Abstract
In young women, estradiol and progesterone primarily control reproduction, but they also affect fluid regulation. Estradiol lowers the operating point for osmoregulation of arginine vasopressin and thirst and increases plasma volume. Although total body water and sodium content are only mildly affected, data presented in this article suggest that reproductive hormones alter homeostatic set points for body fluid and tonicity.
Keywords: estrogens, 17 β-estradiol, progesterone, gonadotropin-releasing hormone antagonist, gonadotropin-releasing hormone agonist, osmoreceptors, renin-angiotensin-aldosterone system
INTRODUCTION
There are clear sex differences in risk factors for a number of syndromes and diseases in addition to sex differences in the function of many physiological systems. For example, premenopausal women have delayed and less severe manifestation of cardiovascular diseases than do men (13), and although there continues to be some controversy surrounding hormone replacement in postmenopausal women, hormone replacement therapy is usually associated with improved cardiovascular function in perimenopausal and postmenopausal women (6,19). One mechanism by which estrogens or progesterone may impact physiological systems is through regulation of body fluids and sodium content. Receptors for estrogens and progesterone are found in nonreproductive tissue involved in fluid regulation, such as the hypothalamus (10,21), the cardiovascular system (6,19), and the kidney tubules (6). Moreover, the impact of sex hormones on body fluid and sodium regulation has important implications for a number of syndromes for which women are at risk, including orthostatic hypotension, insulin resistance, polycystic ovary syndrome, and neurological consequences from postoperative (8) and exercise-associated hyponatremia (1).
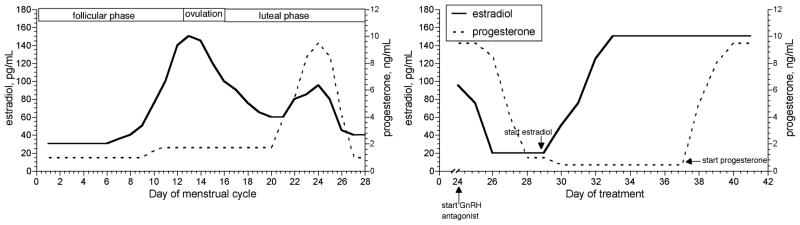
The study of reproductive hormone effects on physiological systems is complex in young women because of hormonal fluctuations over the course of the menstrual cycle (Fig. 1). In our early studies, we determined reproductive hormone effects on body water regulation system in young women by examining responses to fluid perturbations either during the different phases of the menstrual cycle or during administration of oral contraceptives. However, when examining the interaction of these hormones and body fluid regulation under these study paradigms, estradiol or progesterone were elevated or suppressed simultaneously, so we did not isolate their individual effects. A series of studies described in this article used a newer paradigm to control the levels of sex hormones through the use of either a gonadotropin-releasing hormone (GnRH) agonist or antagonist (Fig. 1) to suppress reproductive function (and thus estrogens, progesterone, and gonadotropins). These protocols, in effect, pharmacologically “oophorectomize” subjects for a short easily reversible period. The suppression is followed by controlled administration of estradiol, progesterone, or combined estradiol-progesterone to physiological levels. By selectively administering estradiol and/or progesterone while the subjects were suppressed, we isolated the effects of these hormones on the fluid and sodium regulatory systems during acute fluid or osmotic challenges. In general, our studies have shown that these hormones are associated with acute perturbations in body fluid regulation such as altering the threshold for osmotically induced arginine vasopressin (AVP, the primary hormone involved in the regulation of renal free water) release and thirst onset, as well as alterations in the sodium-regulation hormones atrial natriuretic peptide (ANP), renin, and aldosterone. Despite these effects, however, water and sodium regulation seem only minimally effected by estradiol or progesterone (or both) administration, suggesting that in young and healthy women, these hormones alter the homeostatic set point around which these systems are regulated rather than induce excess fluid or sodium retention or loss. This article will also address the potential impact of these sex hormones on plasma volume and fluid distribution within the extracellular fluid (ECF) space.
Figure 1.
Changes in 17 β-estradiol and progesterone across the menstrual cycle (left). Changes in 17 β-estradiol and progesterone during treatment with a gonadotropin-releasing hormone (GnRH) antagonist beginning on day 25 of a normal menstrual cycle, followed by treatment with two 17 β-estradiol patches (0.1 mg each), and oral progesterone (200 mg/d).
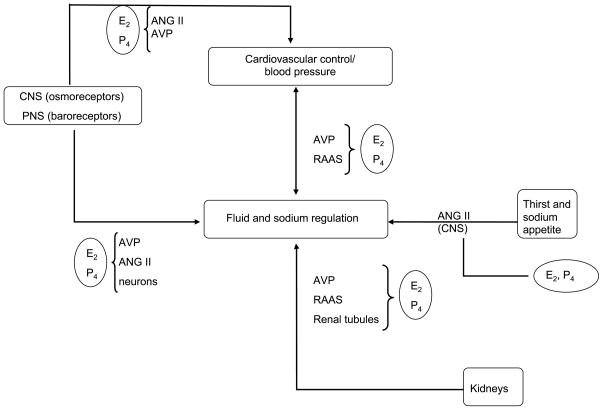
Both estradiol and progesterone can influence the complex and integrated neural and hormonal systems that have evolved to control thirst, fluid intake, sodium appetite, and renal fluid and sodium regulation (Fig. 2). These systems are sensitive to stimuli that arise from fluid deficits in the ECF space or due to excess blood sodium, tonicity, or osmolality. Arginine vasopressin is synthesized in the cell bodies of nuclei located in the anterior hypothalamus, and axons from these areas project into the posterior pituitary where AVP is stored and released in response to stimulation of central osmoreceptors. Increases in plasma osmolality are sensed by central osmo-Na+ receptors, nerves responsible for detecting the concentration of the intravascular fluid. Arginine vasopressin and thirst are highly sensitive to changes in plasma osmolality, requiring only a 2%–3% change in osmolality to induce thirst or AVP increases (4,23,26). Thirst and AVP are also sensitive to volume stimuli via peripheral baroreceptors, but approximately 10% loss in plasma volume is required before any increase in either thirst or AVP is seen. Thus, these fluid regulatory mechanisms are far more sensitive to osmotic than volume stimuli.
