Abstract
IL-12p70 is an immunoregulatory cytokine that has been shown to induce IL-10 production from CD4+ T cells, yet the underlying cellular mechanisms controlling this process are poorly understood. In the present study, we demonstrate that IL-12p70 induces IL-10 production from human memory CD4+ T cells via a PI3K-dependent signaling mechanism. Specifically, stimulation of human memory CD4+ T cells in the presence of IL-12p70 lead to increased PI3K activity and the subsequent phosphorylation and inactivation of the downstream constitutively active serine/threonine kinase, glycogen synthase kinase-3β (GSK3β). Inhibition of PI3K prevented the inactivation of GSK3β by IL-12p70, as well as the subsequent ability of IL-12p70 to augment IL-10 levels by memory CD4+ T cells. Moreover, ectopic expression of a constitutively active form of GSK3β abrogated the ability of IL-12p70 to increase IL-10 production by TCR-stimulated CD4+ T cells. In contrast, direct inhibition of GSK3 mimicked the effect of IL-12p70 on IL-10 production by memory CD4+ T cells. Analysis of downstream transcription factors identified that the ability of IL-12p70 to inactivate GSK3β lead to increased levels of c-Jun. The ability of IL-12p70 to inactivate GSK3β and induce c-Jun levels was required for IL-12 to augment IL-10 production by human memory CD4+ T cells, since small interfering RNA-mediated gene silencing of c-Jun abrogated this process. These studies identify the cellular mechanism by which IL-12 induces IL-10 production from human memory CD4+ T cells.
Interleukin 12p70 is a covalently linked heterodimeric cytokine composed of the p35 and p40 subunits and is produced by multiple cellular lineages such as macrophages and dendritic cells (1). Although IL-12p70 is known as an immunoregulatory cytokine that can induce IFN-γ production from NK cells, B cells, APCs, and T cells, IL-12 can also influence the gene expression profiles of a variety of other genes, including T cell-derived IL-10 (2–6). IL-12’s induction of IL-10 from T cells has been suggested to function as a negative feedback mechanism, as IL-10 can suppress the production of IL-12 from APCs (7–11). In fact, studies by Chang et al. have identified that the magnitude of the IL-10 recall response of memory CD4+ T cells is dependent on the presence of IL-12 (9). The ability of IL-12 to induce the production of IL-10 has also been reported in clinical trials assessing the efficacy of systemic administration of IL-12 for cancer immunotherapy (12). Although the cellular source of IL-10 is unclear from these clinical trials, in vitro studies using human or murine T cells have demonstrated that IL-12 can condition differentiated CD4+ T cells into producing IL-10 (8, 10). Despite the evidence demonstrating the ability of IL-12 to promote IL-10 production from T cells, the cellular mechanisms involved in this process are largely unknown.
Recognition of IL-12 by T cells requires the expression of the IL-12β1 and β2 receptor chains (13). Although these receptors lack intrinsic enzymatic activity, the receptor associated Jak family members, Jak2 and Tyk2, become phosphorylated and activated upon ligand binding, leading to the phosphorylation of key tyrosine residues located within the intracellular domains of the IL-12 receptor complex (14). These phosphorylated tyrosines can then serve as docking sites for other signaling proteins containing Src homology 2 domains such as the members of the STATs (15). IL-12 receptor signaling has also been shown to activate other kinases such as PI3K (16). Interestingly, the ability of human memory CD4+ T cells to produce IL-10 upon TCR stimulation has been shown to be dependent upon PI3K activity (17, 18). In fact, the ability of costimulatory signals such as CD28 to augment IL-10 production from human memory CD4+ T cells is dependent on the PI3K signaling pathway (18). Specifically, activation of the PI3K pathway in human memory CD4+ T cells leads to the inactivation of glycogen synthase kinase-3β (GSK3β)3 and the subsequent production of IL-10 (18). In contrast, active GSK3β in human memory CD4+ T cells was found to suppress the IL-10 recall response of human memory CD4+ T cells upon TCR stimulation (18). However, whether IL-12 induces PI3K activity in human memory CD4+ T cells and whether the ability of IL-12 to promote IL-10 production from human memory CD4+ T cells is dependent on the PI3K signaling pathway have not been investigated.
Here we report that the stimulation of human memory CD4+ T cells in the presence of IL-12 leads to enhanced IL-10 production. We demonstrate that the ability of IL-12 to enhance IL-10 production from memory CD4+ T cells requires an intact PI3K signaling pathway. In this regard, we show that IL-12 activates PI3K in human memory CD4+ T cells and that the subsequent PI3K-mediated inactivation of the downstream kinase GSK3β is required for IL-12 to promote IL-10 production from memory CD4+ T cells. The inactivation of GSK3β by IL-12 was shown to lead to the increased levels of the transcription factor c-Jun. The ability of IL-12 to enhance the cellular levels of c-Jun was shown to be required for IL-12 to promote IL-10 production from human memory CD4+ T cells. These findings identify the molecular mechanism by which IL-12 promotes IL-10 production from memory CD4+ T cells.
Materials and Methods
Media
Cells were cultured in RPMI 1640 medium supplemented with 10% FBS, 50 μM 2-ME, 1 mM sodium pyruvate, 2 mM L-glutamine, 20 mM HEPES, 50 U/ml penicillin, and 50 μg/ml streptomycin.
Reagents
Anti-CD3 (OKT3), human recombinant IL-12p70, human recombinant IL-23, IL-10 ELISA kits, IL-10 ELISPOT kits, and flow cytometry-grade anti-human IL-10 were obtained from eBioscience. SB216763, LiCl, and wortmannin/LY294002 were purchased from Tocris Bioscience, Calbiochem, and LC Laboratories, respectively. Nontargeting pools of small interfering RNA (siRNA) and a mixture of four prevalidated siRNA duplexes specific for c-Jun and GSK3β (ON-TARGETplus) were purchased from Dharmacon. TaqMan probes were purchased from Applied Biosystems. Western blot Abs for human phospho-GSK3β (S9), phospho-Akt (S473), GSK3β, ERK1/2, c-Jun, and β-catenin were obtained from Cell Signaling Technologies. FACE PI3K p85 ELISA kit, which detects phosphotyrosines within YXXM motifs, was obtained from Active Motif as used as directed by the manufacturer. Flow cytometry grade anti-human c-Jun was purchased from Santa Cruz Biotechnology.
Isolation of memory CD4+ T cells
PBMCs were obtained from the venous blood of normal adult donors after isolation of the leukocyte layer and elimination of RBCs using a Ficoll density gradient (University of Louisville, Institutional Review Board, Human Subjects Protection Program, study no. 503.05). Memory CD4+ T cells (CD45RA−CD45RO+) and naive CD4+ T cells (CD45RA+ CD45RO+) were purified from PBMCs by negative selection using kits from Miltenyi Biotec. Memory and naive CD4+ T cells were further purified using a FACSVantage cell sorter (purity > 99%).
