Abstract
Background
Severe asthma subjects have increased physiologically measured air trapping. However, a similar study using CT measures of air trapping has not been performed. This study was designed to address two hypotheses: 1) air trapping, measured by multi-detector CT quantitative methodology, would be a predictor of a more severe asthma phenotype; and 2) historical, clinical, allergic, or inflammatory risk factors could be identified via multivariate analysis.
Methods
Multi-detector CT scanning of a subset of the Severe Asthma Research Program subjects was performed at functional residual capacity. Air trapping was defined as 9.66% or more of the lung tissue less than −850 HU. Subjects who were defined as air trappers were then compared to non-trappers on clinical and demographic factors using both univariate and multivariate statistical analysis.
Results
Air trappers were significantly more likely to have a history of asthma-related hospitalizations, ICU visits and/or mechanical ventilation. Duration of asthma (OR 1.42, 95% CI 1.08–1.87), history of pneumonia (OR 8.55, 95% CI 2.07–35.26), high levels of airway neutrophils (OR 8.67, 95% CI 2.05–36.57), air flow obstruction (FEV1/FVC) (OR 1.61, 95% CI 1.21–2.14) and atopy (OR 11.54, 95% CI 1.97–67.70), were identified as independent risk factors associated with the air trapping phenotype.
Conclusions
Quantitative CT determined air trapping in asthmatic subjects identifies a group of individuals with a high risk of severe disease. Several independent risk factors for the presence of this phenotype were identified, perhaps most interestingly history of pneumonia, neutrophilic inflammation, and atopy.
INTRODUCTION
Physiologically defined air-trapping has long been considered a risk factor for severe forms of obstructive airways disease.1,2 Air trapping is defined physiologically as an increase in residual volume (RV) or by the relationship of RV to total lung capacity (TLC). It can now also be defined and objectively quantified using multi-detector CT (MDCT) imaging and quantitative software analysis. Software programs, that identify the lung field within a stack of CT images, quantify the amount of lung tissue that falls within a range of Hounsfield units (HU), producing a histogram curve of lung voxels. Lower (negative) values represent the least dense (more air-like) areas, while higher numbers represent voxels containing not only air but parenchyma, blood, fibrotic tissue, inflamed parenchyma, etc.3–19 In emphysema, previous studies have suggested that CT images obtained with the lungs held at near TLC with density thresholds of −970 to −910 HU are representative of severe to mild emphysematous regions which were respectively identified on pathologic specimens.3,4,16,19 The normal specific volume of the lung at TLC is 6.0 ml/gm, corresponding to a CT density of −856 HU.3,13 The notion that at functional residual capacity (FRC) the normal specific volume and hence CT density should be less than the TLC value suggests that −850 HU may also be a reasonable threshold for air trapping measured at FRC. The −856 HU cut-off MDCT density has been previously used to quantify air trapping in asthmatic children.5 If pulmonary airways within the lung boarders are included within the voxel count, it is clear that a certain percentage of the lung will always fall below these cut-off values.
Although severe asthma has been associated with air trapping measured plethysmographically, little is understood regarding factors predisposing to this condition. In asthma, there is a strong relationship between FEV1 values and RV,20–22 suggesting airway obstruction is inversely related to air trapping. However, no previous studies have integrated a range of risk factors, including those related to allergy, past medical events, co-morbid conditions and inflammatory processes.
The current study addresses two hypotheses: 1) air trapping, measured by MDCT quantitative methodology, would be a predictor of a more severe asthma phenotype; and 2) independent historical, clinical, allergic, or inflammatory risk factors could be identified in a multivariate analysis as a means of identifying risk factors for this phenotype. One hundred twenty well-characterized asthmatic and normal subjects from the NIH Severe Asthma Research Program (SARP) underwent MDCT scans at FRC and TLC (TLC; data not included in this analysis) between October of 2002 and June of 2006. CT images were compared across subject groups for air trapping calculated within the FRC data sets. After identifying the air-trapping phenotype, a multivariate analysis identified risk factors associated with this phenotype.
METHODS
Study design
As part of SARP, subjects underwent a history, physical examination, allergy skin testing, laboratory tests (including sputum analysis and IgE levels), pulmonary function tests and exhaled nitric oxide (FeNO) testing, completed questionnaires on demographic factors, medication use and medical history, and had a chest MDCT prior to fiberoptic bronchoscopy (bronchoscopy methods are described in the online supplement). All procedures were performed following the SARP protocol. Details and descriptions of the SARP cohort have been previously described.23 The study was approved by each site’s Institutional Review Board and monitored by an independent Data and Safety Monitoring Board.
Human subjects
SARP subjects who underwent MDCT imaging studies were included into this study. The number is much lower than the total number of SARP subjects, as not all SARP sites were performing MDCT. Subjects were 13–60 years old and non-smokers (smoking history <5 pack-years and no smoking within past year). Normal subjects were in good health with normal lung function and a negative methacholine bronchoprovocation (provocative concentration of methacholine causing a 20% decline in forced expiratory volume in one second (FEV1) (PC20) > 16 mg/ml). All asthmatics had physician diagnosed asthma, no concurrent lung disease, and a positive methacholine bronchoprovocation (PC20 ≤ 8mg/ml) or ≥ 15% improvement in FEV1 post-bronchodilator. Asthma subjects were classified as severe or non-severe as previously described.23 Severe asthma subjects met ATS workshop refractory asthma criteria.24 All asthmatics who did not meet criteria for severe asthma were classified as non-severe asthmatics.
