Abstract
Carotenoid turnover was investigated in mature leaves of Arabidopsis (Arabidopsis thaliana) by 14CO2 pulse-chase labeling under control-light (CL; 130 μmol photons m−2 s−1) and high-light (HL; 1,000 μmol photons m−2 s−1) conditions. Following a 30-min 14CO2 administration, photosynthetically fixed 14C was quickly incorporated in β-carotene (β-C) and chlorophyll a (Chl a) in all samples during a chase of up to 10 h. In contrast, 14C was not detected in Chl b and xanthophylls, even when steady-state amounts of the xanthophyll-cycle pigments and lutein increased markedly, presumably by de novo synthesis, in CL-grown plants under HL. Different light conditions during the chase did not affect the 14C fractions incorporated in β-C and Chl a, whereas long-term HL acclimation significantly enhanced 14C labeling of Chl a but not β-C. Consequently, the maximal 14C signal ratio between β-C and Chl a was much lower in HL-grown plants (1:10) than in CL-grown plants (1:4). In lut5 mutants, containing α-carotene (α-C) together with reduced amounts of β-C, remarkably high 14C labeling was found for α-C while the labeling efficiency of Chl a was similar to that of wild-type plants. The maximum 14C ratios between carotenes and Chl a were 1:2 for α-C:Chl a and 1:5 for β-C:Chl a in CL-grown lut5 plants, suggesting high turnover of α-C. The data demonstrate continuous synthesis and degradation of carotenes and Chl a in photosynthesizing leaves and indicate distinct acclimatory responses of their turnover to changing irradiance. In addition, the results are discussed in the context of photosystem II repair cycle and D1 protein turnover.
Carotenoids are classified as accessory pigments in photosynthesis because they augment light harvesting in the blue spectral region by transferring the absorbed light energy to chlorophyll (Chl). However, the universal occurrence of carotenoids in photosynthetic cells, from bacteria to higher plants, indicates their essential roles, rather than mere accessory roles, in photosynthesis. Under excess light, carotenoids provide protection against photooxidative damage by facilitating dissipation of excitation energy from singlet- or triplet-state Chl and scavenging highly reactive singlet oxygen, which is generated through interaction between triplet excited Chl and oxygen (Demmig-Adams, 1990; Müller et al., 2001). These photoprotective functions make carotenoids indispensable for oxygenic photosynthesis, as demonstrated by lethal effects of inhibitors of carotenoid biosynthesis in plants (Bramley, 1993). Regulation of light harvesting and photoprotection by carotenoids requires their close proximity as well as the proper orientation to Chl molecules in pigment-protein complexes of PSI and PSII. Furthermore, a small fraction of non-protein-bound carotenoids serves as antioxidants in the lipid phase of photosynthetic membranes (Havaux and Niyogi, 1999; Havaux et al., 2004) and influences the structure and fluidity of the lipid bilayer (Gruszecki and Strzałka, 2005). Despite these and other lines of defense, the PSII reaction center polypeptide D1, and to a lesser extent also D2, undergo frequent photooxidative damage and repair in the light (Melis, 1999; Baena-González and Aro, 2002). When the repair process cannot keep up with the rate of photodamage, the overall quantum yield of PSII declines.
Carotenoids are derived from isoprenoid precursors in plastids (for reviews on carotenoid biosynthesis in plants, see Lichtenthaler, 1999; Hirschberg, 2001; DellaPenna and Pogson, 2006; Giuliano et al., 2008; Tanaka et al., 2008; Cazzonelli and Pogson, 2010). Following the formation of linear C40 lycopene, the pathway splits into two branches of major cyclic carotenoids: the β,β-branch gives rise to β-carotene (β-C) having two β-rings, whereas the β,ϵ-branch leads to formation of α-carotene (α-C) having one β-ring and one ϵ-ring. Hydroxylation of β-C and α-C produces the xanthophylls zeaxanthin (Z) and lutein (L), respectively. In the β,β-branch, epoxidation of the β-rings of Z results in successive synthesis of antheraxanthin (A) and violaxanthin (V); subsequently, V can be converted to neoxanthin (N), the last carotenoid product of the β,β-branch. Except for some species (García-Plazaola et al., 2007), L does not undergo β-ring epoxidation and the β,ϵ-branch thus stops with L, the most abundant carotenoid in leaves.
Each of these carotenoids occupies specific binding sites in the photosynthetic apparatus to fulfill distinct roles. In both PSI and PSII, carotenes (α-C and β-C) are generally bound in core complexes, which also harbor Chl a molecules, while the majority of xanthophylls (L, Z, A, V, and N) are bound in light-harvesting antenna complexes together with Chl a and Chl b molecules (Bassi et al., 1993; Lee and Thornber, 1995). Accumulation of β-C in core complexes is a common feature of diverse photosynthetic organisms, whereas the occurrence of α-C in addition to β-C is restricted to certain taxa. For higher plants, α-C has been found in leaves of some, but not all, shade-tolerant species (Thayer and Björkman, 1990; Demmig-Adams and Adams, 1992; Demmig-Adams, 1998; Matsubara et al., 2009). Based on this photoacclimatory behavior, it has been proposed that α-C may function as a light-harvesting pigment while β-C may contribute to photoprotection (Krause et al., 2001), presumably by scavenging singlet oxygen and mediating a cyclic electron transfer around PSII (Tracewell et al., 2001; Telfer, 2005).
Pronounced light-dependent changes are also observed for xanthophyll composition in light-harvesting antenna complexes. In a short term (minutes to hours), operation of the xanthophyll cycle, involving Z, A, and V, modulates levels of Z in a light-dependent manner. It is widely accepted that Z is able to enhance the dissipation of excess light energy from singlet excited Chl while V is not (Demmig-Adams, 1990; Müller et al., 2001). Long-term acclimation (days) to strong irradiance typically results in an increased pool size of the xanthophyll-cycle pigments (V + A + Z) and downsizing of PSII antenna, as indicated by a greater Chl a-to-Chl b ratio (Demmig-Adams and Adams, 1992; Demmig-Adams, 1998; Matsubara et al., 2009). Based on the observed changes in steady-state amounts of xanthophylls and carotenes following irradiance shifts, alterations in the balance between biosynthesis and degradation, or turnover, have been implicated as a mechanism for long-term adjustment of carotenoid levels in leaves (Förster et al., 2009). However, just how much biosynthesis and degradation of different carotenoids occurs in photosynthesizing green leaves is an open question to date.
In order to gain insight into carotenoid turnover of mature leaves, we conducted 14CO2 pulse-chase labeling experiments with Arabidopsis (Arabidopsis thaliana) plants. Carotenoid turnover has been studied in algae in the past by applying [14C]bicarbonate (Blass et al., 1959; Grumbach et al., 1978); for example, no more than 1% to 2% of the photosynthetically incorporated 14C was allocated to the lipophilic fraction containing Chl and carotenoid in Chlorella pyrenoidosa after a 2-h pulse application (Grumbach et al., 1978). Even lower labeling efficiency is expected for photosynthetic pigments in nongrowing green leaves, in which pigment turnover takes place almost exclusively as part of the maintenance and acclimation of photosynthetic membranes. To overcome this intrinsic but anticipated difficulty, a 14CO2 application setup was established for efficient and reproducible 14CO2 incorporation in detached leaves of Arabidopsis during a short (30-min) pulse period. Moreover, a method of pigment separation was developed for 14C detection in concentrated leaf pigment extracts using a radio-HPLC system. Because carotenoid composition exhibits marked sun-shade responses in leaves (Demmig-Adams and Adams, 1992; Demmig-Adams, 1998; Matsubara et al., 2009), 14CO2 labeling patterns were studied in three different sets of Arabidopsis plants: (1) plants grown under 130 μmol photons m−2 s−1 (control light [CL]) and exposed to CL during a chase period of up to 10 h (CL plants); (2) plants acclimated to 1,000 μmol photons m−2 s−1 (high light [HL]) for 2 weeks and exposed to HL during the chase period (HL plants); and (3) plants grown under CL but exposed to HL during the chase period (CL→HL plants). These treatments simulated short-term (CL→HL) and long-term (CL or HL) responses to irradiance. Finally, as 14C was found to be rapidly incorporated in β-C and Chl a molecules in leaves of wild-type plants, in which β-C represents the only carotene species, 14C labeling experiments were also conducted with leaves of lut5 mutants containing both α-C and β-C (Fiore et al., 2006; Kim and DellaPenna, 2006) to compare turnover of the two carotenes.