Figure 2.
Schematic to illustrate the complex control of fluid and sodium balance and the multiple ways in which estradiol (E2) and progesterone (P4) may influence these processes. AVP indicates arginine vasopressin; ANG II, angiotensin II; CNS, central nervous system; PNS, peripheral nervous system; RAAS, renin-angiotensin-aldosterone system.
The impact of estrogen and progesterone on osmotic control of thirst and AVP release can be studied by examining changes in the slope and intercept of the P[AVP]/osmolality and thirst/osmolality relationships (4,23,26) (Figs. 2–4). A steeper slope indicates a heightened sensitivity of the central osmoreceptors that stimulate thirst sensation and cause the release of AVP from the hypothalamus and posterior pituitary; a leftward shift in the intercept indicates an earlier threshold for the onset of thirst or the release of AVP. In the series of studies described herein, we used both hypertonic saline infusion and dehydration to increase plasma osmolality under different sex hormone conditions to determine the effects of these hormones on both the sensitivity and threshold for these linear relationships. These studies also examined the way in which sex hormones may affect the global response to acute perturbations in sodium or water content, including activation of the renin-angiotensin-aldosterone system (RAAS) and changes in ANP secretion.
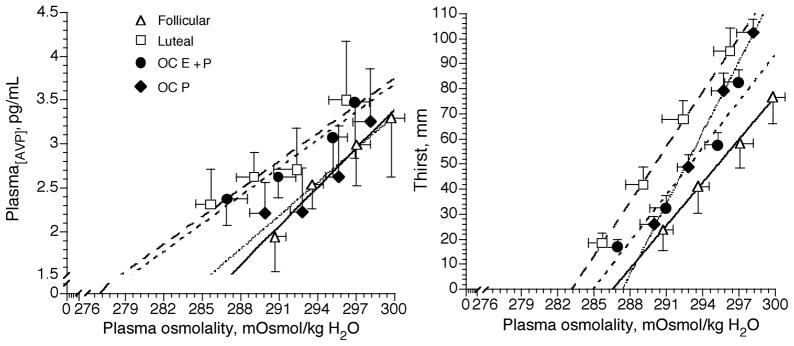
Figure 4.
Plasma arginine vasopressin (AVP) concentration and thirst as a function of plasma osmolality during 120 min of exercise-induced dehydration in the follicular (solid lines) and luteal (thick dashed lines) phases of the menstrual cycle and during combined (estradiol + progestin (thin dashed lines, OC E+P)) and (progestin-only (dotted lines, OC P)) oral contraceptive administration (23,25). Note the high estrogen conditions (luteal and OC E+P) shifted the P[AVP]-POsm curves to the left relative to the follicular phase. This shift also occurred during OCP but to a much lesser extent. (Reprinted from Stachenfeld, N.S., C.S. Silva, D.L. Keefe, C.A. Kokoszka, and E.R. Nadel. Effects of oral contraceptives on body fluid regulation. J. Appl. Physiol. 87:1016–1025, 1999. Copyright © 1999 The American Physiological Society. Used with permission.)
SEX DIFFERENCES
Estrogen receptors are present in the hypothalamic nuclei of animals known to produce AVP (10,21), and a number of studies have demonstrated sex differences in the AVP neuron activity and size in these nuclei (12). Resting P[AVP] is greater in men than in women (although only in the follicular phase (5,26)), and men have greater AVP sensitivity and blood pressure responses to hypertonic saline infusion (26). Moreover, women have greater water turnover in both the midluteal and follicular phases of the menstrual cycle compared with men after a water load (5). Importantly, in addition to the described sex difference in fluid regulation, these studies demonstrate two important points regarding the study of sex differences: first, the discovery of sex differences in these systems may be dependent on the phase of the menstrual cycle when the studies take place; and second, in some cases, the differences within the women at different phases of the cycle were far greater than those between the sexes (26).
