Summary
Th17 cells play key roles in mediating autoimmunity, inflammation and mucosal host defense against pathogens. To determine whether naturally occurring Treg (nTreg) limit Th17-mediated pulmonary inflammation, OVA-specific CD4+ Th17 cells and expanded CD4+CD25+Foxp3+ nTreg were cotransferred into BALB/c mice that were then exposed to OVA aerosols. Th17 cells, when transferred alone, accumulated in the lungs and posterior mediastinal LN and evoked a pronounced airway hyperreactivity (AHR) and neutrophilic inflammation, characterized by B cell recruitment and elevated IgA and IgM levels. Cotransfer of antigen-specific nTreg markedly reduced the Th17-induced pulmonary inflammation and associated neutrophilia, B cell influx and polymeric Ig levels in the airways, but did not inhibit AHR. Moreover, the regulation appeared restricted to the site of mucosal inflammation, since transfer of nTreg did not affect the Th17 response developing in the lung draining LN, as evidenced by unaltered levels of IL-17 production and low numbers of Foxp3+ Treg. Our findings suggest a crucial role for Th17 cells in mediating airway B cell influx and IgA response and demonstrate that antigen-specific nTreg suppress Th17-mediated lung inflammation. These results provide new insights into how Th17 responses are limited and may facilitate development of novel approaches for controlling Th17-induced inflammation.
Keywords: Th17 cells, regulatory T cells, B cells, lung inflammation, regulation
Introduction
Effector CD4+ T cells, typically, are characterized as Th1, Th2 and Th17 on the basis of their cytokine profiles. Th1 cells secrete IFN-γ and are essential for controlling intracellular pathogens, whereas Th2 cells, which produce IL-4, IL-5, and IL-13, clear helminth infections and orchestrate the inflammatory response in asthma. CD4+ Th17 cells, which are characterized by their secretion of a distinctive set of effector cytokines including IL-17, IL-17F, IL-21 and IL-22, mediate autoimmunity and inflammation but also play a crucial role in mucosal host defense against diverse range of pathogens [1, 2]. Th17 cell differentiation in mice requires the inductive cytokines TGF-β and IL-6, but also IL-23 for maturation. Moreover, the differentiation of CD4+Foxp3+ Treg and Th17 cells by TGF-β appear to be reciprocally related, since TGF-β alone promotes expression of the transcription factor Foxp3, whereas the combination of IL-6 and TGF-β suppresses Foxp3 expression and enhances IL-17 production [3]. Other cytokines such as IFN-γ and IL-4 have been suggested to negatively regulate the generation of IL-17-producing Th17 cells [4]. The receptor for IL-17 is ubiquitously expressed in lung, spleen, kidney, and liver, as well as various epithelial cells, isolated fibroblasts and B cells [5]. IL-17 has been implicated in the recruitment of neutrophils and subsequent eradication of extracellular microorganisms [6]. Moreover, IL-17 or IL-17F cooperate with IL-22 to enhance the expression of antimicrobial peptides [7].
Tight regulatory mechanisms are essential for maintenance of immune homeostasis and control of exaggerated inflammatory responses by effector T cells. Foxp3+ Treg can be divided into two major subsets, namely naturally occurring (thymus-derived) CD4+ cells (nTreg) that express CD25, the α chain of IL-2R, and adaptive CD4+CD25+ cells that are induced in vivo from CD25- precursors or ex vivo with IL-2 and TGF-β (iTreg) [8-10]. Although both nTreg and iTreg require IL-2 and TGF-β for their maintenance, the two subsets display different modes of generation and costimulatory requirements [10]. Importantly, the stability of their suppressive function in the presence of IL-6 or IL-4 differs, since nTreg are converted to IL-17 producing (Th17) cells in the presence of IL-6 [11], while the generation of iTreg is inhibited by IL-4 [12].
We have previously demonstrated that in the lung, in addition to eliciting airway recruitment of neutrophils, Th17 cells play a crucial function in mucosal immune defense, by promoting B cell recruitment and enhanced polymeric Ig receptor (pIgR)-mediated transcytosis of polymeric Igs into the airway lumen [13]. In the present study, we examined the effectiveness of nTreg in the regulation of Th17-mediated responses in the lung.
