Abstract
Aminopeptidase M1 (APM1) is essential for embryonic, vegetative, and reproductive development in Arabidopsis (Arabidopsis thaliana). Here, we show that, like mammalian M1 proteases, APM1 appears to have distinct enzymatic and protein-protein interaction domains and functions as a homodimer. Arabidopsis seedlings treated with ezetimibe, an inhibitor of M1 protein-protein interactions, mimicked a subset of apm1 phenotypes distinct from those resulting from treatment with PAQ-22, an inhibitor of M1 catalytic activity, suggesting that the APM1 catalytic and interaction domains can function independently. apm1-1 knockdown mutants transformed with catalytically inactive APM1 did not prevent seedling lethality. However, apm1-2 has a functional enzymatic domain but lacks the carboxyl (C) terminus, and transformation with catalytically inactive APM1 rescued the mutant. Overexpression of human insulin-responsive aminopeptidase/oxytocinase rescued all apm1 phenotypes, suggesting that the catalytic activity was sufficient to compensate for loss of APM1 function, while overexpression of catalytically inactive insulin-responsive aminopeptidase/oxytocinase only rescued apm1-2. Increased catalytic activity alone is not sufficient to compensate for loss of APM1 function, as overexpression of another Arabidopsis M1 family member lacking an extended C terminus did not rescue apm1-1. The protein interactions facilitating enzymatic activity appear to be dependent on the C terminus of APM1, as transformation with an open reading frame containing an internal deletion of a portion of the C terminus or a point mutation in a dileucine motif did not rescue the mutant. These results suggest that both the catalytic and interaction domains are necessary for APM1 function but that APM1 function and dimerization do not require these domains to be present in the same linear molecule.
Eukaryotic M1 peptidases regulate a wide range of physiological response and developmental pathways and play essential roles in embryogenesis, reproduction, cell cycle progression, and cell viability (Constam et al., 1995; Brooks et al., 2003; Sanchez-Moran et al., 2004; Lyczak et al., 2006; Matsui et al., 2006; Walling, 2006; Peer et al., 2009). Thus far, two M1 aminopeptidases in Arabidopsis (Arabidopsis thaliana) have been characterized. Meiotic prophase aminopeptidase 1 (MPA1, M1.05.1, At1g63770) is required for correct meiotic recombination in both male and female gametophytes (Sanchez-Moran et al., 2004), and the membrane-associated, puromycin-sensitive aminopeptidase M1 (APM1, M1.10.1, At4g33090; Murphy and Taiz, 1999a; Murphy et al., 2002) is required for embryonic and seedling development as well as cell cycle progression (Peer et al., 2009). APM1 loss-of-function alleles show pleiotropic phenotypes including embryonic and seedling lethality, dwarfism, loss of apical dominance, reduced seed yield, aberrant planes of cell division, and cell cycle arrest (Peer et al., 2009). These phenotypes suggest functional analogy between APM1 and mammalian puromycin-sensitive aminopeptidase (PSA), which has been shown to function in both cell cycle progression (Constam et al., 1995) and degradation of MHC peptides (Towne et al., 2008). PSA knockout mice have fewer viable embryos, reduced litter size, and are smaller and less fertile or infertile compared with wild-type mice (Osada et al., 2001a, 2001b; Towne et al., 2008). In Caenorhabditis elegans, the APM1/PSA homolog PAM-1 has been shown to be necessary for oocyte-to-embryo transition as well as the establishment of anterior-posterior polarity (Lyczak et al., 2006). The catalytic domain of APM1 also shares sequence similarity with the mammalian insulin-responsive aminopeptidase/oxytocinase (IRAP), which processes signaling peptides like tachykinin and oxytocin (Tsujimoto et al., 1992; Herbst et al., 1997; Albiston et al., 2004), leukotriene A4 hydrolase, which activates the leukotriene A4 receptor at the cell surface (Rudberg et al., 2002), and aminopeptidase N (AP-N/CD13), which functions in cell surface uptake of cholesterol (Kramer et al., 2005). However, there are no reports linking these mammalian proteins to cell cycle progression.
M1 peptidases are defined by two motifs in the catalytic domain: a zinc coordination motif, HEXXH-(X18)-E, and a substrate-binding motif, GXMEN. Mutation of the zinc coordination motif of M1 proteins decreases or abolishes aminopeptidase activity (Medina et al., 1991; Vazeux et al., 1998; Laustsen et al., 2001; Pham et al., 2007). GXMEN is an anionic coordination motif that recognizes the free N terminus of substrates that is located 22 to 32 residues N terminal to the zinc coordination sequence and distinguishes the M1 aminopeptidase family from other gluzincins (Hooper, 1994; Rawlings and Barrett, 1995). Mutation of the GXMEN motif, particularly the Glu residue, also decreases or abolishes aminopeptidase activity (Luciani et al., 1998; Vazeux et al., 1998; Laustsen et al., 2001). Arabidopsis APM1 contains both of these motifs and exhibits enzymatic activity similar to other M1 aminopeptidases (Murphy and Taiz, 1999b; Murphy et al., 2002).
Eukaryotic M1 peptidases are either membrane anchored or peripheral membrane proteins containing a hydrophobic interaction domain (Dyer et al., 1990; Cadel et al., 1997; Keller, 2004; Peer et al., 2009). Subcellular localization of mammalian M1 peptidases such as IRAP and AP-N is important to their function, and perturbation of their subcellular localization either changes or inhibits their function and is an underlying cause of multiple pathologies (Maianu et al., 2001; Alfalah et al., 2006). Sequence homology comparisons, biochemical analyses, and subcellular localization studies indicate that APM1, like IRAP and AP-N, is membrane associated (Murphy and Taiz, 1999a; Murphy et al., 2000, 2002; Peer et al., 2009).
A subgroup of mammalian M1 metallopeptidases including IRAP and AP-N contains dileucine and acidic cluster protein interaction domains that function in trafficking events in the endomembrane system and at the plasma membrane (Rasmussen et al., 2000; Katagiri et al., 2002; Cowburn et al., 2006). Dysfunction of protein-protein interactions mediated by the C terminus of these proteins is implicated in multiple human pathologies including metastasis, tumor cell invasion, and angiogenesis (Mina-Osorio and Ortega, 2005). Independent function of M1 interaction domains is suggested by evidence that a functional catalytic domain is not essential to AP-N mediation of viral particle endocytosis and cholesterol at the cell surface (Kramer et al., 2005). However, independent function cannot be generalized, as IRAP-mediated exocytosis of plasma membrane proteins requires a functional catalytic domain (Albiston et al., 2004). Furthermore, comparisons of APM1 with IRAP must be restricted to the catalytic and C-terminal interaction domains, as IRAP regulation of GLUT4 exocytosis requires a unique 110-amino acid N-terminal extension of the protein (Hou et al., 2006) not found in APM1 or other members of the M1 family.
Dimerization mediated by disulfide linkages and noncovalent interactions is a structural feature of the many M1 metallopeptidases (Hesp and Hooper, 1997; Papadopoulos et al., 2001; Ofner and Hooper, 2002). AP-N was reported to form a noncovalent homodimer (Hussain et al., 1981), and IRAP is thought to form covalently bound homodimers and heterodimers (Itoh et al., 1997; Bernier et al., 1998; Matsumoto et al., 2000; Mustafa et al., 2001). Homodimerization is thought to be essential to catalytic activity, although several studies have suggested that it may also play a role in eukaryotic M1 protein trafficking (Danielsen, 1990, 1994).
In Arabidopsis, three loss-of-function alleles have been described. Heterozygous apm1 mutants are dominant or semidominant, as they exhibit similar phenotypes (including embryonic abortion, postgermination root arrest, and agravitropic root growth) to those observed in homozygotes (Peer et al., 2009). Almost no APM1 transcript is detected in apm1-1, while apm1-2 produces a truncated protein containing the catalytic domain but lacking the putative C-terminal protein interaction domain, and apm1-3 harbors a point mutation in the C-terminal protein interaction domain; all exhibit the same mutant phenotypes (Peer et al., 2009). However, the phenotypes observed are not merely a result of reduced overall hydrolytic capacity, as apm1-2 exhibits the same phenotypes but lacks the C-terminal protein interaction domain. Therefore, APM1 appears to require both catalytic and protein-protein interaction domains for full functionality.
While analyses of apm1 loss-of-function alleles have provided insight into APM1 function, questions regarding the sequence and domain structure of the protein remain outstanding. Here, we analyze which structural and functional elements are required for APM1 dimerization and function. We examine whether the HXLXH and GXMEN motifs contribute equally to APM1 function and whether M1 aminopeptidase function is conserved across plant and animal kingdoms. To resolve these questions, we transformed apm1 mutants with modified forms of the APM1 open reading frame and analyzed the complementation of apm1 embryo-lethal (seed set) and seedling-lethal (postgermination root arrest) phenotypes to dissect the contribution of APM1 subdomains to overall function. We repeated these analyses using selected mammalian and plant M1 constructs to assess the extent of conservation of M1 protein function. The data reported here indicate that (1) APM1 has two functional domains that are required for its activity, (2) APM1 functions as a dimer, and (3) the two domains do not need to be present in the same linear molecule for APM1 functionality.
RESULTS
Sequence Comparisons and Activity Cluster APM1 with PSA/PAM-Like M1 Proteins
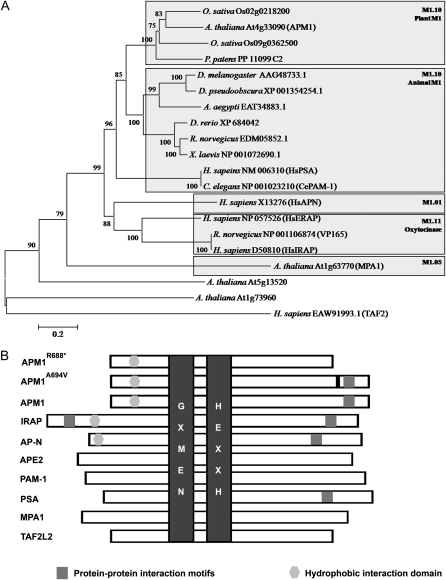
A phylogenetic analysis of M1 amino acid sequences was conducted to place Arabidopsis M1 proteases in an evolutionary context. The M1 family contains members from all kingdoms (except viruses; Fig. 1; Supplemental Fig. S1). In Arabidopsis, there are three canonical M1 aminopeptidases that have the characteristic M1 aminopeptidase motifs (Rasmussen et al., 2000; Murphy et al., 2002; Albiston et al., 2004): APM1, MPA1, and another M1 aminopeptidase encoded at the locus At5g13520 (Supplemental Fig. S1).
Figure 1.
