Abstract
Objective
A systematic review of weaning and extubation for pediatric patients on mechanical ventilation.
Data Selection
Pediatric and Adult Literature, English language
Study Selection
Invited review
Data Sources
Literature review using National Library of Medicine PubMed from January 1972 until April 2008, earlier cross-referenced article citations, the Cochrane Database of Systematic Reviews and the Internet.
Conclusions
Despite the importance of minimizing time on mechanical ventilation, only limited guidance on weaning and extubation is available from the pediatric literature. A significant proportion of patients being evaluated for weaning are actually ready for extubation, suggesting that weaning is often not considered early enough in the course of ventilation. Indications for extubation are even less clear, although a trial of spontaneous breathing would seem a prerequisite. Several indices have been developed in an attempt to predict weaning and extubation success but the available literature would suggest they offer no improvement over clinical judgment. Extubation failure rates range from 2–20% and bear little relationship to the duration of mechanical ventilation. Upper airway obstruction is the single most common cause of extubation failure. A reliable method of assessing readiness for weaning and predicting extubation success is not evident from the pediatric literature.
Keywords: weaning, extubation, mechanical ventilation, respiratory support, spontaneous breathing
INTRODUCTION
Respiratory disorders are the main cause of respiratory failure in children. Unfortunately, respiratory failure fits no well-defined clinical description; it may have an abrupt onset, or may occur insidiously with gradual and progressive deterioration of pulmonary function. Insufficient alveolar ventilation from any cause results in hypoxemia and hypercapnia which may contribute to further depression of ventilation, culminating in frank respiratory failure. The major intervention to prevent morbidity and death is mechanical ventilation. The average pediatric intensive care unit (PICU) has about 30% (range 20 – 64%) of its patients mechanically ventilated, for a mean of 5–6 days1;2.
While mechanical ventilation is often life saving, it can be associated with complications such as ventilator-induced lung injury and nosocomial pneumonia. Endotracheal tubes (ETT) are uncomfortable for patients and increase the need for sedatives. An ETT in the upper airway can be associated with airway injury, particularly in mobile young patients. Moreover, positive pressure ventilation may contribute to cardiovascular instability from heart-lung interactions. Therefore it is important that mechanical ventilation be discontinued as soon as the patient is capable of sustaining spontaneous breathing. However, the experience in adults suggests that premature extubation may also be problematic and result in emergent reintubation with attendant complications, including the potential of catastrophic morbidity3. A high mortality rate has been documented in both pediatric4 and adult5;6 patients who have required reintubation after extubation failure. Extubation failure is independently associated with a fivefold increased risk of death in pediatric patients7. Consequently, while expeditious weaning and extubation are the goal, premature extubation can be lethal.
Over 50% of ventilated PICU patients will have been extubated by 48 hours after admission, but the rest often require prolonged ventilator support. Failed planned extubations in the latter group average 8.0% (unpublished observations from Kurachek et al7) but range up to 20% in some studies. Conversely, 50% of unplanned extubations end in success8, implying that some patients could be extubated earlier. Both premature and delayed extubation increase morbidity and mortality as well as costs. Initiation of weaning and timing of extubation have been largely neglected in the pediatric literature. This review examines available data in children and adults and makes recommendations for weaning and optimal timing of extubation in children receiving ventilation for respiratory failure.
CONCEPTS OF WEANING, SPONTANEOUS BREATHING AND EXTUBATION READINESS TRIALS
Overview of Factors Impacting Weaning
There is no standard method of weaning. Indeed, there is disagreement about when the onset of weaning actually occurs and no validated, objective criteria as to when a patient can be extubated. For definitions of common terms regarding weaning and extubation readiness see Table 1.
Table 1.
Definitions – summary of terms
|
Weaning is the transition from ventilatory support to completely spontaneous breathing, during which time the patient assumes the responsibility for effective gas exchange while positive pressure support is withdrawn. Note that spontaneous breathing is a prerequisite for weaning to begin and decreasing ventilator support is not the sole criterion of successful weaning. |
|
Extubation is the removal of the endotracheal tube. Criteria for extubation include spontaneous ventilation, hemodynamic stability, intact airway reflexes, and manageable airway secretions. Success is defined as 48 hours of spontaneous breathing without positive pressure support. Early extubation failure is defined as that which occurs within 6 hours of extubation; intermediate extubation failure is that which occurs from 6 to 24 hours of extubation; and late extubation failure is defined as that which occurs from 24 to 48 hours of extubation. |
|
Spontaneous Breathing Test (SBT) is a subjective determination of whether the underlying disease process necessitating mechanical ventilation has improved sufficiently to allow the patient adequate gas exchange with spontaneous breathing. |
|
Extubation Readiness Test (ERT) is a formal trial of spontaneous breathing to evaluate readiness for discontinuation of the endotracheal tube and/or ventilatory support. This may be performed with variable pressure support assist but we propose spontaneous breathing be evaluated for 2 hours on CPAP ≤5 cmH2O or T-piece (ZEEP). Criteria of failure, both subjective and objective, are proposed in Table 3 |
|
Ventilator Free Days (VFD) is an outcome measure consisting of the number of days in a given time period (conventionally 28 days) the patient does not require ventilator support. Successful discontinuation of ventilator support requires a minimum of 48 hours without positive pressure ventilation. Patients who die are considered to have zero ventilator free days. |
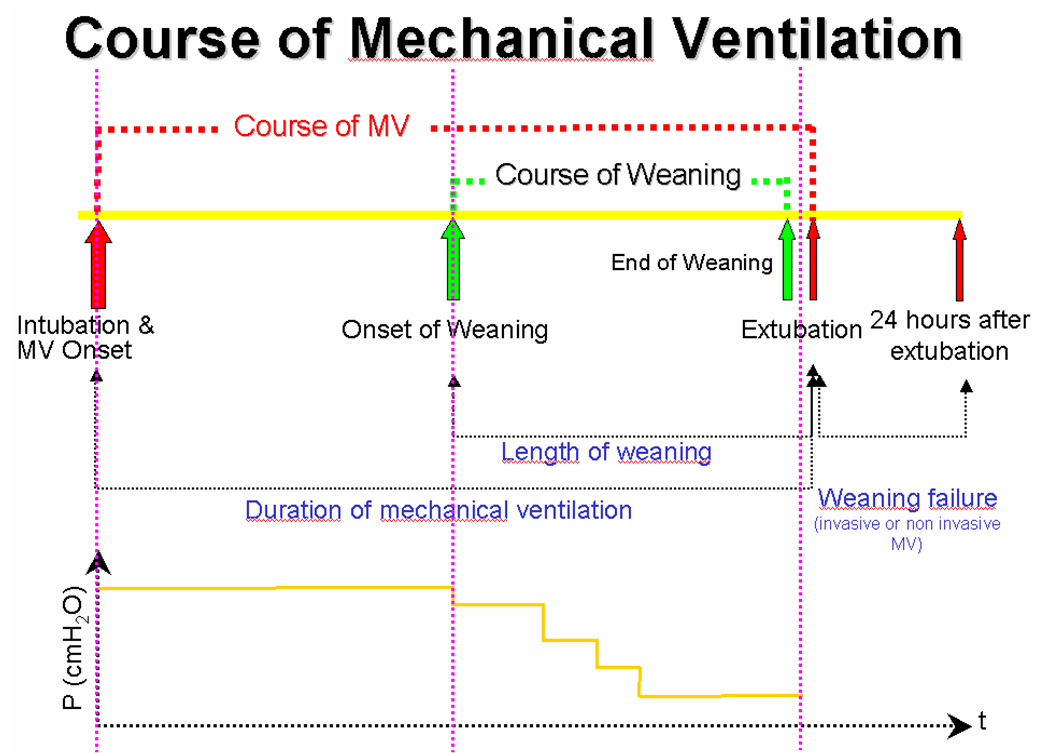
The course of mechanical ventilation begins with intubation and the onset of ventilator support (Figure 1). As the disease progresses, mechanical ventilation is fine-tuned to provide effective gas exchange. When the acute phase of the disease subsides, noted by a decrease in the mean airway pressure required, weaning begins. The end of weaning can be defined as the time at which the patient’s spontaneous breathing alone can provide effective gas exchange, although how this point can best be determined is unclear. At the end of weaning is extubation, or the act of removal of the ETT.
Figure 1.
A schematic of the time and pressure courses of mechanical ventilation, along with the defined phases, in a PICU patient.
The length of weaning depends on a number of factors, among them fluid status. When total body water increases, lung compliance decreases due to increased lung water, chest wall and diaphragm edema. In adults with ARDS it is clear that the injured lungs should be managed dry9;10. Patients managed with a conservative fluid regime had fewer mechanical ventilation days and a quicker return of normal lung function than those receiving a more liberal regime9. The importance of fluid balance in children is not as clear. A retrospective study of cumulative fluid balance showed no effect on weaning or extubation success11. However, two retrospective studies of children with multiple-organ failure showed survival may be associated with less fluid overload (in the setting of continuous renal replacement therapy)12;13. Similarly, a review by Swaniker, et al14 of 128 children placed on extracorporeal life support for respiratory failure showed increased survival with fluid removal and return to dry body weight.
PEEP is another factor that may impact the length of weaning. Early institution of PEEP generally improves oxygenation in ARDS but neither decreases the incidence of ARDS15 nor changes the weaning times or outcomes if a low tidal volume lung protective strategy is used16.
Sedation further complicates weaning and extubation17–19. Over-sedation may depress central respiratory drive whereas under-sedation can leave a child restless. Thrashing movements can result in airway trauma from the ETT. Two groups have shown an association between sedation level and extubation readiness18;19 but this has not been validated prospectively in infants and children. Sedation assessment tools are being developed for this purpose17;20.
Pulmonary hypertension is another important factor in determining readiness for weaning because of its effect on the patient's oxygenation21;22. Supplemental oxygen and ventilator support are the mainstays of treatment for pulmonary hypertension and there is reluctance to withdraw these too quickly in the absence of direct measures of pulmonary arterial pressure or resistance.