T cell stimulations
T cells (2 × 105 cells) were pretreated for 2 h with media containing DMSO (0.1%), wortmannin, NaCl, LiCl, or SB216763 at the indicated final concentration and transferred to 96-well flat bottom plates preincubated with PBS or anti-CD3 (3 μg/ml). Precoated anti-CD3 plates were prepared by incubating the tissue culture plate overnight at 4°C with PBS containing the indicated concentration of anti-CD3 Abs.
Flow cytometry and cytokine assays
Cell-free supernatants were analyzed for IL-10 by ELISA according to the manufacturer’s recommendations (eBioscience). Intracellular IL-10/c-Jun was assessed by adding monesin during the last 6 h of a 72 h stimulation. Cells were subsequently fixed in 4% paraformaldehye, permealized using eBiosicence Perm buffer, and incubated with anti-human IL-10 and/or anti-human c-Jun for 30 min. Samples were washed in excess Perm buffer and analyzed immediately by flow cytometry. Total GSK3β levels were assessed by flow cytometry by transferring cultured cells to 5-ml polystyrene round-bottom tubes and fixing with formaldehye to a final concentration of 4% directly into the media for 10 min at room temperature. Cells were washed once in PBS and resuspended in 90% methanol and incubated on ice for 10 min. Cells were washed in PBS containing 2% FCS and then resuspended in PBS containing 2% FCS and anti-GSK3β and incubated at room temperature for 30 min. Cells were washed twice in PBS containing 2% FCS and analyzed immediately by flow cytometry. IL-10 cytokine-secreting cells measured by ELISPOT were performed as directed by the manufacturer with the addition that the anti-CD3 Ab was added with the capture Ab. ELISPOTs were developed on day 4 postculture and analyzed by Cellular Technology.
Transfections and immunblots
Memory CD4+ T cells were transfected with nontargeting siRNA, siRNA-GSK3β, pcDNA3-GSK3β (S9A), or pcDNA3 (empty vector control) using the Lipofectamine RNAiMAX (Invitrogen) or Lipofectamine LTX (Invitrogen) following the manufacturer’s protocol. The plasmid pcDNA3-GSK3-β (S9A) was obtained from Addgene (plasmid no. 14754) and originally created by Dr. James Woodgett’s laboratory (19). The levels of GSK3β protein were assessed by flow cytometry on day 3. Whole-cell lysates were prepared as previously described (20). Twenty micrograms of total cellular protein from each group was suspended in lithium dodecyl sulfate (LDS) buffer, heated for 10 min at 70°C, resolved by LDS-PAGE, and then transferred to polyvinylidene difluoride membranes using the Novex system (Invitrogen). Probing and visualization of immunoreactive bands were performed using the ECL Plus kit (Amersham Pharmacia) by following the manufacturer’s protocol. Images were acquired using the Kodak Image Station 4000 MM system (Eastman Kodak).
Real-time PCR
RNA extraction and first-strand cDNA synthesis was performed using the 5 Prime PerfectPure RNA Cultured Cell kit and High-Capacity cDNA Archive kit (Applied Biosystems). Real-time PCR was performed using an ABI 7500 system. GAPDH mRNA levels were determined for each time point and used as the endogenous control. Fold increase was calculated according to the ΔΔCT method (21).
Statistical analysis
Data are expressed as means ± SEM. Statistical significance between groups was evaluated by ANOVA and the Tukey multiple comparison test using the Instat program. Differences between groups were considered significant at p < 0.05.
Results
PI3K activity is required for IL-12 to induce IL-10 production from memory CD4+ T cells
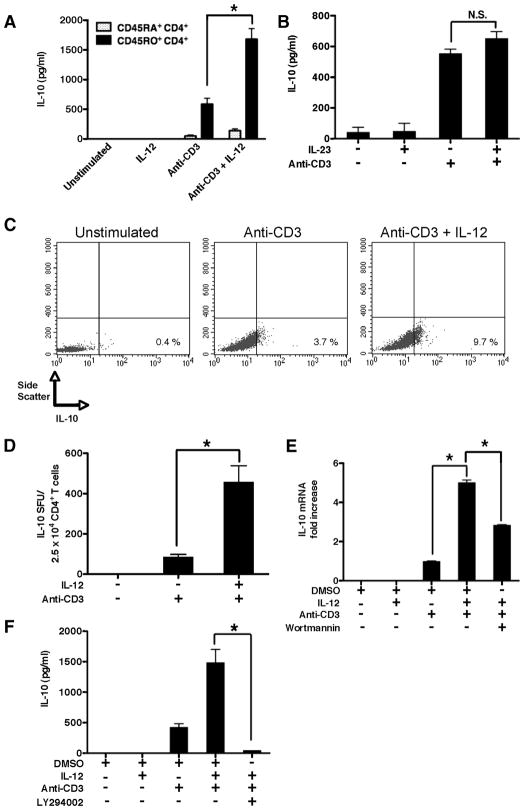
Since human differentiated CD4+ T cells have been shown to be induced to produce IL-10 upon stimulation in the presence of IL-12 (8, 10), we initially investigated whether freshly isolated, cell-sorted human memory CD4+ T cells (CD45RO+CD45RA−) displayed similar properties. TCR stimulation of human memory CD4+ T cells led to the production of IL-10 that was significantly enhanced when TCR stimulation occurred in the presence of IL-12 (Fig. 1A). In contrast, IL-12 did not induce any detectable levels of IL-10 in the absence of TCR stimulation, nor did IL-23, which shares the IL-12p40 subunit with IL-12, significantly enhance IL-10 in the presence or absence of anti-CD3 (Fig. 1, A and B). We also investigated whether IL-12 led to the enhanced frequencies of IL-10-producing memory CD4+ T cells. Similar to IL-10 protein levels, IL-12 alone was not sufficient to induce IL-10-producing memory CD4+ T cells, nor did it induce T cell blasts, as demonstrated by the lack of change in the forward and side scatter profiles (data not shown). However, memory CD4+ T cells TCR stimulated in the presence of IL-12 led to the enhanced frequencies of memory CD4+ T cells expressing IL-10, as compared with cells stimulated in the absence of IL-12 (Fig. 1, C and D).
FIGURE 1.
