Abstract
Glycine protects mammalian intestine against oxidative damage caused by ischaemia-reperfusion (IR) injury and prevents or reverses experimentally-induced colitis. However the mechanism of protection remains largely unknown. The objectives of the current study were to demonstrate directly glycine-mediated protection of human intestinal epithelial cells and to determine the requirement for glycine uptake by the specific transporter GLYT1. Exogenous glycine protected human intestinal Caco-2 and HCT-8 cells against the oxidative agent tert-butylhydroperoxide and reduced the intracellular concentration of reactive oxygen species, when applied prior to but not concomitant with the oxidative challenge. Glycine given prior to oxidative challenge preserved intracellular glutathione concentration but had no effect on the rate of glycine uptake. Protection was dependent on GLYT1 activity, being blocked by a specific GLYT1 inhibitor, supporting a requirement for intracellular glycine accumulation. Maintained intracellular glutathione content is indicated as a mechanism through which the protective effect may in part be mediated. However expression of the genes encoding GLYT1 and the glutathione synthesising enzymes glutamate-cysteine ligase, both catalytic and modifier subunits, and glutathione synthetase was not altered by glycine or tert-butylhydroperoxide, suggesting transcriptional regulation is not involved. This work has demonstrated a novel role of GLYT1 in intestine and shown that intestinal epithelial cells respond directly to oxidative challenge without reliance on extra-epithelial tissues or functions such as neurone, blood-flow or immune responses for antioxidant defence. The protective actions of glycine and maintenance of epithelial antioxidant defences suggest it may be beneficial in treatment of inflammatory bowel disease.
Introduction
Glycine is a well-documented cytoprotective agent. During the last 10–20 years numerous studies have reported the protective effective of glycine in a broad range of tissues, including kidney, liver and lung, and against a number of injurious agents (Weinberg et al. 1990; Silva et al. 1991; Ligumsky et al. 1995; Ikejima et al. 1996; Zhong et al. 1996). A small scale clinical trial has shown beneficial effects of glycine in preventing reperfusion injury of hepatocytes following human liver transplantation (Schemmer et al. 2002) and the outcome of a follow-up, large scale trial is awaited (Luntz et al. 2005). In the intestine, the protective effect of glycine against oxidative damage caused by ischaemia–reperfusion (IR) injury has been demonstrated in a variety of animal studies (Mangino et al. 1996; Iijima et al. 1997; Lee et al. 2001, 2002; Kallakuri et al. 2003), including that sustained during intestinal transplantation (Schaefer et al. 2008). Additionally in rats subjected to abdominal irradiation glycine supplementation preserved colonic wall thickness and morphology (Diestel et al. 2007), while dietary glycine also prevented or reversed experimentally-induced colitis, suggesting a possible beneficial effect of glycine supplementation in inflammatory disease of the bowel (Tsune et al. 2003).
Several mechanisms have been implicated in contributing to the cytoprotective effects of glycine. These include stimulation of glutathione synthesis (Jackson, 1986; Jackson et al. 2004), inhibition of glycine-gated chloride channels (glycine receptors) (Zhong et al. 2003), inhibition of macrophage activation (Schilling & Eder, 2004) and stimulation of heat-shock proteins (Nissim et al. 1992). However, the process by which glycine exerts its protective effect remains uncertain and the available evidence suggests the likelihood of multiple mechanisms, which may be either tissue specific or dependent on the nature of the injurious agent (Sogabe et al. 1996; Deters et al. 1998). In the intestine, studies indicate protection by glycine from damage caused during mesenteric ischaemia is by inhibition of apoptosis (Jacob et al. 2003), while others have shown that glycine protection against intestinal IR injury is by a mechanism consistent with glycine uptake (Lee et al. 2002).
Glycine is a substrate for a number of membrane transport systems in the intestine that may facilitate cellular uptake. System GLY is unique among glycine transporters in that it is highly substrate specific and has a high affinity for glycine. Two genes, GLYT1 (SLC6A9) and GLYT2 (SLC6A5), that encode system GLY-like activity have been identified, and both are members of the sodium- and chloride-dependent neurotransmitter transporter family, SLC6. We have established previously the expression, localization and functional activity of the glycine-specific transporter GLYT1 in human small intestine and model enterocytes, Caco-2 cells. GLYT1 is localized predominantly at the basolateral membrane of enterocytes and functions primarily to import glycine into the cell, suggesting a role in meeting essential requirements of the enterocyte, rather than in nutrient absorption (Christie et al. 2001). Consistent with this likely role is the observed increase in GLYT1 expression in the proximal intestine of rats transferred to total parenteral nutrition (Howard et al. 2004).
Since glycine uptake is implicated in the cytoprotective mechanism of glycine and GLYT1 has been identified as an important and specific uptake transport system in the intestinal epithelium, we have investigated the relationship between glycine cytoprotection and GLYT1 in the human intestinal cell lines, Caco-2 and HCT-8. HCT-8 cells derive from a human ileo-caecal adenocarcinoma (Tompkins et al. 1974) and, like the Caco-2 cell line (Pinto et al. 1983), exhibit structural and functional characteristics comparable to those of intestinal epithelial cells. HCT-8 cells form high resistance, polarised monolayers in culture with apical microvilli, intercellular junctional complexes and brush-border enzymes (Allen et al. 1991). However, whereas differentiated Caco-2 cells resemble the absorptive enterocytes of the small intestine, HCT-8 cells are more comparable to colonocytes of the distal intestine and have proved a useful model to study gut barrier function and mucosal inflammation (Merendino et al. 1999; Marion et al. 2005; Leblond et al. 2006), bacterial invasion (Rogers et al. 2003; Luck et al. 2005; Hashim et al. 2006) and cell signalling (Merchant et al. 2005).
Methods
Materials
[U-14C]Glycine (specific activity, 103 mCi mmol−1) and [2-3H]glycine (specific activity, 15.7 Ci mmol−1) were obtained from GE Healthcare (Little Chalfont, UK). Cell culture media and supplements were from Sigma (Poole, UK). Tissue culture plastics and Transwell filter inserts were obtained from Corning B.V. (Amsterdam, the Netherlands). Caco-2 and HCT-8 cell lines were obtained from ATCC (Rockville, MD, USA). Amino acids and fetal bovine serum were purchased from Sigma. The specific GLYT1 and GLYT2 inhibitors, N-[3-(4′-fluorophenyl)-3-(4′-phenylphenoxy)-propyl]sarcosine (ALX-5407/NFPS) and O-[(2-benzyloxyphenyl-3-fluorophenyl)methyl]-l-serine) (ALX-1393), respectively, were purchased from Sigma. Glutathione detection kit (ApoGSH Glutathione Colorimetric Detection Kit) was from BioVision (San Francisco, CA, USA). SV Total RNA extraction kit, Moloney murine leukaemia virus reverse transcriptase (MMuLV-RT), ribonuclease inhibitor (RNasin), deoxynucleotide triphosphates (dNTPs) and pGEM-T-easy cloning kit were from Promega (Southampton, UK). Hexanucleotide primer was from GE Healthcare and TAq DNA polymerase was from ABgene (Glasgow, UK). Goat anti-rat GLYT1 antibody was purchased from Autogen Bioclear (Calne, Wiltshire, UK). The anti-rat antibodies cross-react with human GLYT1. AlexaFluor 488-labelled donkey anti-goat IgG was from Molecular Probes/Invitrogen (Glasgow, UK). Vectashield mounting medium was from Vector Laboratories (Burlingame, CA, USA). All other reagents were from Sigma.
Cell culture
Caco-2 cells (passage number 26–36; ATCC no. HTB-37) were cultured in Dulbecco's modified Eagle's medium (DMEM) with 4.5 g l−1 glucose, supplemented with 1.2% (w/v) non-essential amino acids, 10% (v/v) fetal calf serum, 100 U ml−1 penicillin and 100 μg ml−1 streptomycin sulphate. HCT-8 cells (passage number 23–40; ATCC no. CCL-244) were cultured in RPMI-1640, supplemented with 2 mmol l−1 glutamine, 100 μg ml−1 streptomycin sulphate, 100 U ml−1 penicillin, 5% (v/v) fetal calf serum and 1 mmol l−1 sodium pyruvate. Glycine concentration in each medium was at physiological levels (0.03 and 0.01 g l−1 for DMEM and RPMI respectively). Cells were maintained at 37°C in a 5% CO2: 95% air incubator in 162 cm2 flasks (Corning).
For uptake experiments cells were seeded onto 12 mm polycarbonate filters (Transwells, Corning) at 5 × 104 cells per well and grown at 37°C, 5% CO2, in humidified air with medium replenished every 72 h. Cell confluence and functional integrity of cell layers was determined by measuring transepithelial resistance (RT) using an electrical voltohmeter (EVOM, World Precision Instruments Inc., Sarasota, FL, USA). Experiments were performed when RT exceeded 200 Ω cm2 after subtraction of standard filter RT (100 Ω cm2), usually after 14 days for Caco-2 and 7 days for HCT-8 cells. For experiments, cells were transferred into serum-free medium containing the appropriate additive or into amino acid-free, serum-free medium for the required period. For the duration of the experiment, control cells were maintained in serum-free medium.
For cytotoxicity studies, glutathione measurement and determination of reactive oxygen species (ROS), cells were seeded in 96-well plates at a concentration of 1 × 104 cells per well. All experiments were performed 2–3 days after seeding, when cells were 80–90% confluent, and 18–24 h after replenishing the medium. For fluorescence assays (ROS and glutathione (GSH) measurement) solid black plates were used. Clear plates were used for cytotoxicity studies and to determine cell confluence at the time of experimentation.
