Abstract
Objective
Aberrant expression of the cell surface proteoglycan, syndecan-1, is found in many malignancies. The current study describes the immunohistochemical study of syndecan-1 expression in normal, hyperplastic, and malignant endometrial tissues for evaluation of application as a parameter of cancer progression in patients with endometrial hyperplasia.
Methods
Immunohistochemical staining of syndecan-1 was performed in 101 formalin fixed, paraffin embedded sections of normal, hyperplastic, and malignant endometrial tissues. We analyzed specimens from patients with normal endometrium (NE, N=10) as controls, and those of simple hyperplasia (SH, N=20), complex hyperplasia without atypia (CH, N=20), atypical hyperplasia (AH, N=20), and endometrial cancer (EC, N=31).
Results
The mean rank of expression scores based on the frequency of syndecan-1 staining were 31.6, 20.5, 52.9, 72.1, and 62.1 for NE, SH, CH, AH and EC, respectively (p<0.001). Syndecan-1 expression was significantly greater in CH (p<0.001) or AH (p<0.001) than in SH, and significantly greater in AH compared to CH (p=0.028). Syndecan-1 is more frequently expressed in CH (p=0.042), AH (p<0.001), or EC (p=0.002) than in NE. Syndecan-1 expression did not differ significantly between NE and SH (p=0.248).
Conclusion
Syndecan-1 expression appears to be useful as a predictive indicator in endometrial hyperplasia.
Keywords: Syndecan-1, Endometrial hyperplasia, Endometrial cancer
INTRODUCTION
Endometrial cancer is the most common disease among gynecological cancers and is one of the main health concerns worldwide.1,2 In the United States alone, 41,200 women develop endometrial cancer, and about 7,350 women die from this disease annually.2 According to the Annual Report of Gynecologic Cancer Registry Program in Korea, the incidence of endometrial cancer in Korean women is recently increasing.1 Endometrial cancer may occur de novo, but can also arise within preexisting premalignant lesions exhibiting advanced degrees of hyperplasia.3 In 1994, according to the WHO classification, simple and complex types of hyperplasia are further subdivided into those with typical or atypical architecture.4 This is the most important distinction from the stand point of clinical management, because the risk of malignant transformation of hyperplasia to invasive carcinoma increases according to the classification of hyperplasia from 2 to 23% in premalignant lesions exhibiting without atypia to with atypia, respectively.5 The progression of a lesion from a premalignant state to endometrial cancer is believed to be the outcome of a series of metagenesis driven by various risk factors.3 The identification of specific intracellular events in carcinogenesis is a necessary prerequisite to the identification of therapeutics that target and interrupt specific steps in the progression of cancer.6
Previous studies have suggested a correlation between the expression of syndecan-1 and the development of cancer characteristics in the human endometrium.7 However, it is not yet clear whether syndecan-1 is closely correlated with the onset of tumorigenesis or if it plays a role as a positive predictive marker in cancer development. For these reasons, we sought to determine if quantitative analysis of syndecan-1 correlated with the histological classification of endometrial hyperplasia by evaluating syndecan-1 expression in endometrial hyperplasia and stage I, grade I cancer tissues by determining the percentage of positively stained cells to yield a quantitative value for syndecan-1 expression. Our results suggest the role of syndecan-1 as a predictive factor of progression from hyperplasia to endometrial cancer.
MATERIALS AND METHODS
1. Patients and tissue samples
Tissue samples were obtained from the paraffin-embedded endometrium of study 101 patients who underwent endometrial curettage and/or hysterectomy and were followed up at the Department of Obstetrics and Gynecology of Ajou University Hospital, Suwon, Korea between 1995 and 2007. The patient population consisted of individuals including endometrial hyperplasia (N=60). Endometrial data obtained were classified into five groups: normal endometrium (NE, N=10) as the control group, simple hyperplasia (SH, N=20), complex hyperplasia without atypia (CH, N=20), complex hyperplasia with atypia or atypical hyperplasia (AH, N=20), and endometrial cancer (EC, N=31) with grade 1 differentiation. NE was obtained from patients with intramural or subserosal myomas showing grossly and microscopically intact endometrium. Specimen was obtained from representative endometrial pathologies: hysterectomized endometrium (N=63) or endometrial curettage (N=38).
