Abstract
Knowledge of vapor pressures of high molar mass organics is essential to predicting their behavior in combustion systems as well as their fate and transport within the environment. This study involved polycyclic aromatic compounds (PACs) containing halogen hetero-atoms, including bromine and chlorine. The vapor pressures of eight PACs, ranging in molar mass from (212–336) g·mol−1, were measured using the isothermal Knudsen Effusion technique over the temperature range of (296–408) K. These compounds included those with few or no data available in the literature, namely: 1,4-dibromonaphthalene; 5-bromoacenaphthene; 9-bromoanthracene; 1,5-dibromoanthracene; 9,10-dibromoanthracene; 2-chloroanthracene; 9,10-dichloroanthracene and 1-bromopyrene. Enthalpies of sublimation of these compounds were determined via application of the Clausius-Clapeyron equation. An analysis is presented on the effects of the addition of halogen hetero-atoms to pure polycyclic aromatic hydrocarbons using these data as well as available literature data. As expected, the addition of halogens onto these PACs increases their enthalpies of sublimation and decreases their vapor pressures as compared to the parent compounds.
Keywords: vapor pressure, polycyclic aromatic hydrocarbons, halogen, hetero-atom, Knudsen effusion
1. Introduction
A variety of applications requires vapor pressure data for predicting many phenomena; e.g. the pharmaceutical industry uses vapor pressures in developing inhalation delivery systems and for estimating the potential hazards associated with the inhalation of toxic vapors [1]. The vapor pressures of polycyclic aromatic compounds (PACs) are important in flame modeling [2] and in determining thermal remediation conditions for sites contaminated with coal and other fossil fuels, such as former manufactured gas plants [3]. There exist relatively few vapor pressure and thermodynamic data concerning PACs.
This study focused on those PACs containing chlorine and bromine atoms, persistent organic pollutants (POPs) included in many toxic risk assessments [4]. Polycyclic aromatic hydrocarbons are found at processing sites for fossil fuels. The PAHs may undergo a variety of halogenation mechanisms to yield PACs in various processes of their environments. For example, Hu et al. [5] show that the presence of bromine ions significantly increases the reaction rate of the cholorination of pyrene, while simultaneously producing brominated pyrenes, including 1-bromopyrene. The promotion of soot formation in flames by chlorine and chlorinated hydrocarbons is linked to PAH formation, as PAHs are believed to be soot precursors [6]. In the presence of bromine and chlorine, it may be possible to promote the formation of halogenated and non-halogenated PAHs [5].
A further step in this research was to analyze available literature data on halogenated PACs in conjunction with the newly obtained data set to investigate whether trends exist with the substitution of halogens onto these environmentally critical aromatic compounds. An extensive literature exists on group contribution methods for predicting phase equilibrium for organic compounds, yet many of these models fall short when polycyclic aromatics are considered [7]. Thus, we aim towards a future ability to describe, systematically, the thermodynamic effect of halogen substitution on the vapor pressures of PACs, for which the presently reported data would be very valuable.
To perform this work, we use the Knudsen effusion technique, which enables the measurement of vapor pressures of high molar mass, semi-volatile organic compounds indirectly via the molecular effusion of a vapor through an orifice under a high vacuum at low to moderate temperatures. This technique eliminates the need for the high temperatures required to measure low volatility compound vapor pressures directly, as high temperatures result in the thermal degradation of these compounds.
The Knudsen effusion method is used to determine indirectly vapor pressures by measuring the molecular leak rate from an effusion cell through a small orifice, without disturbing the thermal and chemical equilibrium within the cell. Application of the Knudsen theory stipulates the conditions of thermal and chemical equilibrium within the sample cell. The rate of molecular effusion through the pinhole leak (measured as the mass loss of sample from the cell) equals the rate at which molecules would strike an area of wall equal to the area of the hole, if the hole were not present; the long mean free path of the vapor molecules as compared to the radius of the orifice justifies this assumption. The use of high vacuum (<10−7 Torr) guarantees this required long mean free path, enabling measurements of vapor pressures as low as 10−6 Torr, at experimental temperatures sufficiently low enough to prevent thermal degradation of the sample. The vapor pressures measured are actually sublimation vapor pressures because of the low temperatures and solid state of the polycyclic aromatic compounds examined. By assuming a constant enthalpy of sublimation, ΔsubH, over the temperature ranges employed, the Clausius-Clapeyron equation models the vapor pressure data as a function of temperature:
| (1) |
where P° is the saturation vapor pressure, T is the absolute temperature, Δsub S is the entropy of sublimation, and R is the universal gas constant. This integrated form of the Clausius-Clapeyron equation results in a fairly linear vapor pressure curve of ln P° vs. 1/T, well representing data in the pressure region of 10−6 to 10−3 Torr.
