Abstract
Land plants must balance CO2 assimilation with transpiration in order to minimize drought stress and maximize their reproductive success. The ratio of assimilation to transpiration is called transpiration efficiency (TE). TE is under genetic control, although only one specific gene, ERECTA, has been shown to regulate TE. We have found that the α-subunit of the heterotrimeric G protein in Arabidopsis (Arabidopsis thaliana), GPA1, is a regulator of TE. gpa1 mutants, despite having guard cells that are hyposensitive to abscisic acid-induced inhibition of stomatal opening, have increased TE under ample water and drought stress conditions and when treated with exogenous abscisic acid. Leaf-level gas-exchange analysis shows that gpa1 mutants have wild-type assimilation versus internal CO2 concentration responses but exhibit reduced stomatal conductance compared with ecotype Columbia at ambient and below-ambient internal CO2 concentrations. The increased TE and reduced whole leaf stomatal conductance of gpa1 can be primarily attributed to stomatal density, which is reduced in gpa1 mutants. GPA1 regulates stomatal density via the control of epidermal cell size and stomata formation. GPA1 promoter::β-glucuronidase lines indicate that the GPA1 promoter is active in the stomatal cell lineage, further supporting a function for GPA1 in stomatal development in true leaves.
Land plants, in particular plants that utilize C3 photosynthesis, must balance CO2 acquisition with water loss in order to maximize fitness. The water loss cost per unit of biomass acquired can be expressed as transpiration efficiency (TE; also referred to as water-use efficiency), the ratio of CO2 assimilation (A) to transpiration. TE strongly correlates with the δ13C of plant tissue, the ratio of 13C to 12C relative to a standard (Farquhar et al., 1982, 1989; Dawson et al., 2002). The physiological basis of this correlation is that in plants there is diffusional and biochemical discrimination against 13C, the heavier and less abundant stable isotope of carbon. Discrimination against 13C decreases with decreasing internal CO2 concentration (Ci), which can result from either increased A or reduced stomatal conductance (gs; Farquhar et al., 1982). While it is known that gs (a main factor controlling transpiration) correlates with A (Wong et al., 1979), genetic variation for TE and/or δ13C has been documented in a number of species (Farquhar and Richards, 1984; Virgona et al., 1990; Ehleringer et al., 1991; Comstock and Ehleringer, 1992; Hammer et al., 1997; Lambrides et al., 2004). In Arabidopsis (Arabidopsis thaliana), multiple quantitative trait loci associated with TE have been identified, indicating that TE is under genetic control (Juenger et al., 2005; Masle et al., 2005; McKay et al., 2008). However, only one gene, ERECTA, has been specifically identified as a regulator of TE (Masle et al., 2005). ERECTA encodes a Leu-rich repeat receptor-like kinase (Torii et al., 1996) and regulates TE via the control of stomatal density, gs, mesophyll cell proliferation, and photosynthetic capacity (Masle et al., 2005).
Heterotrimeric G proteins are GTP-binding proteins that function in the transduction of extracellular signals into intracellular responses. In its inactive state, the G protein classically exists as a trimer consisting of an α-subunit (Gα) bound to GDP, a β-subunit (Gβ), and a γ-subunit (Gγ). When a ligand binds to a G protein-coupled receptor (GPCR), a conformational change occurs in the G protein, resulting in the exchange of GDP for GTP and the dissociation of Gα-GTP from the Gβγ dimer. The G protein subunits remain active until the intrinsic GTPase activity of Gα results in the hydrolysis of GTP to GDP and the reassociation of the inactive trimer. The Arabidopsis genome contains canonical Gα and Gβ genes, GPA1 and AGB1, and two genes known to encode Gγs, AGG1 and AGG2 (Assmann, 2002). One likely GPCR, GCR1, has been functionally characterized (Pandey and Assmann, 2004), and additional GPCRs have been predicted using bioinformatics (Moriyama et al., 2006; Gookin et al., 2008) and interaction with GPA1 in yeast-based protein-protein interaction assays (Gookin et al., 2008). Recently, a new class of G proteins, GPCR-type G proteins (GTG1 and GTG2), have been identified in Arabidopsis that also serve as one class of abscisic acid (ABA) receptors (Pandey et al., 2009).
Despite the paucity of heterotrimeric G protein subunit genes in the Arabidopsis genome as compared with mammalian systems, functional studies of heterotrimeric G protein mutants suggest that G protein function is diverse in Arabidopsis. G proteins have been shown to function in developmental processes and hormonal and environmental signaling, including stomatal aperture regulation (Perfus-Barbeoch et al., 2004; Joo et al., 2005; Chen et al., 2006; Pandey et al., 2006; Trusov et al., 2006; Warpeha et al., 2007; Fan et al., 2008; Zhang et al., 2008a, 2008b). In response to drought stress, ABA concentration increases in the leaves (Davies and Zhang, 1991; Davies et al., 2005), where it promotes stomatal closure and inhibits stomatal opening (Schroeder et al., 2001). The G protein α- and β-subunit mutants, gpa1 and agb1, respectively, are hyposensitive to ABA inhibition of stomatal opening while displaying wild-type ABA promotion of stomatal closure (Wang et al., 2001; Fan et al., 2008). ABA inhibits stomatal opening in part by inhibiting inward-rectifying K+ channels, reducing K+ influx and therefore water entry into the cell (Schroeder et al., 2001). ABA inhibition of inward K+ channel activity is reduced in both gpa1 and agb1 mutants (Wang et al., 2001; Fan et al., 2008). agg1 and agg2 mutants show no altered regulation of ABA-induced stomatal movements or ion channel activities, suggesting that the genome contains additional unknown Gγ(s) or that heterotrimeric G protein signaling in plants does not always operate according to the mammalian paradigm (Trusov et al., 2008). gcr1 mutants are hypersensitive to both ABA inhibition of opening and ABA promotion of stomatal closure (Pandey et al., 2006). gtg1 gtg2 double mutants show a wild-type response for ABA inhibition of stomatal opening and are hyposensitive in ABA promotion of stomatal closure (Pandey et al., 2009).
While the altered stomatal sensitivities of the G protein mutants to ABA suggest that heterotrimeric G proteins may function in the regulation of whole plant water status, few experiments have been performed at the whole leaf or whole plant level. gpa1 mutants in the Wassilewskija background display increased water loss from excised leaves (Wang et al., 2001); however, there are no published reports of experiments assessing whole plant water status in gpa1 or agb1 mutants. gcr1 mutants show reduced water loss from excised leaves, drought tolerance, and improved recovery following the cessation of drought stress (Pandey and Assmann, 2004). In addition to their altered guard cell sensitivities to ABA, gpa1, agb1, and gcr1 mutants are hypersensitive to ABA inhibition of root and seedling development (Pandey et al., 2006), which could have impacts on whole plant water status. Finally, it has been recently reported that gpa1 and agb1 mutants have reduced and increased stomatal densities, respectively, in cotyledons (Zhang et al., 2008a). While stomatal density of leaves can be an important component of whole plant water status, the study by Zhang et al. (2008a) was performed on cotyledons only, whose developmental programs are often independent from those of true leaves (Chandler, 2008). Therefore, it is difficult to infer how this cotyledon phenotype will affect water relations at the whole plant level. Taken together, the stomatal aperture, electrophysiology, and tissue-specific ABA phenotypes of the G protein mutants, in addition to the possibility for altered stomatal density in the G protein mutant leaves, make it difficult to predict how G proteins contribute to the regulation of whole-plant TE. For example, the ABA-hyposensitive stomatal phenotype of gpa1 could result in increased transpiration, possibly reducing TE under certain conditions. Conversely, if gpa1 mutant leaves have reduced stomatal density, transpiration may be reduced, which could enhance TE under a range of conditions. Previous attempts to address the contributions of G proteins to whole plant transpiration, TE, and drought response using excised leaf/rosette assays to measure water loss are not sufficient, because both transpiration and A must be taken into account. Therefore, we investigated the role of GPA1 in regulating TE under ample water and drought stress conditions and in the presence of ABA. We have identified GPA1 as a negative regulator of TE in Arabidopsis via the control of gs and stomatal proliferation.
