Abstract
Precise spatial and temporal expression of the recently identified G-protein coupled receptor GPR54 is critical for proper reproductive function and metastasis suppression. However, regulatory factors that control GPR54 expression remain unknown. Thus, the identification of these cis-acting DNA elements can provide insight into the role of GPR54 in reproduction and cancer. Using luciferase reporter, electrophoretic mobility shift, and chromatin immunoprecipitation assays, we demonstrate that three SP1 sites and a partial estrogen response element modulate mouse GPR54 (mGPR54) promoter activity. Supporting experiments show transcription factor SP1 binds directly to the mGPR54 promoter region and activates gene expression. In conclusion, these novel findings now identify factors that regulate activity of the mGPR54 promoter, and these factors are highly conserved across multiple mammalian species.
Keywords: GPR54, Hypothalamic-Pituitary-Gonadal (HPG) axis, Metastasis, Reproductive development, SP1, ERE
1. Introduction
G-protein coupled receptor GPR54 (also known as KISS1R), together with its endogenous ligand kisspeptin (KP), play pivotal roles in reproduction and cancer metastasis [1-8]. KP-GPR54 were originally postulated to be metastasis suppressors, based on subtractive hybridization and migration studies [4,9,10]. Clinical reports also demonstrate an inverse correlation exists between KP-GPR54 expression levels and metastasis severity [5,11]. Subsequent to these studies, KP-GPR54 signaling in the hypothalamic-pituitary-gonadal axis was shown to be critical for initiation of puberty, ovulation, estrous cycle, and pregnancy [2,3,6,12-14]. Patients with inactivating mutations in GPR54 display idiopathic hypogonadotropic hypogonadism, a condition with low levels of circulating sex hormones, underdeveloped reproductive organs and infertility [1]. In support of these findings, GPR54 knockout (KO) mice phenocopy patients with GPR54 loss-of-function mutations [1,3] and have been used to study GPR54's role in ovulation and estrous cycle. These reports have spurred intense study into this intriguing new area of reproductive and cancer biology.
To date, relatively little information is known about the mechanisms controlling expression of GPR54. Preliminary studies suggest that tight regulation of both KP and GPR54 expression is essential to maintain proper function of the reproductive system, and to prevent migration of metastatic cancer cells. For example, only a few select tissues express GPR54 and KP, including the hypothalamus, pituitary, gonads, placenta, liver, small intestine, pancreas and spleen [5,7,15]. Moreover, KP and GPR54 expression changes dramatically during the course of pregnancy, with striking increases observed in plasma KP and GPR54 mRNA levels, followed by a rapid return to baseline levels post-partum [14,16]. Additionally, KP-GPR54 are potential markers of metastasis severity as mRNA levels of both are significantly decreased in a variety of highly metastatic cancers, including melanoma, epithelial ovarian, pancreatic, thyroid, gastric, esophageal, choriocarcinoma, endometrial, bladder, and uveal melanoma [5,17]. Taken together, these data demonstrate the importance for proper control of KP-GPR54 expression in cancer and reproduction, and therefore, it is highly important that we begin to elucidate the molecular mechanisms regulating the expression of these tightly controlled genes.
Thus far, a few studies have attempted to identify the mechanisms regulating expression of the gene encoding kisspeptin, KISS1. Interestingly, the mechanisms controlling KISS1 expression appear to be tissue specific. For example, SP1/AP-2α increases KISS1 expression in breast cancer cells (MCF-7), whereas estrogen receptor α (ER α) and SP1 must form a complex to enhance KISS1 expression in hypothalamic cells (GT1-7) [18,19]. Presumably, this tissue-specific regulation permits differential expression levels between these highly divergent tissues, although further studies are required to validate this hypothesis. Unfortunately, little to no information exists about the molecular mechanisms controlling expression of the GPR54 gene, KISS1R. Discovering the factors that provide tissue specific control of KP-GPR54 expression will provide knowledge that permits exogenous manipulation of KP-GPR54 expression levels in cancer and reproductive disease.
This manuscript provides novel information detailing the molecular mechanism controlling mouse GPR54 (mGPR54) gene transcription. We have used promoter element prediction software, luciferase reporter assays, electrophoretic mobility shift assays, and chromatin immunoprecipitation to characterize the DNA elements and trans-acting factors regulating mGPR54 expression in the mouse pituitary cell line, AtT-20, which endogenously expresses mGPR54 at physiological levels. Using these multiple approaches, our data strongly suggest that specificity protein-1 (SP1) and sex steroids play important but opposing roles in controlling GPR54 expression.