CT technique
Subjects underwent MDCT spiral scans of the chest with 4, 16 or 64 detector rows (GE Light Speed Ultra, GE Lightspeed 16, Siemens Volume Zoom, Siemens Sensation 16, Siemens Sensation 64 multidetector CT scanners). Suspended expiratory measurements at FRC were obtained at the following settings: GE: 1.675–1.75 pitch, 0.6 sec rotation time, 120 kV, 50–100 mAs, detector collimation 0.625 and 1.25 mm, 0.625–1.25 mm reconstructed slice thickness, medium smooth “standard” reconstruction algorithm; Siemens: 1.5 pitch, 0.5 sec rotation time, 120 kV, 50 mAs, detector collimation of 0.75 mm, 1mm reconstructed slice thickness, slice interval = field of view (mm)/512, and a medium smooth reconstruction algorithm (Siemens B31f) – effective mAs = 33 (low radiation dose). The radiation dose from the low dose CT scans (one at TLC and at FRC) ranged from 1.55 mSv effective dose to 1.70 mSv effective dose. The radiation dose from the higher dose CT scans ranged from 4.0 to 7.6 mSv effective dose. The higher effective doses occurred in larger female subjects. The total radiation dose (TLC and FRC combined) from the low dose CT scans ranged from 1.55 mSv effective dose to 1.70 mSv effective dose while the total radiation dose from the higher dose CT scans ranged from 4.0 to 7.6 mSv effective dose. The higher effective doses occurred in larger female subjects. The average dose per person from all sources of natural radiation is about 300 mrem or 3 mSv per year.25 Thus a low dose volume MDCT scan (suitable for the measure of air trapping) as used in these analyses is equivalent to approximately 30% of the radiation an individual is naturally exposed to in a year, while the high dose is equivalent to 1.5 to 2 years of natural radiation exposure.
MDCT evaluation software
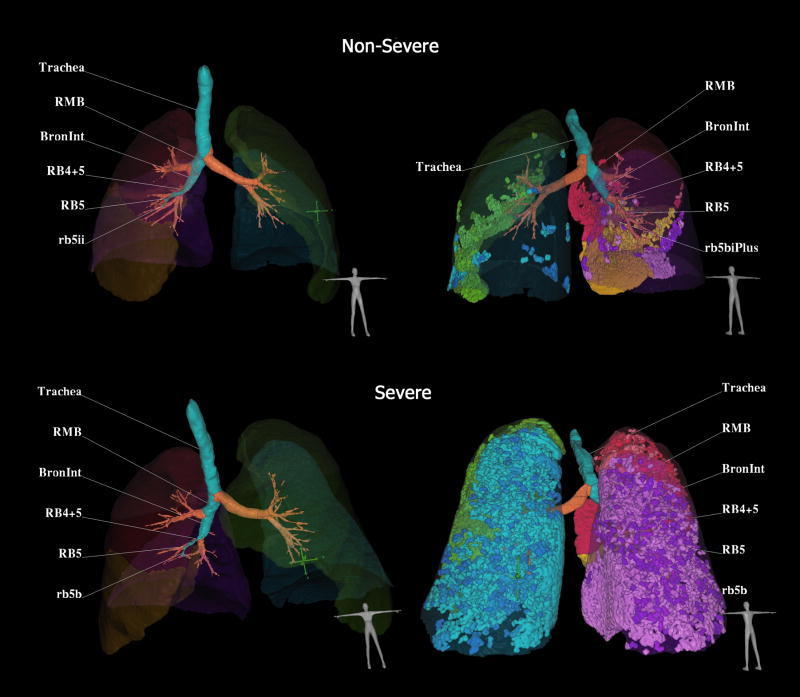
MDCT scans were obtained and analyzed using automated, lung parenchymal evaluation software. This software, using an approach built on the density mask technique, segments the lung from the rest of the thoracic anatomy and generates histogram curves of the lung voxels to analyze the percent of lung tissue between different MDCT voxel numbers, expressed in HUs (Pulmonary Profiler, VIDA Diagnostics, Iowa City, IA).26 A review of the software capabilities and a validation has been published elsewhere.27 The specific MDCT measurements used in the data analysis included percent low attenuating area (%LAA) less than −850 HU, %LAA −900 HU, %LAA −950 HU. The measurements were performed by a trained technician at the University of Iowa, Carver College of Medicine in a blinded manner. In Figure 1, a CT-derived image of the lung and airways (left column) for a severe (lower row) and a non-severe (upper row) asthmatic are illustrated. Trapped air defined as voxels within the lung field falling below −850 are highlighted in the right column. By clicking on any airway path, the software labels the bronchial segments along the path of interest. There is a marked increase in air trapping in the severe asthmatic.
Figure 1.
CT-derived three-dimension display of the lungs, airways and regions of air trapping. Example comparison of two asthmatic subjects falling in the non-severe (upper row) or severe (lower row) categories. In the left column, the lung lobes and airway tree are shown from a ventral view. In the right column, the air trapping is depicted, color coded by lobe and displayed from the dorsal aspect. Software allows one to click on an airway path of interest and airway segment labels are automatically generated. Trapped air defined as voxels within the lung field falling below −850 are highlighted and coded by lobe in the left column. The severe asthma subject has 21.0% of lung less than −850 HU as compared to the non-severe asthma subject with 4.75% of lung less than −850 HU. Images from Pulmonary Workstation 2.0 (Vida Diagnostics, Coralville, Iowa)
Subject classification
The lung percentage less than −850 HU units was dichotomized using a median split of the full cohort (n=120, median = 9.66%). Because airways within the lung boundaries are included in the VIDA software version of the density mask5,14,28–30 it is expected that all subjects will have some voxels falling within range of interest. Subjects above the median were defined as air trappers and compared to those below the median (non-trappers). Airway neutrophil and eosinophil variables were calculated based on sputum and bronchoavelor lavage (BAL) data. Sixty-six of the 90 asthma subjects had either sputum or BAL inflammatory data, and were classified as neutrophil positive if either sputum or BAL neutrophils were above the asthmatic median of either distribution (sputum or BAL). If levels were below the median, subjects were considered neutrophil negative. Subjects were classified as eosinophil positive and negative in the same manner. Atopy was defined by the presence of one or more positive allergy skin test.