Cytotoxicity studies
For cytotoxicity studies, culture medium was removed and the cells rinsed three times in phosphate buffered saline (PBS). tert-Butyl hydroperoxide (t-BOOH), an organic hydroperoxide that induces oxidative stress (Tormos et al. 2004), was added to serum-free culture medium to the desired concentration and the cells incubated in this for the required time (1–24 h). For cells grown on filters the electrical resistance was measured both prior to and at the completion of the treatment period and the treatment medium was present in both apical and basal compartments. To investigate the cytoprotective effects of amino acid pre-treatment, cells were incubated in serum-free culture medium containing 1 or 5 mmol l−1 glycine or alanine for 24 h, washed three times with PBS and then incubated in serum-free medium containing 150 μmol l−1 t-BOOH for a further 24 h. For concurrent treatment with glycine and t-BOOH, cell monolayers were washed three times in PBS and incubated in serum-free medium containing glycine (1 or 5 mmol l−1) and t-BOOH (150 μmol l−1) for 24 h. At the end of treatment, cells were washed three times in Krebs buffer and viability assessed. Control cells were subjected to the same wash protocol as samples and transferred to serum-free medium for the treatment period. When required, mannitol was added to the medium to maintain osmolarity.
MTT assay
Cell viability was measured using the 3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide (MTT) assay (Mosmann, 1983). Briefly, HCT-8 cells were dispensed into 96-well plates at a concentration of 1 × 104 cells per well. Following treatment the cells were rinsed with PBS. MTT reagent (0.25 mg ml−1) was added to the cells and incubated for 4 h. One hundred microlitres of dimethyl sulfoxide was added to the reaction followed by 25 μl of Sorensen's glycine buffer (0.1 mol l−1 glycine, 0.1 mol l−1 NaCl, pH 10.5) to solubilise the cells. Absorbance at 570 nm was measured to calculate cell survival.
Reactive oxygen species measurement
Reactive oxygen species (ROS) were detected and quantified using the Image-IT Live reactive oxygen species detection kit (Invitrogen, Paisley, UK). Briefly, following induction of ROS with t-BOOH, cells were incubated with 25 μmol l−1 5-(and-6)-carboxy-2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA) in Hanks’ buffered salt solution for 30 min at 37°C, and nuclei were counterstained with Hoechst 33342, which binds DNA, and fluorescence measured at 495/592 nm and 350/461 nm (excitation/emission for the fluorescein and Hoechst dyes, respectively).
Measurement of reduced glutathione concentration
To determine glutathione levels cells were rinsed twice with PBS and detached from the plate with trypsin, centrifuged at 700 g for 5 min at 4°C, and the cell pellet resuspended in 0.5 ml ice-cold PBS. Glutathione concentration (μg mg−1 protein) in the cell pellet was measured using the ApoGSH Glutathione Colorimetric Detection Kit (BioVision, Mountain View, CA, USA).
Protein determination
Protein content of the cells was determined in monolayers, which were grown under identical conditions to those used for experiments. Cells were lysed for 20 min on ice in 2% Nonidet P-40, 0.2% SDS, 1 mmol l−1 dithiothreitol (DTT) in PBS and the lysate homogenized by passing through a 25 gauge needle several times. The homogenate was centrifuged at 13,000 g for 15 min to remove insoluble debris and total cellular protein was determined using a modified Bradford assay (Sigma).
Reverse transcription and polymerase chain reaction (RT-PCR)
Total RNA was extracted from confluent monolayers of HCT-8 and Caco-2 cells grown on polycarbonate supports using the SV Total RNA extraction kit. Concentration of RNA was quantified by measuring absorbance at 260 nm, and RNA integrity was verified by electrophoresis on a 1% (w/v) agarose formaldehyde gel stained with ethidium bromide. Reverse transcription (RT) was performed by incubating 1 μg total RNA, 500 ng random hexamers, 0.5 mmol l−1 each of the four dNTPS, 1× reaction buffer, 20 U RNasin, and 100 U MMuLV RT in a final volume of 20 μl at 42°C for 1 h. Oligonucleotide primers used for the detection of GLYT1 (sequence: 5′, ACTCAGTTCTGCCTCCTGGAGAC; 3′, AGCCTGGGTACTGGTAGTGG) were synthesized by VHBio (Newcastle upon Tyne, UK). To demonstrate expression of GLYT1 in human large intestine, PCR was performed on a panel of intestinal cDNA samples (Human Digestive System Multiple Tissue cDNA (MTC) Panel; Clontech). For comparison purposes, samples of human ileum, kidney and liver from the same panel were included in the PCR. Each cDNA sample in the panel is derived from a pool of non-diseased tissues from several donors (n= 2–39) harvested post mortem following sudden death.
PCR reactions were performed in a 25 μl total volume containing 2 μl RT product, 0.2 mmol l−1 dNTPs, 1 μmol l−1 each primer, 2 mmol l−1 MgCl2, 1× PCR buffer and 1.25 U TAq DNA polymerase (ThermoStart TAq, ABgene). All PCR reactions used a hot-start procedure (incubation at 95°C for 15 min to activate the enzyme before beginning cycling), 32 cycles of denaturation at 95°C for 30 s, annealing at 65°C for 30 s, extension at 72°C for 30 s, and a final incubation at 72°C for 10 min. PCR products were electrophoresed on 1.5% (wt/vol) agarose/Tris-borate EDTA gels, stained with ethidium bromide, and analysed on an AlphaInnotech Gel Document system. PCR products were characterized fully either by direct sequencing or cloning in pGem-T-easy (Promega) followed by sequence analysis (Pinnacle, Newcastle University, Newcastle upon Tyne, UK).
Quantitative PCR (qPCR)
The effect of glycine and t-BOOH on abundance of mRNAs encoding GLYT1 and enzymes involved in the synthesis of glutathione (glutamate-cysteine ligase catalytic and modifier subunits (GCLC and GCLM, respectively) and glutathione synthetase (GS)) was measured using relative quantitative real-time PCR. Primers for each mRNA target were designed using D-LUX designer software (Invitrogen), and one primer in each pair was labelled with a suitable fluorophore. Primers were chosen so that amplicons were less than 200 bp in length and spanned at least one exon–exon junction to avoid amplification of residual DNA carried over in the RNA preparation (Table 1). Confluent monolayers of cells grown on 12 mm Transwells were treated with glycine or t-BOOH as described and RNA extracted using the SV total RNA kit (Promega), which includes a DNAse 1 digest to remove contaminating genomic DNA. RNA integrity was determined on an Agilent Bioanalyser and only those samples with a RIN ≥8 were used in PCR analysis. Reverse transcription was performed on 0.5 μg total RNA as described above using RNaseH+ MMuLV RT (Promega) and 1 μl of the product was amplified in 1× Lightcycler 480 Probes Master (Roche) with 0.5 μmol l−1 each primer (total reaction volume 10 μl) on a 96-well Lightcycler 480 (Roche). Titrations were carried out to confirm that the volume of RT product included in PCR was not inhibitory. The PCR, performed in duplicate on each sample, involved an initial denaturation step (95°C, 10 min) and 45 cycles of 95°C for 10 s, 60°C for 20 s, and 72°C for 1 s when fluorescence was measured. Following amplification, melt curve analysis was carried out to determine the specificity of products. Representative PCR products were cloned and sequenced for each target gene and the melt temperature of these used to identify the correct product in subsequent amplifications. Relative mRNA concentrations (arbitrary units) were derived from concentration curves made from serial dilutions of the appropriate cloned PCR product. In all cases PCR efficiency approached 2 (>1.9) and was consistent between runs. Data were normalised to the reference genes GAPDH and hATP5B, selected using the GeNorm programme (Vandesompele et al. 2002) (purchased from PrimerDesign, Southampton, UK).
Table 1.
Oligonucleotide primers used in real-time PCR assays. Primer sequences are written 5′–3′ with exon location and predicted Tm and product size
| cDNA (Genbank accession no.) | Sequence | Tm | Exon location | Product size (bp) |
|---|---|---|---|---|
| GLYT1 (NM_006934) | Forward CCATGTTCAAAGGAGTGGGCTA | 62 | 6 | 72 |
| Reverse TGACCACATTGTAGTAGATGCCG | 63 | 7 | ||
| GCLC (NM_000178) | Forward CTTGTAGTCAGGATGGTTTGCG | 62 | 1 | 171 |
| Reverse TCCTGGACTGATCCCAATTCTG | 62 | 2 | ||
| GCLM (NM_001498) | Forward GCAAATGCAGTCAAATCTGGTG | 60 | 4 | 194 |
| Reverse TCCTTGGAGCATTTACAGCCTTAC | 64 | 5 | ||
| GS (NM_002061) | Forward GTAACCTGCACCAACAATGACG | 64 | 8 | 91 |
| Reverse CGGTGCCAGCTTAGGAATAACC | 64 | 9 |
Immunohistochemistry
HCT-8 cell monolayers (7 days after seeding) grown on filter supports (Transwell; Corning) or 10 μm frozen sections of human colon tissue (purchased from Medical Solutions plc, Peterborough, UK) were fixed for 30 min on ice in 2% (w/v) paraformaldehyde in PBS and permeabilised with 0.1% (v/v) Triton X-100 in PBS for 30 min at room temperature. Samples were blocked with 10% donkey serum in PBS (v/v) for 30 min at 4°C and after a PBS wash incubated with goat anti-rat GLYT1 antibody (1/50 dilution in PBS) overnight at 4°C. Samples were washed with PBS before incubation with an AlexaFluor 488-labelled donkey anti-goat IgG (1/80 dilution in PBS) for 1 h at 4°C. After washing in PBS samples were incubated with ethidium homodimer 1 (1 μg ml−1 in PBS) for 5 min, washed and mounted in Vectashield mounting medium (Vector Laboratories) and visualized using a Leica TCS-NT confocal laser-scanning microscope (CLSM).