RESULTS
gpa1 Mutants Have Increased TE under Ample and Low Soil Water Conditions
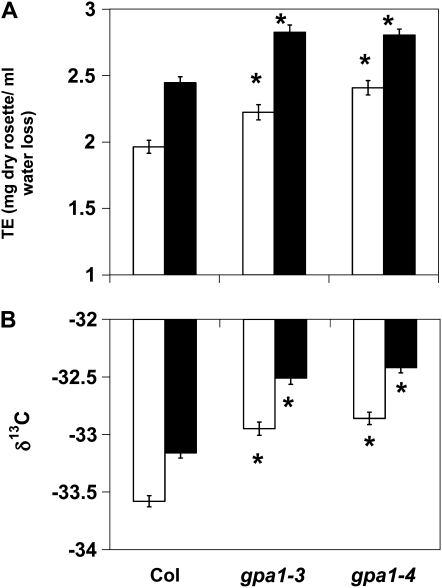
Given the involvement of GPA1 in the regulation of stomatal movements and ABA signaling (Wang et al., 2001; Pandey et al., 2006), TE was measured on gpa1-3, gpa1-4, and ecotype Columbia (Col) under soil water conditions equivalent to 90% (ample water) or 30% (drought stress) of the soil water-carrying capacity. ANOVA found significant effects for both genotype and water level and no significant genotype-treatment interaction (Supplemental Table S1). As would be expected, drought stress significantly increased TE for all genotypes (Fig. 1A). Interestingly, gpa1 mutants displayed increased TE under both ample water and drought stress conditions (Fig. 1A). Under ample water conditions, gpa1 mutants had approximately a 12% increase in TE compared with Col (P = 0.0107 for gpa1-3, P < 0.0001 for gpa1-4). Under drought stress, gpa1 mutants had a 14% increase in TE compared with Col (P < 0.0001 for both gpa1 alleles).
Figure 1.
gpa1 mutants have increased TE and increased δ13C (reduced discrimination) compared with Col. Mean TE (A) and mean δ13C values of rosette tissue (B) of gpa1 and Col under ample water (white bars) and drought stress (black bars) conditions are shown. Error bars represent se. Asterisks indicate that means differ significantly from the mean of Col within the treatment (P < 0.05).
In order to corroborate the whole plant TE phenotypes of the gpa1 mutants, stable carbon isotope analysis was performed on a subset of plants from the TE experiment (Fig. 1B). Consistent with the whole-plant TE data, the drought stress treatment resulted in an increase in the ratio of 13C to 12C (i.e. drought stress resulted in reduced discrimination of 13C) in the rosette tissue of all genotypes and gpa1 mutants had increased ratios of 13C to 12C (reduced discrimination of 13C) compared with Col under both drought stress and ample soil water conditions (P < 0.0001 for all gpa1 versus Col comparisons).
gpa1 Mutants Have Reduced Carbon Isotope Discrimination When Treated with ABA
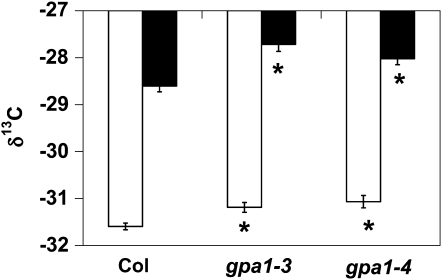
To directly assess the role of ABA in the regulation of TE by GPA1, the rosettes of gpa1 and Col were exogenously treated with ABA and carbon isotope analysis was performed. gpa1 and Col plants were grown under ample soil water conditions (90% of the soil water-carrying capacity) in a similar fashion as in the TE experiments. Carbon isotope analysis was performed on rosettes following 4 weeks of exogenous 25 μm ABA treatment (Fig. 2). Because the plants were sprayed with ABA dissolved in water, whole plant TE could not be reliably calculated. ABA treatment significantly and dramatically increased the ratio of 13C to 12C (reduced discrimination against 13C) in the rosette tissue. The effect of ABA treatment (Fig. 2) on carbon isotope ratio was much stronger than the effect of drought stress (Fig. 1B) for all genotypes. Unexpectedly, given that gpa1 mutants are hyposensitive to ABA inhibition of stomatal opening (albeit wild type for ABA promotion of stomatal closure), gpa1 mutants had reduced discrimination against 13C in rosette tissue compared with Col, even in this experiment where 25 μm ABA was applied directly to the leaves (P < 0.001 for gpa1-3 and P = 0.004 for gpa1-4).
Figure 2.
gpa1 mutants have increased δ13C (reduced discrimination) in the absence and presence of ABA as compared with Col. Mean δ13C values of rosette tissue from gpa1 and Col plants treated with no ABA (white bars) or 25 μm ABA (black bars) are shown. Error bars represent se. Asterisks indicate that means differ significantly from the mean of Col (P < 0.05).
gpa1 Mutants Show a Wild-Type A-Ci Response But a Reduced gs-Ci Response
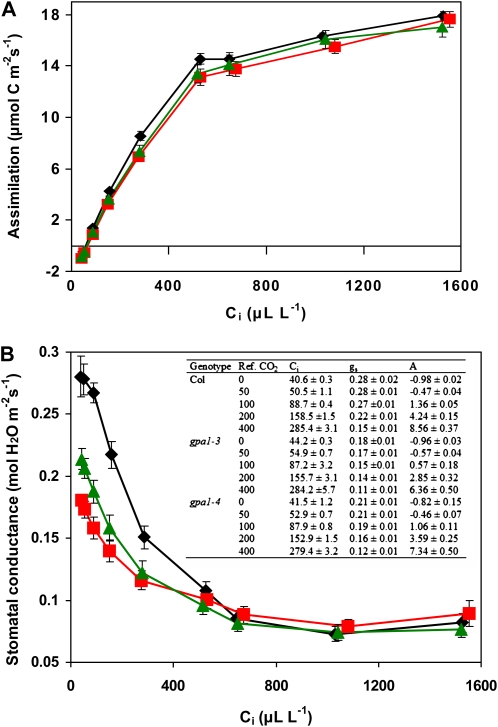
Gas-exchange analysis was performed to determine whether the increased TE of the gpa1 mutants was due to an effect of the mutation that enhanced A, reduced gs, or caused both of these processes. A and gs were measured under a range of external CO2 concentrations under non-light-limiting conditions in intact leaves. A-Ci curves indicate that the gpa1 mutants resemble the wild type in their A responses (Fig. 3A). Maximum rate of carboxylation (Vcmax) and maximum rate of photosynthetic electron transport (Jmax) were calculated for each individual plant curve, and no significant differences were found between the gpa1 mutants and Col for either parameter (Supplemental Fig. S1).
Figure 3.
gpa1 mutants show wild type A-Ci but altered gs-Ci responses. Photosynthesis (A) and stomatal conductance (B) of gpa1-3 (squares), gpa1-4 (triangles), and Col (diamonds) at different internal CO2 concentrations are shown. The inset table in B shows Ci, gs, and A means and se for the lowest five external CO2 concentrations for all genotypes. Significant differences are observed for gs of gpa1 versus Col and for gs at the five lowest values of Ci (P < 0.05). [See online article for color version of this figure.]