2. Materials and Methods
Promoter Analysis and Constructs
Genomic sequence surrounding GPR54 in the Mus musculus genome was analyzed using UCSC Genome Browser (http://genome.ucsc.edu/). mGPR54 is on chromosome 10qC1 from 79,379,716 to 79,384,928. This study focused on the region spanning 79,379,668 to 79,381,420 on chromosome 10, which lies 5’ to the mGPR54 gene.
Cell Culture
AtT-20/D16v-F2 cells were propagated in Dulbecco's modified Eagle's medium containing 10% horse serum, 100 μg/ml streptomycin, and 100 units/ml penicillin at 37 °C in 5% CO2.
Luciferase Assay
Cells were seeded at 300,000 cells/well in 24 well plates. pGL3-promoter construct (400 ng) and prL-CMV construct (4 ng) were transfected using Lipofectamine (Invitrogen, Carlsbad, CA). In studies with SP1 overexpression (400 ng), pCDNA 3.1 + was used as negative control and to normalize quantities of total DNA transfected. Promoter constructs were reduced to 200 ng per well with SP1 overexpression. Cells were collected after 48 hrs and assayed using the dual-luciferase kit (Promega, Catalogue # E2940, Madison, WI). Ratio of firefly to renilla luciferase activity was calculated, normalized to empty vector and then represented as a percentage of full-length promoter activity.
Electrophoretic Mobility Shift Assay (EMSA)
Nuclear extracts were prepared according to [20]. Labeled probe (α -32P dCTP) was generated via PCR with primers referenced in Supplemental Table. 10 μl binding reactions consisted of 5X buffer (50% glycerol, 250 mM KCl, 50 mM HEPES (pH 7.9), 1 mM EDTA, 25 mM MgCl2), 5 mM DTT, 2 μg poly dI.dC dI,dC, 100 ng ssDNA, and 5 μg nuclear extract were incubated on ice for 30 mins. SP1 probe (~30,000 cpm) was added and incubated for 20 mins at RT. SP1 antibody (sc-59x from Santa Cruz), IgY antibody (LN050920, Genway) or cold competitor was pre-incubated in relevant reactions (Santa Cruz, CA; San Diego, CA). Mutant competitor (MT) had three SP1 sites where site directed mutagenesis was done (GGGCGG→GTACGG). DNA-protein complexes were resolved on 5% TBE PAGE (Ready Gels, Bio-Rad, Hercules, CA) at 70V in 1X TBE for 100 min at RT. The gels were dried and exposed to CLXposure film at −80°C for 3-6 hrs (ThermoScientific, Waltham, MA).
Chromatin Immunoprecipitation (ChIP)
Preparation of chromatin and immunoprecipitation (IP) was done according to [20]. 120 μl of chromatin was used per IP and 4μg of SP1 antibody or no antibody (ChIPAb+ from Upstate, Lake Placid, NY). Results were analyzed via qPCR with SYBR Green (Stratagene, La Jolla, CA) with primers from EMSA SP1 probe.
3. Results
3.1 Genomic Arrangement of GPR54
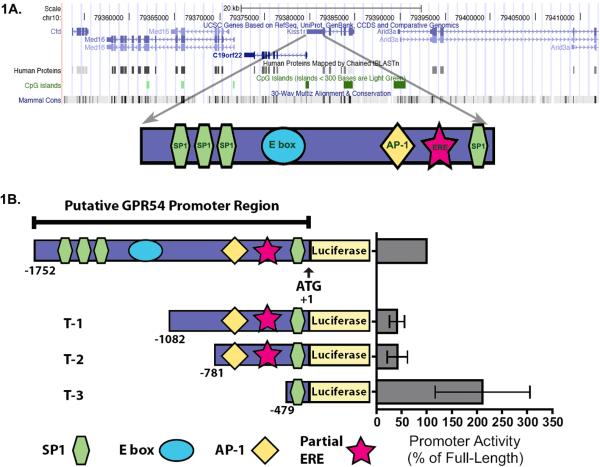
Following analysis using the UCSC genome browser, (http://genome.ucsc.edu/), we discovered the genomic arrangement surrounding GPR54 is highly conserved between human, rat, and mouse (Fig. 1A). Interestingly, both the gene order (Med16, C19orf22, GPR54, and Arid3a), and positioning of CpG islands (two within the GPR54 sequence, one located in the promoter region and one in the fifth exon) are roughly equivalent between human, rat, and mouse. The sequence 1752 bp 5’ to the ATG of mouse GPR54 was chosen as the putative mGPR54 promoter; this region spans the sequence from the 5’ end of C19orf22 (gene on opposite strand of DNA), to the start site of translation for mGPR54. Preliminary sequence analysis of the 1752 bp genomic sequence identified the following promoter elements: four SP1 sites, one E box, one AP-1 site, and one partial estrogen response element (ERE) (Sup. Fig.1). Interestingly, the three SP1 promoter elements clustered at the 5’ end of the promoter region are conserved among all three species (Sup. Fig. 2.).