ESTRADIOL AND PROGESTERONE EFFECTS ON BODY FLUID REGULATION
In our early studies, we administered oral contraceptives to young women and then evaluated their responses to hypertonic saline infusion (4,23), progressive exercise-induced dehydration (23,25), and a subsequent rehydration period to determine estrogen effects on the body water regulation system (Figs. 2 and 3). Combined oral contraceptive agents deliver pharmacological levels of estrogens that exhibit 6–10 times the estrogenic activity provided by endogenous estrogens, whereas progestin-only pills contain no estrogens. Thus, these two oral contraceptive preparations differ significantly in their estrogenic activity, providing conditions in which to isolate estrogen effects on body fluid regulation, albeit in the face of high plasma and tissue progestin levels. Our major finding was that administration of oral contraceptive pills containing estradiol lowered the osmotic threshold for AVP and thirst stimulation during hypertonic saline infusion (Fig. 3) and dehydration (Fig. 4). Interestingly, there were no effects on free water clearance either during hypertonic saline infusion, dehydration, or rehydration, suggesting that the shift in osmotic regulation of AVP and thirst represented a shift in body water regulation to a lower plasma osmolality operating point. Although sodium excretion was slightly reduced during the luteal phase of the menstrual cycle, neither the combined nor the progestin-only oral contraceptives increased sodium retention or blood pressure or stimulated the RAAS. These data in young women differed from our earlier findings in postmenopausal women, in whom 17 β-estradiol administration reduced the osmotic threshold for AVP release during hypertonicity, but free water and sodium retention also increased, so did not indicate a shift in the operating point for body fluid regulation but rather led to greater fluid retention (22).
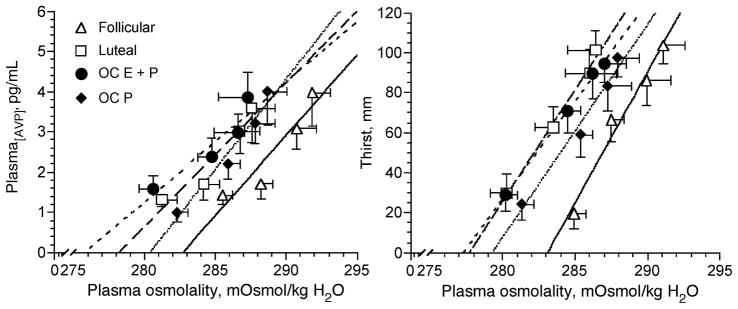
Figure 3.
Plasma arginine vasopressin (AVP) concentration and thirst as a function of plasma osmolality during 120 min of hypertonic (3% NaCl) saline infusion in the follicular (solid lines) and luteal (thick dashed lines) phases of the menstrual cycle and during combined (estradiol + progestin (thin dashed lines, OC E+P)) and (progestin-only (dotted lines, OC P)) oral contraceptive administration (4,23,26). Note the high estrogen conditions (luteal and OC E+P) shifted the P[AVP]-POsm curves to the left relative to the follicular phase. (Reprinted from Calzone, W.L., C. Silva, D.L. Keefe, and N.S. Stachenfeld. Progesterone does not alter osmotic regulation of AVP. Am. J. Physiol. Regul. Integr. Comp. Physiol. 281:R2011–R2020, 2001. Copyright © 2001 The American Physiological Society. Used with permission.)
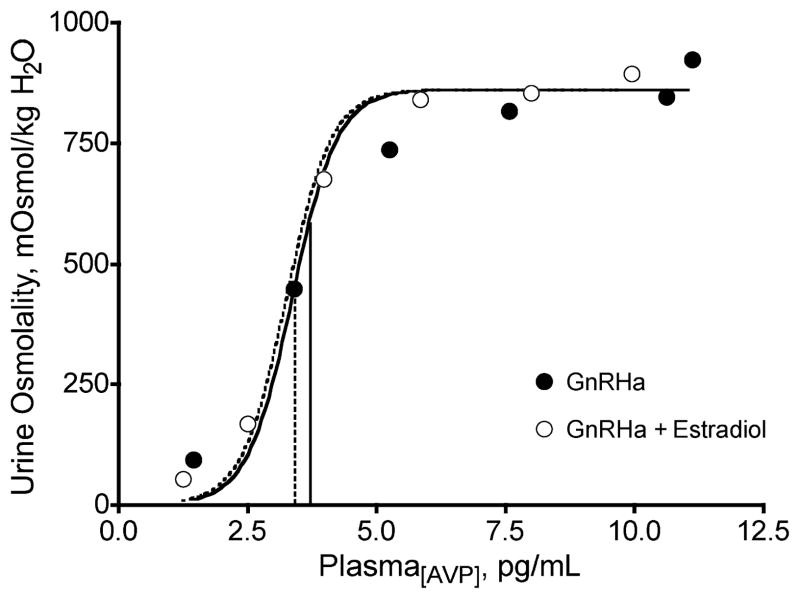
Figure 6.
Mean (n = 8) urine osmolality as a function of mean plasma arginine vasopressin (AVP) concentration in response to graded infusions of synthetic AVP during suppression with a gonadotropin-releasing hormone agonist (GnRHa), with and without 17 β-estradiol administration (23,24,28). [Adapted from Stachenfeld, N.S., H.S. Taylor, C.A. Leone, and D.L. Keefe. Oestrogen effects on urine concentrating response in young women. J. Physiol. 552(Pt 3):869–880, 2003. Copyright © 2002 Blackwell Publishing. Used with permission.]