Stomatal conductance data taken simultaneously with the photosynthetic data in Figure 3A indicate that gpa1 mutants have altered gs response to Ci compared with the wild type (Fig. 3B). At high values of Ci (over 600 μL L−1), gs is at a baseline minimum value for all genotypes (approximately 0.075 mol water m−2 s−1). As Ci is reduced, gs increases for all genotypes; however, in gpa1 mutants, this increase is attenuated. At values of Ci corresponding to external CO2 concentrations at or below ambient CO2 level, gpa1 mutants have significantly reduced gs compared with Col. At minimum values of Ci (approximately 40 μL L−1), gpa1-3 and gpa1-4 show 35% (P < 0.001) and 25% (P = 0.004) reductions, respectively, in gs compared with Col.
gpa1 Mutants Are More Sensitive to Low-CO2-Induced Stomatal Opening
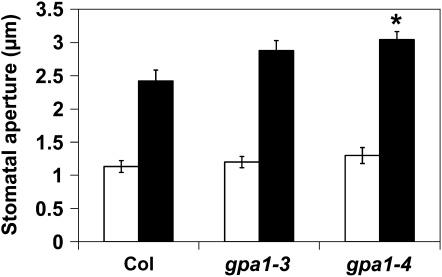
One hypothesis for the altered gs responses to Ci observed in the gpa1 mutants is that the mutants may be hyposensitive to low-CO2-induced stomatal opening. To investigate this hypothesis, we performed stomatal aperture measurements under reduced CO2 and ambient CO2 conditions. Incubation of leaves in buffer that had been bubbled with CO2-free air resulted in increased stomatal opening in all genotypes compared with leaves incubated in buffer equilibrated to ambient CO2 levels (Fig. 4). gpa1 mutants, however, had slightly enhanced stomatal opening compared with Col (Fig. 4; P = 0.06 for gpa1-3, P = 0.008 for gpa1-4). Control experiments using Col leaves incubated in buffer bubbled with ambient CO2 air or with no bubbling showed no significant differences in stomatal aperture; therefore, bubbling alone did not induce stomatal opening. These results suggests that gpa1 mutant guard cells are actually somewhat more sensitive to low-CO2-induced stomatal opening and that altered CO2 sensing or response by the guard cells is not limiting whole leaf gs in gpa1 mutants at values of Ci below 250 μL L−1.
Figure 4.
gpa1 mutants are moderately hypersensitive to low-CO2-induced stomatal opening. Mean stomatal apertures from abaxial epidermal peels after incubation of leaves in low-CO2 buffer (bubbled continuously with CO2-free air; black bars) or ambient CO2 buffer (ambient CO2 of approximately 400 μL L−1; white bars) are shown. Errors bars represent se, and the asterisk indicates a significant difference from Col. P = 0.06 for gpa1-3 and P = 0.008 for gpa1-4 for gpa1 low-CO2 aperture size versus Col low-CO2 aperture size.
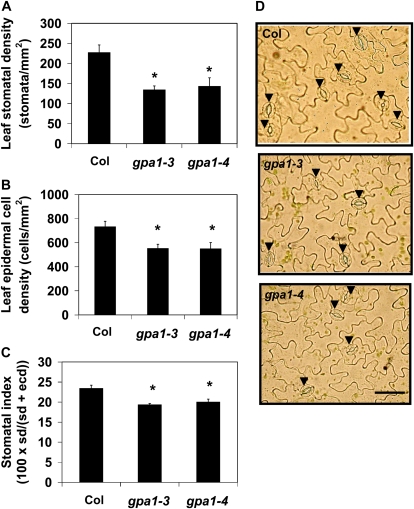
gpa1 Mutants Have Reduced Stomatal Density and Stomatal Index in Mature Leaves
Another hypothesis to explain the reduced gs of gpa1 mutants is that stomatal density is reduced in mature leaves of gpa1 plants. In fact, we found that gpa1 mutants do have approximately 50% fewer stomata on fully expanded leaves compared with Col (Fig. 5, A and D; P = 0.0001 for gpa1-3, P < 0.0001 for gpa1-4). The reduction in stomatal density can be attributed to both increased cell size of epidermal cells and reduced formation of stomata. Epidermal cell density was reduced in gpa1 mutants by 20% compared with Col (P = 0.0203 for gpa1-3, P = 0.0027 for gpa1-4), indicating that the mutants have larger leaf epidermal cells than the wild type (Fig. 5B). Stomatal index, the number of stomata relative to total cell number in the epidermis, was also significantly reduced in gpa1 mutants (P < 0.0001 for both gpa1 alleles), suggesting a role for GPA1 in stomatal development and/or proliferation in true leaves (Fig. 5C).
Figure 5.
gpa1 mutants have reduced stomatal density (sd; A), epidermal cell density (ecd; B), and stomatal index (C). Shown are mean values with error bars indicating se. Asterisks indicate that means differ significantly from the mean of Col (P < 0.05). Photographs of representative epidermal peels of Col and gpa1 mutants are shown in D. Arrowheads indicate stomata. Bar = 50 μm. [See online article for color version of this figure.]
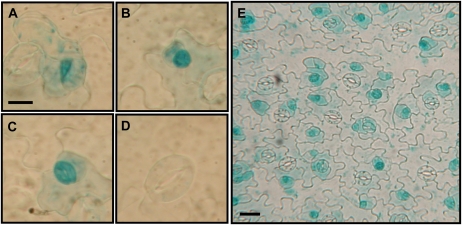
GPA1prom::GUS Activity Is Observed in Stomatal Precursor Cells and Immature Guard Cells
The stomatal density results supported a role for GPA1 in stomatal development. Therefore, we created GPA1prom::GUS reporter lines to examine GPA1 promoter activity in stomata and stomatal precursor cells. Analysis of multiple independent transgenic lines expressing the GPA1prom::GUS construct shows GUS activity in meristemoids (Fig. 6A), guard mother cells (Fig. 6B), and immature stomata (Fig. 6C) of developing true leaves. GUS activity decreases as stomata develop and is absent or faint in the majority of mature stomata (Fig. 6D). The epidermal cells directly adjacent to the immature stomata also show reporter gene activity, albeit weaker than that of the developing stomatal complex (Fig. 6E). These results indicate that GPA1 promoter activity is associated with developing stomatal complexes.
Figure 6.
GPA1prom::GUS activity is observed in stomatal precursor cells and immature stomata. Images are from an epidermal peel of a true leaf of a 15-d-old soil-grown seedling. A, Meristemoid. B, Guard mother cell. C, Immature stomate. D, Mature stomate. Bar = 10 μm. E, GUS activity in developing stomatal complexes and neighboring epidermal cells. Bar = 20 μm.