RESULTS
Maximal PSII Efficiency and Carotenoid Composition in Leaves of Wild-Type Plants
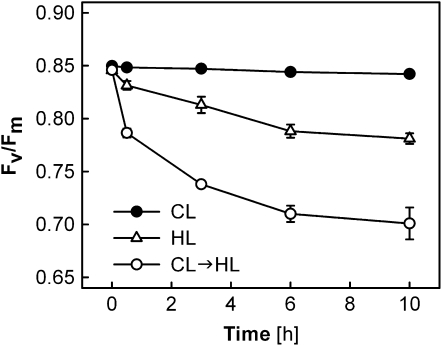
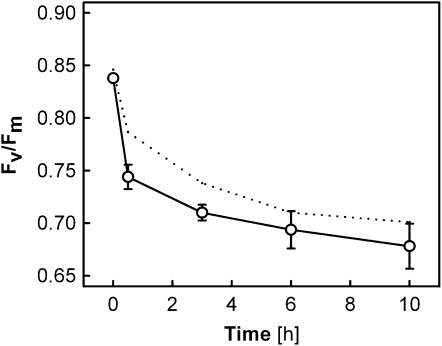
Measurements of Chl a fluorescence were performed in leaves of wild-type Arabidopsis under CL, HL, or CL→HL conditions (Fig. 1). The maximal PSII efficiency (Fv/Fm) remained high in the CL plants when light conditions were not changed, whereas the CL→HL plants showed a significant decrease of Fv/Fm after the transfer to HL at 0 h; the Fv/Fm values of the CL→HL plants decreased rapidly in the first 30 min, then slowly but continuously to values of less than 0.7 after 10 h. The initial rapid decrease accounted for nearly 50% of the PSII impairment measured at 10 h. The HL plants exhibited much less reduction of Fv/Fm during the HL treatment.
Figure 1.
Fv/Fm in leaves of Arabidopsis wild-type plants during different light treatments. Plants acclimated and subjected to 130 μmol photons m−2 s−1 (CL; black circles), plants acclimated and subjected to 1,000 μmol photons m−2 s−1 (HL; white triangles), and plants acclimated to CL and transferred to HL at 0 h (CL→HL; white circles) were used. All data are means ± se (n = 6). Error bars are shown when they are larger than the symbols.
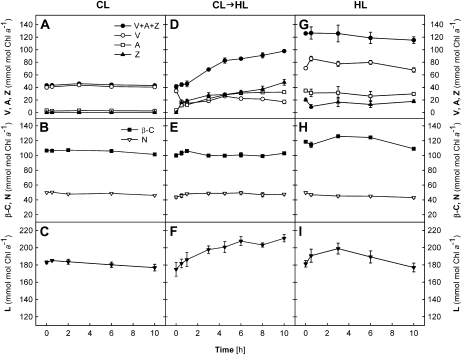
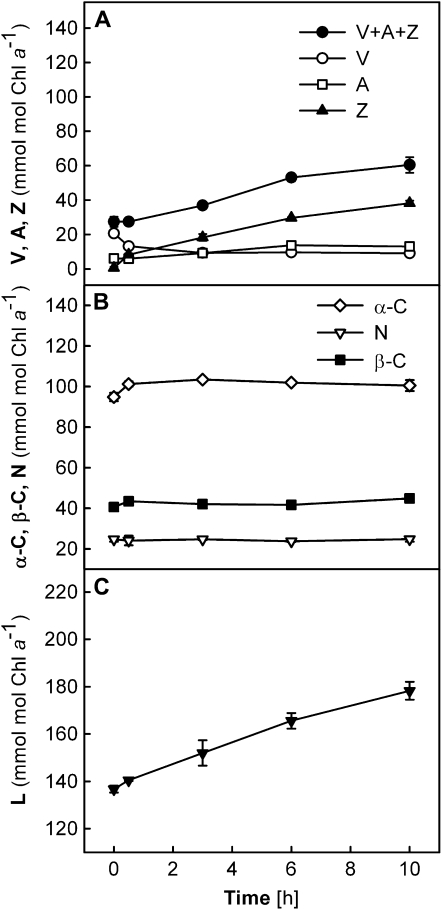
Carotenoid compositions were also analyzed (Fig. 2) during the Fv/Fm measurements (Fig. 1) and are expressed relative to Chl a (mmol mol−1 Chl a). The Chl a content per unit of leaf area did not alter significantly in different treatments throughout the 10-h experiments (data not shown). In the CL plants, V was the major xanthophyll-cycle pigment that was present besides L, N, and β-C; no Z and only a trace of A could be detected (Fig. 2, A–C). The levels of all carotenoid pigments remained unchanged in the CL plants, which also showed no change in Fv/Fm (Fig. 1).
Figure 2.
Changes in carotenoid contents of wild-type leaves during different light treatments. A to C, CL plants. D to F, CL→HL plants. G to I, HL plants. Carotenoid contents are given relative to the Chl a contents (mmol mol−1 Chl a). Chl contents per unit of leaf area did not change during the experiment for both Chl a and Chl b (data not shown), with average ratios of Chl a to Chl b of 3.8 ± 0.03 in the CL and CL→HL plants and 4.77 ± 0.06 in the HL plants. All data are means ± se (n = 3). Error bars are shown when they are larger than the symbols.
In the CL→HL plants, pigment composition changed considerably during the exposure to HL. In parallel to the rapid decrease of Fv/Fm in the first 30 min (Fig. 1), A + Z were quickly formed at the expense of V via operation of the xanthophyll cycle (Fig. 2D). However, about half of the V pool (approximately 20 mmol mol−1 Chl a) remained unconverted to A + Z, presumably representing the V molecules that are not accessible to deepoxidase enzymes (Färber et al., 1997). Notably, the levels of A + Z continued to rise, resulting in an increase of the xanthophyll-cycle compounds V + A + Z by a factor of 2.5 after 10 h (Fig. 2D). Also, L increased in the CL→HL plants by approximately 25% by the end of the experiment (Fig. 2F), whereas the contents of N and β-C stayed constant (Fig. 2E) at the levels of the CL plants (Fig. 2B).
The HL plants had a V + A + Z pool three times greater than that of the CL plants, and no obvious change in V + A + Z was observed during the HL treatment (Fig. 2G). Substantial amounts of A + Z were found initially, but no further deepoxidation occurred during the experiment and V remained the major xanthophyll-cycle pigment in the HL plants. As seen in the CL and CL→HL plants, no change in N or β-C was measured (Fig. 2H). The L content increased slightly in the first few hours but then returned to the initial level in the second half of the treatment (Fig. 2I).
14C-Labeled Pigments in Leaves of Wild-Type Plants
The increase of V + A + Z and L in the CL→HL plants (Fig. 2, D and F), which started after the maximal light-induced deepoxidation in the xanthophyll cycle, suggests enhanced de novo synthesis of these xanthophylls in response to excess light. In order to examine the synthesis and degradation of carotenoid pigments in mature leaves of Arabidopsis, pulse-chase labeling experiments were conducted with 14CO2. After a 30-min pulse of 14CO2 under CL, leaves were exposed to either CL or HL in ambient air for different durations (chase). The incorporation of 14C in photosynthetic pigments was determined by radio-HPLC analysis, and the radioactivity of each pigment was expressed relative to the Chl a content.
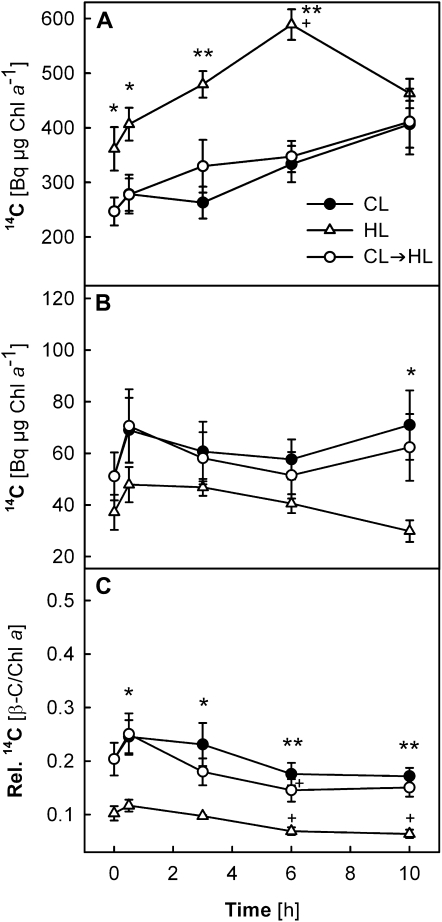
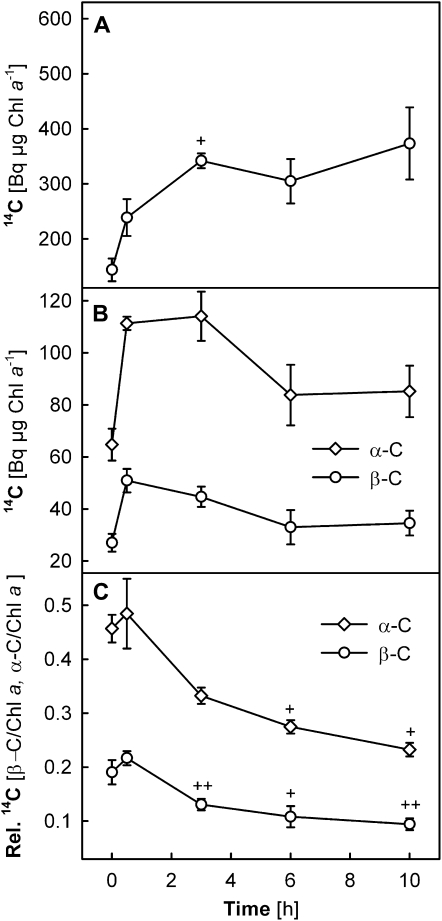
Chl a and β-C showed high radioactivity in all samples (Fig. 3, A and B, respectively), but little label was found in Chl b or the xanthophylls (see Fig. 7 below). Interestingly, the 14C radiolabel of Chl a and β-C was very similar in the CL and CL→HL plants, suggesting that light conditions during the chase period did not affect the 14C levels of these pigments. In the HL plants, significantly higher radioactivity was detected for Chl a (150%–190% of the CL plants after 6 h), while somewhat less 14C was found in β-C (50%–80% of the CL plants). As a result, the 14C ratio β-C/Chl a was much lower in the HL plants (maximum 0.1) than in the CL or CL→HL plants (maximum 0.25; Fig. 3C). The 14C distribution between Chl a and β-C thus seemed to depend on long-term light acclimation states of the leaves.