2. Experimental
2.1. The Knudsen Effusion Technique
The present implementation of the Knudsen effusion method is described in previous publications [3,8]. Measurements were made under isothermal conditions, using an Omega type K thermocouple, calibrated to ±0.1K, located directly above the effusion cell opening. The cell was suspended on one arm of a Cahn 2000 microbalance with a sensitivity of 0.5μg and hangs inside a black copper capsule within the glass-enclosed thermo-gravimetric apparatus (TGA). The balance is interfaced with a National Instruments Data Acquisition Board recording a signal into a National Instruments Labview 7.0 program, continuously monitoring the mass loss and temperature as a function of time. A BOC Edwards turbo-molecular pump maintains the backpressure in the TGA at 10−8 Torr. A Stanford Mass Spectrometer is located after the effusion cell opening to measure the composition of the effusing vapor, used to ensure there is no degradation of the sample and to check the purity of the compounds. A condenser slightly downstream of the mass spectrometer opening helps maintain the high vacuum outside of the cell.
2.2. Materials Examined
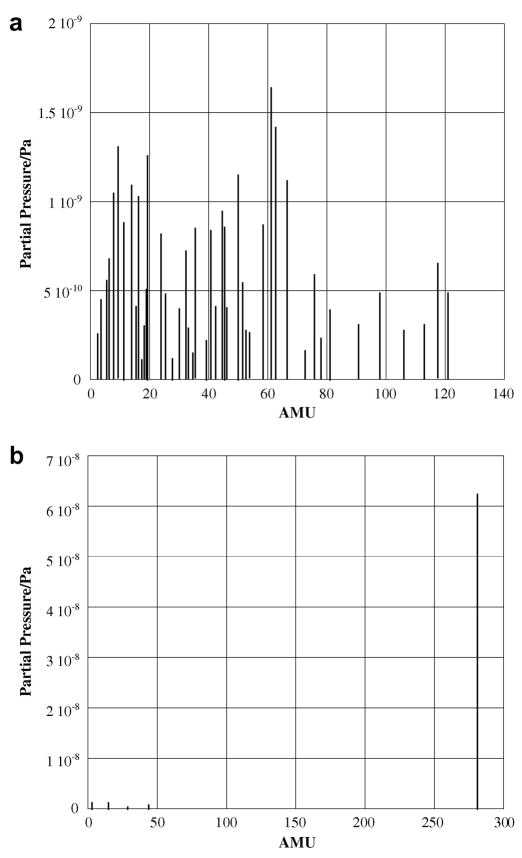
For each compound examined, vapor pressure results were confirmed by a minimum of two independent sets of measurements, in two different effusion cells, to ensure reproducibility. The polycyclic aromatic compounds examined range in molar mass from (212–336) g·mol−1, and were all obtained from TCI America at a minimum purity of 94%. The compounds were placed into the effusion cells straight from the supply container; at least 6% (by mass) of each compound was sublimed before commencing data collection to ensure removal of any volatile impurities, and data recording halted with at least 6% (by mass) remaining to account for any nonvolatile impurities. This method was confirmed by the use of the mass spectrometer to measure the spectra of the effusing vapor. Figure 1, below, shows the spectra of 1-bromopyrene after approximately 1% mass loss and again at 6% mass loss. From these data, we conclude that the fractional sublimation is sufficient to remove the impurities present in the compounds; the impurities all appear to be more volatile than the compound under investigation. These measurements were taken for the four least pure compounds: 5-bromoanthracene, 9-bromoanthracene, 9,10-dichloroanthracene, and 1-bromopyrene. For each compound, the least volatile impurity measured was at the mass of the parent aromatic compound (at 202 atomic mass units for 1-bromopyrene, the mass of the parent compound, pyrene, and so on.) After 6% of the mass was sublimed for each compound, at both the high and low temperatures measured for each compound, over 99% of the vapor phase composition from that point was the halogenated PAH, with the balance hydrogen, nitrogen and oxygen. Furthermore, the melting points of the four least pure compounds were measured using a Digimelt capillary melting point apparatus, for which all fell within their reported literature range; for example, the supplier reports a melting point for 9,10-dichloranthracene of T = 483 K, which we measured at T = 482.53 K, suggesting that any impurities present are likely volatile in nature. This was confirmed for the three other compounds.