As we indicated earlier, although the studies with oral contraceptives in young women indicated a role for estrogens in the osmotic regulation of AVP, even without a strong impact on renal free water clearance, our studies did not isolate estrogen effects because progestins were present at high levels under both oral contraceptive conditions. Moreover, the level of estradiol delivered through the oral contraceptives is much greater than endogenously produced estrogens at any point in the menstrual cycle. To better isolate estradiol effects from those of progesterone or progestins, the studies described below used a GnRH agonist (GnRHa) and, in later studies, an antagonist, to suppress both estradiol and progesterone. While the women were suppressed, we administered estradiol and progesterone to attain levels of these hormones similar to those occurring over a normal menstrual cycle in young and healthy women.
GnRH ANALOG (LEUPROLIDE ACETATE) ADMINISTRATION
In studies in which we used a GnRHa, subjects received leuprolide acetate (0.5 mg/d; TAP Pharmaceuticals Inc., Deerfield, IL) each day for 5–6 wk (23,24,28). This analog possesses greater receptor binding and decreased degradation than endogenous GnRH and acts as a potent inhibitor of gonadotropin secretion. When leuprolide acetate is given continuously, it down-regulates the hypothalamic-pituitary-ovarian axis, with internalization and uncoupling of the GnRH receptors at the hypothalamic level. After an initial stimulation, chronic administration suppresses GnRH-controlled steroidogenesis, leading to low or undetectable estradiol and progesterone plasma concentrations within 14 d. The GnRHa administration began 2–7 d after the subjects’ luteinizing hormone peak. This peak precedes ovulation, usually days 12–14 of a 28-d menstrual cycle, and was determined individually by the use of ovulation prediction kits (OvuQuick; Quidel Corp, San Diego, CA).
GnRH ANTAGONIST (GANIRELIX ACETATE) ADMINISTRATION
In our later studies when a GnRH antagonist became available, women self-administered the GnRH antagonist ganirelix acetate (250 μg/d; Organon, Roseland, NJ) for 9–16 d (27,29) to suppress the production of estradiol and progesterone for the duration of the study. Ganirelix acetate is a synthetic decapeptide with high antagonistic activity against naturally occurring GnRH. Ganirelix acetate is derived from native GnRH with substitutions at positions 1, 2, 3, 6, 8, and 10. When ganirelix acetate is given in therapeutic doses, it acts by competitively blocking the GnRH receptors on the pituitary gonadotroph and subsequent transductions pathway, inducing a rapid and reversible suppression of gonadotropin secretion (17). In young cycling women, continued administration of ganirelix acetate leads to suppression of estrogens and progesterone to postmenopausal levels. These decreases occur after 36–48 h of administration, and the suppression of the hypothalamic-pituitary-ovarian axis is reversed upon cessation of drug therapy (17). The GnRH antagonist administration began approximately 9–10 d after the subjects’ luteinizing hormone peak, determined individually by the use of ovulation prediction kits.
In studies using either the agonist or antagonist, the subjects self-administered daily subcutaneous injections of the GnRH agonist/antagonist. We chose the daily injection method because it is easily discontinued in the event of uncomfortable side effects, such as headaches, breast tenderness, or vasomotor symptoms, and the suppression of the hypothalamic-pituitary-ovarian axis is reversed rapidly upon cessation of drug therapy. Although both the agonist and antagonist were equally as effective at suppressing estrogens and progesterone, we currently use the GnRH antagonist exclusively because the suppression occurs much faster, making our studies easier and more convenient for the subjects and for us. Most importantly, the shorter suppression time with the antagonist (48 h vs 3–4 wk) reduced the number of side effects (primarily vasomotor symptoms and breast tenderness) seen during the agonist administration.
REPRODUCTIVE HORMONE ADD-BACK
Once the women were suppressed, they either received 4 d of 17 β-estradiol (transdermal patch, 0.1 mg/d each) or 4 d of progesterone (either vaginal gel, 90 mg twice per day, or Prometrium (27,29)) or a combined treatment of 17 β-estradiol with progesterone administration, depending on the specific hypothesis being tested. Experimental protocols were performed while the subjects were first suppressed and then during hormone administration. These designs permitted within- and between-subject comparisons concerning hormone effects on osmotic regulation of AVP, thirst, and sodium and fluid regulation.
REPRODUCTIVE HORMONE EFFECTS ON OSMOREGULATION WITH HYPERTONIC SALINE INFUSION
For these series of experiments, we used hypertonic saline (3% NaCl) infusion rather than dehydration because the greater plasma hypertonicity associated with this technique induced powerful and linear thirst responses and increases in P[AVP]. Moreover, hypertonic saline infusion increases plasma osmolality by as much as 16–20 mOsmol/kg H2O during a 2-h infusion in a far more dramatic and linear fashion than that seen during dehydration. Hypertonic saline infusion is quite a different state than dehydration, despite the large increases in thirst and AVP because a large intravascular fluid expansion (~20%) develops as water is drawn from cells in response to the increased osmotic pressure in fluid surrounding them. Nevertheless, under these conditions, the osmotic stimulus overwhelms the inhibitory input by the plasma volume expansion as seen by the large thirst, P[AVP] (Fig. 3), and renal responses (4,23).