IL-12 induces IL-10 production from human memory CD4+ T cells in a PI3K-dependent manner. A, Cell-sorted autologous human naive (CD45RA+CD45RO−) and memory (CD45RO+CD45RA−) CD4+ T cells were stimulated with plate-bound anti-CD3 (3 μg/ml) in the presence or absence of human IL-12p70 (500 pg/ml) and cell-free supernatants were analyzed by ELISA on day 3. B, Cell-sorted human memory CD4+ T cells were stimulated with plate-bound anti-CD3 (3 μg/ml) in the presence or absence of human IL-23 (500 pg/ml) and cell-free supernatants were analyzed by ELISA on day 3. C, Cell-sorted human memory CD4+ T cells were stimulated with plate-bound anti-CD3 (3 μg/ml) in the presence or absence of human IL-12p70 (500 pg/ml). Monensin was added during the last 6 h of a 72-h culture and cells were analyzed for intracellular IL-10 by flow cytometry. D, Cell-sorted human memory CD4+ T cells were stimulated with immobilized anti-CD3 (3 μg/ml) in the presence or absence of IL-12 (500 pg/ml) and analyzed for spot-forming units by ELISPOT on day 4. E, Cell-sorted human memory CD4+ T cells were pretreated with a PI3K inhibitor (wortmannin; 100 nM) or DMSO (0.1%) alone and stimulated with plate-bound anti-CD3 (3 μg/ml) in the presence or absence of human IL-12p70. IL-10 mRNA levels were analyzed by real-time PCR on day 3 and related to the endogenous housekeeping gene GAPDH. F, Cell-sorted human memory CD4+ T cells were pretreated with a PI3K inhibitor (LY294002; 25μM) or DMSO (0.1%) alone and stimulated with plate-bound anti-CD3 (3 μg/ml) in the presence or absence of human IL-12p70 and cell-free supernatants were analyzed by ELISA on day 3. Results are representative of at least three individual experiments. *, p < 0.05 determined by ANOVA and post hoc Tukey test.
Analysis of mRNA levels also demonstrated that TCR stimulation in the presence of IL-12 led to a significant increase in the steady-state levels of IL-10 mRNA, as compared with cells stimulated in the absence of IL-12 (Fig. 1E). IL-12 was not sufficient to induce detectable increases in the steady-state levels of IL-10 mRNA in the absence of TCR-stimulation, as compared with resting memory CD4+ T cells (Fig. 1E).
Past studies have demonstrated that the ability of human memory CD4+ T cells to produce IL-10 upon anti-CD3 or anti-CD3/CD28 stimulation requires an active PI3K signaling pathway (17, 18). Thus, we next investigated whether PI3K activity was required for IL-12 to promote IL-10 production from TCR-stimulated human memory CD4+ T cells. Indeed, inhibition of PI3K activity significantly suppressed the steady-state levels of IL-10 mRNA in human memory CD4+ T cells stimulated in the presence of anti-CD3 and IL-12 (Fig. 1E). Inhibition of PI3K also abrogated the levels of secreted IL-10 produced by human memory CD4+ T cells stimulated in the presence of IL-12 (Fig. 1F). Thus, IL-12 can promote IL-10 production from human memory CD4+ T cells and this effect requires PI3K activity.
IL-12 induces PI3K activity in human memory CD4+ T cells
The finding that PI3K activity was required for the optimal induction of IL-10 from memory cells stimulated in the presence of IL-12 prompted us to next investigate whether IL-12 signaling led to the activation of the PI3K pathway in human memory CD4+ T cells. IL-12 has been previously shown to induce PI3K activity in murine CD4+ T cells (16), yet it is unknown whether IL-12 can induce PI3K activity in human memory CD4+ T cells. The activation of class IA PI3Ks is dependent on the phosphorylation of tyrosines present within YXXM motifs that can then be recognized by the Src homology 2 domain(s) of the p85 subunit of PI3K (22). Therefore, we initially assessed the relative levels of phospho-YXXM motifs present in cells stimulated with IL-12 using a cell-based ELISA. Administration of exogenous IL-12 to human memory CD4+ T cells increased the cellular levels of phospho-YXXM motifs within 30 min of its addition, and these levels were further enhanced at 60 min poststimulation (Fig. 2A). In contrast, administration of exogenous IL-23 to human memory CD4+ T cells did not significantly change the cellular levels of phospho-YXXM motifs (Fig. 2A). We next assessed whether IL-12 affected the activity of kinases downstream of PI3K. Addition of IL-12 enhanced the levels of phospho-Akt (Ser473), which is required for optimal Akt activity (Fig. 2B) (23). Moreover, IL-12 also enhanced the levels of phospho-GSK3β (S9), which is a phospho-specific site that represses GSK3β activity (Fig. 2B) (24). Indeed, the presence of IL-12 led to the enhanced levels of β-catenin, which is a protein destabilized by active GSK3 (Fig. 2B) (25). However, stimulation of memory cells with IL-23 did not alter the levels of phospho-Akt, phospho-GSK3β, or of β-catenin (data not shown). Taken together, these findings demonstrate that IL-12 enhances PI3K activity and results in the subsequent inactivation of GSK3β in human memory CD4+ T cells.
FIGURE 2.
IL-12 activates the PI3K pathway in human memory CD4+ T cells. A, Cell-sorted human memory CD4+ T cells were cultured in the presence or absence of IL-12p70 (500 pg/ml) or IL-23 (500 pg/ml) and cells were analyzed for the amount of phospho-tyrosine present in YXXM motifs at the indicated time points by ELISA. B, Cell-sorted human memory CD4+ T cells were cultured in the presence or absence of IL-12p70 (500 pg/ml) for 2 h. Whole-cell lystes were probed for phospho-Akt (S473), phospho-GSK3β (S9), β-catenin, and total ERK1/2 by Western blot. Results are representative of at least three individual experiments. *, p < 0.05 determined by ANOVA and post hoc Tukey test.
The ability of IL-12 to induce IL-10 production from human memory CD4+ T cells requires GSK3β inactivation
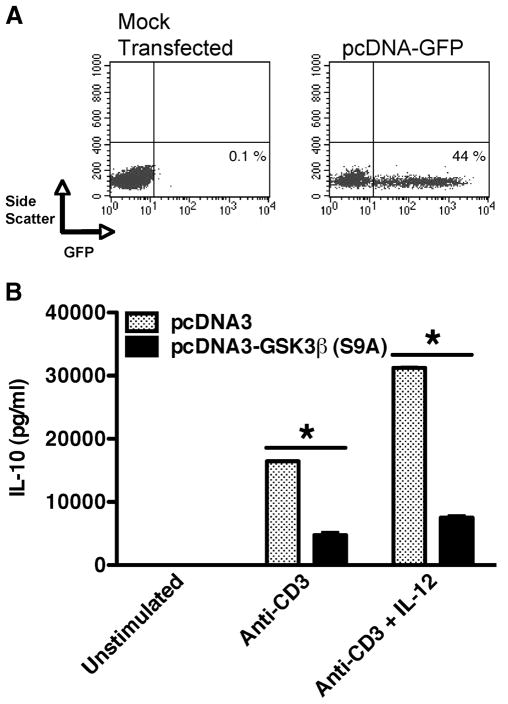
Because the presence of IL-12 increased the levels of phospho-GSK3β (S9), we next investigated whether the repression of GSK3β activity was required for IL-12 to promote IL-10 production from human memory CD4+ T cells. Thus, we ectopically expressed a constitutively active GSK3β(S9A) mutant and assessed the ability of IL-12 to induce IL-10 production from memory CD4+ T cells. Expression of the pcDNA vector in memory CD4+ T cells was confirmed by transfecting memory CD4+ T cells with pcDNA-GFP. Transfection of human memory CD4+ T cells with pcDNA-GFP resulted in >40% of memory CD4+ T cells expressing GFP, as compared with mock-transfected cells (Fig. 3A). We next transfected memory CD4+ T cells with either pcDNA (control vector) or pcDNA-GSK3β (S9A). TCR stimulation of memory CD4+ T cells transfected with the empty vector produced IL-10, which was significantly augmented when stimulated in the presence of IL-12 (Fig. 3B). In contrast, memory CD4+ T cells ectopically expressing a constitutively active GSK3β(S9A) were significantly impaired in their ability to produce IL-10 in the presence of IL-12 (Fig. 3B). These data demonstrate that the IL-12-mediated production of IL-10 is dependent on the ability of IL-12 to inactivate GSK3β.