Figure 1.
a: Mass spectrum of 1-bromopyrene following 1% mass loss with multiple low-volatile impurities. B: Mass spectrum of 1-bromopyrene following 6% mass loss yielding only 1-brompyrene, hydrogen, nitrogen and oxygen and water.
3. Results
The experimental technique was validated by comparing vapor pressures obtained using the present technique applied to fluorene, anthracene, and pyrene, and comparing these to available literature values, spanning a temperature range of 298K to 381K [8].
Table 1 presents data obtained using isothermal steps. These data were examined using the Knudsen effusion equation (equation 1, above) to obtain the enthalpies and entropies of sublimation of each compound, displayed in table 2, which also details the statistical significance of these results; a 95% confidence interval was calculated for each set of experimental results using linear regression.
Table 1.
Measured vapor pressures of halogenated PACs
|
1,4-dibromonaphthalene | |||
| T/K | Pvap/Pa | T/K | Pvap/Pa |
| 297.4 | 0.0193 | 307.9 | 0.0688 |
| 297.8 | 0.0195 | 313.5 | 0.127 |
| 301.2 | 0.0288 | 318.8 | 0.225 |
| 303.4 | 0.0384 | 320.6 | 0.269 |
| 304.6 | 0.0445 | 322.7 | 0.325 |
| 306.9 | 0.0599 | ||
|
5-bromoacenaphthene | |||
| T/K | Pvap/Pa | T/K | Pvap/Pa |
| 295.5 | 0.0392 | 314.4 | 0.344 |
| 298.5 | 0.0563 | 316.6 | 0.435 |
| 303.6 | 0.107 | 318.9 | 0.560 |
| 305.7 | 0.144 | 319.2 | 0.553 |
| 308.0 | 0.184 | 321.2 | 0.725 |
| 311.9 | 0.277 | 321.4 | 0.679 |
|
9-bromoanthracene | |||
| T/K | Pvap/Pa | T/K | Pvap/Pa |
| 315.6 | 0.0060 | 342.1 | 0.102 |
| 319.0 | 0.0097 | 342.3 | 0.116 |
| 321.8 | 0.0116 | 344.1 | 0.136 |
| 322.9 | 0.0157 | 344.3 | 0.141 |
| 325.5 | 0.0187 | 347.1 | 0.208 |
| 329.2 | 0.0295 | 348.2 | 0.229 |
| 330.8 | 0.0340 | 352.1 | 0.353 |
| 333.1 | 0.0479 | 355.7 | 0.450 |
| 336.2 | 0.0628 | 362.8 | 0.830 |
| 339.2 | 0.0905 | 364.0 | 0.966 |
| 339.9 | 0.0904 | 367.8 | 1.48 |
| 340.0 | 0.0919 | ||
|
1,5-dibromoanthracene | |||
| T/K | Pvap/Pa | T/K | Pvap/Pa |
| 358.0 | 0.0068 | 378.3 | 0.050 |
| 358.2 | 0.0072 | 379.8 | 0.0746 |
| 360.6 | 0.0085 | 381.8 | 0.0718 |
| 362.3 | 0.0112 | 383.2 | 0.1041 |
| 363.3 | 0.0133 | 385.4 | 0.1269 |
| 365.1 | 0.015 | 386.5 | 0.1162 |
| 366.7 | 0.018 | 388.0 | 0.1593 |
| 368.5 | 0.0223 | 391.2 | 0.2126 |
| 370.8 | 0.0283 | 392.7 | 0.2427 |
| 372.5 | 0.033 | 395.0 | 0.2976 |
| 373.1 | 0.0376 | 398.5 | 0.3977 |
| 375.6 | 0.0412 | 402.8 | 0.5508 |
| 377.8 | 0.048 | 407.2 | 0.7873 |
| 377.9 | 0.0631 | 407.8 | 0.710 |
| 378.0 | 0.0668 | ||