Figure 3.
A and B, Changes in incorporated 14C radioactivity in Chl a (A) and β-C (B) in leaves of wild-type plants under CL, HL, and CL→HL conditions. C, Ratio between 14C radioactivities of the two pigments (β-C/Chl a). Detached leaves were subjected to 14CO2 administration under CL for 30 min (pulse period) and subsequently exposed to either CL or HL for up to 10 h (chase period, starting at 0 h). The 14C radioactivities of Chl a (A) and β-C (B) were normalized to the Chl a content measured in the same samples (Bq μg−1 Chl a). Asterisks above the symbols indicate significant differences between the CL and HL plants at each time point; no significant difference was found between data of the CL and CL→HL plants. Plus signs above the symbols show significant differences between the time points within each treatment (i.e. after 3-, 6-, or 10-h chase compared with 0.5 h). ** P < 0.01, * and + P < 0.05. All data are means ± se (n = 3–5).
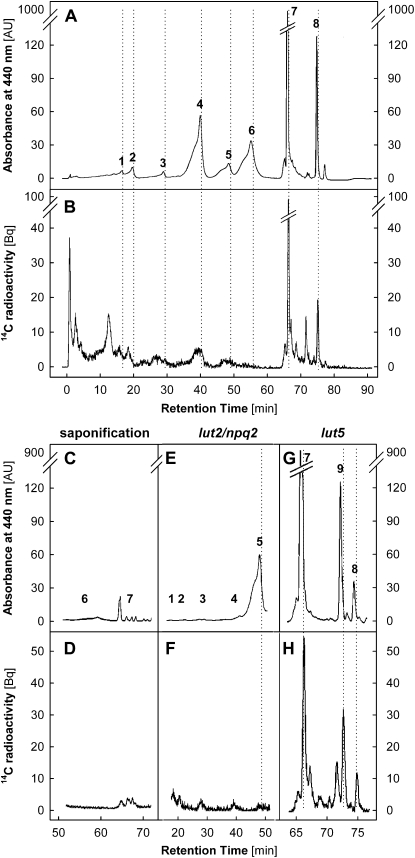
Figure 7.
Radio-HPLC analysis of photosynthetic pigments. A, A chromatogram (detection at 440 nm) of pigments extracted from a leaf of wild-type Arabidopsis grown in CL and exposed to HL for 3 h. Peak 1, V; peak 2, N; peak 3, A; peak 4, L; peak 5, Z; peak 6, Chl b; peak 7, Chl a; peak 8, β-C. AU, Arbitrary units. B, Simultaneous radiogram of 14C-labeled compounds. Dotted lines indicate the expected positions of pigment peaks in the radiogram, with a 20-s offset compared with the corresponding peaks in the chromatogram due to the sequential detection by the UV-visible light and radio detectors. Radioactivity was hardly detectable at peak positions 2, 5, and 6. C and D, Saponification eliminated Chl peaks 6 and 7 in the chromatogram (C) as well as the prominent peak 7 in the corresponding radiogram (D). E and F, Lack of xanthophyll pigments at peaks 1 to 4 concomitant with pronounced accumulation of Z at peak 5 in lut2/npq2 mutants (E) did not eliminate wild-type levels of radiolabeling at less than 40 min (F). G and H, Appearance of peak 9 (α-C) at the expense of peak 8 (β-C) in lut5 mutants (G) was accompanied by the appearance of a new peak in the radiogram at the expected position of α-C together with a smaller peak of β-C (H).
The high 14C radiolabeling of Chl a and β-C just after the pulse suggests a rapid flux of fixed 14C into Chl and carotenoid biosynthesis in mature leaves. The differences in 14C labeling between the HL and CL plants arose mostly during the 30-min pulse. Afterward, the temporal profiles of 14C radioactivity were similar in the CL and HL plants, except for the last time point at 10 h, when radioactivity started to decrease in both Chl a and β-C in the HL plants. In the CL and CL→HL plants, 14C radio signal continued to increase (Chl a) or remained nearly unchanged (β-C; Fig. 3, A and B). The labeling intensity of β-C quickly reached a plateau in all samples already after 0.5 h of chase (Fig. 3B).
Maximal PSII Efficiency and Carotenoid Composition in Leaves of lut5 Mutants
The steady-state level of carotene changed little in leaves in the short term (Fig. 2E), whereas radio-HPLC data revealed the dynamic nature of β-C homeostasis in photosynthesizing leaves (Fig. 3B). This finding prompted us to examine the incorporation of 14C in photosynthetic pigments of the lut5 mutants, which, as a result of a mutation to a heme-containing β-ring hydroxylase gene (Fiore et al., 2006; Kim and DellaPenna, 2006), accumulate a large amount of α-C along with a reduced amount of β-C in leaves. If the roles of the two carotenes in photosynthesis are not identical, as has been proposed by Krause et al. (2001), they may undergo different turnover during illumination. The lut5 mutants were grown under CL and exposed to HL as in the CL→HL experiment of the wild-type plants. Similar to the situation in the wild type, Fv/Fm decreased rapidly in the first 30 min of HL, followed by a slow decrease until 10 h (Fig. 4). The initial reduction of Fv/Fm was faster in the lut5 mutants compared with the wild-type plants (compare with Kim et al., 2009).
Figure 4.
Fv/Fm in leaves of lut5 mutants during HL exposure. Plants grown in CL were transferred to HL at 0 h, as in the CL→HL treatment of the wild-type plants in Figure 1. All data are means ± se (n = 6). For comparison, the data of the wild-type CL→HL plants are also shown (dotted line).
The lut5 mutants are characterized by marked accumulation of α-C concomitant with reduced amounts of all other carotenoid pigments (Fiore et al., 2006; Kim and DellaPenna, 2006). Leaves of the CL-grown lut5 mutants contained approximately 70% V + A + Z, 40% β-C, 50% N, and 80% L with respect to the levels in the CL-grown wild-type plants (compare Figs. 2 and 5). The α-C level found in the lut5 mutants (Fig. 5B) was similar to that of β-C in the wild-type plants (Fig. 2E). The V + A + Z pool of lut5 leaves increased continuously from 0.5 to 10 h under HL (Fig. 5A), as observed in leaves of the CL→HL plants of the wild type, but the increase was less pronounced in the lut5 mutants than in the wild-type plants (by a factor of 2 versus 2.5 in the wild type; Fig. 5A). Concomitantly, a slightly larger increase in L was found in lut5 (+30% versus +25% in the wild type; Fig. 5C) during the 10-h HL treatment. As was the case for β-C in the wild-type plants (Fig. 2E), neither of the two carotenes in lut5 showed a significant change in the steady-state level during the experiment (Fig. 5B).
Figure 5.
Changes in carotenoid contents of lut5 leaves during HL exposure. Plants grown in CL were transferred to HL at 0 h, as in the CL→HL treatment of the wild-type plants in Figure 2, D to F. Carotenoid contents are given relative to the Chl a contents (mmol mol−1 Chl a). Chl contents per unit of leaf area did not change during the experiment for both Chl a and Chl b (data not shown), with an average ratio of Chl a to Chl b of 4.17 ± 0.05. Data are means ± se (n = 3). Error bars are shown when they are larger than the symbols.
14C-Labeled Pigments in Leaves of lut5 Mutants
A 14CO2 labeling experiment was conducted for the lut5 mutants using the same pulse-chase protocol as for the CL→HL plants of the wild type (Fig. 3). As seen in the wild-type plants, rapid and strong incorporation of 14C was found in lut5 for Chl a (Fig. 6A) and carotenes (Fig. 6B), while radioactivity was hardly detectable for Chl b and xanthophylls (data not shown). Although the 14C radioactivity of Chl a just after the 30-min pulse (i.e. at 0 h) was somewhat lower in lut5 than in the wild type, the values reached the wild-type level between 0.5 and 3 h of chase (compare Figs. 3A and 6A).