Results and discussion
Foxp3+ nTreg limit Th17-mediated airway neutrophilic inflammation but not AHR
To model Th17-mediated pulmonary inflammation, we used polarized OVA-specific CD4+ Th17 cells that were injected into BALB/c mice and subsequently exposed to aerosolized OVA. Effector Th17 cells were generated from naïve DO11.10 CD4+ T cells by culture with OVA323-339 peptide in the presence of IL-6, TGF-β and IL-23. CD4+ T cells expressing the OVA-specific transgenic TCR can be enumerated using the anti-clonotypic antibody KJ1-26. In keeping with previous reports [1], under Th17-polarizing conditions, high numbers of IL-17-producing cells were generated (33.3% were stained intracellularly for IL-17) which were CD4+KJ1-26+ and CD62L- (Fig. 1A). Moreover, these CD4+ Th17 cells secreted high levels of IL-17 and IL-21, but also significant amounts of IL-22, in response to T cell receptor cross-linking (Supplementary Figure 1A). Interestingly, 8-day polarized CD4+ Th17 effector cells generally comprised of both CD62L+ (<20%) and CD62L- (>80%) subpopulations. The production of IL-17 and IL-21 and subsequent inflammatory potential of Th17 cells was restricted to CD4+CD62L- cells, since only recipients of unsorted or CD4+CD62L- Th17 cells displayed pronounced peribronchial inflammation, with increased neutrophil, lymphocyte and macrophage infiltration into the bronchoalveolar lavage fluid (BAL). Conversely, mice injected with CD4+CD62L+ cells had negligible inflammation in the lung mucosa and BAL (Supplementary Figure 1A, B and C). To examine the ability of OVA-specific CD4+CD25+Foxp3+ nTreg to regulate Th17-mediated lung responses, nTreg were first purified and expanded in culture for 8 days. Typically, >90% of the expanded CD4+ Treg expressed Foxp3 which were KJ1-26+. Moreover, Foxp3 expression was restricted to the Treg population, since polarized Th17 cells did not express Foxp3 (Supplementary Figure 2A and B). The expanded Foxp3+ nTreg were then cotransferred with CD4+CD62L- Th17 cells into BALB/c hosts that were then exposed to OVA aerosols for 7 days. Following OVA inhalation, Th17 cells caused a pronounced peribronchial and perivascular inflammation (Fig. 1B) with a marked infiltration of neutrophils into the airways (Fig. 1C) that was significantly inhibited by antigen-specific nTreg. Control animals or mice injected solely with Treg did not develop any inflammation.
Figure 1.
IL-17-expressing CD4+ Th17 cells mediate a marked airway inflammation to inhaled OVA that is suppressed by antigen-specific Foxp3+ nTreg. DO11.10 CD4+ Th17 cells alone, expanded CD4+Foxp3+ nTreg alone or Th17 cells plus nTreg were adoptively transferred into BALB/c mice followed by exposure to OVA aerosols for 7 days (control mice did not receive cells). (A) Analysis of differentiated DO11.10 Th17 cells by FACS for the coexpression of intracellular IL-17 with CD4, KJ1-126, or CD62L. (B) Histological analysis of lung tissue was performed using H&E staining. (C) BAL cell differential counts were determined by light microscopic evaluation of Hema3-stained cytospin preparations. Results are expressed as absolute cell number of lymphocytes (lym), neutrophils (neu) and macrophages (mac). (D) AHR was determined in response to methacholine inhalation by measurement of RL and Cdyn as described in the Materials and methods. Data show mean ± SEM (n=5-7 separate experiments). *p<0.05, compared to OVA-challenged Th17 group, Mann-Whitney U test. FACS and histology results are representative of four independent experiments.
Interestingly, the onset of the inflammation in Th17 recipients was associated with a marked increase in AHR compared to control mice (none), as measured by airway resistance (RL) and dynamic compliance (Cdyn) (Fig. 1D). However, in contrast to the Th17-mediated pulmonary inflammatory response, which was strongly inhibited by nTreg, Th17-induced airway resistance and dynamic compliance was not regulated by treatment with Treg (Fig. 1D).
