Abstract
Glutamate-induced rise in the intracellular Ca2+ level is thought to be a major cause of excitotoxic cell death, but the mechanisms that control the Ca2+ overload are poorly understood. Using immunocytochemistry, electrophysiology and Ca2+ imaging, we show that activation of ionotropic glutamate receptors induces a selective internalization of Cav1.3 L-type Ca2+ channels in salamander retinal neurons. The effect of glutamate on Cav1.3 internalization was blocked in Ca2+-free external solution, or by strong buffering of internal Ca2+ with BAPTA. Downregulation of L-type Ca2+ channel activity in retinal ganglion cells by glutamate was suppressed by inhibitors of dynamin-dependent endocytosis. Stabilization of F-actin by jasplakinolide significantly reduced the ability of glutamate to induce internalization suggesting it is mediated by Ca2+-dependent reorganization of actin cytoskeleton. We showed that the Cav1.3 is the primary L-type Ca2+ channel contributing to kainate-induced excitotoxic death of amacrine and ganglion cells. Block of Cav1.3 internalization by either dynamin inhibition or F-actin stabilization increased vulnerability of retinal amacrine and ganglion cells to kainate-induced excitotoxicity. Our data show for the first time that Cav1.3 L-type Ca2+ channels are subject to rapid glutamate-induced internalization, which may serve as a negative feedback mechanism protecting retinal neurons against glutamate-induced excitotoxicity.
Introduction
Glutamate is the major excitatory neurotransmitter in the central nervous system. In the vertebrate retina, glutamate is released from photoreceptors and bipolar cell terminals and exerts its actions by activating postsynaptic ionotropic (iGluR) and/or metabotropic (mGluR) receptors that are expressed in most, if not all, retinal cells (reviewed by Thoreson & Witkovsky, 1999). Overstimulation of glutamate receptors (GluRs), however, can lead to excitotoxic neuronal injury and death (Choi, 1988). Although the molecular basis of excitotoxic action of glutamate is not well understood, a strong relationship between glutamate-triggered neuronal injury and excessive Ca2+ influx has been established (Arundine & Tymianski, 2003). The major Ca2+ entry pathways contributing to the glutamate-induced rise in [Ca2+]i of central and retinal neurons are Ca2+-permeable AMPA/kainate and NMDA receptors (NMDARs), as well as voltage-gated Ca2+ channels, which are indirectly activated by glutamate-induced depolarization. Consistent with this, blockers of voltage-gated L-type Ca2+ channels (LTCC) can be neuroprotective by reducing the amount of Ca2+ loading incurred by GluR stimulation (Sucher et al. 1997; Sattler & Tymianski, 2001; Uemura & Mizota, 2008).
In the vertebrate retina, Ca2+ entry through voltage-gated LTCCs plays a fundamental role in many physiological processes including neurotransmitter release, activation of intracellular signalling, regulation of excitability and gene expression (reviewed by Akopian & Witkovsky, 2002). Three subtypes of LTCC, CaV1.2, CaV1.3 and CaV1.4, with different biophysical and pharmacological properties and different cellular distribution are expressed in the mammalian retina (Kamphuis & Hendriksen, 1998; Taylor & Morgans, 1998; Henderson et al. 2001). The Cav1.4 subtype has been localized primarily to rod and bipolar cell terminals, and its role in the synaptic transmission and in incomplete congenital stationary night blindness has been well established (Strom et al. 1998; Morgans, 1999; Bech-Hansen et al. 2000). Although Cav1.2 and Cav1.3 subtypes have been localized to both synaptic and nuclear layers encompassing cell bodies and processes of ganglion, amacrine and Müller cells (Firth et al. 2001; Henderson et al. 2001; Cristofanilli et al. 2007), their role in the retinal function is unclear. In the present study we tested the hypothesis that activation of glutamate receptors causes a rapid internalization of Cav1.3 LTCCs. Our results suggest that LTCC internalization acts synergistically with the glutamatergic input to regulate the amount of the excitatory drive of retinal ganglion cells (RGCs). We also provide evidence that this mechanism can compensate for overstimulation of GluRs in RGCs, resulting in a decrease in the magnitude of voltage-activated Ca2+ elevations. This serves as a negative-feedback mechanism, which provides an adaptational component to the inner retinal circuitry as well as protection against glutamate excitotoxicity.
Methods
Animals
The handling and the maintenance of animals met the National Institutes of Health guidelines and were approved by Institutional Care and Use Committee at NYU School of Medicine. The number of animals used and their suffering were minimized. Salamanders (Ambystoma tigrinum) were anaesthetized using tricaine methanosulfonate (100 mg ml−1) until the animal no longer reacted to tactile stimulation, then were decapitated and double pithed.
Electrophysiology
The general procedures for preparing dissociated cells and slices of salamander retina for patch clamp and immunocytochemistry have been published (Cristofanilli et al. 2007). Briefly, salamander eyes were cut in half, and the retina was dissected out either for preparation of dissociated cells or for retinal slices. The normal Ringer solution contained (in mm): NaCl 100, KCl 2.5, CaCl2 2, MgCl2 2 and Hepes 10, adjusted to pH 7.5 with NaOH. Standard internal solution contained (in mm): potassium gluconate 100, MgCl2 2, CaCl2 0.2, EGTA 2, Hepes 10, ATP 2 and GTP 0.1, adjusted to pH 7.3. The precise composition of the intracellular and bath solutions varied according to the requirements of the experiment. Whole-cell voltage-gated Ca2+ currents (ICa) were recorded using Patchmaster software and an EPC-10 amplifier (HEKA Electronic, Germany) and analysed with Igor Pro5 software. Capacitance and series resistances were compensated automatically. Membrane currents were sampled at 5 kHz and filtered at 1.0 kHz. A standard voltage protocol was used to elicit voltage-dependent ICa in the presence of TTX (1 μm) and TEA (20 mm, replacing equimolar NaCl) in bath solution to block voltage-gated Na+ and K+ currents, respectively. In addition, Ω-conotoxin GVIA (800 nm) and Ω-agatoxin IVA (1 μm) were included in the bath solution to block N- and P/Q-type Ca2+ currents, respectively. Unless otherwise noted, ICa was recorded with 5–10 mm Ca2+ (but not Ba2+) in the external solution as a charge carrier to allow full expression of Ca2+-dependent processes. Currents were recorded with low-resistance electrodes (5–6 MΩ), filled with pipette solution consisting of (mm): 100 CsCl, 10 KCl, 0.5 CaCl2, 2 MgCl2, 0.5 EGTA, 2 ATP, 0.1 GTP and 10 Hepes, buffered to pH 7·4 with KOH. Summary data are presented as mean ±s.e.m. Statistical comparisons among groups were performed with Student's unpaired t test and differences were considered significant at the P < 0.05 level.
Immunocytochemistry
The detailed procedure for labelling dissociated cells and slices of salamander retina for different Ca2+ channel subtypes is similar to those described previously (Cristofanilli et al. 2007). Briefly, isolated retinas were incubated for 1 h at room temperature (RT) with papain solution (14 U ml−1, Worthington Biochemicals, Lakewood, NJ, USA) in 14 ml of 0.5 mm calcium Ringer solution containing 6 mg cysteine, 1 mm sodium pyruvate, 16 mm d-glucose and 1.1 mm EDTA. After rinses in normal Ringer solution, retinas were triturated and cells plated on poly-l-lysine-coated coverslips to settle for 30–40 min before treatments.