We performed a quantitative analysis of syndecan-1 expression employing a scoring method of the immunohistochemical staining frequency. Histopathologic classification of endometrial hyperplasia was based on the "International Society of Gynecological Pathologists" criteria. The histological grades according to the International Federation of Gynecology and Obstetrics (FIGO) staging classification were as follows: 31 patients in this study were grade 1. Surgical staging was reviewed based on the FIGO staging system and all of the endometrial cancer patients were stage I disease. Endometrial hyperplasia specimens were further subdivided by the presence of atypia and degree of differentiation. In all cases, atypical cells were identified on the original H&E sections (Fig. 1). Follow-up for all patients who had endometrial hyperplasia in the endometrial curettage specimen, as evidenced by staining with syndecan-1, was obtained when possible. We retrospectively reviewed patient charts to identify patient's demographic factors.
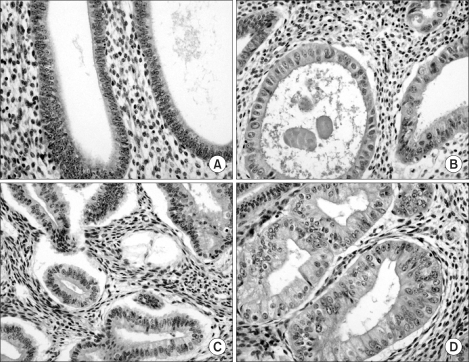
Fig. 1.
Sections of formalin-fixed, paraffin-embedded samples of endometrial tissues were stained with eosin and hematoxylin for hyperplasia classification (×400). (A) Simple hyperplasia without atypia. (B) Simple hyperplasia with atypia. (C) Complex hyperplasia without atypia. (D) Complex hyperplasia with atypia.
2. Immunohistochemical study
Serial paraffin sections were cut to 4 µm in thickness for immunohistochemical study of syndecan-1 expression in endometrial hyperplasia and endometrial cancer cells. Paraffin-embedded sections from clinical endometrial tissue samples were subjected to immunostaining for syndecan-1 using anti human syndecan-1 mouse B-B4 monoclonal antibody and DAB visualization, as described in the manufacturer's manual. In brief, after deparaffination and treatment of 3% H2O2 for 5 min, the tissue samples were blocked with a 10% serum in the blocking solution of the Histostain®-Plus Bulk Kit (Biocompare, San Francisco, CA, USA) for 1 hr and incubated overnight at 4℃ with anti syndecan-1 mouse monoclonal (B-B4 clone) antibodies at 1/400 dilution in the antibody diluent. For either tissue samples, DAB color reaction was performed with the Histostain®-Plus Bulk Kit before being examined under an Olympus microscope D50 (Olympus, Tokyo, Japan) and photographed using an AxioCam MRc5 camera (Carl Zeiss AG, Oberkochen, Germany). Then each stained section was counterstained with Mayer's hematoxylin. Also, the sections from the same tissues were stained with Mayer's hematoxylin and eosin for histological classification.
3. Staining evaluation
The level of syndecan-1 immunoreactivity in endometrial cells was expressed by scoring the percentage of syndecan-1 positive cells into four groups: 0, negative of cells stained; 1, <33% of cells stained; 2, 33 to 66% of cells stained; and 3, >66% of the cells stained.7 Microscopic analyses were evaluated independently by one pathologist (HJH) with no prior knowledge of the clinical data.