In summary, effector Th17 cells, in response to antigen inhalation, caused a significant elevation in the numbers of airway neutrophils, lymphocytes and macrophages, accompanied with an increase in AHR. Antigen-specific Foxp3+ nTreg markedly limited the Th17-induced neutrophilic inflammation taking place in the lung mucosa. The nTreg, however, acted on the inflammatory phase alone since their suppressive properties failed to impact on the level of AHR.
Transfer of Foxp3+ nTreg inhibits Th17 responses in the lung but not draining LN
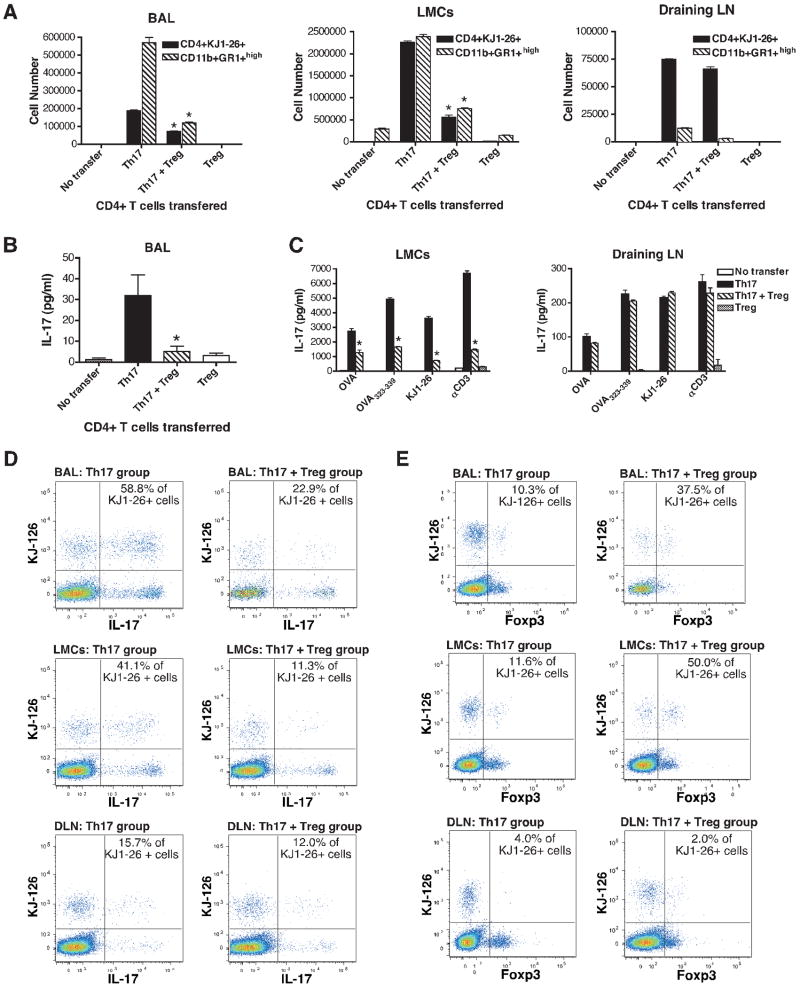
The suppression of Th17-mediated inflammatory processes in the airways following cotransfer of nTreg with Th17 cells was associated with significant reduction in the number of CD4+KJ1-26+ T cells and CD11b+Gr-1+high neutrophils present in the BAL, lung mononuclear cells (LMC) but not lung draining LN (Fig. 2A). Consistently, BAL IL-17 levels and the production of this cytokine by stimulated LMC from Th17 recipient mice were significantly inhibited by treatment with nTreg (Fig. 2B and C). The level of IL-17 production following stimulation of draining LN was low compared to LMC (Fig. 2C).
Figure 2.