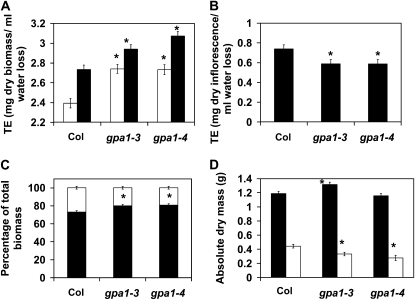
gpa1 Mutants Show Reduced Biomass Allocation to the Inflorescence
We have reported that gpa1 mutants have reduced fitness under both well-watered and drought stress conditions (Nilson and Assmann, 2010). In order to potentially reconcile the increased TE phenotype with the reduced fitness observation, we performed an additional TE experiment examining TE in both vegetative and reproductive phases of Arabidopsis. Interestingly, consistent with our previous TE experiments, gpa1 mutants showed increased TE compared with the wild type when harvested prior to bolting (Fig. 7A; P < 0.0001 for both gpa1 alleles) and also showed increased TE when plants were harvested 5 weeks after bolting, when all plants were flowering and setting seed (P = 0.004 for gpa1-3, P < 0.001 for gpa1-4). However, calculation of TE for the inflorescence only (inflorescence dry weight per total water transpired) showed that gpa1 mutants have reduced inflorescence TE compared with the wild type (Fig. 7B; P = 0.0407 for gpa1-3, P = 0.0116 for gpa1-4). Additionally, analysis of dry biomass partitioning between the rosette and the inflorescence showed that gpa1 mutants allocate less biomass to the inflorescence compared with wild-type plants (Fig. 7C; P = 0.0049 for gpa1-3, P = 0.0004 for gpa1-4). These data suggest that despite the increased TE of gpa1, reduced biomass partitioning to the inflorescence has negative fitness consequences for gpa1 mutants.
Figure 7.
gpa1 mutants have increased TE before and after flowering but reduced inflorescence TE and reduced biomass allocation to the inflorescence compared with Col. A, TE of Col and gpa1 mutants before flowering (white bars) and after flowering (black bars). B, Inflorescence TE of Col and gpa1 mutants. C and D, Biomass partitioning (C) and absolute biomass (D) for Col and gpa1 mutants for rosette (black bars) and inflorescence (white bars) tissues. Errors bars represent se, and asterisks indicate significant mean differences from Col.
DISCUSSION
GPA1 Regulation of TE
Increasing global populations necessitate the development of crop species that can thrive in inhospitable environments, such as drought-prone areas. Breeding programs focusing on different plant physiological traits, including high TE/low 13C discrimination, have been successful at developing high-yielding, drought-tolerant crop cultivars (Condon et al., 2002; Rebetzke et al., 2002; Richards, 2006). Understanding the genetic basis of TE and other plant traits that contribute to plant survival and productivity under lowered water availability will benefit both traditional breeding programs and biotechnological crop enhancement. Quantitative trait loci that affect TE have been identified in Arabidopsis (Juenger et al., 2005; Masle et al., 2005), but only one specific gene, ERECTA, has been shown to regulate TE (Masle et al., 2005). Here, we identify a second gene, GPA1, as a regulator of TE in Arabidopsis. Despite the hyposensitivity of gpa1 guard cells to ABA-induced inhibition of stomatal opening (Wang et al., 2001; Fan et al., 2008), gpa1 mutants have increased vegetative TE and reduced carbon isotope discrimination compared with Col under both ample water and drought conditions. gpa1 mutants also have reduced carbon isotope discrimination when ABA is directly applied to leaves. Since the increased TE of gpa1 mutants could be due to reduced transpiration, enhanced photosynthesis, or both of these processes, we examined a number of physiological (A, gs, stomatal aperture size) and developmental (stomatal index and density, epidermal cell size) traits of gpa1 mutants in order to gain insight into the mechanism by which GPA1 regulates TE.
GPA1 Regulation of Leaf Stomatal Density
A number of leaf morphological traits can affect the rate of water loss from the leaf by affecting properties such as the boundary layer, cuticular conductance, or stomatal conductance. Such traits include leaf thickness and anatomy and the distribution and density of stomata on leaf surfaces. Therefore, we examined gpa1 for altered leaf developmental traits that could contribute to the enhanced TE and reduced gs observed for gpa1. Histological analysis of cross-sections of gpa1 and Col leaves found no significant differences in leaf thickness or anatomy (Supplemental Fig. S2). Recently, it has been shown that gpa1 mutants have reduced stomatal density in cotyledons (Zhang et al., 2008a). However, because cotyledons and leaves have at least partially independent developmental programs (Chandler, 2008), it could not be concluded from Zhang et al. (2008a) that mature gpa1 leaves would also have reduced stomatal densities. We did find, though, that gpa1 mutants have approximately 50% fewer stomata on leaves compared with Col, which is similar to the reduction in density observed in cotyledons (Zhang et al., 2008a). Taking our data as a whole, reduced stomatal density in gpa1 is likely the major contributing factor to gpa1 having reduced gs and increased TE compared with Col.
It has been reported that gpa1 mutants have reduced cell division in shoots; gpa1 mutants have fewer and larger hypocotyl cells and leaf epidermal cells (Ullah et al., 2001). Additionally, inducible overexpression of GPA1 has been shown to cause ectopic cell division in seedlings (Ullah et al., 2001). The epidermal cell density of gpa1 was significantly reduced in mature leaves, indicating that gpa1 mutants have larger epidermal cells in mature leaves. Therefore, increased epidermal cell size and/or reduced epidermal cell formation contribute to the reduced stomatal density observed in gpa1. Stomatal index was also attenuated in mature leaves of gpa1, indicating that there is also reduced stomata formation. However, the reduction in stomatal index (approximately 15%) in mature leaves, while significant, was less than the reduction in stomatal index (28%) reported for gpa1 cotyledons (Zhang et al., 2008a). While Zhang et al. (2008a) report that the reduced stomatal density in cotyledons could be explained entirely by reduced stomata formation, we found a more complex developmental role for GPA1 in regulating stomatal density in leaves: GPA1 modulates both stomatal formation and epidermal cell division/size.
GPA1 has been shown to be expressed in roots, shoots, reproductive organs, and guard cell protoplasts (Ma et al., 1990; Weiss et al., 1993; Huang et al., 1994; Wang et al., 2001; Chen et al., 2006; Fan et al., 2008), but GPA1 expression in developing stomata, to our knowledge, has never been assessed. Using GPA1prom::GUS reporter lines, we found significant GUS activity in stomatal precursor cells, including meristemoids, guard mother cells, and immature stomata. Reporter activity is strongest in immature stomata and is severely reduced in mature guard cells. The finding of GPA1 promoter activity in developing stomata, in addition to the reduced stomatal densities of gpa1 mutants in mature leaves, suggests that GPA1 is a positive regulator of stomatal density in leaves.
It is interesting that the one other identified genetic regulator of TE in Arabidopsis, ERECTA, also functions in stomatal development, albeit as a negative regulator (Masle et al., 2005; Shpak et al., 2005). Heterotrimeric G proteins have additional overlapping functions with ERECTA, including in flower, fruit, and leaf development, cell division, and pathogen responses (Lease et al., 2001; Shpak et al., 2004; Llorente et al., 2005), and double mutant analysis of erecta agb1 mutants show that ERECTA and AGB1 likely function in the same pathway in regulating fruit shape (Lease et al., 2001). Additionally, both GPA1 and ERECTA are membrane-localized proteins with putative functions in signal transduction (Torii et al., 1996; Weiss et al., 1997; Adjobo-Hermans et al., 2006; Chen et al., 2006; Wang et al., 2008). Unfortunately, we were unable to assess whether or not ERECTA and GPA1 function in the same pathway to regulate TE and/or stomatal density, because our attempts to isolate F2 recombinants that are heterozygous for both erecta and gpa1 were not successful, likely because of the tight linkage between the two loci (1.7 centimorgan genetic distance and 7 kb physical distance). Yeast-based biochemical interaction assays between ERECTA and GPA1 were inconclusive, and there was no identifiable phosphorylation of GPA1 or ABG1 by the kinase domain of ERECTA in in vitro phosphorylation assays (data not shown). Further investigation is needed in order to ascertain if GPA1 and ERECTA function in the same pathway to regulate TE and stomatal development.