To trigger internalization, cells were exposed for 5–10 min to glutamate, kainate or NMDA in normal external solution prior to fixing and labelling with anti-Cav1.2 or anti-Cav1.3 antibodies. To block endocytosis, cells were pre-incubated for 1 h with 50 μm myristoylated dynamin-inhibitory peptide (Tocris, Ellisville, MO, USA). For immunostaining of Cav1.3 and Cav1.2 channels, cells were permeabilized with 0.1% Triton X-100 for 5 min in PBS, pre-incubated in blocking solution (4% donkey serum 0.1% Triton X-100 in PBS) for 1 h at RT then incubated overnight at 4°C with polyclonal rabbit anti-Cav1.3 (Chemicon, Temecula, CA, USA; at 1:200) or anti-Cav1.2 (Alomone Labs, Israel; at 1:200) antibodies diluted 1:200 in blocking solution. Cells were rinsed three times in PBS and incubated with corresponding secondary antibodies diluted in blocking solution: Cy3 (Jackson Immunoresearch, West Grove, PA, USA; at 1:200); or Alexa Fluor 488, (Molecular Probes, Eugene, OR, USA; at 1:1000). Cells were then rinsed three times in PBS and coverslipped with Prolong Gold Antifade Mounting Medium (Invitrogen, Carlsbad, CA, USA) and observed with a confocal laser-scanning microscope (Nikon Eclipse C-1, Nikon, Japan). Specificity of the antibody for salamander retina was tested by (a) Western blot analysis (Cristofanilli et al. 2007), (b) omitting the primary antibody, and (c) pre-absorbing the primary antibodies with their respective control peptides (online Supplementary Fig. 1). For pre-absorption experiments, 1 μg of anti-Cav1.3 or anti-Cav1.2 antibodies were incubated for 1 h with 1 μg of corresponding control peptides at room temperature (according to the manufacturer's instructions) before making the final dilutions. We also demonstrated that Cav1.3 antibody recognizes the intended channel by utilizing a Cav1.3 knockout mouse (Busquet et al. 2009). Images of a single focal plane through the centre of the cells were acquired using 60× or 100× oil-immersion objective lens and EZ-C1 (Nikon) software. Changes in the subcellular distribution of Cav1.2 and Cav1.3 channels were assessed by measuring separately the optical fluorescence densities for the total (Ft) and the cytosolic (Fc) areas, using Metamorph software (Universal Imaging Co., Downington, PA, USA). To control for day-to-day variations in staining intensity, treated specimens always were compared with controls prepared the same day under identical fixation, permeabilization, staining and microscopy conditions. Control experiments consist of omitting the primary antibody, using knockout mice animals, and pre-absorbing the primary antibodies with their respective control peptides. For quantification, the membrane fluorescence (Fm) was defined as the difference between Ft and Fc, and the degree of internalization was assessed by measuring the ratio of Fc/Fm (Tombler et al. 2006). Fluorescence intensity profiles were obtained by scans of pixel intensity along a line drawn through the cell perikarya by using Metavue software (Universal Imaging Co.). All data are presented as mean ±s.e.m. Levels of significance were assessed using Student's paired t test with P < 0.05 considered as significant.
Assessment of cell death
Eyecups of salamander were treated with agonists at various concentrations in normal Ringer solution, for 30–60 min, incubated in agonist-free medium for 4–6 h, and cell viability was evaluated by a live/dead assay (Molecular Probes). Samples were loaded with the fluorescent dyes calcein-AM (2 μm) and ethidium homodimer (4 μm), for 30 min in darkness. After washing out excess dyes with normal Ringer solution, eyecups were cryoprotected in 20% sucrose dissolved in PBS at 4°C overnight. Cryostat sections of 15–18 μm were cut and fluorescence images were obtained with a confocal microscope. Live cells were detected by the presence of ubiquitous intracellular esterase activity, determined by the enzymatic conversion of the virtually non-fluorescent cell-permeant calcein AM to green fluorescence. Dead or damaged cells were identified by the uptake of ethidium homodimer-1, a red nuclear dye that is taken up only by damaged or dying cells with permeant membranes. For each treatment group, 15–30 retinal sections (from 3 independent experiments) comparable in size and location were processed. The number of dead cells was counted manually in several visual fields, averaged per slice and plotted as mean ±s.e.m. Statistical significance between groups was determined by one-way ANOVA followed by Tukey post hoc test. The differences were considered significant if P < 0.05.
Results
Glutamate induces selective internalization of Cav1.3 channels in salamander retinal neurons
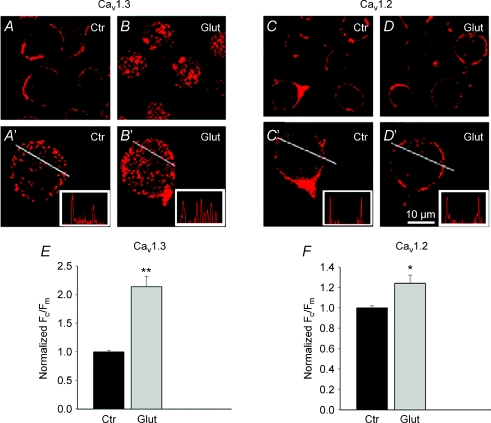
To investigate whether activation of GluRs alters the number of surface-expressed Cav1.3 channels on plasma membrane, dissociated retinal neurons were stimulated for 5 min with 100 μm glutamate prior to fixation and subsequent immunostaining with anti-Cav1.3 antibody.
Parallel immunostaining was performed on control cells incubated in normal Ringer solution. The analysis presented below is confined to non-photoreceptor and non-bipolar cells, which were easily identifiable by their morphology, and excluded based on the distinct shape of their ellipsoids and Landolt's clubs (Szikra & Krizaj, 2006). As a criterion for selecting ganglion cells, we used a minimum cell body diameter of 15 μm. Confocal immunofluorescence images through the perikarya of presumed amacrine and ganglion cells show that under control conditions, Cav1.3 labelling appeared as puncta outlining the cell somas, indicative of a surface localization (Fig. 1A and A′). Upon exposure to glutamate, the fluorescence signal appeared to be more intense in the cytoplasm (Fig. 1B and B′), suggesting that activation of GluRs induced Cav1.3 channel internalization. For quantification, we measured Ft and Fc for each cell, and calculated the membrane intensity per pixel as Fm=Ft−Fc (details in Methods). The ratio of Fc/Fm was used to evaluate internalization (Tombler et al. 2006). In this series of experiments, glutamate increased the mean Fc/Fm ratio from 0.62 ± 0.09 to 1.33 ± 0.12 (n= 50, P < 0.05). The Fc/Fm ratios for control and glutamate-treated cells are presented in Fig. 1E and F.
Figure 1. Glutamate induces selective internalization of Cav1.3 channels in salamander retinal neurons.
Confocal immunofluorescence images of cells incubated in normal Ringer solution (A, A′, C, C′), exposed for 5 min to 100 μm glutamate (B, B′, D, D′), then fixed and labelled for Cav1.3 or Cav1.2. Representative cells from 3 independent experiments were mounted in upper panels. Lower panels show magnified images of individual cells from corresponding upper panels. Glutamate induced internalization of Cav1.3 (B and B′) but had no significant effect on subcellular distribution of Cav1.2 (D and D′). E and F, the degree of internalization is presented as Fc/Fm, normalized to untreated cells. Bars: mean ±s.e.m. (n= 15–30; P < 0.01).
In contrast to its prominent effect on Cav1.3 distribution, glutamate had a relatively small effect on subcellular distribution of Cav1.2 channels. Confocal images of retinal cells labelled with anti-Cav1.2 antibodies in control Ringer solution (Fig. 1C and C′), and after exposure to glutamate (Fig. 1D and D′), showed a significant but much smaller difference in Cav1.2 immunolabelling. A slight increase in Fc/Fm (from 0.57 ± 0.04 to 0.71 ± 0.05) (Fig. 1F), suggests a potential yet minor effect of glutamatergic stimulation on internalization of Cav1.2. Intensity profiles (insets in upper panels) corroborate these data, suggesting that in salamander retinal neurons, LTCCs undergo subtype-selective modulation by glutamate.