Transfer of Foxp3+ nTreg inhibits Th17 responses in the lung mucosa but not draining LN. DO11.10 CD4+ Th17 cells alone, expanded CD4+Foxp3+ nTreg alone or Th17 cells plus nTreg were adoptively transferred into BALB/c mice followed by exposure to OVA aerosols for 7 days (control mice did not receive cells). (A) Number of CD4+KJ-126 T cells and Gr-1+ neutrophils in the BAL, LMC and draining LN cells from recipients of Th17 cells alone, Th17 plus nTreg, nTreg alone or control mice (no transfer) was analyzed by FACS, and the results expressed as total cell number per mouse. (B) IL-17 levels in the BAL were determined by ELISA. (C) IL-17 production by LMC or draining LN cells was measured by ELISA after restimulation with OVA protein (1 mg/ml), OVA323-339 peptide (1 μg/ml), KJ1-26 antibody (5 μg/ml) or immobilized anti-CD3 (5 μg/ml) for 24 h. FACS analysis of IL-17 (D) or Foxp3 (E) expression by intracellular staining and their coexpression with KJ1-26 in BAL cells and cells isolated from lungs (LMC) and draining LN (DLN). Data show mean ± SEM (n=4-8 separate experiments). *p<0.05, compared to OVA-challenged Th17 group, Mann-Whitney U test. FACS results are representative of three independent experiments.
In mice that received both CD4+ Th17 and nTreg, it was important to discriminate between the different cell types expressing KJ1-26. To facilitate this, cells isolated from lungs, draining LN and BAL were analyzed for IL-17 and Foxp3 expression by intracellular staining. Intracellular staining of the CD4+ Th17 cells prior to injection revealed that a high proportion stained for IL-17 (Fig.1A) but not Foxp3 expression (Supplementary Figure 2B). Following transfer of the cells into BALB/c hosts and OVA aerosol challenge, IL-17 expressing KJ1-26+ cells were present in the BAL, LMC and, to a lesser extent, draining LN from Th17 recipients, which ranged from 15.7 to 58.8% (Fig. 2D). In marked contrast, the proportion of KJ1-26+ cells expressing IFN-γ was negligible (3.6%, 1.62% and 1.75% of KJ1-26+ cells in BAL, LMC and draining LN, respectively) suggesting that the OVA-specific T cells retained their Th17 phenotype in vivo. The cotransfer of nTreg with Th17 cells markedly reduced the proportion of IL-17 expressing KJ1-26+ cells present in BAL and LMC (Fig. 2D). However, no effect was observed in the draining LN, suggesting that the transferred nTreg either failed to suppress or were not present in this location. Interestingly, the proportion of KJ1-26+Foxp3+ cells in the BAL and LMC from mice cotransferred with Th17 cells plus nTreg ranged from 37.5 to 50.0% demonstrating that OVA-specific nTreg were recruited to the lungs and airways (Fig. 2E). This was markedly different from the 2.0% of KJ1-26+ cells that coexpressed Foxp3 evident in the draining LN, implying that low numbers of the transferred Foxp3+ nTreg entered this site.
In summary, OVA-specific IL-17-producing Th17 cells migrated to the airway, lung tissue and, to a lesser extent, posterior mediastinal draining LN in response to antigen inhalation and elicited marked pulmonary inflammation. Transfer of Foxp3+ nTreg markedly suppressed the Th17-induced airway inflammation and IL-17 production in the lung. However, the Th17 response developing in the draining LN was unaffected, as evidenced by unaltered levels of IL-17 and low numbers of Foxp3+ Treg migrating to this site.
Foxp3+ nTreg suppress airway Th17-mediated B cell influx and polymeric Ig response
We have observed two striking aspects of the Th17-mediated airway inflammation are evident that distinguished it from other types of inflammation. Firstly, there was enhanced expression pIgR by the airway epithelium that was associated with a pronounced increase in the level of secretory IgA (SIgA) and IgM in the BAL [13]. Secondly, the onset of lung mucosal Th17- but not Th1- or Th2-mediated inflammation was associated with the recruitment of CD19+CD5-Class II+ B cells which were present in large numbers in the BAL and most evident after 7 days of OVA challenge (Fig. 3A). It is likely that the infiltrating CD19+ cells are either B2 cells or B1b, since they did not express CD5. Histological analysis revealed a marked peribronchial CD19+ B cell infiltration in the Th17 recipients, with formation of B cell aggregates (Fig. 3B). Interestingly, the cotransfer of antigen-specific CD4+Foxp3+ nTreg with Th17 cells caused a marked reduction in the Th17-mediated recruitment of CD19+ B cells into the BAL and lung mucosa (Fig. 3C). Moreover, the suppression mediated by Treg was accompanied with a significant decrease in the level of IgA, IgM but not IgG1, IgG2a (Fig. 3D), IgG2b and IgG3 present in the BAL (data omitted for clarity). More importantly, there was a marked reduction in the level of SIgA in the BAL (Fig. 3D). The transfer of nTreg alone did not affect the level of airway SIgA or IgM. It is likely that the inhibition of B cell recruitment and levels of polymeric Igs in the airways reflected the reduced Th17 response rather than Treg directly acting on the B cells.