Measurement of glycine uptake
Epithelial uptake measurements were performed essentially as described previously (Christie et al. 2001). Cell monolayers (grown on 12 mm diameter filters) were washed three times in 250 ml Krebs buffer (buffer composition in mmol l−1: NaCl, 140; KCl, 5.4; CaCl2, 2.8; MgSO4, 1.2; NaH2PO4, 0.3; Hepes, 10; glucose, 5; pH to 7.4 at 37°C with Tris base), Na+-free Krebs buffer (composition as above except that choline chloride replaced NaCl and NaH2PO4 was omitted), or Cl−-free Krebs buffer (composition as for Krebs buffer except that NaCl, KCl and CaCl2 were replaced with the corresponding gluconate salt) as stated. The experimental composition of buffer in both the apical and basal compartments was identical. Radiolabelled glycine was used at tracer concentration (0.5 μCi ml−1), with glycine added to give a final concentration of 5 μmol l−1 (unless stated). 3H- and 14C-labelled glycine were used to measure apical and basolateral uptake, respectively, in the same cell population. Cells were incubated routinely for 10 min, unless otherwise stated, after which time the cells were washed three times in 250 ml volumes of ice-cold Krebs buffer, Na+-free, or Cl−-free buffer (pH 7.4) to remove any loosely associated radiolabel. The filter was removed from the insert and cell monolayer-associated radioisotope was measured using dual-label scintillation counting, with appropriate corrections for quench, channel spillover, and efficiency, using a Beckman LS 5000CE liquid scintillation counter (Beckman Instruments, High Wycombe, UK).
For determination of glycine uptake kinetics, accumulation was measured as described (Christie et al. 2001) over a glycine concentration range of 0–1 mmol l−1 and in the presence of mannitol to ensure equal osmolarity. Unlabelled glycine at 1 mmol l−1 was added to measure non-mediated (non-saturable) uptake. Carrier-mediated uptake was calculated by subtracting non-mediated from total uptake. Values given in the text and figures are for mediated uptake only. Plots of uptake rate versus glycine concentration were fitted by non-linear regression to Michaelis–Menten kinetics. Each experiment, in which each measurement was made on three separate filters, was repeated at least twice.
Statistical analysis
Experiments were performed routinely in triplicate and each experiment was repeated at least twice. Results are expressed as means ±s.e.m. Statistical comparisons were made using one-way ANOVA and Dunnett's post hoc test (Prism, Graphpad Software Inc., San Diego, CA) or by Student's paired t test. Differences between means were considered significant at P < 0.05. Kinetic constants for Michaelis–Menten kinetics were calculated by nonlinear regression analysis using commercially available software (Prism).
Results
Effects of glycine on t-BOOH-induced cell injury
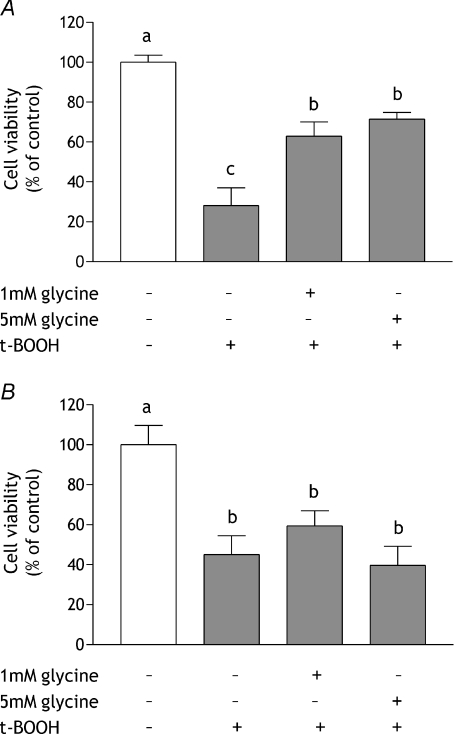
Cell viability, assessed by measuring the ability of cells to reduce MTT, was used as an indicator of cytotoxicity. The protective effects of glycine against oxidative stress were investigated by either incubating colonocyte-like HCT-8 cells with glycine for 24 h prior to exposure to 150 μmol l−1 t-BOOH for 24 h or by concurrent exposure to glycine and t-BOOH for 24 h. At this concentration t-BOOH generally produced a reduction in cell viability of 60–70%. Pre-incubation with 1 mmol l−1 or 5 mmol l−1 glycine reduced significantly the damage caused by subsequent exposure to t-BOOH; cell viability was increased from 28 ± 2% of control following t-BOOH, to 63 ± 7% of control with 1 mmol l−1 glycine before t-BOOH exposure (Fig. 1A). In contrast, concurrent glycine addition was unable to ameliorate the reduction in cell viability induced by t-BOOH (Fig. 1B). Exposing cells to glycine alone, without t-BOOH exposure, had no effect, beneficial or detrimental, on cell viability (cell viability in the presence of 1 mmol l−1 glycine: 98 ± 4% of control (n= 6), 5 mmol l−1 glycine: 101.2 ± 5.2% (n= 6)). To investigate the specificity of the response the effects of pre-treatment with the amino acid alanine, which is structurally similar to glycine, were investigated. Cells were incubated in medium containing 3 mmol l−1l-alanine for 24 h, after which the culture medium was removed and replaced with medium containing 150 μmol l−1 t-BOOH for 24 h: the same protocol as used previously for glycine. In cells exposed to t-BOOH alone, viability was reduced to 42 ± 8% of control and alanine pre-treatment did not alter this (viability in alanine pre-treated cells: 41 ± 2%).
Figure 1. Effect of pre- or concurrent treatment with glycine on cell viability in response to t-BOOH treatment.
A, glycine pre-treatment: HCT-8 cells were incubated in serum-free medium or serum-free medium containing either 1 mmol l−1 or 5 mmol l−1 glycine for 24 h after which they were transferred to serum-free medium containing 150 μmol l−1 t-BOOH for 24 h. B, concurrent glycine treatment: confluent monolayers of HCT-8 cells were incubated in serum-free medium containing 150 μmol l−1 t-BOOH or serum-free medium containing 150 μmol l−1 t-BOOH and either 1 mmol l−1 or 5 mmol l−1 glycine for 24 h. Cell viability was measured using the MTT assay. Results are shown as a percentage of viability in control cells (open bars), which were maintained in normal medium throughout with changes at appropriate time points, and are means ±s.e.m. of three independent experiments performed on 12 replicates. In each chart, means without a common letter are statistically different (P < 0.05).
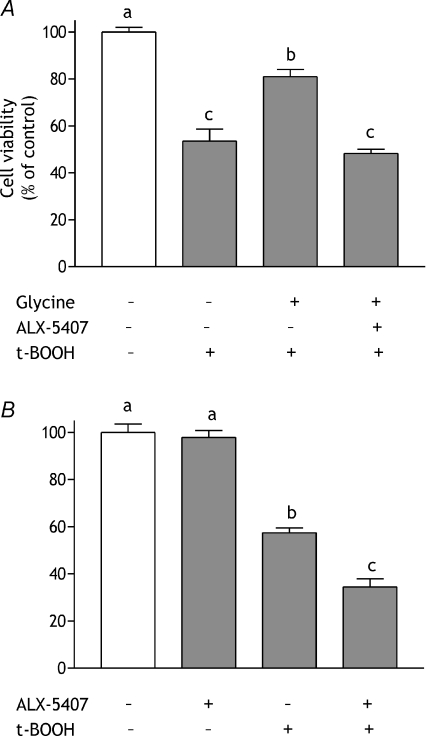
The requirement for glycine pre-incubation prior to oxidative challenge was indicative of a role for glycine uptake in the protective mechanism. Therefore, the involvement of GLYT1 was investigated. Transporter activity was inhibited by incubating HCT-8 cells with 30 nmol l−1 ALX-5407, a GLYT1 specific inhibitor (Atkinson et al. 2001), prior to glycine or t-BOOH treatment (see below). Incubation with ALX-5407 for 15 min prior to glycine pre-treatment completely blocked the beneficial effects of glycine (Fig. 2A). When given alone, i.e. in the absence of glycine supplementation, GLYT1 inhibition exacerbated the effects of t-BOOH but had no effect in unchallenged cells (Fig. 2B).
Figure 2. Effect of the GLYT1 inhibitor ALX-5407 on glycine-induced cell protection.
A, HCT-8 cells were incubated in serum-free medium containing 5 mmol l−1 glycine or 5 mmol l−1 glycine plus 30 nmol l−1 ALX-5407 for 24 h after which they were transferred to serum-free medium containing 150 μmol l−1 t-BOOH for 24 h. Data are compared with those obtained from cells maintained in serum-free medium for 48 h (open bar) and from cells exposed to 150 μmol l−1 t-BOOH following incubation in serum-free medium for 24 h. B, cells were incubated in serum-free medium with or without 30 nmol l−1 ALX-5407 for 24 h after which they were transferred to serum-free medium containing 150 μmol l−1 t-BOOH for 24 h. Control cells (open bar) were maintained in serum-free medium throughout. Cell viability was assessed using the MTT assay. Results are expressed as percent of control value and are means ±s.e.m. from 3 independent experiments performed in triplicate. Means without a common letter are statistically different (P < 0.05).