4. Statistical analysis
Distribution of the patients' characteristics was presented as mean (SD) for continuous variables, and frequency (%) for categorical variables including immunohistochemical staining. Fisher's exact test was used to determine the correlation between the two categorical variables. The Mann-Whitney U-test or Kruskall-Wallis test was used to compare the mean or median values between the two or more groups. A p-value <0.05 was considered statistically significant.
RESULTS
1. Patient characteristics
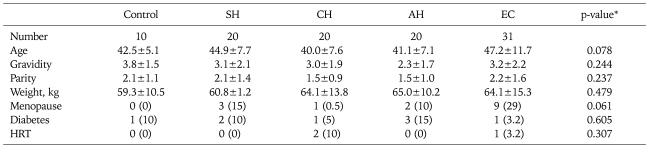
The mean age of included subjects at the time of diagnosis was 44 years (range, 21 to 71 years). There were no significant differences in age, gravidity, parity, body weight, menopausal status, diabetes status, and hormone replacement therapy status between the study groups. Demographic data of the patients are summarized in Table 1.
Table 1.
Demographic data of patients by histologic dating
Values are presented as mean±SD or no. (%).
AH: atypical hyperplasia (complex hyperplasia with atypia), CH: complex hyperplasia without atypia, EC: endometrial cancer, HRT: hormone replacement therapy, SH: simple hyperplasia.
*No significant difference were observed between the groups (p>0.05).
2. Expression of syndecan-1
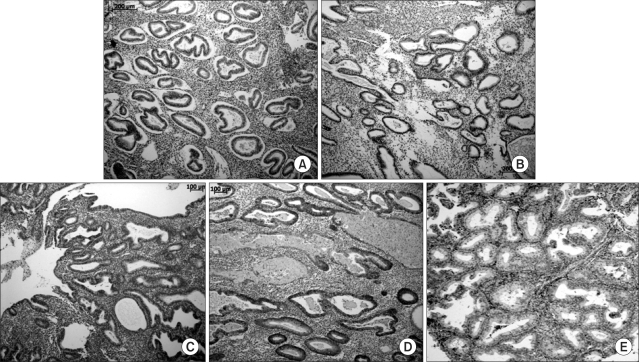
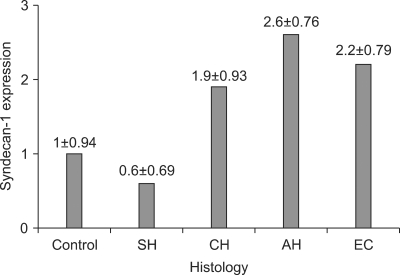
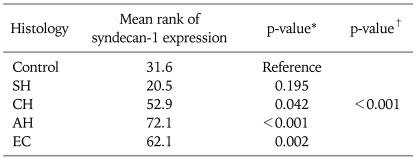
The immunostaining of syndecan-1 in the control, endometrial hyperplasias and endometrial cancer tissues are shown in Fig. 2. The mean score of syndecan-1 immunoreactivity was 1, 0.6, 1.9, 2.6, and 2.2 in NE, SH, CH, AH, and EC, respectively (Fig. 3). The mean rank score of staining according to classification of endometrial hyperplasia is shown in Table 2. In other words, the mean ranks of expression scores based on the frequency of syndecan-1 staining were 31.6, 20.5, 52.9, 72.1, and 62.1 with NE, SH, CH, AH, and EC, respectively (Table 2). The differences were statistically significant (p<0.001).
Fig. 2.
Immunohistochemical staining for syndecan-1 (×100). (A) Normal endometrium showing negative syndecan-1 staining (frequency score: 0). (B) Simple hyperplasia (frequency score: 1). (C) Complex hyperplasia without atypia (frequency score: 2). (D) Complex hyperplasia with atypia (frequency score: 3). (E) Almost tumor cells show positive syndecan-1 expression (frequency score: 3).
Fig. 3.
Immunostaining mean scores of syndecan-1 expression in normal control and in specimens of simple hyperplasia (SH), complex hyperplasia without atypia (CH), complex hyperplasia with atypia (AH), and endometrial cancer (EC).