Leaf-Level Gas Exchange, Stomatal Aperture Regulation, and Stomatal Density
The increased TE exhibited by gpa1 mutants could have been a consequence of enhanced A, reduced gs, or both these processes. Gas-exchange analysis revealed that gpa1 mutants had wild-type A versus Ci responses but had reduced gs compared with Col under ambient and below-ambient CO2 levels. This indicated that reduced gs is the primary means by which gpa1 mutants have increased TE. We observed that stomatal density contributes to the reduced gs of gpa1, but we also needed to evaluate the possibility that gpa1 stomata failed to open in response to low CO2 concentrations or had slower kinetics of response, a phenomenon that would also contribute to reduced gs. However, we found that gpa1 mutant stomata did not fail to open in response to low CO2 and in fact had the opposite phenotype: gpa1 mutants have larger stomatal apertures compared with the wild type when leaves with closed stomata are exposed to low-CO2 buffer. In other words, low-CO2 sensing is altered in gpa1, but gpa1 mutants are somewhat hypersensitive, not hyposensitive, for low-CO2-induced opening. Therefore, the reduced gs and increased TE observed in gpa1 are most likely consequences of gpa1 mutants having reduced stomatal density. Gas-exchange analysis of the Arabidopsis sdd1-1 mutant, which has an elevated stomatal density, showed that gs was affected more by the sdd1-1 mutation than A, although the extent of this difference depended on the light intensity (Schlüter et al., 2003). The relative effects of density on gs and A in that report are consistent with our data on the gpa1 mutants. However, we cannot absolutely rule out the possibility that the gpa1 mutation also enhances A by some mechanism that was not operative/observable under our assay conditions.
gpa1 mutants also have stomata that are opened more widely than the wild type, as measured in stomatal aperture assays, when closed stomata are treated with ABA during light-induced stomatal opening (Wang et al., 2001; Fan et al., 2008). Therefore, GPA1 functions in both ABA and CO2 signaling. To our knowledge, there are no previous reports of the involvement of heterotrimeric G proteins in CO2 sensing in either plant or animal systems. Growing evidence suggests that CO2 signaling and ABA signaling may operate in part via shared signaling components (for review, see Vavasseur and Raghavendra, 2005). In particular, the 2C Ser-Thr protein phosphatases ABI1 and ABI2 appear to function in both ABA and CO2 signaling (Webb and Hetherington, 1997; Leymarie et al., 1998a, 1998b). abi1-1 and abi2-1 mutants, which are ABA insensitive, are also insensitive to high-CO2-induced and extracellular-Ca2+-induced stomatal closure, suggesting that CO2, ABA, and extracellular Ca2+ signaling converge at these signaling nodes (Webb and Hetherington, 1997). Interestingly, CO2 and ABA may also converge in mediating stomatal density. Recently, it has been proposed by Lake and Woodward (2008) that changes in stomatal density due to altered CO2 concentration and humidity may be signaled via ABA regulation of stomatal aperture and transpiration rate.
The gpa1 plant's altered CO2 and ABA stomatal aperture responses will tend to result in wider stomatal apertures and thus could presumably partially compensate for their reduced stomatal density, allowing a closer approximation of a wild-type level of leaf water status than would be possible in the absence of such compensation. This phenomenon of stomatal aperture compensation for changes in stomatal density has been reported for the stomatal development mutant sdd1, which has increased stomatal density, and for SDD1-overexpressing lines, which have reduced stomatal densities (Berger and Altmann, 2000; Bussis et al., 2006). However, gpa1 does not fully compensate for the reduced stomatal density, since at low values of Ci, gpa1 mutants have markedly reduced gs as compared with the wild type.
Recently, Zhao et al. (2010) found using quantitative proteomic techniques that 17 out of 18 proteins enriched in the guard cells of gpa1 mutants are members of the Arabidopsis chloroplast proteome. GPA1 has also been shown to interact with THF1, a plastid membrane protein that has a putative function in Glc signaling, in yeast and root epidermal cells (Huang et al., 2006). Zhao and colleagues (2010) speculate that, in wild-type plants, GPA1 may negatively regulate guard cell photosynthesis in response to Glc levels by suppressing photosynthesis-related proteins. An alternative hypothesis, given the reduced stomatal densities of gpa1 mutants and the possibility of stomatal aperture compensation for density under certain environmental conditions (low CO2, ABA), is that the increase in photosynthesis-related proteins is utilized in gpa1 to produce the energy and solutes required to drive more extreme stomatal movements, such is the case for low-CO2-induced stomatal opening (Fig. 4).
GPA1, Vegetative TE, and Inflorescence TE
We have found that gpa1 mutants have increased vegetative TE under well-watered and drought stress conditions and when ABA is applied directly to the leaves. We have also found that total aboveground biomass TE is increased in gpa1 even when the plants are harvested after 5 weeks of flowering. However, inflorescence TE is reduced in gpa1 mutants compared with Col, and gpa1 mutants do not appear to have an increase in seed production when grown under well-watered, moderate drought, or severe drought conditions (Nilson and Assmann, 2010). One possible explanation for the lack of a fitness (seed production) benefit is altered biomass partitioning in gpa1 mutants. Indeed, as reported here, despite the increased TE of gpa1 plants, gpa1 mutants allocate a smaller proportion of their total biomass to the inflorescence compared with Col. Survival under low-water conditions can also be considered a measure of plant fitness. Although survival was not examined in this study, the increased TE of gpa1 mutants may result in gpa1 mutants having enhanced survival under severe water limitation. Finally, analysis of gpa1 mutants indicates that GPA1 functions in a number of different stress responses (pathogens, ozone, reactive oxygen species), leaf and flower development, and hormonal signaling (Perfus-Barbeoch et al., 2004; Joo et al., 2005; Llorente et al., 2005; Pandey et al., 2006; Trusov et al., 2006; Zhang et al., 2008b). Therefore, pleiotropic effects on fitness caused by the gpa1 mutation may counteract any benefit achieved from enhanced TE. In future experiments, it will be interesting to explore the mechanistic basis of the stomatal proliferation phenotype that underlies the TE effect and to assess whether other G protein subunits also contribute to the regulation of TE.
MATERIALS AND METHODS
Plant Material and Growth Conditions
All Arabidopsis (Arabidopsis thaliana) seeds used in these experiments were collected from cogrown Col, gpa1-3, and gpa1-4 parent plants whose genotypes were confirmed via PCR of genomic DNA. Seeds were stratified on wet filter paper at 4°C in the dark for 48 h prior to planting to synchronize germination. Plants were grown in 8-cm2 pots in soil mix (Miracle-Gro Potting Mix; Scotts) augmented with perlite, unless otherwise specified. Plants were grown in walk-in growth chambers (Controlled Environments Limited) under extended short-day light conditions (12 h of light, 21°C/12 h of dark, 19°C) at a light intensity of 110 to 120 μmol m−2 s−1 and 65% relative humidity.