Type of GluR responsible for Cav1.3 internalization
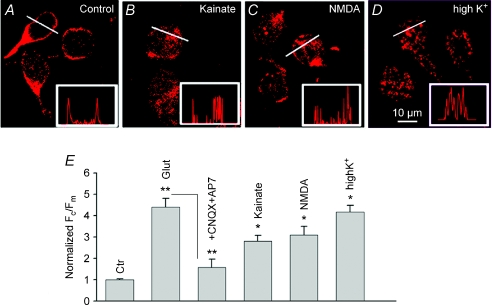
The effect of glutamate on the plasma membrane–cytosolic switch of Cav1.3 immunofluorescence was attenuated in the combined presence of CNQX (10 μm) and AP-7 (100 μm), indicative of ionotropic AMPA and/or NMDA receptor stimulation. To determine which subtype of iGluR is responsible for internalization of Cav1.3, cells were exposed either to kainate (100 μm) or to NMDA (100 μm, in Mg2+-free Ringer solution). In these experiments, CdCl2 (100 μm) was added to the Ringer solution to exclude contribution from voltage-gated Ca2+ channels. Both agonists induced a qualitatively similar but quantitatively lesser effect on Cav1.3 distribution compared with glutamate (Fig. 2A–C). Quantification revealed an increase in Fc/Fm by kainate and NMDA from a control value of 0.7 ± 0.03 (n= 50) to 1.9 ± 0.3 (n= 25, P < 0.05) and 2.2 ± 0.4 (n= 50, P < 0.05), respectively, compared with 2.6 ± 0.11 (n= 70, P < 0.05) induced by glutamate application.
Figure 2. Activation of ionotropic glutamate receptors or prolonged depolarization induces internalization of Cav1.3 channels in salamander third-order retinal cells.
Confocal immunofluorescence images of cells labelled for Cav1.3 in normal Ringer solution (A), after 5 min exposure to 100 μm kainate (B), 100 μm NMDA (in Mg2+-free external solution) (C), or 30 mm KCl (D). CdCl2 (100 μm) was used together with glutamate receptor agonists to exclude contribution of Ca2+ entry through voltage-gated channels. Intensity profiles (insets) along the white lines show changes in membrane and cytoplasmic labelling. E, the total integrated intensity (Ft) and cytoplasmic intensity (Fc) for each cell was measured, and the membrane intensity per pixel was calculated as Fm=Ft−Fc. Degree of internalization assessed as a ratio of Fc/Fm. Bars represent the normalized (to control (Ctr)) Fc/Fm±s.e.m. (n= 20–40, P < 0.05).
The modest effect of NMDA compared to glutamate might be associated with the partial blockage of NMDARs by Cd2+, which at a concentration of 1 mm was shown to suppress NMDA-evoked currents in rabbit retinal amacrine cells (Zhou & Fain, 1995).
To determine whether internalization can be triggered by tonic electrical activity, dissociated cells were exposed for 5 min to 30 mm KCl in the presence of the N/P/Q channel antagonist ω-conotoxin MVIIC (1 μm). This protocol caused robust internalization of Cav1.3 channels (Fig. 2D), increasing Fc/Fm to 4.2 ± 0.3 (n= 30, P < 0.05). The normalized values of Fc/Fm are presented in Fig. 2E. These data suggest that in salamander retinal neurons, internalization of LTCC is activity dependent. Although the exact identity of targeted cells was not established in these studies, the sensitivity of Cav1.3 distribution to NMDA suggests an involvement of third-order (amacrine and/or ganglion) retinal neurons (Shen & Slaughter, 1998). GluR agonists had no effect on subcellular distribution of Cav1.3 channels in photoreceptors, consistent with the absence of glutamate-evoked [Ca2+]i responses in salamander photoreceptors (n= 20, Supplementary Fig. 2).
We next tested whether Cav1.3 internalization can be induced by activation of mGluRs. The predominant mGluR in salamander ganglion cells has been identified as a group III mGluR, activation of which stimulates Ca2+ release from IP3-sensitive stores (Shen & Slaughter, 1998). l-AP4 (100 μm), an agonist of group III metabotropic receptors, failed to induce detectable internalization. Thus, the normalized (to control) value of Fc/Fm in the presence of l-AP4 was 0.97 + 0.04 (P > 0.5, n= 25), indicating that activation of iGluRs, rather than mGluRs, is responsible for glutamate-induced internalization (Supplementary Fig. 3).
Glutamate-induced internalization depends on dynamin activity
There are several different mechanisms by which cells internalize receptor and channel proteins into transport vesicles derived from the plasma membrane. Clathrin-mediated endocytosis, which strictly depends on GTPase activity of dynamin, is the best characterized mechanism for the removal of receptor and channel proteins from the plasma membrane into the cell interior (reviewed by Le Roy & Wrana, 2005).
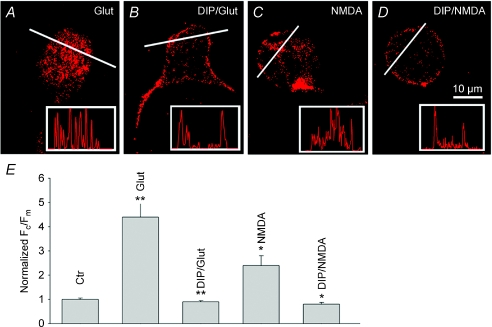
To determine whether the internalization of Cav1.3 channels depends on dynamin activity, we used a myristoylated dynamin-inhibitory peptide (DIP) that blocks endocytosis of receptor and channel proteins by interfering with the binding of amphiphysin with dynamin (Wigge et al. 1997). Dissociated cells were incubated for 1 h in Ringer solution without (Fig. 3A and C) or with 50 μm DIP (Fig. 3B and D), prior to glutamate or NMDA exposure. Cells were then washed, fixed and labelled for Cav1.3.
Figure 3. Internalization of Cav1.3 channels depends on dynamin activity.
Salamander retinal cells were incubated either in normal Ringer solution (A and C), or Ringer solution containing 50 μm DIP (B and D), prior to exposure for 5 min to glutamate or NMDA. Cells were then fixed and labelled for Cav1.3. Intensity profiles for corresponding cells are shown in insets. E, the bar graph shows no significant difference in Fc/Fm ratio in control cells vs. DIP-treated cells exposed to glutamate or NMDA. Bars represent mean Fc/Fm±s.e.m. (n= 20–30, P < 0.01).
Intensity profiles (insets) and quantitative analysis of Fc/Fm ratios (Fig. 3E) show that DIP greatly suppressed both glutamate- and NMDA-induced internalization. The effect of DIP was specific, as its inactive form (DIPctr) failed to suppress glutamate-induced internalization. These data suggest that glutamate-induced internalization of Cav1.3 is mediated by clathrin-dependent endocytosis.
Ca2+ entry from the extracellular space is essential for glutamate-induced internalization
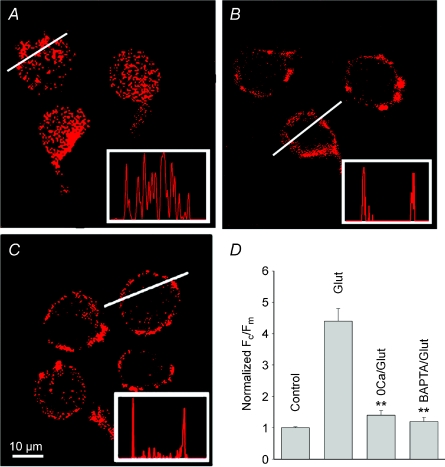
Activation of iGluRs and mGluRs in third-order retinal neurons induces a large Ca2+ influx from extracellular space and release from IP3-sensitive internal Ca2+ stores, respectively (Shen & Slaughter, 1998; Akopian et al. 2006; Hartwick et al. 2008). To determine whether Ca2+ plays a role in the glutamate-induced internalization, retinal cells were incubated for 10–15 min in Ringer solution in either the absence (Fig. 4A) or presence (Fig. 4B) of 10 μm BAPTA-AM, prior to glutamate exposure. Cells were subsequently labelled for Cav1.3 and processed to assess the internalization. Whereas treatment with BAPTA-AM alone had no effect on Cav1.3 distribution, it prevented glutamate-induced internalization. This finding suggests that [Ca2+]i elevation within the cytosol represents the paramount stimulus for Cav1.3 internalization. In cells treated with BAPTA-AM and exposed to glutamate, the Fc/Fm ratio of 0.77 ± 0.13 (n= 40; P > 0.5) was not significantly different from 0.69 ± 0.04, observed in control cells.
