Despite the importance of reactive oxygen species (ROS) in plant immunity, stress signaling, and development (Mori and Schroeder, 2004; Gadjev et al., 2006), the molecular identities of Ca2+-permeable channels responding to ROS have not been established. Here, we propose annexins as candidate channel proteins. Elevation of cytoplasmic free calcium ([Ca2+]cyt) as a regulatory step in plant immunity, stress adaptation, and development will rely on spatiotemporal control of Ca2+-permeable channel activity at endomembranes and the plasma membrane (PM; McAinsh and Pittman, 2009). Higher plant genomes encode multiple potential Ca2+-permeable channel subunits that could contribute to cell- and stimulus-specific patterns of [Ca2+]cyt elevation (McAinsh and Pittman, 2009; Ward et al., 2009). Numerous factors are involved in modulating channel events, including voltage, membrane stretch, pH, phosphorylation, G proteins, amino acids, cyclic nucleotides, and ROS (Ward et al., 2009). Identifying ROS-responsive Ca2+-permeable channels and their encoding genes will help elucidate ROS-Ca2+ signaling networks. Annexins could be able to form a ROS-stimulated passive Ca2+ transport pathway. Here, we examine the evidence leading to this proposal and show that plant annexins are capable of forming a Ca2+-permeable conductance in an oxidized membrane simulating ROS signaling conditions.
ANNEXINS AS CALCIUM TRANSPORTERS
In animals, annexin A5 is one of several annexins that can form a Ca2+-permeable channel in nonoxidized membranes but is also the likely candidate for mediating peroxide-induced Ca2+ influx across the PM of chicken DT40 cells (Kubista et al., 1999; Kourie and Wood, 2000; Gerke and Moss, 2002). Unlike conventional channels, annexins are soluble phospholipid-binding proteins that can undergo conditional association with or insertion into membranes, directly from the soluble phase (Kourie and Wood, 2000; Gerke and Moss, 2002; Gorecka et al., 2007; Mortimer et al., 2008; Laohavisit et al., 2009; Laohavisit and Davies, 2009). The presence of a hydrophilic pore at the center of the molecule is proposed to be the structural basis for annexin Ca2+ channel activity (Gerke and Moss, 2002). Plant annexins (usually around 32–36 kD; Mortimer et al., 2008) now also appear capable of mediating passive, channel-like Ca2+ transport (Hofmann et al., 2000; Mortimer et al., 2008; Laohavisit et al., 2009). Pepper (Capsicum annuum) annexin (CaANN24) mediates Ca2+ influx into artificial vesicles (Hofmann et al., 2000). A maize (Zea mays) annexin preparation (containing the ZmANN33 and ZmANN35 doublet) was found to promote Ca2+ influx into root epidermal protoplasts (Laohavisit et al., 2009). It also formed a Ca2+-permeable cation conductance in planar lipid bilayers that resembled plant PM Ca2+-permeable nonselective cation channel conductances in terms of Ca2+ permeability, voltage dependence, and inhibitor sensitivity (Laohavisit et al., 2009).
Animal annexin A5 has been shown to bind preferentially to malondialdehyde (MDA)-modified lipid (Balasubramanian et al., 2001). MDA is a reactive aldehyde that typifies peroxidized membranes, and its binding to A5 helps to explain the latter's contribution to peroxide-induced Ca2+ transport in vivo (Kubista et al., 1999). MDA is a typical breakdown product of peroxidized polyunsaturated fatty acids in plant membranes and triggers transcriptional stress responses (Weber et al., 2004; Farmer and Davoine, 2007; Yamauchi et al., 2008). Here, we have examined whether annexin-mediated Ca2+ transport is supported in bilayers containing MDA as a test of annexin competence in linking ROS and Ca2+ signaling and examined the impact of MDA on the physical properties of lipid mixtures.