Figure 6.
A and B, Changes in incorporated 14C radioactivity in Chl a (A) and α-C and β-C (B) in leaves of lut5 mutants under CL→HL conditions. C, Ratios between 14C radioactivity of carotenes and Chl a (β-C/Chl a or α-C/Chl a). The experimental protocol was as described for the CL→HL treatment of wild-type plants (compare with Fig. 3). The 14C radioactivities of Chl a or α-C and β-C were normalized to the Chl a contents measured in the same samples (Bq μg−1 Chl a). Plus signs above the symbols indicate significant differences between the time points within each treatment (i.e. after 3-, 6-, or 10-h chase compared with 0.5 h). ++ P < 0.01, + P < 0.05. All data are means ± se (n = 3).
The 14C incorporation in β-C was approximately 35% lower in the lut5 mutants compared with the wild type (compare Figs. 3B and 6B). Nevertheless, taking into account that the β-C content was reduced by 60% in lut5 (Fig. 5B), relatively more 14C was incorporated in β-C molecules in lut5 than in wild-type leaves. Strikingly high 14C labeling was found for α-C, which is present only in the lut5 mutants (Fig. 6B). The 14C radioactivity was up to 60% higher for α-C in lut5 than for β-C in the wild type (compare Figs. 3B and 6B), in spite of their similar steady-state levels (both approximately 100 mmol mol−1 Chl a). When α-C and β-C were added together, the radioactivity of carotenes in the lut5 mutants (Fig. 6B) was more than twice as high as that of β-C in the wild-type plants (Fig. 3B), even though the total amount of carotenes was increased by no more than 40% in lut5 (Fig. 5B). The 14C distribution between the two carotenes in lut5 (Fig. 6B) was nearly proportional to their relative steady-state amounts (Fig. 5B).
The 14C labeling of α-C and β-C in lut5 leaves (Fig. 6B) exhibited similar time-course patterns as observed for β-C in wild-type leaves (Fig. 3B). Unlike in the wild type, however, the radioactivity of carotenes started to decrease in lut5 with increasing duration of the HL exposure. The wild-type level of 14C incorporation in Chl a (Fig. 6A), together with much greater incorporation in carotenes (Fig. 6B), resulted in remarkably high relative radioactivity of carotenes with respect to that of Chl a in lut5 leaves (Fig. 6C). Notably, the ratio between the 14C radioactivities of α-C and Chl a approached 0.5 after 30 min of chase. On the other hand, the relative radioactivity of β-C to that of Chl a was similar in the lut5 mutants and the wild-type plants (compare Figs. 3C and 6C). Hence, for a given amount of 14C incorporation in Chl a molecules, a greater amount of 14C was incorporated in carotenes in the lut5 mutants compared with the wild-type plants grown under the same CL condition.
DISCUSSION
Continuous Turnover of Chl a and β-C in Photosynthesizing Green Leaves
The 14C pulse-chase labeling experiments revealed the dynamic nature of photosynthetic pigment maintenance in mature leaves of Arabidopsis. After the 30-min pulse in CL, Chl a and β-C showed rapid incorporation of 14C from photosynthetic CO2 fixation (Fig. 3, A and B). Both Chl a and β-C are bound in the core complex of PSII (Bassi et al., 1993; Loll et al., 2005), the reaction center of which is susceptible to photoinactivation. The D1 protein of the PSII reaction center undergoes a continuous repair cycle in all light intensities (Tyystjärvi and Aro, 1996) involving PSII disassembly, D1 degradation, insertion of a newly synthesized D1 into the existing PSII, and PSII reassembly (Baena-González and Aro, 2002). Previous studies have shown that translation elongation of mRNA and membrane insertion of new D1 protein depend on the availability of assembly partners (Zhang et al., 1999) and ligation of Chl a (Kim et al., 1994; He and Vermaas, 1998) and β-C (Trebst and Depka, 1997). The rapid and concomitant 14C labeling of Chl a and β-C found in this study (Fig. 3, A and B) demonstrates a continuous flux of newly fixed carbon into Chl a and β-C in photosynthesizing leaves, which most likely is important for the PSII repair cycle. The 14C labeling of Chl a and β-C, but not Chl b and xanthophylls bound in light-harvesting antenna complexes (Bassi et al., 1993), is in line with the report by Feierabend and Dehne (1996) for green leaves of rye (Secale cereale) seedlings, in which a 4-h incubation with δ-[3H]aminolevulinic acid under illumination resulted in Chl labeling in PSII core complexes but not in light-harvesting antennae and PSI. Chl b is synthesized from Chl a (or chlorophyllide a) by chlorophyllide a oxygenase (Tanaka and Tanaka, 2005). When the availability of Chl b exceeds the amount needed for import and stabilization of light-harvesting antenna proteins, surplus Chl b molecules seem to trigger chlorophyllide a oxygenase protein degradation (Yamasato et al., 2005) and thereby suppress Chl b biosynthesis. The little 14C incorporation in Chl b concomitant with strong Chl a labeling found in our experiments may indicate such suppression of Chl b synthesis in Arabidopsis leaves under CL and HL conditions.
Notably, up to 10 h in HL did not affect the 14C signal of Chl a and β-C in the CL→HL plants (Fig. 3), suggesting that this short-term exposure to HL did not alter the turnover of these pigments. Consistent with this notion, previous studies documented no change in D1 protein turnover in leaves of Brassica napus and Arabidopsis when irradiance was suddenly increased from growth light to higher light intensities (Sundby et al., 1993; Russell et al., 1995). Accumulation of photoinactivated PSII due to enhanced photodamage, without corresponding up-regulation of repair, could explain the impaired Fv/Fm measured in the CL→HL plants in this study (Fig. 1). In fact, the rate coefficient of PSII repair can decrease under excess light (Lee et al., 2001; He and Chow, 2003), presumably because oxidative conditions inhibit the translation of D1 (Nishiyama et al., 2001).
A different picture emerged for the HL plants. The Fv/Fm decrease in the HL plants was much less pronounced than in the CL→HL plants during the same HL treatment (Fig. 1). Strong 14C labeling of Chl a recorded in the HL plants throughout the chase period (Fig. 3A), together with the signal decline at 10 h, suggest higher Chl a turnover in these plants compared with the CL plants. High-light-induced up-regulation of PSII repair, especially at the step of D1 cleavage by FtsH and Deg proteases (Kato and Sakamoto, 2009), represents one of the vital photoacclimatory responses in leaves (Tyystjärvi et al., 1992; Aro et al., 1994). Furthermore, the ratio of Chl a to Chl b (see legend to Fig. 2) indicated downsizing of PSII antennae in the HL plants, another predominant acclimatory response in thylakoid membranes under strong light (Anderson et al., 1988). Smaller PSII antennae, and thus the presence of a relatively greater fraction of Chl a molecules in the core complexes, may partly account for the higher 14C signal of Chl a (normalized to the Chl a content) in the HL plants. Yet, the difference in labeling intensities of Chl a between the CL and HL plants (Fig. 3A) seems too large to be attributed solely to such normalization effects.
The continuous increase of 14C in Chl a up to 6 or 10 h in leaves of the HL or CL plants, respectively (Fig. 3A), points to an ongoing flux of fixed 14C into Chl biosynthesis, or recycling of chlorophyllide and/or phytol moieties from hydrolyzed, 14C-labeled Chl a molecules (Vavilin and Vermaas, 2007). In contrast, the radio signal of β-C reached a peak in all samples after 0.5 h of chase and, what is more, the values were always a little lower in the HL plants than in the CL plants (Fig. 3B). The 14C ratio β-C:Chl a was no more than 1:10 in the HL plants, or less than half the values of the CL and CL→HL plants (Fig. 3C). These results may imply that the HL plants had higher rates of D1 and Chl a turnover, but a lower rate of β-C turnover, than the CL plants. Given the proposed photoprotective functions of β-C in the PSII reaction center (Tracewell et al., 2001; Telfer, 2005), the apparent down-regulation of β-C turnover in the HL plants raises several questions. Does acclimation to strong irradiance lead to less frequent loss of β-C? Do β-C molecules fulfill the same functions in leaves acclimated to contrasting irradiance? The photoacclimatory behavior of β-C turnover in leaves merits further investigation.