Figure 4. Calcium influx is essential for glutamate-induced internalization.
Glutamate-induced internalization of Cav1.3 channels observed in normal Ringer solution (2 mm CaCl2) (A), was prevented by pre-treatment of cells for 10 min with 10 μm BAPTA-AM (B), or by removal of Ca2+ from external solution (C). Intensity profiles are shown in insets. D, the bar graph represents normalized (to control) Fc/Fm values obtained under different experimental conditions. Data values represent mean Fc/Fm±s.e.m. (n= 20–50, P < 0.001).
We next examined whether Ca2+ influx from extracellular space or its release from internal stores, e.g. by Ca2+-induced Ca2+ release (CICR), plays a role in Cav1.3 internalization. Depletion of IP3-sensitive Ca2+ stores by 1 μm thapsigargin or antagonism of store-operated Ca2+ entry by 2-aminoethoxydiphenyl borate (100 μm 2-APB; Maruyama et al. 1997; Szikra et al. 2008), had no effect on glutamate-induced internalization (n= 8, P > 0.3, not shown). To test the role of Ca2+ entry, dissociated cells were exposed to glutamate in Ca2+-free external solution (Fig. 4C). The effect of glutamate was attenuated in the absence of external Ca2+. Thus, in the presence of glutamate the Fc/Fm ratio in Ca2+-free solution fell to 1.4 ± 0.15 (n= 25, P < 0.05) from 3.1 ± 0.4 in normal Ringer solution (Fig. 4D). These data indicate that Ca2+ entry via voltage-gated Ca2+ channels and/or iGluR-activated channels rather than its release from internal stores is essential for triggering internalization.
Correlation between internalization and inhibition of L-type Ca2+ current by glutamate
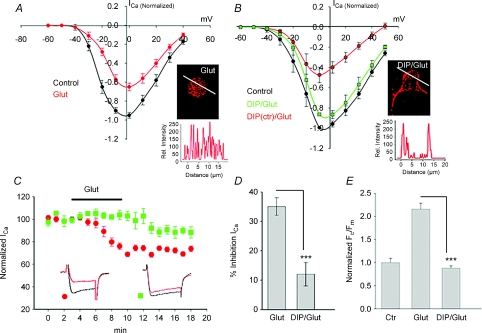
While the immunocytochemical data demonstrate glutamate-induced internalization of Cav1.3 LTCC, the following key questions remain to be answered: (i) Are these the same channels that are responsible for Ca2+ entry during cell depolarization, and (ii) Is internalization accompanied by downregulation of LTCC activity? To address these questions, the inhibitory action of glutamate on L-type ICa was measured in control and DIP-treated salamander retinal slices. Whole-cell Ca2+ currents were recorded from RGCs in which N- and P/Q-channels were suppressed by a cocktail consisting of ω-conotoxin GVIA (800 nm) and ω-agatoxin IVA (1 μm) in the bath solution. Patch pipette solution contained 1 μm thapsigargin and 100 μm 2-APB to suppress contributions from Ca2+ stores and store-operated channels, respectively. We used Ca2+ rather than Ba2+ as charge carrier, because Ba2+ obscures functionally important interactions between Ca2+ and Ca2+-dependent intracellular signalling.
In control slices, application of 100 μm glutamate reduced L-type ICa by 33 ± 3% (n= 16, P < 0.05), without altering the voltage dependence of I–V relationships or current kinetics (Fig. 5A and C).
Figure 5. Correlation between channel internalization and inhibition of L-type current by glutamate.
A, I–V relationships of whole-cell L-type ICa recorded from ganglion cells in salamander retinal slices from a holding potential of −60 mV to +40 mV in 10 mV increments in control, and in the presence of 100 μm glutamate. B, the inhibitory effect of glutamate on ICa was attenuated in cells pre-treated with DIP, but not with its scrambled analogue (DIPctr). Immunofluorescence confocal images of cells under indicated conditions are illustrated in insets. C, time course of the normalized peak ICa (mean ±s.e.m.) recorded with the patch pipette containing either DIP or its scrambled inactive form (DIPctr) in the presence of glutamate in the bath solution. The bar graphs summarize the effect of DIP on glutamate-induced inhibition of ICa (D), and internalization of Cav1.3 (E). Values represent mean ±s.e.m. (n= 7–16, P < 0.05).
The inhibitory action of glutamate could not be relieved by large depolarizing pre-pulses (500 ms to +80 mV, n= 3) that usually used to relieve voltage-dependent inhibition of ICa by activation of G protein-coupled receptors, suggesting this phenomenon was independent of transmembrane voltage. In addition, an overlap between normalized activation curves obtained before and after glutamate application was observed, indicating that glutamate-induced suppression of ICa did not result from a shift in the channel's voltage dependence (not shown). The inhibitory action of glutamate was suppressed by 10 mm BAPTA in the patch pipette solution (data not shown). Combined, these data indicate that activation of GluRs reduces the activity of LTCCs in RGCs in a Ca2+-dependent and voltage-independent manner, which is in agreement with earlier reports (Taschenberger & Grantyn, 1998; Shen & Slaughter, 1998).
Glutamate-induced reduction in Ca2+ channel activity may arise from removal of plasma membrane channels by the endocytic pathway (Jarvis & Zamponi, 2007). To test this possibility, the inhibitory action of glutamate on ICa was examined in slices pre-treated with DIP. Block of endocytosis by DIP suppressed the inhibitory action of glutamate by ∼60% from a mean inhibition of 33 ± 3% to 12 ± 4% (n= 8, P < 0.05) (Fig. 5B and C). The effect of DIP was specific, as its inactive form (DIPctr) was ineffective (Fig. 5B). Confocal immunofluorescence images of Cav1.3-labelled cells and intensity profiles under corresponding conditions are illustrated in insets. Quantification showed good correlation between glutamate-induced inhibition of ICa (Fig. 5D) and internalization of Cav1.3 channels (Fig. 5E). The residual 12 ± 4% inhibition of ICa in DIP-treated cells although small was statistically significant, suggesting that additional mechanisms, acting independently of endocytosis, may also be involved.
Role of the actin cytoskeleton in the glutamate-induced internalization
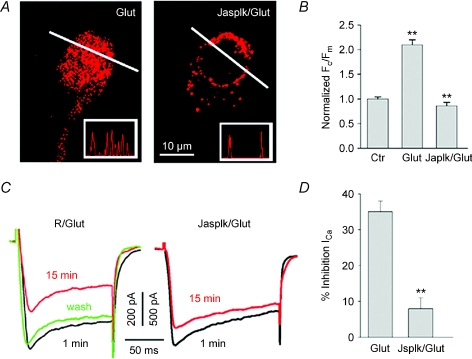
We next addressed the potential mechanism underlying the Ca2+-dependent internalization of Cav1.3 channels by glutamate. Ca2+ influx could induce LTTC internalization through actin depolymerization which would facilitate dissociation of channel proteins from their anchoring sites at the plasma membrane (Lanzetti, 2007). Cells were incubated for 30 min prior to glutamate exposure with Ringer solution containing 10 μm of the F-actin stabilizer jasplakinolide (Fig. 6A, Jasplk/Glut). Cells were subsequently washed, labelled for Cav1.3 and processed to assess internalization. Whereas treatment of cells with jasplakinolide alone had no effect on Cav1.3 distribution, it significantly reduced the promotion of internalization by glutamate, which is apparent from intensity profiles illustrated in insets. The Fc/Fm ratio of 0.75 ± 0.07 (n= 25) in the presence of glutamate in jasplakinolide-treated cells, was comparable to the values of 0.67 ± 0.09 (n= 50, P > 0.5) observed in normal Ringer solution (Fig. 6B).