GENERATION OF A RECTIFYING Ca2+-PERMEABLE CURRENT
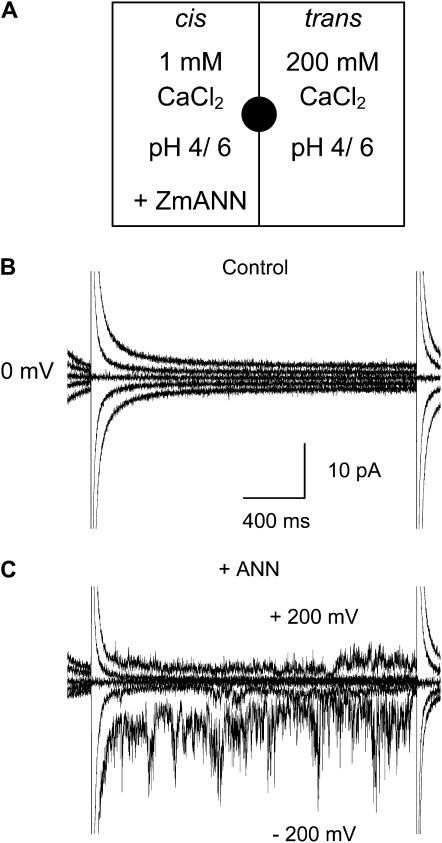
Maize annexin and lipids were prepared as described by Laohavisit et al. (2009), and 3 μg of preparation was incorporated into planar lipid bilayers. These comprised a ternary mixture of MDA-conjugated 1-palmitoyl-2-oleoyl-phosphatidylethanolamine (POPE; Balasubramanian et al., 2001), 1-palmitoyl-2-oleoyl-phosphatidylcholine (POPC; to stabilize the bilayer), and 1-palmitoyl-2-oleoyl-phosphatidylserine (POPS; to promote annexin binding; Gerke and Moss, 2002) in a 5:3:2 ratio. Experimental conditions and amounts of preparation used were identical to those used previously (Laohavisit et al., 2009), with the exception of bilayer composition. Addition of annexin was to the cis-chamber (equivalent to cytosol), which comprised 1 mm CaCl2, pH 4 or 6. The trans-chamber (equivalent to apoplast) comprised 200 mm CaCl2, pH 4 or 6 (Fig. 1A). Qualitatively, this Ca2+ gradient reflects that across the PM, into which maize annexins partition (Carletti et al., 2008), while acidic pH promotes plant and animal annexin transport activity in bilayers (Langen et al., 1998; Gorecka et al., 2007). Low pH also protonates MDA and increases its reactivity (Farmer and Davoine, 2007). Results were from at least four different protein preparations.
Figure 1.
Maize annexin preparation forms a Ca2+-permeable conductance in MDA-modified planar lipid bilayers. A, Schematic of experimental conditions. Highly purified maize annexin preparation (3 μg) was added to the cis-chamber. Chambers were separated by a MDA-POPE:POPC:POPS (5:3:2) bilayer formed across a hole (200 μm diameter) in the septum (black circle). Positive charge flowing from trans to cis is plotted as negative current, while that flowing from cis to trans is plotted as positive current. B, Representative current traces recorded in response to the step voltage protocol applied at −200, −100, and +100 mV from a 0-mV baseline prior to annexin addition; both chambers were at pH 6. C, Representative current traces recorded in response to voltage applied at −200, −100, and +100 mV from a 0-mV baseline, generated after addition of highly purified maize annexin (+ ANN) preparation. Currents at remaining voltages are not shown for clarity.
With pH 6 in both bilayer compartments, a conductance was observed in five out of 12 attempts (42%; Figs. 1C and 2A); with pH 4 in both compartments, activity was observed in nine out of 22 attempts (41%; Fig. 2A). This compares with six out of 12 attempts in the previous study with a non-MDA bilayer (Laohavisit et al., 2009). Activity was observed more quickly (20–60 min at −150 mV holding voltage) compared with the non-MDA bilayer (40–60 min; Laohavisit et al., 2009). The conductance was time independent (Fig. 1C), and heat-inactivated annexin did not support current (n = 5; Fig. 2A). Inward currents are consistent with cations moving into the cis-compartment, equivalent to cations moving into the cell across the PM. Ca2+ is the likely charge carrier in this system, since current was evident at the equilibrium potential for Cl− (ECl = –140 mV, ECa = +50 mV).
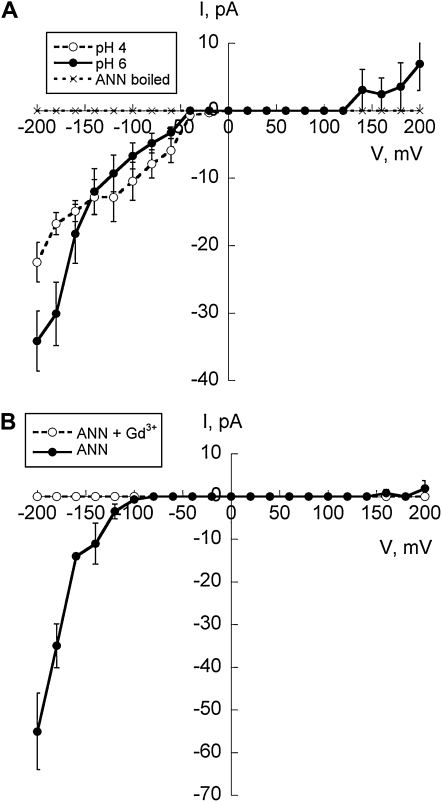
Figure 2.
