first by design, then by disease site, with consideration of what has been learned, the design and rationale for investigation in progress, and future directions.
2. Single agent VEGF inhibitor trials: standard phase II studies
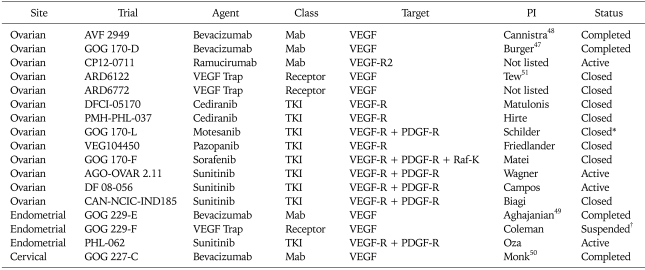
As demonstrated in Table 2, 17 NCI registered trials have evaluated single agent anti-VEGF activity in patients with recurrent ovarian, endometrial and cervical carcinomas. Most of these have utilized two-stage designs in patients with NCI Response Evaluation Criteria in Solid Tumor (RECIST)46 measurable disease and have evaluated primary endpoints of response rate, PFS, or the combination. Thus far, the only completed, published (or accepted for publication) trials are those involving bevacizumab; these have demonstrated single agent activity in EOC,47,48 endometrial49 and cervical carcinoma.50
Table 2.
US NCI registered single agent phase II trials of VEGF inhibitors in recurrent gynecologic cancers
NCI: National Cancer Institute, VEGF: vascular endothelial growth factor, PI: principal investigator, MAb: monoclonal antibody, TKI: tyrosine kinase inhibitor.
*Secondary to toxicity, †Suspended after 1st stage, interim analysis pending.
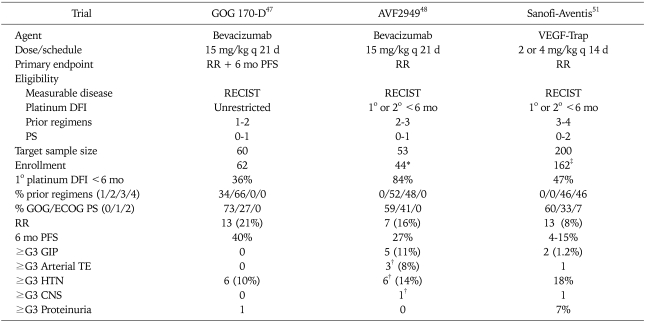
For EOC, the design and results of three, phase II trials of VEGF neutralizing agent monotherapy have been reported, two examining bevacizumab47,48 and one investigating VEGF-Trap (preliminary results published in abstract form only).51 As shown in Table 3, all three trials enrolled patients with RECIST measurable recurrent or persistent disease, yet demonstrated variable clinical activity of single agent therapy, with response rates ranging from 8% to 21% and 6-month PFS rates ranging from 4% to 40%. A recent report from the sponsor stated that the VEGF-Trap trial failed to achieve its primary endpoint of demonstrating either dose level to achieve a RECIST response rate statistically greater than 5%.52 Although it is unscientific to draw conclusions upon comparisons among separate phase II trials (and only preliminary data are available for the VEGF-Trap trial), one may hypothesize that clinical activity may be more favorable for patients with a longer interval from the completion of last platinum-containing chemotherapy, fewer prior cytotoxic regimens, and better performance status. In contradistinction, in the Gynecologic Oncology Group (GOG) trial47 an internal exploratory regression analysis failed to demonstrate any relationship of these known prognostic factors (or age) with PFS. To date there are no published pre-clinical or clinical studies prospectively comparing the anti-tumor activities of bevacizumab and VEGF-Trap.
Table 3.
Phase II trials of VEGF neutralizing single agent therapy in patients with epithelial ovarian and primary peritoneal cancer
RR: response rate, PFS: progression free survival, RECIST: Response Evaluation Criteria in Solid Tumor, PS: performance status, DFI: disease-free interval, GOG: Gynecologic Oncology Group, ECOG: Eastern Cooperative Oncology Group, GIP: gastrointestinal perforation, TE: thrombo-embolism, CNS: central nervous system.
*Trial terminated prematurely secondary to unacceptable frequency of gastrointestinal perforations, †Event fatal in one case, ‡Preliminary analysis.