Table 2.
Mean rank of syndecan-1 expression according to histology
AH: atypical hyperplasia (complex hyperplasia with atypia), CH: complex hyperplasia without atypia, EC: endometrial cancer, SH: simple hyperplasia.
*The Mann-Whitney U-test and †Kruskall-Wallis test was used to compare the mean values between the two or more groups.
In the subgroup analysis, syndecan-1 expression was significantly greater in CH (N=20, p<0.001) or AH (N=20, p<0.001) than SH (N=20). Syndecan-1 expression was significantly greater in AH compared to CH (p=0.028). Syndecan-1 expression was significantly greater in CH (p=0.042), AH (p<0.001), or EC (p=0.002) compared to NE. However, these differences in expression of syndecan-1 were not identified between SH and NE (p=0.248). Also, no significant difference was observed in syndecan-1 expression between AH and EC (p=0.085).
DISCUSSION
A number of immunohistochemical studies have demonstrated the aberrant expression or immunoreactivity of syndecan-1 in many malignancies, as well as a correlation of syndecan-1 expression with neoplastic progression, and inverse significance has been found in different tumors. For example, the expression of syndecan-1 in carcinomas of the head and neck regions,8 esophagus,9 larynx,10 liver,11 lung,12 colon,13 and uterine cervix14 was related to low clinical stage, favorable outcome, and better differentiation, whereas inverse results are noted in malignancies of the nasopharynx,15 breast,16 prostate,17 and thyroid.18
In endometrial cancer, there have been recent histological studies on endometrial tissues suggesting that the expression of syndecan-1 is closely correlated with the gain of carcinogenesis in the human endometrium.7
Syndecan belongs to the heparan sulfate proteoglycan family found as components of cell surface and take part in cell-cell interaction, cell-matrix adhesion, and growth factor signaling.19 Thereby, syndecan is known to regulate cell differentiation, proliferation, migration, and homeostasis.20-22 It has been reported that syndecan-1 may be a critical molecule in maintaining viability signals in endometrial cancer.7
Because syndecan-1 expression has a strong association with endometrial cancer,7 this study examined the expression of syndecan-1 in endometrial hyperplasia specimens by immunohistochemical stain which quantitavely analyzed syndecan-1.
Also, syndecan-1 expression was evaluated for significance according to the histologic types of endometrial hyperplasia, because the risk of endometrial hyperplasia progressing to carcinoma is related to the presence and severity of cytologic atypia.23,24 Kurman et al.4 found that progression to carcinoma occurred in 1% of patients with SH, 3% of patients with CH, 8% of patients with atypical SH, and 29% of patients with AH. As the above results indicate, there was significant difference in syndecan-1 expression according to the histologic classification of endometrial hyperplasia. Syndecan-1 expression was significantly greater in AH than SH or CH. Our results therefore further suggest that syndecan-1 expression is positively correlated with the risk of progression of endometrial hyperplasia to endometrial cancer. Thus, syndecan-1 immunoreactivity in endometrial hyperplasia appears to be a useful indicator of high risk for development of endometrial cancer.
Small sample size was a study limitation. Additional possible limitations of this study were sample selection and other confounders because of retrospective sample selection. We made an effort to minimize these limitations as much as possible.
Future studies will determine whether the extent of syndecan-1 expression in endometrial cancer correlates with prognosis. Also, there is a need for a prospective study for syndecan-1 as a reliable biomarker of aggressiveness in endometrial hyperplasia. Our findings lend support to a suggestion that the risk of endometrial hyperplasia progressing to carcinoma is related to the presence and severity of cytologic atypia. In addition, the current findings, which revealed that aberrant syndecan-1 expression is a property of both complex atypical hyperplasia and invasive carcinoma of the endometrium, raise the possibility that some or all foci diagnosed as complex atypical hyperplasia in fact may be endometrial adenocarcinoma in situ. Thus, syndecan-1 may be used as a diagnostic indicator of progression to endometrial cancer.