Whole Plant TE Experiments
The protocol was modified from Juenger et al. (2005). Plants were grown in a 16:8:1 volume mixture of potting mix, fritted clay (Turface Greens Grade; Profile Products), and perlite. The carrying capacity of the soil mix was determined following a 24-h gravimetric drain of saturated soil. A known dry weight of soil mixture was placed in 250-mL plastic containers with four holes punched in the bottom. A circle of landscape fabric was placed at the bottom of each pot to prevent soil loss. Water was added to the pots to either 90% (ample water) or 30% (drought stress; approximately −1.4 MPa [soil psychrometer; Wescor]) of the soil water-carrying capacity and sealed with a layer of Parafilm and a tight-fitting lid with a small central hole. One stratified seed was placed on the soil surface, centered under the hole of the lid. Pots were weighed every 2 to 3 d, and water was added to the pots with a syringe to return soil to the appropriate water level. Blank pots containing no plants indicated that evaporative losses from the soil under these conditions were minimal. Eight-week-old plants were harvested prior to bolting for vegetative TE measurements. For the phase change TE experiment, the plants were harvested at 13 weeks; all plants were flowering and setting seed at harvest. The rosette and inflorescence (where applicable) were removed and dried at 70°C until a constant weight was achieved for dry weight determination. TE was calculated by dividing the dry weight of the rosette, inflorescence, or entire aboveground biomass where applicable (mg) by the total volume of water transpired (mL). For the ample water/drought stress TE experiment, three genotype × water level replicates were planted in each of seven blocks. Plant mortality resulted in final genotype × water level replicates ranging from 13 to 21 plants, for a total 120 plants. For the phase change TE experiment, eight blocks were planted, each block containing six replicate genotypes. Four blocks were harvested prior to bolting and four blocks after flowering. Plant mortality resulted in final sample sizes ranging from 20 to 24 plants per genotype per harvest time, resulting in 135 plants.
Exogenous ABA Treatment
Plants were grown as described for the TE experiments at 90% of the soil water-carrying capacity in five blocks containing six genotype × ABA concentration replicates (a total of 174 plants after plant mortality). After 4 weeks of growth, plants were sprayed twice weekly with either ABA solution (25 μm ABA, 0.05% ethanol, and 0.02% Silwett L-77) or control solution (0.05% ethanol and 0.02% Silwett L-77). After 4 weeks of ABA application, rosettes were harvested for carbon isotope analysis.
Carbon Isotope Analysis
Each dried rosette was ground to a fine powder using a mortar and pestle and sent to the Cornell Stable Isotope Laboratory (http://www.cobsil.com), where the carbon isotope ratios of the samples (Rs) were determined. The ratios given are relative to the V-PDB standard (RPDB), where δ13C (‰) = (Rs/RPDB − 1) × 1,000. Carbon isotope ratio values rather than carbon isotope discrimination are shown because the carbon signature of the growth chamber air is unknown. Sample size was 12 to 15 genotype × water level replicates for the ample water/drought stress experiment and 11 to 12 genotype × ABA concentration replicates for the ABA treatment experiment.
Stomatal Density and Stomatal Index Measurements
The abaxial epidermis was peeled from fully expanded leaves of 7-week-old plants, wet mounted, and photographed at 400× power using a digital camera mounted to a Nikon Diaphot 300 microscope. Stomatal density and epidermal cell density were determined for each image using Image J. The scale was determined by photographing a slide micrometer. Two or three leaves were sampled per plant, and approximately 24 images were analyzed for cell density per plant. Stomatal index (100 × stomatal density/[stomatal density + epidermal cell density]) was calculated for each image. For each plant, the density and index values were averaged, and the mean value was used for statistical analysis. Six replicate plants were measured for each genotype.
GPA1prom::GUS Lines and GUS Staining
The 1,500-bp region directly upstream of the GPA1 translational start site was amplified using Accuprime Pfx Supermix (Invitrogen) with the forward primer 5′-CTCGAGTTTAAGTGGTTAGGGAAGCTATGTATT-3′ and the reverse primer 5′-GCGGCCGCGATTGTTTCTATATCCCCACAG-3′. The fragment was blunt cloned into the TOPO PCR Blunt II vector (Invitrogen), sequenced, and subcloned into the binary GUS reporter vector pORE R1 at the XhoI and NotI sites (Coutu et al., 2007). pORE R1 GPA1prom::GUS was transformed into Agrobacterium tumefaciens cells (C58C1), and a modified floral dip method was used to infect the floral buds with the transformed Agrobacterium (Clough and Bent, 1998). Seeds were plated on 0.5× Murashige and Skoog medium, 50 μg mL−1 kanamycin, and 0.8% agar to select for transformants. All transformants were confirmed via PCR genotyping.
GUS staining was performed on multiple independent T1 lines. The abaxial epidermis of developing leaves was removed with forceps and immediately placed in acetone on ice. Samples were vacuum infiltrated with acetone for 10 min and fixed at room temperature for 30 min. The acetone was removed, and samples were vacuum infiltrated on ice with GUS staining buffer (50 mm NaPO4, pH 7.2, 0.2% Triton X-100, 2 mm potassium ferrocyanide, and 2 mm potassium ferricyanide) for 10 min. The staining buffer was removed, and the samples were infiltrated under vacuum with GUS staining buffer containing 2 mm 5-bromo-4-chloro-3-indolyl-β-glucuronic acid in dimethylformamide for 20 min on ice. Samples were incubated overnight at 37°C in the dark. Samples were dehydrated in an ethanol series (20%, 35%, and 50%) for 30 min at room temperature for each concentration. The samples were fixed with 50% ethanol, 3.7% formaldehyde, and 5% acetic acid for 30 min at room temperature and then placed in 70% ethanol. Samples were mounted in 70% ethanol and 30% glycerol and examined with a light microscope.
Gas-Exchange Analysis
Gas-exchange analysis was performed on intact, fully expanded leaves of 7-week-old plants using a Licor-6400 photosynthesis system (Licor Biosciences) equipped with the Licor-6400-40 leaf chamber fluorometer. Plants were kept in the growth chamber during all gas-exchange measurements. Light curves performed on wild-type plants prior to A-Ci analyses showed that a light level of 1,000 μmol m−2 s−1 was appropriate for non-light-limiting and nonphotoinhibitory conditions (data not shown). The following variables were held constant during gas-exchange analyses: vapor pressure difference (1.2 kPa), leaf temperature (23°C), and light intensity (1,000 μmol m−2 s−1, 10% blue light). A and gs were measured at the following external CO2 values in the order shown: 2,000, 1,500, 1,000, 800, 400, 200, 100, 50, and 0 μL L−1. Because flow rate was varied in order to maintain a constant vapor pressure difference, it took 10 to 30 s to initially reach each CO2 concentration. Each CO2 level was then held for 2 min to allow A to adjust to the new CO2 concentration before matching of infrared gas analyzers and data logging. Infrared gas analyzers were matched, and data were logged every 15 s for another 2 min at each CO2 level. Estimates of Vcmax and Jmax were calculated for each A-Ci curve using a Web-based curve-fitting utility (Sharkey et al., 2007). For each genotype, gas-exchange measurements were performed on 10 to 12 replicate plants. Data shown are averaged curves where average gs or A is plotted against average Ci.
Stomatal Aperture Assay
Leaves from 6-week-old plants were excised prior to the beginning of the day's light cycle and incubated in buffer (10 mm KCl, 7.5 mm iminodiacetic acid, 10 mm MES, pH 6.15, with KOH) in the dark for 2 h to ensure stomatal closure (Leymarie et al., 1998a). A Licor-6400 was used to scrub CO2 and pump CO2-free air into a 600-mL glass beaker containing 150 mL of buffer (flow rate of 500 μmol s−1). The buffer was equilibrated with CO2-free air overnight. The leaves were placed in the low-CO2 buffer or ambient-CO2 buffer (approximately 400 μL L−1), abaxial side down, in the dark. For the low-CO2 treatment, CO2-free air was continuously pumped into the buffer during the leaf incubation. After 1 h of incubation, the epidermis of the leaves was removed and wet mounted onto slides. Eight genotype × treatment replicates were assayed over a 4-d period. Photographs were obtained using a digital camera mounted to a Nikon Diaphot 300 microscope. Stomatal aperture measurements were performed blind using Image J; a slide micrometer was photographed at the same resolution for scale.