The Sources of the Increases in V + A + Z and L in HL
Light-induced deepoxidation in the xanthophyll cycle provides Z molecules for the dissipation of excess light energy (Demmig-Adams, 1990) and protection of membrane lipids against photooxidation (Havaux and Niyogi, 1999; Havaux et al., 2004). A large xanthophyll-cycle pool size, which allows rapid formation of many Z molecules upon light exposure, is a common pigment feature of sunlit leaves (Demmig-Adams and Adams, 1992; Demmig-Adams, 1998; Matsubara et al., 2009). The Arabidopsis plants in this study also exhibited a marked increase of V + A + Z during the 2-week acclimation to the HL condition (Fig. 2, A and G). In the short term, HL exposure induced deepoxidation of V to A and Z in the CL→HL plants in the first 30 min, then an increase in V + A + Z to reach 80% of the HL plant level after 10 h (Fig. 2D). This increase in V + A + Z was accompanied by an increase in L by 30 mmol mol−1 Chl a (Fig. 2F). The involvement of L in energy dissipation has been demonstrated in leaves of higher plant species having the L epoxide cycle (Matsubara et al., 2008) as well as transgenic and mutant plants of Arabidopsis accumulating extra L molecules at the expense of V (Pogson and Rissler, 2000) or in the absence of Z and A (Li et al., 2009). De novo synthesis of both V + A + Z and L was a feature of light acclimation in avocado (Persea americana) leaves, and unlike the xanthophyll cycles, relationships with carotenoid precursors were not stoichiometric (Förster et al., 2009). In our study, the total increase in V + A + Z and L in the CL→HL plants during the 10-h HL treatment was 90 mmol mol−1 Chl a, which suggests the synthesis of these pigments following the maximal deepoxidation in the xanthophyll cycle.
Contrary to our expectation, however, the radio-HPLC analysis did not provide evidence for 14C incorporation in xanthophylls in the CL→HL plants throughout the 10-h chase. Although we cannot rule out the possibility that minor radioactivities of xanthophylls were masked by the interfering compounds in our radio-HPLC analysis (Fig. 7), it is unlikely that such weak 14C incorporation gave rise to the observed increase in V + A + Z and L. Two scenarios could explain the apparent lack of xanthophyll labeling in our experiments. First, HL-induced de novo synthesis of xanthophylls may have started after the 14C-labeled precursor pool had been used up and replaced by nonlabeled precursors formed during the chase period under normal air. The fact that the radioactivity of carotenes reached the maximal intensity 0.5 h after the 14C pulse labeling (Figs. 3B and 6B) suggests that newly fixed carbon is quickly metabolized and enters the carotenoid biosynthetic pathway in chloroplasts of photosynthesizing leaves. An important implication of this scenario is that short-term high-light exposure enhances de novo synthesis of these xanthophylls without affecting β-C turnover (Fig. 3B), which suggests compartmentalization of xanthophyll and carotene biosynthesis. Notably, a recent study in Arabidopsis has shown that an irradiance increase from 150 to 1,000 μmol photons m−2 s−1 rapidly induces (within 10–60 min) transcriptional up-regulation of a gene encoding nonheme diiron β-ring hydroxylase (Cuttriss et al., 2007), which catalyzes the hydroxylation of β-C to Z (Kim et al., 2009). Pulse-chase labeling with 14CO2 not before but during the HL-induced accumulation of V + A + Z and L could test if de novo biosynthesis of these xanthophylls is enhanced after transfer to strong light.
Additionally, one could postulate the existence of xanthophyll precursor pools that are not directly linked with photosynthetic CO2 fixation. A potential candidate for such a pool is carotene molecules in PSII core complexes. It has been proposed for Chlamydomonas reinhardtii that hydroxylation of β-C, which is released from the PSII reaction center during D1 turnover, may contribute to Z formation under strong light, along with deepoxidation of V to Z in the xanthophyll cycle (Depka et al., 1998). Since β-C molecules are bound not only in the reaction center but also in peripheral regions of the core antenna complexes CP47 and CP43 (Loll et al., 2005), it is possible that some of these β-C molecules in the PSII core complexes dissociate from apoproteins during disassembly and become available for hydroxylation. According to this hypothesis, preexisting, nonlabeled carotene molecules in PSII core complexes may have served as a precursor pool for the additional xanthophylls in our experiments. Although possible, this cannot be the only mechanism of the HL-induced xanthophyll accumulation in leaves of Arabidopsis. While the accumulation of L was slightly enhanced in lut5 leaves containing α-C (Fig. 5, B and C), which is the precursor of L, it also happened in the wild-type plants having no α-C (Fig. 2, E and F). If we attribute the HL-induced increase of V + A + Z in the CL→HL plants (approximately 60 mmol mol−1 Chl a by 10 h; Fig. 2D) solely to β-C from the PSII repair cycle, it follows that nearly 60% of the steady-state amount of β-C (Fig. 2E) must have been hydroxylated during the 10-h HL exposure. Assuming a PSII:PSI ratio of 1.5 to 1.7 (Fan et al., 2007) and 11 β-C and 100 Chl a molecules for monomeric PSII (with two trimeric major light-harvesting antenna complexes) and 26 β-C and 160 Chl a molecules for PSI (Bassi et al., 1993; Ballottari et al., 2004; Liu et al., 2004; Loll et al., 2005), the observed V + A + Z increase would correspond to, for instance, one photoinactivation-repair cycle of the entire PSII population and thereby hydroxylation of all the nonlabeled (and nonbleached) β-C from PSII, including nine β-C molecules in CP47 and CP43. If the two β-C molecules in the PSII reaction center are the only substrates for hydroxylase during D1 turnover, five photoinactivation-repair cycles of the entire PSII population would be needed. Although such high rates of D1 turnover may happen in leaves under strong irradiance (Chow et al., 2002), we then would expect to see some radioactivity appearing at Z during the 10-h chase due to hydroxylation of 14C-labeled β-C molecules. This was not the case.
Whatever the mechanism(s) and precursor pool(s) for HL-induced formation of additional xanthophylls, an important question arises as to the fate of carotenes in PSII under nonstressful conditions, as in the CL plants. Note that PSII photoinactivation and D1 turnover (Tyystjärvi and Aro, 1996; Lee et al., 2001) as well as Chl and carotene biosynthesis (Fig. 3) continuously take place in the light, while steady-state levels of these molecules do not change in the short term (Fig. 2, B, E, and H). If carotenes in photoinactivated PSII are neither bleached (Tracewell et al., 2001; Telfer 2005) nor hydroxylated during the repair cycle (Depka et al., 1998) under nonstressful irradiance, they may be degraded by carotenoid cleavage dioxygenase enzymes (CCD; Auldridge et al., 2006b). It has been shown that the expression level of certain CCDs can influence the carotenoid turnover rate in seeds, fruits, flower petals, and roots (Auldridge et al., 2006a, 2006b; Ohmiya et al., 2006; García-Limones et al., 2008). For chloroplasts of green leaves, on the other hand, not much is known about the occurrence and pathway of carotenoid catabolism, with the exception of abscisic acid biosynthesis (Milborrow, 2001). Plants lacking CCDs could offer an interesting system to examine carotenoid degradation in chloroplasts and leaves.
Different Turnover of α-C and β-C
The lack of the lut5 gene product, a heme-containing cytochrome P450 monooxygenase that catalyzes β-ring hydroxylation of α-C, results in the accumulation of α-C in PSII and PSI as well as reduced levels of all other carotenoids in leaves of Arabidopsis (Fiore et al., 2006; Kim and DellaPenna, 2006). Physiologically, leaves of the lut5 mutants have a smaller capacity of light-induced thermal energy dissipation compared with the wild-type plants (Dall'Osto et al., 2007; Kim et al., 2009) and show more rapid decrease of Fv/Fm when exposed to strong light. While these changes do not cause severe photoinhibition and photodamage under moderately high light (750 or 1,000 μmol photons m−2 s−1; Dall'Osto et al., 2007; Kim et al., 2009), leaves of the lut5 mutants bleach after transfer to higher irradiance (1,800 μmol photons m−2 s−1; Kim et al., 2009).
In this study, the Fv/Fm decrease following the transfer from CL to HL (from 130–1,000 μmol photons m−2 s−1) was not so pronounced in lut5 leaves, although it happened faster than in the wild type (Fig. 4). Under this “tolerable” HL condition, the 14CO2 pulse-chase labeling experiment revealed strikingly high 14C incorporation in carotenes of lut5 leaves. Enhanced radioactivity of carotenes (especially α-C) was observed initially when the leaves were still under CL (Fig. 6B), indicating high carotene turnover even under nonstressful environments. Importantly, as the labeling of Chl a was comparable in the two genotypes (compare Figs. 3A and 6A), the lut5 mutation seems to specifically increase carotene turnover, but not Chl a turnover. Judging by the lack of 14C incorporation in L and Z, high carotene turnover in lut5 did not apparently result in enhanced hydroxylation of 14C-labeled α-C and β-C to L and Z, respectively. It can be hypothesized that α-C is more prone to degradation by photooxidation (Tracewell et al., 2001; Telfer, 2005) or enzymatic cleavage (Auldridge et al., 2006) than β-C. High degradation and turnover rates of carotenes, in addition to the smaller capacity of photoprotective light energy dissipation (Dall'Osto et al., 2007; Kim et al., 2009), may contribute to the high-light sensitivity of the lut5 mutants.