Figure 6. Stabilization of F-actin prevents glutamate-induced Cav1.3 internalization.
A, cells were incubated in either normal Ringer solution (left panel), or in Ringer solution containing 10 μm jasplakinolide (right panel) prior exposing to 100 μm glutamate. B, normalized Fc/Fm±s.e.m. (n= 25–50, P < 0.005) measured for jasplakinolide-treated (Jasplk/Glut) and untreated (Glut) cells exposed to glutamate. C, traces of L-type ICa recorded from control (R/Glut) or jasplakinolide-treated (Jasplk/Glut) ganglion cells in salamander retinal slices before (1 min), and after (15 min) application of glutamate. D, the bar graph quantifies effect of F-actin stabilization on glutamate-induced inhibition of ICa. Each bar represents mean ±s.e.m. (n= 6, P < 0.005).
To further establish a link between glutamate-induced suppression of ICa and internalization of Ca2+ channels, we asked whether stabilization of F-actin that prevents channel internalization, is also able to suppress the inhibitory action of glutamate on ICa. Retinal slices were pre-incubated in Ringer solution containing 10 μm jasplakinolide. A comparison of ICa traces obtained before and after glutamate application (Fig. 6C) shows that stabilization of F-actin resulted in a dramatic decrease in the inhibitory action of glutamate from a mean of 33 ± 3% to 8 ± 3% (Fig. 6C and D, n= 6, P < 0.05). These findings support the hypothesis that Ca2+-dependent actin reorganization plays a key role in both glutamate-induced internalization and downregulation of Ca2+ channel activity.
Ca2+ channel internalization protects RGCs against glutamate excitotoxicity
Ischaemic stress, associated with overactivation of GluRs, excess Ca2+ entry into cells and excitotoxic neuronal death have been suggested as the final common pathway in many CNS pathologies including Alzheimer disease, Huntington's disease and ocular disorders such as diabetic retinopathy and glaucoma (Arundine & Tymianski, 2003; Schmidt et al. 2008). Although excitotoxicity appears to be primarily triggered by unrestricted Ca2+ entry through both voltage-gated LTCCs and iGluR-activated channels, the mechanisms ultimately leading to cell death and those preserving cells from excitotoxic damage are not well understood. We hypothesized that internalization of LTCCs serves as a negative feedback mechanism protecting retinal neurons against glutamate-induced excitotoxicity.
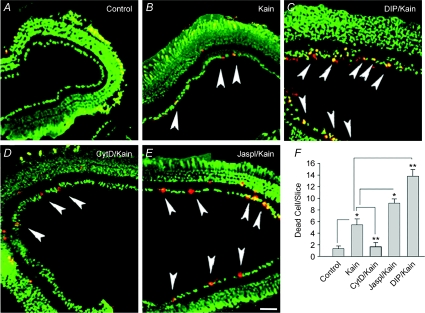
To test this idea, we studied the excitotoxic effect of GluR agonists on RGCs in retinal slices using a live/dead viability assay. Exposure of eyecups to 0.5–1 mm glutamate failed to induce any marked cell death, possibly due to the presence of powerful uptake systems (data not shown). Hence, kainate that is not taken up by glutamate transporters was used to induce cell death in the inner retina. Eyecups were exposed for 30 min to 100 μm kainate, incubated in agonist-free solution for 2–6 h, and examined for cell death by staining with ethidium homodimer. Kainate promoted cell death primarily in the ganglion cell layer, although a few dead cells were also observed in the inner nuclear layer. In salamander retina, the majority of cells in the ganglion cell layer have been characterized as ganglion cells (Lukasiewicz & Werblin, 1988), with only ∼4% constituting displaced amacrine cells (Zhang et al. 2004). This indicates that kainate targeted predominantly ganglion cells. The number of dead RGCs in control slices and those exposed to kainate (Fig. 7A and B, arrowheads) were 1.4 ± 0.4 and 5.3 ± 0.9 cells per slice (n= 30 slices, P < 0.005), respectively. Whereas treatment with DIP alone had no effect on cell death in slices, it significantly increased the number of kainate-induced dead cells to 13.8 ± 1.2 cells per slice (n= 16 slices, P < 0.005). Application of the AMPA receptor antagonist CNQX (25 μm) abolished the excitotoxic action of kainate (not shown). Pre-treatment of retinal slices for 30 min with 10 μm jasplakinolide (Fig. 7E) modestly increased vulnerability of RGCs (n= 17 slices, P < 0.05), whereas prior incubation with the F-actin disrupter, cytochalasin D (Fig. 7D), markedly reduced kainate-induced cell death (n= 15 slices, P < 0.01). Quantification of these results is presented as a bar graph in Fig. 7F. Collectively, these data support our hypothesis that LTCC internalization acting as negative-feedback protects RGCs against glutamate excitotoxicity.
Figure 7. Internalization of Cav1.3 channels reduced vulnerability of salamander RGCs to kainate-induced excitotoxicity.
Live/dead viability assay performed on retinal slices in normal Ringer solution (Control, A) exhibited healthy cells (coloured in green) throughout the retina. B, exposure of slices for 30 min to 100 μm kainate (Kain) promoted cell death in the ganglion cell layer (GCL) (arrowheads) and also few cells in the inner nuclear layer. C, prior incubation of slices for 30–40 min with 50 μm DIP (DIP/Kain) significantly enhanced kainate-induced cell death in the GCL. D, pre-incubation of slices with 10 μm cytochalasin D (CytD/Kain) markedly reduced kainate-induced RGC death. E, in contrast, stabilization of F-actin by 10 μm jasplakinolide (Jaspl/Kain) increased the number of kainate-induced dead cells (E). F, quantification summarizes these data indicating the role of endocytosis and cytoskeletal dynamics in excitotoxic RGC death. Each bar represents the number of dead cells per slice ±s.e.m. (n= 16–20 slices, *P < 0.05; **P < 0.01; 3 independent experiments). Statistical analysis was performed using ANOVA, followed by Tukey's post hoc test. Only cells in the GCL were considered for statistical analysis. Calibration bar, 50 μm.
Discussion
We report here on a novel mechanism whereby internalization of Cav1.3 LTCC controls glutamate-induced Ca2+ overload into retinal neurons. Our data show that activation of iGluRs followed by Ca2+ influx and F-actin reorganization are responsible for triggering clathrin-mediated internalization of Cav1.3 channels. We also provide experimental evidence for the neuroprotective role of Cav1.3 channel internalization in kainate-induced excitotoxicity, with implications for our understanding of the mechanisms associated with inner retinal dysfunction in several severe blinding diseases.