Differences in diaphragmatic function may relate to longer weaning times in infants and young children. Accessory respiratory muscles are not as developed as in older children23. As diaphragmatic dysfunction develops with prolonged mechanical ventilation the duration of weaning can increase.
Steroids may play a role in weaning and extubation by reducing tracheal inflammation associated with tracheal injuries from the ETT, as they do in another cause of subglottic edema in children, croup{Tibballs, 1992 4140 /id}. One randomized, controlled trial in children24 showed steroids prevented upper airway obstruction (UAO) while the only other such study25 did not. Studies in adults have shown the same dichotomy. It is noteworthy that the successful RCTs in both adults and children have started steroids 6 to 24 hours before extubation while the unsuccessful ones have started the drug under 6 hours before extubation.
Finally, other factors are probably important to the weaning process, but there is a dearth of research in these areas and they are not further discussed. These include disease reversibility (rapid – RSV bronchiolitis versus slow – RSV pneumonia/ARDS26), cardiac function, post-operative, neurological and nutritional status.
Predictive Indices for Weaning
Several indices have been developed to predict success in weaning and extubation. While these indices have been variably used in research, they have not found common use in clinical care—likely because of their complexity and lack of proven benefit over clinical judgment.
Rapid Shallow Breathing Index (RSBI=f/Vt) The RSBI was devised by Yang and Tobin27 and found to be a good discriminator of weaning success and failure. This test has become widely used in practice and research with varying success. Recently, the issue has been revisited in a meta-analysis of 41 RSBI studies28. An editorial that accompanied the meta-analysis28 suggests that during weaning, the f/VT index can be thought of as a screening test with high sensitivity and low specificity, and therefore should be used early in the course of MV to identify patients who can breathe on their own. Specificity is obtained by applying a confirmatory test such as esophageal pressure trend measurements29 which are easy to apply in a PICU setting30.
CROP Index (Dynamic Compliance × Maximal Negative Inspiratory Pressure × (PaO2/PAO2) / Respiratory Rate) Thiagarajan et al.31 found that spontaneous respiratory rate ≤ 45/minute, spontaneous tidal volume ≥ 5.5 mL/kg, RSBI ≤ 8 breaths/min/ml/kg body weight and CROP Index ≥ 0.15 mL/kg body weight/breaths/minute were good predictors of successful extubation. Baumeister et al18 used a modified RSBI and CROP indices to predict successful extubation. Their threshold values (RSBI ≤ 11, CROP index ≥ 0.1 mL/kg body weight/breaths/minute) differed from Thiagarajan’s. As with adults, conflicting studies by others18;32;33 found that those indices did not reliably predict extubation outcome in children. Manczur et al.33 studied 47 patients under CPAP. Seven (14.9%) failed extubation with low tidal volume (<6 mL/kg) and minute ventilation (<180 mL/kg) associated with failure. RSBI did not predict outcome.
Volumetric Capnography Hubble et al.34 used volumetric capnography to predict successful extubation in 45 children. A volumetric capnogram plots CO2 concentration in airway gas against expired volume. The slope of an expired, single-breath CO2 waveform can be used to calculate the physiologic deadspace (VD/VT). They found that VD/VT ≤ 0.50 reliably predicted extubation success with 75% sensitivity and 92% specificity, while a VD/VT > 0.65 identified patients at risk for failure. Volumetric capnography requires an arterial or capillary blood gas.
Techniques of weaning
The most common approach to weaning infants and children is gradual reduction of ventilator support. Weaning with intermittent mandatory ventilation (IMV) or synchronized IMV (SIMV) occurs by reducing the ventilator rate. With pressure-support (PS) ventilation, the inspiratory pressure is initially set to provide the required support and then reduced gradually. PS is often combined with IMV/SIMV during weaning. Volume support (VS) and volume-assured pressure support (VAPS) are special forms of PS available in certain ventilators that guarantee a minimal tidal volume per assisted breath. Weaning with VS is semi-automatic, where the PS level required to maintain a certain tidal volume is reduced automatically as respiratory mechanics improve. Extubation occurs from a low level of ventilator support or after an extubation readiness test (ERT) (see later in this review). Unlike in adults, it seems it is common practice to extubate infants and children from a low level of ventilator support4.
A second school of thought recommends moderate amounts of ventilator support to rest the patient’s respiratory muscles and performing a daily ERT. Mechanical ventilation is discontinued if the ERT is passed35;36. This approach has been more commonly used to wean adult patients than children.
In a small number of patients, weaning is attempted with alternating periods of complete ventilatory support and graded spontaneous breathing with assistance. This “sprinting” is performed on the theory that the respiratory muscles can be slowly trained to sustain complete spontaneous breathing. There is currently little evidence that such an approach is an effective way of training muscles. There are also no data comparing such an approach with more traditional approaches of weaning37. A recent multicenter randomized control trial comparing three modes of weaning found that there were no significant differences between having no protocol, or weaning by PS or VS19.
Adult trials have often used standardized weaning protocols38;39 to minimize the time on a ventilator and provide uniform decisions about weaning (see a recent review by Fessler and Brower40). Studies in children have begun to follow suit2;41–43 and the utility of ventilator protocols in this age group has been reviewed44. These weaning trials embraced a daily ERT and all have used Ventilator Free Days (VFD) (see Table 1 for definition) as their primary outcome. The concept of VFD45 is implicitly based on having a low failed extubation rate from any cause other than the original cause of respiratory failure. This standard is likely to be adopted in pediatric trials2 but may be inappropriate since there is not only a higher rate of failed extubations in this group, but up to 40% may involve UAO7. Thus, for pediatric research, it may be important to define the end of successful weaning in a way short of extubation. Whether the extubation is successful or not may be of secondary importance. This approach was taken by Schultz et al in their pediatric weaning study46 and was allowed in the ARDSnet low tidal volume trial36 in adults, both of which studies allowed achievement of minimum support settings short of extubation. Ideally, the timing of extubation should coincide with the determination that the patient is ready to sustain adequate gas exchange by spontaneous breathing alone. It is clear from the published studies that there is no such pediatric standard.
Adult Studies of Weaning and Extubation
In 2001, a Task Force facilitated by the American College of Chest Physicians, the American Association for Respiratory Care and the American College of Critical Care Medicine published Evidence-Based Guidelines for Weaning and Discontinuing Ventilatory Support47. They classified adult studies on weaning from mechanical ventilation into: 1) trials of discontinuation assessment strategies or ERTs; 2) controlled trials of stepwise reduction in mechanical support; and 3) controlled trials of alternative discontinuation strategies.
In a study by Esteban et al.3, 2-hour trials of unassisted breathing using PS of 7 cmH2O were compared to T-piece alone. More patients in the PS group tolerated the trial and were extubated at the end of the trial than the T-piece group (86% vs 78%; relative risk of failure, 0.64; 95% CI, 0.43 to 0.94). There was no difference in the rate of reintubation. A second similar study by Esteban et al.48, also showed no difference in reintubation rates between groups. However, the shorter T-piece trial benefited patients by reducing ICU and hospital duration (2 days and 5 days shorter, respectively).
Five RCTs compared alternative methods of reducing ventilatory support in patients in whom clinicians thought extubation was still several days away35;49–52. The most informative results come from the two largest studies by Esteban49 and Brochard35. Both showed that when patients were first evaluated for extubation using a T-piece, about 76% could be extubated without weaning. The remaining patients were randomized to be weaned using two hour spontaneous breathing trials with several different modalities: multiple daily T-piece/CPAP breathing; PS mode; and SIMV. The trial by Esteban et al49 also included a fourth arm, once-daily T-piece tests. There was no difference in the duration of ventilation between T-piece and PS, the trends going in opposite directions in the two studies: Esteban et al49 favored weaning with T-piece while Brochard et al35 favored PS. Both studies showed shorter duration of ventilation with T-piece compared to SIMV. In the comparison of PS to SIMV, both studies found trends in favor of PS, although the effect in the study by Brochard35 was much larger. Jounieaux et al50 randomized 19 patients to SIMV+PS versus SIMV alone. The duration of the weaning process was approximately 1 day shorter in the group that received PS.
Two adult studies examined the use of noninvasive positive pressure ventilation (NPPV) to facilitate stepwise reductions in ventilator support for patients hospitalized with COPD exacerbations who failed a 2-h T-piece trial51;52. The control strategies in both studies included PS with or without CPAP. In the larger study, Nava et al.51 found that NPPV reduced the duration of mechanical ventilation and ICU stay. The study by Girault52 did not show differences in ICU or hospital stay but NPPV did allow earlier extubation and decreased duration of ventilator support.
Several adult studies have shown that protocol-based weaning, whether conducted by physicians or non-physicians, results in earlier and faster weaning with no increase in complications. On average, protocol-based weaning results in a reduction of 1–2 days in weaning time53–56.
Pediatric Studies of Weaning and Extubation
Pediatric studies relating to weaning and extubation fall into two broad categories – those that describe practice (usually within a single PICU)7;22;57;58 and those which seek to identify predictors of successful extubation, usually retrospectively4;18;31–34;59–61. Less commonly, prospective studies19 have been done where patients are randomized and extubated after reaching a predetermined physiological goal. A number of studies give data about failed extubations and the major ones are listed in Table 2.
Table 2.