Imputation of cellular data
For subjects with lung inflammatory cell data, logistic regression analysis identified variables that significantly predicted neutrophillic inflammation. The model was applied to subjects with missing neutrophil data (n=28), and the predicted probabilities used to classify these subjects as neutrophil positive or negative.
Association with lung function
Initial correlations between lung function (specifically FEV1/FVC as the most definitive parameter to measure airflow limitation) and the percent of lung at −850, −900 and −950 HU (at both FRC and TLC) were evaluated using Spearman’s correlations in the asthma subjects (FRC: −850 HU: −0.583, −900 HU: −0.514, −950 HU: −0.403 p<0.0001 for each; TLC: −850 HU: −0.362 p<0.001, −900 HU: −0.318 p=0.002, −950 HU: −0.199 p=0.06). Correlations between lung function and percent of lung density were stronger at all densities in FRC scans (indicative of air trapping) as compared to TLC scans. Additionally, correlations at −850 HU were stronger than at −900 or −950 HU. Therefore, further studies were performed using −850 HU at FRC.
Chi-square tests determined associations between air trapping and severe asthma and its outcomes (such as intensive care unit admission). Logistic regression analysis was used to evaluate univariate associations among variables for air trapping and to determine a group of risk factors associated with air trapping in asthmatic subjects. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated between air trapping and significant covariates (p<0.05). Confounding was examined as change in magnitude of the estimates.31 Table 5 (online supplement) illustrates variables of interest and their univariate associations with air trapping. All analyses were conducted with SAS (version 9.1).
RESULTS
One hundred-twenty SARP subjects were studied (60 severe persistent asthma, 34 not-severe asthma subjects, and 26 normal controls). Subject demographics are listed in Table 1.
Table 1.
Summary statistics of demographic and clinical variables for severe asthmatics, mild/moderate asthmatics, and normal controls
| Demographic and Clinical Variables | Severe Asthmatics | Non-Severe Asthmatics | Normal Controls |
|---|---|---|---|
| Number of study subjects | 60 | 34 | 26 |
| Categorical Variables | Number (Percent) | Number (Percent) | Number (Percent) |
| Female gender | 33 (55.0%) | 20 (58.8%) | 17 (65.4%) |
| Percent of Lung Less than −850 HU above median | 37 (61.7%) | 14 (41.2%) | 9 (34.6%) |
| Atopic | 46 (76.7%) | 31(91.2%) | 8 (30.8%) |
| Current Use of Oral Steroids | 26 (43.3%) | 0 (0%) | 0 (0%) |
| Current Use of Inhaled Steroids | 59 (98.3%) | 18 (52.9%) | 0 (0%) |
| Continuous Variables | Mean (Standard Deviation) | Mean (Standard Deviation) | Mean (Standard Deviation) |
| Age | 37.5 (13.3) | 34.3 (10.7) | 30.3 (7.8) |
| Percent less −850 | 20.2 (16.7) | 12.1 (12.0) | 12.3 (16.7) |
| FEV1 percent predicted | 62.7 (22.1) | 79.7 (16.6) | 99.7 (10.0) |
| FEV1/FVC (× 100) | 62.6 (13.0) | 70.1 (11.6) | 84.9 (6.3) |
| IgE level | 441.6 (694.8) | 229.7 (295.0) | 93.5 (171.5) |
Air trapping and severity of illness
The relationship of severe asthma and its outcomes with air trapping is illustrated in Figure 2. More subjects who were air trappers were severe asthmatics, although the relationship was not statistically significant (p=0.058). However, air trappers were significantly more likely to have a history of asthma-related hospitalizations, intensive care unit (ICU) visits, and/or mechanical ventilation compared to subjects classified as non-trappers. These differences suggest that MDCT-measured air trapping may identify a different and more severe phenotype of asthma. We therefore built an explanatory model of air trapping to identify clinical variables that were risk factors for air trapping.
Figure 2.
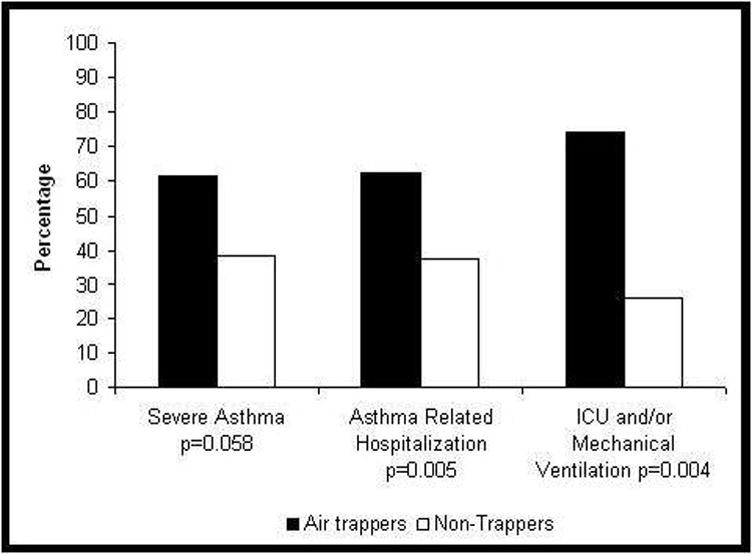
Association between air trapping and presence of severe asthma or severe asthma exacerbations.
Univariate analysis of risk factors associated with air trapping
Subjects considered air trappers were compared to the non-trapping group using univariate oddsratios (Table 3). Air trapping subjects had greater air flow limitation (FEV1 and FVC percent predicted and FEV1/FVC). Subjects with air trapping were likely to be male, older, and have a longer duration of asthma. Air trappers were more likely to report a clinical history of pneumonia and to be atopic than subjects who were not classified as air trappers. Presence of airway neutrophils was marginally associated with air trapping, while airway eosinophils were not associated. The risk of air trapping was inversely associated with FeNO (OR= 0.85, 95% CI: 0.72–0.995 for each 10 ppb increase) in a subset of subjects (n=60), but due to the low number of subjects with FeNO values, it was not considered when determining the final model.