Figure 3.
Antigen-specific Foxp3+ nTreg suppress airway B cell accumulation and polymeric Ig response. DO11.10 CD4+ Th17 cells plus CD4+Foxp3+ Treg, Th17 cells alone or Treg alone were adoptively transferred into BALB/c mice. Control mice did not receive cells (no transfer). CD4+ Th1 and Th2 cells were also transferred into BALB/c hosts. All the mice were then exposed to OVA aerosols for 7 days. (A) Number of CD19+ B cells expressing MHC class II or CD5 in the BAL from Th17, Th2 or Th1 recipients was compared and analyzed by FACS, and results expressed as cell number per mouse. (B) Lung CD19+ B cells were analyzed by immunofluorescence staining of tissue cryosections from Th17 recipient or control mice. (C) Number of MHC class II+CD19+ B cells present in the BAL and LMC from recipients of Th17 cells, Th17 cells plus Treg, Treg alone or control mice was analyzed by FACS and results expressed as cell number per mouse. (D) Levels of IgA, IgM, IgG1, IgG2a and SIgA in BAL from recipients of Th17 cells, Th17 cells plus Treg, Treg alone or control mice were determined by ELISA. Data show mean ± SEM (n=4-7 separate experiments). *p<0.05, compared to OVA-challenged Th17 group, Mann-Whitney U test. Histology results are representative of four independent experiments.
Concluding remarks
In summary, effector CD4+ Th17 cells evoked an increase in AHR and elicited pulmonary inflammation that was typified by neutrophilia, B cell recruitment and elevated airway SIgA and IgM levels. Cotransfer of antigen-specific Foxp3+ nTreg with Th17 cells markedly suppressed Th17-mediated lung inflammatory responses but did not reduce AHR. This regulation appeared to be restricted to the lung, since limited numbers of Treg migrated to the draining LN and Th17 responses were unaffected at this site. Conceivably, OVA-specific nTreg present in the lung mucosal environment suppressed Th17 cells by acting at the level of the CD4+ Th17 cells or, alternatively, promoted suppression by conditioning the airway to promote unresponsiveness, possibly by modifying airway dendritic cell function. This is particularly significant given that the suppression of airway Th2 responses by cotransferring nTreg has proved unsuccessful (our unpublished observation) or required additional treatment of mice with phosphodiesterase inhibitors [14]. It is likely that activation by OVA is a prelude to suppression, since it has been previously demonstrated that antigen recognition by Treg is a prerequisite to immune suppression in vivo [15]. It is particularly noteworthy that, TGF-β, in addition to promoting isotope switching of B cells to IgA production, Th17 differentiation requires this cytokine. Given the pivotal role that Th17 cells subsequently plays in promoting airway B cell recruitment and transcytosis of SIgA, this common dependence of TGF-β appears to form the basis of a coherent mucosal immune response where the production and subsequent transport of IgA are dependent on a common cytokine. In summary, our findings provide new insights into how Th17 responses are regulated and may facilitate the development of novel approaches for controlling Th17-mediated lung inflammatory responses and autoimmune disorders.