Phylogenetic analysis of M1 peptidases. A, Unrooted dendrogram of selected M1 peptidases from animals, archaea, and plants. Peptidases are grouped according to sequence similarity to a representative of a given group. Protein sequences were aligned with MEGA 4.0 using an opening gap penalty of 10 and a gap extension penalty of 0.2. The neighbor-joining method of MEGA was used to construct the dendrogram with 1,000 bootstrap replicates (indicated on branches). For more information, see “Materials and Methods.” B, Schematic representation of selected M1 peptidases. The characteristic M1 domains are highlighted: substrate recognition (GXMEN) and metal ion coordination (HEXXH) domains (dark gray), protein-protein interaction domain (medium gray), and hydrophobic interaction domain that facilitates association to the membrane (light gray). The black bar represents a point mutation.
MPA1 (M1.05.1, At1g63770) is most similar to the prokaryotic M1 subfamily (M1.05; Fig. 1A; Supplemental Fig. S1A). MPA1 is expressed in meiocytes, associates with meiotic chromatin, and functions in both male and female meiosis in Arabidopsis (Sanchez-Moran et al., 2004; Pradillo et al., 2007). Shorter and longer isoforms of the protein (M1.05.1.1 and M1.05.1.2) have been identified based on cDNA transcripts. Little is known about the activities of the other canonical M1 aminopeptidase encoded by At5g13520, referred to here as TAF2-LIKE2 (TAF2L2). TAF2L2 contains putative PDZ and nuclear localization motifs and groups most closely to human M1 TAF2 (M1.972), a protein of unknown function named for its partial homology to a TATA-binding protein-associated factor (TAF), which functions in the modification of transcription factors to facilitate complex assembly and transcription initiation. A noncanonical M1-like protein encoded at the locus At5g52910 also exhibits homology to the human TAF2 protein but has HEXXH-(X25)-E instead of the canonical HEXXH-(X18)-E in its apparent catalytic domain.
Phylogenetic and biochemical analyses (Murphy and Taiz 1999a; Murphy et al., 2002; Smith et al., 2003; http://merops.sanger.ac.uk/) classify APM1 as the single Arabidopsis member of the M1.10 plant-animal (PSA/APM/PAM-1) subfamily of M1 metallopeptidases in Arabidopsis (Fig. 1A; Supplemental Fig. S1A). The M1.10 subfamily is closely related to the mammalian cystinyl-AP M1.11 subfamily characterized by IRAP, which mediates interactions with AS160 in GLUT4 trafficking, and ERAP, which functions in N-terminal peptide processing within the endoplasmic reticulum. APM1 exhibits only 24% identity with MPA1 isoforms in the conserved catalytic domain and even less similarity outside of this region. APM1 also contains a PSA/PAM-like C-terminal domain containing putative protein-protein interaction motifs not found in MPA1 and TAF2L2 (Fig. 1B). Although there are no members of the AP-N (M1.01) and IRAP/ERAP (M1.11) subfamilies in Arabidopsis (Fig. 1A; Supplemental Fig. S1), APM1 does share a number of sequence identities with AP-N and IRAP not seen in other members of the M1.10 subfamily (Murphy et al., 2002; Fig. 1B).
Dimerization of APM1
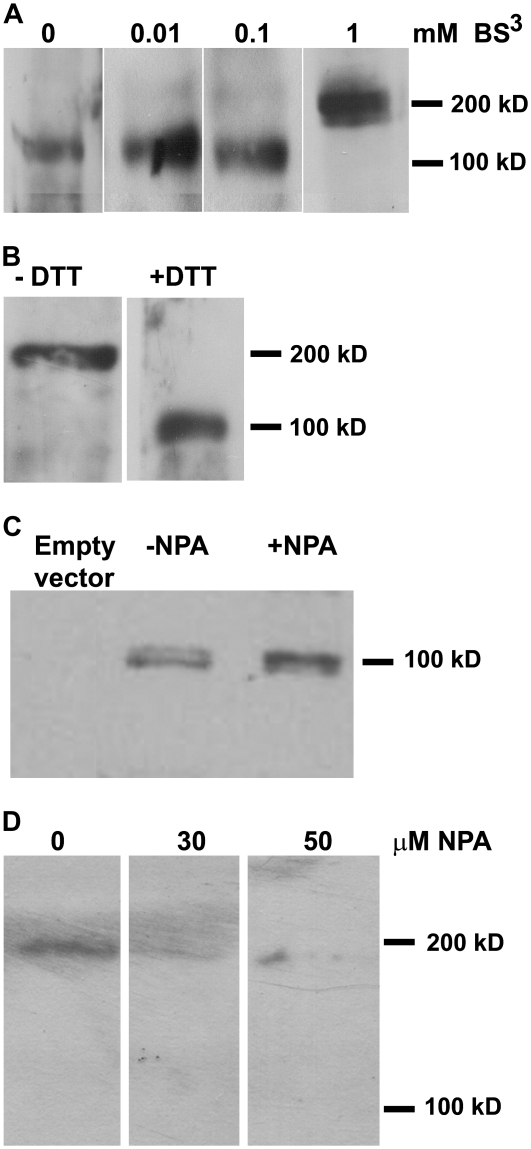
Some other plant aminopeptidases have been shown to require oligomerization for activity (Matsui et al., 2006). We used reducing and cross-linking reagents to examine potential APM1 oligomerization. Microsomal membranes isolated from 5-d-old Arabidopsis were cross-linked using bis-(bulfosuccinimidyl) suberate (BS3). These samples were subjected to SDS-PAGE and western-blot analyses, which revealed an approximately 103-kD band in low concentrations or absence of BS3 and an approximately 206-kD band in the presence of 1 mm BS3. Mass spectrometric sequence analysis of the approximately 206-kD band identified only APM1 sequences in tryptic fragments, indicating that a homodimer was formed (Fig. 2A). To confirm that APM1 forms a dimer, whole microsomal membranes prepared with and without the thiol-reducing agent dithiothreitol (DTT) were analyzed via Blue Native PAGE followed by western blotting. This analysis confirmed that APM1 forms a dimer (approximately 206-kD band) in planta and that disruption of thiol linkages reduces the protein to monomeric form (approximately 103-kD band; Fig. 2B). This suggests that APM1 dimerization or dimer stability involves disulfide bonds.
Figure 2.
APM1 can form a dimer. A, Western blot of 10 μg of whole microsomal membranes from 5-d-old wild-type seedlings. Microsomal membranes were cross-linked with three different concentrations of BS3, run on 8% SDS-PAGE, and incubated with anti-APM1. B, Western blot of 10 μg of whole microsomal membranes prepared with and without a reducing agent (DTT), run on Blue Native PAGE, and incubated with anti-APM1. C, Western blot of 10 μg of in vitro translation of recombinant APM1 in rabbit recticulocytes, with and without NPA, analyzed via SDS-PAGE, and incubated with anti-APM1. D, Western blot of 15 μg of whole microsomal membranes from 5-d-old wild-type seedlings treated with 0, 30, or 50 μm NPA, analyzed via Blue Native PAGE, and incubated with anti-APM1.
However, fractions solubilized from Arabidopsis microsomal membranes containing purified APM1 and aminopeptidase P1 (M24, At4g36760) were previously shown to exhibit increased Tyr aminopeptidase activity when treated with the thiol-reducing agent DTT (Murphy et al., 2002). Examination of recombinant APM1 in vitro translation products synthesized as described previously (Murphy et al., 2002) showed that the aminopeptidase activity against peptide substrates or aminoacyl-fluoromethylcoumarin conjugates was not altered by increasing concentrations of DTT (P > 0.05, Table I). This evidence suggests that APM1 monomers may retain hydrolase activity.
Table I. Activity of the recombinant APM1 in vitro translation product.
Baseline is the activity of the in vitro translation of the empty vector control. AFC, 7-Amino-4-trifluoromethyl-coumarin.
| Peptide Substrate | AFC, Ala, or Tyr Released |
||
| 0 mm DTT | 1 mm DTT | 10 mm DTT | |
| nmol mg−1 min−1 | |||
| AlaGlyGly | 36 ± 3.3 | 34 ± 5.7 | 31 ± 8.3 |
| TyrGlyGly | 40 ± 2.9 | 33 ± 6.4 | 32 ± 9.5 |
| Ala-AFC | 39 ± 7.2 | 38 ± 9.4 | 36 ± 11.3 |
| Tyr-AFC | 67 ± 5.1 | 70 ± 11.6 | 63 ± 14.8 |
APM1 was originally identified via its affinity for the auxin transport inhibitor 1-naphthylphthalamic acid (NPA) and its ability to slowly hydrolyze this compound (Murphy and Taiz, 1999a, 1999b; Murphy et al., 2002). NPA is structurally similar to phthalamide inhibitors of M1 enzymatic activity in mammals and plants (Komoda et al., 2001; Murphy et al., 2002; Kakuta et al., 2003), is able to inhibit APM1 aminopeptidase activity when used at high concentrations, and exhibits increasing inhibition of APM1 activity over time (Murphy and Taiz, 1999a; Murphy et al., 2002). Both northern-blot analysis (Murphy et al., 2002) and quantitative real-time PCR analysis (Table II) showed that APM1 expression increased significantly after treatment with NPA (P < 0.001). SDS-PAGE and western-blot analysis of recombinant APM1 produced by short-term in vitro translation (Murphy et al., 2002) in the presence of NPA indicated that NPA does not directly inhibit APM1 protein synthesis (Fig. 2C). However, ProAPM1:YFP-APM1 signals in Arabidopsis roots rapidly disappear after treatment with NPA at concentrations much lower than those required to inhibit APM1 enzymatic activity (Peer et al., 2009), and APM1 signals (monomer or dimer) could not be observed in Blue Native PAGE/western-blot analyses of microsomal membranes isolated from 5-d-old Arabidopsis seedlings treated with NPA for 1 h (Fig. 2D). These results suggest that NPA affects APM1 protein stability in addition to enzymatic activity.
Table II. Quantitative real-time PCR analysis of APM1 expression in 5-d-old seedlings following NPA treatment.
Data are means and sd of five biological replicates.
| Parameter | NPA |
|||
| 0 μm | 5 μm | 30 μm | 50 μm | |
| Relative abundance | 8.5 ± 0.17 | 8.6 ± 0.28 | 9.7 ± 0.42a | 11.7 ± 0.85a |
P < 0.05, ANOVA followed by Dunnet's posthoc analysis.