Table 3.
Univariate Odds Ratios-Among Asthmatics for Air Trapping
| Variable | Odds Ratio (95% Confidence Interval) | Unit increase |
|---|---|---|
| FEV1/FVC | 1.64 (1.31–2.05) | 5% decrease |
| Asthma Duration | 1.36 (1.13–1.64) | 5 year increase |
| Neutrophils above the median | 2.21 (0.75.055) | |
| History of Pneumonia | 3.54 (1.45–8.60) | |
| Atopy | 5.09 (1.52–17.09) | |
| Sex | 0.43 (0.19–0.996) | |
| FEV1 % predicted | 1.32 (1.16–1.50) | 5% decrease |
| FVC % predicted | 1.20 (1.05–1.36) | 5% decrease |
| FeNO | 0.85 (0.72–0.995) | 10 unit increase |
| Smoking | 1.29 (0.44–3.73) | |
| Eosinophils above the median | 0.80 (0.24–2.68) | |
| Paternal history of allergies | 0.58 (0.21–1.59) | |
| Paternal history of asthma | 1.07 (0.43–2.63) | |
| GERD | 2.07 (0.85–5.08) | |
| Age | 1.68 (1.17–2.40) | 10 year increase |
| Oral Steroid Use | 1.88 (0.74–4.82) |
Multivariate logistic regression analysis
Duration of asthma, history of pneumonia, high levels of neutrophils in the airway, air flow obstruction as measured by FEV1/FVC and atopy were identified as risk factors associated with the air-trapping phenotype (Table 4). For each 5 year increase in asthma duration, there was a 42%increase in the odds of air trapping. A 5% decrease in FEV1/FVC corresponded to a 61% increase in the odds of air trapping. Subjects with a history of pneumonia were at increased risk of air trapping compared to those with no history. Airway neutrophils above the median in sputum or BAL were also associated with an increased risk of air trapping. Atopic subjects were more likely to be air trappers than non-atopic subjects. Although the estimates were relatively large, the confidence intervals were wide for pneumonia, neutrophils, and atopy, all reflective of the relatively small sample size.
Table 4.
Results From Multivariate Logistic Regression Model
| Variable | Odds Ratio (95% Confidence Interval) |
|---|---|
| Duration of Asthma (5 year increase) | 1.42 (1.09–1.87) |
| FEV1/FVC (5% decrease) | 1.61 (1.21–2.15) |
| History of Pneumonia | 8.55 (2.07–35.26) |
| Neutrophillic Inflammation in Airway above the Median | 8.66 (2.05–36.57) |
| Atopy | 11.54 (1.97–67.70) |
The model building process was also completed using only those subjects with measured neutrophil data; the terms were identical, and coefficients were not different from those in the presented model (data not shown).
Air trapping in normal controls
Almost 35% of the normal subjects were classified as air trappers (Table 1). Power limited the analysis of clinical variables with air trapping using multivariate models. However, the odds of air trapping increased significantly with increasing levels of airway obstruction even though FEV1/FVC values were within the normal range. Females were less likely to be air trappers than males although the difference was not statistically significant (p=0.11). No other variables associated with air trapping in asthmatics were associated with air trapping in the normal group.
DISCUSSION
This is the first large study of CT measured air-trapping in a range of extensively characterized asthmatic subjects to identify independent risk factors for the air trapping phenotype, a phenotype associated with the most severe form of asthma. This assessment of air trapping was quantitatively and objectively performed using a histogram based assessment of lung densities (VI-DA Diagnostics, Coralville Iowa) based on the density mask, but which employs a more sophisticated method for identifying lung boundaries.26 Muller et al.30 developed the original concept for the density mask based on early observations which demonstrated that lung volume 32 and regional air content, or density,33 could be accurately assessed via CT. This density mask method identified the lung field and a density threshold within the lung field to count emphysema-like lung voxels. Since then, the histogram of voxel density within the lung field has been widely used to identify emphysema-like lung and fibrosis as reviewed in28 and, in the case of this study, trapped air when the lung is imaged at low lung volumes.14 The histogram-based assessment of the lung used here replaces the former “density mask” approach, but the essence of the measurement remains the same.
In this study, air trapping subjects were defined as individuals with ≥9.66% of their total lung volume at FRC <−850 HU. While this density is not as extreme as the −910 to −970 HU threshold applied to COPD/emphysema, previous reports suggest that this degree of hyperlucency (<−850 HU) should only be seen at TLC as this density is measuring a fully distended alveolus.3 Additionally, higher correlations with lung function were seen at the −850 HU threshold than at either −900 HU or −950 HU, suggesting that −850 HU may be a more appropriate threshold for asthma. As asthma, even in severe cases, is not pathologically an “emphysematous” process involving alveolar septal destruction, the better discrimination of our data at this higher cut-off is not surprising.
This threshold applied to asthma, identified a marginally more severe cohort using the ATS Refractory Asthma definition, but who were much more likely to have had a history of a severe and/or near fatal asthma event, similar to previous reports for physiologic measures of air trapping.1 A recent study, from this cohort, reported that severe asthmatics had a greater component of physiologically measured air trapping relative to airflow limitation than milder subjects and concluded that air trapping is broadly associated with severe asthma.22 Further, Mitsunobu et al. assessed air trapping using MDCT and reported that the relative area of the lungs less than −950 HU correlated with air flow limitation and with severity of asthma. Therefore, our findings are not completely unexpected.34 Unlike previous studies, the SARP database contains a multitude of variables which were then utilized to determine risk factors for air trapping on MDCT scan using a multivariate modeling approach.
