Abstract
Ethylene serves as an important hormone controlling several aspects of plant growth and development, including fruit ripening and leaf and petal senescence. Ethylene is perceived following its binding to membrane-localized receptors, resulting in their inactivation and the induction of ethylene responses. Five distinct types of receptors are expressed in Arabidopsis (Arabidopsis thaliana), and mutant receptors have been described that repress ethylene signaling in a dominant negative manner. One such mutant, ethylene resistant1-1 (etr1-1), results in a strong ethylene-insensitive phenotype in Arabidopsis. In this study, regulated expression of the Arabidopsis etr1-1 in tomato (Solanum lycopersicum) was achieved using an inducible promoter. In the absence of the inducer, transgenic seedlings remained sensitive to ethylene, but in its presence, a state of ethylene insensitivity was induced, resulting in the elongation of the hypocotyl and root in dark-grown seedlings in the presence of ethylene, a reduction or absence of an apical hook, and repression of ethylene-inducible E4 expression. The level of ethylene sensitivity could be controlled by the amount of inducer used, demonstrating a linear relationship between the degree of insensitivity and etr1-1 expression. Induction of etr1-1 expression also repressed the epinastic response to ethylene as well as delayed fruit ripening. Restoration of ethylene sensitivity was achieved following the cessation of the induction. These results demonstrate the ability to control ethylene responses temporally and in amount through the control of mutant receptor expression.
Ethylene is a gaseous hormone affecting plant development and plant responses to adverse environmental conditions (Burg, 1973; Yang and Hoffman, 1984; Bleecker and Kende, 2000; Klee, 2002, 2004; Wang et al., 2002; Lin et al., 2009). Ethylene can influence germination, sex determination, organ elongation, leaf and flower senescence, fruit ripening, programmed cell death, organ abscission, and pathogen responses (Feldman, 1984; Ecker and Davis, 1987; Mattoo and Suttle, 1991; Abeles et al., 1992; Fray and Grierson, 1993; Grbic and Bleecker, 1995; John et al., 1995; Young et al., 1997; Llop-Tous et al., 2000; Ciardi et al., 2001; Tieman et al., 2001; Whitelaw et al., 2002; Kevany et al., 2007, 2008). In several dicotyledonous species, exposure of etiolated seedlings to ethylene results in a triple response phenotype that includes inhibition of hypocotyl and root elongation, radial expansion of the hypocotyl and roots, and the formation of an exaggerated apical hook with unexpanded cotyledons (Neljubow, 1901). This growth response to ethylene is thought to aid the seedling in emerging from soil.
Ethylene is produced from Met that is first converted to S-adenosylmethionine by S-adenosylmethionine synthase, which is then converted to 1-aminocyclopropane-1-carboxylate (ACC) by ACC synthase. ACC oxidase generates ethylene by oxidizing ACC in a reaction that also produces CO2 and HCN (Yang and Hoffman, 1984). Ethylene is perceived following its binding to endoplasmic reticulum-localized receptors (Chen et al., 2002), of which five different types (i.e. ETR1, ERS1, EIN4, ETR2, and ERS2) are present in Arabidopsis (Arabidopsis thaliana) and six are present in tomato (Solanum lycopersicum; Wilkinson et al., 1995; Lashbrook et al., 1998; Chang and Shockey, 1999; Tieman and Klee, 1999; Tieman et al., 2000; Chang and Stadler, 2001; Wang et al., 2002; Klee, 2004; Stepanova and Alonso, 2005; Lin et al., 2009). In the absence of ethylene, the receptors are functionally active and, through their interaction with the CTR1 Raf-like kinase, maintain it in an active state to repress the activity of the downstream components of ethylene signaling (Kieber et al., 1993; Clark et al., 1998). Thus, ethylene receptors function as negative regulators (Hua and Meyerowitz, 1998). Ethylene binding to the N-terminal membrane domain inhibits receptor activity, thereby inactivating CTR1 and relieving the repression of the downstream components of the signaling pathway. As a result, EIN2 is activated and a transcriptional cascade involving the EIN3/EIL and ERF transcription factors is initiated (Chao et al., 1997; Solano et al., 1998; Alonso et al., 1999). Mutations affecting ethylene receptor function have been characterized in species including Arabidopsis and tomato. One class of mutants is characterized by constitutive signaling by the receptor, resulting in a dominant negative effect (Bleecker et al., 1988; Chang et al., 1993; Hua et al., 1995, 1998). One such mutant, etr1-1, has a Cys-to-Tyr mutation at residue 65 in the N-terminal transmembrane domain and results in a strong ethylene-insensitive phenotype (Bleecker et al., 1988; Guzmán and Ecker, 1990; Chang et al., 1993; Chen and Bleecker, 1995). This mutation affects the binding of a single Cu (I) cofactor that is required for ethylene binding and therefore fails to bind ethylene (Schaller and Bleecker, 1995; Hall et al., 1999; Rodríguez et al., 1999; Wang et al., 2006). Consequently, etr1-1 mutant plants are unable to perceive or respond to ethylene. Expression of etr1-1 in species including tomato, tobacco (Nicotiana tabacum), and petunia (Petunia hybrida) results in ethylene insensitivity (Wilkinson et al., 1997; Knoester et al., 1998), demonstrating that the Arabidopsis mutant receptor can function in heterologous species. Whether the etr1-1 mutant receptor exerts dominance primarily through a continued interaction with CTR1 in the presence of ethylene or by maintaining endogenous receptors in an active state in Arabidopsis or other species remains to be determined. Also unknown is whether a state of ethylene insensitivity conferred by the expression of etr1-1 is “all or nothing” (i.e. whether ethylene insensitivity requires a threshold of etr1-1 expression before a state of ethylene insensitivity is achieved, or whether the level of sensitivity to ethylene is determined by the level of etr1-1 expression).
Because of the economic value associated with the processes controlled by ethylene, the ability to control responses to this hormone has received considerable attention. In this report, whether a state of ethylene insensitivity can be controlled in tomato through the inducible expression of the dominant negative mutant Arabidopsis etr1-1 was investigated. Moreover, whether ethylene insensitivity was achieved following a threshold of etr1-1 expression or if the degree of ethylene insensitivity achieved correlated with the level of etr1-1 expression was determined. Induction of etr1-1 expression was made possible through the use of an insect steroid hormone-regulated (i.e. ecdysone) promoter. Growth of transgenic seedlings was inhibited by ethylene in the absence of induction, whereas a state of ethylene insensitivity was achieved following the induction of etr1-1 expression. The degree of ethylene insensitivity observed was dependent on the level of inducer used, correlating with the level of etr1-1 expression, and was similar in hemizygous and homozygous seedlings. Leaf epinasty and fruit ripening in response to ethylene was also repressed following induction of etr1-1 expression. The state of ethylene insensitivity could be reversed by withdrawing the inducer. These results demonstrate that a regulated state of ethylene insensitivity is achieved through the controlled expression of etr1-1.