With regard to adverse effects, when taken together, these trials demonstrated that single agent anti-VEGF therapy was tolerable in general, with the expected frequency of events. The exception to this was higher than expected rate of GIP in the third line Genentech AVF 2,949 trial of bevacizumab in platinum-resistant patients.48 This trial was terminated prematurely because of 5 GIPs reported out of the first 44 patients enrolled. In October 2005, the US FDA released an Action Letter53 alerting investigators and patients to this risk, even though a black box warning was already present on the package insert. At that time, the rates of GIP were highly variable among trials, (as a distinct example, there were no events in 62 patients treated on the GOG phase II bevacizumab trial). As mentioned previously, a 2007 review of GIP by Han et al.54 the aggregate number of events reported in published bevacizumab clinical trials and institutional off-label case series of EOC was 16 (5.2%) events in 308 patients. Based on this and other historical analyses, the risk factors for GIP remain unclear; clearly large scale prospective investigation would more reliably answer this question.
3. Consolidation therapy trials
Despite the fairly obvious rationale for such an approach, the investigation of VEGF inhibitors for their potential to extend PFS or OS in high risk patients considered to be in complete remission following standard therapies has been quite limited. To date there are only two registered trials of this nature. These are active phase II randomized trials in patients with EOC. One is a placebo controlled trial of single agent sorafenib; the other is a trial of bevacizumab +/- erlotinib (an epidermal growth factor receptor 1 Ib).
4. Combinations with cytotoxic regimens
The rationale for combining cytotoxic drugs with anti-VEGF therapy stemmed initially from additive and in some cases synergistic interaction in pre-clinical models. While an additive effect might be explained by complementary, independent anti-tumor activity, multiple purported mechanisms exist to promote synergistic interaction, including sensitization to apoptosis, reversal of cytotoxic drug resistance, and increased tumor access to chemotherapeutics secondary to vascular normalization (see above).30 It has also been hypothesized that combining VEGF targeted agents with frequently administered low dose, so called metronomic chemotherapy may have additive or synergistic anti-angiogenic or anti-tumor effects.
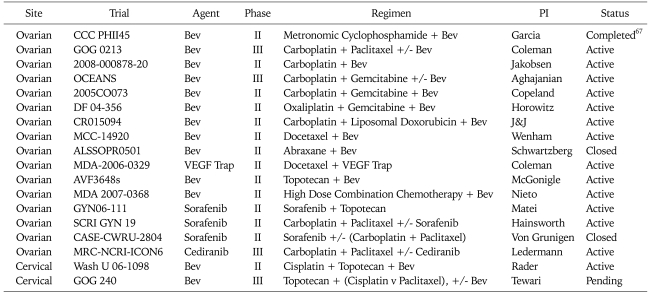
Given the results of pre-clinical studies referenced above and multiple positive phase III trials (all with bevacizumab) in non-gynecologic cancers,55-58 it is not surprising that trials investigating anti-VEGF and cytotoxic therapy combinations in patients with gynecologic tumors have been pursued with vigor. These are listed in Table 4 and Table 5.
Table 4.
National Cancer Institute registered trials of VEGF inhibitors in combination with cytotoxic agents for recurrent gynecologic cancers
VEGF: vascular endothelial growth factor, PI: principal investigator, Bev: bevacizumab.
Table 5.
US National Cancer Institute registered front-line trials of VEGF inhibitors in combination with cytotoxic agents for gynecologic cancers
VEGF: vascular endothelial growth factor, PI: principal investigator, Bev: bevacizumab.
While it would appear reasonable for cytotoxic agents considered active in gynecologic cancers to be combined with anti-VEGF agents in phase II trials, many such trials have perhaps been initiated without evidence of safety or preclinical evidence of benefit, in a "cart before horse" fashion. This enthusiasm has also been reflected in multiple published multi-institutional historic cohort studies of VEGF inhibitors in recurrent EOC, endometrial and cervical cancer reflecting the utilization of VEGF inhibitors in combination with cytotoxic agents in routine clinical practice.59-66 It is unclear whether combination therapy is a better choice than single agent therapy in this setting without controlled clinical trials data.
Perhaps the first phase II trial of combined cytotoxic and anti-VEGF bevacizumab and low dose oral cyclophosphamide in 70 patients with recurrent or persistent EOC.67 Patients were treated with bevacizumab at 10 mg/kg every 14 days with 50 mg of daily oral cyclophosphamide. Based on similar eligibility criteria, patient and disease characteristics were similar to that of the GOG 170-D population. This regimen was associated with a toxicity profile similar to the single agent bevacizumab trial with the exception of 2 cases of G4 cerebral ischemia, 2 cases of pulmonary hypertension, 4 GI perforation events and 3 treatment related deaths. With regard to efficacy, 56% of patients were progression-free as of 6 months and 24% had partial clinical responses. These findings provide rationale for a phase II randomized trial of combination versus single agent therapy, but early stopping rules for excessive toxicity in such trials would be important.