Expression and localisation of GLYT1 in human large intestine and HCT-8 cells
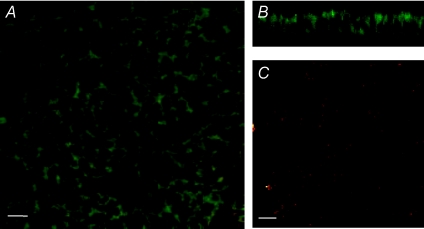
The ability of ALX-5407 to antagonise the protective effects of glycine is indicative of a role for GLYT1 in HCT-8 cells. Previously we have shown GLYT1 expression in human small intestine and the Caco-2 cell model, but this had not been demonstrated in large intestine or the HCT-8 model. PCR amplification of cDNA derived from HCT-8 cell mRNA with oligonucleotide primers specific to human GLYT1 mRNA generated a single product of 409 bp, which on sequencing was found to be identical to human GLYT1, transcript variant 1 (Genbank accession no.: NM_006934) between bases 1437 and 1845, confirming GLYT1 gene expression in this cell line. Confocal immunofluorescence microscopy localized GLYT1 protein to HCT-8 cell apical, lateral and basal plasma membranes. Control reactions, in which the GLYT1-specific antibody was omitted, showed negligible staining (Fig. 3).
Figure 3. CLSM images of fixed and permeabilised HCT-8 cells showing GLYT1 staining (green) throughout the cell membrane.
Shown are a horizontal section (A) taken below the apical plasma membrane and a vertical section (B) of a polarised monolayer of cells. In the control reaction (C) the GLYT1 specific antibody was omitted. The monlayers were also stained with ethidium homodimer (red, shown in A and C). Bar = 10 μm (A and C).
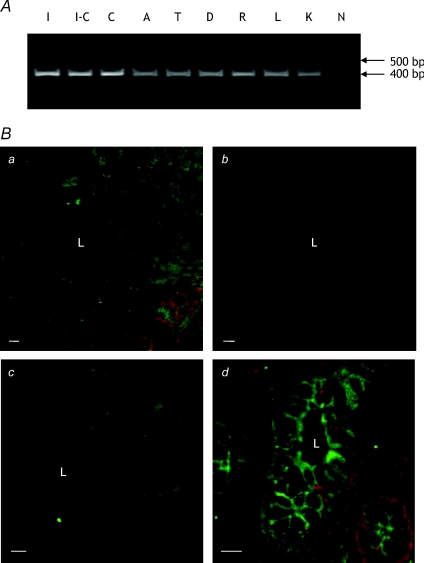
PCR analysis of a human digestive system cDNA panel showed expression of GLYT1 throughout the large intestine. GLYT1 mRNA concentration did not vary greatly but was slightly higher in caecum, where it was similar to that in human ileum (Fig. 4). In human colon, GLYT1 protein was localised to the membrane of cells lining the crypts with strong staining at both apical and basal surfaces. Sections from three separate individuals and from ascending, descending and transverse colon showed identical patterns of immunofluorescence (Fig. 4, results shown only for descending colon). In the absence of anti-GLYT1 antibody, staining of human colonic tissue by the AlexaFluor 488-conjugated secondary antibody was negligible, showing that staining of the test sample was specific for GLYT1.
Figure 4. Expression and localisation of GLYT1 in human large intestine.
A, agarose gel elctrophoresis of products from PCR amplification of human intestinal cDNAs using GLYT1-specific primers. A single PCR product of ∼400 bp is visible for each sample. Lanes are: I, ileum; I-C, ileo-caecum; C, caecum; A, ascending colon; T, transverse colon; D, descending colon; R, rectum; L, liver; K, kidney; N, negative control (ultra-pure water replaced cDNA in the reaction). B, CLSM images of frozen sections of human descending colon stained for GLYT1. a, optical section (low power view) showing GLYT1 expression in both apical and basal membranes of cells throughout the colonic crypt. c–d, optical sections (high power views), again showing GLYT1 expression in both apical and basolateral membranes of cells in the crypt base. In the absence of anti-GLYT1 antibody (b) staining by the AlexaFluor 488-conjugated detecting antibody is negligible. a and b, which show the same region of consecutive sections, were collected and displayed using identical parameters. L, crypt lumen. Bar = 50 μm (a and b); 10 μm (c and d).
GLYT1 functional activity in HCT-8 cells
Glycine uptake in polarised layers of HCT-8 cells was fivefold greater across the basolateral than the apical membrane (basal uptake: 59.4 ± 3.2 pmol cm−2 (10 min)−1; apical: 11.8 ± 1.6; means ±s.e.m., n= 6), comparable to the asymmetry of glycine uptake reported in Caco-2 cells (Christie et al. 2001). Consequently, further investigations concentrated on basolateral glycine uptake. This comprised three components identified by differing ion dependence: Na+ dependent, Cl− dependent and uptake dependent upon both Na+ and Cl−. Na+- and Cl−-dependent uptake, characteristic of GLYT1, accounted for 78.6 ± 6.30% of total glycine uptake and was approximately fivefold higher than that in the presence of Na+ alone (15.3 ± 1.32% of total glycine uptake).
Kinetic analysis, as described in detail by Christie et al. (2001) and performed in the presence of 5 mmol l−1l-alanine to block Na+ only dependent glycine uptake, showed that Na+- and Cl−-dependent glycine uptake followed Michaelis–Menten kinetics describing one-site binding with an apparent Michaelis constant (Km) of 103 ± 4 μmol l−1 (n= 6) and a maximum velocity (Vmax) of 18.5 ± 0.4 pmol cm−2 min−1. In these conditions, Na+- and Cl−-dependent glycine uptake was inhibited only by glycine, sarcosine and glycine ethyl ester, consistent with the known specificity of GLYT1; l-serine and gaba had no inhibitory effect. Finally, the contribution of GLYT1 was confirmed by use of specific inhibitors of GLYT1 and GLYT2, ALX-5407 and ALX-1393 (Xu et al. 2005), respectively, on glycine uptake. Monolayers were incubated with the appropriate inhibitor for 15 min prior to measurement of glycine uptake. ALX-5407, in concentrations ranging from 10 nmol l−1 to 1 μmol l−1, inhibited significantly Na+- and Cl−-dependent basolateral glycine uptake. Inhibition was approximately 40% at 10 nmol l−1 and was maximal at 30 nmol l−1 and above, reducing glycine uptake to 20–30% of that in control cells (Fig. 5). Non-linear regression analysis showed ALX-5407 to be a high affinity antagonist of Na+- and Cl−-dependent glycine uptake with an EC50 value of 9.6 ± 0.23 nmol l−1. ALX-1393 did not inhibit Na+- and Cl−-dependent glycine uptake over the same concentration range (Fig. 5), consistent with the lack of expression of GLYT2 in HCT-8 cells (results not shown). Thus, expression of GLYT1 mRNA and protein, the Na+ and Cl− dependence of glycine uptake, substrate specificity, kinetic characteristics and sensitivity to a specific GLYT1 inhibitor indicate functional system GLY and specifically GLYT1 activity in HCT-8 cells. As GLYT1 accounts for around 70% of Na+- and Cl−-dependent glycine uptake, which in turn represents nearly 80% of total glycine uptake, this is the dominant mechanism for glycine uptake in these cells confirming that this cell line, like Caco-2, is a suitable tool to investigate the relationship between GLYT1 mediated glycine uptake and cytoprotective activity of glycine.
Figure 5. Inhibition of Na+- and Cl−-dependent basolateral glycine uptake by a GLYT1-specific inhibitor in HCT-8 cells.
Effect of GLYT1 inhibitor, ALX-5407, and GLYT2 inhibitor, ALX-1393, on basolateral, alanine-insensitive, Na+- and Cl−-dependent glycine uptake in HCT-8 cells. Glycine uptake was measured in Krebs buffer in the presence of 5 μmol l−1 glycine, 5 mmol l−1 alanine and increasing concentrations of either ALX-5407 (□) or ALX-1393 (▪) over 10 min. Results are expressed as means with error bars of ±s.e.m., n= 6.
Effect of glycine and t-BOOH on glycine uptake
Basolateral glycine uptake was measured in cells treated with glycine for 4 h, t-BOOH for 1 h or a combination of glycine followed by t-BOOH. Glycine had a concentration-dependent effect, with 5 mmol l−1 glycine increasing uptake, measured over 15 min, by approximately 40%. In contrast t-BOOH decreased glycine uptake regardless of whether cells had been pre-treated with glycine or not (Fig. 6).
Figure 6. Effect of glycine and t-BOOH on basolateral glycine uptake.
Caco-2 cells were exposed to 5 mmol l−1 glycine, 150 μmol l−1 t-BOOH or glycine followed by t-BOOH, and basolateral glycine uptake measured over 15 min in Krebs buffer containing 5 mmol l−1 alanine. Results are expressed as means ±s.e.m., n= 6. Means without a common letter are statistically different (P < 0.05).