RESULTS
Induced Expression of etr1-1 Results in Regulated Ethylene Insensitivity
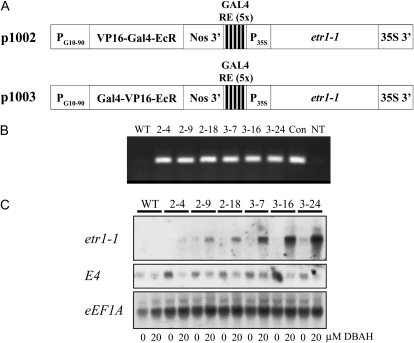
The ecdysone receptor (EcR) is a nuclear transcription factor in arthropods that is activated by ecdysteroids (Riddiford et al., 2000). EcR is composed of an N-terminal transcriptional activation domain (referred to as the A/B domain), a DNA-binding domain (i.e. the C domain), a linker region (i.e. the D region), a domain to which the ligand binds (i.e. the E domain), and, in some receptors, a C-terminal region (i.e. the F domain). Domains D to F have been used to generate inducible promoters for use in plants that are activated by ecdysone (Padidam et al., 2003). As ecdysone agonist-inducible expression has been shown to provide low basal expression in the absence of induction and a high level of expression following induction (Padidam et al., 2003), a Choristoneura fumiferana EcR-based, agonist-inducible system was used to regulate etr1-1 expression. The coding region of the Arabidopsis mutant ethylene receptor, etr1-1, was placed under the control of a minimal cauliflower mosaic virus 35S promoter in which five copies of a 17-bp GAL4 response element had been introduced (Fig. 1A), as described previously (Padidam et al., 2003). Polyadenylation was controlled by the 35S 3′ region introduced downstream of the etr1-1 coding region. Induction from the modified 35S promoter was achieved by the expression of the ligand-binding region of the EcR translationally fused to the GAL4 DNA-binding and VP16 activation domains. These latter domains were N-terminally fused to EcR in either a VP16/GAL4 (i.e. in p1002) or GAL4/VP16 (i.e. in p1003) orientation (Fig. 1A). Expression of this fusion was controlled by the G10-90 promoter and the Nos 3′ region. Both gene cassettes were introduced into the pBIN19 binary vector for Agrobacterium tumefaciens-mediated transformation of tomato hypocotyls. Following transformation, the presence of the etr1-1 transgene in regenerated plants was confirmed by PCR (Fig. 1B).
Figure 1.
Induction of etr1-1 expression from p1002 and p1003 in tomato. A, p1002 and p1003 constructs illustrating the expression of the VP16-GAL4-EcR (p1002) and GAL4-VP16-EcR (p1003) fusions from the PG10-90 promoter and the expression of etr1-1 from the DBAH-inducible, modified 35S promoter. B, PCR amplification of the etr1-1 transgene from three lines containing p1002 (i.e. 2-4, 2-9, 2-18) and three lines containing p1003 (i.e. 3-7, 3-16, 3-24), confirming the presence of the transgene in the transformants. PCR analysis of wild-type seeds (WT) and a reaction containing no template (NT) were included as negative controls. PCR amplification of the etr1-1 transgene from p1003 was included as a positive control (Con). C, Northern analysis of seedlings of the same p1002 and p1003 lines germinated in the dark for 14 d on medium with 20 μm ACC and with or without 20 μm DBAH. The level of etr1-1 expression in the absence or presence of the inducer was measured, as was the expression of the ethylene-inducible E4 mRNA using the same membrane after it had been stripped. Expression of eEF1A mRNA was determined from the same membrane as an RNA-loading control.
To investigate the induction of etr1-1 expression and ethylene insensitivity, T2 seeds from three independent transformants homozygous for p1002 (i.e. lines 2-4, 2-9, 2-18) or p1003 (i.e. lines 3-7, 3-16, 3-24) were germinated on medium containing 20 μm ACC in the presence or absence of the nonsteroidal ecdysone agonist 3,5-dimethyl-benzoic acid N-(1-ethyl-2,2-dimethyl-propyl)-N′-(3S-hydroxymethyl-5-methyl-2,3-dihydro-benzo[1,4]dioxine-6-carbonyl) hydrazide (DBAH) at a concentration of 20 μm, and RNA was extracted for northern analysis. In the presence of the inducer, expression of etr1-1 was observed with substantially higher levels present in seedlings containing the GAL4/VP16 orientation (i.e. in p1003) of the EcR steroid fusion protein (Fig. 1C). For example, following normalization to translation elongation factor 1A (eEF1A) mRNA expression, which was used as the RNA-loading control (Fig. 1C), etr1-1 expression from lines 3-7, 3-16, and 3-24 induced with 20 μm DBAH was 2.17-, 4.20-, and 11.0-fold greater, respectively, than that from line 2-9, which had a similar level of expression as that in line 2-18. This was despite little to no expression of etr1-1 in the absence of the inducer in p1003-containing lines, although a low basal level of etr1-1 expression was detected in uninduced 3-24 seedlings (Fig. 1C). These results suggest that the GAL4/VP16 orientation results in stronger induction from the modified 35S promoter.
To examine whether the expression of etr1-1 was sufficient to prevent the induction of ethylene-regulated gene expression in seedlings grown on ACC, northern analysis of E4 mRNA, previously shown to be ethylene inducible in leaves (Barry et al., 2001), was performed using the same membrane after it had been stripped. E4 mRNA expression was substantially higher in transgenic seedlings grown on 20 μm ACC in the absence of DBAH than in its presence and was equally high in wild-type seedlings grown on 20 μm ACC whether or not the inducer was present when normalized to eEF1A mRNA expression.
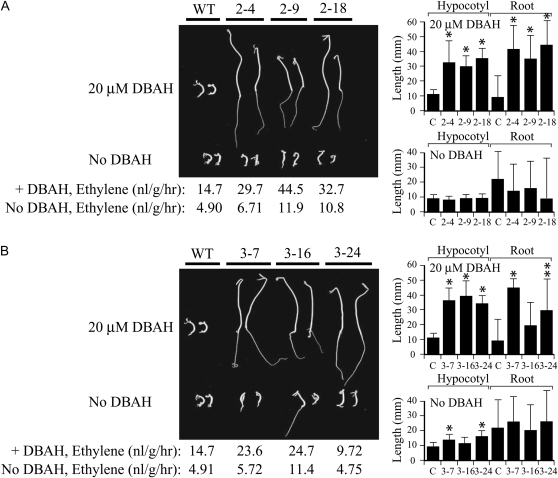
To determine whether the induction of etr1-1 expression resulted in repression of ethylene responses at the whole plant level, the triple response of the seedlings to ethylene was examined. The triple response is an ethylene-mediated response of dark-grown seedlings characterized by the radial expansion of the hypocotyl, the inhibition of root and hypocotyl elongation, and the presence of an exaggerated apical hook (Neljubow, 1901). Wild-type seedlings grown in the dark on 20 μm ACC in the presence or absence of DBAH exhibited these characteristics, including inhibition of hypocotyl and root growth and a pronounced apical hook (Fig. 2A). The growth of the hypocotyl and root in seedlings homozygous for p1002 (i.e. 2-4, 2-9, 2-18) was substantially greater than in wild-type seedlings when etr1-1 expression had been induced (i.e. grown in the presence of DBAH; Fig. 2A). An absence of an exaggerated apical hook was observed in p1002 seedlings in which etr1-1 expression was induced (Fig. 2A). In the absence of induction (i.e. grown in the absence of DBAH), the length of the hypocotyl in p1002 seedlings was not significantly different from that in wild-type seedlings, and although the average root length in p1002 seedlings was greater than in wild-type seedlings, this difference was not significant (Fig. 2A). Moreover, p1002 seedlings displayed an exaggerated apical hook when grown in the absence of DBAH. Similar results were obtained for seedlings homozygous for p1003 (i.e. 3-7, 3-16, 3-24; Fig. 2B).
Figure 2.
Induction of etr1-1 expression from p1002 and p1003 in tomato confers a state of ethylene insensitivity. Seeds from the same lines used in Figure 1 containing p1002 (A) or p1003 (B) were germinated in the dark for 14 d on medium with 20 μm ACC and with or without 20 μm DBAH to assay for their triple response. Two representative seedlings are shown for each line. Quantitative measurements for hypocotyl and root lengths with sd are shown in the histograms to the right of each panel. Ten to 20 seedlings were used for each measurement. Measurements of ethylene evolution from seedlings grown in the absence or presence of the inducer are shown below the triple response panels. The P values represent the statistical significance between each starred bar relative to the wild-type (WT) control: * P < 0.001, ** P < 0.005.
Ethylene production was higher in wild-type seedlings grown in the presence of DBAH relative to those grown in the absence of the inducer (Fig. 2), suggesting that DBAH may induce ethylene biosynthesis. Even higher levels of ethylene production were observed for seedlings containing p1002 or p1003, particularly when etr1-1 expression had been induced, suggesting that the state of ethylene insensitivity may have further induced ethylene biosynthesis, as has been observed in petunia expressing etr1-1 (Wilkinson et al., 1997). Despite some increase in ethylene production, p1002 and p1003 seedlings expressing the etr1-1 transgene exhibited little response to ethylene.