Additional reports of outcomes for patients with epithelial ovarian and primary peritoneal cancers treated with bevacizumab include at least three historical case series of patients treated outside clinical trials with single agent therapy or in combination with cytotoxic drugs, suggesting activity in more heavily pre-treated patients with recurrent disease61,63,66 and two single arm phase II studies demonstrating the feasibility of the combination of traditional carboplatin-paclitaxel chemotherapy combined with bevacizumab in front line therapy.68,69
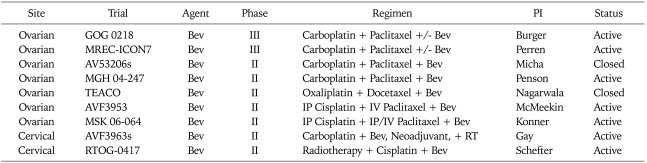
Taken together, these results support the need for randomized trials to determine relative efficacy and toxicity. As shown in Table 4, four such studies are in progress for recurrent EOC. One example is GOG 213, a phase III trial of second line therapy with carboplatin and paclitaxel, with or without bevacizumab, in patients with initial platinum-free intervals of at least 6 months, with a primary endpoint of overall survival. To address the potential added benefit of secondary cytoreductive surgery, in this study, patients who are deemed to be "surgical candidates" undergo secondary randomization to surgery versus no surgery. Another is a placebo controlled trial of carboplatin and gemcitabine with or without bevacizumab in a similar population, with primary endpoint of PFS.
As demonstrated in Table 5, currently, two phase III trials of bevacizumab in front-line therapy are in progress - GOG 0218, activated September 2005 and ICON7, activated October 2006. Both trials include six cycles of standard platinum-taxane chemotherapy, but there are important differences between the two trials which should be noted. GOG 218 is a three-arm, placebo-controlled trial, whose primary objective is to determine whether the addition of bevacizumab (15 mg/kg every 21 days) to standard cytotoxic therapy, when administered concurrently, or concurrently plus extended for an additional 16 cycles, will produce an improvement in PFS. It is limited to patients with stage III or IV disease. In contrast, ICON-7 is a two-arm trial without a placebo, with the experimental arm containing bevacizumab (7.5 mg/kg every 21 days) concomitantly with cytotoxic therapy, then extended for 12 cycles, also with the primary endpoint of PFS. The patient population for the ICON trial includes all patients with at least high risk early stage disease. As far as secondary endpoints are concerned, both trials will systematically examine quality of life, and while translational research will be performed in the context of GOG 218 and a pharmaco-economic analysis is planned for ICON-7.
Clinical trials combining anti-VEGF agents with cytotoxic drugs in the treatment of endometrial and cervical cancers are relatively scarce when compared with those for EOC. Only one trial is currently active in the NCI Database, a GOG phase III randomized trial of cisplatin plus paclitaxel with or without bevacizumab vs. the non-platinum doublet, topotecan plus paclitaxel, with or without bevacizumab.
With respect to endometrial cancer, the reason for the paucity of phase III trials has mostly to do with impact on public health in industrialized nations, but there may be other explanations - relatively few indications for systemic therapy, and the observation that epithelial ovarian cancers and advanced endometrial cancers appear to be similar with respect to histologic cell types (endometrioid, serous, clear cell) and biologic behavior. Hence, the development of systemic therapy for patients with advanced endometrial cancers has tended to shadow the development of systemic therapy for patients with epithelial ovarian cancers.
The situation for carcinomas of the cervix is even more pronounced, with only 4,070 cancer deaths estimated for 2009.1 Again, in 2006, of the 9,710 annual cases of cervical cancer in the U.S., over half were classified as localized, with over 90% of patients cured using standard modalities.2 However, given that this disease is a major cause of morbidity and mortality in less industrialized regions, novel approaches to systemic therapy are still needed. Given the potential for anti-VEFG inhibitors to restore microcirculation, phase I and II trials are in progress to explore the interaction between such agents and standard chemo-radiation in the management of patients with advanced disease.