Statistical Analyses
All statistical analyses were carried out using Minitab 14 Statistical Software. General linear model ANOVA was used for all TE and δ13C analyses with Dunnett- or Tukey-corrected multiple comparisons. For all ANOVAs, data and residuals were examined to confirm that all ANOVA assumptions were met. ANOVA tables can be found in Supplemental Tables S1 to S7. Student's t test was used to test all other means comparisons for statistical significance.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Jmax and Vcmax for Col and gpa1 mutants.
Supplemental Figure S2. Leaf anatomy of Col and gpa1-3.
Supplemental Tables S1 to S7. ANOVA tables.
Supplementary Material
Acknowledgments
We thank Prof. Roger Koide and Ms. Liza Wilson for soil water potential measurements.
References
- Adjobo-Hermans MJ, Goedhart J, Gadella TW., Jr (2006) Plant G protein heterotrimers require dual lipidation motifs of Gα and Gγ and do not dissociate upon activation. J Cell Sci 119: 5087–5097 [DOI] [PubMed] [Google Scholar]
- Assmann SM. (2002) Heterotrimeric and unconventional GTP binding proteins in plant cell signaling. Plant Cell (Suppl) 14: S355–S373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger D, Altmann T. (2000) A subtilisin-like serine protease involved in the regulation of stomatal density and distribution in Arabidopsis thaliana. Genes Dev 14: 1119–1131 [PMC free article] [PubMed] [Google Scholar]
- Bussis D, von Groll U, Fisahn J, Altmann T. (2006) Stomatal aperture can compensate altered stomatal density in Arabidopsis thaliana at growth light conditions. Funct Plant Biol 33: 1037–1043 [DOI] [PubMed] [Google Scholar]
- Chandler JW. (2008) Cotyledon organogenesis. J Exp Bot 59: 2917–2931 [DOI] [PubMed] [Google Scholar]
- Chen JG, Gao Y, Jones AM. (2006) Differential roles of Arabidopsis heterotrimeric G-protein subunits in modulating cell division in roots. Plant Physiol 141: 887–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Comstock JP, Ehleringer JR. (1992) Correlating genetic variation in carbon isotopic composition with complex climatic gradients. Proc Natl Acad Sci USA 89: 7747–7751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condon AG, Richards RA, Rebetzke GJ, Farquhar GD. (2002) Improving intrinsic water-use efficiency and crop yield. Crop Sci 42: 122–131 [DOI] [PubMed] [Google Scholar]
- Coutu C, Brandle J, Brown D, Brown K, Miki B, Simmonds J, Hegedus DD. (2007) pORE: a modular binary vector series suited for both monocot and dicot plant transformation. Transgenic Res 16: 771–781 [DOI] [PubMed] [Google Scholar]
- Davies WJ, Kudoyarova G, Hartung W. (2005) Long-distance ABA signaling and its relation to other signaling pathways in the detection of soil drying and the mediation of the plant's response to drought. J Plant Growth Regul 24: 285–295 [Google Scholar]
- Davies WJ, Zhang JH. (1991) Root signals and the regulation of growth and development of plants in drying soil. Annu Rev Plant Physiol Plant Mol Biol 42: 55–76 [Google Scholar]
- Dawson TE, Mambelli S, Plamboeck AH, Templer PH, Tu KP. (2002) Stable isotopes in plant ecology. Annu Rev Ecol Syst 33: 507–559 [Google Scholar]
- Ehleringer JR, Klassen S, Clayton C, Sherrill D, Fullerholbrook M, Fu QN, Cooper TA. (1991) Carbon isotope discrimination and transpiration efficiency in common bean. Crop Sci 31: 1611–1615 [Google Scholar]
- Fan LM, Zhang W, Chen JG, Taylor JP, Jones AM, Assmann SM. (2008) Abscisic acid regulation of guard-cell K+ and anion channels in Gβ- and RGS-deficient Arabidopsis lines. Proc Natl Acad Sci USA 105: 8476–8481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar GD, Ehleringer JR, Hubick KT. (1989) Carbon isotope discrimination and photosynthesis. Annu Rev Plant Physiol Plant Mol Biol 40: 503–537 [Google Scholar]
- Farquhar GD, Oleary MH, Berry JA. (1982) On the relationship between carbon isotope discrimination and the inter-cellular carbon-dioxide concentration in leaves. Aust J Plant Physiol 9: 121–137 [Google Scholar]
- Farquhar GD, Richards RA. (1984) Isotopic composition of plant carbon correlates with water-use efficiency of wheat genotypes. Aust J Plant Physiol 11: 539–552 [Google Scholar]
- Gookin TE, Kim J, Assmann SM. (2008) Whole proteome identification of plant candidate G-protein coupled receptors in Arabidopsis, rice, and poplar: computational prediction and in-vivo protein coupling. Genome Biol 9: R120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer GL, Farquhar GD, Broad IJ. (1997) On the extent of genetic variation for transpiration efficiency in sorghum. Aust J Agric Res 48: 649–655 [Google Scholar]
- Huang H, Weiss CA, Ma H. (1994) Regulated expression of the Arabidopsis G protein α subunit gene GPA1. Int J Plant Sci 155: 3–14 [Google Scholar]
- Huang J, Taylor JP, Chen JG, Uhrig JF, Schnell DJ, Nakagawa T, Korth KL, Jones AM. (2006) The plastid protein THYLAKOID FORMATION1 and the plasma membrane G-protein GPA1 interact in a novel sugar-signaling mechanism in Arabidopsis. Plant Cell 18: 1226–1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo JH, Wang S, Chen JG, Jones AM, Fedoroff NV. (2005) Different signaling and cell death roles of heterotrimeric G protein α and β subunits in the Arabidopsis oxidative stress response to ozone. Plant Cell 17: 957–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juenger TE, Mckay JK, Hausmann N, Keurentjes JJB, Sen S, Stowe KA, Dawson TE, Simms EL, Richards JH. (2005) Identification and characterization of QTL underlying whole-plant physiology in Arabidopsis thaliana: δ13C, stomatal conductance and transpiration efficiency. Plant Cell Environ 28: 697–708 [Google Scholar]
- Lake JA, Woodward FI. (2008) Response of stomatal numbers to CO2 and humidity: control by transpiration rate and abscisic acid. New Phytol 179: 397–404 [DOI] [PubMed] [Google Scholar]
- Lambrides CJ, Chapman SC, Shorter R. (2004) Genetic variation for carbon isotope discrimination in sunflower: association with transpiration efficiency and evidence for cytoplasmic inheritance. Crop Sci 44: 1642–1653 [Google Scholar]
- Lease KA, Wen J, Li J, Doke JT, Liscum E, Walker JC. (2001) A mutant Arabidopsis heterotrimeric G-protein β subunit affects leaf, flower, and fruit development. Plant Cell 13: 2631–2641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leymarie J, Lasceve G, Vavasseur A. (1998a) Interaction of stomatal responses to ABA and CO2 in Arabidopsis thaliana. Aust J Plant Physiol 25: 785–791 [Google Scholar]
- Leymarie J, Vavasseur A, Lasceve G. (1998b) CO2 sensing in stomata of abi1-1 and abi2-1 mutants of Arabidopsis thaliana. Plant Physiol Biochem 36: 539–543 [Google Scholar]
- Llorente F, Alonso-Blanco C, Sanchez-Rodriguez C, Jorda L, Molina A. (2005) ERECTA receptor-like kinase and heterotrimeric G protein from Arabidopsis are required for resistance to the necrotrophic fungus Plectosphaerella cucumerina. Plant J 43: 165–180 [DOI] [PubMed] [Google Scholar]