Footnotes
The authors declare there are no conflicts of interest.
References
- 1.Lee SE, Kim JW, Park NH, Song YS, Kang SB, Lee HP. Contemporary trends of endometrial cancer in Korean women. Korean J Gynecol Oncol. 2005;16:215–220. [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, et al. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106–130. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 3.Silverberg SG, Mutter GL, Kurman RJ, Kubik-Huch RA, Nogales F, Tavassoli FA. Tumors of the uterine corpus: epithelial tumors and related lesions. In: Tavassoli FA, Stratton MR, editors. WHO classification of tumors: pathology and genetics of tumors of the breast and female genital organs. Lyon: IARC Press; 2003. pp. 221–232. [Google Scholar]
- 4.Kurman RJ, Kaminski PF, Norris HJ. The behavior of endometrial hyperplasia: a long-term study of "untreated" hyperplasia in 170 patients. Cancer. 1985;56:403–412. doi: 10.1002/1097-0142(19850715)56:2<403::aid-cncr2820560233>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 5.Kendall BS, Ronnett BM, Isacson C, Cho KR, Hedrick L, Diener-West M, et al. Reproducibility of the diagnosis of endometrial hyperplasia, atypical hyperplasia, and well-differentiated carcinoma. Am J Surg Pathol. 1998;22:1012–1019. doi: 10.1097/00000478-199808000-00012. [DOI] [PubMed] [Google Scholar]
- 6.Druker BJ, Lydon NB. Lessons learned from the development of an abl tyrosine kinase inhibitor for chronic myelogenous leukemia. J Clin Invest. 2000;105:3–7. doi: 10.1172/JCI9083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi DS, Kim JH, Ryu HS, Kim HC, Han JH, Lee JS, et al. Syndecan-1, a key regulator of cell viability in endometrial cancer. Int J Cancer. 2007;121:741–750. doi: 10.1002/ijc.22713. [DOI] [PubMed] [Google Scholar]
- 8.Anttonen A, Kajanti M, Heikkila P, Jalkanen M, Joensuu H. Syndecan-1 expression has prognostic significance in head and neck carcinoma. Br J Cancer. 1999;79:558–564. doi: 10.1038/sj.bjc.6690088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mikami S, Ohashi K, Usui Y, Nemoto T, Katsube K, Yanagishita M, et al. Loss of syndecan-1 and increased expression of heparanase in invasive esophageal carcinomas. Jpn J Cancer Res. 2001;92:1062–1073. doi: 10.1111/j.1349-7006.2001.tb01061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pulkkinen JO, Penttinen M, Jalkanen M, Klemi P, Grenman R. Syndecan-1: a new prognostic marker in laryngeal cancer. Acta Otolaryngol. 1997;117:312–315. doi: 10.3109/00016489709117794. [DOI] [PubMed] [Google Scholar]
- 11.Matsumoto A, Ono M, Fujimoto Y, Gallo RL, Bernfield M, Kohgo Y. Reduced expression of syndecan-1 in human hepatocellular carcinoma with high metastatic potential. Int J Cancer. 1997;74:482–491. doi: 10.1002/(sici)1097-0215(19971021)74:5<482::aid-ijc2>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 12.Anttonen A, Heikkila P, Kajanti M, Jalkanen M, Joensuu H. High syndecan-1 expression is associated with favourable outcome in squamous cell lung carcinoma treated with radical surgery. Lung Cancer. 2001;32:297–305. doi: 10.1016/s0169-5002(00)00230-0. [DOI] [PubMed] [Google Scholar]