Glycine reduces ROS concentration in t-BOOH-treated intestinal cells
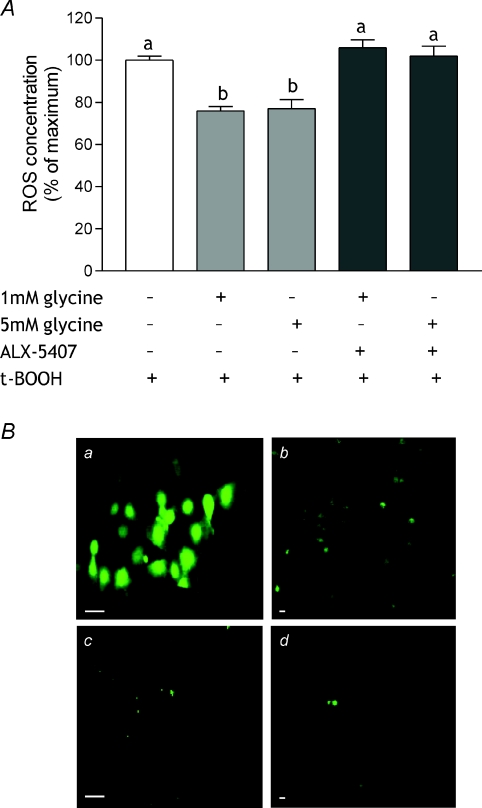
To demonstrate the generation of ROS in cultured cells in response to t-BOOH treatment, confluent monolayers of Caco-2 and HCT-8 cells grown on 22 mm coverslips were pre-loaded with H2DCFDA, incubated with 100 μmol l−1 t-BOOH for 60 min and visualised by CLSM. ROS, evident as green fluorescence throughout the cell (Fig. 7), were identified in a subset, approximately 20–30% of the total population, of cells exposed to t-BOOH, and did not differ between the cell types. Control cells, also loaded with H2DCFDA, were maintained in normal culture medium and showed little fluorescence.
Figure 7. Effect of glycine and GLYT-1 inhibition on ROS concentration.
A, Caco-2 cells were incubated in serum-free medium containing 1 or 5 mmol l−1 glycine with or without 30 nmol l−1 ALX-5407 prior to incubation with t-BOOH and determination of ROS concentration. Control cells (maximum ROS concentration, open bar) were exposed only to t-BOOH. Results are expressed as a percentage of maximum and are means ±s.e.m. of three independent experiments performed in triplicate. Bars without a common letter are statistically different (P < 0.05). B, CLSM images of Caco-2 (a and c) and HCT-8 cells (b and d) preloaded with carboxy-H2DCFDA and exposed to 100 μmol l−1 t-BOOH (A and B) or normal medium (C and D) for 1 h. In the presence of ROS the fluorescein compound is oxidised to carboxy-DCF and emits bright green fluorescence. Images are shown at ×100 (a and c) and ×20 (b and d) magnification. Bar = 10 μm.
To quantify the effect of glycine pre-treatment on ROS generation, the assay was adapted to a 96-well plate format and total fluorescence measured. For comparison purposes, the fluorescence of H2DCFDA following t-BOOH treatment was normalised to Hoechst 33342 (DNA) fluorescence and set at 100%. Pre-treating cells with 1 or 5 mmol l−1 glycine reduced ROS concentration in response to t-BOOH challenge, indicated by a decrease in relative fluorescence, to ∼75% of maximum (P < 0.05) (Fig. 7). Pre-treatment with 1 or 5 mmol l−1l-alanine had no effect (ROS concentrations: 97.3 ± 2.95 and 99.6 ± 3.40% of maximum, respectively). When GLYT-1 was inhibited by incubation with 30 nmol l−1 ALX-5407 for 15 min prior to glycine pre-treatment, ROS concentration remained high indicating that GLYT1 inhibition prevented glycine protection (Fig. 7).
Glycine preserves intracellular glutathione during oxidative stress
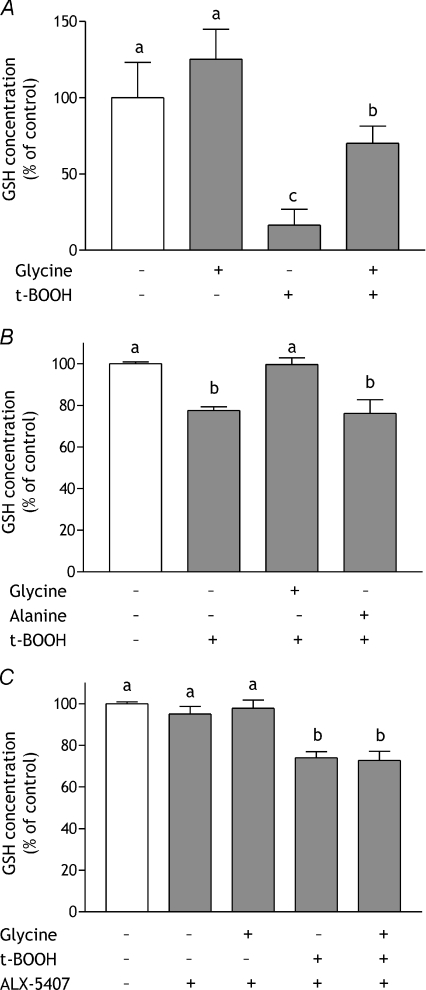
Exposure of HCT-8 cells to 150 μmol l−1 t-BOOH for 24 h resulted in a >80% decrease in cellular total GSH content (Fig. 8A). This was largely prevented by pre-treatment with 5 mmol l−1 glycine for 24 h before challenge, maintaining GSH concentration at ∼70% of that in control cells. Cells exposed to 5 mmol l−1 glycine for 24 h, without subsequent exposure to t-BOOH, showed no significant increase in glutathione content (Fig. 8A). In Caco-2 cells, less extreme conditions which had no effect on cell viability, 100 μmol l−1 t-BOOH for 60 min, were associated with a smaller decrease in GSH concentration (to 77.5 ± 1.8% of control, P < 0.05) (Fig. 8B). Glycine pre-treatment (5 mmol l−1, 4 h) prevented this and returned GSH concentration to control level (99.6 ± 3.2% of control; P < 0.01 compared with t-BOOH-treated). l-Alanine had no effect on GSH concentration, either in control or challenged cells (Fig. 8B). Inhibiting GLYT1 with ALX-5407 prior to glycine loading, in line with its prevention of glycine protection and ROS generation (Figs 2 and 7), prevented glycine maintenance of GSH concentration (Fig. 8C). Pre-treatment with ALX-5407 alone did not exacerbate the effect of t-BOOH (Fig. 8C) and in unchallenged cells the inhibitor had no effect on GSH concentration either when given alone or with glycine supplementation.
Figure 8. Effect of glycine and GLYT-1 inhibition on intracellular GSH concentration during oxidative stress.
GSH concentration was measured in HCT-8 cell monolayers incubated in serum-free medium or serum-free medium containing 5 mmol l−1 glycine for 24 h followed by a further 24 h in serum-free medium or serum-free medium containing 150 μmol l−1 t-BOOH (A), Caco-2 cell monolayers incubated with serum-free medium alone or containing 5 mmol l−1 glycine or l-alanine for 4 h prior to exposure to 100 μmol l−1 t-BOOH for 1 h (B), and Caco-2 cells incubated in serum-free medium containing 30 nmol l−1 ALX-5407 with or without 5 mmol l−1 glycine for 4 h and subsequent exposure to 100 μmol l−1 t-BOOH for 1 h (C). Results are shown relative to cells incubated in serum-free medium throughout (open bars) and are means ±s.e.m. of three independent experiments performed in quadruplicate. Means without a common letter are statistically different (P < 0.05).
Abundance of mRNAs encoding GLYT1 and GSH-related enzymes is not regulated by t-BOOH or glycine
Previous studies have indicated that stress results in up-regulation of GLYT1 gene expression (Harding et al. 2003) and that stimulation of GSH synthesis involves increased expression of GCLC and, to a lesser extent, GCLM and GS (Lu, 2009). Therefore we examined the effect of glycine and t-BOOH on abundance of these mRNAs. In Caco-2 cells, treatment with 5 mmol l−1 glycine for 4 h, 100 μmol l−1 t-BOOH for 1 h or combined glycine pre-treatment and subsequent t-BOOH had no significant effect on abundance of any of the GSH-related mRNAs investigated. Similarly, GLYT1 mRNA abundance was not altered significantly by t-BOOH although treatment with glycine, with or without subsequent t-BOOH, resulted in a non-significant decrease to approximately 75% of that in control cells (Table 2). Comparable results were obtained in HCT-8 cells treated with 4 mmol l−1 glycine or 50–200 μmol l−1 t-BOOH for periods of 2–24 h (data not shown).
Table 2.
Relative mRNA abundance of GLYT1 and GSH-related enzymes in t-BOOH- and glycine-treated Caco-2 cells
| Treatment |
||||
|---|---|---|---|---|
| Control | Glycine | t-BOOH | Glycine + t-BOOH | |
| GLYT1 | 100 ± 6 | 75 ± 5.1 | 102.8 ± 13.4 | 77.5 ± 11.4 |
| GCLC | 100 ± 1.9 | 105 ± 4.1 | 108.4 ± 2.9 | 103 ± 6 |
| GCLM | 100 ± 3.4 | 102 ± 7.8 | 111.3 ± 8.9 | 120 ± 7.1 |
| GS | 100 ± 5.3 | 122 ± 10.8 | 126.3 ± 10.2 | 108 ± 9.5 |
Values (expressed as percentage of control) are given as means ±s.e.m. n= 9–12 per group and are means of 3–4 experiments. In all cases P > 0.05 compared to control.