Rectification, effect of pH, and pharmacology. A, Comparison of IV relationships generated by 3 μg of annexin (ANN) preparation (added to the cis-chamber) at different pH values across the MDA-POPE bilayer. In these experiments, the Ca2+ gradient was the same as in Figure 1. For pH 4, only values from −200 to 0 mV were determined. Data are mean ± se (n = 5 for pH 6; n = 3 for pH 4; n = 5 for heat-inactivated preparation at pH 6). With pH 4 across the MDA bilayer, mean current evoked at −200 mV was −23 ± 3 pA, a 32% decrease compared with pH 6 but not significantly different. B, IV relationships generated by 3 μg of annexin preparation (cis-chamber), MDA-POPE bilayer with a Ca2+ gradient of cis 1 μm and trans 5 mm, pH 6 (n = 3). Predicted ECa was +103 mV; ECl was −215 mV. No clear Erev was seen due to rectification. Mean current evoked at −200 mV was −55 ± 9 pA. Addition of 50 μm Gd3+ to the trans-chamber abolished current (n = 3).
The mean ± se inward current of −34 ± 5 pA at −200 mV and pH 6 (n = 5) was not significantly different from the non-MDA bilayer (−30 ± 4 pA; n = 6; Laohavisit et al., 2009). However, the MDA bilayer supported a very different current-voltage (IV) relationship. With a non-MDA bilayer at pH 6, maize annexin generated a near ohmic IV relationship with clear outward current at positive voltages, and reanalyzing those data yields an r2 value of 0.998 estimated from −200 mV to +200 mV (Laohavisit et al., 2009). In contrast, the inward current observed in this study with MDA was nonlinear, restricted in magnitude over the 0- to −150-mV range compared with the non-MDA bilayer (Laohavisit et al., 2009) and with notable hyperpolarization-activated current emergent at −150 mV (n = 5; Fig. 2A). Outward current was severely restricted such that it only became apparent at voltages more positive than 120 mV (Fig. 2A). No clear reversal potential was observed here, in contrast to the non-MDA bilayer reported previously, where the mean ± se reversal voltage of the conductance was 9 ± 3 mV (Laohavisit et al., 2009). At pH 6 with MDA, mean ± se outward current at +200 mV was 7 ± 4 pA (n = 5), which is significantly smaller (P = 0.014, Student's t test) compared with that supported by the non-MDA bilayer at the same pH (29 ± 6 pA; n = 6; Laohavisit et al., 2009). A more physiological Ca2+ gradient across the MDA bilayer (trans, 5 mm; cis, 1 μm; pH 6) still supported an inwardly rectifying current in four out of 11 attempts (36%; Fig. 2B). Addition of 50 μm Gd3+ as a cation channel blocker to the trans-chamber (Laohavisit et al., 2009) abolished current (Fig. 2B; n = 3), confirming that annexin at the cis-face caused current across the bilayer.
MDA EFFECTS ON LIPID AND BILAYER CHARACTERISTICS
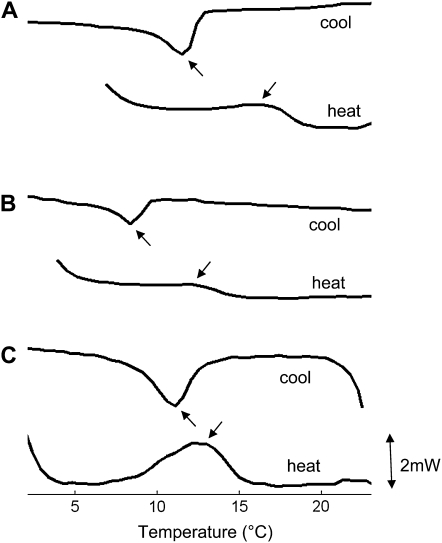
Changes in the bilayer composition resulted in rectification of the inward current and suppression of the outward current. Lipid bilayers can exist in many physical phase states. The principal phase transition separates the gel-like states below the melting transition (Tm) from fluid-like states above Tm (Lewis et al., 2007). The gel states are generally 1 order of magnitude more rigid than the fluid states (Mecke et al., 2003). Differential scanning calorimetry (DSC) revealed that MDA lowered the Tm of POPE (POPE Tm = 23°C ± 2°C; MDA-POPE Tm = 13°C ± 2°C; Fig. 3). The POPE:POPC:POPS ternary lipid mixture had a transition temperature approximately 4°C higher than MDA-POPE:POPC:POPS: (Tm = 16°C ± 2°C versus Tm = 12°C ± 2°C, respectively). However, both values are still at least 5°C below experimental conditions (22°C–25°C); thus, both were in a fluid state. Therefore, changes in annexin transport properties cannot be explained by significant modification of phase state by MDA. However, within a few degrees on either side of the gel fluid temperature, there can be large variation in bending rigidity (Méléard et al., 1997; Mecke et al., 2003). Further study is required to determine whether changes in bending rigidity by MDA are relevant to annexin transport characteristics.