Natural accumulation of α-C is found mainly in leaves under shaded or deeply shaded environments (Thayer and Björkman, 1990; Demmig-Adams and Adams, 1992; Demmig-Adams, 1998; Matsubara et al., 2009). A recent large pigment survey of tropical forest species, commonly containing α-C in leaves, indicated a selection pressure that generally suppresses α-C accumulation in sun-exposed conditions (Matsubara et al., 2009). Since the balance between the two carotenes shifts from α-C to β-C with increasing growth irradiance (Thayer and Björkman, 1990; Matsubara et al., 2009), it has been proposed that β-C may contribute to efficient photoprotection whereas α-C may function as light-harvesting pigment (Krause et al., 2001). Our results from the lut5 mutants, which demonstrate different turnover of α-C and β-C during the PSII repair cycle, are consistent with this notion and underscore the interface between PSII photoprotection/photoinactivation and the regulation of carotenoid biosynthesis during photoacclimation.
CONCLUSION
The 14CO2 pulse-chase labeling experiments revealed “hidden” phenotypes of pigment turnover (Chl a, α-C, and β-C) in mature, photosynthesizing leaves of Arabidopsis. Despite their constant steady-state levels, these pigments undergo continuous turnover even under nonstressful light conditions. Turnover rates of Chl a and carotenes seem to be influenced by photoacclimation states of leaves and by the carotene species. On the other hand, de novo synthesis of xanthophylls (V + A + Z and L) could not be demonstrated by the pulse-chase protocol used for 14CO2 labeling, even though steady-state amounts of these pigments substantially increased in response to high-light stress during the chase, which very likely involves xanthophyll biosynthesis. Most importantly, our results emphasize the need to consider carotenoid biosynthesis in the course of photoprotection in relation to pigment dynamics during the D1 repair cycle following PSII photoinactivation. Together, these findings invite further investigation into the regulatory mechanisms of synthesis and degradation of photosynthetic pigments, which are essential for the maintenance and acclimation of photosynthetic membranes in leaves.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Arabidopsis (Arabidopsis thaliana) Columbia-0 wild type and lut2/npq2 and lut5 mutants (mutant seeds kindly provided by Roberto Bassi, University of Verona) were grown in soil (ED 73 Einheitserde; Balster Einheitserdewerk) in a growth cabinet with a 12-h/12-h day/night photoperiod under a constant relative air humidity of 50% and at 22°C/18°C (day/night) air temperature. Photosynthetically active photon flux density of approximately 130 μmol photons m−2 s−1 (CL) was provided by a combination of FLUORA and warm white fluorescent tubes (Osram). At the beginning and at the end of the day period, the light intensity in the growth cabinet was gradually increased or decreased over 1 h to simulate sunrise and sunset. After germination and 3 weeks of cultivation under the above CL condition, some plants were transferred to HL condition (1,000 μmol photons m−2 s−1) provided by Master HPI-T Plus lamps (Philips) without changing the photoperiod and the relative air humidity but at a constant air temperature of 19°C. These plants were acclimated to the HL condition for about 2 weeks before experiments. Mature leaves (up to four leaves per plant) of 5- to 7-week-old plants were used for all experiments.
Light Treatments and Chl a Fluorescence Measurements
A set of plants grown under the CL condition was exposed to HL (CL→HL plants) in the growth chamber, starting after the sunrise period. For comparison, another set of the CL-grown plants were kept under the CL condition (CL plants). All HL-grown plants were subjected to the HL condition (HL plants). After different durations of the CL→HL, CL, or HL treatment, mature leaves were detached from the plants, placed on a moist tissue, and dark adapted for 15 min using leaf clips. Fv/Fm (fluorescence nomenclature according to van Kooten and Snel, 1990) was determined by measuring Chl a fluorescence in the dark-adapted leaves with a Handy PEA (Hansatech).
Analysis of Photosynthetic Pigments
For pigment analysis, leaf discs (1.13 cm2) were taken directly from attached leaves during the light treatments without dark adaptation. The discs were frozen in liquid nitrogen and stored at −20°C for up to 2 weeks until acetone extraction. Pigment extraction and the HPLC analysis were performed according to the method described by Matsubara et al. (2005) using an Allsphere ODS-1 C18 column (5 μm, 250 × 4.6 mm; Alltech) and a corresponding guard column (5 μm, 7.5 × 4.6 mm; Alltech). Pigments were detected by a Waters PAD-996 UV/visible light detector (Waters), and peak areas were integrated at 440 nm with Waters Empower software. Contents of different carotenoids were expressed relative to the Chl a amount (mmol mol−1 Chl a) for each sample.
Isotope Labeling with 14CO2
Labeling with 14CO2 was performed in a gas-circuit system including a valve for opening or closing the system, a reaction vessel, a gas pump (1.3 L min−1 flow rate), a dew-point trap (kept at 5°C–10°C), and a leaf chamber (12 cm3); the total volume of the system was approximately 20 cm3. Aqueous sodium [14C]carbonate solution (7.4 MBq; GE Healthcare) was injected into 20 μL of perchloric acid (70%) and heated to 90°C in the sealed reaction vessel, ensuring fast liberation of 14CO2. After 25 min of application, remaining 14CO2 in the system was absorbed by soda lime granules (Carbosorb; BDH Laboratory Supplies) for 5 min before opening the leaf chamber.
Four detached leaves were placed side by side in the sealed leaf chamber and supplied with water through the petioles from cavities of the chamber bottom. After the 1-h “sunrise” period (see above), 14CO2 administration started under the CL condition at 19°C. Immediately after 14CO2 labeling, the leaves were floated on water in a petri dish with the adaxial side facing ambient air and, during this chase period, were subjected to either the CL or HL in the growth chamber, corresponding to the CL→HL, CL, and HL treatments of the fluorescence experiment described above. Whole lamina of 14CO2-labeled leaves were sampled at different times of the chase and frozen in liquid nitrogen for radio-HPLC analysis.
Radio-HPLC Analysis of 14C-Labeled Pigments
Pigments were extracted from whole lamina first with 1.2 mL of acetone, followed by two-phase extraction (ethyl acetate and water) twice according to the method of Pogson et al. (1996). The extracts were concentrated to a final volume of 500 μL under a nitrogen gas stream and dim laboratory light. The concentrated extracts were either immediately analyzed by radio-HPLC or stored at −20°C for less than 5 h until analysis.
Analytical HPLC was carried out by a PU-1850 HPLC system (Jasco) equipped with an autosampler (Gynkotek) and a UV/visible light detector (Jasco), followed by a radio monitor (radioflow detector LB 509) with a solid yttrium-gadolinium scintillator cell (YG-150-S-4; both from Berthold Technologies). This setup for sequential detection with a UV/visible light detector and a radio detector resulted in a constant offset of approximately 20 s (Fig. 7, A and B).
In order to obtain good signal-to-noise ratios in the radioactivity detection, 100 μL of concentrated leaf extracts was injected into the HPLC system. An HPLC method using a Prontosil reverse-phase C30 column (3 μm, 250 × 4.6 mm; Bischoff) and a corresponding guard column (3 μm, 10 × 4.0 mm; Bischoff) was established for the radio-HPLC analysis; the amounts of pigments per injection were too large for separation with the HPLC method based on the C18 column described above. Elution was carried out at room temperature and a flow rate of 1.2 mL min−1 with methanol:water (87:13 [v/v]; solvent A) and 100% tert-butyl methyl ether (solvent B) according to the following program: isocratic at 85:15 for 19 min, followed by a linear gradient to 80:20 in 1 min, then isocratic at 80:20 for 38 min, a linear gradient to 40:60 in 3 min, isocratic at 40:60 for 17 min, and finally a linear gradient back to 85:15 in 3 min. The column was equilibrated for 20 min before each injection.
Peak integration was performed with RadioStar software (Berthold Technologies) for both UV/visible light chromatograms (440 nm) and radiograms. Peak areas of the radiogram were normalized to the Chl a content given by the corresponding 440-nm chromatogram and expressed as Bq μg−1 Chl a.
Identification of Photosynthetic Pigments in the Radiograms
Chl peaks were confirmed by saponification of acetone extracts (1.2 mL) with 0.5 g of Ambersep 900 OH (Sigma-Aldrich) on ice for 20 min, as described by Larsen and Christensen (2005). Saponification efficiently removed Chl a and Chl b from the extract (Fig. 7C), resulting in disappearance of the Chl a peak in the radiogram (Fig. 7D). No radio signal was detected for Chl b in any samples. The saponification procedure did not affect carotenoid contents (data not shown).