Internalization of Cav1.3 is mediated by Ca2+ entry and F-actin reorganization
In vertebrate retinal amacrine and ganglion cells, activation of iGluRs induces Ca2+ influx through Ca2+-permeable AMPA/KA and NMDA-gated channels as well as voltage-operated Ca2+ channels activated by incipient depolarization (Lu et al. 1996; Akopian et al. 2006; Hartwick et al. 2008). In addition, Ca2+-permeable store-operated channels (SOC) might be transiently activated following kainate-induced depletion of ER stores. Activation of G protein-coupled group III mGluRs, on the other hand, elevates [Ca2+]i by triggering Ca2+ release from IP3-sensitive and/or ryanodine-sensitive internal stores. Three lines of evidence argue that Ca2+ entry through Ca2+-permeable iGluRs and voltage-gated LTCCs, rather than its release from internal stores, is responsible for glutamate-induced internalization of Cav1.3: (i) the effect of glutamate was prevented by removal of Ca2+ from external solution, (ii) activation of group III mGluRs by l-AP4, which stimulates Ca2+ release from IP3-sensitive internal stores in retinal neurons (Shen & Slaughter, 1998), failed to induce Cav1.3 internalization, (iii) depletion of ryanodine and IP3-sensitive internal stores by thapsigargin, or block of Ca2+ entry via SOC and/or TRP channels by 2-APB (Maruyama et al. 1997; Szikra et al. 2008), had no effect on glutamate-induced internalization. We therefore propose that the effect of Ca2+ is mediated by F-actin reorganization, as stabilization of F-actin by jasplakinolide prevented glutamate-mediated Cav1.3 internalization in presumed RGCs. This idea is supported by our previous findings indicating that Ca2+ influx causes actin depolymerization, which in turn reduces voltage-operated Ca2+ entry in salamander RGCs (Schubert & Akopian, 2004; Cristofanilli & Akopian, 2006). Similar dependence of Cav1.3 internalization on Ca2+ influx and baseline [Ca2+]i has been shown recently to mediate glucose-evoked insulin release in pancreatic β-cells (Huang et al. 2004). Taken together, these observations suggest that regulated endocytosis of LTCCs serves as a powerful negative feedback mechanism to limit Ca2+ loads imposed upon cells during sustained depolarization.
Although previous studies reported downregulation of L-type ICa in RGCs by iGluR activation (Taschenberger & Grantyn, 1998; Shen & Slaughter, 1998), it is not known whether such modulation is specific for channel subtype, or if the reduction in channel activity is accompanied by alterations in the number of channels in the plasma membrane. We show that iGluR agonists selectively target Cav1.3 channels without affecting the subcellular distribution of Cav1.2, suggesting that LTCCs in the retina can undergo subtype-selective modulation. A potential mechanism for this process might involve the ProSAP/Shank family of scaffolding proteins which link GluRs and the C-terminal of the Cav1.3 channels to the PSD-95/SAP90 complex, which act as a scaffold for various neurotransmitter receptors, ion channels, or other signaling molecules, and is coupled both to regulators of F-actin and the endocytic machinery via dynamin (Okamoto et al. 2001; Olson et al. 2005). The stability of plasma membrane Cav1.2 clusters during glutamatergic stimulation was displayed in central neurons, in which both targeting and expression of Cav1.2 channels failed to change following activation of NMDARs (Di Biase et al. 2009; but see Green et al. 2007). We therefore propose that glutamate-induced Ca2+ entry disrupts F-actin, which in turn triggers removal of Cav1.3 channels from plasma membrane by endocytosis.
Internalization underlies an inhibition of L-type Ca2+ channel activity by glutamate
Our electrophysiological data strongly indicate that dynamin-dependent internalization of Cav1.3 channels is the major underlying mechanism for glutamate-induced inhibition of L-type currents in salamander RGCs. This conclusion is supported by the following lines of evidence: (i) both glutamate-induced internalization of Cav1.3 channels and inhibition of L-type currents in RGCs were attenuated by dynamin inhibition; (ii) removal of extracellular Ca2+, or strong buffering of internal Ca2+ by BAPTA, abolished the Cav1.3 channel internalization and suppressed the inhibitory action of glutamate on ICa; (iii) stabilization of F-actin by jasplakinolide significantly reduced the effect of glutamate on both ICa inhibition and channel internalization. Given that AMPARs do not cycle in intact light-adapted retinas (Xia et al. 2006), it is unlikely that DIP interfered with internalization of AMPARs (Luscher et al. 1999). Our results are in agreement with the generally recognized role of F-actin in controlling endocytosis and the delivery of ion channel proteins into and out of the plasma membrane (Tombler et al. 2006; Lanzetti, 2007).
Implications for glutamate-induced excitotoxicity
Overstimulation of GluRs followed by excess Ca2+ entry is thought to be associated with cellular degeneration, remodelling and cell death in many ocular pathologies such as diabetic retinopathy and glaucoma (Tezel & Wax, 2004; Osborne et al. 2008), whereas antagonism of L-type channels was shown to exert protective effects (Koseki et al. 2008; Sakamoto et al. 2009). Results presented in this study are consistent with earlier reports in central neurons demonstrating a link between endocytosis or F-actin dynamics and excitotoxic cell death (Furukawa et al. 1997). The proposed mechanism may also be helpful for understanding the controversy regarding involvement of glutamate excitotoxicity in RGC death in glaucoma, as several studies failed to detect substantial elevations in glutamate levels in the glaucomatous eye (Salt & Cordeiro, 2006). Our results suggest that the excitotoxic damage caused by initial ischaemia is not determined exclusively by elevated levels of intraretinal glutamate, but also depends upon: (i) the relative proportion of Cav1.3 (over Cav1.2) channels within a given cell, (ii) the functional state of the endocytic machinery, and (iii) the actin cytoskeleton dynamics. Recent studies demonstrated that LTCC blockers reduce intraocular pressure in the primate and human eye (Tian et al. 2000; Wang et al. 2008), stabilize the progression of glaucoma (Mikheytseva et al. 2004), induce an improvement in visual field parameters in glaucoma patients (Luksch et al. 2005; Koseki et al. 2008), and protect RGCs against hypoxia/ischaemia (Uemura & Mizota, 2008; Sakamoto et al. 2009). Conversely, facilitation of Ca2+ influx through LTCCs is likely to worsen the clinical picture via Ca2+ overloads in RGCs. It is therefore plausible that LTCC internalization plays a protective role by reducing excessive excitation and by preserving the metabolic capital of the cell that would have been expended combating Ca2+ overloads. Our findings, therefore, are likely to point at a universal mechanism that underlies neuroprotection in degenerative diseases in the retina and across the CNS.
In summary, we show that activation of iGluRs triggers clathrin-mediated selective internalization of Cav1.3 LTCC in a subset of retinal amacrine and ganglion neurons. This process is mediated by Ca2+-dependent F-actin reorganization. This internalization mechanism may serve as a negative feedback during light–dark adaptation to modulate the excitability of inner retinal circuits and to ensure constant output under different illumination conditions. Our data also suggest that internalization of LTTCs could control glutamate-induced deregulation of Ca2+ homeostasis.
Acknowledgments
We thank Dr J. Striessnig and A. M. Rajadhyaksha for critically reading the manuscript. This research was supported by NIH grants EY13870 to D.K. and EY12497 to A.A. P.B. wishes to acknowledge support from the Institute of Biomolecular Chemistry, Hungarian Academy of Sciences, Budapest, Hungary.
Glossary
Abbreviations
- CytD
cytochalasin D
- DIP
dynamin inhibitory peptide
- iGluR
ionotropic glutamate receptor
- LTCC
L-type Ca2+ channel
- mGluR
metabotropic glutamate receptor
- RGC
retinal ganglion cell
Author contributions
F.M.: collection, analysis and interpretation of data; final approval of the manuscript. P.B. and D.K.: drafting and revising the manuscript, final approval of the manuscript; A.A.: conception and design of the experimental protocol; collection, analysis and interpretation of data; drafting and revising the manuscript; final approval of the manuscript.
Author's present address
F. Mizuno: Department of Neurobiology, Duke University Medical Center. Durham, NC 27710, USA.
Supplemental material
Fig.1. Confocal immunofluorescence images of salamander retinal neurons labeled for Cav1.3 (green) or Cav1.2 (red). For negative controls, the primary antibodies were either omitted from the procedure or were pre-absorbed with the appropriate peptides. Nonspecific staining of photoreceptor outer segments is marked by arrowheads in preabsorbing fluorescence and corresponding DIC image.