5. Combinations with other biologic targeted agents
Although conceptually attractive, the evaluation of rational combinations of anti-VEGF and other biologic agents is in its infancy. One avenue of pursuit has been cross-talk between the VEGF and EGFR-1 pathway. However, thus far preliminary data suggest lack of additional benefit for anti-EGFR-1 drugs.70 Another trial is evaluating the combination of bevacizumab with Sorafenib, attempting to exploit simultaneous blockade of VEGF and its receptors, as well as other pathways potentially involved in ovarian cancer progression.
SUMMARY AND FUTURE DIRECTIONS
In summary, VEGF appears to be a driving force in the biology of tumor progression for the most common gynecologic malignancies, likely related to multiple mechanisms. VEGF neutralizing therapy has demonstrated clinical benefit in phase II trials for all three disease sites. The addition of these agents to standard therapy is now being explored in phase II randomized and phase III trials, with results from those in patients with EOC maturing within the coming year. Said with cautious optimism, VEGF-targeted therapeutics might one day represent another standard modality to complement surgery, cytotoxic chemotherapy, and radiotherapy in the control of these diseases.
There are several future directions which should be considered to be of high priority within the next ten years. These include both clinical-pathologic and pre-clinical investigation on the mechanisms of gastrointestinal perforation in order to identify true predictors of this complication; hypothesis driven correlative laboratory research in the context of phase III trials in order to identify factors predictive of efficacy for anti-VEGF agents; studies to determine mechanisms resistance or escape from anti-VEGF therapy; and clinical trials to determine the potential utility of continuing versus discontinuing anti-VEGF therapy after disease progression.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigel C, et al. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106–130. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 3.Byrne AT, Ross L, Holash J, Nakanishi M, Hu L, Hofmann JI, et al. Vascular endothelial growth factor-trap decreases tumor burden, inhibits ascites, and causes dramatic vascular remodeling in an ovarian cancer model. Clin Cancer Res. 2003;9:5721–5728. [PubMed] [Google Scholar]
- 4.Hu L, Hofmann J, Zaloudek C, Ferrara N, Hamilton T, Jaffe RB. Vascular endothelial growth factor immunoneutralization plus Paclitaxel markedly reduces tumor burden and ascites in athymic mouse model of ovarian cancer. Am J Pathol. 2002;161:1917–1924. doi: 10.1016/S0002-9440(10)64467-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luo JC, Toyoda M, Shibuya M. Differential inhibition of fluid accumulation and tumor growth in two mouse ascites tumors by an antivascular endothelial growth factor/permeability factor neutralizing antibody. Cancer Res. 1998;58:2594–2600. [PubMed] [Google Scholar]
- 6.Luo JC, Yamaguchi S, Shinkai A, Shitara K, Shibuya M. Significant expression of vascular endothelial growth factor/vascular permeability factor in mouse ascites tumors. Cancer Res. 1998;58:2652–2660. [PubMed] [Google Scholar]
- 7.Mesiano S, Ferrara N, Jaffe RB. Role of vascular endothelial growth factor in ovarian cancer: inhibition of ascites formation by immunoneutralization. Am J Pathol. 1998;153:1249–1256. doi: 10.1016/S0002-9440(10)65669-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Urick ME, Giles JR, Johnson PA. VEGF expression and the effect of NSAIDs on ascites cell proliferation in the hen model of ovarian cancer. Gynecol Oncol. 2008;110:418–424. doi: 10.1016/j.ygyno.2008.05.018. [DOI] [PubMed] [Google Scholar]