Figure 1. Analysis of the mGPR54 gene.
A, Mus musculus chromosome 10qC1 genomic arrangement is presented. The gene order is conserved between mouse and human (Med16, C19orf22, GPR54, and Arid3a). Two CpG islands are contained within the GPR54 sequence, one in the promoter region and one in the fifth exon. The sequence 1752 bp 5’ to the ATG of mouse GPR54 was chosen as the putative mGPR54 promoter; this region spans the sequence from the 5’ end of C19orf22 to the start site of translation for mGPR54 (N.B. C19orf22 is on opposite strand of DNA). Preliminary analysis identified the following promoter elements: four SP1 sites, one E box, one AP-1 site, and one partial estrogen response element (ERE). The SP1 and partial ERE sites are conserved in both the mouse and human promoters. (not shown here, see Sup. Fig. 2.). B, Activity of mGPR54 truncation constructs were examined using luciferase reporter assays. Activity was normalized to % of full-length construct in each assay, (n = 2-5, 3 replicates each, and expressed as average ± SEM).
3.2. Features of the 5’- flanking promoter region
Next, we addressed the relative contribution of the identified elements to control mGPR54 promoter activity using a dual-luciferase assay. AtT-20 cells were used as the model cell line for these experiments. These cells are a mouse pituitary adenoma derived cell line that express low levels of mGPR54, which we confirmed using quantitative reverse-transcriptase polymerase chain reaction (data not shown). Following transfection into AtT-20 cells, we observed that the full-length putative mGPR54 promoter increased luciferase reporter activity ~15-fold over activity observed with empty vector. The activity of the full-length promoter was used to normalize all subsequent experiments and data are expressed as percentage of full-length promoter. To assess the potential importance of the elements identified in the putative promoter for regulating mGPR54 gene activity, we sequentially truncated the promoter into constructs T-1 (−1082 to +1), T-2 (−781 to +1), and T-3 (−479 to +1) and measured the ability of each construct to drive luciferase activity in comparison to full-length construct. First, we found that the T-1 construct displayed significantly decreased promoter activity, suggesting that activating elements are located within the region −1752 to −1083 of the promoter (Fig. 1B). Indeed, sequence analysis revealed that three SP1 sites and a single E box are found between bps −1752 to −1083. Next, we observed that the T-2 construct showed a similar level of promoter activity compared to T-1, suggesting that no elements are located between bps −1082 to −782 that contribute to promoter activity. However, we found that construct T-3 caused a significant increase in luciferase activity, suggesting a repressive element exists between bps −781 to −480. Further sequence examination revealed the presence of a partial ERE and a single AP-1 binding site in this region. To test the contribution of the partial ERE to promoter activity, we destroyed the consensus sequence (AGGTCA→ACATCA) [21]. Construct ΔERE showed increased promoter activity, suggesting estrogen receptor (i.e. ER-α, ER-β)-mediated transcriptional repression (Fig. 2A).
Figure 2. Contribution of SP1 elements and partial ERE to mGPR54 promoter activity.
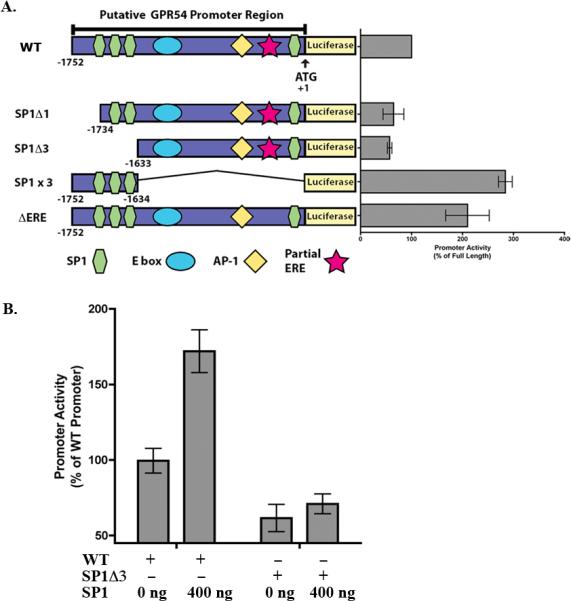
A, Activities of mGPR54 deletion constructs were examined in AtT-20 cells. Data are normalized to % of activity observed using full-length mGRP54, (n = 2-3, 3 replicates each and expressed as average ± SEM). B Activity of full-length mGPR54 promoter construct or SP1Δ3 construct co-transfected with 0 or 400 ng SP1 in AtT-20 cells. Data are normalized to % of activity observed using full-length promoter, (n=3, 3 replicates each and expressed as average ± SEM).