Fig.2. Subcellular distribution of Cav1.3 was determined by fixing and labeling dissociated cells either following 5 min application of glutamate (A), or after 20-30 min washing glutamate from Ringer solution (B). Representative cells selected from 3 different experiments are mounted in panels A and B. Fluorescence and corresponding DIC images of third-order retinal neurons (marked by arrowheads) are presented (C, D). Glutamate had no effect on subcellular distribution of Cav1.3 in photoreceptors (arrows). Non-specific staining of outer segments is characteristic for salamander photoreceptors.
Fig.3. Activation of group III metabotropic receptors by L-AP4 failed to induce detectable internalization of Cav1.3 channels.
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer-reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors
References
- Akopian A, Szikra T, Cristofanilli M, Krizaj D. Glutamate-induced Ca2+ influx in third-order neurons of salamander retina is regulated by the actin cytoskeleton. Neuroscience. 2006;138:17–24. doi: 10.1016/j.neuroscience.2005.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akopian A, Witkovsky P. Calcium and retinal function. Mol Neurobiol. 2002;25:95–114. doi: 10.1385/MN:25:2:113. [DOI] [PubMed] [Google Scholar]
- Arundine M, Tymianski M. Molecular mechanisms of calcium-dependent neurodegeneration in excitotoxicity. Cell Calcium. 2003;34:325–337. doi: 10.1016/s0143-4160(03)00141-6. [DOI] [PubMed] [Google Scholar]
- Bech-Hansen NT, Naylor MJ, Maybaum TA, Sparkes RL, Koop B, Birch DG, Bergen AA, Prinsen CF, Polomeno RC, Gal A, Drack AV, Musarella MA, Jacobson SG, Young RS, Weleber RG. Mutations in NYX, encoding the leucinerich proteoglycan nyctalopin, cause X-linked complete congenital stationary night blindness. Nat Genet. 2000;26:319–323. doi: 10.1038/81619. [DOI] [PubMed] [Google Scholar]
- Busquet P, Khoi Nguyen N, Schmid E, Tanimoto N, Seeliger MW, Ben-Yosef T, Mizuno F, Akopian A, Striessnig J, Singewald N. CaV1.3 L-type Ca2+ channels modulate depression-like behaviour in mice independent of deaf phenotype. Int J Neuropsychopharmacol. 2009;11:1–15. doi: 10.1017/S1461145709990368. [DOI] [PubMed] [Google Scholar]
- Choi DW. Glutamate neurotoxicity and diseases of the nervous system. Neuron. 1988;1:623–634. doi: 10.1016/0896-6273(88)90162-6. [DOI] [PubMed] [Google Scholar]
- Cristofanilli M, Akopian A. Calcium channel and glutamate receptor activities regulate actin organization in salamander retinal neurons. J Physiol. 2006;575:543–554. doi: 10.1113/jphysiol.2006.114108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristofanilli M, Mizuno F, Akopian A. Disruption of actin cytoskeleton causes internalization of Cav1.3 (alpha 1D) L-type calcium channels in salamander retinal neurons. Mol Vis. 2007;13:1496–1507. [PubMed] [Google Scholar]
- Di Biase V, Obermair GJ, Szabo Z, Altier C, Sanguesa J, Bourinet E, Flucher BE. Stable membrane expression of postsynaptic CaV1.2 calcium channel clusters is independent of interactions with AKAP79/150 and PDZ proteins. J Neurosci. 2009;28:13845–13855. doi: 10.1523/JNEUROSCI.3213-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firth SI, Morgan IG, Boelen MK, Morgans CW. Localization of voltage-sensitive L-type calcium channels in the chicken retina. Clin Exp Ophthalmol. 2001;29:183–187. doi: 10.1046/j.1442-9071.2001.00401.x. [DOI] [PubMed] [Google Scholar]
- Furukawa K, Fu W, Li Y, Witke W, Kwiatkowski DJ, Mattson MP. The actin-severing protein gelsolin modulates calcium channel and NMDA receptor activities and vulnerability to excitotoxicity in hippocampal neurons. J Neurosci. 1997;17:8178–8186. doi: 10.1523/JNEUROSCI.17-21-08178.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green EM, Barrett CF, Bultynck G, Shamah SM, Dolmetsch RE. The tumor suppressor eIF3e mediates calcium-dependent internalization of the L-type calcium channel CaV1.2. Neuron. 2007;55:615–632. doi: 10.1016/j.neuron.2007.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwick AT, Hamilton CM, Baldridge WH. Glutamatergic calcium dynamics and deregulation of rat retinal ganglion cells. J Physiol. 2008;586:3425–3446. doi: 10.1113/jphysiol.2008.154609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson D, Doerr TA, Gottesman J, Miller RF. Calcium channel immunoreactivity in the salamander retina. Neuroreport. 2001;12:1493–1499. doi: 10.1097/00001756-200105250-00039. [DOI] [PubMed] [Google Scholar]
- Huang L, Bhattacharjee A, Taylor JT, Zhang M, Keyser BM, Marrero L, Li M. [Ca2+]i regulates trafficking of Cav1.3 (α1D Ca2+ channel) in insulin-secreting cells. Am J Physiol Cell Physiol. 2004;286:C213–C221. doi: 10.1152/ajpcell.00346.2003. [DOI] [PubMed] [Google Scholar]
- Jarvis SE, Zamponi GW. Trafficking and regulation of neuronal voltage-gated calcium channels. Curr Opin Cell Biol. 2007;19:474–482. doi: 10.1016/j.ceb.2007.04.020. [DOI] [PubMed] [Google Scholar]
- Kamphuis W, Hendriksen H. Expression patterns of voltage-dependent calcium channel alpha 1 subunits (α1A–α1E) mRNA in rat retina. Brain Res Mol Brain Res. 1998;55:209–220. doi: 10.1016/s0169-328x(97)00363-x. [DOI] [PubMed] [Google Scholar]
- Koseki N, Araie M, Tomidokoro A, Nagahara M, Hasegawa T, Tamaki Y, Yamamoto S. A placebo-controlled 3-year study of a calcium blocker on visual field and ocular circulation in glaucoma with low–normal pressure. Ophthalmology. 2008;115:2049–2057. doi: 10.1016/j.ophtha.2008.05.015. [DOI] [PubMed] [Google Scholar]
- Lanzetti L. Actin in membrane trafficking. Curr Opin Cell Biol. 2007;19:453–458. doi: 10.1016/j.ceb.2007.04.017. [DOI] [PubMed] [Google Scholar]
- Le Roy C, Wrana JL. Clathrin- and non-clathrin-mediated endocytic regulation of cell signalling. Nat Rev Mol Cell Biol. 2005;6:112–126. doi: 10.1038/nrm1571. [DOI] [PubMed] [Google Scholar]
- Lu YM, Yin HZ, Chiang J, Weiss JH. Ca permeable AMPA/kainite and NMDA channels: high rate of Ca influx underlies potent induction of injury. J Neurosci. 1996;16:5457–5465. doi: 10.1523/JNEUROSCI.16-17-05457.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukasiewicz P, Werblin F. A slowly inactivating potassium current truncates spike activity in ganglion cells of the tiger salamander retina. J Neurosci. 1988;12:4470–4481. doi: 10.1523/JNEUROSCI.08-12-04470.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luksch A, Rainer G, Koyuncu D, Ehrlich P, Maca T, Gschwandtner ME, Vass C, Schmetterer L. Effect of nimodipine on ocular blood flow and colour contrast sensitivity in patients with normal tension glaucoma. Br J Ophthalmol. 2005;89:21–25. doi: 10.1136/bjo.2003.037671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüscher C, Xia H, Beattie EC, Carroll RC, von Zastrow M, Malenka RC, Nicoll RA. Role of AMPA receptor cycling in synaptic transmission and plasticity. Neuron. 1999;24:649–658. doi: 10.1016/s0896-6273(00)81119-8. [DOI] [PubMed] [Google Scholar]