Discussion
Multiple studies have established that glycine is able to protect intact intestine from injury caused by ischaemia–reperfusion (Mangino et al. 1996; Iijima et al. 1997; Lee et al. 2001, 2002; Schaefer et al. 2008) and it has also been demonstrated that it can prevent or reverse the damage caused during the induction of experimental colitis by TNBS and dextran sulphate or abdominal irradiation in rodents (Tsune et al. 2003). However, the underlying molecular mechanisms remain unknown. Therefore, to provide a more tractable system for such studies, we have established human cell models of oxidative intestinal injury, and used these to investigate glycine mediated intestinal cell protection. Moreover, utilisation of these intestinal cell models allowed isolation of the effects to intrinsic properties and functions of the epithelial cells, distinct from any confounding effects on extra-epithelial tissues or functions, such as neurone, blood flow or immune responses.
The oxidising agent t-BOOH induced ROS formation in both Caco-2 and HCT-8 cells and resulted in a decrease in cell viability. Glycine, given prior to t-BOOH challenge, reduced ROS concentration and protected cells against damage. However, it had no protective effect when administered simultaneously with t-BOOH, consistent with a requirement for glycine accumulation by the cells. That this is indeed the case was confirmed by inclusion of the GLYT1-specific inhibitor ALX-5407, which blocked the protective effects of prior exposure to exogenous glycine against t-BOOH challenge. These data, supported by the demonstration of expression of GLYT1 mRNA and protein in human colon, where nutrient absorption is minimal and is thus unlikely to be involved in assimilation of dietary glycine, suggest that GLYT1 is essential for cytoprotection by glycine and important in preserving intestinal cell health.
Intestinal cells are exposed to constant and high levels of oxidising agents and so require an efficient mechanism for preserving cellular redox potential. Glutathione, the most abundant antioxidant of the intestinal mucosa, is generated in intestinal cells. It is required for intestinal function (Martensson et al. 1990) and at times of prolonged or severe stress the supply of its component amino acids, including glycine (Jackson, 1986), becomes essential. Additionally, glycine supplementation has been shown to increase hepatocyte, erythrocyte and plasma glutathione activity and protect against alcohol-induced liver injury in rats (Senthilkumar et al. 2004). Therefore, we examined the effect of glycine exposure on glutathione concentration in intestinal cells during oxidative stress. Prolonged exposure to t-BOOH reduced dramatically glutathione concentration and this effect was reversed partially by pre-treatment with glycine; less severe treatment had a lesser effect that was completely reversible, suggesting that accumulated glycine is used to replenish glutathione.
In tissues such as kidney and liver, glycine cytoprotection appears to be independent of glutathione levels (Dickson et al. 1992; Weinberg, 1992) and instead works by activation of the glycine receptor (GlyR), a glycine-gated chloride channel (Gundersen et al. 2005). However, as this requires extracellular glycine (Dong et al. 2001), it is unlikely to be the active mechanism in intestinal epithelial cells where we have clearly shown a dependence on GLYT1-mediated glycine uptake. Furthermore, we also demonstrated a requirement for glycine administration prior to challenge which is not essential for GlyR mediated protection (Miller et al. 1994), and found that l-alanine was ineffective as a cytoprotectant. l-Alanine is a weak agonist of the glycine receptor (Rajendra et al. 1997) and has been shown to have similar, although lesser, protective effects to glycine in tissues where GlyR protection has been described (Silva et al. 1991; Weinberg et al. 1992; Marsh et al. 1993; Zhang et al. 2003).
Evidence of GlyR-independent mechanisms of glycine cytoprotection has also been found in other tissues. High concentrations of glycine (10–30 mm compared with 1–5 mm used here) protected lung endothelial cells against gabexate mesilate-induced injury and appeared to work by blocking the opening of membrane channels and pores in a glycine receptor-independent mechanism (Aki et al. 2008). Cytoprotection of liver cells by glycine has been observed in the absence of chloride and calcium ions (Frank et al. 2000) in an as yet unexplained process. These differences suggest multiple but not exclusive modes of action. Indeed, our observations in intestinal cells differ from those of Katayama & Mine (2007) who demonstrated increased glutathione content and glutathione reductase activity in oxidatively-stressed Caco-2 cells pre-treated with alanine but showed no cytoprotection, indicated by a reduction in IL-8 secretion, by glycine. However, there were significant methodological differences between that work and ours, including differences in cell culture conditions, nature of oxidative challenge and measured indicators of stress, supporting earlier conclusions that the mechanism of protection is dependent on both cell/tissue type and the nature of the challenge (Sogabe et al. 1996; Deters et al. 1998).
That we have shown GLYT1 dependence and increased glutathione concentration in two human intestinal cell lines grown under different conditions suggests that this is a general mechanism relevant to the whole of the intestine. Moreover evidence from other work supports a more widespread role for GLYT1 in cytoprotection. GLYT1 mRNA expression was up-regulated in mouse fibroblasts subjected to endoplasmic reticulum stress, mediated by the transcription factor ATF-4, which plays a central role in several stress response pathways (Harding et al. 2003). Enhanced expression of other amino acid transporters, particularly of xCT, the light chain of the transporter X−c important in providing cells with cysteine for glutathione synthesis, was also noted suggesting that part of the cellular response to stress is to increase the availability of specific amino acids required for the synthesis of essential proteins and peptides.
In view of this and having shown altered glycine transport in response to glycine and t-BOOH we examined the effect of the various treatments on GLYT1 mRNA abundance in our cell models. However, it was not changed by any of the treatments, suggesting that in this system GLYT1 transcriptional regulation is not a significant factor. Furthermore as the antibody used in the localisation studies proved unsuitable for Western blot analysis it was also not possible to demonstrate whether the observed changes in GLYT1 activity following glycine or t-BOOH treatment were due to alterations in concentration of the GLYT1 protein within the cell membrane. However, that GLYT1 activity was reduced by t-BOOH may in part explain the reduced effectiveness of glycine when co-administered with t-BOOH.
Many previous studies have established a link between expression of the glutathione-related enzymes glutamate-cysteine ligase (GCL) and GS with GSH concentration and shown that expression of the genes encoding these molecules is upregulated in response to stress and leads to stimulation of GSH synthesis. GCL catalyses the first step of GSH synthesis, joining cysteine and glutamate in a γ-linkage to form γ-glutamylcysteine (γ-GC). It is a heterodimer composed of a catalytic (GCLC) and a modifier (GCLM) subunit. Cellular GSH content is correlated closely with GCLC and, to a lesser extent, GCLM mRNA and protein content (Morales et al. 1997; Krzywanski et al. 2004; Suh et al. 2004; Takamura et al. 2006). GS catalyses the second and final step of GSH synthesis: the addition of glycine to γ-GC. Although cysteine availability and GCLC expression are generally thought to be the rate limiting factors in GSH synthesis, evidence of co-ordinate regulation of GS, GCLC and GCLM and work showing that increasing expression of both GS and GCLC increases GSH synthesis to a greater extent than increasing that of GCLC alone suggests that GS may be of greater significance than previously thought (Lu, 2009). As there appears to be a close correlation between expression of these enzymes and de novo synthesis of GSH we examined mRNA abundances to determine if upregulation of transcription was required for the maintenance of GSH concentration by glycine during oxidative challenge. However, none of the treatments, glycine, t-BOOH or a combination of the two (glycine followed by t-BOOH), had an effect on abundance of any of the mRNAs, suggesting that in this case, and as found with GLYT1, transcriptional regulation of these enzymes is not essential for glycine protection in this model. GCL activity is regulated by GSH in a negative feedback loop and by phosphorylation/dephosphorylation (Suh et al. 2004; Lu, 2009). Thus it remains possible that de novo synthesis of GSH rather than preservation of existing supply is involved in glycine protection of intestinal cells.
It has been suggested that the fate of amino acids taken up by intestinal cells is determined by the site of acquisition, i.e. those absorbed from the gut lumen are used in different processes than those acquired from the serosal fluid, and that this is independent of the availability of amino acid at the opposing surface (Reeds et al. 2000). It is thus likely that the ‘source’ of a nutrient and the mechanism by which it is acquired are dictated by the specific use for which it is required. On the basis of the experimental evidence presented here, we suggest that glycine acquired across the basolateral membrane of intestinal cells by GLYT1 is used in epithelial cell defence and in maintaining intracellular antioxidant potential. Further knowledge of the precise mechanism by which glycine protects intestine and the role of GLYT1, including regulation of expression and activity, may reveal potential for treatment of oxidative intestinal injury, including inflammatory bowel disease.
Acknowledgments
This work was supported by a grant from the Wellcome Trust (070194), with additional support in the form of studentships from the MRC (I.T.) and BBSRC (S.M.W.). The authors thank Maxine E. Geggie for expert technical support.
Glossary
Abbreviations
- CLSM
confocal laser scanning microscopy
- GSH
glutathione
- GCL
glutamate-cysteine ligase
- GCLC
GCL catalytic subunit
- GCLM
GCL modifier subunit
- GS
GSH synthetase
- γ-GC
γ-glutamylcysteine
- IR
ischaemia–reperfusion
- ROS
reactive oxygen species
Author contributions
A.H., I.T., S.J., S.M.W. and B.H.H. contributed to the conception and design of experiments. A.H., I.T. and S.J. contributed to collection, analysis and interpretation of data and A.H., D.F. and B.H.H. were involved with drafting and revising the article.