Putative Protein-Protein Interaction Domains
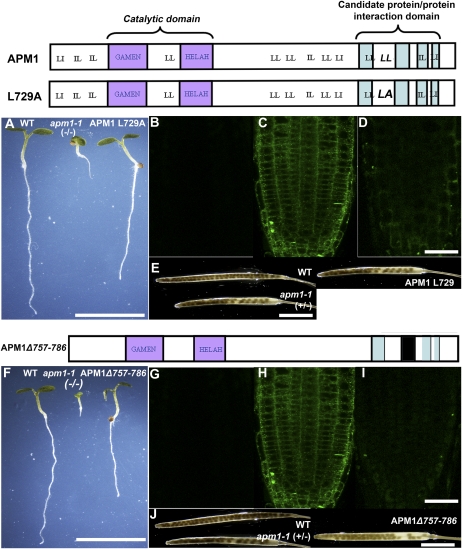
Critical regions in the C terminus that are required for APM1 function were determined by mutational analysis of amino acids and domains. To confirm that the C-terminal region is required for APM1 function, a truncated form of APM1, similar to that of apm1-2, was generated (APM1Δ720–880) and transformed into apm1-1 (+/−), and the transformants were genotyped and analyzed. Transformation with this construct failed to complement postgermination root arrest, lack of apical dominance, and embryonic defects in homozygotes and produced a dominant negative phenotype in heterozygotes, in which the root growth arrest phenotype was not rescued (Supplemental Figs. S2 and S3). This supports the hypothesis that amino acid residues in this region of the C terminus are required for APM1 function throughout development.
Dileucine motifs have been shown to play a role in IRAP protein trafficking through protein-protein interactions (Hou et al., 2006; Watson et al., 2008). Dileucine and similar motifs in APM1 were identified and mutated to Ala to determine whether these motifs mediated APM1 protein-protein interactions. These constructs were under the control of a dexamethasone (Dex)-inducible promoter; effects of Dex dosage on the apm1-1 arrested root growth, and embryonic abortion phenotypes in lines with these constructs are shown in Supplemental Figures S4 to S6. Of the 13 motifs identified, only one pair (LL728,729), when mutated, was unable to complement homozygous apm1-1 root growth arrest (dead at 5 d after germination) or embryonic abortion phenotypes (significantly fewer seeds than in the wild type; P < 0.05; Fig. 3, A and E; Supplemental Fig. S5). The dosage effects of APM1L729A on the apm1-1 arrested root growth phenotype indicate that the construct was expressed (Supplemental Fig. S4). The remaining 12 mutated dileucine motifs were able to complement the apm1-1 mutant and therefore apparently are not critical elements for APM1 function (Supplemental Figs. S5 and S6). YFP-APM1L729A transformant roots showed faint membrane and cytosolic yellow fluorescent protein (YFP) signals compared with controls (Fig. 3, B–D). However, western-blot analysis of YFP-APM1L729A microsomal membranes did not show a signal indicating the presence of the protein (Supplemental Fig. S7A), suggesting that the protein may be degraded due to a decrease in protein stability. These results suggest that this dileucine pair is necessary for APM1 function or stability and that LL728,729 may also function in conjunction with another protein-protein interaction domain within APM1.
Figure 3.
Alterations of the protein-protein interaction domain reduce phenotypic complementation. The YFP-APM1L729A mutation is indicated by LA in the top cartoon, and the YFP-APM1Δ757–786 deletion is indicated by a black box in the bottom cartoon. A, Five-day-old wild-type (WT), apm1-1, and YFP-APM1L729A seedlings germinated and grown on 10 μm Dex. B to D, Confocal laser scanning microscopy analysis of YFP-APM1L729A. B, Autofluorescence control. C, Pro35S:YFP-APM1. D, YFP-APM1L729A. E, Seed set of YFP-APM1L729A plants induced with 10 μm Dex. F, Five-day-old wild-type, apm1-1, and YFP-APM1Δ757–786 seedlings germinated and grown on 10 μm Dex. G to I, Confocal laser scanning microscopy analysis of YFP-APM1Δ757–786. G, Autofluorescence. H, Pro35S:YFP-APM1. I, YFP-APM1Δ757–786. J, Seed set of YFP-APM1Δ757–786 plants induced with 10 μm Dex. Bars = 5 cm (A and F), 5 cm (B–D, E, and J), and 50 μm (G–I). [See online article for color version of this figure.]
Through bioinformatic analyses, we identified four candidate protein-protein interaction domains (see “Materials and Methods”). Only one of the internal deletion constructs, APM1Δ757–786, in the region most similar to the protein-protein interaction GLUT4/AS160 trafficking domain in the C terminus of IRAP was unable to complement homozygous apm1-1 root growth arrest (dead at 5 d after germination) or embryonic abortion (significantly fewer seeds than in the wild type; P < 0.05) phenotypes (Fig. 3, F and J; Supplemental Fig. S5), whereas the other deletion constructs rescued the mutant (Supplemental Figs. S5 and S6). These constructs were under the control of a Dex-inducible promoter, and Dex dosage effects on the apm1-1 phenotypes are shown in Supplemental Figure S4. Expression analysis revealed that the YFP-APM1Δ757–786 construct was expressed (Supplemental Fig. S7C). Faint membrane and cytosolic YFP signals were observed in YFP-APM1Δ757–786 transformant roots compared with control roots (Fig. 3, G–I), and western-blot analysis of YFP-APM1Δ757–786 microsomal membranes did not show a signal (Supplemental Fig. S7B). These results suggest that, like APM1L729A, this mutation results in decreased protein stability. Together, these results suggest that these regions of APM1 are involved in protein stability and/or function, which may include protein-protein interactions and/or trafficking of APM1 to the correct location for it to function.
The APM1 Catalytic Domain Is Required for Development
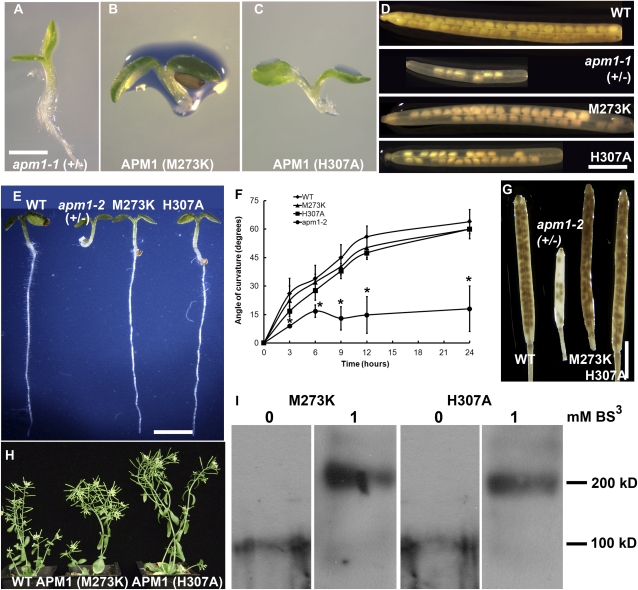
To investigate the roles of the two APM1 catalytic domains for APM1 function, point mutations in the substrate-binding GAMEN and zinc-binding HELAH motifs were generated by site-directed mutagenesis. apm1-1 (+/−) seedlings were transformed with ProAPM1:YFP-APM1M273K or ProAPM1:YFP-APM1H307A, and the resulting transformants were genotyped and analyzed. Both point mutation constructs failed to complement the root growth arrest and embryonic abortion phenotypes in homozygotes (Fig. 4, A–D). This suggests that the HELAH and GAMEN motifs are both required for APM1 function. However, it appears that the defects in embryo development and subsequent seed set are partially compensated for by the expression of APM1 with an altered GAMEN motif (Fig. 4D; Supplemental Fig. S4), suggesting that either substrate specificity or catalytic activity is less important for the progression of embryogenesis. Both point mutation transformants exhibited upturned leaf margins in apm1-1, although this phenotype was not observed in apm1-2 (+/−) transformed with these constructs.
Figure 4.
Catalytically inactive APM1 rescues apm1-2 but not apm1-1. A to D, Complementation analyses of apm1-1 with mutated catalytic residues. Five-day-old seedlings. A, apm1-1 (+/−). B, apm1-1 (+/−) transformed with ProAPM1:APM1M273K (M273K). C, apm1-1 (+/−) transformed with ProAPM1:APM1H307A (H307A). D, Seed set of wild-type (WT) and mutant plants. E to L, Complementation analyses of apm1-2 (−/−) with mutated catalytic residues. E, Five-day-old seedlings. F, Gravitropic bending in apm1-2 (−/−) transformed with ProAPM1:APM1M273K (M273K) or ProAPM1:APM1H307A (H307A). G, Seed set. H, Four-week-old plants. I, Western-blot analyses of 10 μg of whole microsomal membranes prepared from apm1-2 5-d-old seedlings expressing either ProAPM1:APM1M273K or ProAPM1:APM1H307A cross-linked with 1 mm BS3, run on 8% SDS-PAGE, and incubated with anti-GFP. In the absence of BS3, only monomers were observed (approximately 105 kD). Bars = 1 mm (A–C), 15 mm (D), 1 mm (E and J), 1.5 cm (F–I), and 3 cm (K). [See online article for color version of this figure.]
In apm1-2, a truncated protein (APM1R688*) is generated that includes the catalytic domain but lacks the protein-protein interaction domain. When apm1-2 (+/−) was transformed with ProAPM1:YFP-APM1M273K or ProAPM1:YFP-APM1H307A, both constructs were able to complement the arrested root growth, loss of apical dominance, and embryonic abortion and gravitropic response phenotypes of the homozygous mutants (Fig. 4, E–H). BS3 cross-linking showed that both of the catalytically inactive APM1 proteins formed homodimers (approximately 206 kD) as well as heterodimers with APM1R688* (approximately 237 kD; Fig. 4I). Mass spectrometric analysis of BS3-treated microsomal membranes confirmed that APM1 heterodimers were formed with the mutated APM1 sequences.
M1 Catalytic Activity Alone Is Not Sufficient to Rescue apm1
The TAF2L2 M1 aminopeptidase possesses the two signature conserved catalytic motifs but lacks a homologous C-terminal protein-protein interaction domain present in APM1. TAF2L2 has aminopeptidase activity (L. Walling, unpublished data, referenced in Walling, 2006), although its function in planta is currently unknown. To determine if additional M1 aminopeptidase activity alone is sufficient to rescue any of the apm1 phenotypes, apm1-1 (+/−), apm1-2 (+/−), and apm1-3 (−/−) were transformed with full-length TAF2L2 cDNA fused to GFP under the control of a 35S promoter (Pro35S:GFP-TAF2L2). Overexpression of TAF2L2 did not rescue apm1-1 or apm1-2. However, the resulting apm1-3 (APM1A694V) Pro35S:GFP-TAF2L2 transformants exhibited wild-type root length and gravitropic response (P > 0.05; Supplemental Fig. S8, A and G), although the loss of apical dominance and embryonic abortion phenotypes were not rescued (P < 0.05; Supplemental Figs. S4 and S8, E and F). No change in leaf morphology was observed during vegetative growth (Supplemental Fig. S8, B–D). Western-blot analysis of BS3 cross-linked microsomal membranes showed that GFP-TAF2L2 was present as a monomer (approximately 80 kD), a homodimer (approximately 160 kD), and a heterodimer with APM1 (approximately 180 kD; Supplemental Fig. S8H).