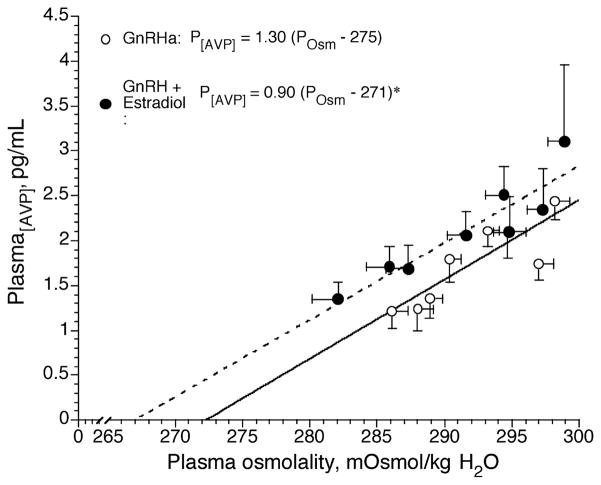
We used the GnRH analog (suppression) add-back protocol to examine the effect of estradiol with and without progesterone on osmotic regulation of AVP. Under these conditions, we found that estradiol administration to GnRH-suppressed young women shifted the osmotic threshold for the release of AVP to a lower plasma osmolality (Fig. 5) similar to our earlier hypertonic saline infusion and dehydration studies (Figs. 3 and 4), and again, this shift was not accompanied with any change in the sensitivity of this relationship (Fig. 5). Taken together, these series of studies demonstrate that high estradiol levels in blood and tissue reduce the increase in plasma osmolality required to induce AVP release from the anterior pituitary.
Figure 5.
Plasma arginine vasopressin (AVP) concentration as a function of plasma osmolality during 120 min of hypertonic (3% NaCl) saline infusion during suppression of reproductive function with a gonadotropin-releasing hormone agonist (GnRHa), with (dashed line) and without (solid line)17 β-estradiol administration (4,23,26). Note the high estrogen condition shifted the P[AVP]-POsm curve to the left relative to the GnRH agonist-alone. (Reprinted from Stachenfeld, N.S., and D.L. Keefe. Estrogen effects on osmotic regulation of AVP and fluid balance. Am. J. Physiol. Endocrinol. Metab. 283:E711–E721, 2002. Copyright © 2002 The American Physiological Society. Used with permission.)
Although the mechanism for the estradiol effects in humans is, for the moment, elusive, we speculate that the estradiol-related shift in osmotic regulation of AVP release occurs via the central nervous system. Estrogens readily cross the blood-brain barrier and may modulate osmotic AVP release directly via actions within the hypothalamus. Estradiol receptors have been identified in the hypothalamic nuclei of neurophysin- and AVP-producing cells in animals (16) and humans (11,12). Moreover, estrogens up-regulate osmotic stimulation of vasopressinergic neuronal activity in the supraoptic nucleus (SON) of brain slices of ovariectomized rats (2).
The lower osmotic AVP threshold in the hypothalamus associated with estradiol administration may be related to the particular subtypes of estrogen receptors involved. Two primary subtypes of estrogen receptors have been identified in humans. Estrogen receptor–β (ERβ) inhibits (11), and estrogen receptor–α (ERα) stimulates, AVP neuronal activity in the SON (20). Thus, the ultimate effect of estradiol on osmotic regulation of AVP may be dependent on the extent to which each receptor is expressed in the SON as well as the affinity of that receptor for 17 β-estradiol. Young women express primarily ERβ in the AVP-producing neurons in the SON (11), so we should expect a later, rather than an earlier, osmotic threshold for AVP release during 17 β-estradiol administration. However, the ERβ receptors have a lower affinity for 17 β-estradiol relative to the ERα (14), so stimulation of the AVP neurons in the SON may have predominated when we administered 17 β-estradiol, despite the greater prevalence of the ERβ subtype. Moreover, the GnRHa may have interfered with ERβ function (3). The impact of estrogen receptor function in AVP regulation in humans has not been tested, but the animal data may suggest that greater 17 β-estradiol affinity for ERα in the hypothalamus contributes to an earlier osmotic threshold for AVP release. This interpretation is also consistent with data demonstrating greater plasma AVP levels at rest and during hypertonic saline infusion in postmenopausal women in response to 17 β-estradiol administration (22) as ERα predominate (11) and GnRH levels are typically high.
EFFECTS OF ESTROGEN ON BODY FLUID REGULATION
As stated earlier, we interpret the estrogen-associated lowering of the osmotic threshold for AVP release as a lowering of the osmoregulatory set point rather than a change in the fluid regulation because the greater P[AVP] did not seem to contribute significantly to fluid retention. Although there were small increases in overall fluid retention during estradiol administration, renal free water clearance was unaffected by estrogen administration. This lack of effect on renal free water clearance suggested to us that estrogens may alter renal sensitivity to AVP or even interfere with AVP action in the kidney (9) as has been noted in collecting ducts of rat kidneys (9). We also observed little change in free water clearance in young women during combined estradiol and progesterone administration despite greater P[AVP], supporting reduced renal response to AVP. Moreover, although osmotically controlled secretion of AVP is greater in the midluteal phase of the menstrual cycle (4,7,25) (concomitant with increases in estrogens and progesterone), the luteal phase is usually not accompanied by greater water retention (4,7,25).