|
9,10-dibromoanthracene | |||
| T/K | Pvap/Pa | T/K | Pvap/Pa |
| 359.1 | 0.0090 | 377.2 | 0.0546 |
| 360.4 | 0.0103 | 377.4 | 0.0630 |
| 361.4 | 0.0126 | 378.3 | 0.0655 |
| 364.9 | 0.0171 | 381.3 | 0.0905 |
| 365.5 | 0.0167 | 381.7 | 0.0923 |
| 368.7 | 0.0255 | 381.9 | 0.0964 |
| 369.7 | 0.0297 | 386.1 | 0.133 |
| 372.8 | 0.0394 | 386.4 | 0.142 |
| 372.9 | 0.0399 | 391.1 | 0.210 |
| 373.8 | 0.0445 | 391.7 | 0.208 |
|
2-chloroanthracene | |||
| T/K | Pvap/Pa | T/K | Pvap/Pa |
| 331.4 | 0.0083 | 358.2 | 0.124 |
| 333.6 | 0.0097 | 358.9 | 0.135 |
| 338.9 | 0.0208 | 361.4 | 0.179 |
| 341.2 | 0.0240 | 363.0 | 0.195 |
| 343.0 | 0.0270 | 363.9 | 0.217 |
| 349.4 | 0.0571 | 367.2 | 0.284 |
| 351.6 | 0.0729 | 368.1 | 0.314 |
| 352.6 | 0.0876 | 370.4 | 0.355 |
| 355.4 | 0.103 | 371.8 | 0.400 |
| 355.6 | 0.100 | ||
|
9,10-dichloroanthracene | |||
| T/K | Pvap/Pa | T/K | Pvap/Pa |
| 316.0 | 2.7E-04 | 348.9 | 0.0172 |
| 330.8 | 0.0020 | 353.4 | 0.0370 |
| 330.9 | 0.0022 | 353.6 | 0.0394 |
| 337.7 | 0.0047 | 355.8 | 0.0374 |
| 339.1 | 0.0057 | 357.9 | 0.0546 |
| 341.7 | 0.0071 | 363.5 | 0.0847 |
| 344.3 | 0.0087 | 364.4 | 0.0779 |
| 345.6 | 0.0127 | 367.5 | 0.1216 |
| 347.3 | 0.0144 | 368.5 | 0.1645 |
| 348.3 | 0.0167 | 376.0 | 0.2362 |
|
1-bromopyrene | |||
| T/K | Pvap/Pa | T/K | Pvap/Pa |
| 320.8 | 0.0018 | 355.4 | 0.0606 |
| 339.4 | 0.0152 | 358.3 | 0.0783 |
| 339.5 | 0.0137 | 358.6 | 0.0901 |
| 347.1 | 0.0308 | 362.3 | 0.112 |
| 347.3 | 0.0282 | 363.4 | 0.155 |
| 351.4 | 0.0508 | 363.4 | 0.128 |
| 353.8 | 0.0508 | 363.4 | 0.160 |
| 353.8 | 0.0497 | 365.9 | 0.180 |
| 355.3 | 0.0594 | 367.9 | 0.233 |
Table 2.
Enthalpies and entropies of sublimation of halogenated PACs as determined via the Clausius-Clapeyron equation.
| Compound | CAS Reg. No. | Molecular Weight g mol−1 | Min. Purity % | Temp Range K | Δ sub H kJ mol−1 | ΔsubS kJ K−1mol−1 | Molecular Structure |
|---|---|---|---|---|---|---|---|
| naphthalene [10] C10H8 | 91-20-3 | 128.17 | >99 | 258–314 | 73.3± 0.61 | 0.266 | |
| 2-chloronaphthalene [12] C10H7Cl | 91-58-7 | 162.62 | >99 | 280–329 | 75.5 ± 0.63 | 0.261 | |
| 2-bromonaphthalene [12] C10H7Br | 580-13-2 | 207.07 | >99 | 280–328 | 80.4 ±0.68 | 0.268 | |
| 1,4-dibromonaphthalene C10H6Br2 | 83-53-4 | 285.96 | 98 | 297–322 | 90.8 ± 1.7 | 0.272 | 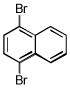 |
| acenaphthene [9] C12H10 | 83-32-9 | 154.21 | 99 | 299–320 | 78.7 ± 2.2 | 0.254 |  |
| 5-bromoacenaphthene C12H9Br | 2051-98-1 | 233.10 | >94 | 296–321 | 87.4 ± 2.6 | 0.269 | 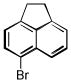 |