Graduated Loss of Sensitivity to Ethylene Is Determined by the Level of etr1-1 Expression
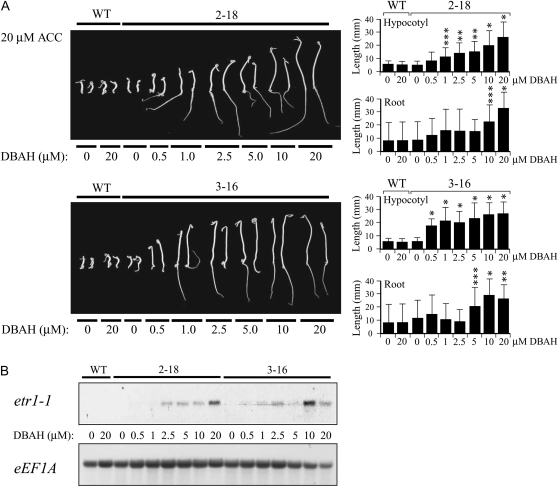
Previous studies on ethylene receptor mutations that confer insensitivity to the hormone did not examine whether the dominant effect of the mutant receptor varied as a function of mutant receptor expression or whether ethylene insensitivity was achieved only once a threshold of mutant receptor expression had been reached. The use of an inducible promoter permitted a dose-response analysis of the effect of etr1-1 expression on the level of ethylene insensitivity achieved. Consequently, the growth of seedlings germinated in the dark on medium containing 20 μm ACC and different levels of the inducer was examined for a representative p1002 line (i.e. 2-18) and a representative p1003 line (i.e. 3-16). In the absence of DBAH, the lengths of the hypocotyls and roots in 2-18 seedlings were not significantly different from wild-type seedlings (Fig. 3A). However, the lengths of their hypocotyls and roots increased as a function of DBAH concentration up to 20 μm, the highest level tested. The exaggeration of the apical hook was gradually reduced with increasing DBAH concentration up to 20 μm, at which point no hook was evident. etr1-1 expression in line 2-18 also increased as a function of DBAH concentration, with the highest level achieved at 20 μm of the inducer (Fig. 3B).
Figure 3.
The level of etr1-1 expression from p1002 and p1003 in tomato determines the level of ethylene insensitivity. A, etr1-1 expression in line 2-18 seedlings (containing p1002) or in line 3-16 seedlings (containing p1003) was induced by the concentration of DBAH indicated on medium containing 20 μm ACC. The seedlings were grown in the dark for 10 d. Wild-type seeds (WT) were included as a control. Two representative seedlings are shown for each line. Quantitative measurements for hypocotyl and root lengths with sd are shown in the histograms to the right of each panel. Ten to 20 seedlings were used for each measurement. B, Northern analysis of the seedlings in A. The level of etr1-1 expression in the absence or presence of the inducer at the concentrations indicated was measured, as was the expression of eEF1A to serve as a control for RNA loading. The P values represent the statistical significance between each starred bar relative to the 0-μm DBAH control: * P < 0.001, ** P < 0.005, *** P < 0.05.
As with line 2-18, hypocotyl and root lengths in seedlings from line 3-16 were not significantly different from wild-type seedlings but increased as a function of DBAH concentration (Fig. 3A). etr1-1 expression also increased as a function of DBAH concentration, with the highest level achieved at 10 to 20 μm DBAH once normalized to eEF1A mRNA expression (Fig. 3B). The exaggeration of the apical hook was also lessened commensurate with the increase in the growth of the hypocotyl and root as etr1-1 expression was induced (Fig. 3A). Maximum hypocotyl growth in 3-16 seedlings was achieved at a lower concentration of the inducer (0.5–1 μm) than observed for 2-18 seedlings, whereas maximum root growth was not achieved until 5 to 10 μm DBAH was used (Fig. 3A). The lower level of inducer required to achieve greater hypocotyl and root growth in line 3-16 relative to line 2-18 correlated with the greater degree of induction of etr1-1 expression in line 3-16. For example, etr1-1 expression was detected in line 3-16 treated with as little as 0.5 μm DBAH, whereas expression of etr1-1 was not observed in line 2-18 until 1 to 2.5 μm DBAH was used (Fig. 3B). Moreover, following induction with 20 μm DBAH and normalization to eEF1A mRNA expression, etr1-1 expression in line 3-16 was 2.9-fold greater than in line 2-18 (Fig. 3B). These results demonstrate that the degree of insensitivity to ethylene conferred by the etr1-1 receptor is a graduated response determined by the level of its expression.
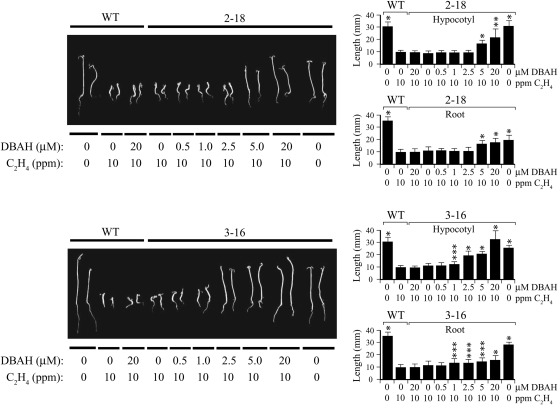
To confirm that the results with ACC were due to insensitivity to ethylene itself, the same lines were germinated on vermiculite in the dark for 2 d in air, at which point they were transferred to 10 μL L−1 ethylene for an additional 10 d of growth in the dark. As observed with growth on ACC, in the absence of DBAH, the lengths of the hypocotyls and roots in seedlings from lines 2-18 and 3-16 were not significantly different from wild-type seedlings when exposed to ethylene (Fig. 4). The hypocotyl and root lengths did increase, however, as a function of DBAH concentration up to 20 μm. A higher level of the inducer was required to achieve a detectable decrease in ethylene sensitivity in both lines when exposed to ethylene (Fig. 4) versus growth on ACC (Fig. 3A). Nevertheless, 2-18 and 3-16 seedlings grown in the presence of 20 μm DBAH and 10 μL L−1 ethylene had little to no apical hook and substantially greater hypocotyl and root growth relative to their growth in the absence of the inducer, where both lines exhibited an exaggerated apical hook, short hypocotyls and roots, and radial thickening of the hypocotyl (Fig. 4). Consequently, a graduated loss of sensitivity to exogenous ethylene was achieved by increasing etr1-1 expression.
Figure 4.
Induction of etr1-1 expression from p1002 and p1003 in tomato determines the level of sensitivity to exogenous ethylene. Seeds from line 2-18 (containing p1002), line 3-16 (containing p1003), or the wild type (WT) were germinated in the dark in air for 2 d in vermiculite containing the concentration of DBAH indicated and then grown in either air or 10 μL L−1 ethylene as indicated for an additional 10 d. Two representative seedlings are shown for each line. Quantitative measurements for hypocotyl and root lengths with sd are shown in the histograms to the right of each panel. Ten to 20 seedlings were used for each measurement. The P values represent the statistical significance between each starred bar relative to the 0-μm DBAH control in the presence of 10 μL L−1 ethylene: * P < 0.001, ** P < 0.005, *** P < 0.05.
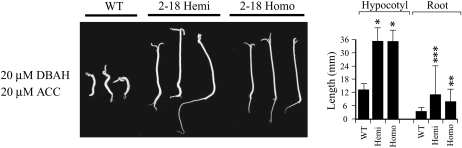
Because the etr1-1 receptor is dominant, the effect of inducing etr1-1 expression in seedlings that are hemizygous for the etr1-1 transgene could be compared with those that are homozygous for the transgene. To examine this, hemizygous etr1-1 F1 seeds generated from a cross between homozygous 2-18 plants and wild-type plants were grown in the dark for 10 d on medium containing 20 μm ACC and 20 μm DBAH, and their growth was compared with homozygous 2-18 seedlings as well as wild-type seedlings. Hemizygous 2-18 seedlings exhibited a similar degree of ethylene insensitivity as homozygous 2-18 seedlings, including increased hypocotyl and root growth relative to wild-type seedlings and a lack of an apical hook (Fig. 5). These results indicate that the level of ethylene insensitivity achieved following full induction of the etr1-1 transgene that is present in a hemizygous state can be as great as that achieved in plants homozygous for the transgene.