- Ma H, Yanofsky MF, Meyerowitz EM. (1990) Molecular cloning and characterization of GPA1, a G protein α subunit gene from Arabidopsis thaliana. Proc Natl Acad Sci USA 87: 3821–3825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masle J, Gilmore SR, Farquhar GD. (2005) The ERECTA gene regulates plant transpiration efficiency in Arabidopsis. Nature 436: 866–870 [DOI] [PubMed] [Google Scholar]
- McKay JK, Richards JH, Nemali KS, Sen S, Mitchell-Olds T, Boles S, Stahl EA, Wayne T, Juenger TE. (2008) Genetics of drought adaptation in Arabidopsis thaliana. II. QTL analysis of a new mapping population, Kas-1 X Tsu-1. Evolution 62: 3014–3026 [DOI] [PubMed] [Google Scholar]
- Moriyama E, Strope P, Opiyo S, Chen Z, Jones A. (2006) Mining the Arabidopsis thaliana genome for highly-divergent seven transmembrane receptors. Genome Biol 7: R96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilson SE, Assmann SM. (2010) Heterotrimeric G proteins regulate reproductive trait plasticity in response to water availability. New Phytol 185: 734–746 [DOI] [PubMed] [Google Scholar]
- Pandey S, Assmann SM. (2004) The Arabidopsis putative G protein-coupled receptor GCR1 interacts with the G protein α subunit GPA1 and regulates abscisic acid signaling. Plant Cell 16: 1616–1632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey S, Chen JG, Jones AM, Assmann SM. (2006) G-protein complex mutants are hypersensitive to abscisic acid regulation of germination and postgermination development. Plant Physiol 141: 243–256 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Pandey S, Nelson DC, Assmann SM. (2009) Two novel GPCR-type G proteins are abscisic acid receptors in Arabidopsis. Cell 136: 136–148 [DOI] [PubMed] [Google Scholar]
- Perfus-Barbeoch L, Jones AM, Assmann SM. (2004) Plant heterotrimeric G protein function: insights from Arabidopsis and rice mutants. Curr Opin Plant Biol 7: 719–731 [DOI] [PubMed] [Google Scholar]
- Rebetzke GJ, Condon AG, Richards RA, Farquhar GD. (2002) Selection for reduced carbon isotope discrimination increases aerial biomass and grain yield of rainfed bread wheat. Crop Sci 42: 739–745 [Google Scholar]
- Richards RA. (2006) Physiological traits used in the breeding of new cultivars for water-scarce environments. Agric Water Manage 80: 197–211 [Google Scholar]
- Schlüter U, Muschak M, Berger D, Altmann T. (2003) Photosynthetic performance of an Arabidopsis mutant with elevated stomatal density (sdd1-1) under different light regimes. J Exp Bot 54: 867–874 [DOI] [PubMed] [Google Scholar]
- Schroeder JI, Allen GJ, Hugouvieux V, Kwak JM, Waner D. (2001) Guard cell signal transduction. Annu Rev Plant Physiol Plant Mol Biol 52: 627–658 [DOI] [PubMed] [Google Scholar]
- Sharkey TD, Bernacchi CJ, Farquhar GD, Singsaas EL. (2007) Fitting photosynthetic carbon dioxide response curves for C3 leaves. Plant Cell Environ 30: 1035–1040 [DOI] [PubMed] [Google Scholar]
- Shpak ED, Berthiaume CT, Hill EJ, Torii KU. (2004) Synergistic interaction of three ERECTA-family receptor-like kinases controls Arabidopsis organ growth and flower development by promoting cell proliferation. Development 131: 1491–1501 [DOI] [PubMed] [Google Scholar]
- Shpak ED, McAbee JM, Pillitteri LJ, Torii KU. (2005) Stomatal patterning and differentiation by synergistic interactions of receptor kinases. Science 309: 290–293 [DOI] [PubMed] [Google Scholar]
- Torii KU, Mitsukawa N, Oosumi T, Matsuura Y, Yokoyama R, Whittier RF, Komeda Y. (1996) The Arabidopsis ERECTA gene encodes a putative receptor protein kinase with extracellular leucine-rich repeats. Plant Cell 8: 735–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trusov Y, Rookes JE, Chakravorty D, Armour D, Schenk PM, Botella JR. (2006) Heterotrimeric G proteins facilitate Arabidopsis resistance to necrotrophic pathogens and are involved in jasmonate signaling. Plant Physiol 140: 210–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trusov Y, Zhang W, Assmann SM, Botella JR. (2008) Gγ1 + Gγ2 ≠ Gβ: heterotrimeric G protein Gγ-deficient mutants do not recapitulate all phenotypes of Gβ-deficient mutants. Plant Physiol 147: 636–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullah H, Chen JG, Young JC, Im KH, Sussman MR, Jones AM. (2001) Modulation of cell proliferation by heterotrimeric G protein in Arabidopsis. Science 292: 2066–2069 [DOI] [PubMed] [Google Scholar]
- Vavasseur A, Raghavendra AS. (2005) Guard cell metabolism and CO2 sensing. New Phytol 165: 665–682 [DOI] [PubMed] [Google Scholar]
- Virgona JM, Hubick KT, Rawson HM, Farquhar GD, Downes RW. (1990) Genotypic variation in transpiration efficiency, carbon-isotope discrimination and carbon allocation during early growth in sunflower. Aust J Plant Physiol 17: 207–214 [Google Scholar]
- Wang SY, Assmann SM, Fedoroff NV. (2008) Characterization of the Arabidopsis heterotrimeric G protein. J Biol Chem 283: 13913–13922 [DOI] [PubMed] [Google Scholar]
- Wang XQ, Ullah H, Jones AM, Assmann SM. (2001) G protein regulation of ion channels and abscisic acid signaling in Arabidopsis guard cells. Science 292: 2070–2072 [DOI] [PubMed] [Google Scholar]
- Warpeha KM, Upadhyay S, Yeh J, Adamiak J, Hawkins SI, Lapik YR, Anderson MB, Kaufman LS. (2007) The GCR1, GPA1, PRN1, NF-Y signal chain mediates both blue light and abscisic acid responses in Arabidopsis. Plant Physiol 143: 1590–1600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb AA, Hetherington AM. (1997) Convergence of the abscisic acid, CO2, and extracellular calcium signal transduction pathways in stomatal guard cells. Plant Physiol 114: 1557–1560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss CA, Huang H, Ma H. (1993) Immunolocalization of the G protein α subunit encoded by the GPA1 gene in Arabidopsis. Plant Cell 5: 1513–1528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss CA, White E, Huang H, Ma H. (1997) The G protein α subunit (GPA1) is associated with the ER and the plasma membrane in meristematic cells of Arabidopsis and cauliflower. FEBS Lett 407: 361–367 [DOI] [PubMed] [Google Scholar]
- Wong SC, Cowan IR, Farquhar GD. (1979) Stomatal conductance correlates with photosynthetic capacity. Nature 282: 424–426 [Google Scholar]
- Zhang L, Hu G, Cheng Y, Huang J. (2008a) Heterotrimeric G protein α and β subunits antagonistically modulate stomatal density in Arabidopsis thaliana. Dev Biol 324: 68–75 [DOI] [PubMed] [Google Scholar]
- Zhang W, He SY, Assmann SM. (2008b) The plant innate immunity response in stomatal guard cells invokes G-protein-dependent ion channel regulation. Plant J 56: 984–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Stanley B, Zhang W, Assmann SM. (2010) ABA-regulated G protein signaling in Arabidopsis guard cells: a proteomic perspective. J Proteome Res (in press) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.