| anthracene [9] C14H10 | 120-12-7 | 178.23 | 99 | 322–348 | 98.5 ± 3.3 | 0.271 | |
| 9-bromoanthracene C14H9Br | 1564-64-3 | 257.13 | 95 | 316–368 | 100.5 ± 1.8 | 0.276 | 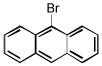 |
| 1,5-dibromoanthracene C14H8Br2 | 3278-82-8 | 336.02 | 98 | 358–408 | 116.7 ± 3.0 | 0.285 | 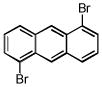 |
| 9,10-dibromoanthracene C14H8Br2 | 523-27-3 | 336.02 | 98 | 359–392 | 114.2 ± 2.8 | 0.279 | 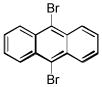 |
| 2-chloroanthracene C14H9Cl | 17135-78-3 | 212.67 | >98 | 331–372 | 99.3 ± 2.7 | 0.260 | |
| 9,10-dichloroanthracene C14H9Cl | 605-48-1 | 247.12 | >96 | 316–376 | 113.9 ± 4.5 | 0.293 | 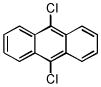 |
| pyrene [9] C16H10 | 129-00-0 | 202.26 | 99 | 322–381 | 97.8 ± 3.3 | 0.266 | 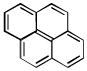 |
| 1-bromopyrene C16H9Br | 1714-29-0 | 281.15 | 94 | 321–366 | 99.2 ± 4.3 | 0.257 | 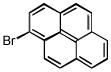 |
Figures 2 through 5 demonstrate the effects of the addition of halogen hetero-atoms on the vapor pressures of parent PACs. We begin by examining the effect on the enthalpy of sublimation with the substitution of chlorine and bromine onto naphthalene, for which the parent enthalpy of sublimation falls around 73.3 kJ•mol−1 over the temperature range of 258K to 314K [10]. The enthalpy of sublimation of 2-bromonaphthalene measured by Ribeiro da Silva, Ferrão and Lopes [11] was (81.2±1.0) kJ•mol−1 at T = (280–328) K, in good accord with that (80.4±0.68 kJ•mol−1) measured by Verevkin [12]. These data show an increase in enthalpy of approximately 10% (or 7 to 8 kJ•mol−1) with the substitution of a single bromine onto the 2-position of naphthalene. Our measured value for the enthalpy of sublimation of 1,4-dibromonaphthalene is (90.8±1.7) kJ•mol−1, an increase of 17.5•kJ mol−1, or about 24% over pure naphthalene; thus, we note that the effect of each bromine substituent is in the range of (7–10) kJ•mol−1. Verevkin measured the enthalpy of sublimation of 2-chloronaphthalene as (75.5±0.631) kJ•mol−1, showing a very slight increase as compared to the parent naphthalene [11]. Figure 2 illustrates these observations in a Clausius-Clapeyron plot.
Figure 2.
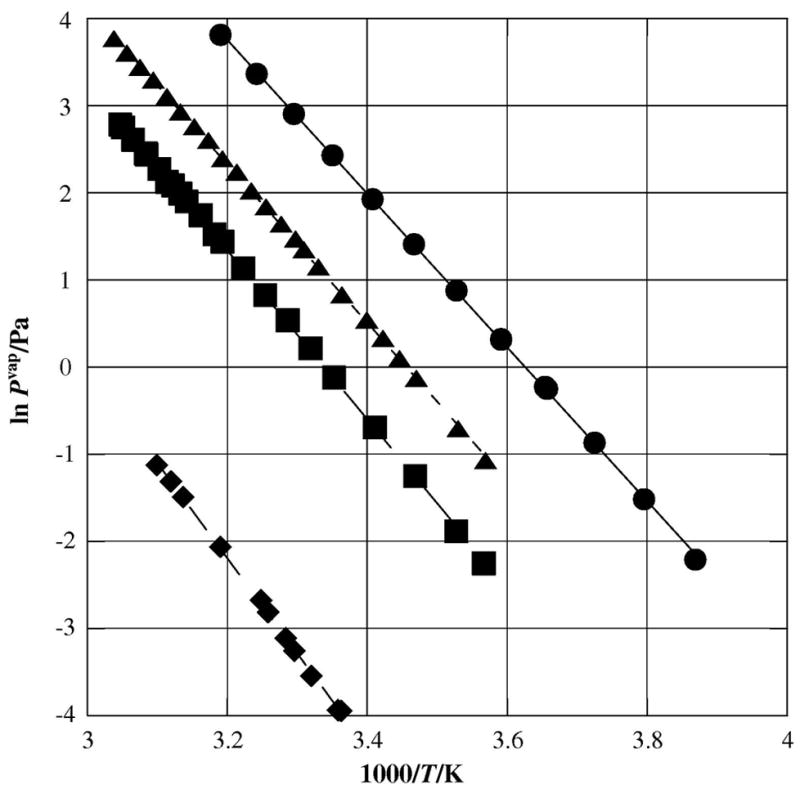
Plot of sublimation vapor pressures against reciprocal temperature for the halogenated naphthalenes; λ naphthalene [10] ν 2-bromonaphthalene [12] υ 1,4-dibromonaphthalene (this study); σ 2-chloronaphthalene [12]
Figure 5.