The 14CO2 labeling was also conducted for Arabidopsis carotenoid mutants lut2/npq2 and lut5. In the lut2/npq2 mutants (Havaux et al., 2004), constitutively containing a large amount of Z but lacking all other xanthophylls (V, A, N, and L; Fig. 7E), the radiogram revealed interfering lipophilic compounds in the region where these xanthophylls are expected (Fig. 7F). No radio peak of Z appeared, despite the strong accumulation of this pigment in the lut2/npq2 mutants. In the lut5 mutants, which accumulate α-C at the expense of β-C (Fig. 7G; Fiore et al., 2006, Kim and DellaPenna, 2006), a new peak was observed in the radiogram at the expected position of α-C together with a smaller peak of β-C (Fig. 7H).
Statistical Data Analysis
14C labeling data were statistically tested by t test. For the experiments with the wild-type plants, differences between the CL and HL plants or the CL and CL→HL plants were tested for statistical significance at each sampling time point. Additionally, statistical significance was also checked for time-course variations within each treatment by comparing data after 3, 6, and 10 h of chase with 0.5 h. The values at 0.5 h were chosen as reference since the 14C intensity of carotenes reached the maximal levels at this time point. Time-course changes in the experiment with the lut5 mutants were also statistically tested.
Acknowledgments
We thank Roberto Bassi and Luca Dall'Osto for kindly providing the seeds of lut5 and lut2/npq2 mutants. Valuable suggestions of Barry Osmond during the manuscript preparation are much appreciated. K.G.B. acknowledges the support for her Ph.D. thesis at the Heinrich-Heine-Universität Düsseldorf.
References
- Anderson JM, Chow WS, Goodchild DJ. (1988) Thylakoid membrane organisation in sun/shade acclimation. Aust J Plant Physiol 15: 11–26 [Google Scholar]
- Aro EM, McCaffery S, Anderson JM. (1994) Recovery from photoinhibition in peas (Pisum sativum L.) acclimated to varying growth irradiances: role of D1 protein turnover. Plant Physiol 104: 1033–1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auldridge ME, Block A, Vogel JT, Dabney-Smith C, Mila I, Bouzayen M, Magallanes-Lundback M, DellaPenna D, McCarty DR, Klee HJ. (2006a) Characterization of three members of the Arabidopsis carotenoid cleavage dioxygenase family demonstrates the divergent roles of this multifunctional enzyme family. Plant J 45: 982–993 [DOI] [PubMed] [Google Scholar]
- Auldridge ME, McCarty DR, Klee HJ. (2006b) Plant carotenoid cleavage oxygenases and their apocarotenoid products. Curr Opin Plant Biol 9: 315–321 [DOI] [PubMed] [Google Scholar]
- Baena-González E, Aro EM. (2002) Biogenesis, assembly and turnover of photosystem II units. Philos Trans R Soc Lond B Biol Sci 357: 1451–1459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballottari M, Govoni C, Caffarri S, Morosinotto T. (2004) Stoichiometry of LHCI antenna polypeptides and characterization of gap and linker pigments in higher plants photosystem I. Eur J Biochem 271: 4659–4665 [DOI] [PubMed] [Google Scholar]
- Bassi R, Pineau B, Dainese P, Marquardt J. (1993) Carotenoid-binding proteins of photosystem II. Eur J Biochem 212: 297–303 [DOI] [PubMed] [Google Scholar]
- Blass U, Anderson JM, Calvin M. (1959) Biosynthesis and possible functional relationships among the carotenoids; and between chlorophyll a and chlorophyll b. Plant Physiol 34: 329–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramley PM. (1993) Inhibition of carotenoid biosynthesis. Young AJ, Britton G, , Carotenoids in Photosynthesis. Chapman and Hall, London, pp 127–159 [Google Scholar]
- Cazzonelli CI, Pogson BJ. (2010) Source to sink: regulation of carotenoid biosynthesis in plants. Trends Plant Sci (in press) [DOI] [PubMed] [Google Scholar]
- Chow WS, Lee HY, Park YI, Park YM, Hong YN, Anderson JM. (2002) The role of inactive photosystem-II-mediated quenching in a last-ditch community defence against high light stress in vivo. Philos Trans R Soc Lond B Biol Sci 357: 1441–1449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuttriss AJ, Chubb AC, Alawady A, Grimm B, Pogson BJ. (2007) Regulation of lutein biosynthesis and prolamellar body formation in Arabidopsis. Funct Plant Biol 34: 663–672 [DOI] [PubMed] [Google Scholar]
- Dall'Osto L, Fiore A, Cazzaniga S, Giuliano G, Bassi R. (2007) Different roles of α- and β-branch xanthophylls in photosystem assembly and photoprotection. J Biol Chem 282: 35056–35068 [DOI] [PubMed] [Google Scholar]
- DellaPenna D, Pogson BJ. (2006) Vitamin synthesis in plants: tocopherols and carotenoids. Annu Rev Plant Biol 57: 711–738 [DOI] [PubMed] [Google Scholar]
- Demmig-Adams B. (1990) Carotenoids and photoprotection in plants: a role for the xanthophyll zeaxanthin. Biochim Biophys Acta 1020: 1–24 [Google Scholar]
- Demmig-Adams B. (1998) Survey of thermal energy dissipation and pigment composition in sun and shade leaves. Plant Cell Physiol 39: 474–482 [Google Scholar]
- Demmig-Adams B, Adams WW III. (1992) Carotenoid composition in sun and shade leaves of plants with different life forms. Plant Cell Environ 15: 411–419 [Google Scholar]
- Depka B, Jahns P, Trebst A. (1998) β-Carotene to zeaxanthin conversion in the rapid turnover of the D1 protein of photosystem II. FEBS Lett 424: 267–270 [DOI] [PubMed] [Google Scholar]
- Fan DY, Hope AB, Smith PJ, Jia H, Pace RJ, Anderson JM, Chow WS. (2007) The stoichiometry of the two photosystems in higher plants revisited. Biochim Biophys Acta 1767: 1064–1072 [DOI] [PubMed] [Google Scholar]
- Färber A, Young AJ, Ruban AV, Horton P, Jahns P. (1997) Dynamics of xanthophyll-cycle activity in different antenna subcomplexes in the photosynthetic membranes of higher plants: the relationship between zeaxanthin conversion and nonphotochemical fluorescence quenching. Plant Physiol 115: 1609–1618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feierabend J, Dehne S. (1996) Fate of the porphyrin cofactors during the light-dependent turnover of catalase and of the photosystem II reaction-center protein D1 in mature rye leaves. Planta 198: 413–422 [Google Scholar]
- Fiore A, Dall'Osto L, Fraser PD, Bassi R, Giuliano G. (2006) Elucidation of the β-carotene hydroxylation pathway in Arabidopsis thaliana. FEBS Lett 580: 4718–4722 [DOI] [PubMed] [Google Scholar]
- Förster B, Osmond CB, Pogson BJ. (2009) De novo synthesis and degradation of Lx and V cycle pigments during shade and sun acclimation in avocado leaves. Plant Physiol 149: 1179–1195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Limones C, Schnabele K, Blanco-Portales R, Bellido ML, Caballero JL, Schwab W, Munoz-Blanco J. (2008) Functional characterization of FaCCD1: a carotenoid cleavage dioxygenase from strawberry involved in lutein degradation during fruit ripening. J Agric Food Chem 56: 9277–9285 [DOI] [PubMed] [Google Scholar]
- García-Plazaola JI, Matsubara S, Osmond CB. (2007) The lutein epoxide cycle in higher plants: its relationships to other xanthophyll cycles and possible functions. Funct Plant Biol 34: 759–773 [DOI] [PubMed] [Google Scholar]
- Giuliano G, Tavazza R, Diretto G, Beyer P, Taylor MA. (2008) Metabolic engineering of carotenoid biosynthesis in plants. Trends Biotechnol 26: 139–145 [DOI] [PubMed] [Google Scholar]
- Grumbach KH, Lichtenthaler HK, Erismann KH. (1978) Incorporation of 14CO2 in photosynthetic pigments of Chlorella pyrenoidosa. Planta 140: 37–43 [DOI] [PubMed] [Google Scholar]
- Gruszecki WI, Strzałka K. (2005) Carotenoids as modulators of lipid membrane physical properties. Biochim Biophys Acta 1740: 108–115 [DOI] [PubMed] [Google Scholar]
- Havaux M, Dall'Osto L, Cuine S, Giuliano G, Bassi R. (2004) The effect of zeaxanthin as the only xanthophyll on the structure and function of the photosynthetic apparatus in Arabidopsis thaliana. J Biol Chem 279: 13878–13888 [DOI] [PubMed] [Google Scholar]
- Havaux M, Niyogi KK. (1999) The violaxanthin cycle protects plants from photooxidative damage by more than one mechanism. Proc Natl Acad Sci USA 96: 8762–8767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Chow WS. (2003) The rate coefficient of repair of photosystem II after photoinactivation. Physiol Plant 118: 297–304 [Google Scholar]