We next tested the hypothesis that estradiol administration would attenuate the urine-concentrating response compared with GnRHa alone, that is, that estrogens reduce renal tubular sensitivity to AVP. Using the GnRH analog protocol with estradiol administration, we infused synthetic AVP (Pitressin) during serial 60-min periods. Vasopressin was infused at the following infusion rates: 10, 35, 100, 150, and 200 μU Pitressin·min−1·kg−1 body weight. At the end of each hour — or each level of infusion, the subject provided a urine sample, from which we determined urine osmolality. The subjects performed this study twice — once while taking the GnRHa alone; the second, when taking the GnRHa with 17 β-estradiol. This gradual infusion rate provided excellent dose-response curves with which to examine renal tubular sensitivity to AVP (Fig. 6; n = 8). The similar EC50 of the urine osmolality-P[AVP] response curve between GnRHa alone and estradiol administration conditions indicated no estradiol effect on AVP-mediated renal concentrating response. However, in the physiological range where AVP has the greatest renal concentrating effect, urine osmolality was significantly lower during estradiol treatment (Fig. 6). Thus, although the overall dose-response curve suggests no change in sensitivity, there may be some attenuation in renal concentrating response at physiological levels of P[AVP]. Studies infusing Pitressin at rates designed to invoke static and physiological P[AVP] would be more definitive.
SODIUM RETENTION
When examining the studies as a whole, overall changes in water or fluid retention were consistently present during estradiol and/or progesterone administration, but these differences were small in healthy young women. We suspect that the small but consistently present water retention associated with estradiol administration may be a function of greater sodium retention rather than AVP-induced increases in free water retention (23,25). For the most part, our studies have shown that increases in sodium retention are independent of RAAS stimulation or ANP (23,25). Although plasma renin activity was greater at baseline during estradiol administration, the greater plasma renin activity did not persist through hypertonic saline infusion, and there was little effect on aldosterone, suggesting minimal RAAS contribution to the greater sodium retention. Conversely, others have shown that estradiol administration can activate the RAAS by enhancing angiotensinogen synthesis, inhibiting angiotensin-converting enzyme activity, and augmenting plasma and tissue levels of renin (15). Under these circumstances, the greater plasma renin activity might cause a decrease in renal blood flow but not necessarily a change in angiotensin II or aldosterone (15). An explanation for the discrepancy between their findings and our own may be the route of estradiol administration. Our studies used the subcutaneous patch delivery system (which bypasses liver metabolism for the most part), whereas earlier studies (15) used an oral administrative route. Because circulating angiotensinogen is synthesized primarily in the liver, and oral estradiol is metabolized in the liver, increases in plasma angiotensin II and aldosterone may be a consequence only of oral estradiol administration.
SEX HORMONES AND BODY FLUID DISTRIBUTION
Clinical reports of edema in association with sex hormone administration, the premenstrual period, and pregnancy suggest that estradiol and progesterone may play important roles in body fluid distribution. Estrogens tend to increase, whereas progesterone tends to decrease, plasma volume (22,23,25) through effects on capillary fluid dynamics or Starling forces (18,24,30). Because estrogens and progesterone increase renal sodium reabsorption (23,25), the plasma volume increases are a function of ECF volume expansion to some extent, but plasma volume seems to increase disproportionally to ECF volume (27). We conducted studies to determine whether estradiol and progesterone induced preferential water movement into and storage in the plasma over other ECF compartments.
The intravascular and interstitial fluid compartments make up approximately 20% and 80%, respectively, of the ECF, and movement across these compartments is governed by changes in Starling forces, which include oncotic (due to proteins) and hydrostatic pressures. Plasma proteins diffuse slowly across the capillary endothelium, so changes in plasma protein content will change the fluid distribution of the extracellular compartment unlike changes due to sodium, which alter the total ECF. For example, an increase in plasma proteins increases oncotic pressure and favors fluid movement into the plasma, whereas plasma protein escape increases interstitial fluid. Thus, in response to acute plasma volume loss, Starling forces adjust to favor plasma proteins and fluid movement out of interstitial and into vascular compartments, thereby selectively restoring plasma volume. Sodium and other electrolytes do not act as effective osmotic agents between the vascular and interstitial compartments because they freely diffuse across capillary endothelium, so changes in the total ECF are related to changes in sodium content.
The measurement of transcapillary escape of albumin from the plasma is an important indicator of the oncotic force within the plasma: a lower transcapillary escape of albumin is usually associated with an increase in plasma albumin content and plasma oncotic pressure. We determined trans-capillary protein movement and plasma volume with Evans Blue dye dilution and ECF using inulin washout (27,29). We executed these protocols in two different groups, one group received progesterone-only administration followed by combined estradiol-progesterone administration (27), and a second group received estradiol-only administration followed by combined estradiol-progesterone administration (29). The subjects were suppressed with the GnRH antagonist under both protocols.
Plasma volume increased during estradiol administration relative to GnRH antagonist alone. This plasma volume expansion occurred despite an ECF contraction and was a clear linear function of lower transcapillary escape rate of albumin, which decreased in direct proportion to the increases in plasma volume. In contrast to studies using oral contraceptives (4,25), progesterone administration also increased plasma volume. Unlike the estradiol administration, the progesterone-related plasma volume increase was associated with a proportional increase in ECF volume and, to some extent, associated with lower albumin transcapillary escape rates (27). Thus, the progesterone-associated increase in plasma volume was achieved through the combined mechanisms of greater protein retention in the vascular space as well as overall expansion of ECF, whereas the estradiol-associated increase in plasma volume was a function solely of greater fluid retained in the vascular space. Combined estradiol-progesterone administration caused the greatest increase in plasma volume and maintained ECF volume at a level similar to that during GnRH antagonist administration alone because of the combined mechanisms of plasma protein and ECF sodium and fluid maintenance (27,29).