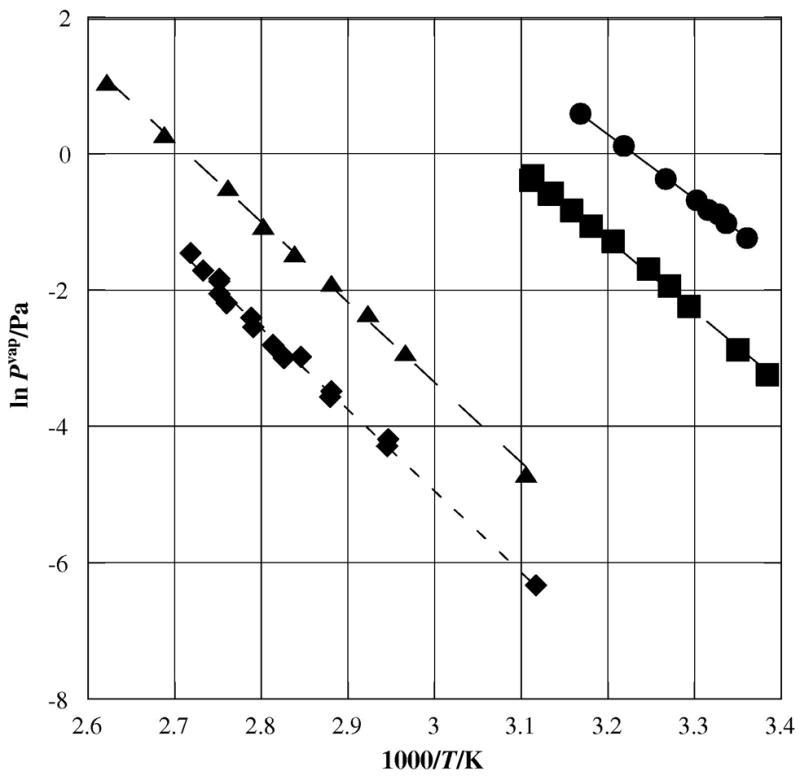
Plot of sublimation vapor pressures against temperature for 5-bromoacenaphthene and 1-bromopyrene compared to parent PAHs determined via the Knuden effusion technique; λ acenaphthene [8]; ν 5-bromoacenaphthene; σ pyrene[9]; υ 1-bromopyrene.
The same trend is observed with the addition of one chlorine to anthracene, creating 2-chloroanthracene; we measured the enthalpy of sublimation at (99.3± 2.6) kJ•mol−1, compared to pure anthracene {ΔHsub=(98.5±3.3) kJ•mol−1, [8]}. The sublimation enthalpy showed very little change upon substitution as seen in figure 3. With the addition of two chlorines, the enthalpy of sublimation of 9,10-dichloranthracene is measured at 113.9± kJ•mol−1, an increase of approximately 14%, or 15.4 kJ•mol−1 from the parent molecule.
Figure 3.
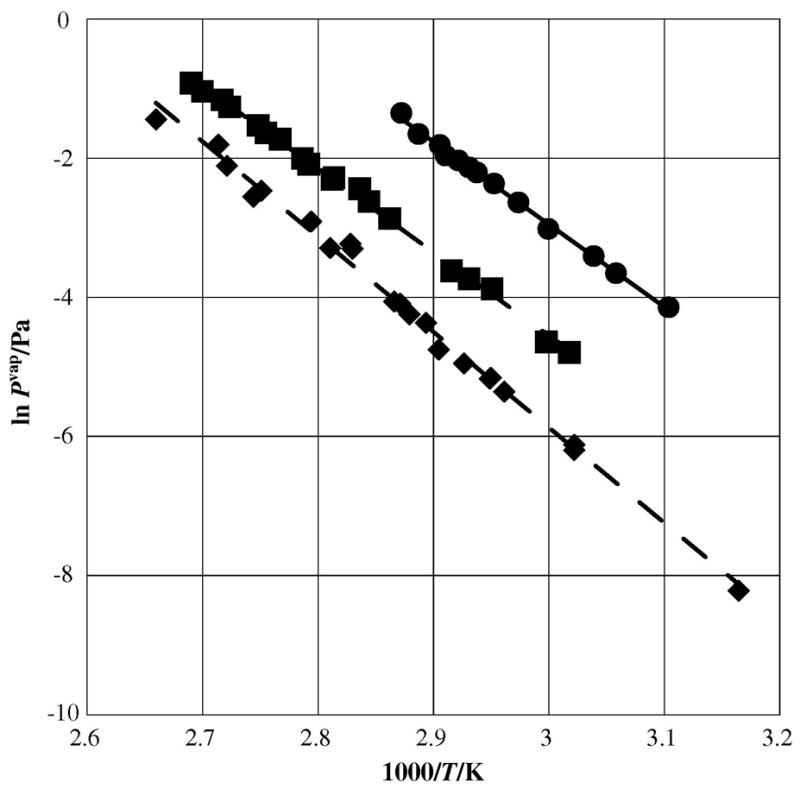
Plot of sublimation vapor pressures against reciprocal temperature for chlorinated anthracenes as measured by the Knudsen effusion technique; λ anthracene [9]; ν 2-chloroanthracene; ◆ 9,10-dichloroanthracene
We see only a slightly higher enthalpy of sublimation for 1,5-dibromoanthracene and 9,10-dibromoanthracene at 116.7±3.0 kJ•mol−1 and 114.2±2.7 kJ•mol−1, respectively than for the 9,10-dichloroanthracene at 113.9±1.5 kJ•mol−1, even though bromine is a significantly larger atom than chlorine (refer to table 2). It does not appear that the position of the halogens on the dibrominated anthracenes alters the enthalpy of sublimation to a statistically significant degree, as illustrated in figure 4. Again in this case, the addition of a single bromine atom to anthracene does not appear to influence the enthalpy of sublimation very much.