3.3 Role of SP1 sites in mGPR54 expression
Thus far, our data suggest that SP1 may be involved in regulating mGPR54 gene transcription because removal of bps −1752 to −1083 containing three SP1 sites caused a decrease in reporter activity. The first construct we created to test this hypothesis involved deletion of the first SP1 site at −1735 (SP1Δ1), which resulted in ~40% decrease in activity relative to full-length promoter. Next, we deleted all three SP1 sites located at bps −1735, −1713, and −1639 (SP1Δ3). If multiple SP1 sites are involved in regulation of mGPR54 gene activity, we would expect to observe a further reduction in activity. Indeed, we observed a further 10% decrease, suggesting that multiple SP1 sites are involved (Fig. 2A). We confirmed these results by creating a construct that only contains the three SP1 sites (SP1x3, bp −1752 – −1634). This construct caused a significant increase in gene activity greater then that observed in full-length promoter alone (Fig. 2A). This intriguing result strongly suggests that opposing elements are located within the putative promoter, presumably to permit dual control of GPR54 gene activity and precise regulation of expression levels in a tissue-selective manner.
Our deletion constructs suggest SP1 increases mGPR54 gene activity, and if so, one would expect that we can exogenously drive GPR54 gene expression by overexpressing SP1. To test this possibility, we co-transfected AtT-20 cells with saturating concentrations of SP1 cDNA and full-length GPR54 promoter. We observed an approximate 200% increase in activity of the full-length promoter following co-transfection with SP1. Conversely, we would expect SP1 overexpression to have no effect when SP1 sites are deleted. Indeed, co-expressing SP1 cDNA with the SP1Δ3 construct resulted in no detectable differences in gene activity (Fig. 2B). Taken together, these results strongly imply that SP1 functions as an activator of mGPR54 gene transcription.
3.4 SP1 directly binds to mGPR54 promoter
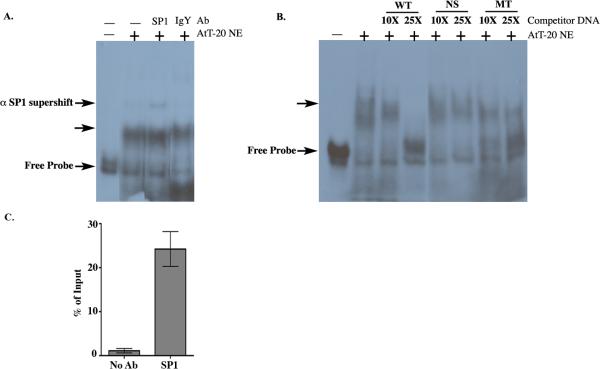
Thus far, we have found that deleting SP1 sites or overexpressing SP1 affects GPR54 gene activity. Our working hypothesis is that SP1 is binding directly to the mGPR54 gene promoter to increase transcription, although we can not rule out the possibility that SP1 indirectly regulates mGPR54 activity. Therefore, we tested the ability of SP1 to interact with the mGPR54 putative promoter using electrophoretic mobility shift assays (EMSAs). A 151 bp probe with the three SP1 elements located between bps −1752 to −1601 of the mGPR54 promoter was used for these experiments (Fig. 3A). Following incubation of AtT-20 nuclear extract with [α −32P] labeled probe, we observed formation of a specific complex that increased with increasing concentrations of nuclear extract (Fig. 3B). Next, we tested if SP1 was present in the mobility shift complex using a commercially available SP1 antibody. A modest, but significant super-shift of the DNA-protein complex was noted with the SP1 antibody. As a negative control, we demonstrated that anti-IgY antibody is unable to supershift the complex (Fig. 4A). The specificity of the binding activity was further investigated using competition assays. A significant reduction in DNA-protein complex formation was observed when saturating concentrations of cold competitor containing SP1 sites was added to the reaction. No such competition was detected with neither a nonspecific fragment lacking SP1 sites (NS), nor a fragment with mutated SP1 sites (MT) (Fig. 4B).
Figure 3. AtT-20 Nuclear Extract binds to mGPR54 gene.