Days of Mechanical Ventilation and Rates of Failed Extubation
| Author | Tests done pre or post extubation decision * |
N | Variables | FE (%) | LMV (d) |
|---|---|---|---|---|---|
| Khan, 199660 | Retrospective | 208 | RSBI, Vt/kg | 16 | 5.1 |
| Baumeister, 199718 | Retrospective | 47 | RSBI <11 | 19 | ? |
| Thiagarajan, 199932 | Retrospective | 227 | RSBI <8 | 11 | 4.9 |
| Farias, 199864 | Prospective | 84 | SBT: T-piece | 16 | 6.0 |
| Manczur, 200034 | Retrospective | 47 | OI, VT/kg, MV | 14 | ? |
| Hubble, 200035 | Retrospective | 45 | Vd/Vt <0.5 | 4 | 5.9 |
| Venkataraman, 200033 | Retrospective | 312 | RSBI, Vt/kg | 16 | 5.1 |
| Edmunds, 200159 | Retrospective | 632 | Clinical | 4.9 | 6.5 |
| Farias, 200261 | Prospective | 418 | SBT: T-piece | 14 | 6.5 |
| Randolph, 200219 | Prospective | 313 | SBT; PS/PEEP | 16 | 7.0 |
| Harrison, 200222 | Prospective | 202 | SBT; PS/PEEP | 10 | ? |
| Kurachek, 20037 | Prospective | 2794 | Clinical | 6.2 | 4.8 |
| Baisch, 200558 | Retrospective | 3193 | Clinical – 5 y | 4.1 | 5.9 |
| Fontela, 200562 | Retrospective | 124 | Clinical | 10.5 | 5.8 |
| Chavez, 200665 | Retrospective | 70 | SBT: T-piece | 7.8 | 3.3 |
Retrospective and prospective refer here as to whether the physiological measurements were taken after or before a decision had been made clinically to extubate the patients, respectively.
While protocol-based weaning results in faster, earlier weaning with better outcomes in adults, the data is less solid for children. No advantage over clinical weaning was shown in one prospective study19 but shorter weaning (by 12 hours) against historical controls was shown in another62. A study by Shultze et al showed shorter weaning times on a protocol which was respiratory therapist initiated (10 versus 0.8 hours), but surprisingly had no overall difference in hours of mechanical ventilation46.
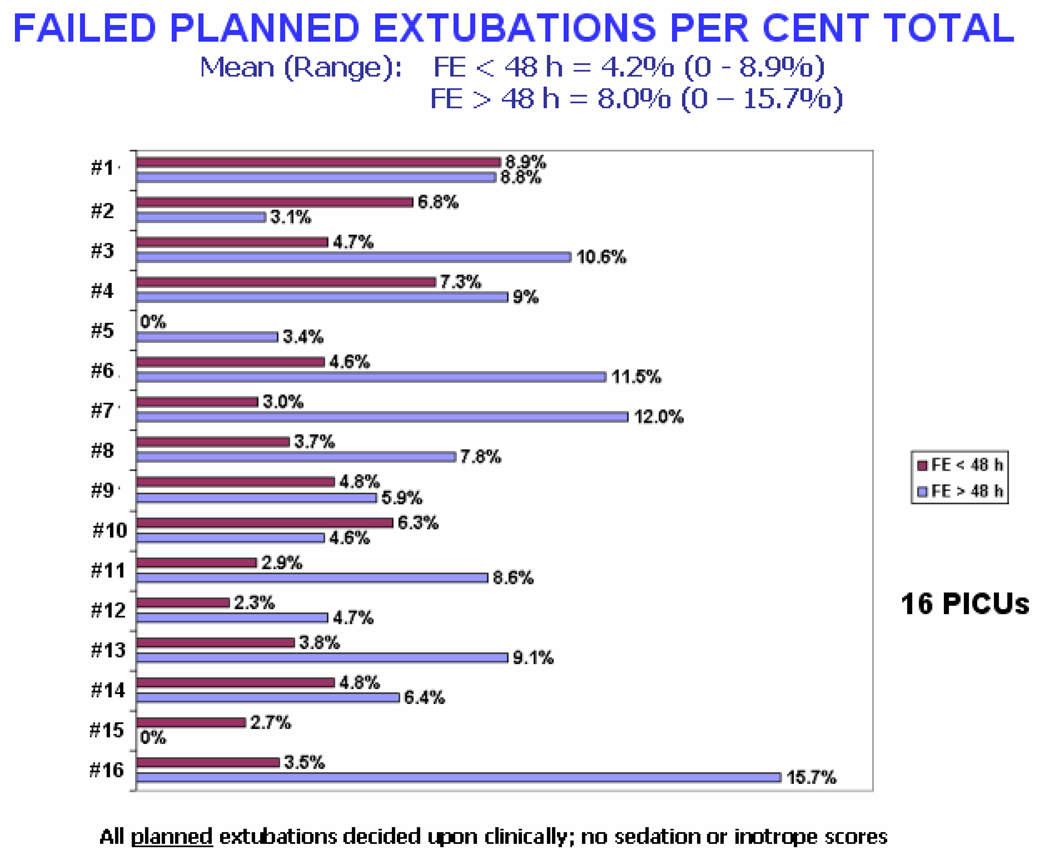
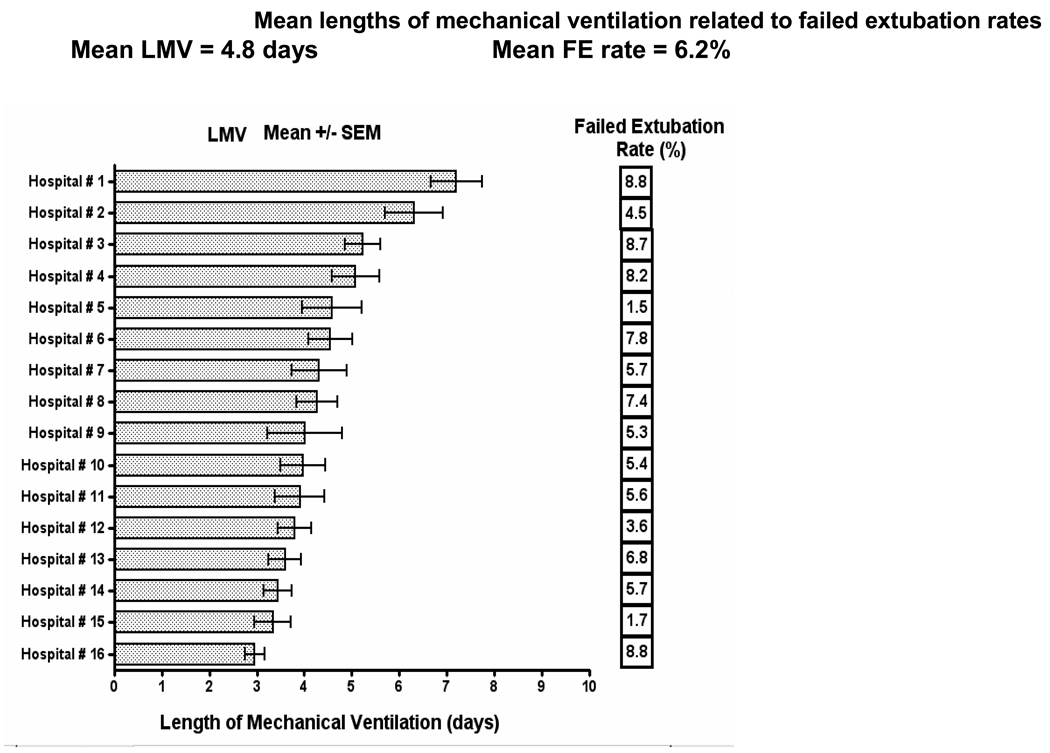
A comprehensive and prospective study on intubation and extubation practices from 16 U.S. PICUs gave valuable insight into the variability of length of mechanical ventilation and failed extubations7. Defining extubation failure as reintubation within 24 hours, they reported a failure rate of 6.2% (range 1.5 – 8.8%) in 1459 patients intubated for at least 48 hours and ventilated for a mean of 4.8 days (range 3 – 7 days). Risk factors for extubation failure included age < 24 months, dysgenetic or syndromic condition, chronic respiratory disorder, chronic neurologic condition, and the need to replace the ETT on admission for any reason. UAO accounted for 37% of failed extubations. Previously unpublished data from this study showed that, contrary to common perception, there was no relationship between the duration of mechanical ventilation and rates of failed extubation, even when excluding patients ventilated for less than 48 hours (Figure 2 and Figure 3). In this study patients who failed extubation and required reintubation went on to have longer average durations of ventilation.
Figure 2.
The failure rate of planned extubations of patients within the first 48 hours of arrival in the PICU is, on average, half that of the rate for patients ventilated for longer than 48 hours. This presents previously unpublished data from the 2003 report of Kurachek et al7 of extubation practices in 16 PICUs across the United States.
Figure 3.
The rates of failed extubation in 16 PICUs across the United States along with their average number of days of mechanical ventilation. There are marked variations in the lengths of ventilation and also the failed extubation rates, with no relationship between the two, i.e. longer ventilation does not result in fewer failed extubations and vice versa. This presents previously unpublished data from the report of Kurachek et al7.
Among single center studies, Edmunds et al.58 reported a 7.9% failed extubation rate in 280 patients intubated for at least 48 hours. They used clinical criteria to determine the appropriate time to extubate and found that a higher incidence of extubation failure was associated with longer durations of ventilation. UAO accounted for almost 25% of failures. In 2005, Fontela et al61 studied 124 infants and children intubated for at least 12 hours. They excluded patients who failed extubation due to UAO. Extubation failure, defined as re-intubation within 48 hours, was associated with younger age, mean OI > 5, longer duration of mechanical ventilation (>15 days), increased sedation (>10 days), and use of inotropes. Baisch and colleagues57, in 2005, reported a 4.1% extubation failure rate within 48 hours in 3193 infants and children. Extubation failures were younger (median age of 6.5 months vs. 21.3 months), had longer durations of intubation, PICU, and hospital stay but no difference in mortality.
Many studies have described extubation practices in pediatrics, most of them conducted in a single PICU7;18;19;22;31;32;34;57–60;63;64. Only two were multi-centered, one reporting clinical practice over 16 PICUs7 and the other utilizing an ERT as part of a weaning protocol in 10 PICUs19. Most studies reporting clinical practice outcomes suggest that a failed extubation rate less than 10% is the norm as supported by the Kurachek study7. Trials using an ERT all report a higher rate of failed extubation – around 14 – 20% in the Randolph study19. The disparity may be, in part, caused by the inclusion of patients extubated in less than 24 – 48 hours. When the failed extubation data from the 16 PICUs is re-analyzed excluding those patients, the failed extubation rate increases from a mean of 6.2% to 8%.