Based on univariate analysis, numerous factors were associated with the air trapping phenotype including airway obstruction, measured by FEV1/FVC. We chose FEV1/FVC (among the multiple related spirometric values available) as the FEV1% predicted is low in restrictive, as well as obstructive disease, while the FEV1/FVC decreases only with increasing airflow limitation. This relationship has been reported in physiologically measured air trapping1 and in air trapping measured by MDCT–albeit, based on univariate analysis.34 A variety of other factors, including longer duration of disease, male sex, and lower FeNO were either marginally or significantly related. The association of air trapping with increased age and longer disease duration suggest a contribution of remodeling over time, while the relationship with male sex could be explained by the greater prevalence of asthma in early childhood in boys than in girls or a greater susceptibility to elements of the remodeling process. Interestingly, when matched for severity, male asthmatics have lower FEV1, as well as lower FEV1/FVC, than females.35 The relationship of air trapping with lower FeNO is surprising but may suggest that, in this cohort, NO has a bronchodilating effect36 that limits the degree of air trapping seen. However, because of its limited sample size, FeNO was not considered in the multivariate analysis. Further studies are needed to determine if it is protective against air trapping. Despite the potential relationship with FeNO, eosinophils were not associated with air trapping. The relationship of eosinophils to airway obstruction has been variable across studies,1,37–40 therefore, further study of this relationship is required
A multivariate analysis was undertaken selecting factors in the univariate analyses associated with air trapping (p<0.20). In the multivariate analysis, FEV1/FVC, duration of disease, reported history of pneumonia, neutrophillic airway inflammation, and atopy were identified as independent risk factors. Some univariately associated variables were not significant in the multivariate model, likely due to the overlapping nature of these variables. Among the risk factors, perhaps the most interesting are history of pneumonia, neutrophilic inflammation, and atopy. Because this is a cross-sectional study, causal relationships can not be presumed, with the observed relationships as likely to be a consequence of air trapping as causes. Despite these uncertainties, the results remain provocative. Consistent with our finding of an association of the more severe air trapping phenotype with history of pneumonia, analysis of the entire SARP database (>400 subjects) determined pneumonia to be independently associated with asthma severity (OR=3.30 95%CI:1.92–5.69).23 More severe disease may increase the risk for developing pneumonia due to poor secretion clearance and immunosuppression by corticosteroids (CS). An analysis of a large healthcare database found that asthmatics are at higher risk of development of pneumonia.41 Inhaled CS (ICS) as a risk factor for pneumonia is also becoming increasingly identified. ICS use has been associated with an increased risk for pneumonia in prospective studies of COPD.42,43 All severe asthma subjects in this study were on high ICS doses which could have contributed to a higher pneumonia risk. Only longitudinal studies will confirm (or refute) that relationship.
Another interesting risk factor was airway neutrophilia. The observation that pneumonia and neutrophils are independent risk factors for air trapping suggests that historical pneumonia is not driving the neutrophilia, nor is the neutrophilia likely a residual of pneumonia. It is possible that neutrophlia is a by-product of high corticosteroid use in this population. Corticosteroids inhibit neutrophil apoptosis and enhance their activity and survival which may explain their increase.44,45 Unfortunately, despite greater than 100 patients in this trial, power limitations restricted our ability to adjust the model for corticosteroids. Whether caused by more severe disease or its treatment, higher levels of lung neutrophils could lead to air trapping. Neutrophils produce enzymes, including elastases and metalloproteinases which contribute to elastin (and other matrix elements) breakdown observed in fatal asthma and severe cases of asthma.46–48 These airway and perhaps parenchymal changes could alter elastic recoil properties and lead to a more “emphysematous-like” pattern and increased air trapping. An emphysematous-like pattern seen on CT in chronic asthma subjects has been reported in other studies.49
The final risk factor of interest was atopy. Had the analysis included non-asthmatics, this association would not have been surprising, as atopy is strongly associated with asthma. However, the analysis was restricted to asthmatics, 82% of whom were atopic. Non-atopic asthmatics are a mix of individuals including aspirin sensitive to post-viral adult onset asthmatics.38,50 The multivariate analysis is adjusted for asthma duration so the relationship cannot be attributed to non-atopic asthmatics having a shorter disease duration. The relationship between atopy and air trapping has not been extensively evaluated. One study reported more extensive airway remodeling (assessed by high resolution CT) among non-atopic individuals than atopic individuals.51 This study did not specifically assess air trapping and only qualitative analyses were conducted. Further studies are needed to determine whether the remodeling process associated with non-atopic differs from atopic asthma, leading to differences in radiologic and physiologic changes.
Finally, a large percentage of normal controls met the threshold for air trapping. It is unclear whether these subjects are at increased risk for asthma, have genetic predisposition to air trapping, or had some past insult which induced these changes. Although these subjects had normal pulmonary function testing and negative methacholine challenges, they had a lower FEV1/FVC and tended to be males, both seen in the asthmatics with air trapping. Studies of air trapping in normal subjects are needed to determine if air trapping is a risk factor for asthma development.
There are limitations to this report. Although one of the largest MDCT studies of asthma, a larger sample size would have provided increased power perhaps resulting in more stable estimates and ability to evaluate other factors. Additionally, airway neutrophil data were unavailable for 23% of subjects. We imputed missing airway neutrophil data to consider this variable in the multivariate logistic regression model. The imputation may have resulted in misclassification, however including imputed values did not change the results obtained using subjects with actual neutrophil data. In contrast, the strengths include the large well-characterized and diverse population, the availability of lung inflammatory markers and the quantitative measure of air trapping. Finally, the multivariate analysis included a range of data allowing for examination of confounding.
This study supports the utility of MDCT scanning to identify a group of asthmatics at risk of severe disease, particularly intensive healthcare utilization. Independent risk factors were identified, including history of pneumonia, neutrophilic inflammation, and atopy. Further prospective studies to evaluate the role of these factors in development of this phenotype are needed.