Figure 3.
Typical DSC thermograms of lipid suspensions. A, POPE:POPC:POPS. B, MDA-POPE:POPC:POPS. C, POPS. Arrows identify melting (Tm) and crystallization (Tc) transitions. Curves are averages over three repeated heating or cooling steps (10°C min−1). The vertical (excess heat) scale is constant throughout. Measured transition temperatures are as follows: POPE:POPC:POPS, Tm = 16°C ± 2°C, Tc = 11.5°C ± 1°C; MDA-POPE:POPC:POPS, Tm = 12°C ± 2°C, Tc = 8.5°C ± 1°C; POPS, Tm = 13°C ± 1°C, Tc = 11°C ± 1°C; POPE, Tm = 23°C ± 2°C, Tc = 19.5°C ± 1°C; MDA-POPE, Tm = 13°C ± 2°C, Tc = 6°C ± 2°C. Measured values of Tm for POPE and POPS are approximately 1°C below the main fluid gel transition temperatures in the literature (Silvius, 1982), so Tm is taken as a proxy for this transition. Tc is dependent on the scanning rate, falling by approximately 1°C at 20°C min−1 (data not shown), consistent with Lewis et al. (2007). Lipid mixtures have weaker and broader peaks than pure lipids. MDA-POPE behaves more like a lipid mixture, probably due to by-products of the lipid/MDA reaction (Balasubramanian et al., 2001). DSC thermograms were obtained with a Perkins-Elmer Pyrus I heat conduction machine. The protocol followed Borochov et al. (1994): lipids (2 mg) were deposited directly onto DSC pans in chloroform solution, and solvent was removed under nitrogen, before resuspension in buffer (30 mL, 1 mm CaCl2, pH 6). The sample was equilibrated for 2 h at 50°C before scanning against buffer alone.
Lipid mixtures can also phase separate into coexisting regions of different composition and different mechanical properties. To test for the possibility of large-scale phase separation, which could certainly influence protein activity, giant unilamellar vesicles (10–20 μm diameter) were formed (by electroformation; Angelova et al., 1992) from the two ternary mixtures incorporating 5% (w/v) Texas Red-1,2-dihexadecanoyl-sn-glycero-3-phosphoethanolamine. Large-scale (greater than 1 μm) phase separation would be expected to result in dark or bright spots on the vesicle surface, viewed by fluorescence microscopy (Veatch and Keller, 2005). Neither mixture supported such patterning (5°C–50°C range) either with Texas Red or by observation of the blue-green autofluorescence of MDA-POPE (data not shown). These observations do not necessarily preclude phase separation without segregation of the fluorescent components or the presence of phase separation into submicrometer domains that could influence annexin activity.
The possibilities remain that either MDA changes the membrane as a whole (but we restrict this to the possibility of subtle modulations of the bending rigidity or membrane viscosity, which could affect membrane protein conformation; Andersen et al., 2007) or that its presence affects annexin directly. Reaction of MDA with phosphatidylethanolamine gives a negatively charged lipid-MDA adduct that binds annexin A5 in a similar manner to phosphatidylserine, which is also negatively charged (Balasubramanian et al., 2001). Here, MDA-POPE and POPS had very similar Tm (Tm = 13°C ± 2°C and Tm = 13°C ± 1°C, respectively). This supports the equivalence of these two lipids in protein interactions. It is possible that an MDA-modified electrostatic environment alone causes annexin to rectify the current. This could be due to selective recruitment or activity of one of the annexin doublet members (ZmANN33 or ZmANN35). This now requires further experimentation using heterologously expressed annexin.