Figure 5.
Induction of etr1-1 expression from tomato hemizygous (Hemi) for the etr1-1 transgene confers a similar level of ethylene insensitivity as tomato homozygous (Homo) for the etr1-1 transgene. Line 2-18 was used to generate seeds hemizygous for the etr1-1 transgene following crosses with wild-type (WT) plants. Hemizygous 2-18 seeds, homozygous 2-18 seeds, and wild-type seeds were germinated in the dark for 10 d on medium with 20 μm ACC and 20 μm DBAH to assay for their triple response. Three representative seedlings are shown for each line. Quantitative measurements for hypocotyl and root lengths with sd are shown in the histograms to the right of each panel. Ten to 20 seedlings were used for each measurement. The P values represent the statistical significance between each starred bar relative to the wild-type control: * P < 0.001, ** P < 0.005, *** P < 0.05.
Induction of etr1-1 Expression Affords a High Level of Insensitivity
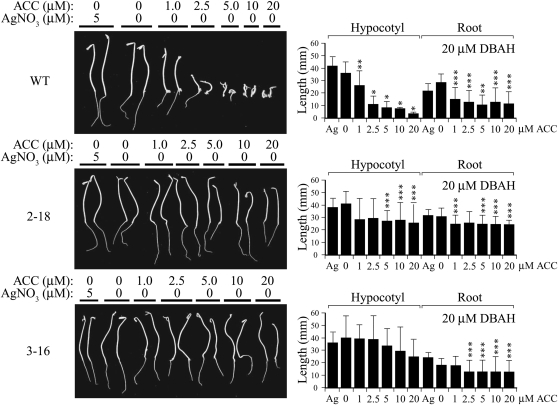
To determine the degree to which the induction of etr1-1 expression confers a state of ethylene insensitivity, the growth of 2-18 and 3-16 seedlings was compared with wild-type seedlings in the triple response assay on medium containing 20 μm DBAH and different levels of ACC. Hypocotyl growth was greatest for wild-type seedlings grown in the presence of Ag2+ (Fig. 6), which confers a state of ethylene insensitivity by replacing the copper cofactor present in the ethylene-binding site of the receptor, which perturbs the binding site sufficiently that ethylene binding is uncoupled from signal output (Rodríguez et al., 1999). In the absence of Ag2+, hypocotyl growth of wild-type seedlings was reduced relative to growth on Ag2+, although this difference was not significant. In the absence of Ag2+, hypocotyl and root growth of wild-type seedlings was increasingly inhibited and the apical hook became increasingly exaggerated as the concentration of ACC in the medium increased from 1.0 to 20 μm (Fig. 6).
Figure 6.
Induction of etr1-1 expression confers ethylene insensitivity over a range of ACC concentrations. Seeds of line 2-18 (containing p1002) and line 3-16 (containing p1003) were germinated on 20 μm DBAH and either 5 μm AgNO3 or ACC at the concentrations indicated and grown in the dark for 14 d. Wild-type seeds (WT) were included as a negative control. Two representative seedlings are shown for each line. Quantitative measurements for hypocotyl and root lengths with sd are shown in the histograms to the right of each panel. Ten to 20 seedlings were used for each measurement. The P values represent the statistical significance between each starred bar relative to growth on 5 μm AgNO3: * P < 0.001, ** P < 0.005, *** P < 0.05.
Growth of 2-18 seedlings in the presence of Ag2+ was similar to its growth when etr1-1 expression was induced by 20 μm DBAH, suggesting that etr1-1 expression conferred a similar degree of ethylene insensitivity as did Ag2+ (Fig. 6). Hypocotyl and root growth was only slightly reduced in the presence of increasing concentrations of ACC, and there was little evidence of an exaggerated apical hook (Fig. 6). Similar results were obtained for 3-16 seedlings in that hypocotyl and root growth was somewhat reduced in the presence of ACC (Fig. 6). The slight inhibition of hypocotyl growth observed for 2-18 seedlings at low ACC concentrations (1.0–2.5 μm) was not observed for 3-16 seedlings, consistent with the higher level of etr1-1 induction in 3-16 seedlings (Fig. 1C). These results demonstrate that the induction of etr1-1 expression confers a state of ethylene insensitivity that is largely maintained even in the presence of high concentrations of ACC.
Control of the Epinastic Response and Fruit Ripening through Induction of etr1-1 Expression
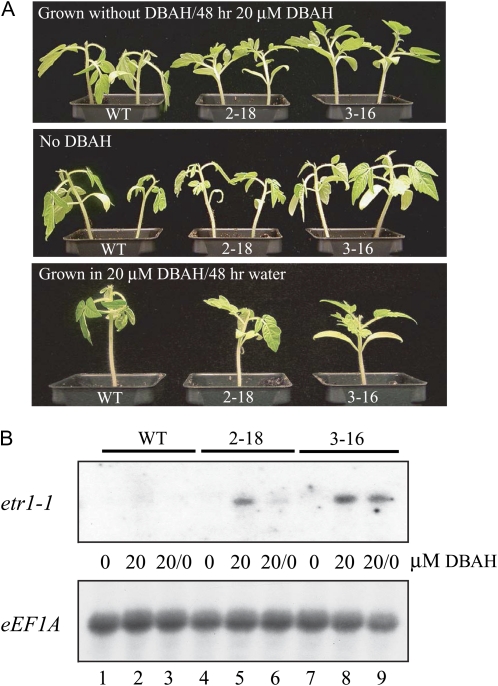
The ethylene-induced epinastic response in leaves results in their downward curvature, resulting from auxin-mediated differential growth between the adaxial and abaxial sides of the petiole or a differential sensitivity of adaxial/abaxial tissues to ethylene and its effect on cell expansion (Palmer, 1972; Kang, 1979). In contrast to germinating seedlings in the triple response assay, which involves inhibition of the growth of the hypocotyl and root, the petiole and leaf blade are existing organs; therefore, the duration of a state of ethylene insensitivity following the induction of etr1-1 expression once the inducer has been withdrawn can be examined without the complication of the generation of new cells during the induction or withdrawal periods. To establish whether the epinastic response is regulated by the induction of etr1-1 expression, 3-week-old 2-18 and 3-16 plants grown in the absence of the inducer were treated with 20 μm DBAH for 2 d prior to exposure to 5 μL L−1 ethylene for 48 h. Following induction of etr1-1 expression, 2-18 and 3-16 plants exhibited no epinastic response to the ethylene treatment, whereas wild-type plants showed a strong epinastic response (Fig. 7A, top). Analysis of these plants revealed that etr1-1 expression was induced in line 2-18 (Fig. 7B, lane 5) and in line 3-16 (Fig. 7B, lane 8) but not in wild-type plants (Fig. 7B, lane 2). In contrast, 3-week-old 2-18 and 3-16 plants grown in the absence of the inducer prior to exposure to 5 μL L−1 ethylene for 48 h exhibited a strong epinastic response, as did wild-type plants (Fig. 7A, middle), and no etr1-1 expression was detected in line 2-18 (Fig. 7B, lane 4), line 3-16 (Fig. 7B, lane 7), or in wild-type plants (Fig. 7B, lane 1). To examine whether sensitivity to ethylene could be restored in 2-18 and 3-16 plants in which etr1-1 expression had been induced, 2-18, 3-16, and wild-type plants grown in 20 μm DBAH for 3 weeks were watered without inducer for 2 d and then treated with 5 μL L−1 ethylene for 48 h. The 2-18 plants exhibited epinasty following the ethylene treatment as did wild-type plants, whereas no epinastic response to the ethylene treatment was observed in 3-16 plants (Fig. 7A, bottom). The level of etr1-1 expression was significantly lower in DBAH-grown 2-18 plants that were watered without inducer for 2 d (Fig. 7B, lane 6) than in water-grown 2-18 plants that were treated with inducer for 2 d (Fig. 7B, lane 5), consistent with some restoration of its sensitivity to ethylene. The observation that wild-type plants exhibited an even stronger epinastic response to ethylene suggests that the residual level of etr1-1 expression detected in these 2-18 plants continued to provide a degree of insensitivity to the ethylene treatment. In contrast, the level of etr1-1 expression was reduced by only 2.1-fold in DBAH-grown 3-16 plants that were watered without inducer for 2 d (Fig. 7B, lane 9) relative to water-grown 3-16 plants that were treated with inducer for 2 d (Fig. 7B, lane 8), such that significant levels of etr1-1 mRNA remained in DBAH-grown 3-16 plants watered without inducer for 2 d. These data are consistent with the higher level of induction of etr1-1 expression observed in 3-16 leaves, where etr1-1 expression was 1.81-fold greater than in 2-18 leaves, following normalization to eEF1A mRNA expression, and they indicate that the level of etr1-1 expression detected in DBAH-grown 3-16 plants watered without inducer for 2 d continued to provide a state of ethylene insensitivity.