By homology, TAF2L2 also belongs to the TATA-binding protein-associated factor family. GFP-TAF2L2 subcellular localization showed GFP signals in the cytosol and nucleus, and nuclear localization was confirmed by colocalization with 4′,6-diamino-phenylindole (DAPI; Supplemental Fig. S8I). However, unequivocal endosomal or plasma membrane localization was not observed. Although TAF2L2 was able to form a heterodimer with APM1A694V and rescue the root growth arrest phenotype, the catalytic activity of TAF2L2 alone is not sufficient to compensate for the lack of APM1 function.
Catalytic and Protein-Protein Interaction Activities Are Required for APM1 Function
Selective M1 protease inhibitors were used to biochemically separate the catalytic and protein interaction activities of APM1. We could thereby assess which apm1 seedling phenotypes might be attributed to a lack of APM1 hydrolytic activity and which might be more associated with altered C-terminal protein-protein interactions or subcellular trafficking of APM1. Ezetimibe, a mammalian intestinal cholesterol uptake inhibitor, binds the C terminus of AP-N without inhibiting hydrolytic activity (Kramer et al., 2005). Treatment of wild-type seedlings with ezetimibe phenocopied the root and cotyledon defects seen in apm1-1 (+/−) and apm1-2 (+/−) (Supplemental Fig. S9, A–C). ProAPM1:YFP-APM1 subcellular localization in Arabidopsis root tips appeared to be unaltered after ezetimibe treatment compared with untreated controls (Supplemental Fig. S9, H and I).
PAQ-22 is an enzymatic inhibitor that specifically inhibits the hydrolytic activity of M1 aminopeptidases and has been shown to inhibit APM1 enzymatic activity (Komoda et al., 2001; Murphy et al., 2002; Kakuta et al., 2003). Wild-type seedlings treated with PAQ-22 also exhibited truncated roots as well as the enlarged hypocotyl cells observed in apm1 mutants (Supplemental Fig. S9, D–G; Peer et al., 2009). However, ProAPM1:YFP-APM1 did not appear to be mislocalized in Arabidopsis root tips after PAQ-22 treatment (Supplemental Fig. S9, H and J). These results suggest that both the protein-protein interaction and catalytic domains are essential for APM1 function, but inhibiting either activity alone did not alter localization.
Conservation of M1 Function between the Plant and Animal Kingdoms
Human IRAP is one of the best characterized members of the M1 metallopeptidase family, and apart from a unique 110-amino acid N-terminal extension, it exhibits sequence and enzymatic similarities to APM1. An IRAP cDNA under the control of a 35S promoter was used to transform apm1-1 (+/−) and rescued all of the apm1-1 (−/−) phenotypes (arrested root growth, gravitropic response, loss of apical dominance, and defects in embryo development; Fig. 5, A–C; Supplemental Figs. S4 and S10, A–E). This suggests that there is conservation of M1 function between the plant and animal kingdoms. To determine if expression of a catalytically inactive IRAP would rescue any of the apm1 phenotypes, apm1-1 (+/−) was transformed with a mutated, catalytically inactive form of an IRAP cDNA (IRAPm; Ye et al., 2007) under the control of a 35S promoter. This transformation did not rescue the arrested root phenotype, loss of apical dominance, or embryonic abortion phenotypes of apm1-1 homozygotes and heterozygotes (Fig. 5, A–C), as was the case with transformants created with catalytically inactive APM1 (Fig. 4, A–D; Supplemental Fig. S3).
Figure 5.
IRAP can rescue apm1. A to C, Phenotypic analyses of apm1-1 (+/−) transformed with IRAP or the catalytically inactive IRAPm. A, Five-day-old seedlings. B, Three-week-old plants. C, Seed set. D to F, Phenotypic analyses of apm1-2 (−/−) transformed with IRAP or the catalytically inactive IRAPm. D, Five-day-old seedlings. E, Three-week-old plants. F, Seed set. Bars = 5 mm (A and D), 3 cm (B and E), and 5 mm (C and F). WT, Wild type. [See online article for color version of this figure.]
Transformation of apm1-2 (+/−) with either the wild-type cDNA of IRAP or IRAPm was also able to rescue all of the apm1-2 (−/−) phenotypes (arrested root growth, gravitropic response, loss of apical dominance, and defects in embryo development; Fig. 5, D–F; Supplemental Figs. S3 and S10, A–J). A leaf phenotype similar to what was seen with catalytically inactive APM1 transformants was also observed.
In western-blot analyses of heterologously expressed IRAP in Arabidopsis, IRAP (approximately 165-kD monomer) was found in microsomal membranes (Supplemental Fig. S10K). Following proteinase K digestion, full-length IRAP was observed in the microsomal membrane fractions (Supplemental Fig. S10K), suggesting that IRAP is a peripheral membrane protein in Arabidopsis. APM1 in the wild type is shown for reference in Supplemental Figure S10L. APM1-IRAP heterodimers formed in apm1-1 (APM1-IRAP; approximately 265 kD) and apm1-2 (APM1R668*-IRAP; approximately 237 kD), in the absence and presence of BS3 (Supplemental Fig. S10L), and was confirmed by sequence analysis of the bands by mass spectrometry. This suggests that APM1-IRAP forms a stable heterodimer even in the absence of cross-linking reagent, resulting in rescued apm1 phenotypes.
To further investigate if the unique N-terminal AS160/GLUT4 protein-protein interaction domain and the transmembrane domain (IRAP1–109) were sufficient to rescue apm1, apm1-1 (+/−) was transformed with a cDNA encoding this region (see “Materials and Methods”) under the control of a 35S promoter. This construct failed to rescue the apm1 mutant phenotypes (Supplemental Fig. S11, A–F), suggesting that the conserved catalytic and C-terminal protein-protein interaction domains of IRAP were responsible for the observed activity and likely mediate dimerization with APM1.
An APM1-IRAP chimera was generated where the N-terminal portion of APM1, containing the first 210 amino acid residues, was fused to IRAP, lacking the first 110 amino acid residues. Transformation with this construct rescued homozygous apm1-1, confirming that the protein-protein interaction domain of the C terminus of IRAP can compensate for APM1 function (Supplemental Fig. S11, G–K). These results support the hypothesis that the M1 catalytic and C-terminal protein-protein interaction domain functions are conserved between plants and animals.
Subcellular Localization of APM1 Is Important for Its Activity
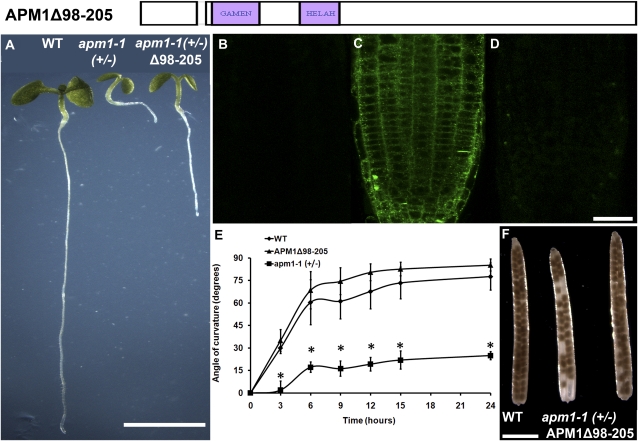
M1 peptidases are either peripheral membrane or membrane-anchored proteins (Dyer et al., 1990; Cadel et al., 1997; Keller, 2004; Peer et al., 2009). APM1 has previously been shown to be peripherally associated with the plasma membrane and endomembrane structures (cytosolic face; Murphy et al., 2002; Peer et al., 2009), indicating that it is a peripheral membrane protein. Sequence comparisons were used to identify a conserved N-terminal hydrophobic region in the protein (Fig. 1B). An APM1 cDNA in which this putative hydrophobic interacting domain was deleted (APM1Δ98–205) was used to transform apm1-1 (+/−). Transformation with this construct was not able to restore wild-type growth or gravitropic response in apm1-1 homozygote plants (Fig. 6, A, E, and F). Transformants showed a faint YFP cytosolic signal indicative of altered subcellular localization (Fig. 6, B–D). These results indicate that this hydrophobic region is required for APM1 membrane association and therefore subsequent localization to the plasma membrane.
Figure 6.
Membrane association of APM1 is important for its function. A, Five-day-old seedlings of wild-type (WT), apm1-1 (+/−), and APM1Δ98–205 plants. B to D, Localization of APM1Δ98–205. B, Autofluorescence control. C, Pro35S:YFP-APM1. D, Pro35S:YFP-APM1Δ98–205. E, Seed set of wild-type, apm1-1 (+/−), and Pro35S:YFP-APM1Δ98–205 plants. F, Gravitropic response. Bars = 5 mm (A), 50 μm (B–D), and 5 mm (E). [See online article for color version of this figure.]
DISCUSSION
The results presented here indicate that APM1 functions as a dimer and requires both catalytic and protein interaction domains for full functionality. As is seen in other M1 proteases (Danielsen, 1990, 1994; Papadopoulos et al., 2001; Ofner and Hooper, 2002; Ye et al., 2007), dimer formation is necessary for enzymatic and/or cellular trafficking of the protein. In the proposed model (Supplemental Fig. S12), native APM1 forms a homodimer and localizes to sites of action on the surface of endomembrane compartments and the plasma membrane (Peer et al., 2009). In apm1-1 (+/−), some APM1 protein can form a homodimer, but due to the low levels present in the mutant (Peer et al., 2009), not all of the APM1 protein can dimerize and the residual monomers have cytosolic localization. In apm1-2 (+/−), in which a truncated form of APM1 is generated (APM1R688*), a dimer can form either with itself (APM1R688*-APM1R688*) or with full-length APM1 (APM1-APM1R688*). Although the apm1-2 monomeric truncated protein may be more stable in the cell, the resulting dimers are predicted to be less stable than complete homodimers and would be likely to dissociate more readily.
The C-terminal domains of some mammalian M1 aminopeptidases have been shown to enhance enzymatic activity and also to function as intramolecular chaperones required for correct protein folding and trafficking to the plasma membrane (Ofner and Hooper, 2002; Rozenfeld et al., 2004). APM1 may also have intrachaperone activity, which may account for the severity of the apm1-2 phenotype, since apm1-2 contains a catalytic domain but lacks the C-terminal domain. Chaperone-dependent activation of APM1 may involve additional chaperone activities, as APM1 copurified with the cellular chaperone/PPIase FKBP42/TWISTED DWARF1 (TWD1; Murphy et al., 2002; Geisler et al., 2003) and a strong interaction was observed between APM1 and TWD1 in split ubiquitin and coimmunoprecipitation assays (O.R. Lee and A.S. Murphy, unpublished data).