Spontaneous Breathing Trials (SBT) and Extubation Readiness Tests (ERT)
In 2001, Farias et al.4 compared spontaneous breathing trials (SBT) using PS of 10 cmH2O versus a T-piece. The use of PS was, in their reasoning, to overcome the resistance of the ETT. The 257 subjects had to tolerate the two hours long trial (either on PS or T-piece) to be considered for extubation. The primary physician could terminate the SBT for objective (e.g. increased RR or SpO2 < 90%) or subjective signs (e.g. diaphoresis or increased respiratory work) of poor tolerance. There were no differences in the rate of extubation failure within 48 hours (15.1% vs 12.8%) or SBT failure (20.8% vs 22.7%). The study concluded that an SBT conducted on PS of 10 cmH2O was as effective as an SBT utilizing a T-piece. In 2002, the same authors60 studied 418 patients intubated for at least 48 hours utilizing a SBT for 2 hours by either T-piece or PS of 10 cmH2O. Of the 323 patients (77%) who passed the SBT and were extubated, 14% were re-intubated within 48 hours. Respiratory rate, tidal volume, RSBI, and maximal negative inspiratory pressure (PImax) were all poor predictors of extubation outcome. In both studies patients underwent a SBT only when the primary physician deemed them ready and this may not have been the earliest point at which a SBT could have been performed. In adults, Esteban found that two-thirds of patients passed an SBT before weaning had even begun3. If the SBT had been performed earlier in the Farias study there might have been an increase in the SBT failure rate in the T-piece group when compared to the PS group.
Chavez et al.64, in 2006, used a 15-minute SBT to determine extubation readiness in pediatric patients. The SBT was performed when the attending intensivist deemed the patient ready for extubation and consisted of providing a continuous flow rate (3 L/min for infants and 10 L/min for older children) via an anesthesia bag adjusted to provide a CPAP of 5 cmH2O. Of the 70 patients, 64 (91%) passed and, of those, 5 (7.8%) subsequently failed extubation (1 reintubation, 4 required non-invasive ventilation). The failed extubation rate was no better than historical rates where extubation was based on clinical decision alone. Although the SBT had high sensitivity (95%) and positive predictive value (92%), the high success rate could have been simply because all the patients enrolled in the study were deemed ready for extubation by the clinicians. In essence, the SBT did not contribute to predicting a successful extubation compared to clinical decision alone.
El-Khatib and colleagues65 evaluated the accuracy of the initial negative inspiratory pressure (PI) to PImax ratio in predicting extubation outcome for 50 infants and children. They concluded that the PI / PImax ratio could not be used to predict extubation outcome in pediatric patients, and further stated that indices predicting extubation outcome in adults should not be extrapolated to infants and children before testing and validation.
Venkataraman and co-workers59 prospectively evaluated predictors of successful extubation in infants and children. In 1996 they examined 208 infants and children on MV for at least 24 hours. They excluded premature infants and those with neuromuscular disease and defined extubation failure as re-intubation within 48 hours. Factors associated with extubation failure included: decreased tidal volume to inspiratory time (VT/Ti), signifying a decreased central drive possibly related to sedation; decreased spontaneous VT, signifying a decreased effort of breathing; and a higher PIP associated with a low dynamic compliance, signifying an increased load on the respiratory muscles. Additional parameters included higher FIO2, mean airway pressure, oxygenation index (OI= Mean Airway Pressure × FiO2/PaO2), and fraction of the total minute ventilation provided by the ventilator (FrVe). The overall extubation failure rate was 16.3%. Dividing patients into low-risk (< 10%) and high-risk groups (>25%), the FrVe for the low-risk group was < 20% and was >30% for the high-risk group. The RSBI and CROP index were not good predictors of extubation outcome in this study. Venkataraman et al32 validated their earlier study with 312 patients who had a similar extubation failure rate of 16%.
There has been only one prospective study evaluating weaning protocols and an ERT19. In 2002, Randolph et al examined the effect of weaning protocols on extubation outcome in 313 patients intubated for at least 24 hours from 10 PICUs. Of 313 subjects, 183 (58%) failed an initial ERT consisting of a two hour SBT on PEEP of 5 cmH2O and FIO2 ≤ 0.5. Failure was defined as a SpO2 < 95%, an exhaled tidal volume < 5 mL/kg ideal body weight, or a respiratory rate outside the normal range for age. Patients passing the initial ERT were switched to PS and the PS adjusted for ETT size (ETT size 3.0–3.5 = PS of 10 cmH2O; ETT size 4.0–4.5 = PS of 8 cmH2O; ETT size ≥ 5.0 = PS of 6 cmH2O). Patients who failed were randomized to three groups for subsequent weaning: PS, VS, and no protocol. There were no significant differences in the extubation failure rate or duration of weaning among the three groups. Increased sedative use in the first 24 hours of weaning predicted failure. This study was important for several reasons. Although it defined an ERT, it failed to consider previously described predictors of extubation success. Similar to other studies looking at predictors of extubation success, the failed extubation rate was in the 14–20% range – significantly higher than the range found with clinical determination of extubation readiness (2–9%)7;57;58. The protocol also used high amounts of PS, ostensibly to overcome the resistance imposed by the ETT. This likely amounted to continuing mechanical ventilation and may have lead to an overestimation of readiness for extubation.
Criteria for Readiness for Extubation
Readiness for extubation implies that weaning is completed, the patient is sufficiently awake with intact airway reflexes, is hemodynamically stable, and has manageable secretions. Extubation failure has been variably defined as re-intubation within 24–72 hours. Clinical and laboratory signs predictive of extubation failure are given in Table 3. Tests commonly employed to assess extubation readiness include testing for a leak around the ETT (“leak test”) and assessing respiratory muscle strength by measuring negative inspiratory force (NIF). In some cases it may be desirable to extubate a patient who has not completed weaning, and subsequently support them with noninvasive ventilation.
Table 3. Criteria for Extubation Readiness Test Failure.
Proposed Criteria for Failure During 2 Hours on CPAP < 5 cmH2O or T-piece
| Clinical Criteria: |
|
| Laboratory Criteria: |
|
Leak Test
UAO has been stated as a cause of up to 37% of failed extubations in children7. UAO is infrequently reported as a cause of extubation failure in adults but a recent investigation suggests that it may be as common in adults as in children66. Cuffed ETTs have been cited as a cause of increased UAO on extubation but Newth et al.67 found no difference in the incidence of failed extubations over all age groups between those intubated with appropriately sized cuffed or uncuffed tubes.
The “leak test”68–71,whereby air is heard to leak around the ETT at low pressure, usually less than 20–25 cmH2O, is commonly employed to predict UAO after extubation. Finholt et al72 showed the leak test was reproducible only under conditions of neuromuscular blockade with the head in a neutral position – hardly the condition for imminent extubation of a child. In a survey of Pediatric Critical Care Fellowship Directors in North America 76% of the responders taught and performed the leak test and would recommend delay of extubation (and prescription of steroids) if there was no leak under 30 cmH2O70. Steroids are a confounding factor in that they appear to reduce stridor, but their effect on reducing extubation failure is controversial25;73. In a recent study, Mhanna et al74 demonstrated that a leak test of less than 20 mmHg (27.2 cmH2O) was better at predicting stridor in children older than 7 years of age than those younger, but in neither case had very good sensitivity. In a prospective, blinded study of 50 pediatric patients, Wratney et al analyzed the change in airway leak as measured at the time of intubation and extubation as a predictor of extubation outcome. They found that measuring the leak serially over time was a better predictor of extubation success than of extubation failure75.
From these data, one can conclude that if an audible leak (to the ear, not the stethoscope) can be heard at < 25 cmH2O in a patient with the head in neutral position this is probably good news. However, extubation should not be delayed if the test is negative and all other conditions for extubation are favorable. Many patients, particularly those with numerous secretions and/or prolonged intubated, will have a “seal” around their ETT which will be coughed out once the ETT is removed.
Negative Inspiratory Force
Common procedures used to evaluate the respiratory muscles are maximum inspiratory pressure (MIP) and maximum expiratory pressure (MEP). However, there is little consensus concerning these methods. Measurement of MIP is the most clinically relevant because the inspiratory muscles (the major one of which is the diaphragm) carry the largest burden of ventilatory work, even when the patient’s primary problem is airflow obstruction. The measurement of MEP is also useful, however, for differentiating generalized neuromuscular weakness from specific weakness of the diaphragm or other inspiratory muscles. In the PICU, MIP is sometimes referred to as negative inspiratory force (NIF). This is inappropriate in that it removes the essential elements of “maximal” and “low lung volume” from the intent of the procedure. True maximal NIF can be produced only when the subject inspires from residual volume – a condition rarely met in intubated patients. NIF of at least −30 cmH2O has been found predictive of extubation success in adults76;77 and also in children as part of the CROP index18;31 or as a stand-alone test under rigorous conditions with CO2 stimulation78. However, Venkataraman et al59 did not find this test useful for prediction as part of the CROP index or as a single test. In the PICU, the test is usually performed quickly at the bedside with an uncalibrated manometer and with both inspiration and exhalation obstructed, i.e. an unvalidated technique.
From these data, one can conclude that it is probably reassuring if a spontaneously breathing patient has a routinely obtained NIF of at least −30 cmH2O but it is unreliable and not validated in children as a test of extubation readiness. Although the measurement of a true MIP in critically ill patients should be a useful indicator of global respiratory muscle function, it is highly dependent on numerous variables and there is no accepted, standardized approach. Consistently low values (i.e. above −15 cmH2O), irrespective of technique, are unlikely to be associated with successful weaning.