Figure 7.
Induction of etr1-1 expression regulates the epinastic response in tomato. A, Top, line 2-18 (containing p1002), line 3-16 (containing p1003), and wild-type (WT) plants grown in the absence of DBAH for 3 weeks were treated with 20 μm DBAH for 2 d before treatment with 5 μL L−1 ethylene for an additional 2 d. The epinastic response can be seen from the downward curvature of wild-type leaves. Middle, line 2-18, line 3-16, and wild-type plants grown in the absence of DBAH for 3 weeks were treated with water for 2 d before treatment with 5 μL L−1 ethylene for an additional 2 d. Bottom, line 2-18, line 3-16, and wild-type plants grown in the presence of DBAH for 3 weeks were treated with water for 2 d before treatment with 5 μL L−1 ethylene for an additional 2 d. B, Northern analysis of the seedlings in A. Plants grown in the absence of DBAH and watered with 20 μm DBAH for 2 d before the ethylene treatment are indicated by 20 μm DBAH. Plants grown in the absence of DBAH and given water for 2 d before the ethylene treatment are indicated by 0 μm DBAH. Plants grown in the presence of 20 μm DBAH and given water for 2 d before the ethylene treatment are indicated by 20/0 μm DBAH. Northern analysis of eEF1A expression was included as a control for RNA loading.
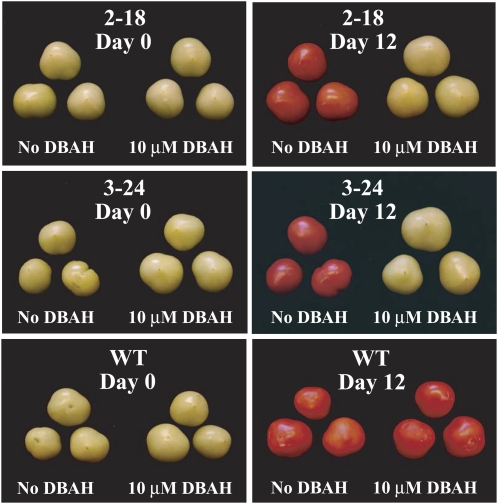
Ethylene regulates fruit ripening in tomato (Lin et al., 2009). To investigate whether the induction of etr1-1 expression would delay fruit ripening, 2-18 and 3-24 plants were grown to maturity in the absence of the inducer. Fruit from line 2-18, line 3-24, and wild-type plants were treated with water or 10 μm DBAH prior to breaker stage. At this stage, all fruit were similar in size and appearance (Fig. 8). Following 12 d, fruit from wild-type plants had ripened whereas those from 2-18 and 3-24 plants remained largely green (Fig. 8).
Figure 8.
Induction of etr1-1 expression regulates fruit ripening in tomato. Fruit from line 2-18 (containing p1002), line 3-24 (containing p1003), and wild-type (WT) plants were treated with water or 10 μm DBAH prior to breaker stage (day 0). The degree of ripening following 12 d is shown (day 12).
DISCUSSION
Although expression of etr1-1 in Arabidopsis and in other species including tomato results in a state of ethylene insensitivity (Wilkinson et al., 1997; Knoester et al., 1998), whether different degrees of reduced sensitivity to ethylene could be achieved through regulating etr1-1 expression or whether ethylene insensitivity following etr1-1 expression was an “all-or-nothing” effect was unknown. Through the regulated induction of etr1-1 expression, this study shows that the response to ethylene can be modulated from full sensitivity to virtually complete insensitivity to the hormone. This was achieved through the use of an inducer system based on the constitutive expression of the ligand-binding domain of the C. fumiferana EcR fused with the GAL4 DNA-binding domain and the herpes simplex virus VP16 acidic activation domain that is activated only in the presence of the inducer. Of the two orientations tested, use of the GAL4-VP16-EcR orientation in p1003 resulted in a greater degree of induction of etr1-1 expression than did VP16-GAL4-EcR in p1002, resulting in a reduction in ethylene sensitivity at lower concentrations of the inducer than was observed with p1002. The greater degree of induction of etr1-1 expression from p1003 suggests that the GAL4-VP16-EcR orientation provides a higher level of activator function, either through improved folding or increased stability of the fusion protein. The degree of ethylene insensitivity achieved correlated well with the level of etr1-1 transcript levels up to the point where full insensitivity was achieved, suggesting that etr1-1 protein, although not measured due to a lack of antiserum for the protein, correlated with etr1-1 transcript levels.
Micro-Tom, a dwarf cultivar, has been used as a model for molecular research in tomato (Meissner et al., 1997; Eyal and Levy, 2002). Micro-Tom contains mutations in the SELF-PRUNING and DWARF genes and a mutation that causes a reduction in internode length without altering the level of gibberellic acid (Martí et al., 2006). Because of the mutation in the DWARF gene, Micro-Tom is defective in brassinosteroid biosynthesis and dark-grown seedlings exhibit a weak photomorphogenic phenotype (Martí et al., 2006). Nevertheless, Micro-Tom seedlings exhibited a typical triple response to ethylene when grown in the dark and an epinastic response to ethylene when grown in the light, demonstrating that, despite the mutations affecting the growth of Micro-Tom, this variety exhibits normal responses to ethylene in the assays employed in this study.
In the absence of the inducer, seedlings containing p1002 or p1003 were highly responsive to ethylene, resulting in a virtual wild-type triple response phenotype. In a small number of p1003 seedlings, some root elongation was observed in the absence of DBAH (Fig. 2B) when grown in the presence of 20 μm ACC. As growth on 20 μm ACC inhibits root growth of wild-type seedlings, this may indicate some leaky expression of etr1-1 in the absence of DBAH, as can be seen in line 3-24 (Fig. 1C). No expression of etr1-1 was observed in p1002 seedlings in the absence of DBAH, suggesting that a higher level of basal expression from the GAL4-VP16-EcR orientation of p1003 in some transgenic lines may accompany the higher level of induction observed for this construct.
The observation that etr1-1 functioned in tomato to confer a state of complete ethylene insensitivity is consistent with the previous observation that its constitutive expression in tomato resulted in ethylene insensitivity (Wilkinson et al., 1997). As ETR1 is known to interact with CTR1 (Clark et al., 1998), the fact that etr1-1 functioned in tomato indicates that the Arabidopsis etr1-1 receptor can interact with the tomato homolog of CTR1. The observation that degrees of reduced sensitivity to ethylene could be achieved through the regulated expression of etr1-1 suggests that, even at suboptimal levels of etr1-1 expression in tomato, the etr1-1 mutant receptor can compete with the endogenous ethylene receptors to interact with the CTR1 tomato homolog. Although the stoichiometry of ethylene receptors and CTR1 in tomato is unknown, this result suggests that if the available CTR1 tomato homolog is associated with the endogenous ethylene receptors, etr1-1 is able to compete effectively for interaction with CTR1 and maintain the protein in an active state to repress the activation of the downstream components of ethylene signaling. This indicates that maintaining some CTR1 in an active state through a low level of etr1-1 expression is sufficient to reduce a plant's sensitivity to ethylene. It is also possible that etr1-1 receptor protein may interact with endogenous tomato ethylene receptors to maintain them in an active state.