- 9.Verheul HM, Hoekman K, Jorna AS, Smit EF, Pinedo HM. Targeting vascular endothelial growth factor blockade: ascites and pleural effusion formation. Oncologist. 2000;5(Suppl 1):45–50. doi: 10.1634/theoncologist.5-suppl_1-45. [DOI] [PubMed] [Google Scholar]
- 10.Abulafia O, Triest WE, Sherer DM. Angiogenesis in primary and metastatic epithelial ovarian carcinoma. Am J Obstet Gynecol. 1997;177:541–547. doi: 10.1016/s0002-9378(97)70143-1. [DOI] [PubMed] [Google Scholar]
- 11.Alvarez AA, Krigman HR, Whitaker RS, Dodge RK, Rodriguez GC. The prognostic significance of angiogenesis in epithelial ovarian carcinoma. Clin Cancer Res. 1999;5:587–591. [PubMed] [Google Scholar]
- 12.Gasparini G, Bonoldi E, Viale G, Verderio P, Boracchi P, Panizzoni GA, et al. Prognostic and predictive value of tumour angiogenesis in ovarian carcinomas. Int J Cancer. 1996;69:205–211. doi: 10.1002/(SICI)1097-0215(19960621)69:3<205::AID-IJC10>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 13.Goodheart MJ, Ritchie JM, Rose SL, Fruehauf JP, De Young BR, Buller RE. The relationship of molecular markers of p53 function and angiogenesis to prognosis of stage I epithelial ovarian cancer. Clin Cancer Res. 2005;11:3733–3742. doi: 10.1158/1078-0432.CCR-04-0056. [DOI] [PubMed] [Google Scholar]
- 14.Hollingsworth HC, Kohn EC, Steinberg SM, Rothenberg ML, Merino MJ. Tumor angiogenesis in advanced stage ovarian carcinoma. Am J Pathol. 1995;147:33–41. [PMC free article] [PubMed] [Google Scholar]
- 15.Chen CA, Cheng WF, Lee CN, Wei LH, Chu JS, Hsieh FJ, et al. Cytosol vascular endothelial growth factor in endometrial carcinoma: correlation with disease-free survival. Gynecol Oncol. 2001;80:207–212. doi: 10.1006/gyno.2000.6048. [DOI] [PubMed] [Google Scholar]
- 16.Kaku T, Kamura T, Kinukawa N, Kobayashi H, Sakai K, Tsuruchi N, et al. Angiogenesis in endometrial carcinoma. Cancer. 1997;80:741–747. doi: 10.1002/(sici)1097-0142(19970815)80:4<741::aid-cncr13>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 17.Obermair A, Tempfer C, Wasicky R, Kaider A, Hefler L, Kainz C. Prognostic significance of tumor angiogenesis in endometrial cancer. Obstet Gynecol. 1999;93:367–371. doi: 10.1016/s0029-7844(98)00417-7. [DOI] [PubMed] [Google Scholar]
- 18.Salvesen HB, Iversen OE, Akslen LA. Prognostic significance of angiogenesis and Ki-67, p53, and p21 expression: a population-based endometrial carcinoma study. J Clin Oncol. 1999;17:1382–1390. doi: 10.1200/JCO.1999.17.5.1382. [DOI] [PubMed] [Google Scholar]
- 19.Wagatsuma S, Konno R, Sato S, Yajima A. Tumor angiogenesis, hepatocyte growth factor, and c-Met expression in endometrial carcinoma. Cancer. 1998;82:520–530. doi: 10.1002/(sici)1097-0142(19980201)82:3<520::aid-cncr14>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 20.Bremer GL, Tiebosch AT, van der Putten HW, Schouten HJ, de Haan J, Arends JW. Tumor angiogenesis: an independent prognostic parameter in cervical cancer. Am J Obstet Gynecol. 1996;174:126–131. doi: 10.1016/s0002-9378(96)70384-8. [DOI] [PubMed] [Google Scholar]
- 21.Cooper RA, Wilks DP, Logue JP, Davidson SE, Hunter RD, Roberts SA, et al. High tumor angiogenesis is associated with poorer survival in carcinoma of the cervix treated with radiotherapy. Clin Cancer Res. 1998;4:2795–2800. [PubMed] [Google Scholar]
- 22.Obermair A, Wanner C, Bilgi S, Speiser P, Kaider A, Reinthaller A, et al. Tumor angiogenesis in stage IB cervical cancer: correlation of microvessel density with survival. Am J Obstet Gynecol. 1998;178:314–319. doi: 10.1016/s0002-9378(98)80018-5. [DOI] [PubMed] [Google Scholar]