MISPERCEPTIONS ABOUT THE IMPACT OF ENDOTRACHEAL TUBES ON WEANING AND SPONTANEOUS BREATHING TRIALS
Many clinicians believe that, for an infant or young child, respiring through a small ETT is akin to breathing through a straw thereby imposing an unacceptable work of breathing. This notion is contrary to both clinical observation and physiology. Data from Keidan and colleagues79 show the work of breathing through an ETT (without PEEP) to be half the effort required for the mask and oropharyngeal airway. They also found that breathing spontaneously with a face mask in place was even more work when there was no oropharyngeal airway79. A 3 kg infant accepts a 3.0 mm ID ETT whereas an adult of 60 kg can tolerate a 9.0 mmID ETT -- a 20 times increase in body size but only a 3 times increase in ETT size. The subglottic area of the infant is also 20 times greater in proportion to body size than that of an adult80. Nonetheless, the inverse 4th power relationship of airflow resistance to radius dictates that the infant ETT has a much higher “resting” resistance, but it is irrelevant because of the shorter ETT and low flows generated by the infant compared to the adult (vide infra). The net effect is that the infant is breathing through a hose rather than a straw when compared to the adult.
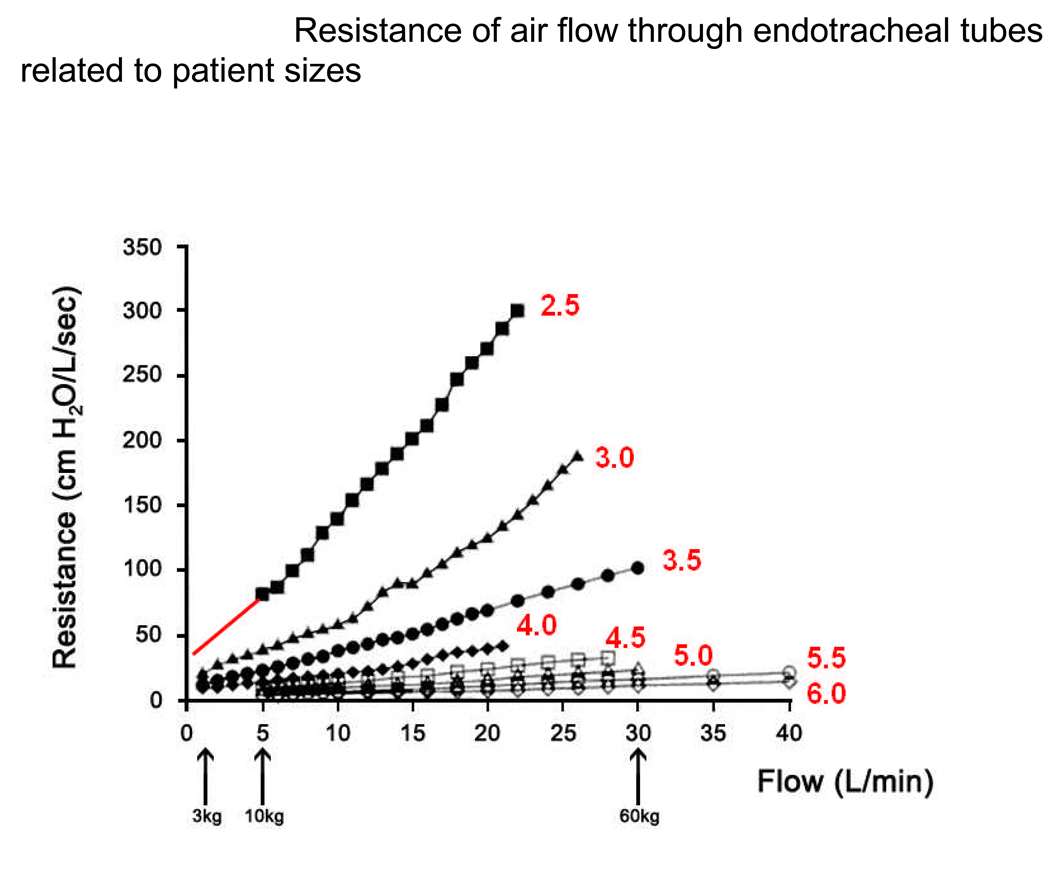
The main determinants of ETT resistance are internal diameter and length. However, the resistance of a tube to airflow must incorporate the notion of resistance at a flow that is physiologically relevant. This is sometimes ignored in clinically relevant papers81. Peak and mid-inspiratory flows in humans are approximately 0.5 L/kg/min30. When related to a 60 kg adult, this gives flows of about 30 L/min with a resistance of 10 cmH2O/L/sec in even a 6.5 mmID ETT (Figure 4). By comparison, a 3 kg infant breathing through a 3.0 mmID ETT has inspiratory flows of about 1.5 L/min and a resistance of 15–20 cmH2O/L/sec, almost double that of the adult but clinically and physiologically irrelevant when considering the inspiratory resistance in the normal infant is already 80 – 90 cmH2O/L/sec30.
Figure 4.
The resistance of air flow through an endotracheal tube, modified from the work of Manczur et al81. The various endotracheal sizes are identified by red lettering, representing the internal diameters of the tubes in millimeters. As flow rates (in liters/minute) are increased there is an increase in resistance, which becomes greater the smaller the internal diameter of the ETT. Thus, small diameter (i.e. pediatric) ETTS have become associated with high airflow resistances and the notion of “breathing through a straw” was (wrongly) conceived.
Peak inspiratory flow rates are approximately 0.5 L/kg ideal body weight/min at all ages in humans. On the abscissa, the 3 arrows (from right) represent the peak flows for a 60 kg adult (intubated with a 6.5 – 8.5 mm ID ETT), a 10 kg child (using a 4 – 4.5 mm ID ETT) and a newborn infant.of 3 kg (with a 3 mm ID ETT), respectively. All resistances generated at these flows are well within normal limits and are, in fact, all less than 50 cmH2O/L/s). Note, for simplicity of interpretation, the linear data for the 2.5 mm ID ETT have been extended into the lower flow range where it had not been measured by the authors of the original paper. (Modified from Manczur et al. with permission)
The notion that resistance increases in smaller ETTs because of conversion from laminar to turbulent flow was investigated by Jarreau and colleagues in 199982. They found that flow in smaller ETTs of 2.5 – 3.5 mmID was laminar, not turbulent. Flow limitation in ETTs was studied by Hammer and Newth in Rhesus monkeys about the size of human infants83. They showed that even in the smallest ETT studied (3.0 mmID), limitation of flow occurred only at about 400 mL/s over most of vital capacity. This is the equivalent of 24 L/min or 8 L/kg/min in a 3 kg infant – well above the 1.5 L/min peak and mid-inspiratory flows normally achieved by infants of this size (vide infra).
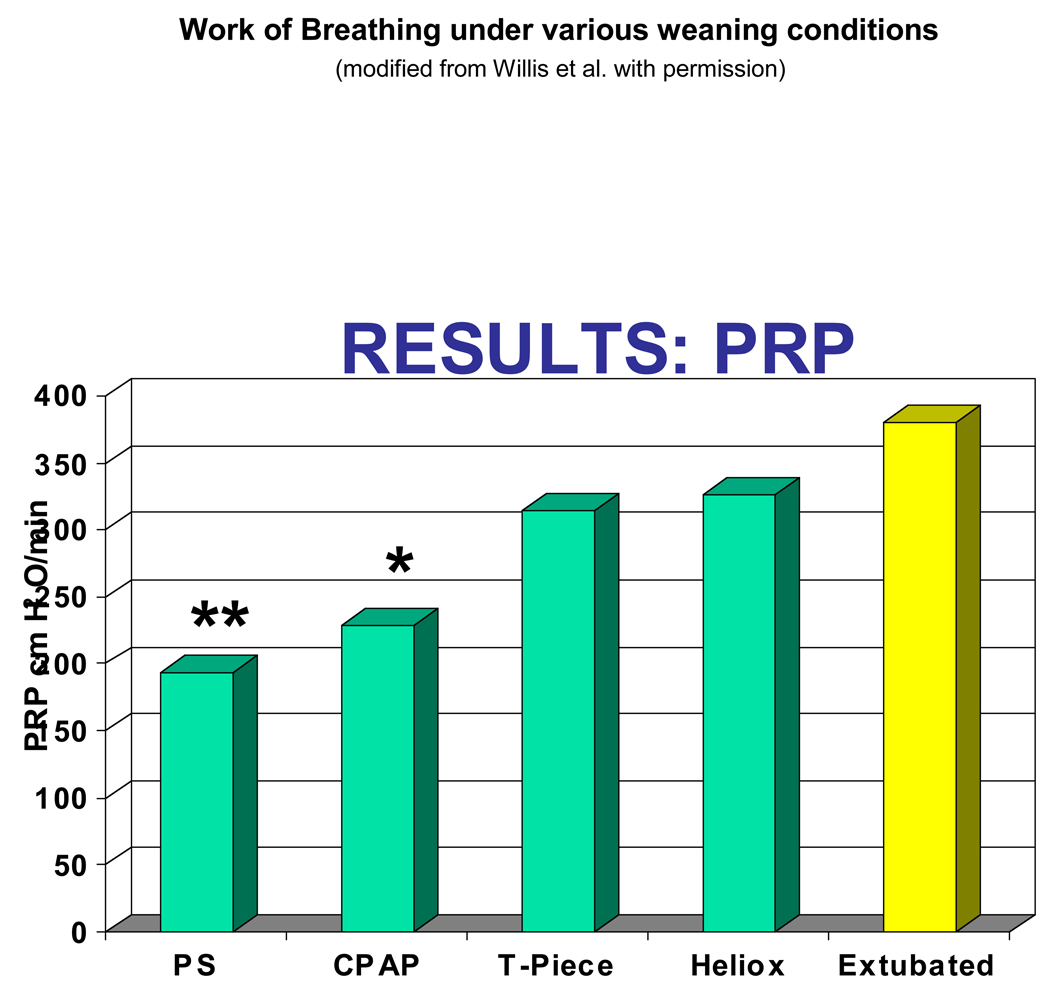
Willis et al84 quantified the work of breathing (as measured by a surrogate, the pressure-rate product) of 22 patients. They found no difference between CPAP and PS of 5 cmH2O. Both provided a decreased work of breathing than either T-piece (with or without heliox) or the extubated patient (Figure 5). Patients on T-piece had less work of breathing than when extubated. Takeuchi et al85 showed that the work of breathing thru an ETT for infants was only marginally higher than that after extubation. They also showed that 4 cmH2O PS was more than enough to offset the marginal increases in work of breathing through a 3.5–4.5 mmID ETT and was equivalent to breathing without the ETT85. Farias et al, in a series of studies involving 634 infants and children4;60;63, demonstrated that for an ERT a trial of spontaneous breathing lasting up to 2 hours could be safely undertaken on a T-piece.