References
- Aki T, Egashira N, Yamauchi Y, Hama M, Yano T, Itoh Y, Yamada T, Oishi R. Protective effects of amino acids against gabexate mesilate-induced cell injury in porcine aorta endothelial cells. J Pharmacol Sci. 2008;107:238–245. doi: 10.1254/jphs.08053fp. [DOI] [PubMed] [Google Scholar]
- Allen CN, Harpur ES, Gray TJB, Hirst BH. Toxic effects of non-steroidal anti-inflammatory drugs in a human intestinal epithelial cell line (HCT-8), as assessed by the MTT and neutral red assays. Toxicology in Vitro. 1991;5:183–191. doi: 10.1016/0887-2333(91)90016-7. [DOI] [PubMed] [Google Scholar]
- Atkinson BN, Bell SC, De Vivo M, Kowalski LR, Lechner SM, Ognyanov VI, Tham CS, Tsai C, Jia J, Ashton D, Klitenick MA. ALX 5407: a potent, selective inhibitor of the hGlyT1 glycine transporter. Mol Pharmacol. 2001;60:1414–1420. doi: 10.1124/mol.60.6.1414. [DOI] [PubMed] [Google Scholar]
- Christie GR, Ford D, Howard A, Clark MA, Hirst BH. Glycine supply to human enterocytes mediated by high-affinity basolateral GLYT1. Gastroenterology. 2001;120:439–448. doi: 10.1053/gast.2001.21207. [DOI] [PubMed] [Google Scholar]
- Deters M, Siegers CP, Strubelt O. Influence of glycine on the damage induced in isolated perfused rat liver by five hepatotoxic agents. Toxicology. 1998;128:63–72. doi: 10.1016/s0300-483x(98)00048-1. [DOI] [PubMed] [Google Scholar]
- Dickson RC, Bronk SF, Gores GJ. Glycine cytoprotection during lethal hepatocellular injury from adenosine triphosphate depletion. Gastroenterology. 1992;102:2098–2107. doi: 10.1016/0016-5085(92)90338-y. [DOI] [PubMed] [Google Scholar]
- Diestel CF, Marques RG, Lopes-Paulo F, Paiva D, Horst NL, Caetano CE, Portela MC. Role of L-glutamine and glycine supplementation on irradiated colonic wall. Int J Colorectal Dis. 2007;22:1523–1529. doi: 10.1007/s00384-007-0341-8. [DOI] [PubMed] [Google Scholar]
- Dong Z, Venkatachalam MA, Weinberg JM, Saikumar P, Patel Y. Protection of ATP-depleted cells by impermeant strychnine derivatives: implications for glycine cytoprotection. Am J Pathol. 2001;158:1021–1028. doi: 10.1016/S0002-9440(10)64049-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank A, Rauen U, de Groot H. Protection by glycine against hypoxic injury of rat hepatocytes: inhibition of ion fluxes through nonspecific leaks. J Hepatol. 2000;32:58–66. doi: 10.1016/s0168-8278(00)80190-7. [DOI] [PubMed] [Google Scholar]
- Gundersen RY, Vaagenes P, Breivik T, Fonnum F, Opstad PK. Glycine – an important neurotransmitter and cytoprotective agent. Acta Anaesthesiol Scand. 2005;49:1108–1116. doi: 10.1111/j.1399-6576.2005.00786.x. [DOI] [PubMed] [Google Scholar]
- Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, Calfon M, Sadri N, Yun C, Popko B, Paules R, Stojdl DF, Bell JC, Hettmann T, Leiden JM, Ron D. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell. 2003;11:619–633. doi: 10.1016/s1097-2765(03)00105-9. [DOI] [PubMed] [Google Scholar]
- Hashim A, Mulcahy G, Bourke B, Clyne M. Interaction of Cryptosporidium hominis and Cryptosporidium parvum with primary human and bovine intestinal cells. Infect Immun. 2006;74:99–107. doi: 10.1128/IAI.74.1.99-107.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard A, Goodlad RA, Walters JR, Ford D, Hirst BH. Increased expression of specific intestinal amino acid and peptide transporter mRNA in rats fed by TPN is reversed by GLP-2. J Nutr. 2004;134:2957–2964. doi: 10.1093/jn/134.11.2957. [DOI] [PubMed] [Google Scholar]
- Iijima S, Shou J, Naama H, Calvano SE, Daly JM. Beneficial effect of enteral glycine in intestinal ischemia/reperfusion injury. J Gastrointest Surg. 1997;1:53–60. doi: 10.1007/s11605-006-0011-0. [DOI] [PubMed] [Google Scholar]
- Ikejima K, Iimuro Y, Forman DT, Thurman RG. A diet containing glycine improves survival in endotoxin shock in the rat. Am J Physiol Gastrointest Liver Physiol. 1996;271:G97–103. doi: 10.1152/ajpgi.1996.271.1.G97. [DOI] [PubMed] [Google Scholar]
- Jackson AA. Blood glutathione in severe malnutrition in childhood. Trans R Soc Trop Med Hyg. 1986;80:911–913. doi: 10.1016/0035-9203(86)90256-7. [DOI] [PubMed] [Google Scholar]
- Jackson AA, Gibson NR, Lu Y, Jahoor F. Synthesis of erythrocyte glutathione in healthy adults consuming the safe amount of dietary protein. Am J Clin Nutr. 2004;80:101–107. doi: 10.1093/ajcn/80.1.101. [DOI] [PubMed] [Google Scholar]
- Jacob T, Ascher E, Hingorani A, Kallakuri S. Glycine prevents the induction of apoptosis attributed to mesenteric ischemia/reperfusion injury in a rat model. Surgery. 2003;134:457–466. doi: 10.1067/s0039-6060(03)00164-8. [DOI] [PubMed] [Google Scholar]
- Kallakuri S, Ascher E, Pagala M, Gade P, Hingorani A, Scheinman M, Mehraein K, Jacob T. Protective effect of glycine in mesenteric ischemia and reperfusion injury in a rat model. J Vasc Surg. 2003;38:1113–1120. doi: 10.1016/s0741-5214(03)00939-x. [DOI] [PubMed] [Google Scholar]
- Katayama S, Mine Y. Antioxidative activity of amino acids on tissue oxidative stress in human intestinal epithelial cell model. J Agric Food Chem. 2007;55:8458–8464. doi: 10.1021/jf070866p. [DOI] [PubMed] [Google Scholar]
- Krzywanski DM, Dickinson DA, Iles KE, Wigley AF, Franklin CC, Liu RM, Kavanagh TJ, Forman HJ. Variable regulation of glutamate cysteine ligase subunit proteins affects glutathione biosynthesis in response to oxidative stress. Arch Biochem Biophys. 2004;423:116–125. doi: 10.1016/j.abb.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Leblond J, Hubert-Buron A, Bole-Feysot C, Ducrotte P, Déchelotte P, Coëffier M. Regulation of proteolysis by cytokines in the human intestinal epithelial cell line HCT-8: role of IFNγ. Biochimie. 2006;88:759–765. doi: 10.1016/j.biochi.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Lee MA, McCauley RD, Kong SE, Hall JC. Pretreatment with glycine reduces the severity of warm intestinal ischemic-reperfusion injury in the rat. Ann Plast Surg. 2001;46:320–326. doi: 10.1097/00000637-200103000-00020. [DOI] [PubMed] [Google Scholar]
- Lee MA, McCauley RD, Kong SE, Hall JC. Influence of glycine on intestinal ischemia-reperfusion injury. J Parenter Enteral Nutr. 2002;26:130–135. doi: 10.1177/0148607102026002130. [DOI] [PubMed] [Google Scholar]
- Ligumsky M, Sestieri M, Okon E, Ginsburg I. Antioxidants inhibit ethanol-induced gastric injury in the ratRole of manganese, glycine, and carotene. Scand J Gastroenterol. 1995;30:854–860. doi: 10.3109/00365529509101591. [DOI] [PubMed] [Google Scholar]
- Lu SC. Regulation of glutathione synthesis. Molecular Aspects of Medicine. 2009;30:42–59. doi: 10.1016/j.mam.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck SN, Bennett-Wood V, Poon R, Robins-Browne RM, Hartland EL. Invasion of epithelial cells by locus of enterocyte effacement-negative enterohemorrhagic Escherichia coli. Infect Immun. 2005;73:3063–3071. doi: 10.1128/IAI.73.5.3063-3071.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luntz SP, Unnebrink K, Seibert-Grafe M, Bunzendahl H, Kraus TW, Buchler MW, Klar E, Schemmer P. HEGPOL: randomized, placebo controlled, multicenter, double-blind clinical trial to investigate hepatoprotective effects of glycine in the postoperative phase of liver transplantation [ISRCTN69350312] BMC Surg. 2005;5:18. doi: 10.1186/1471-2482-5-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangino JE, Kotadia B, Mangino MJ. Characterization of hypothermic intestinal ischemia-reperfusion injury in dogs. Effects of glycine. Transplantation. 1996;62:173–178. doi: 10.1097/00007890-199607270-00005. [DOI] [PubMed] [Google Scholar]
- Marion R, Coeffier M, Lemoulan S, Gargala G, Ducrotte P, Dechelotte P. L-Arginine modulates CXC chemokines in the human intestinal epithelial cell line HCT-8 by the NO pathway. Biochimie. 2005;87:1048–1055. doi: 10.1016/j.biochi.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Marsh DC, Vreugdenhil PK, Mack VE, Belzer FO, Southard JH. Glycine protects hepatocytes from injury caused by anoxia, cold ischemia and mitochondrial inhibitors, but not injury caused by calcium ionophores or oxidative stress. Hepatology. 1993;17:91–98. [PubMed] [Google Scholar]