Figure 4.
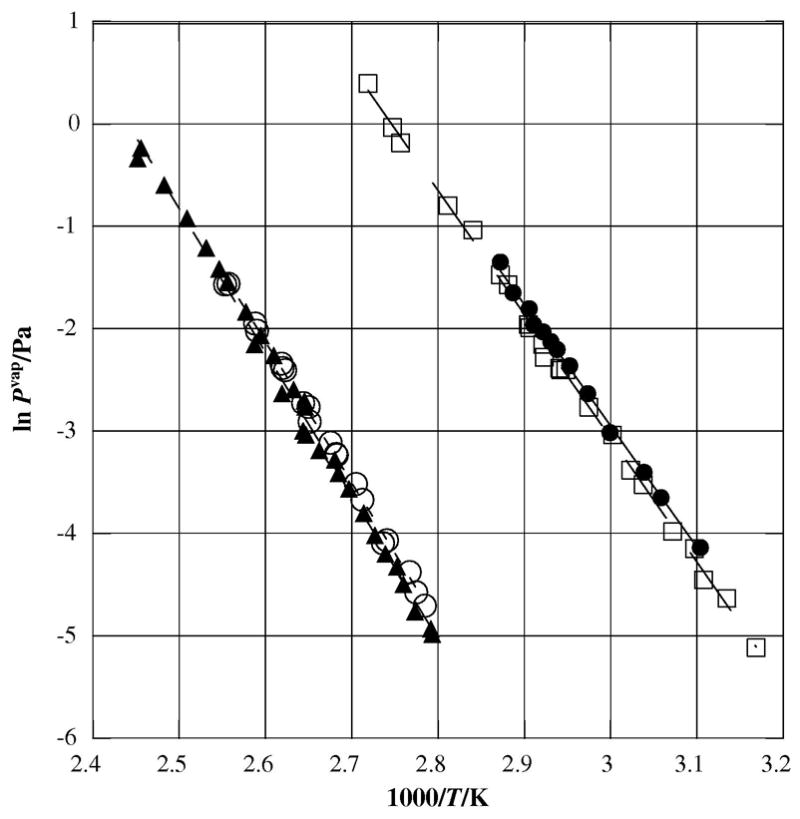
Plot of sublimation vapor pressures against reciprocal temperature for brominated anthracenes as measured by the Knudsen effusion technique; λ anthracene [9]; θ 9-bromoanthracene; σ 1,5-dibromoanthracene; μ 9,10-dibromoanthracene
For pyrene, a four-ring PAC, the addition of one bromine atom does not change the measured enthalpy of sublimation by a significant amount, as may be seen in table 2. The vapor pressure of 1-bromopyrene was notably lower than for unsubstituted pyrene, as shown in figure 5.
The vapor pressure of acenaphthene was significantly lowered by the addition of a single bromine atom, decreasing by almost a full order of magnitude at room temperature. In this case, the substituted acenaphthene shows a higher enthalpy of sublimation than did the unsubstituted (87.4±2.6) kJ•mol−1 compared to (78.7±2.2) kJ•mol−1).