CONSEQUENCES FOR ROS- AND Ca2+-BASED SIGNALING
Membranes are key sources of signaling lipids (Ng et al., 2001; Farmer and Davoine, 2007; Matos and Pham-Thi, 2009), including oxidized lipids (Mueller et al., 2008), but relatively few studies have addressed the impact of bilayer composition on plant channel function (Lee et al., 1994; Kaile, 1997; Klüsener et al., 1997). As annexins formed an inwardly directed Ca2+-permeable conductance in MDA-containing bilayers, it is feasible that annexins contribute to the inwardly directed and ROS-activated PM Ca2+ conductances identified in plants (Mori and Schroeder, 2004; Demidchik et al., 2007, 2009). The stress conditions that increase plant annexin abundance and recruitment to membranes (e.g. exogenous abscisic acid, drought, salinity, metal stress [Bianchi et al., 2002; Repetto et al., 2003; Lee et al., 2004; Vandeputte et al., 2007; Konopka-Postupolska et al., 2009]) can also result in ROS production (Demidchik et al., 2003; Boudsocq and Laurière, 2005; Konopka-Postupolska et al., 2009; Rodríguez-Serrano et al., 2009) and the lipid peroxidation that is typified by increases in MDA content (Demiral and Turkan, 2005; Collin et al., 2008). This suggests that annexins could function in integrating ROS and Ca2+ in stress signaling. Additionally, annexins are found at growth points such as root hair and pollen tube apices (for review, see Mortimer et al., 2008; Laohavisit and Davies, 2009) at which ROS can regulate [Ca2+]cyt, pointing to annexins as a putative ROS-regulated Ca2+ influx pathway for polar growth. This becomes more credible with the finding here that maize annexin was transport competent at 1 μm Ca2+, in the range of [Ca2+]cyt associated with polar growth (Wymer et al., 1997). Additionally, ROS appear to promote the association of maize annexin with lipids (Mortimer et al., 2009).
Annexin involvement in such pathways is likely to be complex, as the annexins identified to date as transport competent (CaANN24, ZmANN33/35, and Arabidopsis thaliana annexin 1 [AtANN1]) also exhibit peroxidase activity in soluble or membrane-bound form (Hofmann et al., 2000; Gorecka et al., 2007; Konopka-Postupolska et al., 2009; Laohavisit et al., 2009; Mortimer et al., 2009). Manipulation of AtANN1 abundance inversely correlates with abscisic acid-induced ROS accumulation in guard cells, but the mode of action remains unclear (Konopka-Postupolska et al., 2009). It has been proposed that annexin peroxidase activity could protect membranes against peroxidation (Jami et al., 2008) or locally terminate a peroxide-based signal in membrane microdomains (Mortimer et al., 2009). The two AtANN1 Cys residues are S-glutathionylated in vivo on abscisic acid treatment, resulting in a halving of the protein's affinity for Ca2+ (Konopka-Postupolska et al., 2009). Were AtANN1 able to act as a Ca2+ transporter, this could restrict further Ca2+-dependent recruitment from the cytosol to membranes and act as a “brake” on any Ca2+ transport activity, helping to terminate [Ca2+]cyt elevation. Furthermore, AtANN1 has been identified in a proteomic study of S-nitrosylated proteins (Lindemayr et al., 2005), suggesting that AtANN1 could lie downstream of nitric oxide. The effects of S-nitrosylation of the two Cys residues on AtANN1 in vitro activities have not been determined but could link the ROS/Ca2+ signaling networks with those of nitric oxide.
FUTURE PROSPECTS
The inward rectification observed in this study would restrict annexin-mediated Ca2+ influx at the PM to a narrow voltage range, typical of the “hyperpolarized” state, with little or no influx in the “depolarized” and “K” states (Tyerman et al., 2001). Hence, membrane composition could help determine specific [Ca2+]cyt “signature” signaling responses (McAinsh and Pittman, 2009) mediated by annexins. Stress-induced membrane peroxidation has been linked to changes in PM H+-ATPase activity (Veselov et al., 2002; Cheng et al., 2009), which could affect hyperpolarization and annexin-mediated Ca2+ influx. That plant annexins can be cytosolic, organellar, or extracellular (for review, see Mortimer et al., 2008; Laohavisit et al., 2009) raises the possibility that their movement to the membrane phase to mediate Ca2+ transport has consequence for a range of signaling contexts, including those involving ROS.