The hypothesis that the APM1 catalytic domain does not function independently is further supported by a lack of apm1-1 and apm1-2 complementation by transformation with a C-terminal deletion construct of APM1 (APM1Δ757–786), APM1L729A, or overexpression of the M1 aminopeptidase TAF2L2, which lacks the C-terminal protein interaction domain. In all cases, a lack of intact APM1 or a lack of the C-terminal interaction domain in the endogenous or recombinant protein fails to rescue mutant phenotypes. Only in the case of apm1-3, which harbors an A→V point mutation in the C terminus, is partial rescue observed. Rescue cannot be accomplished by increasing catalytic activity alone.
Although both the catalytic and protein interaction domains of APM1 are required for its function, these domains do not need to be in the same linear protein. Only one copy of each is sufficient for APM1 function, as illustrated by the ability of the catalytically inactive forms of APM1 and IRAP to rescue apm1-2 phenotypes. In the proposed model, when apm1-1 is transformed with either APM1 or IRAP, the mutant phenotypes are rescued (Supplemental Fig. S12). This can be a result of either the recombinant protein forming homodimers with itself or, in the case of IRAP, forming heterodimers with APM1. When catalytically inactive APM1 or IRAP is expressed in apm1-1 (+/−), the mutant phenotype is not rescued, indicating insufficient formation of enzymatically active dimers to rescue the apm1 phenotype. However, when transformed into apm1-2, the catalytically inactive proteins were able to rescue the mutant phenotype, presumably through their ability to form enzymatically active heterodimers with the truncated protein (Supplemental Fig. S12). Mutations in APM1 that alter its subcellular localization result in an inability to rescue apm1 phenotypes, highlighting localization as an important factor in the function of APM1.
CONCLUSION
The severe morphological and cell cycle defects observed in apm1 mutants, the lack of mutant rescue by overexpression of conserved M1 catalytic domains, and the association of APM1 with important membrane signaling complexes suggest that APM1 is not merely a component of the protein turnover machinery of plant cells. APM1 and MPA1 appear to function analogously with animal M1 peptidases that act in protein and peptide hormone maturation/degradation as well as the regulation of embryogenesis, reproduction, meiotic and mitotic cell cycle progression, and cell viability. Activity and correct localization of APM1 in these processes appear to require dimerization events mediated by the C-terminal protein interaction domain. Finally, the results presented here suggest that APM1 function is more analogous to PSA/PAM1/AP-N than IRAP and suggest that a mechanism analogous to the IRAP-GLUT4 interaction does not occur in plants. Future studies will determine the targets and partners of APM1 that are downstream regulators or structural components of developmental processes.
MATERIALS AND METHODS
Phylogenetic Analysis
To identify all M1 aminopeptidase sequences in the Arabidopsis (Arabidopsis thaliana) genome and in other fully or partially sequenced genomes, nonredundant protein sequences with the M1 protein sequence in their definition fields were obtained from GenBank (Benson et al., 2000). Arabidopsis nonredundant protein sequences were also blasted using subdomains of APM1 and MPA1. All alignments were performed using the default parameters in MEGA (Kumar et al., 2004 [pairwise alignment, with a gap opening penalty of 10 and gap extension penalty 0.1; multiple alignment, with a gap opening penalty of 10 and gap extension penalty 0.2; protein weight matrix gonnet; residue-specific penalties on, hydrophilic penalties on, gap separation distance of 4; and gap separation off, negative matrix off, and delay divergent cutoff {%} of 30]), which uses ClustalW as a base alignment algorithm (Thompson et al., 2002): matrix, gonnet250; gapopen, 10; no endgap, yes; gap ext, 0.2; gap dist, 4; iteration, 1; numiter, 1; output format, phylip; output order, aligned; tree type, phylip; correct dist, off; ignore gaps, off; clustering, NJ. Files were converted to the nexus format and run in MrBayes using ngen = 1,000,000, aamodel = fixed, samplefreq = 100. Alignments were to generate unrooted neighbor-joining trees, also using the default parameters (bootstrap of 1,000 and random seed of 4,148, complete deletion of gaps and missing data, and using Poisson correction for the amino acid model) to P < 0.01 The alignments calculated bootstrapping values and only used genes for which transcripts were available. Current MEROPS alignments were assembled in consultation with the authors of this paper. Our approach also contained structural characterization modeled on mammalian PSA to determine the validity of the alignment based on helices and catalytic sites.
Growth Conditions
Seedlings were grown on 1% phytagar plates, quarter-strength Murashige and Skoog (MS) basal salts, pH 5.5, 22°C and 14 h, and 100 μmol m−2 s−1 except as indicated for specific treatments. apm1 mutants were transferred to 1% phytagar plates, quarter-strength MS basal salts, pH 5.5, supplemented with 0.5% Suc to induce adventitious root formation from the hypocotyls prior to transfer to soil; however, a robust root system was never formed. Growth conditions for plants on soil were grown in the greenhouse under natural light conditions, and in the winter the daylength was extended to 16 h with HID lights (150 μmol m−2 s−1). For more information, see http://www.hort.purdue.edu/hort/facilities/greenhouse/hlaTech.shtml.
Generation of Constructs
Site-directed mutagenesis was carried out by generation of two fragments that were then used in a three-way ligation reaction to generate either Pro35S:APM1-YFP or ProAPM1:YFP-APM1 with the mutated catalytic site, G273K or H307A. In this procedure, one of the two mutagenic primers and one of the external primers were used to amplify the PCR products. All PCR-derived products were sequenced completely for correct sequence determination: gene-specific forward primer 5′-GATTCTTCCCCATGGAGTGGG-3′ (NcoI restriction site) and gene specific reverse primer 5′-GCGCTTTTCATATGAACCACAAC-3′ (NdeI restriction site). To generate the G273K construct, the following primers were used: 5′-GCCGGCGCTAAGGAGAATTAT-3′ and 5′-ATAATTCTCCTTAGCGCCGGC-3′; while the H307A construct was generated using 5′-GGCCAGTTCTGCAGCAACTAC-3′ and 5′-GTAGTTGCTGCAGAACTGGCC-3′ primers. These constructs were then transformed into Agrobacterium tumefaciens C58 pGV3850 (Zambrisky et al., 1983) by electroporation. The apm1-1 (+/−) and apm1-2 (+/−) mutants were transformed by the floral dip method (Clough and Bent, 1998), and transformants were selected using Basta (50 ppm) and genotyped for the apm1-1 T-DNA mutation or the apm1-2 point mutation as follows. PCR with Lba1 primer 5′-TGGTTCACGTAGTGGGCCATCG-3′ and 5′-TGATGAAGCTACGTCCAACATGGCGG-3′ was used to determine if the transformant contained a T-DNA mutation. To determine if the seedling was homozygous for the T-DNA mutation, PCR with primers 5′-TGATGAAGCTACGTCCAACATGGCGG-3′ and 5′-CTTTTATAATACGAGGGTTGTAAGC-3′ was used. To determine if the transformant contained the point mutation (apm1-2), an amplicon was generated using gene-specific primers (5′-TTCATTGGCGTTTTCCAGTTTGCTG-3′ and 5′-TTACACCGGAAAGTCCATAAAGTC-3′) and subsequently digested using XmnI. (This mutation resulted in a loss of the XmnI restriction site.) Seventeen independent lines were isolated for both constructs, and all 17 exhibited similar phenotypes. Three were used for detailed phenotypic analyses.
A truncated form of APM1 (APM1Δ720–880) was generated by digesting Pro-35S:YFP-APM (Peer et al., 2009) with BstBI and BlpI, blunt ending, and subsequent self-ligation. To generate APM1Δ98–205, an internal deletion of APM1 was amplified by PCR from APM1 cDNA using the primers 5′-CCATGGTTTGTTTGACTACGTGG-3′ with an EcoRI site and 5′-GAATTCCATCGGATGTATGATCTTCCACG-3′ with an NcoI site. This fragment was subcloned into the Pro-35S:YFP-APM construct (Peer et al., 2009). Constructs were transformed into plants, and plants were screened and genotyped as above. Five independent lines were isolated for Pro35S:YFP-APM1Δ720–880, and all five exhibited similar phenotypes. Three were used for detailed phenotypic analyses. Two independent lines were isolated for Pro35S:YFP-APM1Δ98–205, and both exhibited similar phenotypes, with one line exhibiting a stronger signal when viewed with the confocal microscope. This line was used for detailed phenotypic analyses.
Point mutations of the dileucine residues and internal deletions of the putative protein-protein interaction domains were generated using primers (Supplemental Table S1), subcloning corresponding fragments in pENTR:YFP-APM1 and subsequently into pOpOn2.1 using reagents and protocols from Invitrogen. Constructs were transformed into plants, and plants were screened and genotyped as above. For all constructs, at least six independent lines were isolated, and all exhibited similar phenotypes. Four lines were used for detailed phenotypic analyses.
A full-length IRAP cDNA was amplified by PCR from pCI-IRAP (Albiston et al., 2004) using the primers 5′-CACCATGAATAGAAGCTCAGGCCTTCGG-3′ and 5′-CTACAGCCACCATGTGAGACTTTTGAGG-3′, then subcloned into pENTR-D/TOPO and subsequently into the Gateway binary vector pGWB15 using reagents and protocols from Invitrogen. The same procedure was employed for the catalytically inactive form of IRAP (Albiston et al., 2004). Constructs were transformed into plants, and plants were screened and genotyped as above. Five independent lines were isolated for Pro35S:IRAP and Pro35S:IRAPm, and all exhibited similar phenotypes. Three lines were used for detailed phenotypic analyses.
The N terminus of IRAP (1–109) was amplified by PCR from pCI-IRAP (Albiston et al., 2004) using the primers 5′-AAAAAGCAGGCTGGATGAATAGAAGCTC-3′ and 5′-AGAAAGCTGGGTTTAGAATGTCATCGAGG-3′, which were subcloned into pDONR Zeo and subsequently into the Gateway binary vector pEARLEYGATE 104 using reagents and protocols from Invitrogen. Constructs were transformed into plants, and plants were screened and genotyped as above. Four independent lines were isolated for Pro35S:IRAP (1–109), and all exhibited similar phenotypes. Three lines were used for detailed phenotypic analyses.
The chimera between APM1 and IRAP was generated. Two fragments were generated using PCR as follows. The first fragment was generated using 5′-GAATTCATGGATCAGTTCAAAGG-3′ (EcoRI restriction site) and 5′-GGATCCTTGATAGGAGACAATC-3′ (BamHI restriction site) and used to amplify the first 210 amino acid residues. The second fragment was generated using 5′-GAATCCTTCAGGGGTTCTGTGAC-3′ (BamHI restriction site) and 5′-CCCGGGCTACAGCCACCATGTG-3′ (XmaI restriction site) to amplify the region of the CDS of IRAP without the first 110 amino acid residues. These fragments were subcloned into pBI121 in a three-way ligation reaction. Constructs were transformed into plants, and plants were screened and genotyped as above. Eight independent lines were isolated for Pro35S:YFP-APM1Δ98–205, and all exhibited similar phenotypes. Three lines were used for detailed phenotypic analyses.