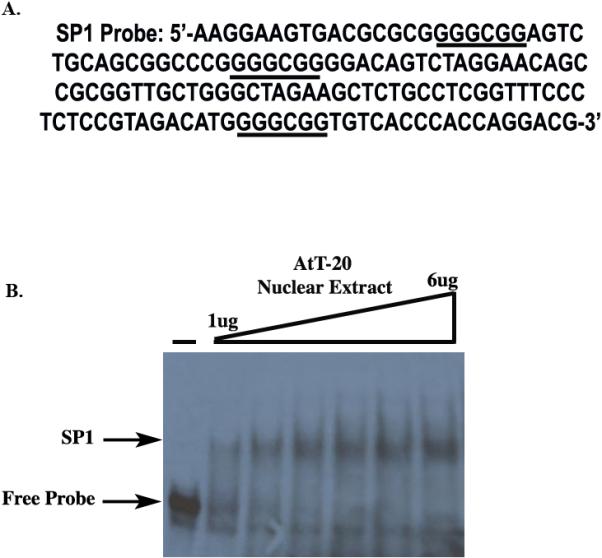
A, Nucleotide sequence of the SP1 probe used for EMSA. B, Increasing concentrations of AtT-20 nuclear extract (NE) were tested to determine optimal concentration for maximum EMSA shift. (-) does not contain AtT-20 NE.
Figure 4. SP1 binds directly to mGPR54 gene.
A, SP1 probe was incubated without (-) or with (+) 5 ug of AtT-20 nuclear extract (NE) containing SP1 antibody (SP1) or IgY antibody (IgY). EMSA reveals a supershift in the presence of SP1 antibody, but not IgY. DNA-protein complex indicated by unlabeled arrow. B, SP1 probe was incubated without (-) or with (+) 5 ug of AtT-20 NE containing wild type (WT), nonspecific (NS), or mutant SP1 sites (MT) cold probe. 10X and 25X concentrations of cold competitors were used where indicated. C, Chromatin isolated from AtT-20 cells was incubated with no antibody or SP1 antibody, Enrichment at SP1 sites was calculated as % of input. Data presented are from 2-3 replicates within one chromatin preparation and representative of 2 experiments.
Finally, we wished to confirm that SP1 binds to the mGPR54 promoter in vivo using chromatin immunoprecipitation. Sheared chromatin purified from AtT-20 cells was incubated with either SP1 antibody or no antibody, and the amount of mGPR54 promoter DNA encompassing the three SP1 sites (−1752 to −1601) was assessed by quantitative-PCR. A ~25% enrichment in mGPR54 promoter DNA was observed in the SP1-antibody immnuoprecipitates in comparison to immunoprecipitates containing no antibody (Fig. 4C). Taken together, our data strongly suggest that SP1 specifically binds to the proximal promoter region of mGPR54 in AtT-20 cells and directly regulates GPR54 transcription.
4. Discussion
To date, few studies have identified factors controlling GPR54 gene expression. Our experiments have identified a 1752 bp region as the mGPR54 promoter, shown repression of mGPR54 promoter activity by a partial ERE, and activation of mGPR54 expression by transcription factor SP1 via three SP1 binding sites clustered at the 5’ end. Additionally, the arrangement of the SP1 binding sites is conserved across species, suggesting their key part in GPR54 regulation.
Our studies demonstrate that SP1 regulates GPR54 gene activity by binding directly to GC boxes in the promoter region [22]. Several of the known characteristics of SP1 indicate this transcription factor uses a complex mechanism to control the expression of genes important in reproduction and cancer [6,23]. For example, activation of SP1 increases the expression of VEGF and EGFR, two proteins important for angiogenesis [24-26]. Also, SP1 can regulate the methylation state of CpG islands in promoter regions [27]. Interestingly, a recent study examined the methylation status of CpG islands in highly metastatic cancer cell biopsies to determine which genes were highly repressed [28]. Both KISS1 and GPR54 genes were found to be CpG methylated and repressed, in agreement with the hypothesis that these genes must be inactivated to suppress their anti-metastatic properties. Thus, it would be interesting to determine in future studies if SP1 is involved in controlling the methylation status of the KISS1 and GPR54 genes, and to understand the molecular mechanism by which this process occurs in both reproductive and cancer cells.
The discovery that a partial ERE can regulate GPR54 gene expression supports the findings of previous studies in the field. For example, estradiol has been implicated in repression of GPR54 expression in several studies performed in vivo and in vitro. Navarro et al., recently showed that GPR54 transcript levels in the hypothalamus increased in female rats following ovariectomization, and more conclusively, GPR54 expression then decreased upon estrogen replacement [6]. Additionally, Richard et al. revealed GPR54 expression in the female rat pituitary can be repressed by administering estradiol [29]. Moreover, estradiol treatment of human fetal GnRH neuroblasts causes a dose-dependent decrease in both KISS1 and GPR54 expression levels [30]. Our data both support and extend these previous findings suggesting that estrogen acts as a repressor of GRP54 gene activity, because disruption of the partial ERE resulted in an increase in GPR54 promoter activity. Future studies will determine the subtype of estrogen receptor involved in this mechanism, and if these effects are tissue specific.