Acknowledgments
We thank Henk Miedema and Phillip J. White for advice on bilayers and Paul Hopkinson for help with DSC experiments. We acknowledge colleagues whose work was not cited due to space restrictions.
References
- Andersen OS, Bruno MJ, Sun H, Koeppe RE., II (2007) Single-molecule methods for monitoring changes in bilayer elastic properties. AM Dopico, ed, Methods in Molecular Biology 400: Methods in Membrane Lipids. Humana Press, Totowa, NJ, pp 543–570 [DOI] [PubMed] [Google Scholar]
- Angelova MI, Soleau S, Méléard P, Faucon JF, Bothorel P. (1992) Preparation of giant vesicles by external AC electric fields: kinetics and applications. Prog Colloid Polym Sci 89: 127–131 [Google Scholar]
- Balasubramanian K, Bevers EM, Willems GM, Schroit AJ. (2001) Binding of annexin V to membrane products of lipid peroxidation. Biochemistry 40: 8672–8676 [DOI] [PubMed] [Google Scholar]
- Bianchi MW, Damerval C, Vartanian N. (2002) Identification of proteins regulated by cross-talk between drought and hormone pathways in Arabidopsis wild-type and auxin-insensitive mutants, axr1 and axr2. Funct Plant Biol 29: 55–61 [DOI] [PubMed] [Google Scholar]
- Borochov N, Wachtel EJ, Bach D. (1994) Phase behavior of mixtures of cholesterol and saturated phosphatidylglycerols. Chem Phys Lipids 76: 85–92 [DOI] [PubMed] [Google Scholar]
- Boudsocq M, Laurière C. (2005) Osmotic signaling in plants: multiple pathways mediated by emerging kinase families. Plant Physiol 138: 1185–1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carletti P, Masi A, Spolaore B, De Laureto PP, De Zorzi M, Turetta L, Ferretti M, Nardi S. (2008) Protein expression changes in maize roots in response to humic substances. J Chem Ecol 34: 804–818 [DOI] [PubMed] [Google Scholar]
- Cheng Y, Qi Y, Zhu Q, Wang N, Zhao X, Chen H, Cui X, Xu L, Zhang W. (2009) New changes in the plasma-membrane-associated proteome of rice roots under salt stress. Proteomics 9: 3100–3114 [DOI] [PubMed] [Google Scholar]
- Collin VC, Eymery F, Genty B, Rey P, Havaux M. (2008) Vitamin E is essential for tolerance of Arabidopsis thaliana to metal-induced oxidative stress. Plant Cell Environ 31: 244–257 [DOI] [PubMed] [Google Scholar]
- Demidchik V, Shabala S, Davies JM. (2007) Spatial variation in H2O2 response of Arabidopsis thaliana root epidermal Ca2+ flux and plasma membrane Ca2+ channels. Plant J 49: 377–386 [DOI] [PubMed] [Google Scholar]
- Demidchik V, Shabala SN, Coutts KB, Tester MA, Davies JM. (2003) Free oxygen radicals regulate plasma membrane Ca2+- and K+-permeable channels in plant root cells. J Cell Sci 116: 81–88 [DOI] [PubMed] [Google Scholar]
- Demidchik V, Shang Z, Shin R, Thompson E, Rubio L, Laohavisit A, Mortimer JC, Chivasa S, Slabas AR, Glover BJ, et al. (2009) Plant extracellular ATP signaling by plasma membrane NADPH oxidase and Ca2+ channels. Plant J 58: 903–913 [DOI] [PubMed] [Google Scholar]
- Demiral T, Turkan I. (2005) Comparative lipid peroxidation, antioxidant defense systems and proline content in roots of two rice cultivars differing in salt tolerance. Environ Exp Bot 53: 247–257 [Google Scholar]
- Farmer EE, Davoine C. (2007) Reactive electrophile species. Curr Opin Plant Biol 10: 380–386 [DOI] [PubMed] [Google Scholar]
- Gadjev I, Vanderauwera S, Gechev TS, Laloi C, Minkov IV, Shulaev V, Apel K, Inzé D, Mittler R, van Breusegem F. (2006) Transcriptomic footprints disclose specificity of reactive oxygen species signaling in Arabidopsis. Plant Physiol 141: 436–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerke V, Moss SE. (2002) Annexins: from structure to function. Physiol Rev 82: 331–371 [DOI] [PubMed] [Google Scholar]
- Gorecka K, Thouverey C, Buchet R, Pikula S. (2007) Potential role of annexin AnnAt1 from Arabidopsis thaliana in pH-mediated cellular response to environmental stimuli. Plant Cell Physiol 48: 792–803 [DOI] [PubMed] [Google Scholar]