- 23.Ino K, Shibata K, Kajiyama H, Yamamoto E, Nagasaka T, Nawa A, et al. Angiotensin II type 1 receptor expression in ovarian cancer and its correlation with tumour angiogenesis and patient survival. Br J Cancer. 2006;94:552–560. doi: 10.1038/sj.bjc.6602961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paley PJ, Staskus KA, Gebhard K, Mohanraj D, Twiggs LB, Carson LF, et al. Vascular endothelial growth factor expression in early stage ovarian carcinoma. Cancer. 1997;80:98–106. doi: 10.1002/(sici)1097-0142(19970701)80:1<98::aid-cncr13>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 25.Shen GH, Ghazizadeh M, Kawanami O, Shimizu H, Jin E, Araki T, et al. Prognostic significance of vascular endothelial growth factor expression in human ovarian carcinoma. Br J Cancer. 2000;83:196–203. doi: 10.1054/bjoc.2000.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang S, Robinson JB, Deguzman A, Bucana CD, Fidler IJ. Blockade of nuclear factor-kappaB signaling inhibits angiogenesis and tumorigenicity of human ovarian cancer cells by suppressing expression of vascular endothelial growth factor and interleukin 8. Cancer Res. 2000;60:5334–5339. [PubMed] [Google Scholar]
- 27.Mu J, Abe Y, Tsutsui T, Yamamoto N, Tai XG, Niwa O, et al. Inhibition of growth and metastasis of ovarian carcinoma by administering a drug capable of interfering with vascular endothelial growth factor activity. Jpn J Cancer Res. 1996;87:963–971. doi: 10.1111/j.1349-7006.1996.tb02127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sher I, Adham SA, Petrik J, Coomber BL. Autocrine VEGF-A/KDR loop protects epithelial ovarian carcinoma cells from anoikis. Int J Cancer. 2009;124:553–561. doi: 10.1002/ijc.23963. [DOI] [PubMed] [Google Scholar]
- 29.Xu L, Yoneda J, Herrera C, Wood J, Killion JJ, Fidler IJ. Inhibition of malignant ascites and growth of human ovarian carcinoma by oral administration of a potent inhibitor of the vascular endothelial growth factor receptor tyrosine kinases. Int J Oncol. 2000;16:445–454. doi: 10.3892/ijo.16.3.445. [DOI] [PubMed] [Google Scholar]
- 30.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 31.Price DJ, Miralem T, Jiang S, Steinberg R, Avraham H. Role of vascular endothelial growth factor in the stimulation of cellular invasion and signaling of breast cancer cells. Cell Growth Differ. 2001;12:129–135. [PubMed] [Google Scholar]
- 32.Chen H, Ye D, Xie X, Chen B, Lu W. VEGF, VEGFRs expressions and activated STATs in ovarian epithelial carcinoma. Gynecol Oncol. 2004;94:630–635. doi: 10.1016/j.ygyno.2004.05.056. [DOI] [PubMed] [Google Scholar]
- 33.Fan F, Wey JS, McCarty MF, Belcheva A, Liu W, Bauer TW, et al. Expression and function of vascular endothelial growth factor receptor-1 on human colorectal cancer cells. Oncogene. 2005;24:2647–2653. doi: 10.1038/sj.onc.1208246. [DOI] [PubMed] [Google Scholar]
- 34.Boocock CA, Charnock-Jones DS, Sharkey AM, McLaren J, Barker PJ, Wright KA, et al. Expression of vascular endothelial growth factor and its receptors flt and KDR in ovarian carcinoma. J Natl Cancer Inst. 1995;87:506–516. doi: 10.1093/jnci/87.7.506. [DOI] [PubMed] [Google Scholar]
- 35.Wu Y, Hooper AT, Zhong Z, Witte L, Bohlen P, Rafii S, et al. The vascular endothelial growth factor receptor (VEGFR-1) supports growth and survival of human breast carcinoma. Int J Cancer. 2006;119:1519–1529. doi: 10.1002/ijc.21865. [DOI] [PubMed] [Google Scholar]
- 36.Bevacizumab: anti-VEGF monoclonal antibody, avastin, rhumab-VEGF. Drugs R D. 2002;3:28–30. doi: 10.2165/00126839-200203010-00006. [DOI] [PubMed] [Google Scholar]
- 37.Aflibercept: AVE 0005, AVE 005, AVE0005, VEGF Trap - Regeneron, VEGF Trap (R1R2), VEGF Trap-Eye. Drugs R D. 2008;9:261–269. doi: 10.2165/00126839-200809040-00006. [DOI] [PubMed] [Google Scholar]
- 38.Krupitskaya Y, Wakelee HA. Ramucirumab, a fully human mAb to the transmembrane signaling tyrosine kinase VEGFR-2 for the potential treatment of cancer. Curr Opin Investig Drugs. 2009;10:597–605. [PubMed] [Google Scholar]
- 39.Lindsay CR, MacPherson IR, Cassidy J. Current status of cediranib: the rapid development of a novel anti-angiogenic therapy. Future Oncol. 2009;5:421–432. doi: 10.2217/fon.09.18. [DOI] [PubMed] [Google Scholar]