Numerous studies have implied that GPR54 and KISS1 are co-regulated with precise temporal and spatial accuracy, as both partners in this signaling system are required for the estrous cycle, ovulation, pregnancy, and reproduction [5,6,8,31]. Here, we have characterized the promoter elements that contribute to regulation of GPR54 expression. Consistent with KISS1 regulation [18,19,32], we have found that a partial ERE represses expression and that SP1 elements recruit SP1 to activate transcription of GPR54, and these findings are the first documenting cis-acting DNA regulatory elements for GPR54. This information provides a starting point for understanding the precise regulation of KP-GPR54 signaling system in reproduction and cancer.
Supplementary Material
Acknowledgements
We thank Dr. Robert Steiner, Dr. Kyung-Soon Lee, and Susan Kloet (University of Washington) for helpful discussion.
JSL was supported in part by PHS NRSA T32 GM07270 from NIGMS. MCD was supported in part by NIH 5 T32 GM07750. JLW was supported in part by NIH Grant T32 HD07453. This research was supported in part by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD)–National Institutes of Health (NIH) through cooperative agreements U54HD12629 (to the University of Washington Center for Research in Reproduction and Contraception).
Abbreviations
- SP1
specificity protein-1
- mGPR54
Mus musculus GPR54
- KP
kisspeptin
- GPCR
G-protein-coupled-receptor
- AP-1
Activating protein-1
- ERE
estrogen response element
- bp
base pair
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O'Rahilly S, Carlton MB, Crowley WF, Aparicio SA, Colledge WH. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349:1614–1627. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- 2.Gottsch ML, Cunningham MJ, Smith JT, Popa SM, Acohido BV, Crowley WF, Seminara S, Clifton DK, Steiner RA. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology. 2004;145:4073–4077. doi: 10.1210/en.2004-0431. [DOI] [PubMed] [Google Scholar]
- 3.Funes S, Hedrick JA, Vassileva G, Markowitz L, Abbondanzo S, Golovko A, Yang S, Monsma FJ, Gustafson EL. The KiSS-1 receptor GPR54 is essential for the development of the murine reproductive system. Biochem Biophys Res Commun. 2003;312:1357–1363. doi: 10.1016/j.bbrc.2003.11.066. [DOI] [PubMed] [Google Scholar]
- 4.Ohtaki T, Shintani Y, Honda S, Matsumoto H, Hori A, Kanehashi K, Terao Y, Kumano S, Takatsu Y, Masuda Y, Ishibashi Y, Watanabe T, Asada M, Yamada T, Suenaga M, Kitada C, Usuki S, Kurokawa T, Onda H, Nishimura O, Fujino M. Metastasis suppressor gene KiSS-1 encodes peptide ligand of a G-protein-coupled receptor. Nature. 2001;411:613–617. doi: 10.1038/35079135. [DOI] [PubMed] [Google Scholar]
- 5.Makri A, Pissimissis N, Lembessis P, Polychronakos C, Koutsilieris M. The kisspeptin (KiSS-1)/GPR54 system in cancer biology. Cancer Treat Rev. 2008;34:682–692. doi: 10.1016/j.ctrv.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 6.Navarro VM, Castellano JM, Fernández-Fernández R, Barreiro ML, Roa J, Sanchez-Criado JE, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M. Developmental and hormonally regulated messenger ribonucleic acid expression of KiSS-1 and its putative receptor, GPR54, in rat hypothalamus and potent luteinizing hormone-releasing activity of KiSS-1 peptide. Endocrinology. 2004;145:4565–4574. doi: 10.1210/en.2004-0413. [DOI] [PubMed] [Google Scholar]
- 7.Kotani M, Detheux M, Vandenbogaerde A, Communi D, Vanderwinden JM, Le Poul E, Brézillon S, Tyldesley R, Suarez-Huerta N, Vandeput F, Blanpain C, Schiffmann SN, Vassart G, Parmentier M. The metastasis suppressor gene KiSS-1 encodes kisspeptins, the natural ligands of the orphan G protein-coupled receptor GPR54. J Biol Chem. 2001;276:34631–34636. doi: 10.1074/jbc.M104847200. [DOI] [PubMed] [Google Scholar]