Materials and methods
Mice and preparation of DO11.10 CD4+ Th17 cells
Animal studies were approved by Institutional Animal Care and Use Committee and performed according to NIH guidelines. BALB/c and DO11.10 transgenic mice (Jackson Laboratory) were used throughout (6-8 wk old) and housed in specific pathogen-free facilities. To prepare CD4+ Th17 cells, CD4+ T cells were purified from peripheral LN cells obtained from DO11.10 mice by negative selection and depletion of CD8+ cells using MACS beads (Miltenyi Biotech). CD4+ T cells (5 × 105/ml) were incubated for 4 days in the presence of OVA323-339 peptide (1 μg/ml, Mimotopes), IL-6 (10 ng/ml, R&D Systems), TGF-β (2 ng/ml, Sigma), anti-IL-4 (5 μg/ml, 11B11, from ATCC), anti-IFN-γ (5 μg/ml, R4-6A2, ATCC) and mitomycin C-inactivated splenic APCs (1 × 106/ml). After 4 days, cells were then restimulated as before but in the presence of IL-23 (10 ng/ml, R&D) for further 4 days. Eight-day polarized CD62L- Th17 cells were purified (by magnetic bead sorting using anti-CD62L FITC, BD Biosciences) and anti-FITC beads (Miltenyi Biotech) prior to in vivo analysis. Polarized DO11.10 Th2 or Th1 cells were prepared as described previously [16].
Transfer of polarized DO11.10 CD4+CD62L- Th17 cells
Eight-day polarized DO11.10 CD4+CD62L- Th17 cells (8 × 106 cells/mouse) were adoptively transferred into BALB/c animals by injection i.v. Mice (4-6 per group) were then intranasally challenged by exposure to aerosolized solutions of OVA (0.5%, Sigma) for 20 min/day, over 7 consecutive days using a Wright's nebulizer. Control mice were exposed to OVA aerosols but did not receive DO11.10 T cells.
Preparation and cotransfer of expanded DO11.10 Foxp3+ nTreg
Natural Treg were purified from DO11.10 peripheral LN cells using CD4+CD25+ Treg cell isolation kit (Miltenyi Biotech). The limited numbers of CD4+CD25+Foxp3+ nTreg in DO11.10 made a complete analysis of the properties of these cells difficult. Consequently, purified CD4+CD25+ nTreg were expanded as described before [17] in the presence of APCs (1 × 106 cells/ml), OVA peptide (1 μg/ml), IL-2 (10 ng/ml), IL-4 (2 ng/ml) and anti-IFN-γ Ab (5 μg/ml). From a starting population of approx. 3 × 106 nTreg, after 8 days in culture, cell yield was typically 30-50 × 106 cells and the viability was >95%. Eight-day expanded CD4+ nTreg were harvested and a sample is taken for Foxp3 staining using mouse Treg Flow kit (Biolegend) to verify expression of Foxp3+ (>90%). DO11.10 CD4+CD62L- Th17 cells alone (6 × 106 cells/mouse), CD4+Foxp3+ nTreg alone (4 × 106/mouse) or Th17 plus nTreg (ratio 1.5:1.0) were adoptively transferred into BALB/c mice and exposed to OVA aerosols as described above.
Assessment of lung function
After 7 days of OVA challenge, anesthetized and tracheotomized mice were mechanically ventilated and exposed to increasing concentrations of aerosolized methacholine (0, 1.5, 3, 6, 12, and 24 mg/ml). Each aerosol was delivered for a period of 3 min followed by a second 3 min period and lung function was assessed by measurement of pulmonary RL and Cdyn (Buxco Electronics).
Level of pulmonary inflammation
BAL was collected, pooled and cell differential counts were determined by light microscopic evaluation of Hema3-stained cytospin preparations and expressed as absolute cell numbers as described before [16]. Lung tissue was obtained for histological analysis or dispersed by collagenase (Sigma) to prepare LMC. The number of OVA-specific T cells, neutrophils and B cells present in BAL, LMC or lung draining posterior mediastinal LN was analyzed by FACS.
FACS analysis
BAL, LMC, and draining LN cells were stained and analyzed on a FACSAria (BD Biosciences,) to enumerate CD4+ T cells (using anti-CD4 mAb GK1.5-APC-Cy7, BD Biosciences) and OVA-specific T cells (KJ1-26, anti-TCR PE, Caltag Laboratories), or Gr-1+ neutrophils (anti-Ly-6G mAb, BD Biosciences) and CD11b+ cells (anti-CD11b FITC, Miltenyi Biotech). B cells were analyzed anti-CD19 clone 6D5, anti-CD5 clone 53-7.3 (Biolegend) and anti-I-A/I-E clone M5/114 (BD Biosciences).