A full-length At5g13520 cDNA was amplified by PCR from pUNI-At5g13520 using the primers 5′-AGAAAGCTGGGTTTAGATGTACTTAG-3′ and 5′-CTACAGCCACCATGTGAGACTTTTGAGG-3′, then subcloned into pDONR Zeo and subsequently into the Gateway binary vector pEARLEYGATE 104 using reagents and protocols from Invitrogen. Constructs were transformed into plants, and plants were screened and genotyped as above. Transformants from apm1-3 were genotyped as follows. An amplicon was generated using gene-specific primers (5′-TTCATTGGCGTTTTCCAGTTTGCTG-3′ and 5′-TTACACCGGAAAGTCCATAAAGTC-3′) and subsequently digested using AluI. (This mutation resulted in a loss of the AluI restriction site.) Three independent lines were isolated for Pro35S:YFP-APM1Δ98–205, and all exhibited similar phenotypes. All three lines were used for detailed phenotypic analyses.
Inducible Constructs
Point mutations of the dileucine residues were generated as follows. Dileucine residues were identified and primers were mutated to selected Leu residues and Ala residues using Primer Generator (http://www.med.jhu.edu/medcenter/primer/primer.cgi; Supplemental Table S1). These primers were used to generate mutagenic fragments and were subcloned into pENTR-TOPO/D YFP-APM1 (Peer et al., 2009) using the unique restriction sites generated with each point mutation. Point mutations that were after the first 300 amino acid residues used the following primer combination to generate the mutagenic fragments: forward primer 5′-GATTCTTCCCCATGGAGTGGG-3′ (NcoI restriction site) and reverse primer 5′-TTAGTTTGAAGAGAGCTGAGCAACG-3′ (BlpI restriction site). Point mutations that were before the first 300 amino acid residues used the following primer combination to generate the mutagenic fragments: forward primer 5′-GGAATTCATGGATCAGTTCAAAGGTGAGCC-3′ (EcoRI restriction site) and reverse primer 5′-GCGCTTTTCATATGAACCACAACAC-3′ (NdeI restriction site). These fragments were then subcloned into pENTR-TOPO/D YFP-APM1 in a three-way ligation reaction (Peer et al., 2009) and subsequently subcloned into Gateway binary vector pOpON2.1 using reagents and protocols from Invitrogen. These were subsequently subcloned into pOpON using reagents and protocols from Invitrogen. Constructs were transformed into plants, and plants were screened and genotyped as above. Resulting transformants were selected on quarter-strength MS plates supplemented by kanamycin (50 mg mL−1) and genotyped as described above.
Dex was dissolved in either dimethyl sulfoxide or ethanol and kept as a 20 mm stock at −20°C. Unless otherwise stated, 10 μm Dex was used for experiments. Dex was added to quarter-strength MS medium with 1% (w/v) phytagar plates, pH 5.5, to achieve induction during seed germination or after seedling transfer in sterile conditions. For application to soil-grown plants, transgenic plants carrying the inducible construct were watered with varying concentrations of Dex (0–10 μm) every 3 d.
Transgenic seeds [apm1-1 (+/−) transformed with pOpON YFP-APM] were sown on plates supplemented with 10 μm Dex (as outlined above), and every day, 10 to 20 seedlings were transferred to plates without Dex for 5 d. Six-day-old seedlings were imaged using a Nikon SMZ-U research-grade stereomicroscope. The reciprocal experiment was repeated where transgenic seeds were sown on plates without Dex, and every day, 10 to 20 seedlings were transferred to plates with 10 μm Dex for 5 d. These experiments were repeated three times using three lines each time.
Identification of Candidate Protein-Protein Interaction Domains
Candidate protein-protein interaction domains were identified using prediction programs that first identified hydrophobic regions in the C terminus of APM1, which were further analyzed for putative protein-protein interaction sites. Initial identification of the three hydrophobic regions was carried out using prediction programs: the Phobius prediction program identified a region between 803 and 822 as a possible candidate (Keller, 2004), and PredictProtein identified regions from 692 to 706 and 821 to 835 (Rost et al., 2004). A fourth region (757–786) was identified due to it being most similar to the GLUT4 trafficking domain located in the N-terminal region of IRAP. These regions were compared with results obtain from PredictProtein, which identified putative protein-protein interaction motifs using the default settings (stretch = 5, crowd predictions = 7, gap = 8, and itr = 51). Since these putative interaction regions contained protein-protein interaction motifs, they were subsequently targeted for deletion analyses. These regions were deleted using primers (Supplemental Table S1) that were used to generate fragments with XmaI restriction sites at either end: 5′-GTTCTCCTGGGGAAGGACAA-3′ and 5′-TTAGTTTGAAGAGAGCTGAGCAACG-3′. These fragments were then subcloned into pENTR-TOPO/D YFP-APM1 in a three-way ligation reaction (Peer et al., 2009) and subsequently subcloned into pOpON using reagents and protocols from Invitrogen. Transgenic plants were generated as outlined above. Other domain analyses used National Center for Biotechnology Information conserved domains (http://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi?RID=9AZGSHK4014&mode=all) and WoLFPSORTII (http://wolfpsort.org/results/pa47e8359bfb59d24baa870103166382a.detailed1.html#queryProtein).
Expression Analysis
Quantitative Real-Time PCR Analysis of APM1 Expression in 5-d-Old NPA-Treated Seedlings
RNA was extracted with the RNeasy kit (Qiagen) from 5-d-old seedlings. cDNA was prepared from total RNA with Bioscript Reverse Transcriptase (Bioline). Transcript levels were determined on a Bio-Rad iCycler IQ using iQ Sybr Green Supermix (Bio-Rad) in 20-μL reactions. An aliquot of the reverse transcriptase reaction was used as template. PCR conditions were as follows: 1.5 min at 95°C (one cycle); 30 s at 95°C, 30 s at 54°C, and 30 s at 72°C (45 cycles). Gene-specific primers used were as follows: 5′-TTTTGGCTGATAGGAACACT-3′ and 5′-GTGAAGTAGCTTGGAAATGG-3′. Each reverse transcriptase sample was assayed in triplicate. Data were further analyzed in Excel. Transcript levels were normalized to β-tubulin6 (efficiency 1.07) and GADPH (efficiency 1.05) and quantitated using Pfaffl (2001).
Expression Analysis of APM1 in Dex-Inducible Constructs
RNA was extracted with the RNeasy kit (Qiagen) from 5-d-old seedlings grown on Dex (Peer et al., 2009). cDNA was prepared from total RNA with Bioscript Reverse Transcriptase (Bioline) from YFP-APM1, APM1L729A, and APM1Δ757–786 lines. Since APM1 is expressed in these lines, albeit at a low level, it was not possible to use quantitative real-time PCR, because this method cannot distinguish between the endogenous transcript and the transgene simultaneously. APM1 and APM1Δ757–786 can be distinguished by electrophoresis, since APM1Δ757–786 produces a shorter transcript; however, the point mutation cannot be distinguished from the wild-type transcript.
Mass Spectroscopy Analyses
Mass spectroscopy analyses were as described by Titapiwatanakun et al. (2009).
Microscopy
Five-day-old seedlings were imaged on a LSM710 confocal laser scanning microscope as outlined by Peer et al. (2009). For DAPI staining, seedlings were incubated in DAPI (1 mg mL−1) for 5 min and rinsed three times in water before being visualized on the LSM710-META confocal laser scanning microscope.
Gravitropism Assay
For analyzing the response to a gravity stimulus, 5-d-old seedlings were gravistimulated by rotating the plate by 90° for 30 min. To analyze the response of the transgenic plant to a gravity stimulus, seedlings were grown on quarter-strength MS medium with 1% (w/v) phytagar, pH 5.2, for 5 d (unlike assays conducted at 4 d in Peer et al., 2009). Plates were kept in a vertical position. After reorienting the plates by 90°, the root tip position was marked every 3 h over a 24-h time period. The angles of curvature were measured by the ImageJ program, and the data were analyzed by Microsoft Excel. The averages were calculated from 50 to 68 seedlings.
Inhibitor Studies
For inhibitor studies, wild-type, apm1-1 (+/−), and apm1-2 (+/−) seedlings were germinated on quarter-strength MS (pH 5.5), 1% phytagar plates supplemented with either 10 nm PAQ-22 or 50 nm ezetimibe (Zetia). Five-day-old seedlings were imaged using a Nikon E800 microscope. For NPA assays, seedlings were grown as above for 5 d, then transferred to plates containing 30 or 50 μm NPA for 1 h prior to microsomal membrane preparation as described by Peer et al. (2009).
Cross-Linking
Microsomal membrane preparations were isolated, without reducing agent, as outlined by Peer et al. (2009) and were cross-linked to BS3, as outlined in the manufacturer's instructions (Pierce Biotechnology). The proteasome inhibitor, MG132, was included in the grinding buffer to ensure that proteolysis was not a factor affecting dimerization. This method was used in analyses of AUX/IAA proteins to prevent degradation (Gray et al., 2001). Stock solutions of 0.1, 1, and 10 mm were prepared in water, which was then added to the membrane preparations to final concentrations of 0.01, 0.1, and 1 mm, respectively. Samples were incubated for 2 h on ice with 1 μm MG132 before being quenched with 30 mm Tris, pH 7.5, for 10 min on ice. Samples were then run on SDS-PAGE or Blue Native gels followed by western-blot analyses as outlined by Peer et al. (2009).
Proteinase K Digestion
Microsomal membrane preparations were isolated and digested with proteinase K (100 μg mL−1; Sigma) for 1 h on ice. Phenylmethylsulfonyl fluoride (to a final concentration of 1 mm) was added to terminate the reaction with incubation for 20 min on ice. Samples were run on an 8% SDS-PAGE gel followed by western-blot analyses with anti-hemagglutinin (Santa Cruz Biotechnology; 1:500 dilution in 5% skim milk in Tris-buffered saline, 0.1% Tween 20, 16 h, 4°C) followed by incubation with the secondary antibody (1:5,000, 1 h, room temperature) and incubation with enhanced chemiluminescence (Pierce) for 5 min.
Blue Native PAGE Analyses
Microsomal membrane preparations were isolated, with and without reducing agent, as outlined by Peer et al. (2009). Membrane preparations were run on Blue Native PAGE Novex 4% to 16% Bis-Tris gradient gels (Invitrogen) as outlined in the manufacturer's instructions (Invitrogen). Western-blot analyses were carried out as outlined by Wittig et al. (2006), using the APM1 antibody generated by Murphy et al. (2002) as outlined by Peer et al. (2009). Blots were incubated using anti-GFP (Santa Cruz Biotechnology; 1:1,000 dilution in 5% skim milk in Tris-buffered saline, 0.1% Tween 20, 16 h, 4°C) followed by incubation with the secondary antibody (1:5,000, 1 h, room temperature) and incubation with enhanced chemiluminescence (Pierce) for 5 min.