Under conditions in which the etr1-1 transgene was fully induced (i.e. growth in the presence of 20 μm DBAH), a state of complete ethylene insensitivity was achieved in seedlings that were either hemizygous or homozygous for the etr1-1 transgene. The full state of insensitivity achieved in each case argues that the level of transgene expression in each exceeded that needed to confer a complete state of ethylene insensitivity. Any difference in transgene expression between a hemizygous line and a homozygous line, however, may confer different degrees of ethylene sensitivity at lower levels of transgene induction through the use of suboptimal levels of inducer. These results indicate that the level of ethylene insensitivity achieved following full induction of the etr1-1 transgene when present in a hemizygous state can be as great as that achieved in plants homozygous for the transgene.
A state of ethylene insensitivity following the induction of etr1-1 expression was observed in seedlings, in leaves, and during fruit ripening, suggesting that the induction of etr1-1 expression resulted in ethylene insensitivity in diverse organs. As seedlings undergo rapid growth, the generation of new cells complicates attempts to determine how rapidly a state of ethylene insensitivity is achieved following exposure to the inducer or how long a state of ethylene insensitivity is maintained following withdrawal of the inducer. In contrast, the lack of cell division in mature leaves and an assay based on their epinastic response to ethylene provided the means to examine this question. Exposure to the inducer for 2 d was sufficient to induce etr1-1 expression in leaves of p1002 or p1003 plants and confer complete insensitivity to ethylene. Withdrawal of the inducer for 2 d from plants grown in its presence was sufficient to reduce etr1-1 expression in leaves of p1002 plants and restore a degree of sensitivity to ethylene, demonstrating that ethylene sensitivity can be restored in preexisting cells following cessation of the induction of etr1-1 expression. Although a reduction in etr1-1 expression was also observed in p1003 leaves, because of the higher level of induction with this construct, the residual level of etr1-1 expression remaining after 2 d without inducer remained sufficient to maintain a state of ethylene insensitivity in p1003 plants. These results indicate that the period of protection against ethylene conferred by etr1-1 expression following its induction is determined by the level of etr1-1 expression provided by the construct. The data also indicate that the turnover of etr1-1 receptor protein occurs within 2 d, although the precise rate of turnover will require western analysis.
Because of the importance that ethylene plays in aspects of plant development or in responses to adverse environmental conditions, the ability to induce a state of ethylene insensitivity in crop species at will, to modulate the sensitivity to ethylene incrementally, or to restore ethylene sensitivity following withdrawal of the inducer could provide the means to control ethylene responses in order to achieve greater control over developmental processes such as fruit ripening or stress responses that affect crop yields.
MATERIALS AND METHODS
Plasmid Constructs
A modified EcR-based inducible system (Padidam et al., 2003) was used for the regulation of etr1-1 expression in Arabidopsis (Arabidopsis thaliana). The chimeric activator gene cassette contains the EcR ligand-binding region transcriptionally fused to the 441-bp GAL4 DNA-binding domain (amino acids 1–147; Laughon and Gesteland, 1984), the 264-bp VP16 acidic activation domain (amino acids 413–490) from Herpes simplex virus (Dalrymple et al., 1985), and the D to F regions containing the ligand-binding domain (amino acids 206–539) of EcR from Choristoneura fumiferana (spruce budworm; Perera et al., 1999), introduced between the constitutive G10-90 promoter (P10–90; Ishige et al., 1999) and the nopaline synthase terminator sequence (Nos 3′). In p1002, the VP16 domain is positioned N terminally followed by the GAL4 and EcR domains (VGE), whereas in p1003, the GAL4 domain is positioned N terminally followed by the VP16 and EcR domains (GVE; Fig. 1A). The GAL4-responsive etr1-1 expression cassette contains five copies of the 17-bp GAL4 response element (5×GAL4 RE; Giniger et al., 1985) and a 53-bp minimal promoter from the cauliflower mosaic virus 35S promoter (P35S) positioned upstream of the etr1-1 coding region and the 35S terminator region (35S 3′; Fig. 1A). The cassettes were introduced in the binary plasmid pBIN19 for subsequent transformation of tomato by Agrobacterium tumefaciens. All gene sequences were confirmed by DNA sequencing.
Plant Material and Transformation
Tomato (Solanum lycopersicum ‘Micro-Tom’) transformation was performed using Agrobacterium as described (McCormick, 1991). Briefly, cotyledon explants excised from 7-d-old seedlings were placed with their adaxial surface up in D1 medium (4.3 g L−1 Murashige and Skoog [MS] salts, 30 g of Glc, 1× Gamborg's B5 vitamins, 1 mg L−1 zeatin, and 0.8% agar, pH 5.8) and incubated at 24°C for 2 d at 50 to 60 μE m−2 s−1 prior to a 20-min incubation with Agrobacterium treated with 375 μm acetosyringone. The cotyledon explants were placed on Whatman filter paper in D1 medium with their abaxial side up and incubated at 24°C for 2 d at 50 to 60 μE m−2 s−1. The cotyledons were transferred (abaxial side up) to 2Z medium (4.3 g L−1 MS salts, 20 g of Suc, 2 mg L−1 zeatin, 100 mg L−1 inositol, 1× Nitsch vitamins, 0.5 mg L−1 folic acid, and 0.8% agar, pH 6.0) containing 500 mg L−1 carbenicillin and 100 mg L−1 kanamycin and incubated for 10 d at 24°C at 50 to 60 μE m−2 s−1. The cotyledon explants were transferred to 1Z medium (same as 2Z medium but with 1 mg L−1 zeatin) and at 3-week intervals thereafter. Regenerated shoots were rooted on MSSV medium (4.3 g L−1 MS salts, 30 g of Suc, 1× Nitsch vitamins, 0.5 mg L−1 folic acid, and 0.8% agar, pH 6.0) supplemented with 125 mg L−1 carbenicillin, 25 mg L−1 kanamycin, and 2 mg L−1 indole-3 butyric acid. Plants were transferred to soil and grown in commercial soil in a greenhouse supplied with charcoal-filtered air. Plants were grown under natural light conditions in a 10-h-light/14-h-dark cycle.
The nonsteroidal ecdysone agonist DBAH (RheoGene) dissolved in dimethyl sulfoxide was diluted in water and used to induce the modified 35S promoter at the concentrations indicated. DBAH was included in water agar for the triple response assays or applied during watering for soil-grown seedlings or plants. For epinasty assays, soil-grown seedlings were watered with either water or DBAH for 3 weeks. They were then watered with water or DBAH for an additional 2 d prior to their exposure to 5 μL L−1 ethylene. For triple response assays, seeds were placed on water agar containing, where indicated, DBAH and ACC and grown in the dark for the time indicated.