Figure 5.
The Work of Breathing (as approximated using pressure-rate product by Willis et al.84) measured in 17 infants and young children under the various randomly applied conditions (from left to right) of: Pressure supported breaths (5cm H2O), continuous positive airway pressure (4 cm H2O), T-piece with oxygen, T-piece with Heliox, and postextubation. As demonstrated, the work of breathing rose slightly for each condition, but always remained low and was statistically significantly lower only for breaths supported with pressure support and CPAP. (Modified from Willis et al. with permission)
While it has become fashionable to use PS with PEEP rather than CPAP or T-piece breathing to overcome ETT resistance, it is clear the evidence shows the increased resistance is minimal and the additional work of breathing negligible. If an infant or young child cannot sustain a SBT on CPAP or a T-piece for several hours, they are as likely to fail extubation as with PS applied. Additionally, adding PS is likely to mask respiratory insufficiency and contribute to a higher failed extubation rate.
FUTURE RESEARCH IN PEDIATRIC WEANING AND EXTUBATION
Despite better understanding of how to avoid lung injury with positive pressure ventilation, the goal remains to minimize time on mechanical ventilation. Optimized weaning strategies and tools to predict extubation readiness are means to that goal. Several areas of investigation are suggested:
Extubation predictors in the PICU In order to promote research in weaning, SBTs and ERTs, a score could be devised similar to the PRISM III for predicted mortality but that would give case-mix adjusted extubation failure rates. Failed planned extubation (or, conversely, successful planned extubation) predictors could be developed as quality indicators for ventilation outcomes. Once case-mix adjusted extubation failure rates were validated, if there prove to be differences among PICUs (as preliminary data suggests), then practice patterns could be investigated to identify those that are successful and those that are not.
Spontaneous Breathing Trials Given that even a CPAP trial gives more support to a weaning infant than he or she needs if ready for extubation, SBTs utilizing T-piece trials versus CPAP with and without “minimal PS” should be rigorously undertaken. Such trials should comply with the canons of diagnostic test evaluation grounded on Bayes’ Theorem (conditional independence, pre-test probability, test-referral bias and spectrum bias)28;86.
Postextubation Incidence of Acute Upper Airway Obstruction The incidence of post-extubation UAO as a cause of extubation failure seems to be very high, approximately 40% of cases in the Kurachek study7. Thus, it is appropriate to determine the true incidence of UAO in pediatric practice following extubation using objective measuring methods such as esophageal flow-pressure loops for evidence of flow-limitation utilizing noninvasive respiratory inductance technology for the flow component. This would allow not only further evaluation of case-mix adjusted extubation failure rates (vide supra), but also a firm, objective basis for the application of innovative therapies for reducing subglottic edema such as continuous vaporization of alpha-agonists and the respective roles of heliox and CPAP in lessening intrinsic PEEP.
The Role of Corticosteroids in Extubation Success or Failure Steroids are widely used but their role in extubation is ill-understood (vide infra).
SUMMARY OF RELEVANT PEDIATRIC AND ADULT PATIENT STUDIES
Mechanical ventilation is often life saving but is associated with risks. Risks can be reduced by weaning and extubation as soon as the patient is able to support his/her breathing. Key points include
Not all patients require gradual weaning. Both adult35;49 and pediatric19;60 studies have shown that when patients pass a SBT and are subjected to an ERT, 50–75% of the patients are deemed ready to extubate.
There are no infallible predictive tests for successful extubation. The RSBI has become moderately popular but since there is a wide range of age groups with different respiratory rates it may not be a good predictor of extubation success or failure in the pediatric population33. Whether age-specific f/VT ratio is better is currently unknown. This area is fertile ground for future research. Identifying predictors of successful weaning and extubation would likely shorten the duration of ventilation and prove not only to decrease lengths of stay, but potentially reduce ventilator associated lung injury.
Adult studies show that T-piece or PS trials for an ERT are equally effective; IMV or SIMV are not deemed as useful. Pediatric studies have led to similar conclusions 4;60;63.
Use of a weaning protocol results in faster weaning in adults. While the data are less clear in children, it is likely a consistent approach to ventilator weaning will shorten ventilator time and result in better outcomes
A recent Cochrane Review on the role of steroids concludes “Using corticosteroids to prevent (or treat) stridor after extubation has not proven effective for neonates, children or adults. However, given the consistent trend towards benefit, this intervention does merit further study”.{Markovitz, 2008 4207 /id}
Acknowledgements
The authors are grateful for the editorial assistance of Drs. Kang and Khemani (Children’s Hospital Los Angeles, University of Southern California), Dr. Brigham Willis (St. Joseph’s Hospital, Phoenix) and Dr. Neal Patel (Monroe Carell Children’s Hospital, Vanderbilt University).
This work was supported by cooperative agreements from the National Institute of Child Health and Human Development and the Department of Health and Human Services (U10HD050096, U10HD049981, U10HD500009, U10HD049945, U10HD049983, U10HD050012 and U01HD049934).
Bibliography
- 1.Khemani RG, Markovitz BP, Curley MAQ. Epidemiologic factors of mechanically ventilated PICU patients in the United States. Pediatr Crit Care Med. 2007;8:A39. [Google Scholar]
- 2.Curley MA, Hibberd PL, Fineman LD, et al. Effect of prone positioning on clinical outcomes in children with acute lung injury: a randomized controlled trial. JAMA. 2005;294(2):229–237. doi: 10.1001/jama.294.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Esteban A, Alia I, Gordo F, et al. The Spanish Lung Failure Collaborative Group. Extubation outcome after spontaneous breathing trials with T-tube or pressure support ventilation. Am J Respir Crit Care Med. 1997;156(2 Pt 1):459–465. doi: 10.1164/ajrccm.156.2.9610109. [DOI] [PubMed] [Google Scholar]
- 4.Farias JA, Retta A, Alia I, et al. A comparison of two methods to perform a breathing trial before extubation in pediatric intensive care patients. Intensive Care Med. 2001;27(10):1649–1654. doi: 10.1007/s001340101035. [DOI] [PubMed] [Google Scholar]
- 5.Epstein SK, Ciubotaru RL, Wong JB. Effect of failed extubation on the outcome of mechanical ventilation. Chest. 1997;112(1):186–192. doi: 10.1378/chest.112.1.186. [DOI] [PubMed] [Google Scholar]
- 6.Epstein SK, Nevins ML, Chung J. Effect of unplanned extubation on outcome of mechanical ventilation. Am J Respir Crit Care Med. 2000;161(6):1912–1916. doi: 10.1164/ajrccm.161.6.9908068. [DOI] [PubMed] [Google Scholar]
- 7.Kurachek SC, Newth CJ, Quasney MW, et al. Extubation failure in pediatric intensive care: A multiple-center study of risk factors and outcomes. Crit Care Med. 2003;31(11):2657–2664. doi: 10.1097/01.CCM.0000094228.90557.85. [DOI] [PubMed] [Google Scholar]
- 8.Little LA, Koenig JC, Jr, Newth CJ. Factors affecting accidental extubations in neonatal and pediatric intensive care patients. Crit Care Med. 1990;18:163–165. doi: 10.1097/00003246-199002000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Wiedemann HP, Wheeler AP, Bernard GR, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354(24):2564–2575. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 10.Ware LB, Matthay MA. Alveolar fluid clearance is impaired in the majority of patients with acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;163(6):1376–1383. doi: 10.1164/ajrccm.163.6.2004035. [DOI] [PubMed] [Google Scholar]
- 11.Randolph AG, Forbes PW, Gedeit RG, et al. Cumulative fluid intake minus output is not associated with ventilator weaning duration or extubation outcomes in children. Pediatr Crit Care Med. 2005;6(6):642–647. doi: 10.1097/01.pcc.0000185484.14423.0d. [DOI] [PubMed] [Google Scholar]
- 12.Foland JA, Fortenberry JD, Warshaw BL, et al. Fluid overload before continuous hemofiltration and survival in critically ill children: a retrospective analysis. Crit Care Med. 2004;32(8):1771–1776. doi: 10.1097/01.ccm.0000132897.52737.49. [DOI] [PubMed] [Google Scholar]