Supplementary Material
Table 2.
Summary statistics of demographic and clinical variables by air trapping status
| Demographic and Clinical Variables | Trappers | Non-trappers |
|---|---|---|
| Number of study subjects | 51 | 43 |
| Categorical Variables | Number (Percent) | Number (Percent) |
| Female gender | 24 (47.1%) | 29 (67.4%) |
| Current use of oral steroids | 17 (33.3%) | 9 (20.9%) |
| Current use of inhaled steroids | 41 (87.2%) | 36 (76.7%) |
| High level of neutrophils | 25 (78.1%) | 21 (61.8%) |
| High level of eosinophils | 9 (28.1%) | 10 (29.4%) |
| Ever smoked | 10 (20.0%) | 7 (16.3%) |
| History of GERD | 22 (44.9%) | 11 (28.2%) |
| History of pneumonia | 33 (70.2%) | 16 (40.0%) |
| Severe Asthma | 37 (72.6%) | 23 (53.5%) |
| Atopic | 47 (92.2%) | 30 (69.8%) |
| Continuous Variables | Mean (Standard Deviation) | Mean (Standard Deviation) |
| Age | 39.8 (12.1) | 32.3 (11.8) |
| Percent less −850 | 28.0 (13.9) | 4.5 (2.4) |
| Duration of asthma | 27.2 (13.7) | 17.9 (10.6) |
| Age at onset of asthma | 12.6 (14.2) | 14.3 (14.9) |
| FEV1 percent predicted | 59.4 (21.0) | 80.1( 16.9) |
| FVC percent predicted | 78.6 (20.8) | 90.2 (15.7) |
| FEV1/FVC × 100 | 59.5 (11.8) | 72.3 (10.8) |
| Positive skin reactions (number) | 4.1 (2.6) | 3.1 (3.2) |
| Percent eosinophils (sputum) | 4.1 (5.5) | 6.2 (19.5) |
| Percent eosinophils (BAL) | 1.9 (7.1) | 0.8 (1.4) |
| Percent neutrophils (sputum) | 41.8 (22.6 | 22.6 (19.1) |
| Percent neutrophils (BAL) | 6.0 (6.1) | 1.3 (1.9) |
| IgE level | 257.8 (327.5) | 450.5 (735.6) |
| FeNO | 30.7 (26.2) | 52.9 (48.2) |
Acknowledgments
American Academy of Allergy, Asthma and Immunology’s Strategic Training in Allergy Research (ST*AR) Award
Grant Support: National Institutes of Health HL69149, HL64368, HL69349, HL69170, HL-69155, HL69174, HL69130, HL69167, HL69116, HL69174-05
Footnotes
No authors have conflicts of interest to disclose.
References
- 1.Wenzel S, Schwartz L, Langmack E, et al. Evidence that severe asthma can be divided pathologically into two inflammatory subtypes with distinct physiologic and clinical characteristics. Am J Respir Crit Care Med. 1999;160:1001–1008. doi: 10.1164/ajrccm.160.3.9812110. [DOI] [PubMed] [Google Scholar]
- 2.Martinez FJ, Foster G, Curtis JL, et al. Predictors of Mortality in Patients with Emphysema and Severe Airflow Obstruction. Am J Respir Crit Care Med. 2006;173:1326–1334. doi: 10.1164/rccm.200510-1677OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coxson HO, Rogers RM, Whittall KP, et al. A Quantification of the Lung Surface Area in Emphysema Using Computed Tomography. Am J Respir Crit Care Med. 1999;159:851–856. doi: 10.1164/ajrccm.159.3.9805067. [DOI] [PubMed] [Google Scholar]
- 4.Madani A, Zanen J, de Maertelaer V, et al. Pulmonary emphysema: objective quantification at multi-detector row CT--comparison with macroscopic and microscopic morphometry. Radiology. 2006;238:1036–1043. doi: 10.1148/radiol.2382042196. [DOI] [PubMed] [Google Scholar]
- 5.Jain N, Covar R, Gleason M, et al. Quantitative Computed Tomography Detects Peripheral Airway Disease in Asthmatic Children. Pediatr Pulmonol. 2005;40:211–218. doi: 10.1002/ppul.20215. [DOI] [PubMed] [Google Scholar]
- 6.Bakker M, Stolk J, Putter H, et al. Variability in densitometric assessment of pulmonary emphysema with computed tomography. Investigative Radiology. 2005;40:777–783. doi: 10.1097/01.rli.0000186418.31139.21. [DOI] [PubMed] [Google Scholar]
- 7.Newell J, Hogg JC, Snider GL. Report of a workshop: quantitative computed tomography scanning in longitudinal studies of emphysema. Eur Respir J. 2004;23:769–775. doi: 10.1183/09031936.04.00026504. [DOI] [PubMed] [Google Scholar]
- 8.Bankier AA, Madani A, Gevenois PA. CT quantification of pulmonary emphysema: assessment of lung structure and function. Crit Rev Comput Tomogr. 2002;43:399–417. [PubMed] [Google Scholar]
- 9.Gierada DS, Yusen RD, Pilgram TK, et al. Repeatability of quantitative CT indexes of emphysema in patients evaluated for lung volume reduction surgery. Radiology. 2001;220:448–454. doi: 10.1148/radiology.220.2.r01au46448. [DOI] [PubMed] [Google Scholar]
- 10.Gierada DS, Yusen RD, Villanueva IA, et al. Patient selection for lung volume reduction surgery: An objective model based on prior clinical decisions and quantitative CT analysis. Chest. 2000;117:991–998. doi: 10.1378/chest.117.4.991. [DOI] [PubMed] [Google Scholar]
- 11.Mergo PJ, Williams WF, Gonzalez-Rothi R, et al. Three-dimensional volumetric assessment of abnormally low attenuation of the lung from routine helical CT: inspiratory and expiratory quantification. Am J Roentgenol. 1998;170:1355–1360. doi: 10.2214/ajr.170.5.9574615. [DOI] [PubMed] [Google Scholar]