CONCLUSIONS
We have found both estradiol and progesterone to have important effects on body fluid and sodium regulation, primarily by shifting leftward the set point for osmotic regulation thirst and AVP both during exercise and osmotic stimulation with hypertonic saline infusion. In the case of young healthy women, the shift in osmoregulation seems to have only small effects on overall body water and sodium retention but alters body water distribution within the ECF space. In future studies that target younger and older populations susceptible to hyponatremia, the contributions of these sex hormones will be important considerations in understanding this apparent dysregulation of water and sodium.
Acknowledgments
I gratefully acknowledge the intellectual contributions Drs. Ethan Nadel (posthumous), Gary Mack, and Wendy Calzone, M.S.; the technical assistance of Cheryl Leone, M.A.; the clinical support of Drs. Hugh Taylor and Celso Silva; and the cooperation of the volunteer subjects. These studies were supported, in part, by National Heart Lung Blood Institute R01 HL62240 and R01 HL71159, as well as the U.S. Army Medical and Research and Materiel Command under contract DAMD17-96-C-6093.
References
- 1.Almond CSD, Shin AY, Fortescue EB, Mannix RC, Wypij D, Binstadt BA, Duncan CN, Olson DP, Salerno AE, New-burger JW, Greenes DS. Hyponatremia among runners in the Boston Marathon. N Engl J Med. 2005;352:1550–1556. doi: 10.1056/NEJMoa043901. [DOI] [PubMed] [Google Scholar]
- 2.Barron WM, Schreiber J, Lindheimer MD. Effect of ovarian sex steroids on osmoregulation and vasopressin secretion in the rat. Am J Physiol. 1986;250(4 Pt 1):E352–E361. doi: 10.1152/ajpendo.1986.250.4.E352. [DOI] [PubMed] [Google Scholar]
- 3.Byers M, Kuiper GG, Gustafsson JA, Park-Sarge OK. Estrogen receptor-beta mRNA expression in rat ovary: down regulation by gonadotropins. Molecular Endocrinol. 1997;11:172–182. doi: 10.1210/mend.11.2.9887. [DOI] [PubMed] [Google Scholar]
- 4.Calzone WL, Silva C, Keefe DL, Stachenfeld NS. Progesterone does not alter osmotic regulation of AVP. Am J Physiol Regul Integr Comp Physiol. 2001;281:R2011–R2020. doi: 10.1152/ajpregu.2001.281.6.R2011. [DOI] [PubMed] [Google Scholar]
- 5.Claybaugh JR, Sato AK, Crosswhite LK, Hassell LH. Effects of time of day, gender, and menstrual cycle phase on the human response to oral water load. Am J Physiol Regul Integr Comp Physiol. 2000;279:R966–R973. doi: 10.1152/ajpregu.2000.279.3.R966. [DOI] [PubMed] [Google Scholar]
- 6.Dubey RK, Jackson EK. Estrogen-induced cardiorenal protection: potential cellular, biochemical and molecular mechanisms. Am J Physiol Renal Physiol. 2001;280:F365–F388. doi: 10.1152/ajprenal.2001.280.3.F365. [DOI] [PubMed] [Google Scholar]
- 7.Forsling ML, Akerlund M, Strömberg P. Variations in plasma concentrations of vasopressin during the menstrual cycle. J Endocrinol. 1981;89:263–266. doi: 10.1677/joe.0.0890263. [DOI] [PubMed] [Google Scholar]
- 8.Fraser CL, Arieff AI. Epidemiology, pathophysiology, and management of hyponatremic encephalopathy. Am J Med. 1997;102:67–77. doi: 10.1016/s0002-9343(96)00274-4. [DOI] [PubMed] [Google Scholar]
- 9.Hatano T, Ogawa K, Kanda K, Seo H, Masui N. Effect of ovarian steroids on cyclicadenosine 3′:5′ monophosphate production stimulated by arginine vasopressin in rat renal monolayer cells. Endocrinol Japon. 1988;35:267–274. doi: 10.1507/endocrj1954.35.267. [DOI] [PubMed] [Google Scholar]
- 10.Heritage AS, Stumpf WE, Sar M, Grant LD. Brainstem catecholamine neurons are target sites for sex steroid hormones. Science. 1980;207:1377–1379. doi: 10.1126/science.7355296. [DOI] [PubMed] [Google Scholar]
- 11.Ishunina TA, Kruijver FPM, Balesar R, Swaab DF. Differential expression of estrogen receptor alpha and beta immunoreactivity in the human supraoptic nucleus in relation to sex and aging. J Clin Endocrinol Metab. 2000;85:3283–3291. doi: 10.1210/jcem.85.9.6826. [DOI] [PubMed] [Google Scholar]
- 12.Ishunina TA, Swaab DF. Vasopressin and oxytocin neurons of the human supraoptic and paraventricular nucleus; size changes in relation to sex and age. J Clin Endocrinol Metab. 1999;84:4637–4644. doi: 10.1210/jcem.84.12.6187. [DOI] [PubMed] [Google Scholar]