Northern Analysis
RNA was extracted by quick freezing the plant material in liquid nitrogen, grinding to a fine powder, and resuspending in 600 μL of extraction buffer (100 mm Tris [pH 8.0], 50 mm EDTA, 200 mm NaCl, 1% SDS, and 10 μL mL−1 β-mercaptoethanol). Following centrifugation, the supernatant was extracted with 700 μL of phenol:chloroform (1:1) and centrifuged to separate the phases. The supernatant was back extracted with chloroform, and the RNA was precipitated from the aqueous phase and resuspended in 600 μL of water. A PCR-generated etr1-1 fragment was radiolabeled with dCTP using the Prime-a-Gene labeling system (Promega) and used for hybridization with the membrane overnight at 39°C in 5× SSPE (150 mm NaCl, 10 mm NaH2PO4·H2O, and 1 mm EDTA), 5× Denhardt's solution, 50% formamide, and 1.5% SDS. Blots were washed for 30 min at 25°C in 1× SSPE/0.5% SDS, 30 min at 37°C in 0.5× SSPE/0.5% SDS, and 30 min at 60°C in 0.5× SSPE/0.5% SDS. The membrane was then exposed to film overnight at −80°C with an intensifier screen. Each northern-blot procedure was repeated at least twice. The same membrane was stripped in hybridization buffer at 65°C for 30 to 60 min until no signal could be detected. Where indicated, the membrane was reprobed for E4 and eEF1A mRNA using similar conditions, except that the hybridization with eEF1A mRNA was performed at 44°C.
PCR Analysis
DNA was isolated from total nucleic acid following its precipitation, resuspension in 10 mm Tris-HCl, 1 mm EDTA, pH 7.5, and treatment with RNase. PCR amplification was performed in 25-μL reactions containing 1× PCR buffer, 1 unit of HotStarTaq DNA polymerase (Qiagen), 250 μm deoxyribonucleotide triphosphates, 10 μm forward and reverse primers, and 50 ng of genomic DNA. Reactions were carried out using the following conditions: 95°C/5 min (one cycle); 95°C/30 s, 55°C/30 s, and 72°C/1 min (35 cycles); and a final extension at 72°C/5 min (one cycle). The upstream primer, AtETR1-F2, is 5′-CTACCAAATCGCTCTCCGTATTCACGAG-3′, and the downstream primer, AtETR1-R3, is 5′-TTACTCGTACAGTACCCGGGGCTCGAGAAG-3′.
Ethylene Determination
Ethylene was measured from whole seedlings that were placed in glass vials and capped with a rubber septum. Following a 2-h incubation, 0.9 mL of head space was sampled from each vial and the ethylene content was measured using a 6850 series gas chromatography system (Hewlett-Packard) equipped with a HP Plot alumina-based capillary column (Agilent Technologies), which can detect as little as 10 nL L−1 ethylene. The ethylene peak was identified as that which had the same retention time as pure ethylene. Tissue fresh weight was measured for each sample. Three to four replicates were measured, and the average and sd are reported.
Acknowledgments
We thank Christian Caldwell for technical assistance. Construction of p1002 and p1003 was provided by RheoGene, Inc.
References
- Abeles FB, Morgan PW, Saltveit ME., Jr (1992) Ethylene in Plant Biology, Ed 2 Academic Press, San Diego [Google Scholar]
- Alonso JM, Hirayama T, Roman G, Nourizadeh S, Ecker JR. (1999) EIN2, a bifunctional transducer of ethylene and stress responses in Arabidopsis. Science 284: 2148–2152 [DOI] [PubMed] [Google Scholar]
- Barry CS, Fox EA, Yen H, Lee S, Ying T, Grierson D, Giovannoni JJ. (2001) Analysis of the ethylene response in the epinastic mutant of tomato. Plant Physiol 127: 58–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleecker AB, Estelle MA, Somerville C, Kende H. (1988) Insensitivity to ethylene conferred by a dominant mutation in Arabidopsis thaliana. Science 241: 1086–1088 [DOI] [PubMed] [Google Scholar]
- Bleecker AB, Kende H. (2000) Ethylene: a gaseous signal molecule in plants. Annu Rev Cell Dev Biol 16: 1–18 [DOI] [PubMed] [Google Scholar]
- Burg SP. (1973) Ethylene in plant growth. Proc Natl Acad Sci USA 70: 591–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Kwok SF, Bleecker AB, Meyerowitz EM. (1993) Arabidopsis ethylene-response gene ETR1: similarity of product to two-component regulators. Science 262: 539–544 [DOI] [PubMed] [Google Scholar]
- Chang C, Shockey JA. (1999) The ethylene-response pathway: signal perception to gene regulation. Curr Opin Plant Biol 2: 352–358 [DOI] [PubMed] [Google Scholar]
- Chang C, Stadler R. (2001) Ethylene hormone receptor action in Arabidopsis. Bioessays 23: 619–627 [DOI] [PubMed] [Google Scholar]
- Chao Q, Rothenberg M, Solano R, Roman G, Terzaghi W, Ecker JR. (1997) Activation of the ethylene gas response pathway in Arabidopsis by the nuclear protein ETHYLENE-INSENSITIVE3 and related proteins. Cell 89: 1133–1144 [DOI] [PubMed] [Google Scholar]
- Chen YF, Randlett MD, Findell JL, Schaller GE. (2002) Localization of the ethylene receptor ETR1 to the endoplasmic reticulum of Arabidopsis. J Biol Chem 277: 19861–19866 [DOI] [PubMed] [Google Scholar]
- Chen QG, Bleecker AB. (1995) Analysis of ethylene signal-transduction kinetics associated with seedling-growth response and chitinase induction in wild-type and mutant Arabidopsis. Plant Physiol 108: 597–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciardi JA, Tieman DM, Jones JB, Klee HJ. (2001) Reduced expression of the tomato ethylene receptor gene LeETR4 enhances the hypersensitive response to Xanthomonas campestris pv. vesicatoria. Mol Plant Microbe Interact 14: 487–495 [DOI] [PubMed] [Google Scholar]
- Clark KL, Larsen PB, Wang X, Chang C. (1998) Association of the Arabidopsis CTR1 Raf-like kinase with the ETR1 and ERS ethylene receptors. Proc Natl Acad Sci USA 95: 5401–5406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalrymple MA, McGeoch DJ, Davison AJ, Preston CM. (1985) DNA sequence of the herpes simplex virus type 1 gene whose product is responsible for transcriptional activation of immediate early promoters. Nucleic Acids Res 13: 7865–7879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecker JR, Davis RW. (1987) Plant defense genes are regulated by ethylene. Proc Natl Acad Sci USA 84: 5202–5206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyal E, Levy AA. (2002) Tomato mutants as tools for functional genomics. Curr Opin Plant Biol 5: 112–117 [DOI] [PubMed] [Google Scholar]
- Feldman LJ. (1984) Regulation of root development. Annu Rev Plant Physiol 35: 223–242 [DOI] [PubMed] [Google Scholar]
- Fray RG, Grierson D. (1993) Molecular genetics of tomato fruit ripening. Trends Genet 9: 438–443 [DOI] [PubMed] [Google Scholar]
- Giniger E, Varnum S, Ptashne M. (1985) Specific DNA binding of GAL4, a positive regulatory protein of yeast. Cell 40: 767–774 [DOI] [PubMed] [Google Scholar]
- Grbic V, Bleecker AB. (1995) Ethylene regulates the timing of leaf senescence in Arabidopsis. Plant J 8: 595–602 [Google Scholar]
- Guzmán P, Ecker JR. (1990) Exploiting the triple response of Arabidopsis to identify ethylene-related mutants. Plant Cell 2: 513–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall AE, Chen QG, Findell JL, Schaller GE, Bleecker AB. (1999) The relationship between ethylene binding and dominant insensitivity conferred by mutant forms of the ETR1 ethylene receptor. Plant Physiol 121: 291–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua J, Chang C, Sun Q, Meyerowitz EM. (1995) Ethylene insensitivity conferred by Arabidopsis ERS gene. Science 269: 1712–1714 [DOI] [PubMed] [Google Scholar]