FIGURE 3.
GSK3 inactivation is required for IL-12 to promote IL-10 production from human memory CD4+ T cells. A, Human memory CD4+ T cells were mock transfected or transfected with pcDNA-GFP and cells were analyzed for GFP expression 24 h later by flow cytometry. B, Human memory CD4+ T cells were transfected with 50 ng of pcDNA (empty vector control) or pcDNA-GSK3β (S9A) and cells were stimulated 24 h posttransfection with immobilized anti-CD3 (3 μg/ml) and cell-free supernatants were analyzed for IL-10 by ELISA 72 h postactivation. Results are representative of at least three individual experiments. *, p < 0.05 determined by ANOVA and post hoc Tukey test.
Direct inactivation of GSK3 augments the cellular levels of c-Jun in TCR-activated memory CD4+ T cells
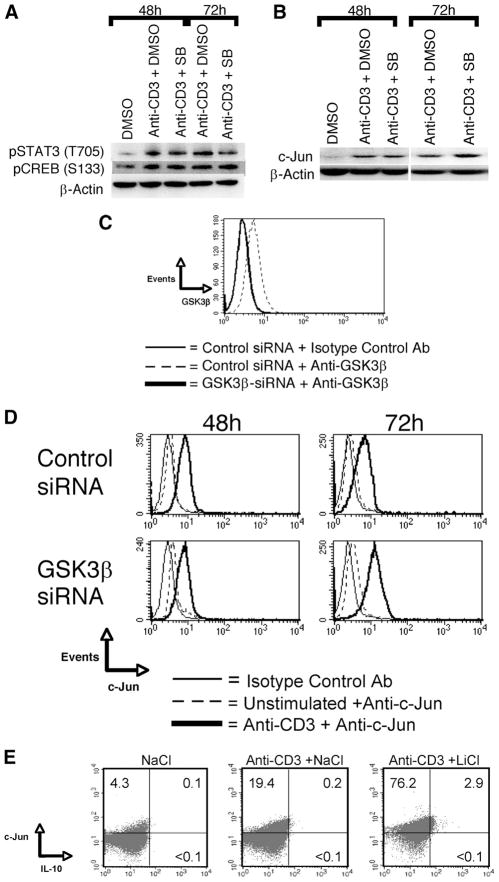
We next examined how GSK3 activity was influencing the activity of transcription factors known to be involved in the regulation of IL-10. The time points of 48 and 72 h post-TCR activation were investigated, as these time points were previously shown to correspond to increased IL-10 mRNA levels upon TCR stimulation in the presence of GSK3β inactivation (18). We initially examined whether GSK3 activity was increasing the levels of phospho-CREB (S133) that have been shown to be the mechanism by which GSK3 regulates IL-10 production by TLR-stimulated innate immune cells (26). Repression of GSK3 activity in the presence of TCR stimulation did not lead to increases in the cellular levels of phospho-CREB (S133) in memory CD4+ T cells at either 48 or 72h post-TCR activation (Fig. 4A). Next we examined whether GSK3 activity was affecting the levels of phospho-STAT3, as it has been shown to be subject to GSK3 regulation in certain innate cells and has been demonstrated to play a fundamental role in IL-10 production by human monocytes (27, 28). However, no net change in phospho-STAT3 (Tyr) was observed at either time point examined in GSK3-inhibited cells stimulated via the TCR, as compared with cells stimulated in the absence of GSK3 inhibition (Fig. 4A). GSK3 has also been shown to regulate c-Jun stability by C-terminal phosphorylation and play a role in IL-10 production by both innate and adaptive immune cells (29, 30). Inhibition of GSK3 using the pharmacological inhibitors SB216763 (Fig. 4B) or LiCl (Fig. 4E) led to the enhanced levels of c-Jun in TCR-activated memory CD4+ T cells, as compared with cells stimulated via the TCR alone. Knockdown of GSK3β in human memory CD4+ T cells (Fig. 4C) resulted in increased levels of c-Jun 72 h post-TCR activation, as compared with cells transfected with nontargeting siRNA pools (Fig. 4D). No change in c-Jun levels was observed upon pharmacological inhibition in the absence of TCR stimulation (data not shown). Moreover, IL-10-producing cells induced upon GSK3 repression coexpressed high levels of c-Jun protein, as compared with cells stimulated in the absence of GSK3 inhibition (Fig. 4E). These data demonstrate that the levels of c-Jun in human memory CD4+ T cells are subject to regulation by GSK3β.
FIGURE 4.
GSK3β inactivation leads to enhanced cellular levels of c-Jun in TCR-activated memory CD4+ T cells. A and B, Memory CD4+ T cells were pretreated for 2 h with DMSO (0.1%) or with SB216763 (6 μM) and then transferred to plates precoated with anti-CD3 (3 μg/ml). On the indicated day, whole-cell lysates were prepared and analyzed for phospho-STAT3 (T705), phospho-CREB (S133), c-Jun, or β-actin. C, Human memory CD4+ T cells were transfected with a nontargeting pool of siRNA or a pool of GSK3β siRNA and the levels of endogenous GSK3β were assessed by flow cytometry 48 h posttransfection. D, Memory CD4+ T cells were transfected with equal amounts of nontargeting siRNA GSK3α-specific siRNA or GSK3β-specific siRNA. On day 3, transfected cells were transferred to anti-CD3-coated plates (3 μg/ml) and analyzed for cellular levels of c-Jun 48 or 72 h postactivation by flow cytometry. E, Memory CD4+ T cells were pretreated in NaCl (6 mM) or LiCl (6 mM) for 2 h and then transferred to plates coated with anti-CD3 (3 μg/ml). Monensin was added during the last 6 h of a 72-h culture and cells were analyzed for intracellular c-Jun and IL-10 by flow cytometry. Results are representative of three individual experiments.