- Hofmann A, Proust J, Dorowski A, Schantz R, Huber R. (2000) Annexin 24 from Capsicum annuum: x-ray structure and biochemical characterization. J Biol Chem 275: 8072–8082 [DOI] [PubMed] [Google Scholar]
- Jami SK, Clark GB, Turlapati SW, Handley C, Roux SJ, Kirti PB. (2008) Ectopic expression of an annexin from Brassica juncea confers tolerance to abiotic stress treatments in transgenic tobacco. Plant Physiol Biochem 46: 1019–1030 [DOI] [PubMed] [Google Scholar]
- Kaile A. (1997) Cholesterol-modulated ion channel activity in tonoplast vesicles: a planar lipid bilayer study. J Exp Bot 48: 979–983 [Google Scholar]
- Klüsener B, Boheim G, Weiler EW. (1997) Modulation of the ER Ca2+ channel BCC1 from tendrils of Bryonia dioica by divalent cations, protons and H2O2. FEBS Lett 407: 230–234 [DOI] [PubMed] [Google Scholar]
- Konopka-Postupolska D, Clark G, Goch G, Debski J, Floras K, Cantero A, Fijolek B, Roux S, Henning J. (2009) The role of annexin 1 in drought stress in Arabidopsis thaliana. Plant Physiol 150: 1394–1410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourie JI, Wood HB. (2000) Biophysical and molecular properties of annexin-formed channels. Prog Biophys Mol Biol 73: 91–134 [DOI] [PubMed] [Google Scholar]
- Kubista H, Hawkins TE, Moss SE. (1999) Annexin V mediates a peroxide-induced Ca2+-influx in B-cells. Curr Biol 9: 1403–1406 [DOI] [PubMed] [Google Scholar]
- Langen R, Isas JM, Hubbell WL, Haigler HT. (1998) A transmembrane form of annexin XII detected by site-directed spin labelling. Proc Natl Acad Sci USA 95: 14060–14065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laohavisit A, Davies JM. (2009) Multifunctional annexins. Plant Sci 177: 532–539 [Google Scholar]
- Laohavisit A, Mortimer JC, Demidchik V, Coxon KM, Stancombe MA, Macpherson N, Brownlee C, Hofmann A, Webb AAR, Miedema H, et al. (2009) Zea mays annexins modulate cytosolic free Ca2+ and generate a Ca2+-permeable conductance. Plant Cell 21: 479–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Lee EJ, Yang EJ, Lee JE, Park AR, Song WH, Park OK. (2004) Proteomic identification of annexins, calcium-dependent membrane binding proteins that mediate osmotic stress and abscisic acid signal transduction in Arabidopsis. Plant Cell 16: 1378–1391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Lee HJ, Crain RC, Lee A, Korn SJ. (1994) Polyunsaturated fatty acids modulate aperture and two distinct K+ channel currents in guard cells. Cell Signal 6: 181–186 [DOI] [PubMed] [Google Scholar]
- Lewis RNAH, Mannock DA, McElhaney RN. (2007) Differential scanning calorimetry in the study of lipid phase transitions in model and biological membranes. Dopico AM, , Methods in Molecular Biology 400: Methods in Membrane Lipids. Humana Press, Totowa, NJ, pp 171–195 [DOI] [PubMed] [Google Scholar]
- Lindemayr C, Saalbach G, Durner J. (2005) Proteomic identification of S-nitrosylated proteins in Arabidopsis. Plant Physiol 137: 921–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matos AR, Pham-Thi A-T. (2009) Lipid deacylating enzymes in plants: old activities, new genes. Plant Physiol Biochem 47: 491–503 [DOI] [PubMed] [Google Scholar]
- McAinsh MR, Pittman JL. (2009) Shaping the calcium signature. New Phytol 181: 275–294 [DOI] [PubMed] [Google Scholar]
- Mecke KR, Charitat T, Graner F. (2003) Fluctuating lipid bilayer in an arbitrary potential: theory and experimental determination of bending rigidity. Langmuir 19: 2080–2087 [Google Scholar]