- 8.Hiden U, Bilban M, Knöfler M, Desoye G. Kisspeptins and the placenta: regulation of trophoblast invasion. Rev Endocr Metab Disord. 2007;8:31–39. doi: 10.1007/s11154-007-9030-8. [DOI] [PubMed] [Google Scholar]
- 9.Lee JH, Miele ME, Hicks DJ, Phillips KK, Trent JM, Weissman BE, Welch DR. KiSS-1, a novel human malignant melanoma metastasis-suppressor gene. J Natl Cancer Inst. 1996;88:1731–1737. doi: 10.1093/jnci/88.23.1731. [DOI] [PubMed] [Google Scholar]
- 10.Masui T, Doi R, Mori T, Toyoda E, Koizumi M, Kami K, Ito D, Peiper SC, Broach JR, Oishi S, Niida A, Fujii N, Imamura M. Metastin and its variant forms suppress migration of pancreatic cancer cells. Biochem Biophys Res Commun. 2004;315:85–92. doi: 10.1016/j.bbrc.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 11.Shoji S, Tang XY, Umemura S, Itoh J, Takekoshi S, Shima M, Usui Y, Nagata Y, Uchida T, Osamura RY, Terachi T. Metastin inhibits migration and invasion of renal cell carcinoma with overexpression of metastin receptor. Eur Urol. 2009;55:441–449. doi: 10.1016/j.eururo.2008.02.048. [DOI] [PubMed] [Google Scholar]
- 12.Matsui H, Takatsu Y, Kumano S, Matsumoto H, Ohtaki T. Peripheral administration of metastin induces marked gonadotropin release and ovulation in the rat. Biochem Biophys Res Commun. 2004;320:383–388. doi: 10.1016/j.bbrc.2004.05.185. [DOI] [PubMed] [Google Scholar]
- 13.Thompson EL, Patterson M, Murphy KG, Smith KL, Dhillo WS, Todd JF, Ghatei MA, Bloom SR. Central and peripheral administration of kisspeptin-10 stimulates the hypothalamic-pituitary-gonadal axis. J Neuroendocrinol. 2004;16:850–858. doi: 10.1111/j.1365-2826.2004.01240.x. [DOI] [PubMed] [Google Scholar]
- 14.Horikoshi Y, Matsumoto H, Takatsu Y, Ohtaki T, Kitada C, Usuki S, Fujino M. Dramatic elevation of plasma metastin concentrations in human pregnancy: metastin as a novel placenta-derived hormone in humans. J Clin Endocrinol Metab. 2003;88:914–919. doi: 10.1210/jc.2002-021235. [DOI] [PubMed] [Google Scholar]
- 15.Muir AI, Chamberlain L, Elshourbagy NA, Michalovich D, Moore DJ, Calamari A, Szekeres PG, Sarau HM, Chambers JK, Murdock P, Steplewski K, Shabon U, Miller JE, Middleton SE, Darker JG, Larminie CG, Wilson S, Bergsma DJ, Emson P, Faull R, Philpott KL, Harrison DC. AXOR12, a novel human G protein-coupled receptor, activated by the peptide KiSS-1. J Biol Chem. 2001;276:28969–28975. doi: 10.1074/jbc.M102743200. [DOI] [PubMed] [Google Scholar]
- 16.Janneau JL, Maldonado-Estrada J, Tachdjian G, Miran I, Motté N, Saulnier P, Sabourin JC, Coté JF, Simon B, Frydman R, Chaouat G, Bellet D. Transcriptional expression of genes involved in cell invasion and migration by normal and tumoral trophoblast cells. J Clin Endocrinol Metab. 2002;87:5336–5339. doi: 10.1210/jc.2002-021093. [DOI] [PubMed] [Google Scholar]
- 17.Katagiri F, Nagai K, Kida A, Tomita K, Oishi S, Takeyama M, Doi R, Fujii N. Clinical significance of plasma metastin level in pancreatic cancer patients. Oncol Rep. 2009;21:815–819. [PubMed] [Google Scholar]
- 18.Mitchell DC, Abdelrahim M, Weng J, Stafford LJ, Safe S, Bar-Eli M, Liu M. Regulation of KiSS-1 metastasis suppressor gene expression in breast cancer cells by direct interaction of transcription factors activator protein-2alpha and specificity protein-1. J Biol Chem. 2006;281:51–58. doi: 10.1074/jbc.M506245200. [DOI] [PubMed] [Google Scholar]
- 19.Li D, Mitchell D, Luo J, Yi Z, Cho SG, Guo J, Li X, Ning G, Wu X, Liu M. Estrogen regulates KiSS1 gene expression through estrogen receptor alpha and SP protein complexes. Endocrinology. 2007;148:4821–4828. doi: 10.1210/en.2007-0154. [DOI] [PubMed] [Google Scholar]