- 13.Isles CG, Hole DJ, Hawthorne VM, Lever AF. Relation between coronary risk and coronary mortality in women of the Renfew and Paisley survey: comparison with men. Lancet. 1992;339:702–706. doi: 10.1016/0140-6736(92)90599-x. [DOI] [PubMed] [Google Scholar]
- 14.Kuiper GG, Carlsson B, Enmark E, Häggblad J, Nilsson S, Gustafsson JA. Comparsion of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1997;138:863–870. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- 15.Kuroski de Bold ML. Estrogen, natriuretic peptides and the renin-angiotensin system. Cardiovasc Res. 1999;41:524–531. doi: 10.1016/s0008-6363(98)00324-1. [DOI] [PubMed] [Google Scholar]
- 16.Li X, Schwartz P, Rissman E. Distribution of estrogen receptor-beta-like immunoreactivity in the rat forebrain. Neuroendocrinology. 1997;66:63–67. doi: 10.1159/000127221. [DOI] [PubMed] [Google Scholar]
- 17.Oberyé JJL, Mannaerts BMJL, Huisman JAM, Timmer CJ. Pharmacokinetic and Pharmacodynamic characteristics of ganirelix (Antagon/Orgalutran). Part II. Dose-proportionality and gonadotropin suppression after multiple doses of ganirelix in healthy female volunteers. Fertil Steril. 1999;72:1006–1012. doi: 10.1016/s0015-0282(99)00414-8. [DOI] [PubMed] [Google Scholar]
- 18.Oian P, Tollan A, Fadnes HO, Noddeland H, Maltau JM. Transcapillary fluid dynamics during the menstrual cycle. Am J Obstet Gynecol. 1987;156:952–955. doi: 10.1016/0002-9378(87)90364-4. [DOI] [PubMed] [Google Scholar]
- 19.Orshal JM, Khalil RA. Gender, sex hormones, and vascular tone. Am J Physiol Regul Integr Comp Physiol. 2004;286:R233–R249. doi: 10.1152/ajpregu.00338.2003. [DOI] [PubMed] [Google Scholar]
- 20.Paech K, Webb P, Kuiper G, Nilsson S, Gustafsson JA, Kushner PJ, Scanlan TS. Differential ligand activation of estrogen receptors ERalpha and ERbeta at AP1 sites. Science. 1997;277:1508–1510. doi: 10.1126/science.277.5331.1508. [DOI] [PubMed] [Google Scholar]
- 21.Sar M, Stumpf WE. Simultaneous localization of [3H]estradiol and neurophysin I or arginine vasopressin in hypothalamic neurons demonstrated by a combined technique of dry-mount autoradiography and immunohistochemistry. Neurosci Lett. 1980;17:179–184. doi: 10.1016/0304-3940(80)90081-6. [DOI] [PubMed] [Google Scholar]
- 22.Stachenfeld NS, DiPietro L, Palter SF, Nadel ER. Estrogen influences osmotic secretion of AVP and body water balance in postmenopausal women. Am J Physiol. 1998;274(1 Pt 2):R187–R195. doi: 10.1152/ajpregu.1998.274.1.R187. [DOI] [PubMed] [Google Scholar]
- 23.Stachenfeld NS, Keefe DL. Estrogen effects on osmotic regulation of AVP and fluid balance. Am J Physiol Endocrinol Metab. 2002;283:E711–E721. doi: 10.1152/ajpendo.00192.2002. [DOI] [PubMed] [Google Scholar]
- 24.Stachenfeld NS, Keefe DL, Palter SF. Estrogen and progesterone effects on transcapillary fluid dynamics. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1319–R1329. doi: 10.1152/ajpregu.2001.281.4.R1319. [DOI] [PubMed] [Google Scholar]
- 25.Stachenfeld NS, Silva CS, Keefe DL, Kokoszka CA, Nadel ER. Effects of oral contraceptives on body fluid regulation. J Appl Physiol. 1999;87:1016–1025. doi: 10.1152/jappl.1999.87.3.1016. [DOI] [PubMed] [Google Scholar]
- 26.Stachenfeld NS, Splenser AE, Calzone WL, Taylor MP, Keefe DL. Sex differences in osmotic regulation of AVP and renal sodium handling. J Appl Physiol. 2001;91:1893–1901. doi: 10.1152/jappl.2001.91.4.1893. [DOI] [PubMed] [Google Scholar]
- 27.Stachenfeld NS, Taylor HS. Progesterone increases plasma volume independent of estradiol. J Appl Physiol. 2005;98:1991–1997. doi: 10.1152/japplphysiol.00031.2005. [DOI] [PubMed] [Google Scholar]
- 28.Stachenfeld NS, Taylor HS, Leone CA, Keefe DL. Oestrogen effects on urine concentrating response in young women. J Physiol. 2003;552(Pt 3):869–880. doi: 10.1113/jphysiol.2003.046920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stachenfeld NS, Taylor HS. Effects of estrogen and progesterone administration on extracellular fluid. J Appl Physiol. 2004;96:1011–1018. doi: 10.1152/japplphysiol.01032.2003. [DOI] [PubMed] [Google Scholar]
- 30.Tollan A, Kvenild K, Strand H, Oian P, Maltau JM. Increased capillary permeability for plasma proteins in oral contraceptive users. Contraception. 1992;45:473–481. doi: 10.1016/0010-7824(92)90160-u. [DOI] [PubMed] [Google Scholar]