- 40.Sloan B, Scheinfeld NS. Pazopanib, a VEGF receptor tyrosine kinase inhibitor for cancer therapy. Curr Opin Investig Drugs. 2008;9:1324–1335. [PubMed] [Google Scholar]
- 41.Strumberg D. Preclinical and clinical development of the oral multikinase inhibitor sorafenib in cancer treatment. Drugs Today (Barc. 2005;41:773–784. doi: 10.1358/dot.2005.41.12.937959. [DOI] [PubMed] [Google Scholar]
- 42.Izzedine H, Buhaescu I, Rixe O, Deray G. Sunitinib malate. Cancer Chemother Pharmacol. 2007;60:357–364. doi: 10.1007/s00280-006-0376-5. [DOI] [PubMed] [Google Scholar]
- 43.AVSTIN (bevacizumab): Full prescribing information, including boxed warnings [Internet] South San Francisco: Genentech Inc.; c2009. [cited 2010 Jan 20]. Available from: http://www.gene.com/gene/products/information/oncology/avastin/insert.jsp. [Google Scholar]
- 44.US National Cancer Institute. Bethesda: US National Cancer Institute; c2010. [cited 2010 Jan 20]. Common toxicity criteria [Internet] Available from: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm. [Google Scholar]
- 45.US National Cancer Institute. Bethesda: US National Cancer Institute; c2010. [cited 2010 Jan 20]. Clinical trials [Internet] Available from: http://www.cancer.gov/clinicaltrials. [Google Scholar]
- 46.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 47.Burger RA, Sill MW, Monk BJ, Greer BE, Sorosky JI. Phase II trial of bevacizumab in persistent or recurrent epithelial ovarian cancer or primary peritoneal cancer: a Gynecologic Oncology Group Study. J Clin Oncol. 2007;25:5165–5171. doi: 10.1200/JCO.2007.11.5345. [DOI] [PubMed] [Google Scholar]
- 48.Cannistra SA, Matulonis UA, Penson RT, Hambleton J, Dupont J, Mackey H, et al. Phase II study of bevacizumab in patients with platinum-resistant ovarian cancer or peritoneal serous cancer. J Clin Oncol. 2007;25:5180–5186. doi: 10.1200/JCO.2007.12.0782. [DOI] [PubMed] [Google Scholar]
- 49.Aghajanian C, Sill MW, Darcy K, Greer B, McMeekin DS, Rose PG, et al. A phase II evaluation of bevacizumab in the treatment of recurrent or persistent endometrial cancer: a Gynecologic Oncology Group (GOG) Study. J Clin Oncol. 2009;27(15S):abstr 5531. doi: 10.1200/JCO.2010.32.6397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Monk BJ, Sill MW, Burger RA, Gray HJ, Buekers TE, Roman LD. Phase II trial of bevacizumab in the treatment of persistent or recurrent squamous cell carcinoma of the cervix: a gynecologic oncology group study. J Clin Oncol. 2009;27:1069–1074. doi: 10.1200/JCO.2008.18.9043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tew WP, Colombo N, Ray-Coquard I, del Campo J, Scambia G, Spriggs D. VEGF-Trap for patients (pts) with recurrent platinum-resistant epithelial ovarian cancer (EOC) : preliminary results of a randomized, multicenter phase II study. J Clin Oncol. 2007;25(18S):abstr 5508. [Google Scholar]
- 52.CNBC News [Internet]. Sanofi, regeneron cancer drug misses study goal (21 May 2008) Englewood Cliffs (NJ): CNBC Inc.; c2009. [cited 2008 May 21]. Available from: http://www.cnbc.com/id/24755167. [Google Scholar]
- 53.National Cancer Institute. Investigational Drug Branch, U.S. Department of Health & Human Services, Cancer Therapy Evaluation Program (CTEP): Bevacizumab IND action letter. Bethesda: National Cancer Institute; 2005. [Google Scholar]
- 54.Han ES, Monk BJ. What is the risk of bowel perforation associated with bevacizumab therapy in ovarian cancer? Gynecol Oncol. 2007;105:3–6. doi: 10.1016/j.ygyno.2007.01.038. [DOI] [PubMed] [Google Scholar]
- 55.Giantonio BJ, Catalano PJ, Meropol NJ, O'Dwyer PJ, Mitchell EP, Alberts SR, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007;25:1539–1544. doi: 10.1200/JCO.2006.09.6305. [DOI] [PubMed] [Google Scholar]