- Maruyama T, Kanaji T, Nakade S, Kanno T, Mikoshiba K. 2APB, 2-aminoethoxydiphenyl borate, a membrane-penetrable modulator of Ins(1,4,5)P3-induced Ca2+ release. J Biochem. 1997;122:498–505. doi: 10.1093/oxfordjournals.jbchem.a021780. [DOI] [PubMed] [Google Scholar]
- Mikheytseva IN, Kashintseva LT, Krizhanovsky GN, Kopp OP, Lipovetskaya EM. The influence of the calcium channel blocker verapamil on experimental glaucoma. Int Ophthalmol. 2004;25:75–79. doi: 10.1023/b:inte.0000031737.08988.b0. [DOI] [PubMed] [Google Scholar]
- Morgans CW. Localization of the 1F calcium channel subunit in the rat retina. Invest Ophthalmol Vis Sci. 1999;42:2414–2418. [PubMed] [Google Scholar]
- Okamoto PM, Gamby C, Wells D, Fallon J, Vallee RB. Dynamin isoform-specific interaction with the shank/ProSAP scaffolding proteins of the postsynaptic density and actin cytoskeleton. J Biol Chem. 2001;276:48458–48465. doi: 10.1074/jbc.M104927200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson PA, Tkatch T, Hernandez-Lopez S, Ulrich S, Ilijic E, Mugnaini E, Zhang H, Bezprozvanny I, Surmeier DJ. G-protein-coupled receptor modulation of striatal CaV1.3 L-type Ca2+ channels is dependent on a Shank-binding domain. J Neurosci. 2005;25:1050–1062. doi: 10.1523/JNEUROSCI.3327-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne NN, Wood JP, Chidlow G, Bae JH, Melena J, Nash MS. Ganglion cell death in glaucoma: what do we really know? Brit J Ophthalmol. 2008;83:980–986. doi: 10.1136/bjo.83.8.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto K, Kawakami T, Shimada M, Yamaguchi A, Kuwagata M, Saito M, Nakahara T, Ishii K. Histological protection by cilnidipine, a dual L/N-type Ca2+ channel blocker, against neurotoxicity induced by ischemia-reperfusion in rat retina. Exp Eye Res. 2009;88:974–982. doi: 10.1016/j.exer.2008.12.011. [DOI] [PubMed] [Google Scholar]
- Salt TE, Cordeiro MF. Glutamate excitotoxicity in glaucoma: throwing the baby out with the bathwater? Eye. 2006;20:730–731. doi: 10.1038/sj.eye.6701967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattler R, Tymianski M. Molecular mechanisms of glutamate receptor-mediated excitotoxic neuronal cell death. Mol Neurobiol. 2001;24:107–129. doi: 10.1385/MN:24:1-3:107. [DOI] [PubMed] [Google Scholar]
- Schmidt KG, Bergert H, Funk RH. Neurodegenerative diseases of the retina and potential for protection and recovery. Curr Neuropharmacol. 2008;6:164–178. doi: 10.2174/157015908784533851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert T, Akopian A. Actin filaments regulate voltage-gated ion channels in salamander retinal ganglion cells. Neuroscience. 2004;125:583–590. doi: 10.1016/j.neuroscience.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Shen W, Slaughter MM. Metabotropic and ionotropic glutamate receptors regulate calcium channel currents in salamander retinal ganglion cells. J Physiol. 1998;510:815–828. doi: 10.1111/j.1469-7793.1998.815bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strom TM, Nyakatura G, Apfelstedt-Sylla E, et al. An L-type calcium-channel gene mutated in incomplete X-linked congenital stationary night blindness. Nat Genet. 1998;19:260–263. doi: 10.1038/940. [DOI] [PubMed] [Google Scholar]
- Sucher NJ, Lipton SA, Dreyer EB. Molecular basis of glutamate toxicity in retinal ganglion cells. Vision Res. 1997;37:3483–3493. doi: 10.1016/S0042-6989(97)00047-3. [DOI] [PubMed] [Google Scholar]
- Szikra T, Cusato K, Thoreson WB, Barabas P, Bartoletti TM, Krizaj D. Depletion of calcium stores regulates calcium influx and signal transmission in rod photoreceptors. J Physiol. 2008;586:4859–4875. doi: 10.1113/jphysiol.2008.160051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szikra T, Krizaj D. The dynamic range and domain-specific signals of intracellular calcium in photoreceptors. Neuroscience. 2006;141:143–155. doi: 10.1016/j.neuroscience.2006.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taschenberger H, Grantyn R. Interaction of calcium-permeable non-N-methyl-D-aspartate receptor channels with voltage-activated potassium and calcium currents in rat retinal ganglion cells in vitro. Neuroscience. 1998;84:877–896. doi: 10.1016/s0306-4522(97)00541-1. [DOI] [PubMed] [Google Scholar]
- Taylor WR, Morgans C. Localization and properties of voltage-gated calcium channels in cone photoreceptors of Tupaia belangeri. Vis Neurosci. 1998;15:541–552. doi: 10.1017/s0952523898153142. [DOI] [PubMed] [Google Scholar]
- Tezel G, Wax MB. Hypoxia-inducible factor 1α in the glaucomatous retina and optic nerve head. Arch Ophthalmol. 2004;122:1348–1356. doi: 10.1001/archopht.122.9.1348. [DOI] [PubMed] [Google Scholar]
- Thoreson WB, Witkovsky P. Glutamate receptors and circuits in the vertebrate retina. Prog Retin Eye Res. 1999;18:765–810. doi: 10.1016/s1350-9462(98)00031-7. [DOI] [PubMed] [Google Scholar]
- Tian B, Kaufman PL. Cytoskeletal involvement in the regulation of aqueous humor outflow. Invest Ophthalmol Vis Sci. 2000;41:619–623. [PubMed] [Google Scholar]
- Tombler E, Cabanilla NJ, Carman P, Permaul N, Hall JJ, Richman RW, Lee J, Rodriguez J, Felsenfeld DP, Hennigan RF, Diversé-Pierluissi MA. G protein-induced trafficking of voltage-dependent calcium channels. J Biol Chem. 2006;281:1827–1839. doi: 10.1074/jbc.M508829200. [DOI] [PubMed] [Google Scholar]
- Uemura A, Mizota A. Retinal concentration and protective effect against retinal ischemia of nilvadipine in rats. Eur J Ophthalmol. 2008;18:87–93. doi: 10.1177/112067210801800115. [DOI] [PubMed] [Google Scholar]
- Wang RF, Gagliuso DJ, Podos SM. Effect of flunarizine, a calcium channel blocker, on intraocular pressure and aqueous humor dynamics in monkeys. J Glaucoma. 2008;17:73–78. doi: 10.1097/IJG.0b013e318133a845. [DOI] [PubMed] [Google Scholar]
- Wigge P, Kohler K, Vallis Y, Doyle CA, Owen D, Hunt SP, McMahon HT. Amphiphysin heterodimers: potential role in clathrin-mediated endocytosis. Mol Biol Cell. 1997;8:2003–2015. doi: 10.1091/mbc.8.10.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y, Carroll RC, Nawy S. State-dependent AMPA receptor trafficking in the mammalian retina. J Neurosci. 2006;26:5028–5036. doi: 10.1523/JNEUROSCI.0169-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Yang Z, Wu SM. Immuocytochemical analysis of spatial organization of photoreceptors and amacrine and ganglion cells in the tiger salamander retina. Vis Neurosci. 2004;21:157–166. doi: 10.1017/s0952523804042075. [DOI] [PubMed] [Google Scholar]
- Zhou ZJ, Fain GL. Neurotransmitter receptors of starburst amacrine cells in rabbit retinal slices. J Neurosci. 1995;15:5334–5345. doi: 10.1523/JNEUROSCI.15-07-05334.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.