- 12.Gould GA, MacNee W, McLean A, et al. CT measurements of lung density in life can quantitate distal airspace enlargement--an essential defining feature of human emphysema. Am Rev Respir Dis. 1988;137:380–392. doi: 10.1164/ajrccm/137.2.380. [DOI] [PubMed] [Google Scholar]
- 13.Coxson HO, Mayo JR, Behzad H, et al. Measurement of lung expansion with computed tomography and comparison with quantitative histology. J Appl Physiol. 1995;79:1525–1530. doi: 10.1152/jappl.1995.79.5.1525. [DOI] [PubMed] [Google Scholar]
- 14.Newman K, Lynch D, Newman L, et al. Quantitative Computed Tomography Detects Air Trapping due to Asthma. Chest. 1994;106:105–109. doi: 10.1378/chest.106.1.105. [DOI] [PubMed] [Google Scholar]
- 15.Miniati M, Filippi E, Falaschi F, et al. Radiologic evaluation of emphysema in patients with chronic obstructive pulmonary disease. Chest radiography versus high resolution computed tomography. Am J Respir Crit Care Med. 1995;151:1359–1367. doi: 10.1164/ajrccm.151.5.7735585. [DOI] [PubMed] [Google Scholar]
- 16.Gevenois PA, De Vuyst P, Sy M, et al. Pulmonary emphysema: quantitative CT during expiration. Radiology. 1996;199:825–829. doi: 10.1148/radiology.199.3.8638012. [DOI] [PubMed] [Google Scholar]
- 17.Gierada DS, Slone RM, Bae KT, et al. Pulmonary emphysema: comparison of preoperative quantitative CT and physiologic index values with clinical outcome after lung-volume reduction surgery. Radiology. 1997;205:235–242. doi: 10.1148/radiology.205.1.9314991. [DOI] [PubMed] [Google Scholar]
- 18.Johnson JL, Kramer SS, Mahboubi S. Air trapping in children: evaluation with dynamic lung densitometry with spiral CT. Radiology. 1998;206:95–101. doi: 10.1148/radiology.206.1.9423657. [DOI] [PubMed] [Google Scholar]
- 19.Bankier AA, De Maertelaer V, Keyzer C, et al. Pulmonary Emphysema: Subjective Visual Grading versus Objective Quantification with Macroscopic Morphometry and Thin-Section CT Densitometry. Radiology. 1999;211:851–858. doi: 10.1148/radiology.211.3.r99jn05851. [DOI] [PubMed] [Google Scholar]
- 20.Walamies M. Diagnostic role of residual volume in paediatric patients with chronic symptoms of the lower airways. Clin Physiol. 1998;18:49–54. doi: 10.1046/j.1365-2281.1998.00072.x. [DOI] [PubMed] [Google Scholar]
- 21.Brown R, Pearse D, Pyrgos G, et al. The structural basis of airways hyperresponsiveness in asthma. J Appl Physiol. 2006;101:30–39. doi: 10.1152/japplphysiol.01190.2005. [DOI] [PubMed] [Google Scholar]
- 22.Sorkness RL, Bleecker ER, Busse WW, et al. Lung function in adults with stable but severe asthma: air trapping and incomplete reversal of obstruction with bronchodilation. J Appl Physiol. 2008;104:394–403. doi: 10.1152/japplphysiol.00329.2007. [DOI] [PubMed] [Google Scholar]
- 23.Moore WC, Bleecker ER, Curran-Everett D, et al. Characterization of the severe asthma phenotype by the National Heart, Lung, and Blood Institute’s Severe Asthma Research Program. J Allergy Clin Immunol. 2007;119:405–413. doi: 10.1016/j.jaci.2006.11.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wenzel S, Fahy J, Irvin C, et al. Proceeding of the ATS workshop on refractory asthma: current understanding, recommendations, and unanswered questions. Am J Resp Crit Care Med. 2000;162:2341–2351. doi: 10.1164/ajrccm.162.6.ats9-00. [DOI] [PubMed] [Google Scholar]
- 25.Huda W, Vance A. Patient radiation doses from adult and pediatric CT. Am J Roentgenol. 2007;188:540–546. doi: 10.2214/AJR.06.0101. [DOI] [PubMed] [Google Scholar]
- 26.Hu S, Hoffman EA, Reinhardt JM. Automatic lung segmentation for accurate quantitation of volumetric X-ray CT images. IEEE Trans Med Imaging. 2001;20:490–498. doi: 10.1109/42.929615. [DOI] [PubMed] [Google Scholar]
- 27.Hoffman E, Clough A, Christensen G, et al. The comprehensive imaging-based analysis of the lung: a forum for team science. Acad Radiol. 2004;11:1370–1380. doi: 10.1016/j.acra.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 28.Hoffman EA, Reinhardt JM, Sonka M, et al. Characterization of the interstitial lung diseases via density-based and texture-based analysis of computed tomography images of lung structure and function. Acad Radiol. 2003;10:1104–1118. doi: 10.1016/s1076-6332(03)00330-1. [DOI] [PubMed] [Google Scholar]
- 29.van Beek E, Hoffman E. Functional Imaging: CT and MRI. Clin Chest Med. 2008;29:195–216. doi: 10.1016/j.ccm.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Müller NL, Staples CA, Miller RR, et al. “Density mask”. An objective method to quantitate emphysema using computed tomography. Chest. 1988;94:782–787. doi: 10.1378/chest.94.4.782. [DOI] [PubMed] [Google Scholar]