IL-12 enhances the frequency of c-Jun expressing memory CD4+ T cells
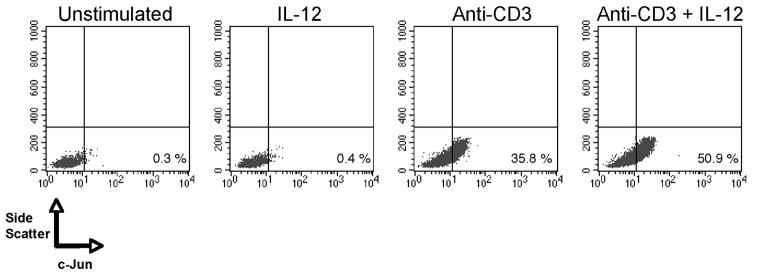
Because direct inactivation of GSK3β led to the increased cellular levels of c-Jun, taken together with our findings that the repression of GSK3β activity by IL-12 was necessary for IL-12 to induce IL-10 production from human memory CD4+ T cells, we next investigated if the stimulation of human memory CD4+ T cells in the presence of exogenous IL-12 increased the frequency of cells containing detectable levels of c-Jun protein. In the absence of TCR stimulation, IL-12 did not result in any detectable change in the levels of c-Jun protein in human memory CD4+ T cells (Fig. 5). However, higher frequencies of c-Jun containing cells were observed in TCR-stimulated memory CD4+ T cells stimulated in the presence of IL-12, as compared with cells stimulated by the TCR alone (Fig. 5). Thus, IL-12 enhances the frequency of memory CD4+ T cells expressing c-Jun.
FIGURE 5.
IL-12 enhances c-Jun levels in TCR-activated memory CD4+ T cells. Memory CD4+ T cells were activated with immobilized anti-CD3 (3 μg/ml) in the presence or absence of IL-12 (500 pg/ml). Cells were analyzed 72 h later for intracellular c-Jun by flow cytometry. Results are representative of three individual experiments.
Induction of IL-10 from memory CD4+ T cells by IL-12 is dependent on c-Jun levels
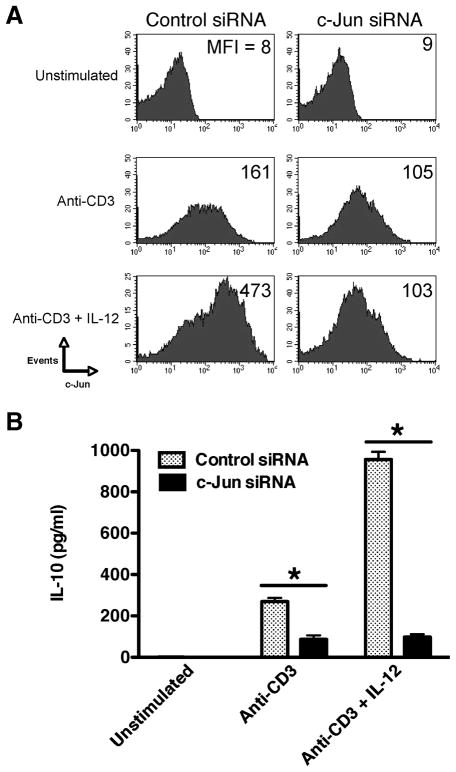
We next examined whether the ability of IL-12 to augment c-Jun levels played a functional role in the capacity of IL-12 to induce IL-10 production by human memory CD4+ T cells. Because c-Jun was induced upon TCR activation, memory CD4+ T cells were transfected with c-Jun-specific or nontargeting siRNA pools 24 h post-TCR activation. Preliminary experiments revealed that the transfection of memory CD4+ T cells immediately before TCR activation resulted in poor knockdown of total c-Jun levels that were likely due to the reported instability of siRNA in recently activated T cells (31). Transfection of memory CD4+ T cells with c-Jun-specific siRNA pools decreased the cellular levels of c-Jun by 72 h, as compared with cells transfected with control siRNA pools (Fig. 6A). Moreover, transfection of memory CD4+ T cells with c-Jun-specific siRNAs resulted in a 4-fold reduction in the cellular levels of c-Jun upon stimulation in the presence of IL-12, as compared with cells transfected with non-targeting siRNA pools (Fig. 6A).
FIGURE 6.
IL-12 induction of IL-10 from memory CD4+ T cells requires c-Jun. Memory CD4+ T cells were stimulated with immobilized anti-CD3 (3 μg/ml) and transfected with either nontargeting siRNA pools (denoted as Control siRNA) or c-Jun-specific siRNA pools 24 h postactivation. A, Memory CD4+ T cells were analyzed for intracellular c-Jun by flow cytometry 72 h postactivation. B, Cell-free supernatants were analyzed for IL-10 levels by ELISA 72 h postactivation. Results are representative of three individual experiments. *, p < 0.05 determined by ANOVA and post hoc Tukey test.
We next assessed the levels of IL-10 produced from TCR-stimulated memory CD4+ T cells transfected with either c-Jun or non-targeting siRNA pools in the presence or absence of IL-12. Memory CD4+ T cells transfected with nontargeting siRNA pools produced IL-10 upon TCR stimulation, which was significantly enhanced in the presence of IL-12 (Fig. 6B). In contrast, cells transfected with c-Jun-specific siRNA failed to up-regulate IL-10 production upon stimulation in the presence of IL-12 (Fig. 6B). Therefore, these findings show that the ability of IL-12 to augment IL-10 production from human memory CD4+ T cells is dependent on the ability of IL-12 to increase the cellular levels of c-Jun.
Discussion
In the present study, we identified and characterized the cell signaling pathway by which IL-12 induced IL-10 production from TCR-activated human memory CD4+ T cells. Activating human memory CD4+ T cells in the presence of IL-12p70 led to increased PI3K activity in which the inhibition of PI3K prevented the ability of IL-12p70 to inactivate the constitutively active kinase GSK3β and subsequently abrogated the IL-12-induced IL-10 production from memory CD4+ T cells. Furthermore, ectopic expression of a constitutively active form of GSK3β attenuated the increased levels of IL-10 produced by CD4+ T cells stimulated in the presence of IL-12p70. In contrast, direct inhibition of GSK3β mimicked the effects of IL-12p70 on the levels of IL-10 produced by memory CD4+ T cells. By analyzing several transcription factors downstream of GSK3β, we observed that memory CD4+ T cells activated in the presence of IL-12 contained higher levels of c-Jun and this effect was dependent on the inactivation of GSK3β. The functional role for the elevated levels of c-Jun was demonstrated by the finding that siRNA-mediated gene silencing of c-Jun attenuated the ability of IL-12 to enhance IL-10 production by memory CD4+ T cells.