- 56.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 57.Miller KD, Chap LI, Holmes FA, Cobleigh MA, Marcom PK, Fahrenbacher L, et al. Randomized phase III trial of capecitabine compared with bevacizumab plus capecitabine in patients with previously treated metastatic breast cancer. J Clin Oncol. 2005;23:792–799. doi: 10.1200/JCO.2005.05.098. [DOI] [PubMed] [Google Scholar]
- 58.Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 59.Bidus MA, Webb JC, Seidman JD, Rose GS, Boice CR, Elkas JC. Sustained response to bevacizumab in refractory well-differentiated ovarian neoplasms. Gynecol Oncol. 2006;102:5–7. doi: 10.1016/j.ygyno.2006.03.048. [DOI] [PubMed] [Google Scholar]
- 60.Burger RA. Experience with bevacizumab in the management of epithelial ovarian cancer. J Clin Oncol. 2007;25:2902–2908. doi: 10.1200/JCO.2007.12.1509. [DOI] [PubMed] [Google Scholar]
- 61.Cohn DE, Valmadre S, Resnick KE, Eaton LA, Copeland LJ, Fowler JM. Bevacizumab and weekly taxane chemotherapy demonstrates activity in refractory ovarian cancer. Gynecol Oncol. 2006;102:134–139. doi: 10.1016/j.ygyno.2006.01.030. [DOI] [PubMed] [Google Scholar]
- 62.Monk BJ, Choi DC, Pugmire G, Burger RA. Activity of bevacizumab (rhuMAB VEGF) in advanced refractory epithelial ovarian cancer. Gynecol Oncol. 2005;96:902–905. doi: 10.1016/j.ygyno.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 63.Monk BJ, Han E, Josephs-Cowan CA, Pugmire G, Burger RA. Salvage bevacizumab (rhuMAB VEGF)-based therapy after multiple prior cytotoxic regimens in advanced refractory epithelial ovarian cancer. Gynecol Oncol. 2006;102:140–144. doi: 10.1016/j.ygyno.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 64.Richardson DL, Backes FJ, Seamon LG, Zanagnolo V, O'Malley DM, Cohn DE, et al. Combination gemcitabine, platinum, and bevacizumab for the treatment of recurrent ovarian cancer. Gynecol Oncol. 2008;111:461–466. doi: 10.1016/j.ygyno.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 65.Wright JD, Viviano D, Powell MA, Gibb RK, Mutch DG, Grigsby PW, et al. Bevacizumab combination therapy in heavily pretreated, recurrent cervical cancer. Gynecol Oncol. 2006;103:489–493. doi: 10.1016/j.ygyno.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 66.Wright JD, Hagemann A, Rader JS, Viviano D, Gibb RK, Norris L, et al. Bevacizumab combination therapy in recurrent, platinum-refractory, epithelial ovarian carcinoma: a retrospective analysis. Cancer. 2006;107:83–89. doi: 10.1002/cncr.21969. [DOI] [PubMed] [Google Scholar]
- 67.Garcia AA, Hirte H, Fleming G, Yang D, Tsao-Wei DD, Roman L, et al. Phase II clinical trial of bevacizumab and low-dose metronomic oral cyclophosphamide in recurrent ovarian cancer: a trial of the California, Chicago, and Princess Margaret Hospital phase II consortia. J Clin Oncol. 2008;26:76–82. doi: 10.1200/JCO.2007.12.1939. [DOI] [PubMed] [Google Scholar]
- 68.Micha JP, Goldstein BH, Rettenmaier MA, Genesen M, Graham C, Bader K, et al. A phase II study of outpatient first-line paclitaxel, carboplatin, and bevacizumab for advanced-stage epithelial ovarian, peritoneal, and fallopian tube cancer. Int J Gynecol Cancer. 2007;17:771–776. doi: 10.1111/j.1525-1438.2007.00886.x. [DOI] [PubMed] [Google Scholar]
- 69.Penson RT, Cannistra SA, Seiden MV, Krasner CN, Matulonis UA, Horowitz NS, et al. Phase II study of carboplatin, paclitaxel and bevacizumab as first line chemotherapy and consolidation for advanced mullerian tumors. J Clin Oncol. 2006;24(18S):abstr 5020. doi: 10.1200/JCO.2009.22.7900. [DOI] [PubMed] [Google Scholar]
- 70.Nimeiri HS, Oza AM, Morgan RJ, Friberg G, Kasza K, Faoro L, et al. Efficacy and safety of bevacizumab plus erlotinib for patients with recurrent ovarian, primary peritoneal, and fallopian tube cancer: a trial of the Chicago, PMH, and California Phase II Consortia. Gynecol Oncol. 2008;110:49–55. doi: 10.1016/j.ygyno.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]