- 31.Hosmer D, Lemeshow S. Applied Logistic Regression. 2. New York: Wiley and Sons, Inc; 2000. [Google Scholar]
- 32.Hoffman E, Sinak L, Robb R, et al. Noninvasive quantitative imaging of shape and volume of lungs. J Appl Physiol. 1983;54:1414–1421. doi: 10.1152/jappl.1983.54.5.1414. [DOI] [PubMed] [Google Scholar]
- 33.Hoffman EA. Effect of body orientation on regional lung expansion: a computed tomo-graphic approach. J Appl Physiol. 1985;59:468–480. doi: 10.1152/jappl.1985.59.2.468. [DOI] [PubMed] [Google Scholar]
- 34.Mitsunobu F, Mifune T, Ashida K, et al. Influence of age and disease severity on high resolution CT lung densitometry in asthma. Thorax. 2001;56:851–856. doi: 10.1136/thorax.56.11.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee JH, Haselkorn T, Chipps BE, et al. Gender differences in IgE-mediated allergic asthma in the epidemiology and natural history of asthma: Outcomes and Treatment Regimens (TENOR) study. J Asthma. 2006;43:179–184. doi: 10.1080/02770900600566405. [DOI] [PubMed] [Google Scholar]
- 36.Brown RH, Mitzner W. Airway response to deep inspiration: role of nitric oxide. Eur Respir J. 2003;22:57–61. doi: 10.1183/09031936.03.00090403. [DOI] [PubMed] [Google Scholar]
- 37.ten Brinke A, Grootendorst DC, Schmidt JT, et al. Chronic sinusitis in severe asthma is related to sputum eosinophilia. J Allergy Clin Immunol. 2002;109:621–626. doi: 10.1067/mai.2002.122458. [DOI] [PubMed] [Google Scholar]
- 38.Miranda CC, Busacker AA, Balzar SS, et al. Distinguishing severe asthma phenotypes: role of age at onset and eosinophilic inflammation. J Allergy Clin Immunol. 2004;113:101–108. doi: 10.1016/j.jaci.2003.10.041. [DOI] [PubMed] [Google Scholar]
- 39.Crimi E, Spanevello A, Neri M, et al. Dissociation between Airway Inflammation and Airway Hyperresponsiveness in Allergic Asthma. Am J Respir Crit Care Med. 1998;157:4–9. doi: 10.1164/ajrccm.157.1.9703002. [DOI] [PubMed] [Google Scholar]
- 40.Virchow JC, Jr, Hölscher U, Virchow C., Sr Sputum ECP levels correlate with parameters of airflow obstruction. Am Rev Respir Dis. 1992;146:604–606. doi: 10.1164/ajrccm/146.3.604. [DOI] [PubMed] [Google Scholar]
- 41.Talbot TR, Hartert TV, Mitchel E, et al. Asthma as a Risk Factor for Invasive Pneumo-coccal Disease. N Engl J Med. 2005;352:2082–2090. doi: 10.1056/NEJMoa044113. [DOI] [PubMed] [Google Scholar]
- 42.Calverley PM, Anderson JA, Celli B, et al. Salmeterol and Fluticasone Propionate and Survival in Chronic Obstructive Pulmonary Disease. N Engl J Med. 2007;356:775–789. doi: 10.1056/NEJMoa063070. [DOI] [PubMed] [Google Scholar]
- 43.Ernst P, Gonzalez AV, Brassard P, et al. Inhaled Corticosteroid Use in Chronic Obstructive Pulmonary Disease and the Risk of Hospitalization for Pneumonia. Am J Respir Crit Care Med. 2007;176:162–166. doi: 10.1164/rccm.200611-1630OC. [DOI] [PubMed] [Google Scholar]
- 44.Cox G. Glucocorticoid treatment inhibits apoptosis in human neutrophils. Separation of survival and activation outcomes. J Immunol. 1995;154:4719–4725. [PubMed] [Google Scholar]
- 45.Liles WC, Dale DC, Klebanoff SJ. Glucocorticoids inhibit apoptosis of human neutrophils. Blood. 1995;86:3181–3188. [PubMed] [Google Scholar]
- 46.Mauad T, Silva LFF, Santos MA, et al. Abnormal Alveolar Attachments with Decreased Elastic Fiber Content in Distal Lung in Fatal Asthma. Am J Respir Crit Care Med. 2004;170:857–862. doi: 10.1164/rccm.200403-305OC. [DOI] [PubMed] [Google Scholar]
- 47.Mauad T, Xavier AC, Galtarossa S, et al. Elastosis and Fragmentation of Fibers of the Elastic System in Fatal Asthma. Am J Respir Crit Care Med. 1999;160:968–975. doi: 10.1164/ajrccm.160.3.9809088. [DOI] [PubMed] [Google Scholar]
- 48.Pham DM, Chu HW, Martin RJ, et al. Increased matrix metalloproteinase-9 with elasto-lysis in nocturnal asthma. Ann Allergy Asthma Immunol. 2003;90:72–78. doi: 10.1016/S1081-1206(10)63617-4. [DOI] [PubMed] [Google Scholar]
- 49.Biernacki W, Redpath AT, Best JJ, et al. Measurement of CT lung density in patients with chronic asthma. Eur Respir J. 1997;10:2455–2459. doi: 10.1183/09031936.97.10112455. [DOI] [PubMed] [Google Scholar]
- 50.Szczeklik A, Stevenson D. Aspirin-induced asthma: advances in pathogenesis, diagnosis, and management. J Allergy Clin Immunol. 2003;111:913–921. doi: 10.1067/mai.2003.1487. [DOI] [PubMed] [Google Scholar]
- 51.Paganin F, Séneterre E, Chanez P, et al. Computed tomography of the lungs in asthma: influence of disease severity and etiology. Am J Respir Crit Care Med. 1996;152:110–114. doi: 10.1164/ajrccm.153.1.8542102. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.