Although most reports regarding the functional role of the PI3K/Akt pathway in T cells have linked this pathway to either cellular proliferation or survival, the production of IL-10 by human T cells has also been shown to be dependent on the PI3K pathway (32). In this regard, GSK3, whose activity can be regulated by Akt, was initially shown to function as a negative regulator of T cell proliferation and IL-2 production (33–35). However, it has also been reported that the capacity of the costimulatory molecule CD28 to augment IL-10 production from human memory CD4+ T cells is dependent on the PI3K signaling pathway (17, 18). Our laboratory extended these observations by showing that GSK3 exhibited distinct effects on naive and memory CD4+ T cells. Specifically, GSK3 functioned primarily as a negative regulator of proliferation in human naive CD4+ T cells, similar to the findings of others demonstrating that GSK3 controls T cell proliferation (33–35). However, GSK3β did not exhibit any discernible effects on memory CD4+ T cell proliferation, but it did negatively regulate their production of IL-10 (18). In the present study, we did not observe significant changes in the proliferation of memory CD4+ T cells activated in the presence of IL-12 despite their increased production of IL-10. These findings contrast those of Yoo et al., who reported that IL-12 promoted CD4+ T cell proliferation (16). The discrepancy between our study and that of Yoo et al. is likely due to the source of CD4+ T cells, as we utilized human memory CD4+ T cells in place of murine CD4+ T cells. In this regard, we did not observe enhanced IL-10 production from naive CD4+ T cells stimulated in the presence of IL-12. Therefore, because GSK3β plays discrete roles depending on the antigenic experience of human CD4+ T cells (18), the consequence of GSK3 inactivation by way of IL-12 and PI3K promotes distinct phenotypic profiles in human naive and memory CD4+ T cells.
Several reports have linked the regulation of Jun proteins to the production of effector cytokines produced by T cells (36, 37). In particular, overexpression of c-Jun in murine differentiated CD4+ T cells has been shown to promote their ability to produce IL-10 (37). However, whether c-Jun is involved in the regulation of IL-10 by human CD4+ T cells has not been previously investigated. The present report provides the first evidence demonstrating the importance of c-Jun levels in the IL-10 recall response of human memory CD4+ T cells, and it further identifies the upstream signaling components involved in the regulation of c-Jun in IL-12-stimulated memory CD4+ T cells. De novo synthesized c-Jun is highly unstable and has been estimated to possess a half-life of <60 min in T cells (36). In fact, the present study demonstrated that resting memory CD4+ T cells contained very low to nondetectable levels of c-Jun protein. We did not observe enhanced levels of c-Jun in memory CD4+ T cells cultured in the presence of IL-12 or upon GSK3 inactivation in the absence of TCR stimulation. Thus, it is likely that TCR stimulation induces human memory CD4+ T cells to transcribe c-Jun. Because GSK3β can phosphorylate and destabilize c-Jun, unrepressed GSK3β likely functions to suppress the endogenous levels of c-Jun in TCR-stimulated human memory CD4+ T cells (29, 30). Our data support this concept, as the direct inactivation of GSK3β in human memory CD4+ T cells via either pharmacological inhibition or genetic silencing augmented the cellular c-Jun levels present in memory CD4+ T cells upon TCR activation. Because TCR stimulation itself is a poor inactivator of GSK3β in memory CD4+ T cells (18), accessory signals that stimulate PI3K activity are likely required to achieve full GSK3β inactivation for the optimal stabilization of c-Jun protein and the production of IL-10 during human memory CD4+ T cell recall responses. In this regard, the present study demonstrated that the capacity of IL-12 to augment IL-10 production by memory CD4+ T cells was dependent on its ability to stimulate PI3K and inactivate GSK3β.
Interestingly, while studying the effects of IL-12 on human memory CD4+ T cells, we also examined whether the closely related cytokine IL-23 behaved in a similar fashion with respect to the induction of IL-10 from memory CD4+ T cells. We found that IL-23 did not induce IL-10 production from human memory CD4+ T cells. Moreover, we observed that IL-23 failed to induce the phosphorylation of YXMM motifs in memory CD4+ T cells and was unable to promote the phosphorylation of GSK3β and Akt (our unpublished observations). Thus, despite sharing the IL-12β1 receptor chain and IL-12p40 subunit, IL-23 and IL-12 do not share the ability to promote PI3K activity and IL-10 production in human memory CD4+ T cells. The differential ability to induce PI3K and subsequent inactivation of GSK3β observed between IL-12 and IL-23 explains why IL-12, but not IL-23, was able to promote IL-10 production from human memory CD4+ T cells.
The fact that GSK3 activity did not influence CREB activity may seem surprising given that this pathway was previously implicated in the regulation of IL-10 production by human monocytes, dendritic cells, and B cells (26, 38). However, it is important to contrast the distinct phenotypic differences associated with GSK3 repression in other cellular lineages such as human monocytes and memory CD4+ T cells. For example, the relative activity of GSK3 does not significantly impact the production of proinflammatory cytokines such as IFN-γ, IL-5, or IL-17A by TCR-stimulated memory CD4+ T cells, whereas GSK3 activity does influence the production of proinflammatory cytokines such as IL-1, IL-6, TNF, and IL-12 by TLR-stimulated innate immune cells (26). Because CREB can displace NF-κB p65 from CBP, GSK3 activity can influence a larger array of cytokines in human monocytes than in memory CD4+ T cells, due to the NF-κB dependence of many proinflammatory cytokines (18, 26, 39). Second, the kinetics of IL-10 production by human monocytes, as compared with human memory CD4+ T cells, is vastly different, occurring as much as 48 h later in T cells (11, 18). Thus, although the ability of GSK3β to control the production of IL-10 does appear to be conserved among multiple cell types comprising both the innate and adaptive immune compartments, the underlying molecular mechanism by which GSK3 controls this process can vary among cell types.
Numerous reports have confirmed the finding that IL-12 can promote IL-10 production in vitro and in vivo in both mice and humans, yet the cellular mechanisms responsible for this effect have remained obscure (8–11, 40–43). The work presented in this study demonstrates the importance of PI3K signaling pathway, and its capacity to regulate the transcription factor c-Jun is responsible for the ability of IL-12 to promote IL-10 production from human memory CD4+ T cells. Understanding the downstream signaling pathways controlling IL-10 production provides a logical strategy for designing strategies to combat immune-related inflammatory diseases.
Footnotes
This research was supported by Grant R01DE017680 from the National Institute of Dental Research.