- Hua J, Meyerowitz EM. (1998) Ethylene responses are negatively regulated by a receptor gene family in Arabidopsis thaliana. Cell 94: 261–271 [DOI] [PubMed] [Google Scholar]
- Hua J, Sakai H, Nourizadeh S, Chen QG, Bleecker AB, Ecker JR, Meyerowitz EM. (1998) EIN4 and ERS2 are members of the putative ethylene receptor gene family in Arabidopsis. Plant Cell 10: 1321–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishige F, Takaishi M, Foster R, Chua NH, Oeda K. (1999) A G-box motif (GCCACGTGCC) tetramer confers high levels of constitutive expression in dicot and monocot. Plant J 18: 443–448 [Google Scholar]
- John I, Drake R, Farrell A, Cooper W, Lee P, Horton P, Grierson D. (1995) Delayed leaf senescence in ethylene-deficient ACC-oxidase antisense tomato plants: molecular and physiological analysis. Plant J 7: 483–490 [Google Scholar]
- Kang BG. (1979) Epinasty. Haupt W, Feinleib ME, , Physiology of Movements: Encyclopedia of Plant Physiology, Vol VII. Springer, Berlin, pp 647–667 [Google Scholar]
- Kevany BM, Taylor MG, Klee HJ. (2008) Fruit-specific suppression of the ethylene receptor LeETR4 results in early-ripening tomato fruit. Plant Biotechnol J 6: 295–300 [DOI] [PubMed] [Google Scholar]
- Kevany BM, Tieman DM, Taylor MG, Cin VD, Klee HJ. (2007) Ethylene receptor degradation controls the timing of ripening in tomato fruit. Plant J 51: 458–467 [DOI] [PubMed] [Google Scholar]
- Kieber JJ, Rothenberg M, Roman G, Feldman KA, Ecker JR. (1993) CTR1, a negative regulator of the ethylene response pathway in Arabidopsis, encodes a member of the Raf family of protein kinases. Cell 72: 427–441 [DOI] [PubMed] [Google Scholar]
- Klee HJ. (2002) Control of ethylene-mediated processes in tomato at the level of receptors. J Exp Bot 53: 2057–2063 [DOI] [PubMed] [Google Scholar]
- Klee HJ. (2004) Ethylene signal transduction: moving beyond Arabidopsis. Plant Physiol 135: 660–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoester M, van Loon LC, van den Heuvel J, Hennig J, Bol JF, Linthorst HJ. (1998) Ethylene-insensitive tobacco lacks nonhost resistance against soil-borne fungi. Proc Natl Acad Sci USA 95: 1933–1937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lashbrook CC, Tieman DM, Klee HJ. (1998) Differential regulation of the tomato ETR gene family throughout plant development. Plant J 15: 243–252 [DOI] [PubMed] [Google Scholar]
- Laughon A, Gesteland R. (1984) Primary structure of the Saccharomyces cerevisiae GAL4 gene. Mol Cell Biol 4: 260–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Z, Zhong S, Grierson D. (2009) Recent advances in ethylene research. J Exp Bot 60: 3311–3336 [DOI] [PubMed] [Google Scholar]
- Llop-Tous I, Barry CS, Grierson D. (2000) Regulation of ethylene biosynthesis in response to pollination in tomato flowers. Plant Physiol 123: 971–978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martí E, Gisbert C, Bishop GJ, Dixon MS, García-Martínez JL. (2006) Genetic and physiological characterization of tomato cv. Micro-Tom. J Exp Bot 57: 2037–2047 [DOI] [PubMed] [Google Scholar]
- Mattoo AK, Suttle JC. (1991) The Plant Hormone Ethylene. CRC Press, Boca Raton, FL [Google Scholar]
- McCormick S. (1991) Transformation of tomato with Agrobacterium tumefaciens. Lindsey K, , Plant Tissue Culture Manual: Fundamentals and Applications. Kluwer Academic Publishers, Boston, pp 1–9 [Google Scholar]
- Meissner R, Jacobson Y, Melamed S, Levyatuv S, Shalev G, Ashri A, Elkind Y, Levy AA. (1997) A new model system for tomato genetics. Plant J 12: 1465–1472 [Google Scholar]
- Neljubow DN. (1901) Uber die horizontale Nutation der Stengel von Pisum sativum und einiger Anderen. Pflanzen Beih Bot Zentralbl 10: 128–139 [Google Scholar]
- Padidam M, Gore M, Lu DL, Smirnova O. (2003) Chemical-inducible, ecdysone receptor-based gene expression system for plants. Transgenic Res 12: 101–109 [DOI] [PubMed] [Google Scholar]
- Palmer JH. (1972) Roles of ethylene and indole-3-acetic acid in petiole epinasty in Helianthus annuus and the modifying influence of gibberellic acid. J Exp Bot 23: 733–743 [Google Scholar]
- Perera SC, Sundaram M, Krell PJ, Retnakaran A, Dhadialla TS, Palli RS. (1999) An analysis of ecdysone receptor domains required for heterodimerization with ultraspiracle. Arch Insect Biochem Physiol 41: 61–70 [Google Scholar]
- Riddiford LM, Cherbas P, Truman JW. (2000) Ecdysone receptors and their biological actions. Vitam Horm 60: 1–73 [DOI] [PubMed] [Google Scholar]
- Rodríguez FI, Esch JJ, Hall AE, Binder BM, Schaller GE, Bleecker AB. (1999) A copper cofactor for the ethylene receptor ETR1 from Arabidopsis. Science 283: 996–998 [DOI] [PubMed] [Google Scholar]
- Schaller GE, Bleecker AB. (1995) Ethylene-binding sites generated in yeast expressing the Arabidopsis ETR1 gene. Science 270: 1809–1811 [DOI] [PubMed] [Google Scholar]
- Solano R, Stepanova A, Chao Q, Ecker JR. (1998) Nuclear events in ethylene signaling: a transcriptional cascade mediated by ETHYLENE-INSENSITIVE3 and ETHYLENE-RESPONSE-FACTOR1. Genes Dev 12: 3703–3714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanova AN, Alonso JM. (2005) Arabidopsis ethylene signaling pathway. Sci STKE 276: cm4. [DOI] [PubMed] [Google Scholar]
- Tieman DM, Ciardi JA, Taylor MG, Klee HJ. (2001) Members of the tomato LeEIL (EIN3-like) gene family are functionally redundant and regulate ethylene responses throughout plant development. Plant J 26: 47–58 [DOI] [PubMed] [Google Scholar]
- Tieman DM, Klee HJ. (1999) Differential expression of two novel members of the tomato ethylene-receptor family. Plant Physiol 120: 165–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tieman DM, Taylor MG, Ciardi JA, Klee HJ. (2000) The tomato ethylene receptors NR and LeETR4 are negative regulators of ethylene response and exhibit functional compensation within a multigene family. Proc Natl Acad Sci USA 97: 5663–5668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang KL, Li H, Ecker JR. (2002) Ethylene biosynthesis and signaling networks. Plant Cell (Suppl) 14: S131–S151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Esch JJ, Shiu SH, Agula H, Binder BM, Chang C, Patterson SE, Bleecker AB. (2006) Identification of important regions for ethylene binding and signaling in the transmembrane domain of the ETR1 ethylene receptor of Arabidopsis. Plant Cell 18: 3429–3442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitelaw CA, Lyssenko NN, Chen L, Zhou D, Mattoo AK, Tucker ML. (2002) Delayed abscission and shorter internodes correlate with a reduction in the ethylene receptor LeETR1 transcript in transgenic tomato. Plant Physiol 128: 978–987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson JQ, Lanahan MB, Clark DG, Bleecker AB, Chang C, Meyerowitz EM, Klee HJ. (1997) A dominant mutant receptor from Arabidopsis confers ethylene insensitivity in heterologous plants. Nat Biotechnol 15: 444–447 [DOI] [PubMed] [Google Scholar]
- Wilkinson JQ, Lanahan MB, Yen HC, Giovannoni JJ, Klee HJ. (1995) An ethylene-inducible component of signal transduction encoded by never-ripe. Science 270: 1807–1809 [DOI] [PubMed] [Google Scholar]
- Yang SF, Hoffman NE. (1984) Ethylene biosynthesis and its regulation in higher plants. Annu Rev Plant Physiol 35: 155–189 [Google Scholar]
- Young TE, Gallie DR, DeMason DA. (1997) Ethylene-mediated programmed cell death during maize endosperm development of wild-type and shrunken2 genotypes. Plant Physiol 115: 737–751 [DOI] [PMC free article] [PubMed] [Google Scholar]