FACS analysis of intracellular IL-17, IFN-γ and Foxp3 expression
For analysis of intracellular IL-17 or IFN-γ, LMC, draining LN, BAL or polarized Th17 cells (1 × 106 cells) were first stimulated with 50 ng/ml PMA + 500 ng/ml ionomycin (Sigma) in the presence of 1μl of the protein transport inhibitor BD GolgiPlug containing brefeldin A (BD Biosciences) for 4 h at 37 °C. Cells (0.5 × 106) were then blocked using 2.4G2 supernatant (ATCC) and stained with CD62L, CD4 (BD Biosciences), or KJ1.26 mAb (eBiosciences). Following treatment with fixation and permeabilization buffers (Biolegend), cells were intracellularly stained using IL-17 mAb, IFN-γ mAb or isotype control (BD Biosciences) and analyzed by FACSAria.
For intracellular Foxp3 analysis, LMC, draining LN, BAL, polarized Th17 or expanded Treg were first stained with CD4/CD25 mAb, then fixed and permeabilized using Foxp3 staining kit (Biolegend). After permeabilization, the cells were intracellularly stained using Foxp3 mAb or isotype control and analyzed by FACSAria.
Lung histology
Lung tissue was fixed in Histochoice (Amresco) and embedded in paraffin using a Shandon Citadel tissue processor (Thermo Fisher Scientific). Microtome sections were cut at 5 μm thickness and stained with H&E using a Shandon Varistain 24-4 (Thermo Fisher Scientific). For immunofluorescent staining of B cells, lungs were frozen in Tissue-Tek OCT (Sakura). Cryosections were mounted on glass slides and stained with biotinylated anti-CD19 mAb (BD Biosciences) and Alexa Flour 488-conjugated avidin (Invitrogen).
Measurement of IL-17, IL-21 and IL-22 production
To measure Th17 cytokines, CD4+CD62L-, CD62L+ or unsorted or Th17 cells (5 × 105/ml) were stimulated with whole OVA protein (1 mg/ml) or OVA323-339 peptide (1 μg/ml, in the presence of 106/ml Thy-1 depleted spleen cells pretreated with mitomycin C), immobilized anti-CD3 (2 or 5 μg/ml, 2C11, ATCC) and KJ-126 (5 μg/ml, ATCC). Similarly, LMC or lung draining LN cells were stimulated with OVA protein, OVA peptide, anti-CD3 or KJ1-26. All supernatants were harvested after 24 h stimulation and measurement of IL-17, IL-21 or IL-22 was performed by ELISA (R&D Systems). IL-17 levels in BAL were measured by commercially available ELISA kit (e-Bioscience).
ELISA measurement of Ig and SIgA
Measurement of total IgA or IgM, IgG1, IgG2a, IgG2b and IgG3 levels in BAL was performed using Immunoglobulin isotype panel ELISA kit and standards (SouthernBiotech), according to manufacturer's instructions. For SIgA measurement, plates were coated with anti-pIgR (2 μg/ml) overnight, followed by incubation with BAL samples (1:50 dilution), then HRP-anti-IgA (SouthernBiotech) and finally TMB substrate (BD Biosciences). No SIgA standard is available, however, results are corrected per μg of BAL protein and expressed as fold increase from control values.
Statistical analysis
Data are expressed as means ± SE. Comparisons were analyzed for statistical significance by the Mann Whitney test, with p values <0.05 being considered significant.
Supplementary Material
Acknowledgments
We thank M. Buford (Buxco), P. Shaw (FACS Core), L. Herritt and D. Brooks (Histology Core) for their technical assistance. This work was supported by grants from NHLBI, NIH (R01-HL079189 to Roberts, K) and COBRE (P20RR017670 to Holian, A).