Abbreviations used in this paper: GSK3β, glycogen synthase kinase-3β; siRNA, small interfering RNA.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Gubler U, Chua AO, Schoenhaut DS, Dwyer CM, McComas W, Motyka R, Nabavi N, Wolitzky AG, Quinn PM, Familletti PC. Coexpression of two distinct genes is required to generate secreted bioactive cytotoxic lymphocyte maturation factor. Proc Natl Acad Sci USA. 1991;88:4143–4147. doi: 10.1073/pnas.88.10.4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Micallef MJ, Ohtsuki T, Kohno K, Tanabe F, Ushio S, Namba M, Tanimoto T, Torigoe K, Fujii M, Ikeda M, et al. Interferon-γ-inducing factor enhances T helper 1 cytokine production by stimulated human T cells: synergism with interleukin-12 for interferon-γ production. Eur J Immunol. 1996;26:1647–1651. doi: 10.1002/eji.1830260736. [DOI] [PubMed] [Google Scholar]
- 3.Lauwerys BR, Renauld JC, Houssiau FA. Synergistic proliferation and activation of natural killer cells by interleukin 12 and interleukin 18. Cytokine. 1999;11:822–830. doi: 10.1006/cyto.1999.0501. [DOI] [PubMed] [Google Scholar]
- 4.Yoshimoto T, Okamura H, Tagawa YI, Iwakura Y, Nakanishi K. Interleukin 18 together with interleukin 12 inhibits IgE production by induction of interferon-γ production from activated B cells. Proc Natl Acad Sci USA. 1997;94:3948–3953. doi: 10.1073/pnas.94.8.3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fukao T, Frucht DM, Yap G, Gadina M, O’shea JJ, Koyasu S. Inducible expression of Stat4 in dendritic cells and macrophages and its critical role in innate and adaptive immune responses. J Immunol. 2001;166:4446–4455. doi: 10.4049/jimmunol.166.7.4446. [DOI] [PubMed] [Google Scholar]
- 6.Watford WT, Hissong BD, Bream JH, Kanno Y, Muul L, O’shea JJ. Signaling by IL-12 and IL-23 and the immunoregulatory roles of STAT4. Immunol Rev. 2004;202:139–156. doi: 10.1111/j.0105-2896.2004.00211.x. [DOI] [PubMed] [Google Scholar]
- 7.Sica A, Saccani A, Bottazzi B, Polentarutti N, Vecchi A, Damme JV, Mantovani A. Autocrine production of IL-10 mediates defective IL-12 production and NF-κB activation in tumor-associated macrophages. J Immunol. 2000;164:762–767. doi: 10.4049/jimmunol.164.2.762. [DOI] [PubMed] [Google Scholar]
- 8.Windhagen A, Anderson DE, Carrizosa A, Williams RE, Hafler DA. IL-12 induces human T cells secreting IL-10 with IFN-γ. J Immunol. 1996;157:1127–1131. [PubMed] [Google Scholar]
- 9.Chang HD, Helbig C, Tykocinski L, Kreher S, Koeck J, Niesner U, Radbruch A. Expression of IL-10 in Th memory lymphocytes is conditional on IL-12 or IL-4, unless the IL-10 gene is imprinted by GATA-3. Eur J Immunol. 2007;37:807–817. doi: 10.1002/eji.200636385. [DOI] [PubMed] [Google Scholar]
- 10.Gerosa F, Paganin C, Peritt D, Paiola F, Scupoli MT, Aste-Amezaga M, Frank I, Trinchieri G. Interleukin-12 primes human CD4 and CD8 T cell clones for high production of both interferon-γ and interleukin-10. J Exp Med. 1996;183:2559–2569. doi: 10.1084/jem.183.6.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meyaard L, Hovenkamp E, Otto SA, Miedema F. IL-12-induced IL-10 production by human T cells as a negative feedback for IL-12-induced immune responses. J Immunol. 1996;156:2776–2782. [PubMed] [Google Scholar]
- 12.Del Vecchio M, Bajetta E, Canova S, Lotze M, Wesa A, Parmiani G, Anichini A. Interleukin-12: biological properties and clinical application. Clin Cancer Res. 2007;13:4677–4685. doi: 10.1158/1078-0432.CCR-07-0776. [DOI] [PubMed] [Google Scholar]
- 13.Presky DH, Yang H, Minetti LJ, Chua AO, Nabavi N, Wu CY, Gately MK, Gubler U. A functional interleukin 12 receptor complex is composed of two β-type cyotkine receptor subunits. Proc Natl Acad Sci USA. 1996;93:14002–14007. doi: 10.1073/pnas.93.24.14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bacon CM, McVicar DW, Ortaldo JR, Rees RC, O’shea JJ, Johnston JA. Interleukin 12 (IL-12) induces tyrosine phosphorylation of JAK2 and TYK2: differential use of Janus family tyrosine kinase by IL-2 and IL-12. J Exp Med. 1995;181:399–404. doi: 10.1084/jem.181.1.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacobson NG, Szabo SJ, Weber-Nordt RM, Zhong Z, Schreiber RD, Darnell JE, Murphy KM. Interleukin 12 signaling in T helper type 1 (Th1) cells involves tyrosine phosphorylation of signal transducer and activator of transcription (Stat)3 and Stat4. J Exp Med. 1995;181:1755–1762. doi: 10.1084/jem.181.5.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoo JK, Cho JH, Lee SW, Sung YC. IL-12 provides proliferation and survival signals to murine CD4+ T cells through phoshatidylinositol 3-kinase/Akt signaling pathway. J Immunol. 2002;169:3637–3643. doi: 10.4049/jimmunol.169.7.3637. [DOI] [PubMed] [Google Scholar]
- 17.Okamoto N, Tezuka K, Kato M, Abe R, Tsuji T. PI3-kinase and MAP-kinase signaling cascades in AILIM/ICOS- and CD28-costimulated T-cells have distinct functions between cell proliferation and IL-10 production. Biochem Biophys Res Commun. 2003;310:691–702. doi: 10.1016/j.bbrc.2003.09.065. [DOI] [PubMed] [Google Scholar]
- 18.Garcia CA, Benakanakere MB, Alard P, Kosiewicz MM, Kinane DF, Martin M. Antigenic experience dictates functional role of glycogen synthase kinase-3 in human CD4+ T cell responses. J Immunol. 2008;181:8363–8371. doi: 10.4049/jimmunol.181.12.8363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stambolic V, Woodgett JR. Mitogen inactivation of glycogen synthase kinase-3β in intact cells via serine 9 phosphorylation. Biochem J. 1994;303:701–704. doi: 10.1042/bj3030701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martin M, Schifferle RE, Cuesta N, Vogel SN, Katz J, Michalek SM. Role of the phosphatidylinositol 3 kinase-Akt pathway in the regulation of IL-10 and IL-12 by Porphyromonas gingivalis lipopolysaccharide. J Immunol. 2003;171:717–725. doi: 10.4049/jimmunol.171.2.717. [DOI] [PubMed] [Google Scholar]