- Méléard P, Gerbaud C, Pott T, Fernandes-Puente L, Bivas I, Mitov M, Dufourcq J, Bothorel P. (1997) Bending elasticities of model membranes: influences of temperature and sterol content. Biophys J 72: 2616–2629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori IC, Schroeder JI. (2004) Reactive oxygen species activation of plant Ca2+ channels: a signaling mechanism in polar growth, hormone transduction, stress signaling and hypothetically mechanotransduction. Plant Physiol 135: 702–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortimer JC, Coxon KM, Laohavisit A, Davies JM. (2009) Heme-independent soluble and membrane-associated peroxidase activity of a Zea mays annexin preparation. Plant Signal Behav 4: 428–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortimer JC, Laohavisit A, Macpherson N, Webb A, Brownlee C, Battey NH, Davies JM. (2008) Annexins: multifunctional components of growth and adaptation. J Exp Bot 59: 533–544 [DOI] [PubMed] [Google Scholar]
- Mueller S, Hilbert B, Dueckershoff K, Roitsch T, Krischke M, Mueller MJ, Berger S. (2008) General detoxification and stress responses are mediated by oxidized lipids through TGA transcription factors in Arabidopsis. Plant Cell 20: 768–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng CKY, Carr K, McAinsh MR, Powell B, Hetherington AM. (2001) Drought-induced guard cell signal transduction involves sphingosine-1-phosphate. Nature 410: 596–599 [DOI] [PubMed] [Google Scholar]
- Repetto O, Bestel-Corre G, Dumas-Gaudot E, Berta G, Gianinazzi-Pearson V, Gianinazzi S. (2003) Targeted proteomics to identify cadmium-induced protein modifications in Glomus mosseae-inoculated pea roots. New Phytol 157: 555–567 [DOI] [PubMed] [Google Scholar]
- Rodríguez-Serrano M, Romero-Puertas MC, Pazmiño DM, Testillano PS, Risueño MC, del Rio LA, Sandalio LM. (2009) Cellular response of pea plants to cadmium toxicity: cross talk between reactive oxygen species, nitric oxide, and calcium. Plant Physiol 150: 229–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvius JR. (1982) Thermotropic phase transitions of pure lipids in model membranes and their modifications by membrane proteins. Jost PC, Griffith OH, , Lipid-Protein Interactions. John Wiley & Sons, New York, pp 239–281 [Google Scholar]
- Tyerman SD, Beilby M, Whittington J, Juswono U, Newman I, Shabala S. (2001) Oscillations in proton transport revealed from simultaneous measurements of net current and net proton fluxes from isolated root protoplasts: MIFE meets patch clamp. Aust J Plant Physiol 28: 591–604 [Google Scholar]
- Vandeputte O, Lowe YO, Burssens S, van Raemdonck D, Hutin D, Boniver D, Geelen D, El Jaziri M, Baucher M. (2007) The tobacco Ntann12 gene, encoding an annexin, is induced upon Rhodoccocus fascians infection and during leafy gall development. Mol Plant Pathol 8: 185–194 [DOI] [PubMed] [Google Scholar]
- Veatch SL, Keller SL. (2005) Seeing spots: complex phase behavior in simple membranes. Biochim Biophys Acta 1746: 172–185 [DOI] [PubMed] [Google Scholar]
- Veselov AP, Kurganova LN, Likhacheva AV, Sushkova UA. (2002) Possible regulatory effects of lipid peroxidation on the H+-ATPase activity of the plasmalemma under stress conditions. Russ J Plant Physiol 49: 385–389 [Google Scholar]
- Ward JM, Maser P, Schroeder JI. (2009) Plant ion channels: gene families, physiology and functional genomic analyses. Annu Rev Physiol 71: 59–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber H, Chételat A, Reymond P, Farmer EE. (2004) Selective and powerful stress gene expression in Arabidopsis in response to malondialdehyde. Plant J 37: 877–888 [DOI] [PubMed] [Google Scholar]
- Wymer CI, Bibikova TN, Gilroy S. (1997) Cytoplasmic free calcium distributions during development of root hairs of Arabidopsis thaliana. Plant J 12: 427–439 [DOI] [PubMed] [Google Scholar]
- Yamauchi Y, Furutera A, Seki K, Toyoda Y, Tanaka K, Sugimoto Y. (2008) Malondialdehyde generated from peroxidized linoleic acid causes protein modification in heat-stressed plants. Plant Physiol Biochem 46: 786–796 [DOI] [PubMed] [Google Scholar]