- He Q, Vermaas W. (1998) Chlorophyll a availability affects psbA translation and D1 precursor processing in vivo in Synechocystis sp. PCC 6803. Proc Natl Acad Sci USA 95: 5830–5835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschberg J. (2001) Carotenoid biosynthesis in flowering plants. Curr Opin Plant Biol 4: 210–218 [DOI] [PubMed] [Google Scholar]
- Kato Y, Sakamoto W. (2009) Protein quality control in chloroplasts: a current model of D1 protein degradation in the photosystem II repair cycle. J Biochem 146: 463–469 [DOI] [PubMed] [Google Scholar]
- Kim J, DellaPenna D. (2006) Defining the primary route for lutein synthesis in plants: the role of Arabidopsis carotenoid β-ring hydroxylase CYP97A3. Proc Natl Acad Sci USA 103: 3474–3479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Eichacker LA, Rüdiger W, Mullet JE. (1994) Chlorophyll regulates accumulation of the plastid-encoded chlorophyll proteins P700 and D1 by increasing apoprotein stability. Plant Physiol 104: 907–916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Smith JJ, Tian L, DellaPenna D. (2009) The evolution and function of carotenoid hydroxylases in Arabidopsis. Plant Cell Physiol 50: 463–479 [DOI] [PubMed] [Google Scholar]
- Krause GH, Koroleva OY, Dalling JW, Winter K. (2001) Acclimation of tropical tree seedlings to excessive light in simulated tree-fall gaps. Plant Cell Environ 24: 1345–1352 [Google Scholar]
- Larsen E, Christensen LP. (2005) Simple saponification method for the quantitative determination of carotenoids in green vegetables. J Agric Food Chem 53: 6598–6602 [DOI] [PubMed] [Google Scholar]
- Lee AI, Thornber JP. (1995) Analysis of the pigment stoichiometry of pigment-protein complexes from barley (Hordeum vulgare). The xanthophyll cycle intermediates occur mainly in the light-harvesting complexes of photosystem I and photosystem II. Plant Physiol 107: 565–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HY, Hong YN, Chow WS. (2001) Photoinactivation of photosystem II complexes and photoprotection by non-functional neighbours in Capsicum annuum L. leaves. Planta 212: 332–342 [DOI] [PubMed] [Google Scholar]
- Li Z, Ahn TK, Avenson TJ, Ballottari M, Cruz JA, Kramer DM, Bassi R, Fleming GR, Keasling JD, Niyogi KK. (2009) Lutein accumulation in the absence of zeaxanthin restores nonphotochemical quenching in the Arabidopsis thaliana npq1 mutant. Plant Cell 21: 1798–1812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenthaler HK. (1999) The 1-deoxy-d-xylulose-5-phosphate pathway of isoprenoid biosynthesis in plants. Annu Rev Plant Physiol Plant Mol Biol 50: 47–65 [DOI] [PubMed] [Google Scholar]
- Liu ZF, Yan HC, Wang KB, Kuang TY, Zhang JP, Gui LL, An XM, Chang WR. (2004) Crystal structure of spinach major light-harvesting complex at 2.72 Å resolution. Nature 428: 287–292 [DOI] [PubMed] [Google Scholar]
- Loll B, Kern J, Saenger W, Zouni A, Biesiadka J. (2005) Towards complete cofactor arrangement in the 3.0 Å resolution structure of photosystem II. Nature 438: 1040–1044 [DOI] [PubMed] [Google Scholar]
- Matsubara S, Krause GH, Aranda J, Virgo A, Beisel KG, Jahns P, Winter K. (2009) Sun-shade patterns of leaf carotenoid composition in 86 species of neotropical forest plants. Funct Plant Biol 36: 20–36 [DOI] [PubMed] [Google Scholar]
- Matsubara S, Krause GH, Seltmann M, Virgo A, Kursar TA, Jahns P, Winter K. (2008) Lutein epoxide cycle, light harvesting and photoprotection in species of the tropical tree genus Inga. Plant Cell Environ 31: 548–561 [DOI] [PubMed] [Google Scholar]
- Matsubara S, Naumann M, Martin R, Nichol C, Rascher U, Morosinotto T, Bassi R, Osmond B. (2005) Slowly reversible de-epoxidation of lutein-epoxide in deep shade leaves of a tropical tree legume may ‘lock-in’ lutein-based photoprotection during acclimation to strong light. J Exp Bot 56: 461–468 [DOI] [PubMed] [Google Scholar]
- Melis A. (1999) Photosystem-II damage and repair cycle in chloroplasts: what modulates the rate of photodamage in vivo? Trends Plant Sci 4: 130–135 [DOI] [PubMed] [Google Scholar]
- Milborrow BV. (2001) The pathway of biosynthesis of abscisic acid in vascular plants: a review of the present state of knowledge of ABA biosynthesis. J Exp Bot 52: 1145–1164 [PubMed] [Google Scholar]
- Müller P, Li XP, Niyogi KK. (2001) Non-photochemical quenching: a response to excess light energy. Plant Physiol 125: 1558–1566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama Y, Yamamoto H, Allakhverdiev SI, Inaba M, Yokota A, Murata N. (2001) Oxidative stress inhibits the repair of photodamage to the photosynthetic machinery. EMBO J 20: 5587–5594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmiya A, Kishimoto S, Aida R, Yoshioka S, Sumitomo K. (2006) Carotenoid cleavage dioxygenase (CmCCD4a) contributes to white color formation in chrysanthemum petals. Plant Physiol 142: 1193–1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogson B, McDonald KA, Truong M, Britton G, DellaPenna D. (1996) Arabidopsis carotenoid mutants demonstrate that lutein is not essential for photosynthesis in higher plants. Plant Cell 8: 1627–1639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogson JB, Rissler HM. (2000) Genetic manipulation of carotenoid biosynthesis and photoprotection. Philos Trans R Soc Lond B Biol Sci 355: 1395–1403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell AW, Critchley C, Robinson SA, Franklin LA, Seaton G, Chow WS, Anderson JM, Osmond CB. (1995) Photosystem II regulation and dynamics of the chloroplast D1 protein in Arabidopsis leaves during photosynthesis and photoinhibition. Plant Physiol 107: 943–952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundby C, McCaffery S, Anderson JM. (1993) Turnover of the photosystem II D1 protein in higher plants under photoinhibitory and nonphotoinhibitory irradiance. J Biol Chem 268: 25476–25482 [PubMed] [Google Scholar]
- Tanaka R, Tanaka A. (2005) Effects of chlorophyllide a oxygenase overexpression on light acclimation in Arabidopsis thaliana. Photosynth Res 85: 327–340 [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Sasaki N, Ohmiya A. (2008) Biosynthesis of plant pigments: anthocyanins, betalains and carotenoids. Plant J 54: 733–749 [DOI] [PubMed] [Google Scholar]
- Telfer A. (2005) Too much light? How β-carotene protects the photosystem II reaction centre. Photochem Photobiol Sci 4: 950–956 [DOI] [PubMed] [Google Scholar]
- Thayer SS, Björkman O. (1990) Leaf xanthophyll content and composition in sun and shade determined by HPLC. Photosynth Res 23: 331–343 [DOI] [PubMed] [Google Scholar]
- Tracewell CA, Vrettos JS, Bautista JA, Frank HA, Brudvig GW. (2001) Carotenoid photooxidation in photosystem II. Arch Biochem Biophys 385: 61–69 [DOI] [PubMed] [Google Scholar]
- Trebst A, Depka B. (1997) Role of carotene in the rapid turnover and assembly of photosystem II in Chlamydomonas reinhardtii. FEBS Lett 400: 359–362 [DOI] [PubMed] [Google Scholar]
- Tyystjärvi E, Aliyrkko K, Kettunen R, Aro EM. (1992) Slow degradation of the D1 protein is related to the susceptibility of low-light-grown pumpkin plants to photoinhibition. Plant Physiol 100: 1310–1317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyystjärvi E, Aro EM. (1996) The rate constant of photoinhibition, measured in lincomycin-treated leaves, is directly proportional to light intensity. Proc Natl Acad Sci USA 93: 2213–2218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kooten O, Snel JFH. (1990) The use of chlorophyll fluorescence nomenclature in plant stress physiology. Photosynth Res 25: 147–150 [DOI] [PubMed] [Google Scholar]
- Vavilin D, Vermaas W. (2007) Continuous chlorophyll degradation accompanied by chlorophyllide and phytol reutilization for chlorophyll synthesis in Synechocystis sp PCC 6803. Biochim Biophys Acta 1767: 920–929 [DOI] [PubMed] [Google Scholar]
- Yamasato A, Nagata N, Tanaka R, Tanaka A. (2005) The N-terminal domain of chlorophyllide a oxygenase confers protein instability in response to chlorophyll b accumulation in Arabidopsis. Plant Cell 17: 1585–1597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang LX, Paakkarinen V, van Wijk KJ, Aro EM. (1999) Co-translational assembly of the D1 protein into photosystem II. J Biol Chem 274: 16062–16067 [DOI] [PubMed] [Google Scholar]