- 13.Goldstein SL, Somers MJ, Baum MA, et al. Pediatric patients with multi-organ dysfunction syndrome receiving continuous renal replacement therapy. Kidney Int. 2005;67(2):653–658. doi: 10.1111/j.1523-1755.2005.67121.x. [DOI] [PubMed] [Google Scholar]
- 14.Swaniker F, Kolla S, Moler F, et al. Extracorporeal life support outcome for 128 pediatric patients with respiratory failure. J Pediatr Surg. 2000;35(2):197–202. doi: 10.1016/s0022-3468(00)90009-5. [DOI] [PubMed] [Google Scholar]
- 15.Pepe PE, Hudson LD, Carrico CJ. Early application of positive end-expiratory pressure in patients at risk for the adult respiratory distress syndrome. N Engl J Med. 1984;311:281–286. doi: 10.1056/NEJM198408023110502. [DOI] [PubMed] [Google Scholar]
- 16.Brower RG, Lanken PN, MacIntyre N, et al. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med. 2004;351(4):327–336. doi: 10.1056/NEJMoa032193. [DOI] [PubMed] [Google Scholar]
- 17.Alexander E, Carnevale FA, Razack S. Evaluation of a sedation protocol for intubated critically ill children. Intensive Crit Care Nurs. 2002;18(5):292–301. doi: 10.1016/s0964339702000502. [DOI] [PubMed] [Google Scholar]
- 18.Baumeister BL, el-Khatib M, Smith PG, et al. Evaluation of predictors of weaning from mechanical ventilation in pediatric patients. Pediatr Pulmonol. 1997;24:344–352. doi: 10.1002/(sici)1099-0496(199711)24:5<344::aid-ppul7>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 19.Randolph AG, Wypij D, Venkataraman ST, et al. Effect of mechanical ventilator weaning protocols on respiratory outcomes in infants and children: a randomized controlled trial. JAMA. 2002;288(20):2561–2568. doi: 10.1001/jama.288.20.2561. [DOI] [PubMed] [Google Scholar]
- 20.Curley MA, Harris SK, Fraser KA, et al. State Behavioral Scale: a sedation assessment instrument for infants and young children supported on mechanical ventilation. Pediatr Crit Care Med. 2006;7(2):107–114. doi: 10.1097/01.PCC.0000200955.40962.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davis S, Worley S, Mee RB, et al. Factors associated with early extubation after cardiac surgery in young children. Pediatr Crit Care Med. 2004;5(1):63–68. doi: 10.1097/01.PCC.0000102386.96434.46. [DOI] [PubMed] [Google Scholar]
- 22.Harrison AM, Cox AC, Davis S, et al. Failed extubation after cardiac surgery in young children: Prevalence, pathogenesis, and risk factors. Pediatr Crit Care Med. 2002;3(2):148–152. doi: 10.1097/00130478-200204000-00011. [DOI] [PubMed] [Google Scholar]
- 23.Honma Y, Wilkes D, Bryan MH, et al. Rib cage and abdominal contributions to ventilatory response to CO2 in infants. J Appl Physiol. 1984;56:1211–1216. doi: 10.1152/jappl.1984.56.5.1211. [DOI] [PubMed] [Google Scholar]
- 24.Anene O, Meert KL, Uy H, et al. Dexamethasone for the prevention of postextubation airway obstruction: a prospective, randomized, double-blind, placebo- controlled trial [see comments] Crit Care Med. 1996;24(10):1666–1669. doi: 10.1097/00003246-199610000-00011. [DOI] [PubMed] [Google Scholar]
- 25.Tellez DW, Galvis AG, Storgion SA, et al. Dexamethasone in the prevention of postextubation stridor in children. J Pediatr. 1991;118:289–294. doi: 10.1016/s0022-3476(05)80505-0. [DOI] [PubMed] [Google Scholar]
- 26.Hammer J, Newth CJL. Respiratory Syncytial Virus Infection in Children. In: Vincent J-L, editor. 1997 Yearbook of Intensive Care and Emergency Medicine. Springer: Heidelberg; 1997. pp. 625–637. [Google Scholar]
- 27.Yang KL, Tobin MJ. A prospective study of indexes predicting the outcome of trials of weaning from mechanical ventilation. N Engl J Med. 1991;324:1445–1450. doi: 10.1056/NEJM199105233242101. [DOI] [PubMed] [Google Scholar]
- 28.Tobin MJ, Jubran A. Variable performance of weaning-predictor tests: role of Bayes' theorem and spectrum and test-referral bias. Intensive Care Med. 2006 doi: 10.1007/s00134-006-0439-4. [DOI] [PubMed] [Google Scholar]
- 29.Jubran A, Grant BJ, Laghi F, et al. Weaning prediction: esophageal pressure monitoring complements readiness testing. Am J Respir Crit Care Med. 2005;171(11):1252–1259. doi: 10.1164/rccm.200503-356OC. [DOI] [PubMed] [Google Scholar]
- 30.Argent AC, Newth CJ, Klein M. The mechanics of breathing in children with acute severe croup. Intensive Care Med. 2008;34(2):324–332. doi: 10.1007/s00134-007-0910-x. [DOI] [PubMed] [Google Scholar]
- 31.Thiagarajan RR, Bratton SL, Martin LD, et al. Predictors of successful extubation in children. Am J Respir Crit Care Med. 1999;160(5 Pt 1):1562–1566. doi: 10.1164/ajrccm.160.5.9810036. [DOI] [PubMed] [Google Scholar]
- 32.Venkataraman ST, Khan N, Brown A. Validation of predictors of extubation success and failure in mechanically ventilated infants and children. Crit Care Med. 2000;28(8):2991–2996. doi: 10.1097/00003246-200008000-00051. [DOI] [PubMed] [Google Scholar]
- 33.Manczur TI, Greenough A, Pryor D, et al. Comparison of predictors of extubation from mechanical ventilation in children. Pediatr Crit Care Med. 2000;1(1):28–32. doi: 10.1097/00130478-200007000-00005. [DOI] [PubMed] [Google Scholar]
- 34.Hubble CL, Gentile MA, Tripp DS, et al. Deadspace to tidal volume ratio predicts successful extubation in infants and children. Crit Care Med. 2000;28(6):2034–2040. doi: 10.1097/00003246-200006000-00059. [DOI] [PubMed] [Google Scholar]
- 35.Brochard L, Rauss A, Benito S, et al. Comparison of three methods of gradual withdrawal from ventilatory support during weaning from mechanical ventilation. Am J Respir Crit Care Med. 1994;150(4):896–903. doi: 10.1164/ajrccm.150.4.7921460. [DOI] [PubMed] [Google Scholar]
- 36.The Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000 May 4;342(18):1301–1308. doi: 10.1056/NEJM200005043421801. 2000; 342:1301-1308. [DOI] [PubMed] [Google Scholar]
- 37.Venkataraman ST. Weaning and extubation in infants and children: religion, art, or science. Pediatr Crit Care Med. 2002;3(2):203–205. doi: 10.1097/00130478-200204000-00026. [DOI] [PubMed] [Google Scholar]
- 38.Morris AH, Wallace CJ, Menlove RL, et al. Randomized clinical trial of pressure-controlled inverse ratio ventilation and extracorporeal CO2 removal for adult respiratory distress syndrome. Am J Respir Crit Care Med. 1994;149:295–305. doi: 10.1164/ajrccm.149.2.8306022. [DOI] [PubMed] [Google Scholar]
- 39.McKinley BA, Moore FA, Sailors RM, et al. Computerized decision support for mechanical ventilation of trauma induced ARDS: results of a randomized clinical trial. J Trauma. 2001;50(3):415–424. doi: 10.1097/00005373-200103000-00004. [DOI] [PubMed] [Google Scholar]
- 40.Fessler HE, Brower RG. Protocols for lung protective ventilation. Crit Care Med. 2005;33 3 Suppl:S223–S227. doi: 10.1097/01.ccm.0000155919.53727.d5. [DOI] [PubMed] [Google Scholar]
- 41.Willson DF, Thomas NJ, Markovitz BP, et al. Effect of exogenous surfactant (calfactant) in pediatric acute lung injury: a randomized controlled trial. JAMA. 2005;293(4):470–476. doi: 10.1001/jama.293.4.470. [DOI] [PubMed] [Google Scholar]
- 42.Jouvet P, Farges C, Hatzakis G, et al. Weaning children from mechanical ventilation with a computer-driven system (closed-loop protocol): A pilot study*. Pediatr Crit Care Med. 2007;8(5):425–432. doi: 10.1097/01.PCC.0000282157.77811.F9. [DOI] [PubMed] [Google Scholar]
- 43.Restrepo RD, Fortenberry JD, Spainhour C, et al. Protocol-driven ventilator management in children: comparison to nonprotocol care. J Intensive Care Med. 2004;19(5):274–284. doi: 10.1177/0885066604267646. [DOI] [PubMed] [Google Scholar]
- 44.Graham AS, Kirby AL. Ventilator management protocols in pediatrics. Respir Care Clin N Am. 2006;12(3):389–402. doi: 10.1016/j.rcc.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 45.Schoenfeld DA, Bernard GR. Statistical evaluation of ventilator-free days as an efficacy measure in clinical trials of treatments for acute respiratory distress syndrome. Crit Care Med. 2002;30(8):1772–1777. doi: 10.1097/00003246-200208000-00016. [DOI] [PubMed] [Google Scholar]
- 46.Schultz TR, Lin RJ, Watzman HM, et al. Weaning children from mechanical ventilation: a prospective randomized trial of protocol-directed versus physician-directed weaning. Respir Care. 2001;46(8):772–782. [PubMed] [Google Scholar]
- 47.Meade M, Guyatt G, Sinuff T, et al. Trials comparing alternative weaning modes and discontinuation assessments. Chest. 2001;120 6 Suppl:425S–437S. doi: 10.1378/chest.120.6_suppl.425s. [DOI] [PubMed] [Google Scholar]
- 48.Esteban A, Alia I, Tobin MJ, et al. Spanish Lung Failure Collaborative Group. Effect of spontaneous breathing trial duration on outcome of attempts to discontinue mechanical ventilation. Am J Respir Crit Care Med. 1999;159(2):512–518. doi: 10.1164/ajrccm.159.2.9803106. [DOI] [PubMed] [Google Scholar]
- 49.Esteban A, Frutos F, Tobin MJ, et al. Spanish Lung Failure Collaborative Group. A comparison of four methods of weaning patients from mechanical ventilation. N Engl J Med. 1995;332:345–350. doi: 10.1056/NEJM199502093320601. [DOI] [PubMed] [Google Scholar]