- Martensson J, Jain A, Meister A. Glutathione is required for intestinal function. Proc Natl Acad Sci U S A. 1990;87:1715–1719. doi: 10.1073/pnas.87.5.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchant NB, Rogers CM, Trivedi B, Morrow J, Coffey RJ. Ligand-dependent activation of the epidermal growth factor receptor by secondary bile acids in polarizing colon cancer cells. Surgery. 2005;138:415–421. doi: 10.1016/j.surg.2005.06.030. [DOI] [PubMed] [Google Scholar]
- Merendino N, Dwinell MB, Varki N, Eckmann L, Kagnoff MF. Human intestinal epithelial cells express receptors for platelet-activating factor. Am J Physiol Gastrointest Liver Physiol. 1999;277:G810–818. doi: 10.1152/ajpgi.1999.277.4.G810. [DOI] [PubMed] [Google Scholar]
- Miller GW, Lock EA, Schnellmann RG. Strychnine and glycine protect renal proximal tubules from various nephrotoxicants and act in the late phase of necrotic cell injury. Toxicol Appl Pharmacol. 1994;125:192–197. doi: 10.1006/taap.1994.1064. [DOI] [PubMed] [Google Scholar]
- Morales A, Garcia-Ruiz C, Miranda M, Mari M, Colell A, Ardite E, Fernandez-Checa JC. Tumor necrosis factor increases hepatocellular glutathione by transcriptional regulation of the heavy subunit chain of γ-glutamylcysteine synthetase. J Biol Chem. 1997;272:30371–30379. doi: 10.1074/jbc.272.48.30371. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Nissim I, Hardy M, Pleasure J, States B. A mechanism of glycine and alanine cytoprotective action: stimulation of stress-induced HSP70 mRNA. Kidney Int. 1992;42:775–782. doi: 10.1038/ki.1992.347. [DOI] [PubMed] [Google Scholar]
- Pinto M, Robine-Leon S, Appay M, Kedinger M, Triadou N, Dussaulx E, Lacroix B, Simon-Assam P, Haffen K. Enterocyte-like differentiation and polarisation of the human colon carcinoma cell line Caco-2 in culture. Biol Cell. 1983;47:323–330. [Google Scholar]
- Rajendra S, Lynch JW, Schofield PR. The glycine receptor. Pharmacol Ther. 1997;73:121–146. doi: 10.1016/s0163-7258(96)00163-5. [DOI] [PubMed] [Google Scholar]
- Reeds PJ, Burrin DG, Stoll B, van Goudoever JB. Role of the gut in the amino acid economy of the host. In: Fürst P, Young V, editors. Nestlé Nutrition Institute Workshop Series: Clinical & Performance Program vol. 3, Proteins, Peptides and Amino Acids in Enteral Nutrition. Basel: Vevey/S. Karger AG; 2000. pp. 25–46. [DOI] [PubMed] [Google Scholar]
- Rogers TJ, Paton AW, McColl SR, Paton JC. Enhanced CXC chemokine responses of human colonic epithelial cells to locus of enterocyte effacement-negative shiga-toxigenic Escherichia coli. Infect Immun. 2003;71:5623–5632. doi: 10.1128/IAI.71.10.5623-5632.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer N, Tahara K, Schuchtrup S, Websky MV, Overhaus M, Schmidt J, Wirz S, Abu-Elmagd KM, Kalff JC, Hirner A, Turler A. Perioperative glycine treatment attenuates ischemia/reperfusion injury and ameliorates smooth muscle dysfunction in intestinal transplantation. Transplantation. 2008;85:1300–1310. doi: 10.1097/TP.0b013e31816c576f. [DOI] [PubMed] [Google Scholar]
- Schemmer P, Golling M, Kraus T, Mehrabi A, Mayatepek E, Herfarth C, Klar E. Extended experience with glycine for prevention of reperfusion injury after human liver transplantation. Transplant Proc. 2002;34:2307–2309. doi: 10.1016/s0041-1345(02)03247-5. [DOI] [PubMed] [Google Scholar]
- Schilling T, Eder C. A novel physiological mechanism of glycine-induced immunomodulation: Na+-coupled amino acid transporter currents in cultured brain macrophages. J Physiol. 2004;559:35–40. doi: 10.1113/jphysiol.2004.070763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senthilkumar R, Sengottuvelan M, Nalini N. Protective effect of glycine supplementation on the levels of lipid peroxidation and antioxidant enzymes in the erythrocyte of rats with alcohol-induced liver injury. Cell Biochem Funct. 2004;22:123–128. doi: 10.1002/cbf.1062. [DOI] [PubMed] [Google Scholar]
- Silva P, Rosen S, Spokes K, Epstein FH. Effect of glycine on medullary thick ascending limb injury in perfused kidneys. Kidney Int. 1991;39:653–658. doi: 10.1038/ki.1991.78. [DOI] [PubMed] [Google Scholar]
- Sogabe K, Roeser NF, Venkatachalam MA, Weinberg JM. Differential cytoprotection by glycine against oxidant damage to proximal tubule cells. Kidney Int. 1996;50:845–854. doi: 10.1038/ki.1996.384. [DOI] [PubMed] [Google Scholar]
- Suh JH, Shenvi SV, Dixon BM, Liu H, Jaiswal AK, Liu RM, Hagen TM. Decline in transcriptional activity of Nrf2 causes age-related loss of glutathione synthesis, which is reversible with lipoic acid. Proc Natl Acad Sci U S A. 2004;101:3381–3386. doi: 10.1073/pnas.0400282101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamura Y, Fatma N, Kubo E, Singh DP. Regulation of heavy subunit chain of γ-glutamylcysteine synthetase by tumor necrosis factor-α in lens epithelial cells: role of LEDGF/p75. Am J Physiol Cell Physiol. 2006;290:C554–566. doi: 10.1152/ajpcell.00398.2005. [DOI] [PubMed] [Google Scholar]
- Tompkins WA, Watrach AM, Schmale JD, Schultz RM, Harris JA. Cultural and antigenic properties of newly established cell strains derived from adenocarcinomas of the human colon and rectum. J Natl Cancer Inst. 1974;52:1101–1110. doi: 10.1093/jnci/52.4.1101. [DOI] [PubMed] [Google Scholar]
- Tormos C, Javier Chaves F, Garcia MJ, Garrido F, Jover R, O’Connor JE, Iradi A, Oltra A, Oliva MR, Saez GT. Role of glutathione in the induction of apoptosis and c-fos and c-jun mRNAs by oxidative stress in tumor cells. Cancer Letters. 2004;208:103–113. doi: 10.1016/j.canlet.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Tsune I, Ikejima K, Hirose M, Yoshikawa M, Enomoto N, Takei Y, Sato N. Dietary glycine prevents chemical-induced experimental colitis in the rat. Gastroenterology. 2003;125:775–785. doi: 10.1016/s0016-5085(03)01067-9. [DOI] [PubMed] [Google Scholar]
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3 doi: 10.1186/gb-2002-3-7-research0034. RESEARCH0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg JM. Glutathione and glycine in acute renal failure. Ren Fail. 1992;14:311–319. doi: 10.3109/08860229209106635. [DOI] [PubMed] [Google Scholar]
- Weinberg JM, Varani J, Johnson KJ, Roeser NF, Dame MK, Davis JA, Venkatachalam MA. Protection of human umbilical vein endothelial cells by glycine and structurally similar amino acids against calcium and hydrogen peroxide-induced lethal cell injury. Am J Pathol. 1992;140:457–471. [PMC free article] [PubMed] [Google Scholar]
- Weinberg JM, Venkatachalam MA, Garzo-Quintero R, Roeser NF, Davis JA. Structural requirements for protection by small amino acids against hypoxic injury in kidney proximal tubules. FASEB J. 1990;4:3347–3354. doi: 10.1096/fasebj.4.15.2253849. [DOI] [PubMed] [Google Scholar]
- Xu TX, Gong N, Xu TL. Inhibitors of GlyT1 and GlyT2 differentially modulate inhibitory transmission. Neuroreport. 2005;16:1227–1231. doi: 10.1097/00001756-200508010-00019. [DOI] [PubMed] [Google Scholar]
- Zhang K, Weinberg JM, Venkatachalam MA, Dong Z. Glycine protection of PC-12 cells against injury by ATP-depletion. Neurochem Res. 2003;28:893–901. doi: 10.1023/a:1023275426637. [DOI] [PubMed] [Google Scholar]
- Zhong Z, Jones S, Thurman RG. Glycine minimizes reperfusion injury in a low-flow, reflow liver perfusion model in the rat. Am J Physiol Gastrointest Liver Physiol. 1996;270:G332–338. doi: 10.1152/ajpgi.1996.270.2.G332. [DOI] [PubMed] [Google Scholar]
- Zhong Z, Wheeler MD, Li X, Froh M, Schemmer P, Yin M, Bunzendaul H, Bradford B, Lemasters JJ. L-Glycine: a novel antiinflammatory, immunomodulatory, and cytoprotective agent. Curr Opin Clin Nutr Metab Care. 2003;6:229–240. doi: 10.1097/00075197-200303000-00013. [DOI] [PubMed] [Google Scholar]