- 21.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 22.Deane JA, Fruman DA. Phosphoinositide 3-kinase: diverse roles in immune cell activation. Annu Rev Immunol. 2004;22:563–598. doi: 10.1146/annurev.immunol.22.012703.104721. [DOI] [PubMed] [Google Scholar]
- 23.Woodgett JR. Recent advances in the protein kinase B signaling pathway. Curr Opin Cell Biol. 2005;17:150–157. doi: 10.1016/j.ceb.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 24.Cohen P, Frame S. The renaissance of GSK3. Nat Rev Mol Cell Biol. 2001;2:769–776. doi: 10.1038/35096075. [DOI] [PubMed] [Google Scholar]
- 25.Ikeda S, Kishida S, Yamamoto H, Murai H, Koyama S, Kikuchi A. Axin, a negative regulator of the Wnt signaling pathway, forms a complex with GSK3β and β -catenin and promotes GSK3b-dependent phosphoylation of β -catenin. EMBO J. 1998;17:1371–1384. doi: 10.1093/emboj/17.5.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martin M, Rehani K, Jope RS, Michalek SM. Toll-like receptor-mediated cytokine production is differentially regulated by glycogen synthase kinase 3. Nat Immunol. 2005;6:777–784. doi: 10.1038/ni1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beurel E, Jope RS. Differential regulation of STAT family members by glycogen synthase kinase-3. J Biol Chem. 2008;283:21934–21944. doi: 10.1074/jbc.M802481200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benkhart EM, Siedlar M, Wedel A, Werner T, Ziegler-Heitbrock LHW. Role of Stat3 in lipopolysaccharide-induced IL-10 gene expression. J Immunol. 2000;165:1612–1617. doi: 10.4049/jimmunol.165.3.1612. [DOI] [PubMed] [Google Scholar]
- 29.Wei W, Jin J, Schlisio S, Harper JW, Kaelin WG. The v-Jun point mutation allows c-Jun to escape GSK3-dependent recognition and destruction by the Fbw7 ubiquitin ligase. Cancer Cell. 2005;8:25–33. doi: 10.1016/j.ccr.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 30.Wang H, Garcia CA, Rehani K, Cekic C, Alard P, Kinane DF, Mitchell T, Martin M. IFN-β production by TLR4-stimulated innate immune cells is negatively regulated by GSK3-β. J Immunol. 2008;181:6797–6802. doi: 10.4049/jimmunol.181.10.6797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mantei A, Rutz S, Janke M, Kirchhoff D, Jung U, Patzel V, Vogel U, Rudel T, An dreou I, Weber M, Scheffold A. siRNA stabilization prolongs gene knockdwon in primary T lymphocytes. Eur J Immunol. 2008;38:2616–2625. doi: 10.1002/eji.200738075. [DOI] [PubMed] [Google Scholar]
- 32.Kane LP. The PI-3 kinase/Akt pathway and T cell activation: pleiotropic pathways downstream of PIP3. Immunol Rev. 2003;192:7–20. doi: 10.1034/j.1600-065x.2003.00008.x. [DOI] [PubMed] [Google Scholar]
- 33.Appleman LJ, van Puijenbroek AA, Shu KM, Nadler LM, Boussiotis VA. CD28 costimulation mediates down-regulation of p27kip1 and cell cycle progression by activation of the PI3K/PKB signaling pathway in primary human T cells. J Immunol. 2002;168:2729–2736. doi: 10.4049/jimmunol.168.6.2729. [DOI] [PubMed] [Google Scholar]
- 34.Diehn M, Alizadeh AA, Rando OJ, Liu CL, Stankunas K, Botstein D, Crabtree GR, Brown PO. Genomic expression programs and the integration of the CD28 costimulatory signal in T cell activation. Proc Natl Acad Sci USA. 2002;99:11796–11801. doi: 10.1073/pnas.092284399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ohteki T, Parsons M, Zakarian A, Jones RG, Nguyen LT, Woodgett JR, Ohashi PS. Negative regulation of T cell proliferation and interleukin 2 production by the serine threonine kinase GSK-3. J Exp Med. 2000;192:99–104. doi: 10.1084/jem.192.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gao M, Labuda T, Xia Y, Gallagher E, Fang D, Liu YC, Karin M. Jun turnover is controlled through JNK-dependent phoshorylation of the E3 ligase Itch. Science. 2004;306:271–275. doi: 10.1126/science.1099414. [DOI] [PubMed] [Google Scholar]
- 37.Wang ZY, Sato H, Kusam S, Sehra S, Toney LM, Dent AL. Regulation of IL-10 gene expression in Th2 cells by Jun proteins. J Immunol. 2005;174:2098–2105. doi: 10.4049/jimmunol.174.4.2098. [DOI] [PubMed] [Google Scholar]
- 38.Lambert SL, Martinez OM. Latent membrane protein 1 of EBV activates phosphatidylinositol 3-kinase to induce production of IL-10. J Immunol. 2007;179:8225–8234. doi: 10.4049/jimmunol.179.12.8225. [DOI] [PubMed] [Google Scholar]
- 39.Parry GC, Mackman N. Role of cyclic AMP response element-binding protein in cyclic AMP inhibition of NF-κB-mediated transcription. J Immunol. 1997;159:5450–5456. [PubMed] [Google Scholar]
- 40.Bajetta E, Del Vecchio M, Mortarini R, Nadeau R, Rakhit A, Rimassa L, Fowst C, Borri A, Anichini A, Parmiani G. Pilot study of subcutaneous recombinant human interleukin 12 in metastatic melanoma. Clin Cancer Res. 1998;4:75–85. [PubMed] [Google Scholar]
- 41.Motzer RJ, Rakhit A, Thompson JA, Nemunaitis J, Murphy BA, Ellerhorst J, Schwartz LH, Berg WJ, Bukowski RM. Randomized multicenter phase II trial of subcutaneous recombinant human interleukin-12 verson interferon-α2a for patients with advanced renal cell carcinoma. J Interferon Cytokine Res. 2001;21:257–263. doi: 10.1089/107999001750169934. [DOI] [PubMed] [Google Scholar]
- 42.Segal BM, Dweyer BK, Shevach EM. An interleukin (IL)-10/IL-12 immunoregulatory circuit controls susceptibility to autoimmune disease. J Exp Med. 1998;187:537–546. doi: 10.1084/jem.187.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Portielje JE, Lamers CH, Kruit WH, Sparreboom A, Bolhuis RL, Stoter G, Huber C, Gratama JW. Repeated administrations of interleukin (IL)-12 are associated with persistently elevated plasma levels of IL-10 and declining IFN-γ, tumor necrosis factor-α, IL-6, and IL-8 responses. Clin Cancer Res. 2003;9:76–83. [PubMed] [Google Scholar]