In Vitro Translation
In vitro translation was as described by Murphy et al. (2002). Enzymatic assays were performed as described by Murphy and Taiz (1999b) and Murphy et al. (2002), except that phenylisothiocyante derivatization in tripeptide hydrolysis assays was replaced with Accutag and peptides were incubated for only 15 min. Protein was incubated in DTT solution as indicated for 1 h and 6°C before assay. DTT was also present in the assay buffer. The baseline is empty vector control protein in the same buffer.
Sequence data from this article can be found in the GenBank/EMBL data libraries under the following accession numbers: Arabidopsis APM1 (At4g33090), Neosartorya fischeri XP_001267120, Aspergillus fumigatus XP_751922.1, Aspergillus clavatus XP_001271795.1, Aspergillus niger CAC_38353.1, Ashbya gossypii NP_982415.1, Pichia stipitis XP_001385797.2, Phyllostachys angusta AAL83915.1, Saccharomyces cerevisiae AAG01326.1, Physcomitrella patens PP_11099_C2, Oryza sativa Os090362500, O. sativa Os02g0218200, Dictyostelium discoideum XP_646344.1, Danio rerio XP 684042, Homo sapiens PSA NM_006310, Caenorhabditis elegans PAM-1 NP_001023210, Xenopus laevis NP_001072690.1, Rattus norvegicus EDM05852.1, Aedes aegypti EAT34883.1, Drosophila pseudoobscura XP_001354254.1, Drosophila melanogaster AAG48733.1, Trypanosoma cruzi strain XP_809697.1, H. sapiens AP-N X13276, H. sapiens IRAP D50810, H. sapiens ERAP NP 057526, R. norvegicus VP165 NP 001106874, Candidatus nitrosopumilus maritimus ZP_02023923.1, Cenarchaeum symbiosum A ABK77113.1, O. sativa Os03g0819100, A. fumigatus XP_751922, Aspergillus terreus XP_001214491, Aspergillus oryzae XP_001821168, Penicillium marneffei XP_002150453, Sclerotinia sclerotiorum XP_001594581, Botryotinia fuckeliana XP_001548342, Podospora anserina XP_001908716, Neurospora crassa XP_959172, Coccidiodes immitis XP_001241125, P. marneffei XP_002149108, A. fumigatus XP_747910, N. fischeri XM_001266098, Aspergillus nidulans XP_661886, A. niger XP_001393995, A. terreus XP_001216601, C. symbiosum A YP_875417.1, C. nitrosopumilus maritimus SCM1 YP_001582291.1, Bos taurus AAB28170.1, B. taurus NP 776523.2, H. sapiens AAH06199.3, H. sapiens AAD17527.1, H. sapiens CAG33409.1, H. sapiens NP_056991.2, H. sapiens TAF2 EAW91993.1, Arabidopsis TAF2L2 At5g13520, Arabidopsis MPA1 At1g63770, Arabidopsis At5g52910, Arabidopsis At4g29490, Arabidopsis At1g73960, Arabidopsis At4g36760, Arabidopsis At2g45240, Arabidopsis At1g13270, Solanum lycopersicum AAO15916.1, and Arabidopsis At2g24200.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Phylogenetic analysis of M1 peptidases.
Supplemental Figure S2. The C terminus of APM1 is required for APM1 function.
Supplemental Figure S3. Seed set quantification from transgenic plants.
Supplemental Figure S4. Site-directed mutagenesis of LL729,730 and deletion of the APM1 protein-protein interaction domain (APMΔ757–786) did not rescue apm1.
Supplemental Figure S5. Seed set quantification from apm1-1 (−/−) carrying mutated dileucine or internal deletions of APM1.
Supplemental Figure S6. Site-directed mutagenesis of LL809,810 (APML809A) rescued apm1.
Supplemental Figure S7. APM1 protein abundance is decreased in APML729A and APM1Δ757–786 transformants.
Supplemental Figure S8. Increased M1 enzymatic activity is not sufficient to rescue apm1-3.
Supplemental Figure S9. Catalytic and protein-protein interaction domains have distinct functions.
Supplemental Figure S10. IRAP can rescue apm1-1, and IRAP can form a heterodimer with APM1.
Supplemental Figure S11. Overexpression of APM1-IRAP chimera, but not IRAP1–109, is sufficient to rescue apm1-1.
Supplemental Figure S12. Model of APM1 subcellular localization based on dimerization though protein-protein interaction motifs.
Supplemental Table S1. Primers used to generate constructs.
Supplementary Material
References
- Albiston AL, Ye S, Chai SY. (2004) Membrane bound members of the M1 family: more than aminopeptidases. Protein Pept Lett 11: 491–500 [DOI] [PubMed] [Google Scholar]
- Alfalah M, Krahn MP, Wetzel G, von Hörsten S, Wolke C, Hooper N, Kalinski T, Krueger S, Naim HY, Lendeckel U. (2006) A mutation in aminopeptidase N (CD13) isolated from a patient suffering from leukemia leads to an arrest in the endoplasmic reticulum. J Biol Chem 281: 11894–11900 [DOI] [PubMed] [Google Scholar]
- Benson DA, Karsch-Mizrachi I, Lipman DJ, Ostell J, Rapp BA, Wheeler DL. (2000) GenBank. Nucleic Acids Res 28: 15–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernier SG, Bellemare JM, Escher E, Guillemette G. (1998) Characterization of AT4 receptor from bovine aortic endothelium with photosensitive analogues of angiotensin IV. Biochemistry 37: 4280–4287 [DOI] [PubMed] [Google Scholar]
- Brooks DR, Hooper NM, Isaac RE. (2003) The Caenorhabditis elegans orthologue of mammalian puromycin-sensitive aminopeptidase has roles in embryogenesis and reproduction. J Biol Chem 278: 42795–42801 [DOI] [PubMed] [Google Scholar]
- Cadel S, Foulon T, Viron A, Balogh A, Midol-Monnet S, Noel N, Cohen P. (1997) Aminopeptidase B from the rat testis is a bifunctional enzyme structurally related to leukotriene-A4 hydrolase. Proc Natl Acad Sci USA 94: 2963–2968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. (1998) Floral dip: a simplified method for Agrobacterium mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Constam DB, Tobler AR, Rensing-Ehl A, Kemler I, Hersh LB, Fontana A. (1995) Puromycin-sensitive aminopeptidase: sequence analysis, expression, and functional characterization. J Biol Chem 270: 26931–26939 [DOI] [PubMed] [Google Scholar]
- Cowburn AS, Sobolewski A, Reed BJ, Deighton J, Murray J, Cadwallader KA, Bradley JR, Chilvers ER. (2006) Aminopeptidase N (CD13) regulates tumor necrosis factor-alpha-induced apoptosis in human neutrophils. J Biol Chem 281: 12458–12467 [DOI] [PubMed] [Google Scholar]
- Danielsen EM. (1990) Perturbation of intestinal microvillar enzyme biosynthesis by amino acid analogs: evidence that dimerization is required for the transport of aminopeptidase N out of the endoplasmic reticulum. J Biol Chem 265: 14566–14571 [PubMed] [Google Scholar]
- Danielsen EM. (1994) Dimeric assembly of enterocyte brush border enzymes. Biochem 33: 1599–1605 [DOI] [PubMed] [Google Scholar]
- Dyer SH, Slaughter CA, Orth K, Moomaw CR, Hersh LB. (1990) Comparison of the soluble and membrane-bound forms of the puromycin-sensitive enkephalin-degrading aminopeptidases from rat. J Neurochem 54: 547–554 [DOI] [PubMed] [Google Scholar]
- Geisler M, Kolukisaoglu HU, Bouchard R, Billion K, Berger J, Saal B, Frangne N, Koncz-Kalman Z, Koncz C, Dudler R, et al. (2003) TWISTED DWARF1, a unique plasma membrane-anchored immunophilin-like protein, interacts with Arabidopsis multidrug resistance-like transporters AtPGP1 and AtPGP19. Mol Biol Cell 14: 4238–4249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray WM, Kepinski S, Rouse D, Leyser O, Estelle M. (2001) Auxin regulates SCF(TIR1)-dependent degradation of AUX/IAA proteins. Nature 414: 271–276 [DOI] [PubMed] [Google Scholar]
- Herbst A, Wolner-Hanssen P, Ingemarsson I. (1997) Risk factors for acidemia at birth. Obstet Gynecol 90: 125–130 [DOI] [PubMed] [Google Scholar]
- Hesp JR, Hooper NM. (1997) Proteolytic fragmentation reveals the oligomeric and domain structure of porcine aminopeptidase A. Biochemistry 36: 3000–3007 [DOI] [PubMed] [Google Scholar]
- Hooper NM. (1994) Families of zinc metalloproteases. FEBS Lett 354: 1–6 [DOI] [PubMed] [Google Scholar]
- Hou JC, Suzuki N, Pessin JE, Watson RT. (2006) A specific dileucine motif is required for the GGA-dependent entry of newly synthesized insulin-responsive aminopeptidase into the insulin-responsive compartment. J Biol Chem 281: 33457–33466 [DOI] [PubMed] [Google Scholar]
- Hussain MM, Tranum-Jensen J, Noren O, Sjostrom H, Christiansen K. (1981) Reconstitution of purified amphiphilic pig intestinal microvillus aminopeptidase: mode of membrane insertion and morphology. Biochem J 199: 179–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh C, Watanabe M, Nagamatsu A, Soeda S, Kawarabayashi T, Shimeno H. (1997) Two molecular species of oxytocinase (L-cystine aminopeptidase) in human placenta: purification and characterization. Biol Pharm Bull 20: 20–24 [DOI] [PubMed] [Google Scholar]
- Kakuta H, Tanatani A, Nagasawa K, Hashimoto Y. (2003) Specific nonpeptide inhibitors of puromycin-sensitive aminopeptidase with a 2,4(1H,3H)-quinazolinedione skeleton. Chem Pharm Bull 55: 1273–1282 [DOI] [PubMed] [Google Scholar]
- Katagiri H, Asano T, Yamada T, Aoyama T, Fukushima Y, Kikuchi M, Kodama T, Oka Y. (2002) Acyl-coenzyme A dehydrogenases are localized on GLUT4-containing vesicles via association with insulin-regulated aminopeptidase in a manner dependent on its dileucine motif. Mol Endocrinol 16: 1049–1059 [DOI] [PubMed] [Google Scholar]
- Keller SK. (2004) Role of the insulin-regulated aminopeptidase IRAP in insulin action and diabetes. Biol Pharm Bull 27: 761–764 [DOI] [PubMed] [Google Scholar]
- Komoda M, Kakuta H, Takahashi H, Fujimoto Y, Kadoya S, Kato F, Hashimoto Y. (2001) Specific inhibitor of puromycin-sensitive aminopeptidase with a homophthalimide skeleton: identification of the target molecule and a structure-activity relationship study. Bioorg Med Chem 9: 121–131 [DOI] [PubMed] [Google Scholar]