- 50.Jounieaux V, Duran A, Levi-Valensi P. Synchronized intermittent mandatory ventilation with and without pressure support ventilation in weaning patients with COPD from mechanical ventilation. Chest. 1994;105(4):1204–1210. doi: 10.1378/chest.105.4.1204. [DOI] [PubMed] [Google Scholar]
- 51.Nava S, Ambrosino N, Clini E, et al. Noninvasive mechanical ventilation in the weaning of patients with respiratory failure due to chronic obstructive pulmonary disease. A randomized, controlled trial. Ann Intern Med. 1998;128(9):721–728. doi: 10.7326/0003-4819-128-9-199805010-00004. [DOI] [PubMed] [Google Scholar]
- 52.Girault C, Daudenthun I, Chevron V, et al. Noninvasive ventilation as a systematic extubation and weaning technique in acute-on-chronic respiratory failure: a prospective, randomized controlled study. Am J Respir Crit Care Med. 1999;160(1):86–92. doi: 10.1164/ajrccm.160.1.9802120. [DOI] [PubMed] [Google Scholar]
- 53.Ely EW, Baker AM, Dunagan DP, et al. Effect on the duration of mechanical ventilation of identifying patients capable of breathing spontaneously. N Engl J Med. 1996;335:1864–1869. doi: 10.1056/NEJM199612193352502. [DOI] [PubMed] [Google Scholar]
- 54.Kollef MH, Shapiro SD, Silver P, et al. A randomized, controlled trial of protocol-directed versus physician-directed weaning from mechanical ventilation. Crit Care Med. 1997;25:567–574. doi: 10.1097/00003246-199704000-00004. [DOI] [PubMed] [Google Scholar]
- 55.Strickland JH, Jr, Hasson JH. A computer-controlled ventilator weaning system. A clinical trial. Chest. 1993;103(4):1220–1226. doi: 10.1378/chest.103.4.1220. [DOI] [PubMed] [Google Scholar]
- 56.Marelich GP, Murin S, Battistella F, et al. Protocol weaning of mechanical ventilation in medical and surgical patients by respiratory care practitioners and nurses: effect on weaning time and incidence of ventilator-associated pneumonia. Chest. 2000;118(2):459–467. doi: 10.1378/chest.118.2.459. [DOI] [PubMed] [Google Scholar]
- 57.Baisch SD, Wheeler WB, Kurachek SC, et al. Extubation failure in pediatric intensive care incidence and outcomes. Pediatr Crit Care Med. 2005;6(3):312–318. doi: 10.1097/01.PCC.0000161119.05076.91. [DOI] [PubMed] [Google Scholar]
- 58.Edmunds S, Weiss I, Harrison R. Extubation failure in a large pediatric ICU population. Chest. 2001;119(3):897–900. doi: 10.1378/chest.119.3.897. [DOI] [PubMed] [Google Scholar]
- 59.Khan N, Brown A, Venkataraman ST. Predictors of extubation success and failure in mechanically ventilated infants and children. Crit Care Med. 1996;24:1568–1579. doi: 10.1097/00003246-199609000-00023. [DOI] [PubMed] [Google Scholar]
- 60.Farias JA, Alia I, Retta A, et al. An evaluation of extubation failure predictors in mechanically ventilated infants and children. Intensive Care Med. 2002;28(6):752–757. doi: 10.1007/s00134-002-1306-6. [DOI] [PubMed] [Google Scholar]
- 61.Fontela PS, Piva JP, Garcia PC, et al. Risk factors for extubation failure in mechanically ventilated pediatric patients. Pediatr Crit Care Med. 2005;6(2):166–170. doi: 10.1097/01.PCC.0000154922.65189.48. [DOI] [PubMed] [Google Scholar]
- 62.Hughes MR, Smith CD, Tecklenburg FW, et al. Effects of a weaning protocol on ventilated pediatric intensive care unit (PICU) patients. Top Health Inf Manage. 2001;22(2):35–43. [PubMed] [Google Scholar]
- 63.Farias JA, Alia I, Esteban A, et al. Weaning from mechanical ventilation in pediatric intensive care patients. Intensive Care Med. 1998;24:1070–1075. doi: 10.1007/s001340050718. [DOI] [PubMed] [Google Scholar]
- 64.Chavez A, dela Cruz R, Zaritsky A. Spontaneous breathing trial predicts successful extubation in infants and children. Pediatr Crit Care Med. 2006;7(4):324–328. doi: 10.1097/01.PCC.0000225001.92994.29. [DOI] [PubMed] [Google Scholar]
- 65.el-Khatib MF, Baumeister B, Smith PG, et al. Inspiratory pressure/maximal inspiratory pressure: does it predict successful extubation in critically ill infants and children? Intensive Care Med. 1996;22:264–268. doi: 10.1007/BF01712248. [DOI] [PubMed] [Google Scholar]
- 66.Francois B, Bellissant E, Gissot V, et al. 12-h pretreatment with methylprednisolone versus placebo for prevention of postextubation laryngeal oedema: a randomised double-blind trial. Lancet. 2007;369(9567):1083–1089. doi: 10.1016/S0140-6736(07)60526-1. [DOI] [PubMed] [Google Scholar]
- 67.Newth CJ, Rachman B, Patel N, et al. The use of cuffed versus uncuffed endotracheal tubes in pediatric intensive care. J Pediatr. 2004;144(3):333–337. doi: 10.1016/j.jpeds.2003.12.018. [DOI] [PubMed] [Google Scholar]
- 68.Adderley RJ, Mullins GC. When to extubate the croup patient: the "leak: test. Can J Anaesth. 1987;34:304–306. doi: 10.1007/BF03015171. [DOI] [PubMed] [Google Scholar]
- 69.Pettignano R, Holloway SE, Hyman D, et al. Is the leak test reproducible? South Med J. 2000;93(7):683–685. [PubMed] [Google Scholar]
- 70.Foland JA, Super DM, Dahdah NS, et al. The use of the air leak test and corticosteroids in intubated children: a survey of pediatric critical care fellowship directors. Respir Care. 2002;47(6):662–666. [PubMed] [Google Scholar]
- 71.De BY, De BD, Moraine JJ, et al. The cuff leak test to predict failure of tracheal extubation for laryngeal edema. Intensive Care Med. 2002;28(9):1267–1272. doi: 10.1007/s00134-002-1422-3. [DOI] [PubMed] [Google Scholar]
- 72.Finholt DA, Henry DB, Raphaely RC. Factors affecting leak around tracheal tubes in children. Can Anaesth Soc J. 1985;32(4):326–329. doi: 10.1007/BF03011335. [DOI] [PubMed] [Google Scholar]
- 73.Markovitz BP, Randolph AG. Corticosteroids for the prevention of reintubation and postextubation stridor in pediatric patients: A meta-analysis. Pediatr Crit Care Med. 2002;3(3):223–226. doi: 10.1097/00130478-200207000-00003. [DOI] [PubMed] [Google Scholar]
- 74.Mhanna MJ, Zamel YB, Tichy CM, et al. The "air leak" test around the endotracheal tube, as a predictor of postextubation stridor, is age dependent in children. Crit Care Med. 2002;30(12):2639–2643. doi: 10.1097/00003246-200212000-00005. [DOI] [PubMed] [Google Scholar]
- 75.Wratney AT, Cheifetz IM. Extubation criteria in infants and children. Respir Care Clin N Am. 2006;12(3):469–481. doi: 10.1016/j.rcc.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 76.Sahn SA, Lakshminarayan S, Petty TL. Weaning from mechanical ventilation. JAMA. 1976;235(20):2208–2212. [PubMed] [Google Scholar]
- 77.Truwit JD, Marini JJ. Validation of a technique to assess maximal inspiratory pressure in poorly cooperative patients. Chest. 1992;102(4):1216–1219. doi: 10.1378/chest.102.4.1216. [DOI] [PubMed] [Google Scholar]
- 78.Gozal D, Shoseyov D, Keens TG. Inspiratory pressures with CO2 stimulation and weaning from mechanical ventilation in children. Am Rev Respir Dis. 1993;147:256–261. doi: 10.1164/ajrccm/147.2.256. [DOI] [PubMed] [Google Scholar]
- 79.Keidan I, Fine GF, Kagawa T, et al. Work of breathing during spontaneous ventilation in anesthetized children: a comparative study among the face mask, laryngeal mask airway and endotracheal tube. Anesth Analg. 2000;91(6):1381–1388. doi: 10.1097/00000539-200012000-00014. [DOI] [PubMed] [Google Scholar]
- 80.Eckenhoff JE. Some anatomic considerations of the infant larynx influencing endotracheal anesthesia. Anesthesiology. 1951;12:401–410. doi: 10.1097/00000542-195107000-00001. [DOI] [PubMed] [Google Scholar]
- 81.Manczur T, Greenough A, Nicholson GP, et al. Resistance of pediatric and neonatal endotracheal tubes: influence of flow rate, size, and shape. Crit Care Med. 2000;28(5):1595–1598. doi: 10.1097/00003246-200005000-00056. [DOI] [PubMed] [Google Scholar]
- 82.Jarreau PH, Louis B, Dassieu G, et al. Estimation of inspiratory pressure drop in neonatal and pediatric endotracheal tubes. J Appl Physiol. 1999;87(1):36–46. doi: 10.1152/jappl.1999.87.1.36. [DOI] [PubMed] [Google Scholar]
- 83.Hammer J, Newth CJ. Influence of endotracheal tube diameter on forced deflation flow-volume curves in rhesus monkeys. Eur Respir J. 1997;10:1870–1873. doi: 10.1183/09031936.97.10081870. [DOI] [PubMed] [Google Scholar]
- 84.Willis BC, Graham AS, Yoon E, et al. Pressure-rate products and phase angles in children on minimal support ventilation and after extubation. Intensive Care Med. 2005;31(12):1700–1705. doi: 10.1007/s00134-005-2821-z. [DOI] [PubMed] [Google Scholar]
- 85.Takeuchi M, Imanaka H, Miyano H, et al. Effect of patient-triggered ventilation on respiratory workload in infants after cardiac surgery. Anesthesiology. 2000 Nov;93(5):1238–1244. doi: 10.1097/00000542-200011000-00017. 2001; 93:1238-44. [DOI] [PubMed] [Google Scholar]
- 86.Connors AF., Jr A fresh look at the weaning process. Intensive Care Med. 2006;32(12):1928–1929. doi: 10.1007/s00134-006-0440-y. [DOI] [PubMed] [Google Scholar]