- 13.Fujiya M, Watari J, Ashida T, Honda M, Tanabe H, Fujiki T, et al. Reduced expression of syndecan-1 affects metastatic potential and clinical outcome in patients with colorectal cancer. Jpn J Cancer Res. 2001;92:1074–1081. doi: 10.1111/j.1349-7006.2001.tb01062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Numa F, Hirabayashi K, Kawasaki K, Sakaguchi Y, Sugino N, Suehiro Y, et al. Syndecan-1 expression in cancer of the uterine cervix: association with lymph node metastasis. Int J Oncol. 2002;20:39–43. [PubMed] [Google Scholar]
- 15.Chen CL, Ou DL. Expression of syndecan-1 (CD138) in nasopharyngeal carcinoma is correlated with advanced stage and poor prognosis. Hum Pathol. 2006;37:1279–1285. doi: 10.1016/j.humpath.2006.04.024. [DOI] [PubMed] [Google Scholar]
- 16.Barbareschi M, Maisonneuve P, Aldovini D, Cangi MG, Pecciarini L, Angelo Mauri F, et al. High syndecan-1 expression in breast carcinoma is related to an aggressive phenotype and to poorer prognosis. Cancer. 2003;98:474–483. doi: 10.1002/cncr.11515. [DOI] [PubMed] [Google Scholar]
- 17.Zellweger T, Ninck C, Mirlacher M, Annefeld M, Glass AG, Gasser TC, et al. Tissue microarray analysis reveals prognostic significance of syndecan-1 expression in prostate cancer. Prostate. 2003;55:20–29. doi: 10.1002/pros.10209. [DOI] [PubMed] [Google Scholar]
- 18.Ito Y, Yoshida H, Nakano K, Takamura Y, Miya A, Kobayashi K, et al. Syndecan-1 expression in thyroid carcinoma: stromal expression followed by epithelial expression is significantly correlated with dedifferentiation. Histopathology. 2003;43:157–164. doi: 10.1046/j.1365-2559.2003.01656.x. [DOI] [PubMed] [Google Scholar]
- 19.Bernfield M, Kokenyesi R, Kato M, Hinkes MT, Spring J, Gallo RL, et al. Biology of the syndecans: a family of transmembrane heparan sulfate proteoglycans. Annu Rev Cell Biol. 1992;8:365–393. doi: 10.1146/annurev.cb.08.110192.002053. [DOI] [PubMed] [Google Scholar]
- 20.Soukka T, Pohjola J, Inki P, Happonen RP. Reduction of syndecan-1 expression is associated with dysplastic oral epithelium. J Oral Pathol Med. 2000;29:308–313. doi: 10.1034/j.1600-0714.2000.290704.x. [DOI] [PubMed] [Google Scholar]
- 21.Mukunyadzi P, Liu K, Hanna EY, Suen JY, Fan CY. Induced expression of syndecan-1 in the stroma of head and neck squamous cell carcinoma. Mod Pathol. 2003;16:796–801. doi: 10.1097/01.MP.0000081731.17549.53. [DOI] [PubMed] [Google Scholar]
- 22.Kurokawa H, Matsumoto S, Murata T, Yamashita Y, Tomoyose T, Zhang M, et al. Immunohistochemical study of syndecan-1 down-regulation and the expression of p53 protein or Ki-67 antigen in oral leukoplakia with or without epithelial dysplasia. J Oral Pathol Med. 2003;32:513–521. doi: 10.1034/j.1600-0714.2003.00117.x. [DOI] [PubMed] [Google Scholar]
- 23.Hunter JE, Tritz DE, Howell MG, DePriest PD, Gallion HH, Andrews SJ, et al. The prognostic and therapeutic implications of cytologic atypia in patients with endometrial hyperplasia. Gynecol Oncol. 1994;55:66–71. doi: 10.1006/gyno.1994.1249. [DOI] [PubMed] [Google Scholar]
- 24.Amant F, Moerman P, Neven P, Timmerman D, Van Limbergen E, Vergote I. Endometrial cancer. Lancet. 2005;366:491–505. doi: 10.1016/S0140-6736(05)67063-8. [DOI] [PubMed] [Google Scholar]