- 20.Wang EH, Tjian R. Promoter-selective transcriptional defect in cell cycle mutant ts13 rescued by hTAFII250. Science. 1994;263:811–814. doi: 10.1126/science.8303298. [DOI] [PubMed] [Google Scholar]
- 21.Klein-Hitpass L, Ryffel GU, Heitlinger E, Cato AC. A 13 bp palindrome is a functional estrogen responsive element and interacts specifically with estrogen receptor. Nucleic Acids Res. 1988;16:647–663. doi: 10.1093/nar/16.2.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lania L, Majello B, De Luca P. Transcriptional regulation by the Sp family proteins. Int J Biochem Cell Biol. 1997;29:1313–1323. doi: 10.1016/s1357-2725(97)00094-0. [DOI] [PubMed] [Google Scholar]
- 23.Saffer JD, Jackson SP, Annarella MB. Developmental expression of Sp1 in the mouse. Mol Cell Biol. 1991;11:2189–2199. doi: 10.1128/mcb.11.4.2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shi Q, Le X, Abbruzzese JL, Peng Z, Qian CN, Tang H, Xiong Q, Wang B, Li XC, Xie K. Constitutive Sp1 activity is essential for differential constitutive expression of vascular endothelial growth factor in human pancreatic adenocarcinoma. Cancer Res. 2001;61:4143–4154. [PubMed] [Google Scholar]
- 25.Xie K, Wei D, Huang S. Transcriptional anti-angiogenesis therapy of human pancreatic cancer. Cytokine Growth Factor Rev. 2006;17:147–156. doi: 10.1016/j.cytogfr.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 26.Salvatori L, Pallante P, Ravenna L, Chinzari P, Frati L, Russo MA, Petrangeli E. Oestrogens and selective oestrogen receptor (ER) modulators regulate EGF receptor gene expression through human ER alpha and beta subtypes via an Sp1 site. Oncogene. 2003;22:4875–4881. doi: 10.1038/sj.onc.1206784. [DOI] [PubMed] [Google Scholar]
- 27.Suske G. The Sp-family of transcription factors. Gene. 1999;238:291–300. doi: 10.1016/s0378-1119(99)00357-1. [DOI] [PubMed] [Google Scholar]
- 28.Yamashita S, Tsujino Y, Moriguchi K, Tatematsu M, Ushijima T. Chemical genomic screening for methylation-silenced genes in gastric cancer cell lines using 5-aza-2'-deoxycytidine treatment and oligonucleotide microarray. Cancer Sci. 2006;97:64–71. doi: 10.1111/j.1349-7006.2006.00136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Richard N, Galmiche G, Corvaisier S, Caraty A, Kottler ML. KiSS-1 and GPR54 genes are co-expressed in rat gonadotrophs and differentially regulated in vivo by oestradiol and gonadotrophin-releasing hormone. J Neuroendocrinol. 2008;20:381–393. doi: 10.1111/j.1365-2826.2008.01653.x. [DOI] [PubMed] [Google Scholar]
- 30.Morelli A, Marini M, Mancina R, Luconi M, Vignozzi L, Fibbi B, Filippi S, Pezzatini A, Forti G, Vannelli GB, Maggi M. Sex Steroids and Leptin Regulate the “First Kiss” (KiSS 1/G-Protein-Coupled Receptor 54 System) in Human Gonadotropin-Releasing-Hormone-Secreting Neuroblasts. J Sex Med. 2008;5:1097–1113. doi: 10.1111/j.1743-6109.2008.00782.x. [DOI] [PubMed] [Google Scholar]
- 31.Roa J, Vigo E, Castellano JM, Gaytan F, Navarro VM, Aguilar E, Dijcks FA, Ederveen AG, Pinilla L, van Noort PI, Tena-Sempere M. Opposite Roles of Estrogen Receptor (ER) {alpha} and ER{beta} in the Modulation of Luteinizing Hormone Responses to Kisspeptin in the Female Rat: Implications for the Generation of the Preovulatory Surge. Endocrinology. 2008 doi: 10.1210/en.2007-1540. [DOI] [PubMed] [Google Scholar]
- 32.Mitchell DC, Stafford LJ, Li D, Bar-Eli M, Liu M. Transcriptional regulation of KiSS-1 gene expression in metastatic melanoma by specificity protein-1 and its coactivator DRIP-130. Oncogene. 2007;26:1739–1747. doi: 10.1038/sj.onc.1209963. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.