- Kramer W, Girbig F, Corsiero D, Pfenninger A, Frick W, Jahne G, Rhein M, Wendler W, Lottspeich F, Hochleitner EO, et al. (2005) Aminopeptidase N (CD13) is a molecular target of the cholesterol absorption inhibitor ezetimibe in the enterocyte brush border membrane. J Biol Chem 280: 1306–1320 [DOI] [PubMed] [Google Scholar]
- Kumar S, Tamura K, Nei M. (2004) MEGA3: integrated software for Molecular Evolutionary Genetics Analysis and sequence alignment. Brief Bioinform 5: 150–163 [DOI] [PubMed] [Google Scholar]
- Laustsen PG, Vang S, Kristensen T. (2001) Mutational analysis of the active site of human insulin-regulated aminopeptidase. Eur J Biochem 268: 98–104 [DOI] [PubMed] [Google Scholar]
- Luciani N, Marie-Claire C, Ruffet E, Beaumont A, Roques BP, Fournié-Zaluski MC. (1998) Characterization of Glu350 as a critical residue involved in the N-terminal amine binding site of aminopeptidase N (EC 3.4.11.2): insights into its mechanism of action. Biochemistry 37: 686–692 [DOI] [PubMed] [Google Scholar]
- Lyczak R, Zweier L, Group T, Murrow MA, Snyder C, Kulovitz L, Beatty A, Smith K, Bowerman B. (2006) The puromycin-sensitive aminopeptidase PAM-1 is required for meiotic exit and anteroposterior polarity in the one-cell Caenorhabditis elegans embryo. Development 33: 4281–4292 [DOI] [PubMed] [Google Scholar]
- Maianu L, Keller SR, Garvey WT. (2001) Adipocytes exhibit abnormal subcellular distribution and translocation of vesicles containing glucose transporter 4 and insulin-regulated aminopeptidase in type 2 diabetes mellitus: implications regarding defects in vesicle trafficking. J Clin Endocrinol Metab 86: 5450–5456 [DOI] [PubMed] [Google Scholar]
- Matsui M, Fowler JH, Walling LL. (2006) Leucine aminopeptidases: diversity in structure and function. Biol Chem 387: 1535–1544 [DOI] [PubMed] [Google Scholar]
- Matsumoto H, Rogi T, Yamashiro K, Kodama S, Tsuruoka N, Hattori A, Takio K, Mizutani S, Tsujimoto M. (2000) Characterization of a recombinant soluble form of human placental leucine aminopeptidase/oxytocinase expressed in Chinese hamster ovary cells. Eur J Biochem 267: 46–52 [DOI] [PubMed] [Google Scholar]
- Medina JF, Rådmark O, Funk CD, Haeggström JZ. (1991) Molecular cloning and expression of mouse leukotriene A4 hydrolase cDNA. Biochem Biophys Res Commun 176: 1516–1524 [DOI] [PubMed] [Google Scholar]
- Mina-Osorio P, Ortega E. (2005) Aminopeptidase N (CD13) functionally interacts with FcgammaRs in human monocytes. J Leukoc Biol 77: 1008–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy A, Peer WA, Taiz L. (2000) Regulation of auxin transport by aminopeptidases and endogenous flavonoids. Planta 211: 315–324 [DOI] [PubMed] [Google Scholar]
- Murphy A, Taiz L. (1999a) Localization and characterization of soluble and plasma membrane aminopeptidase activities in Arabidopsis seedlings. Plant Physiol Biochem 37: 431–443 [Google Scholar]
- Murphy A, Taiz L. (1999b) Naphthylphthalamic acid is enzymatically hydrolyzed at the hypocotyl-root transition zone and other tissues of Arabidopsis thaliana seedlings. Plant Physiol Biochem 37: 413–430 [Google Scholar]
- Murphy AS, Hoogner KR, Peer WA, Taiz L. (2002) Identification, purification, and molecular cloning of N-1-naphthylphthalmic acid-binding plasma membrane-associated aminopeptidases from Arabidopsis. Plant Physiol 128: 935–950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafa T, Chai SY, Mendelsohn FA, Moeller I, Albiston AL. (2001) Characterization of the AT4 receptor in a human neuroblastoma cell line (SK-N-MC). J Neurochem 76: 1679–1687 [DOI] [PubMed] [Google Scholar]
- Ofner LD, Hooper NM. (2002) The C-terminus domain, but not the interchain disulphide, is required for the activity and intracellular trafficking of aminopeptidase A. Biochem J 362: 191–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osada T, Watanabe G, Kondo S, Toyoda M, Sakaki Y, Takeuchi T. (2001a) Male reproductive defects caused by puromycin-sensitive aminopeptidase deficiency in mice. Mol Endocrinol 15: 960–971 [DOI] [PubMed] [Google Scholar]
- Osada T, Watanabe G, Sakaki Y, Takeuchi T. (2001b) Puromycin-sensitive aminopeptidase is essential for the maternal recognition of pregnancy in mice. Mol Endocrinol 15: 882–893 [DOI] [PubMed] [Google Scholar]
- Papadopoulos T, Kelly JA, Bauer K. (2001) Mutational analysis of the thyrotropin-releasing hormone-degrading ectoenzyme: similarities and differences with other members of the M1 family of aminopeptidases and thermolysin. Biochem 40: 9347–9355 [DOI] [PubMed] [Google Scholar]
- Peer WA, Hosein FN, Bandyopadhyay A, Makam SN, Otegui M, Lee G, Blakeslee JJ, Cheng Y, Titapiwatanakun B, Yakubov B, et al. (2009) Mutation of the membrane-associated M1 protease APM1 results in distinct embryonic and seedling developmental defects. Plant Cell 21: 1693–1721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl MW. (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29: 2002–2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham VL, Cadel MS, Gouzy-Darmon C, Hanquez C, Beinfeld MC, Nicolas P, Etchebest C, Foulon T. (2007) Aminopeptidase B, a glucagon-processing enzyme: site directed mutagenesis of the Zn2+-binding motif and molecular modelling. BMC Biochem 8: 21–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradillo M, López E, Romero C, Sánchez-Morán E, Cuñado N, Santos JL. (2007) An analysis of univalent segregation in meiotic mutants of Arabidopsis thaliana: a possible role for synaptonemal complex. Genetics 175: 505–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlings ND, Barrett AJ. (1995) Evolutionary families of metallopeptidases. Methods Enzymol 248: 183–228 [DOI] [PubMed] [Google Scholar]
- Rasmussen TE, Pedraza-Diaz S, Hardre R, Laustsen PG, Carrion AG, Kristensen T. (2000) Structure of the human oxytocinase/insulin-regulated aminopeptidase gene and localization to chromosome 5q21. Eur J Biochem 267: 2297–2306 [DOI] [PubMed] [Google Scholar]
- Rost B, Yachdav G, Liu J. (2004) The PredictProtein server. Nucleic Acids Res 32: W321–W326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozenfeld R, Muller L, Messari SE, Llorens-Cortes C. (2004) The C-terminal domain of aminopeptidase A is an intramolecular chaperone required for the correct folding, cell surface expression, and activity of this monozinc aminopeptidase. J Biol Chem 279: 43285–43295 [DOI] [PubMed] [Google Scholar]
- Rudberg PC, Tholander F, Thunnissen MM, Haeggström JZ. (2002) Leukotriene A4 hydrolase/aminopeptidase: glutamate 271 is a catalytic residue with specific roles in two distinct enzyme mechanisms. J Biol Chem 277: 1398–1404 [DOI] [PubMed] [Google Scholar]
- Sanchez-Moran E, Jones G, Franklin F, Santos J. (2004) A puromycin-sensitive aminopeptidase is essential for meiosis in Arabidopsis thaliana. Plant Cell 16: 2895–2909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AP, Nourizadeh SD, Peer WA, Xu J, Bandyopadhyay A, Murphy AS, Goldsbrough PB. (2003) Arabidopsis AtGSTF2 is regulated by ethylene and auxin, and encodes a glutathione S-transferase that interacts with flavonoids. Plant J 36: 433–442 [DOI] [PubMed] [Google Scholar]
- Thompson JD, Gibson TJ, Higgins DG. (2002) Multiple sequence alignment using ClustalW and ClustalX. Curr Protoc Bioinformatics Chapter 2: Unit 2.3 [DOI] [PubMed] [Google Scholar]
- Titapiwatanakun B, Blakeslee JJ, Bandyopadhyay A, Yang H, Mravec J, Sauer M, Cheng Y, Adamec J, Nagashima A, Geisler M, et al. (2009) ABCB19/PGP19 stabilises PIN1 in membrane microdomains in Arabidopsis. Plant J 57: 27–44 [DOI] [PubMed] [Google Scholar]
- Towne CF, York IA, Neijssen J, Karow ML, Murphy AJ, Valenzuela DM, Yancopoulos GD, Neefjes JJ, Rock KL. (2008) Puromycin-sensitive aminopeptidase limits MHC class I presentation in dendritic cells but does not affect CD8 T cell responses during viral infections. J Immunol 180: 1704–1712 [DOI] [PubMed] [Google Scholar]
- Tsujimoto M, Mizutani S, Adachi H, Kimura M, Nakazato H, Tomoda Y. (1992) Identification of human placental leucine aminopeptidase as oxytocinase. Arch Biochem Biophys 292: 388–392 [DOI] [PubMed] [Google Scholar]
- Vazeux G, Iturrioz X, Corvol P, Llorens-Cortes C. (1998) A glutamate residue contributes to the exopeptidase specificity in aminopeptidase A. Biochem J 334: 407–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walling LL. (2006) Recycling or regulation? The role of amino-terminal modifying enzymes. Curr Opin Plant Biol 9: 227–233 [DOI] [PubMed] [Google Scholar]
- Watson RT, Hou JC, Pessin JE. (2008) Recycling of IRAP from the plasma membrane back to the insulin-responsive compartment requires the Q-SNARE syntaxin 6 but not the GGA adaptors. J Cell Sci 121: 1243–1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittig I, Braun H, Schägger H. (2006) Blue native PAGE. Nat Methods 1: 418–428 [DOI] [PubMed] [Google Scholar]
- Ye S, Chai SY, Lew RA, Albiston AL. (2007) Insulin-regulated aminopeptidase: analysis of peptide substrate and inhibitor binding to the catalytic domain. Biol Chem 388: 399–403 [DOI] [PubMed] [Google Scholar]
- Zambrisky P, Joos H, Genetello C, Leemans J, van Montagu M, Schell J. (1983) Ti plasmid vector for the introduction of DNA into plant cells without alteration of their normal regeneration capacity. EMBO J 2: 2143–2150 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.