Abbreviations
- AHR
airway hyperreactivity
- BAL
bronchoalveolar lavage fluid
- LMC
lung mononuclear cells
- nTreg
naturally occurring Treg
- SIgA
secretory IgA
Footnotes
Conflict of interest: The authors declare no financial or commercial conflict of interest.
References
- 1.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 2.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 3.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 4.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yao Z, Fanslow WC, Seldin MF, Rousseau AM, Painter SL, Comeau MR, Cohen JI, Spriggs MK. Herpesvirus Saimiri encodes a new cytokine, IL-17, which binds to a novel cytokine receptor. Immunity. 1995;3:811–821. doi: 10.1016/1074-7613(95)90070-5. [DOI] [PubMed] [Google Scholar]
- 6.Ye P, Rodriguez FH, Kanaly S, Stocking KL, Schurr J, Schwarzenberger P, Oliver P, Huang W, Zhang P, Zhang J, Shellito JE, Bagby GJ, Nelson S, Charrier K, Peschon JJ, Kolls JK. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med. 2001;194:519–527. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M, Fouser LA. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006;203:2271–2279. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shevach EM, DiPaolo RA, Andersson J, Zhao DM, Stephens GL, Thornton AM. The lifestyle of naturally occurring CD4+ CD25+ Foxp3+ regulatory T cells. Immunol Rev. 2006;212:60–73. doi: 10.1111/j.0105-2896.2006.00415.x. [DOI] [PubMed] [Google Scholar]
- 9.Sakaguchi S. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 10.Horwitz DA, Zheng SG, Gray JD. Natural and TGF-beta-induced Foxp3(+)CD4(+) CD25(+) regulatory T cells are not mirror images of each other. Trends Immunol. 2008;29:429–435. doi: 10.1016/j.it.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 11.Zheng SG, Wang J, Horwitz DA. Cutting edge: Foxp3+CD4+CD25+ regulatory T cells induced by IL-2 and TGF-beta are resistant to Th17 conversion by IL-6. J Immunol. 2008;180:7112–7116. doi: 10.4049/jimmunol.180.11.7112. [DOI] [PubMed] [Google Scholar]
- 12.Dardalhon V, Awasthi A, Kwon H, Galileos G, Gao W, Sobel RA, Mitsdoerffer M, Strom TB, Elyaman W, Ho IC, Khoury S, Oukka M, Kuchroo VK. IL-4 inhibits TGF-beta-induced Foxp3+ T cells and, together with TGF-beta, generates IL-9+ IL-10+ Foxp3(-) effector T cells. Nat Immunol. 2008;9:1347–1355. doi: 10.1038/ni.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jaffar Z, Ferrini ME, Herritt LA, Roberts K. Cutting edge: Lung mucosal Th17-mediated responses induce polymeric Ig receptor expression by the airway epithelium and elevate secretory IgA levels. J Immunol. 2009;182:4507–4511. doi: 10.4049/jimmunol.0900237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bopp T, Dehzad N, Reuter S, Klein M, Ullrich N, Stassen M, Schild H, Buhl R, Schmitt E, Taube C. Inhibition of cAMP degradation improves regulatory T cell-mediated suppression. J Immunol. 2009;182:4017–4024. doi: 10.4049/jimmunol.0803310. [DOI] [PubMed] [Google Scholar]
- 15.Huter EN, Stummvoll GH, DiPaolo RJ, Glass DD, Shevach EM. Cutting edge: antigen-specific TGF beta-induced regulatory T cells suppress Th17-mediated autoimmune disease. J Immunol. 2008;181:8209–8213. doi: 10.4049/jimmunol.181.12.8209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jaffar Z, Wan KS, Roberts K. A key role for prostaglandin I2 in limiting lung mucosal Th2, but not Th1, responses to inhaled allergen. J Immunol. 2002;169:5997–6004. doi: 10.4049/jimmunol.169.10.5997. [DOI] [PubMed] [Google Scholar]
- 17.Jaffar Z, Sivakuru T, Roberts K. CD4+CD25+ T cells regulate airway eosinophilic inflammation by modulating the Th2 cell phenotype. J Immunol. 2004;172:3842–3849. doi: 10.4049/jimmunol.172.6.3842. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.