4. Discussion
Overall, the successive addition of halogens to polycyclic aromatic compounds always decreases the vapor pressure as compared to the unsubstituted compound. As expected, the more halogens substituted, the lower the vapor pressure. Single chlorine substitution had a significantly smaller effect on vapor pressure than did bromine substitution at the same position for naphthalene, even though the enthalpy of sublimation was not much influenced by the chlorine heteroatom. The vapor pressure at T = 298 K for 2-chloronapthalene was measured by Verevkin to be 2.49 Pa, compared to 0.84 Pa for 2-bromonaphthalene. For anthracene at the same temperature, two bromines at the 9,10 positions yielded a vapor pressure of 3.68*10−6 Pa and two chlorines at the same positions of 2.17*10−5 Pa.
In the case of the smaller naphthalene, the difference in vapor pressures appears to be due more to enthalpic considerations, whereas for the larger anthracene, the difference is more attributable to entropic effects. One observable trend among the PACs composed of only fused benzene rings (naphthalene, anthracene and pyrene) is that the relative effect of halogen addition decreases as the parent compound’s molecular size increases; the change in enthalpy of sublimation with the addition of one bromine increases in naphthalene by +7.1kJ·mol−1, in anthracene by 2.0kJ·mol−1 and in pyrene by 1.4 kJ·mol−1. The addition of one bromine to acenaphthene causes an increase in the ΔHsub by 8.7 kJ·mol−1, comparable to that in naphthalene.
In all instances, the hetero-atom substitution decreased the vapor pressure compared to the parent compound. The magnitude of the effect was, however, not always easily predictable based upon the above observations. For example, addition of a single bromine to anthracene had a much more modest effect on vapor pressure than did addition of a single bromine to either the smaller naphthalene or the larger pyrene. In naphthalene, the reduction is almost purely of enthalpic origin. For pyrene, the entropic change is larger for the unsubstituted than for the substituted compound. In the intermediate anthracene case, neither the enthalpic nor entropic effects are large; the vapor pressure is not significantly influenced by the single substitution.
From these data, we conclude that the effect of a single bromine or chlorine substitution decreases as the parent molecule increases in size. In addition, we only see a significant difference in the change in enthalpy of sublimation with the addition of a single bromine atom versus one chlorine for naphthalene, the smallest of the PACs measured. The difference in enthalpy between substituting either chlorine or bromine onto anthracene or pyrene was significantly smaller. Larger thermodynamic effects occur with the addition of two halogens, though it appears from the dibrominated anthracenes that the carbon position to which the halogens are substituted does not strongly influence the change in vapor pressure.
Acknowledgments
The project described was supported by Grant Number 5 P42 ES013660 from the National Institute of Environmental Health Sciences (NIEHS), NIH and the contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIEHS, NIH.
The authors appreciate the help of Daniel Lim at Brown University in measuring the melting points of the halogenated PAHs and Professor G. Diebold for the use of his Digimelt apparatus.
Contributor Information
Jillian L. Goldfarb, Email: Jillian_Goldfarb@brown.edu.
Eric M. Suuberg, Email: Eric_Suuberg@brown.edu.
References
- 1.Tesconi M, Yalkowsky SH. J Pharmaceutical Sci. 1998;87:1512–1520. doi: 10.1021/js980231s. [DOI] [PubMed] [Google Scholar]
- 2.Wang H, Frenklach M. Combustion and Flame. 1994;96:163–170. [Google Scholar]
- 3.Oja V, Suuberg EM. J Chem Eng Data. 1998;43:486–492. [Google Scholar]
- 4.Eljarrat E, Barcelo D. Trends in Analytical Chemistry. 2003;22:655–665. [Google Scholar]
- 5.Hu J, Jin X, Kunikane S, Terao Y, Aizawa T. Environ Sci Technol. 2006;40:487–493. doi: 10.1021/es0516108. [DOI] [PubMed] [Google Scholar]
- 6.Huang J, Senkan SM. Proc Comb Inst. 1996;26:2335–2341. [Google Scholar]
- 7.Coutsikos P, Voutsas E, Magoulas K, Tassios DP. Fluid Phase Equilibria. 2003;207:263–281. [Google Scholar]
- 8.Oja V, Suuberg EM. Anal Chem. 1997;69:4619–4626. [Google Scholar]
- 9.Goldfarb JL, Suuberg EM. J Chem Eng Data. Under Review. [Google Scholar]
- 10.Ruzicka K, Fulem M, Ruzicka V. J Chem Eng Data. 2005;50:1956–1970. [Google Scholar]
- 11.Ribeiro da Silva MAV, Ferrão MLCCH, Lopes AJM. J Chem Thermodynamics. 1993;25:229–235. [Google Scholar]
- 12.Verevkin SP. J Chem Thermodynamics. 2003;35:1237–1251. [Google Scholar]